I am grateful to the Epidemiology and Prevention Council for the honor and the opportunity to speak today. Henry Blackburn entitled his introduction to the first Ancel Keys lecture, “Ancel Keys, pioneer” and summarized Keys's astonishing array of work in nutrition and physiology, methods and prevention, including the diet-heart hypothesis and efforts to bridge biology, preventive medicine and public health (2). As a result of his work and the work of many others over the last 50 years, the mortality rate from coronary heart disease (CHD) declined by about 50% between 1980 and 2000, attributable in part to changes in medical treatments after coronary events and in part to changes in levels of risk factors in the population (3). In this lecture, as an admiring follower and a northwest settler, I consider some potential next steps in CHD prevention.
This talk has two related parts. The first reviews how we approve preventive drug therapies in America. High quality-drug evaluations serve as one essential foundation for future CHD prevention efforts. The second part considers two potential approaches to the use of drug therapies in intermediate-risk individuals for the primary prevention of CHD. The first involves using novel biomarkers to improve risk prediction and target treatment to those newly identified as high-risk individuals. The second involves simply expanding the eligibility for well-evaluated drugs to the entire group of intermediate-risk individuals. A comparative effectiveness trial evaluating the risks and benefits of these two approaches would help guide future prevention efforts.
Distributions of serum cholesterol
Ancel Keys was among the first to put forward hypotheses about population-level and individual-level causes of cardiovascular disease (2). In Keys's Seven Countries Study, the distribution curves scarcely overlap for total cholesterol levels in individuals from South Japan and East Finland [Supplemental figure 1 (4)]. Within populations, however, even though cholesterol is strongly associated with cardiovascular events, the cholesterol-level overlap between those who do and do not go on to have a CHD event is substantial [Supplemental figure 2 (5)].
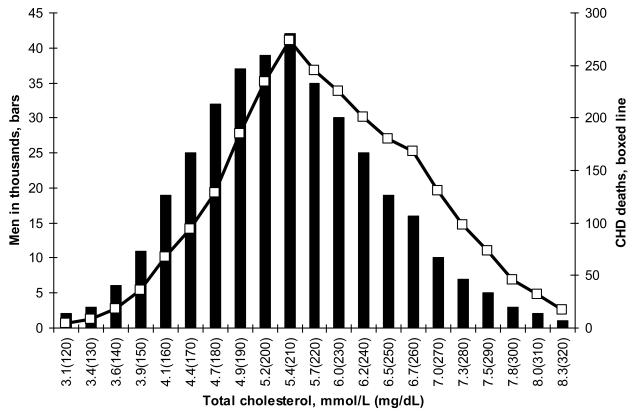
Geoffrey Rose distinguished between two methods of cardiovascular-disease prevention. The aim of the population-based strategy is to reduce level of a risk factor in many people and thus shift its distribution. Interventions such as smoking cessation and low-fat diets, which restore biologic normality for the species, can be “presumed to be safe” (5). Rose used the term “prevention paradox” to describe the fact that for mass preventive measures, the number needed to treat to prevent one event would be high and provide “little [benefit] to each participating individual” (5). In contrast, the high-risk strategy focuses on the individuals who have high levels of a risk factor and frequently relies on drugs treatments. The use of preventive drug therapies in individuals with extreme levels of risk factors represents an efficient use of resources in individuals most likely to benefit; but this high-risk strategy entails another paradox, the “treatment paradox”: because many events occur among those with average or intermediate levels of risk, the high-risk approach may do little to reduce the overall burden of CHD in the population [Figure 1 (6,7)].
Figure 1. Distributions of cholesterol levels and age-adjusted CHD deaths.
The bars in Figure 1 represent the number of MRFIT screenees with cholesterol levels between 3.1 mmol [120 mg/dL] and 8.3 mmol/L [320 mg/dL] (6). Six-year age-adjusted CHD mortality rates range from 3.16 (cholesterol <= 4.3 mmol/L [167 mg/dL]) to 13.05 (cholesterol >= 6.8 mmol/L [264 mg/dL]) (7). The open boxes indicate the number of age-adjusted CHD deaths that occurred over a six-year period among screenees within each group. Compared with the distribution of the population (bars), the distribution of the deaths (line) is shifted to the right. About 25% of the screenees have cholesterol levels >= 6.2 mmol/dL [240 mg/dL], and about 38% of the CHD deaths occur among those with cholesterol levels >= 6.2 mmol/L [240 mg/dL]. The other 62% of CHD deaths occurred among those with cholesterol levels < 6.2 mmol/L [240 mg/dL].
In the early 1980s, the available lipid-lowering drug therapies were limited. The results of the WHO clofibrate study (8) profoundly influenced Rose's thinking about drug therapies (9). In this randomized trial, clofibrate not only reduced the incidence of ischemic heart disease but also increased the risk of total mortality. Indeed, for every 15 CVD events that might be prevented by clofibrate, there were about 11 extra deaths. This risk-benefit profile argued against the widespread use of clofibrate as a “long-term mass preventive medication” (9) in the primary prevention setting, which includes low-risk people who are well, who are without clinical disease, and who are even without symptoms. As another side effect, the mixed results of the clofibrate trial would later prompt the US Food and Drug Administration (FDA) to request or require large long-term trials to evaluate new lipid-lowering drugs such as the statins.
Drug approval and rosiglitazone for the treatment of type 2 diabetes
Drug therapies for risk factors such as high levels of blood pressure, lipids and glucose represent, in Geoffrey Rose's terms, a high-risk approach to a population-based problem. The purpose of these therapies is to prevent cardiovascular events, yet the FDA generally approves new medications for these conditions on the basis of premarket studies that use biomarkers or surrogate end points. This approach typically reduces the costs of development and the time to approval, but often provides incomplete information about the risks and benefits of new drug therapies (10).
In observational studies of patients with type 2 diabetes, the relative risk for cardiovascular events associated with a 1 percent increment in glycated hemoglobin is 1.18 (11). These data suggest that in clinical trials, a 1% reduction in glycated hemoglobin would be expected to reduce the relative risk of cardiovascular events by about 15%. Do drug-treatment effects on glycated hemoglobin serve as a valid and accurate proxy for their effects on the incidence of cardiovascular events?
In a meta-analysis of the three recent trials of intensive treatment of patients with type 2 diabetes (12-15), the relative risk reduction was only 6% (95% confidence interval [CI], 14% to −2%). This 95% CI excludes the expected value of 15%, and the observed benefit for the prevention of cardiovascular events is less than half the predicted risk reduction. The reasons are not clear. Perhaps aggressive lowering of glucose does little to influence the adverse effects of insulin resistance, obesity, and inflammation typically associated with type 2 diabetes (16). Additionally, the increased risk of hypoglycemia associated with intensive treatment may precipitate cardiovascular events or cause deaths that are mistakenly attributed to cardiovascular disease. Finally, drug therapies often have a number of actions, some of which may be lead to serious adverse events.
Rosiglitazone, used in both of the US trials (13,15), activates genes that influence the control of glucose and lipids. Approved in 1999, the drug was heavily promoted and rapidly became a blockbuster. Between 2000 and 2006, the 58 million rosiglitazone prescriptions sold in the US represent about 4.8 million person years of use (17). The manufacturer's internal meta-analysis in August 2005 (18), like Nissen's meta-analysis published almost two years later (19), suggested that rosiglitazone might be associated with an increased rather than a decreased risk of myocardial infarction (hazard ratio = 1.31; 95% CI = 1.01 to 1.70). The history of its evaluation illustrates some of the pitfalls of the current system.
In the Phase III trials, the use of rosiglitazone at 4 and 8 mg doses reduced both fasting glucose and glycated hemoglobin. The same doses, however, increased both LDL cholesterol and body weight. For instance in one trial (20), the total cholesterol difference between rosiglitazone 4 mg and placebo is 0.6 mmol/L [23 mg/dL]--a difference larger than the total cholesterol difference of 0. 4 mmol/L [15 mg/dL] between controls and cases with myocardial infarction (21). Based on the evidence from these several biomarkers, it is difficult to predict the overall expected effect on the risk of cardiovascular events. The FDA medical reviewer expressed concern about the potential “deleterious long term effects [of rosiglitazone] on the heart,” and he recommended a postmarketing study to evaluate “the CVD risk” as “a condition of approval” (22). The sponsor conducted 3 Phase IV trials.
In the ADOPT study (23), patients with new-onset diabetes were randomized to receive rosiglitazone, metformin, or glyburide as monotherapy, and the primary outcome was length of time before the first-line treatment failed to provide adequate glycemic control. MI and stroke events were not included or prospectively evaluated as a primary or secondary outcome except incidentally as reported in adverse-event forms.
In the DREAM study (24), patients with impaired glucose tolerance or impaired fasting glucose were randomized to receive rosiglitazone or placebo, and the primary outcome was the development of chemical diabetes or death. In this study of low-risk patients, the evaluation of cardiovascular events was a secondary outcome, and although underpowered, the results were not reassuring (HR for MI = 1.66, 95% CI = 0.73-3.80; HR for heart failure = 7.03, 95% CI = 1.60 to 30.9). The DREAM trial represents an effort, based solely on biomarkers, to transform a pre-disease state into a treatable condition. Both ADOPT and DREAM targeted marketing or marketable issues, the durability of monotherapy and drug treatment for pre-disease. At the same time, they conspicuously avoided a direct well-powered answer the cardiovascular risk-benefit question posed by the FDA medical officer.
In the RECORD trial, patients with type 2 diabetes on metformin or sulfonylurea were randomized to the addition of rosiglitazone or to active therapy with metformin plus a sulfonylurea (25). The primary outcome was cardiovascular hospitalization or death. During the trial, the investigators had expected more than 1000 events in the control group, but they ascertained only 322: about two-thirds of the expected events were missing. The low event rate in an open trial with a non-inferiority design raises questions about the conduct of the RECORD trial, which also documented increased risks of heart failure and fracture (26). The analysis of the outcome of MI in the RECORD trial could not exclude a 63% increase in risk (HR = 1.14, 95% CI = 0.80 to 1.63).
In summary, these 3 postmarket trials provide no clear answer to question about the MI risk-benefit profile. If rosiglitazone increases the MI risk by the sponsor's estimate of 30%, the prescriptions dispensed between 2000 to 2006 might have been the occasion of an additional 20,000 heart attacks in the US. With several notable exceptions such as ALLHAT (27) and the WHI (28), the NIH has largely turned the evaluation of drug treatments over to industry. The duty to provide a return on investment to shareholders tends to create for industry an asymmetric interest in safety and efficacy. As a result, the design, conduct and reporting of some industry-funded studies--the rosiglitazone and ezetimibe trials are examples--remain an enduring public-health problem (29-31). In short, the current approach to the evaluation of the efficacy and safety of medications occasionally provides an unreliable patchwork of evidence about health outcomes and incomplete information about the risk-benefit profiles of some new drug therapies.
Although flawed, the current drug-evaluation system is quirky and can work fairly well for drugs such as the statins that turn out to be safe and effective. The favorable risk-benefit profile in secondary prevention studies encouraged trials in primary prevention. Over the years, the indications for the statins expanded. In 2008, sales of atorvastatin reached $5.8 billion in the US. In a recent meta-analysis that included 10 primary-prevention trials and more than 70,000 patients (32), statins were associated with major reductions in the risk of coronary events, stroke, and total mortality. The CHD relative risk reduction is typically 25 to 30% and similar among various risk groups. For this reason, the number needed to treat to prevent one CHD event depends largely on the baseline absolute risk.
Next steps in CHD prevention
Let me turn now to two potential approaches to prevent CHD among intermediate-risk patients. The National Cholesterol Education Program (NCEP) guidelines (33), simplified here, serve as a framework. A treatable of LDL cholesterol depends in part on the patient's 10-year risk of cardiovascular disease as assessed by the Framingham Risk Score (FRS). High-risk patients with a 10-year risk > 20% merit aggressive treatment, but the approach to those at intermediate risk, with a 10-year risk of 10% to 20%, is less clear and depends in part on LDL level. Efforts to improve risk prediction use novel biomarkers or measures of subclinical disease to reclassify “intermediate risk” persons into a high-risk category that merits drug treatment. In contrast, expanding the eligibility for drug treatment involves shifting the treat-or-not boundary well into the “intermediate risk” category.
Risk prediction models
The Framingham Risk Score (FRS), which is well validated, uses traditional risk factors, including age, sex, blood pressure, lipids, and smoking (34,35). Efforts to improve risk prediction add novel biomarkers or measures of subclinical disease to the FRS. The quality of a risk-score model depends on several measures. Calibration evaluates the degree to which the observed and predicted events rates are similar. Discrimination assesses how well the model distinguishes between those who do and do not have an event. The C-statistic is analogous to the area under the receiver operating curve (ROC), which plots the sensitivity of the test against the false positive rate. The AUC provides an estimate of the probability that the model assigns a higher risk to those who develop CHD than to those who do not. Finally, reclassification methods evaluate the performance of the model at clinically relevant boundaries such as treatment thresholds.
Coronary artery calcium [CAC], a measure of subclinical disease, is strongly associated with the incidence of cardiovascular events (36,37). The relative risks [RR] comparing a CAC score of >=300 with a score of 0 are in the range of 3.9 to 6.8, much larger than the relative risks for many traditional risk factors such as smoking, which doubles the risk of CHD; but the improvement in AUC occasioned by the addition of imaging-study results to a model that includes traditional risk factors alone is still modest, increasing the AUC from 0.79 to 0.83 in one study (37).
In a recent meta-analysis (38), C-reactive protein (CRP) was associated with a modest increase in the risk of CHD (RR = 1.6, 95% CI = 1.4 to 1.8 for CRP > 3 compared with < 1). In 8 studies that evaluated the improvement in discrimination achieved by the Framingham risk score when CRP is added to the model (39), the increment in the AUC is small or absent at two significant digits (range of AUC change, 0.00 to 0.02). For an independent risk factor, the weak evidence of model improvement may seem to be counter-intuitive. But because the values of those who do and do not go on to have a CHD event overlap so extensively, CRP in particular and risk factors in general do not function well as diagnostic tests.
Reclassification methods are another approach to evaluate the addition of a new marker to a model that includes traditional risk factors. In one study (39), the 10 year risk was estimated for the Framingham risk score alone and the Framingham risk score plus CRP. For those without CHD, 331 moved to a higher risk category and 325 to a lower; for those with CHD, 37 were moved to a higher risk group and 23 were moved to a lower risk group. Overall, there were slightly more patients reclassified in the correct direction, and the net reclassification was 8.5% (95% CI, −1.3% to 18.3%). The US Preventive Services Task Force recently reported similar findings for CRP (38).
Because the CRP-CHD association is strong, graded and continuous, a large number of events occur among the majority of people with average or intermediate CRP values. As a consequence, the model improvement attributable to CRP is small, and its predictive utility limited (40). As a general internist, I use intermediate-risk status in a patient, not as an occasion for new diagnostic tests, but as an opportunity for shared decision making. What are patients' preferences about various forms of treatment, concerns about outcomes, fears about side effects, and their ability or willingness to pay?
The prediction problem arises from the fact that continuous risk factors such as CRP do not function well as diagnostic tests (41). The distributions of risk factor levels in those with and without disease overlap to a considerable extent, especially for odds ratios typically seen for most cardiovascular disease risk factors (41). The odds ratios for CRP and smoking are in the range of 1.5 to 3. Even risk factors such as coronary calcium with odds ratios as high as 7 have widely overlapping distributions, produce AUC levels closer to 0.5 than to 1.0, and improve discrimination only marginally. In contrast, odds ratios in the range of 50 to 400 have distributions that differ markedly, and their risk factors perform better as diagnostic tests with AUC levels much closer to 1.0. Known CVD risk factors, however, are not associated with odds ratios of this magnitude. What about the use of multiple independent risk factors?
With two independent risk factors, each associated with a detection rate or sensitivity of 15%, it is tempting to think that the detection rate would be twice as high--30% rather than 15% (42). Doubling the detection rate in this manner, however, also doubles the false positive rate. If we wish to hold the false positive rate constant at 5%, the increment in detection achieved by a model that includes two independent risk factors is in fact much more modest, increasing from 15% for one risk factor up to 22% for the model with two independent risk factors. For risk factors, each with an odds ratio of 3 comparing the first and fifth quintiles, it would require 15 independent risk factors to achieve an overall model sensitivity of 40%. For risk factors with an odds ratio of 5 each, it would require 15 independent risk factors to achieve an overall model sensitivity of almost 80% (42). Given the available risk factors, including measures of subclinical disease, the epidemiological limits of risk prediction are formidable.
Expanded use of drug therapies
Expanding the eligibility for drug therapies to intermediate-risk individuals is an alternative approach. The definition of a treatable level of CHD risk depends on the drug efficacy, safety, and cost. Shifting the treatable level to include intermediate-risk individuals means that the number needed to treat to prevent one event increases, so that safety and cost as well as efficacy become important. With more than 20 trials in the 30 years since Geoffrey Rose expressed concern about “mass preventive medication” (9), the efficacy and safety of the statins, in contrast to clofibrate, are now well established (32,43).
Trade statins cost $3 to $4 per day. At a local northwest pharmacy, generic statins cost 11 cents per day--a forty-fold difference in cost. The availability of generic statins has markedly reduced the costs to prevent one event. For a patient with a 20% 10-year risk and under the assumption of a 25% relative risk reduction, the use of rosuvastatin costs about $288,000 to prevent one event; but for a patient with a 10% 10-year risk, the use of a generic statin now requires an investment of about $16,000 to prevent one event.
George Diamond estimated the likely cost-effectiveness of three prevention strategies for 50 million patients at intermediate risk: 1) treat everyone at intermediate risk; 2) use the NCEP methods; or 3) use electron beam computed tomography (EBCT) to evaluate CAC and identify high-risk “treatable” individuals (44). In this model, he assumed that statins cost $2 per day. The treatment savings come from the prevention of events that are assumed to cost the medical care system $100,000 each. Under these assumptions, the treat-everyone approach is the most expensive option with a net cost of $21 billion compared with net costs of $8 billion for the NCEP screening methods and $17 billion for the EBCT methods. At a cost of 11 cents per day for generic statins, the treat-everyone approach turns out to be the least expensive method. Indeed, the expanded-treatment approach is estimated to save $13 billion dollars compared with a cost savings of $2 billion for the NCEP approach and an increase in costs of $9 billion with the EBCT method.
Targeting high-risk individuals with ever more aggressive therapies may provide marginal additional benefit for the individuals concerned, but these high-risk approaches do little to reduce the overall burden of CVD in the intermediate-risk population (Figure 1)--the “treatment paradox.” With the efficacy and safety of the statins well established and with the low costs of the generic statins, it may be time to evaluate a broader use of statins in intermediate-risk individuals.
Proposed comparative effectiveness trial
Last year, Michael Lauer called for new evidence from randomized trials about the risks and benefits associated with coronary-calcium screening (45). The Institute of Medicine identified a comparative effectiveness study of CVD risk stratification methods as one its 100 high-priority studies (46). No comparison group was identified. In the trial proposed here, eligible subjects would include those at intermediate risk or perhaps defined simply by age. Patients would be randomized either to (1) a risk stratification approach that used CRP, imaging studies or any other risk factor and treated the high-risk patients identified by these methods; or (2) low-dose statin therapy (43). This arm might include low-dose diuretics (47) or other elements of a “polypill” (48), and it might include a factorial design to evaluate various methods of the delivery of population-based CVD risk screening, counseling and treatment.
Concluding observations
In conclusion, the current system of drug evaluation relies on industry, which has an asymmetric interest in efficacy and safety. On occasion, industry's approach to safety issues has represented a menace to the health of the public (49,50). In other instances, it has been difficult to obtain reliable and valid estimates of the risk-benefit profile of drugs such as rosiglitazone or ezetimibe. High-quality drug evaluations are essential to next-generation prevention efforts, especially those that consider expanding eligibility for “mass preventive medication” to the large number of individuals with “normal,” “average,” or “intermediate” levels of risk factors.
The current model of medical care in America seems to be inclined toward resource-intensive individualized risk management, the use of expensive vascular evaluations that incorporate new biomarkers and imaging tools, and an aggressive lowering of treatment targets for individuals deemed to be high risk. An alternative that may be effective for primary prevention is a primary-care model that starts with individual patients, obtains simple estimates of their cardiovascular risk and offers appropriate treatments that are known to be safe and effective to a wide spectrum of individuals. My hope is that this talk may promote discussion about one or more potential comparative effectiveness trials of public health importance.
Supplementary Material
Acknowledgements
I wish to thank Donna Arnett, Greg Burke, Curt Furberg, Phil Greenland, Thomas Lumley, Vasan Ramachandran, David Siscovick, Russ Tracy, Noel Weiss for their thoughtful comments on a draft of the lecture.
Funding sources: This research was supported in part by grants HL74745, and HL080295, HL085251, HL087652 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the author and does not necessarily represent the views of the National Heart, Lung, And Blood Institute or the National Institutes of Health or the views of the scientists who were kind enough to review earlier drafts.
Footnotes
Disclosure or conflicts: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Senior JR. Unintended hepatic adverse events associated with cancer chemotherapy. Toxicologic Pathol. 2010 doi: 10.1177/0192623309351719. in press. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn H. Introduction to Ancel Keys Lecture: Ancel Keys, Pioneer. Circulation. 1991:1402–4. doi: 10.1161/01.cir.84.3.1402. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in US deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 4.Rose G. Ancel Keys Lecture. Circulation. 1991;84:1405–9. doi: 10.1161/01.cir.84.3.1405. [DOI] [PubMed] [Google Scholar]
- 5.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 6.Martin MJ, Browner WS, Wentworth D, Hulley SB, Kuller LH. Serum cholesterol, blood pressure, and mortality: Implications from a cohort of 361,622 men. Lancet. 1986;ii:933–936. doi: 10.1016/s0140-6736(86)90597-0. [DOI] [PubMed] [Google Scholar]
- 7.Stamler J, Wentworth D, Neaton JD, for the MRFIT Research Group Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 1986;256:2823–2828. [PubMed] [Google Scholar]
- 8.Report from the Committee of Principal Investigators A co-operative trial in the primary prevention of ischaemic heart disease using clofibrate. Br Heart J. 1978;40:1069–1118. doi: 10.1136/hrt.40.10.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose G. Strategy of prevention: Lessons from cardiovascular disease. Br Med J. 1981;282:1847–1851. doi: 10.1136/bmj.282.6279.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Psaty BM, Weiss NS, Furberg CD, koepsell TD, Siscovick DS, Rosendaal FR, Smith NL, Heckbert SR, Kaplan RC, Lin D, Fleming TR, Wagner EH. Surrogate endpoints, health outcomes, and the drug approval process for the treatment of risk factors for cardiovascular disease. JAMA. 1999;282:786–790. doi: 10.1001/jama.282.8.786. [DOI] [PubMed] [Google Scholar]
- 11.Selvin E, Marionoulos S, Berkenbilt G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kelly T, Bazzano L, Fonseca V, Thethi T, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- 13.The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The ADVANCE Collaborative Group Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 15.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Hurang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 16.Goodarzi M, Psaty BM. Glucose lowering to control macrovascular disease in type 2 diabetes: treating the wrong surrogate end point? JAMA. 2008;300:2051–3. doi: 10.1001/jama.2008.510. [DOI] [PubMed] [Google Scholar]
- 17.Floyd J, Barbehenn E, Lurie P, Wolfe S. Case series of liver failure associated with rosiglitazone and pioglitazone. Pharmacoepidemiol Drug Saf. 2009;18:1238–43. doi: 10.1002/pds.1804. [DOI] [PubMed] [Google Scholar]
- 18.GlaxoSmithKline Study No ZM2005/00181/01: Avandia Cardiovascular Event Modeling Project. Http://ctr.gsk.co.uk/Summary/Rosiglitazone/III_CVmodeling.pdf.
- 19.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2472. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 20.Levovitz HE, Dole JF, Patwardhan R, Rappaport eb, Freed MI. Rosiglitazone monotherpy is effective in patients with type 2 diabetes. J Clin Endocrin Metabol. 2001;86:280–8. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 21.Psaty BM, Smith NL, Heckbert SR, Vos HL, Lemaitre RN, Reiner AP, Siscovick DS, Bis J, Lumley T, Longstreth WT, Jr, Rosendaal FR. Diuretic therapy, the alpha-adducin gene variant, and the risk of myocardial infarction or stroke in persons with treated hypertension. JAMA. 2002;287:1680–1689. doi: 10.1001/jama.287.13.1680. [DOI] [PubMed] [Google Scholar]
- 22.Misbin RI. Medical office review of Avandia: application number: 021071. Center for Drug Evaluation and Research; Http://oversight.house.gov/story.asp?ID=1325. [Google Scholar]
- 23.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. for the ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 24.The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 25.Home PD, Pocock SJ, Beck-Nielson H, Curtis PS, Gamis R, Haenfeld M, Jones NP, Komajda M, McMurray JJV, for the RECORD Study Team Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 26.Psaty BM, Prentice RL. Variation in event rates in trials of patients with type 2 diabetes. JAMA. 2009;302:1698–1700. doi: 10.1001/jama.2009.1497. [DOI] [PubMed] [Google Scholar]
- 27.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium-channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 28.Writing Group for the Women's Health Initative Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 29.Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, Califf R. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359:1357–66. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 30.Fleming T. Identifying and addressing safety signals in clinical trials. N Engl J Med. 2008;359:1400–2. doi: 10.1056/NEJMe0807372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor A, Nissen S. Preliminary observations from preliminary trial results: have we finally had enough? Circ Cardiovasc Qual Outcomes. 2008;1:54–57. doi: 10.1161/CIRCOUTCOMES.108.811901. [DOI] [PubMed] [Google Scholar]
- 32.Brugts J, Yetgin T, Hoeks S, Gotto AM, Shepherd J, Westendorp RGJ, de Craen AJM, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Summary of the Second Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) JAMA. 1993;269:3015–3023. [PubMed] [Google Scholar]
- 34.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 35.Pencina M, D'Agostino R, Sr, Larson M, Massaro J, Vasan R. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–84. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham Score for Risk Prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 37.Detrano R, Guerci A, Carr J, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 38.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analysis for the US Preventive Services Task Force. Ann Intern Med. 2009;151:483–95. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 39.Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, Smeeth L, Deanfield JE, Lowe GD, Rumley A, Gowkes FGR, Humphries SE, Hingorani AD. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38:217–31. doi: 10.1093/ije/dyn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasan RS. Commentary: C-reactive protein and risk prediciton--moving beyond associations to assessing predictive utility and clinical usefulness. Int J Epidemiol. 2009;38:231–4. doi: 10.1093/ije/dyn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pepe M, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ration in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 42.Wald NJ, Morris JK, Rish S. The efficacy of combining risk factors as a screening test. J Med Screen. 2005;12:197–201. doi: 10.1258/096914105775220642. [DOI] [PubMed] [Google Scholar]
- 43.Mills E, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52:1769–81. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 44.Diamond GA, Kaul S. The things to come of SHAPE: cost and effectiveness of cardiovascular prevention. Am J Cariol. 2007;99:1013–1015. doi: 10.1016/j.amjcard.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 45.Lauer MS. Discarding logic: 2008 Ancel Keys Memorial Lecture. Circulation. 2009;119:1533–37. doi: 10.1161/CIRCULATIONAHA.108.842765. [DOI] [PubMed] [Google Scholar]
- 46.Institute of Medicine Committee on Comparative Effectiveness Research Prioritization . Initial national priorities for comparative effectiveness research: report brief. Institute of Medicine of the National Academies; 2009. [Google Scholar]
- 47.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 48.Yusuf S, Pais P, Afzal R, Xavier D, Teo K, Eikelboom J, Sigamani A, Mohan V, Gupta R, Thomas N, for the Indian Polycap Study Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double blind, randomised trial. Lancet. 2009;373:1341–51. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
- 49.Psaty BM, Furberg CD, Ray WA, Weiss NS. Potential for conflict of interest in the evaluation of suspected adverse drug reactions: use of cerivastatin and risk of rhabdomyolysis. JAMA. 2004;292:2622–2631. doi: 10.1001/jama.292.21.2622. [DOI] [PubMed] [Google Scholar]
- 50.Psaty BM, Kronmal RA. Reporting mortality findings in trials of rofecoxib for Alzheimer disease or cognitive impairment. JAMA. 2008;299:1813–1817. doi: 10.1001/jama.299.15.1813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.