Abstract
In vitro assembly or complementation of a hybrid assimilatory nitrate reductase was attained by mixing a preparation of nitrate-induced N. crassa mutant nit-1 specifically with acid-treated (pH 2.5) bovine milk or intestinal xanthine oxidase, rabbit liver aldehyde oxidase, or chicken liver xanthine dehydrogenase. The complementation reaction specifically required induced nit-1, the only nitrate reductase mutant of Neurospora that lacked xanthine dehydrogenase and was unable to use hypoxathine or nitrate as a sole nitrogen source. The complementing activities of the above acid-treated enzymes correspond to their xanthine or aldehyde oxidizing activity profiles on sucrose density gradients. The resulting soluble, reduced nicotinamide adenine dinucleotide phosphate (NADPH)-nitrate reductases are the same as the Neurospora wild type enzyme in sucrose density gradient profile, molecular weight, substrate affinities, and sensitivity to inhibitors and temperature. By analogy to a similar in vitro complementation of nitrate reductase in mixtures of induced nit-1 and individual nonalleic Neurospora mutants, or uninduced wild type, the complemented nitrate apparently consists of an inducible protein subunit (possessing inducible NADPH-cytochrome c reductase) furnished by nit-1 and a subunit from the acid-treated xanthine or aldehyde oxidizing system which can substitute for the constitutive component furnished by the other mutants or uninduced wild type. The data suggest that Neurospora nitrate reductase and the xanthine oxidizing system and aldehyde oxidase of animals, all of which are molybdenum-containing enzymes catalyzing the reduction of nitrate to nitrite, share a highly similar protein subunit.
Full text
PDF

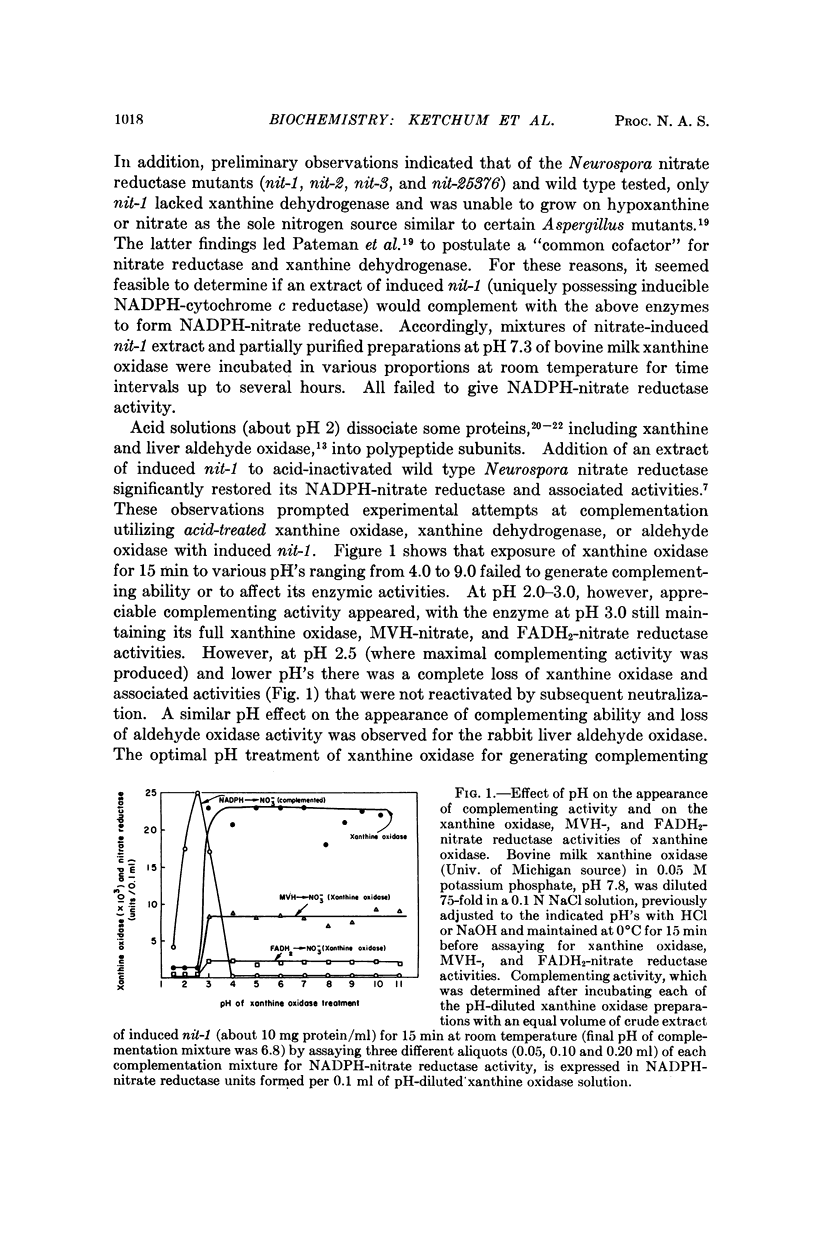

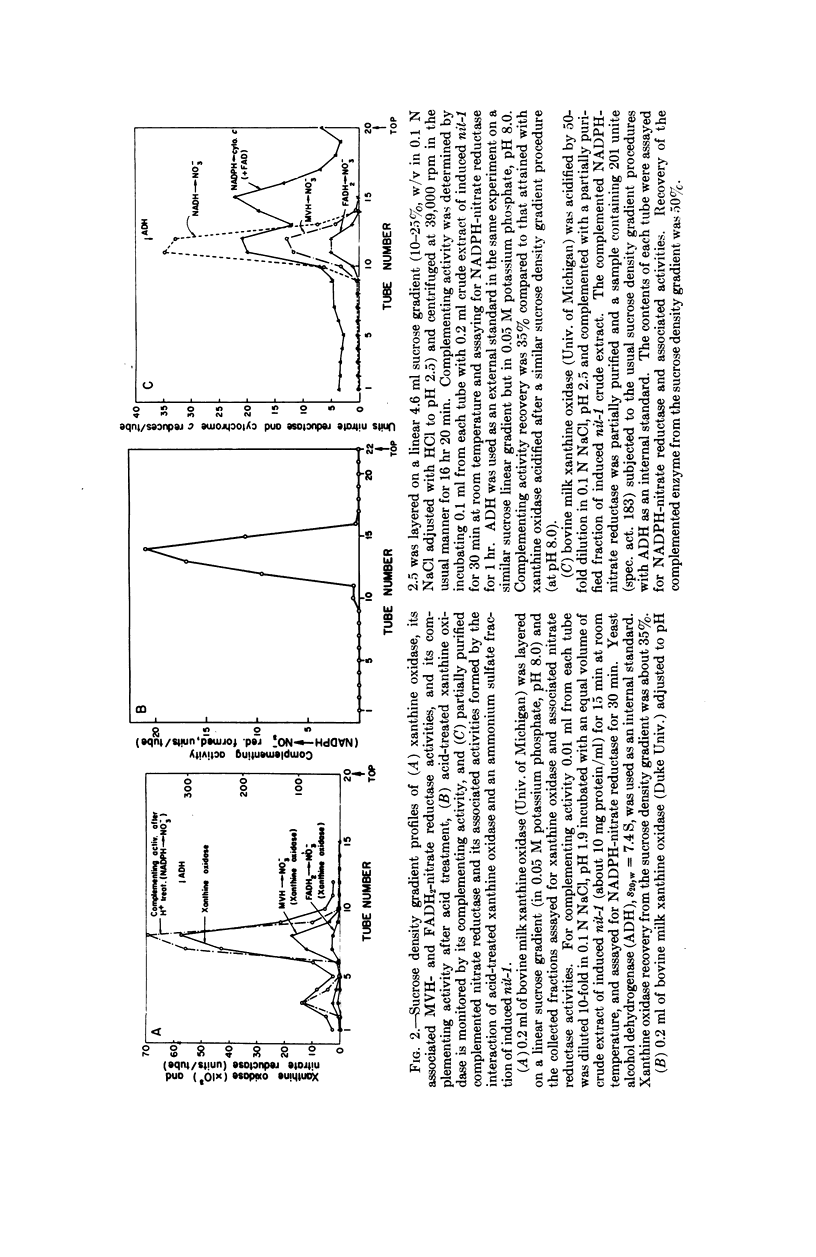



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Weber G. The reversible acid dissociation and hybridization of lactic dehydrogenase. Arch Biochem Biophys. 1966 Sep 26;116(1):207–223. doi: 10.1016/0003-9861(66)90028-2. [DOI] [PubMed] [Google Scholar]
- Andrews P., Bray R. C., Edwards P., Shooter K. V. The chemistry of xanthine oxidase. 11. Ultracentrifuge and gel-filtration studies on the milk enzyme. Biochem J. 1964 Dec;93(3):627–632. doi: 10.1042/bj0930627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. A., Koshland D. E., Jr Specificity in the assembly of multisubunit proteins. Proc Natl Acad Sci U S A. 1969 Sep;64(1):247–254. doi: 10.1073/pnas.64.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEAL W. C., RUTTER W. J., MASSEY V., VAN HOLDE K. E. Reversible alteration of the structure of enzymes in acidic solution. Biochem Biophys Res Commun. 1963 Jan 18;10:49–54. doi: 10.1016/0006-291x(63)90266-3. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Further purification and properties of Neurospora nitrate reductase. J Biol Chem. 1969 Jun 10;244(11):2870–2882. [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman E, Mitchell H K. Mutants of Drosophila Melanogaster Deficient in Xanthine Dehydrogenase. Genetics. 1959 Mar;44(2):153–162. doi: 10.1093/genetics/44.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C., McELROY W. D. Neurospora nitrate reductase: the role of phosphate flavine and cytochrome c reductase. Arch Biochem Biophys. 1958 Feb;73(2):466–483. doi: 10.1016/0003-9861(58)90290-x. [DOI] [PubMed] [Google Scholar]
- Massey V., Brumby P. E., Komai H. Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem. 1969 Apr 10;244(7):1682–1691. [PubMed] [Google Scholar]
- NASON A., EVANS H. J. Triphosphopyridine nucleotide-nitrate reductase in Neurospora. J Biol Chem. 1953 Jun;202(2):655–673. [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A., McELROY W. D. Molybdenum and nitrate reductase. I. Effect of molybdenum deficiency on the Neurospora enzyme. J Biol Chem. 1954 Mar;207(1):341–351. [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Mechanism of action of nitrate reductase from Neurospora. J Biol Chem. 1954 Nov;211(1):183–197. [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Molybdenum and nitrate reductase. II. Molybdenum as a constituent of nitrate reductase. J Biol Chem. 1954 Mar;207(1):353–360. [PubMed] [Google Scholar]
- Nason A., Antoine A. D., Ketchum P. A., Frazier W. A., 3rd, Lee D. K. Formation of assimilatory nitrate reductase by in vitro inter-cistronic complementation in Neurospora crassa. Proc Natl Acad Sci U S A. 1970 Jan;65(1):137–144. doi: 10.1073/pnas.65.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. A., Handler P. Preparation of bovine xanthine oxidase and the subunit structures of some iron flavoproteins. J Biol Chem. 1968 Oct 25;243(20):5368–5373. [PubMed] [Google Scholar]
- PATEMAN J. A., COVE D. J., REVER B. M., ROBERTS D. B. A COMMON CO-FACTOR FOR NITRATE REDUCTASE AND XANTHINE DEHYDROGENASE WHICH ALSO REGULATES THE SYNTHESIS OF NITRATE REDUCTASE. Nature. 1964 Jan 4;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., FRIDOVICH I., HANDLER P. Hepatic aldehyde oxidase. I. Purification and properties. J Biol Chem. 1962 Mar;237:922–928. [PubMed] [Google Scholar]
- REITHEL F. J. THE DISSOCIATION AND ASSOCIATION OF PROTEIN STRUCTURES. Adv Protein Chem. 1963;18:123–226. doi: 10.1016/s0065-3233(08)60269-7. [DOI] [PubMed] [Google Scholar]
- Rajagopalan K. V., Handler P. Purification and properties of chicken liver xanthine dehydrogenase. J Biol Chem. 1967 Sep 25;242(18):4097–4107. [PubMed] [Google Scholar]
- STELLWAGEN E., SCHACHMAN H. K. The dissociation and reconstitution of aldolase. Biochemistry. 1962 Nov;1:1056–1069. doi: 10.1021/bi00912a016. [DOI] [PubMed] [Google Scholar]


