Abstract
In the present study, we have evaluated a boronated dendrimer-epidermal growth factor (BD-EGF) bioconjugate as a molecular targeting agent for boron neutron capture therapy (BNCT) of the human EGFR gene-transfected F98 rat glioma, designated F98EGFR. EGF was chemically linked to a heavily boronated polyamidoamine dendrimer (BD) by means of the heterobifunctional reagent, mMBS. Biodistribution studies were carried out at 6 h and 24 h following intratumoral (i.t.) injection or intracerebral (i.c.) convection enhanced delivery (CED) of 125I-labeled or unlabeled BD-EGF (40 μg 10B/10 μg EGF) to F98 glioma bearing rats. At 24 h. there was 43% more radioactivity in EGFR(+) tumors following CED compared to i.t. injection, and a doubling of the tumor boron concentration (22.3 μg/g vs. 11.7 μg/g). CED of BD-EGF resulted in a 7.2× increase in the volume of distribution within the infused cerebral hemisphere and a 1.9× increase in tumor uptake of BD-EGF compared with i.t. injection. Based on these favorable bio-distribution data, BNCT was carried out at the Massachusetts Institute of Technology nuclear reactor 14 days following i.c. tumor implantation and 24 h. after CED of BD-EGF. These animals had a MST of 54.1 ± 4.7 days compared to 43.0 ± 2.8 days following i.t. injection. Rats that received BD-EGF by CED in combination with i.v. boronophenylalanine (BPA), which has been used in both experimental and clinical studies, had a MST of 86.0 ± 28.1 days compared to 39.8 ± 1.6 days for i.v. BPA alone (P < 0.01), 30.9 ± 1.4 days for irradiated controls and 25.1 ± 1.0 days for untreated controls (overall P < 0.0001). These data have demonstrated that the efficacy of BNCT was significantly increased (P < 0.006), following i.c CED of BD-EGF compared to i.t injection, and that the survival data were equivalent to those previously reported by us using the boronated anti-human-EGF mAb, C225 (cetuximab).
Keywords: Convection enhanced delivery, Boronated EGF, Boron neutron capture therapy, F98 rat glioma
Introduction
The gene encoding the epidermal growth factor receptor (EGFR) and its mutant isoform, EGFRvIII, frequently are overexpressed in malignant gliomas and both are low or undetectable in normal brain [1, 2]. Based on these differences in receptor expression, we have carried out studies using either boronated EGF [3–5] or anti-EGFR monoclonal antibodies (mAbs) [6–9] as molecular targeting agents for boron neutron capture therapy. BNCT is a binary system based on the selective uptake of sufficient amounts of boron-10 (~109 atoms/cell) by tumor cells, followed by irradiation with low energy (<0.025 eV), thermal neutrons. The resulting nuclear capture and fission reactions produce α particles and recoiling 7Li nuclei, which have high linear energy transfer (LET) and pathlengths of approximately 9 μm and 5 μm, respectively. Each component can be manipulated independently, so that the interval between administration of the capture agent and neutron irradiation can be adjusted to an optimal time at which the differential between boron concentration levels in normal tissues and tumor are maximized. For BNCT to be successful, there must be selective accumulation of 10B in the tumor, low levels in blood, endothelial cells and normal brain, and a sufficient thermal neutron fluence must be delivered to the tumor site. These requirements are discussed in detail in several recent reviews [10–12] and a recently published monograph [13].
One of the major challenges for effectively treating high grade gliomas with BNCT is how to selectively deliver a sufficient amount of 10B to individual tumor cells to sustain a lethal 10B(n,α)7Li capture reaction. Using F98 rat glioma cells, which had been transfected with the wildtype human EGFR gene and has been designated F98EGFR, we have observed enhanced survival of glioma bearing rats following direct intratumoral (i.t.) injection of boronated EGF either alone or in combination with boronophenylalanine (BPA) [3, 5]. In order to increase the tumor up take of boronated EGF (BD-EGF), in the present study we have employed convection enhanced delivery (CED) to improve targeting of the EGFR (+) F98EGFR glioma [4]. CED is an innovative method for local drug delivery to brain tumors by which a pressure gradient, or bulk flow, is used to drive an infusate through the extracellular fluid compartment [14, 15]. It allows delivery of the infusate to the tumor and surrounding brain at much higher concentrations than could be achieved by systemic administration. As demonstrated in animal studies [4, 16, 17], and in several clinical trials [18–20], CED has not only increased the delivery of both low and high molecular weight agents, but also has improved their therapeutic efficacy.
In the present study, we have compared the efficacy of BNCT following either i.t. injection or CED of BD-EGF in rats bearing i.c. F98EGFR gliomas. As described in detail in the following report, CED of BD-EGF was therapeutically more effective than i.t. injection for BNCT of F98EGFR glioma-bearing rats.
Materials and methods
Production and characterization of F98EGFR-expressing glioma cells
The F98 rat glioma cell line (#CRL-2397, American Type Culture Collection, Manassus, VA) was derived from a brain-tumor bearing CD-Fischer rat, whose mother had received N-ethyl-N-nitrosourea during pregnancy and it has been propagated in vitro and in vivo for almost 50 years [21]. F98 cells were transfected with the pLXIN vector containing a wild-type EGFR, cDNA, as described by us in detail elsewhere [3]. One cell line, designated F98EGFR, was selected for the studies described in this report.
Expression of EGFR on F98EGFR cells was quantified by means of fluorescence activated cell sorting (FACS). Monoclonal antibody (mAb), EGFR-1 (BD PharMingen, San Jose, CA) was used to determine EGFR site density. This is an IgG2b mAb directed against the extracellular domain of wild-type EGFR and is non-reactive with EG-FRvIII. Wildtype F98 (F98WT) and F98EGFR cell lines were analyzed by quantitative FACS, as described in detail elsewhere [22]. Incubation of the bead sample with an identical aliquot of fluoresceinated mAb, used for cell analysis, permitted the extrapolation of the number of antibody molecules bound per cell. The optimal concentrations of the fluoresceinated mAbs were determined by repeated titrations of mAb EGFR-1 versus NR6 W cells, which expressed wild-type EGFR [23]. A concentration of 10 μg/ml of mAb EGFR-1 fully saturated all available receptor sites on 106 cells. Background binding was determined by inclusion of fluoresceinated, irrelevant IgG1 and IgG2b as isotype controls and the EGFR negative cell line, NR6 M. The number of EGFR sites expressed by the transfected F98EGFR cells was determined by quantitative FACS using the Quantum Simply Cellular system (Flow Cytometry Standards Corp., San Juan, Puerto Rico.
Tumor implantation and MRI studies
All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996) and a protocol was approved by the Institutional Laboratory Animal and Use Committee (ILUAC) of The Ohio State University. Fischer rats, purchased from the Animal Production Branch, National Cancer Institute, Frederick, MD, and weighing 200–220 g, were anesthetized with a 1.2:1 mixture of ketamine/xylazine. Following this, 103 tumor cells were implanted stereotactically i.c. into the right caudate nucleus, as previously described [24]. A small plastic screw (Arrow Machine Manufacturing, Inc., Richmond, VA) with an entry port, which allowed insertion of a 27 gauge needle, was embedded into the calvarium prior to implantation. In order to compare the growth rates of F98WT and F98EGFR gliomas, tumor bearing rats were imaged using a Varian Unity Inova Magnetic Resonance System (Varian, Palo Alto, CA) beginning 8 days after implantation. The tumor volume was measured using T2- and T1-weighted magnetic resonance imaging (MRI) every second day [25]. The T2-weighted images were acquired using multislice fast spin echo (FSE), as described by us in detail elsewhere [26].
Immunohistochemical characterization of the F98EGFR glioma
Rats bearing i.c. implants of either F98WT or F98EGFR gliomas were euthanized at that point in time when they showed clinical signs of a progressively growing brain tumor [27]. The brains were removed and fixed in 10% buffered formalin, following which they were embedded in paraffin. Four μM sections were cut and stained with hematoxylin and eosin (H&E) for routine histologic examination. Immunostaining for wildtype EGFR, using the anti-EGFR mAb, EGFR-1 (Zymed, South San Francisco, CA), was carried out on formalin-fixed paraffin embedded tissue that were cut at 4 m, as per instructions of the manufacturer, using the anti-EGFR1 mAb at a 1:1,000 dilution. Slides then were washed in phosphate buffered saline (PBS) and the appropriate dilution of biotinylated rabbit anti-mouse IgG (DAKO Corp., Carpinteria, CA) was applied. They then were incubated for 30 min. at ambient temperature, washed again in PBS (pH = 7.4), and exposed to horseradish peroxidase-avidin complex (ImmunoPure Ultra-Sensitive ABC Staining kit and Metal Enhanced DAB Substrate kit, Pierce) for 30 min., washed with PBS, and developed with diaminobenzidine.
Preparation of boronated EGF
A fourth-generation polyamido amine (PAMAM) dendrimer, which is composed of repetitive amino groups arranged in a starburst pattern, was boronated with a boron-10 enriched (>98% 10B) methylisocyanato polyhedral borane anion, Na(CH3)3NB10H8NCO, to yield a boronated dendrimer (BD) using a procedure described in detail elsewhere [28]. EGF was derivatized with the heterobifunctional reagent m-maleimidobenzoyl-N-hydroxysulfosuccinimide ester (mMBS) (Pierce Chemical Co., Rockford, IL) and linked to sulfhydryl-containing BD to yield a BD-EGF bioconjugate. Boron was quantified by means of direct current plasma-atomic emission spectroscopy (DCP-AES) using a Spectraspan VB spectrometer (Applied Research Laboratories, La Brea, CA), as previously described [29]. Fractions, containing peak concentrations of both protein and boron, were pooled and used in the studies described below.
Biodistribution of BD-EGF following either convection enhanced delivery or intratumoral injection
For radiolocalization studies, BD-EGF was reacted with Bolton-Hunter reagent to introduce a phenolic function into the bioconjugate. It then was radioiodinated with 125I-NaI by means of a procedure described by us in detail [28]. 125I labeled BD-EGF was shown to be stable and was not dehalogenated for at least one week when kept at 4°C. F98WT or F98EGFR cells (105 per 10 μl) were implanted stereotactically into the right caudate nucleus of Fischer rats. Twelve to 14 days later biodistribution studies were initiated. Rats were anesthetized with a mixture of ketamine/xylazine, following which intratumoral (i.t.) injection and CED of 125I-BD-EGF were performed via a 27 gauge needle that had been inserted into the entry port of the plastic screw. This had been embedded in the calvarium at the time of tumor implantation. Intratumoral injection of 10 μl of the bioconjugate was carried out over a 2 min time period by means of a 25 μl Hamilton syringe. CED was carried out using a syringe pump (Harvard Apparatus Co, Cambridge, MA) to deliver 10 μl of BD-EGF at a flow rate of 0.33 μl/min for 30 min. Rats received 5 μCi of 125I-labeled BD-EGF (40 μg of boron/10 μg EGF) by either CED or i.t. injection and were euthanized either 6 or 24 h later. Tumor, normal brain, blood and other tissue samples were taken and biodistribution was determined by means of γ-scintillation counting using a well counter. Tissue samples were counted along with triplicate samples of the injectate in order to correct for decay of the isotope before γ counting. In a separate study to quantify the uptake of boron in tumor and normal tissues, the animals received non-radiolabeled BD-EGF, either alone or in combination with i.v. BPA, i.t. injection or CED. The animals were euthanized 24 h following administration. Boron concentrations were determined by means of DCP-AES [29], and the percent injected dose per gram (% ID/g) was calculated.
Boron neutron capture therapy experiments
BNCT was performed 14 days following stereotactic implantation of 103 F98EGFR glioma cells into male Fischer rats. Animals were transported to the Nuclear Reactor Laboratory at the Massachusetts Institute of Technology (MIT) and then randomized on the basis of weight into experimental groups of 8–11 rats each as follows: Group 1, i.t injection of BD-EGF and BNCT; Group 2, CED of BD-EGF and BNCT; Group 3, i.t. injection of BD-EGF plus i.v. BPA and BNCT; Group 4, CED of BD-EGF plus i.v. BPA and BNCT; Group 5, i.v. BPA and BNCT; Group 6, CED of saline and neutron irradiation; and Group 7, CED of BD-EGF. BNCT was initiated 24 h after i.t. injection or CED of 10 μl of BD-EGF (40 μg of 10B/10 μg of EGF) and 2.5 h after i.v. administration via the penile vein of BPA (500 mg/kg body weight) (Katchem, Ltd., Prague, Czech Republic). All irradiated rats were anesthetized with a 1.2:1 mixture of ketamine and xylazine. BNCT was carried out, as described in detail elsewhere [6] at the MITR-II research reactor in the M011 irradiation facility. This produces a beam of thermal neutrons of high purity and intensity with no measurable fast neutron component.
Dosimetry
Dosimetric measurements were performed on both dead rats and phantoms made from type 6 nylon, using bare gold foils and a graphite walled ionization chamber (V = 0.1 cm3) flushed with reagent grade CO2 [30]. The measured dose rates in brain (2.2% nitrogen by weight), normalized to the reactor operating at a power of 5 MW, were 18.5 cGy/min for the 1H(n,γ)2H capture reaction with tissue hydrogen, 7.7 cGy/min for the 14N(n,p)14C capture reaction with tissue nitrogen and 3.4 cGy/min per μg 10B in tumor and normal tissues. As previously described in a preceding section for dosimetric calculations, boron concentrations were determined in tumor normal brain and blood in a separate group of animals 24 h after administration of BD-EGF and 2.5 h after i.v. injection of BPA. Animal irradiations were performed with the reactor operating at a power between 4.0 and 4.8 MW. These took between 6.9 and 8.6 min to deliver a thermal neutron fluence of 2.64 × 1012 n.cm−2 to complete previous dose prescriptions [5, 6]. After completion of BNCT, the animals were held at MIT for ~3 days to allow induced radioactivity to decay, following which they were returned to The Ohio State University in Columbus, OH for clinical monitoring.
Monitoring of clinical status and neuropathologic evaluation
All animals were weighed three times per week and their clinical status was evaluated at the same time. Once the animals had progressively growing tumors, as evidenced by the combination of sustained weight loss (20% of body weight over 3 d), ataxia and peri-orbital hemorrhage, they were euthanized in order to minimize discomfort. Survival times were determined by adding 1 day to the time between tumor implantation and euthanization by exposure to halothane vapor. Rats surviving >180 days were designated long term survivors and were euthanized. The brains of all animals in the therapy studies were removed after death, fixed in 10% buffered formalin, and then cut coronally at the level of the optic chiasm and 2 mm anterior and posterior to it. Tissue sections through the tumor were embedded in paraffin, cut at 4 μm, stained with H&E, and examined microscopically to assess histopathologic changes. The tumor size index was determined from H&E stained coronal sections of brain, using a semiquantitative grading scale ranging from 0 to 4. Each section was scored as follows: 0, no tumor; 1, very small (i.e., microscopic, <1 mm); 2, small (~1–3 mm); 3, large (~4–7 mm); and 4, massive (>8 mm).
Statistical evaluation of data
The means and standard deviations (SD) were computed for boron concentrations in the tumor, brain around tumor (BAT), ipsilateral (tumor bearing) and contralateral (non-tumor bearing) cerebral hemispheres, and blood and the T:Br concentration ratios were calculated for each group. The Wilcoxin–Gehan rank sum test [31] was used to evaluate survival data following implantation of logarithmically incremental numbers of F98WT or F98EGFR glioma cells. To study the effects of BNCT on survival of F98 glioma bearing rats, the mean survival time (MST), standard error (SE), and median survival time (MeST) were calculated for each group using the Kaplan–Meier estimate. Kaplan–Meier and Cox survival curves also were plotted for each group. An overall log rank test was performed to test for equality of survival curves over the seven groups. The Wald test was used for these comparisons, with a Bonferroni method of adjustment for the multiple comparisons [32]. The percent increased life span (%ILS) was determined from the following equations where “t” designates treated and “u” designates untreated animals:
Results
Characterization of the F98EGFR rat glioma cell line
F98 glioma clones, which stably expressed the non-phosphorylated human EGFR gene, were used for quantitative FACS analysis. Data on the expression of EGFR in F98EGFR and F98WT glioma cells are shown in Fig. 1. Since mere visual inspection of the peaks was not accurate in quantitative FACS, regression analysis of the data was carried out and compared with data obtained with calibrated fluorescent beads. Localization of EGFR on the cell surface was confirmed and quantified by FACS using mAb EGFR-1. The F98EGFR glioma expressed 1.2 × 105 receptor sites per cell compared with undetectable levels on F98WT cells (i.e., in the range of 104 per cell). Quantitative FACS analysis revealed that F98EGFR cells were positive with mAb EGFR-1 but not with mAb L8A4, which was reactive with EGFRvIII. Both mAbs bound to F98WT in amounts similar to background levels of control IgG1.
Fig. 1.
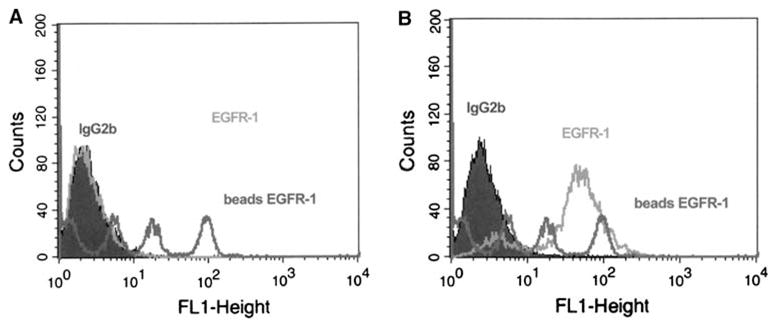
Flow cytometric analysis. Quantitative FACS analysis revealed that mAb EGFR-1 bound to F98WT cells in amounts similar to background levels of a control IgG1 antibody (A) and F98EGFR cells were positive with mAb EGFR-1 (B) and expressed 1.2 × 105 receptor sites per cell compared with undetectable levels on F98WT cells (i.e., in the range of 104 per cell)
Neuropathologic evaluation and immunohistochemical characterization of the F98EGFR glioma
The morphology of the F98EGFR glioma was indistinguishable on H&E-stained sections from that of the F98WT glioma, as was previously described by us [33]. The tumor was composed of a mixed population of spindle shaped cells with fusiform nuclei, frequently forming a whorled pattern of growth, and a smaller subpopulation of polygonal cells with round to oval nuclei. Infiltrating islands of tumor cells were seen at varying distances in the adjacent white matter, and usually, they surrounded a central capillary. Immunostaining of sections of the F98EGFR glioma with EGFR-1 mAb was strongly positive while in contrast F98WT tumors were uniformly negative.
Determination of in vivo tumor growth rate
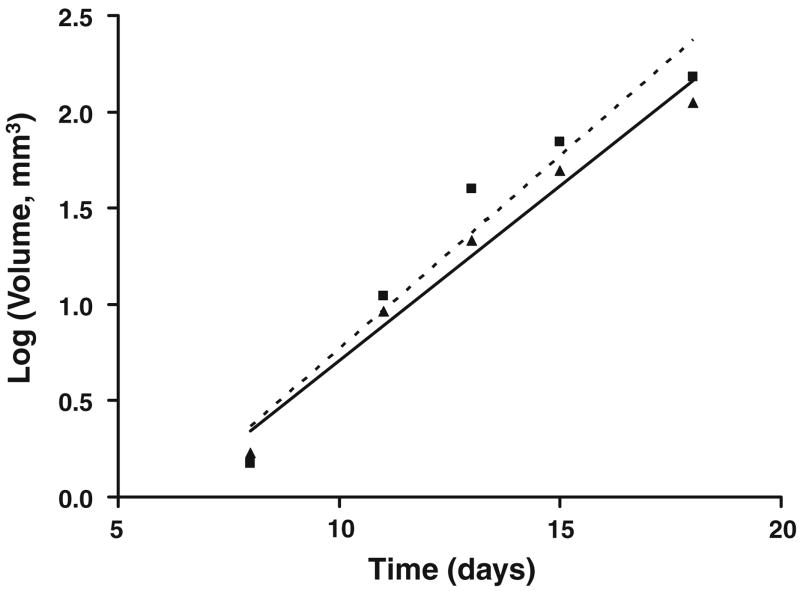
MRI studies were carried out in a small subset of animals to non-invasively quantify tumor growth rates as a function of time. Plots of the mean tumor volume versus time for the F98EGFR and F98WT gliomas are shown in Fig. 3, and these were statistically equivalent (P > 0.25). Based on MRI measurements, the mean tumor volumetric doubling times (± SE) for the F98WT and F98EGFR gliomas were 59.8 ± 4.8 and 53.2 ± 6.2 h (Fig. 2), respectively, which were not significantly different (P > 0.05).
Fig. 3.
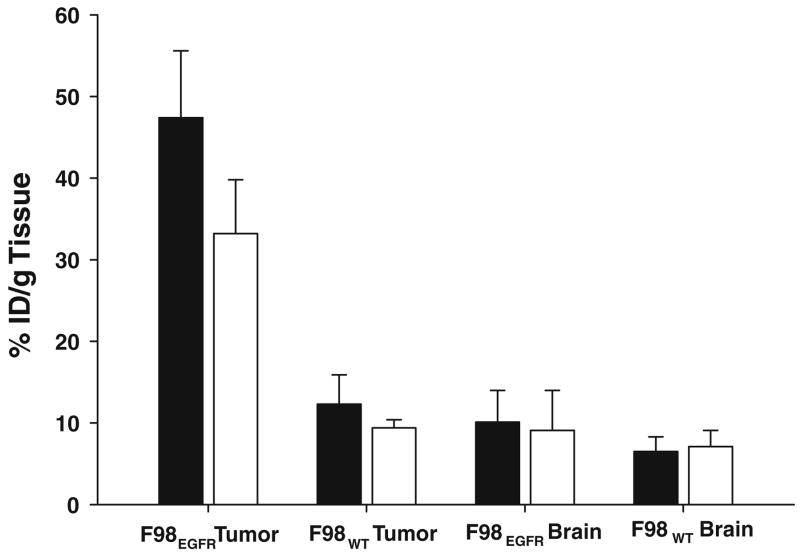
Tumor and brain uptake of 125I-BD-EGF at 24 h. following either i.t. injection (open square) or CED (filled square) into rats bearing either F98WT or F98EGFR gliomas. Following CED, 47.5 % ID/g of BD- EGF was retained in F98EGFR gliomas compared to 33.2 % ID/g following i.t. injection (P <0.01). In contrast, following either CED or i.t. injection of the BD- EGF, only 12.3% and 9.4% ID/g were retained in F98WT gliomas, respectively, which was significantly different (P < 0.003) from that detected in F98EGFR gliomas. CED of BD-EGF resulted in a 7.2× increase in the volume of distribution within the infused cerebral hemisphere and a 1.9× increase in tumor uptake of BD-EGF compared with i.t. injection, thereby demonstrating the superiority of CED as a mode of administration of the bioconjugate
Fig. 2.
Magnetic resonance imaging studies were carried out in a small subset of animals to non-invasively quantify tumor growth rates as a function of time. Plots of the mean tumor volume versus time for the F98EGFR (filled triangle) and F98WT (filled square) gliomas have been plotted, and these were statistically equivalent (P >0.25). Based on MRI measurements, the mean tumor volumetric doubling times (± SE) for the F98WT and F98EGFR gliomas were 59.8 ± 4.8 and 53.2 ± 6.2 hours, respectively, which were not significantly different (P > 0.05)
Biodistribution of BD-EGF following CED
As determined by γ-scintillation counting of individual animals, between 1 and 6 h following i.t. injection or CED, 65–73% ID/g of BD-EGF was non-specifically localized in F98EGFR and F98WT tumors and the differences between the CED and i.t. injection group were not significant (P > 0.2) (data not shown). However, as shown in Fig. 3, by 24 h following CED, 47.5% ID/g of BD-EGF was retained in F98EGFR gliomas compared to 33.2% ID/g following i.t. injection (P < 0.01), indicating that retention of the bioconjugate was dependent upon EGFR expression. In contrast, following either CED or i.t. injection of BD-EGF in F98WT glioma bearing rats, only 12.3% and 9.4% ID/g, respectively, were retained in the gliomas. These were significantly different (P < 0.003) from the values observed in F98EGFR glioma bearing animals. In contrast, the amounts of BD-EGF in brain and blood were not significantly different for CED and i.t. injection groups.
Boron concentrations and dosimetry
Tumor, normal brain and blood boron concentrations at 24 h following either CED or i.t. injection of BD-EGF (40 μg of 10B/10 μg EGF) are summarized in Table 1. The tumor boron concentration in rats that received CED of BD-EGF was 22.3 μg/g compared with 11.7 μg/g for i.t. injection (P < 0.005). The corresponding boron concentrations in normal brain of the ipisilateral (tumor bearing) cerebral hemisphere were 3.1 μg/g and 2.9 μg/g, respectively. Boron concentrations following either CED or i.t. injection of BD-EGF in the contralateral (non-tumor bearing) cerebral hemisphere, blood, liver, kidneys and spleen all were at undetectable levels (<0.5 μg/g). Dosimetric calculations were based on mean boron concentrations of tumor, brain and blood at 24 h following CED of BD-EGF and 2.5 h after i.v. administration of BPA in a separate group of untreated animals. Based on these total boron concentrations, the mean absorbed physical dose delivered to F98EGFR tumors was 6.6 Gy following CED of BD-EGF, 4.4 Gy following i.t. injection and 5.0 for i.v. BPA alone and 10.0 Gy in combination with CED of BD-EGF and 7.4 Gy in combination with i.t. BD-EGF. The normal brain doses ranged from 1.9 to 3.2 Gy. All radiation doses were expressed as the physical absorbed doses and no attempt was made to apply biological weighting factors due to a lack of information on the in vivo cellular and subcellular distribution of BD-EGF.
Table 1.
Boron concentrations and physical radiation doses delivered to tumor, brain and blood at 24 h following either i.t. injection or CEF of BD-EGF
| Boron concentrations (μg/g)a |
Physical dose (Gy)c |
|||||
|---|---|---|---|---|---|---|
| Test group | Tumor | Brainb | Blood | Tumor | Brain | Blood |
| BD-EGF/CED | 22.3 ± 4.3 | 3.1 ± 1.5 | < 0.5 | 6.6 | 2.6 | 1.9 |
| BD-EGF/i.t. | 11.7 ± 1.6 | 2.9 ± 1.0 | < 0.5 | 4.4 | 2.6 | 1.9 |
| BPA/i.v. | 14.4 ± 6.7 | 4.9 ± 1.4 | 6.2 ± 2.0 | 5.0 | 3.0 | 3.2 |
| BD-EGF/CED + BPA/i.v. | 36.2 ± 6.9 | 5.1 ± 1.5 | 5.5 ± 3.2 | 10.0 | 3.1 | 3.1 |
| BD-EGF/i.t. +BPA/i.v. | 25.6 ± 6.4 | 6.0 ± 3.2 | 6.4 ± 0.8 | 7.4 | 3.2 | 3.3 |
| Radiation controls | None | None | None | 1.9 | 1.9 | 1.9 |
| Untreated controls | None | None | None | 0 | 0 | 0 |
Boron content was quantified by means of DCP-AES. These values were obtained from rats that had received i.t. BD-EGF (40 μg10B/10 μg EGF) alone 24 h earlier, or in combination with i.v. BPA (500 mg/kg b.w. equivalent to 24 mg B/kg b.w.), which was administered 2.5 h prior to euthanization
Boron concentrations in the tumor bearing (ipsilateral) cerebral hemisphere following excision of the tumor
Physical dose estimates include contributions from fast neutrons, γ photons, and 14N (n,p)14C and 10B (n,α) 7Li reactions
Therapeutic response of glioma bearing rats following BNCT
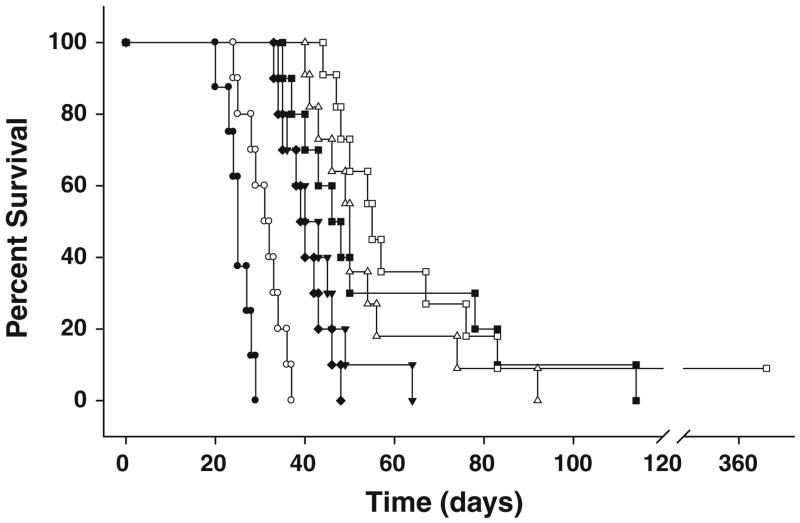
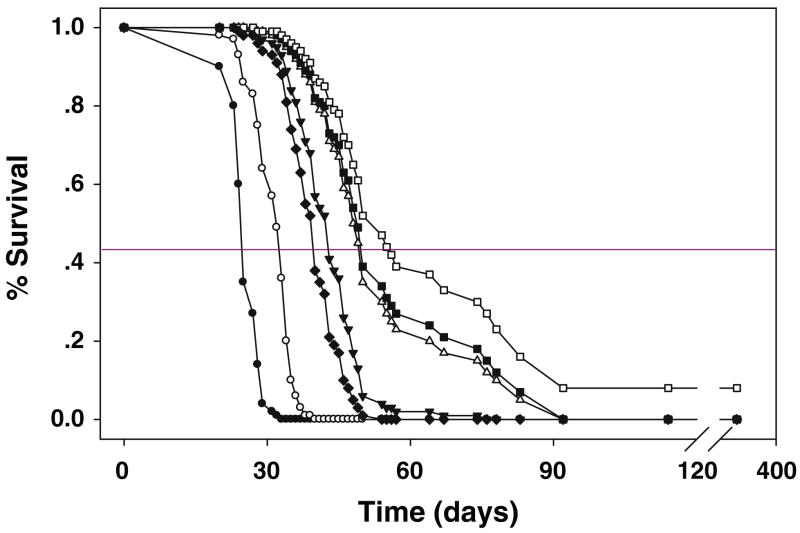
All animals in a pilot study to determine tolerance to BNCT following CED of BD-EGF lost ~10% of their b.w. within 7–10 days after treatment. Rats that received CED of 40 μg of 10B/10 μg BD-EGF and 500 mg/kg b.w. of BPA i.v. lost <10% of their b.w., but regained it within 2 weeks. Based on these results, this dose of BD-EGF was selected for therapy studies. All rats tolerated BNCT without any untoward effects and 3–7 days later they were returned to Columbus, Ohio for clinical monitoring. Survival data are summarized in Table 2 and Kaplan–Meier and Cox survival plots for BNCT treated animals and irradiated controls are shown in Figs. 4 and 5, respectively. Untreated control rats had a MST ± SE of 25.1 ± 1.0 days compared to a modest increase of 30.9 ± 1.4 days for the irradiated controls. Animals that received BD-EGF by CED, followed by BNCT, had a MST of 54.1 ± 4.7 days (range 40–92 days) compared to 43.0 ± 2.8 days for i.t. injection of BD-EGF. Animals that had received BD-EGF by CED in combination with i.v. BPA had a MST of 86.0 ± 28.1 days (range 44–350 days) with one rat surviving >365 days compared to 39.8 ± 1.6 days (range 33–48 days) for i.v. BPA alone (P < 0.01), 30.9 ± 1.4 days for irradiated controls and 25.1 ± 1.0 days for untreated controls. The corresponding % ILS were 243% for the combination versus 116% for CED of BD-EGF and 71% for i.t. injection of BD-EGF. The test for equality of the survival curves indicated that overall, the differences were highly significant (P < 0.0001). The results from these comparisons conclusively demonstrated that the therapeutic effect of CED of BD-EGF was significantly better than that obtained following i.t injection (P < 0.006).
Table 2.
Survival times of F98 glioma-bearing rats following eitherCED or i.t. injection of BD-EGF with or without i.v. BPA and BNCT
| Test group | Na | Range | Survival time (days) |
% Increased life spanb |
||
|---|---|---|---|---|---|---|
| Mean ± SE | Median | Mean | Median | |||
| BD-EGF/i.t. | 11 | 34–64 | 43.0 ± 2.8 | 42 | 71 | 66 |
| BD-EGF/CED | 11 | 40–92 | 54.1 ± 4.7 | 50 | 116 | 100 |
| BD-EGF/i.t + BPA/i.v. | 10 | 35–114 | 57.4 ± 8.2 | 47 | 129 | 88 |
| BD-EGF/CED + BPA/i.v. | 10 | 44–365(1) | 86.0 ± 28.1 | 55 | 243 | 120 |
| BPA/i.v. | 10 | 33–48 | 39.8 ± 1.6 | 40 | 59 | 58 |
| Irradiated controls | 10 | 24–37 | 30.9 ± 1.4 | 32 | 23 | 26 |
| Untreated controls | 8 | 20–29 | 25.1 ± 1.0 | 25 | – | – |
The number in parentheses were the number of rats surviving >365 days
N designate the number of animals per group
Percent of increased life span was defined relative to mean and median survival times of untreated controls
Fig. 4.
Kaplan–Meier survival plots for F98EGFR glioma-bearing rats. Survival time in days after implantation have been plotted for untreated animals (filled circle), irradiation (open circle), i.v. BPA (filled diamond), i.t. BD-EGF (inverted filled triangle), CED BD-EGF (open triangle), i.t. BD-EGF + BPA (filled square), CED BD-EGF + BPA (open square).F98EGFR glioma bearing rats that received BD-EGF, administered i.c. by means of CED in combination with i.v. BPA had a MST of 86 days compared to 57 days for rats that received i.t. BD-EGF and i.v. BPA. The improved survival of animals that received BD-EGF by CED versus i.t. injection (MST 43 days) correlated with a doubling of the tumor boron concentration compared to that obtained following i.t. injection (22.3 μg/g vs. 11.7 μg/g)
Fig. 5.
Cox survival plots for F98EGFR glioma-bearing rats. Survival times in days after implantation have been plotted for untreated animals (filled circle), irradiation (open circle), i.v. BPA (filled diamond), i.t. BD-EGF (inverted filled triangle), CED BD-EGF (open triangle), i.t. BD-EGF + BPA (filled square), CED BD-EGF + BPA (open square). Cox’s method performs a simultaneous fit of the survival curves using all of the data points with a partial likelihood approach. Therefore, the number of data points in each curve includes all of the death times, rather than those animals in a specific group
Histopathologic evaluation
The brains of all animals were subjected to histopathologic examination. Animals that received boronated EGF either alone or in combination with BPA had almost equivalent tumor size indices (3.6, 3.8 and 3.8). Control animals that received either non-boronated dendrimer, which had been conjugated to EGF, or BD-EGF without neutron irradiation had smaller tumor size indices (3.4 and 3.0, respectively). The fact that these animals died with smaller tumors than those that had received BNCT suggests that they may have had more cerebral edema. Histopathologically, the appearance of the tumor in the various treatment groups was similar to that previously described by us for animals that had received the boronated mAb L8A4 [8]. Briefly summarized, irrespective of the treatment, almost all of the tumors were highly infiltrative of surrounding white matter with islands of malignant cells at some distance from the main tumor mass. Small zones of necrosis were seen either centrally or at the periphery of the main tumor mass. There was considerable cellular pleomorphism ranging from spindle-shaped cells showing a storiform pattern of growth to contiguous sheets or clusters of small round tumor cells showing a highly infiltrative pattern of growth.
Discussion
As previously reported by us [3, 5], i.t. injection of BD-EGF, either alone or in combination with i.v. administration of BPA to F98EGFR glioma bearing rats, followed by BNCT, resulted in a significant prolongation in survival times compared with those observed in animals bearing EGFR (−) F98WT tumors. These studies provided proof-of- principle for molecular targeting of an EGFR (+) tumor with a boronated bioconjugate and provided a platform to extend our studies to evaluate mAbs as boron delivery agents for BNCT of the F98EGFR and F98npEGFRvIII gliomas [6–9].
In the present study, we have evaluated CED of BD-EGF as a molecular targeting agent for BNCT of the EGFR positive glioma, F98EGFR. The best survival data were obtained using BD-EGF, administered i.c. to glioma bearing rats by means of CED in combination with i.v. BPA. These animals had a MST of 86 days compared to 54 days for those that received i.t. BD-EGF and i.v. BPA. The improved survival of animals that received BD-EGF by CED versus i.t. injection (MST 43 days) correlated with a doubling of the tumor boron concentration compared to that obtained following i.t. injection (22.3 μg/g vs. 11.7 μg/g). CED of BD-EGF resulted in a 7.2 fold increase in the volume of distribution within the infused cerebral hemisphere and a 1.9 fold increase in tumor uptake of BD-EGF compared with i.t. injection [4]. The survival data that we have obtained using BD-EGF, administered by CED, are among the best that we ever have observed with the F98 glioma model [34], and are far superior to those obtained following i.v. delivery of BPA and equivalent to those obtained following i.c. delivery of BPA [27]. Furthermore, BD-EGF in combination with i.v. BPA resulted in MSTs that were comparable to those obtained by its i.c. administration of BPA combined with blood-brain barrier disruption (95 vs. 86 days) [27]. Although the tumor boron concentrations obtained with BD-EGF were only one-third of those obtained with BD-C225 [7], the MSTs of rats that received BD-C225 and BD-EGF were identical. What are the possible explanations for this? The most obvious is that BD-EGF was more readily internalized [35] than BD-C225 [28], and therefore this resulted in more favorable microdosimetry (i.e. intracellular versus extracellular localization of boron). In addition, the lower M.W. of EGF (6,000 Da), compared to that of C225 (150,000 Da), should have resulted in a better dispersion and cellular uptake of BD-EGF within the tumor following CED [14]. However, the broad range in survival times of rats that received the bioconjugate either alone or in combination with i.v. administration of BPA strongly suggests that there was considerable variability in the intra-tumoral uptake and cellular distribution of boron within tumor cells, even when it was administered by CED.
Clinically, CED has been and is being used to deliver a variety of agents to patients after surgical resection of their brain tumors to eradicate residual infiltrative tumor cells [18–20, 36]. Between one and three catheters have been inserted into the resection cavity, and infusion volumes as high as 420 ml have been administered over a 3-week interval without any significant adverse effects [20, 37, 38]. If CED were to be used clinically for the administration of boronated EGF or mAbs directed against either wildtype EGFR or EGFRvIII, it probably would have to be carried out over a longer period of time than a few days in order to maximize dispersion of the bioconjugate within the tumor following which there would have to be a long enough interval of time prior to the initiation of BNCT in order to allow for clearance of the bioconjugate from normal brain. After localization of the bioconjugate in the brain inter-stitium, additional movement within the brain or tumor occurs by diffusion, and the intra-tumoral oncotic pressure can significantly limit the volume of diffusion (Vd) [39, 40]. For high molecular weight (HMW) agents, such as BD-EGF, there is less diffusion within the brain or tumor relative to tissue clearance compared to low molecular weight (LMW) agents and this further reduces Vd. The small Vd and steep concentration gradients associated with diffusion severely limit the effectiveness of diffusive drug delivery for the regional therapy of brain tumors, and hence the importance of maximizing the convective phase of delivery. Studies by Boucher and Jain have demonstrated that the interstitial pressure and diffusion coefficients vary from one experimental tumor model to another, as well as within the tumor itself [39, 40]. This can produce significant variations in diffusion-driven drug concentrations within the tumor [41]. Although there still may be variations in concentrations of the infusate within the tumor after CED, these are much less than those that would occur after either systemic administration or direct i.t. injection. For example, CED, which produces high-flow microinfusion with volumetric inflow rates of 0.33 μl/min over 30 min, can deliver the same amount of agent to much larger volumes of brain and brain tumor [14, 42] than would otherwise be possible by direct interstitial injection, which has low-flow (diffusion) and a smaller Vd [43] The present study has shown that CED can improve the delivery of BD-EGF to much larger volumes of brain and tumor than could be achieved by i.t. injection with a significant pharmacodynamic advantage over systemic administration, where concentrations of HMW agents attain brain concentrations that are only 0.01–0.0001% of the plasma concentration [44, 45]. Although CED currently is the best method that we have to disperse both LMW and HMW agents within the brain, clearly there is a significant need for improvement. If we cannot obtain adequate dispersion and cellular and subcellular distribution of these agents in a rat brain, which weighs only ~1.2 g, it is even less likely that they can be adequately dispersed in a human brain that weighs ~1,200 g.
A major question that must be considered when using a HMW boron delivery agents for BNCT is whether the boronated ligand has a sufficiently high affinity and specificity for the receptor to permit in vivo cellular targeting. Bioconjugates produced by covalently coupling EGF and BSH to an allylated 70 kDa dextran chain had decreased specificity for EGFR as additional BSH groups were attached [46]. In contrast, using BD we have not seen a reduction in specificity of BD-EGF, although the Ka was reduced from 108 M−1 to 107 M−1 [35] Delivery of mAbs or EGF-based bioconjugates to brain tumors is a particularly challenging problem because only small quantities can be expected to localize within the tumor after systemic administration [4, 47, 48]. Another point to consider is that in the present study, we have used tumor cells that uniformly expressed EGFR. However, we know that there is considerable variability in EGFR expression in human gliomas [2, 49] and, therefore, it is highly unlikely that targeting this receptor alone would be sufficient. This is supported by our data on the treatment of composite tumors [9] that were composed of a mixed population of cells expressing either EGFR or EGFRvIII [8].
At this juncture, our own pre-clinical studies on molecular targeting of EGFR using boronated EGF and mAbs have reached the point where a Phase I clinical trial to evaluate the biodistribution of these agents would be worthwhile [50]. This could be in patients with either GBMs or squamous cell carcinomas of the head and neck [11, 51], both of which express amplified EGFR. However, as previously stated, BD-EGF and boronated mAbs are not stand alone boron delivery agents, but should be used in combination with BPA and/or sodium borocaptate (BSH). Currently, there are several ongoing clinical trials using BNCT to treat patients with recurrent, therapeutically refractory carcinomas of the head and neck and oral cancer [52, 53], and this logically would be a good starting point to evaluate either locally or systemically administered boronated EGFR targeting agents in combination with BPA or BSH. Since the anti-EGFR mAb cetuximab now is in wide clinical use [54–57], this would be the most logical choice as a targeting agent. If such studies proved to be therapeutically effective, then it would be appropriate to initiate clinical studies in patients with GBM who are candidates for BNCT.
Acknowledgments
We thank Ms. Michelle Van Fossen for secretarial assistance in the preparation of this manuscript. The studies described in this report were supported by N.I.H. grants 1R01 CA098945 (R.F. Barth), the Roswell Park Alliance Foundation (R.A. Fenstermaker), and the United States Department of Energy thorugh the program of Innovations in Nuclear Infrastructure and Education, Office of Nuclear Energy, Science and Technology (contract no. DE-FG07-02ID14420DE-FG07-02, K14420), and the Office of Environmental and Biological Research (contract no. DE-FG02-02ER63358).
Footnotes
Presented in part at the 100th Annual Meeting of the American Association for Cancer Research, Denver, CO, April 18–22, 2009.
References
- 1.Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- 2.Sauter G, Maeda T, Waldman FM, Davis RL, Feuerstein BG. Patterns of epidermal growth factor receptor amplification in malignant gliomas. Am J Pathol. 1996;148:1047–1053. [PMC free article] [PubMed] [Google Scholar]
- 3.Barth RF, Yang W, Adams DM, Rotaru JH, Shukla S, Sekido M, Tjarks W, Fenstermaker RA, Ciesielski M, Nawrocky MM, Coderre JA. Molecular targeting of the epidermal growth factor receptor for neutron capture therapy of gliomas. Cancer Res. 2002;62:3159–3166. [PubMed] [Google Scholar]
- 4.Yang W, Barth RF, Adams DM, Ciesielski MJ, Fenstermaker RA, Shukla S, Tjarks W, Caligiuri MA. Convection-enhanced delivery of boronated epidermal growth factor for molecular targeting of EGF receptor-positive gliomas. Cancer Res. 2002;62:6552–6558. [PubMed] [Google Scholar]
- 5.Yang W, Barth RF, Wu G, Bandyopadhyaya AK, Thirumamagal BT, Tjarks W, Binns PJ, Riley K, Patel H, Coderre JA, Ciesielski MJ, Fenstermaker RA. Boronated epidermal growth factor as a delivery agent for neutron capture therapy of EGF receptor positive gliomas. Appl Radiat Isot. 2004;61:981–985. doi: 10.1016/j.apradiso.2004.05.071. [DOI] [PubMed] [Google Scholar]
- 6.Barth RF, Wu G, Yang W, Binns PJ, Riley KJ, Patel H, Coderre JA, Tjarks W, Bandyopadhyaya AK, Thirumamagal BT, Ciesielski MJ, Fenstermaker RA. Neutron capture therapy of epidermal growth factor (+) gliomas using boronated cetuximab (IMC-C225) as a delivery agent. Appl Radiat Isot. 2004;61:899–903. doi: 10.1016/j.apradiso.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Yang W, Barth RF, Kawabata S, Swindall M, Bandy-opadhyaya AK, Tjarks W, Khorsandi B, Blue TE, Ferketich AK, Yang M, Christoforidis GA, Sferra TJ, Binns PJ, Riley KJ, Ciesielski MJ, Fenstermaker RA. Molecular targeting and treatment of an epidermal growth factor receptor-positive glioma using boronated cetuximab. Clin Cancer Res. 2007;13:1260–1268. doi: 10.1158/1078-0432.CCR-06-2399. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Barth RF, Wu G, Kawabata S, Sferra TJ, Bandy-opadhyaya AK, Tjarks W, Ferketich AK, Moeschberger ML, Binns PJ, Riley KJ, Coderre JA, Ciesielski MJ, Fenstermaker RA, Wikstrand CJ. Molecular targeting and treatment of EG-FRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin Cancer Res. 2006;12:3792–3802. doi: 10.1158/1078-0432.CCR-06-0141. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Wu G, Barth RF, Swindall MR, Bandyopadhyaya AK, Tjarks W, Tordoff K, Moeschberger M, Sferra TJ, Binns PJ, Riley KJ, Ciesielski MJ, Fenstermaker RA, Wikstrand CJ. Molecular targeting and treatment of composite EGFR and EG-FRvIII-positive gliomas using boronated monoclonal antibodies. Clin Cancer Res. 2008;14:883–891. doi: 10.1158/1078-0432.CCR-07-1968. [DOI] [PubMed] [Google Scholar]
- 10.Barth RF, Coderre JA, Vicente MG, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 11.Barth RF. Boron neutron capture therapy at the crossroads: challenges and opportunities. Appl Radiat Isot. 2009;67:S3–S6. doi: 10.1016/j.apradiso.2009.03.102. [DOI] [PubMed] [Google Scholar]
- 12.Vicente MGH, editor. Anti-Cancer Agents in Med Chem. Vol. 6. 2006. Boron in medicinal chemistry; pp. 73–181. [Google Scholar]
- 13.Altieri S, Barth RF, Bortolussi S, Zonta A. Thirteenth International Congress on Neutron Capture Therapy. Appl Radiat Isot. 2009;67:S1–S2. doi: 10.1016/j.apradiso.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol. 1999;277:R1218–1229. doi: 10.1152/ajpregu.1999.277.4.R1218. [DOI] [PubMed] [Google Scholar]
- 15.Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mardor Y, Rahav O, Zauberman Y, Lidar Z, Ocherashvilli A, Daniels D, Roth Y, Maier SE, Orenstein A, Ram Z. Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res. 2005;65:6858–6863. doi: 10.1158/0008-5472.CAN-05-0161. [DOI] [PubMed] [Google Scholar]
- 17.Thomale UW, Tyler B, Renard V, Dorfman B, Chacko VP, Carson BS, Haberl EJ, Jallo GI. Neurological grading, survival, MR imaging, and histological evaluation in the rat brainstem glioma model. Childs Nerv Syst. 2009;25:433–441. doi: 10.1007/s00381-008-0767-5. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson S, Lesniak MS. Convection enhanced drug delivery of novel therapeutic agents to malignant brain tumors. Curr Drug Deliv. 2007;4:169–180. doi: 10.2174/156720107780362302. [DOI] [PubMed] [Google Scholar]
- 19.Lopez KA, Waziri AE, Canoll PD, Bruce JN. Convection-enhanced delivery in the treatment of malignant glioma. Neurol Res. 2006;28:542–548. doi: 10.1179/016164106X116836. [DOI] [PubMed] [Google Scholar]
- 20.Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, Friedman HS, Greer K, Herndon JEII, Kunwar S, McLendon RE, Paolino A, Petry NA, Provenzale JM, Reardon DA, Wong TZ, Zalutsky MR, Pastan I, Bigner DD. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10:320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth RF, Kaur B. Rat brain tumor models in experimental neurooncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009 doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57:4130–4140. [PubMed] [Google Scholar]
- 23.Wikstrand CJ, Hale LP, Batra SK, Hill ML, Humphrey PA, Kurpad SN, McLendon RE, Moscatello D, Pegram CN, Reist CJ, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55:3140–3148. [PubMed] [Google Scholar]
- 24.Yang W, Barth RF, Carpenter DE, Moeschberger ML, Goodman JH. Enhanced delivery of boronophenylalanine for neutron capture therapy by means of intracarotid injection and blood-brain barrier disruption. Neurosurgery. 1996;38:985–992. doi: 10.1097/00006123-199605000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Ross BD, Zhao YJ, Neal ER, Stegman LD, Ercolani M, Ben-Yoseph O, Chenevert TL. Contributions of cell kill and posttreatment tumor growth rates to the repopulation of intracerebral 9L tumors after chemotherapy: an MRI study. Proc Natl Acad Sci U S A. 1998;95:7012–7017. doi: 10.1073/pnas.95.12.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Barth RF, Wu G, Ciesielski MJ, Fenstermaker RA, Moffat BA, Ross BD, Wikstrand CJ. Development of a syngeneic rat brain tumor model expressing EGFRvIII and its use for molecular targeting studies with monoclonal antibody L8A4. Clin Cancer Res. 2005;11:341–350. [PubMed] [Google Scholar]
- 27.Barth RF, Yang W, Rotaru JH, Moeschberger ML, Joel DD, Nawrocky MM, Goodman JH, Soloway AH. Boron neutron capture therapy of brain tumors: enhanced survival following intracarotid injection of either sodium borocaptate or boron-ophenylalanine with or without blood-brain barrier disruption. Cancer Res. 1997;57:1129–1136. [PubMed] [Google Scholar]
- 28.Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MJ, Fenstermaker RA. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjug Chem. 2004;15:185–194. doi: 10.1021/bc0341674. [DOI] [PubMed] [Google Scholar]
- 29.Barth RF, Adams DM, Soloway AH, Mechetner EB, Alam F, Anisuzzaman AKM. Determination of boron in tissues and cells using direct-current plasma atomic emission spectroscopy. Anal Chem. 1991;63:890–893. doi: 10.1021/ac00009a010. [DOI] [PubMed] [Google Scholar]
- 30.Rogus RD, Harling OK, Yanch JC. Mixed field dosimetry of epithermal neutron beams for boron neutron capture therapy at the MITR-II research reactor. Med Phys. 1994;21:1611–1625. doi: 10.1118/1.597267. [DOI] [PubMed] [Google Scholar]
- 31.Madsen RW, Moeschberger ML. Statistical concepts. Prentice-Hall; Englewood Cliffs, New Jersey: 1986. [Google Scholar]
- 32.Klein JP, Moeschberger ML. Survival analysis techniques for censored and truncated data. 2. Springer; New York: 2003. [Google Scholar]
- 33.Clendenon NR, Barth RF, Gordon WA, Goodman JH, Alam F, Staubus AE, Boesel CP, Yates AJ, Moeschberger ML, Fairchild RG, et al. Boron neutron capture therapy of a rat glioma. Neurosurgery. 1990;26:47–55. doi: 10.1097/00006123-199001000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Barth RF, Yang W, Coderre JA. Rat brain tumor models to assess the efficacy of boron neutron capture therapy: a critical evaluation. J Neurooncol. 2003;62:61–74. doi: 10.1007/BF02699934. [DOI] [PubMed] [Google Scholar]
- 35.Capala J, Barth RF, Bendayan M, Lauzon M, Adams DM, Soloway AH, Fenstermaker RA, Carlsson J. Boronated epidermal growth factor as a potential targeting agent for boron neutron capture therapy of brain tumors. Bioconjug Chem. 1996;7:7–15. doi: 10.1021/bc950077q. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami M, Kawakami K, Puri RK. Interleukin-4-Pseudomonas exotoxin chimeric fusion protein for malignant glioma therapy. J Neurooncol. 2003;65:15–25. doi: 10.1023/a:1026294416718. [DOI] [PubMed] [Google Scholar]
- 37.Raghavan R, Brady ML, Rodriguez-Ponce MI, Hartlep A, Pedain C, Sampson JH. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus. 2006;20:12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- 38.Sampson JH, Raghavan R, Brady ML, Provenzale JM, Herndon JEII, Croteau D, Friedman AH, Reardon DA, Coleman RE, Wong T, Bigner DD, Pastan I, Rodriguez-Ponce MI, Tanner P, Puri R, Pedain C. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro Oncol. 2007;9:343–353. doi: 10.1215/15228517-2007-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46:149–168. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 40.Boucher Y, Salehi H, Witwer B, Harsh GRt, Jain RK. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br J Cancer. 1997;75:829–836. doi: 10.1038/bjc.1997.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251–4255. [PubMed] [Google Scholar]
- 42.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharma-codynamics. Am J Physiol. 1994;266:R292–305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 43.Kroll RA, Pagel MA, Muldoon LL, Roman-Goldstein S, Neuwelt EA. Increasing volume of distribution to the brain with interstitial infusion: dose, rather than convection, might be the most important factor. Neurosurgery. 1996;38:746–752. discussion 752–744. [PubMed] [Google Scholar]
- 44.Faillot T, Magdelenat H, Mady E, Stasiecki P, Fohanno D, Gropp P, Poisson M, Delattre JY. A phase I study of an anti-epidermal growth factor receptor monoclonal antibody for the treatment of malignant gliomas. Neurosurgery. 1996;39:478–483. doi: 10.1097/00006123-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Yang W, Barth RF, Leveille R, Adams DM, Ciesielski M, Fen-stermaker RA, Capala J. Evaluation of systemically administered radiolabeled epidermal growth factor as a brain tumor targeting agent. J Neurooncol. 2001;55:19–28. doi: 10.1023/a:1013017821166. [DOI] [PubMed] [Google Scholar]
- 46.Gedda L, Olsson P, Ponten J, Carlsson J. Development and in vitro studies of epidermal growth factor-dextran conjugates for boron neutron capture therapy. Bioconjug Chem. 1996;7:584–591. doi: 10.1021/bc9600473. [DOI] [PubMed] [Google Scholar]
- 47.Brady LW, Miyamoto C, Woo DV, Rackover M, Emrich J, Bender H, Dadparvar S, Steplewski Z, Koprowski H, Black P, et al. Malignant astrocytomas treated with iodine-125 labeled monoclonal antibody 425 against epidermal growth factor receptor: a phase II trial. Int J Radiat Oncol Biol Phys. 1992;22:225–230. doi: 10.1016/0360-3016(92)91009-c. [DOI] [PubMed] [Google Scholar]
- 48.Kalofonos HP, Pawlikowska TR, Hemingway A, Courtenay-Luck N, Dhokia B, Snook D, Sivolapenko GB, Hooker GR, McKenzie CG, Lavender PJ, et al. Antibody guided diagnosis and therapy of brain gliomas using radiolabeled monoclonal antibodies against epidermal growth factor receptor and placental alkaline phosphatase. J Nucl Med. 1989;30:1636–1645. [PubMed] [Google Scholar]
- 49.Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS, Bigner DD. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 1990;50:8017–8022. [PubMed] [Google Scholar]
- 50.Yang W, Barth RF, Wu G, Tjarks W, Binns P, Riley K. Boron neutron capture therapy of EGFR or EGFRvIII positive gliomas using either boronated monoclonal antibodies or epidermal growth factor as molecular targeting agents. Appl Radiat Isot. 2009;67:S328–S331. doi: 10.1016/j.apradiso.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Baumann M, Krause M. Targeting the epidermal growth factor receptor in radiotherapy: radiobiological mechanisms, preclinical and clinical results. Radiother Oncol. 2004;72:257–266. doi: 10.1016/j.radonc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Kato I, Fujita Y, Maruhashi A, et al. Effectiveness of boron neutron capture therapy for recurrent head and neck malignancies. Appl Radiat Isot. 2009 doi: 10.1016/j.apradiso.2009.03.103. (in press) [DOI] [PubMed] [Google Scholar]
- 53.Mrhalova M, Plzak J, Betka J, Kodet R. Epidermal growth factor receptor—its expression and copy numbers of EGFR gene in patients with head and neck squamous cell carcinomas. Neo-plasma. 2005;52:338–343. [PubMed] [Google Scholar]
- 54.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 55.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kroning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA., III EPIC: phase III trial of cetuximab plus irinotecan after fluoro-pyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 57.Kimura Y, Ariyoshi Y, Shimahara M, Miyatake S, Kawabata S, Ono K, Suzuki M, Maruhashi A. Boron neutron capture therapy for recurrent oral cancer and metastasis of cervical lymph node. Appl Radiat Isot. 2009;67:S47–S49. doi: 10.1016/j.apradiso.2009.03.019. [DOI] [PubMed] [Google Scholar]