Abstract
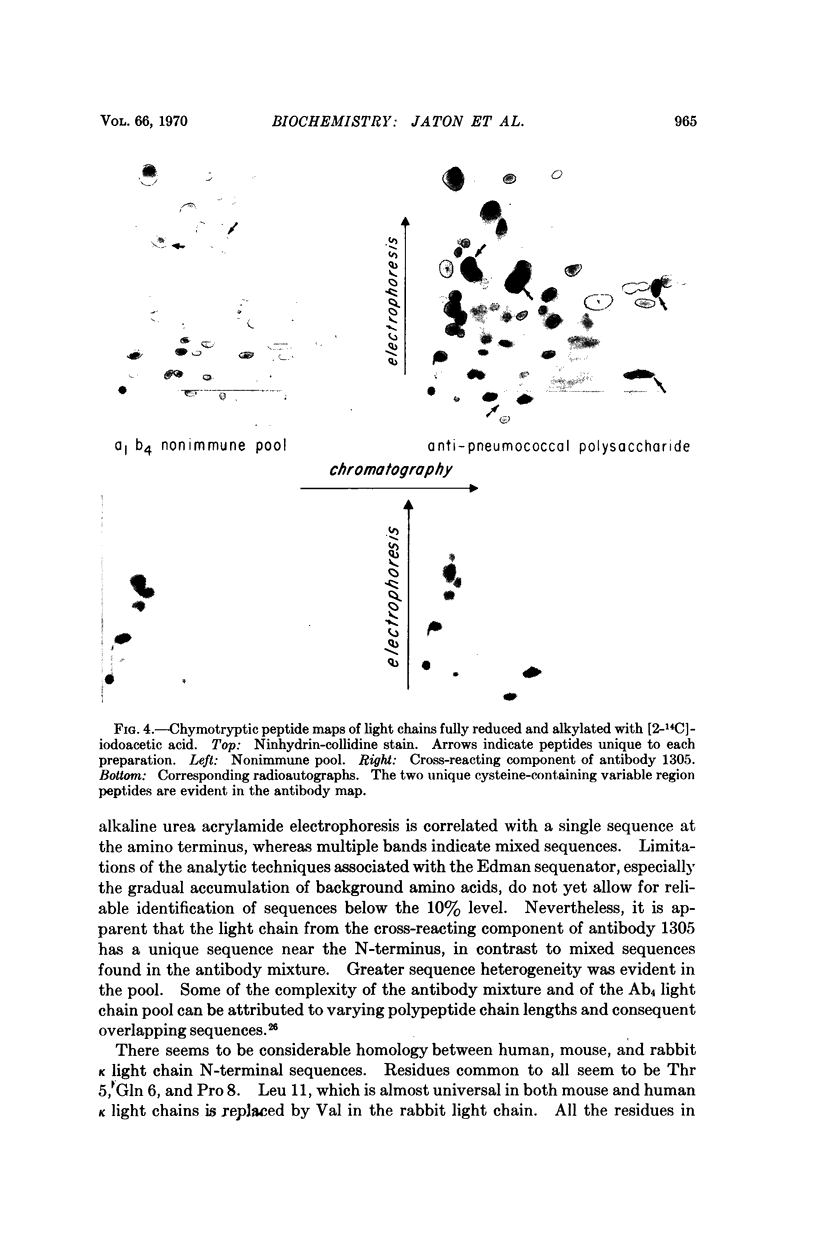
Antibodies of sufficient homogeneity for sequence studies were readily obtained in high concentrations from rabbits immunized with pneumococcal vaccines. By taking advantage of slightly differing immunologic specificity for Type III and Type VIII capsular polysaccharides, an antibody with unique electrophoretic mobility could be isolated from serum containing several distinct antibody components by using appropriate cross-reacting immunoadsorbents. A unique sequence for the N-terminal 11 amino acid residues of the light chain of the antibody was found, in contrast to several sequences in the antibody mixture from which this component was isolated. The sequence of a nonimmune light chain pool demonstrates even greater heterogeneity. Chymotryptic peptide maps of the antibody light chain show two unique cysteine-containing variable region peptides not seen in maps of nonimmune light chain pool of the same allotypic specificity as that of the antibody light chain. The experimental approach described here may provide further insight into the structure-function relationship of several homogeneous antibodies of closely related specificity for the same polysaccharide antigen.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn M., Notani G., Rice S. A. Characterization of the antibody to the C-carbohydrate produced by a transplantable mouse plasmacytoma. Immunochemistry. 1969 Jan;6(1):111–123. doi: 10.1016/0019-2791(69)90183-9. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Eisen H. N., Simms E. S., Potter M. Mouse myeloma proteins with antihapten antibody acitivity. The protein produced by plasma cell tumor MOPC-315. Biochemistry. 1968 Nov;7(11):4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Kinetics of labeling and isolation of a labeled peptide. Biochemistry. 1970 Mar 3;9(5):1267–1278. doi: 10.1021/bi00807a031. [DOI] [PubMed] [Google Scholar]
- Haber E. Antibodies of restricted heterogeneity for structural study. Fed Proc. 1970 Jan-Feb;29(1):66–71. [PubMed] [Google Scholar]
- Haber E. Immunochemistry. Annu Rev Biochem. 1968;37:497–520. doi: 10.1146/annurev.bi.37.070168.002433. [DOI] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Krause R. M. Experimental approaches to homogenous antibody populations. Factors controlling the occurrence of antibodies with uniform properties. Fed Proc. 1970 Jan-Feb;29(1):59–65. [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Pincus J. H., Jaton J. C., Bloch K. J., Haber E. Antibodies to type III and type VIII pneumococcal polysaccharides: evidence for restricted structural heterogeneity in hyperimmunized rabbits. J Immunol. 1970 May;104(5):1143–1148. [PubMed] [Google Scholar]
- Pincus J. H., Jaton J. C., Bloch K. J., Haber E. Properties of structurally restricted antibody to type VIII pneumococcal polysaccharide. J Immunol. 1970 May;104(5):1149–1154. [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Potter M., Leon M. A. Three IgA myeloma immunoglobulins from the BALB/ mouse: precipitation with pneumococcal C polysaccharide. Science. 1968 Oct 18;162(3851):369–371. doi: 10.1126/science.162.3851.369. [DOI] [PubMed] [Google Scholar]
- Van Orden H. O., Carpenter F. H. Hydrolysis of phenylthiohydantoins of amino acids. Biochem Biophys Res Commun. 1964;14:399–403. doi: 10.1016/0006-291x(64)90075-0. [DOI] [PubMed] [Google Scholar]