Abstract
We report the synthesis of novel acid-responsive therapeutic nanoparticles (NPs) with sub-100 nm size consisting of polymer-cisplatin conjugates. The uniqueness of these drug delivery polymeric NPs lies in the covalent conjugation of each cisplatin drug to the hydrophobic segment of two biocompatible diblock copolymer chains through hydrazone bond, resulting in highly differential drug release profile at different environmental acidity. We demonstrate that the synthesized polymer-cisplatin conjugates can readily precipitate to form sub-100 nm NPs in aqueous solution due to their very low critical micellar concentration (CMC). The resulting NPs show well controlled cisplatin loading yield, excellent acid-responsive drug release kinetics, and enhanced in vitro cytotoxicity against ovarian cancer cells as compared to free cisplatin. As an environmentally sensitive drug delivery vehicle, these NPs can potentially minimize the drug loss during NP circulation in the blood where the pH value is neutral and trigger rapid intracellular drug release after the NPs are endocytosed by the target cells. This characteristic drug release profile holds the promise to suppress cancer cell chemoresistance by rapidly releasing a high dose of chemotherapy drugs inside the tumor cells, thereby improving the therapeutic efficacy of the drug payload.
Keywords: Polymeric nanoparticle, Cisplatin, Drug delivery, Controlled release, Stimuli-responsive
Introduction
Cis-diamminedichloroplatinum(II), known as cisplatin, has been widely used in the clinic to treat a variety of cancers such as ovarian, breast, bladder, head and neck, and small cell lung cancer because of its potent activity to cross-link DNA upon entering the cells.1 It preferentially binds to the N7 atoms of guanine bases in DNA double-helix strands, thereby preventing the strands from uncoiling and separating. This prohibits the division of the cells and ultimately results in cellular apoptosis.2–4 However, cisplatin is vulnerable to attack by plasma proteins, particularly those containing thiol groups, such as human serum albumin.5 It has been reported that one day after cisplatin administration, 65–98% of the platinum in the blood was protein bound.6, 7 This undesirable protein binding deactivated the drugs leading to less therapeutic efficacy and accounted for some severe side effects of cisplatin therapy.8–10
To improve the therapeutic index of cisplatin while minimizing its adverse side effects, cisplatin analogue Pt(IV) prodrugs have been synthesized.11–14 These Pt(IV) prodrugs usually have less potency and toxicity than Pt (II) before they are reduced to their corresponding Pt (II) derivatives by cysteine or other stimuli. In these Pt(IV) prodrugs, two additional coordination sites become available which offer great synthetic flexibility for further chemical modification. For instance, axial oxidation of cisplatin yields a dihydroxy product, cis,cis,trans- Pt(NH3)2Cl2(OH)2, which can be further esterified with carboxyl or acid anhydride groups to provide different functionality. Ang et al. have synthesized ethacraplatin via the acylation of cis,cis,trans-Pt(NH3)2Cl2(OH)2 with an excess of ethacrynic acid chloride and demonstrated the capacity of ethacraplatin to overcome glutathione-s-transferase mediated drug resistance.11 More recently, Mukhopadhyay et al. have modified cisplatin with acid anhydride, followed by conjugation with targeting peptides for selective delivery of Pt(IV) to angiogenic tumor vasculature.15 These cisplatin analogue Pt (IV) prodrugs mainly have been applied directly as small molecule drugs for cancer treatment.
A further approach to improve the therapeutic efficacy of these cisplatin analogue Pt(IV) prodrugs is to load them into a drug nanocarrier,16, 17 for example a polymeric nanoparticle (NP), and then preferentially deliver the drug-loaded nanocarriers to the cells or tissues of interest instead of administering free drugs. Many advantages of using polymeric NPs to deliver drugs have been recognized in the past decades.18, 19 It prolongs the half-life of drugs in the systemic circulation, releases drugs at a sustained rate or in an environmentally responsive manner, delivers drugs in a target manner to minimize systemic side effects, and delivers two or more drugs simultaneously for combination therapy to generate a synergistic effect and suppress drug resistance.19 However, loading cisplatin into polymeric NPs is challenging because of the poor solubility of cisplatin in organic solvents and its partial solubility in water.12, 20 A pioneer study by Dhar et al. has physically encapsulated cisplatin analogue Pt(IV) prodrugs into polymeric NPs by attaching two linear hexyl chains to the Pt(IV) prodrugs and thus converting the prodrug to a more hydrophobic derivative. They have successfully demonstrated the enhanced therapeutic efficacy of the Pt(IV) prodrug-encapsulated NPs against prostate cancer. 12
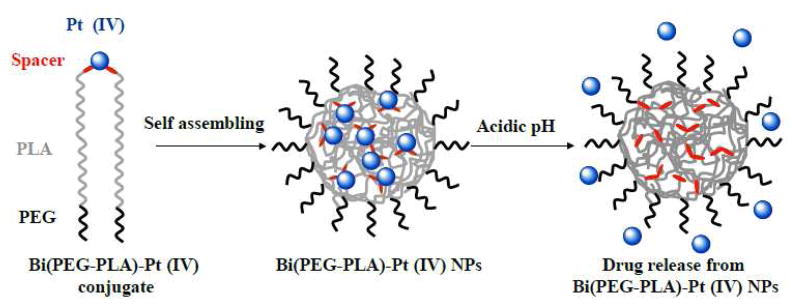
Here we hypothesized that by covalently conjugating Pt(IV) prodrugs to the building blocks of polymeric NPs through a stimuli-sensitive bond (e.g., an acid-responsive bond), one is able to not only load cisplatin analogue Pt(IV) prodrugs into polymeric NPs but also control cisplatin release kinetics from the NPs in an environmentally sensitive manner. This would allow for minimizing the amount of drugs leaching out of the NPs during their circulation in the blood (pH=7.4), and enable rapid intracellular drug release when the NPs are endocytosed by the target cells (pH=5~6 21). To this end, we synthesized a hydrazine terminated poly(ethylene glycol)-b-poly(l-lactide) (PEG-PLA-NH-NH2) copolymer and a levulinic acid modified cisplatin analogue Pt(IV) prodrug. Subsequently, these two compounds were covalently conjugated to each other with a stoichiometric ratio of 2:1 through acid-responsive hydrazone bond, resulting in a polymer-cisplatin prodrug conjugate, Bi(PEG-PLA)-Pt(IV). This conjugate was then precipitated to form sub-100 nm polymeric NPs as illustrated in Figure 1. We demonstrated that these polymer-cisplatin prodrug conjugate NPs had well controlled drug loading yield, excellent acid-responsive drug release characteristics and potent cytotoxicity against ovarian cancer.
Figure 1.
Schematic illustrations of the structure of polymer-cisplatin prodrug conjugate, the formation of Bi(PEG-PLA)-Pt(IV) NPs through self-assembling, and acid-responsive drug release from the NPs
Results and discussions
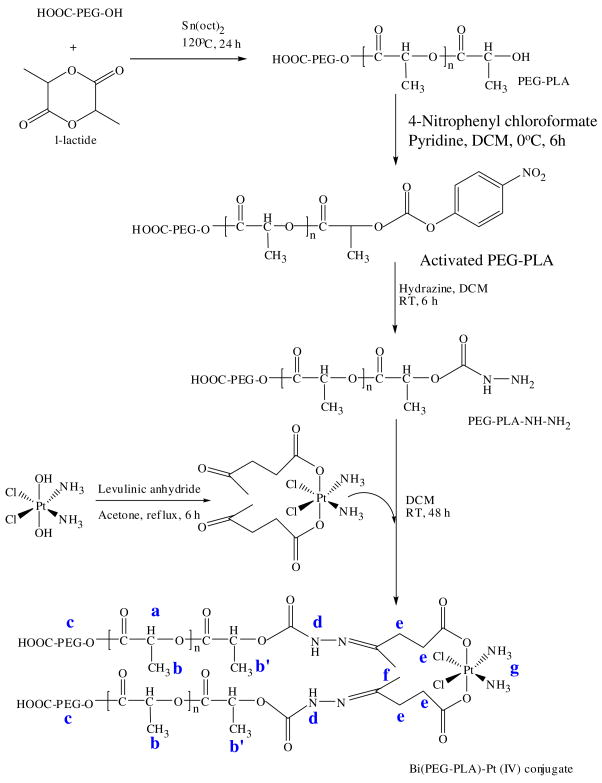
In the study, we first synthesized a Bi(PEG-PLA)-Pt(IV) polymer-cisplatin prodrug conjugate following the protocol illustrated in Figure 2. Briefly, carboxyl-functionalized poly(ethylene glycol) (COOH-PEG-OH, Mn = 3500 gmol−1) was used as macroinitiator for the ring opening polymerization of l-lactide in the presence of stannous octoate as a catalyst. The resultant poly(ethylene glycol)-b-poly(l-lactide) (PEG-PLA) was characterized by gel permeation chromatography (GPC) with a molecular weight of 10000 gmol−1 and a polydispersity index of 1.12. Then the PEG-PLA was activated by 4-nitrophenyl chloroformate and derivatized to its hydrazine derivative (PEG-PLA-NH-NH2). Subsequently, this hydrazine terminated polymer was reacted with pre-synthesized cis,trans,cis-PtCl2(OCOCH2CH2COCH3)2 (NH3)2 cisplatin analogue prodrug, in which levulinic acid was used as a spacer, resulting in a Bi(PEG-PLA)-Pt(IV).
Figure 2.
Schematic description of the synthesis of Bi(PEG-PLA)-Pt(IV) polymer-prodrug conjugate.
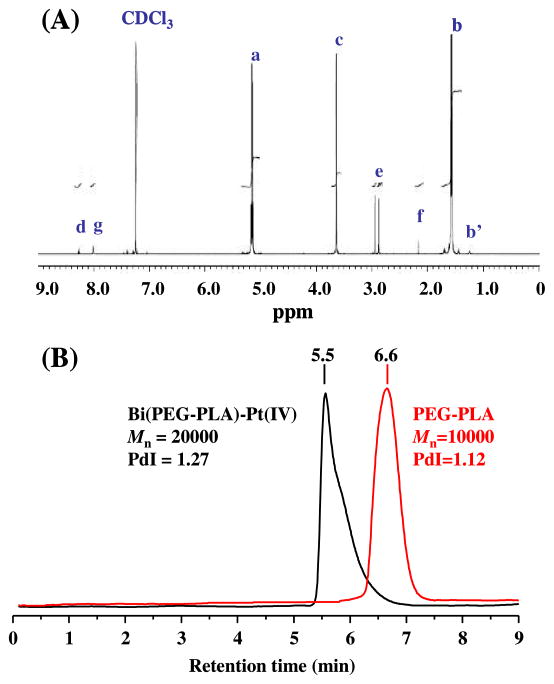
The entire reaction processes were monitored by 1H-NMR spectroscopy and GPC. The 1H NMR spectra of all intermediate products were provided in the Supporting Information (Figure S1–S4). As indexed in Figure 3A, the 1H-NMR spectrum of the final polymer-cisplatin prodrug conjugate included all characteristic resonance peaks of the PEG-PLA polymer, the levulinic acid spacer, and the Pt(IV) prodrug, for example, the characteristic peaks of −NH-N= at δ 8.28 ppm, −CH2 of levulinic acid at δ 2.1 and 2.95 ppm, and −NH3 of cisplatin at δ 8.0 ppm. A considerable 1H NMR resonance shift was observed for the −CH2 of levulinic acid and the −NH3 of cisplatin in the final product as compared to those in PtCl2(OCOCH2CH2COCH3)2(NH3)2 (Figure S4). This is likely due to the presence of two polyester chains that sandwich the Pt(IV) metal from the axial position in the Bi(PEG-PLA)-Pt(IV) conjugate. Such conformation creates local magnetic field inhomogeneity accounting for the resonance shift. In addition, the quadrupolar effect of the 14N nucleus may also contribute to the resonance shift because it makes protons resonating in a broad spectrum.14 The formation of Bi(PEG-PLA)-Pt(IV) conjugate was further confirmed by the GPC measurements. As shown in Figure 3B, the characteristic peak of PEG-PLA at 6.6 min disappeared in the chromatogram of the polymer-cisplatin prodrug conjugate. Instead, a dominant peak appeared at the retention time of 5.5 min, corresponding to the molecular weight of about 20000 gmol−1. This clearly indicates the formation of Bi(PEG-PLA)-Pt(IV) conjugate, which contains two PEG-PLA polymer chains.
Figure 3.
Characterization of Bi(PEG-PLA)-Pt(IV) polymer-prodrug conjugate. (A) 1H-NMR spectrum of the synthesized Bi(PEG-PLA)-Pt(IV) conjugate. (B) GPC chromatogram of PEG-PLA polymer and Bi(PEG-PLA)-Pt (IV) conjugate.
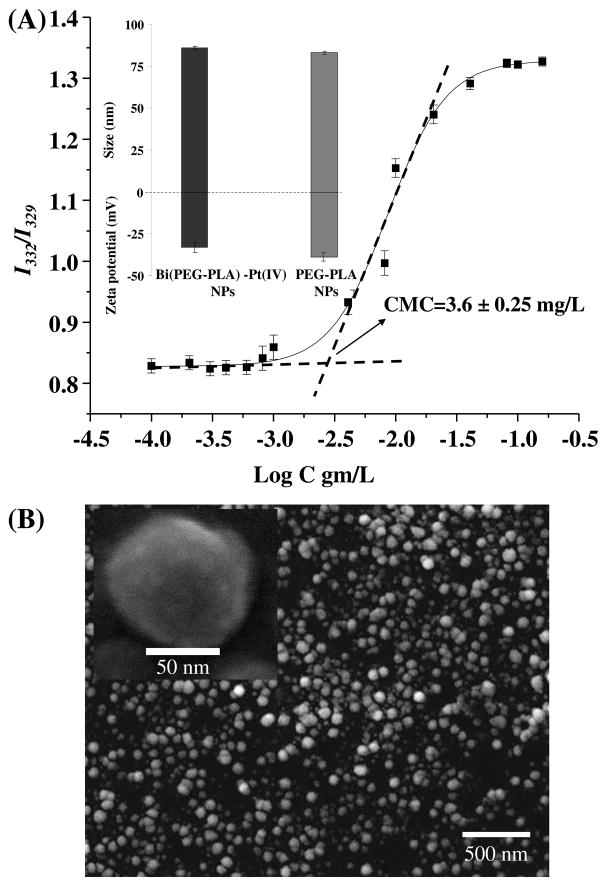
Next we measured the critical micellar concentration (CMC) of the synthesized Bi(PEG-PLA)-Pt(IV) conjugate to evaluate their feasibility of forming NPs. CMC was determined using pyrene as a hydrophobic probe that has been widely used for this purpose because of its characteristic fluorescence spectra sensitive to environmental polarity.22 The fluorescence emission of pyrene was fixed at 390 nm while its excitation spectra were monitored at various concentrations of Bi(PEG-PLA)-Pt(IV) conjugate. The intensity ratio at 332 nm and 329 nm was plotted against polymer-cisplatin prodrug concentration in a semi-log graph. As shown in Figure 4A, the CMC of the Bi(PEG-PLA)-Pt(IV) conjugate was 3.6±0.25 mg/L. This very low CMC value indicates that the conjugate is prone to form NPs via precipitation method. Indeed, dynamic light scattering (DLS) measurement showed that the self-assembled Bi(PEG-PLA)-Pt(IV) conjugate NPs had an unimodal size distribution with an averaged diameter of 86±2.0 nm (Figure 4A inset), which was consistent with the findings from SEM images (Figure 4B). The surface zeta potential of the NPs was about −33±1.2 mV (Figure 4A inset). We further found that the size and surface zeta potential of the polymer-prodrug conjugate NPs were similar to those of the corresponding PEG-PLA polymeric NPs, 83±2.0 nm and −36±2.0 mV, respectively. This suggests that conjugation of cisplatin prodrug to the PEG-PLA polymer chain has negligible effect on formation of the polymeric NPs.
Figure 4.
(A) Determination of the critical micelle concentration (CMC) of Bi(PEG-PLA)-Pt(IV) conjugate using pyrene probe. Inset: NP size, surface zeta potential of Bi(PEG-PLA)-Pt(IV) NPs and PEG-PLA NPs measured by DLS. (B) Representative SEM image of Bi(PEG-PLA)-Pt(IV) conjugate NPs. Inset: High resolution SEM image of a single Bi(PEG-PLA)-Pt(IV) NP.
After having prepared Bi(PEG-PLA)-Pt(IV) NPs, we then quantified cisplatin loading yield of the NPs and cisplatin release kinetics from the NPs at different pH values using inductively coupled plasma optical emission spectrometry (ICP-OES). Here the drug loading yield is defined as the weight ratio of the cisplatin payload to the NPs including both polymer excipients and cisplatin. The drug release kinetics represents how fast the drugs leak out of the NPs, plotting as the weight ratio of the accumulative released cisplatin to the total cisplatin payload against time. As shown in Figure 5A, when more Pt(IV) prodrugs were used to react with PEG-PLA during the polymer-prodrug conjugation process, a higher cisplatin drug loading yield was achieved. For example, the initial Pt(IV)/PEG-PLA reaction molar ratio of 2:1, 4:1, and 6:1 resulted in a final cisplatin drug loading yield of 0.35±0.01 wt%, 0.89±0.02 wt%, and 1.05±0.03 wt% (mean±SD, n=3), respectively. However, when the Pt(IV)/PEG-PLA molar ratio was higher than 6:1 (e.g., 8:1), no considerable drug loading yield increase occurred. This is likely due to the saturation of polymer chains, in which all PEG-PLA polymers have been conjugated with Pt(IV) prodrugs. Figure 5B showed the cisplatin release kinetics from the Bi(PEG-PLA)-Pt(IV) NPs at three distinct pH values, pH=5.0, 6.0, and 7.4, respectively. The cisplatin release rate from the NPs at pH=5.0 and 6.0 was significantly faster than at pH=7.4. When the cisplatin loading yield was 1.05 wt% (Figure 5B), it took the Bi(PEG-PLA)-Pt(IV) NPs around 4 hrs and 6 hrs to release 50% of total cisplatin payload at pH=5.0 and 6.0, respectively, versus 22 hrs at pH=7.4. The contrast of the cisplatin release rate was even more sharply within the first a few hrs. For example, during the first 2 hrs period, 17% and 15% of the cisplatin payload was released at pH=5.0 and 6.0, respectively, while only 2% was released at pH=7.4. These results suggest that cisplatin release kinetics from the Bi(PEG-PLA)-Pt(IV) NPs is pH-dependent. This is mainly because the cisplatin analogue Pt(IV) prodrugs were covalently conjugated to the polymer chains through hydrazone bond, which is an acid-labile bond. At pH=5~6, hydrazone bond can be easily cleaved within a few minutes to free the drugs which will diffuse out of the NPs. In contrast, this bond is relatively stable at pH=7.4.23 The observed sustained cisplatin release at pH=7.4 may be due to the degradation of the PLA polymers, to which the cisplatin analogue prodrugs were covalently linked. As a biodegradable polymer, PLA ester can be hydrolyzed to small segments or monomers at both neutral pH and acidic pH.24 Here we incubated the PEG-PLA NPs in pH=5.0, 6.0, and 7.4 PBS solutions at 37 °C, respectively. At each time point, an aliquot of the PEG-PLA NPs were collected to measure the polymer molecular weight (Mw) using GPC. As shown in Figure 5B inset, after 50 hrs incubation, the polymer Mw decreased by a factor of 20%, 18%, and 8 % at pH=5.0, pH=6.0, and pH=7.4, respectively. This data reasonably explains the sustained drug release kinetics at neutral pH as shown in Figure 5B but also raises a concern that the observed rapid drug release at pH=5.0 and 6.0 might be because of fast PLA degradation at acidic pH rather than the cleavage of hydrazone bond. However, negligible difference of Mw loss was observed within the first 24 hrs of incubation at these three pH values. This confirms that the drug burst at pH=5.0 and 6.0 during the first a few hrs is due to the cleavage of the hydrazone bond but not polymer degradation.
Figure 5.
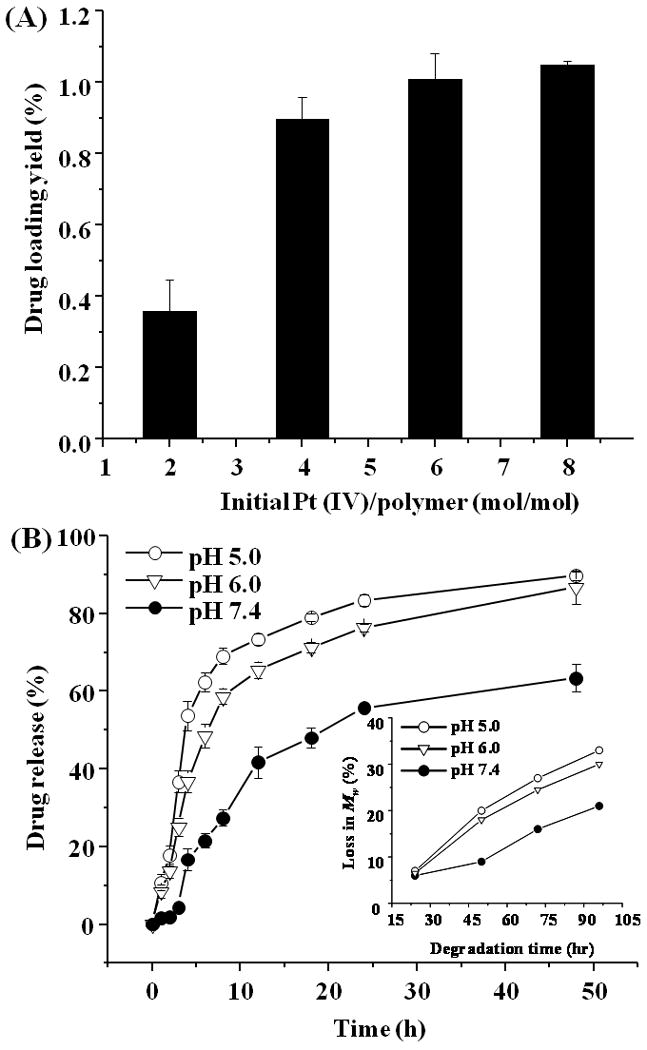
(A) Cisplatin loading yield of Bi(PEG-PLA)-Pt(IV) NPs at various initial Pt(IV)/PEG-PLA reaction molar ratios. (B) Cisplatin release profile from Bi(PEG-PLA)-Pt(IV) NPs at pH=5.0 (open circles), pH=6.0 (open triangles), and pH=7.4 (solid circles) PBS buffer at 37 °C. Inset: Hydrolytic degradation rate of PEG-PLA NPs at pH=5.0, pH=6.0, and pH=7.4, respectively.
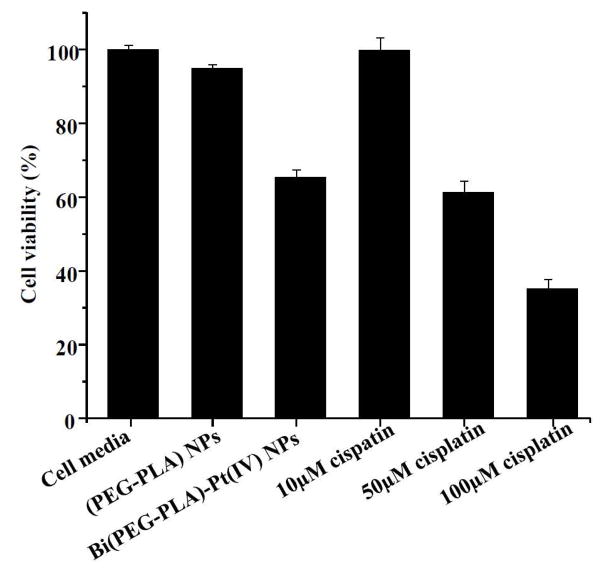
Lastly, we examined the in vitro cellular cytotoxicity of the synthesized acid-responsive Bi(PEG-PLA)-Pt(IV) NPs. To this end, we chose A2780 human ovarian carcinoma cell line as a model cancer cell because of the well-known toxicity of cisplatin against ovarian cancer. Following a well established cellular cytotoxicity measurement protocol,25, 26 the A2780 cells were incubated with Bi(PEG-PLA)-Pt(IV) NPs for 4 hrs. After the incubation, the excess NPs were removed and the cells were washed three times with fresh buffer followed by the addition of fresh culture media. Subsequently the cells were incubated for 72 hrs before being assessed by MTT assay. Cell culture media and PEG-PLA NPs (without cisplatin analogue prodrugs) were used as negative controls. Free cisplatin drug at different concentrations (10 μM, 50 μM, and 100 μM respectively) served as positive controls. As shown in Figure 6, the cell viability of the Bi(PEG-PLA)-Pt(IV) NPs decreased to about 65% after 4 hrs incubation. In contrast, PEG-PLA NPs had negligible cytotoxicity against ovarian cancer cells, similar as the cell media. The cell viability of free cisplatin at 10 μM, 50 μM, and 100 was 99%, 62%, and 35%, respectively. Based on the cisplatin loading yield of 1.05 wt% measured in Figure 5A, we calculated that the amount of cisplatin loaded in the Bi(PEG-PLA)-Pt(IV) NPs for this cytotoxicity study was equivalent to 7 μM free cisplatin. Surprisingly, the NPs with an equivalent 7 μM free cisplatin had cellular cytotixicity against ovarian cancer cells as high as 50 μM free cisplatin. To ensure that the measured cytotoxicity was due to the internalized NPs but not the free drugs in the media released from the NPs, the culture media were filtered through a membrane with a molecular weight cut-off of 10 KDa after the 4 hrs incubation with Bi(PEG-PLA)-Pt(IV) NPs. The filtrate was collected to quantify Pt content using ICP-OES. Negligible amount of free Pt drug (0.05 μM) was observed in the media, which was consistent with the slow drug release profile of the NPs at pH=7.4. This approximate 7 fold cytotoxicity increase of Bi(PEG-PLA)-Pt(IV) NPs might be attributed to the burst drug release in the acidic intracellular environment. Upon internalization, the acid-responsive NPs caused a surge in intracellular drug concentration that possibly overwhelmed some chemoresistance mechanisms of tumor cells, such as the P-glycoprotein (P-gp) membrane proteins mediated drug efflux mechanism.27 Our results are consistent with an earlier study by Xu et al., who have examined the activity of cisplatin encapsulated in different NP systems and have observed enhanced cytotoxicity of those NPs with fast cisplatin release profile. 28
Figure 6.
MTT assay to measure the cytotoxicity of Bi(PEG-PLA)-Pt(IV) NPs against A2780 human ovarian carcinoma cell line in comparison with cell culture media, PEG-PLA NPs and free cisplatin (10 μM, 50 μM and 100 μM). The amount of cisplatin loaded in the Bi(PEG-PLA)-Pt(IV) NPs was equivalent to 7 μM free cisplatin drug. All samples were incubated with cells for 4 hrs, and the cells were subsequently washed and incubated for a total of 72 hrs before assessing cell viability in each group (n=6).
Conclusions
In conclusion, a novel acid-responsive Bi(PEG-PLA)-Pt(IV) polymer-cisplatin prodrug conjugate NP was synthesized as a new cisplatin delivery vehicle. The uniqueness of this drug delivery NP system is that the cisplatin analogue prodrug was covalently linked to the hydrophobic segment of two PEG-PLA copolymer chains through pH-sensitive hydrazone bond. We demonstrated that sub-100 nm Bi(PEG-PLA)-Pt(IV) NPs were readily formed due to the very low CMC of the Bi(PEG-PLA)-Pt(IV) conjugate. These NPs had well controlled cisplatin loading yield and showed excellent acid-responsive drug release kinetics, leading to enhanced in vitro cytotoxicity against tumor cells as compared to free cisplatin. As an environmentally sensitive drug delivery vehicle, these NPs can potentially minimize the drug loss during their circulation in the blood where the pH value is neutral and trigger rapid intracellular drug release when the NPs are endocytosed by the target cells. This characteristic drug release kinetics may suppress cancer cell chemoresistance and improve the therapeutic efficacy of the drug payload. We also speculate that by attaching targeting ligands onto the surface of these NPs, one can improve the binding specificity of the NPs, thereby further enhancing the therapeutic index of these polymer-prodrug conjugate NPs. Moreover, although cisplatin was selected as a specific model drug in this study, the concept and technique of developing acid-responsive drug delivery polymeric NPs by covalently conjugating drug molecules to polymer chains through double or multiple stimuli-responsive linkers can be generalized to deliver many other types of therapeutic or diagnostic agents, including both hydrophilic and hydrophobic agents.
Experimental Section
Materials
L-lactide (cis-3,6-dmethyl-1,4-dioxan-2,5-dione), stannous octoate, K2PtCl4, AgNO3, and all other chemicals for wet chemistry synthesis of cisplatin were purchased from Sigma-Aldrich. Poly(ethylene glycol) (Mn=3500 gmol−1) was obtained from JenKem Technology, Shanghai, China. Cell culture reagents and media were purchased from Media Tech. 3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyl-tetrazolium bromide (MTT) cell proliferation assay kit was obtained from Promega Corporation, USA. A2780 human ovarian carcinoma cells were received from Dr. Stephen Howell in the Moores Cancer Center at UCSD.
Synthesis of PEG-PLA and activated PEG-PLA copolymers
The PEG-PLA diblock copolymer was synthesized by ring opening polymerization (ROP) and activated using 4-nitrophenyl chloroformate. Briefly, l-lactide (1.6 g, 11.1 mmol, purified by recrystallization in ethyl acetate) and COOH-PEG3500-OH (0.2 g, 0.057 mmol) were heated to 120 °C under nitrogen atmosphere for complete melting. Then stannous octoate in anhydrous toluene was added into the melting mixture with a monomer/catalyst molar ratio of 500:1 to initiate ROP. Polymerization was carried under reflux condition at 120 °C under nitrogen atmosphere for 24 hrs. After the completion of the reaction, the crude product was cooled to room temperature, stirred with cold water to hydrolyze unreacted l-lactide monomers, and extracted with chloroform. The synthesized PEG-PLA diblock polymers were then purified by precipitation in cold diethyl ether and dried under vacuum. This washing process was repeated for three times. To activate the obtained PEG-PLA for further conjugation, three grams of the diblock copolymers were dissolved in 50 mL of methylene chloride (MC) and activated by 141.0 mg of 4-nitrophenyl chloroformate while adding 99.0 mg of pyridine (stoichiometric molar ratio, PPEG-PLA : 4-nitrophenyl chloroformate : pyridine = 1:3:5) in a drop-wise manner at 0 °C. Then the reaction was carried out for 12 hrs at room temperature in nitrogen. The activated PEG-PLA diblock copolymers were recovered by precipitation in ice cold diethyl ether and dried under reduced pressure. The final activated polymers and all polymer intermediates were characterized by Varian Mercury 400 nuclear magnetic resonance (NMR) spectroscopy. 1H NMR (CDCl3, 400 MHz, δ ppm) spectra of PEG-PLA: 1.2 (t, −CH3 PLA end group, J=7.0 Hz), 1.55 (m, −CH3, PLA repeating unit), 3.47 (q, −CH2, PEG end group, J=7.0 Hz), 3.63 (m, −CH2, PEG repeating unit) 5.15 (m, −CH, PLA repeating unit). 1H NMR (CDCl3, 400 MHz, δ ppm) spectra of the activated PEG-PLA: 1.2 (t, −CH3 PLA end group, J=7.0 Hz), 1.55 (m, −CH3, PLA repeating unit), 3.47 (q, −CH2, PEG end group, J=7.0 Hz), 3.63 (m, −CH2, PEG repeating unit) 5.15 (m, −CH, PLA repeating unit), 7.93 (t, aromatic −CH, J= 6.9 Hz), 8.78 (d, aromatic −CH, J=5.0 Hz). The molecular weight of the synthesized polymer was determined using gel permeation chromatography (GPC) (Viscotek, USA). For the GPC measurements, tetrahydrofuran (THF) was used as a mobile phase with a flow rate of 1 mL/min. Weight average molecular weights as well as polydispersity indices were calculated from a calibration curve using a series of polystyrene standards.
Synthesis of cis-trans-cis PtCl2(OCOCH2CH2COCH3)2(NH3)2 prodrug
Cisplatin and PtCl2(OH)2(NH3)2 were first synthesized following a previously published protocol.29 The obtained cis-trans-cis PtCl2(OH)2(NH3)2 was then used to prepare cis-trans-cis PtCl2(OCOCH2CH2COCH3)2(NH3)2. An excess of levulinic anhydride was added to an acetone solution containing 100 mg (0.3 mmol) of PtCl2(OH)2(NH3)2 in reflux condition. After 12 hrs of reaction, cold water was added to hydrolyze excess levulinic anhydride. The reaction mixture was left at 2°C for 16 hrs. The acetone was removed from the reaction mixture under reduced pressure leaving a white residue. The residue was purified by washing with water, ethanol, and ether in that order, resulting in a final product yield of 39.0%. 1H NMR (d6-DMSO, 500 MHz, δ ppm): 2.13 (s, 3H, −CH3), 2.72 (s, 2H, −CH2), 6.2–6.8 (br, 3H, NH3).
Synthesis of Bi(PEG-PLA)-Pt(IV) conjugate
The activated PEG-PLA (100 mg) was dissolved in 20 mL dimethylformamide (DMF) and reacted with hydrazine (stoicheometric ratio of activated PEG-PLA:hydrazine=1:10) in nitrogen at room temperature for 12 hrs. By precipitating in ice chilled diethyl ether, the resulting hydrazine functionalized PEG-PLA diblock copolymer (PEG-PLA-NH-NH2) was retrieved. The PEG-PLA-NH-NH2 was then mixed with PtCl2(OCOCH2CH2COCH3)2(NH3)2 with a stoicheometric ratio of 1:8 in 30 mL of MC/DMF (1:1 volume ratio) in the presence of 3 Ǻ molecular sieves. The reaction was allowed to proceed for 48 hrs at 50 °C under reflux in nitrogen. The crude product Bi(PEG-PLA)-Pt(IV) conjugate, was purified by repeated precipitation in diethyl ether and was subsequently extracted with water and chloroform. The purified Bi(PEG-PLA)-Pt(IV) conjugate was kept at −20 °C for further use. 1H NMR (CDCl3, 400 MHz, δ ppm): 1.2 (t, −CH3 PLA end group, J=7.0 Hz), 1.55 (m, −CH3, PLA repeating unit), 2.1 (s, −CH3, spacer), 2.9 (d, −CH2 spacer, J=2.8 Hz), 3.47 (q, −CH2, PEG end group, J=7.0 Hz), 3.63 (m, −CH2, PEG repeating unit) 5.15 (m, −CH, PLA repeating unit), 8.0 (br, 3H, −NH3), 8.2 (br, 1H, −NH). The conjugation yield was determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Intensity of spectral line at 265.945 nm was measured for all samples and standards. The platinum level in the samples was determined by comparing the measured intensity with a standard curve of platinum.
Preparation of Bi(PEG-PLA)-Pt(IV) conjugate NPs
The NPs were prepared via a nanoprecipitaton method following a previously published protocol.30 10 mg of Bi(PEG-PLA)-Pt(IV) conjugate were dissolve in 3 mL of acetonitrile and added to a vial containing 10 mL of water under constant stirring. After the completion of the nanoprecipitation the organic solvent was evaporated in the hood for 2 hrs and under reduced pressure for additional 3 hrs to insure the complete removal of acetonitrile. Then the NP solutions were washed three times using an Amicon Ultra-4 centrifugal filter (Millipore, Billerica, MA) with a molecular weight cutoff of 10 KDa. The NP size and surface zeta potential were obtained from three repeat measurements using a dynamic light-scattering (Malvern Zetasizer, ZEN 3600) with backscattering angle of 173°. The morphology and particle size were further characterized using scanning electron microscopy (SEM). Samples for SEM were prepared by dropping 5 μL of NPs solutions onto a polished silicon wafer. After drying the droplet at room temperature overnight, the sample was coated with chromium and then imaged by SEM.
Drug loading yield and drug release studies
To measure the drug loading yield and release profile of cisplatin from the Bi(PEG-PLA)-Pt(IV) NPs, 100 μL of the prepared NP solutions were loaded into each Slide-A-Lyzer MINI dialysis microtube with a molecular weight cutoff of 3.5 KDa (Pierce, Rockford, IL). The NPs were then dialyzed against pH=5.0, pH=6.0, and pH=7.4 PBS buffer, respectively, at 37 °C. PBS buffers were changed every 12 hrs during the whole dialysis process. At each predetermined time point, NP solutions from three mini dialysis units were collected separately for drug quantification. The concentration of cisplatin was quantified using ICP-OES as described above.
Polymer degradation study
Hydrolytic degradation of PEG-PLA NPs was studied by incubating the NPs in pH=5.0, pH=6.0, and pH=7.4 PBS buffer at 37 °C. At each time point, an aliquot of NPs was collected, extracted with chloroform and reprecipitated in cold diethyl ether. The change of polymer molecular weight was determined by using GPC.
Cell viability assay
Cytotoxicity of Bi(PEG-PLA)-Pt(IV) NPs was assessed against A2780 human ovarian carcinoma cell line using the MTT assay. First, A2780 human ovarian carcinoma cells were seeded (2×104) in 96-well plates and incubated for 24 hrs. Next, the medium was replaced with 150 μL of fresh medium and incubated with 50 μL of Bi(PEG-PLA)-Pt(IV) NPs for 4 hrs. Then the excess NPs were removed and cells were washed three times with fresh buffer followed by the addition of fresh medium. The plates were then incubated for 72 hrs and measured by MTT reagent following a protocol provided by the manufacturer. Fresh cell media and PEG-PLA NPs were used as negative controls. Free cisplatin at various concentrations was used as positive controls.
Supplementary Material
Acknowledgments
We thank Drs. Andrew Kummel, Stephen Howell and Sadik Esener for valuable discussion, and Dr. William Trogler for the use of GPC. This work is supported by the National Institute of Health grant U54CA119335 and the University of California San Diego.
Footnotes
Supporting information available
1H-NMR spectra of PEG-PLA, activated PEG-PLA, PEG-PLA-NH-NH2, PtCl2(OCOCH2CH2,COCH3)2(NH3)2, and Pt calibration curve of ICP-OES. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wang D, Lippard SJ. Cellular Processing of Platinum anticancer Drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Galanski M, Arion VB, Jakupec MA, Keppler BK. Recent Developments in the Field of Tumor-Inhibiting Metal Complexes. Curr Pharm Des. 2003;9:2078–2089. doi: 10.2174/1381612033454180. [DOI] [PubMed] [Google Scholar]
- 3.Pasini A, Zunino F. New Cisplatin Analogues - on the Way to Better Antitumor Agents. Angew Chem Int Ed. 1987;26:615–624. [Google Scholar]
- 4.Wong E, Giandomenico CM. Current Status of Platinum-Based Antitumor Drugs. Chem Rev. 1999;99:2451–2466. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 5.Calderone V, Casini A, Mangani S, Messori L, Orioli PL. Structural Investigation of Cisplatin-Protein Interactions: Selective Platination of His19 in a Cuprozinc Superoxide Dismutase. Angew Chem Int Ed. 2006;45:1267–1269. doi: 10.1002/anie.200502599. [DOI] [PubMed] [Google Scholar]
- 6.DeConti RC, Toftness BR, Lange RC, Creasey WA. Clinical and Pharmacological Studies with Cis-diamminedichloroplatinum (II) Cancer Res. 1973;33:1310–1315. [PubMed] [Google Scholar]
- 7.Ivanov AI, Christodoulou J, Parkinson JA, Barnham KJ, Tucker A, Woodrow J, Sadler PJ. Cisplatin Binding Sites on Human Albumin. J Biol Chem. 1998;273:14721–14730. doi: 10.1074/jbc.273.24.14721. [DOI] [PubMed] [Google Scholar]
- 8.Andrews PA, Wung WE, Howell SB. A High-Performance Liquid Chromatographic Assay with Improved Selectivity for Cisplatin and Active Platinum (II) Complexes in Plasma Ultrafiltrate. Anal Biochem. 1984;143:46–56. doi: 10.1016/0003-2697(84)90556-6. [DOI] [PubMed] [Google Scholar]
- 9.Borch RF, Pleasants ME. Inhibition of Cis-platinum Nephrotoxicity by Diethyldithiocarbamate Rescue in a Rat Model. Proc Natl Acad Sci USA. 1979;76:6611–6614. doi: 10.1073/pnas.76.12.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolman RC, Deacon GB, Hambley TW. Studies of the Binding of a Series of Platinum(IV) Complexes to Plasma Proteins. J Inorg Biochem. 2002;88:260–267. doi: 10.1016/s0162-0134(01)00360-9. [DOI] [PubMed] [Google Scholar]
- 11.Ang WH, Khalaila I, Allardyce CS, Juillerat-Jeanneret L, Dyson PJ. Rational Design of Platinum(IV) Compounds to Overcome Glutathione-S-Transferase Mediated Drug Resistance. J Am Chem Soc. 2005;127:1382–1383. doi: 10.1021/ja0432618. [DOI] [PubMed] [Google Scholar]
- 12.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted Delivery of Cisplatin to Prostate Cancer Cells by Aptamer Functionalized Pt(IV) Prodrug-PLGA-PEG Nanoparticles. Proc Natl Acad Sci USA. 2008;105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohya Y, Oue H, Nagatomi K, Ouchi T. Design of Macromolecular Prodrug of Cisplatin Using Dextran with Branched Galactose Units as Targeting Moieties to Hepatoma Cells. Biomacromolecules. 2001;2:927–933. doi: 10.1021/bm010053o. [DOI] [PubMed] [Google Scholar]
- 14.Ang WH, Pilet S, Scopelliti R, Bussy F, Juillerat-Jeanneret L, Dyson PJ. Synthesis and Characterization of Platinum(IV) Anticancer Drugs with Functionalized Aromatic Carboxylate Ligands: Influence of the Ligands on Drug Efficacies and Uptake. J Med Chem. 2005;48:8060–8069. doi: 10.1021/jm0506468. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay S, Barnes CM, Haskel A, Short SM, Barnes KR, Lippard SJ. Conjugated Platinum(IV)-Peptide Complexes for Targeting Angiogenic Tumor Vasculature. Bioconjug Chem. 2008;19:39–49. doi: 10.1021/bc070031k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, Nishio K, Matsumura Y, Kataoka K. Novel Cisplatin-Incorporated Polymeric Micelles Can Eradicate Solid Tumors in Mice. Cancer Res. 2003;63:8977–8983. [PubMed] [Google Scholar]
- 17.Nishiyama N, Yokoyama M, Aoyagi T, Okano T, Sakurai Y, Kataoka K. Preparation and Characterization of Self-Assembled Polymer-Metal Complex Micelle from Cis-dichlorodiammineplatinum(II) and Poly(ethylene glycol)-Poly(aβ, -aspartic acid) Block Copolymer in an Aqueous Medium. Langmuir. 1999;15:377–383. [Google Scholar]
- 18.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 20.Fujiyama J, Nakase Y, Osaki K, Sakakura C, Yamagishi H, Hagiwara A. Cisplatin Incorporated in Microspheres: Development and Fundamental Studies for Its Clinical Application. J Control Release. 2003;89:397–408. doi: 10.1016/s0168-3659(03)00131-7. [DOI] [PubMed] [Google Scholar]
- 21.Modi S, Swetha MG, Goswami D, Gupta GD, Mayor S, Krishnan Y. A DNA Nanomachine That Maps Spatial and Temporal pH Changes Inside Living Cells. Nat Nanotechnol. 2009;4:325–330. doi: 10.1038/nnano.2009.83. [DOI] [PubMed] [Google Scholar]
- 22.Ray GB, Chakraborty I, Moulik SP. Pyrene Absorption Can Be a Convenient Method for Probing Critical Micellar Concentration (cmc) and Indexing Micellar Polarity. J Coll Inter Sci. 2006;294:248–254. doi: 10.1016/j.jcis.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” Drug Delivery Systems: Double-Targeted pH-Responsive Pharmaceutical Nanocarriers. Bioconjug Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal Gene Silencing Using Biodegradable Polymer Nanoparticles Densely Loaded with Small-Interfering RNA. Nat Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong HL, Bendayan R, Rauth AM, Wu XY. Simultaneous Delivery of Doxorubicin and GG918 (Elacridar) by New Polymer-Lipid Hybrid Nanoparticles (PLN) for Enhanced Treatment of Multidrug-Resistant Breast Cancer. J Control Release. 2006;116:275–284. doi: 10.1016/j.jconrel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. Reversal of Multidrug Resistance by Transferrin-Conjugated Liposomes Co-encapsulating Doxorubicin and Verapamil. J Pharm Pharmaceut Sci. 2007;10:350–357. [PubMed] [Google Scholar]
- 27.Gottesman MM. Mechanisms of Cancer Drug Resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 28.Xu P, Van Kirk EA, Murdoch WJ, Zhan Y, Isaak DD, Radosz M, Shen Y. Anticancer Efficacies of Cisplatin-Releasing pH-Responsive Nanoparticles. Biomacromolecules. 2006;7:829–835. doi: 10.1021/bm050902y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuroda R, Ismail IM, Sadler PJ. X-ray and NMR Studies of Rrans-Dihydroxo-platinum(IV) Antitumor Complexes. J Inorg Biochem. 1984;22:103–117. doi: 10.1016/0162-0134(84)80019-7. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Formulation of Functionalized PLGA-PEG Nanoparticles for In Vivo Targeted Drug Delivery. Biomaterials. 2007;28:869–876. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.