Abstract
Study Objective:
To study 5-year change in computed tomography (CT)-derived visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) associated with sleep duration in 2 minority groups.
Design:
Longitudinal epidemiologic study.
Setting:
Three US communities.
Participants:
African Americans (N = 332) and Hispanic Americans (N = 775), aged 18-81 years, participating in the IRAS Family Study.
Interventions:
none
Measurements and Results:
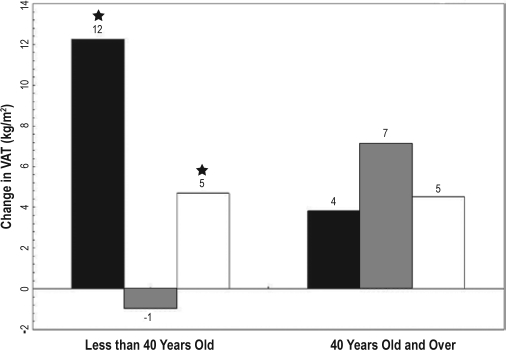
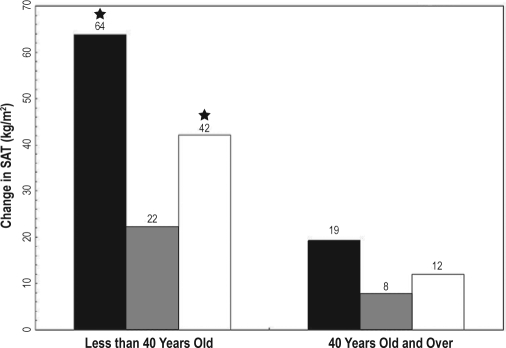
Abdominal CT scans and BMI obtained at a 5-year interval. Sleep duration was assessed by questionnaire at baseline and categorized as ≤5 h, 6-7 h, and ≥8 h. Generalized estimating equations assessed the association between sleep duration and 5-year fat accumulation with adjustment for age, race, gender, study site, baseline fat measure, physical activity, total calories, smoking status, and education. Age interacted with sleep duration to predict change in fat measures (P < 0.01). In those younger than 40 years, ≤5 h of sleep was related to a greater accumulation of BMI (1.8 kg/m2, P < 0.001), SAT (42 cm2, P < 0.0001), and VAT (13 cm2, P > 0.01), compared to sleep duration between 6 and 7 h. Eight hours or more of sleep was also significantly related to a greater accumulation of BMI (0.8 kg/m2, P < 0.001), SAT (20 cm2, P < 0.01) and VAT (6 cm2, P < 0.05) compared to sleep duration between 6 and 7 h. No significant relationship existed between sleep duration and fat depot change in participants older than 40 years old.
Conclusions:
In this minority cohort, extremes of sleep duration are related to increases in BMI, SAT, and VAT in persons younger than 40 years old.
Citation:
Hairston KG; Bryer-Ash M; Norris JM; Haffner S; Bowden DW; Wagenknecht LE. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study.
Keywords: Sleep duration, abdominal fat
THERE EXISTS AN EPIDEMIC OF OBESITY IN THE WESTERN WORLD THAT IS AFFECTING ADULTS AND CHILDREN ALIKE. IN PARTICULAR, CENTRAL obesity has been associated with hypertension, dyslipidemia, insulin resistance, fatty liver disease, and type 2 diabetes.1 Unfortunately, the current arsenal of therapeutic agents for weight loss and weight loss management is small and ineffective. While research has continued to elucidate the untoward consequences of obesity—specifically central adiposity—there has been limited advance in the understanding of why certain populations accumulate adipose tissue centrally.
In concert with the increase in obesity, there has been a decrease in the daily sleep duration over the last several years. Only 38% of American adults reported obtaining 8 h of sleep in 2001, and that number decreased to 28% by 2009.2 Over the last decade, evidence has suggested a role for short sleep duration as a risk factor for weight gain and obesity. The proposed mechanisms include reduced physical activity due to sleep deprivation3 and increased caloric intake secondary to neurohormonal changes. 4Furthermore, longer duration of sleep (> 8 h) is correlated with depression, a known predictor of weight gain.5
While the current literature reports an inverse relationship between sleep duration and obesity in children6,7 and adults,6–8 the current literature has either been cross-sectional in design or used general measures of adiposity such as BMI and weight. Few studies have examined precise measures of central adiposity. Additionally, they have not studied large numbers of under-represented minorities, groups at high risk of obesity.
The IRAS Family study employed computed tomography (CT) scanning of the abdomen in a large cohort of African American and Hispanic American men and women. This study is longitudinal and has obtained self-report measures of sleep duration. Our goal is to determine whether sleep duration is associated with 5-year changes in CT-derived visceral (VAT) and subcutaneous adipose tissue (SAT) areas.
METHODS
The IRAS Family Study was designed to explore genetic and epidemiologic contributions to abdominal adiposity and glucose homeostasis traits among Hispanic and African Americans using a family-based design. The study was an extension of the Insulin Resistance Atherosclerosis Study (IRAS), in which the primary objective was to determine the relationship between insulin resistance and atherosclerosis in 1625 individuals. In the IRAS Family Study, family members of the IRAS cohort were recruited to participate in a baseline clinical examination between 1999 and 2002. Additional families were recruited from the general population to supplement the IRAS families. Ascertainment and recruitment of families were based upon family size, and not on extreme phenotype (e.g., diabetes, obesity). Hispanic families were recruited from San Antonio, TX, and the San Luis Valley, CO. African American families were recruited from Los Angeles, CA. Follow-up examinations were conducted approximately 5 years after baseline examinations. The institutional review boards at the respective institutions approved the protocol, and written informed consent was given by each participant.
Of the initial 1856 participants, aged 18 to 81 years, who attended the baseline IRAS Family Study examination, 1427 participated in the follow-up examination. Overall, there were 405 (21.8%) who did not attend the follow-up visit and 24 (1.3%) who died between visits. Those who died in the interim were older and sicker, and the non-attenders at follow-up had lesser abdominal fat areas compared with those who attended. For this report, we also excluded 320 participants who had incomplete data, namely incomplete CT measures at baseline or follow-up. Thus, this report is based on 1107 people: 332 African Americans and 775 Hispanics.
Measurements followed identical protocols at the baseline and follow-up visits. Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as weight (kg)/height (m)2. Abdominal fat mass was measured at the L2/L3 and L4/L5 vertebral regions by CT under a common protocol at each of the 3 sites. The effective whole-body radiation dose for this study did not exceed 100 mrem. Exclusions for the CT scan included the inability to lay supine, weight exceeding the limit for the CT table (generally 350 to 400 pounds), and pregnancy. All subjects were gowned, and all tight undergarments were removed. Patients were placed in a supine position with the feet or head directed toward the gantry and with the arms above the head. Care was taken to position the patient symmetrically on the CT table. No pads or cushions, other than the standard table pad, were used.
All patients received an anterior-posterior scout of the abdomen and pelvis (diaphragm through symphysis pubis) followed by 3 axial images. Optimal parameters for the scout varied with CT model and patient body habitus. After the scout was obtained, the L4-L5 disc space was located by counting the lumbar vertebra with L1 being the first non-rib bearing vertebra. If there were more or fewer than 5 non rib-bearing lumbar vertebrae, the disc space closest to the iliac crest was considered to be L4-L5. The L2-L3 disc was identified as the second one above L4-L5. Three axial images were acquired. A single 10 mm-thick image was obtained through the L2-L3 disc space, followed by a single 10 mm-thick axial image through the L4-L5 disc space during suspended respiration.
Scans were read centrally using IDL Version 6.3 software (Research Systems, Inc; Boulder, CO). The reading required that a border was drawn within the muscle separating the visceral fat from subcutaneous fat to grossly separate these compartments. Next, the following areas were outlined and factored out of the visceral fat computation: liver area, intra-abdominal vessels, spleen, segment aorta/iliac arteries, bowel fat, and kidney fat. Once these areas were excluded, the areas of VAT and SAT were calculated. VAT and SAT at the L2-L3 and L4-L5 levels were highly correlated. Thus, we chose to use L4-L5 for consistency with the literature, as this slice is highly correlated to visceral fat total volume.9
Baseline sleep duration (hours) was assessed by questionnaire and categorized as ≤5 h, 6-7 h (reference), or ≥ 8 h. The sleep question read, “On average, about how many hours of sleep do you get a night?” Dietary intake was assessed using the Block Brief 2000, a short, retrospective, 1-year, semi-quantitative, food frequency interview. We selected total kilo-calories and several macronutrient measures to incorporate in our statistical models including percent calories from sweets, soluble fiber, total protein, and saturated fat. Smoking status was assessed by questionnaire and categorized as never, current, and former. An estimate of usual frequency of participation in vigorous activities was used with a defined response set ranging from “rarely or never” to “5 or more times per week.” An estimate of energy expenditure was derived from a modified 1-year recall of physical activities. The unit of measurement is kcal energy expended per kg body weight per year. Essentially, each activity group consists of activities requiring similar energy expenditure estimated as METS. For this purpose, 1 MET is equal to 1 kcal expended/kg body weight/h and is also equal to the ratio of active energy expenditure to resting energy expenditure, where resting energy expenditure is assumed to be equal to 1 MET. Accounting for hours of sleep (1 MET) and assuming that time not reported in sleep or in moderate or vigorous activity groups is spent in light activity (1.5 METS), all time can be accounted for by level of energy expenditure, and an estimate of total energy expenditure can be derived. Energy expenditure as estimated from this instrument ought to be consistent with responses to both the overall ranking of activity and with the frequency of participation in vigorous activity. We used education as a surrogate for socioeconomic status (SES), based on the literature.10 Education (highest grade or year of school completed) was assessed by questionnaire and then grouped into 3 categories, “less than high school,” “high school graduate,” and “more than high school.”
SAS version 9.1 (SAS Institute, Cary, NC) was used for all statistical analyses. Data are presented as N (percent) for categorical variables and mean (SD) for continuous variables. Univariate comparisons across sleep duration categories were calculated using χ2 tests of association for categorical variables, and one-way analyses of variance (ANOVAs) for continuous variables. In order to directly compare the impact of various sleep durations on accumulation of fat between the subcutaneous and visceral fat depots, we calculated percent change.
Three separate outcomes were modeled to test for associations with baseline sleep duration using GEE1 linear regression with exchangeable correlation to account for family structure: 5-year change in BMI, 5-year change in SAT, and 5-year change in VAT. Sleep duration was modeled as a categorical variable, and 6-7 h was set as the reference based on a review of previous literature and the distribution of sleep duration in the cohort.11 Two models were fit for each outcome. In the initial models (Model A), sleep duration was adjusted for age, gender, race, center, and the baseline fat measure (baseline BMI for the BMI change model, baseline VAT for the VAT change model, or baseline SAT for the SAT change model). The second set of models (Model B) additionally adjusted for smoking status, physical activity (frequency of participation in vigorous activities), total calories (log transformed), education, and macronutrient intake or kcal energy expended per kg body weight per year. Interactions between gender/sleep duration on fat accumulation and race/sleep duration on fat accumulation were examined and found to be non-significant in all cases; thus our regression analyses included participants of both ethnic groups and genders, with adjustment for these characteristics. The interaction between age and sleep duration was significant for each of the fat change models; thus an age-sleep duration interaction was included in the models (age dichotomized at 40 years, the approximate median of the data). In the results we present (1) the adjusted mean fat accumulation for each sleep category (Table 2 and Figures); and (2) the effect size contrasted to the reference group (text below).
Table 2.
Sleep duration as a predictor of five-year change in BMI and CT-measured adipose tissue areas
| Model A* | BMI(kg/m2) |
SAT(cm2) |
VAT(cm2) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Age:<40 y | LS Mean | Standard Error | Pairwise P-Value | LS Mean | Standard Error | Pairwise P-Value | LS Mean | Standard Error | Pairwise P-Value |
| ≤ 5 h | 2.70 | 0.37 | <0.0001 | 68.21 | 9.79 | 0.0001 | 11.89 | 3.92 | 0.0013 |
| 6–7 h (reference) | 0.91 | 0.14 | - | 26.93 | 4.26 | - | −1.46 | 1.85 | - |
| ≥ 8 h | 1.81 | 0.21 | 0.0004 | 47.54 | 5.93 | 0.0030 | 5.64 | 2.30 | 0.0114 |
| Age:≥40 y | |||||||||
| ≤ 5 h | 0.68 | 0.24 | 0.5018 | 18.20 | 4.97 | 0.1077 | 4.42 | 3.73 | 0.5522 |
| 6–7 h (reference) | 0.49 | 0.15 | - | 8.58 | 3.47 | - | 6.90 | 2.09 | - |
| ≥ 8 h | 0.60 |
0.20 |
0.6118 |
13.52 |
4.85 |
0.3999 |
4.85 |
3.03 |
0.5685 |
| P-value for interaction | 0.0013 | 0.0210 | 0.0093 | ||||||
| Model B** | |||||||||
| Age: < 40 y | |||||||||
| ≤ 5 h | 2.59 | 0.38 | <0.0001 | 63.79 | 9.60 | <0.0001 | 12.25 | 3.89 | 0.0024 |
| 6–7 h (reference) | 0.80 | 0.17 | - | 22.22 | 5.00 | - | −0.96 | 2.31 | - |
| ≥8 h | 1.57 | 0.24 | 0.0025 | 42.13 | 6.97 | 0.0046 | 4.70 | 2.59 | 0.0399 |
| Age:≥ 40 y | |||||||||
| ≤ 5 h | 0.73 | 0.24 | 0.3755 | 19.64 | 5.17 | 0.0520 | 3.82 | 3.97 | 0.4473 |
| 6–7 h (reference) | 0.48 | 0.16 | - | 7.74 | 3.75 | - | 7.14 | 2.08 | - |
| ≥ 8 h | 0.60 |
0.22 |
0.5854 |
11.98 |
5.68 |
0.4978 |
4.51 |
3.12 |
0.4781 |
| P-value for interaction | 0.0036 | 0.0397 | 0.0103 | ||||||
Model A adjusted for age, gender, center/race, baseline fat, and age by sleep duration interaction
Model B is Model A plus adjustment for smoking status, physical activity, total calories and education
RESULTS
The cohort was 62% female and 30% African American. The mean age at baseline was 41.7 years with a range of 18 to 81 years. Mean sleep duration was 6.7 h, with 17% reporting ≤ 5 h, 55% reporting 6-7 h, and 28% reporting ≥ 8 h. In those younger than 40 years old, short sleep duration (≤ 5 h) was most frequently reported by Hispanic men (30%), and long sleep duration (≥ 8 h) was most frequently reported by Hispanic women (53%) (Table 1a). In this sample, 67% never smoked, while 22% were current smokers. Only 11% participated in vigorous activity ≥5 times per week, with most (37%) reporting 2-4 times per week. Participants reporting ≤ 5 h of sleep consumed more total calories (2224 kcal) than those reporting 6-7 h (1920 kcal) or ≥ 8 h (2199 kcal). This pattern was consistent with percent of calories from sweets and saturated fat (grams). Regarding education, high school graduates were the largest percentage of those reporting ≤ 5 h (56%) and 6 to 7 h (54%). In the ≥ 40 years group, short sleep duration was most frequently reported by African American women (36%), and long sleep duration was most frequently reported by Hispanic women (59%). (Table 1b.)
Table 1a.
Baseline characteristics by sleep duration for participants aged less than 40 years (mean and SD, unless noted)
| Overall | ≤ 5 h | 6 to 7 h | ≥ 8 h | P-Value* | |
|---|---|---|---|---|---|
| N | 522 | 71 | 281 | 170 | |
| Age, years | 30.3 (6.0) | 31.9 (5.5) | 30.1 (5.7) | 29.9 (6.5) | 0.0177 |
| African American men, N (%) | 54 (10.3) | 16 (22.5) | 31 (11.0) | 7 (4.1) | <0.0001 |
| African American women, N (%) | 86 (16.5) | 19 (26.8) | 47 (16.7) | 20 (11.8) | <0.0001 |
| Hispanic men, N (%) | 167 (32.0) | 21 (29.6) | 93 (33.1) | 53 (31.2) | <0.0001 |
| Hispanic women, N (%) | 215 (41.2) | 15 (21.1) | 110 (39.2) | 90 (52.9) | <0.0001 |
| BMI, kg/m2 | |||||
| Baseline | 28.3 (6.5) | 29.7 (7.3) | 28.6 (6.3) | 27.2 (6.3) | 0.0278 |
| Follow-up | 29.7 (6.8) | 32.5 (7.9) | 29.5 (6.5) | 29.0 (6.6) | 0.0080 |
| Absolute change | 1.5 (3.0) | 2.8 (3.1) | 0.9 (2.9) | 1.8 (2.9) | <0.0001 |
| Percent change | 5.8 (10.6) | 9.9 (11.7) | 3.8 (9.6) | 7.4 (10.9) | <0.0001 |
| SAT, cm2 | |||||
| Baseline | 326 (175) | 346 (198 ) | 333 (176) | 306 (162) | 0.1860 |
| Follow-up | 367 (181) | 416 (219) | 362 (175)≥ | 356 (170) | 0.1111 |
| Absolute change | 41 (79) | 70 (80) | 29 (76) | 49 (77) | 0.0006 |
| Percent changev | 20 (35)v | 25 (31) | 16 (33) | 24 (38) | 0.0221 |
| VAT, cm2 | |||||
| Baseline | 81 (49) | 85 (53) | 82 (47) | 78 (49) | 0.3052 |
| Follow-up | 88 (50) | 102 (57) | 85 (48) | 87 (50) | 0.0450 |
| Absolute change | 7 (30) | 17 (29) | 3 (30) | 9 (29) | 0.0063 |
| Percent change | 18 (47) | 32 (45) | 13 (45) | 22 (50) | 0.0045 |
| Total calories, kcal | 2,053 (1,059) | 2,224 (1,003) | 1,920 (965) | 2,199 (1,196) | 0.0074 |
| % cal from sweets | 11.7 (8.9) | 13.2 (8.5) | 11.7 (8.9) | 11.1 (8.9) | 0.1629 |
| Soluble fiber (beans, vegs, fruit) , g | 11.0 (7.6) | 10.9 (6.0) | 10.4 (7.4) | 12.2 (8.3) | 0.0412 |
| Protein, g | 83.8 (44.8) | 90.5 (44.6) | 78.0 (39.1) | 90.5 (52.1) | 0.0076 |
| Saturated fat, g | 29.6 (17.2) | 32.3 (16.2) | 27.5 (15.4) | 31.9 (19.8) | 0.0130 |
| Smoking status, N (%) | |||||
| Current | 117 (22.4) | 19 (26.8) | 50 (17.8) | 48 (28.2) | 0.2814 |
| Former | 55 (10.5) | 9 (12.7) | 38 (13.5) | 8 (4.7) | 0.2814 |
| Non-smoker | 350 (67.0) | 43 (60.6) | 193 (68.7) | 114 (67.1) | 0.2814 |
| Vigorous Physical Activity, N (%) | |||||
| Rarely/Never | 77 (14.8) | 13 (18.3) | 36 (12.8) | 28 (16.5) | 0.9231 |
| 1-3 times / month | 117 (22.4) | 12 (16.9) | 69 (24.6) | 36 (21.2) | 0.9231 |
| 1 time/week | 75 (14.4) | 8 (11.3) | 42 (15.0) | 25 (14.7) | 0.9231 |
| 2-4 times / week | 195 (37.4) | 29 (40.9) | 105 (37.4) | 61 (35.9) | 0.9231 |
| 5+ times / week | 58 (11.1) | 9 (12.7) | 29 (10.3) | 20 (11.8) | 0.9231 |
| Education N (%) | |||||
| < High School | 136 (12.8) | 26 (14.9) | 59 (10.1) | 51 (16.8) | |
| High School Graduate | 516 (48.7) | 97 (55.8) | 313 (53.8) | 106 (35.0) | 0.0268 |
| > High School | 407 (38.4) | 51 (29.3) | 210 (36.1) | 146 (48.2) | |
| Total Energy Expenditure (kcal/kg/year) | 16,693(4,222) | 17,954(4,552) | 16,598(3,966) | 16,324(4,416) | 0.0316 |
P-values for continuous variables are based on linear GEE models that adjust for family structure.
P-values for ordinal categorical variables (smoking status, physical activity and education) are based on multinomial GEE models that adjust for family structure. The P-value for race/gender is based on χ2 test.
Table 1b.
Baseline characteristics by sleep duration for participants aged 40 years or older (mean and SD, unless noted)
| Overall | ≤ 5 h | 6 to 7 h | ≤ 8 h | P-value* | |
|---|---|---|---|---|---|
| N | 585 | 113 | 325 | 147 | |
| Age, years | 51.9 (9.1) | 53.7 (10.2) | 51.2 (8.3) | 52.1 (9.6) | 0.0455 |
| African American men, N (%) | 72 (12.3) | 21 (18.6) | 48 (14.8) | 3 (2.0) | < 0.0001 |
| African American women, N (%) | 120 (20.5) | 41 (36.3) | 63 (19.4) | 16 (10.9) | < 0.0001 |
| Hispanic men, N (%) | 129 (22.1) | 13 (11.5) | 75 (23.1) | 41 (27.9) | < 0.0001 |
| Hispanic women, N (%) | 264 (45.3) | 38 (33.6) | 139 (42.8) | 87 (59.2) | < 0.0001 |
| BMI, kg/m2 | |||||
| Baseline | 29.6 (5.9) | 31.4 (7.1) | 29.3 (5.6) | 28.8 (5.0) | 0.0131 |
| Follow-up | 30.1 (6.1) | 32.1 (7.5) | 29.8 (5.6) | 29.3 (5.8) | 0.0041 |
| Absolute change | 0.6 (2.6) | 0.8 (2.5) | 0.5 (2.7) | 0.5 (2.3) | 0.6569 |
| Percent change | 2.2 (9.1) | 2.6 (7.9) | 2.4 (9.9) | 1.7 (7.9) | 0.5566 |
| SAT, cm2 | |||||
| Baseline | 361 (156) | 392 (173 ) | 352 (154) | 358 (145) | 0.0670 |
| Follow-up | 374 (160) | 412 (183) | 363 (156) | 368 (149) | 0.0262 |
| Absolute change | 13 (66) | 20 (61) | 11 (68) | 10 (64) | 0.3161 |
| Percent changev | 6 (21) | 7 (19) | 6 (22) | 5 (21) | 0.6696 |
| VAT, cm2 | |||||
| Baseline | 126 (59) | 139 (65) | 121 (57) | 127 (59) | 0.0070 |
| Follow-up | 127 (62) | 136 (61) | 124 (61) | 125 (64) | 0.1016 |
| Absolute change | 1 (37) | −3 (35) | 3 (37) | −2 (39) | 0.2446 |
| Percent change | 5 (34) | 2 (27) | 7 (35) | 3 (38) | 0.1757 |
| Total calories, kcal | 1,611 (815) | 1,614 (970) | 1,583 (738) | 1,671 (850) | 0.5662 |
| % cal from sweets | 12.0 (10.2) | 12.2 (10.8) | 11.8 (10.3) | 12.4 (9.5) | 0.8294 |
| Soluble fiber (beans, vegs, fruit) , g | 11.3 (7.3) | 11.2 (8.4) | 11.3 (6.6) | 11.2 (7.7) | 0.9754 |
| Protein, g | 65.7 (34.9) | 62.9 (40.3) | 65.2 (31.8) | 68.9 (36.8) | 0.4349 |
| Saturated fat, g | 22.4 (13.1) | 22.7 (15.0) | 21.7 (11.7) | 23.9 (14.3) | 0.2564 |
| Smoking status, N (%) | |||||
| Current | 134 (22.9) | 36 (31.9) | 67 (20.6) | 31 (21.1) | 0.0119 |
| Former | 172 (29.4) | 39 (34.5) | 92 (28.3) | 41 (27.9) | 0.0119 |
| Non-smoker | 279 (47.7) | 38 (33.6) | 166 (51.1) | 75 (51.0) | 0.0119 |
| Vigorous Physical Activity, N (%) | |||||
| Rarely/Never | 186 (31.8) | 46 (40.7) | 89 (27.4) | 51 (34.7) | 0.1567 |
| 1-3 times / month | 127 (21.7) | 21 (18.6) | 69 (21.2) | 37 (25.2) | 0.1567 |
| 1 time/week | 66 (11.3) | 7 (6.2) | 47 (14.5) | 12 (8.2) | 0.1567 |
| 2-4 times / week | 153 (26.2) | 23 (20.4) | 90 (27.7) | 40 (27.2) | 0.1567 |
| 5+ times / week | 53 (9.1) | 16 (14.2) | 30 (9.2) | 7 (4.8) | 0.1567 |
| Education N (%) | |||||
| < High School | 136 (12.8) | 26 (14.9) | 59 (10.1) | 51 (16.8) | |
| High School Graduate | 516 (48.7) | 97 (55.8) | 313 (53.8) | 106 (35.0) | 0.0268 |
| > High School | 407 (38.4) | 51 (29.3) | 210 (36.1) | 146 (48.2) | |
| Total Energy Expenditure (kcal/kg/year) | 15,741(3,570) | 15,743(3,916) | 15,922(3,414) | 15,339(3,623) | 0.2596 |
P-values for continuous variables are based on linear GEE models that adjust for family structure.
P-values for ordinal categorical variables (smoking status, physical activity and education) are based on multinomial GEE models that adjust for family structure. The P-value for race/gender is based on a χ2 test.
The minimally adjusted model for change in BMI revealed that, in the younger age group, ≤ 5 h of sleep was associated with an increase of 1.79 kg/m2 ± 0.37 kg/m2 over 5 years (P < 0.001), and ≥ 8 h was associated with an increase of 0.90 kg/m2 ± 0.0.21 kg/m2(P < 0.001), when compared to 6 to 7 h (Table 2). For SAT, ≤ 5 h of sleep was associated with an increase of 41 cm2 over 5 years (P < 0.001), and ≥ 8 h was associated with an increase of 21 cm2(P < 0.01) when compared to 6 to 7 h. For VAT, ≤ 5 h of sleep was associated with an increase of 13 cm2 over 5 years (P < 0.01), and ≥ 8 h was associated with an increase of 7 cm2(P < 0.05) when compared to 6 to 7 h. Adjustment for additional covariates in Model B resulted in some slight attenuation of the associations. However, in all cases, short and long sleep duration were still significantly associated with increased accumulation of fat relative to sleep duration of 6 to 7 h (Figures 1 and 2). In addition, short sleep duration had the greatest effect on fat accumulation in each fat depot. However, the relative impact of short sleep did not differ between the depots (increases of 25% and 32% for SAT vs VAT, respectively, Table 1a).
Figure 1.
Five-year change in visceral adipose tissue by age ■ ≤ 5 h,  6 to 7 h, □ ≤ 8 h; VAT (visceral adipose tissue); (★) Indicates significant difference from 6 to 7 h (P > 0.01). Model adjusted for age, gender, center/race, baseline fat, and age.
6 to 7 h, □ ≤ 8 h; VAT (visceral adipose tissue); (★) Indicates significant difference from 6 to 7 h (P > 0.01). Model adjusted for age, gender, center/race, baseline fat, and age.
Figure 2.
Five-year change in subcutaneous adipose tissue by age ■ ≤ 5 h,  6 to 7 h, □ ≥ 8 h; SAT (subcutaneous adipose tissue); (★) Indicates significant difference from 6 to 7 h (P > 0.01). Model adjusted for age, gender, center/race, baseline fat, and age.
6 to 7 h, □ ≥ 8 h; SAT (subcutaneous adipose tissue); (★) Indicates significant difference from 6 to 7 h (P > 0.01). Model adjusted for age, gender, center/race, baseline fat, and age.
In the older age group, there was no significant association between BMI, SAT, or VAT and sleep duration in either model (Table 2). However, similar trends persisted for SAT and BMI, with short and long sleep resulting in greater accumulation of fat relative to sleep durations of 6 to 7 h.
DISCUSSION
This is the first epidemiologic study to investigate the relationship between five year changes in CT measured abdominal adiposity with sleep duration in a large cohort of African American and Hispanic men and women. We observed in participants younger than 40 years of age that extremes of sleep duration were associated with increases in BMI, SAT, and VAT fat areas over a 5-year period. Short sleep (≤ 5 h per night) was associated with the greatest accumulation of fat in each depot. No association between sleep duration and change in fat measures was observed in those older than 40 years of age. Our study is unique in its longitudinal design, its use of precise measures of abdominal fat areas by CT, and the examination of a large minority cohort who are known to be disproportionately affected by metabolic disorders such as insulin resistance and diabetes.
Existing literature has clearly documented the relationship of visceral fat accumulation and metabolic disorders, such as insulin resistance, impaired fasting glucose and incident type 2 diabetes.12,14–15 Emerging literature has also shown that extremes in sleep duration are associated longitudinally with increases in BMI and insulin resistance, impaired fasting glucose, and incident type 2 diabetes.16–20 Cross-sectionally, others have shown a clear relationship between visceral and subcutaneous fat accumulation and short sleep duration. It is a logical conclusion, therefore, that extremes in sleep duration may result in increased incidence of metabolic derangements via visceral fat accumulation. Thus far, few studies have shown the relationship between longitudinal changes in precise measures of abdominal adiposity and extremes in sleep duration. The results of this study allow the connection between sleep duration, visceral fat accumulation and glucose derangement to be made.
Sleep duration has been shown to be related to the incidence of metabolic conditions including insulin resistance, metabolic syndrome, and incident diabetes.21 Chaput found the adjusted odds ratio for type 2 diabetes (DM) /impaired glucose tolerance (IGT) was 1.58 (1.13–2.31) for those with 9-10 h of sleep and 2.09 (1.34–2.98) for those with 5-6 h of sleep, relative to 7-8 hours after adjustment for potential confounding variables. The short and long sleepers presented with significantly higher total insulin area under the curve (P < 0.05), whereas total glucose AUC was not different between the three sleep duration groups.22 Gottlieb found in men, specifically, that shorter sleep duration (≤ 6 h of sleep) resulted in twice the rate of incident type 2 diabetes as that in the reference group (7-8 h). Compared with those sleeping 7 to 8 h per night, subjects sleeping ≤ 5 h and 6 h per night had adjusted odds ratios for DM of 2.51 (95% confidence interval, 1.57–4.02) and 1.66 (95% confidence interval, 1.15–2.39), respectively. Adjusted odds ratios for IGT were 1.33 (95% confidence interval, 0.83–2.15) and 1.58 (95% confidence interval, 1.15–2.18), respectively. Subjects sleeping ≥ 9 h per night also had increased odds ratios for DM and IGT. These associations persisted when subjects with insomnia symptoms were excluded.23
In our study, sleep duration was not associated with accumulation of abdominal fat in those older than 40 years of age. This is in contrast to one previous study, which showed that older adults did have differential rates of abdominal depot accumulation based on sleep duration.19 Our study may differ because it is a minority cohort, or because the rate of fat accumulation in the older age group was quite small. Our unique observation suggests that targeted interventions should focus on young adults.
In addition to targeting the younger age group, our work, consistent with others, suggests that ethnic minorities are at high risk for extremes of sleep duration. Previous studies have shown that ethnic minorities, African Americans in particular, consistently report shorter sleep durations than their white or Hispanic counterparts. Hale found that black respondents, aged 18 and older, had an increased risk of being short (≤ 6 h/night) and long (≥ 8 h/night) sleepers (OR = 1.41,95% CI = 1.27–1.57 and OR = 1.62, 95% CI = 1.40–1.88, respectively) relative to white respondents.24 In that study, living in an inner city was associated with increased risk of short sleeping and reduced risk of long sleeping, compared to non-urban areas, a finding that possibly explained some of the higher risk of short sleeping among blacks who are more likely to live in the inner city. Similar results were seen in other studies investigating African American children and young adults.25,26 Similarly, our analysis showed that the women in these ethnic minority groups were at particularly high risk for extremes in sleep duration. The younger African American women were one of the largest groups (27%) of participants reporting ≤ 5 h of sleep second to Hispanic men (30%). Although the adipose accumulation–sleep duration association did not differ by racial group, more African American women than Hispanic women were in the shorter sleep duration category, which we have shown to be associated with higher adiposity accumulation.
These levels of adipose tissue accumulation associated with extremes in sleep duration in the younger age group are clinically significant, being consistent with the effect sizes associated with future risk of type 2 diabetes found in other studies.12–15,27 Boyko and colleagues observed that those persons who progressed to type 2 diabetes had VAT areas that were 20 cm2 larger than those who did not progress to type 2 diabetes.12 Similar results were seen in the Health ABC study where an additional 35 cm2 in VAT area differentiated those developing diabetes from those not developing type 2 diabetes.27 In our study, the increases in VAT and SAT among those with short sleep duration were 12 cm2 and 69 cm2. These increases are similar with previously determined effect sizes, thereby suggesting that this accumulation of abdominal fat may lead to adverse metabolic consequences in these young adults.
Identifying extremes in sleep duration as a potential mechanism for increased visceral fat accumulation in young people and its subsequent risk of metabolic derangements will have direct clinical and research implications. In our study, there was no evidence that short sleep duration differentially impacted the VAT vs SAT depots. It was evident, however, that short sleep tended to impact adiposity more than long sleep. The proposed mechanisms by which shorter sleep impacts fat depots are 2-fold: (1) increased caloric intake via increased hunger caused by increased leptin/ghrelin and increased opportunity to sleep; and (2) reduced energy expenditure via altered thermoregulation and increased fatigue.8 Both increased caloric intake and decreased vigorous activity were observed in our short sleep group.
In clinical practice, discussion about and encouragement of adequate sleep duration should be just as important as healthy diet and increased physical activity. This will be particularly relevant for people as they make life transitions such as college, marriage, and childbearing, because these times are often associated with sleep deprivation in younger ages. In the research arena, since it is becoming clear that sleep duration has significant impact on glucose regulation, blood pressure and other hormone levels,17,28,29 it will be crucial to adjust for sleep duration in studies related to metabolism and its regulation.
There were a few limitations of our study. First, our exposure assessment was somewhat limited. Since this study was not designed to characterize sleep, we only assessed quantity of sleep through self-report, not quality of sleep, differential sleep patterns based on day of week, or use of sleep aids, nor did we inquire about a sleep apnea diagnosis, or perform a sleep study. Studies have shown that self-report is a reasonable measure of actual sleep but people can overestimate the amount of sleep received compared to measured amounts.30,31 We also did not obtain data on important confounders such as depressive symptoms, a well-known correlate of sleep disorders32 and obesity.33 However, our findings persisted after adjustment for a number of potential confounders including physical activity, dietary patterns, and SES.
In summary, our study is the first to describe the effect of sleep duration on changes in CT-derived abdominal fat depots in a large minority cohort. In the younger age group, we observed significant 5-year increases in BMI, VAT, and SAT with 5 hours or less and 8 hours or more of sleep compared to 6-7 hours. Other reports have observed that extremes in sleep duration are related to poor metabolic outcomes including increased rates of obesity, insulin resistance, and type 2 diabetes. Short sleep has become more common in the US, and minorities are disproportionately affected. As we continue to explore the reasons for the rapidly climbing obesity and diabetes rates among young people, particularly ethnic minority groups, our results linking extremes in sleep duration with increases in abdominal fat areas may explain a component of the increase.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bryer-Ash has received research support from Proctor and Gamble, Sanofi-Aventis, and Eli Lilly; has participated in speaking engagements fro Merck and Pfizer; is on the advisory board of Novo-Nordisk; and has financial interest in Amylin, Pfizer, and Dexcom Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported in part by NIH grants HL060894, HL060931, HL060944, HL061019, and HL061210.
REFERENCES
- 1.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: the healthy transitions study. Obesity. 2001;9:10–6. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]
- 2.Foundation NS. Washington, DC: 2009 Sleep in America Poll; 2009. [Google Scholar]
- 3.Dinges DF Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 4.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief Communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 5.Herva A, Laitinen J, Miettunen J, et al. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes. 2005;30:520–7. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- 6.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: A prospective study from birth to 9.5 years. J Pediatr. 2004;145:20–5. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Hasler GG. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 8.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han TS KI, Walsh K, Greene RM, Lean ME. Relationship between volumes and areas from single transverse scans of intra-abdominal fat measured by magnetic resonance imaging. Int J Obes Relat Metab Disord. 1997;21:1161–6. doi: 10.1038/sj.ijo.0800530. [DOI] [PubMed] [Google Scholar]
- 10.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56:769–84. doi: 10.1016/s0277-9536(02)00073-4. [DOI] [PubMed] [Google Scholar]
- 11.Ohayon MM, Vecchierini M. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–9. [PubMed] [Google Scholar]
- 12.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 13.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and sub-cutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto WY, Jablonski KA, Bray GA, et al. for the Diabetes Prevention Program Research Group. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes. 2007;56:1680–5. doi: 10.2337/db07-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metab Clin Exp. 1987;36:54–9. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio FP TF, Kandala NB, Currie A, Peile E, Stranges S. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large US sample. Sleep. 2007;12:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohatsu ND, Tsai R, Young T, et al. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166:1701–5. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Garcia E, Faubel R, Leon-Munoz L, Zuluaga MC, Banegas JR, Rodriguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87:310–6. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 20.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19:351–7. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Chaput JP, Després JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50:2298–304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 24.Hale L DD. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 26.Nunes J, Jean-Louis G, Zizi F, et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc. 2008;100:317–22. doi: 10.1016/s0027-9684(15)31244-x. [DOI] [PubMed] [Google Scholar]
- 27.Kanaya AM, Wassel Fyr C, Vittinghoff E, et al. Adipocytokines and incident diabetes mellitus in older adults: the independent effect of plasminogen activator inhibitor 1. Arch Intern Med. 2006;166:350–6. doi: 10.1001/archinte.166.3.350. [DOI] [PubMed] [Google Scholar]
- 28.Chaput J-P, Despres J-P, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec Family Study. Obesity (Silver Spring) 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 29.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Berg JF, Knvistingh Neven A, Tulen JHM, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes. 2008;32:1083–90. doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 32.Van Moffaert M. Sleep disorders and depression: the ‘chicken and egg’ situation. J Psychosom Res. 1994;38:9–13. doi: 10.1016/0022-3999(94)90131-7. discussion 12-3. [DOI] [PubMed] [Google Scholar]
- 33.Vgontzas AN, Bixler EO, Chrousos GP, Pejovic S. Obesity and sleep disturbances: meaningful sub-typing of obesity. Arch Physiol Biochem. 2008;114:224–36. doi: 10.1080/13813450802521507. [DOI] [PubMed] [Google Scholar]