Abstract
In Arabidopsis (Arabidopsis thaliana), a family of six genes encodes acyl-coenzyme A-binding proteins (ACBPs). A member of this family, ACBP1, contains an amino-terminal transmembrane domain that targets it to the plasma membrane and the endoplasmic reticulum. To investigate ACBP1 function, ACBP1-overexpressing transgenic Arabidopsis plants were characterized using lipid analysis. ACBP1 overexpressors showed reduction in several species of diunsaturated phosphatidylcholine (PC), prompting us to investigate if they were altered in response to freezing stress. ACBP1 overexpressors demonstrated increased freezing sensitivity accompanied by a decrease in PC and an increase in phosphatidic acid (PA), while acbp1 mutant plants showed enhanced freezing tolerance associated with PC accumulation and PA reduction. We also showed binding of a recombinant eukaryotic ACBP (ACBP1) to PA, indicative of the possibility of enhanced PA interaction in ACBP1 overexpressors. Since phospholipase Dα1 (PLDα1) is a major enzyme promoting the hydrolysis of PC to PA, PLDα1 expression was examined and was observed to be higher in ACBP1 overexpressors than in acbp1 mutant plants. In contrast, the expression of PLDδ, which plays a positive role in freezing tolerance, declined in the ACBP1 overexpressors but increased in acbp1 mutant plants. Given that ACBP1 is localized to the endoplasmic reticulum and plasma membrane, it may regulate the expression of PLDα1 and PLDδ by maintaining a membrane-associated PA pool through its ability to bind PA. Moreover, both genotypes showed no alterations in proline and soluble sugar content or in cold-regulated (COR6.6 and COR47) gene expression, suggesting that the ACBP1-mediated response is PLD associated and is independent of osmolyte accumulation.
Arabidopsis (Arabidopsis thaliana) acyl-CoA-binding proteins (ACBPs) are conserved at the acyl-CoA-binding domain and range in size from 10.4 to 73.1 kD (Leung et al., 2004). They include membrane-associated ankyrin repeat-containing ACBP1 and ACBP2, extracellularly targeted ACBP3, Kelch motif-containing ACBP4 and ACBP5, and cytosolic 10-kD ACBP6 (for review, see Xiao and Chye, 2009). These proteins are localized in various subcellular compartments, and their recombinant derivatives have been shown to bind different acyl-CoA esters (Engeseth et al., 1996; Chye, 1998; Chye et al., 1999, 2000; Leung et al., 2004, 2006; Gao et al., 2008; Li et al., 2008a; Xiao et al., 2008b, 2009). Previous studies have indicated that ACBP6 overexpression in transgenic Arabidopsis enhances freezing tolerance accompanied by an up-regulation of phospholipase D δ (PLDδ) expression (Chen et al., 2008). These findings prompted us to investigate whether other ACBPs are involved in cold stress.
Low temperature is a major environmental factor that restricts the geographical distribution and productivity of plants. Under low-temperature stress, complex processes including changes in gene regulation occur, culminating in cold acclimation and the acquirement of cold and freezing tolerance in some plants (Thomashow, 1999). Since the ability to manipulate cold tolerance leads to implications in agriculture and food production, intensive investigations in recent years have led to the identification and characterization of genes that confer cold and freezing tolerance.
Initial studies on cold-regulated gene expression in Arabidopsis have revealed that the family of CBF (also known as DREB1) transcription factors regulates many cold-inducible genes (Thomashow, 1999; Yamaguchi-Shinozaki and Shinozaki, 2006; Maruyama et al., 2009). The overexpression of CBFs (CBF1, CBF2, or CBF3) in Arabidopsis up-regulated cold-regulated (COR) gene expression and concomitantly improved freezing tolerance (Jaglo-Ottosen et al., 1998; Liu et al., 1998; Kasuga et al., 1999; Gilmour et al., 2004). Constitutive expression of the COR genes also improves freezing tolerance (Artus et al., 1996; Steponkus et al., 1998), while their disruption dramatically decreases tolerance (Knight et al., 1999, 2009; Boyce et al., 2003). Some studies have shown that CBF expression is regulated by other transcription factors, such as ICE1, which elevates the expression of CBF3 and COR to enhance freezing tolerance (Chinnusamy et al., 2003, 2007; Miura et al., 2007). Other independent pathways known to affect freezing tolerance are related to the accumulation of osmolytes such as Pro and soluble sugars (Xin and Browse, 1998; Gilmour et al., 2000; Rajashekar et al., 2006).
PLDα1 and PLDδ have been implicated in mediating freezing tolerance. Previous studies have demonstrated that PLDα1-deficient Arabidopsis plants are more tolerant to freezing and show decreased membrane lipid hydrolysis of phosphatidylcholine (PC) to phosphatidic acid (PA) and an accumulation of osmolytes in comparison with the wild type (Welti et al., 2002; Rajashekar et al., 2006). PLDδ enhances freezing tolerance (Li et al., 2004) by binding microtubules and interaction with the cytoskeleton, thereby stabilizing the plasma membrane (Gardiner et al., 2001; Li et al., 2004, 2008b).
In Arabidopsis, the overexpression of ACBP6 was observed to enhance freezing tolerance (Chen et al., 2008). While ACBP6 is a 10-kD cytosolic protein, the larger ankyrin repeat-containing ACBP1 has been subcellularly localized to the plasma membrane and endoplasmic reticulum (ER; Chye, 1998; Chye et al., 1999; Li and Chye, 2003). In this report, we show that the acbp1 mutant is tolerant to freezing stress. We suggest that reduction in PLDα1 expression and decrease in the hydrolysis of PC to PA likely enhances membrane stability in the acbp1 mutant plants, resulting in enhanced cold acclimation and freezing tolerance. Moreover, increased expression of PLDδ in the acbp1 mutant plants may enhance tolerance by stabilization of the membranes. We also demonstrate here that a recombinant eukaryotic ACBP (ACBP1) can bind PA and that ACBP1 can possibly regulate the expression of PLDα1 and PLDδ through its interaction with membrane-associated PA.
RESULTS
ACBP1 Overexpressors Show Alterations in PC Molecular Species
ACBP1-overexpressing transgenic lines (ox-1 and ox-2) expressing the full-length ACBP1 cDNA from the cauliflower mosaic virus (CaMV) 35S promoter were generated, tested, and confirmed to accumulate the 37-kD ACBP1 protein by western-blot analysis in an earlier study (Xiao et al., 2008a). When these lines grown at 23°C were subjected to lipid analysis (Tables I and II), we observed that two species of diunsaturated PC, 36:5 PC and 38:6 PC, were significantly lower (P < 0.05) in the ACBP1 overexpressors (ox-1 and ox-2) than in the wild type. This result indicates that ACBP1 may play a role in the freezing response, since diunsaturated PCs are known to reduce the formation of the hexagonal II (HII) phase and enhance freezing tolerance in plants (Uemura and Steponkus, 1994). Hence, we next investigated if the ACBP1 overexpressors and acbp1 mutant plants would perform differently from the wild type under freezing stress.
Table I. Changes in PC species of wild-type (Col-0) and ACBP1-overexpressing (ox-1 and ox-2) plants grown at 23°C or CA followed by freezing treatment.
The values are means ± sd (nmol g−1 dry weight; n = 3). Significant differences (P < 0.05) from the wild type in the same experiment are indicated in boldface.
| PC Species | 23°C |
−8°C |
||||
| Wild Type | ox-1 | ox-2 | Wild Type | ox-1 | ox-2 | |
| 32:0 | 6.5 ± 1.5 | 5.2 ± 1.0 | 4.6 ± 1.4 | 6.5 ± 1.8 | 4.7 ± 1.3 | 5.3 ± 0.4 |
| 34:4 | 134.1 ± 9.0 | 117.1 ± 15.4 | 126.2 ± 4.8 | 132.7 ± 21.5 | 76.7 ± 19.0a | 89.9 ± 13.3a |
| 34:3 | 3,690.4 ± 180.7 | 3,768.6 ± 629.4 | 4,176.2 ± 69.2b | 3,568.3 ± 487.3 | 3,030.0 ± 552.7 | 3,035.0 ± 594.3 |
| 34:2 | 3,123.3 ± 275.4 | 3,344.1 ± 414.6 | 3,288.5 ± 300.1 | 2,614.0 ± 440.2 | 2,139.7 ± 477.3 | 2,206.6 ± 227.9 |
| 34:1 | 299.8 ± 57.2 | 279.1 ± 42.3 | 307.1 ± 63.8 | 145.2 ± 37.0 | 110.2 ± 15.3 | 131.9 ± 15.9 |
| 36:6 | 1,886.2 ± 184.7 | 1,651.7 ± 179.7 | 1,892.0 ± 97.2 | 1,786.0 ± 204.7 | 1,330.9 ± 217.4a | 1,347.7 ± 312.3 |
| 36:5 | 4,934.5 ± 294.0 | 4,151.1 ± 470.8a | 4,271.8 ± 471.2a | 3,620.0 ± 590.8 | 2,439.9 ± 689.8a | 2,696.8 ± 382.7a |
| 36:4 | 2,424.8 ± 165.5 | 2,137.7 ± 205.5 | 2,183.0 ± 232.2 | 1,753.6 ± 332.3 | 1,104.6 ± 265.6a | 1,160.8 ± 205.5a |
| 36:3 | 1,020.3 ± 156.3 | 983.4 ± 184.3 | 1,022.4 ± 117.2 | 527.3 ± 79.6 | 387.9 ± 61.4a | 411.1 ± 66.8 |
| 36:2 | 365.4 ± 39.7 | 485.7 ± 70.5b | 436.2 ± 101.4 | 225.4 ± 46.0 | 220.6 ± 40.9 | 226.9 ± 16.3 |
| 36:1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 38:6 | 15.4 ± 2.2 | 11.2 ± 2.0a | 11.1 ± 1.7a | 12.0 ± 3.4 | 8.7 ± 1.9 | 8.7 ± 2.1 |
| 38:5 | 46.9 ± 2.0 | 39.9 ± 6.5 | 34.3 ± 4.2a | 34.0 ± 6.8 | 24.2 ± 5.7 | 21.3 ± 4.8a |
| 38:4 | 48.6 ± 10.5 | 48.7 ± 7.6 | 42.6 ± 8.7 | 35.0 ± 5.5 | 22.8 ± 4.8a | 23.1 ± 2.2a |
| 38:3 | 44.4 ± 6.2 | 34.7 ± 9.5 | 33.1 ± 7.4 | 22.3 ± 4.5 | 16.5 ± 5.5 | 17.0 ± 3.7 |
| 38:2 | 25.9 ± 6.9 | 24.1 ± 2.3 | 14.8 ± 4.2a | 18.9 ± 1.3 | 15.5 ± 4.0 | 14.8 ± 3.1a |
| 40:5 | 1.5 ± 1.6 | 2.5 ± 1.4 | 1.2 ± 0.9 | 5.7 ± 1.3 | 3.3 ± 1.3a | 3.6 ± 1.8 |
| 40:4 | 4.9 ± 1.8 | 10.4 ± 4.9 | 5.4 ± 5.0 | 10.6 ± 1.6 | 5.9 ± 1.4a | 5.4 ± 1.4a |
| 40:3 | 3.7 ± 1.7 | 8.0 ± 5.4 | 4.1 ± 2.5 | 10.1 ± 1.5 | 5.0 ± 1.2a | 3.3 ± 0.9a |
| 40:2 | 2.2 ± 2.4 | 2.5 ± 2.2 | 4.5 ± 0.9 | 4.4 ± 1.2 | 2.4 ± 1.7 | 3.9 ± 1.4 |
Value lower than the wild type in the same experiment (P < 0.05).
Value higher than the wild type in the same experiment (P < 0.05).
Table II. Total amount of each head group class in rosettes of wild-type (Col-0) and ACBP1-overexpressing (ox-1 and ox-2) plants grown at 23°C or CA followed by freezing treatment.
The values are means ± sd (nmol mg−1 dry weight; n = 3). Significant differences (P < 0.05) from the wild type in the same experiment are indicated in boldface. DGDG, Digalactosyldiacylglycerol.
| Lipid Class | 23°C |
−8°C |
||||
| Wild Type | ox-1 | ox-2 | Wild Type | ox-1 | ox-2 | |
| PC | 18.1 ± 1.01 | 17.1 ± 2.13 | 17.9 ± 1.02 | 14.5 ± 2.12 | 11.0 ± 2.20 | 11.4 ± 1.49a |
| PA | 0.2 ± 0.03 | 0.2 ± 0.02 | 0.2 ± 0.02 | 5.5 ± 1.74 | 8.7 ± 1.89 | 9.8 ± 2.33b |
| DGDG | 37.5 ± 1.93 | 36.7 ± 2.19 | 38.5 ± 1.23 | 41.6 ± 2.22 | 44.9 ± 1.02 | 43.9 ± 2.50 |
| MGDG | 184.0 ± 8.32 | 184.3 ± 12.12 | 177.8 ± 7.64 | 133.5 ± 10.86 | 143.6 ± 11.18 | 137.6 ± 10.35 |
| PG | 12.3 ± 0.36 | 12.6 ± 1.55 | 13.5 ± 0.81 | 16.5 ± 0.86 | 16.0 ± 0.45 | 15.8 ± 0.88 |
| PE | 6.2 ± 0.80 | 6.4 ± 0.26 | 5.6 ± 0.29 | 7.6 ± 1.25 | 7.4 ± 1.43 | 8.2 ± 1.23 |
| PI | 6.7 ± 0.29 | 6.2 ± 0.52 | 6.3 ± 0.34 | 6.8 ± 0.42 | 6.3 ± 0.13 | 6.4 ± 0.35 |
| PS | 1.1 ± 0.08 | 1.0 ± 0.11 | 1.1 ± 0.04 | 0.8 ± 0.22 | 0.6 ± 0.13 | 0.8 ± 0.11 |
| LysoPG | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.01 |
| LysoPC | 0.02 ± 0.00 | 0.04 ± 0.00b | 0.03 ± 0.00b | 0.17 ± 0.01 | 0.30 ± 0.10 | 0.36 ± 0.15 |
| LysoPE | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.31 ± 0.09 | 0.34 ± 0.06 | 0.40 ± 0.07 |
Value lower than the wild type in the same experiment (P < 0.05).
Value higher than the wild type in the same experiment (P < 0.05).
The acbp1 Knockout Mutant Shows Enhanced Freezing Tolerance, While ACBP1-Complemented Plants Are Freezing Sensitive
A T-DNA knockout mutant of ACBP1 (designated acbp1) from Syngenta (SAIL_653_B06) that had been previously characterized (Xiao et al., 2008a) was used to investigate the role of ACBP1 in freezing stress. This mutant was complemented by a construct expressing ACBP1 from the CaMV 35S promoter in Agrobacterium tumefaciens-mediated transformation as reported by Xiao et al. (2008a). An ACBP1-complemented transgenic line (cACBP1-2) that was confirmed to accumulate the 37-kD ACBP1 protein by western-blot analysis (Xiao et al., 2008a) was subsequently used in the freezing treatment.
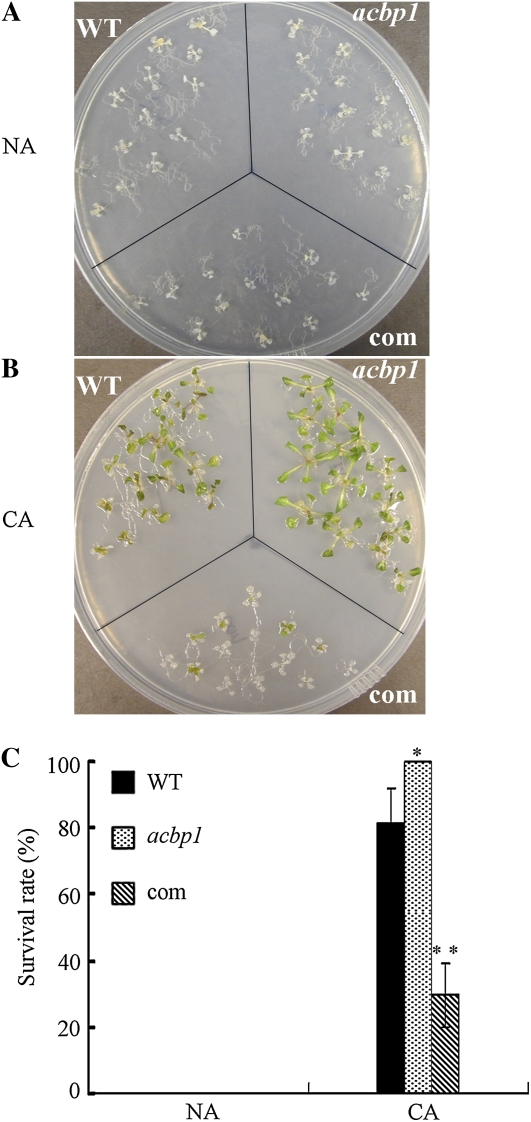
To investigate the role of ACBP1 in freezing tolerance, nonacclimated (NA) and cold-acclimated (CA) 11-d-old seedlings of acbp1 mutant and ACBP1-complemented seedlings grown on Murashige and Skoog (MS) medium were treated at −12°C for 1 h using the wild type as a control. Exposure at −12°C killed all NA seedlings (Fig. 1A). With CA, ACBP1-complemented seedlings showed enhanced tolerance over NA; however, the survival rate of complemented seedlings (30%) was significantly (P < 0.01) lower than in the wild type (82%; Fig. 1, A and B). In contrast, all acbp1 mutant seedlings survived in comparison with only 82% (P < 0.05) of the wild type (Fig. 1, B and C).
Figure 1.
The acbp1 mutant seedlings display enhanced freezing tolerance, while ACBP1-complemented plants show increased freezing sensitivity. A and B, NA and CA 11-d-old wild-type (WT), acbp1 mutant, and ACBP1-complemented (com) seedlings after treatment at −12°C for 1 h. After thawing overnight at 4°C, the plates were transferred to a growth chamber (16-h-light [23°C]/8-h-dark [21°C] photoperiods) for a 7-d recovery before photography. C, Survival rate of NA and CA wild-type, acbp1 mutant, and ACBP1-complemented seedlings shown in A and B. Asterisks denote significant differences from the wild type (** P < 0.01, * P < 0.05). Values are means ± sd(n = 3).
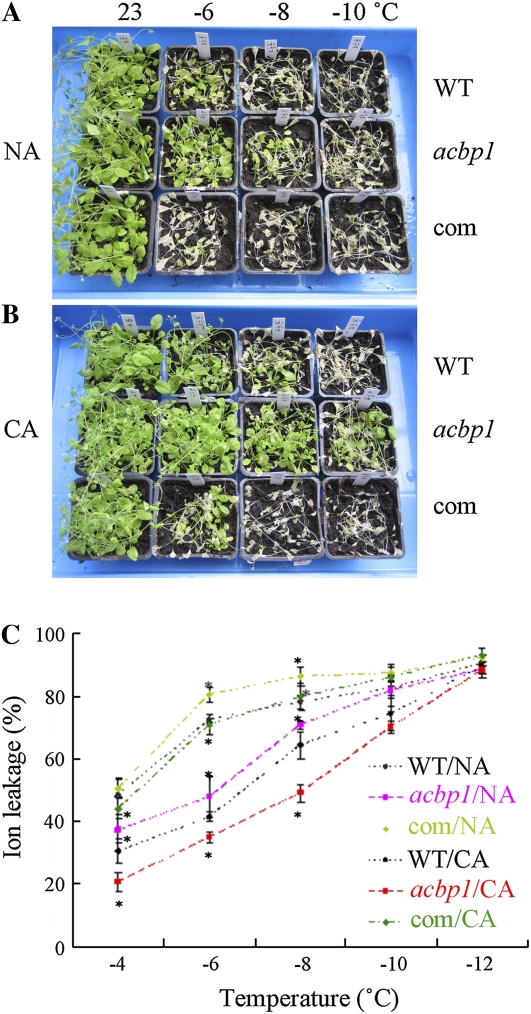
Five-week-old mature plants were also subjected to freezing treatment. NA acbp1 mutant plants tolerated temperatures of −6°C and −8°C better than NA wild-type or ACBP1-complemented plants (Fig. 2A). As shown in Figure 2B, more CA acbp1 mutant plants survived at −6°C, −8°C, and −10°C than either CA wild-type or ACBP1-complemented plants. Both NA and CA ACBP1-complemented plants displayed greater freezing sensitivity than the wild type (Fig. 2, A and B).
Figure 2.
The acbp1 mutant plants are more tolerant to freezing stress, while ACBP1-complemented plants show increased freezing sensitivity. A and B, NA and CA 5-week-old wild-type (WT), acbp1 mutant, and ACBP1-complemented (com) plants after freezing treatment at the indicated temperatures. The plants were photographed after thawing overnight at 4°C and recovery in a growth chamber (16-h-light [23°C]/8-h-dark [21°C] photoperiods) for 7 d. C, Electrolyte leakage measurement of NA and CA wild-type, acbp1 mutant, and ACBP1-complemented plants after freezing treatment at the indicated temperatures lasting 1 h followed by thawing at 4°C overnight. Asterisks denote significant differences from the wild type (* P < 0.05). Values are means ± sd(n = 3).
Electrolyte leakage was measured with freezing-treated leaves from NA and CA wild-type, acbp1 mutant, and ACBP1-complemented plants. Results showed that the ionic leakage at −4°C, −6°C, and −8°C of NA and CA acbp1 mutant plants was significantly lower (P < 0.05) than in the wild type (Fig. 2C). For NA Arabidopsis, ionic leakage of ACBP1-complemented plants was significantly greater than in the wild type following treatment at −6°C and −8°C (P < 0.05; Fig. 2C). For CA plants, ionic leakage of ACBP1-complemented lines was significantly greater than in the wild type following treatment at −4°C, −6°C, and −8°C (P < 0.05; Fig. 2C).
ACBP1 Overexpressors Are More Sensitive to Freezing Stress
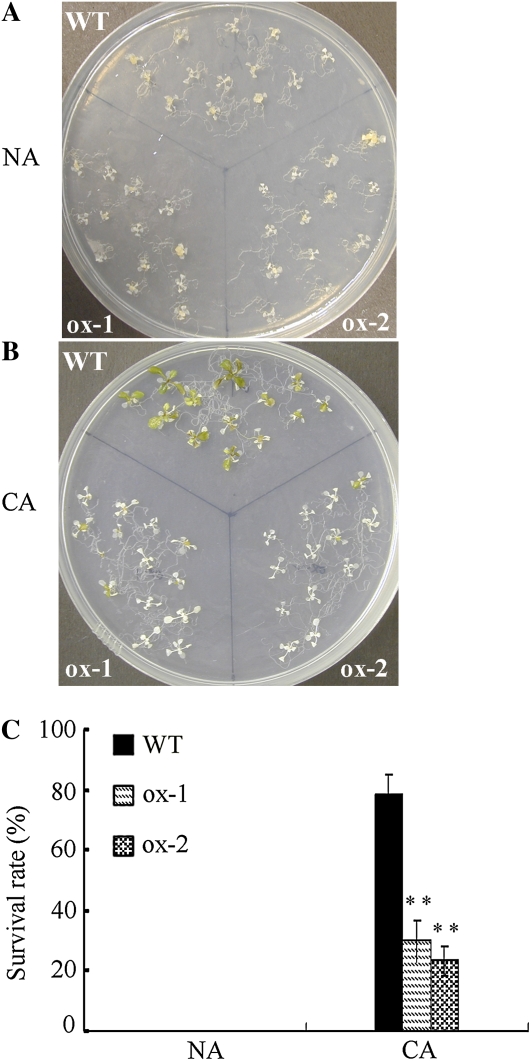
NA and CA 11-d-old seedlings of the wild type and ACBP1 overexpressors (ox-1 and ox-2) were grown on MS medium and treated at −12°C for 1 h to investigate the effects of freezing treatment on seedling development. For NA seedlings, −12°C was lethal to both the wild type and ACBP1 overexpressors (Fig. 3A). After CA at 4°C for 3 d, the survival rate of ACBP1-overexpressing seedlings increased to 30% (ox-1) and 23% (ox-2) but were still significantly (P < 0.01) lower than in the wild type (78%; Fig. 3, B and C).
Figure 3.
ACBP1-overexpressing seedlings show increased sensitivity to freezing stress in comparison with the wild type. A and B, NA and CA 11-d-old wild-type (WT) and ACBP1-overexpressing (ox-1 and ox-2) seedlings after treatment at −12°C for 1 h. Plates were thawed overnight at 4°C following a 7-d recovery in a growth chamber (16-h-light [23°C]/8-h-dark [21°C] photoperiods) before photography. C, Survival rate of NA and CA wild-type and ACBP1-overexpressing seedlings after freezing displayed in A and B. Asterisks denote significant differences from the wild type (** P < 0.01). Values are means ± sd(n = 3).
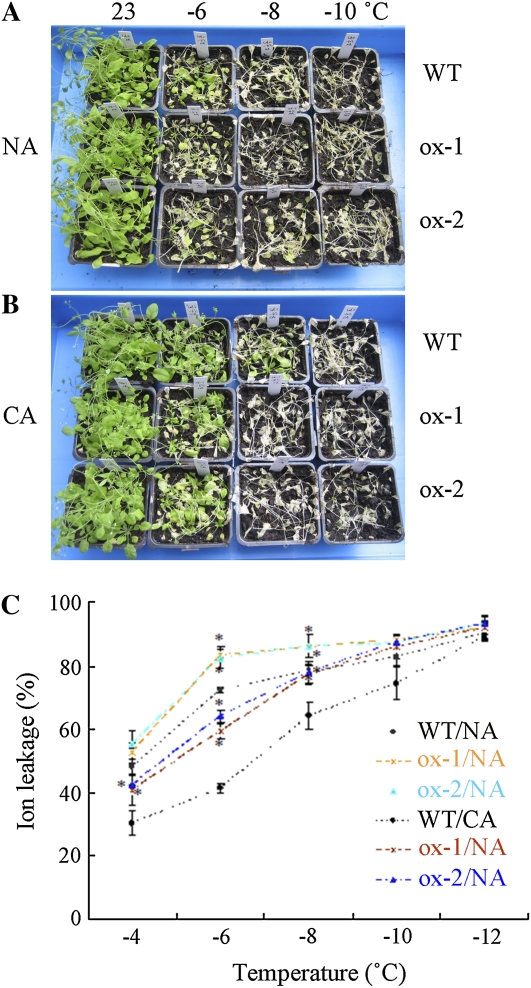
To test the effect of freezing treatment on mature plants, 5-week-old wild-type and ACBP1-overexpressing plants from NA and CA sets were examined. When the temperature reached −6°C, NA wild-type plants were more freezing tolerant than NA ACBP1 overexpressors (Fig. 4A). However, when the temperature was lowered to −8°C and −10°C, most wild-type plants and ACBP1 overexpressors were killed (Fig. 4A). After CA at 4°C for 3 d, the wild type and ACBP1 overexpressors were better protected against freezing stress (Fig. 4B). Most (93%) CA wild-type plants survived at −6°C, while the survival rates of ACBP1 overexpressors (ox-1 and ox-2) were 62% (P < 0.01) and 67% (P < 0.05), respectively. Few (4%) CA ACBP1 overexpressors survived at −8°C, while 56% of the wild type remained viable (Fig. 4B). These results suggested that ACBP1 overexpressors are more sensitive to freezing stress at −6°C and −8°C and that CA enhanced freezing tolerance in the wild type. Both NA and CA ACBP1-overexpressing plants displayed a phenotype similar to ACBP1-complemented plants under freezing stress (Figs. 2, A and B, and 4, A and B), confirming that the accumulation of ACBP1 led to greater freezing sensitivity.
Figure 4.
ACBP1-overexpressing plants are more sensitive to freezing stress in comparison with the wild type. A and B, NA and CA 5-week-old wild-type (WT) and ACBP1-overexpressing (ox-1 and ox-2) plants after freezing treatment at the indicated temperatures. Plants were photographed after a 7-d recovery in a growth chamber (16-h-light [23°C]/8-h-dark [21°C] photoperiods). C, Electrolyte leakage of NA and CA wild-type and ACBP1-overexpressing plants after freezing treatment at the indicated temperatures lasting 1 h followed by thawing at 4°C overnight. Asterisks denote significant differences from the wild type (* P < 0.05). Values are means ± sd(n = 3).
Subsequently, freezing injury was evaluated by taking electrolyte leakage measurements of NA and CA freezing-treated leaves from wild-type and ACBP1-overexpressing plants (Fig. 4C). For NA Arabidopsis, ionic leakage was greater in ACBP1 overexpressors than in the wild type following treatment at −6°C and −8°C (P < 0.05; Fig. 4C). For CA plants, ionic leakage was greater in ACBP1 overexpressors than in the wild type following treatment at −4°C, −6°C, and −8°C (P < 0.05; Fig. 4C).
The Expression of ACBP1 Is Not Induced by Cold Treatment
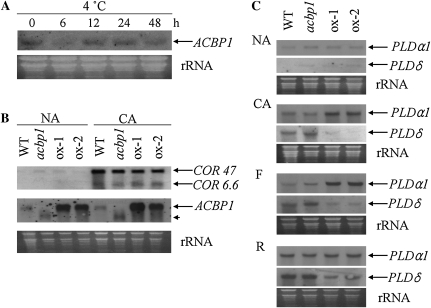
The expression profiles of ACBP1 during cold acclimation were examined by northern-blot analysis. Total RNA was extracted from 5-week-old wild-type Arabidopsis exposed to 4°C for 0, 6, 12, 24, and 48 h. As shown in Figure 5A, the expression of ACBP1 was first down-regulated after 6 h of cold treatment and then increased to the untreated level 12 and 24 h after treatment. At 48 h, the expression of ACBP1 was slightly weaker than at 0 h (Fig. 5A) but rose to a similar level after cold acclimation for 3 d (Fig. 5B). Similar expression profiles were observed in microarray data (http://bar.utoronto.ca/). These results indicate that the expression of ACBP1 is not cold inducible.
Figure 5.
The expression of ACBP1, COR genes, PLDα1, and PLDδ in wild-type (WT), acbp1 mutant, and ACBP1-overexpressing (ox-1 and ox-2) plants. A, Cold induction of ACBP1 expression. Total RNA (20 μg lane−1) was isolated from wild-type Arabidopsis rosettes at the indicated hours (h) after treatment. B, Total RNA (20 μg lane−1) was prepared from rosettes of wild-type, acbp1 mutant, and ACBP1-overexpressing plants before (NA) or after (CA) 3 d of cold acclimation at 4°C. The blots were hybridized with digoxigenin-labeled probes for COR6.6, COR47, and ACBP1. C, The transcript levels of PLDα1 and PLDδ in the wild type, acbp1, and ACBP1 overexpressors. Total RNA (20 μg lane−1) was prepared from wild-type, acbp1, ox-1, and ox-2 rosettes harvested before (NA) or after (CA) 3 d of cold acclimation, followed by freezing at −8°C for 1 h (F) and recovery (R; the temperature was raised to 4°C at 1°C h−1 and held at 4°C for 12 h before sampling). All bottom panels show ethidium bromide-stained rRNA, indicating the relative amounts of total RNA loaded per lane.
Freezing Tolerance of the acbp1 Mutant and Sensitivity of ACBP1 Overexpressors Are Independent of COR Gene Expression
To obtain further insight into ACBP1 function in freezing stress, the expression of ACBP1 and two COR genes (COR6.6 and COR47) was measured in 5-week-old Arabidopsis plants by northern-bolt analysis. After cold acclimation (4°C for 3 d), ACBP1 was not induced in the wild type (Fig. 5B), in contrast to the cold-induced ACBP6 (Chen et al., 2008). Without CA, the COR6.6 and COR47 transcripts were barely detectable in the wild type, the acbp1 mutant, and ACBP1 overexpressors (Fig. 5B). After cold acclimation for 3 d, the mRNA levels of COR6.6 and COR47 increased in all three genotypes. Although cold acclimation enhanced freezing tolerance of acbp1 mutant plants, the expression levels of COR6.6 and COR47 were similar among wild-type, acbp1 mutant, and ACBP1-overexpressing plants (Fig. 5B), suggesting that tolerance of the acbp1 mutant and sensitivity of ACBP1 overexpressors are not dependent on cold-induced COR gene expression.
Tolerance of the acbp1 Mutant and Sensitivity of ACBP1 Overexpressors Are Correlated to the Expression of PLDα1 and PLDδ
PLDα1 and PLDδ are important in mediating freezing tolerance in Arabidopsis. Previous studies have demonstrated that PLDα1-deficient Arabidopsis plants are more tolerant to freezing (Welti et al., 2002; Rajashekar et al., 2006). In contrast, knockout of PLDδ decreased and its overexpression increased freezing tolerance (Li et al., 2004). We have shown that the overexpression of the 10-kD ACBP6 resulted in enhanced freezing tolerance that was correlated to elevated PLDδ expression (Chen et al., 2008).
To determine whether tolerance in the acbp1 mutant and sensitivity in ACBP1 overexpressors are associated with PLD expression, the expression of PLDα1 and PLDδ in the wild type, acbp1 mutant, and ACBP1 overexpressors (ox-1 and ox-2) was investigated by northern-blot analysis using PCR-generated digoxigenin-labeled cDNA probes. As shown in Figure 5C, ACBP1 overexpressors (ox-1 and ox-2) showed higher levels of PLDα1 mRNA than the wild type at CA and freezing stages, while the expression of PLDα1 was lower in the acbp1 mutant than in the wild type. In contrast, the mRNA levels of PLDδ were lower in ox-1 and ox-2 plants than in the wild type at CA, freezing, and postfreezing recovery stages, while its expression in the acbp1 mutant was higher than in the wild type at CA and freezing stages (Fig. 5C). However, the expression of PLDα1 and PLDδ was similar in all three genotypes when nonacclimated (Fig. 5C).
Changes in Lipid Molecular Species after Freezing Treatment of CA Wild-Type, acbp1 Mutant, ACBP1-Overexpressing, and ACBP1-Complemented Plants
Lipid profiling of Arabidopsis plants was carried out by electrospray ionization tandem mass spectrometry. In the absence of freezing treatment (23°C), the ACBP1 overexpressors, acbp1 mutant, and ACBP1-complemented plants all showed significantly higher LysoPC content than the wild type (Tables II and III). However, they showed no significant differences in LysoPC when compared with the wild type after CA and freezing treatment at −8°C (Tables II and III). Results indicate that after CA and freezing treatment, the total amount of PA significantly increased in the ACBP1 overexpressor ox-2 (Table II) and the ACBP1-complemented line (Table III) when compared with the wild type. Although the difference in PA content between the wild type and the ACBP1 overexpressor ox-1 was not significant, total PA increased 43.5-fold in ox-1 compared with 27.5-fold in the wild type after CA and freezing treatment (Table II). Thus, ox-1 accumulated 58% more PA than the wild type, while ox-2 and complemented plants accumulated 78% and 75% more PA, respectively (Tables II and III). Particularly, several PA species were significantly higher in ox-1, ox-2, and the ACBP1-complemented line than in the wild type, including 34:6 PA, 34:3 PA, 34:2 PA, 36:6 PA, and 36:2 PA (Tables IV and V). After CA and freezing treatment, the acbp1 mutant accumulated significantly less (51%) PA than the wild type (Table III). Specifically, 32:0 PA, 34:4 PA, 34:3 PA, 36:6 PA, and 36:5 PA were reduced in the acbp1 mutant (Table V).
Table III. Total amount of each head group class in rosettes of wild-type (Col-0), acbp1 mutant, and ACBP1-complemented (com) plants grown at 23°C or CA followed by freezing treatment.
The values are means ± sd (nmol mg−1 dry weight; n = 3). Significant differences (P < 0.05) from the wild type in the same experiment are indicated in boldface. DGDG, Digalactosyldiacylglycerol.
| Lipid Class | 23°C |
−8°C |
||||
| Wild Type | acbp1 | com | Wild Type | acbp1 | com | |
| PC | 18.1 ± 1.01 | 17.5 ± 2.03 | 16.2 ± 0.22a | 14.5 ± 2.12 | 18.4 ± 2.07b | 8.9 ± 1.41a |
| PA | 0.2 ± 0.03 | 0.2 ± 0.07 | 0.3 ± 0.14 | 5.5 ± 1.74 | 2.8 ± 0.35a | 9.6 ± 0.69b |
| DGDG | 37.5 ± 1.93 | 34.2 ± 2.74 | 36.7 ± 0.80 | 41.6 ± 2.22 | 41.0 ± 1.31 | 44.3 ± 1.94 |
| MGDG | 184.0 ± 8.32 | 168.6 ± 11.44 | 169.1 ± 5.27a | 133.5 ± 10.86 | 139.9 ± 7.72 | 135.9 ± 9.93 |
| PG | 12.3 ± 0.36 | 11.1 ± 1.72 | 12.0 ± 0.18 | 16.5 ± 0.86 | 16.7 ± 1.02 | 13.6 ± 1.65a |
| PE | 6.2 ± 0.80 | 5.7 ± 0.60 | 5.5 ± 0.12 | 7.6 ± 1.25 | 8.9 ± 1.12 | 6.3 ± 1.40 |
| PI | 6.7 ± 0.29 | 6.5 ± 0.48 | 5.8 ± 0.12 | 6.8 ± 0.42 | 7.1 ± 0.42 | 6.4 ± 0.30 |
| PS | 1.1 ± 0.08 | 1.1 ± 0.10 | 1.0 ± 0.08 | 0.8 ± 0.22 | 1.1 ± 0.16 | 0.7 ± 0.07 |
| LysoPG | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 |
| LysoPC | 0.02 ± 0.00 | 0.03 ± 0.00b | 0.03 ± 0.01b | 0.17 ± 0.01 | 0.13 ± 0.06 | 0.38 ± 0.27 |
| LysoPE | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.31 ± 0.09 | 0.23 ± 0.04 | 0.44 ± 0.03b |
Value lower than the wild type in the same experiment (P < 0.05).
Value higher than the wild type in the same experiment (P < 0.05).
Table IV. Changes in PA species of wild-type (Col-0) and ACBP1-overexpressing (ox-1 and ox-2) plants grown at 23°C or CA followed by freezing treatment.
The values are means ± sd (nmol g−1 dry weight; n = 3). Significant differences (P < 0.05) from the wild type in the same experiment are indicated in boldface.
| PA Species | 23°C |
−8°C |
||||
| Wild Type | ox-1 | ox-2 | Wild Type | ox-1 | ox-2 | |
| 32:0 | 0 | 0 | 0 | 2.26 ± 1.2 | 3.9 ± 2.0 | 4.6 ± 3.2 |
| 34:6 | 0.2 ± 0.4 | 0.1 ± 0.2 | 0 ± 0.1 | 395.7 ± 132.9 | 786.0 ± 224.3a | 835.9 ± 59.0a |
| 34:5 | 0 | 0 ± 0.2 | 0 | 12.4 ± 5.0 | 11.7 ± 14.6 | 20.7 ± 11.6 |
| 34:4 | 0.3 ± 0.5 | 0.6 ± 0.5 | 1.7 ± 1.3 | 169.3 ± 77.6 | 275.9 ± 53.7 | 233.6 ± 97.9 |
| 34:3 | 48.9 ± 3.4 | 63.6 ± 3.7a | 67.0 ± 7.6a | 1,121.7 ± 334.7 | 1,904.6 ± 355.2a | 2,083.4 ± 375.9a |
| 34:2 | 55.7 ± 7.2 | 62.1 ± 10.8 | 59.9 ± 10.3 | 1,034.7 ± 334.7 | 1,759.2 ± 410.1a | 1,894.8 ± 582.9a |
| 34:1 | 1.1 ± 2.3 | 1.1 ± 1.4 | 2.2 ± 1.2 | 61.5 ± 28.2 | 83.3 ± 24.7 | 112.6 ± 35.8 |
| 36:6 | 6.7 ± 4.7 | 8.3 ± 3.8 | 13.8 ± 3.5a | 524.9 ± 145.4 | 842.0 ± 179.1a | 955.8 ± 99.9a |
| 36:5 | 32.8 ± 7.6 | 32.0 ± 3.1 | 32.4 ± 5.3 | 1,127.6 ± 354.1 | 1,626.7 ± 357.2 | 1,885.3 ± 495.5a |
| 36:4 | 22.3 ± 5.6 | 17.6 ± 5.3 | 25.5 ± 5.7 | 799.5 ± 269.4 | 1,071.2 ± 240.4 | 1,309.6 ± 497.9 |
| 36:3 | 4.9 ± 3.1 | 8.3 ± 5.3 | 6.8 ± 2.1 | 149.6 ± 59.3 | 230.6 ± 53.3 | 287.4 ± 85.7a |
| 36:2 | 2.7 ± 2.6 | 3.6 ± 2.5 | 2.9 ± 3.4 | 73.1 ± 24.8 | 136.7 ± 36.4a | 146.1 ± 44.3a |
Value higher than the wild type in the same experiment (P < 0.05).
Table V. Changes in PA species of wild-type (Col-0), acbp1 mutant, and ACBP1-complemented (com) plants grown at 23°C or CA followed by freezing treatment.
The values are means ± sd (nmol g−1 dry weight; n = 3). Significant differences (P < 0.05) from the wild type in the same experiment are indicated in boldface.
| PA Species | 23°C |
−8°C |
||||
| Wild Type | acbp1 | com | Wild Type | acbp1 | com | |
| 32:0 | 0 | 0 | 0.1 ± 0.3 | 2.26 ± 1.2 | 0.3 ± 0.1a | 3.7 ± 0.7 |
| 34:6 | 0.2 ± 0.4 | 0 | 0.4 ± 0.6 | 395.7 ± 132.9 | 269.3 ± 26.6 | 751.8 ± 60.0b |
| 34:5 | 0 | 0 | 0 | 12.4 ± 5.0 | 8.1 ± 4.5 | 24.3 ± 17.4 |
| 34:4 | 0.3 ± 0.5 | 1.5 ± 1.6 | 1.8 ± 2.3 | 169.3 ± 77.6 | 52.0 ± 16.8a | 195.5 ± 42.2 |
| 34:3 | 48.9 ± 3.4 | 51.1 ± 12.5 | 99.3 ± 52.5 | 1,121.7 ± 334.7 | 516.9 ± 75.1a | 1,985.0 ± 134.1b |
| 34:2 | 55.7 ± 7.2 | 54.6 ± 19.2 | 89.8 ± 28.9 | 1,034.7 ± 334.7 | 563.7 ± 90.0 | 1,838.8 ± 191.0b |
| 34:1 | 1.1 ± 2.3 | 1.3 ± 1.1 | 3.8 ± 5.5 | 61.5 ± 28.2 | 22.4 ± 13.1 | 82.2 ± 3.5 |
| 36:6 | 6.7 ± 4.7 | 9.9 ± 6.0 | 21.2 ± 15.8 | 524.9 ± 145.4 | 257.1 ± 3.8a | 946.3 ± 65.4b |
| 36:5 | 32.8 ± 7.6 | 37.5 ± 13.1 | 50.5 ± 18.7 | 1,127.6 ± 354.1 | 561.5 ± 89.5a | 2,014.7 ± 139.6b |
| 36:4 | 22.3 ± 5.6 | 31.8 ± 20.8 | 38.5 ± 9.3b | 799.5 ± 269.4 | 406.0 ± 82.5 | 1,305.3 ± 90.9b |
| 36:3 | 4.9 ± 3.1 | 8.5 ± 3.1 | 11.9 ± 8.0 | 149.6 ± 59.3 | 76.0 ± 12.9 | 283.1 ± 29.2b |
| 36:2 | 2.7 ± 2.6 | 2.0 ± 2.1 | 5.3 ± 3.6 | 73.1 ± 24.8 | 43.1 ± 8.9 | 140.0 ± 15.9b |
Value lower than the wild type in the same experiment (P < 0.05).
Value higher than the wild type in the same experiment (P < 0.05).
In contrast, PC levels declined 20% in the wild type after CA and freezing, while the decrease was 36%, 36%, and 45% in ox-1, ox-2, and ACBP1-complemented lines, respectively (Tables II and III). In this case, ox-1, ox-2, and the ACBP1-complemented line accumulated 24%, 21%, and 39% less PC than the wild type, respectively (Tables II and III). In particular, 34:4 PC, 36:5 PC, 36:4 PC, 38:4 PC, 40:4 PC, and 40:3 PC in ACBP1-overexpressing (ox-1 and ox-2) and ACBP1-complemented plants were significantly lower (P < 0.05) than in the wild type (Table VI). Among these PC species, 34:4 PC (16:1–18:3), 36:5 PC (18:3–18:2), 36:4 PC (18:2–18:2 > 18:1–18:3), and 38:4 PC (20:1–18:3 > 20:2–18:2) are diunsaturated species (Devaiah et al., 2006). After CA and freezing treatment, acbp1 mutant plants accumulated 27% more PC than the wild type. Several species of PC were significantly higher (P < 0.05) in the acbp1 mutant than in the wild type (Table III). These include 34:4 PC, 34:2 PC, 36:5 PC, 36:4 PC, 36:2 PC, 38:5 PC, 38:4 PC, 38:3 PC, and 40:4 PC (Table VI). In addition, 34:4 PC (16:1–18:3), 36:5 PC (18:3–18:2), 36:4 PC (18:2–18:2 > 18:1–18:3), 38:5 PC (20:3–18:3), 38:4 PC (20:1–18:3 > 20:2–18:2), and 38:3 PC (20:1–18:2 > 20:0–18:3) are diunsaturated species (Devaiah et al., 2006). Prior to CA and freezing treatment, LysoPC was significantly higher (P < 0.05) in the ACBP1 overexpressors (ox-1 and ox-2), acbp1 mutant, and the ACBP1-complemented line than in the wild type (Tables II and III). Following CA and freezing, no significant differences in LysoPC were evident among all phenotypes (Tables II and III). More significant changes occurred in the ACBP1-complemented line than in the wild type (Table III). Before CA and freezing, total PC and monogalactosyldiacylglycerol (MGDG) were significantly lower in the ACBP1-complemented line than in the wild type, while phosphatidylglycerol (PG) and lysophosphatidylethanolamine (LysoPE) in the ACBP1-complemented line were significantly lower and higher than in the wild type after CA and freezing, respectively (Table III). Nevertheless, these changes were not observed in the ACBP1 overexpressors (ox-1 and ox-2; Table II).
Table VI. Changes in PC species of wild-type (Col-0), acbp1 mutant, and ACBP1-complemented (com) plants grown at 23°C or CA followed by freezing treatment.
The values are means ± sd (nmol g−1 dry weight; n = 3). Significant differences (P < 0.05) from the wild type in the same experiment are indicated in boldface.
| PC Species | 23°C |
−8°C |
||||
| Wild Type | acbp1 | com | Wild Type | acbp1 | com | |
| 32:0 | 6.5 ± 1.5 | 7.4 ± 1.4 | 5.0 ± 2.3 | 6.5 ± 1.8 | 5.0 ± 4.8 | 4.1 ± 3.1 |
| 34:4 | 134.1 ± 9.0 | 129.8 ± 8.7 | 108.0 ± 2.6a | 132.7 ± 21.5 | 162.6 ± 8.7b | 52.2 ± 36.9a |
| 34:3 | 3,690.4 ± 180.7 | 3,376.6 ± 235.5 | 3,711.3 ± 172.9 | 3,568.3 ± 487.3 | 4,027.9 ± 426.4 | 1,730.1 ± 1,180.1a |
| 34:2 | 3,123.3 ± 275.4 | 3,443.0 ± 142.9 | 2,822.0 ± 126.7 | 2,614.0 ± 440.2 | 3,401.0 ± 402.0b | 1,289.1 ± 892.9a |
| 34:1 | 299.8 ± 57.2 | 379.6 ± 59.7 | 288.7 ± 55.7 | 145.2 ± 37.0 | 162.2 ± 6.0 | 77.8 ± 52.5 |
| 36:6 | 1,886.2 ± 184.7 | 1,796.2 ± 206.5 | 1,823.2 ± 188.7 | 1,786.0 ± 204.7 | 2,084.9 ± 386.7 | 728.9 ± 495.0a |
| 36:5 | 4,934.5 ± 294.0 | 4,713.7 ± 665.5 | 3,944.8 ± 211.5a | 3,620.0 ± 590.8 | 5,032.5 ± 597.8b | 1,595.2 ± 1,113.4a |
| 36:4 | 2,424.8 ± 165.5 | 2,741.6 ± 182.6 | 2,018.4 ± 222.0a | 1,753.6 ± 332.3 | 2,364.6 ± 317.8b | 727.7 ± 514.9a |
| 36:3 | 1,020.3 ± 156.3 | 1,092.8 ± 110.7 | 920.0 ± 38.2 | 527.3 ± 79.6 | 597.9 ± 35.9 | 269.1 ± 181.6a |
| 36:2 | 365.4 ± 39.7 | 411.7 ± 48.3 | 390.1 ± 57.4 | 225.4 ± 46.0 | 310.2 ± 39.7b | 129.3 ± 87.6 |
| 36:1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 38:6 | 15.4 ± 2.2 | 14.8 ± 2.1 | 14.0 ± 5.7 | 12.0 ± 3.4 | 14.2 ± 2.3 | 5.3 ± 3.5 |
| 38:5 | 46.9 ± 2.0 | 46.0 ± 4.7 | 36.3 ± 1.8a | 34.0 ± 6.8 | 47.2 ± 7.3b | 14.1 ± 9.8a |
| 38:4 | 48.6 ± 10.5 | 57.6 ± 16.7 | 38.7 ± 10.0 | 35.0 ± 5.5 | 50.9 ± 3.0b | 16.2 ± 11.8a |
| 38:3 | 44.4 ± 6.2 | 38.4 ± 11.9 | 31.4 ± 4.5a | 22.3 ± 4.5 | 30.1 ± 3.1b | 11.4 ± 7.7a |
| 38:2 | 25.9 ± 6.9 | 24.4 ± 4.9 | 15.0 ± 4.5a | 18.9 ± 1.3 | 24.6 ± 4.7 | 9.0 ± 8.0 |
| 40:5 | 1.5 ± 1.6 | 3.5 ± 0.5 | 3.0 ± 2.6 | 5.7 ± 1.3 | 7.8 ± 1.7 | 1.1 ± 1.5a |
| 40:4 | 4.9 ± 1.8 | 8.1 ± 3.9 | 8.6 ± 1.5a | 10.6 ± 1.6 | 16.1 ± 2.6b | 2.7 ± 2.8a |
| 40:3 | 3.7 ± 1.7 | 9.6 ± 3.0b | 7.0 ± 2.4 | 10.1 ± 1.5 | 11.0 ± 4.1 | 2.5 ± 1.7a |
| 40:2 | 2.2 ± 2.4 | 1.9 ± 1.6 | 2.0 ± 1.6 | 4.4 ± 1.2 | 4.5 ± 2.0 | 1.1 ± 1.0a |
Value lower than the wild type in the same experiment (P < 0.05).
Value higher than the wild type in the same experiment (P < 0.05).
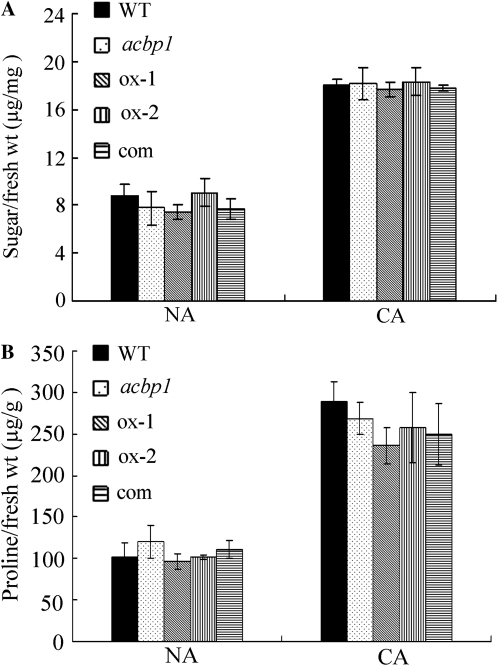
No Change in Soluble Sugar and Pro Accumulation during Cold Acclimation in the acbp1 Mutant
The accumulation of soluble sugar and Pro is associated with enhanced freezing tolerance in Arabidopsis and tobacco (Nicotiana tabacum; Xin and Browse, 1998; Gilmour et al., 2000; Rajashekar et al., 2006; Zhao et al., 2009a). In the absence of cold acclimation, similar levels of soluble sugar were observed in wild-type, acbp1 mutant, ACBP1-overexpressing, and ACBP1-complemented plants (Fig. 6A). After 3 d of cold acclimation at 4°C, soluble sugar content in all genotypes increased by 2-fold (Fig. 6A). Pro content increased by 1.8-fold in the wild type after cold acclimation (Fig. 6B). Similar increases occurred in the other genotypes, but there were no significant differences between them (Fig. 6B).
Figure 6.
Changes in soluble sugar (A) and Pro (B) after cold acclimation of wild-type (WT), acbp1 mutant, ACBP1-complemented (com), and ACBP1-overexpressing (ox-1 and ox-2) plants. Values are means ± sd(n = 3).
His6-ACBP1 Binds PA in Vitro
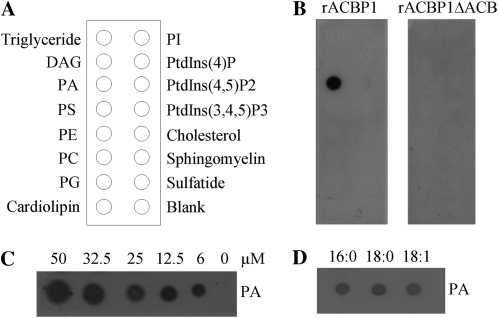
To explore the interaction between ACBP1 and the phospholipids (PLs) PA and PC, which influence freezing tolerance, we tested the binding of purified recombinant ACBP1 (Chye, 1998) to various lipids. We have previously shown using similar filter-binding assays that recombinant ACBP6 binds PC in vitro (Chen et al., 2008). When we used a protein-lipid overlay assay of commercially available membrane lipid strips, recombinant ACBP1 (rACBP1) was observed to specifically bind PA but not other lipids, including diacylglycerol, phosphatidylserine (PS), PE, PC, PG, phosphatidylinositol (PI), phosphatidylinositol 4-phosphate, phosphatidylinositol 4,5-bisphosphate, phosphatidylinositol 3,4,5-trisphosphate, and 3-sulfogalactosylceramide, which were simultaneously tested (Fig. 7, A and B).
Figure 7.
Interaction of recombinant ACBP1 with PA. A, Diagram of lipid species on membrane lipid strips (Echelon Biosciences). B, Binding of full-length (rACBP1) and deletion mutant (rACBP1 Δ ACB) ACBP1 to lipids on membrane lipid strips in A. The strips were incubated with 1 μ g mL−1 purified rACBP1 or rACBP1 Δ ACB protein, and their binding was detected by immunoblotting with HRP-conjugated anti-penta-His antibodies. C, rACBP1/PA binding on filters. Serial concentrations (0, 6, 12.5, 25.0, 32.5, and 50 μm) of 16:0-PA were spotted onto nitrocellulose and incubated with 1 μ g mL−1 purified rACBP1 protein. The rACBP1/PA binding was detected by immunoblotting with HRP-conjugated anti-penta-His antibodies. D, Effect of PA acyl species on rACBP1/PA binding. Twenty micromolar lipids (16:0-PA, 18:0-PA, and18:1-PA) spotted onto nitrocellulose were incubated with 1 μ g mL−1 purified rACBP1 protein. The rACBP1/PA binding was detected by immunoblotting with HRP-conjugated anti-penta-His antibodies. DAG, Diacylglycerol; PtdIns(4)P, phosphatidylinositol 4-phosphate; PtdIns(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PtdIns(3,4,5)P3, phosphatidylinositol 3,4,5-trisphosphate; sulfatide, 3-sulfogalactosylceramide.
To examine the significance of the acyl-CoA-binding domain of ACBP1 in mediating PA binding, a 20-kD His-tagged fusion protein lacking the ACBP domain (rACBP1 Δ ACB) was expressed, purified from Escherichia coli (data not shown), and tested using the protein-lipid binding assay. As shown in Figure 7B, deletion of the acyl-CoA-binding domain in ACBP1 abolished PA binding. Furthermore, results from blots containing serial dilutions of PA (16:0) indicated that rACBP1 binds PA in a dose-dependent manner (Fig. 7C). As the PL species used in Figure 7, A and B, were 16:0, the binding of other fatty acid species of PA to rACBP1 was subsequently tested. Results indicated that rACBP1 binds all species of PA (16:0-PA, 18:0-PA, and 18:1-PA) tested.
DISCUSSION
Upon freezing, the plasma membrane of the plant cell is most susceptible to injury (Steponkus, 1984; Uemura et al., 2006). Its destabilization and disruption is believed to be the primary cause of freezing injury in plants (Steponkus, 1984). Hence, stabilization of plant membranes against cellular dehydration would alleviate such injury. Plants have developed an innate ability to combat freezing after exposure to low nonfreezing temperature, and this is defined as cold acclimation (Thomashow, 1999). During cold acclimation in rye (Secale cereale), the plasma membrane increases cryostability by reducing the formation of nonlamellar phase (like HII phase) lipids, which prevent expansion-induced lysis (Uemura and Steponkus, 1994). This is accompanied by changes in lipid composition that modulate membrane stabilization (Thomashow, 1999). Increases in the proportion of PL in the plasma membrane during cold acclimation are commonly observed in various herbaceous and woody species, such as rye (Uemura and Steponkus, 1994), oat (Avena sativa; Uemura and Steponkus, 1994), seashore paspalum (Paspalum vaginatum; Cyril et al., 2002), Arabidopsis (Uemura et al., 1995; Welti et al., 2002), and mulberry (Morus bombycis; Yoshida, 1984). Under low-temperature stress, an increase in the ratio of unsaturated PL is a crucial factor in maintaining the biological functions of membranes (Nishida and Murata, 1996). Some studies have shown that an increase in unsaturated PG enhanced cold tolerance in transgenic tobacco (Ishizaki-Nishizawa et al., 1996; Sakamoto et al., 2003) and rice (Oryza sativa; Ariizumi et al., 2002), while a decrease in unsaturated PG impaired the recovery of photosynthesis after low-temperature photoinhibition in transgenic tobacco (Moon et al., 1995; Maréchal et al., 1997). Other than PL increases during cold acclimation, an accumulation of diunsaturated PC species may lead to a decreased propensity for freeze-induced formation of the nonlamellar phase and minimize the incidence of expansion-induced lysis (Uemura and Steponkus, 1994).
In this study, lipid analysis revealed that the freezing-tolerant acbp1 mutant accumulated more diunsaturated PC (34:4 PC, 36:5 PC, 36:4 PC, 38:5 PC, 38:4 PC, and 38:3 PC) following freezing treatment, while ACBP1-overexpressing and ACBP1-complemented plants displayed reductions in diunsaturated PC (34:4 PC, 36:5 PC, 36:4 PC, and 38:4 PC). Such differences in diunsaturated PC content may provide a plausible explanation for the varied response to freezing treatment in the acbp1 mutant, ACBP1 overexpressors, and ACBP1-complemented plants. In addition, after treatment, varying levels of diunsaturated PC in these three genotypes corresponded to distinct differences in the transcription of PLDα1. PLDα1 is the most abundant PLD gene product in plants and accounts for approximately half of the hydrolysis of PC to PA and more than half of the PA generated under freezing stress (Welti et al., 2002; Li et al., 2008b). PC is believed to be a major substrate for PLDα1 during freezing stress, and in vitro experiments have confirmed that PC is preferentially hydrolyzed in comparison with PE (Pappan et al., 1998; Welti et al., 2002). Northern-blot analysis revealed that the expression of PLDα1 was down-regulated in the acbp1 mutant at CA and freezing stages, while ACBP1 overexpressors showed enhanced PLDα1 expression at the same stages. Correspondingly, after cold acclimation followed by freezing treatment, acbp1 mutant plants accumulated more PC and less PA than wild-type plants in comparison with reduced PC and elevated PA in ACBP1 overexpressors (ox-1 and ox-2). Interestingly, previous studies of PLDα1-deficient Arabidopsis suggest that the suppression of PLDα1 resulted in PC increase and PA decrease, similar to what was observed in the acbp1 mutant plants (Welti et al., 2002; Li et al., 2008b). Under freezing stress, PLDα1-deficient plants were more tolerant due to a reduced ratio of PA to PC (Welti et al., 2002; Li et al., 2008b). This low ratio of PA to PC likely enhances membrane stability and improves freezing tolerance (Welti et al., 2002; Li et al., 2008b). Similarly, a low ratio of PA to PC in acbp1 mutant plants may possibly be attributed to enhanced freezing tolerance. In contrast, ACBP1-overexpressing and ACBP1-complemented plants with a high ratio of PA to PC were observed to be more sensitive to freezing.
Besides PLDα1, PLDδ is another phospholipase involved in mediating the plant freezing response, as demonstrated in the PLDδ knockout mutant and PLDδ overexpressors in Arabidopsis (Li et al., 2004). In our earlier study, ACBP6-overexpressing Arabidopsis attained freezing tolerance through the increased expression of PLDδ, while the acbp6 mutant was freezing sensitive due to a decrease in PLDδ expression (Chen et al., 2008). Other reports suggest that PLDδ contributes to approximately 20% of total PA following freezing, which would promote nonlamellar phase membrane lipids and inhibit the function of phospholipase A (Li et al., 2004, 2008b). In addition, the activation of PLD may cause the reorganization of microtubules (Dhonukshe et al., 2003). Thus, PLDδ may contribute to membrane stabilization through its ability to bind tubulin (Gardiner et al., 2001; Li et al., 2004, 2008b). Results from this study support a role for PLDδ in mediating freezing tolerance in the acbp1 mutant and in freezing sensitivity in ACBP1 overexpressors.
Furthermore, in vitro filter-binding assays on the binding of His-tagged ACBP1 to PL showed that it binds PA. ACBP1 is localized at the plasma membrane and ER (Chye, 1998; Chye et al., 1999; Li and Chye, 2003), while PLDα1 is also associated with the plasma and intracellular membranes (Fan et al., 1999). Similarity in their membrane localization suggests that ACBP1 could maintain a membrane-associated PA pool resulting from the PLDα1-promoted hydrolysis of PC to PA. Previous studies have shown that PA is a considerable negative curvature and fusigenic lipid (Kooijman et al., 2005). It has also been suggested that freeze-induced PA species may harm cell membranes by promoting the formation of HII phase due to the cone-like molecular shape of PA (Li et al., 2008b). Interaction of ACBP1 with membrane-associated PA may possibly promote the formation of the nonlamellar phase, which could result in a reduction in membrane stability and freezing sensitivity.
It has been reported that the yeast 10-kD ACBP can regulate the expression of genes in stress responses involving catalase and heat shock proteins as well as those related to lipid metabolism, such as genes encoding OLE1 (stearoyl-CoA desaturase), INO1 (myoinositol-3-phosphate synthase), PSD1 (PS decarboxylase 1), OPI3 (methylene-fatty acyl-phospholipid synthase), and CHO2 (PE N-methyltransferase; Feddersen et al., 2007). It has been suggested that the yeast ACBP-acyl-CoA ester complex modulates gene expression directly or indirectly by the donation of acyl-CoA esters (Feddersen et al., 2007). Thus, ACBP1 could possibly resemble the yeast 10-kD ACBP in regulating gene expression. Furthermore, we have previously suggested that ACBP1 may be able to transfer acyl-CoA esters from the ER to the plasma membrane to form a membrane-associated acyl-CoA pool (Chye, 1998; Chye et al., 1999; Li and Chye, 2003). Possibly, together with the PA-binding ability of ACBP1, the expression levels of PLDα1 and PLDδ could be regulated by the sequestering action of PA or acyl-CoAs maintained by ACBP1.
Our previous study indicated that the overexpression of cytosolic ACBP6 enhances freezing tolerance in Arabidopsis by the up-regulation of PLDδ (Chen et al., 2008) together with an increased accumulation of Glc and Pro (Q.-F. Chen and M.-L. Chye, unpublished data). In this study, we further investigated the role of an Arabidopsis membrane-associated ACBP, ACBP1, in the freezing response. Our data from ion leakage experiments suggest that the key point in acquiring freezing tolerance in the acbp1 mutant is possibly related to its enhanced membrane stability. In contrast to ACBP6, a mutation in ACBP1 suppressed PLDα1 expression and the hydrolysis of PC to PA, possibly protecting the plasma membrane. In the acbp1 mutant, PLDδ expression is up-regulated, which may then enhance freezing tolerance by its positive role in stabilizing membranes under freezing stress. The differences in the regulation of PLDα1 and PLDδ between ACBP1 and ACBP6 may be due to their differences in lipid binding and subcellular localization. Additionally, the ACBP1-mediated freezing response is independent of osmolyte accumulation, in contrast to ACBP6. Furthermore, PA, to which recombinant ACBP1 binds, as shown in this study, is known to be an important stress-signaling lipid in plants (Testerink and Munnik, 2005). Also, membrane-associated ACBP1 possesses C-terminal ankyrin repeats that may interact with protein partners, as has been demonstrated with ACBP2 (Li and Chye, 2004; Gao et al., 2008), which is highly conserved to ACBP1. Hence, ACBP1 could be involved in other plant stress responses besides freezing stress (as has been shown herein) and heavy metal tolerance (as has been demonstrated previously; Xiao et al., 2008a).
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Treatments
The acbp1 knockout mutant, ACBP1 overexpressors, and ACBP1-complemented plants were generated from Arabidopsis (Arabidopsis thaliana ecotype Columbia [Col-0]) in an earlier study (Xiao et al., 2008a). The loss, and the overexpression and complementation, of ACBP1 were confirmed by northern- and western-blot analyses as reported by Xiao et al. (2008a). As described previously (Xiao et al., 2008a), ACBP1-overexpressing transgenic lines were generated by Agrobacterium tumefaciens-mediated transformation. Three independent T2 transgenic lines were observed to overexpress ACBP1 mRNA. Two lines (ox-1 and ox-2) in the T2 population showed an approximately 3:1 (resistant:sensitive) segregation ratio on selective medium, indicating the presence of a single copy of the 35S::ACBP1 transgene. These two lines were subsequently confirmed to accumulate the 37-kD ACBP1 protein by western-blot analysis (Xiao et al., 2008a). T4 transgenic plants from these two lines were used in this study. Northern-blot analysis revealed that these two T4 lines were stable after several generations (Fig. 5B). Arabidopsis seeds were surface sterilized and planted on MS medium (Murashige and Skoog, 1962) containing 2% Suc. The plates were incubated at 4°C for 2 d and then transferred to a growth chamber under 16-h-light (23°C)/8-h-dark (21°C) cycles. Plants were also grown in soil under the same conditions.
NA plants or seedlings were grown in the growth chamber under 16-h-light (23°C)/8-h-dark (21°C) cycles until treatment. For cold acclimation, 5-week-old plants in soil pots and 11-d-old seedlings on agar plates were transferred from the growth chamber to a 4°C cold room under white light for 3 d prior to treatment or harvest.
For freezing treatment, NA and CA plants or seedlings were transferred to a growth chamber (Watlow series 942) and subjected to a temperature drop from 4°C to −2°C at 2°C h−1. The temperature was held at −2°C for 2 h, and ice crystals were placed on the soil or plates to induce crystallization and prevent supercooling. Subsequently, the temperature was lowered to −12°C at 2°C h−1. After 1 h at the final temperature, the plants or seedlings were thawed at 4°C overnight. The plants were photographed after recovery for 7 d under the normal growth conditions described above.
Lipid Profiling
Lipid extraction was performed following the protocol provided by the Kansas Lipidomics Research Center. After CA treatment for 3 d at 4°C, 5-week-old plants were frozen at −8°C for 2 h, and rosettes from three plants were harvested. NA plants were incubated in a growth chamber at 23°C until rosettes were harvested and transferred to 3 mL of isopropanol containing 0.01% butylated hydroxytoluene at 75°C for 15 min, after which 1.5 mL of chloroform and 0.6 mL of water were added. Tubes were then shaken for 1 h, and the extract was moved for lipid analysis. The remaining tissue was subject to reextraction with chloroform:methanol (2:1) with 0.01% butylated hydroxytoluene four to five times, each with 30 min of agitation, until the tissue became completely white. The remaining plant tissue was incubated overnight at 105°C and subsequently weighed to obtain the dry weight. Finally, the combined extracts were washed with 1 mL of 1 m KCl followed by 2 mL of water. The solvent was evaporated under nitrogen, and samples were mailed by courier for lipid profiling at the Kansas Lipidomics Research Center.
Electrolyte Leakage
To measure ionic leakage, Arabidopsis rosettes from NA and CA plants were collected after freezing at the indicated temperature for 1 h and then incubated at 4°C for 24 h. Deionized water was added and gently agitated at 23°C for 1 h. Ionic leakage of the solution was measured using a conductivity meter (YSI model 55). Total ionic strength was determined after heating the solution in a 100°C water bath for 10 min and cooling to 23°C as described previously (Welti et al., 2002).
RNA Gel-Blot Analysis
Total RNA was prepared from rosettes of NA or CA 5-week-old plants collected in liquid nitrogen at the indicated times following treatment using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Twenty micrograms of total RNA per sample was separated on a 1.5% agarose gel containing 6% formaldehyde. RNA was transferred to Hybond-N membranes (Amersham) by capillary action. To generate probes, the PCR Digoxigenin Probe Synthesis Kit was used following the manufacturer's instructions (Roche).
The gene-specific primers used were ML179 (5′-AATCTTTGGTTTGATCTTCGC-3 ′) and ML759 (5′-GTCTACAATTGGAATCCTTCTTCTC-3 ′) for ACBP1, ML882 (5′-CAGAGACCAACAAGAATGCC-3 ′) and ML883 (5′-CGTAGTACATCTAAAGGGAG-3 ′) for COR6.6, ML884 (5′-CAAGATTACTCTGCTAGAGGAGC-3 ′) and ML885 (5′-GTATACGATGAGTGTTATGGG-3 ′) for COR47, ML921 (5′-TATGCGACGATTGATCTGCA-3 ′) and ML922 (5′-CTGAGAGCCTGAATCACATC-3 ′) for PLDα1, and ML923 (5′-AGCGACTCTAGCTCGAACAC-3 ′) and ML924 (5′-CAAGCATAAGAAGAACCCAG-3 ′) for PLDδ. Northern-blot analysis was performed using the Digoxigenin Nucleic Acid Detection Kit (Roche) according to standard procedures.
Sugar and Pro Measurements
Sugar measurements were carried out according to Li et al. (2004). Rosettes from NA or CA 5-week-old plants were harvested, weighed, and ground to powder in liquid nitrogen and then incubated in 75% ethanol overnight with gentle shaking. After centrifugation at 20,000g, 20 μ L of each extract was incubated with 1,000 μ L of anthrone reagents (0.15% [w/v] anthrone, 72% [v/v] H2SO4, and 28% [v/v] water) at 100°C for 1 h. The soluble sugar value was expressed as Glc equivalents and measured at 625 nm. Pro measurements were carried out according to a previously described method (Bates et al., 1973; Zhao et al., 2009b). Harvested rosettes from 5-week-old plants were weighed and subjected to extraction using 3% sulfosalicylic acid. After filtration, 2 mL of each filtrate was incubated with 2 mL of glacial acetic acid and 2 mL of acid ninhydrin reagent (2.5% [w/v] ninhydrin, 60% [v/v] glacial acetic acid, and 40% [v/v] 6 m phosphoric acid) at 100°C for 1 h. The reaction was terminated in an ice bath. Toluene (4 mL) was added into the extractions followed by vigorous shaking for 20 s. After incubation at 23°C for 24 h, A520 was measured to obtain values of Pro.
Purification of Recombinant Proteins rACBP1 and rACBP1 Δ ACB for Protein-Lipid Binding Assays
Expression and purification of His-tagged ACBP1 recombinant protein rACBP1 were performed according to Chye (1998). To construct the plasmid for rACBP1 Δ ACB expression, a 4.5-kb PstI-PstI fragment from pAT61 (ACBP1 coding sequence without the transmembrane domain in pRSET B vector; Chye, 1998) was recovered and self-ligated to generate plasmid pAT473. Purification of rACBP1 Δ ACB was carried out under native conditions as described (Chye, 1998). The protein-lipid overlay assay for rACBP1 and rACBP1 Δ ACB proteins on membrane lipid strips (Echelon Biosciences; catalog no. P-6002) was performed according to the manufacturer's instructions. His6-ACBP1 was used to test the binding of various lipids on filters following Zhang et al. (2004) with minor modifications. Various concentrations of lipids were spotted on nitrocellulose membranes followed by incubation at room temperature for 1 h in the dark. The lipid-bound filter was blocked in Tris-buffered saline (TBS) with 1% nonfat milk for 1 h and incubated with 1 μ g mL−1 purified His6-ACBP1 protein in blocking buffer for 2 h. The filter was then gently washed three times, each for 10 min, with TTBS (TBS plus 0.1% Tween 20). After incubation with the horseradish peroxidase (HRP)-conjugated anti-His6 antibodies (1:2,000; Qiagen) for 1 h at room temperature, the filter was again washed three times with TTBS, each for 10 min, and subjected to detection using the ECL Western-Blotting Detection Kit (Amersham) following the manufacturer's protocols.
Sequence data from this article are available in the GenBank/EMBL data libraries under accession numbers NM_124726 (ACBP1), NM_121602 (COR6.6), NM_101894 (COR47), NM_112443 (PLDα1), and NM_119745 (PLDδ).
Acknowledgments
We thank Prof. Ruth Welti and Ms. Mary Roth (Kansas Lipidomics Research Center, Kansas State University) for lipid profiling.
References
- Ariizumi T, Kishitani S, Inatsugi R, Nishida I, Murata N, Toriyama K. (2002) An increase in unsaturation of fatty acids in phosphatidylglycerol from leaves improves the rates of photosynthesis and growth at low temperatures in transgenic rice seedlings. Plant Cell Physiol 43: 751–758 [DOI] [PubMed] [Google Scholar]
- Artus NN, Uemura M, Steponkus PL, Gilmour SJ, Lin C, Thomashow MF. (1996) Constitutive expression of the cold-regulated Arabidopsis thaliana COR15 α gene affects both chloroplast and protoplast freezing tolerance. Proc Natl Acad Sci USA 93: 13404–13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207 [Google Scholar]
- Boyce JM, Knight H, Deyholos M, Openshaw MR, Galbraith DW, Warren G, Knight MR. (2003) The sfr6 mutant of Arabidopsis is defective in transcriptional activation via CBF/DREB1 and DREB2 and shows sensitivity to osmotic stress. Plant J 34: 395–406 [DOI] [PubMed] [Google Scholar]
- Chen QF, Xiao S, Chye ML. (2008) Overexpression of the Arabidopsis 10-kilodalton acyl-coenzyme A-binding protein, ACBP6, enhances freezing tolerance. Plant Physiol 148: 304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK. (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu JH, Zhu JK. (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12: 444–451 [DOI] [PubMed] [Google Scholar]
- Chye ML. (1998) Arabidopsis cDNA encoding a membrane-associated protein with an acyl-CoA binding domain. Plant Mol Biol 38: 827–838 [DOI] [PubMed] [Google Scholar]
- Chye ML, Huang BQ, Zee SY. (1999) Isolation of a gene encoding Arabidopsis membrane-associated acyl-CoA binding protein and immunolocalization of its gene product. Plant J 18: 205–214 [DOI] [PubMed] [Google Scholar]
- Chye ML, Li HY, Yung MH. (2000) Single amino acid substitutions at the acyl-CoA-binding domain interrupt 14[C]palmitoyl-CoA binding of ACBP2, an Arabidopsis acyl-CoA-binding protein with ankyrin repeats. Plant Mol Biol 44: 711–721 [DOI] [PubMed] [Google Scholar]
- Cyril J, Powell GL, Duncan RP, Baird WV. (2002) Changes in membrane polar lipid fatty acids of seashore paspalum in response to low temperature exposure. Crop Sci 42: 2031–2037 [Google Scholar]
- Devaiah SP, Roth MR, Baughman E, Li MY, Tamura P, Jeannotte R, Welti R, Wang XM. (2006) Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase D α 1 knockout mutant. Phytochemistry 67: 1907–1924 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Laxalt AM, Goedhart J, Gadella TWJ, Munnik T. (2003) Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell 15: 2666–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeseth NJ, Pacovsky RS, Newman T, Ohlrogge JB. (1996) Characterization of an acyl-CoA-binding protein from Arabidopsis thaliana. Arch Biochem Biophys 331: 55–62 [DOI] [PubMed] [Google Scholar]
- Fan L, Zheng SQ, Cui DC, Wang XM. (1999) Subcellular distribution and tissue expression of phospholipase D α , D β , and D γ in Arabidopsis. Plant Physiol 119: 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feddersen S, Neergaard TBF, Knudsen J, Faergeman NJ. (2007) Transcriptional regulation of phospholipid biosynthesis is linked to fatty acid metabolism by an acyl-CoA-binding-protein-dependent mechanism in Saccharomyces cerevisiae. Biochem J 407: 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Xiao S, Li HY, Tsao SW, Chye ML. (2008) Arabidopsis thaliana acyl-CoA-binding protein ACBP2 interacts with a heavy-metal-binding farnesylated protein AtFP6. New Phytol 181: 89–102 [DOI] [PubMed] [Google Scholar]
- Gardiner JC, Harper JDI, Weerakoon ND, Collings DA, Ritchie S, Gilroy S, Cyr RJ, Marc J. (2001) A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell 13: 2143–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Fowler SG, Thomashow MF. (2004) Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol 54: 767–781 [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF. (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124: 1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki-Nishizawa O, Fujii T, Azuma M, Sekiguchi K, Murata N, Ohtani T, Toguri T. (1996) Low-temperature resistance of higher plants is significantly enhanced by a nonspecific cyanobacterial desaturase. Nat Biotechnol 14: 1003–1006 [DOI] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280: 104–106 [DOI] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17: 287–291 [DOI] [PubMed] [Google Scholar]
- Knight H, Mugford SG, Ülker B, Gao D, Thorlby G, Knight MR. (2009) Identification of SFR6, a key component in cold acclimation acting post-translationally on CBF function. Plant J 58: 97–108 [DOI] [PubMed] [Google Scholar]
- Knight H, Veale EL, Warren GJ, Knight MR. (1999) The sfr6 mutation in Arabidopsis suppresses low-temperature induction of genes dependent on the CRT/DRE sequence motif. Plant Cell 11: 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman EE, Chupin V, Fuller NL, Kozlov MM, Kruijff BD, Burger KNJ, Rand PR. (2005) Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry 44: 2097–2102 [DOI] [PubMed] [Google Scholar]
- Leung KC, Li HY, Mishra G, Chye ML. (2004) ACBP4 and ACBP5, novel Arabidopsis acyl-CoA-binding proteins with Kelch motifs that bind oleoyl-CoA. Plant Mol Biol 55: 297–309 [DOI] [PubMed] [Google Scholar]
- Leung KC, Li HY, Xiao S, Tse MH, Chye ML. (2006) Arabidopsis ACBP3 is an extracellularly targeted acyl-CoA-binding protein. Planta 223: 871–881 [DOI] [PubMed] [Google Scholar]
- Li HY, Chye ML. (2003) Membrane localization of Arabidopsis acyl-CoA binding protein ACBP2. Plant Mol Biol 51: 483–492 [DOI] [PubMed] [Google Scholar]
- Li HY, Chye ML. (2004) Arabidopsis acyl-CoA-binding protein ACBP2 interacts with an ethylene-responsive element-binding protein, AtEBP, via its ankyrin repeats. Plant Mol Biol 54: 233–243 [DOI] [PubMed] [Google Scholar]
- Li HY, Xiao S, Chye ML. (2008a) Ethylene- and pathogen-inducible Arabidopsis acyl-CoA-binding protein 4 interacts with an ethylene-responsive element binding protein. J Exp Bot 59: 3997–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Li MY, Zhang WH, Welti R, Wang XM. (2004) The plasma membrane-bound phospholipase D δ enhances freezing tolerance in Arabidopsis thaliana. Nat Biotechnol 22: 427–433 [DOI] [PubMed] [Google Scholar]
- Li WQ, Wang RP, Li MY, Li LX, Wang CM, Welti R, Wang XM. (2008b) Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. J Biol Chem 283: 461–468 [DOI] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal E, Block MA, Dorne AJ, Douce R, Joyard J. (1997) Lipid synthesis and metabolism in the plastid envelope. Physiol Plant 100: 65–77 [Google Scholar]
- Maruyama K, Takeda M, Kidokoro S, Yamada K, Sakuma Y, Urano K, Fujita M, Yoshiwara K, Matsukura S, Morishita Y, et al. (2009) Metabolic pathways involved in cold acclimation identified by integrated analysis of metabolites and transcript regulated by DREB1 and DREB2. Plant Physiol 150: 1972–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Jin JB, Lee JY, Yoo CY, Stirm V, Miura T, Ashworth EN, Bressan RA, Yun DJ, Hasegawa PM. (2007) SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 19: 1403–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon BY, Higashi SI, Gombos Z, Murata N. (1995) Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc Natl Acad Sci USA 92: 6219–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nishida I, Murata N. (1996) Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol 47: 541–568 [DOI] [PubMed] [Google Scholar]
- Pappan K, Austin-Brown S, Chapman KD, Wang XM. (1998) Substrate selectivities and lipid modulation of plant phospholipase D α , - β , and - γ. Arch Biochem Biophys 353: 131–140 [DOI] [PubMed] [Google Scholar]
- Rajashekar CB, Zhou HE, Zhang YW, Li WQ, Wang XM. (2006) Suppression of phospholipase Dα1 induces freezing tolerance in Arabidopsis: response of cold-responsive genes and osmolyte accumulation. J Plant Physiol 163: 916–926 [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Sulpice R, Hou CX, Kinoshita M, Higashi SI, Kanaseki T, Nonaka H, Moon BY, Murata N. (2003) Genetic modification of the fatty acid unsaturation of phosphatidylglycerol in chloroplasts alters the sensitivity of tobacco plants to cold stress. Plant Cell Environ 27: 99–105 [Google Scholar]
- Steponkus PL. (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol 35: 543–584 [Google Scholar]
- Steponkus PL, Uemura M, Joseph RA, Gilmour SJ, Thomashow MF. (1998) Mode of action of the COR15a gene on the freezing tolerance of Arabidopsis thaliana. Proc Natl Acad Sci USA 95: 14570–14575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testerink C, Munnik T. (2005) Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10: 368–375 [DOI] [PubMed] [Google Scholar]
- Thomashow MF. (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50: 571–599 [DOI] [PubMed] [Google Scholar]
- Uemura M, Joseph RA, Steponkus PL. (1995) Cold acclimation of Arabidopsis thaliana. Plant Physiol 109: 15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Steponkus PL. (1994) A contrast of the plasma membrane lipid composition of oat and rye leaves in relation to freezing tolerance. Plant Physiol 104: 479–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Tominaga YK, Nakagawara C, Shigematsu S, Minami A, Kawamura Y. (2006) Responses of the plasma membrane to low temperatures. Physiol Plant 126: 81–89 [Google Scholar]
- Welti R, Li WQ, Li MY, Sang YM, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang XM. (2002) Profiling membrane lipids in plant stress responses: role of phospholipase D α in freezing-induced lipid changes in Arabidopsis. J Biol Chem 277: 31994–32002 [DOI] [PubMed] [Google Scholar]
- Xiao S, Chen QF, Chye ML. (2009) Light-regulated Arabidopsis ACBP4 and ACBP5 encode cytosolic acyl-CoA-binding proteins that bind phosphatidylcholine and oleoyl-CoA ester. Plant Physiol Biochem 47: 926–933 [DOI] [PubMed] [Google Scholar]
- Xiao S, Chye ML. (2009) An Arabidopsis family of six acyl-CoA-binding proteins has three cytosolic members. Plant Physiol Biochem 47: 479–484 [DOI] [PubMed] [Google Scholar]
- Xiao S, Gao W, Chen QF, Ramalingam S, Chye ML. (2008a) Overexpression of membrane-associated acyl-CoA-binding protein ACBP1 enhances lead tolerance in Arabidopsis. Plant J 54: 141–151 [DOI] [PubMed] [Google Scholar]
- Xiao S, Li HY, Zhang JP, Chan SW, Chye ML. (2008b) Arabidopsis acyl-CoA-binding proteins ACBP4 and ACBP5 are subcellularly localized to the cytosol and ACBP4 depletion affects membrane lipid composition. Plant Mol Biol 68: 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Browse J. (1998) eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc Natl Acad Sci USA 95: 7799–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57: 781–803 [DOI] [PubMed] [Google Scholar]
- Yoshida S. (1984) Chemical and biophysical changes in the plasma membrane during cold acclimation of mulberry bark cells (Morus bombycis Koidz. cv Goroji). Plant Physiol 76: 257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Qin CB, Zhao J, Wang XM. (2004) Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101: 9508–9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LN, Liu FX, Xu WY, Di C, Zhou SX, Xue YB, Yu JJ, Su Z. (2009a) Increased expression of OsSPX1 enhances cold/subfreezing tolerance in tobacco and Arabidopsis thaliana. Plant Biotechnol J 7: 550–561 [DOI] [PubMed] [Google Scholar]
- Zhao MG, Chen L, Zhang LL, Zhang WH. (2009b) Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol 151: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]