Abstract
Apoptosis induction by BH3 mimetics is a therapeutic strategy for human cancer. These mimetics exert single-agent activity in cells “primed” for cell death. Primed cells are dependent upon antiapoptotic Bcl-2 proteins for survival, and are characterized by the ability of the BH3-mimetic to induce cytochrome c release from their isolated mitochondria. Our aim was to examine the single-agent activity of obatoclax, a BH3 mimetic in cholangiocarcinoma cell lines. In clonogenic assays, inhibition of colony formation was observed by obatoclax treatment. Despite single-agent activity by obatoclax, the mitochondria from these cells did not release cytochrome c following incubation with this BH3 mimetic. However, immunofluorescence and cell fractionation studies identified Bax activation and translocation to mitochondria following treatment with obatoclax. shRNA targeted knockdown of Bax doubled the IC50 for obatoclax, but did not abrogate its cytotoxicity, while knockdown of Bak did not alter the IC50. In a cell free system, obatoclax induced an activating conformational change of Bax which was attenuated by site-directed mutagenesis of a previously identified protein activation site. Finally, the drug also elicited a significant in vivo response in a rodent model of this disease. In conclusion, single agent obatoclax treatment results in Bax activation which contributes, in part, to cell death in cholangiocarcinoma cells. These data indicate that BH3 mimetics may also function as direct activators of Bax and induce cytotoxicity in cells not otherwise primed for cell death.
Introduction
Oncogene and multiple stress pathways active in human cancers predispose malignant cells to death by apoptosis1. As a result, cancers often acquire mechanisms to evade apoptosis in order to develop and progress2. Apoptosis is regulated by Bcl-2 family proteins, a protein family consisting of three subsets: pro-survival proteins such as Mcl-1, Bcl-2, A1, and Bcl-XL; the pro-apoptotic multi-domain proteins Bax and Bak; and BH3-only proteins such as Bim, Bid, Bad, Noxa, Bmf, Hrk, Bik, and PUMA3. The BH3-only proteins act as initiators of the core cell death machinery. These proteins may be classified as activators, which by directly triggering Bax or Bak activation induce mitochondrial dysfunction and cellular demise, or sensitizers, which sensitize the cell to apoptotic cues by inhibiting the function of pro-survival Bcl-2 proteins. Bim, Bid, and possibly PUMA are classified as activators, while the other BH3-only proteins are viewed as sensitizers4-6.
Given the ability of the BH3-only proteins to initiate cell death pathways, the pharmaceutical industry has developed an array of BH3-only protein mimetics7. However, to date, none of these has proven to be a direct activator. The binding profiles of these mimetics are not uniform and resistance to these drugs has been documented. For example, overexpression of Mcl-1 is now a recognized mechanism of resistance to the BH3-only protein mimetic ABT-737, as this mimetic specifically binds Bcl-2 and Bcl-XL, but not Mcl-18, 9. Obatoclax, a recently developed BH3 mimetic, has a different binding specificity than ABT-737 as it binds to all pro-survival members of the Bcl-2 protein family, including Mcl-110. This property of obatoclax provides a potential therapeutic advantage for this drug.
ABT-737, which functions as a sensitizing BH3 mimetic, exerts single agent activity in selected malignant cell lines despite its inability to directly activate Bax or Bak. Work by Letai and co-workers suggests that the apparent paradox of direct cytotoxicity by a sensitizing BH3-mimetic in the absence of additional, exogenously applied apoptotic stimuli can be explained if certain cells are “primed” for cell death.11 The mitochondrial anti-apoptotic proteins of such cells are near capacity in their ability to sequester the activator BH3-only proteins Bim, PUMA, and Bid. Additional BH3-only proteins or mimetics displace pre-existing, bound activators from this sequestration, liberating them to trigger Bax or Bak oligomerization on the mitochondrial membrane12. The isolated mitochondria of such cells release cytochrome c in the presence of a BH3 mimetic13. In contrast, the mitochondria of non-primed cells are not “loaded” with activator BH3-only proteins bound to anti-apoptotic proteins; and isolated mitochondria from these cells do not release cytochrome c upon exposure to a BH3 mimetic.
Direct Bax activation is a common mechanism for cell death by pro-apoptotic stimuli14. Bax exists in the cytosol as an inactive, globular protein. Recent data suggest activator BH3-only proteins may directly activate Bax by binding to a site distinct from its BH3 groove, and mutation of lysine 21 (K21E) abolishes Bax activation15. Following activating signals, the protein undergoes a conformational change exposing both its N- and C-terminal domains16. This conformational activation facilitates its translocation to mitochondria, where its C-terminal transmembrane domain inserts into the outer mitochondrial membrane. Further stimuli promote its homo-oligomerization forming channels or pores in the mitochondrial outer membrane14, 17. These pores result in the egress of cytochrome c and other pro-apoptotic proteins from the inter-mitochondrial membrane space into the cytosol, triggering cell death cascades.
Cholangiocarcinoma, a devastating neoplasm18, is resistant to current medical therapies, likely due to abundant Mcl-1 expression19. Because obatoclax binds Mcl-1 in addition to other anti-apoptotic Bcl-2 proteins, we examined this BH3 mimetic as a potential therapeutic agent for cholangiocarcinoma. In these studies, obatoclax was observed to exert single agent activity in human cholangiocarcinoma cell lines, as well as in a syngeneic rat orthotopic model of cholangiocarcinoma. Despite the single agent activity, the mitochondria of these cells were not “primed” for cell death. Instead, obatoclax treatment was associated with Bax activation, suggesting that obatoclax may function as a direct activator of this pro-apoptotic protein.
Material and Methods
Obatoclax
Obatoclax was supplied by GeminX Biotechnologies, Inc. (Montreal, Canada). For in vitro studies, obatoclax was dissolved at 20 mM in DMSO and aliquoted. Aliquots were further diluted in sterile water immediately prior to each experiment. The maximal DMSO concentration was 0.1% (v/v), which was used as a vehicle control in respective in vitro experiments. For in vivo experiments obatoclax was prepared according to the supplier's protocol.
Cell lines and culture
The human cholangiocarcinoma cell lines HuCCT-120, KMCH21, and Mz-ChA-122 along with the rat cholangiocarcinoma cell line BDEneu23, 24 were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, 100μg/mL gentamicin, and 100 μg/L insulin.
Clonogenic and cell death assays
Clonogenic assays were performed as previously described25. For colony quantification, 6-well plates were stained with coomassie blue and colonies counted using ImageJ software.26 The IC50 was calculated as indicated using Calcusyn software (Biosoft, Cambridge, UK). Apoptosis was confirmed by examining nuclear morphology using fluorescent microscopy following staining with 4′,6-diamidino-2-phenylindole (DAPI) as previously described27.
Immunoblot analysis
Whole cell lysates were prepared as previously described in detail25, proteins resolved by SDS-PAGE and transferred to nitrocellulose membrane. Membranes were blotted with primary antibody at the indicated dilutions. Antibodies used were: Bcl-2 (N-19 Santa Cruz, 1:1000), Bcl-Xl (S-18 Santa Cruz 1:1000), Bax (N-20 Santa Cruz, 1:5000), Bak (G-23 Santa Cruz, 1:5000), Bim (BD Pharmigen 1:500), Bid (R&D Systems 1:1000), Noxa (ProSci 1:500), PUMA (Sigma 1:1000), Actin (C-11 Santa Cruz 1:1000). Horseradish peroxidase-conjugated secondary antibodies for mouse (Invitrogen), rabbit (Invitrogen), and goat (Biosource) were used at a concentration of 1:2000. Proteins were visualized with enhanced chemiluminescence reagents (Hyglo Quick Spray, Denville Scientific) and Kodak X-OMAT film.
Mitochondria isolation
Mitochondria were isolated from cells by nitrogen cavitation and differential centrifugation as previously described28. Briefly, cells were lifted from a minimum of five 150 mm culture plates (∼90% confluent) in MA buffer [100 mM sucrose, 1 mM EGTA, 20 mM MOPS (pH 7.4), 1 g/L BSA, and Complete protease inhibitors (Roche Diagnostics)] followed by centrifugation at 800 × g for 10 min. Pellets were resuspended in 3 ml of MA buffer and nitrogen cavitation was completed (420 lb/in2 for 10 minutes) on ice. The cell lysate was centrifuged at 2500 × g for 5 minutes to remove cellular debris. The clarified supernatant was centrifuged at 16,000 × g for 15 minutes to pellet mitochondria. The pellet was resuspended in MA buffer and protein concentration quantified via the Bradford assay.
Cytochrome C release assay
Isolated mitochondria (100 μg of protein per reaction) were resuspended in 50 μL of MA buffer. Vehicle, drug, tBid (20 nM), or lysis buffer was added and the reaction incubated at 37° C for 30 minutes. Following incubation, the suspension was centrifuged at 16,000 × g for 15 minutes. The mitochondrial pellet and supernatant were separated. Samples were subjected to immunoblot analysis for cytochrome c (Santa Cruz, 1:1000).
Bax immunofluorescence
Immunofluorescence for the active conformation of Bax was performed as previously described by us29. The Bax 6A7 antibody (Exalpha, 1:100) was used as a primary antibody, and secondary staining was performed with an Alexa 633-conjugated anti-mouse antibody (1:1000, Invitrogen). Images were obtained using an inverted confocal microscope (LSM510; Carl Zeiss Microimaging, Inc. Thornwood, New Jersey) equipped with a 40× lens using LSM510 imaging software (Carl Zeiss Microimaging, Inc.) employing excitation and emission wavelengths of 633 nM and 656 nM, respectively.
Bax mitochondrial translocation, insertion, and oligomerization
For Bax translocation, mitochondria were isolated from cells as described above (five 150 mm dishes at 90% confluency per condition). The mitochondria were resuspended in lysis buffer on ice for 15 min and, following centrifugation at 14,000 × g for 15 min, the supernatant was resolved by SDS-PAGE. Immunoblot analysis for Bax (N-20 Sigma, 1:5000) and, as a control for protein loading, cytochrome c oxidase (Sigma, 1:1000), was completed.
For Bax mitochondrial insertion, mitochondria were isolated from cells as described above (five 150 mm dishes at 90% confluency per condition). Following protein concentration determination, 500 μg of mitochondrial protein was resuspended in 500 μl 0.1 M Na2CO3, pH 12 (Sigma), and incubated on ice for 20 min. The suspension was then centrifuged at 100,000 × g for 1 hour to collect mitochondrial membranes. The pellet was resuspended in 500 μl MB buffer [210 mM mannitol, 70 mM sucrose, 1 mM EDTA, 10 mM Hepes (pH 7.5)] with 2% CHAPS, incubated on ice for 1 hour, sonicated and centrifuged at 100,000 × g for 30 min. The supernatant (integral membrane proteins) was analyzed by SDS-PAGE and immunoblot analysis for Bax (N-20 Sigma, 1:5000). VDAC (Rockland, 1:1000) served as a loading control for integrated proteins.
Bax activation in a cell-free system
Recombinant human Bax was prepared as previously described using the IMPACT system (New England Biolabs)30. The recombinant human Bax construct was a generous gift from Dr. Nico Tjandra. Protein concentration was determined using Bradford Reagent (Sigma). A 20 μL reaction of recombinant Bax protein (300 ng) in column buffer (20mM Hepes, 500mM NaCl, pH 8.5) was incubated with vehicle, increasing concentrations of obatoclax, or 5% NP40 detergent at 37°C for 1 hour. Following the incubation 2 μl was removed from each reaction to assay as an input, then 250 μL of 3% BSA in PBS was added to each reaction with 15 μL of 6A7 anti-Bax antibody (Santa Cruz) and incubated for 1 hour at 4°C. Protein A agarose beads (60 μL) were then added to each reaction and the mixture was incubated at 4°C overnight. The beads were pelleted, washed with PBS, and boiled in SDS-sample buffer. The supernatant was subjected to immunoblot analysis for Bax (N-20 Santa Cruz, 1:2000). Site directed mutagenesis of the lysine 21 residue to glutamic acid in the Bax construct was completed using the Quickchange II kit (Stratagene). The K21E product was confirmed by sequencing and the protein preparation and reaction undertaken as above. Oligomerization was assayed by incubating 5 μg of recombinant protein in column buffer (50 μl reaction), with vehicle or increasing concentrations of obatoclax at 37°C for 1 hour. Bis[sulfosuccinimidyl] suberate (BS3) (Thermo Scientific) was then added at a concentration of 0.25 mM for 30min at room temperature. The reaction was quenched with 20 mM Tris-HCl (pH 8.0) at room temperature for 15 min and the reactions assayed by immunoblotting with the Bax N-20 antibody.
Bax and Bak targeted knockdown by shRNA
shRNA constructs for Bax and Bak were generated and validated as previously described31. HuCCT-1 cells were stably transfected with the shRNA constructs for Bax and Bak using Fugene HD reagent (Roche). Cells were selected with puromycin (0.5mg/L) in normal growth medium. Knockdown of Bax and Bak expression was confirmed in clonal populations by immunoblot analysis. Cells were then used in clonogenic assays as previously described.
In vivo studies
All animal experimentation described in this study was performed in accordance with and approved by the Institutional Animal Care and Use Committee. In vivo cell transplantation was carried out in adult Fischer 344 male rats (Harlan, Indianapolis, IN) with initial mean body weights ranging between 200 and 250 g as previously decribed in detail by us32. For survival and tumor size studies a subcutaneous port (Instech Laboratories, Plymouth Meeting, PA) was implanted on the low back of the rodent and the attached catheter tunneled into the peritoneal cavity. The gastroduodenal artery was cannulated in a retrograde fashion and the catheter doubly secured in the artery with 5-0 silk sutures. The catheter was flushed and locked with heparinized saline (500 units/ml). The incisions were closed with running 5-0 chromic suture. Buprenorphine (0.05 mg/kg SubQ) was used for post-operative analgesia. For apoptosis studies, obatoclax (1.5 mg/kg) or vehicle was given intravenously once daily for 5 consecutive days starting 14 days after tumor implantation. Twenty-four hours after receiving the fifth treatment the rats were euthanized and the livers removed for analysis. For tumor size and survival analysis, obatoclax (1.5mg/kg) or vehicle was given via hepatic artery infusion (through the subcutaneous port) once daily for 5 consecutive days starting 10 days after tumor implantation. TUNEL assays were carried out using the In Situ Cell Death Detection Kit (Roche) according to the supplier's protocol, as previously described by us32. Slides were viewed on an epifluorescence microscope (Nikon Eclipse TE200, 60× objective) and positive cells counted in 10 high-power fields.
Statistical Analysis
Data represent at least 3 independent experiments using cells from a minimum of three separate isolations and are expressed as the means ± standard deviations. Differences between groups were compared using 2-tailed Student t-tests or chi-square tests. Survival was analyzed by Kaplan-Meier method and groups compared by log-rank analysis.
Results
Single agent activity of obatoclax in cholangiocarcinoma cells
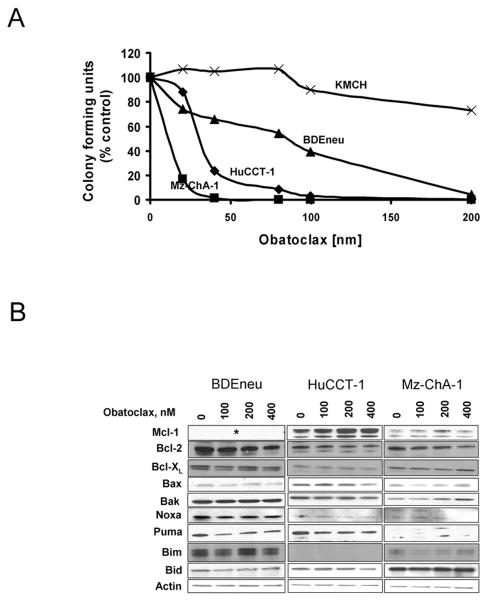
Clonogenic assays were undertaken to examine the single-agent activity of obatoclax in four cholangiocarcinoma cell lines; three human cell lines HuCCT-1, KMCH, and Mz-ChA-1, and the rat cholangiocarcinoma cell line BDEneu. The HuCCT-1, Mz-chA-1, and BDEneu cell lines were sensitive to obatoclax inhibition of colony formation in a concentration-dependent manner (Fig. 1A). A greater than 50% inhibition of colony formation was observed at concentrations between 5-100 nM in all three cell lines with complete inhibition observed at 200 nM obatoclax. The cholangiocarcinoma cell line KMCH was not sensitive to obatoclax as a single agent at these doses, consistent with our previously published observations25. The sensitivity of the human cholangiocarcinoma cell lines was directly proportional to the level of Mcl-1 expression, with KMCH cells containing the highest level of Mcl-1 expression (supplemental Fig. 1). Obatoclax concentrations of 400 nM and greater did inhibit colony formation of KMCH cells, suggesting the cytoprotective effect of Mcl-1 can be overcome with higher drug concentrations (data not shown). Because either cell cycle arrest or apoptosis can inhibit colony formation in clonogenic assays, apoptosis by obatoclax was confirmed in BDEneu cells (supplemental Fig. 2). In this context, we have shown that obatoclax displays single agent activity against several cholangiocarcinoma cell lines.
Figure 1.
Obatoclax has single agent activity against cholangiocarcinoma which is not associated with altered BCL-2 protein expression (A,B) Cell death was quantified by clonogenic assay (A) in four cholangiocarcinoma cell lines (HuCCT-1, Mz-ChA-1, BDEneu, KMCH-1) treated with obatoclax at doses ranging from 0 to 800 nM for 48 h. Results are colony forming units as a percentage of control and represent means from three separate trials. (B) Immunoblotting of three obatoclax-sensitive cholangiocarcinoma cell lines treated with vehicle or increasing doses of obatoclax (100nM-400nM) for 24 hours. (*) No commercially available antibody for rat Mcl-1.
Cholangiocarcioma mitochondria are not primed for death
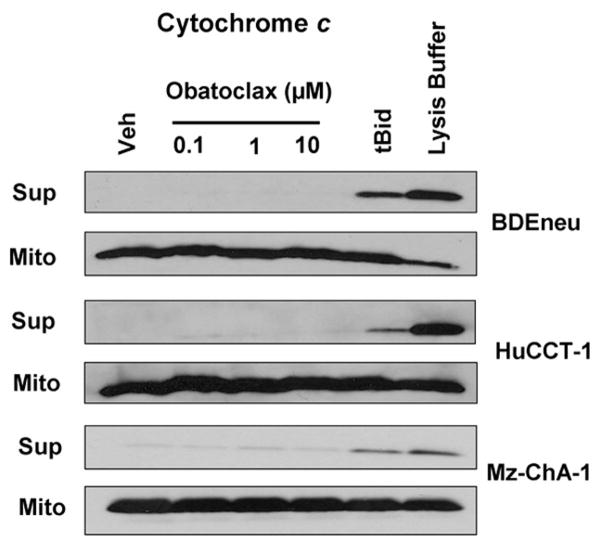
To exclude altered expression of pro-apoptotic or anti-apoptotic Bcl-2 proteins as a mechanism for cell death by obatoclax, immunoblot analysis for these proteins was performed. No consistent changes in expression of the Bcl-2 family proteins were observed with obatoclax treatment in any of the three obatoclax-sensitive cell lines (Fig. 1B). Given that expression of the Bcl-2 family proteins was unaltered in obatoclax treated cells, we sought to further examine the mechanism for single agent cytotoxicity by this BH3 mimetic. As single agent activity by obatoclax suggests that cholangiocarcinoma cells are primed for cell death, we next examined the ability of this BH3 mimetic to directly induce cytochrome c release in isolated mitochondria. Unexpectedly, addition of obatoclax to mitochondria isolated from all three sensitive cholangiocarcinoma cell lines failed to directly induce cytochrome c release, although cytochrome c was readily identified in the supernatant when the mitochondria were incubated with tBid protein or lysed by detergent (Fig. 2). These data suggest that the mitochondria from these cholangiocarcinoma cell lines are not primed for death, but rather obatoclax-induced mitochondrial dysfunction occurs through a more complex cascade involving cytosolic components.
Figure 2.
Mitochondria from cholangiocarcinoma cells are not primed for death. Immunoblot for cytochrome c from supernatant and mitochondrial pellets indicating no release of cytochrome c with obatoclax treatment in isolated mitochondria. Mitochondria were incubated with tBid (20 nM) and lysed with detergent as positive controls.
Obatoclax treatment leads to Bax activation and translocation to mitochondria
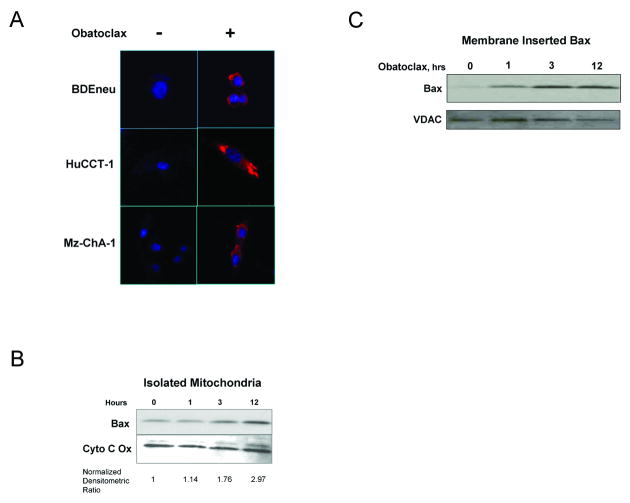
Bax is a cytosolic protein which potently triggers cell death upon its activation. Because BH3 proteins, and therefore, potentially a BH3 mimetic may directly activate Bax33, 34, we examined the activation and subcellular localization of Bax following treatment with obatoclax. Bax activation by obatoclax was by immunofluorescence for an active conformation of Bax, using the 6A7 antibody, in all three cholangiocarcinoma cell lines (Fig. 3A). A 3-fold increase in mitochondria-associated Bax was also observed in isolated mitochondria following obatoclax treatment (Fig. 3B). The translocation of Bax to mitochondria was associated with its insertion in to the mitochondrial membrane, as the association was resistant to bicarbonate treatment (Fig. 3C). Collectively these data suggest obatoclax either directly or indirectly promotes Bax activation.
Figure 3.
Obatoclax treatment is associated with Bax activation, mitochondrial translocation, and membrane insertion. (A-D) Immunofluorescence for active Bax (red) (A) using a conformation specific antibody in HuCCT-1, Mz-ChA-1, and BDEneu cells treated with 400nM obatoclax for 24 hours demonstrated Bax activation in treated cells. Nuclei are stained with DAPI. (B) With obatoclax treatment of BDEneu cells, Bax was recruited to mitochondria. Normalized densitometric ratios are provided beneath the figure. (C) Bicarbonate treatment of mitochondria isolated from treated and untreated BDEneu cells demonstrated rapid membrane insertion of Bax with obatoclax treatment.
Direct activation of Bax by obatoclax
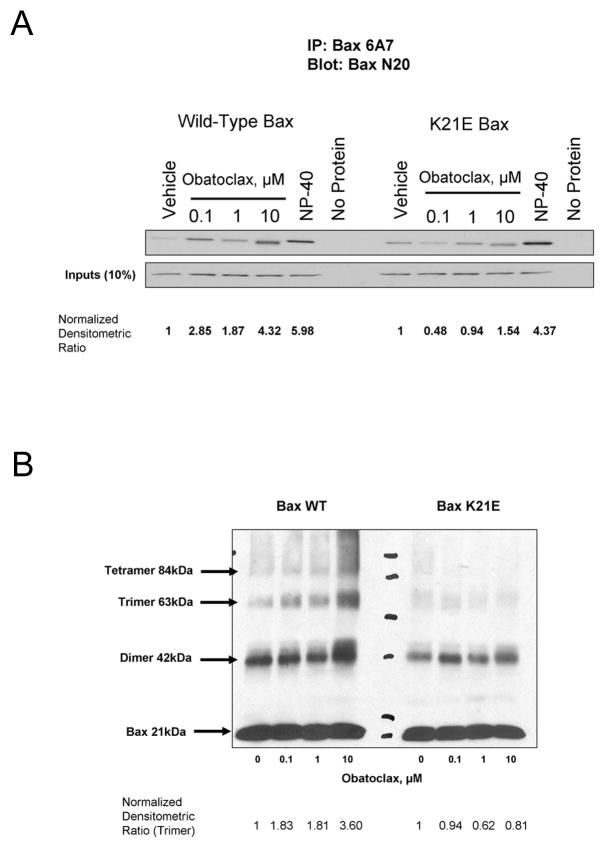
In order to ascertain whether the activation of Bax by obatoclax may be via a direct mechanism, recombinant Bax was incubated with obatoclax in a cell-free reaction. Following incubation with obatoclax the active conformation of Bax could be immunoprecipitated with the Bax 6A7 antibody (Fig 4A). The amount of active Bax recovered with obatoclax treatment increased in a concentration dependent manner. This conformational change in Bax was not observed in vehicle treated protein; however, treatment with the detergent NP-40, which induces activating-like conformational changes in Bax, also lead to recovery of active Bax with the 6A7 antibody. To further demonstrate the specificity of this activation, site directed mutagenesis of the lysine 21 residue, K21E, was performed. This residue has previously been demonstrated to be an important residue in a Bax activation site15. The activation of the K21E Bax was attenuated as demonstrated by a decrease in the amount of immunoprecipated active Bax following obatoclax treatment as compared to detergent treated protein (Fig 4A). Additionally, oligomerization of wild-type Bax (trimers and tetramers) in the cell-free system could be demonstrated in a concentration dependent manner with obatoclax treatment and cross-linking (Fig 4B), however the higher order multiples were significantly decreased or absent in the mutant protein. Unexpectedly, a protein complex consistent with Bax dimerization was observed with cross-linker alone. In this cell free system, obatoclax treatment promotes Bax activation.
Figure 4.
Obatoclax activation of Bax takes place through a direct mechanism, which is attenutated by site directed mutagenesis of the lysine 21 residue. Wild-type (A) recombinant human Bax or K21E mutant Bax was incubated with increasing doses of obatoclax. An increasing amount of active Bax was immunoprecipitated with obatoclax treatment in wild-type Bax, which was attenuated in the K21E mutant protein. Cross-linking of recombinant wild-type Bax protein (B) demonstrated increasing multimers with obatoclax treatment, which were not present in mutant protein. Normalized densitometric ratios are provided beneath the figure.
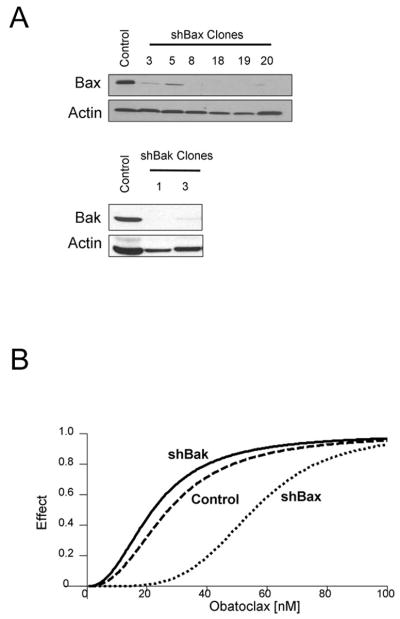
Cell death by obatoclax is facilitated by Bax
To further elucidate the contribution of Bax in obatoclax induced apoptosis, HuCCT-1 cells stably expressing shRNA for Bax or Bak were generated. The specificity and efficiency of the shRNA knockdown of the respective targets in these cells was confirmed by immunoblot analysis (Fig 5A). The median dose effect (Dm) for obatoclax induced cell death, a calculation of the median-effect (surrogate for LD50 in cell death assays) was compared between wild type cells and those in which Bax or Bak had been selectively knocked down. For wild-type HuCCT-1 cells the Dm was 27.0 nM and the Bak knockdown cells had a similar Dm of 21.8 nM. In contrast, the Dm for obatoclax doubled to 55.3 nM in HuCCT-1 cells stably expressing the shBax construct, indicating a protective effect by reducing Bax expression (Fig 5B). These data provide further evidence suggesting Bax contributes to obatoclax-induced cell death.
Figure 5.
Targeted knockdown of Bak and Bax by shRNA demonstrated a Bax-dependent effect on cell death (A,B) shRNA specific for Bak and Bax were stably transfected into HuCCT-1 cells (A). shBak clones (1,3) and shBax clones (18,19) were subjected to increasing concentrations of obatoclax in clonogenic assays. Median dose-effect (Dm) was calculated using Calcusyn software and is plotted in a dose-effect plot (B), indicating an increase in Dm for the shBax clones, with unchanged Dm in the shBak clones as compared to controls. Results from the clonogenic assay were colony forming units as a percentage of control and included three separate trials for each clone.
In vivo activity of Obatoclax
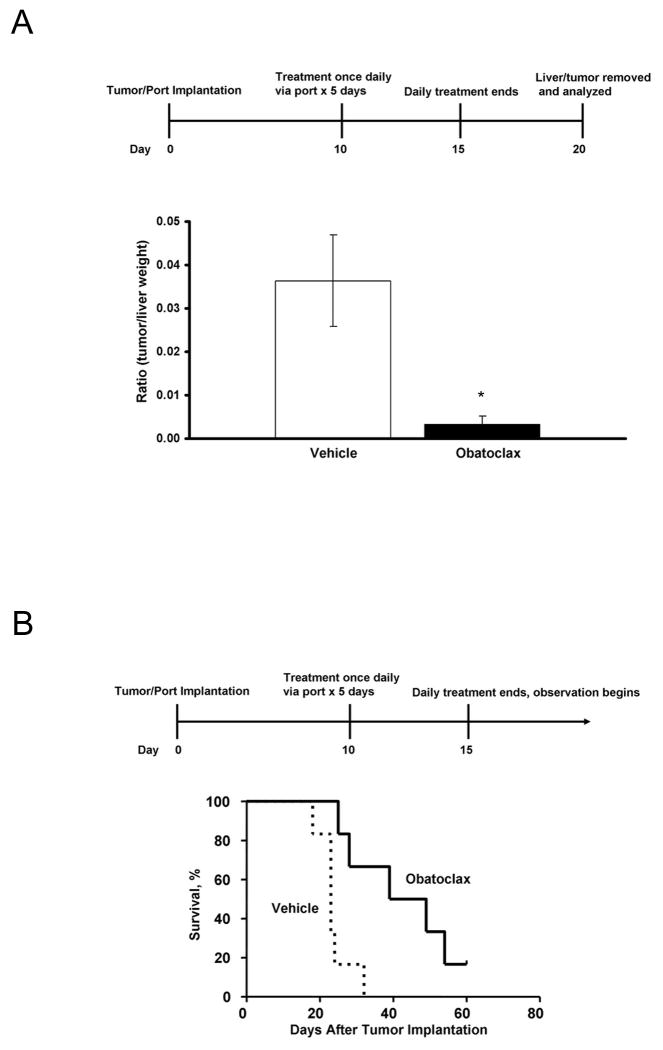
Given the in vitro activity of obatoclax against cholangiocarcinoma cell lines, we sought to test the in vivo effects of this drug using a recently characterized syngeneic rat orthotopic model of cholangiocarcinoma. Tumors were removed following treatment with obatoclax or vehicle for 5 days. TUNEL staining demonstrated a significant increase in the number of cells undergoing apoptosis in tumors removed from obatoclax treated rats compared to controls (12.3 +/- 1.9 cells per high power field vs. 5.8 +/- 3, p=0.05) with no increase in apoptosis in normal hepatocytes (1 +/- 0.1 cells per high power field vs. 0.9 +/- 0.2, p=0.5). To further examine the in vivo efficacy of obatoclax, the drug was given via a hepatic artery injection to ensure adequate tumor exposure to the drug. An initial group of rats (n=8) had their livers removed 5 days after completing treatment, while a second group was observed for survival (n=12). The mean tumor weights in animals treated with obatoclax was 10-fold lower than vehicle treated controls, 0.04 g (+/- 0.03 g) vs. 0.38 g (+/- 0.10 g), p<0.001. This relationship also held true when the ratio of tumor weight to liver weight was calculated (Figure 6A); a 12-fold decrease in this ratio was observed in obatoclax-treated animals compared to controls, 0.003 (+/- 0.002) vs. 0.036 (+/- 0.010), p<0.001. This decrease in tumor size also correlated with increased survival in obatoclax treated rats (Figure 6B). The median survival increased from 23 days in control animals to 44 days in obatoclax-treated animals, p=0.005.
Figure 6.
Obatoclax has single agent activity in vivo. A significant decrease in tumor size was noted after intra-arterial injection of obatoclax for 5 days, *p<0.001 (A). Survival was improved in animals treated with obatoclax by intra-arterial injection (B), p=0.005.
Discussion
The results of this study provide new insight into the mechanisms of obatoclax-mediated cell death. The results indicate that obatoclax: i) exerts single agent activity in cholangiocarcinoma cell lines; ii) induces cell death by promoting Bax activation; and iii) is efficacious in an in vivo model of cholangiocarcinoma. Each of these results is discussed in greater detail below.
In the current study, we observed maximal single agent obatoclax activity between concentrations of 50-100 nM in two human cell lines, and at 200 nM in a third rat cell line (Fig. 1A). Obatoclax has been tested in small, phase I studies of patients with advanced hematologic malignancies35, 36. In these studies multiple dosing regimens were tried with doses ranging from 3.5 to 40 mg/m2. The reported recommended phase II dose was 28 mg/m2, which corresponds to a mean maximal plasma concentration of 73 ng/ml when infused over a 3 hour period35. This peak plasma concentration is equivalent to approximately 175 nM. Thus, biologically active concentrations of obatoclax observed in our in vitro studies are achievable at doses employed in humans.
Obatoclax, as a single agent, inhibited colony formation in clonogenic assays. Although cell cycle arrest will also manifest as an inhibition of colony formation in this assay, we also confirmed the occurrence of apoptosis as demonstrated by morphology and dependence on Bax. The BH3 mimetic, ABT-737 also exerts single agent activity in selected malignant cell lines37. These cells are under BH3-only protein stress as the mitochondria isolated from such cell lines are already “loaded” with BH3-only proteins bound to anti-apoptotic proteins. The activator BH3-only proteins released from their sequestration by the BH3 mimetic then induce mitochondrial dysfunction with cytochrome c release11. However, we failed to observe this phenomenon with obatoclax in mitochondria isolated from our cell lines. We, therefore, examined the possibility that the cells are not under BH3-only protein stress, but that obatoclax delivers a sufficient, activating BH3 stimulus for the cells to undergo apoptosis. If this was true, the stimulus should directly result in Bax or Bak activation. Interestingly, we observed Bax activation in obatoclax treated cells as manifest by an activating conformational change, translocation, and insertion into mitochondria. These data suggest obatoclax provides a sufficient BH3 stimulus to promote Bax activation. As Mcl-1 can antagonize Bax activation38, it is likely that the ability of obatoclax to bind Mcl-1 also contributes to Bax activation in these cells.
Although Bak and Bax are often thought to be functionally redundant proteins in mediating outer mitochondrial membrane permeabilization during apoptosis39, several observations suggest specificity for signaling through one or the other in a cell type, context, and stimulus specific manner 40, 41. Our studies are consistent with a preferential specificity for Bax in obatoclax-mediated cell death in cholangiocarcinoma cell lines. For example, Bax knockdown by shRNA doubled the median dose effect for obatoclax (Fig. 5B). The cell free system studies and activation assays also suggest obatoclax has the potential to promote activating conformational changes in Bax. To our knowledge, this is the first study to suggest a BH3-only protein mimetic may function as a direct activating stimulus for Bax activation. This observation distinguishes obatoclax from ABT-737, which like the BH3 domain peptide of Bad, only binds the pro-survival Bcl-2 proteins Bcl-2 and Bcl-XL37, and acts as a sensitizing but not activating BH3 stimulus. Obatoclax treatment lead to significant apoptotic stimulating activity, decreased tumor size, and improved survival in our in vivo syngeneic, orthotopic rat model of cholangiocarcinoma.24 Not only does this model reflect a similar molecular signature as human cholangiocarcinoma, but the syngeneic, orthotopic model avoids the problems of immunocompromise and incompatibilities of the tumor microenvironment problematic in human xenograft models. Analogous to our in vitro studies, obatoclax demonstrated single agent activity in vivo suggesting the in vitro studies are applicable to the more complex native environment of these cancers.
While our studies suggest that obatoclax can directly activate Bax in cell-free systems and cholangiocarcinoma cell lines, it is unlikely that this is the only mechanism for obatoclax-induced cell death. Previous studies have demonstrated the ability of obatoclax to induce cell death in Bak/Bax double-deficient mouse embryonic fibroblasts25, 42. Likely, there are as yet to be determined additional mechanisms for obatoclax-induced cell death.
In summary, obatoclax displays single agent activity in human cholangiocarcinoma cells and a rodent model of cholangiocarcinoma that mimics the human disease. The mechanism of cell death appears to be at least partially dependent upon Bax activation, and the data support a process where obatoclax appears to directly activate this pro-apoptotic protein. These data extend our knowledge by which BH3-agonists promote cell death in cancer cells. Development of BH3 mimetics which directly activate Bax appear to be feasible. Finally our observations also suggest obatoclax warrants further evaluation for the treatment of human cholangiocarcinoma.
Supplementary Material
Acknowledgments
We thank Erin Nystuen-Bungum for excellent secretarial support, and Dr. Scott H. Kaufmann for carefully reviewing and editing the manuscript.
This work was supported by NIH Grants R01 DK59427 to GJG, R01 CA 83650, R01 CA 39225 (AES), the optical microscopy core of PO DK84567, the Mayo Clinic Clinician Investigator Program, (RLS), and the Mayo Foundation.
References
- 1.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136(5):823–37. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 4.Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(51):20327–32. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbour AM, Heraud JE, Daunt CP, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death & Differentiation. 2009;16(4):555–63. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]
- 6.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nature Reviews Cancer. 2008;8(2):121–32. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 7.Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death & Differentiation. 2008;15(6):977–87. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–88. doi: 10.1016/j.ccr.2006.10.006. see comment. [DOI] [PubMed] [Google Scholar]
- 9.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–99. doi: 10.1016/j.ccr.2006.08.027. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–65. doi: 10.1016/j.ccr.2006.03.027. see comment. [DOI] [PubMed] [Google Scholar]
- 12.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. Journal of Clinical Investigation. 2007;117(1):112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12(2):171–85. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):3668–72. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavathiotis E, Suzuki M, Davis ML, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455(7216):1076–81. doi: 10.1038/nature07396. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Tu HC, Ren D, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Molecular Cell. 2009;36(3):487–99. doi: 10.1016/j.molcel.2009.09.030. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goping IS, Gross A, Lavoie JN, et al. Regulated targeting of BAX to mitochondria. Journal of Cell Biology. 1998;143(1):207–15. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. Journal of the National Cancer Institute. 2006;98(12):873–5. doi: 10.1093/jnci/djj234. see comment. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128(7):2054–65. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Miyagiwa M, Ichida T, Tokiwa T, Sato J, Sasaki H. A new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In Vitro Cellular & Developmental Biology. 1989;25(6):503–10. doi: 10.1007/BF02623562. [DOI] [PubMed] [Google Scholar]
- 21.Murakami T, Yano H, Maruiwa M, Sugihara S, Kojiro M. Establishment and characterization of a human combined hepatocholangiocarcinoma cell line and its heterologous transplantation in nude mice. Hepatology. 1987;7(3):551–6. doi: 10.1002/hep.1840070322. [DOI] [PubMed] [Google Scholar]
- 22.Knuth A, Gabbert H, Dippold W, et al. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. Journal of Hepatology. 1985;1(6):579–96. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 23.Lai GH, Zhang Z, Shen XN, et al. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129(6):2047–57. doi: 10.1053/j.gastro.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Sirica AE, Zhang Z, Lai GH, et al. A novel “patient-like” model of cholangiocarcinoma progression based on bile duct inoculation of tumorigenic rat cholangiocyte cell lines. Hepatology. 2008;47(4):1178–90. doi: 10.1002/hep.22088. [DOI] [PubMed] [Google Scholar]
- 25.Mott JL, Bronk SF, Mesa RA, Kaufmann SH, Gores GJ. BH3-only protein mimetic obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Molecular Cancer Therapeutics. 2008;7(8):2339–47. doi: 10.1158/1535-7163.MCT-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasband WS. ImageJ. Bethesda, MD: U.S. National Institutes of Health; 19972008. [Google Scholar]
- 27.Werneburg NW, Guicciardi ME, Bronk SF, Kaufmann SH, Gores GJ. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. Journal of Biological Chemistry. 2007;282(39):28960–70. doi: 10.1074/jbc.M705671200. [DOI] [PubMed] [Google Scholar]
- 28.Guicciardi ME, Bronk SF, Werneburg NW, Yin XM, Gores GJ. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology. 2005;129(1):269–84. doi: 10.1053/j.gastro.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. Journal of Biological Chemistry. 2006;281(17):12093–101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103(4):645–54. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clinical Cancer Research. 2009;15(1):150–9. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology. 2009 doi: 10.1002/hep.23214. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H, Rafiuddin-Shah M, Tu HC, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature Cell Biology. 2006;8(12):1348–58. doi: 10.1038/ncb1499. see comment. [DOI] [PubMed] [Google Scholar]
- 34.Lovell JF, Billen LP, Bindner S, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135(6):1074–84. doi: 10.1016/j.cell.2008.11.010. see comment. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien SM, Claxton DF, Crump M, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113(2):299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schimmer AD, O'Brien S, Kantarjian H, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clinical Cancer Research. 2008;14(24):8295–301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 37.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clinical Cancer Research. 2009;15(4):1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–9. doi: 10.1126/science.1133289. see comment. [DOI] [PubMed] [Google Scholar]
- 39.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–30. doi: 10.1126/science.1059108. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmati PG, Gillissen B, von Haefen C, et al. Adenovirus-mediated overexpression of p14(ARF) induces p53 and Bax-independent apoptosis. Oncogene. 2002;21(20):3149–61. doi: 10.1038/sj.onc.1205458. [DOI] [PubMed] [Google Scholar]
- 41.Wang GQ, Gastman BR, Wieckowski E, et al. A role for mitochondrial Bak in apoptotic response to anticancer drugs. Journal of Biological Chemistry. 2001;276(36):34307–17. doi: 10.1074/jbc.M103526200. [DOI] [PubMed] [Google Scholar]
- 42.Vogler M, Weber K, Dinsdale D, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death & Differentiation. 2009;16(7):1030–9. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.