Abstract
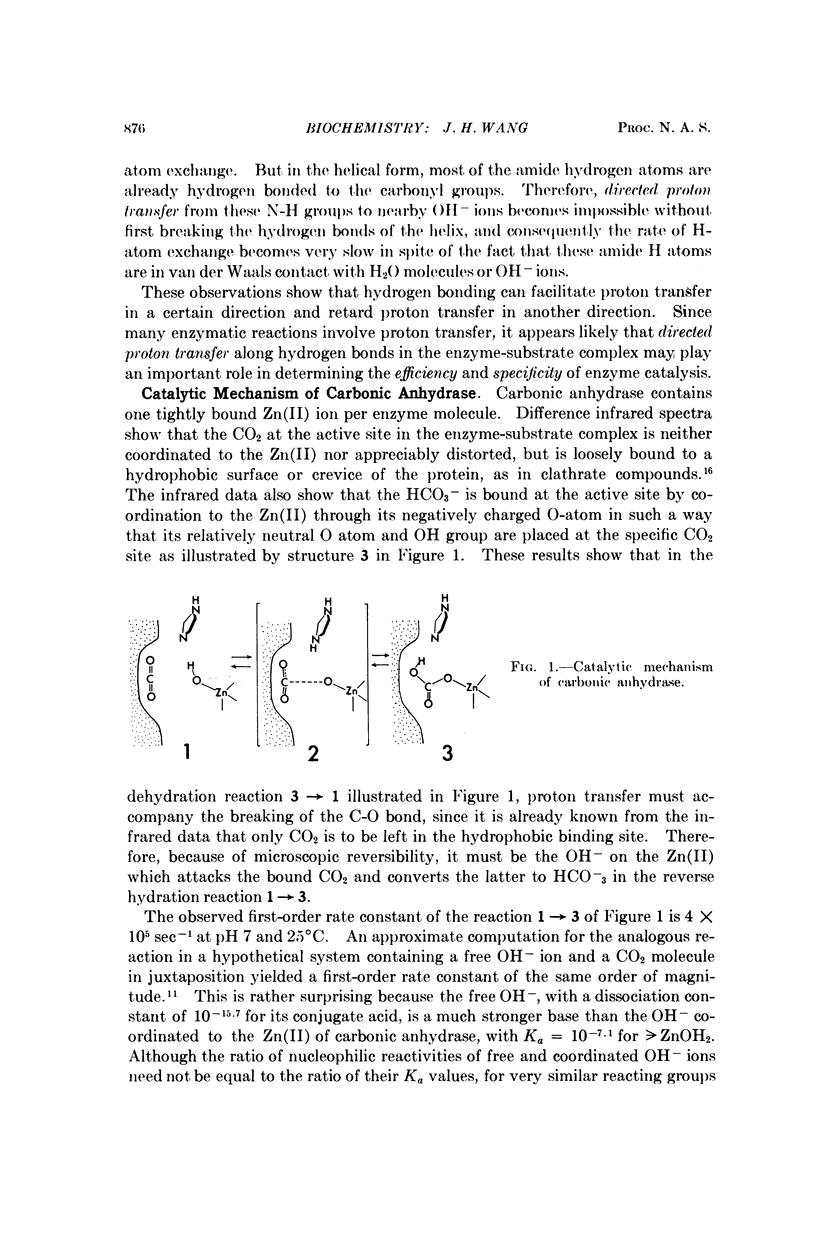
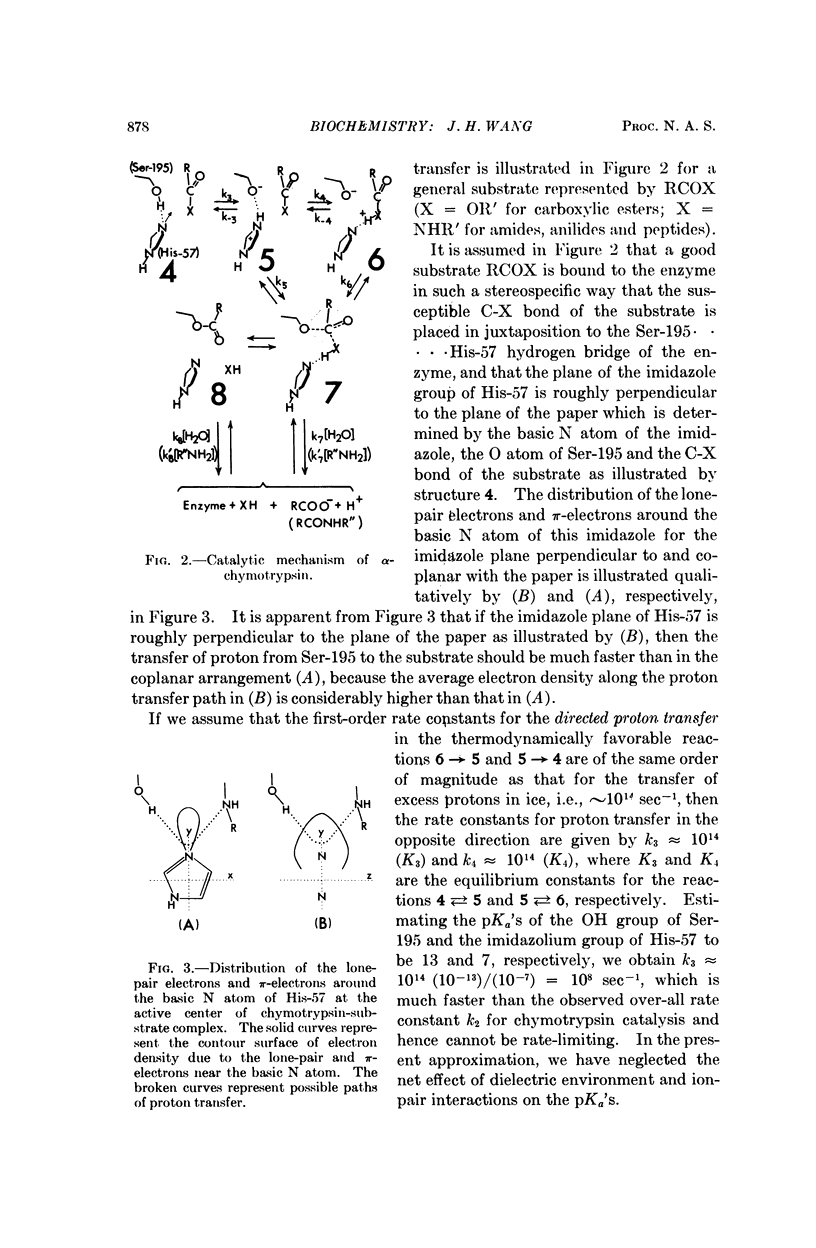
Hydrogen bonding can facilitate proton transfer in certain directions and retard proton transfer in certain other directions. By assuming that directed proton transfer along strategically oriented hydrogen bonds in the enzyme-substrate complex plays an important role in determining the efficiency and specificity of the enzyme, we present a unified interpretation for the reported observations on carbonic anhydrase and α-chymotrypsin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. L., Gibian M. J., Whelan D. J. The alkaline pH dependence of chymotrypsin reactions: postulation of a pH-dependent intramolecular competitive inhibition. Proc Natl Acad Sci U S A. 1966 Sep;56(3):833–839. doi: 10.1073/pnas.56.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Bruice T. C., Pandit U. K. INTRAMOLECULAR MODELS DEPICTING THE KINETIC IMPORTANCE OF "FIT" IN ENZYMATIC CATALYSIS. Proc Natl Acad Sci U S A. 1960 Apr;46(4):402–404. doi: 10.1073/pnas.46.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNNINGHAM L. W. Proposed mechanism of action of hydrolytic enzymes. Science. 1957 Jun 7;125(3258):1145–1146. doi: 10.1126/science.125.3258.1145. [DOI] [PubMed] [Google Scholar]
- Gutfreund H., Sturtevant J. M. THE MECHANISM OF CHYMOTRYPSIN-CATALYZED REACTIONS. Proc Natl Acad Sci U S A. 1956 Oct;42(10):719–728. doi: 10.1073/pnas.42.10.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY B. S. AMINO-ACID SEQUENCE OF BOVINE CHYMOTRYPSINOGEN-A. Nature. 1964 Mar 28;201:1284–1287. doi: 10.1038/2011284a0. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S., KILBY B. A. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem J. 1954 Feb;56(2):288–297. doi: 10.1042/bj0560288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himoe A., Hess G. P. On the elucidation of the pH dependence of chymotrypsin catalyzed reactions at alkaline pH. Biochem Biophys Res Commun. 1966 May 3;23(3):234–239. doi: 10.1016/0006-291x(66)90533-x. [DOI] [PubMed] [Google Scholar]
- Hollands T. R., Voynick I. M., Fruton J. S. Action of pepsin on cationic synthetic substrates. Biochemistry. 1969 Feb;8(2):575–585. doi: 10.1021/bi00830a017. [DOI] [PubMed] [Google Scholar]
- Inagami T., York S. S., Patchornik A. An electrophilic mechanism in the chymotrypsin-catalyzed hydrolysis of anilide substrates. J Am Chem Soc. 1965 Jan 5;87(1):126–127. doi: 10.1021/ja01079a027. [DOI] [PubMed] [Google Scholar]
- Koshland D. E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc Natl Acad Sci U S A. 1958 Feb;44(2):98–104. doi: 10.1073/pnas.44.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- OOSTERBAAN R. A., VAN ADRICHEM M. E. Isolation of acetyl peptides from acetylchymotrypsin. Biochim Biophys Acta. 1958 Feb;27(2):423–425. doi: 10.1016/0006-3002(58)90359-7. [DOI] [PubMed] [Google Scholar]
- Parker L., Wang J. H. On the mechanism of action at the acylation step of the alpha-chymotrypsin-catalyzed hydrolysis of anilides. J Biol Chem. 1968 Jul 10;243(13):3729–3734. [PubMed] [Google Scholar]
- Riepe M. E., Wang J. H. Infrared studies on the mechanism of action of carbonic anhydrase. J Biol Chem. 1968 May 25;243(10):2779–2787. [PubMed] [Google Scholar]
- SCHAFFER N. K., SIMET L., HARSHMAN S., ENGLE R. R., DRISKO R. W. Phosphopeptides from acid-hydrolyzed P32-labeled diisopropylphosphoryl chymotrypsin. J Biol Chem. 1957 Mar;225(1):197–206. [PubMed] [Google Scholar]
- SMITH E. L. Catalytic action of the metal peptidases. Fed Proc. 1949 Sep;8(3):581–588. [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]


