Abstract
Both food-storing behaviour and the hippocampus change annually in food-storing birds. Food storing increases substantially in autumn and winter in chickadees and tits, jays and nutcrackers and nuthatches. The total size of the chickadee hippocampus increases in autumn and winter as does the rate of hippocampal neurogenesis. The hippocampus is necessary for accurate cache retrieval in food-storing birds and is much larger in food-storing birds than in non-storing passerines. It therefore seems probable that seasonal change in caching and seasonal change in the hippocampus are causally related. The peak in recruitment of new neurons into the hippocampus occurs before birds have completed food storing and cache retrieval for the year and may therefore be associated with spacing caches, encoding the spatial locations of caches, or creating a neuronal architecture involved in the recollection of cache sites. The factors controlling hippocampal plasticity in food-storing birds are not well understood. Photoperiodic manipulations that produce change in food-storing behaviour have no effect on either hippocampal size or neuronal recruitment. Available evidence suggests that changes in hippocampal size and neurogenesis may be a consequence of the behavioural and cognitive involvement of the hippocampus in storing and retrieving food.
Keywords: neurogenesis, hippocampus, photoperiod, memory, hoarding, caching
1. Introduction
Food storing is seasonal in most birds that exhibit the behaviour, with the greatest amount of storing occurring in autumn and winter and the least in spring and summer. Food-storing passerines, specifically chickadees and tits, jays and nutcrackers and nuthatches, are non-migratory north temperate zone residents with broad diets. For these birds, food storing occurs at a time of year when invertebrate food is scarce and most plants have completed seed production. Both the abundance and predictability of food have been shown to influence birds’ decision to cache food (Pravosudov 1985; McNamara et al. 1990; Lucas & Walter 1991; Hurly 1992; Lucas et al. 1993; Lucas 1994; Pravosudov & Grubb 1997). Food storing functions to reduce both the within-day variation in food availability and the longer term variation in food availability that occurs across the autumn, winter and early spring.
For some food-storing birds, caching is a very short-term proposition. Chickadees and tits recover much of the food they store within a few days (Cowie et al. 1981; Stevens & Krebs 1986), while leaving some for much longer periods of 40 days or more (Brodin & Ekman 1994). For Clark's nutcrackers (Nucifraga columbiana), storing occurs in autumn when pine seed is abundant. Birds create caches and then move to lower elevations for the winter. Nutcrackers do not retrieve and use this supply of cached food until they return to high elevations and begin breeding early in spring, before other sources of food are available (Tomback 1978).
Chickadees, tits and corvids recover their stored food by remembering where they have hidden their caches. For review see Shettleworth (2003), Sherry & Hoshooley (2007) and Balda et al. (1997). The hippocampus plays a crucial role in memory for the spatial locations of caches. Lesions of the hippocampus reduce the accuracy of cache retrieval to chance levels, without reducing the tendency to make caches or to search for them (Sherry & Vaccarino 1989). Hippocampal lesions in food-storing birds disrupt performance on other spatial tasks, too, without impairing the ability to form simple associations (Sherry & Vaccarino 1989; Hampton & Shettleworth 1996). The hippocampuses of food-storing and non-storing birds differ in a number of ways. Food-storing birds have larger hippocampuses than non-storing species (Krebs et al. 1989; Sherry et al. 1989) and show higher rates of neurogenesis than non-storing species (Hoshooley & Sherry 2007).
Along with seasonal changes in food-storing behaviour, there also occur seasonal changes in the brains of food-storing birds. The hippocampus undergoes change in size and neurogenesis that is correlated with the seasonal pattern in food-storing. Because the retrieval of food caches is hippocampus-dependent and because the hippocampus differs between food-storing and non-storing birds, the co-occurrence of seasonal change in the hippocampus and seasonal change in food storing raises a variety of questions about the evolutionary and developmental relations between behaviour and the brain of food-storing birds. We will review what is known about seasonal change in the hippocampus of food-storing birds, particularly the chickadees and tits, and discuss some general considerations about the function and control of seasonal plasticity in the brains of these birds.
There has been a major revision of avian brain nomenclature in recent years that reflects a new understanding of the organization of the avian brain and homology between the brains of birds and mammals (Reiner et al. 2004; Avian Brain Nomenclature Consortium 2005). Some of the brain regions we mention were renamed in this revision. The current revised name of these areas will be used with the former name given in parentheses at the first occurrence.
2. Seasonal change in food-storing behaviour
Food storing is clearly seasonal in chickadees and tits. Storing occurs at high levels in autumn and winter and low levels in spring and summer, but a more detailed examination of this pattern reveals variability in both the timing and duration of seasonal food storing (Pravosudov 2006). One of the earliest reports of seasonal food storing by chickadees described an October peak in birds storing food in Ithaca, NY (Odum 1942). This widely cited study, however, provides only a few lines of text which mention anecdotally that storing began in mid-October and continued into November. Other studies report a seasonal peak in food storing in the autumn, in both captive birds (Ludescher 1980) and in the wild (Haftorn 1956; Nakamura & Wako 1988). A comprehensive examination of available data on seasonality of food storing by chickadees and tits shows that while food storing does tend to reach maxima in autumn and early winter, the seasonal peak can span several months (Pravosudov 2006). Furthermore, some studies show a second spring peak in storing activity. It seems probable that for individual birds, food storing varies with year-to-year variation in food availability and predictability, energy expenditure, as well as social factors such as winter flock demography and dominance structure. While the data reviewed by Pravosudov (2006) show that there are indeed seasonal maxima in storing, these peaks are not always fixed in the calendar and can be quite broad, in contrast to other well-known changes in the behaviour of song birds such as the more precisely timed onset of song production and the initiation of reproductive behaviour and nesting (Tramontin & Brenowitz 2000).
3. Seasonal change in hippocampal size
Seasonal change in the size of the hippocampus in food-storing birds was first described by Smulders et al. (1995). Black-capped chickadees (Poecile atricapillus) in Ithaca, NY showed a peak in relative hippocampal size in October, at the same time of year that food storing was reported to be greatest in this population of chickadees. No seasonal change was found in two visual areas measured as control regions, the nucleus rotundus and the entopallial nucleus (ectostriatum).
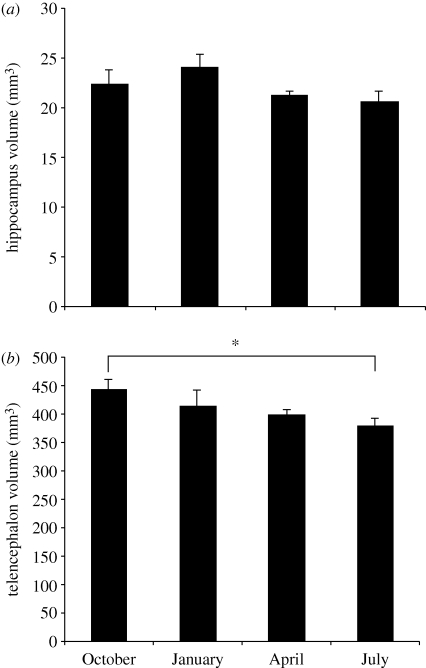
There is variability, however, in the seasonal timing of this size change in the hippocampus. In a study of black-capped chickadees collected in southern Ontario we found birds had a larger hippocampus from February to April than from October to November (Hoshooley & Sherry 2007). In another study of birds from the same population we found no seasonal difference in hippocampal size in birds collected in October, January, April and July (Hoshooley et al. 2007; see figure 1). The balance of evidence seems to indicate that in some years there is no seasonal change in total hippocampal size, while for those years in which seasonal change in hippocampal size has been reported, the greatest hippocampal size can occur in autumn (Smulders et al. 1995) or in late winter and early spring (Hoshooley & Sherry 2007). We will return to possible reasons for this variability below, after describing what is known about seasonal variation in hippocampal neurogenesis.
Figure 1.
(a) Hippocampus volume and (b) telencephalon volume in black-capped chickadees collected at four times during the year. Sample sizes are: October n = 6, January n = 5, April n = 6, July n = 7. There are no significant differences between months in hippocampal size or size of the hippocampus relative to the telencephalon. Asterisk indicates a significant difference between months (post hoc test p < 0.05). Error bars = 1 s.e.m. Adapted from Hoshooley et al. (2007).
4. Seasonal change in hippocampal neurogenesis
Seasonal change in hippocampal neurogenesis was first described by Barnea & Nottebohm (1994) in a population of chickadees in eastern New York state. Birds were given injections of tritiated thymidine, [3H]thymidine, which is incorporated into the nucleus of mitotic cells, and were then released back into the wild. Birds given [3H]thymidine in October had more labelled hippocampal cells when captured six weeks later than birds injected in August or February/March. There was no seasonal difference in the incorporation of new cells into the hyperpallium apicale (hyperstriatum accessorium), a structure lateral and adjacent to the hippocampus, indicating that the pattern in neurogenesis observed in the hippocampus did not occur throughout the hyperpallium. There was no significant difference in the total number of neurons in the hippocampus at the times of year sampled, indicating that hippocampal neurogenesis did not result in an increase in the total number of hippocampal neurons but must be, instead, part of a process of neuron replacement.
Barnea & Nottebohm (1994) also labelled dividing cells in captive chickadees in both October and May. Captive birds, like birds released into the wild, showed more new neurons in the hippocampus in October than in May, but in both groups, the number of labelled cells was about half that of birds spending the interval between [3H]thymidine injection and sacrifice in the wild. We will return to this captivity effect, below.
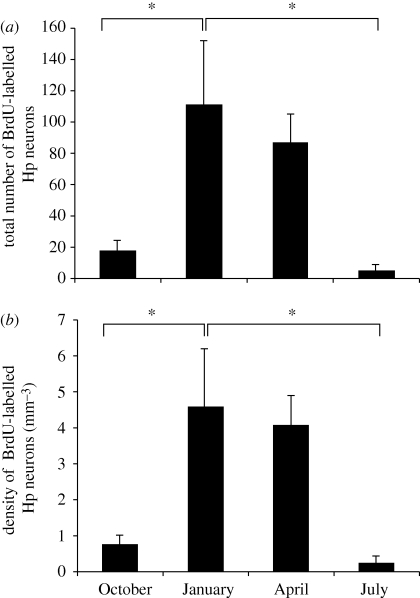
In a study of the annual cycle of food storing and hippocampal plasticity in birds collected in southern Ontario, we found that while chickadees brought into captivity in October were more likely to store food than birds captured at other times of year, the greatest number of new neurons appeared in the hippocampus in January and April with very low levels of hippocampal neurogenesis in October and July (Hoshooley et al. 2007; see figure 2). In a further study of birds taken from the same population of chickadees, relatively high levels of hippocampal neurogenesis were also observed in October/November and in February/April, with no significant difference in hippocampal neurogenesis between these two times of year (Hoshooley & Sherry 2007). The sites where chickadees were collected in the studies by Barnea & Nottebohm (1994), Smulders et al. (1995), Hoshooley et al. (2007) and Hoshooley & Sherry (2007) are all found between 41° and 43° N latitude so it is unlikely that population difference of the kind described by Roth & Pravosudov (2009) is responsible for differences observed in the timing or occurrence of seasonal hippocampal change.
Figure 2.
(a) Total number of BrdU-labelled hippocampal (Hp) cells and (b) mean density of BrdU-labelled hippocampal cells in black-capped chickadees collected at four times of year. BrdU was administered on the day following capture and birds were sacrificed 6 days later. Sample sizes as for figure 1. Month of capture significantly affects both the total number of labelled cells and the density of labelled cells (F3,17 > 5.1, p < 0.01). Asterisks indicate significant differences between months (post hoc tests p < 0.05). Error bars = 1 s.e.m. Adapted from Hoshooley et al. (2007).
5. Neurogenesis in the avian brain
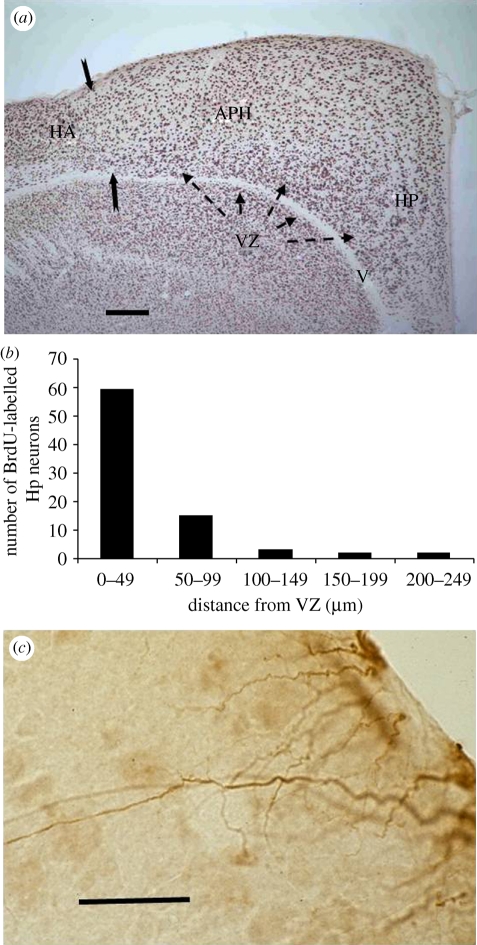
In birds, new neurons originate in a zone lining the lateral ventricles. This region is known by various names, usually the ventricular zone in birds, but in mammals the subventricular zone, periventricular zone or subependymal zone. The birth of new cells that eventually mature into neurons occurs throughout the avian ventricular zone, although there are neurogenic ‘hotspots’ with higher mitotic activity (Alvarez-Buylla et al. 1990). The ventricular zone is the only neurogenic region in the avian brain (figure 3), in contrast to the mammalian brain in which new neurons are produced in both the subventricular zone and the subgranular zone of the dentate gyrus of the hippocampus. Reptiles, like birds, have a single neurogenic region in the brain—also the ventricular zone—while fish and amphibians have multiple neurogenic regions in the adult brain. In teleost fish, over 100 neurogenic regions have been described (Lindsey & Tropepe 2006). The broad evolutionary trend in adult neurogenesis in the vertebrate brain has been a reduction in the number of neurogenic zones.
Figure 3.
(a) Black-capped chickadee hippocampus. The avian hippocampus consists of the hippocampus (HP) and the area parahippocampalis (APH). Solid arrows mark the boundary with hyperpallium apicale (HA). The ventricular zone (VZ), shown by dashed arrows, is the region of stem cell division that produces both neurons and radial glial cells. Cells are labelled for the neuronal nuclei specific protein NeuN. V, ventricle (scale bar, 200 µm). (b) Distances new neurons have travelled from the VZ six days following administration of the mitotic cell division marker BrdU, are shown in the bar graph. (c) Radial glial processes of cells labelled for the glial fibrillary acidic protein (GFAP). Glial cell bodies remain in the VZ while new neurons move along the radial processes into the hippocampus and other regions of the telencephalon (scale bar, 30 µm). (a) Adapted from Sherry & Hoshooley (2009). (b) Adapted from Hoshooley et al. (2007).
Much of what is known about the origin and migration of new neurons in the adult avian brain, from the ventricular zone where they originate to the telencephalic areas where they are later detected, comes from studies of the song control system of the canary (Alvarez-Buylla & Nottebohm 1988; Alvarez-Buylla et al. 1988, 1998; Kirn et al. 1999). The term ‘neurogenesis’ is often used loosely to refer to the process that includes the production of new cells from stem cell progenitors, the differentiation of these cells into neuronal phenotypes, their migration to target brain areas, and eventual incorporation of these new neurons into neural circuits. Some authors reserve the term ‘neuronal recruitment’ for the final inclusion of new neurons in their destination areas (e.g. Barnea & Nottebohm 1994). In any case, the generation of new neurons is clearly a complex process that involves both phenotypic transformation and spatial displacement of post-mitotic cells over a time course of days to weeks. The following summarizes the central features of neurogenesis in the avian brain.
New neurons can be identified in the adult brain in several ways. [3H]thymidine and 5-bromo-2′-deoxyuridine (BrdU) are incorporated into DNA in the cell nucleus at the S-phase of cell division, replacing thymidine during DNA synthesis, and such cells can later be identified by autoradiography or immunohistochemistry, respectively. Both of these cell division markers label cells for about 2 h after they are injected systemically (Cameron & McKay 2001)—perhaps for an even shorter time in birds (Alvarez-Buylla et al. 1990)—and so the time at which cell division occurred can be determined with some precision. When determining the time of cell division is not an objective, multiple injections are often given over a period of several hours or several days in order label a greater number of dividing cells. Other methods that do not require injection of exogenous markers are also used. The protein Ki-67 is expressed by proliferating cells during mitosis and such cells can be identified immunohistochemically (Scholzen & Gerdes 2000). These methods label dividing cells of all kinds, however, and confirming the neuronal identity of labelled cells requires an additional step. Neuronal morphology can be used (e.g. Kirn et al. 1999) as can immunohistochemical double labelling of cells for the neuronal nuclei specific protein NeuN expressed by mature neurons (e.g. Hoshooley & Sherry 2007). The protein doublecortin (DCX) is expressed by immature neurons for several weeks following cell birth and is used increasingly in studies of neurogenesis (Brown et al. 2003). Because this protein is found almost exclusively in neurons it serves as both a cell birth marker and confirmation of neuronal identity, though by its nature it cannot be used to identify new neurons once they mature.
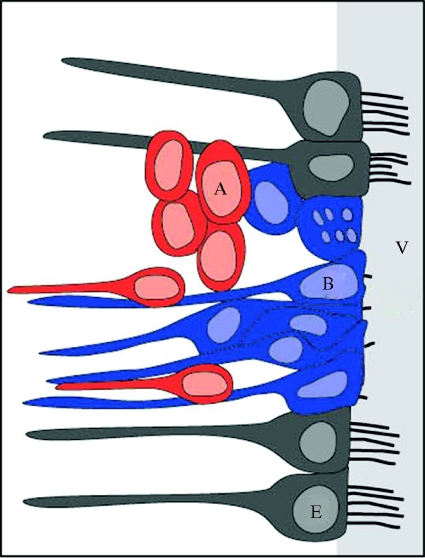
The progenitor cells producing new neurons in the avian ventricular zone are radial glia (Doetsch 2003; figure 4). These cells divide producing new cells that migrate along the radial processes of glial cells with their soma in the ventricular zone and long processes extending into the parenchyma of the adult forebrain. The radial processes of these cells are readily observable in the hippocampus of chickadees by immunohistochemically labelling for glial fibrillary acid protein (GFAP, figure 3).
Figure 4.
The ventricular zone of adult birds showing ependymal cells (E) in grey, radial glial cells (B) in blue and new neurons (A) in red. Ependymal cells have multiple cilia extending into the lateral ventricle (V) while radial glia have only a single cilium. Radial glia divide to produce new neurons which migrate along the processes of radial glial cells (elongated cells shown in red) as well as moving parallel to the ventricle (oval cells shown in red). New neurons are not in contact with the ventricle. Glia nuclei may move close to the ventricle at the time of cell division. Adapted from Doetsch (2003) based on data from Alvarez-Buylla et al. (1998) and García-Verdugo et al. (2002).
Some cells produced in the ventricular zone can be found with the morphology of young migrating neurons in HVC (higher vocal centre) as early as 3 days following labelling with [3H]thymidine (Kirn et al. 1999). There is a marked increase in the number of new neurons in HVC, however, 8–11 days following cell labelling. New neurons originating in the ventricular zone are present in the hippocampus within 7 days following cell division (Barnea & Nottebohm 1994; Hoshooley et al. 2007; see figure 3). Seven to 11 days is thus the approximate time required for a new neuron to move from the ventricular zone to its target area in the avian brain and to differentiate into a neuronal phenotype. Fifteen to 18 days after cell birth, some HVC neurons have established connections with area RA (robust nucleus of the archistriatum, Kirn et al. 1999). By 22 days following labelling, however, the number of new HVC neurons has decreased by half, a process of attrition similar to that occurring early in brain development.
Studies in which birds are sacrificed at varying intervals following injection of cell birth markers such as BrdU and [3H]thymidine thus provide snapshots of different stages of the neurogenic sequence. In one study, new neurons were present in the hippocampus six days following BrdU labelling at various distances from the ventricle (figure 3) but most were within 50 µm from their probable birthplace (Hoshooley et al. 2007). Six weeks following labelling with [3H]thymidine, new hippocampal neurons were found an average 165 µm from the ventricle, though distributed at a range of distances in a broad band parallel to the ventricle (Barnea & Nottebohm 1996). Short survival times following cell labelling probably give a picture of migration and early recruitment in progress while longer survival times show the final destination of postmigratory neurons.
The role of radial glia as neural stem cells in the avian brain is somewhat surprising but parallels the neural stem cell role of glia in the mammalian subventricular and subgranular zones (Doetsch 2003). Since these cells occur throughout the brain, one question of current interest is whether the ventricular, subventricular and subgranular regions are specialized niches that allow cells to act as neural progenitors (Miller & Gauthier-Fisher 2009). In mammals, neural stem cells in the subventricular zone are in contact with both blood vessels and the ventricle and can thus respond to chemical signals from many different sources. Avian neural stem cells, similarly, are in direct contact with the ventricle, extend a single cilium into the ventricle, and are in close contact with ependymal cells possessing multiple ventricular cilia (figure 4). In the mammalian subventricular zone, ependymal cells synthesize proteins that affect cell division and differentiation (Miller & Gauthier-Fisher 2009). The organization of the avian ventricular zone, coupled with current knowledge of the relation between behaviour and adult neurogenesis in birds, may provide an opportunity to better understand the regulation of neural stem cell activity in general.
6. Photoperiodic effects on food storing
Given the seasonality of both food storing and hippocampal plasticity, it is reasonable to suppose that such circannual changes in behaviour and the hippocampus might be under photoperiodic control, as is the case for annual change in reproductive behaviour, song and the song control nuclei of passerines. There have been a number of attempts to induce change in food storing and the hippocampus by manipulation of photoperiod. Shettleworth et al. (1995) found that exposing chickadees taken from the wild in March to long days (14L : 10D followed by 16L : 8D) and warm temperatures (22°C) produced higher levels of food storing after 6–8 weeks than holding birds on short days (8D : 16L) at lower temperatures (17°C). After a period of 12 weeks, however, birds held on short days increased storing to near the level found in long day birds. Subsequent observation of these two groups when tested under identical conditions of day length and temperature suggested that photoperiodic history, rather than day length and temperature at the time of testing determined the level of storing. Shettleworth et al. (1995) interpreted these results as showing that for birds captured in March, a period of long days simulates summer conditions and leads eventually to the initiation of storing as would be expected to occur in autumn. The occurrence of moult in the long day group but not in the short day group in this experiment supports this interpretation. In a further experiment, Shettleworth et al. (1995) repeated a similar photoperiodic manipulation with birds captured in November. They found that storing did not differ between the short day and long day groups but from the beginning of the study birds in both groups stored at a higher level than observed in birds taken from the wild in March. Season of capture was shown in this way to have a major effect on food storing, greater than any effect produced in captivity by manipulation of day length and temperature.
Day length and time available for feeding were varied together in the experiments of Shettleworth et al. (1995) described above. Although the availability of storable sunflower seeds was equated for the groups, long day length meant a longer period of daylight in which to feed on the standard diet available to all birds. The amount of time available for feeding may have influenced fat deposition, body weight, and hence the amount of food stored each day, without there being a direct photoperiodic effect on food-storing behaviour. Karpouzos et al. (2005) separated the effects of day length and time available for feeding by placing birds on short days (9L : 15D), long days (15L : 9D) or a long day photoperiod (15L : 9D) but with only 9 h of food availability. Birds in the latter group thus had a longer daylight period but the same number of hours in which to feed as birds on the short day photoperiod. Although these manipulations had significant effects on body mass and body fat, they had no effect on food storing. Birds on short days, with 9 h in which to feed, reached higher body masses than birds on long days with 15 h in which to feed. Birds on long days with 9 h in which to feed maintained body masses intermediate between the other two groups. The two groups with only 9 h in which to feed had significantly higher fat scores than the long day group. Nevertheless, food storing showed a pattern of gradual increase during the five weeks of the experiment in all groups with no differences between the groups.
7. Photoperiodic effects on the hippocampus
Do manipulations of photoperiod that produce changes in food-storing behaviour also cause changes in the hippocampus? Krebs et al. (1995) performed manipulations of day length similar to those performed by Shettleworth et al. (1995) described above. Chickadees and non-storing house sparrows (Passer domesticus), both captured in March, were placed on either long (16L : 8D) or short (8L : 16D) days. After several months on long days, both chickadees and house sparrows began to moult. After they had experienced several months on long days, chickadees in the long day group also began to store more food than birds kept on short days. The timing of moult and the difference in food storing observed between the groups of chickadees on long and short days were comparable to those observed by Shettleworth et al. (1995). There was no effect of the day length manipulation, however, on the size of the hippocampus in either species. Manipulations of photoperiod that clearly produce eventual changes in food storing—changes of the kind that would be expected to occur at the end of the summer after an extended period on long days—had no detectable effect on the size of the hippocampus.
It is possible that in these studies manipulations of photoperiod were performed with birds that were not in a photosensitive state, ready to respond to changes in day length. Birds’ responses to day length depend not only on the photoperiod they are exposed to, but also on their ability to respond to changes in day length (Dawson et al. 2001). Birds exposed to long summer days, for example, cease to respond to long days with gonadal recrudescence, reproductive behaviour and song and instead enter a period of photorefractoriness. Exposure to short days is necessary to re-establish photosensitivity. MacDougall-Shackleton et al. (2003) therefore compared food storing and hippocampal size in three groups of black-capped chickadees captured in October and November. A photorefractory group was maintained on long days (19L : 5D) simulating summer conditions. A photosensitive group was maintained on short days (8.75L : 15.25D) simulating winter conditions. A subset of these photosensitive birds were then photostimulated by exposure to long days (19L : 5D) simulating spring conditions. Photostimulated males underwent a very large increase in gonad size, confirming that the birds responded physiologically to this manipulation of photoperiod. Females also showed a gonadal response, with greatest ovary size in photostimulated birds, intermediate ovary size in photosensitive birds and smallest ovary size in photorefractory birds, as would be expected to occur in females in the wild. Photorefractory birds exhibited very little food storing, compared with the other two groups. Photosensitive birds continued to store food at elevated levels throughout the experiment while photostimulated birds abruptly decreased the amount of food they stored when photostimulated, as would be expected to occur in chickadees in the wild in spring. There was no change, however, in hippocampal size in any of these groups (MacDougall-Shackleton et al. 2003).
In a subsequent experiment, manipulations of photoperiod that simulated autumn conditions were found to have no effect on the recruitment of new neurons into the hippocampus (Hoshooley et al. 2005). In this experiment, all chickadees were initially maintained on short days (8L : 16D) then transferred to long days, initially (24L : 0D) followed by (15L : 9D), simulating a summer photoperiod. After 71 days, when moult indicated these birds had become photorefractory, some were transferred to short days (8L : 16D), simulating autumn conditions, while controls remained on long (15L : 9D) days. There were no differences in the recruitment of new neurons into the hippocampus among these groups, nor was there any difference among the groups in hippocampal size. The manipulations of photoperiod were not without effect, however, because there was some indication of greater recruitment of new neurons into the hyperpallium apicale adjacent to the hippocampus (Hoshooley et al. 2005).
The general conclusion that emerges from these studies, each examining a different aspect of photoperiodic responsiveness in chickadees and simulating different seasonal conditions, is that while the frequency of food storing can be changed by manipulations of photoperiod, the size of the hippocampus and the recruitment of new neurons into the hippocampus cannot. This is in contrast to field studies that report seasonal changes in both hippocampal size and neuronal recruitment in the wild. Several reasons for this difference between field and laboratory studies have been proposed. One possibility is that the hippocampus, unlike the song control nuclei (Tramontin & Brenowitz 2000), does not respond to changes in photoperiod by a change in size, or in the frequency of neurogenesis, either in the field or in the laboratory. Field studies in which birds are sampled from a wild population at different times of year may not give a portrait of seasonal change in the hippocampus within individuals. Instead, changes in the hippocampus between samples may be due to differential survival in the wild of birds that differ in hippocampal anatomy or to changes in the age structure of the population being sampled. The variability in the annual timing of food storing in the wild, described above, supports the idea that food storing, unlike reproductive behaviour in song birds, is not a circannual cycle controlled by photoperiod.
Alternatively, it is possible that photoperiod does drive changes in hippocampal size and neurogenesis in the wild, but these changes are not observed in captivity, for several reasons. A ‘captivity effect’ on hippocampal size and neuronal recruitment has been reported in a number of studies, the latter not only in the hippocampus of birds (Barnea & Nottebohm 1994; Smulders et al. 2000; Hoshooley & Sherry 2004; LaDage et al. 2009) but also in structures such as the olfactory pathway of the shore crab Carcinus maenas (Hansen & Schmidt 2004). Birds held in captivity have lower rates of neurogenesis than wild caught birds (Barnea & Nottebohm 1994), and the production of new hippocampal neurons is negatively correlated with time in captivity over a 5–20 day period (Hoshooley & Sherry 2004). Experience, activity and enriched environments have positive effects on hippocampal neurogenesis (Kempermann et al. 1998; Gould et al. 1999; van Praag et al. 2005). The hippocampus may, thus, change in response to photoperiod, but only indirectly, as a consequence of a substantial change in food storing behaviour induced by change in day length and perhaps other seasonal factors. In captivity, it may not be possible for birds to engage in enough food storing and cache retrieval to produce the changes in hippocampal size and neurogenesis observed in the wild.
8. Hippocampal neurogenesis in storing and non-storing birds
Although the timing of the appearance of new neurons in the adult chickadee hippocampus is correlated with the onset of food storing, there are many changes occurring in the chickadee's environment and in the behaviour of chickadees in autumn. The social system changes, from breeding pairs that have just completed raising young to winter flocks of 6–8 individuals that defend a shared winter territory (Smith 1991). Home range expands because the winter flock territory is about 3 times the size of the breeding territory. Diet also changes in the autumn, as the proportion of invertebrate food in the diet declines. Furthermore, the appearance of the habitat can change dramatically as deciduous trees lose their leaves and snow covers the ground and tree branches. Other non-migratory residents of the north temperate zone are experiencing the same seasonal changes, however, and many of these birds do not store food. We therefore compared the annual cycle of hippocampal neurogenesis in food-storing chickadees with that of the house sparrow (Passer domesticus). House sparrows, like chickadees, form dominance-structured winter flocks (Smith 1991; Lowther & Cink 1992). The proportion of invertebrate food in the house sparrow diet, though normally lower than that of chickadees, shows an autumn decline parallel to that which occurs in chickadees (Smith 1991; Lowther & Cink 1992). The home range size of both chickadees and house sparrows increases, neither species is migratory, and house sparrows are found throughout all but the extreme northwest part of the black-capped chickadee's geographical range.
We administered the cell bird marker BrdU (5-bromo-2′-deoxyuridine), which, like [3H]thymidine, labels mitotic cells, to chickadees and house sparrows in autumn (October–November) and spring (February–April). Birds were captured and held throughout the study in large outdoor aviaries exposed to natural temperatures and photoperiod. Birds were given 6 BrdU injections over a 2-day period beginning on the first day following capture and sacrificed six weeks later.
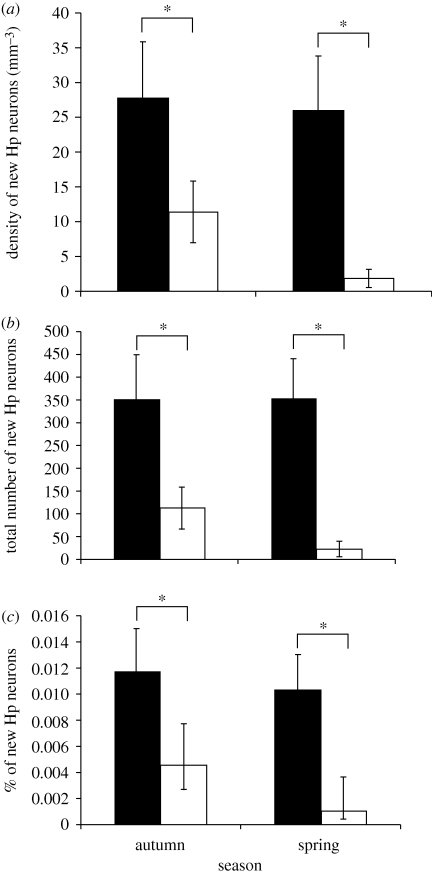
Chickadees showed significantly more neuronal recruitment in the hippocampus than found in house sparrows in both spring and autumn measured as the total number of new hippocampal neurons, the density of new neurons per mm3, and the percentage of hippocampal neurons that were new (figure 5). There was no effect of season, however, and no interaction between season and species, showing that neuronal recruitment was greater in chickadees than in house sparrows in both autumn and spring with no indication of seasonal change in recruitment in either chickadees or house sparrows. Double-labelling with NeuN (neuronal nuclei-specific protein) in a subset of eight birds showed that 32 per cent of all BrdU-labelled cells co-expressed this neuronal marker and this percentage was the same for both chickadees and house sparrows. Counts of NeuN-labelled neurons were highly correlated with counts performed using strictly morphological criteria of neuronal identity (r = 0.908, p < 0.001, n = 5). In the hyperpallium apicale laterally adjacent to the hippocampus there was a low level of neuronal recruitment that did not differ between species and showed no effect of season.
Figure 5.
More new neurons are found in the chickadee hippocampus (solid bar) than in the house sparrow hippocampus (open bar) in both spring and autumn. There was no difference between autumn and spring in hippocampal neurogenesis in chickadees in this study. (a) The density of new hippocampal neurons per mm3, (b) the number of new hippocampal neurons and (c) the per cent of all hippocampal neurons that are new. Birds received BrdU the day following capture and were sacrificed 6 weeks later. Sample sizes are: autumn chickadees, n = 9; autumn house sparrows, n = 5; spring chickadees, n = 6; spring house sparrows, n = 4. For differences between species on all measures, F1,20 > 6.3, p < 0.02. Asterisks indicate significant differences by post hoc tests p < 0.05. Error bars = ±1 s.e.m. Adapted from Hoshooley & Sherry (2007).
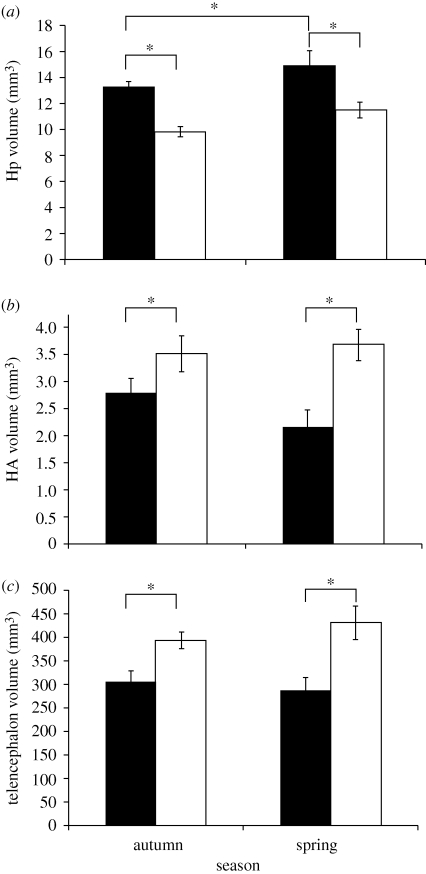
The total number of neurons in the chickadee hippocampus was greater than in the house sparrow, not surprisingly because the chickadee hippocampus is larger (figure 6), despite the larger body size of house sparrows: a mean body weight of 27 g compared with 11 g for chickadees. The chickadee hippocampus thus incorporates new neurons at a substantially higher rate than the hippocampus of the house sparrow. Because both species experience similar changes in social system, home range size, diet and appearance of their habitat—but only chickadees store food—we conclude that this higher rate of hippocampal neurogenesis is associated in some way with food-storing behaviour. This is not, of course, the ideal comparative analysis. Chickadees and house sparrows belong to different families and differ in more ways than food storing. The ideal comparative analysis of the relation between food storing and hippocampal neurogenesis would include many species, both food-storing and non-food-storing and control for phylogeny (Felsenstein 1985; Harvey & Purvis 1991; Sherry 2006). The results of the comparison of black-capped chickadees and house sparrows are suggestive, however, and indicate that a broader comparative analysis of hippocampal neurogenesis in birds would be informative.
Figure 6.
(a) The black-capped chickadee hippocampus (solid bar) is larger than the house sparrow hippocampus (open bar) in both autumn and spring. (b) House sparrows are larger birds with larger brains and thus have a larger hyperpallium apicale (HA) and (c) telencephalon than chickadees. Chickadees, in this study, also had a larger hippocampus in spring than in autumn (a). Samples sizes as for figure 5. Differences betweens species for all three brain areas F1,20 > 12.4, p < 0.002. Asterisks indicate significant differences by post hoc tests, p < 0.05. Error bars = ±1 s.e.m. Adapted from Hoshooley & Sherry (2007).
9. Summing up
What, then, do we know about the seasonal variation in the hippocampus and its relation to seasonal change in food storing? The basic facts are that food storing is elevated in autumn and winter and sometimes has a subsequent spring peak. This maximum can be a broad plateau spanning several months and its exact timing can vary from year to year. The hippocampus is essential for the accurate recovery of caches (Sherry & Vaccarino 1989). The hippocampus also differs in a number of ways between food-storing and non-food-storing birds. Among others, it is both larger and shows greater hippocampal neurogenesis than the hippocampus of non-storing species. Hippocampal size may vary seasonally in food-storing birds and the available evidence indicates that when a seasonal maximum is observed it occurs sometime in the autumn and winter. Seasonal variation in hippocampal neurogenesis is more reliably observed than change in total hippocampal size and also exhibits an autumn or winter maximum, but for neurogenesis, too, there is variation in timing of the seasonal maximum. Given this basic information, what can we reasonably suppose the hippocampus of food-storing birds does, and what does seasonal change in the hippocampus indicate?
If we assume that the hippocampus serves some function that makes food storing advantageous for chickadees, tits and other food-storing birds, what, exactly, might the hippocampus do? It may help food-storing birds space their caches apart to reduce pilfering by other animals (Male & Smulders 2007). Food-storing chickadees and tits disperse their caches quite widely within their home range (Cowie et al. 1981). One way to space caches apart is to remember where earlier caches were put, and the hippocampus may play a role in doing this. Once caches are in place, the hippocampus could play no further role in the food-storing strategy. According to this view, caches could be retrieved by some mechanism that does not require memory (Brodin & Clark 1997; Smulders & Dhondt 1997) and so neurogenic activity and perhaps total size of the hippocampus decreases when food storing begins to decline.
Another possibility is that the hippocampus is important in remembering the locations of caches but its role is encoding, not the retrieval of information from memory about the spatial locations of cache. The hippocampus undergoes neuronal recruitment, and perhaps a size increase, in autumn and winter because that is when caching happens. The hippocampus is not needed for cache retrieval in this scenario and so neurogenesis subsides and the structure decreases in size once food caching declines.
Finally, the hippocampus may be required for both encoding and retrieving information about the locations of caches. This can occur any time from early autumn to late spring and so hippocampal neurogenesis during autumn or winter may create the neuronal architecture that encodes information about cache locations and later retrieves this information from memory. Autumn neurogenesis, and perhaps an increase in hippocampal size, builds a hippocampus that deals with the large numbers of caches that are to be created and retrieved over the ensuing months until invertebrate food is once again available and breeding begins. None of these ideas can be ruled out by the available evidence, but all can be tested using a variety of experimental methods in the field and in the laboratory. The hippocampus of birds and mammals is an ancient structure (Rodríguez et al. 2002) that exhibits diversity in both organization and function (Bingman et al. 2006; Pravosudov et al. 2006; Smulders 2006; Spencer et al. 2008; Eichenbaum & Fortin 2009). Research on seasonal variation in the hippocampus of food-storing birds may help us better understand the evolution of this complex structure.
Acknowledgements
All research was carried out under Canadian Wildlife Service Scientific Collection Permit CA-0031 and University of Western Ontario Animal Use Protocols 2002-084-8 and 2007-001-03.
We would like to thank the members of the Advanced Facility for Avian Research, supported by the Canada Foundation for Innovation, the Ontario Research Fund and the University of Western Ontario, for their many helpful comments and suggestions. This research was supported by Discovery Grants from the Natural Sciences and Engineering Research Council of Canada to DFS and a Canadian Institutes of Health Research Doctoral Fellowship to JSH.
Footnotes
One contribution of 10 to a Theme Issue ‘Integrating ecology, psychology and neurobiology within a food-hoarding paradigm’.
References
- Alvarez-Buylla A., Nottebohm F.1988Migration of young neurons in adult avian brain. Nature 335, 353–354 (doi:10.1038/335353a0) [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Theelen M., Nottebohm F.1988Mapping of radial glia and of a new cell type in adult canary brain. J. Neurosci. 8, 2707–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Theelen M., Nottebohm F.1990Proliferation ‘hotspots’ in adult avian ventricular zone reveal radial cell division. Neuron 5, 101–109 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., García-Verdugo J. M., Mateo A. S., Merchant-Larios H.1998Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J. Neurosci. 18, 1020–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avian Brain Nomenclature Consortium 2005Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 6, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda R. P., Kamil A. C., Bednekoff P. A.1997Predicting cognitive capacities from natural history. Curr. Ornithol. 13, 333–366 [Google Scholar]
- Barnea A., Nottebohm F.1994Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl Acad. Sci. USA 91, 11 217–11 221 (doi:10.1073/pnas.91.23.11217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A., Nottebohm F.1996Recruitment and replacement of hippocampal neurons in young and adult chickadees: an addition to the theory of hippocampal learning. Proc. Natl Acad. Sci. USA 93, 714–718 (doi:10.1073/pnas.93.2.714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingman V. P., Siegel J. J., Gagliardo A., Erichsen J. T.2006Representing the richness of avian spatial cognition: properties of a lateralized homing pigeon hippocampus. Rev. Neurosci. 17, 17–28 [DOI] [PubMed] [Google Scholar]
- Brodin A., Clark C. W.1997Long-term hoarding in the Paridae: a dynamic model. Behav. Ecol. 8, 178–185 (doi:10.1093/beheco/8.2.178) [Google Scholar]
- Brodin A., Ekman J.1994Benefits of food hoarding. Nature 372, 510 (doi:10.1038/372510a0) [Google Scholar]
- Brown J. P., Couillard-Després S., Cooper-Kuhn C. M., Winkler J., Aigner L., Kuhn H. G.2003Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10 (doi:10.1002/cne.10874) [DOI] [PubMed] [Google Scholar]
- Cameron H. A., McKay R. D. G.2001Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435, 406–417 (doi:10.1002/cne.1040) [DOI] [PubMed] [Google Scholar]
- Cowie R. J., Krebs J. R., Sherry D. F.1981Food storing by marsh tits. Anim. Behav. 29, 1252–1259 (doi:10.1016/S0003-3472(81)80077-2) [Google Scholar]
- Dawson A., King V. M., Bently G. E., Ball G. F.2001Photoperiodic control of seasonality in birds. J. Biol. Rhythm 16, 365–380 (doi:10.1177/074873001129002079) [DOI] [PubMed] [Google Scholar]
- Doetsch F.2003The glial identity of neural stem cells. Nat. Neurosci. 6, 1127–1134 (doi:10.1038/nn1144) [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Fortin N. J.2009The neurobiology of memory based predictions. Phil. Trans. R. Soc. B 364, 1183–1191 (doi:10.1098/rstb.2008.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J.1985Phylogenies and the comparative method. Am. Nat. 125, 1–15 (doi:10.1086/284325) [Google Scholar]
- García-Verdugo J. M., Ferrón S., Flames N., Collado L., Desfilis E., Font E.2002The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res. Bull. 57, 165–775 [DOI] [PubMed] [Google Scholar]
- Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J.1999Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265 (doi:10.1038/6365) [DOI] [PubMed] [Google Scholar]
- Haftorn S.1956Contribution to the food biology of tits especially about storing of surplus food. Part IV. A comparative analysis of Parus atricapillus L., P. cristatus L. and P. ater L. Det Kgl Norske Videnskabers Selskabs Skrifter 4, 1–54 [Google Scholar]
- Hampton R. R., Shettleworth S. J.1996Hippocampal lesions impair memory for location but not color in passerine birds. Behav. Neurosci. 110, 831–835 (doi:10.1037/0735-7044.110.4.831) [DOI] [PubMed] [Google Scholar]
- Hansen A., Schmidt M.2004Influence of season and environment on adult neurogenesis in the central olfactory pathway of the shore crab, Carcinus maenas. Brain Res. 1025, 85–97 (doi:10.1016/j.brainres.2004.08.001) [DOI] [PubMed] [Google Scholar]
- Harvey P. H., Purvis A.1991Comparative methods for explaining adaptation. Nature 351, 619–624 (doi:10.1038/351619a0) [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Sherry D. F.2004Neuron production, neuron number, and structure size are seasonally stable in the hippocampus of the food-storing black-capped chickadee (Poecile atricapillus). Behav. Neurosci. 118, 345–355 (doi:10.1037/0735-7044.118.2.345) [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Sherry D. F.2007Greater hippocampal neuronal recruitment in food-storing than in non-food-storing birds. Dev. Neurobiol. 67, 406–414 (doi:10.1002/dneu.20316) [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Phillmore L. S., MacDougall-Shackleton S. A.2005An examination of avian hippocampal neurogenesis in relationship to photoperiod. Neuroreport 16, 987–991 (doi:10.1097/00001756-200506210-00021) [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Phillmore L. S., Sherry D. F., MacDougall-Shackleton S. A.2007Annual cycle of the black-capped chickadee: seasonality of food-storing and the hippocampus. Brain Behav. Evol. 69, 161–168 (doi:10.1159/000096984) [DOI] [PubMed] [Google Scholar]
- Hurly T. A.1992Energetic reserves of marsh tits (Parus palustris): food and fat storage in response to variable food-supply. Behav. Ecol. 3, 181–188 (doi:10.1093/beheco/3.2.181) [Google Scholar]
- Karpouzos H., Hernandez A. M., MacDougall-Shackleton E. A., MacDougall-Shackleton S. A.2005Effects of day-length and food availability on food caching, mass and fat reserves in black-capped chickadees (Poecile atricapillus). Physiol. Behav. 84, 465–469 (doi:10.1016/j.physbeh.2005.01.012) [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G., Gage F. H.1998Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 18, 3206–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn J. R., Fishman Y., Sasportas K., Alvarez-Buylla A., Nottebohm F.1999Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J. Comp. Neurol. 411, 487–494 (doi:10.1002/(SICI)1096-9861(19990830)411:3<487::AID-CNE10>3.0.CO;2-M) [PubMed] [Google Scholar]
- Krebs J. R., Sherry D. F., Healy S. D., Perry V. H., Vaccarino A. L.1989Hippocampal specialization of food-storing birds. Proc. Natl Acad. Sci. USA 86, 1388–1392 (doi:10.1073/pnas.86.4.1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs J. R., Clayton N. S., Hampton R. R., Shettleworth S. J.1995Effects of photoperiod on food-storing and the hippocampus in birds. Neuroreport 6, 1701–1704 [DOI] [PubMed] [Google Scholar]
- LaDage L. D., Roth T. C., Fox R. A., Pravosudov V. V.2009Effects of captivity and memory-based experiences on the hippocampus in mountain chickadees. Behav. Neurosci. 123, 284–291 (doi:10.1037/a0014817) [DOI] [PubMed] [Google Scholar]
- Lindsey B. W., Tropepe V.2006A comparative framework for understanding the biological principles of adult neurogenesis. Progr. Neurobiol. 80, 281–307 (doi:10.1016/j.pneurobio.2006.11.007) [DOI] [PubMed] [Google Scholar]
- Lowther P. E., Cink C. L.1992House sparrow. In The birds of North America No. 12 (eds Poole A., Stettenheim P., Gill F.), 20pp Philadelphia, PA/Washington, DC: The Academy of Natural Sciences/The American Ornithologists' Union [Google Scholar]
- Lucas J. R.1994Regulation of cache stores and body-mass in Carolina chickadees (Parus carolinensis). Behav. Ecol. 5, 171–181 (doi:10.1093/beheco/5.2.171) [Google Scholar]
- Lucas J. R., Walter L. R.1991When should chickadees hoard? Theory and experimental results. Anim. Behav. 41, 579–601 (doi:10.1016/S0003-3472(05)80898-X) [Google Scholar]
- Lucas J. R., Peterson L. J., Boudinier R. L.1993The effects of time constraints and changes in body mass and satiation on the simultaneous expression of caching and diet-choice decisions. Anim. Behav. 45, 639–658 (doi:10.1006/anbe.1993.1080) [Google Scholar]
- Ludescher B.1980Fressen und Verstecken von Sämereien be der Weidenmeise Parus montanus im Jahresverlauf unter konstanten Ernährungsbedingungen. Ökologie der Vögel 2, 135–144 [Google Scholar]
- MacDougall-Shackleton S. A., Sherry D. F., Clark A. P., Pinkus R., Hernandez A. M.2003Photoperiodic regulation of food-storing and hippocampus volume in black-capped chickadees Poecile atricapilla. Anim. Behav. 65, 805–812 (doi:10.1006/anbe.2003.2113) [Google Scholar]
- Male L. H., Smulders T. V.2007Memory for food caches: not just for retrieval. Behav. Ecol. 18, 456–459 (doi:10.1093/beheco/arl107) [Google Scholar]
- McNamara J. M., Houston A. I., Krebs J. R.1990Why hoard? The economics of food storing in tits, Parus spp. Behav. Ecol. 1, 12–23 (doi:10.1093/beheco/1.1.12) [Google Scholar]
- Miller F. D., Gauthier-Fisher A.2009Home at last: neural stem cell niches defined. Cell Stem Cell 4, 507–510 (doi:10.1016/j.stem.2009.05.008) [DOI] [PubMed] [Google Scholar]
- Nakamura H., Wako Y.1988Food storing behaviour of willow tit Parus montanus. J. Yamashina Inst. Ornithol. 20, 1–20 [Google Scholar]
- Odum E. P.1942Annual cycle of the black-capped chickadee—3. Auk 59, 499–531 [Google Scholar]
- Pravosudov V. V.1985Search for and storage of food by Parus cinctus Lapponicus and P. montanus borealis (Paridae). Zool. Zh. 64, 1036–1043 [Google Scholar]
- Pravosudov V. V.2006On seasonality in food-storing behaviour in parids: do we know the whole story? Anim. Behav. 71, 1455–1460 (doi:10.1016/j.anbehav.2006.01.006) [Google Scholar]
- Pravosudov V. V., Grubb T. C.1997Management of fat reserves and food caches in tufted titmice (Parus bicolor) in relation to unpredictable food supply. Behav. Ecol. 8, 332–339 (doi:10.1093/beheco/8.3.332) [Google Scholar]
- Pravosudov V. V., Kitaysky A. S., Omanska A.2006The relationship between migratory behaviour, memory and the hippocampus: an intraspecific comparison. Proc. R. Soc. B 273, 2641–2649 (doi:10.1098/rspb.2006.3624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A., et al. 2004Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 475, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez F., López J. C., Vargas J. P., Broglio C., Gómez Y., Salas C.2002Spatial memory and hippocampal pallium through vertebrate evolution: insights from reptiles and teleost fish. Brain Res. Bull. 57, 499–503 (doi:10.1016/S0361-9230(01)00682-7) [DOI] [PubMed] [Google Scholar]
- Roth T. C., Pravosudov V. V.2009Hippocampal volumes and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc. R. Soc. B 276, 401–405 (doi:10.1098/rspb.2008.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T., Gerdes J.2000The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182, 311–322 (doi:10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9) [DOI] [PubMed] [Google Scholar]
- Sherry D. F.2006Neuroecology. Annu. Rev. Psychol. 57, 167–197 (doi:10.1146/annurev.psych.56.091103.070324) [DOI] [PubMed] [Google Scholar]
- Sherry D. F., Hoshooley J. S.2007Neurobiology of spatial behavior. In The ecology and behavior of chickadees and titmice: an integrated approach (ed. Otter K. A.), pp. 9–23 Oxford, UK: Oxford University Press [Google Scholar]
- Sherry D. F., Hoshooley J. S.2009The seasonal hippocampus of food-storing birds. Behav. Process. 80, 334–338 (doi:10.1016/j.beproc.2008.12.012) [DOI] [PubMed] [Google Scholar]
- Sherry D. F., Vaccarino A. L.1989Hippocampus and memory for food caches in black-capped chickadees. Behav. Neurosci. 103, 308–318 (doi:10.1037/0735-7044.103.2.308) [Google Scholar]
- Sherry D. F., Vaccarino A. L., Buckenham K., Herz R. S.1989The hippocampal complex of food-storing birds. Brain Behav. Evolut. 34, 308–317 (doi:10.1159/000116516) [DOI] [PubMed] [Google Scholar]
- Shettleworth S. J.2003Memory and hippocampal specialization in food-storing birds: challenges for research on comparative cognition. Brain Behav. Evolut. 62, 108–116 (doi:10.1159/000072441) [DOI] [PubMed] [Google Scholar]
- Shettleworth S. J., Hampton R. R., Westwood R. P.1995Effects of season and photoperiod on food storing by black-capped chickadees, Parus atricapillus. Anim. Behav. 49, 989–998 (doi:10.1006/anbe.1995.0128) [Google Scholar]
- Smith S. M.1991The black-capped chickadee Ithaca, NY: Cornell University Press [Google Scholar]
- Smulders T. V.2006A multi-disciplinary approach to understanding hippocampal function in food-hoarding birds. Rev. Neurosci. 17, 53–69 [DOI] [PubMed] [Google Scholar]
- Smulders T. V., Dhondt A. A.1997How much memory do tits need? Trends Ecol. Evol. 12, 417–418 (doi:10.1016/S0169-5347(97)01200-7) [DOI] [PubMed] [Google Scholar]
- Smulders T. V., Sasson A. D., DeVoogd T. J.1995Seasonal variation in hippocampal volume in a food-storing bird, the black-capped chickadee. J. Neurobiol. 27, 15–25 (doi:10.1002/neu.480270103) [DOI] [PubMed] [Google Scholar]
- Smulders T. V., Casto J. M., Nolan V., Ketterson E. D., DeVoogd T. J.2000Effects of captivity and testosterone on the volumes of four brain regions in the dark-eyed junco (Junco hyemalis). J. Neurobiol. 43, 244–253 (doi:10.1002/(SICI)1097-4695(20000605)43:3<244::AID-NEU3>3.0.CO;2-#) [PubMed] [Google Scholar]
- Spencer J. L., Waters E. M., Romeo R. D., Wood G. E., Milner T. A., McEwen B. S.2008Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocrinol. 29, 219–237 (doi:10.1016/j.yfrne.2007.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. A., Krebs J. R.1986Retrieval of stored seeds by marsh tits Parus palustris in the field. Ibis 128, 513–525 (doi:10.1111/j.1474-919X.1986.tb02703.x) [Google Scholar]
- Tomback D. F.1978Foraging strategies of Clark's nutcracker. Living Bird 16, 123–161 [Google Scholar]
- Tramontin A. D., Brenowitz E. A.2000Seasonal plasticity in the adult brain. Trends. Neurosci. 23, 251–258 (doi:10.1016/S0166-2236(00)01558-7) [DOI] [PubMed] [Google Scholar]
- van Praag H., Shubert T., Zhao C. M., Gage F. H.2005Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 38, 8680–8685 [DOI] [PMC free article] [PubMed] [Google Scholar]