Abstract
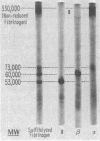
The three unique polypeptide chains of human fibrinogen differ significantly in molecular weight. Cross-linkage of fibrin by fibrin-stabilizing factor results in the rapid formation of cross-links between γ-chains and a slower formation of cross-links between α-chains. β-Chains are not involved directly in the cross-linking of fibrin. Reduced, cross-linked fibrin contains uncross-linked β-chains, dimers of γ-chain, and higher polymers of α-chain. Although it is uncertain whether the γ-γ dimers are formed by chains in different molecules of fibrin, the polymers of α-chain in fibrin can only be accounted for by cross-linkage of α-chains in different molecules. The nature of cross-linkage among the subunits in fibrin can account well for the three-dimensional, covalent structure of cross-linked, insoluble fibrin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Johnson P., Mihalyi E. Physicochemical studies of bovine fibrinogen. I. Molecular weight and hydrodynamic properties of fibrinogen and fibrinogen cleaved by sulfite in 5 M guanidine-HC-l solution. Biochim Biophys Acta. 1965 Jul 22;102(2):467–475. doi: 10.1016/0926-6585(65)90137-8. [DOI] [PubMed] [Google Scholar]
- Lorand L., Chenoweth D., Domanik R. A. Chain pairs in the crosslinking of fibrin. Biochem Biophys Res Commun. 1969 Oct 8;37(2):219–224. doi: 10.1016/0006-291x(69)90722-0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Chenoweth D. Intramolecular localization of the acceptor cross-linking sites in fibrin. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1247–1252. doi: 10.1073/pnas.63.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee P. A., Rogers L. A., Marler E., Hill R. L. The subunit polypeptides of human fibrinogen. Arch Biochem Biophys. 1966 Sep 26;116(1):271–279. doi: 10.1016/0003-9861(66)90033-6. [DOI] [PubMed] [Google Scholar]
- Mills D. A., Triantaphyllopoulos D. C. Distribution of carbohydrate among the polypeptide chains and plasmin digest products of human fibrinogen. Arch Biochem Biophys. 1969 Dec;135(1):28–35. doi: 10.1016/0003-9861(69)90512-8. [DOI] [PubMed] [Google Scholar]
- Takagi T., Iwanaga S. Polypeptide chain involved in the cross-linking of stabilized bovine fibrin. Biochem Biophys Res Commun. 1970 Jan 6;38(1):129–136. doi: 10.1016/0006-291x(70)91094-6. [DOI] [PubMed] [Google Scholar]
- Waugh D. F., Livingstone B. J. Clotting Time and Reaction Velocity in the Interaction of Bovine Fibrinogen and Thrombin. Science. 1951 Feb 2;113(2927):121–124. doi: 10.1126/science.113.2927.121. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]