Abstract
GRP94, the endoplasmic reticulum Hsp90, is a metazoan-restricted chaperone essential for early development in mammals, yet dispensable for mammalian cell viability. This dichotomy suggests that GRP94 is required for the functional expression of secretory and/or membrane proteins that enable the integration of cells into tissues. To explore this hypothesis, we have identified the Drosophila ortholog of GRP94, Gp93, and report that Gp93 is an essential gene in Drosophila. Loss of zygotic Gp93 expression is late larval lethal and causes prominent defects in the larval midgut, the sole endoderm-derived larval tissue. Gp93 mutant larvae display pronounced defects in the midgut epithelium, with aberrant copper cell structure, markedly reduced gut acidification, atypical septate junction structure, depressed gut motility, and deficits in intestinal nutrient uptake. The metabolic consequences of the loss of Gp93-expression are profound; Gp93 mutant larvae exhibit a starvation-like metabolic phenotype, including suppression of insulin signaling and extensive mobilization of amino acids and triglycerides. The defects in copper cell structure/function accompanying loss of Gp93 expression resemble those reported for mutations in labial, an endodermal homeotic gene required for copper cell specification, and α-spectrin, thus suggesting an essential role for Gp93 in the functional expression of secretory/integral membrane protein-encoding lab protein target genes and/or integral membrane protein(s) that interact with the spectrin cytoskeleton to confer epithelial membrane specialization.
Keywords: Drosophila, Gp93, Hsp90, GRP94, HSP90B1, copper cell, midgut, epithelium, endoderm, growth control
Introduction
Hsp90 chaperones regulate the conformational state of proteins functioning in growth control, signaling, and development (Pratt and Toft, 2003; Rutherford et al., 2007; Wandinger et al., 2008). In this capacity, Hsp90 serves critical roles in the maintenance and expression of polymorphic signaling pathway variants that influence morphogenesis and survival (Rutherford et al., 2007; Whitesell and Lindquist, 2005). Metazoans, but not lower eukaryotes, express a paralog of Hsp90, GRP94 (HSP90B1), in the endoplasmic reticulum, the site of secretory and membrane protein biogenesis (Chen et al., 2006; Stechmann and Cavalier-Smith, 2004). At present, relatively little is known regarding the chaperone biology of GRP94, though recent studies have demonstrated an essential role for GRP94 in the functional expression of a subset of metazoan-specific proteins functioning in cell-cell, cell-tissue, and cell-substratum interactions, including the Toll-like receptor family, a subset of integrins, and insulin-like growth factor II (Randow and Seed, 2001; Wanderling et al., 2007; Yang et al., 2007). Hsp90 chaperones thus appear to have dichotomous functions in metazoans, with Hsp90 regulating the functional expression of intracellular signaling pathways associated with growth, survival, and morphogenesis, and GRP94 regulating the functional expression of intercellular signaling pathways and cell surface proteins that enable cells to function in the context of tissues. Consistent with this view, GRP94 expression is not required for mammalian tissue culture cell viability (Randow and Seed, 2001), yet is essential for early development in mouse (Wanderling et al., 2007).
The finding that GRP94 is required for the functional expression of the Toll-like receptor (TLR) family provides intriguing suggestions regarding the functions of the GRP94 proteome in tissue biology (Yang et al., 2007). Toll, the parent member of the Toll/Toll-like family, is a maternal effect gene that serves critical roles in dorsal-ventral patterning in the Drosophila embryo (Hashimoto et al., 1988). The patterning functionality of Toll and its related genes, such as 18-wheeler, reflect a cell adhesion activity provided by the encoded ectodomain (Eldon et al., 1994; Hashimoto et al., 1991). Recent evidence also identifies critical roles for 18-wheeler in cell migration, again consistent with an intrinsic adhesion activity of the TLR ectodomain (Kleve et al., 2006). All TLRs are type I transmembrane proteins whose ectodomains contain extended leucine-rich repeat (LRR) arrays (Eldon et al., 1994; Hashimoto et al., 1991; Kobe and Deisenhofer, 1994; Medzhitov et al., 1997). LRRs can express diverse functions including, but not limited to, hetero- and homotypic fusion, protein-protein interaction, and of particular importance, an innate immune function in the recognition of diverse pathogen-associated molecular patterns (PAMPS) (Bella et al., 2008; Kobe and Deisenhofer, 1994; Medzhitov et al., 1997). Interestingly, and whereas Drosophila Toll and TLRs serve critical functions in early development, these proteins also serve key functions in the recognition and response to fungal pathogens in the larval and adult stages (Lemaitre et al., 1996). The conservation in Toll/TLR structure/function between fly and mammals, and the discovery that GRP94 is essential for TLR family expression, suggests that GRP94 may regulate the functional expression of metazoan-specific proteins functioning in cell/tissue morphogenesis.
To investigate this hypothesis, we have identified the Drosophila GRP94, Gp93, and demonstrate that Gp93 is an essential gene. The loss of zygotic Gp93 expression causes prominent defects in the anterior midgut epithelium, marked by highly aberrant copper cell morphology, decreased gut acidification, irregular septate junction organization, and diminished gut motility. Because copper cell specification and stable differentiation requires expression of the homeotic gene labial (Bienz, 1996), these data suggest essential roles for the Gp93 proteome in cell fate specification and epithelial homeostasis. Coincident with defects in the copper cell region of the gut, Gp93 mutant larvae display substantially reduced dipeptide and cationic amino acid assimilation. Though loss of Gp93 expression has somewhat limited and variable effects on nutrient assimilation, the metabolic consequence is severe; Gp93 mutant larvae display extensive mobilization of fat body triglycerides, lipid droplet accumulation in the oenocytes, and gross deficits in growth which cannot be rescued by nutritional supplementation. These results reveal that Gp93 expression is essential for the integrated secretory (gastric acid) and absorptive (amino acids/dipeptides) functions of the midgut that enable nutrient assimilation-coupled growth control.
Materials and Methods
Drosophila Stocks, P-Element Excision, and Rescue
All marker mutations and balancer chromosomes are described and referenced in the FlyBase Consortium. Crosses were carried out at 25°C in vials containing freshly yeasted fly food (molasses, corn meal, yeast extract, and agar). P{EPgy2}Gp93EY06213, P{tGPH}2; Sb/TM3, Ser, and UAS-Dcr2.D lines were obtained from the Bloomington Drosophila stock center. A UAS-Gp93-hairpin line was obtained from NIG-Fly, Japan. Gal4 lines dilp2, ppl, mex were gifts from Dr. Ping Shen (University of Georgia, Athens, USA), Dr. Alex Gould (National Institute for Medical Research, MRC, UK), and Dr. Graham Thomas (Penn State University, University Park, USA) respectively.
Imprecise P-element excision using the P{EPgy2}Gp93EY06213 line was performed to create deletion mutants within the coding sequence of Gp93 (Salz et al., 1987). 11 Gp93 deletion stocks were screened by PCR and Gp931, Gp932, and Gp933 were fully sequenced. A genomic rescue construct was created with a PCR-generated genomic sequence, cloned into vector pUAST, and subsequently confirmed by automated DNA sequencing. P-element transformations of the rescue construct were performed by standard techniques (Spradling, 1986) at the Duke University Model System Genomics facility. Transformants were isolated and crossed to a Gp93 mutant background.
Western Blot Analysis
Gp93 mutant and heterozygote embryos and larvae were homogenized in SDS-PAGE sample buffer and heated at 95°C for 5 min; soluble lysate protein concentration was determined by BCA assay (Pierce) and 20 μg of each sample was analyzed by SDS-PAGE and immunoblot. Gp93 was detected with a rabbit anti-Gp93 polyclonal serum, raised against a synthetic N-terminal peptide, and Drosophila BiP and Sec61α were identified using polyclonal antisera directed against the mammalian orthologs. Antisera dilutions were 1:1000. For Akt activation assays, where the conversion of Akt to phospho-Akt was assayed by immunoblot, fat bodies from 96 hr after egg deposition (AED) Gp93 mutant and heterozygote larvae were cultured in PBS for 1 hour at 25°C, followed by incubation with or without 20 μg/ml human recombinant insulin for 1 hour at 25°C. Tissue samples were sonicated in SDS-PAGE sample buffer, heated, and lysates were analyzed by reducing 10% SDS-PAGE. Akt and phosphorylated-Akt were detecting using anti-Akt and anti-phospho-Drosophila Akt (Ser 505) antisera (Cell Signaling) respectively.
Larval Growth and Gut Function Assays
To assay larval growth, Gp93 mutant and heterozygote embryos were sorted onto normal fly medium and maintained at 25°C. At the indicated time points, larvae (~50) were collected, pooled, and weighed. To assess gut motility, 56 hr AED Gp93 mutant and heterozygote larvae were maintained overnight on apple juice agar supplemented with yeast paste containing 0.14% (w/w) Fast Green dye. At 72 hr AED, fed larvae (dye-stained yeast in the gut) were moved to apple juice agar supplemented with normal yeast paste and scored at 20 min intervals for loss of green coloring in the gut. Larval foraging behavior was assayed as described in (Kramer et al., 2003). Briefly, 0–2 hour embryos were sorted and placed at the perimeter of 10 cm Petri dishes lined with 20% sucrose-soaked filter paper. Unfertilized egg frequencies were determined and the number of larvae distant from the central food source was assessed at 48, 72, and 96 hr AED. Percentages of wandering larvae were calculated as the number of larvae not on food divided by the total number of hatched embryos, multiplied by 100 (Kramer et al., 2003).
Nutrient assimilation was assayed as the transfer of isotopically labeled glucose, amino acids or dipeptides from the gut to the circulating hemolymph. In these assays, 200 μl of dye-stained food, prepared as described (Voght et al., 2007), and containing 2 μCi [3H]-L-alanyl-L-alanine, 2 μCi L-[4,5–3H(N)]-lysine, 2 μCi L-[3,4,5–3H(N)]-leucine, or 1 μCi [14C(U)]-D-glucose was used for each assay. 76 hr AED Gp93 mutant and heterozygote larvae were placed on the dye/radioisotope-supplemented food for 4 hours at room temperature. Larvae with dye-positive guts were collected, washed in ice cold water, the excess moisture removed, and hemolymph was collected from 8–13 larvae by cuticle puncture and microsyringe sampling. Hemolymph fractions were directly added to scintillation fluid and radioisotope levels determined by liquid scintillation spectrometry (Packard Tri-Carb). Gut acidification and alkalinization studies were performed using the nutrient uptake assay food described above, supplemented with 0.14% (w/v) pH indicator dye (bromphenol blue or phenol red) (Dubreuil et al., 1998). 76 hr AED larvae were fed for 4 hours, washed with PBS and immediately dissected into PBS with 5mM EGTA (Phillips and Thomas, 2006). Gut tissue micrographs were subsequently acquired with a Leica MZ FLIII stereomicroscope.
Lipid Analysis and Trehalose Measurements
Lipid Analysis
Fat bodies from fifteen 96 hr AED Gp93 mutant and heterozygote larvae were dissected and added to 400 μl of methanol:chloroform (2:1). After sonication and centrifugation, supernatant fractions were supplemented with 266 μl chloroform:H2O (1:1), vortexed, and centrifuged. The lower, organic phase, containing the neutral and phospholipid fraction, was removed, dried by vacuum centrifugation and lipids were resuspended in 100 μl chloroform:methanol (2:1). The total organic phosphorous (total lipid phosphorous) was determined by chemical analysis of organic phosphorous (Ames and Dubin, 1960). Five larval equivalents of extracted lipid were analyzed for total triglyceride content (Duke Clinical Chemistry Facility). For thin layer chromatography analysis of triglycerides, lipid extracts (19 nmol lipid phosphorous) were loaded onto silica gel plates, with glyceryl trioleate as a standard, and resolved using a hexanes: diethyl ether: acetic acid (70:30:1) solvent system. Plates were stained with 0.2% Amido Black 10B in 1M NaCl, destained in 1M NaCl, and air dried (Plekhanov, 1999). The dried plated were scanned and relative band intensity determined using ImageJ (NIH).
Trehalose measurements
1 μl hemolymph was collected from a group of 4–6 Gp93 heterozygous or mutant larvae at 96 hr AED and added to 9 μl of buffer (5 mM Tris-HCl, pH 6.6, 137 nM NaCl, 2.7 nM KCl). Samples were heated at 70°C for 5 min, cooled, and incubated with trehalase (Sigma) at 37°C for 12 h (Okamura et al., 2007). Glucose concentrations were determined in triplicate using a glucose assay kit (Sigma).
Histology
96 hr AED Gp93 mutant and heterozygote larval brain tissue were stained with anti-DILP2 antibody (a gift from Dr. Mark Brown, University of Georgia, Athens, USA) as previously described (Cao and Brown, 2001). Images were acquired with a Zeiss LSM510 Meta confocal microscope. To assess endoreplication activity, Gp93 mutant and heterozygote larvae were fed normal fly food supplemented with 100 μg/ml BrdU for 24 hr. Guts were dissected at 120 hr AED and fixed in 4% paraformaldehyde (PFA)/PBS for 1 hour at room temperature. Incorporated BrdU was detected using an anti-BrdU primary antibody (1:500) (BD Pharmigen) and HRP-coupled donkey anti-mouse secondary antibody (1:250) and DAB substrate (Pierce). Tissue was mounted in 50% glycerol and micrographs were acquired with a Zeiss Axiophot microscope. Copper uptake by the copper cells of the middle midgut was observed by feeding 76 hr AED larvae normal fly food supplemented with 500 μM CuSO4 for 24 hours followed by gut dissection and fixation in 4% PFA/PBS for 30 min. Tissue was washed and mounted in 80% glycerol. Micrographs were obtained with a DAPI filter set on a Zeiss Axiophot microscope.
Oenocyte staining
82 hr AED Gp93 mutant and heterozygote larvae were placed on water or 20% sucrose-saturated filter paper for 14 hours. Larvae were filleted at 96 hr AED and fixed in 4% PFA/PBS for 10 min. Pelts were washed with water and stained with Oil Red O as described previously (Gutierrez et al., 2007). Micrographs were acquired with a Zeiss Axiophot microscope.
Antibody staining and image collection
Larval gut and fat body tissue were fixed in 4% PFA/PBS for 30 min and stained using standard histological techniques. Antibodies used were anti-Lamin Dm0 (1:50) (DSHB), anti-discs large (1:1000) (DSHB), and goat anti-mouse AlexaFluor647 secondary antibody (1:200) (Invitrogen). Phalloidin-AlexaFluor 546 (Invitrogen) was used at a dilution of 1:40. Tissues were mounted in 80% glycerol and micrographs acquired with a Zeiss Axiophot microscope or a Zeiss LSM510 Meta confocal microscope. For the LSM510 Meta confocal microscope, images were acquired with a Plan-Neofluar 40X-1.3-N.A.-oil or Plan-Apochromat 63X-1.4-N.A.-oil lens and LSM v4.2 software. Maximum intensity projections were processed through ImageJ (NIH). For the Zeiss Axiophot microscope, images were acquired with a Plan-Apochromat 5X-0.16-N.A., Plan-Neofluar 10X-0.30-N.A., or a Plan-Apochromat 20X-0.60-N.A. lens, using a QImaging Retica EXi digital camera and QCapture software.
Metabolomics
120 Hr AED Gp93 mutant and heterozygote larvae grown on normal fly food at room temperature were pooled and aqueous extracts prepared by homogenization in DD H2O. Protein concentration was determined using a Nano Drop spectrophotometer (Thermo Fisher Scientific). Amino acids and organic acids were analyzed using stable isotope dilution techniques. Amino acid measurements were made by flow injection tandem mass spectrometry using sample preparation methods described previously (Wu et al., 2004). The data were acquired using a Micromass Quattro MicroTM system equipped with a model 2777 autosampler, a model 1525 HPLC solvent delivery system and a data system controlled by MassLynx 4.1 operating system (Waters).
RNA isolation and cDNA microarray analysis
Total RNA from pooled, 72 hr AED Gp93 mutant or heterozygote larvae crosses was obtained by TRIzol extraction (Invitrogen). Total RNA fractions from three independent experiments were obtained and RNA quality was assayed using an Agilent bioanalyzer (Silicon Genetics), as performed in the Duke Microarray Core Facility (DMCF). Amplified mRNA was analyzed on oligonucleotide DNA microarrays using a Drosophila Operon Oligo Set, array ID DO15K (Operon). Total RNA from each sample was reverse transcribed with the fluorescent label Cy3 and single label samples hybridized to the Operon arrays. Detailed protocols for the labeling and sample processing are available on the DMCF Web site (http://microarray.genome.duke.edu/spotted-arrays/protocols).
Data processing and statistical analysis
Genespring GX software (Agilent) was used to assess the statistical significance of expression differences between array data. Samples were normalized to the mean expression intensity of all samples and only those genes with an expression intensity within 20–100% of the mean were considered. Genes that did not show any expression in any of the samples were flagged as “absent” and removed from the data set. Arrays were coded as either Gp93 mutant or heterozygote and principle components analysis was performed on all samples. Pearson correlation values between samples were computed to identify clustering between samples. Unpaired Student t-tests were performed to determine the p-value significance of gene expression differences. An arbitrary cut-off of p < 0.001 was set, and an arbitrary cut-off for expression fold change was set at fold-change > 3.0.
All genes with an expression fold change > 3.0 were analyzed by gene ontology (GO) using GO listings from the Affymetrix GeneChip Drosophila Genome 2.0 array gene list (Affymetrix, Santa Clara, CA). Descriptions of biological process, cellular location and molecular function were compiled for all 210 differentially expressed genes.
Results
Drosophila GRP94/HSP90B1, Gp93, is an essential gene
Gp93 (CG5520) encodes the D. melanogaster ortholog of the endoplasmic reticulum HSP90, GRP94 (HSP90B1). Gp93 is comprised of 5 exons, occupies a 35 kb region of chromosome 3R at cytological location 98B6, and encodes a protein of 787 amino acids with a predicted molecular weight of 90.2 kD. A ClustalW alignment of Gp93 and GRP94 demonstrated a high overall identity (59%) and similarity (77%) of the fly protein to its mammalian counterpart (Fig. S1). Notably, all known protein domains and established catalytic resides are conserved between the fly and mammalian GRP94, including the amino-terminal signal sequence, carboxy-terminal endoplasmic reticulum retention/retrieval motif, N-terminal adenine nucleotide binding pocket, ATPase catalytic residues, and signature helix 1-4-5 subdomain insertion (Dollins et al., 2007; Soldano et al., 2003).
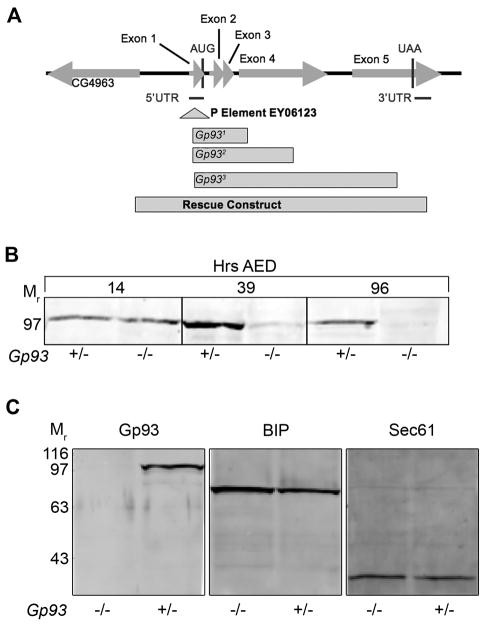
Gp93 deletion mutants were isolated by imprecise P-element excision from an isogenized line, P{EPgy2}Gp93EY06213. For all alleles, 100% of the homozygous mutant animals died at the third instar larval stage, indicating that Gp93 is an essential gene. All deletions included exons 1–4 (Fig. 1A). Complementation between mutant alleles was not observed. To confirm that the mutant lethal phenotype was a consequence of Gp93 deletion, rescue experiments were performed with a genomic rescue construct comprising the complete Gp93 gene by P-element-mediated germline transformation (Spradling, 1986). Full rescue of the mutant phenotype was observed, indicating that the mutant phenotype is solely due to loss of Gp93 expression. This conclusion was further validated by immunoblot analysis of Gp93 content. In the experiment depicted in Fig. 1B, equivalent quantities of total protein from Gp93 heterozygous and mutant embryos (14 hr after egg deposition (AED)), first instar larvae (39 hr AED), and third instar larvae (96 hr AED) were examined. Gp93 was detectable in Gp93 heterozygous and mutant embryos, with the protein present in Gp93 mutant embryos reflecting maternal contributions. In Gp93 mutant larvae, Gp93 was barely detectable at first instar, and undetectable at third instar (Fig. 1B). Efforts to generate Gp93 mutant germline clones were unsuccessful, suggesting critical roles for Gp93 in oogenesis. Loss of Gp93 expression did not result in substantial alterations in ER protein composition, as determined by immunoblot analyses of Gp93, BiP, the ER Hsp70 chaperone, and the protein translocation channel component Sec61α, in Gp93 heterozygote and mutant third instar larval tissue (Fig. 1C).
Figure 1. Identification of the Drosophila GRP94 ortholog, Gp93.
A) Genetic map describing the Gp93 gene region. Drosophila line P{EPgy2}Gp93EY06213 was used for P element excision mutagenesis. A schematic illustration of three deletion lines in the Gp93 locus, Gp931, Gp932, Gp933 and a rescue construct is provided. B) Immunoblot analysis depicting levels of Gp93 in Gp93 heterozygote and mutant 14 hr embryos, first instar larvae, and third instar larvae. C) Immunoblot analysis depicting levels of Gp93, BIP, and Sec61α in third instar Gp93 larvae.
Gp93 mutants display a severe growth defect
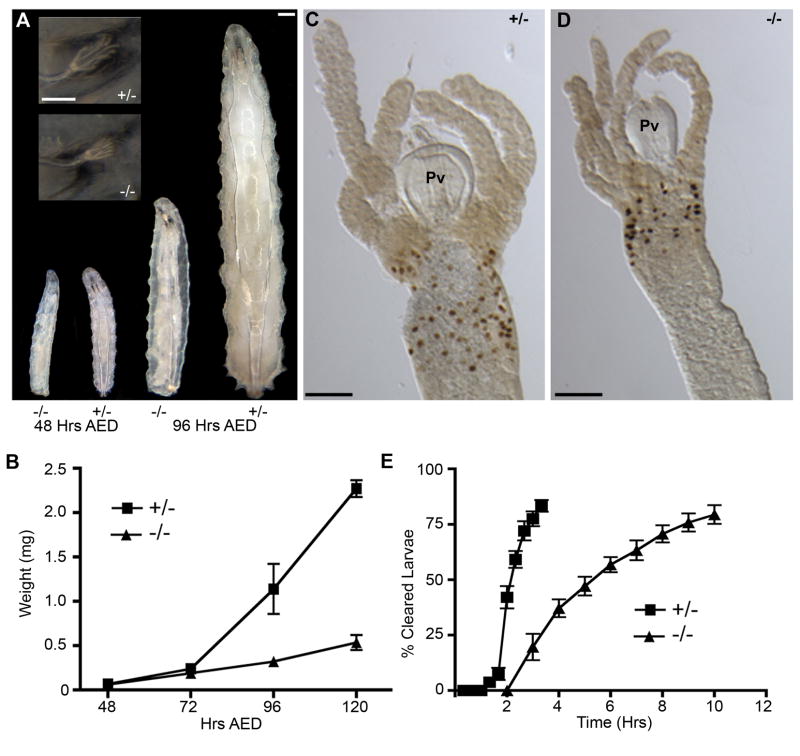
Growth in holometabolous insects is limited to the larval stages and is marked by an early linear phase and a subsequent exponential phase (Bakker, 1959; Church and Robertson, 1966). Visual comparisons of Gp93 mutant larvae indicated that loss of Gp93 expression resulted in a late larval growth defect. In early stages of larval life Gp93 heterozygote and mutant larvae are morphologically identical whereas at later stages, the growth rates diverge, with the Gp93 heterozygote larvae displaying exponential growth and the Gp93 mutant larvae a substantially slower, linear growth rate (Fig. 2A, B). Although growth control in Gp93 mutant larvae is defective, the developmental program is normal; Gp93 mutant larvae molt and on the basis of anterior spiracle (Fig. 2A, inset) and mouth hook (data not shown) morphology, progress through the third instar stage.
Figure 2. Gp93 mutant larvae display reduced gut motility and a growth defect.
A) Bright field images of Gp93 heterozygote and mutant larvae grown on normal fly food for indicated time. Inset shows anterior spiracles at 96 Hrs AED. Scale bars = 200 μm. B) Quantification of larval wet weight at indicated time points. Each point represents the mean weight of 30–80 larvae and error bars represent SEM. C–D) BrdU incorporation in anterior midgut of Gp93 heterozygote and mutant larvae at 120 hrs AED. Pv = proventriculus. Scale bar = 200 μm. E) Quantification of gut clearance rates for Gp93 heterozygote and mutant larvae beginning at 72 hrs AED. Each point represents the mean of 10 experiments containing 4–8 larvae. Error bars represent S.D.
To further evaluate the Gp93 mutant growth phenotype, experiments were conducted to determine if Gp93 mutant larval tissues were capable of endoreplication, the post-first instar cell cycle comprising DNA replication in the absence of cell division, and which is responsible for larval growth (Migeon et al., 1999). In these experiments, third instar Gp93 heterozygote and Gp93 mutant larvae were fed on complete food supplemented with 5-bromodeoxyuridine (BrdU) and larval tissues were processed for histochemical analysis of BrdU incorporation. The data presented in Fig. 2C, D demonstrate that although overall tissue dimensions in Gp93 mutant larvae were substantially smaller than paired Gp93 heterozygote larvae, BrdU incorporation was apparent in both. From these data we conclude that defects in endoreplication are not a proximal cause of the Gp93 mutant larval growth defect.
As in mammals, growth in Drosophila larvae is under hormonal control and includes significant behavioral and gut function components. To assess the contribution of feeding behavior and gut function to the Gp93 mutant larval growth deficit, foraging/food uptake, gut clearance, and larval wandering behavior were examined. To assess foraging/eating behavior, larvae raised on complete food media were transferred onto dye-containing food plates. After a 4 hr interval, the fraction of larvae with pronounced gut staining was determined. In this analysis, the Gp93 heterozygous and mutant larvae were indistinguishable, indicating that the nutrient sensing and foraging behavioral components of animal growth were not markedly affected by the loss of Gp93 expression (Fig. S2A).
To assay gut motility, larvae were fed dye-stained food, transferred to dye-free plates, and the time to gut clearance assayed. As shown in Fig. 2E, gut clearance rates for Gp93 mutant larvae were substantially lower than the heterozygote controls; comparisons at the 3 hr time point, where the heterozygote larvae display maximal clearance, indicate that loss of Gp93 expression was accompanied by a ca. 75% decrease in gut motility. The fractional population exhibiting complete gut clearance was nearly identical for the two genotypes, and so the clearance assay provides an accurate means for comparing relative gut motility. In support of this conclusion, the fraction of Gp93 mutant larvae exhibiting late stage, ecdysone-dependent wandering behavior represented a relatively small (< 25% at 96 hr AED) cohort, and so the observed differences in gut motility are unlikely to reflect ecdysone-dependent differences (Fig. S2B). In summary, loss of Gp93 expression results in a marked loss of gut motility, though foraging and eating behaviors are relatively unaltered. Notably, Gp93 mutant larvae feed continuously, yet are unable to undergo the exponential growth characteristic of third instar larvae. These observations suggest that loss of Gp93 expression disrupts growth control. This hypothesis was examined in studies of gut function and distal growth signaling detailed below.
Loss of Gp93 expression causes a loss of gut acidification, defects in acid-secreting cell structure, and compromises nutrient absorption function
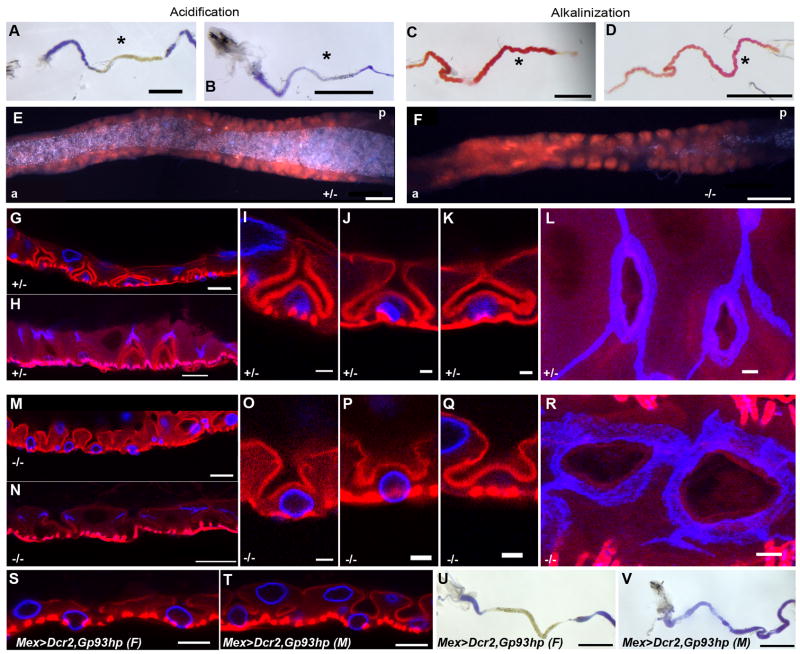
The Drosophila larval gut is segregated into distinct domains, comprised of the ectoderm-derived foregut and hindgut, and the endoderm-derived midgut, which performs critical functions in nutrient processing (Murakami et al., 1999). The Drosophila midgut displays prominent acid and base transport functions; the middle midgut region is highly enriched in acid-secreting copper cells and is a zone of high acidification (Dimitriadis, 1991; Dubreuil et al., 1998; Shanbhag and Tripathi, 2009). In contrast, the posterior midgut is highly alkaline (Dubreuil et al., 1998; Shanbhag and Tripathi, 2009). To assess gastric acid and base secretion, the acidification and alkalinization functions of the middle and posterior midgut were determined by feeding larvae pH indicator-supplemented food. Fast Green dye-supplemented food was used to score larval feeding activity (data not shown). Analysis of gut pH in larvae fed Bromphenol blue-supplemented food identified a substantial defect in midgut acidification in Gp93 mutant larvae, indicative of defects in gastric acid secretion (Fig. 3A,B). In contrast, the posterior midgut of both heterozygote and Gp93 mutant larvae was notably basic, indicating that hindgut bicarbonate transport was functional in the mutant larvae (Fig. 3C,D).
Figure 3. Loss of Gp93 expression causes loss of gastric acid secretion, defects in copper cell structure and aberrant septate junction morphology.
A,B) Bright field images of 80 hr AED Gp93 larval anterior midgut tissue containing bromphenol blue stained yeast paste. C,D) Bright field images of 80 hr AED Gp93 larval posterior midgut tissue containing phenol red (alkalinization indicator)-stained yeast paste. Scale bars in panels A–D = 1 mm. E–F) Digital images of the cuprophilic region of the middle midgut of 96 hr AED Gp93 larvae after a 24 hr feeding period on fly food supplemented with 500μM CuSO4; a=anterior, p=posterior. Scale bars = 100μm. G–R) Confocal micrographic images of the cuprophilic region of the middle midgut of 80 hr AED Gp93 larvae. In panels G, I–K, M, O–Q, red = F actin (phalloidin), blue = lamin Dm0. In panels H, L, N, R, red = F actin (phalloidin), blue = Discs large. L – R) Micrographs of maximum intensity projections of 8.1 μm and 5.8 μm through the apical membrane showing the apical invagination of copper cells. Scale bars in panels I, J, O, P = 20 μm. Scale bars in panels G, H, M, N = 5 μm. S,T) Confocal micrographic images of the cuprophilic region of the middle midgut of female (F) and male (M) 72–80 hr AED mex>Dcr2,Gp93-hairpin larvae. Red = F-actin (phalloidin), blue = lamin Dm0. U,V) Bright field images of female (F) and male (M) third instar mex>Dcr2,Gp93-hairpin larval anterior midgut tissue from larvae fed bromphenol blue-supplemented yeast paste. Scale bars in panels S,T = 20 μm. Scale bars in panels U–V = 1 mm.
The deficits in gut acidification seen in the Gp93 mutant larvae suggested that loss of Gp93 expression disabled the acid-secreting functionality of the copper cells. Comparisons of copper uptake in the copper cell region of heterozygote and Gp93 mutant larvae demonstrated that copper cells were both present and functional in the Gp93 mutant larvae (Fig. 3E, F). As well, the overall tissue organization of the Gp93 mutant larvae copper cell regions was normal, with symmetrically interdigitated interstitial cells present throughout this region.
Similar to gastric parietal cells, copper cells possess characteristic deep invaginations of the apical, proton transporting plasma membrane (Dubreuil et al., 1998; Lee et al., 1993). As shown in Fig. 3G, I, J, K (heterozygote) and M, O, P, Q (mutant), the Gp93 mutant middle midgut epithelium is highly irregular, most notably with respect to copper cell apical plasma membrane morphology. In heterozygote middle midgut tissue, the copper cells, with basal nuclei, are interdigitated between clearly defined interstitial cells, containing apical nuclei, and display the characteristic invaginated apical plasma membrane morphology, with a narrow channel structure connecting the boomerang-shaped apical plasma membrane invagination to the gut lumen (panels I,J,K). In contrast, the copper cell apical plasma membrane invaginations of the Gp93 mutant midgut are distorted, with enlarged, irregular, apical membrane invaginations connecting to the gut lumen (panels O, P, Q). Nuclear location in the Gp93 mutant midgut copper and interstitial cells was identical to the heterozygote tissue.
The cell-cell interface between the copper and interstitial cells is provided by smooth septate junctions. Examination of midgut septate junction morphology identified further defects in the Gp93 mutant midgut epithelium. In control tissue (Fig. 3H, L), septate junctions, marked by staining for the septate junction component Discs large (Dlg), display a prominent apical-basolateral orientation, with a tight organization of Dlg, and extension to the apical membrane bifurcation. In Gp93 mutant midgut epithelium (Fig. 3N, R), the septate junctions were much narrower and exhibited a substantially decreased apical-basolateral orientation. In addition, confocal surface projections demonstrated a loss of Dlg organization (Fig. 3L, R). It is not yet known whether these morphological differences alter epithelial permeability.
To determine if these phenotypes were solely a consequence of the loss of Gp93 expression in the gut, Gp93 expression was silenced in the gut tissue via expression of a Gp93-specific hairpin RNA, using a mex-Gal4 driver line (Philips and Thomas, 2006). In these experiments, copper cell morphology was only slightly affected in female larvae (Fig. 3S) yet severely affected in male larvae (Fig. 3T). Likewise, loss of gut acidification function was apparent in male, but not female larvae (Fig. 3U,V). The mex gene is located on the X chromosome and in male progeny mex-GAL4 expression is elevated, due to X-chromosome dosage compensation. As a consequence, RNAi-mediated suppression of Gp93 expression is more pronounced in male vs. female progeny. These data further establish that midgut Gp93 expression is required for proper copper cell differentiation and acid secretion function.
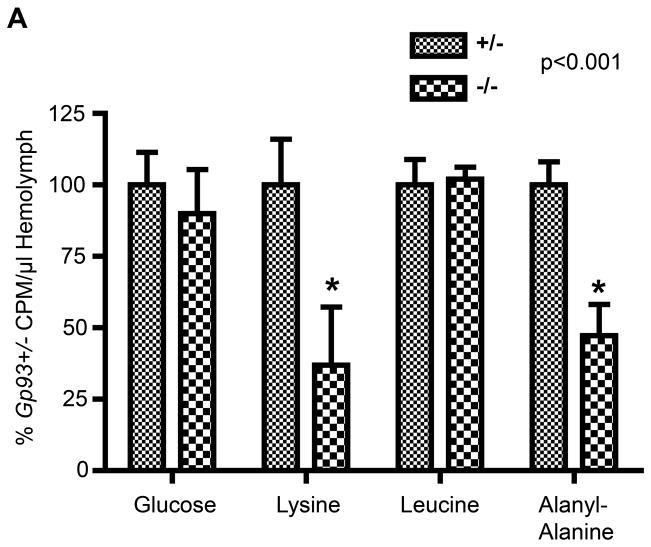
The defective copper cell morphology seen in Gp93 mutant larvae is associated with a significant defect in midgut gastric acid secretion. Because the midgut also serves important functions in nutrient assimilation, and many nutrient transporters utilize the proton electrochemical gradient to drive nutrient uptake, studies were performed to assess nutrient assimilation in the Gp93 heterozygote and mutant larvae. In these experiments, larvae were fed isotopically labeled glucose, lysine, leucine, or the dipeptide alanyl-alanine, and transepithelial transport to the hemolymph assayed. In mammals, di- and tripeptides serve as the primary source of dietary amino acids and are internalized in the gut via the activity of the PepT1 family of peptide transporters (Adibi, 1997). Dipeptide uptake in Drosophila is mediated by the PepT1 ortholog OPT1 (Roman et al., 1998). Following a 4 hour isotope feeding period, larvae were collected and hemolymph collected by cuticle puncture. As shown in Fig. 4, glucose and leucine assimilation activity was identical between Gp93 mutant and heterozygote larvae. In contrast, lysine and alanyl-alanine dipeptide uptake was markedly suppressed in the Gp93 mutant gut, with uptake levels corresponding to 35–40% of those determined in Gp93 heterozygote larvae. Supplementation of the larval food source with 1% tryptone, a peptide-rich casein digest, did not complement the growth defect (data not shown), indicating that cationic amino acid and dipeptide uptake is limiting in the Gp93 mutant gut. This deficiency may reflect defects in the functional maturation of nutrient transporters and/or synergistic consequences of the loss of gastric acid secretion, noting as above that cationic amino acid and dipeptide transporters are commonly dependent upon electrochemical proton gradients as the free energy source for transport (Daniel et al., 2006).
Figure 4. Gp93 loss causes specific nutrient assimilation defects.
A) Quantification of radiolabeled nutrient uptake in 80 hr AED Gp93 larvae. Data represents normalized percent c.p.m. of radiolabeled nutrient per microliter hemolymph ± S.D. of Gp93 heterozygote vs. mutant larval hemolymph values. Each bar represents the mean of 6–12 experiments; error bars represent S.D. Significance determined by one way ANOVA.
Transcriptional response to loss of larval Gp93 expression is biased to metabolism genes
The inability of Gp93 mutant larvae to undergo exponential growth despite continuous feeding suggests critical roles for the Gp93 proteome in nutrient assimilation-linked growth control. The data reported above identify defects in gut function that likely contribute to this deficit. To gain further insight into the metabolic status of Gp93 mutant larvae, cDNA microarray studies were performed, using the Operon spotted array format. After expression percentile and flag filtration screening, 12,477 genes of the 13,664 probed genes were evaluated. Application of significance criteria at p<0.001 and fold-change of greater than 3.0, yielded 210 genes (1.69% of genome) displaying significant transcriptional responses to the loss of Gp93 expression (Fig. S3). Of the 210 differentially expressed genes, 111 have assigned biological functions. When classified by gene ontology (GO) category, this gene cohort was markedly enriched in genes functioning in metabolism, proteolysis, and nutrient transport (Fig. S3A). The most prominent GO category is metabolism (37 genes). Within this cohort, six genes functioning in lipid metabolism were identified, including the triacylglycerol lipases, Lip3, and Lip1 (Fig. S3B). Of the 21 (18.9%) genes involved in proteolysis, three are of the Jonah family of midgut serine proteases (CG10475, CG6580, CG7170) (Ross et al., 2003), and all are down-regulated in Gp93 mutant larvae. Among the 19 (17.1%) genes categorized as encoding transport function and upregulated in the Gp93 mutant larvae is yin (OPT1) (Roman et al., 1998). The neural Lazarillo (NLaz) gene, a member of the Lipocalin family expressed in the developing gut and fat body, also displayed substantial up-regulation. NLaz is a secreted protein that couples stress response signaling to metabolic growth control (Hull-Thompson et al., 2009).
The transcriptional response to loss of Gp93 expression bears similarity to that previously reported to occur in response to starvation (Zinke et al., 2002). Yet, and as noted, Gp93 mutant larvae eat continuously and thus are not per se nutrient-limited. To further evaluate the transcriptional profile of Gp93 mutant larvae, RT-PCR studies were performed on total RNA from Gp93 mutant and heterozygote larvae, analyzing the expression levels of a nutrient starvation signature gene set (Zinke et al., 2002)(Fig. S3C). Loss of Gp93 expression was associated with a substantial upregulation of the starvation marker lipase 3 (lip3) (lane 4), but no detectable upregulation of the starvation markers CG6113 (Lip4) (lane 2) or CPTI (mitochondrial carnitine palamitoyltransferase I) (lane 3), in 72 or 96 hr AED mutant larvae (Fig S3C). These data demonstrate that the midgut epithelial defects that accompany loss of Gp93 expression cause a substantial metabolic stress similar to, but distinct from, that evoked by starvation.
Amino acid profiling of Gp93 mutant larvae: Identification of a catabolic metabolic state
Metazoan growth control is primarily exerted through coordinate regulation of the TOR and insulin/IGF signaling pathways, with TOR regulation representing a cell-autonomous regulatory pathway and insulin/IGF-II signaling, an endocrine/humoral regulatory pathway (Oldham and Hafen, 2003). In fly, as in mammals, TOR activity is regulated by amino acids, via the GTPase, Rheb (Stocker et al., 2003). Also similar to mammals, the Drosophila insulin receptor/phosphatidylinositol 3-kinase (PI3K) pathway integrates cellular metabolism and nutritional status (Britton et al., 2002). To further assess the metabolic status of Gp93 mutant larvae, whole animal amino acid levels were determined in Gp93 mutant and heterozygous larvae (Table I). With the exception of the urea cycle metabolites, ornithine and citrulline, Gp93 mutant larvae display significantly reduced levels of all assayed amino acids, both essential and non-essential. The observed decreases varied from modest (ca. 15%; glycine, serine) to substantial (ca. 50%; tyrosine, methionine, phenylalanine). This pattern, in magnitude and in amino acid identity, is highly reminiscent of that reported previously for Drosophila larvae during starvation (Chen, 1967; Chen, 1955). These data are also consistent with early reports describing the mobilization of body protein to amino acids upon starvation in Popillia japonica and Anomala orientalis (Ludwig and Wugmeister, 1955; Po-Chedley, 1958). The metabolic phenotype of Gp93 mutant larvae also bears similarity to the primary metabolic hallmarks of cachexia, a complex metabolic syndrome characterized by loss of muscle and adipose tissue, and which is unresponsive to feeding.
Table I.
Amino acid analysis of Gp93 mutant and homozygous larvae
| Amino Acid | Gp93+/− (μM) | Gp93−/− (μM) |
|---|---|---|
| Arg | 559.9±56.6 | 341.1±31.5 |
| His | 304.4±21.8 | 205.7±2.7 |
| Leu/Ile | 363.4±38.90 | 149.6±17.7 |
| Met | 143.5±13.4 | 68.9±3.7 |
| Phe | 151.5±17.8 | 63.2±9.5 |
| Val | 384.6±55.6 | 151.3±11.95 |
| Ala | 2075.1±259.1 | 1100.1±257.0 |
| Asx | 324.7±26.0 | 155.4±14.5 |
| Glx | 818.9±224.5 | 679.9±72.7 |
| Pro | 665.95±115.0 | 435.5±23.3 |
| Gly | 646.4±73.8 | 487.9±47.4 |
| Ser | 471.8±44.7 | 414.3±41.9 |
| Tyr | 370.2±36.1 | 38.6±16.8 |
| Orn | 136.0±16.0 | 144.9±20.8 |
| Cit | 3.0±0.7 | 2.5±0.8 |
Concentrations are represented in μM ± S.D.
Loss of Gp93 expression results in triglyceride mobilization
The amino acid data reported above, viewed with respect to the transcriptional profiling, describe an unusual metabolic phenotype where Gp93 mutant animals are in an apparent protein catabolic state, have nutrient-specific defects in gut assimilation, yet feed continuously on nutrient rich food. To determine if the catabolic state extended to triglycerides, the primary storage lipid in Drosophila, gross fat body morphology, fat body triglyceride levels, oenocyte-directed triglyceride mobilization, and hemolymph trehalose concentrations were determined in fed, third instar Gp93 mutant and heterozygote larvae (Fig. 5, Fig. S4, Table II). The insect fat body serves both storage and endocrine functions, broadly overlapping with vertebrate adipose and liver tissue; oenocytes serve hepatocyte-like functions (Butterworth et al., 1965; Gutierrez et al., 2007; Leopold and Perrimon, 2007). As shown in Fig. 5A, fat body tissue in Gp93 heterozygote third instar larvae was highly lobular and opaque. In the depicted light micrograph, the associated salivary gland tissue (arrowhead) is tightly organized and displays a refractive index that differs from the fat body tissue. In contrast, fat body tissue from Gp93 mutant larvae has reduced cellularity and is nearly transparent although the associated salivary gland tissue displays refractive properties similar to Gp93 heterozygote salivary gland tissue (Fig. 5B). The relative transparency of the Gp93 mutant larval fat body tissue was suggestive of reduced levels of storage triglycerides. To assess the triglyceride content of Gp93 heterozygote and mutant larval fat body tissue, fat body tissue was dissected from third instar larvae and the neutral lipid/phospholipid fraction isolated by Folch extraction. Organic phosphate levels were determined, to allow normalization of samples to total phospholipid content, and relative triglyceride levels examined by thin layer chromatography (Fig. S4). Enzymatic analyses indicated that Gp93 mutant fat body tissue contained 20 to 25-fold less triglyceride per unit phospholipid than the paired Gp93 heterozygote fat body tissue (Table II).
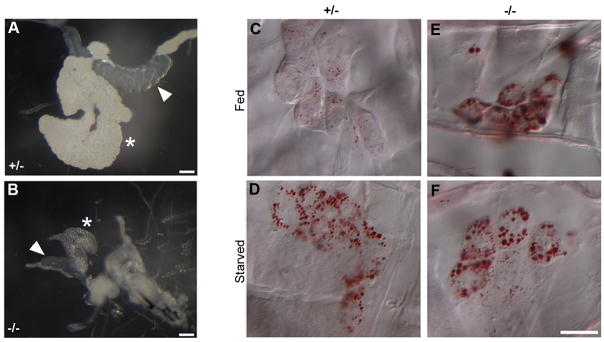
Figure 5. Triglyceride metabolism in Gp93 mutant larvae is similar to the homeostatic response to starvation.
A, B) Bright field images of salivary gland (arrowhead) and fat body (asterisk) of 96 hr AED Gp93 larvae. Scale bars = 100 μm. C–F) Bright field images of 96 hr AED Gp93 larval oenocytes stained with Oil Red O. For panels D, F, larvae were starved for 14 hr; in panels C, E larvae were kept on normal fly food until dissection. Scale bar = 50μm.
Table II.
Triglyceride and trehalose levels are altered in response to loss of Gp93 expression.
| Gp93 Heterozygote | Gp93 Mutant | |
|---|---|---|
| TAG – Clinical (mg/dL) | 109±10 | <5 |
| Trehalose (μg/μl) | 11.71±1.76 | 15.23±0.87 |
Triglyceride data is presented as (mg/dL) ± SEM. Trehalose data represents the mean of triplicate assays and is presented as μg trehalose per μl hemolymph ± S.D.
As reported by Gutierrez et al., nutrient deprivation promotes the mobilization of lipids from the fat body to the oenocytes. Such transfer is promoted during starvation, when amino acid uptake in the fat body is restricted, or when fat body lipolysis is activated. Using the Oil Red O staining method of Gutierrez et al., third instar Gp93 heterozygote larvae show very low oenocyte lipid accumulation (Fig. 5C). Following 12 hr of starvation, however, Gp93 heterozygote oenocytes were Oil Red O positive, with numerous cytoplasmic lipid droplets evident (Fig. 5D). Gp93 mutant oenocytes were Oil Red O positive in both the fed and starved states, indicating that steady state fat body lipolysis activity was elevated in the absence of starvation (Fig. 5E, F). In addition to providing further confirmation of the nutrient-stressed metabolic phenotype of the Gp93-mutant larvae, these data demonstrate that the signaling, secretion and endocytic pathways that direct lipid mobilization and uptake in Gp93 mutant fat body cells and oenocytes were effectively operational.
Insulin receptor (InR) signaling is suppressed in Gp93 mutant fat body tissue
Mobilization of fat body triglycerides occurs in response to diminished insulin signaling and is accompanied by elevations in hemolymph trehalose levels (Colombani et al., 2003; Rulifson et al., 2002). Indeed, hemolymph trehalose concentrations were significantly elevated in the Gp93 mutant larvae (Table II), suggestive of a suppression of insulin signaling (Rulifson et al., 2002). Fat body insulin pathway activity was thus examined, using a GFP-pleckstrin homology domain insulin pathway reporter (tGPH) (Britton et al., 2002). As previously reported, activation of insulin signaling, and the coincident increase in plasma membrane phosphatidylinositol 3,4,5P3 levels, promotes plasma membrane association of the tGPH GFP reporter (Britton et al., 2002). Micrographs depicting tGPH reporter localization, cell size, and nuclear size in fat body tissue of third instar Gp93 larvae are shown in Fig. 6. In Gp93 heterozygote fat body tissue (Fig. 6A–D), prominent tGPH plasma membrane localization was observed and all cells displayed centrally located, symmetrical nuclear profiles. In Gp93 mutant fat body tissue, in contrast, tGPH was diffusely present throughout the cytoplasm (panel E). In addition, the cytoplasm of third instar Gp93 mutant fat body cells contained single, very large, prominent lipid vesicles and the nuclei were small and irregular (panels E–G, merge in H). These data identify a marked suppression of insulin signaling pathway activity in the Gp93 mutant larvae. These findings prompted the hypothesis that insulin receptor surface expression was Gp93-dependent. In this scenario, the systemic metabolic and growth defects observed in the Gp93 mutant larvae would reflect the combined dysregulation of midgut epithelial homeostasis and loss of insulin receptor functional expression.
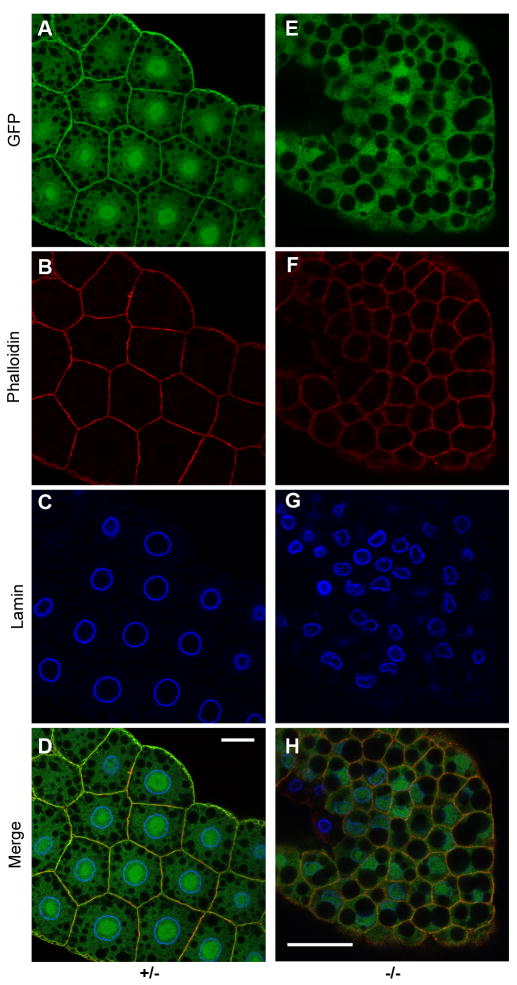
Figure 6. Loss of Gp93 expression causes fat body suppression of insulin pathway signaling.
Insulin receptor pathway reporter tGPH localization in fat body tissue of Gp93 heterozygote (panels A–D) and Gp93 mutant (panels E–H) larvae. tGPH fluorescence is depicted in panels A and E; the cell periphery was identified by staining of cortical actin with phalloidin (panels B and F). Panels C and G depict nuclei, identified by staining of the nuclear lamin Dm0. Panels D and H represent merged images. Scale bars = 20μm.
Insulin signaling pathway function is insensitive to loss of Gp93 expression
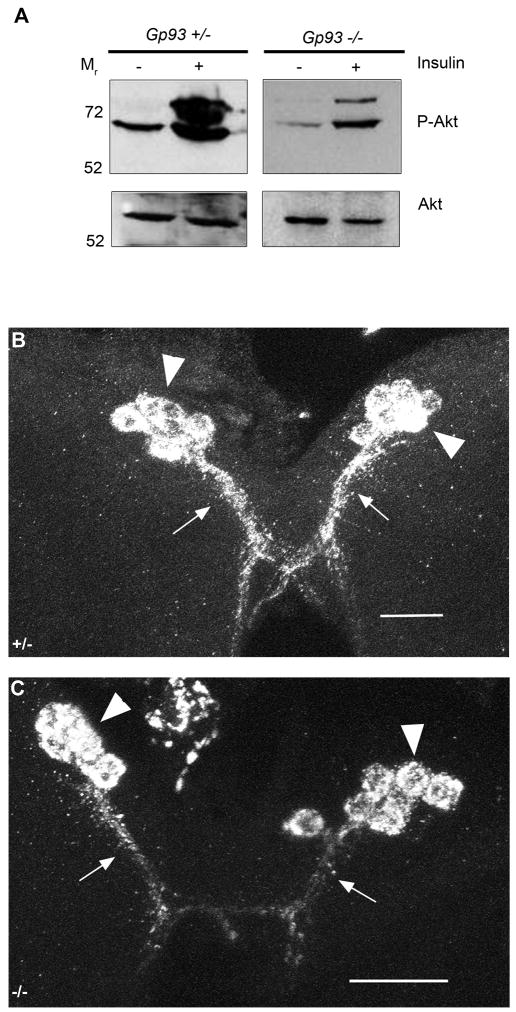
To determine if insulin receptor surface expression required Gp93, fat body tissue was isolated from third instar Gp93 mutant larvae and insulin pathway function assessed in vitro. The data in Fig. 7A demonstrate that insulin elicits activation of insulin pathway signaling, assayed as the conversion of Akt to phospho-Akt, in both Gp93 mutant and heterozygote fat body tissue in vitro. We conclude that the absence of insulin pathway signaling function in the fat body tissue of Gp93 mutant third instar larvae is unlikely to reflect a loss of functional, cell surface InR. Nonetheless, it is possible that InR surface density is substantially reduced in Gp93 mutant fat body tissue and although the tissue is, per se, insulin responsive, the magnitude of the insulin-dependent receptor activation in vivo is insufficient to elicit discernible redistribution of tGPH. For technical reasons, accurate assessments of fat body InR surface density are difficult to obtain. As an alternative approach, InR surface density and signaling activity was assessed in HEK293 cells stably expressing either a GRP94-targeted, validated shRNA or a scrambled control shRNA. InR surface density was determined by flow cytometric analysis (Fig. S5A) and signaling activity by immunoblot analysis of phospho-Akt (Fig. S5B). In these experiments, loss of GRP94 expression was associated with a modest (ca. 20%) reduction in InR surface expression and no change in insulin-dependent elicitation of phospho-Akt. Although these data derive from a mammalian system, the high structural and functional homologies between the insect and mammalian insulin signaling pathways and GRP94 orthologs support the conclusion that the absence of insulin signaling pathway activity in Gp93 mutant fat body tissue is unlikely to be solely due to defects in InR maturation and/or trafficking to the cell surface.
Figure 7. InR function and DILP2 sub cellular localization are unaffected by loss of Gp93 expression.
A) Immunoblot analysis of fat body Akt phosphorylation status in Gp93 heterozygote and mutant larval fat bodies. B–C) DILP2 staining in medial neurosecretory cells in Gp93 heterozygote (B) and Gp93 mutant brain tissue (C). Arrowheads identify cell bodies and arrows the distal axons. Scale bars = 20 μm.
The recent finding that secretion of IGF-II requires GRP94 suggests additional hypotheses for the slow growth phenotype of Gp93 mutant larvae (Wanderling et al., 2007). If, for example, Gp93 participates in the functional maturation of the insect insulin molecules (Drosophila insulin-like peptides or DILPs), loss of Gp93 expression would be expected to disrupt DILP secretion, and thereby yield a slow growth phenotype. At present, methods for quantifying hemolymph DILP levels are unavailable; as an alternative approach DILP2 expression was examined in the median neurosecretory cells (mNSCs) of Gp93 mutant and heterozygote larval brain tissue by immunohistochemistry. In both Gp93 heterozygote (Fig. 7B) and Gp93 mutant (Fig. 7C) larval brain tissue, DILP2-positive mNSCs were identified and prominent staining was observed in both the cell bodies (large arrowheads) and distal axons (small arrows). The presence of DILP2-positive staining in the distal axons indicates that DILP2 has exited the ER and is likely competent for secretion. As a further test of this conclusion, Gp93 expression was reduced in the mNSCs through use of a dilp2-GAL4 driver, to induce expression of a Gp93 RNA hairpin in a UAS-Dicer2 background. The progeny of these crosses progressed to adulthood with no discernible defects, suggesting that Gp93 is not required for DILP2 maturation/secretion (data not shown). However, and as noted earlier (Fig. 3), it is possible that the magnitude of the knockdown obtained with the dilp2-GAL4 driver may be insufficient to affect DILP2 maturation/secretion. These data, in summary, further substantiate the conclusion that defects in midgut epithelial structure/function are the proximal defect in the Gp93 mutant larvae and suggest that the dysregulation of organismal growth control is a downstream consequence of compromised midgut function.
Discussion
We have identified the Drosophila GRP94 ortholog, Gp93, and report that Gp93 is an essential gene. In Drosophila larvae, the loss of zygotic Gp93 expression causes pronounced defects in the midgut epithelium, yielding disrupted copper cell morphology, loss of gastric acid secretion, reduced gut motility, deficits in nutrient assimilation, metabolic growth control and, ultimately, a larval-lethal growth defect.
Gp93 function in epithelial biology
Although Gp93 is ubiquitously expressed, discernible consequences of the loss of zygotic Gp93 expression were restricted, in larvae, to the midgut. This region of the midgut contains the acid-secreting copper cells, in register with adjacent interstitial cells, and in the absence of Gp93 expression, copper cell structure/function is highly aberrant. Copper cells are specified by the homeotic gene labial (lab), the only homeotic gene expressed in the endoderm (Bienz, 1996; Hoppler and Bienz, 1994). In the larval stage, lab expression is essential for both the differentiation of copper cell progenitors to their gastric acid-secreting, highly structured terminal phenotype and the perdurance of the differentiated state (Hoppler and Bienz, 1994). The unusual requirement for homeotic (lab) gene expression in copper cell specification and continued differentiation indicates that lab protein target gene products confer and/or regulate copper cell morphology and function (Hoppler and Bienz, 1994). Interestingly, the midgut epithelial phenotype of Gp93 mutant larvae mirrors mutations in lab, in the loss of copper cell acid secretion functionality and aberrant morphology of the apical membrane domain (Dubreuil et al., 1998; Hoppler and Bienz, 1994). The striking similarities between Gp93 and lab mutants suggest that Gp93 chaperone activity may be essential for the functional expression of lab protein target gene products that confer the unique copper cell apical membrane morphology and function, and enable junctional association with the adjacent interstitial cells.
In addition to similarities with lab mutants, the Gp93 mutant midgut epithelium displays defects similar to those reported for loss of function mutants in the Drosophila α- and βH-spectrin (Dubreuil et al., 1998; Lee et al., 1993; Phillips and Thomas, 2006). In Drosophila, α-spectrin is enriched in septate junctions, apical cell-cell junctions functioning in a manner similar to vertebrate tight junctions, and mutations in α- and βH-spectrin cause defects in nuclear positioning, midgut acidification, and copper cell apical pore enlargement (Dubreuil et al., 1998; Lee et al., 1993). In Gp93 mutant larvae, the defects in epithelial morphology and copper cell apical pore morphology are not accompanied by defects in nuclear positioning, suggesting that the phenotypic similarities between the Gp93 and α- and βH-spectrin mutant phenotypes derive from distinct, but functionally overlapping, molecular origins. Because Gp93 is a resident ER luminal chaperone, the loss of Gp93 expression would not be expected to directly affect α- and βH-spectrin function. However, as spectrin serves multiple functions in, for example, cell adhesion complex assembly and signaling function, as well as epithelial apicobasal membrane domain specification, we speculate that loss of Gp93 expression disables the functional expression of integral membrane proteins that directly or indirectly interact with spectrin, and thereby mimic α- and βH-spectrin mutant phenotypes.
In addition to defects in acid secretion and apical membrane morphology, loss of Gp93 expression in the copper cell is accompanied by aberrant copper cell-interstitial cell septate junction structure, as has also been reported for mutations in the Drosophila, α-spectrin (Dubreuil et al., 1998). Septate junctions function in a manner similar to vertebrate tight junctions to regulate intercellular adhesion, paracellular diffusion and cell polarity. At least 13 gene products comprise the Drosophila septate junction, including α-spectrin, and of these, at least six are integral membrane proteins, the GPI-anchored protein contactin, fasciclin-3, gliotactin, lachesin, neuroglian, and neurexin (Baumgartner et al., 1996; Faivre-Sarrailh et al., 2004; Genova and Fehon, 2003; Llimargas et al., 2004; Patel et al., 1987; Schulte et al., 2003). Because the Gp93-proteome is necessarily limited to secretory and integral membrane proteins, any or all of these gene products may require Gp93 for functional expression, and thus their sorting/assembly into midgut septate junctions may be defective in the Gp93 mutants. Interestingly, of these six gene products, at least five, contactin, fasciclin-3, gliotactin, lachesin, neuroglian, have been demonstrated to display cell adhesion activity (Faivre-Sarrailh et al., 2004; Genova and Fehon, 2003; Llimargas et al., 2004; Patel et al., 1987; Schulte et al., 2003). With it now established that GRP94 is required for Toll-like receptor and integrin expression (Randow and Seed, 2001; Yang et al., 2007), and given that Toll and the Toll-like protein 18-wheeler serve important cell adhesion roles in early Drosophila development (Eldon et al., 1994; Keith and Gay, 1990), we postulate that Gp93(GRP94) chaperone function is uniquely required for the functional expression of protein domains that display adhesion activity and which are critical for multicellularity.
Metabolic consequences of loss of Gp93 expression
The Drosophila insulin signaling pathway is highly sensitive to nutrient status (Baker and Thummel, 2007; Britton et al., 2002). When Drosophila larvae are deprived of dietary protein, for example, insulin signaling pathway function is suppressed and fat body nutrient stores are mobilized (Britton et al., 2002). Paralleling scenarios of nutrient starvation, Gp93 mutant larvae fed complete fly food display a suppression of fat body insulin signaling and greatly enhanced fat body lipid catabolism. Interestingly, restricting the loss of Gp93 expression to the fat body, via a pumpless-GAL4>UAS-Gp93-hairpin, has no discernible effect on larval growth rates, morphology, or development (Table SI). This conclusion is supported by the extensive and metabolically appropriate fat body response to the nutrient-limiting phenotype of Gp93 mutant larvae, yet must be considered conjectural at present, as we have not established that fat body Gp93 is absent in the pumpless-GAL4>UAS-Gp93-hairpin line.
Although the metabolic and transcriptional phenotype of Gp93 mutant larvae bears many similarities with previously characterized metabolic and transcriptional responses to starvation, the Gp93 mutant larval phenotype is expressed in animals that are continuously eating. Furthermore, nutrient assimilation studies demonstrate that although nutrient transport from the gut is suppressed in the mutants, significant nutrient uptake is evident, and in the case of glucose and leucine, indistinguishable from Gp93 heterozygote larvae. These data suggest that the altered midgut function and morphology in Gp93 mutant larvae also includes disruptions in gut-specific nutrient sensing and signaling function. In mammalian systems, nutrient sensing in the gut is mediated by enteroendocrine cells, signaling via peptide hormones including 5-HT, cholecystokinin (CCK), secretin, corticotrophin-releasing factor, somatostatin, orexin, or peptide YY (Tome, 2007). The Drosophila larval midgut contains enteroendocrine cells and these cells are a significant source of peptide hormones that can be presumed to regulate appetite and digestion by similar mechanisms to those occurring in mammals (Micchelli and Perrimon, 2006; Nichols, 2007; Ohlstein and Spradling, 2006; Veenstra, 2009). The growth defect and starvation signaling response seen in the Gp93 mutant larvae may thus reflect defects in enteroendocrine cell-mediated nutrient sensing and signaling functions. As these cells can be considered professional secretory cells, we speculate that Gp93 expression may be required for enteroendocrine cell peptide hormone secretion. Consistent with this view, GRP94 is required for insulin-like growth factor II secretion in mouse (Ostrovsky et al., 2009; Wanderling et al., 2007).
In summary, our investigations into the chaperone biology of GRP94, using Drosophila as a model, have revealed essential roles for Gp93 in midgut epithelial homeostasis and function. Of particular interest, in the larval stage, the loss of Gp93 expression predominately affects the structure/function of the copper cell. Because copper cell structure/function has the highly unusual characteristic of being both specified by, and requiring the continued expression of, the homeotic gene labial, the only homeotic gene expressed in the endoderm, these findings provide a unique model system for the study of Hsp90 chaperone function in cell specification, cell differentiation, and cell-cell interaction.
Supplementary Material
Gp93, human GRP94, human Hsp90, and yeast Hsp90 (Hsc82) amino acid sequences were aligned using ClustalW algorithm with CLC Sequence Viewer (CLC Bio). The characteristic helix 1-4-5 subdomain insertion is highlighted in blue; ER retention signal highlighted in red; signal sequence highlighted in black; ATP binding residue noted with blue asterisk; ATP hydrolysis residues noted with red asterisks.
A) Quantification of feeding behavior in Gp93 heterozygous and mutant larvae at 56 and 80 hr AED. B) Quantification of the fractional population of Gp93 heterozygote and mutant larvae displaying wandering behavior at the indicated time points.
A) Transcriptional response to loss of Gp93 expressed by gene ontogeny (GO) category. B) Genes of known function whose expression are significantly upregulated or downregulated in Gp93 mutant larvae. C) RT-PCR starvation gene signature analysis comparing 72 hr AED Gp93 heterozygote and mutant larvae at the indicated time points. Lane 1 = act, lane 2 = Lip4, lane 3 = CPTI, and lane 4 = Lip3.
Thin layer chromatography analysis of lipid extracts from 96 Hr AED Gp93 heterozygote and mutant larval fat body tissue. Equivalent quantities of total phospholipids, determined by organic phosphorous analysis, were loaded in each lane. Relative amounts were determined by densitometry analysis (ImageJ) of amido-black stained plates.
A) Flow cytometric analysis of InR cell surface localization in GRP94-scrambled (sc) and GRP94-knockdown (kd) HEK-293 cells using an α-InR antibody (1:50). Red (GRP94sc) and orange (GRP94kd) peaks are unstained controls; blue (GRP94sc) and green (GRP94kd) represent antibody stained cell populations. B) Immunoblot analysis depicting phosphorylation of Akt in GRP94sc and GRP94kd HEK-293 cells in response to addition of human insulin.
TABLE S1. Effects of global (tub-Gal4) and fat body restricted (ppl-Gal4) RNai-mediated Gp93 silencing on larval growth.
Acknowledgments
Where noted, monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank (DSHB) developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA. The authors would like to thank Dr. Tim Oliver, Department of Cell Biology, DUMC for helpful advice and support for all confocal microscopy studies and Jamie Roebuck, Model Systems Genomics, Duke University for advice and embryo injections. We also acknowledge the enthusiastic and tireless contributions of Mark Nicchitta to the isolation of Gp93 mutant lines. Supported by grants from the NIH (CA104392, CVN, and DK58398, CBN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology. 1997;113:332–40. doi: 10.1016/s0016-5085(97)70112-4. [DOI] [PubMed] [Google Scholar]
- Ames BN, Dubin DT. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;235:769–75. [PubMed] [Google Scholar]
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–66. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker K. Feeding period, growth and pupation in larvae of Drosophila melanogaster. Entomologia exp appl. 1959;2:171–186. [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–68. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Bella J, Hindle KL, McEwan PA, Lovell SC. The leucine-rich repeat structure. Cell Mol Life Sci. 2008;65:2307–33. doi: 10.1007/s00018-008-8019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Induction of the endoderm in Drosophila. Annu Rev Cell Dev Biol. 1996;7:113–119. [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–49. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- Butterworth FM, Bodenstein D, King RC. Adipose Tissue of Drosophila Melanogaster. I. An Experimental Study of Larval Fat Body. J Exp Zool. 1965;158:141–53. doi: 10.1002/jez.1401580203. [DOI] [PubMed] [Google Scholar]
- Cao C, Brown MR. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res. 2001;304:317–21. doi: 10.1007/s004410100367. [DOI] [PubMed] [Google Scholar]
- Chen B, Zhong D, Monteiro A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genomics. 2006;7:156. doi: 10.1186/1471-2164-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS. Amino Acid and Protein Metabolism in Insect Development. In: Beament JWL, Treherne JE, editors. Advacnes in Insect Physiology. Vol. 3. Academic Press; 1967. pp. 53–114. [Google Scholar]
- Chen PSaHE. Zur Stoffwechselphysiologie der Mutante letalmeander (lme) von Drosophila melanogaster. Revue suisse Zool. 1955;62:338–347. [Google Scholar]
- Church RB, Robertson FW. Biochemical analysis of genetic differences in the growth of Drosophila. Genet Res. 1966;7:383–407. doi: 10.1017/s0016672300009836. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–49. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Daniel H, Spanier B, Kottra G, Weitz D. From bacteria to man: archaic proton-dependent peptide transporters at work. Physiology (Bethesda) 2006;21:93–102. doi: 10.1152/physiol.00054.2005. [DOI] [PubMed] [Google Scholar]
- Dimitriadis VK. Fine structure of the midgut of adult Drosophila auraria and its relationships to the sites of acidophilic secretion. J Insect Physiol. 1991;37:167–177. [Google Scholar]
- Dollins DE, Warren JJ, Immormino RM, Gewirth DT. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell. 2007;28:41–56. doi: 10.1016/j.molcel.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil RR, Frankel J, Wang P, Howrylak J, Kappil M, Grushko TA. Mutations of alpha spectrin and labial block cuprophilic cell differentiation and acid secretion in the middle midgut of Drosophila larvae. Dev Biol. 1998;194:1–11. doi: 10.1006/dbio.1997.8821. [DOI] [PubMed] [Google Scholar]
- Eldon E, Kooyer S, D’Evelyn D, Duman M, Lawinger P, Botas J, Bellen H. The Drosophila 18 wheeler is required for morphogenesis and has striking similarities to Toll. Development. 1994;120:885–99. doi: 10.1242/dev.120.4.885. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–42. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol. 2003;161:979–89. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445:275–80. doi: 10.1038/nature05382. [DOI] [PubMed] [Google Scholar]
- Hashimoto C, Gerttula S, Anderson KV. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: importance of transmembrane signaling for dorsal-ventral pattern formation. Development. 1991;111:1021–8. doi: 10.1242/dev.111.4.1021. [DOI] [PubMed] [Google Scholar]
- Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–79. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Bienz M. Specification of a single cell type by a Drosophila homeotic gene. Cell. 1994;76:689–702. doi: 10.1016/0092-8674(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Hull-Thompson J, Muffat J, Sanchez D, Walker DW, Benzer S, Ganfornina MD, Jasper H. Control of metabolic homeostasis by stress signaling is mediated by the lipocalin NLaz. PLoS Genet. 2009;5:e1000460. doi: 10.1371/journal.pgen.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith FJ, Gay NJ. The Drosophila membrane receptor Toll can function to promote cellular adhesion. EMBO J. 1990;9:4299–306. doi: 10.1002/j.1460-2075.1990.tb07878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleve CD, Siler DA, Syed SK, Eldon ED. Expression of 18-wheeler in the follicle cell epithelium affects cell migration and egg morphology in Drosophila. Dev Dyn. 2006;235:1953–61. doi: 10.1002/dvdy.20820. [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci. 1994;19:415–21. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Kramer JM, Davidge JT, Lockyer JM, Staveley BE. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Dev Biol. 2003;3:5. doi: 10.1186/1471-213X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Coyne RS, Dubreuil RR, Goldstein LS, Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol. 1993;123:1797–809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–8. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- Llimargas M, Strigini M, Katidou M, Karagogeos D, Casanova J. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development. 2004;131:181–90. doi: 10.1242/dev.00917. [DOI] [PubMed] [Google Scholar]
- Ludwig D, Wugmeister M. Respiratory metabolism and the activities of cytochrome oxidase and succinic dehydrogenase during the embryonic development of the Japanese beetle, Popillia japonica Newman. J Cell Physiol. 1955;45:157–65. doi: 10.1002/jcp.1030450202. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Migeon JC, Garfinkel MS, Edgar BA. Cloning and characterization of peter pan, a novel Drosophila gene required for larval growth. Mol Biol Cell. 1999;10:1733–44. doi: 10.1091/mbc.10.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami R, Takashima S, Hamaguchi T. Developmental genetics of the Drosophila gut: specification of primordia, subdivision and overt-differentiation. Cell Mol Biol (Noisy-legrand) 1999;45:661–76. [PubMed] [Google Scholar]
- Nichols R. The first nonsulfated sulfakinin activity reported suggests nsDSK acts in gut biology. Peptides. 2007;28:767–73. doi: 10.1016/j.peptides.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Okamura T, Shimizu H, Nagao T, Ueda R, Ishii S. ATF-2 regulates fat metabolism in Drosophila. Mol Biol Cell. 2007;18:1519–29. doi: 10.1091/mbc.E06-10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Ostrovsky O, Ahmed NT, Argon Y. The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol Biol Cell. 2009;20:1855–64. doi: 10.1091/mbc.E08-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NH, Snow PM, Goodman CS. Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48:975–88. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Thomas GH. Brush border spectrin is required for early endosome recycling in Drosophila. J Cell Sci. 2006;119:1361–70. doi: 10.1242/jcs.02839. [DOI] [PubMed] [Google Scholar]
- Plekhanov AY. Rapid staining of lipids on thin-layer chromatograms with amido black 10B and other water-soluble stains. Anal Biochem. 1999;271:186–7. doi: 10.1006/abio.1999.4127. [DOI] [PubMed] [Google Scholar]
- Po-Chedley DS. Effects of starvation on the free amino acids in larval blood of the oriental beetle, Anomala orientalis. J NY ent Soc. 1958;66:171–176. [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–6. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- Roman G, Meller V, Wu KH, Davis RL. The opt1 gene of Drosophila melanogaster encodes a proton-dependent dipeptide transporter. Am J Physiol. 1998;275:C857–69. doi: 10.1152/ajpcell.1998.275.3.C857. [DOI] [PubMed] [Google Scholar]
- Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–31. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–20. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Rutherford S, Hirate Y, Swalla BJ. The Hsp90 capacitor, developmental remodeling, and evolution: the robustness of gene networks and the curious evolvability of metamorphosis. Crit Rev Biochem Mol Biol. 2007;42:355–72. doi: 10.1080/10409230701597782. [DOI] [PubMed] [Google Scholar]
- Salz HK, Cline TW, Schedl P. Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics. 1987;117:221–31. doi: 10.1093/genetics/117.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J Cell Biol. 2003;161:991–1000. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag S, Tripathi S. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J Exp Biol. 2009;212:1731–44. doi: 10.1242/jeb.029306. [DOI] [PubMed] [Google Scholar]
- Soldano KL, Jivan A, Nicchitta CV, Gewirth DT. Structure of the N-terminal domain of GRP94. Basis for ligand specificity and regulation. J Biol Chem. 2003;278:48330–8. doi: 10.1074/jbc.M308661200. [DOI] [PubMed] [Google Scholar]
- Spradling AC. P element-mediated transformation. In: Roberts D, editor. Drosophila: A Practical Approach. IRL Press; Oxford: 1986. pp. 175–197. [Google Scholar]
- Stechmann A, Cavalier-Smith T. Evolutionary origins of Hsp90 chaperones and a deep paralogy in their bacterial ancestors. J Eukaryot Microbiol. 2004;51:364–73. doi: 10.1111/j.1550-7408.2004.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–65. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- Tome D. From gut nutrient sensing to nutrient perception: a cooperative role involving CCK and 5-HT? Am J Physiol Regul Integr Comp Physiol. 2007;292:R1061–2. doi: 10.1152/ajpregu.00846.2006. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 2009;336:309–23. doi: 10.1007/s00441-009-0769-y. [DOI] [PubMed] [Google Scholar]
- Voght SP, Fluegel ML, Andrews LA, Pallanck LJ. Drosophila NPC1b promotes an early step in sterol absorption from the midgut epithelium. Cell Metab. 2007;5:195–205. doi: 10.1016/j.cmet.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Wanderling S, Simen BB, Ostrovsky O, Ahmed NT, Vogen SM, Gidalevitz T, Argon Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell. 2007;18:3764–75. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–7. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–72. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Wu JY, Kao HJ, Li SC, Stevens R, Hillman S, Millington D, Chen YT. ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest. 2004;113:434–440. doi: 10.1172/JCI19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–26. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinke I, Schutz CS, Katzenberger JD, Bauer M, Pankratz MJ. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J. 2002;21:6162–73. doi: 10.1093/emboj/cdf600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gp93, human GRP94, human Hsp90, and yeast Hsp90 (Hsc82) amino acid sequences were aligned using ClustalW algorithm with CLC Sequence Viewer (CLC Bio). The characteristic helix 1-4-5 subdomain insertion is highlighted in blue; ER retention signal highlighted in red; signal sequence highlighted in black; ATP binding residue noted with blue asterisk; ATP hydrolysis residues noted with red asterisks.
A) Quantification of feeding behavior in Gp93 heterozygous and mutant larvae at 56 and 80 hr AED. B) Quantification of the fractional population of Gp93 heterozygote and mutant larvae displaying wandering behavior at the indicated time points.
A) Transcriptional response to loss of Gp93 expressed by gene ontogeny (GO) category. B) Genes of known function whose expression are significantly upregulated or downregulated in Gp93 mutant larvae. C) RT-PCR starvation gene signature analysis comparing 72 hr AED Gp93 heterozygote and mutant larvae at the indicated time points. Lane 1 = act, lane 2 = Lip4, lane 3 = CPTI, and lane 4 = Lip3.
Thin layer chromatography analysis of lipid extracts from 96 Hr AED Gp93 heterozygote and mutant larval fat body tissue. Equivalent quantities of total phospholipids, determined by organic phosphorous analysis, were loaded in each lane. Relative amounts were determined by densitometry analysis (ImageJ) of amido-black stained plates.
A) Flow cytometric analysis of InR cell surface localization in GRP94-scrambled (sc) and GRP94-knockdown (kd) HEK-293 cells using an α-InR antibody (1:50). Red (GRP94sc) and orange (GRP94kd) peaks are unstained controls; blue (GRP94sc) and green (GRP94kd) represent antibody stained cell populations. B) Immunoblot analysis depicting phosphorylation of Akt in GRP94sc and GRP94kd HEK-293 cells in response to addition of human insulin.
TABLE S1. Effects of global (tub-Gal4) and fat body restricted (ppl-Gal4) RNai-mediated Gp93 silencing on larval growth.