Abstract
By its action on rhodopsin, light triggers the well-known visual transduction cascade, but can also induce cell damage and death through phototoxic mechanisms -- a comprehensive understanding of which is still elusive despite more than 40 years of research. Herein, we integrate recent experimental findings to address several hypotheses of retinal light damage, premised in part on the close anatomical and metabolic relationships between the photoreceptors and the retinal pigment epithelium. We begin by reviewing the salient features of light damage, recently joined by evidence for retinal remodeling which has implications for the prognosis of recovery of function in retinal degenerations. We then consider select factors that influence the progression of the damage process and the extent of visual cell loss. Traditional, genetically-modified, and emerging animal models are discussed, with particular emphasis on cone visual cells. Exogenous and endogenous retinal protective factors are explored, with implications for light damage mechanisms and some suggested avenues for future research. Synergies are known to exist between our long term light environment and photoreceptor cell death in retinal disease. Understanding the molecular mechanisms of light damage in a variety of animal models can provide valuable insights into the effects of light in clinical disorders and may form the basis of future therapies to prevent or delay visual cell loss.
1. Introduction
Retinal photoreceptor cells are uniquely adapted to function over a wide range of ambient light conditions. However, in most species prolonged intense visible light exposure can lead to photoreceptor cell damage. In nocturnal animals, the light intensity required for visual cell damage need be only 2–3 times above normal room lighting (Noell 1965; Noell et al., 1966). Visual cell damage can then progress to cell death and loss of vision, or “retinal light damage” may regress with recovery of function.
For many years, and based primarily on similar end point morphologies, retinal light damage has served as a model for human retinal degenerations arising from environmental insult, aging and genetic disease. For example, there are remarkable similarities between late stage retinal cell remodeling in light damaged rodent retinas and the anatomical changes found in advanced atrophic AMD (Marc et al., 2008). Intense light exposure has also been used as an environmental stressor in testing genetic animal models of photoreceptor cell degeneration. The outcomes of this work have improved our understanding of both visual transduction and retinal degenerations, but a fundamental question remains. How can animal models of retinal degeneration impact our understanding of the effects of light on the progression of human retinal disease? To answer this question, we need detailed knowledge of the molecular basis of retinal light damage in animal models. Toward this goal, good progress has been made in preventing retinal light damage with antioxidants and neurotrophic factors, but we still do not know how visual cell damage leads to visual cell death. Thus, although we have gained insights into the process, the mechanism of light damage still eludes a thorough understanding.
Three light damage hypotheses, originally presented in Noell’s landmark publication (Noell et al., 1966), have guided much of the work in this area. These include: a toxic photoproduct arising from vitamin A during exposure to intense light; a metabolic abnormality resulting from light exposure; and light-induced oxidative reactions. Taken together with a large amount of additional work, we now know that retinal light damage is a multi-factorial process involving both environmental and genetic factors. As in other retinal degenerations, these factors may combine to either predispose or protect photoreceptors from damage and subsequent cell death.
In this chapter, we address several light damage hypotheses as we integrate recent experimental findings into our understanding of retinal phototoxicity. We begin with a description of the morphology and biochemical changes found in retina and rod outer segments (ROS) upon intense light exposure. Next we describe the time course of photoreceptor cell death including its morphological and biochemical hallmarks. One unique feature of light damage in rats reared in darkness is widespread destruction of the retinal pigment epithelium (RPE), while animals reared in dim cyclic light exhibit retinal damage with minor or regionally focused RPE damage (Noell, 1980). Because of the close anatomical and metabolic relationships between photoreceptors and RPE, dysfunction in one leads to degeneration in the other. Therefore, we describe the effects of intense light on RPE cells and the time course of damage in vivo, along with additional insights from in vitro studies. This is followed by a discussion of the long term cellular changes that follow intense light exposure, a process known as retinal remodeling.
Central to all hypotheses of retinal light damage is the importance of rhodopsin as the trigger for photoreceptor cell damage (Noell et al., 1966; Humphries et al., 1997; Grimm et al., 2000c). Furthermore, the action spectrum for retinal light damage is identical to the rhodopsin absorption spectrum (Williams and Howell, 1983). Hence, we discuss rhodopsin as a primary factor in determining the extent of photoreceptor cell loss and examine rhodopsin bleaching intermediates as potential photosensitizers. Looking at other factors that influence light damage, we discuss the potential beneficial and detrimental effects of light rearing environments. This is followed by an examination of potential toxic photoproducts, based on light’s effects on retinol and its metabolites, and possible protective or destructive metabolites of docosahexaenoic acid (DHA).
Much has been learned about visual transduction and light-induced photoreceptor cell damage from studies involving genetically modified animals. We describe some genetic animal models and the impact of intense light on their retinal degenerations. We also touch on differences in outcomes and interpretation based on the use of different species. Animal models with cone cell dominant retinas are less well known and their potential to serve as models for human retinal disease is underappreciated. Yet, the loss of functional cone photoreceptors can be devastating. Therefore, we describe a variety of models now available and discuss some of the problems inherent in attempting to light damage cones.
Finally, we cover the protective effects of exogenous antioxidants and neurotrophic factors with a discussion of insights derived from the timing of treatment relative to light exposure. The innate ability of the retina to resist retinal light damage and its circadian dependent vulnerability are also described, along with approaches for identifying endogenous factors that may contribute to damage or provide protection. In the last 20 years, numerous studies have added to our base of knowledge about light-induced retinal degeneration and contributed to a better understanding of the process. However, the sheer number of manuscripts involving retinal light damage is now so extensive that it is almost impossible to cite all references. Those interested in additional background on this topic are referred to the following earlier publications and their associated references: - Penn and Anderson, 1992; Fain and Lisman, 1993; Organisciak and Winkler, 1994; Rapp, 1995; Remé et al., 1996; Remé et al., 1998a, b; Stone et al., 1999; Organisciak and Sarna, 2001; Organisciak et al., 2003; Rozanowska and Sarna, 2005; Remé, 2005; Wenzel et al., 2005; Wu et al., 2006; and Rozanowska et al., 2009.
2. Features of the Retinal Light Damage Model
2.1 Morphological and Biochemical Findings
The effects of intense light on retinal morphology and biochemistry can be dramatic, because the entire population of visual cells is often adversely affected. In nocturnal rodents, light damage is typically confined to rod photoreceptors and retinal cell loss largely restricted to the outer nuclear layer (ONL). As such, measurements of ONL area, thickness, or counts of visual cell nuclei are frequently used to quantify light’s damaging effects. Retinal DNA measurements and quantitative determinations of visual cell proteins, several days after light exposure, also make for good end point estimates of photoreceptor cell loss. Retinal light damage is a graded response, with areas containing little or no damage adjacent to more severely affected regions. Within the superior hemisphere, light damage is often found to be particularly severe in a region 1–2 mm from the optic nerve head (Rapp and Williams 1980). In moderate light damage, the periphery is generally spared and damage in the inferior hemisphere is less than in the superior hemisphere, or absent altogether. Regional differences in photoreceptor cell light damage have been the topic of several studies since Rapp and Williams (1980) first described it.
Reduced photoreceptor cell damage in the inferior half of the retina has been attributed to shorter ROS and a lower rhodopsin level than found in the superior hemisphere (Battelle and LaVail, 1978; Rapp et al., 1985; Penn and Anderson, 1987). The lack of damage in peripheral retina is generally thought to result from a decrease in retinal irradiance in that area. Other suggestions for “regional” damage or protection are a better intra-retinal circulation in the inferior region, and neuroprotective factor synthesis in response to bright light conditioning (Liu et al., 1998; Li et al., 2003). Stone et al. (1999) found high levels of bFGF in both the inferior region of the retina and in the periphery. The localization of bFGF also correlated with reduced photoreceptor cell light damage in those areas. Stone et al. (1999) concluded that a higher oxygen tension, or preferential light exposure from overhead lighting, was the reason for reduced damage in those areas. Surprisingly, when the room lighting in the animal facility was changed from the ceiling to the side, light damage in the superior hemisphere was largely prevented (Stone et al. 1999). This indicates that the incident angle of light in an animal’s rearing environment can have a dramatic influence on the region of the eye impacted by intense light.
Enhanced rod cell damage in the inferior hemisphere occurs in some light damage animal models (Bush et al., 1991; Hafezi et al., 1997; Wenzel et al., 2000) and may arise for the same reasons, e.g. those related to the delivery of light. Whereas most light exposure chambers are designed to provide a Ganzfeld type illumination, with relatively uniform corneal irradiances, that does not mean that light is uniformly focused on the retina. Using well controlled light rearing environments, Penn and associates (1992) reported that visual cells in the inferior region of the rat retina are preferentially lost with increasing light intensity. This disproportionate light-effect on photoreceptors in the inferior region results in a reduced “local” concentration of rhodopsin, with respect to its concentration in the superior region (Williams et al., 1999). Accordingly, when these animals were subjected to acute intense light, photoreceptors in the superior hemisphere incurred the greatest amount of damage. An adaptive regional modification of rhodopsin levels fits well with the work of Battelle and LaVail (1978) and Rapp et al. (1985) and with the theory of photostasis (Penn and Williams, 1986).This theory predicts that the retina will absorb a relatively constant number of photons per day, which could explain long term, light-mediated adjustments in rhodopsin levels and the regional vulnerability of visual cells to acute intense light treatment. However, photostasis also has its limits.
The extent of photoreceptor cell loss varies with the time of day that light exposure starts (Organisciak et al., 2000). An example of this is shown in Figure 1A, which contains ONL measurements in rats 2 weeks after 8 hours of intense green light beginning at various times (Vaughan et al., 2002). Following light treatment at 1 am, photoreceptor loss in the superior hemisphere is extensive, especially in the “sensitive” region near the optic nerve, while damage in the inferior region is practically absent (Rapp and Williams, 1980; Penn et al., 1992). However, when rats reared under identical dim light conditions were exposed to the same light beginning at 9 am or 5 pm, the decrease in ONL thickness was much less. Since these rats were reared under identical conditions, all had the same initial rhodopsin levels and all were well dark adapted before light exposure. Therefore, although photostasis would predict equivalent light damage, that did not happen. This indicates that, while the total number of photons normally absorbed by the retina each day may be relatively constant, the damaging effects of acute intense light also depend on circadian events or other factors that combine to determine the extent of visual cell loss.
Figure 1.
Morphometric analysis of ONL thickness (Figure 1A) and ROS length (Figure 1B) in retinas from rats treated with light at 1am, 9am or 5pm. Figure 1C is a composite of data from panels A and B comparing relative changes in retina ONL thickness and ROS length for rats treated with light at 1am. Figure 1D is a composite of data from rats exposed to light at 9am. All data are the means of 3 separate determinations for cyclic light reared rats exposed to approximately 1200lux green light for 8 hours. (Adapted from Vaughan et al., Photochem Photobiol. 75: 547–53, 2002)
2.1.1 Intense Light-Induced Changes in Rod Outer Segments
In the retina, rhodopsin bleaching triggers both visual transduction and light’s pathological effects on photoreceptors. This places the initial site of photon absorption and the initiation of light damage at the level of ROS. Histological examination immediately after light exposure reveals that photoreceptor cell damage is initiated in the distal tips of ROS and that with time it progresses to include the entire ROS (Bush et al., 1991; Vaughan, et al., 2002; Remé, 2005). Another, almost immediate, effect is an increase in phagosome numbers in the RPE. Under physiological light conditions, these arise from the distally shed tips of ROS, a process that is circadian in nature (LaVail, 1976; Goldman et al., 1980). These shed disks are normally replaced by newly synthesized disks at the ROS base and rod length remains relatively constant. However, because large numbers of phagosomes are found in RPE when light-damaged ROS disks are engulfed, this can lead to an overall decrease in rod length. Were this a simple transient event, rod length would be expected to return to normal within 1–2 weeks. However, ROS length is more permanently affected by intense light, especially in the superior half of the retina (Figure 1B). A point-by-point comparison between the relative changes in ROS length and ONL thickness reveals that ROS are disproportionately short in photoreceptors adjacent to areas exhibiting severe cell loss (Figure 1C, shaded area). Moderate photoreceptor cell loss in the superior hemisphere also leads to reduced ROS length (Figure 1D). Thus, photoreceptors in retinal regions flanking those with severe or moderate light damage survive, albeit with shortened ROS. Why damaged visual cells survive, and what “tips” the balance from damage to cell death are unknown, but Rapp and coworkers (1994) have shown that moderate UVA damage results in ROS shortening, a process attributed to impaired disk synthesis. Katz et al. (1993) reported that the cross sectional area of ROS also decreases with increasing levels of environmental light. In addition, ROS membrane-rhodopsin packing density depends on rearing light intensity (Penn and Anderson, 1987) and is higher in rats reared in darkness (Organisciak and Noell, 1977). These features may help to explain why rhodopsin recovery in the light damaged retina can be lower than expected from measurements of ONL thickness or residual DNA.
Retinal light damage may begin in the distal tips of ROS but, at the levels of light used to induce damage, rhodopsin bleaching typically exceeds 90% and is continuous throughout the rod. Furthermore, under high light conditions, the optics of illumination is such that rhodopsin bleaching is relatively uniform across the retina (Williams, 1998). How is it then that the bleaching of rhodopsin by intense visible light is responsible for a localized effect on disk morphology and for its effects on ROS length? A possible answer lies in the work of Williams and Penn (1985), who found a more rapid rate of rhodopsin regeneration in the distal tips of ROS than in the proximal disks. They suggested that vitamin A is efficiently delivered to the tips of ROS during dark regeneration, because in rodents the apical processes of RPE cells extend only part way down the length of the rod. During light exposure, this would make repetitive bleaching and regeneration of rhodopsin more likely in distal ROS disks.
Another reason for light’s effects in ROS tips may be related to differences in membrane composition. For example, a gradient of membrane cholesterol content has been found in ROS (Andrews and Cohen, 1979; Caldwell and McLaughlin, 1985). Distal disks have a lower cholesterol/phospholipid ratio than found in disks located near the ROS base (Boesze-Battaglia et al., 1990; ibid1994). Because newly formed ROS disks progressively displace older ones, the distribution of cholesterol is therefore related to disk age. This regional difference in ROS disk sterol content can affect visual transduction. Light stimulated phosphodiesterase activity is lower in nascent ROS disks, compared to those located in the distal region of ROS (Boesze-Battaglia and Albert, 1990). Lower membrane cholesterol in older disks might also affect the shedding of light damaged ROS and impact the level of lipid oxidation. Kayatz et al. (1999) found increased peroxide reactivity in ROS upon intense light exposure, but the oxidation was not focused exclusively in the ROS tips. Furthermore, although photo-damage in rat retina leads to cholesterol oxidation, this occurs primarily in the RPE, rod inner segments and ganglion cell layer (Rodriguez and Fliesler, 2009). The presence of light-induced peroxides in ROS disks of various ages and cholesterol content suggests that ROS fatty acids are the likely target of oxidative attack.
2.1.2 Time Course of Photoreceptor Damage and Cell Death
Rhodopsin’s activation by intense light generates signals that initiate pathological changes in the rod photoreceptor cell body. Although details of this signaling cascade are currently unknown, the entire rod cell, from ROS tip to synaptic terminal, is quickly involved. The rapidity with which this occurs suggests that a diffusible substance is the agent of damage, but this has not yet been demonstrated. Ultimately, however, light damage leads to visual cell death through a series of apoptotic events. One manifestation of apoptosis is the appearance of double stranded DNA breaks, detectable as a “ladder” of 180–200 base pair fragments upon agarose gel electrophoresis. Whereas DNA fragmentation is a relatively late apoptotic event, the onset of DNA ladder formation in the retina begins within hours of light onset and depends on the wavelengths of light used, as well as its intensity (Shahinfar et al., 1991; Remé et al., 1995; Li et al., 1996). In rats, a retinal DNA ladder was detectable after several hours of green light exposure at an intensity of about 3000 lux (Shahinfar et al., 1991; Li et al., 1996). Remé et al. (1995) reported extensive DNA ladder formation after only 2 hours of white light exposure, also at 3000 lux. In each case, TUNEL staining revealed the appearance of fragmented DNA in the ONL which coincided with the presence of DNA ladders. These changes were also found to coincide with, or be preceded by, single-strand DNA breaks (Organisciak et al., 1999a) and nuclear chromatin condensation (Shahinfar et al., 1991; Hafezi et al., 1997a), lending support to the hypothesis of a light-induced diffusible oxidative agent.
Knowing the relative time courses of apoptotic changes and DNA fragmentation can help elucidate the mechanism of light damage by indicating when photoreceptor damage has tipped to the point of initiating cell death. One problem with determining the onset of DNA damage is detecting the low level of strand cleavage that precedes the appearance of DNA ladders, or TUNEL reactivity. Another is the long duration of some intense light exposures, which makes it difficult to know how much light is required to initiate visual cell death. To detect early signs of DNA fragmentation, we sought to accelerate the rate of light damage across the entire retina. Fortunately, Werner Noell provided us with one of his original hyperthermic light exposure chambers (Noell et al., 1966). This allowed us to treat rats for brief periods, and then to assess the near synchronous development of DNA fragmentation occurring in practically all photoreceptors. Figure 2 contains retinal DNA fragmentation patterns after 2 hours of green light, under hyperthermia, followed by various times in darkness at room temperature. Eleven-hundred lux of 490–580nm light were used in these experiments because rhodopsin absorbs maximally in that region of the spectrum. According to Gordon et al. (2002), only about one third of white fluorescent light overlaps with rhodopsin’s absorption spectrum. This means that our green light exposures would be nearly equivalent to 3000 lux of full spectrum white light, but without the potential for spurious effects from short wave length, higher energy light. As shown in Figure 2, DNA fragmentation is clearly present 4 hours after light exposure and is most likely present after only 2 hours. DNA degradation increases thereafter, is at its maximum 1–2 days later, and is practically absent after 4 days. This suggests that the onset of light-induced DNA damage is rapid, occurring in about 2 hours, and that once started DNA degradation continues for about 2 days. At that time, DNA in severely damaged photoreceptor cells has been completely degraded and DNA repair (Gordon et al., 2002) is nearly completed in photoreceptors destined to survive.
Figure 2.
Rats were preheated in darkness in a humidified atmosphere at 97 °C for 2 hours. Core body temperature was 100.5 ° F at the beginning of exposure to 1100 lux green light for 2 hours. After exposure, animals were returned to darkness at room temperature. Retinas were excised from rats at various times thereafter and DNA extracted as described (Organisciak et al., 1999).
2.1.3 Caspases and Other Early Events
Conventional thinking suggests that, if double-strand DNA fragmentation is endonuclease-mediated and detectable within several hours of damaging light exposure, then enzyme activation should be preceded by identifiable up-stream events. During apoptosis, an increase in cellular caspase activity is generally, but not always, regarded as a precursor to DNA degradation. In the case of retinal light damage in rats, activation of caspases-1, 3, 7, 8, and 9 has been reported to occur (Wu et al., 2002; Paterson, 2005; Perche et al., 2007). Evidence supporting the involvement of caspases in light damage includes Western analysis of pro-caspases and their proteolytic cleavage products (Wu et al., 2002; Patterson, 2005). Perche et al. (2007) used synthetic caspase inhibitors and found that caspases-3 and 7 were active during a 24 hour light exposure, while caspases-1 and 4 and calpains were active 1 day later. Increases in caspase mRNAs also occur during prolonged light treatment (Wu et al., 2002; Patterson, 2005). Tomita et al. (2005) reported that caspase-3 mRNA was elevated in rat retina after 6–12 hours of light, but that its enzymatic activity was unaffected. Li et al. (2003) found no increase in retinal caspase-3 protein levels upon bright light adaptation of rats for several days and subsequent intense light treatment. Similarly, following brief periods of intense white light, Donovan et al. (2001) found increased levels of intracellular calcium and superoxide and mitochondrial membrane depolarization in retinal cells from Balb/c mice, but no activation of caspase-3. Inhibition of the neuronal form of nitric oxide synthase (NOS), prevented TUNEL reactivity in the ONL and greatly reduced the effects of light on calcium levels, superoxide production and mitochondria membrane potential. It was suggested that S-nitrosylation of essential cysteine residues by nitric oxide (NO) might actually inhibit caspase activation (Donovan et al., 2001). In the rat light damage model, inhibition of retinal NOS provided structural protection of photoreceptors, but only modest functional protection (Kaldi et al., 2003). In rat brain neurons, NO treatment led to decreases in NADH (Zhang et al., 1994) and ATP, changes which appear to promote necrotic cell death over apoptosis (Leist et al., 1999).
Given the time frame of retinal DNA fragmentation and the differences noted above, at this point only a few general conclusions are possible. First, the role of caspases in retinal light damage appears to be animal model specific. In the rat retina, caspase-3 mRNA levels increase rapidly during intense light and inhibition of caspase activity prevents light damage. In some cases, caspase-3 activation precedes or is coincidental with DNA fragmentation; in others, it does not appear to be active. It is worth noting that Tomita et al. (2005) and Perche et al. (2007) used different strains of albino rats which may exhibit different susceptibilities to retinal light damage (Borges et al., 1990; Iseli et al., 2002). Caspase-1 activation in rats appears to occur much later (Perche et al., 2007), or not at all (Tomita et al., 2005). In mice, caspase-1 mRNA levels increase 6–8 hours after light treatment, indicating a down-stream role in DNA degradation (Grimm et al., 2000b). Second, calpain activity increases 1 day after the end of light treatment in rats (Perche et al., 2007), but its activation may occur sooner in mice. Calcium dependent calpain activation is rapid in vitro, while being inhibited by antioxidants (Sanvicens et al., 2004). The activation of many endonucleases also depends on cellular calcium and antioxidants are known to prevent retinal light damage. This suggests a role for both calcium ion and oxidative stress in the damage process. In support of a role for calcium, rats treated with Flunarizine, which blocks calcium release from intracellular stores, exhibited reduced light damage (Edward et al., 1991). Finally, differences in the light levels required to induce retinal damage also seem to be species specific. Mice generally require more intense light to induce damage than rats, and different light intensity-dependent apoptotic pathways are known to exist (Hao et al., 2002). Apoptotic cell death is an energy dependent process whereas necrosis is affected more by cellular calcium (Yuan et al., 2003). It is likely that a continuum between apoptotic and necrotic photoreceptor death exists and that the balance between caspase and calpain activation depends on the time course of changes in cellular energy and ion levels.
2.2 Damage to the Retinal Pigment Epithelium
The intimate metabolic and morphological relationships between the neuroretina and the RPE, their high oxygen tension, and the rate and duration of photon flux all link damage in one tissue to degeneration in the other. A unifying factor in photoreceptor/RPE damage appears to be light-induced reactive oxygen species, generated by the bleaching of rhodopsin or from compounds in the RPE, such as A2E (bis-retinaldehyde-phosphatidylethanolamine). Retinaldehyde is found in both rhodopsin and A2E, but the preferential absorption of blue light by A2E increases the likelihood for RPE damage from high energy photons (Sparrow et al., 2000). Blue light damage may also involve mitochondrial cytochromes in RPE (King et al., 2004)-or lipofuscin particles which accumulate with age (Rozanowska et al., 1995). High levels of environmental light have been implicated in the accumulation of drusen (Bressler et al., 1995) and photo-oxidized A2E appears to activate the complement system (Zhou et al., 2006), indications that light damage in the RPE may contribute to clinical disease.
In rats exposed to intense green light, the appearance of RPE damage was delayed by a few hours with respect to visual cell damage (Noell et al., 1966). This delay was attributed to a light-induced toxic photoproduct arising in photoreceptors and then diffusing to the RPE. Using an intermittent light exposure paradigm on rats, consisting of multiple 1 hour light/2 hour dark periods, Blanks et al. (1992) found large numbers of RPE phagosomes after each light cycle. Pretreatment with ascorbic acid greatly reduced phagosome accumulation, suggesting that oxidation-induced damage in ROS was involved in their phagocytic removal. A subsequent study confirmed a delay of 5-10 hours between photoreceptor and RPE cell damage in rats exposed to broad band white light (Hafezi et al., 1997a). These authors concluded that a large ROS disk shedding event triggered by intense light could serve as a pathological signal for apoptosis in RPE cells. In this regard, we found massive ROS disk shedding in light damaged rats reared in darkness, along with greater RPE cell damage than seen in rats reared in dim cyclic light (Vaughan et al., 2002). Thus, the removal of light damaged ROS disks may be toxic, leading to oxidative stress in RPE. This phagocytic process could also lead to the accumulation of blue light absorbing molecules such as A2E (Sparrow et al., 2000), or to partially degraded ROS proteins and lipid-protein adducts (Crabb et al., 2002).
2.2.1 In vitro Studies and Insights
Whereas much has been learned from in vivo studies, in vitro the RPE lends itself to studies designed to directly test treatments that promote cellular damage or protection. For example, maintenance of cellular glutathione (GSH) is widely believed to be important in preventing oxidative damage in tissues. Incubation of RPE cells in the presence of zinc induces the rate controlling enzyme in GSH synthesis, apparently through an antioxidant responsive element (ARE) and Nrf-2 (nuclear factor erythroid-2 related factor)(Ha et al., 2006). At physiological levels, zinc prolongs RPE cell life in vitro, while at higher levels it can be toxic (Wood and Osborne, 2003). Yet, even in the presence of high zinc levels, simultaneous incubation of RPE cells with antioxidants reduces its toxic effects (Wood and Osborne, 2003). Pre-incubation of RPE cells with the intracellular antioxidant α-lipoic acid also induces GSH synthesis and reduces cell death induced by peroxide treatment (Voloboueva et al., 2005). The natural substance sulforaphane, found in crucifers such as broccoli, has been shown to induce genes in the RPE, which lead to increases in GSH, detoxification enzymes such as NADH quinone-oxidoreductase (Gao and Talalay, 2004), and thioredoxin, a redox-active protein (Tanito et al., 2005a). Similarly, eriodictyol, a bioflavinoid found in citrus fruits, induces GSH synthesis in ARPE-19 cells, and provides long term protection against oxidative damage (Johnson et al., 2009). Likewise, over-expression of GSH peroxidase-1 and/or -4 in ARPE-19 cells decreased the extent of oxidant-mediated cell death (Lu et al., 2009). Thus, the thiol status of RPE is important in maintaining function and a variety of effectors including oxidative stress (Lin et al., 2002) and the thiol specific oxidant diamide (Obin et al., 1998) can lead to decreases. Zinc ion, natural substances and antioxidants all enhance GSH levels, often through an ARE and transcription factors such as Nrf-2, leading to a reduction in RPE oxidation (Gao and Talalay, 2004; Tanito et al., 2005a; Ha et al., 2006, Johnson et al., 2009). Reducing oxidative stress in RPE, with ascorbic acid, also maintains mitogen-activated protein kinase-1 phosphatase (MKP-1), which inactivates the JNK pathway and prevents photo-damage (Lornejad-Schafer et al., 2009). In total, these data suggest that there are multiple mechanisms of antioxidant action in RPE, involving a decrease in reactive oxygen species and increases in GSH levels which, in turn, affect enzymes and/or pathways that protect the cells against oxidative stress.
2.3 Post Exposure Retinal Remodeling
Retinal remodeling refers to a series of anatomical changes in retinal cells following the loss of relatively large numbers of photoreceptors, occurring over weeks or even months (Marc and Jones, 2003). Three distinct phases have been described from animal models of hereditary retinal degenerations and retinal detachment (Marc and Jones, 2003; Fisher et al., 2005; Marc, in press). Phase 1 events encompass RPE stress and photoreceptor degeneration, such as outer segment shortening, misplaced visual pigment, and synapse abnormalities. Phase 2 events include bona fide photoreceptor death, microglial activity, bipolar cell dendrite retraction, and Müller cell hypertrophy including formation of a seal - as distinct from a scar - in the outer retina. Phase 3 involves aberrant neurite formation and synaptogenesis; by this point, remodeling is thought to be irreversible thus “narrowing the window” for therapeutic intervention (Marc, in press).
We recently documented retinal remodeling in light-damaged retinas of albino rats (Marc et al., 2008). These animals were reared in dim light for 60 days and then subjected to 24–48 hr of intense green light, which typically results in loss of 50–80% photoreceptors. Remodeling in these light-damaged rat eyes exhibited the three phases described above along with some notable additions. First, Phase 3 events transpired on par with the fastest known heritable retinal degenerations. Second, the border between damaged and surviving retinal cells was abrupt. Third, retinal degeneration exceeded the typical Phase 3 events to include breakdown not only of the Müller cell seal but also of the natural blood-retina interface formed by the RPE, Bruch’s membrane, and the choriocapillaris. Glial and neuronal cells migrated through the breach and took up residence in the choroid, ultimately compromising vascular perfusion as noted previously (Tanito et al., 2007). While glial migration has been described before, the extent of neuronal motility and migration is remarkable in the light-damaged rat retina. The local consequences of this are to destroy all retinal architecture, leaving behind a tissue riddled with fluid-filled holes. For this reason, remodeling following light damage has been termed “extreme”; yet similarities with the retinal cell loss phenotype of late stage atrophic age-related macular degeneration is striking (Marc et al., 2008).
Interestingly, survival of even substantially dedifferentiated cones delays Phase 3 remodeling events (Jones and Marc, 2005, Marc, in press). In hereditary retinal degenerations, contact with surviving cones - even profoundly altered cones - seems to induce rod bipolar cells to engage in target switching and become, in essence, OFF-bipolar cells (Marc et al., 2007). Therefore, cone survival may suppress gross remodeling while still significantly corrupting retinal circuitry. These findings are significant with respect to improving the probability of success in retinal transplants. Only by understanding the reasons for, and mechanisms of, retinal remodeling can we discover ways to prevent the anatomical relocation of cells and synapses.
3. Factors Involved in the Light Damage Process
3.1 Rhodopsin and its Bleaching Intermediates
Early studies by Noell et al. (1966), Kaitz and Auerbach (1979), and Williams and Howell (1983) all pointed to rhodopsin bleaching as the trigger for retinal light damage. Retinol availability was also shown to be crucial, as vitamin A deficient rats were protected against light damage (Noell et al., 1971). Genetic deletion of retinal proteins in mice also point to the importance of rhodopsin bleaching and its regeneration in understanding light damage. Rhodopsin knockout (KO) mice, which totally lack the chromophore, and RPE-65 KO mice, which express only the apoprotein opsin (Redmond et al., 1998), are both protected against light damage (Humphries et al., 1997; Grimm et al., 2000c). In mice, RPE-65 protein levels and the rate of rhodopsin regeneration are related and slowing regeneration results in light damage resistance (Wenzel et al., 2001a). Similarly, slowing rhodopsin regeneration about 50 fold by inhibiting the visual cycle with 13-cis retinoic acid prevents light damage in the rat model (Sieving et al., 2001). Thus, a reduced rate of rhodopsin regeneration, elevated opsin levels and the absence of rhodopsin in photoreceptors all lead to protection against light damage. This supports rhodopsin’s key role in mediating photic damage, but raises questions about the identity of any chromophores that might absorb light and their relationship to rhodopsin.
3.1.1. Meta Rhodopsin I, II and III
It is well known that transient photo-intermediates form upon rhodopsin bleaching. Three of these intermediates, Meta rhodopsin I, II and III, each have different absorption spectra and different half-lives (Hofmann, 1986). Their half-lives are also affected by temperature, pH, membrane lipids and the presence or absence of G-proteins. Meta rhodopsin I, II and III exist in an equilibrium that can be altered by subsequent light treatment. Grimm et al. (2000a) reported that absorption of short wavelength blue light (403nm) by Meta II caused a reversal of rhodopsin bleaching, with regeneration occurring independent of the visual cycle. Ritter et al. (2008) concluded that blue light (~400nm) resulted in the conversion of Meta II to Meta III, a 475 nm absorbing intermediate. Whether blue light irradiation reforms native rhodopsin, or whether it leads to Meta III, prolonged blue light should result in the simultaneous presence of all 3 Meta intermediates. However, given their relative half lives (Hofmann, 1986), Meta II and III probably predominate during prolonged light exposure. This may help explain why the retina is susceptible to blue light damage, even though (a) it bleaches rhodopsin less efficiently than does green light (Grimm et al., 2000a) and (b) rhodopsin’s absorption spectrum fits the action spectrum for light damage (Williams and Howell, 1983).
Meta rhodopsin II is unlikely to be a photosensitizer in the visible portion of the spectrum, because it absorbs primarily short wavelength light (lambda max 380 nm) and because green light (490–580 nm) alone causes retinal damage. Although Meta rhodopsin I (lambda max 480 nm) absorbs green light, its ability to absorb short wavelength blue light is limited. On the other hand, Meta rhodopsin III absorbs light over the entire visible spectrum, a process that ultimately leads to the reappearance of Meta II (Ritter et al., 2008) or a Meta II like species (Sommer and Farrens, 2006). In bright light, 30–50% of rhodopsin is converted to Meta III (Lewis et al., 1997). Under physiological bleaching conditions, Meta III has a half-life of minutes to hours, depending on the presence of transducin and/or arrestin (Heck et al., 2003). This relatively long half life may serve to sequester trans-retinal in the vitamin A binding pocket of rhodopsin and help prevent the formation of toxic photoproducts by slowing its release (Ritter et al., 2008). Evidence for the role of a long lived photo-intermediate is found in the early experiments of Noell et al. (1966). They used intermittent light/dark periods to induce retinal damage during hyperthermia and concluded that extensive rhodopsin bleaching sensitizes the retina to damage upon subsequent light exposure. Rats treated with green light displayed extensive photoreceptor cell damage after only three 5-minute light/1 hour dark treatment periods, damage that was greater than found in rats treated with 15 minutes of continuous light (Noell et al., 1966). Similar effects occur in euthermic rats treated with several 1 hour light/2 hour dark periods (Organisciak et al., 1989a; Li et al., 1996). However, rhodopsin regeneration during the intervening dark periods decreased as the number of light cycles increased (Organisciak et al., 1989a). Although this does not prove that Meta rhodopsin III is a photosensitizer, these studies indicate that a long lived photo- intermediate is important in the process of retinal light damage.
3.2 Rearing Light Environments
The long term survival of retinal photoreceptor cells following intense light exposure depends on a variety of external factors. One of these arises from the cell’s ability to adapt in response to changes in light rearing conditions. This photoreceptor cell plasticity was first described by Penn and Williams (1986), who coined the term “photostasis”. Part of photostasis involves homeostatic alterations in rhodopsin levels and ROS length based on light levels in the rearing environment (Penn and Williams, 1986). In subsequent studies, Penn and Anderson and associates reported that light history also affected retinal antioxidant levels, ROS lipid composition and light damage susceptibility (Penn et al., 1987; Penn and Anderson, 1987). More recently, long term bright light conditioning has been shown to alter the expression of mitochondrial enzymes (Huang et al., 2004) and to protect against retinal light damage by decreasing DHA levels (Li et al., 2001). Short term preconditioning with bright light also leads to protection against retinal damage by subsequent intense light treatment (Liu et al., 1998). The benefits of light pre-conditioning appear to be reversible, as demonstrated by the different degrees of retinal light damage in rats changed from bright- to dim- light rearing and vice versa (Penn et al., 1992).
Rats reared in darkness express higher levels of rhodopsin and transducin and lower levels of arrestin than rats reared in dim cyclic light, and are more susceptible to retinal light damage (Organisciak et al., 1991; Farber et al., 1991). The relative levels of these proteins also correlate with the onset of light damage susceptibility in young RCS dystrophic rats (Organisciak et al., 1999b). ROS arrestin and transducin levels also change during light exposure, as they move between the ROS and rod inner segment (RIS) compartments (Philip et al., 1987; Brann and Cohen, 1987; Whelan and McGinnis, 1988; Sokolov et al., 2002). Translocation of transducin out of ROS during light exposure allows photoreceptor function at higher light intensities during prolonged exposure (Sokolov et al., 2002) and could be expected to help reduce photoreceptor light damage.
However, a decrease in photoreceptor arrestin levels can impair the shut-off of activated rhodopsin and lead to the accumulation of phototransduction intermediates and more extensive light damage (Xu et al., 1997). For example, arrestin KO mice have been shown to be highly susceptible to retinal light damage (Chen et al., 1999). Lamb and Pugh (2004) have suggested that this may be due to a build-up of Meta rhodopsin III. In contrast, a high level of arrestin would be expected to decrease both the half-live of Meta rhodopsin III (Lamb and Pugh, 2004; Ritter, 2008) and light damage susceptibility. Thus, photoreceptor damage, in at least one form of light damage (Hao et al., 2002), appears to depend on the levels of visual transduction proteins that compete for binding to the C-terminal of rhodopsin, as well as their sub-cellular locations during light exposure.
3.3 Visual Cycle and Retinoid Derivatives
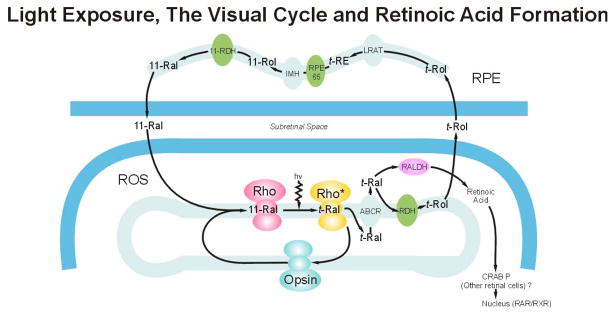
During continuous illumination, a steady state rhodopsin level is achieved by the balance between its bleaching and regeneration. This balance is linked to the visual cycle and the adjacent RPE, where many of the enzymes necessary for retinoid processing and storage are found (Figure 3). In his early work, Noell hypothesized that the membranolytic properties of retinol might be toxic to photoreceptors (Noell et al., 1966). Subsequent studies of the then nascent visual cycle under strong light conditions revealed retinol movement to, and esterification in, the RPE with a time course that precludes its role in photoreceptor phototoxicity (Noell et al., 1971). However, more recent work has revealed the complexity of the rod cell visual cycle and identified retinol dehydrogenase (RDH) activity as a slow step in the cycle (Crouch et al., 1996; Saari, 2000; McBee et al., 2001). Using retinal slices, Chen et al. (2005) found maximal retinol formation 30–60 minutes after the start of rhodopsin bleaching. Under strong bleach conditions in vivo, all-trans retinal -not retinol- rapidly accumulates in ROS (Saari, 2000). During prolonged light exposure, retinyl esters accumulate in RPE and they remain elevated for some time after exposure (Saari et al., 2001). This implicates retinoid isomerization (RPE-65) as a second slow step in the visual cycle (Redmond et al., 1998; Grimm et al., 2000c; Saari et al., 2001).
Figure 3.
Extensive rhodopsin bleaching releases nanomolar amounts of all- trans retinal (t-Ral) which is then transported across the ROS disk membrane by an ATP-binding cassette (ABCR). Most of the t-Ral is converted to all-trans retinol (t-Rol) by RDH. T-Rol is then transported to the RPE where it is either converted back into 11-cis retinal (11-Ral), or stored as retinyl esters (t-RE). The RPE enzymes involved include: lecthin retinol acyl transferase (LRAT), RPE-65, isomerohydrolase (IMH) and 11-cis retinol dehydrogenase (11-RDH). Picomolar levels of t-Ral are converted to trans-retinoic acid by retinaldehyde dehydrogenase (RALDH), which may then be isomerized to 9-cis retinoic acid. RAR/RXR heterodimers are formed after binding retinoic acid and then migrate to the photoreceptor cell nucleus. (Adapted from Saari, Invest Ophthalmol Vis Sci 41; 337–48, 2000).
Although the light-induced accumulation of trans-retinal in ROS is transient- RDH converts it to retinol- retinal is also a substrate for a variety of retinaldehyde dehydrogenases. These enzymes detoxify potentially damaging aldehydes, but also catalyze the irreversible formation of small amounts of retinoic acids during retinal development (McCaffery et al., 1993) and during light exposure (McCaffery et al., 1996; Chrispell et al., 2009). The propensity for retinoic acid formation in retina is not the same in all cases. For example, the specific activity of RDH in ROS from rats reared in darkness is only 75% of that found in cyclic light rats and intense light exposure further inhibits its activity (Darrow et al., 1997). Pico molar levels of all-trans- and 9-cis retinoic acid have been measured in retinas from dark reared rats treated with intense light, while their levels were below detection threshold in cyclic light reared animals (Duncan et al., 2006). Retinas from light exposed transgenic rats expressing P23H rhodopsin also form all-trans retinoic acid (Duncan et al., 2006). Retinoids are well known transcriptional regulators, mediating both retinal cell differentiation and apoptosis during development (Kelly et al., 1994; Streichert et al., 1999; Soderpalm et al., 2000; Cveki and Wang, 2009). The formation of retinoic acids in the retinas of light exposed adult animals, then, links rhodopsin bleaching with transcriptional regulation.
The biological effects of retinoids are mediated through retinoic acid receptors (RAR) and retinoid X receptors (RXR) in a ligand specific manner involving all trans- and/or 9-cis retinoic acid (McCaffery et al., 1993; Janssen et al., 1999; Mori et al., 2001; Cveki and Wang, 2009). RAR and RXR hetero- or homo-dimers bind to retinoic acid response elements in DNA (Kastner et al., 1994) and trigger different cellular signaling pathways. They are also members of a superfamily of nuclear transcription factor regulators that includes steroid and thyroid hormones (Mangelsdorf et al., 1995), vitamin D3 receptors (Johnson et al., 1995) and retinoid related orphan receptors (Andre et al., 1998). These receptors are found in various ocular cell types, including photoreceptors (Jannsen et al., 1999; Mori et al., 2001), and in some instances form heterodimers with RAR or RXR (Johnson et al., 1995; Mangelsdorf et al., 1995).
At this time, it is unknown if retinoic acids are a toxic photoproduct of intense light-induced rhodopsin bleaching. Their actual site(s) of synthesis in retina are also unknown, although we assume it occurs in photoreceptors (see Figure 3); at least one form has been localized to rod inner segments (Chrispell et al., 2009). Nevertheless, given the ability of retinoids to affect cellular signaling and the diversity of RAR and RXR heterodimers, both beneficial and damaging effects might be expected. All-trans-retinoic acid promotes cell survival in retinal cultures from normal mice, but causes degeneration of retinal cells in cultures expressing the P23H rhodopsin mutation (Streichert et al., 1999). Finding retinoic acids in the retinas of light-exposed rats reared in darkness, but not in those reared in dim cyclic light, suggests the intriguing possibility that they may be a signal for cell death. This could account for different light damage susceptibilities in cyclic light and dark reared rats and give rise to potential differences in mechanisms.
3.4 Dietary Docosahexaenoic Acid (DHA): Metabolism vs. Oxidation
3.4.1 DHA Metabolism
ROS disk membranes contain the highest level of DHA of any cellular organelle in the body. Anywhere from 30–50 mol% of ROS fatty acids are DHA, meaning that 1 out of 2 or 3 fatty acids is highly unsaturated. This high degree of polyunsaturation has been the source of much speculation about the role of DHA in retinal degenerations and in retinal light damage in particular. It has been known for some time that a diet deficient in essential fatty acids leads to an abnormal electroretinogram (ERG) in rats (Benolken et al., 1973) and to a prolonged ROS disk membrane renewal time (Anderson et al., 1976). In rhesus monkeys, Neuringer et al. (1986) found that a prenatal diet deficient in linolenic acid (18:3n-3), the essential fatty acid precursor of DHA, led to an increase in long chain n-6 fatty acids, prolonged ERG dark recovery times, and below-normal visual acuity in juvenile animals. Similarly, young rats fed 18:3n-3 deficient diets have depressed levels of DHA in ROS and elevated docosapentaneoic acid (22:5n-6), which replaces DHA during linolenic acid deficiency (Tinoco, 1982).
In numerous studies, DHA-depleted rats were found to exhibit reduced retinal light damage (Organisciak et al., 1987; Bush et al., 1991; ibid 1994; Koutz et al., 1995; Wiegand et al., 1995; Organisciak et al., 1996), although the reasons are not entirely clear. Bush et al. (1994) suggest that protection is attributable to reduced photon absorption by rhodopsin and to a slower than normal rate of rhodopsin regeneration. Remé et al. (1993) tested fish oil vs. soybean oil diets and, as expected, found higher levels of 20:5n-3 (EPA) in retinas of fish oil-fed rats, but no effect on DHA levels. Wiegand et al. (1995) used a variety of diets to alter ROS DHA levels and found both DHA and 22:5n-6 to be equally susceptible to light-induced oxidation. In the study by Remé et al. (1993), fish oil-fed rats exhibited protection against light damage, which was attributed to EPA utilization by a retinal cyclooxygenase and/or lipoxygenase. Organisciak et al. (1996) suggested that a high level of ROS 22:5n-6 might reduce light damage by decreasing the availability of DHA for a retinal DHA lipoxygenase previously identified by Birkle and Bazan (1986).
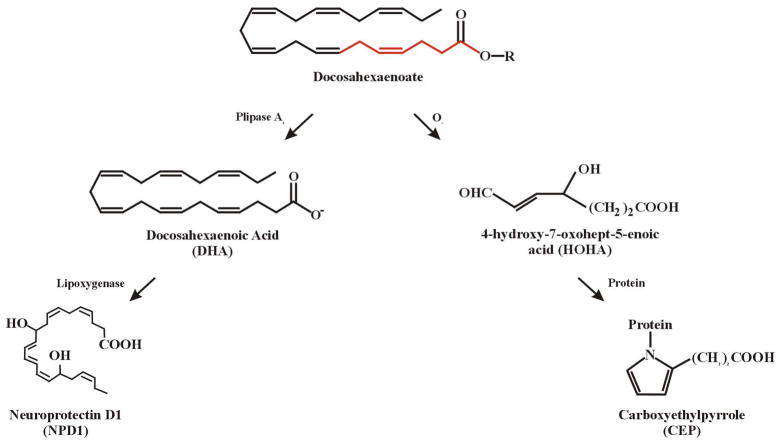
Rats fed diets containing high levels of DHA acquire increased amounts of DHA in ROS membranes, but no apparent increase in peroxidation in vivo (Wang and Saito, 2001). Neuro-2A cells, derived from mouse neuroblastoma, incubated with DHA incorporated the fatty acid into phosphatidyserine, steadily increasing its levels, while apoptotic cell death was prevented (Kim et al., 2000). In mouse brain, free DHA can bind with an RXR (de Urquiza et al., 2000), suggesting an ability to affect gene transcription and cellular signaling in much the same way as do retinoic acids. In ARPE-19 cells, Bazan and associates (Mukherjee et al., 2004) showed that esterified DHA can be converted to a protective docosanoid, called neuroprotectin D-1 (NPD-1), by a combination of phospholipase A2 and 15-lipoxygenase-like enzyme activity (see Figure 4). NPD-1 reduces oxidative stress induced apoptosis in RPE and brain tissue by upregulating the bcl-2 family of survival proteins while downregulating bax and other pro-apoptotic proteins (Bazan, 2005). NPD-1 is produced primarily in the RPE and provides neuroprotection in the retina largely by preventing RPE cell loss. Taken together, these studies indicate that dietary fatty acids can have an impact on light-induced retinal degeneration through mechanisms that involve polyunsaturated fatty acid metabolism.
Figure 4.
Enzymatic and oxidative mechanisms of docosahexaenoate metabolism and degradation. The esterified form of DHA (docosahexaenoate) is converted to free DHA by phospholipase activity. A lipoxygenase then forms picomolar levels of neuroprotectin D-1 (NPD1), primarily in RPE cells. Molecular oxygen reacts with docosahexaenoate to form a 7 carbon reactive aldehyde intermediate (HOHA) that is capable of forming carboxyethylpyrrole (CEP) protein adducts. The first 7 carbons in docosahexaenoate (shown in red) give rise to HOHA; R= glycerophospholpid esterified with DHA.
3.4.2 Unsaturated Fatty Acids and DHA Oxidation
In the retina, polyunsaturated fatty acids are also susceptible to oxidation by molecular oxygen (Remé et al., 1993), UV light (Wang and Saito, 2001) and visible light exposure (Wiegand et al., 1983; Richards et al., 2006). In the case of n-6 and n-3 unsaturated fatty acids, two products of light-induced lipid oxidation are hydroxnonenal (HNE) and hydroxyhexenal (HHE), respectively, which can form covalent adducts with retinal proteins (Tanito et al., 2002; ibid 2005b; ibid 2007). Glycolytic enzymes in the retina appear to be particularly susceptible to HNE modification (Kapphahn et al., 2006) and HHE has been found in ROS of light exposed rats (Tanito et al., 2005a). Recently, Sun et al. (2006) characterized light-induced lipid hydroperoxides formed in vivo in rat retina from n-3 polyunsaturated fatty acids. Several of these peroxide intermediates were shown to affect phagocytosis of ROS fragments by RPE cells (Sun et al., 2006). One intermediate, 4-hydroxy-7-oxohept-5 enoic acid (HOHA; Figure 4) is a specific oxidation fragment of DHA that reacts with protein amino groups to form carboxyethylpyrrole (CEP) protein adducts (Crabb et al., 2002; Gu et al., 2003). Using 2D gel-MS analysis of CEP proteins in light exposed rat retinas, Crabb found several glycolytic enzymes and structural proteins that were adducted with CEP (unpublished). In preliminary studies, we found that CEP-protein adducts were present in rat ROS at all times of the day and night as well as during intense light exposure (Organisciak et al., 2008).
In humans, elevated levels of CEP adducts, and other oxidative protein modifications, were found in drusen from AMD patient eyes compared to eyes from age-matched controls without disease (Crabb et al., 2002). Unknown at this time is the extent of CEP adduction in each protein and the effects of CEP modification on enzyme activity or protein function. The origin(s) of CEP adducts in the RPE also remains an open question, as both ROS and choroidal blood are possible sources. Elevated CEP immunoreactivity and anti-CEP autoantibody levels were found in serum from AMD patients (Gu et al., 2003). Likewise, CEP immunostaining is present in photoreceptors and RPE from Balb/c mice (Gu et al., 2003). Furthermore, although DHA bound to human serum albumin imparted protection against neuronal ischemia (Belayev et al., 2005), immunization of mice with CEP bound to mouse albumin produced an RPE pathology similar to that seen in AMD (Hollyfield et al., 2008). In an ongoing AREDS clinical trial, dietary polyunsaturated fatty acids are now being tested for their potential benefit in AMD patients. It seems reasonable to expect that DHA oxidation and perhaps CEP auto-antibody formation will also occur. Possibly, the cellular, or subcellular, locations of DHA and its metabolites will be important in determining whether beneficial or detrimental effects will be seen.
4. Animal Models and Retinal Light Damage
4.1 Genetic Modifiers
The advent of transgenic animals and gene knockout models has provided new opportunities to examine retinal degenerations based on specific amino acid mutations or the absence of specific proteins. Using these models, both enhanced light damage and protection against light damage have been found. Hao et al. (2002) tested transducin and s-antigen null mice in a light damage paradigm of high intensity and low intensity light. They found visual cell death depended on visual transduction at lower light levels, but was independent of transducin activity at high light levels. As mentioned earlier, arrestin KO mice are highly susceptible to light damage (Chen et al., 1999). In contrast, c-fos KO mice are protected against light-induced retinal cell apoptosis (Hafezi et al., 1997b). Yet the deletion of c-fos did not protect rd mice from retinal degeneration (Hafezi et al., 1998), nor did it delay cell death in rhodopsin null mice (Hobson et al., 2001). This suggests that redundant apoptotic pathways exist, and that they are differentially expressed in genetic animal models and environmentally induced retinal degenerations. Over-expression of the anti-apoptotic protein bcl-2 effectively reduced retinal cell death in the rd mouse and in an opsin mutant (334ter) mouse (Chen et al., 1996). Bcl-2 over-expression also resulted in decreased photoreceptor loss following prolonged light exposure (Chen et al., 1996), but this may have been a result of shortened ROS. Joseph and Li (1996) reported light damage protection in bcl-2 transgenic mice, but they attributed the effect to lower rhodopsin levels than are found in wild type animals.
The relationship between an RPE-65 polymorphism and light damage susceptibility has complicated some genetic studies, but also helped to clarify the role of rhodopsin in light damage. Using quantitative trait loci in several different mouse strains, Danciger et al. (2000) found a good correlation between RPE-65 with methionine 450 and light damage protection versus RPE-65 leucine 450 and enhanced light damage susceptibility. They proposed that methionine oxidation in RPE-65 slows rhodopsin regeneration, thereby reducing the extent of light damage. Subsequently, RPE-65 activity was confirmed to be the rate limiting step in rhodopsin regeneration in mice (Grimm et al., 2000c; Wenzel et al., 2003). More recently, a light sensitive mouse strain (NZW/LacJ) was found to have the protective RPE-65 met450 variant (Danciger et al., 2005), indicating that other light damage genes exist (Danciger et al., 2000; ibid 2005). Frequently, rhodopsin mutations heighten susceptibility to light, be it from chronic dim cyclic light exposure (Naash et al., 1996), or from acute intense light (Wang et al., 1997). Recently, White et al. (2007) found that mice with a T17M rhodopsin mutation, which affects glycosylation, exhibited retinal damage after only a few minutes of intense light treatment. A rhodopsin glycosylation defect in dogs (T4R) also leads to extreme light sensitivity (Gu et al., 2009). The amino terminal region of rhodopsin contains its glycosylation sites and is found on the intradiskal side of ROS membranes. The release of trans-retinal from bleached rhodopsin also appears to be intradiskal, raising the possibility that these light sensitive animal models might exhibit abnormal retinaldehyde metabolism.
4.2 Cone Cell Dominant Retinas
Rhodopsin’s absorption spectrum and the role of phototransduction are central to understanding lightinduced damage in rod photoreceptors. Our understanding of cone phototransduction (Fu and Yau 2008), of cone genes (Corbo et al. 2007), of cone cell biology (Jacobson et al., 2007), and of the diurnal nature of most cone dominant retinas still lags behind our understanding of rods (Mata et al., 2002; Kefalov et al., 2005; Mata et al., 2005; Muniz et al., 2007; reviewed in Mustafi et al., 2009). Discerning pathways that affect cone survival after light insult will be complicated, yet our human reliance on cone-based vision places a compelling focus on cones. How does light damage manifest in cone photoreceptors, and why are cones remarkably resistant to light damage compared to rods?
This issue was addressed early on, when Cicerone (1976) and LaVail (1976a) each noted cone survival in light-damaged albino rat retina, suggesting that cone cells are more resilient than rods in the face of light insult. However, many studies of mouse or rat retina since then have recorded cone death which, depending on light exposure conditions and phenotype, can occur within days (e.g. Krebs et al. 2009) or may be markedly delayed (Tanito et al., 2007a). The consensus is that cone death is secondary to loss of the far more numerous rods, in other words a bystander effect (Chrystostomou et al., 2008, ibid. 2009, Krebs et al., 2009). Rod cell loss could deprive cones of a rod-derived survival factor (Lorentz et al. 2006, Yang et al., 2009) and possibly induce an excess of outer retinal oxygen (Stone et al., 1999) that may be toxic to cones. Progressively impaired choroidal function, likely due to massive neuronal and glial remodeling (Marc et al., 2008), has a time course consistent with the gradual starvation of cones that initially survive light insult (Tanito et al., 2007). These proposed causes of secondary cone cell death in rod-dominant nocturnal rodent retina are not mutually exclusive.
Ripps (2002) has proposed an interesting variant of the bystander effect in which gap junction channels are the route by which a toxic product originating in damaged rods is passed directly to the cytoplasm of otherwise healthy neighboring cones. A gap junction-dependent example of natural cell death has been demonstrated in normal development of the mouse inner retinal layers (Cusato et al., 2003). Rod-cone coupling by gap junctions is also stronger at night than in the daytime (Ribelayga et al., 2008), consistent with potentiated light damage at night, but to our knowledge, there has been no attempt to examine the role of gap junctions in retinal light damage.
Because nocturnal rodent retinas normally have few cones and lack a cone-rich region approximating the human fovea, there have been attempts to enhance the representation of cones in model systems. The 661W cell line isolated from mouse retinal tumors is cone-like (Tan et al., 2004; Al-Ubaidi et al., 2008) and permits in vitro experimental approaches. The 661W cell line has been used recently to model “cone” light damage (Kanan et al., 2007; Yang et al., 2007a), revealing a possible signal pathway between light-damaged “cones” and microglia (Yang et al., 2007b). The Nrl null (Nrl−/−) mouse retina develops a rodless retina enriched in cells that resemble short wavelength absorbing (S-) cones (Mears et al., 2001). Unusual features, such as the “cone” whorls found in the Nrl−/− retina (Mears et al., 2001, Dang et al., 2004), support the notion that Nrl−/− retina may contain “hybrid” photoreceptors (Mustafi et al., 2009). A preliminary study (Glösmann and Peichl, 2007) has showed that S-cones, but not mid wavelength (M-) cones, suffer light damage in albino rats maintained in bright cyclic light (160 lux) for 4 weeks. This finding suggests that S-cone-like photoreceptors in the Nrl−/− mouse retina might be vulnerable to light damage, but this remains to be tested.
Neither of these cone-enhanced systems, nor the rodent retinas from which they were derived, bear much structural resemblance to human central retina in terms of cone numbers or distributions. For example, human retina is estimated to contain 5–6% cones, concentrated in the macular region, while in nocturnal rodents the 1–3% cone population is arrayed essentially uniformly throughout the retina. It has been logically argued that diurnal rodents, including rod- and cone-dominant species, may be more useful models of cone cell biology in health and disease (Table 1). In stark contrast to results from rats and mice, however, the retinas of diurnal rodents have proven markedly difficult to light damage. Collier and his associates used diurnal gray squirrels in light damage studies (Collier et al., 1989). This species has a retina containing 60% cones (Table 1) and has a yellow lens. Only in aphakic animals treated with near-UV wavelength light (366 nm) was photoreceptor light damage achieved. Most recently, David Hicks and associates, at the Cellular and Integrative Neurosciences Institut (CNRS) in Strasbourg, France, have conducted light damage studies on the Nile grass rat. This rodent has 33% cones distributed evenly across its retina (Table 1), making it technically rod-dominant, but it is a diurnal species. Its lens lacks the yellow pigment found in squirrels. Eight hours of 15,000 lux white light in unrestrained animals, or 2 hours of 20,000 lux white light in an anesthetized dilated animal, produced no substantial evidence of light damage; moreover, time of day had no effect. These animals had been reared in cyclic light of a standard rodent colony, including 2 weeks of dim cyclic light (20 lux) and 12 hours in complete darkness prior to intense light exposure. As in the gray squirrel, short wavelength light (410 nm) was required to achieve photoreceptor degeneration in the Nile grass rat, but this also resulted in global retinal destruction quite distinct from the progression of light damage described for rod-dominant rodent models (Boudard and Hicks, manuscript in preparation).
Table 1.
Summary comparison of cone complement of rodent retinas; shaded entries are nocturnal, all others are diurnal (listed in order of increasing approximate cone proportions).
| Common Name | Scientific Name | % Cones | % L/Ma Cones | % Sb Cones | References |
|---|---|---|---|---|---|
| Rat | Rattus norvegicus | 1% | 90% | 10% | Szél and Röhlich 1992 |
| Mouse | Mus musculus | 3% | * | * | Carter-Dawson and LaVail 1979 |
| Agouti | Dasyprocta aguti (also D. leporina) | 8% | 90% | 10% | Rocha et al, 2009 |
| Degu | Octodon degus | 33% | 93% | 7% | Jacobs et al. 2003 |
| Nile grass rat | Arvicanthis ansorgei | 33% | 93% | 7% | Bobu et al. 2006 |
| African grass rat | Arvicanthis niloticus | Gaillard et al. 2008 | |||
| Gray squirrel | Sciurus carolinensis | 60% |
West and Dowling 1975 Blakeslee et al. 1988 |
||
| California ground squirrel | Spermophilus beecheyi | 85% | 93% | 7% |
Long and Fisher 1983 Kryger et al. 1998 |
L/M cones: long (red) or middle (green) wavelength cones.
S cones: short (blue) wavelength cones.
Categorization of adult mouse cones is complicated by opsin coexpression (reviewed in Lukáts et al. 2005).
4.2.1 Primates and Light Damage
Diurnal vs. nocturnal differences in light damage susceptibility are not unique to rodents; such a distinction is also found in primates. Photoreceptor damage from intense visible light has been studied in the nocturnal owl monkey (Fullmer et al., 1978) and in diurnal macaques (Sykes et al., 1978; Tso, 1987). Both are rod-dominant, as are primates typically, although the owl monkey is relatively rod-enriched (Finlay et al., 2008). Both possess a fovea, but only in macaques is there a pure-cone foveola: in the owl monkey fovea, rods still outnumber cones by 14 to 1 (Wikler and Rakic, 1990). Macaques possess M- and S-cones but functional S-cones are absent from the owl monkey retina (Jacobs et al., 1996). Similar white light exposure (ca. 0.2 W/cm2 for 30 minutes) was catastrophic in the nocturnal owl monkey retina (Fuller et al., 1978), but caused negligible photoreceptor damage in the diurnal macaque retina (Tso, 1987). Earlier, Sykes et al. (1981) also studied white light damage in macaque retina, but used longer exposures (12 hr) at an intensity far lower (0.2–0.8 mW/cm2) than that used by Tso (1987). Sykes et al. (1981) were able to distinguish the threshold intensities required for detectable light damage in rods vs. cones, limited to outer segment disruption. Significantly, cone OS exhibited a lower threshold than ROS, opposite what is found in nocturnal rodents and raising questions about the rod-to-cone bystander effect hypothesis with regard to the diurnal primate retina. At intensities high enough to damage both ROS and COS, these authors also found that COS damage occurred at the base whereas ROS damage occurred at the distal tip. The significance of this observation is unclear.
A consistent finding in both rodents and primates is the vulnerability difference between diurnal and nocturnal retinas. The relative resistance of diurnal retinas is likely due to multiple factors that may either operate less robustly in nocturnal retinas, or may be absent from them altogether. An elegant comparative study suggests one feature that could contribute resistance to the rods of diurnal retinas, including the rod-dominant retinas of diurnal primates. Solovei and colleagues (2009) have reported a division in the architecture of rod nuclear chromatin between nocturnal and diurnal mammals. With a computer model, they suggest that rod nuclei in nocturnal species function as collecting lenses, helping to increase photon capture per rod cell compared to that of the rods of diurnal species. Thus, the ROS of nocturnal animals may simply collect more light energy from incident radiation. By having a lower quantum catch, diurnal rods would be less vulnerable to damage and less able to exert a bystander effect on cones, whereas the capture of photons in nocturnal rods would enhance the likelihood of rod and cone damage.
Comparative studies also show that some diurnal retinas are more resistant to light damage than others. Use of very intense monochromatic light exposure on the macaque retina results in partially selective ablation of cone types: blue light irreversibly damages S-cones; green light damages M-cones, which recover about a week later; and red light has no effect (Sperling 1986). Gerald Jacobs and associates at the University of California Santa Barbara used this same approach in the 1970s with California ground squirrel, a strictly diurnal rodent with 85% cones in its retina and a pure-cone central region (Table 1). Even a full day’s exposure to intense monochromatic light failed to produce any cone cell damage in the eyes of anesthetized ground squirrels with dilated pupils (Gerald H. Jacobs, personal communication). These wild-caught animals were reared in ambient southern California conditions, and then when captured maintained under standard cyclic animal room lighting. While it’s tempting to speculate that cone dominance is responsible for the ground squirrel’s resistance, crucially the rod-dominant Nile grass rat appears equally resistant. There are undoubtedly other yet-to-be-discovered protective factors at play in the diurnal rodent retina.
5. Protection Against Retinal Light Damage
5.1 Antioxidants and Ocular Drug Delivery
Natural and synthetic antioxidants prevent retinal light damage and photoreceptor cell loss. This includes the natural L-stereoisomer of ascorbic acid (Organisciak et al., 1985; Li et al., 1985) as well as its D-stereoisomer, which is an antioxidant but not a cofactor for enzyme-mediated hydroxylation (Organisciak et al., 1989b; ibid 1992). The L-stereoisomer of N-acetyl-cysteine (Tanito et al., 2002; Busch et al., 1999) and N-nitro-arginine methyl ester (Goureau et al., 1993; Donovan et al., 2001; Kaldi et al., 2003) also effectively reduce light damage, but their D-stereoisomers are ineffective. Natural substances such as ginkgo biloba extract (Ranchon et al., 1999) probably function directly as antioxidants during light exposure, while others, including saffron (Maccarone et al., 2008) and sulforaphane (Tanito et al., 2005a) induce the synthesis of antioxidative enzymes. Synthetic antioxidants that have also proven effective include WR-77913, a radioprotective dye that quenches singlet oxygen (Remé et al., 1991); the free radical spin trap phenyl-N-tert-butylnitrone (PBN) (Ranchon et al., 2001; Tomita et al., 2005, Tanito et al., 2005b); OT-551, a TEMPOL derivative that catalyzes the degradation of superoxide (Tanito et al., 2007b); and dimethylthiourea (DMTU), a quencher of H2O2 and hydroxyl radicals (Lam et al., 1989; Organisciak et al., 1992; Ranchon et al., 1999; Vaughan et al., 2006). Based on the specificities of antioxidants for different forms of reactive oxygen, it is tempting to implicate particular oxygen radicals in the mechanism of light damage. However, no antioxidant exhibits complete fidelity with a single species of reactive oxygen, making that primarily speculation. Another problem with inferring mechanism is the lack of evidence, in all cases, that an antioxidant actually passed the blood-retinal barrier and was taken up by the tissue. Finally, several different forms of reactive oxygen are probably involved in the damage process, albeit at different times. As an example, macrophages invade damaged tissues and release several different types of reactive oxygen, but their appearance in retina during light damage is relatively late. Still, the effectiveness of a large number of antioxidants is compelling evidence that oxidative stress is an integral part of the light damage process.
Oxidative stress also appears to be an early event in the retinal light damage process. Demontis et al. (2002) detected an increase in light-induced oxidation in isolated rod cells within minutes of light onset. Changes in fluorescence detectable oxidation in the inner and outer segments were attributed to retinaldehyde photoisomerization and mitochondrial metabolism, respectively. In cultured rod cells, Yang et al. (2003) found changes in fluorescence in the mitochondria-rich inner segment ellipsoids, which were induced by blue light and quenched by antioxidants. The rapid appearance of oxidative stress in isolated photoreceptors does not appear to be an in vitro artifact. Retinal ganglion cells in culture also exhibit mitochondrial mediated oxidation and cellular apoptosis, but this requires 2–3 days of intense light (Osborne et al., 2008). In photoreceptor cell inner segments, superoxide dismutase (SOD) and catalase normally reduce the effects of superoxide and H2O2 generated by mitochondrial metabolism (Rao et al., 1985; Atalla et al., 1987). Mittag et al. (1999) found that transgenic mice with a mutated cytoplasmic form of SOD incurred greater retinal light damage than did non-transgenic animals with normal SOD. In the nucleus, immunoreactive HNE and HHE adducts were present 3 hours after intense light exposure and prior to the appearance of TUNEL staining in the ONL (Tanito et al., 2005a). The rapid induction of oxidative stress from intense light may help explain why antioxidants are most effective in vivo if given prior to light treatment. Figure 5 illustrates rhodopsin recovery and retinal morphology in light exposed rats given a single dose of the synthetic antioxidant DMTU. There was almost complete protection when DMTU was administered 30 minutes before the start of light, but the antioxidant was ineffective when given 15–60 minutes after lights on. This early onset of light-induced oxidative damage implicates the initial rate of rhodopsin bleaching in the damage mechanism.
Figure 5.
Retinal morphology and rhodopsin recovery in rats exposed to light. Dark adapted rats were treated with DMTU (1X IP; 500mg/kg) 30 minutes before the start of light, or at various times thereafter. Light exposures (490–580 nm; ~1200lux) began at 1 am and lasted for 8 hours. The animals were then allowed to recover for 2 weeks in darkness before retinal histology or rhodopsin measurements were made. Retinal histology from the superior hemisphere of rats treated 30 minutes before light (A), 60 minutes after the onset of light (B), or given the saline vehicle (C). Arrow heads denote cone nuclei in the ONL (A) or remnant ONL layer after light damage (B, C). Morphometric measurements of ONL thickness, along the vertical meridian, for rats treated as above (D). Average whole eye rhodopsin levels measured in rats (n=4–5) treated with DMTU at various times before or after light exposure (E). The unexposed controls (open square) and light exposed controls (open triangle) were given an equal volume of saline 30 minutes before exposure. (Adapted from Vaughan et al., Photochem Photobiol. 75: 547–53, 2002 and Organisciak et al., Invest Ophthalmol Vis Sci. 41: 3694–3701, 2000).
5.1.1 Retinal Protein Expression and Protection
Photoreceptor cell transcription and protein markers of oxidative stress are also altered by intense light. Retinas from light-exposed rats show an increase in heme oxygenase -1 (HO-1) mRNA and protein levels (Kutty et al., 1995). HO-1 is a 32 kDa inducible stress protein, which converts the pro-oxidant heme to biliverdin, ultimately forming the antioxidant bilirubin (Frei et al., 1989). Bilirubin is oxidatively converted back into biliverdin, which can then be reconverted to bilirubin by biliverdin reductase. Although it is normally found in low concentrations in cells, bilirubin effectively reduces membrane lipid oxidation by this enzymatic recycling process (Sedlak et al., 2009). Induction of HO-1 during light-induced stress, therefore, is a self protective response resulting from a higher level of bilirubin in the retina. The fact that antioxidant pretreatment of rats prevents HO-1 induction during intense light exposure (Kutty et al., 1995) supports the idea that light results in an oxidative stress in the retina.
At the same time, there is a light-induced reduction in mRNA levels for interphotoreceptor cell retinol binding protein (IRBP) (Organisciak et al., 2000). IRBP is constitutively expressed in photoreceptors, so a decrease in its mRNA means that visual cell transcription is affected by intense light. Decreases in the expression of several other visual cell proteins have also been found in arrestin-KO and rhodopsin kinase-KO mice following light exposure (Choi et al., 2001). At the protein level, intense light reduces the activity of RDH, an oxidatively sensitive ROS enzyme, while DMTU prevents that reduction (Darrow et al., 1997). Tomita et al. (2005) found a light dependent increase in the expression of mRNA for caspase-3, which was inhibited by an antioxidant (PBN), but saw no increase in enzyme activity. One possible explanation for the lack of caspase activity is covalent modification by light-induced S-nitrosylation of cysteine residues (Donovan et al., 2001). Another light-induced covalent modification is nitrotyrosine formation. Using 2D gel electrophoresis coupled with MS and nitrotyrosine immunoreactivity, Miyagi et al. (2002) identified the mitochondrial form of glutamate dehydrogenase as being NO modified in both photoreceptors and RPE. Inhibition of NOS prevents retinal light damage in mice and rats (Donovan et al., 2001; Kaldi et al., 2003), suggesting that NO formation is also part of the damage mechanism.
Although rapid changes in the formation of reactive oxygen(s) and/or mitochondrial metabolism can occur during light exposure, longer-term adaptive changes in retinal protein expression also happen. For example, glutathione peroxidase (GPX) is induced in photoreceptors following photic injury (Ohira et al., 2003) or by rearing rats in bright light environments (Penn and Anderson, 1992). In mouse retina, the synthesis of thioredoxin, a 13 kDa protein containing reactive cysteines, is increased by intense light treatment (Tanito et al., 2002). Gosbell et al. (2006) reported that the antioxidative enzyme GPX-1 was also up-regulated in retina by intense light. Unexpectedly, however, GPX-1 null mice incurred less photoreceptor cell damage than did wild type animals. Although this was attributed to a compensatory up-regulation of other antioxidative enzymes, GPX-1 deficient mice also have shorter ROS than normal and diminished ERGs (Gosbell et al., 2006), which could contribute to their relative lack of light damage. Enzymes that utilize GSH appear to be abundant in the inner retinal layers, but relatively deficient in photoreceptor ROS (Atalla et al., 1998; Organisciak et al., 1998) or only transiently induced by light (Ohira et al., 2003). In fact, based on the immuohistological localization of GSH in the retina, it has been proposed that the tripeptide is practically absent from rod and cone photoreceptors (Winkler 2008). If true, this could explain why rod cells are highly vulnerable to light-induced oxidative damage while the inner retina is remarkably resistant. Winkler (2008) also proposes that the approximate 10 day turnover time for newly formed ROS disks is a substitute for the relative lack of antioxidant. However, because GSH also appears to be absent from cones and they are more resistant to light damage, the possibility exists that there are distinctly different light damage mechanisms in rods and cones.
5.2 Neurotrophic Factors and Endogenous Retinal Mechanisms
A wide variety of neuroprotective factors reduce or prevent retinal light damage (LaVail et al., 1992; ibid 1998) and at least some of these are up-regulated by local stress or injury (Faktorovich et al., 1992; Cao et al., 2001). Perhaps the best studied neuroprotective protein is bFGF. Preconditioning rats with short term bright light exposure (Liu et al., 1998; Li et al., 2003), prior optic nerve injury (Bush and Williams, 1991), ischemic preconditioning (Casson et al., 2003) and drugs that prevent retinal ischemia (Agarwal et al., 2002), all reduce the extent of retinal light damage while increasing bFGF expression, or its re-localization (Kostyk et al., 1994). As shown by Stone et al. (1999) and Walsh et al. (2001), the highest regional levels of immunoreactive bFGF in the rat retina correlate with those areas incurring the least amount of light damage. Surprisingly, however, intravitreal injection of bFGF in rats affected the inner retinal layers, by causing a reduction in the ERG b-wave, not the photoreceptor specific a-wave (Stone et al., 1999). Exogenously applied bFGF also fails to prevent photoreceptor cell light damage in mice (LaVail et al., 1998). The reasons for this inefficacy are unknown, but differences in the binding of bFGF to receptors and a reduced response to local injury have been suggested (LaVail et al., 1998). Other factors, including granulocyte colony-stimulating factor (Oishi et al., 2008) and hypoxia-induced erythropoietin (Grimm et al., 2002), are neuroprotective in mice. In the rat light damage model, intravitreal injection of factors derived from RPE (Cao et al., 2001) or lens epithelium (Machida et al., 2001) are effective. Irrespective of these species differences, bFGF and ciliary neurotrophic factor (CNTF) synthesis occurs during light exposure (Gao and Hollyfield, 1996; Walsh et al., 2001; Li et al., 2003) or after drug treatment (Agarwal et al., 2002) and reaches a maximum 2–4 days after light onset or local injury (Wen et al., 1997; Cao et al., 2001). Thus, to achieve neuroprotection from growth factors during acute light exposure, pretreatment would appear to be necessary, much the same as for antioxidants. At the same time, growth factors can also trigger receptor mediated signaling pathways that impart long term protection.
5.2.1 bFGF Receptors and Pathways
In isolated developing photoreceptors, bFGF prolongs cell survival (Fontaine et al., 1998), probably through a tyrosine kinase receptor (Gospodarwicz et al., 1987). In retinal Müller cells, tyrosine kinase receptors (Tkr) are thought to be pro-survival, while the neurotrophin receptor p75 is thought to promote cell death. Blocking the Müller cell p75 receptor provides protection against photoreceptor light damage and stimulates the production of bFGF, while Tkr inhibition decreases bFGF levels and enhances light damage (Harada et al., 2000). However, p75 receptor null mice were not protected against light damage, suggesting an alternative apoptotic pathway (Roher et al., 2003). Exogenous bFGF applied to Müller cells induces c-fos and c-jun expression, perhaps by activating protein kinase C, and increases the denovo synthesis of bFGF (Cao et al., 1998). Further support for a bFGF receptor-mediated protective effect is provided by Campochiaro et al. (1996) who reported age-related photoreceptor cell degeneration in transgenic mice lacking functional FGF receptors. Midkine, a neurotrophic retinoic acid-responsive gene product, provides almost as much protection against light damage as does bFGF (Unoki et al., 1994). It is also of interest that bFGF, midkine and retinoic acid are all formed in the retina and all affect retinal development (Unoki et al., 1994; Zhao and Barnstable, 1996; Stone et al., 1999). Thus, multiple factors elicit short term photoreceptor protection; and multiple long term neuroprotective mechanisms, some of which involve glial cells, can foster protection against environmental light stress or trauma.
5.2.2 Circadian Effects
While neuroprotection is often achieved by exogenous agents or treatments, the retina has also evolved the ability to protect itself with endogenous factors that are expressed in a circadian fashion. A diurnal response to intense light was first reported by Duncan and O’Steen (1985), a finding subsequently confirmed by others (White and Fisher, 1987; Wiechmann and O’Steen, 1992; Bush et al., 1998; Organisciak et al., 2000). These studies all point to specific times of the day when protection against retinal light damage is greatest and other times when light damage is enhanced. Wiechmann and O’Steen (1992) found that repeated injections of melatonin, which is normally secreted at night, led to an increase in retinal light damage. Bush and associates (1998) blocked the melatonin receptor with the antagonist luzindol and found protection against light damage. Thus, the evidence indicates that there is a melatonin dependent propensity for retinal light damage, which is receptor mediated and which correlates with time of day. However, the identities of specific proteins or pathways which either enhance or protect against light damage, and the timing of their expression, are largely unknown.
The synthesis of a number of photoreceptor cell visual transduction proteins occurs in a circadian fashion (Brann and Cohen, 1987; Bowes et al., 1988; Korenbrot and Fernald, 1989; McGinnis et al., 1992; Wiechmann and Sinacola, 1997). Also, RPE phagocytosis of the tips of shed ROS, and the expression of retinal c-fos and c-jun, are light entrained (LaVail, 1976b; Goldman et al., 1980; Yoshida et al., 1993; Imaki et al., 1995). In the ONL, c-fos is normally elevated at night, while its expression in the inner retina, particularly in ganglion cells, is transiently enhanced at light onset (Yoshida et al., 1993). In rats, RPE-65 mRNA levels are also highest at the beginning of the day, but RPE-65 protein levels do not correlate with circadian dependent light damage susceptibility (Beatrice et al., 2003). Other evidence indicates that melanopsin, a protein located within a subset of ganglion cells, serves as a retinal circadian irradiance detector (Provencio et al., 2000; Hattar et al., 2002; Berson et al., 2002). Potential circadian phase resetting cryptochromes are also found in the inner retinal layers (Yagita et al., 2001). Although melanopsin and cryptochromes are both photoreceptive proteins, and even though both may trigger light-induced signals in the retina, the inner nuclear layer (INL) is not usually damaged by visible light. This might be related to inefficient light absorption by these photoreceptive proteins, or to high GSH levels in the INL which serves to block oxidative damage (Winkler 2008).
Despite the relative resistance of the inner retina to light damage, rod photoreceptors are vulnerable to light damage at night while being well protected during the day. In an attempt to understand how changes in retinal gene expression and circadian light damage susceptibility might be related, we performed gene array analysis and confirmed some results by real time PCR. Figure 6 contains rt-PCR mRNA expression profiles for several retinal transcription factors and protein markers of oxidative stress. Rat retinas were collected at 9 am and 5 pm and compared with those obtained at 1 am, the time point of maximum light damage susceptibility (Organisciak et al., 2000). In totally dark adapted rats, the expression of retinal c-fos, c-jun, fra-1, GFAP, HO-1 and macrophage chemoattractant protein-1 (MCP-1) increased 1–4 fold between 1 am and 9 am (Figure 6A). Other rats were treated with intense light for 8 hours, starting at 1 am, to determine the time course of light-induced changes in transcription factors (Figure 6B). Light exposure led to increases in mRNA expression for several transcription factors, and most exceeded the increases found in retinas from dark adapted rats. When rats were given the antioxidant DMTU before light treatment, c-fos and fra-1 mRNA levels decreased 7–10 fold, suggesting that their expression was originally triggered by light-induced oxidative stress. At the same time, the expression of c-jun, NFkB and Atf3, an endoplasmic reticulum factor, were largely unaffected by light.
Figure 6.
Real time, RT-PCR analysis of mRNA levels in rat retinas at different times of the day and night. Rats were dark adapted for 16 hours before sacrifice at 1 am, 9 am, or 5 pm (panel A). Retinas were excised and RNA extracted with a Direct mRNA Kit (Qiagen# 72022) from 9 individual retinas from 9 different animals. Some rats were treated with intense light (1200 lux, 490–580 nm) starting at 1 am (panel B), or starting at different times of the day (panel C). The antioxidant DMTU (500mg/Kg), or saline, was given IP 1 hour before light exposure. Average mRNA values are shown for rats exposed to light from 1 am to 9 am (1–9), 9 am to 5 pm (9-5), or 5 pm to 1 am (5-1). Data from animals pretreated with DMTU are represented by closed triangles. (Adapted from Barsalou et al., Assn Res Vis Ophthalmol., 2004).
As shown by the mRNA expression patterns for protein markers of oxidative stress, the timing of light treatment also affects their synthesis (Figure 6C). For rats treated with 8 hours of light starting at 1 am, the increases in retinal GFAP and MCP-1 expression were significantly greater than for other rats treated with light starting at 9 am or 5 pm. The large increase in expression for MCP-1 between 1 am and 9 am, suggests that a strong signal for macrophage invasion was generated by retinal damage. Antioxidant pretreatment resulted in a dramatic reduction in mRNA levels for MCP-1 and for the Muller cell stress protein GFAP, indicating that their expression was driven by oxidative stress. For HO-1, the light-induced increase in expression was about 10 fold for exposures starting at 1 am and 9 am. These increases were greater than for rats treated with light at 5 pm, the time point when light damage is minimal (Organisciak et al., 2000). This indicates that the effect of oxidative stress in the retina is far greater for light exposures beginning at 1 am than for light treatments at other times during the day. Our findings also confirm some of the changes found in retinal gene array studies using bright light treated albino mice (Chen et al., 2004; Huang et al., 2005) and further suggest that time of day can affect the outcomes of both gene array and PCR measurements.
The synthesis of corticosterone, the rodent equivalent of human cortisol, also occurs on a 24 hour circadian cycle, and an increase in its synthesis is part of the normal stress response. In rats, circulating corticosterone levels were found to vary with time of day, but they did not correlate with the circadian profile of retinal light damage (Vaughan et al., 2002). In mice, Wenzel et al. (2001) found that the stress of overnight fasting induced corticosterone synthesis and glucocorticoid receptor translocation. Both stress induced corticosterone and dexamethasone, a synthetic glucocorticoid, protected against light damage and both inhibited the expression of activator protein-1 (AP-1) (Wenzel et al., 2001b). However, in the T4R rhodopsin mutant dog, brief light exposure resulted in extensive photoreceptor cell damage that was not inhibited by corticoids, including dexamethasone, even though AP-1 and c-fos activation were inhibited (Gu et al., 2009). Heterodimeric complexes of c- jun and c-fos, or fra-1 (Wenzel et al., 2002), form the transcription factor AP-1 and may promote cell death in light exposed mice (Hafazi et al., 1997b). In the dog model this did not happen, providing yet another example of species differences that can involve divergent pathways of light-induced retinal cell death.
6. The Emerging Picture and Future Directions
The effects of intense or prolonged light exposure on the retina vary by species, region of the retina, time of day, diet, prior light rearing history and genetic background. From the perspective of sorting out mechanisms, each variable has proven useful. While the use of different animal models has provided insights into understanding light damage, the data also indicate that damage or cell death mechanisms are not easily extrapolated between species. The notion that “the mouse is not a small rat” was recognized by LaVail in one of the first comprehensive studies relating strain and species differences with the extent of retinal light damage (LaVail et al., 1987). We now know that the L450M amino acid variation in RPE-65 correlates with resistance to light damage in many strains of mice (Danciger et al., 2000). The oxidation of methionine 450 in RPE-65 to methionine sulfoxide is probably beneficial, because it leads to reduced enzyme levels, slower rhodopsin regeneration, and light damage protection (Danciger et al., 2000; Wenzel et al., 2001a). Although the regeneration of rhodopsin correlates with light damage susceptibility in some mice, RPE-65 activity is not the rate limiting step in regenerating rhodopsin in the rat (Iseli et al., 2002). Photobleaching of rhodopsin - and quite possibly the initial rate of bleaching- is a key factor for light damage in rats. The same may hold for exceedingly light sensitive dogs and mice with glycosylation defects in the amino terminal region of rhodopsin (Gu et al., 2009; White et al., 2007). Whatever rhodopsin’s exact role is in light damage, photoreceptors become hyperpolarized by light and remain that way for the duration of exposure. As pointed out by Sieving et al. (2001), prolonged hyperpolarization of rod photoreceptors is insufficient to cause retinal light damage, but light-induced changes in intracellular ions is still an area amenable to study in animal models.
There is also a need for additional comparative light damage studies in a variety of animal models. For example, a comparative proteomics analysis subsequent to a standard light insult of macaque and owl monkey retina might be very informative. The macaque shares its diurnality with the human, and the owl monkey is only recently evolved from a diurnal ancestor (Dyer et al., 2009), so we might thereby gain insights about possible damage, repair, or cell death mechanisms shared (or not) by traditional rodent models and humans. Diurnal rodent studies are more feasible than primate studies and offer a complementary approach to the existing literature, which has focused on discerning damage pathways in vulnerable nocturnal rodents so that protective therapies can be designed. Studies of damage-resistant rodents would provide a useful counterpoint for identifying naturally evolved protective or repair mechanisms that appear to confer even greater bright light tolerance than is already intrinsic to the foveate primate. The diurnal ground squirrel model has an additional feature that must be borne in mind, which is its hibernation physiology. The emerging consensus is that animals that hibernate exhibit neuroprotection from a variety of insults, but our understanding of hibernation’s protective mechanisms is too incomplete to say whether they do, or do not, contribute to light damage resistance. Hibernators spend months in continuous darkness, during which time the metabolic activity of the predominant cones is profoundly suppressed, whereas rods seem to retain mitochondrial activity (Figure 7). Dark-rearing potentiates light damage in many nocturnal rodents, but it is unknown whether hibernation does so in the ground squirrel. Finally, diurnal rodent retinas provide a means for exploring various environmental and hereditary sensitizers to light damage, particularly those that may endanger cones. Only cautious comparisons between species will reduce confusion and advance our understanding of damage mechanisms.
Figure 7.
Confocal microscope image of a labeled vertical section of 13-lined ground squirrel outer retina collected during midwinter torpor. Red, PNA labels the extracellular matrix surrounding cone outer segments (OS). Green, anti-cytochrome oxidase-1 label indicates mitochondrial function. Blue, Anti-rhodopsin labels ROS. Amongst lightly-labeled cone inner segment (IS) ellipsoids, a rod IS ellipsoid retains the robust anti-COX 1 label (arrow) found uniformly in all ellipsoids of retinas collected during summer arousal (data not shown). Magnification bar equal to 20 micrometers. (Adapted from Gruber et al., Assn Res Vis Ophthalmol., 2006).
Compelling evidence exists that intense light leads to oxidative stress in the retina and triggers photoreceptor cell responses involving transcriptional regulation, changes in enzyme activity and the initiation and execution phases of apoptosis by redundant pathways. Antioxidants delivered before light insult, but generally not after, prevent photoreceptor cell damage and the variety of downstream events that follow. The effectiveness of a wide variety of antioxidants indicates that reactive oxygen species are formed during intense light, but whether they are the only trigger for damage still needs to be determined. Also, determining the actual site(s) of antioxidant action is an area in need of further study. In this regard, the intimate metabolic and morphological links between RPE and retinal photoreceptors may be important. For example, antioxidants may enter the RPE more rapidly, and thus react more effectively, than in retina. The expected consequences of this would be a decrease in the formation, and possibly secretion, of reactive oxygen species that could impact photoreceptors. Reactive oxygen is formed during phagocytosis of ROS tips and antioxidants have been shown to inhibit light-induced ROS disk shedding and the appearance of large phagosomes in RPE (Blanks et al., 1992). Given the high oxygen tension and photon flux in the retinal/RPE complex, it is likely that oxidation is ongoing (Atalla et al., 1988), but that under normal circumstances retinal cell death does not occur. Therefore, basal protective mechanisms are probably in place and functional most of the day. However, light exposure that bleaches the vast majority of rhodopsin, especially at night, can overwhelm the photoreceptor’s capacity to prevent damage. If future work confirms that GSH is absent from photoreceptors (Winkler, 2008), this would enhance our understanding of the vulnerability of visual cells to photo-damage and possibly chemical toxicity.
A distinct advantage of the light damage model over animal models expressing hereditary retinal degenerations is the synchronous involvement of practically all photoreceptors in the damage process. This acute form of retinal degeneration provides a good opportunity to “ferret out” the temporal sequence of events leading to cell death. While many of the downstream events in light-induced and hereditary retinal degenerations are similar, significant differences do exist. At this time, the role of AP-1 in promoting retinal cell death seems to be species dependent (Wenzel et al., 2001b; Gu et al., 2009). In another example, antioxidants have not been found to be effective in preventing degeneration in animal models of retinal disease (Kaldi et al., 2003; Ranchon et al., 2003). Neurotrophins seem to be protective in some animal models, but not in others (LaVail et al., 1998) and the order of apoptotic events, including the activation of caspases, are not the same in all species. Also, the apoptotic cell death marker clusterin is expressed in both the light damaged rat and rd mouse retina, but with different kinetics and cellular locations (Wong et al., 1994).
At the same time, synergies can be found between the different types of retinal degenerations. Some null mutants do not exhibit retinal degeneration without the benefit of intense light treatment, while others are exquisitely sensitive to light (LaVail et al., 1999; White et al., 2007; Gu et al., 2009). An important area for future investigation is the synergy between environmental insult and genetic predisposition in retinal degenerations. Along those lines, genomic and proteomic analysis of tissues from different animal models should help uncover the underlying factors that contribute to protection or retinal degeneration. We already have a good start characterizing gene expression profiles in light damage (Choi et al., 2001; Chen et al., 2004; Huang et al., 2005), but detailed proteomic analysis is still needed. While all types of animal models can be criticized for their relevance to human physiology, people with inherited ocular diseases primarily live and work in light environments. The use of intense or prolonged light exposures and genetic animal models can improve our understanding of basic mechanisms in retinal degeneration by exacerbating visual cell death with an acute insult superimposed on a chronic condition. At this time, we need to extrapolate our findings in animal models to human disease, but it may turn out that simple treatments developed to prevent retinal degeneration in animals will form the basis of therapeutic interventions in people.
Acknowledgments
This work was supported by the Ohio Lions Research Foundation, funding from M. Petticrew, Springfield, OH and by NIH grant EY-10959(DTO) and by a Sigma Xi Grant-in-Aid and the UW Oshkosh Collaborative Grant Program (DVK). We thank R. Darrow and L. Barsalou for research assistance, A.R.Gruber for photomicroscopy and Drs. Larry Donoso, Darrell Fleischman and Barry Winkler for their critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. Literature Citations
- Agarwal NJ, Martin E, Krishamoorthy RR, Landers R, Wen R, et al. Levobetaxolol-induced up-regulation of retinal bFGF and CNTF mRNAs and preservation of retinal function against a photic-induced retinopathy. Exp Eye Res. 2002;74:445–53. doi: 10.1006/exer.2001.1145. [DOI] [PubMed] [Google Scholar]
- Al-Ubaidi MR, Matsumoto H, Kurono S, Singh A. Proteomics profiling of the cone photoreceptor cell line, 661W. Adv Exp Med Biol. 2008;613:301–11. doi: 10.1007/978-0-387-74904-4_35. [DOI] [PubMed] [Google Scholar]
- Andre E, Conquest F, Steinmayr M, Stratton C, Porciatti V, et al. Disruption of retinoid-related orphan receptor ⍰ changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998;17:3867–77. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RE, Landis DJ, Dudley PA. Essential fatty acid deficiency and renewal of rod outer segments in the albino rat. Invest Ophthalmol. 1976;15:232–36. [PubMed] [Google Scholar]
- Andrews LD, Cohen AI. Freeze-fracture evidence for the presence of cholesterol in particle-free patches and basal disks and the plasma membrane of retinal rod outer segments of mice. J Cell Biol. 1979;81:215–28. doi: 10.1083/jcb.81.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalla L, Fernandez MA, Rao NA. Immunohistochemical localization of catalase in ocular tissue. Curr Eye Res. 1987;6:1181–87. doi: 10.3109/02713688709025227. [DOI] [PubMed] [Google Scholar]
- Atalla LR, Sevanian A, Rao NA. Hydrogen peroxide localization in ocular tissues: An electron microscopic cytochemical study. Curr Eye Res. 1988;7:931–36. doi: 10.3109/02713688808997249. [DOI] [PubMed] [Google Scholar]
- Atalla LR, Sevanian A, Rao NA. Immunohistochemical localization of glutathione peroxidase in ocular tissue. Curr Eye Res. 1998;7:1023–27. doi: 10.3109/02713688809015149. [DOI] [PubMed] [Google Scholar]
- Barsalou LS, Darrow RM, Organisciak DT. Time-dependent and light-induced changes in retinal gene expression. Assn Res Vis Ophthalmol. 2004 Meeting abstract 683. [Google Scholar]
- Battelle BA, LaVail MM. Rhodopsin content and rod outer segment length in albino rats: Modification by dark adaptation. Exp Eye Res. 1978;26:487–497. doi: 10.1016/0014-4835(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Neuroprotectin D1 (NPD-1): A DHA-derived mediator tthat protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–66. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatrice J, Wenzel A, Remé CE, Grimm C. Increased light damage susceptibility at night does not correlate with RPE65 levels and rhodopsin regeneration in rats. Exp Eye Res. 2003;76:695–700. doi: 10.1016/s0014-4835(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Belayev L, Marcheselli VL, Khoutorova L, Rodriguez de Turco EB, Ginsberg MD, et al. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–23. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- Benolken RM, Anderson RE, Wheeler TG. Membrane fatty acids associated with the electrical response in visual excitation. Science. 1973;182:1253–54. doi: 10.1126/science.182.4118.1253. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–73. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Birkle Dl, Bazan NG. The arachidonic acid cascade and phospholipid and docosahexaenoic acid metabolism in the retina. In: Osborne NN, Chader GJ, editors. Progress in Retinal Research. Pergamon Press; Oxford, England: 1986. pp. 309–22. [Google Scholar]
- Blakeslee B, Jacobs GH, Neitz J. Spectral mechanisms in the tree squirrel retina. J Comp Physiol A. 1988;162:773–80. doi: 10.1007/BF00610966. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Pickford MS, Organisciak DT. Ascorbate treatment prevents the accumulation of phagosomes in RPE in light damage. Invest Ophthalmol Vis Sci. 1992;33:2814–21. [PubMed] [Google Scholar]
- Bobu C, Craft CM, Masson-Pevet M, Hicks D. Photoreceptor organization and rhythmic phagocytosis in the nile rat Arvicanthis ansorgei: a novel diurnal rodent model for the study of cone pathophysiology. Invest Ophthalmol Vis Sci. 2006;47:3109–18. doi: 10.1167/iovs.05-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Albert AD. Cholesterol modulation of photoreceptor function in bovine retinal rod outer segments. J Biol Chem. 1990;265:20727–30. [PubMed] [Google Scholar]
- Boesze-Battaglia K, Fliesler SJ, Albert AD. Relationship of cholesterol content to spatial distribution and age of disk membranes in retinal rod outer segments. J Biol Chem. 1990;265:18867–70. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Hennessey T, Albert AD. Cholesterol heterogeneity in bovine rod outer segment disk membranes. J Biol Chem. 1989;264:8151–55. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Organisciak DT, Albert AD. RCS rat retinal rod outer segment membranes exhibit different cholesterol distributions than those of normal rats. Exp Eye Res. 1994;58:293–300. doi: 10.1006/exer.1994.1020. [DOI] [PubMed] [Google Scholar]
- Borges JM, Edward DP, Tso MOM. A comparative study of photic injury in four inbred stains of albino rats. Curr Eye Res. 1990;9:799–803. doi: 10.3109/02713689008999576. [DOI] [PubMed] [Google Scholar]
- Bowes G, van Veen T, Farber DB. Opsin, G-protein and 48-kDa protein in normal and rd mouse retinas: Developmental expression of mRNAs and proteins and light/dark cycling of mRNAs. Exp Eye Res. 1988;47:369–90. doi: 10.1016/0014-4835(88)90049-8. [DOI] [PubMed] [Google Scholar]
- Brann MR, Cohen LV. Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science. 1987;235:585–87. doi: 10.1126/science.3101175. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Munoz B, Maguire MG, Vitale SE, Schein OD, et al. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities. Waterman study. Arch Ophthalmol. 1995;113:301–08. doi: 10.1001/archopht.1995.01100030055022. [DOI] [PubMed] [Google Scholar]
- Busch EM, Gorgels TGMF, Roberts JE, van Norren D. The effects of two stereoisomers of N-acetylcysteine on photochemical damage by UVA and blue light in rat retina. Photochem Photobiol. 1999;70:353–58. [PubMed] [Google Scholar]
- Bush RA, Malone A, Remé CE, Williams TP. Dietary deficiency of n-3 fatty acids alters rhodopsin content and function in the rat retina. Invest Ophthalmol Vis Sci. 1994;35:91–100. [PubMed] [Google Scholar]
- Bush RA, Remé CE, Malone A. Light damage in the rat retina: The effect of dietary deprivation on n-3 fatty acids on acute structural alterations. Exp Eye Res. 1991;53:741–52. doi: 10.1016/0014-4835(91)90109-r. [DOI] [PubMed] [Google Scholar]
- Bush RA, Suguwara T, Iuvone PM, Sieving PA. The melatonin receptor antagonist luzindol protects photoreceptors from light damage in rat. Invest Ophthalmol Vis Sci. 1998;39:2458–65. [PubMed] [Google Scholar]
- Bush RA, Williams TP. The effect of unilateral optic nerve section on retinal light damage in rats. Exp Eye Res. 1991;52:139–53. doi: 10.1016/0014-4835(91)90254-c. [DOI] [PubMed] [Google Scholar]
- Caldwell RB, McLaughlin BJ. Freeze-fracture study of filipin binding in photoreceptor outer segments and pigment epithelium of dystrophic and normal retinas. J Comp Neurology. 1985;236:523–37. doi: 10.1002/cne.902360408. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Chang M, Ohsao M, Vinores SA, Nie Z, et al. Retinal degeneration in transgenic mice with photoreceptor specific expression of a dominant-negative fibroblast growth factor receptor. J Neurosci. 1996;16:1679–88. doi: 10.1523/JNEUROSCI.16-05-01679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Li F, Steinberg RH, LaVail MM. Induction of c-fos and c-jun mRNA expression by basic fibroblast growth factor in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998;39:565–73. [PubMed] [Google Scholar]
- Cao W, Li F, Steinberg RH, LaVail MM. Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BNDF, GFAP, and IGF-I in the rat retina. Exp Eye Res. 2001;72:591–604. doi: 10.1006/exer.2001.0990. [DOI] [PubMed] [Google Scholar]
- Cao W, Tombran-Tink J, Eliaas R, Sezate S, Mrezek D, et al. In vivo protection of photoreceptors from light damage by pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2001;42:1646–52. [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–62. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Casson RJ, Wood JPM, Melena J, Chidlow G, Osborne NN. The effect of ischemic preconditioning on light-induced photoreceptor injury. Invest Ophthalmol Vis Sci. 2003;44:1348–54. doi: 10.1167/iovs.02-0368. [DOI] [PubMed] [Google Scholar]
- Chen C, Tsina E, Cornwall MC, Crouch RK, Vijayaraghavan S, et al. Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors. Biophys J. 2005;88:2278–87. doi: 10.1529/biophysj.104.054254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Flannery JG, LaVail MM, Steinberg RH, Xu J, et al. BCL-2 overexpression reduces apoptotic photoreceptor cell death in three different retinal degenerations. Proc Natl Acad Sci. 1996;93:7042–47. doi: 10.1073/pnas.93.14.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Simon MI, Matthes MT, Yasumura D, LaVail MM. Increased susceptibility to light damage in an arrestin knockout model of Oguchi disease (stationary night blindness) Invest Ophthalmol Vis Sci. 1999;40:2978–82. [PubMed] [Google Scholar]
- Chen L, Wu W, Dentchev T, Zeng Y, Wang J, et al. Light damage induced changes in mouse retinal genes expression. Exp Eye Res. 2004;29:239–47. doi: 10.1016/j.exer.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Choi S, Hao W, Chen C-K, Simon MI. Gene expression profiles of light-induced apoptosis in arrestin/rhodopsin kinase-deficient mouse models. Proc Natl Acad Sci. 2001;98:13096–101. doi: 10.1073/pnas.201417498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispell JD, Feathers KL, Kane MA, Kim CY, Brooks M, et al. Rdh 12 activity and effects on retinoid processing in the murine retina. J Biol Chem. 2009;284:21468–77. doi: 10.1074/jbc.M109.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou V, Stone J, Stowe S, Barnett NL, Valter K. The status of cones in the rhodopsin mutant P23H-3 retina: light-regulated damage and repair in parallel with rods. Invest Ophthalmol Vis Sci. 2008;49:1116–25. doi: 10.1167/iovs.07-1158. [DOI] [PubMed] [Google Scholar]
- Chrysostomou V, Valter K, Stone J. Cone-rod dependence in the rat retina: variation with the rate of rod damage. Invest Ophthalmol Vis Sci. 2009;50:3017–23. doi: 10.1167/iovs.08-3004. [DOI] [PubMed] [Google Scholar]
- Cicerone CM. Cones survive rods in the light-damaged eye of the albino rat. Science. 1976;194:1183–85. doi: 10.1126/science.996550. [DOI] [PubMed] [Google Scholar]
- Collier RJ, Waldron WR, Zigman S. Temporal sequence of changes to the gray squirrel retina after near-UV exposure. Invest Ophthalmol Vis Sci. 1989;30:631–37. [PubMed] [Google Scholar]
- Corbo JC, Myers CA, Lawrence KA, Jadhav AP, Cepko CL. A typology of photoreceptor gene expression patterns in the mouse. Proc Natl Acad Sci U S A. 2007;104:12069–74. doi: 10.1073/pnas.0705465104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci. 2002;99:14682–87. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch RK, Chader GJ, Wiggert B, Pepperberg DR. Retinoids and the visual cycle. Photochem Photobiol. 1996;64:613–21. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Cusato K, Bosco A, Rozental R, Guimarães CA, Reese BE, et al. Gap junctions mediate bystander cell death in developing retina. J Neurosci. 2003;23:6413–22. doi: 10.1523/JNEUROSCI.23-16-06413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cveki A, Wang W-l. Retinoic acid signaling in mammalian eye development. Exp Eye Res. 2009;89:280–91. doi: 10.1016/j.exer.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danciger M, Matthes MT, Yasamura D, Akhmedov NB, Rickabaugh T, et al. A QTL on distal chromosome 3 that influences the severity of light-induced damage to mouse photoreceptors. Mamm Genome. 2000;11:422–27. doi: 10.1007/s003350010081. [DOI] [PubMed] [Google Scholar]
- Danciger M, Yang H, Handschumacher L, LaVail MM. Constant light-induced retinal damage and the RPE65-met450 variant: Assessment of the NZW/LacJ mouse. Mol Vis. 2005;11:374–79. [PubMed] [Google Scholar]
- Dang L, Pulukuri S, Mears AJ, Swaroop A, Reese BE, et al. Connexin 36 in photoreceptor cells: studies on transgenic rod-less and cone-less mouse retinas. Mol Vis. 2004;10:323–27. [PubMed] [Google Scholar]
- Darrow RA, Darrow RM, Organisciak DT. Biochemical characterization of cell specific enzymes in light-exposed rat retinas. Curr Eye Res. 1997;16:144–51. doi: 10.1076/ceyr.16.2.144.5092. [DOI] [PubMed] [Google Scholar]
- Demontis GC, Longoni B, Marchiafava PL. Molecular steps involved in light-induced oxidative damage to retinal rods. Invest Ophthalmol Vis Sci. 2002;43:2421–27. [PubMed] [Google Scholar]
- deUrquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–44. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- Donovan M, Carmody RJ, Cotter TG. Light -induced photoreceptor apoptosis in vivo requires neuronal nitric-oxide synthase and guanylate cyclase activity and is caspase-3-independent. J Biol Chem. 2001;276:23000–08. doi: 10.1074/jbc.M005359200. [DOI] [PubMed] [Google Scholar]
- Duncan TE, O’Steen WK. The diurnal susceptibility of rat retinal photoreceptors to light-induced damage. Exp Eye Res. 1985;41:497–507. doi: 10.1016/s0014-4835(85)80007-5. [DOI] [PubMed] [Google Scholar]
- Duncan T, Wiggert B, Whittaker N, Darrow R, Organisciak DT. Effect of visible light on normal and P23H-3 transgenic rat retinas: Characterization of a novel retinoic acid derivative present in the P23H-3 retina. Photochem Photobiol. 2006;82:741–45. doi: 10.1562/2005-10-05-RA-712. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Martins R, da Silva Filho M, Muniz JA, Silveira LC, et al. Developmental sources of conservation and variation in the evolution of the primate eye. Proc Natl Acad Sci. 2009;106:8963–68. doi: 10.1073/pnas.0901484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward DP, Lam T-T, Shahinfar S, Li J, Tso MOM. Amelioration of light-induced retinal degeneration by a calcium overload blocker. Arch Ophthalmol. 1991;109:554–62. doi: 10.1001/archopht.1991.01080040122042. [DOI] [PubMed] [Google Scholar]
- Fain GL, Lisman JE. Photoreceptor degeneration in vitamin A deprivation and retinitis pigmentosa: The equivalent light hypothesis. Exp Eye Res. 1993;57:335–40. doi: 10.1006/exer.1993.1132. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12:3554–67. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DB, Seager-Danciger J, Organisciak DT. Levels of mRNA encoding proteins of the cGMP cascade as a function of light environment. Exp Eye Res. 1991;53:781–86. doi: 10.1016/0014-4835(91)90114-t. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Franco EC, Yamada ES, Crowley JC, Parsons M, et al. Number and topography of cones, rods and optic nerve axons in New and Old World primates. Vis Neurosci. 2008;25:289–99. doi: 10.1017/S0952523808080371. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Lewis GP, Linberg KA, Verardo MR. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24:395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Kinkl N, Sahel J, Dreyfus H, Hicks D. Survival of purified rat photoreceptors in vitro is stimulated directly by fibroblast growth factor-2. J Neurosci. 1998;23:9662–72. doi: 10.1523/JNEUROSCI.18-23-09662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci. 1989;86:6377–81. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2008;454:805–19. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller D, Machemer R, Knighton RW. Retinal damage produced by intraocular fiber optic light. Am J Ophthalmol. 1978;85:519–37. doi: 10.1016/s0002-9394(14)75250-x. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Bonfield S, Gilmour GS, Kuny S, Mema SC, et al. Retinal anatomy and visual performance in a diurnal cone-rich laboratory rodent, the Nile grass rat (Arvicanthis niloticus) J Comp Neurol. 2008;510:525–38. doi: 10.1002/cne.21798. [DOI] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Basic fibroblast growth factor: Increased gene expression in inherited and light-induced photoreceptor degeneration. Exp Eye Res. 1996;62:181–89. doi: 10.1006/exer.1996.0022. [DOI] [PubMed] [Google Scholar]
- Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci. 2004;101:10446–51. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glösmann M, Peichl L. Cone damage and ectopic cone opsin in the albino rat retina induced by moderate cyclic light. Assn Res Vis Ophthalmol. 2007 Meeting abstract 1343. [Google Scholar]
- Goldman AI, Teirstein PS, O’Brien PJ. The role of ambient lighting in circadian disc shedding in the rod outer segment of the rat retina. Invest Ophthalmol Vis Sci. 1980;19:1257–67. [PubMed] [Google Scholar]
- Gordon WC, Casey DM, Lukiw WJ, Bazan NG. DNA damage and repair in light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2002;43:3511–21. [PubMed] [Google Scholar]
- Gosbell AD, Stefanovic N, Scurr LL, Pete J, Kola I, et al. Retinal light damage: Structural and functional effects of the antioxidant glutathione peroxidase-1. Invest Ophthalmol Vis Sci. 2006;47:2613–22. doi: 10.1167/iovs.05-0962. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D, Ferrara N, Schweogerer L, Neufeld G. Structural characterization and biological function of fibroblast growth factor. Endoc Rev. 1987;8:95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Goureau O, Jeanny JC, Becquet F, Hartmann MP, Courtois Y. Protection against light-induced retinal degeneration by an inhibitor of NO synthase. Neuro Report. 1993;5:233–336. doi: 10.1097/00001756-199312000-00012. [DOI] [PubMed] [Google Scholar]
- Grimm C, Remé CE, Rol PO, Williams TP. Blue light’s effects on rhodopsin: photoreversal of bleaching in living rat eyes. Invest Ophthalmol Vis Sci. 2000a;41:3984–90. [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–24. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Hafezi F, Remé CE. Gene expression in the mouse retina: The effect of damaging light. Mol Vision. 2000b;6:252–60. [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, et al. Protection of RPE 65 deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat Genet. 2000c;25:63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- Gruber AR, Betts KE, Lewis GP, Fisher SK, Vaughan DK. Photoreceptor antigens are altered during seasonal hibernation of a cone-dominant rodent. Assn Res Vis Ophthalmol Meeting. 2006 Abstract 2845. [Google Scholar]
- Gu D, Beltran WA, Pearce-Kelling S, Li Z, Acland GM, et al. Steroids do not prevent photoreceptor degeneration in the light-exposed T4R rhodopsin mutant dog retina irrespective of AP-1 inhibition. Invest Ophthalmol Vis Sci. 2009;50:3482–94. doi: 10.1167/iovs.08-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Gillette Meer S, Miyagi M, Rayborn ME, Hollyfield JG, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–35. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- Ha K-N, Chen Y, Cai J, Sternberg P. Increased glutathione synthesis through an ARE-Nrf2- dependent pathway by zinc in the RPE: Implication for protection against oxidative stress. Invest Ophthalmol Vis Sci. 2006;47:2709–15. doi: 10.1167/iovs.05-1322. [DOI] [PubMed] [Google Scholar]
- Hafezi F, Abegg M, Grimm C, Wenzel A, Munz K, et al. Retinal degeneration in the rd mouse in the absence of c-fos. Invest Ophtthalmol Vis Sci. 1998;39:2239–44. [PubMed] [Google Scholar]
- Hafezi F, Marti A, Munz K, Remé CE. Light-induced apoptosis: Differential timing in the retina and pigment epithelium. Exp Eye Res. 1997a;64:963–70. doi: 10.1006/exer.1997.0288. [DOI] [PubMed] [Google Scholar]
- Hafezi F, Steinbach JP, Marti A, Munz K, Wang ZQ, et al. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med. 1997b;3:346–49. doi: 10.1038/nm0397-346. [DOI] [PubMed] [Google Scholar]
- Hao W, Wenzel A, Obin MS, Chen C-K, Brill E, et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32:254–60. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26:533–41. doi: 10.1016/s0896-6273(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao H-W, Takao M, Berson DM, Yau K-W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck MS, Schadel A, Maretzki D, Bartl FJ, Ritter E, et al. Signaling states of rhodopsin: Formation of the storage form, metarhodopsin III, from active metarhodopsin II. J Biol Chem. 2003;278:3162–69. doi: 10.1074/jbc.M209675200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann KP. Photoproducts of rhodopsin in the disc membrane. Photochem Photophys. 1986;13:309–27. [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature Med. 2008;14:194–98. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Li F, Alvarez RA, Ash JD, Anderson RE. Down-regulation of ATP synthase subunit-6, cytochrome C oxidase III and NADH dehydrogenase-3 by bright cyclic light. Invest Ophthalmol Vis Sci. 2004;45:2489–96. doi: 10.1167/iovs.03-1081. [DOI] [PubMed] [Google Scholar]
- Huang H, Frank MB, Dozmorov I, Cao W, Cadwell C, et al. Identification of mouse retinal genes differentially regulated by dim and bright cyclic light rearing. Exp Eye Res. 2005;80:727–39. doi: 10.1016/j.exer.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 2005;15:216–19. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Imaki J, Yamashita K, Yamakawa A, Yoshida K. Expression of jun family genes in rat retinal cells: Regulation by light/dark cycle. Mol Brain Res. 1995;30:48–52. doi: 10.1016/0169-328x(94)00270-o. [DOI] [PubMed] [Google Scholar]
- Iseli H-P, Wenzel A, Hafezi F, Remé CE, Grimm C. Light damage susceptibility and RPE-65 in rats. Exp Eye Res. 2002;75:407–13. [PubMed] [Google Scholar]
- Jacobs GH, Calderone JB, Fenwick JA, Krogh K, Williams GA. Visual adaptations in a diurnal rodent, Octodon degus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:347–61. doi: 10.1007/s00359-003-0408-0. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz M, Neitz J. Mutations in S-cone pigment genes and the absence of colour vision in two species of nocturnal primate. Proc Biol Sci. 1996;263:705–10. doi: 10.1098/rspb.1996.0105. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci USA. 2007;104:15123–8. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen JJM, Kuhlmann ED, van Vugt AHM, Winkens HJ, Janssen BPM, et al. Retinoic acid receptors and retinoid X receptors in the mature retina: Subtype determination and cellular distribution. Curr Eye Res. 1999;19:338–47. doi: 10.1076/ceyr.19.4.338.5307. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immunolocalizatioin of the calcitriol receptor, calbindin-D28k and the plasma membrane calcium pump in the human eye. Curr Eye Res. 1995;14:101–08. doi: 10.3109/02713689508999921. [DOI] [PubMed] [Google Scholar]
- Johnson J, Maher P, Hanneken A. The flavinoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Invest Ophthalmol Vis Sci. 2009;50:2398–2406. doi: 10.1167/iovs.08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Li T. Overexpression of bcl-2 or bcl-xL transgenes and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 1996;37:2434–46. [PubMed] [Google Scholar]
- Kaitz M, Auerbach E. Action spectrum for light induced retinal degeneration in dystrophic rats. Vis Res. 1979;19:1041–44. doi: 10.1016/0042-6989(79)90229-3. [DOI] [PubMed] [Google Scholar]
- Kaldi I, Dittmar M, Pierce P, Anderson RE. L-NAME protects against acute light damage in albino rats, but not against retinal degeneration in P23H and S334ter transgenic rats. Exp Eye Res. 2003;76:453–61. doi: 10.1016/s0014-4835(02)00334-2. [DOI] [PubMed] [Google Scholar]
- Kanan Y, Moiseyev G, Agarwal N, Ma JX, Al-Ubaidi MR. Light induces programmed cell death by activating multiple independent proteases in a cone photoreceptor cell line. Invest Ophthalmol Vis Sci. 2007;48:40–51. doi: 10.1167/iovs.06-0592. [DOI] [PubMed] [Google Scholar]
- Kapphahn RJ, Giwa BM, Berg KM, Roehrich H, Feng X, et al. Retinal proteins modified by 4-hydroxynonenal: Identification of molecular targets. Exp Eye Res. 2006;83:165–75. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Katz ML, Stientjes HJ, Gao C-L, Norberg M. Bright environmental light accelerates rhodopsin depletion in retinoid-deprived rats. Invest Ophthalmol Vis Sci. 1993;34:2000–08. [PubMed] [Google Scholar]
- Kayatz P, Heimann K, Schraermeyer U. Ultrastructural localization of light-induced lipid peroxides in the rat retina. Invest Ophthalmol Vis Sci. 1999;40:2314–21. [PubMed] [Google Scholar]
- Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, et al. Breaking the covalent bond--a pigment property that contributes to desensitization in cones. Neuron. 2005;46:879–90. doi: 10.1016/j.neuron.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW, Turner JK, Reh TA. Retinoic acid promotes differentiation of photoreceptors in vitro. Development. 1994;120:2091–102. doi: 10.1242/dev.120.8.2091. [DOI] [PubMed] [Google Scholar]
- Kim H-Y, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3) J Biol Sci. 2000;275:35215–23. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem Photobiol. 2004;79:470–75. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–57. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Kostyk SK, D’Amore PA, Herman IM, Wagner JA. Optic nerve injury alters basic fibroblast growth factor localization in the retina and optic tract. J Neurosci. 1994;14:1441–49. doi: 10.1523/JNEUROSCI.14-03-01441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutz CA, Wiegand RD, Rapp LM, Anderson RE. Effects of dietary fat on the response of the rat retina to chronic and acute light stress. Exp Eye Res. 1995;60:307–16. doi: 10.1016/s0014-4835(05)80112-5. [DOI] [PubMed] [Google Scholar]
- Krebs MP, White DA, Kaushal S. Biphasic photoreceptor degeneration induced by light in a T17M rhodopsin mouse model of cone bystander damage. Invest Ophthalmol Vis Sci. 2009;50:2956–65. doi: 10.1167/iovs.08-3116. [DOI] [PubMed] [Google Scholar]
- Kryger Z, Galli-Resta L, Jacobs GH, Reese BE. The topography of rod and cone photoreceptors in the retina of the ground squirrel. Vis Neurosci. 1998;15:685–91. doi: 10.1017/s0952523898154081. [DOI] [PubMed] [Google Scholar]
- Kutty RK, Kutty G, Wiggert B, Chader GJ, Darrow RM, et al. Induction of heme oxygenase -1 in the retina by intense light: Suppression by the antioxidant dimethylthiourea. Proc Natl Acad Sci. 1995;92:1177–81. doi: 10.1073/pnas.92.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S, Tso MOM, Gurne DH. Amelioration of retinal photic inury in albino rats by dimethylthiourea. Arch Ophthalmol. 1990;108:1751–57. doi: 10.1001/archopht.1990.01070140105039. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN. Dark adaptation and the retinoid cycle of vision. Progress in Retinal and Eye Res. 2004;23:307–80. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: Relationship to cyclic lighting. Science. 1976b;194:1072–74. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Survival of some photoreceptor cells in albino rats following long-term exposure to light. Invest Ophthalmol Vis Sci. 1976a;15:64–70. [PubMed] [Google Scholar]
- LaVail MM, Gorrin GM, Repaci MA, Yasumura D. Light-induced retinal degeneration in albino mice and rats: Strain and species differences. In: Hollyfield JG, Anderson RE, LaVail MM, editors. Degenerative Retinal Disorders; Clinical and Laboratory Investigations. Alan R Liss; New York: 1987. pp. 439–54. [PubMed] [Google Scholar]
- LaVail MM, Gorrin GM, Yasumura D, Matthes MT. Increased susceptibility to constant light in nr and pcr mice with inherited retinal degenerations. Invest Ophthalmol Vis Sci. 1999;40:1020–24. [PubMed] [Google Scholar]
- LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, et al. Multiple growth factors, cytokines, and neuotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci. 1992;89:11249–53. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, Lau-Villacorta C, Unoki K, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- Leist M, Single B, Naumann H, Fava E, Simon B, et al. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp Cell Res. 1999;249:396–403. doi: 10.1006/excr.1999.4514. [DOI] [PubMed] [Google Scholar]
- Lewis JW, van Kuijk FJ, Carruthers JA, Kliger DS. Metarhodopsin III formation and decay kinetics: Comparison of bovine and human rhodopsin. Vision Res. 1997;37:1–8. doi: 10.1016/s0042-6989(96)00138-1. [DOI] [PubMed] [Google Scholar]
- Li F, Cao W, Anderson RE. Protection of photoreceptor cells in adult rats from light-induced degeneration by adaptation to bright cyclic light. Exp Eye Res. 2001;73:569–77. doi: 10.1006/exer.2001.1068. [DOI] [PubMed] [Google Scholar]
- Li F, Cao W, Anderson RE. Alleviation of constant-light-induced photoreceptor degeneration by adaptation of adult albino rat to bright cyclic light. Invest Ophthalmol Vis Sci. 2003;44:4968–75. doi: 10.1167/iovs.03-0140. [DOI] [PubMed] [Google Scholar]
- Li S, Chang C-J, Abler AS, Fu J, Tso MOM, et al. A comparison of continuous versus intermittent light exposure on apoptosis. Curr Eye Res. 1996;15:914–22. doi: 10.3109/02713689609017635. [DOI] [PubMed] [Google Scholar]
- Li YZ, Tso MOM, Wang H-M, Organisciak DT. Amelioration of photic injury in rat retina by ascorbic acid. Invest Ophthalmol Vis Sci. 1985;26:1589–98. [PubMed] [Google Scholar]
- Lin T-K, Hughes G, Muratovska A, Blaikie FH, Brooks PS, et al. Specific modification of mitochondrial protein thiols in response to oxidative stress. J Biol Chem. 2002;277:17048–56. doi: 10.1074/jbc.M110797200. [DOI] [PubMed] [Google Scholar]
- Liu C, Peng M, Laties AM, Wen R. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J Neurosci. 1998;18:1337–44. doi: 10.1523/JNEUROSCI.18-04-01337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KO, Fisher SK. The distributions of photoreceptors and ganglion cells in the California ground squirrel, Spermophilus beecheyi. J Comp Neurol. 1983;221:329–40. doi: 10.1002/cne.902210308. [DOI] [PubMed] [Google Scholar]
- Lorentz O, Sahel J, Mohand-Saïd S, Leveillard T. Cone survival: identification of RdCVF. Adv Exp Med Biol. 2006;572:315–19. doi: 10.1007/0-387-32442-9_44. [DOI] [PubMed] [Google Scholar]
- Lornejad-Schafer MR, Schafer C, Schoffl H, Frank J. Cytoprotective role of mitogen-activated protein kinase phosphatase-1 in light-damaged human retinal pigment epithelial cells. Photochem Photobiol. 2009;85:834–442. doi: 10.1111/j.1751-1097.2008.00479.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Oveson BC, Jo Y-J, Lauer TW, Usui S, et al. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid Redox Signal. 2009;11:715–24. doi: 10.1089/ars.2008.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukáts A, Szabó A, Röhlich P, Vígh B, Szél A. Photopigment coexpression in mammals: comparative and developmental aspects. Histol Histopathol. 2005;20:551–74. doi: 10.14670/HH-20.551. [DOI] [PubMed] [Google Scholar]
- Maccarone R, Di Marco S, Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Invest Ophthalmol Vis Sci. 2008;49:1254–61. doi: 10.1167/iovs.07-0438. [DOI] [PubMed] [Google Scholar]
- Machida S, Chaudhry P, Shinohara T, Singh DP, Reddy VN, et al. Lens epithelium-derived growth factor promotes photoreceptor survival in light-damaged and RCS rats. Invest Ophthalmol Vis Sci. 2001;42:1087–95. [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Umesono K, Blumberge B, et al. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–39. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE. Injury and repair: retinal remodeling. In: Dartt DA, Besharse JC, Dana R, editors. Encyclopedia of the Eye. Elsevier/Academic Press; 2010. In press. [Google Scholar]
- Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol. 2003;28:139–47. doi: 10.1385/MN:28:2:139. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, et al. Extreme retinal remodeling triggered by light damage: Implications for age related macular degeneration. Mol Vis. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Ruiz A, Radu RA, Bui TV, Travis GH. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44:11715–21. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: The inter link of photo transduction and retinoid metabolism in the vertebrate retina. Progress in Retinal and Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Mey J, Drager UC. Light-mediated retinoic acid production. Proc Natl Acad Sci USA. 1996;93:12570–74. doi: 10.1073/pnas.93.22.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery P, Posch KC, Napoli JL, Gudas L, Drager UC. Changing patterns of the retinoic acid system in the developing retina. Dev Biol. 1993;158:390–99. doi: 10.1006/dbio.1993.1197. [DOI] [PubMed] [Google Scholar]
- McGinnis JF, Whelan JP, Donoso LA. Transient, cyclic changes in mouse visual cell gene products during the light-dark cycle. J Neurosci Res. 1992;31:584–90. doi: 10.1002/jnr.490310325. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–52. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Mittag TW, Bayer AU, LaVail MM. Light-induced retinal damage in mice carrying a mutated SOD 1 gene. Exp Eye Res. 1999;69:677–83. doi: 10.1006/exer.1999.0748. [DOI] [PubMed] [Google Scholar]
- Miyagi M, Sakaguchi H, Darrow RM, Yan L, West KA, et al. Evidence that light modulates protein nitration in rat retina. Mol Cellular Proteomics. 2002;1:293–303. doi: 10.1074/mcp.m100034-mcp200. [DOI] [PubMed] [Google Scholar]
- Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest Ophthalmol Vis Sci. 2001;42:1312–18. [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci. 2004;101:8491–96. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz A, Villazana-Espinoza ET, Hatch AL, Trevino SG, Allen DM, et al. A novel cone visual cycle in the cone-dominated retina. Exp Eye Res. 2007;85:175–84. doi: 10.1016/j.exer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Engel AH, Palczewski K. Structure of cone photoreceptors. Progress in Retinal and Eye Res. 2009;28:289–302. doi: 10.1016/j.preteyeres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naash MI, Peachey NS, Zong-Yi L, Gryczan CC, Goto Y, et al. Light-induced acceleration of photoreceptor degeneration in transgenic mice expressing mutant rhodopsin. Invest Ophthalmol Vis Sci. 1996;37:775–82. [PubMed] [Google Scholar]
- Neuringer M, Conner WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal w3 fatty acid deficiency on the retina and brain in rhesus monkeys. Proc Natl Acad Sci. 1986;83:4021–25. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell WK, Delmelle MC, Albrecht R. Vitamin A effect on retina: Dependence on light. Science. 1971;172:72–76. doi: 10.1126/science.172.3978.72. [DOI] [PubMed] [Google Scholar]
- Noell WK, Albrecht R. Irreversible effects of visible light on the retina: role of vitamin A. Science. 1971;172:76–80. doi: 10.1126/science.172.3978.76. [DOI] [PubMed] [Google Scholar]
- Noell WK. There are different kinds of light damage. In: Williams TP, Baker BN, editors. The Effects of Constant Light on Visual Processes. Plenum Press; New York: 1980. pp. 3–28. [Google Scholar]
- Noell WK. Aspects of experimental and hereditary degeneration. In: Graymore C, editor. Biochemistry of the Retina. Academic Press; London: 1965. pp. 51–72. [Google Scholar]
- Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–73. [PubMed] [Google Scholar]
- Obin M, Shang F, Gong X, Handelman G, Blumberg J, et al. Redox regulation of ubiquitin-conjugating enzymes: Mechanistic insights using the thiol-specific oxidant diamide. FASEB J. 1998;12:561–69. doi: 10.1096/fasebj.12.7.561. [DOI] [PubMed] [Google Scholar]
- Ohira A, Tanito M, Kaidzu S, Kondo T. Glutathione peroxidase induced in rat retinas to conteract photic injury. Invest Ophthalmol Vis Sci. 2003;44:1230–36. doi: 10.1167/iovs.02-0191. [DOI] [PubMed] [Google Scholar]
- Oishi A, Otani A, Sasabara M, Kojima H, Nakamura H, et al. Granulocyte colony-stimulating factor protects photoreceptor cells against light-induce damage. Invest Ophthalmol Vis Sci. 2008;49:5629–35. doi: 10.1167/iovs.08-1711. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Bicknell IR, Darrow RM. The effects of L- and D- ascorbic acid administration on retinal tissue levels and light damage in rats. Curr Eye Res. 1992;11:231–41. doi: 10.3109/02713689209001774. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou LS. Light-induced retinal degeneration. In: Levin LA, DiPolo A, editors. Ocular Neuroprotection. Marcel Dekker; New York: 2003. pp. 85–107. [Google Scholar]
- Organisciak DT, Darrow RA, Barsalou L, Darrow RM, Lininger LA. Light-induced damage to the retina: Differential effects of dimethylthiourea on photoreceptor survival, apoptosis and DNA. Photochem Photobiol. 1999a;70:261–68. [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Circadian dependent retinal light damage in rats. Invest Ophthalmol Vis Sci. 2000;41:3694–3701. [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou LS, Renganathan K, Crabb JS, et al. Light exposure alters carboxyethylpyrrole modified protetins in rod outer segments. Assn Res Vis Ophthalmol Meeting. 2008 Abstract 253. [Google Scholar]
- Organisciak DT, Darrow RM, Bicknell IR, Jiang Y-L, Pickford M, et al. Protection against retinal light damage by natural and synthetic antioxidants. In: Anderson RE, Hollyfield JG, LaVail MM, editors. Retinal Degenerations. CRC Press; Boca Raton, FL: 1989b. pp. 189–201. [Google Scholar]
- Organisciak DT, Darrow RM, Darrow RA, Lininger LA. Environmental light and age-related changes in retinal proteins. In: Williams TP, Thistle AB, editors. Photostasis and Related Phenomena. Plenum Press; New York: 1998. pp. 79–92. [Google Scholar]
- Organisciak DT, Darrow RM, Jiang Y-L, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment dososahexaenoate. Invest Ophthalmol Vis Sci. 1996;37:2243–57. [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Jiang Y-L, Marak GE, Blanks JC. Protection by dimethylthiourea against retinal light damage in rats. Invest Ophthalmol Vis Sci. 1992;33:1599–1609. [PubMed] [Google Scholar]
- Organisciak DT, Jiang Y-H, Wang H-M, Pickford M, Blanks JC. Retinal light damage in rats exposed to intermittent light. Invest Ophthalmol Vis Sci. 1989a;30:795–805. [PubMed] [Google Scholar]
- Organisciak DT, Li M, Darrow RM, Farber DB. Photoreceptor cell damage by light in young Royal College of Surgeons rats. Curr Eye Res. 1999b;19:188–96. doi: 10.1076/ceyr.19.2.188.5333. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Noell WK. The rod outer segment phospholipid/opsin ratio of rats maintained in darkness or cyclic light. Invest Ophthalmol. 1977;16:188–90. [PubMed] [Google Scholar]
- Organisciak DT, Sarna T. Genetic, age and light-induced degenerations of the retina and retinal pigment epithelium. In: Coohill TP, Valenzeno DP, editors. Photobiology for the 21st Century. Valdenmar Publ; Overland, KS: 2001. pp. 81–100. [Google Scholar]
- Organisciak DT, Wang H-M, Li YZ, Tso MOM. The protective effect of ascorbate in retinal light damage of rats. Invest Ophthalmol Vis Sci. 1985;26:1580–88. [PubMed] [Google Scholar]
- Organisciak DT, Wang H-M, Noell WK. Aspects of the ascorbate protective mechanism in retinal light damage of rats with normal and reduced ROS docosahexaenoic acid. In: Hollyfield JG, Anderson RE, LaVail MM, editors. Degenerative Retinal Disorders: Clinical and Laboratory Investigations. Alan R. Liss; New York: 1987. pp. 455–68. [PubMed] [Google Scholar]
- Organisciak DT, Winkler BS. Retinal light damage: practical and theoretical considerations. Progress in Retinal and Eye Res. 1994;13:1–29. [Google Scholar]
- Organisciak DT, Xie A, Wang H-M, Jiang Y-L, Darrow RM, et al. Adaptive changes in visual cell transduction protein levels: Effects of light. Exp Eye Res. 1991;53:773–79. doi: 10.1016/0014-4835(91)90113-s. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Li G-Y, Ji D, Mortiboys HJ, Jackson S. Light affects mitochondria to cause apoptosis to cultured cells: Possible relevance to ganglion cell death in certain optic neuropathies. J Neurochem. 2008;105:2013–28. doi: 10.1111/j.1471-4159.2008.05320.x. [DOI] [PubMed] [Google Scholar]
- Patterson Ml. PhD thesis. University of Alberta; Edmonton, Canada: 2005. Definition of the degeneration phenotype in light-induced retinal degeneration in rats, a model system of human retinal dystrophy. [Google Scholar]
- Penn JS, Anderson RE. Effect of light history on rod outer segment membrane composition in the rat. Exp Eye Res. 1987;44:767–78. doi: 10.1016/s0014-4835(87)80040-4. [DOI] [PubMed] [Google Scholar]
- Penn JS, Anderson RE. Effect of light history on the rat retina. In: Osborne N, Chader G, editors. Progress in Retinal Research. Pergamon Press; Oxford, UK: 1992. pp. 75–98. [Google Scholar]
- Penn JS, Naash MI, Anderson RE. Effect of light history on retinal antioxidants and light damage susceptibility. Exp Eye Res. 1987;44:779–88. doi: 10.1016/s0014-4835(87)80041-6. [DOI] [PubMed] [Google Scholar]
- Penn JS, Tolman BL, Thum LA, Koutz CA. Effect of light history on the rat retina: Time course of adaptation and readaptation. Neurochemical Res. 1992;17:91–99. doi: 10.1007/BF00966869. [DOI] [PubMed] [Google Scholar]
- Penn JS, Williams TP. Photostasis: Regulation of daily photon catch by rat retinas in response to various cyclic illuminances. Exp Eye Res. 1986;43:915–28. doi: 10.1016/0014-4835(86)90070-9. [DOI] [PubMed] [Google Scholar]
- Perche O, Doly M, Ranchon-Cole I. Caspase-dependent apoptosis in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:2753–59. doi: 10.1167/iovs.06-1258. [DOI] [PubMed] [Google Scholar]
- Philip NJ, Chang W, Long K. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 1987;225:127–32. doi: 10.1016/0014-5793(87)81144-4. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–05. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranchon I, Chen S, Alvarez K, Anderson RE. Systemic administration of phenyl-N-tert-butylnitrone protects the retina from light damage. Invest Ophthalmol Vis Sci. 2001;42:1375–79. [PubMed] [Google Scholar]
- Ranchon I, Gorrand J-M, Cluzel J, Droy-Lefaix M-T, Doly M. Functional protection of photoreceptors from light-induced damage by dimethylthiourea and ginkgo biloba extract. Invest Ophthalmol Vis Sci. 1999;40:1191–99. [PubMed] [Google Scholar]
- Ranchon I, LaVail MM, Kotake Y, Anderson RE. Free radical trap phenyl-N-tert-butylnitrone protects against light damage but does not rescue P23H and S334ter rhodopsin transgenic rats from retinal degeneration. J Neurosci. 2003;23:1375–79. doi: 10.1523/JNEUROSCI.23-14-06050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NA, Thaete LG, Delmage M, Sevanian A. Superoxide dismutase in ocular structures. Invest Ophthalmol Vis Sci. 1985;26:1778–81. [PubMed] [Google Scholar]
- Rapp LM. Retinal phototoxicity. In: Chang LW, Dyer RS, editors. Handbook of Neurotoxicology. Marcel Dekker; New York: 1995. pp. 963–1003. [Google Scholar]
- Rapp LM, Fisher PL, Dhindsa HS. Reduced rate of rod outer segment disk synthesis in photoreceptor cells recovering from UVA light damage. Invest Ophthalmol Vis Sci. 1994;35:3540–48. [PubMed] [Google Scholar]
- Rapp LM, Naash MI, Wiegand RD, Joel CD, Nielsen JC, et al. Morphological and biochemical comparisons between retinal regions having differing susceptibility to photoreceptor degeneration. In: La Vail MM, editor. Retinal Degeneration: Experimental and Clinical Studies. Alan R Liss; New York: 1985. pp. 421–37. [Google Scholar]
- Rapp LM, Williams TP. A parametric study of retinal light damage in albino and pigmented rats. In: Williams TP, Baker BN, editors. The effects of constant light on visual processes. Plenum Press; New York: 1980. pp. 133–59. [Google Scholar]
- Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, et al. Rpe65 is necessary for production of 11-cis vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–51. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- Remé CE. The dark side of light: Rhodopsin and the silent death of vision. Invest Ophthalmol Vis Sci. 2005;46:2672–82. doi: 10.1167/iovs.04-1095. [DOI] [PubMed] [Google Scholar]
- Remé CE, Braschler UF, Roberts J, Dillon J. Light damage in the rat retina: Effects of a radioprotective agent (WR-77913) on acute rod outer segment disk disruptions. Photochem Photobiol. 1991;54:137–42. doi: 10.1111/j.1751-1097.1991.tb01997.x. [DOI] [PubMed] [Google Scholar]
- Remé CE, Grimm C, Hafezi F, Marti A, Wenzel A. Apoptotic cell death in retinal degenerations. Progress in Retinal and Eye Res. 1998a;17:443–463. doi: 10.1016/s1350-9462(98)00009-3. [DOI] [PubMed] [Google Scholar]
- Remé CE, Hafezi F, Marti A, Munz K, Reinboth JJ. Light damage to the retina and pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium, Current Aspects of Function and Disease. Oxford University Press; Oxford UK: 1998b. pp. 563–86. [Google Scholar]
- Remé CE, Malone A, Jung HH, Wei Q, Munz K. Effect of dietary fish oil on acute light-induced photoreceptor damage in the rat retina. Invest Ophthalmol Vis Sci. 1993;35:78–90. [PubMed] [Google Scholar]
- Remé C, Reiboth J, Clausen M, Hafezi F. Light damage revisited: Converging evidence, diverging views? Graefe’s Arch Clin Exp Ophthalmol. 1996;234:2–11. doi: 10.1007/BF00186512. [DOI] [PubMed] [Google Scholar]
- Remé CE, Weller M, Szczyesny P, Munz K, Hafezi F, et al. Light-induced apoptosis in the rat retina in-vivo. In: Anderson RE, LaVail MM, Hollyfield JG, editors. Degenerative Diseases of the Retina. Plenum Press; New York: 1995. pp. 19–25. [Google Scholar]
- Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MJ, Nagel BA, Fliesler SJ. Lipid hydroperoxide formation in the retina: Correlation with retinal degeneration and light damage in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82:538–41. doi: 10.1016/j.exer.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H. Cell death in retinitis pigmentosa: gap junctions and the ‘bystander’ effect. Exp Eye Res. 2002;74:327–36. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- Ritter E, Elgeti M, Bartl FJ. Activity switches of rhodopsin. Photochem Photobiol. 2008;84:911–20. doi: 10.1111/j.1751-1097.2008.00324.x. [DOI] [PubMed] [Google Scholar]
- Rocha FA, Ahnelt PK, Peichl L, Saito CA, Silveira LC, et al. The topography of cone photoreceptors in the retina of a diurnal rodent, the agouti (Dasyprocta aguti) Vis Neurosci. 2009;26:167–75. doi: 10.1017/S095252380808098X. [DOI] [PubMed] [Google Scholar]
- Rodriguez IR, Fliesler SJ. Photodamage generates 7-keto- and 7-hydroxycholesterol in the rat retina via a free radical-mediated mechanism. Photochem Photobiol. 2009;85:1116–25. doi: 10.1111/j.1751-1097.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Matthes MT, LaVail MM, Reichardt LF. Lack of p75 receptor does not protect photoreceptors from light-induced cell death. Exp Eye Res. 2003;76:125–29. doi: 10.1016/s0014-4835(02)00258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, et al. Blue light-induced reactivity of retinal age pigment. J Biol Chem. 1995;270:18825–30. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- Rozanowska M, Rozanowski B, Boulton M. Smith KC, editor. Light-induced damage to the retina. Photobiology of the Retina. 2009 Photobiological Sciences Online. http://www.photobiology.info/
- Rozanowska M, Sarna T. Light-induced damage to the retina: Role of rhodopsin chromophore revisited. Photochem Photobiol. 2005;81:1305–30. doi: 10.1562/2004-11-13-IR-371. [DOI] [PubMed] [Google Scholar]
- Saari JC. Biochemistry of visual pigment regeneration. Invest Ophthalmol Vis Sci. 2000;41:337–48. [PubMed] [Google Scholar]
- Saari JC, Nawrot M, Kennedy BN, Garwin GG, Hurley JB, et al. Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron. 2001;29:739–48. doi: 10.1016/s0896-6273(01)00248-3. [DOI] [PubMed] [Google Scholar]
- Sanvicens N, Gomez-Vicente V, Masip I, Messeguer A, Cotter TG. Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J Biol Chem. 2004;279:39268–78. doi: 10.1074/jbc.M402202200. [DOI] [PubMed] [Google Scholar]
- Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, et al. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci. 2009;106:5171–76. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinfar S, Edward DP, Tso MO. A pathological study of photoreceptor cell death in retinal photic injury. Cur Eye Res. 1991;10:47–59. doi: 10.3109/02713689109007610. [DOI] [PubMed] [Google Scholar]
- Soderpalm AK, Fox DA, Karlsson J-O, van Veen T. Retinoic acid produces rod photoreceptor selective apoptosis in developing mammalian retina. Invest Ophthalmol Vis Sci. 2000;41:937–47. [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, et al. Massive light-driven translocation of transducin between two major compartments of rod cells: A novel mechanism of light adaptation. Neuron. 2002;35:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Solovei I, Kreysing M, Lanctôt C, Kösem S, Peichl L, et al. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–68. doi: 10.1016/j.cell.2009.01.052. [DOI] [PubMed] [Google Scholar]
- Sommer ME, Farren DL. Arrestin can act as a regulator of rhodopsin photochemistry. Vision Res. 2006;46:4532–46. doi: 10.1016/j.visres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–89. [PubMed] [Google Scholar]
- Sperling HG. Spectral sensitivity, intense spectral light studies and the color receptor mosaic of primates. Vision Res. 1986;26:1557–71. doi: 10.1016/0042-6989(86)90175-6. [DOI] [PubMed] [Google Scholar]
- Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, et al. Mechanisms of photoreceptor death and survival in mammalian retina. Progress in Retinal and Eye Res. 1999;6:689–735. doi: 10.1016/s1350-9462(98)00032-9. [DOI] [PubMed] [Google Scholar]
- Streichert LC, Birnbach CD, Reh TA. A diffusible factor from normal retinal cells promotes rod photoreceptor survival in an in vitro model of retinitis pigmentosa. J Neurobiol. 1999;39:475–90. [PubMed] [Google Scholar]
- Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD-36-mediated phagocytosis by retinal pigment epithelium. J Biol Sci. 2006;281:4222–30. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes SM, Robison WG, Jr, Waxler M, Kuwabara T. Damage to the monkey retina by broad-spectrum fluorescent light. Invest Ophthalmol Vis Sci. 1981;20:425–34. [PubMed] [Google Scholar]
- Szél A, Röhlich P. Two cone types of rat retina detected by anti-visual pigment antibodies. Exp Eye Res. 1992;55:47–52. doi: 10.1016/0014-4835(92)90090-f. [DOI] [PubMed] [Google Scholar]
- Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:764–8. doi: 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005a;46:3859–68. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- Tanito M, Kaidzu S, Anderson RE. Delayed loss of cone and remaining rod photoreceptor cells due to impairment of choroidal circulation after acute light exposure in rats. Invest Ophthalmol Vis Sci. 2007a;48:1864–72. doi: 10.1167/iovs.06-1065. [DOI] [PubMed] [Google Scholar]
- Tanito M, Li F, Elliott MH, Dittmar M, Anderson RE. Protective effect of TEMPOL derivatives against light-induced retinal damage in rats. Invest Ophthalmol Vis Sci. 2007b;48:1900–05. doi: 10.1167/iovs.06-1057. [DOI] [PubMed] [Google Scholar]
- Tanito M, Masutani H, Kim Y-C, Nishikawa M, Ohira A, et al. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Ophthalmol Vis Sci. 2005b;46:979–87. doi: 10.1167/iovs.04-1120. [DOI] [PubMed] [Google Scholar]
- Tanito M, Nishiyama A, Tanaka T, Masutani H, Nakamura H, et al. Change of redox status and modulation by thiol replenishment in retinal photooxidative damage. Invest Ophthalmol Vis Sci. 2002;43:2392–2400. [PubMed] [Google Scholar]
- Tinoco J. Dietary requirements and functions of alpha-linolenic acid in animals. Prog Lipid Res. 1982;21:1–45. doi: 10.1016/0163-7827(82)90015-7. [DOI] [PubMed] [Google Scholar]
- Tomita H, Kotake Y, Anderson RE. Mechanism of protection from light-induced retinal degeneration by the synthetic antioxidant phenyl-N-tert-butylnitrone. Invest Ophthalmol Vis Sci. 2005;46:427–34. doi: 10.1167/iovs.04-0946. [DOI] [PubMed] [Google Scholar]
- Tso MOM. Retinal photic injury in normal and scorbutic monkeys. Trans Am Ophthalmol Soc. 1987;85:498–556. [PMC free article] [PubMed] [Google Scholar]
- Unoki K, Ohba N, Arimura H, Muramatsu H, Muramatsu T. Recue of photoreceptors from the damaging effects of constant light by midkine, a retinoic acid-responsive gene product. Invest Ophthalmol Vis Sci. 1994;35:4063–68. [PubMed] [Google Scholar]
- Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem Photobiol. 2002;75:547–53. doi: 10.1562/0031-8655(2002)075<0547:efacro>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Peachey NS, Richards MJ, Buchan B, Fliesler SJ. Light-induced exacerbation of retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82:296–504. doi: 10.1016/j.exer.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. R-a-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2005;46:4302–10. doi: 10.1167/iovs.04-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh N, Valter K, Stone J. Cellular and subcellular patterns of expression of bFGF and CNTF in the normal and light stressed adult rat retina. Exp Eye Res. 2001;72:495–501. doi: 10.1006/exer.2000.0984. [DOI] [PubMed] [Google Scholar]
- Wang J-Y, Saito M. Dietary supplementation of n-3 fatty acids and hydroperoxide levels in rat retina. Free Rad Res. 2001;35:367–75. doi: 10.1080/10715760100300881. [DOI] [PubMed] [Google Scholar]
- Wang M, Lam TT, Tso MOM, Naash MI. Expression of a mutant opsin gene increases the susceptibility of the retina to light damage. Vis Neurosci. 1997;14:55–62. doi: 10.1017/s0952523800008750. [DOI] [PubMed] [Google Scholar]
- Wen R, Cheng T, Song Y, Matthes MT, Yasumura D, et al. Continuous exposure to bright light upregulates bFGF and CNTF expression in the rat retina. Curr Eye Res. 1998;17:494–500. doi: 10.1076/ceyr.17.5.494.5186. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Marti A, Kueng-Hitz N, Hafezi F, et al. c-fos Controls the “private pathway” of light-induced apoptosis of retinal photoreceptors. J Neurosci. 2000;20:81–88. doi: 10.1523/JNEUROSCI.20-01-00081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Remé CE. The genetic modifier RPE65Leu 450: Effect on light damage susceptibility in c-fos-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:2798–2802. doi: 10.1167/iovs.02-1134. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Remé CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroportection for retinal degeneration. Progress in Retinal and Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Seeliger MW, Jaissle G, Hafezi F, et al. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001b;42:1653–59. [PubMed] [Google Scholar]
- Wenzel A, Iseli HP, Fleischmann A, Hafezi F, Grimm C, et al. Fra-1 substitutes for c-fos in AP-1 mediated signal transduction in retinal apoptosis. J Neurochem. 2002;80:1089–94. doi: 10.1046/j.0022-3042.2002.00807.x. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Remé CE, Williams TP, Hafezi F, Grimm C. The RPE65 leu 450 met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001a;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RW, Dowling JE. Anatomical evidence for cone and rod-like receptors in the gray squirrel, ground squirrel, and prairie dog retinas. J Comp Neurol. 1975;159:439–60. doi: 10.1002/cne.901590402. [DOI] [PubMed] [Google Scholar]
- Whelan JP, McGinnis JF. Light-dependent subcellular movement of photoreceptor proteins. J Neurosci Res. 1988;20:263–70. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- White DA, Hauswirth WW, Kaushal S, Lewin AS. Increased sensitivity to light-induced damage in a mouse model of autosomal dominant retinal disease. Invest Ophthalmol Vis Sci. 2007;48:1942–51. doi: 10.1167/iovs.06-1131. [DOI] [PubMed] [Google Scholar]
- White MP, Fisher LV. Degree of light damage to the retina varies with time of day of bright light exposure. Physiol Behavior. 1987;39:607–13. doi: 10.1016/0031-9384(87)90160-0. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, O’Steen WK. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 1992;33:1894–1902. [PubMed] [Google Scholar]
- Wiechmann AF, Sinacola MK. Diurnal expression of recoverin in the rat retina. Mol Brain Res. 1997;45:321–24. doi: 10.1016/s0169-328x(96)00288-4. [DOI] [PubMed] [Google Scholar]
- Wiegand RD, Giusto NM, Rapp LM, Anderson RE. Evidence for rod outer segment lipid peroxidation following constant illumination of the rat retina. Invest Ophthalmol Vis Sci. 1983;24:1433–35. [PubMed] [Google Scholar]
- Wiegand RD, Koutz CA, Chen H, Anderson RE. Effect of dietary fat and environmental lighting on the phospholipid molecular species of rat photoreceptor membranes. Exp Eye Res. 1995;60:291–306. doi: 10.1016/s0014-4835(05)80111-3. [DOI] [PubMed] [Google Scholar]
- Wikler KC, Rakic P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J Neurosci. 1990;10:3390–401. doi: 10.1523/JNEUROSCI.10-10-03390.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TP. Light history and photostasis. In: Williams TP, Thistle AB, editors. Photostasis and Related Phenomena. Plenum Press; New York: 1998. pp. 17–32. [Google Scholar]
- Williams TP, Howell WL. Action spectrum of retinal light damage in albino rats. Invest Ophthalmol Vis Sci. 1983;24:285–87. [PubMed] [Google Scholar]
- Williams TP, Penn JS. Intracellular topography of rhodopsin regeneration in vertebrate rods. J Gen Physiol. 1985;86:413–22. doi: 10.1085/jgp.86.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TP, Squitieri A, Henderson RP, Webbers JPP. Reciprocity between light intensity and rhodopsin concentration across the rat retina. J Physiology. 1999;516:869–74. doi: 10.1111/j.1469-7793.1999.0869u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS. An hypothesis to account for the renewal of outer segments in rod and cone photoreceptor cells: Renewal as a surrogate antioxidant. Invest Ophthalmol Vis Sci. 2008;49:3259–61. doi: 10.1167/iovs.08-1785. [DOI] [PubMed] [Google Scholar]
- Wong P, Kutty RK, Darrow RM, Shivaram S, Kutty G, et al. Changes in clusterin expression associated with light-induced retinal damage in rats. Biochem Cell Biol. 1994;72:499–503. doi: 10.1139/o94-067. [DOI] [PubMed] [Google Scholar]
- Wood JPM, Osborne NN. Zinc and energy requirements in induction of oxidative stress to retinal pigment epithelial cells. Neurochem Res. 2003;28:1525–33. doi: 10.1023/a:1025622425501. [DOI] [PubMed] [Google Scholar]
- Wu J, Gorman A, Zhou X, Sandra C, Chen E. Involvement of caspase-3 in photoreceptor cell apoptosis induced by in vivo blue light exposure. Invest Ophthalmol Vis Sci. 2002;43:3349–54. [PubMed] [Google Scholar]
- Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Survey Ophthalmol. 2006;51:461–81. doi: 10.1016/j.survophthal.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, et al. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389:505–09. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, vander Horst GTJ, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–81. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- Yang J-H, Basinger SF, Gross RL, Wu SM. Blue light-induced generation of reactive oxygen species in photoreceptor ellipsoids requires mitochondrial electron transport. Invest Ophthalmol Vis Sci. 2003;44:1312–19. doi: 10.1167/iovs.02-0768. [DOI] [PubMed] [Google Scholar]
- Yang LP, Zhu XA, Tso MO. Role of NF-kappaB and MAPKs in light-induced photoreceptor apoptosis. Invest Ophthalmol Vis Sci. 2007a;48:4766–76. doi: 10.1167/iovs.06-0871. [DOI] [PubMed] [Google Scholar]
- Yang LP, Zhu XA, Tso MO. A possible mechanism of microglia-photoreceptor crosstalk. Mol Vis. 2007b;13:2048–57. [PubMed] [Google Scholar]
- Yang Y, Mohand-Said S, Danan A, Simonutti M, Fontaine V, et al. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009;17:787–95. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Kawamura K, Imaki J. Differential expression of c-fos mRNA in rat retinal cells: Regulation by light/dark cycle. Neuron. 1993;10:1049–54. doi: 10.1016/0896-6273(93)90053-t. [DOI] [PubMed] [Google Scholar]
- Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–13. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly (ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–89. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- Zhao S, Barnstable CJ. Differential effects of bFGF on development of the rat retina. Brain Res. 1996;723:169–76. doi: 10.1016/0006-8993(96)00237-5. [DOI] [PubMed] [Google Scholar]
- Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A@E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci. 2006;103:16182–87. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]