Abstract
Accumulation of methionine sulfoxide (Met(O)) is a significant feature of human cataract and previous studies have shown that methionine sulfoxide reductase A (MsrA), which acts to repair Met(O), can defend human lens cells against oxidative stress induced cell death. A key feature of oxidative stress is increased reactive oxygen species (ROS) in association with loss of mitochondrial function. Here, we sought to establish a potential role for MsrA in the accumulation of ROS in lens cells and the corresponding mitochondrial membrane potential in these cells. Targeted gene silencing was used to establish populations of lens cells expressing different levels of MsrA, and the mitochondrial membrane potential and ROS levels of these cell populations were monitored. Decreased MsrA levels were found to be associated with loss of cell viability, decreased mitochondrial membrane potential, and increased ROS levels in the absence of oxidative stress. These effects were augmented upon oxidative stress treatment. These results provide evidence that MsrA is a major determinant for accumulation of ROS in lens cells and that increased ROS levels in lens cells are associated with a corresponding decrease in mitochondrial membrane potential that is likely related to the requirement for MsrA in lens cell viability.
Keywords: lens, cataract, MsrA, mitochondrial function, oxidative stress
1. Introduction
Oxidative stress is thought to be a major factor in the development of age-related cataract which is a leading cause of world blindness (Kupfer et al., 1994; Spector, 1995). A major modification associated with oxidative stress is the oxidation of protein methionine residues to methionine sulfoxide (Met(O)). Met(O) is barely detectable in young lenses but increases in the lens with age (Spector, 1995) suggesting that Met(O) formation is a characteristic of lens aging. In cataract, as much as 60% of membrane bound protein methionines are found as Met(O) (Truscott and Augusteyn, 1977; Garner and Spector, 1980) providing an association between methionine oxidation and cataract development. Met(O) formation is known to cause loss of protein and subsequent cellular function (Brot et al., 1981; Caldwell et al., 1978; Ciorba et al., 1997; Johnson and Travis, 1979; Vogt, 1995; Swaim and Pizzo, 1988) that could be detrimental to lens homeostasis and be a causative factor in cataract formation.
Unlike many protein oxidations, Met(O) residues are repaired by members of the methionine sulfoxide reductase (Msr) enzyme system that can restore their activity. Two separate classes of Msrs, called MsrA and MsrB, have been identified that are specific for repair of Met(O) S-and R-epimers, respectively (Weissbach et al., 2002). Msrs dictate life span in species including yeast, flies and mice (Weissbach et al., 2002). For instance, over-expression of MsrA in transgenic flies renders them more resistant to oxidative stress and dramatically increases their lifespan (Ruan et al., 2002). In yeast and human T-lymphocytes over-expression of MsrA confers direct protection against peroxide-mediated oxidative stress (Moskovitz et al., 1998). By contrast, E. coli and yeast lacking MsrA are more sensitive to oxidative stress (Moskovitz et al., 1995, 1997). Deletion of MsrA in mice results in increased sensitivity to oxidative stress, a shortened lifespan, and neurological impairment (Moskovitz et al., 2001).
Msr activity has been detected in the human lens (Spector et al., 1982) and both MsrA (Kantorow et al., 2004) and MsrB (Marchetti et al., 2005) are required for protection of lens cells against oxidative stress induced lethality. Of the many systems that can defend the lens against oxidative stress damage, many are localized to the mitochondria suggesting that protection or repair of mitochondrial components is important for lens maintenance. MsrA has been localized to the mitochondria (Hansel et al., 2002) and its overexpression in cultured PC12 cells reduced ROS levels and prevented loss of mitochondrial membrane potential under hypoxic and hyperoxic conditions (Yermolaieva et al., 2004) suggesting a major role for MrsA in the maintenance of mitochondrial function under oxidative stress conditions. To date, the effect of MsrA deletion on mitochondrial function has not been reported in lens or other cell types.
The requirement of MsrA for lens cell viability combined with its ability to reduce ROS levels and protect mitochondrial function when overexpressed in other systems suggests that deletion of MsrA could contribute to increased ROS levels and loss of mitochondrial function in lens and other cells. Here, we tested this hypothesis by measuring the mitochondrial membrane potential and ROS levels in populations of human lens cells expressing different amounts of MsrA relative to control cells. Our findings provide evidence that MsrA is required for mitochondrial function and the elimination of ROS in human lens cells even in the absence of exogenously added oxidative stress. These results provide evidence that loss of MsrA mitochondrial repair and/or protection could play an important role in the progression of cataract and other oxidative stress associated diseases.
2. Methods
2.1. Cell culture
Transformed human lens epithelial (HLE) cells (SRA01/04) (Matsui et al., 2003) were grown and cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Gaithersburg, MD) supplemented with 15% fetal bovine serum (Invitrogen), gentamicin (50 units/ml; Invitrogen), penicillin-streptomycin antibiotic mix (50 units/ml, Invitrogen) and Fungizone (5 µl/ml, Invitrogen) at 36.5 °C in the presence of 5% CO2.
2.2. Short interfering RNA (siRNA)-targeted gene silencing
Double-stranded siRNAs specific for MsrA were obtained as previously described (Kantorow et al., 2004). HLE cells were transfected using the Transmessenger Transfection Reagent Kit (Qiagen, Valencia, CA). Briefly, HLE cells were plated in 6-well plates at a density of 500,000 cells per well mock-transfected or transfected with 5 µg siRNAs. RNA was isolated using Trizol (Invitrogen) at 48 h post-transfection. MsrA transcript levels were detected by semi-quantitative RT-PCR using the Superscript One-Step RT-PCR kit (Qiagen) and analyzed relative to GAPDH transcript levels by gel electrophoresis. The primer sequences for MsrA and GAPDH are as follows: MsrA forward 5′-AGTACCTGAGCAAGAACCCCA-3′, MsrA reverse 5′-TCACTCAGACCCCAGAAGACA-3′, GAPDH forward 5′-CCACCCATGGCAAATTCCATGGCA-3′, and GAPDH reverse 5′-TCTAGACGGCAGGTCAGGTCCACC-3′. MsrA transcript was amplified for 32 PCR cycles using an annealing temperature of 56 °C and GAPDH transcript was amplified for 20 PCR using an annealing temperature of 60 °C.
2.3. Cell viability measurements
HLE cells were plated in 96-well plates at a density of 20,000 cells per well and mock-transfected or transfected with 0.3 µg of siRNA per well. At 48 h post-transfection, HLEs were treated with 0 µM or 500 µM TBHP for 2 h in serum-supplemented media. After 24 h, cell viability was monitored using an MTS assay (Cell Titer 96 Aqueous One Solution Cell Proliferation Assay, Promega, Madison, WI) containing 3-(4,5-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium following the manufacturer’s protocols. MTS color change was monitored using a TECAN GENios plate reader set at an absorbance reading of 492 nm.
2.4. Analysis of ROS and mitochondrial membrane potential
HLE cells were plated in 6-well plates and transfected and treated with TBHP described above. For ROS detection, cells were stained with 5 µM H2DCFDA containing 2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen) for 60 min. For mitochondrial membrane potential analysis, HLE cells were plated in 6-well plates and untransfected, mock-transfected and transfected as described above without TBHP treatment. 48 h post-transfection, cells were stained with 5 µM JC-1 containing 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide (Invitrogen) for 20 min. ROS staining and membrane potential changes were examined using a Zeiss inverted microscope with a FITC filter and semi-quantitation of ROS fluorescence and membrane potential loss were determined by densitometry.
3. Results
3.1. MsrA is important for lens cell viability in the absence of exogenously added oxidative stress
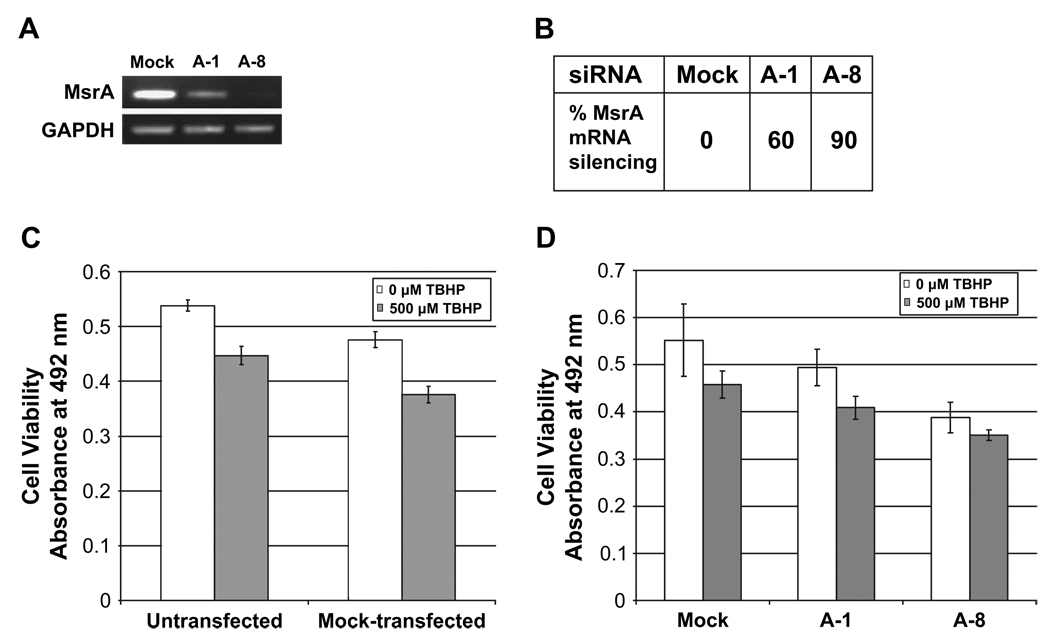
The effect of MsrA deletion on the viability of lens cells was determined using MsrA specific siRNAs to reduce MsrA levels, and subsequent cell viability was monitored by MTS assay. As shown in Figs. 1A and 1B, MsrA-1 siRNA reduced MsrA transcript levels by approximately 60% while MsrA-8 reduced MsrA levels by 90%.
Fig. 1.
Viability of HLE cells treated with MsrA-specific siRNAs under normal and oxidative stress conditions. (A) Ethidium-bromide stained gel showing the levels of MsrA-specific gene suppression at 48 h post-transfection with indicated siRNAs relative to mock-transfected cells. (B) Relative percent of MsrA transcript levels in mock and siRNA-transfected cells determined by densitometry. (C) Representative graph of cell viability measured by MTS assay 48 h between untransfected and mock-transfected cells exposed to 0 µm (white bars) or 500 µm (gray bars) TBHP. Error bars represent standard deviations for three separate assays. (D) Representative graph of cell viability measured by MTS assay 48 h between mock-transfected and siRNA-transfected cells using indicated siRNAs treated with 0 µm (white bars) or 500 µm (gray bars) TBHP. Error bars represent standard deviations for three separate assays.
Using MsrA-1 or MsrA-8 siRNAs respectively, an approximately 11% and 30% decrease in cell viability was detected relative to mock transfected or untransfected cells in the absence of exogenously added oxidative stress (Figs. 1C and 1D). Little or no effect on cell viability was detected between untransfected or mock-transfected cells ruling out the potential loss of cell viability as a consequence of the transfection procedure (Fig. 1C). The addition of 500 µM TBHP further decreased cell viability to 40% using MsrA-1 and 35% using MsrA-8 (Fig. 1D).
3.2. Increased ROS production accompanies silencing of the MsrA gene
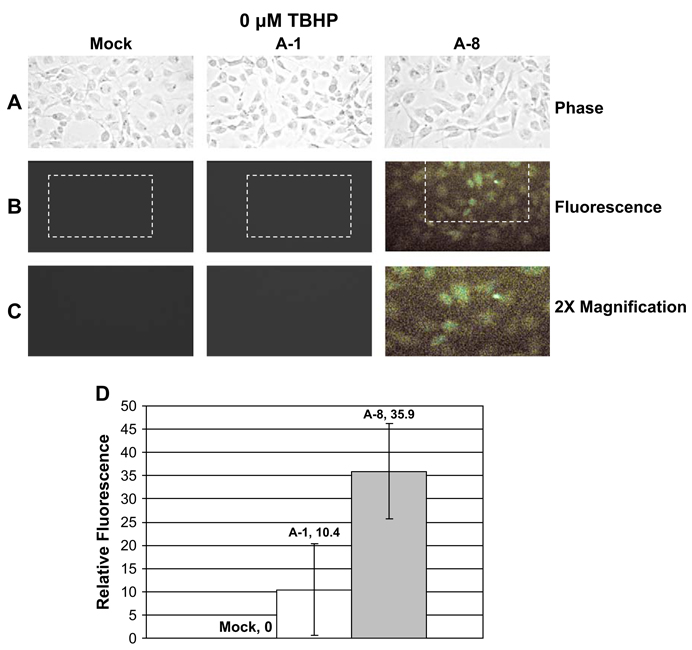
To establish a potential relationship between decreased MsrA levels and increased ROS production, ROS levels were examined between mock transfected and MsrA-1 or MsrA-8 siRNA silenced cells using H2DCFDA, a membrane permeable dye which passively diffuses into cells. Increased levels of ROS cleave the acetate groups resulting in emission of green fluorescence.
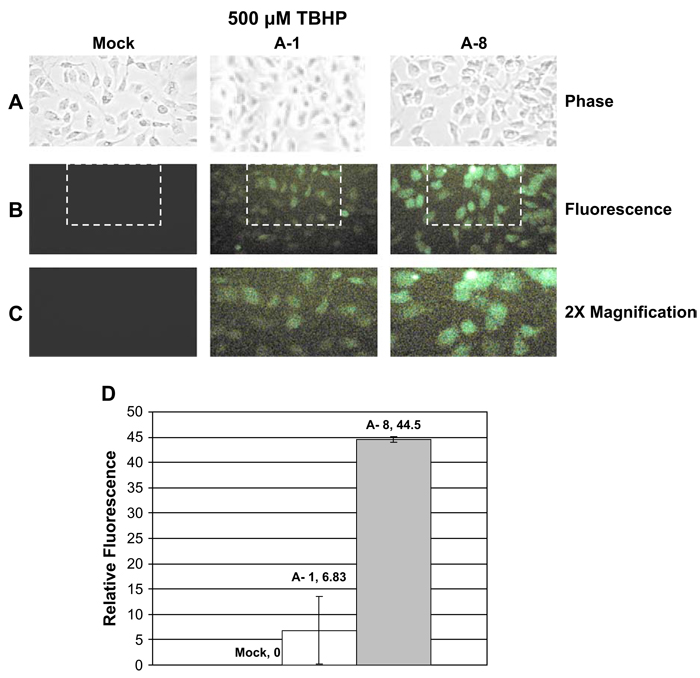
No significant changes in ROS levels were observed in mock or MsrA-1 transfected cells (Fig. 2). However, cells transfected with MsrA-8 which resulted in greater MsrA silencing than MsrA-1, exhibited significant ROS levels in the absence of oxidative stress (Fig. 2). The presence of 500 µM TBHP resulted in even higher ROS levels that was detectable using both siRNAs (Fig. 3). With or without TBHP-oxidative stress treatment, ROS levels detected were proportional to the MsrA levels expressed by each cell population.
Fig. 2.
Detection of ROS in HLE cells treated with MsrA-specific siRNAs under normal conditions. Cells were incubated in the absence of TBHP for 2 h after mock-transfection or siRNA transfection. Cells were stained with H2DCFDA for 60 min to detect ROS by microscopy. (A) Phase contrast. (B) Fluorescence. (C) 2× magnification of indicated area. (D) Representative graph depicting the relative green fluorescence in mock and siRNA-transfected cells derived from three separate experiments.
Fig. 3.
Detection of ROS in HLE cells treated with MsrA-specific siRNAs under oxidative stress conditions. Cells were incubated in the presence of 500 µm TBHP for 2 h after mock-transfection or siRNA-transfection. Cells were stained with H2DCFDA for 60 min to detect ROS by microscopy. (A) Phase contrast. (B) Fluorescence. (C) 2× magnification of indicated area. (D) Representative graph depicting the relative green fluorescence in mock and siRNA-transfected cells derived from three separate experiments.
3.3. Loss of mitochondrial membrane potential accompanies silencing of the MsrA gene
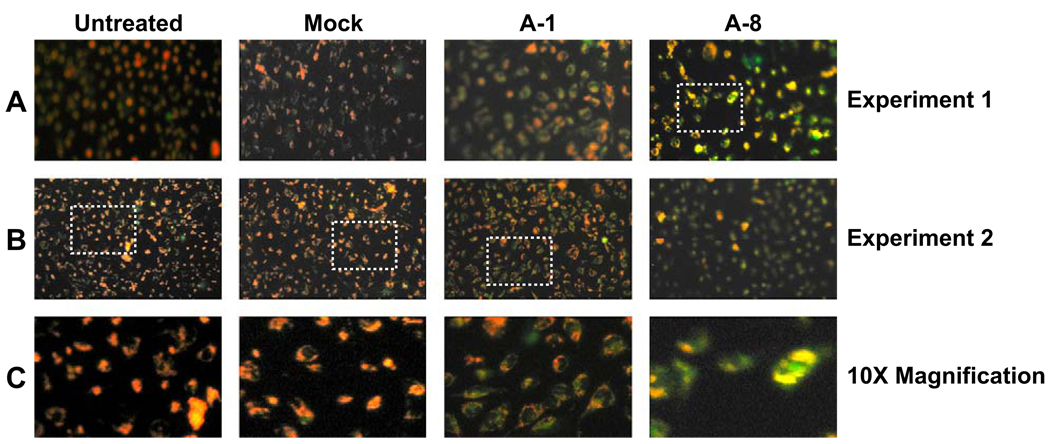
To evaluate the effect of MsrA gene silencing on mitochondrial membrane potential we used a fluorescent dye, JC-1 which exists in the mitochondria as an aggregate that emits red fluorescence and exists in the cytosol as a monomer that emits green fluorescence. The uptake of JC-1 into the mitochondria is dependent on mitochondrial membrane potential so that loss of mitochondrial membrane potential leads to increasing green fluorescence in the cytosol and decreasing mitochondrial red fluorescence.
Inhibition of MsrA gene expression resulted in decreased mitochondrial membrane potential relative to untreated or mock-transfected cells and was proportional to the level of MsrA-gene silencing. Under normal conditions, untreated and mock-transfected cells exhibited aggregation of JC-1 in the mitochondria as evidenced by red staining (Fig. 4, panels A–C untreated cells). By contrast, lens cells transfected with MsrA-1 exhibited loss of membrane potential evidenced by loss of the red mitochondrial staining as a consequence of diffusion of JC-1 into the cytosol and a corresponding increase in green fluorescence due to monomeric JC-1 staining (Fig. 4, panels A–C, MsrA-1). Even more significant loss of mitochondrial membrane potential was observed using MsrA-8 where most of the JC-1 was found in the cytoplasm in the form of diffused green staining (Fig. 4, panels A–C, MsrA-8).
Fig. 4.
Detection of mitochondrial membrane potential in HLE cells treated with MsrA-specific siRNAs in the absence of oxidative stress. Cells were stained with JC-1 for 20 min to detect changes in mitochondrial membrane potential as indicated by red mitochondrial staining (increased potential) or green cytosolic staining (decreased potential). Two separate experiments are shown (A and B).
4. Discussion
Since overexpression of MsrA is associated with decreased ROS and increased mitochondrial function upon oxidative stress conditions, we sought to examine the effects of MsrA deletion on these processes. Since Met(O) is a major characteristic of age-onset cataract and deletion of MsrA cause loss of lens cell viability (Kantorow et al., 2004), lens cells were chosen for these studies. Thus, cell viability, ROS levels and mitochondrial membrane potential were examined in human lens cells expressing decreasing levels of MsrA.
The data demonstrate that loss of MsrA in the absence of exogenous oxidative stress is associated with decreased cell viability (Fig. 1), increased ROS production (Fig. 2 and Fig. 3), and decreased mitochondrial membrane potential (Fig. 4). Addition of TBHP-induced oxidative stress augmented the loss of cell viability and ROS levels (compare Fig. 3 and Fig. 4). These effects were proportional to the level of MsrA in the cells. Collectively, these data support the hypothesis that MsrA is important for mitochondrial function even in the absence of oxidative stress.
We can not rule out the possibility that increased ROS and loss of mitochondrial function upon reduction of MsrA levels in lens cells is an indirect effect arising as a secondary consequence of unknown events that result in damage to mitochondrial components. However, we feel that the present observations are a direct effect of MsrA on the mitochondria since MsrA is found in the mitochondria where it would be expected to act directly on mitochondrial components. Damage to mitochondrial components is known to increase the amount of ROS generated during normal respiration as a consequence of inefficient oxygen conversion. Reduction of MsrA levels could therefore reduce the normal repair of oxidized proteins in the mitochondria initiating a cycle of events leading to increasing mitochondrial damage and, ultimately, apoptotic cell death.
In addition to its established role in protein repair, MsrA has also been proposed to act as a free radical scavenger since each round of methionine oxidation to Met(O) and reduction back to methionine would destroy one equivalent of ROS (Levine et al., 1996). In this mechanism, methionine residues in proteins are functioning as catalytic anti-oxidants. At present we do not know whether the main function of MsrA in mitochondria is to the repair damaged proteins or to act as a ROS scavenger. It would be expected that either of these possibilities would prevent mitochondrial damage and result in decreased ROS.
We acknowledge that the present results obtained using tissue culture cells may not reflect the actual changes that would take place in vivo. Indeed, preliminary examination of the lenses of young MsrA knockout mice have revealed no obvious changes in lens opacity or morphology up to 3 months of age (Kantorow and Giblin, unpublished). However, the same mice exhibit neurological impairment and increased Met(O) content in multiple tissues at the same age (Moskovitz et al., 2001) and exhibit decreased weight and smaller stature. It is possible that lens changes will be observed upon further aging of the knockout mice.
It is also possible that normal oxygen exposure to lens cells could be a source of oxidative stress since the lens in vivo exists in a low oxygen environment. Although this possibility can not be ruled out, we do not believe that this condition contributes to the present data since similar effects have been observed upon deletion of MsrA in retinal cells which are not found in an anoxic environment (Lee et al., 2006). Moreover, no changes in ROS levels or mitochondrial membrane potential were seen in the mock transfected controls.
Regardless of the exact mechanism of MsrA action, the present study provides evidence that MsrA is important for the function of lens cell mitochondria. These data, in combination with data showing increased Met(O) content in aged and cataract lenses (Spector, 1995; Truscott and Augusteyn, 1977; Garner and Spector, 1980) suggests that loss of MsrA activity in combination with the formation of Met(O) and concomitant loss of mitochondrial function could be important factors in the development of cataract and other oxidative stress associated diseases.
Acknowledgments
The authors thank Dr. Venkat Reddy for providing the SRA01/04 lens epithelia cells. This work was supported by National Eye Institute-National Institutes of Health Grant EY13022(MK) and contribution number P200415 from the State of Florida center of Excellence in Biomedical and Marine Biotechnology.
Abbreviations
- Met(O)
methionine sulfoxide
- Msr
methionine sulfoxide reductase
- TBHP
t-butyl hydroperoxide
- ROS
reactive oxygen species
- HLE
human lens epithelial
References
- Brot N, Weissbach L, Werth J, Weissbach H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc. Natl. Acad. Sci. USA. 1981;78:2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P, Luk DC, Weissbach H, Brot N. Oxidation of the methionine residues of Escherichia coli ribosomal protein L12 decreases the protein’s biological activity. Proc. Natl. Acad. Sci. USA. 1978;75:5349–5352. doi: 10.1073/pnas.75.11.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc. Natl. Acad. Sci. USA. 1997;94:9932–9937. doi: 10.1073/pnas.94.18.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner MH, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc. Natl. Acad. Sci. USA. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel A, Kuschel L, Hehl S, Lemke C, Agricola HJ, Hoshi T, Heinemann SH. Mitochondrial targeting of the human peptide methionine sulfoxide reductase (MSRA), an enzyme involved in the repair of oxidized proteins. FASEB J. 2002;16:911–913. doi: 10.1096/fj.01-0737fje. [DOI] [PubMed] [Google Scholar]
- Johnson D, Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J. Biol. Chem. 1979;254:4022–4026. [PubMed] [Google Scholar]
- Kantorow M, Hawse JR, Cowell TL, Benhamed S, Pizarro GO, Reddy VN, Hejtmancik JF. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc. Natl. Acad. Sci. USA. 2004;101:9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer C, Underwood B, Gillen T. Principles and Practice of Ophthalmology Basic Science. Philadelphia: W.B. Saunders Co.; 1994. Leading Causes of Visual Impairment Word Wide; pp. 1249–1255. [Google Scholar]
- Lee JW, Gordiyenko NV, Marchetti M, Tserentsoodol N, Sagher D, Alam S, Weissbach H, Kantorow M, Rodriquez IR. Gene structure, localization and role in oxidative stress of methionine sulfoxide reductase A (MSRA) in the monkey retina. Exp. Eye Res. 2006;82:816–827. doi: 10.1016/j.exer.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Mosoni L, Berlett BS, Statdman ER. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA. 1996;96:1536–1540. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti MA, Pizarro GO, Sagher D, DeAmicis C, Brot N, Hejtmancik JF, Weissbach H, Kantorow M. Methionine sulfoxide Reductases B1, B2, and B3 are present in the human lens and confer oxidative stress resistance to lens cells. Invest. Ophthalmol. Vis. Sci. 2005;46:2107–2112. doi: 10.1167/iovs.05-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Lin LR, Ho YS, Reddy VN. The effects of up-and downregulation of MnSOD enzyme on oxidative stress in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2003;44:3467–3475. doi: 10.1167/iovs.02-0830. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Berlett BS, Poston JM, Stadtman ER. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc. Natl. Acad. Sci. USA. 1997;94:9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Flescher E, Berlett BS, Azare J, Poston JM, Stadtman ER. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc. Natl. Acad. Sci. USA. 1998;95:14071–14075. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Rahman MA, Strassman J, Yancey SO, Kushner SR, Brot N, Weissbach H. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J. Bacteriol. 1995;177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, Hoshi T. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. USA. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- Spector A, Scotto R, Weissbach H, Brot N. Lens methionine sulfoxide reductase. Biochem. Biophys. Res. Commun. 1982;108:429–434. doi: 10.1016/0006-291x(82)91884-8. [DOI] [PubMed] [Google Scholar]
- Swaim MW, Pizzo SV. Methionine sulfoxide and the oxidative regulation of plasma proteinase inhibitors. J. Leukoc. Biol. 1988;43:365–379. doi: 10.1002/jlb.43.4.365. [DOI] [PubMed] [Google Scholar]
- Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim. Biophys. Acta. 1977;492:43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic. Biol. Med. 1995;18:93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- Weissbach H, Etienne F, Hoshi T, Heinemann SH, Lowther WT, Matthews B, St. John G, Nathan C, Brot N. Peptide methionine sulfoxide reductase: structure, mechanism of action, and biological function. Arch. Biochem. Biophys. 2002;397:172–178. doi: 10.1006/abbi.2001.2664. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Xu R, Schinstock C, Brot N, Weissbach H, Heinemann SH, Hoshi T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc. Natl. Acad. Sci. USA. 2004;101:1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]