Abstract
Background
Alternative Promoter (AP) usages have been shown to enable diversified transcriptional regulation of individual gene in a context-specific (e.g., pathway, cell lineage, tissue type, and development stage et. ac.) way. Aberrant uses of APs have been directly linked to mechanism of certain human diseases. However, whether or not there exists a general link between a gene's AP repertoire and its expression diversity is currently unknown. The general relation between a gene's AP repertoire and its disease susceptibility also remains largely unexplored.
Methodology/Principal Findings
Based on the differential expression ratio inferred from all human microarray data in NCBI GEO and the list of disease genes curated in public repositories, we systemically analyzed the general relation of AP repertoire with expression diversity and disease susceptibility. We found that genes with APs are more likely to be differentially expressed and/or disease associated than those with Single Promoter (SP), and genes with more APs are more likely differentially expressed and disease susceptible than those with less APs. Further analysis showed that genes with increased number of APs tend to have increased length in all aspects of gene structure including 3′ UTR, be associated with increased duplicability, and have increased connectivity in protein-protein interaction network.
Conclusions
Our genome-wide analysis provided evidences that increasing alternative promoter repertories is positively associated with differential expression and disease susceptibility.
Introduction
Promoter is the region of DNA consisting of transcriptional regulatory elements required for transcription initiation. Alternative Promoter (AP) usage refers to the control of alternative transcriptional start within a single gene locus by using alternative promoter. AP usage has been observed for many individually characterized genes [1], [2] and recent genomic studies have found that approximately 50% of human genes have at least one AP [3], [4]. The wide-spread AP usage indicates it might play a critical role in shaping human genome and transcriptome [1], [2], [5], [6].
As AP consists of different modules of cis-regulatory elements [7], [8], AP usage has long been explored for the regulation of expression diversity of individual metazoan gene [9]. For example, by selectively using one promoter active in parotid gland and the other active in liver, mammal α-amylase gene shows a more than 100-fold difference of expression level in these two tissues [10]. The number of individually characterized genes with AP driving context-specific (e.g., pathway, cell line, tissue type, development stage, species et. ac.) manner of differential expression has accumulated during the past two decades [1], [2], [5], [6], [11], [12]. This thus raises an interesting question: are genes with AP more likely to be differentially expressed than genes with Single Promoter (SP)? Furthermore, among genes with AP, are genes with more AP more likely to be differentially expressed?
There is also growing evidence that AP usage is linked to disease through aberrant promoter choice and/or genetic defects affecting the functional cis-regulatory element [2], [9]. For example, the upstream promoter of MYC, dominant negative in normal tissue, is aberrantly activated in Burkitt's lymphoma cells due to aberrant translocation of MYC gene locus [13]. A recent survey of mammalian AP showed that the group of putative human cancer related genes (∼2,800) on average have 2 promoters compared with an average 1.5 promoters among the other human genes [2]. However, cancer related genes can be classified into passenger and driver, with the later playing a critically causal instead of passive role in tumor formation and progression [14], [15]. It remains unclear whether there is a general link between a gene's AP repertoire and the likelihood of being cancer driving genes. Furthermore, it remains unclear whether or not there is a positive relationship between the increasing promoter repertoire and the likelihood of being associated with general human diseases.
Results
AP Genes Are More Likely to Be Differentially Expressed
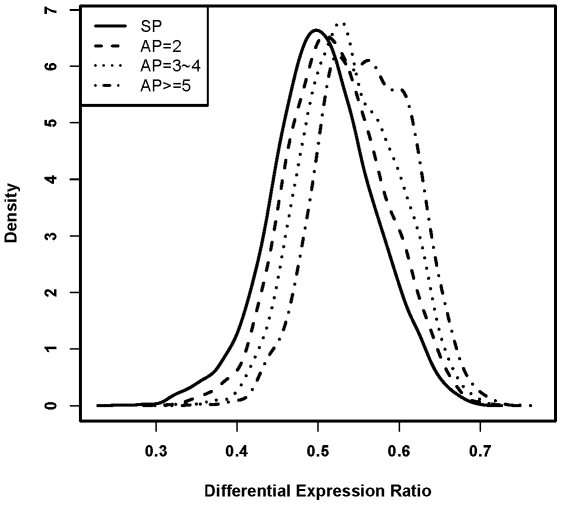
For each human gene, we obtained its Differential Expression Ratio (DER) from the study by Chen et al. [16], [17]. The DER value of a gene is its frequency of differential expression in multiple microarray studies (see Methods section). As DER was derived from all available human microarray datasets deposited at GEO, it provided a comprehensive metric to measure the regulation diversity at expression level. To test the hypothesis whether genes with AP are more likely differentially expressed than genes with SP, we compared the DER between SP and AP genes. Of the genes with SP, the median DER was 0.50. In contrast, the genes with AP have median DER 0.53 (P<2.2e-16, Wilcox rank sum test). To test whether there is a general link between increasing number of promoter and differential expression among genes with AP, we divided the AP genes into three classes based on their number of promoters (AP = 2, AP = 3/4, AP> = 5, see methods). As shown in Figure 1, genes with more AP are more likely to be differentially expressed. The median DER was 0.52 for AP = 2 class (P = 2.2e-16, vs. SP), and increased to 0.54 for AP = 3/4 class (P<2.2e-16, vs. AP = 2 class). The median DER was further increased to 0.56 for AP> = 5 class (P<2.2e-16, comparing with that of AP = 3/4 class). Recent studies have shown that different tissues, cell types, developmental and/or disease stage are often regulated by distinct transcriptional factors, and there is considerable diversity in the composition of cis-regulatory elements in alternative promoters [2], [7], [18]. The increased number of alternative promoters from a single locus will provide increased flexibility and diversity of AP usage, and thereby generate either identical or distinct protein conducts in a tissues, cell lineage, stage, and time point specific manner. Such a diversifying and complex regulation control might contribute to the increased frequency of differential expression observed here for AP genes.
Figure 1. Distribution of differential expression ratio for each gene class.
The figure (density plot) showed that genes with more alternative promoters are more likely to be differentially expressed. SP means gene with single promoter, while AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
AP Genes Are More Likely to Be Disease Susceptible
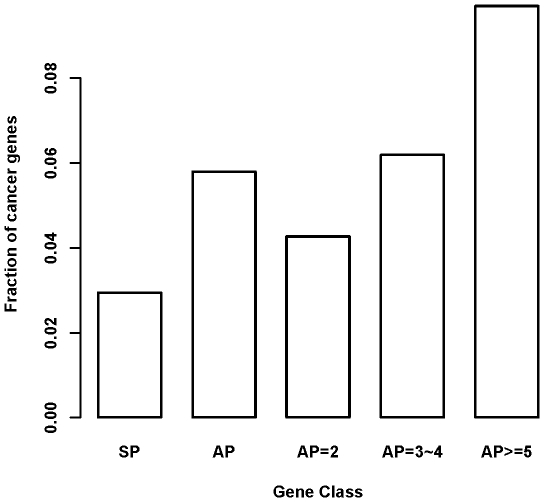
The study by Chen et al [17] has revealed that highly differentially expressed genes are more likely to be associated with disease. As we found that AP genes are more likely to be differentially expressed, it is expected that AP genes are more likely to be involved in disease. To confirm this positive link and quantify the extent to which a gene's promoter repertoire is associated with the likelihood of disease susceptibility, we first compiled a list of 775 human cancer genes which are likely to play casual roles in tumor formation and progression. We built a 2×2 contingency table using the number of cancer-driver gene and non-cancer-driver genes, and tested whether the fraction of cancer-driver genes is significantly increased from SP to AP gene classes using Fisher's exact test. As shown in Figure 2, the fraction of cancer-driver genes in SP class was 2.9%, and increased to 5.8% in AP class, an almost 2-fold increase (P = 2.2e-16). We further compare the fraction of cancer-driver genes between different AP classes. The fraction was found to be 4.3% for AP = 2 class (P = 0.00021, vs. SP), 6.2% for AP = 3/4 class (P = 0.00026, vs. AP = 2), and 9.7% for AP> = 5 class (P = 8.075e-05, vs. AP = 3/4).
Figure 2. Fraction of cancer driver genes for each gene class.
The figure showed that genes with more alternative promoters tend to be enriched with cancer driver gene. The Y-axis is the fraction of genes belonging to cancer driver gene in each gene class. SP means gene with single promoter while AP means gene with alternative promoters. AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
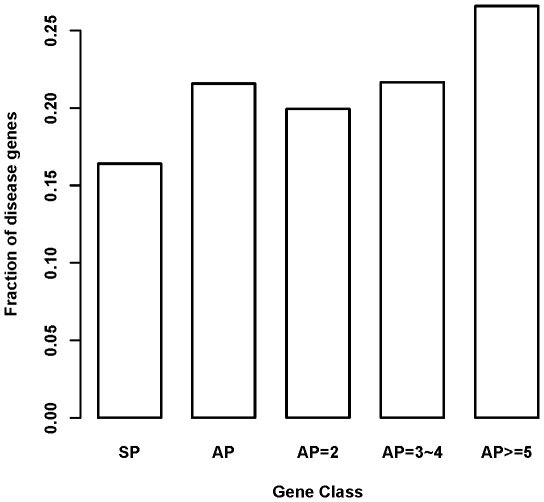
In order to further characterize the general relationship between having increased promoter repertoire and the likelihood of being human disease susceptibility gene, we compiled a list of 3,392 curated human disease-associated genes. We again built 2×2 contingency tables and tested whether there is an increased fraction of disease gene from SP to AP gene classes using Fisher's exact test. As shown in Figure 3, the fraction of disease genes in SP class was 16.4%, and increased to 21.6% in AP class (P = 2.78e-16). The fraction was 19.9% for AP = 2 class (P = 2.497e-06, vs. SP), 21.7% for AP = 3/4 class (P = 0.04481, vs. AP = 2), and 26.6% for AP> = 5 class (P = 0.0004199, vs. AP = 3/4).
Figure 3. Fraction of disease genes for each gene class.
The figure showed that genes with more alternative promoters tend to be enriched with disease gene. The Y-axis is the fraction of genes belonging to disease gene in each gene class. SP means gene with single promoter while AP means gene with alternative promoters. AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
AP Genes Are Longer in All Aspects of Gene Structure
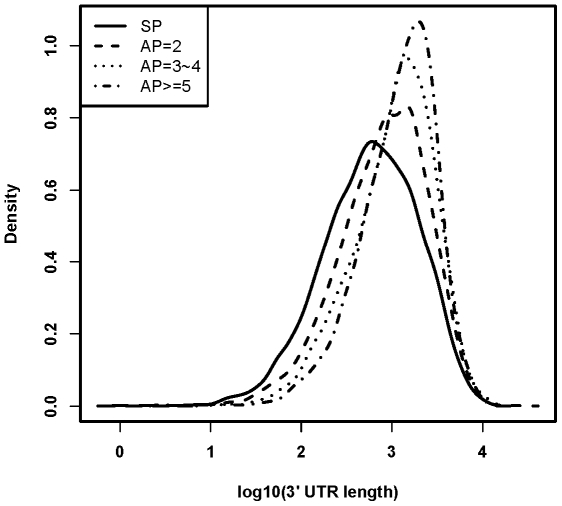
As shown in Table 1 and Figure S1, AP genes are significantly longer than SP genes in all aspects of the gene structure including genomic sequence, coding sequence (CDS), 5′ untranslated regions (5′ UTR), 3′ UTR, total exon, and total intron. AP genes also tend to have more exons and introns. Among AP genes, the class with more AP tends to be longer in all aspects of gene structure than the class with less AP (Table 1 and Figure S1). For example, the median of total intron length is 14.4, 25.2, 43.7 and 87.2 kb for SP, AP = 2, AP = 3∼4 and AP> = 5 gene class, respectively (P<2.2e-16, Wilcox rank sum test). As AP usage will lead to alternative usage of first exon, the increased number of AP will undoubtedly increase the degree of freedom for the extension of transcript region from the 5′ end [3]. However, it is remarkable that 3′ UTR, the region enriched for microRNA binding sites important for post-transcriptional regulation, also tend to be longer as the number of AP increases (Figure 4).
Table 1. Length parameter of each gene class.
| Genomic Sequence | CDS | 5′ UTR | 3′ UTR | Total Exon | Total Intron | # of Exon | # of Intron | |
| SP | 16,835 a | 1,097 | 139 | 599 | 2,158 | 14,370 | 6 b | 5 |
| AP | 41,017 | 1,638 | 183 | 1,062 | 3,274 | 37,314 | 11 | 10 |
| Pvalue c | <2.2e-16* | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 |
| AP = 2 | 28,100 | 1,415 | 172 | 881 | 2,808 | 25,162 | 9 | 8 |
| Pvalue d | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 |
| AP = 3∼4 | 48,058 | 1,763 | 188 | 1,178 | 3,492 | 43,650 | 12 | 11 |
| Pvalue e | <2.2e-16 | <2.2e-16 | 2.153e-05 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 |
| AP> = 5 | 90,787 | 2,296 | 207 | 1,325 | 4,178 | 87,204 | 17 | 16 |
| Pvalue f | <2.2e-16 | <2.2e-16 | 0.000329 | 0.0005684 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 |
The table showed that genes with more alternative promoters tend to have increased length in all aspects of gene structure parameter. SP means gene with single promoter while AP means gene with alternative promoters. AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
: Median length;
: Median count;
: Wilcoxon rank sum test, AP vs. SP.
: Wilcoxon rank sum test, AP = 2 vs. SP.
: Wilcoxon rank sum test, AP = 3∼4 vs. AP = 2.
: Wilcoxon rank sum test, AP> = 5 vs. AP = 3∼4.
: The Wilcoxon rank sum test function in R (wilcox.test) returns “P<2.2e-16” when P is smaller than 2.2e-16.
Figure 4. Length distribution for the 3′ un-translated region (3′ UTR) of each gene class.
The figure (density plot) showed that genes with more alternative promoters tend to have longer 3′ UTR. SP means gene with single promoter, while AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
AP Genes Are Associated with Increased Duplicability
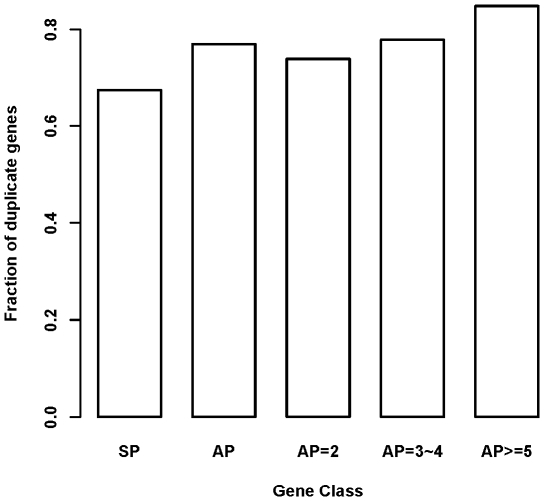
We retrieved 14, 410 unique duplicate genes and 5, 226 unique singleton genes from Ensembl database via BioMart, with the fraction of duplicate gene about 73%. 10,665 of duplicate genes and 4,054 of singleton genes have curated promoter architecture from DBTSS (used in this work), with a similar ratio of duplicate gene (i.e, 72.5%). As shown in Figure 5, duplicate genes comprise 67% of SP genes, but make up 77% of AP genes (P = 1.087e-07, Fisher's exact test). The fraction was 74% for AP = 2 class (P = 0.002138, vs. SP), 78% for AP = 3/4 class (P = 0.08113, vs. AP = 2), and 85% for AP> = 5 class (P = 0.05049, vs. AP = 3/4).
Figure 5. Fraction of duplicate genes for each gene class.
The figure showed that genes with more alternative promoters tend to have increased duplicability. The Y-axis is the fraction of genes belonging to duplicate gene in each gene class. SP means gene with single promoter while AP means gene with alternative promoters. AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
AP Genes Are More Likely to Be Associated with Hub
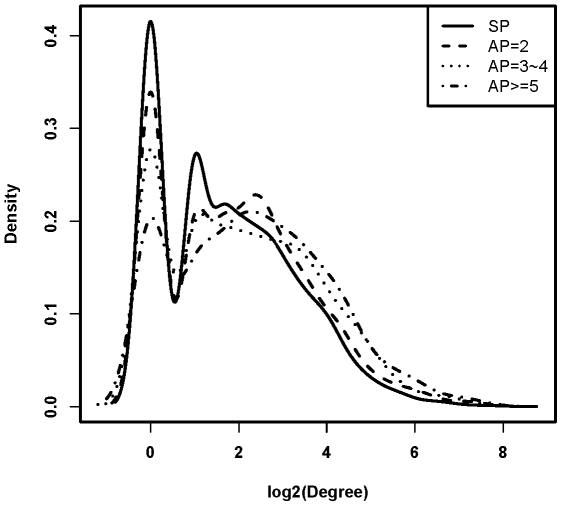
We downloaded the manually curated human protein–protein interaction network from HPRD[19]. We found that the AP genes tend to have significantly more node connectivity (degree) than that of SP genes, and display a much stronger trend as the number of AP increases (Figure 6, P< = 0.01, Wilcoxon rank sum test). The average connectivity of SP genes is 6.5, and increases to 10.5 for AP> = 5 gene class (P<2.2e-16).
Figure 6. Distribution of node connectivity (degree) for each gene class in human protein-protein interaction network.
The figure (density plot) showed that genes with more alternative promoters tend to have increased node connectivity. SP means gene with single promoter, while AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
Example of AP genes
To exemplify the characters of AP genes studied in this work, we described several genes whose alternative promoter usage has been shown in literatures. GNAS (guanine nucleotide binding protein, alpha stimulating activity polypeptide 1), is a G protein involved in hormonal regulation of adenylate cyclase. GNAS has ten potential alternative promoters supported by curated full-length c-DNA clones, and the switched recruitment of four of them has been found to generate multiple protein transcripts involved in metabolic regulation and development (For reviews, see Weinstein et al. [20] and Davuluri et al. [2]). GNAS has a high frequency of differential expression - differentially expressed in more than 69% of GEO dataset in which it was measured (DER value equals to 0.691). Promoter switching of GNAS has been found to plays a role in various diseases and tumorigenesis through loss of imprinting [21], [22], [23]. It is a disease gene of multiple syndromes including Albright hereditary osteodystrophy, pseudopseudohypoparathyroidism and McCune-Albright syndrome [24], [25]. It is a cancer driver gene of pituitary adenoma [26]. It is a duplicate gene, and the paralog is GNAL. The gene length of RUNX1 is 71.5 kb, well above the median of SP gene (16.8 kb). GNAS has 23 interacting partners in the protein-protein interaction network, comparing with an average connectivity of 6.5 for SP genes.
FGFR1 (fibroblast growth factor receptor 1), is a member of the fibroblast growth factor receptor family that binds to both acidic and basic fibroblast growth factors. FGFR1 has seven alternative promoters supported by curated full-length c-DNA clones, and at least of four of them have been shown to control the differential expression in a tissue- and cancer cell- specific manner [27], [28], [29], [30]. We found that FGFR1 is indeed frequently differentially expressed, with the DER value of 0.684. It is a disease gene of a number of syndromes including familial Pfeiffer syndrome [31]. It is cancer driver gene, implicated in the tumorigenesis of hematological malignancies including chronic myeloid leukemia, myeloid hyperplasia and non-Hodgkin's lymphoma [32]. It is a duplicate gene, with its paralogs including RET and FGFR2. FGFR1 has 18 exons and 5.9 kb exon length, comparing with the 6 exons and 2.2 kb exon length for SP gene. The protein-interaction network connectivity of FGFR1 is 36.
PDGFRA (platelet-derived growth factor receptor, alpha polypeptide), is a cell surface tyrosine kinase receptor for members of the platelet-derived growth factor family. The expression of PDGFRA is regulated by four potential alternative promoters, and the switched usage of two of them has been found to be involved in early human embryogenesis [33], [34]. The DER value of PDGFRA is 0.651, indicating that it is differentially expressed in more than 65% of GEO dataset in which it was measured. It is a key disease gene in hematologic disorder, involved in the gene fusions associated with the hypereosinophilic syndrome [35], [36]. It also serves as a well-documented cancer driver gene of gastrointestinal stromal tumor [37]. The paralog of PDGFRA, PDGFRB, has two alternative promoters and is also a cancer driver gene [38]. Compared with SP genes, PDGFA is both longer (69.1 kb) and connected by more interacting partners (24) in the protein-protein interaction network.
Discussion
The functional role of alternative promoter usage in differential expression and/or disease susceptibility has been characterized for a bunch of genes. However, whether there is a positive link between a gene's AP repertoire and its likelihood of being differentially expressed and/or disease associated remains unknown. Based on a systematic analysis of promoter, microarray and disease gene in the public repositories, we found that compared with single-promoter genes, genes with alternative promoters are more likely to be differentially expressed and/or disease associated. Furthermore, our results showed that among AP genes, those with more promoters are more likely differentially expressed and/or disease susceptible.
Gene expression data has been frequently incorporated into the prioritization of disease candidate genes or SNPs. Recent translational study by Chen et al [17] has demonstrated that highly differentially expressed genes are more likely to have variants associated with disease, based on the analysis of all microarray data from GEO database. The finding that there is a positive association between differential expression and disease susceptibility marked a significant step towards the translation of gene expression data into disease gene prioritization. However, the molecular, genetic and genomic mechanism underlying this translation remains to be explored. Our study found that there is a general link between alternative promoter and differential expression and disease susceptibility. We further demonstrate that genes with increased number of alternative promoters are marked with features important to regulation complexity and disease origins, including increased gene length, duplicability and connectivity. While it remains to be explored for the positive prediction value of incorporating alternative promoter repertoire into disease gene prioritization, our results will be useful to understand the genomic mechanism underlying the translation from differential expression to disease susceptibility.
A better characterization of the role of alternative promoter usage on expression diversity and disease susceptibility requires a truly unbiased and comprehensive resource of alternative promoter activity, gene expression change and disease propensity. The DBTSS full-length cDNA derived alternative promoter data are taken from >160 distinct cDNA library of various cell types and tissue, and the GEO derived DER data are calculated based on 4,877 group-versus-group comparisons on 476 human GEO datasets. Although comprehensive, there is a possibility that both DBTSS and GEO data might be biased to certain biological niches. Thus, a future research direction will be to identify the separated effects in the analysis of alternative promoter versus differential expression, by classifying the different kinds of experiment in DBTSS and GEO (e.g., based on tissue, disease condition, and et. ac.). Also, it remains to be explored the effects of adopting alternative metric of differential expression and different definition of alternative promoters (e.g., varied cutoff of TSS clustering, other curated promoter database [39], and et. ac.). Similarly, the OMIM-based disease gene record is far from complete and historically biased to monogenic disorders. A more complete catalog of genes underlying different disease will alleviate the potential analysis bias to certain type of human disorders.
Recent technique developments in high-density promoter microarray and next-generation sequencing have enabled the genome-wide monitoring of alternative promoter activity and transcriptome change under different conditions [5], [6], [40], [41], [42]. Simultaneously, results from multiple genome wide association studies have shed light to the widespread involvement of regulatory variants including alternative promoters in disease association [43], [44], [45], [46]. By integrating the fast-accumulated data from these high-throughput studies and other functional genomics data, we expect that a more complete understanding of the mechanism of and extent to which alternative promoter usage has shaped human transcriptome and diseasome will be achieved.
In summary, based on a systematic analysis of promoter, microarray and disease gene in public repositories, we demonstrated that there exists a general link between a gene's alternative promoter repertoire and its expression diversity and/or disease susceptibility. Our further comparative analyses of AP vs. SP gene reveal several remarkable features of AP genes as a class. First, we found that AP genes tend to have longer length in all aspects of gene structure. As gene length is found to be positively related with the density of functional elements [47], it is reasonable to suggest that AP genes, with increased length in all aspects of gene parameter, subject to more sophisticated regulation besides transcriptional factor mediated promoter binding (e.g., alternative splicing [1], [48], microRNA mediated regulation [49], [50], [51], and et. ac.). Second, we showed that AP genes are associated with increased duplicability. Gene duplication has been widely appreciated as one of the factors underlying genetics variation, phenotypic diversity and disease mechanism [52]. Third, we observed that AP genes tend to have higher connectivity in protein-protein interaction network. The topological centrality of AP genes thus indicates that they play critical role in human physiological system [53]. Collectively, our analysis suggests that increasing AP repertories might be an important factor in shaping human genome, transcriptome and diseasome.
Methods
We retrieved information of promoter annotation from DBTSS (Version 6.0, based on UCSC hg18) [54]. DBTSS determine alternative promoters using clustering of transcriptional start sites (TSS) by 500 bps, with TSS derived from collection (>160 distinct libraries) of experimentally determined 5′-end sequences of full-length cDNA clones. A total of 15,180 human RefSeq genes with curated full-length cDNA derived promoter architecture were obtained, which include 7,291 genes with Single Promoter (SP) and 7,889 genes with Alternative Promoter (AP). Among genes with AP, there are 3, 772 genes with two promoters (AP = 2), 2,941 with three or four (AP = 3∼4), and 1,176 with five or more (AP> = 5). The length parameter of gene structure was based on NCBI Reference Sequence (RefSeq) annotation. The 5′ UTR length is calculated from transcription start position and cording region start, while that of 3′ UTR from transcription end position and cording region end. For genes with multiple transcripts, the longest one is selected for length calculation.
We obtained the differential expression ratio (DER) of human genes from the study by Chen et al. [16], [17]. Briefly, the authors downloaded all curated human microarray-based gene expression datasets from the NCBI Gene Expression Omnibus (GEO) [55], [56], and conducted comprehensive group-versus-group comparisons within each dataset based on GEO annotated experimental variables (e.g., time, treatment, tissue, development stage et ac.) to identify differentially expressed (q value≤0.05, using SAM [57]) genes. For each human gene, the DER was calculated as the count of GEO datasets in which it was differentially expressed divided by the count of GEO datasets in which it was measured [17]. Only genes that were measured in at least 5% of all GEO datasets are included, which include 14,783 (97.4%) of the 15,180 genes with promoter annotations available from the DBTSS database.
We downloaded a manually curated collection of ∼380 human genes whose variants play a causal role in cancer (Cancer Gene Census database [14]). CGC is a regularly updated database to catalogue those genes for which mutations, deletions, and/or translocations have been causally implicated in cancer. We also compiled a set of ∼450 human cancer candidate genes, which are most likely to be key driver genes, based on recent large-scale sequencing of breast, colorectal, pancreatic and brain tumor genomes [15], [58], [59], [60]. The combination of these two datasets resulted in a list of 775 unique cancer driver genes.
We compiled a list of ∼2,380 known disease genes from the Morbid Map (MM) of the Online Mendelian Inheritance in Man (OMIM) [61]. Only the Morbid Map entries with the “(3)” tag, for which there is strong evidence that abnormality of the particular gene is causative to the disorder, were used to derive the list of human disease gene. We also downloaded a list of ∼2,360 human genes with annotated disease-associated variants from the latest Swiss-Prot database [62]. A combination of these two dataset resulted in 3,392 non-redundant human disease genes.
We used BioMart [63]to retrieve the complete set of human duplicate genes from EnsemblCompara GeneTrees database[64]. This corresponds to a total of 14,410 unique genes that have at least one duplicate copy in the human genome, and a total of 5,226 unique known singleton genes that have no duplicate copy.
We downloaded the manually curated human protein–protein interaction network from the Human Protein Reference Database [19], which is composed of 9,306 unique proteins and 35,023 protein–protein interactions (with self-interaction removed). The network degree was calculated using the NetworkAnalyzer plug-in [65] of Cytoscape package [66].
Supporting Information
Length distribution for the gene structure parameter of each gene class. The figure (density plot) showed that genes with more alternative promoters tend to be longer in all aspects of gene structure. SP means gene with single promoter, while AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
(0.27 MB PDF)
Acknowledgments
We wish to thank Drs. Yaoqi Zhou, Yi Xing, Zihua Hu, Marc Halfon and Michael Buck for helpful discussions and comments on this manuscript.
Footnotes
Competing Interests: The author has declared that no competing interests exist.
Funding: This work was supported by Health Research, Incorporated (HRI) affiliated with the Roswell Park Cancer Institute (RPCI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Landry JR, Mager DL, Wilhelm BT. Complex controls: the role of alternative promoters in mammalian genomes. Trends Genet. 2003;19:640–648. doi: 10.1016/j.tig.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Davuluri RV, Suzuki Y, Sugano S, Plass C, Huang TH. The functional consequences of alternative promoter use in mammalian genomes. Trends Genet. 2008;24:167–177. doi: 10.1016/j.tig.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Kimura K, Wakamatsu A, Suzuki Y, Ota T, Nishikawa T, et al. Diversification of transcriptional modulation: Large-scale identification and characterization of putative alternative promoters of human genes. Genome Research. 2006;16:55–65. doi: 10.1101/gr.4039406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek D, Davis C, Ewing B, Gordon D, Green P. Characterization and predictive discovery of evolutionarily conserved mammalian alternative promoters. Genome Research. 2007;17:145–155. doi: 10.1101/gr.5872707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper SJ, Trinklein ND, Anton ED, Nguyen L, Myers RM. Comprehensive analysis of transcriptional promoter structure and function in 1% of the human genome. Genome Res. 2006;16:1–10. doi: 10.1101/gr.4222606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim TH, Barrera LO, Zheng M, Qu C, Singer MA, et al. A high-resolution map of active promoters in the human genome. Nature. 2005;436:876–880. doi: 10.1038/nature03877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juven-Gershon T, Hsu JY, Kadonaga JT. Perspectives on the RNA polymerase II core promoter. Biochem Soc Trans. 2006;34:1047–1050. doi: 10.1042/BST0341047. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Q, Halfon MS. Complex organizational structure of the genome revealed by genome-wide analysis of single and alternative promoters in Drosophila melanogaster. BMC Genomics. 2009;10:9. doi: 10.1186/1471-2164-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayoubi TA, Van De Ven WJ. Regulation of gene expression by alternative promoters. FASEB J. 1996;10:453–460. [PubMed] [Google Scholar]
- 10.Schibler U, Hagenbuchle O, Wellauer PK, Pittet AC. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983;33:501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm BT, Landry JR, Takei F, Mager DL. Transcriptional control of murine CD94 gene: differential usage of dual promoters by lymphoid cell types. J Immunol. 2003;171:4219–4226. doi: 10.4049/jimmunol.171.8.4219. [DOI] [PubMed] [Google Scholar]
- 12.Landry JR, Mager DL. Widely spaced alternative promoters, conserved between human and rodent, control expression of the Opitz syndrome gene MID1. Genomics. 2002;80:499–508. [PubMed] [Google Scholar]
- 13.Marcu KB, Bossone SA, Patel AJ. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- 14.Futreal PA, Coin L, Marshall M, Down T, Hubbard T, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Li L, Butte AJ. AILUN: reannotating gene expression data automatically. Nat Methods. 2007;4:879. doi: 10.1038/nmeth1107-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R, Morgan AA, Dudley J, Deshpande T, Li L, et al. FitSNPs: highly differentially expressed genes are more likely to have variants associated with disease. Genome Biol. 2008;9:R170. doi: 10.1186/gb-2008-9-12-r170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine M, Tjian R. Transcription regulation and animal diversity. Nature. 2003;424:147–151. doi: 10.1038/nature01763. [DOI] [PubMed] [Google Scholar]
- 19.Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, et al. Human Protein Reference Database–2009 update. Nucleic Acids Res. 2009;37:D767–772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstein LS, Xie T, Zhang QH, Chen M. Studies of the regulation and function of the Gs alpha gene Gnas using gene targeting technology. Pharmacol Ther. 2007;115:271–291. doi: 10.1016/j.pharmthera.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalande M. Imprints of disease at GNAS1. J Clin Invest. 2001;107:793–794. doi: 10.1172/JCI12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linglart A, Gensure RC, Olney RC, Juppner H, Bastepe M. A novel STX16 deletion in autosomal dominant pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Genet. 2005;76:804–814. doi: 10.1086/429932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastepe M, Frohlich LF, Linglart A, Abu-Zahra HS, Tojo K, et al. Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type Ib. Nat Genet. 2005;37:25–27. doi: 10.1038/ng1487. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein LS, Yu S. The Role of Genomic Imprinting of Galpha in the Pathogenesis of Albright Hereditary Osteodystrophy. Trends Endocrinol Metab. 1999;10:81–85. doi: 10.1016/s1043-2760(98)00124-6. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Litman D, Rosenberg MJ, Yu S, Biesecker LG, et al. A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J Clin Invest. 2000;106:1167–1174. doi: 10.1172/JCI10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayward BE, Barlier A, Korbonits M, Grossman AB, Jacquet P, et al. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107:R31–36. doi: 10.1172/JCI11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu IM, Touhalisky K, Baran C. Multiple controlling mechanisms of FGF1 gene expression through multiple tissue-specific promoters. Prog Nucleic Acid Res Mol Biol. 2001;70:155–174. doi: 10.1016/s0079-6603(01)70016-5. [DOI] [PubMed] [Google Scholar]
- 28.Chotani MA, Payson RA, Winkles JA, Chiu IM. Human fibroblast growth factor 1 gene expression in vascular smooth muscle cells is modulated via an alternate promoter in response to serum and phorbol ester. Nucleic Acids Res. 1995;23:434–441. doi: 10.1093/nar/23.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu YC, Lee DC, Chen SL, Liao WC, Lin JW, et al. Brain-specific 1B promoter of FGF1 gene facilitates the isolation of neural stem/progenitor cells with self-renewal and multipotent capacities. Dev Dyn. 2009;238:302–314. doi: 10.1002/dvdy.21753. [DOI] [PubMed] [Google Scholar]
- 30.Myers RL, Ray SK, Eldridge R, Chotani MA, Chiu IM. Functional characterization of the brain-specific FGF-1 promoter, FGF-1.B. J Biol Chem. 1995;270:8257–8266. doi: 10.1074/jbc.270.14.8257. [DOI] [PubMed] [Google Scholar]
- 31.Muenke M, Schell U, Hehr A, Robin NH, Losken HW, et al. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat Genet. 1994;8:269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- 32.Roumiantsev S, Krause DS, Neumann CA, Dimitri CA, Asiedu F, et al. Distinct stem cell myeloproliferative/T lymphoma syndromes induced by ZNF198-FGFR1 and BCR-FGFR1 fusion genes from 8p11 translocations. Cancer Cell. 2004;5:287–298. doi: 10.1016/s1535-6108(04)00053-4. [DOI] [PubMed] [Google Scholar]
- 33.Mosselman S, Claesson-Welsh L, Kamphuis JS, van Zoelen EJ. Developmentally regulated expression of two novel platelet-derived growth factor alpha-receptor transcripts in human teratocarcinoma cells. Cancer Res. 1994;54:220–225. [PubMed] [Google Scholar]
- 34.Kraft HJ, Mosselman S, Smits HA, Hohenstein P, Piek E, et al. Oct-4 regulates alternative platelet-derived growth factor alpha receptor gene promoter in human embryonal carcinoma cells. J Biol Chem. 1996;271:12873–12878. doi: 10.1074/jbc.271.22.12873. [DOI] [PubMed] [Google Scholar]
- 35.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 36.Griffin JH, Leung J, Bruner RJ, Caligiuri MA, Briesewitz R. Discovery of a fusion kinase in EOL-1 cells and idiopathic hypereosinophilic syndrome. Proc Natl Acad Sci U S A. 2003;100:7830–7835. doi: 10.1073/pnas.0932698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 38.Simon MP, Pedeutour F, Sirvent N, Grosgeorge J, Minoletti F, et al. Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat Genet. 1997;15:95–98. doi: 10.1038/ng0197-95. [DOI] [PubMed] [Google Scholar]
- 39.Sun H, Palaniswamy SK, Pohar TT, Jin VX, Huang TH, et al. MPromDb: an integrated resource for annotation and visualization of mammalian gene promoters and ChIP-chip experimental data. Nucleic Acids Res. 2006;34:D98–103. doi: 10.1093/nar/gkj096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singer GA, Wu J, Yan P, Plass C, Huang TH, et al. Genome-wide analysis of alternative promoters of human genes using a custom promoter tiling array. BMC Genomics. 2008;9:349. doi: 10.1186/1471-2164-9-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandelin A, Carninci P, Lenhard B, Ponjavic J, Hayashizaki Y, et al. Mammalian RNA polymerase II core promoters: insights from genome-wide studies. Nat Rev Genet. 2007;8:424–436. doi: 10.1038/nrg2026. [DOI] [PubMed] [Google Scholar]
- 43.Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Gobbi M, Viprakasit V, Hughes JR, Fisher C, Buckle VJ, et al. A regulatory SNP causes a human genetic disease by creating a new transcriptional promoter. Science. 2006;312:1215–1217. doi: 10.1126/science.1126431. [DOI] [PubMed] [Google Scholar]
- 45.Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, et al. A genome-wide association study of global gene expression. Nature Genetics. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 46.Goring HHH, Curran JE, Johnson MP, Dyer TD, Charlesworth J, et al. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nature Genetics. 2007;39:1208–1216. doi: 10.1038/ng2119. [DOI] [PubMed] [Google Scholar]
- 47.Sironi M, Menozzi G, Comi GP, Cagliani R, Bresolin N, et al. Analysis of intronic conserved elements indicates that functional complexity might represent a major source of negative selection on non-coding sequences. Hum Mol Genet. 2005;14:2533–2546. doi: 10.1093/hmg/ddi257. [DOI] [PubMed] [Google Scholar]
- 48.Xin D, Hu L, Kong X. Alternative promoters influence alternative splicing at the genomic level. PLoS One. 2008;3:e2377. doi: 10.1371/journal.pone.0002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 50.Yu X, Lin J, Zack DJ, Mendell JT, Qian J. Analysis of regulatory network topology reveals functionally distinct classes of microRNAs. Nucleic Acids Res. 2008;36:6494–6503. doi: 10.1093/nar/gkn712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouhi A, Mager DL, Humphries RK, Kuchenbauer F. MiRNAs, epigenetics, and cancer. Mamm Genome. 2008;19:517–525. doi: 10.1007/s00335-008-9133-x. [DOI] [PubMed] [Google Scholar]
- 52.Conrad B, Antonarakis SE. Gene duplication: a drive for phenotypic diversity and cause of human disease. Annu Rev Genomics Hum Genet. 2007;8:17–35. doi: 10.1146/annurev.genom.8.021307.110233. [DOI] [PubMed] [Google Scholar]
- 53.Ideker T, Sharan R. Protein networks in disease. Genome Res. 2008;18:644–652. doi: 10.1101/gr.071852.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakaguri H, Yamashita R, Suzuki Y, Sugano S, Nakai K. DBTSS: database of transcription start sites, progress report 2008. Nucleic Acids Res. 2008;36:D97–101. doi: 10.1093/nar/gkm901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, et al. NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res. 2009;37:D885–890. doi: 10.1093/nar/gkn764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, et al. NCBI GEO: mining tens of millions of expression profiles–database and tools update. Nucleic Acids Res. 2007;35:D760–765. doi: 10.1093/nar/gkl887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones S, Zhang XS, Parsons DW, Lin JCH, Leary RJ, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parsons DW, Jones S, Zhang XS, Lin JCH, Leary RJ, et al. An integrated genomic analysis of human glioblastoma Multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 61.Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33:D514–517. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu CH, Apweiler R, Bairoch A, Natale DA, Barker WC, et al. The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic Acids Res. 2006;34:D187–191. doi: 10.1093/nar/gkj161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smedley D, Haider S, Ballester B, Holland R, London D, et al. BioMart–biological queries made easy. BMC Genomics. 2009;10:22. doi: 10.1186/1471-2164-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vilella AJ, Severin J, Ureta-Vidal A, Heng L, Durbin R, et al. EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 2009;19:327–335. doi: 10.1101/gr.073585.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Assenov Y, Ramirez F, Schelhorn SE, Lengauer T, Albrecht M. Computing topological parameters of biological networks. Bioinformatics. 2008;24:282–284. doi: 10.1093/bioinformatics/btm554. [DOI] [PubMed] [Google Scholar]
- 66.Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, et al. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc. 2007;2:2366–2382. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Length distribution for the gene structure parameter of each gene class. The figure (density plot) showed that genes with more alternative promoters tend to be longer in all aspects of gene structure. SP means gene with single promoter, while AP = 2, AP = 3∼4, and AP> = 5 means gene with only 2 promoters, 3 or 4 promoters, and at least 5 promoters, respectively.
(0.27 MB PDF)