Abstract
In epidermal and mesophyll cells of Arabidopsis (Arabidopsis thaliana) leaves, nuclei become relocated in response to strong blue light. We previously reported that nuclear positions both in darkness and in strong blue light are regulated by the blue light receptor phototropin2 in mesophyll cells. Here, we investigate the involvement of phototropin and the actin cytoskeleton in nuclear positioning in epidermal cells. Analysis of geometrical parameters revealed that, in darkness, nuclei were distributed near the center of the cell, adjacent to the inner periclinal wall, independent of cell shape. Dividing the anticlinal wall into concave, convex, and intermediate regions indicated that, in strong blue light, nuclei became relocated preferably to a concave region of the anticlinal wall, nearest the center of the cell. Mutant analyses verified that light-dependent nuclear positioning was regulated by phototropin2, while dark positioning of nuclei was independent of phototropin. Nuclear movement was inhibited by an actin-depolymerizing reagent, latrunculin B, but not by a microtubule-disrupting reagent, propyzamide. Imaging actin organization by immunofluorescence microscopy revealed that thick actin bundles, periclinally arranged parallel to the longest axis of the epidermal cell, were associated with the nucleus in darkness, whereas under strong blue light, the actin bundles, especially in the vicinity of the nucleus, became arranged close to the anticlinal walls. Light-dependent changes in the actin organization were clear in phot1 mutant but not in phot2 and phot1phot2 mutants. We propose that, in Arabidopsis, blue-light-dependent nuclear positioning is regulated by phototropin2-dependent reorganization of the actin cytoskeleton.
Positioning organelles is essential for cellular activities. The nucleus changes its position in a programmatic way during development and the cell cycle (Britz, 1979; Nagai, 1993; Chytilova et al., 2000). For example, before asymmetrical divisions that give rise to the formation of root hair cells or guard mother cells, the nucleus migrates to the future division plane (Britz, 1979). In elongating root hair cells of Arabidopsis (Arabidopsis thaliana), the nucleus is maintained at a fixed distance from the apex (Ketelaar et al., 2002).
While the nuclear migrations before mitosis and in root hairs are developmental, nuclear positioning is also regulated environmentally. In the fern, Adiantum capillus-veneris, nuclei in prothallial cells change their intracellular positions in response to light (Kagawa and Wada, 1993, 1995). The nuclei are located along the anticlinal walls in darkness and move toward the outer periclinal walls in weak light and to the anticlinal walls in strong light (Kagawa and Wada, 1993, 1995; Tsuboi et al., 2007). This response is called light-dependent nuclear positioning. Since the response is induced in cells that exhibit neither cell division nor expansion, it is believed to have a physiological role, distinct from the nuclear positioning associated with development.
Recently, light-dependent nuclear positioning was reported in the spermatophyte Arabidopsis (Iwabuchi et al., 2007). In epidermal and mesophyll cells of dark-treated leaves, nuclei are distributed along the inner periclinal wall. Under strong light, they become located along the anticlinal walls. In mesophyll cells, nuclear movement from inner periclinal to anticlinal walls is induced repeatedly and specifically by blue light of high-fluence rate (more than 50 μ mol m−2 s−1) and is regulated by the blue light receptor phototropin2. Interestingly, mesophyll cells of the phot2 mutant have aberrantly positioned nuclei even in darkness. By contrast, the involvement of phototropins in nuclear positioning has not yet been examined for epidermal cells.
Phototropin is a blue light receptor containing two light oxygen voltage domains at the N terminus, which bind an FMN chromophore, and a Ser/Thr kinase domain at the C terminus, which undergoes blue-light-dependent autophosphorylation (Briggs et al., 2001a; Christie, 2007). Arabidopsis possesses phototropins1 and 2 (Huala et al., 1997; Jarillo et al., 2001; Kagawa et al., 2001; Sakai et al., 2001). Phototropins are shown microscopically and biochemically to localize to the plasma membrane region (Briggs et al., 2001b; Sakamoto and Briggs, 2002; Kong et al., 2006) and mediate several responses, including phototropism (Liscum and Briggs, 1995; Sakai et al., 2001), stomatal opening (Kinoshita et al., 2001), and chloroplast movements (Jarillo et al., 2001; Kagawa et al., 2001; Sakai et al., 2001). In general, phototropin1 is more sensitive to light than its paralog and mediates low-fluence-rate light responses, whereas phototropin2 functions predominantly under higher fluence rates (Sakai et al., 2001).
While the photoreceptor eliciting these nuclear movements has been revealed, the motile system responsible for moving the nuclei is still unknown. In general, organelle movements depend on the cytoskeleton, with the specific roles for actin and microtubules dependent on the organelle and species (Wada and Suetsugu, 2004). In land plants, the actin cytoskeleton plays a pivotal role in positioning organelles, including nuclei, chloroplasts, mitochondria, and peroxisomes (Wada and Suetsugu, 2004; Takagi et al., 2009).
The role of the cytoskeleton in developmental nuclear movements has been investigated. In growing root hairs of Arabidopsis, the nuclear movements are driven along actin filaments (Ketelaar et al., 2002), whereas, in tobacco (Nicotiana tabacum) BY-2 cells, the cell-cycle-based nuclear migration before mitosis is found to depend on microtubules (Katsuta et al., 1990). In interphase Spirogyra crassa cells, centering of nuclei is regulated by both actin filaments and microtubules, but in distinct ways (Grolig, 1998). To the best of our knowledge, the cytoskeletal basis of environmentally induced nuclear movements in land plants has not been elucidated.
The best-characterized organelle movements are the light-induced orientation movements of chloroplasts, and although exceptions have been reported, this movement depends on actin (Britz, 1979; Takagi, 2003; Wada et al., 2003). Under weak light, chloroplasts gather at the periclinal walls, perpendicular to the direction of light (accumulation response), whereas under strong light, they become positioned along the anticlinal walls, parallel to the direction of light (avoidance response). Recently, for Arabidopsis, Kadota et al. (2009) characterized the nature of the actin filaments probably involved in these movements. With the onset of either accumulation or avoidance response, short actin filaments appear at the leading edge of each chloroplast.
In Arabidopsis, light-dependent nuclear positioning shows similarities to the chloroplast avoidance response, with regard to the direction of movement, relevant photoreceptor (phototropin2), and effective fluence rate (Iwabuchi and Takagi, 2008). On the other hand, nuclei are larger than chloroplasts and might require thicker, more rigid actin bundles for effective motility. Here, we investigate the involvement of the actin cytoskeleton as well as phototropin in regulatory system for nuclear positioning in epidermal cells of Arabidopsis leaves.
RESULTS
Phototropin2-Dependent Nuclear Positioning in Leaf Epidermis
First, we confirmed distribution pattern of nuclei in epidermal cells of wild-type Arabidopsis. After dark treatment for 16 h, nuclei were rarely if ever near anticlinal walls (Fig. 1A) and were determined to be adjacent to inner periclinal walls by changing the fluorescence microscope focal plane. This position is consistent with a previous report that demonstrated nuclei to be located adjacent to the inner periclinal walls in transverse sections (Iwabuchi et al., 2007). We call this position the dark position. After continuous irradiation with blue light at 100 μ mol m−2 s−1 for 5 h, nuclei became positioned along the anticlinal wall of the cells (Fig. 1B). This position is referred to as the light position.
Figure 1.
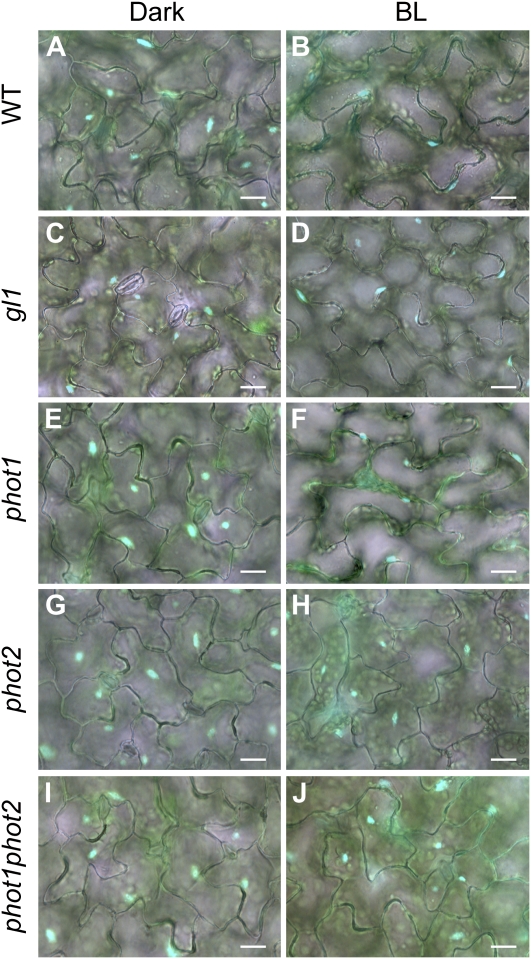
Distribution of nuclei in epidermal cells of Arabidopsis leaves under dark and light treatments. After dark treatment for 16 h (Dark), leaves of the wild type (WT; A and B), gl1 (C and D), phot1 (E and F), phot2 (G and H), and phot1phot2 (I and J) were continuously irradiated with blue light at 100 μ mol m−2 s−1 for 5 h (BL). Nuclei were stained with Hoechst 33342 and are shown in blue. All images are merged bright-field and fluorescence images. Bars = 20 μ m.
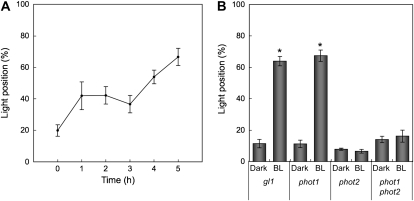
Next, we examined the time course of nuclear positioning in epidermal cells. After dark treatment for 16 h, leaves of the wild-type plants were irradiated with blue light at 100 μ mol m−2 s−1, and the position of the nuclei were recorded over 5 h. The extent of nuclei adopting the light position increased steadily from 20% to 65% over that time (Fig. 2A).
Figure 2.
Quantification of nuclear positioning in epidermal cells. A, Time course of light-dependent nuclear positioning. After the 16-h dark treatment, leaves of the wild-type plants were continuously irradiated with blue light at 100 μ mol m−2 s−1 up to 5 h. The abscissa shows the irradiation time. The ordinate shows the percentage of cells having nuclei located along anticlinal walls. Each plot shows the mean ± se. On average, 150 cells were observed in each of four to five leaves from different plants. B, Nuclear positioning in phototropin mutants. After dark treatment for 16 h (Dark), leaves of the gl1 and phototropin mutants (phot1, phot2, and phot1phot2) were continuously irradiated with blue light at 100 μ mol m−2 s−1 for 5 h (BL). The ordinate shows the percentage of cells having nuclei located along anticlinal walls. Each plot shows the mean ± se. On average, 70 cells were observed in each of five leaves from different plants. An asterisk means that a significant difference was detected between Dark and BL in each type of plant (P < 0.05 with Student's t test).
To investigate the involvement of phototropin in nuclear positioning in epidermal cells, we examined phot1-5, phot2-1, and phot1-5/phot2-1 mutants, the genetic background of which is gl1 and different from that of the phototropin mutants used in a previous study (Iwabuchi et al., 2007). After dark treatment for 16 h, nuclei in epidermal cells of gl1 and all phototropin mutants were in the dark position (Fig. 1, C, E, G, and I). After irradiation with blue light at 100 μ mol m−2 s−1 for 5 h, many nuclei in gl1 and phot1 were in the light position (Fig. 1, D and F). On the other hand, nearly all nuclei in phot2 and phot1phot2 appeared to remain in the dark position (Fig. 1, H and J).
To quantify nuclear movement, we scored the percentage of cells adopting the light position (Fig. 2B). Compared to mesophyll (Supplemental Figs. S1 and S2), nuclei in epidermal cells adopted the light position somewhat less consistently; nevertheless, they were completely unresponsive to blue light without phototropin2. These results indicate that phototropin2 mediates blue-light-dependent nuclear positioning in epidermal cells and mesophyll cells. As reported by Iwabuchi et al. (2007) for phot2 in the Wassilewskija background, many nuclei in gl1/phot2 mesophyll cells were abnormally distributed in darkness, being located along anticlinal and outer periclinal walls, an abnormal positioning that can be seen as a high rate of dark-adapted cells exhibiting the light position (double asterisk in Supplemental Fig. S2). However, in epidermis, the dark position of nuclei in phot2 was not detectably abnormal (Fig. 2B).
Positions of Nuclei in Epidermal Cells in Darkness
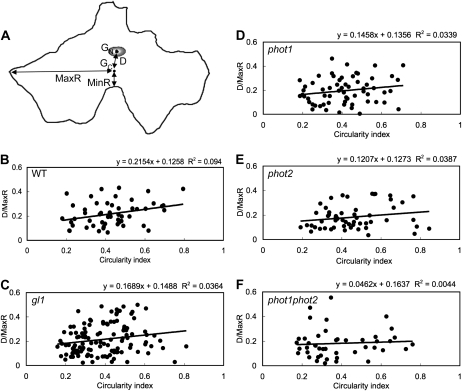
Unlike mesophyll cells, epidermal cells are relatively flat in the plane of the leaf lamina and have jigsaw-like, irregularly shaped anticlinal walls that interlock with those of neighboring cells. Because of the relatively planar shape of epidermal cells compared to that of the mesophyll, we were better able to assess the position of the nucleus geometrically (Fig. 3). We defined the following positional parameters: GN and GC are the centroid of the nucleus and cell, respectively; D is the distance between these centroids; and MinR and MaxR are the shortest and longest distances, respectively, between the cell centroid and an anticlinal wall (Fig. 3A).
Figure 3.
Relationship between nuclear position and cell shape in epidermal cells in darkness. A, Representative epidermal cell. Positional parameters were defined as follows: GN and GC, the centers of the nucleus and the cell; D, the distance between GN and GC; and MinR and MaxR, the shortest and longest distances from the GC to anticlinal wall. B to F, Scatter plot analysis of circularity index versus D/MaxR. Circularity index (abscissa) and D/MaxR (ordinate) were calculated for dark-adapted epidermal cells of the wild type (WT; B), gl1 (C), phot1 (D), phot2 (E), and phot1phot2 (F). The solid line shows linear regression line.
After dark treatment for 16 h, > 80% of nuclei in all lines took on the dark position. Under this condition, there was no significant difference in MinR, MaxR, and D in all plants, compared with gl1, except that the value of MinR in phot1phot2 mutant was significantly smaller than that in gl1 (Table I). When D was normalized against MaxR in each cell, the value of D/MaxR was about 0.2 in all plants and there was no remarkable difference among phototropin mutants (Table I). We further investigated whether the dark position is related to cell shape, taking advantage of the circularity index, which reflects the extent to which a shape approaches a circle (a perfect circle has a circularity index of 1). In all plants, there was little correlation between circularity index and D/MaxR (Fig. 3). These results indicate that nuclei of epidermal cells are located near the centroid of inner periclinal walls in darkness, bearing no relation to cell shape, and without requiring either phototropin.
Table I. Positional parameters for nuclear position in Arabidopsis epidermal cells in darkness.
MinR, MaxR, and D in dark-adapted epidermal cells of the wild-type, gl1, phot1, phot2, and phot1phot2 leaves were measured. D was normalized against MaxR in each cell, respectively. Each value shows the mean ± se. On average, 13 epidermal cells were observed in each of five leaves from different plants. The asterisk shows that the values are significantly different from those of gl1 (P < 0.05, Student's t test).
| Parameter | Wild Type | gl1 | phot1 | phot2 | phot1phot2 |
| MinR (μ m) | 9.97 ± 0.88 | 9.29 ± 0.37 | 8.15 ± 0.49 | 9.69 ± 0.91 | 7.10 ± 0.59 * |
| MaxR (μ m) | 53.33 ± 3.40 | 51.12 ± 3.20 | 50.24 ± 2.46 | 44.50 ± 3.14 | 45.74 ± 3.59 |
| D (μ m) | 11.00 ± 0.86 | 10.36 ± 0.87 | 9.36 ± 1.02 | 7.74 ± 1.26 | 9.42 ± 1.63 |
| D/MaxR | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.19 ± 0.02 | 0.18 ± 0.02 | 0.23 ± 0.05 |
Positions of Nuclei in Epidermal Cells in the Light
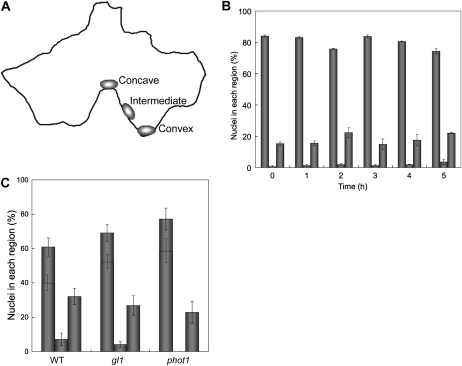
In epidermal cells, the anticlinal walls are highly interdigitated. To know whether the nuclear light position is related to the shape of the cell, we divided the anticlinal wall of each cell into three regions (concave, intermediate, and convex; Fig. 4A) and quantified the percentage of nuclei present in each type of region over time. In wild-type plants irradiated continuously with 100 μ mol m−2 s−1 blue light, once nuclei reached the anticlinal wall, approximately 80% of them were specifically located in a concave region, a proportion that appeared unchanged for the duration of the irradiation (Fig. 4B). These results indicate that under strong blue light, nuclear position is effectively restricted to concave regions of anticlinal walls; moreover, once a nucleus has attained a certain position along the anticlinal wall, it either remains in place or moves rapidly between concave regions.
Figure 4.
Nuclear position in epidermal cells under light treatment. A, Illustration of nuclear position in an epidermal cell, showing the regions of the anticlinal wall classified as concave, intermediate, and convex. B, Extent of nuclei located at the concave region over time. After the 16-h dark treatment, leaves of wild-type plants were continuously irradiated with blue light at 100 μ mol m−2 s−1 up to 5 h. The abscissa shows the irradiation time. The ordinate shows the percentage of nuclei at the anticlinal wall located in concave (left columns), convex (center columns), and intermediate regions (right columns). Each plot shows the mean ± se. On average, 150 cells were observed in each of four to five leaves from different plants. C, Extent of nuclei located at the concave region nearest GC. After the 16-h dark treatment, leaves of the wild type (WT), gl1, and phot1 were continuously irradiated with blue light for 5 h. The ordinate shows the percentage of nuclei at the anticlinal wall located in concave (left columns), convex (center columns), and intermediate regions (right columns). The percentage of cells having nuclei located at the concave region nearest GC is shown in each of the left-hand columns. Each plot shows the mean ± se. On average, 12 cells were observed in each of five leaves from different plants.
Because nuclei in epidermal cells in darkness were located near the cell centroid (GC), we reasoned that they would move to the concave region nearest the centroid if they tended to move the shortest distance in response to light. To test this, for a strong, blue light treatment (100 μ mol m−2 s−1, 5 h), the percentage of nuclei moving to the nearest concave region was assayed (Fig. 4C). In the wild type, as well as in gl1 and phot1, out of all the nuclei located at a concave region, > 65% of them had moved to the nearest concave region. Although the remaining 35% of nuclei were located at more distant concave regions, these regions were always relatively near to the cell's centroid. Nuclei that missed the nearest concave region were frequently observed in small and round cells (for example, cells adjacent to stomata) in which the distances from the centroid to the various concave regions were all about the same. These results suggest that in blue light, nuclei of epidermal cells move reliably to the concave region nearest the cell's centroid.
Effects of Cytoskeleton-Disrupting Reagents on Nuclear Positioning
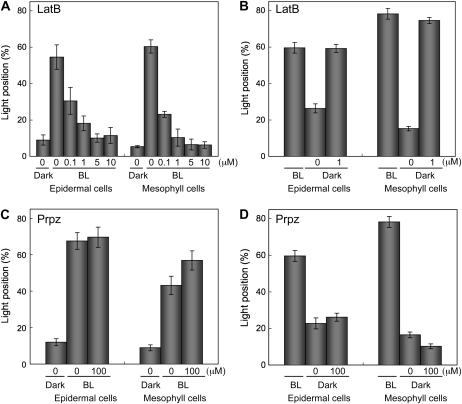
We next addressed the question whether actin filaments or microtubules are involved in nuclear positioning. First, we took advantage of an actin-depolymerizing reagent, latrunculin B, and a microtubule-depolymerizing reagent, propyzamide. In latrunculin B-treated leaves, light-dependent nuclear movement was inhibited in both epidermal and mesophyll cells, in a dose-dependent manner (Fig. 5A). We further examined the effect of latrunculin B on nuclear repositioning following a return to darkness. In this case, nuclei both in epidermal and in mesophyll cells failed to adopt the dark position (Fig. 5B). In contrast, propyzamide, even at 100 μm, inhibited neither the light-dependent nuclear movement nor the return to the dark position (Fig. 5, C and D). These results strongly suggest that nuclear migration between the dark and light positions depends on actin but not microtubules. Neither inhibitor appeared to affect the centripetal distribution of nuclei in plants adapted to darkness for 16 h.
Figure 5.
Effects of latrunculin B and propyzamide on nuclear positioning in epidermal and mesophyll cells. A and C, Nuclear migration from the cell center to anticlinal wall induced by light. Dark-adapted leaves (Dark) of the wild-type plants were treated with 0.1 to 10 μm latrunculin B (LatB; A) or 100 μm propyzamide (Prpz; C) for 1 h before the end of the 16-h dark treatment and then irradiated with blue light at 100 μ mol m−2 s−1 for 5 h (BL) in the presence of each inhibitor. B and D, Nuclear migration from the anticlinal wall to center induced by a return to darkness. Leaves of the wild-type plants irradiated with blue light at 100 μ mol m−2 s−1 for 5 h (BL) were dark adapted for 5 h in the presence of 1 μm latrunculin (B) or 100 μm propyzamide (D). Bars labeled 0 μm represent treatments with 1% and 0.3% DMSO instead of latrunculin B and propyzamide, respectively. Each plot shows the mean ± se. On average, 80 cells were observed in each of five leaves from different plants.
Confirming the conclusion that light-dependent nuclear positioning depends on actin filaments rather than on microtubules,1 μm latrunculin B fully disrupted actin filaments, and 100 μm propyzamide disrupted most microtubules (Supplemental Fig. S3).
Organization of Actin Filaments
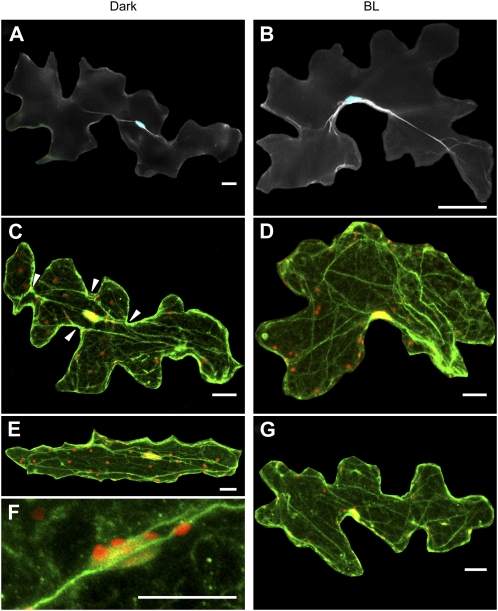
Having implicated actin in nuclear positioning, we next analyzed the structure of actin filaments in both light- and dark-treated epidermal cells. To image actin filaments, we first stained with fluorescent phalloidin. After dark treatment for 16 h, the nucleus, located at the cell center along the inner periclinal wall, was associated with thick actin bundles, which were periclinally arranged roughly in the longitudinal direction of the cell (Fig. 6A). In contrast, after irradiation with blue light at 100 μ mol m−2 s−1 for 5 h, the nucleus was positioned along the anticlinal wall, and thick actin bundles, especially in the vicinity of the nucleus, were arranged close to the anticlinal wall, together with the nucleus (Fig. 6B).
Figure 6.
Organization of actin filaments in epidermal cells under dark and light treatments. After dark treatment for 16 h (Dark), leaves of wild-type plants were continuously irradiated with blue light at 100 μ mol m−2 s−1 for 5 h (BL). A and B, Wide-field fluorescence micrographs of nuclei (blue) and actin filaments (white). Actin filaments were stained with phalloidin. C to G, Confocal fluorescence micrographs of nuclei (red) and actin filaments (green). Actin filaments were stained with anti-actin antibody. Arrowheads in C show concave regions to which actin bundles are connected. Magnified nucleus and actin filaments are shown in F. Cells were scanned from the lower to upper surfaces at 1- μ m intervals and are shown as maximum projections. Nuclei appear yellow because of the overlap of nuclear and actin staining. Plastid-like structures appear red from a combination of their autofluorescence and propidium iodide staining. Bars = 20 μ m.
Using immunofluorescence staining and observation with confocal microscopy, we further investigated the effect of blue light on actin organization. This method generally revealed more and finer actin bundles, which were predominantly longitudinally aligned and associated with nuclei (Fig. 6, C–G). As far as we could tell, actin bundles associated with nuclei in dark-adapted epidermal cells were always arranged along the inner periclinal walls. This is consistent with the fact that nuclei are always located adjacent to the inner periclinal walls in darkness. Such actin bundles were closely associated with the nucleus (Fig. 6F), and, intriguingly, one or both ends of a bundle tended to connect to a concave region of an anticlinal wall (Fig. 6C, arrowheads). This was frequently observed in cells with highly interdigitated, anticlinal walls but was difficult to observe in cells with less interdigitated anticlinal walls; nevertheless, in both types of cell, actin bundles were periclinally arranged roughly parallel to the cell's longest axis (Fig. 6E). With light treatment, rearrangement of actin filaments from periclinal toward anticlinal walls was evident (Fig. 6, D and G). These results indicate that reorganization of actin filaments occurs along with nuclear migration.
In epidermal cells, we unexpectedly found that strong blue light also changed the distribution of small, plastid-like structures, 3.3 ± 0.2 μ m in diameter (mean ± se of 20 structures) and emitting autofluorescence under green light excitation (compare Fig. 6, C and E with D and G). These structures seemed to be associated with actin filaments, as was the case in the nucleus (Fig. 6F).
Actin Organization in Phototropin Mutants
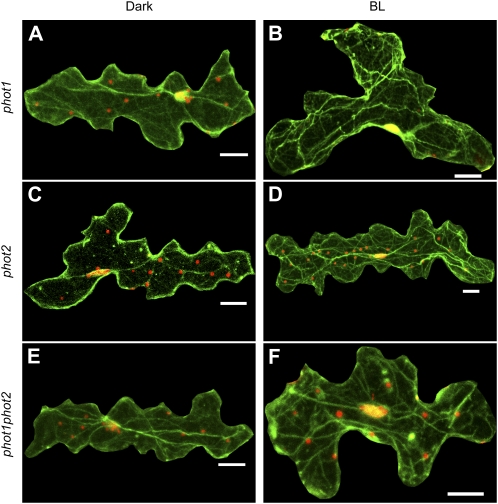
Because the light-dependent nuclear positioning is regulated by phototropin2, we examined organization of actin filaments in epidermal cells of phototropin mutants by immunofluorescence microscopy. As described above, nuclei of the phototropin mutants were positioned at the cell center along the inner periclinal wall in darkness (Fig. 7, A, C, and E). Like the wild-type cells, nuclei were associated with thick actin bundles, which were periclinally arranged roughly parallel to the longest axis of the epidermal cells. After continuous irradiation with blue light at 100 μ mol m−2 s−1 for 5 h, nuclei in phot1 mutant were positioned along the anticlinal wall, and actin bundles in the vicinity of the nucleus were arranged close to anticlinal wall, together with the nucleus (Fig. 7B). In contrast, nuclei of phot2 and phot1phot2 mutants did not appreciably change position, and actin filaments also appeared to be unchanged (Fig. 7, D and F). These results indicate that reorganization of actin filaments is regulated by phototropin2. It was also found that the plastid-like structures were distributed along the anticlinal walls under strong blue light in the wild type and in phot1 but not in phot2 and phot1phot2 (Fig. 7), indicative of the involvement of phototropin2 in their positioning.
Figure 7.
Organization of actin filaments in the epidermal cells of phototropin mutants under dark and light treatments. Confocal fluorescence micrographs of leaves of phot1 (A and B), phot2 (C and D), and phot1phot2 (E and F) at the end of the 16-h dark treatment (Dark) or after a 5-h continuous irradiation with blue light at 100 μ mol m−2 s−1 (BL). Nuclei (red) and actin filaments (green) were imaged as in Figure 6, C to G. Bars = 20 μ m.
DISCUSSION
Nuclear Positioning in Arabidopsis Leaf Cells in Darkness
Using geometrical parameters, we revealed that nuclei of epidermal cells are located near the center of the cell in darkness (Table I). The nuclei are always along the inner periclinal wall and never along the outer one (Iwabuchi et al., 2007). While the maximum distance from cell centroid to cell wall (MaxR, defined in Fig. 4) in all phototropin mutants is more or less the same as that of the control gl1, the minimum distance (MinR) in phot1phot2 is significantly decreased (Table I), implying a role for both phototropins in determining MinR. The significant decrease in the minimum distance might be related to a growth phenotype, insofar as leaves of phot1phot2 strongly curl downward (Sakamoto and Briggs, 2002). Nevertheless, in all the genotypes, nuclei are distributed in darkness around 20% of the maximal distance (Table I), and the dark position has no evident correlation with cell shape (Fig. 3). Taken together, our results indicate that the dark position of nuclei in Arabidopsis leaf epidermal cells is strictly confined to the center of the inner periclinal wall, a confinement that is scarcely if at all regulated by phototropin.
In contrast to epidermal cells, the dark position of nuclei in mesophyll cells is influenced by phototropin2 (Supplemental Fig. S2; Iwabuchi et al., 2007), illustrating that dark positioning is differently regulated in epidermal and mesophyll cells. The difference between these cell types with respect to the involvement of phototropin2 in dark positioning of nuclei might be connected with the presence or absence of chloroplasts. This idea is supported by the fact that phototropin2 regulates dark positioning of nuclei in prothallial cells of A. capillus-veneris (Tsuboi et al., 2007), which also have chloroplasts. In both species, chloroplasts are located at similar regions as nuclei in darkness, and chloroplast positioning in darkness is regulated by phototropin2 (Suetsugu et al., 2005; Iwabuchi et al., 2007; Tsuboi et al., 2007). We hypothesize that copositioning of nuclei and chloroplasts in photosynthetic cells plays an important role even in darkness.
Nuclear Positioning in Arabidopsis Leaf Cells under Blue Light
In epidermal and mesophyll cells, phototropin2 but not phototropin1 is required for nuclear migration under strong blue light (Fig. 2). Since the extent of nuclei located in each region of anticlinal walls is not altered in phot1 epidermis (Fig. 4), it is evident that phototropin1 has little or no influence on blue-light-dependent nuclear positioning in Arabidopsis leaf cells.
Intriguingly, under strong blue light, nuclei in epidermal cells move to a concave region of an anticlinal wall, usually to the concave region nearest the center of the cell (Fig. 4). Moreover, the specific positioning of nuclei seems to be maintained during the irradiation (Fig. 4). We propose two explanations for how nuclei successfully reach the nearest concave region of the cell wall. One is that the nucleus moves from the inner periclinal wall to the anticlinal wall by the shortest path and that, in the majority of cells, the nearest region of anticlinal wall is a concave region. The other is that concave regions are selected specifically for nuclear anchoring. These possibilities are difficult to distinguish, but the former is simpler than the latter and applies directly to cells with less interdigitated anticlinal walls and to mesophyll cells with anticlinal wall of circular shape because nuclei in these cell types are likely to be positioned anywhere along the anticlinal wall.
What is the physiological significance of light-dependent nuclear positioning? Light-dependent nuclear positioning has two notable features: (1) It is induced by so-called strong light, and (2) nuclei move to the anticlinal wall, parallel to the direction of incident light, presumably minimizing the cross section of the nucleus available for light absorption. Therefore, we suggest that nuclei move to avoid DNA damage caused by surplus light, including UV (Iwabuchi and Takagi, 2008). We are currently seeking to test this possibility by using mutants defective in light-dependent nuclear positioning, such as phot2.
Actin and the Nuclear Dark Position
In dark-adapted epidermal cells, actin bundles are associated with both nuclei and anticlinal walls, but mainly near the inner periclinal cell wall (Fig. 6). Furthermore, an intact actin cytoskeleton is required for nuclear migration back to the cell center upon a return to darkness, whereas microtubules are not detectably involved in this movement (Fig. 5, B and D). We therefore conclude that actin bundles play essential roles for nuclear movement back to the inner periclinal wall.
Mechanisms of nuclear positioning associated with developmental processes have been extensively studied in plant and animal cells (Britz, 1979; Reinsch and Gönczy, 1998). In tobacco BY-2 cells, nuclei move from the side of the cell to the center prior to entering M phase, a movement that depends on microtubules (Katsuta et al., 1990). Conversely, when protoplasts prepared from BY-2 cells regenerate a cell wall, nuclei move from cell center to periphery, and this movement depends on actin as well as the cell wall (Katsuta and Shibaoka, 1988). These authors argued that actin filaments are associated with both the nucleus and the plasma membrane and generate tension between them. Tension in actin filaments has also been invoked to explain nuclear positioning in the green alga S. crassa, only in this case, microtubules also contribute to positioning the nucleus (Grolig, 1998). Therefore, not only might the nucleus in Arabidopsis leaf cells move using actin bundles but also the dark position in the cell center might represent an equilibrium position where tension forces balance.
In epidermal cells in darkness, thick actin bundles are periclinally arranged roughly parallel to the longest axis of the cells, and they are frequently connected to concave regions of the anticlinal wall (Fig. 6C). These arrangements of actin filaments are scarcely observed under blue light and to our knowledge not previously reported. Earlier studies of Arabidopsis leaf epidermal cells reported longitudinally aligned actin bundles, as well as fine actin filaments, but localized only in convex regions of the anticlinal walls (Fig. 7 in Fu et al., 2002; Smith, 2003). While the reason for the different localization patterns is not certain, it is possible that previous investigations harvested material in the light. Insofar as spatial organization of actin filaments and microtubules is believed to contribute to morphogenesis of leaf epidermal cells (Smith, 2003), links between actin bundles and concave regions that occur in darkness might be indispensable not only for nuclear positioning but also for cell shaping.
Actin and the Nuclear Light Position
Here, we show that the blue-light-induced nuclear migration from the cell center to the anticlinal wall is actin dependent (Fig. 5A) and that thick actin bundles are always associated with nuclei (Fig. 6). In the light, nuclei are mainly positioned at the concave region of the anticlinal wall nearest to the center of cell. Although, in darkness, actin bundles associated with nuclei are frequently connected to concave regions of anticlinal walls, these regions are spread around the cell periphery and are not necessarily nearest to the cell center. Hence, the nuclear migration may involve a rearrangement of actin bundles. Nuclei migrate in phot1 but in neither phot2 nor phot1phot2, indicating that phototropin2 is the photoreceptor triggering this motile response (Fig. 7). Phototropins are known to mediate blue-light-dependent transient increases in cytosolic calcium concentration in Arabidopsis leaves (Harada et al., 2003; Harada and Shimazaki, 2007). Calcium-sensitive actin-binding proteins, such as villin, might be involved in rearrangement of actin bundles, although the specific mechanisms involved require elucidation.
In principle, cytoskeletal reorganization can be caused by two different mechanisms: by the formation and breakdown of new filaments or by the movement of existing filaments. In the former, rearrangement depends on the activities of multiple actin-binding proteins that include nucleating proteins, such as formin, bundling proteins, such as villin, and depolymerizing proteins, such as cofillin (Staiger and Blanchoin, 2006; Higaki et al., 2007). In the latter mechanism, movement depends on myosin to generate motive force.
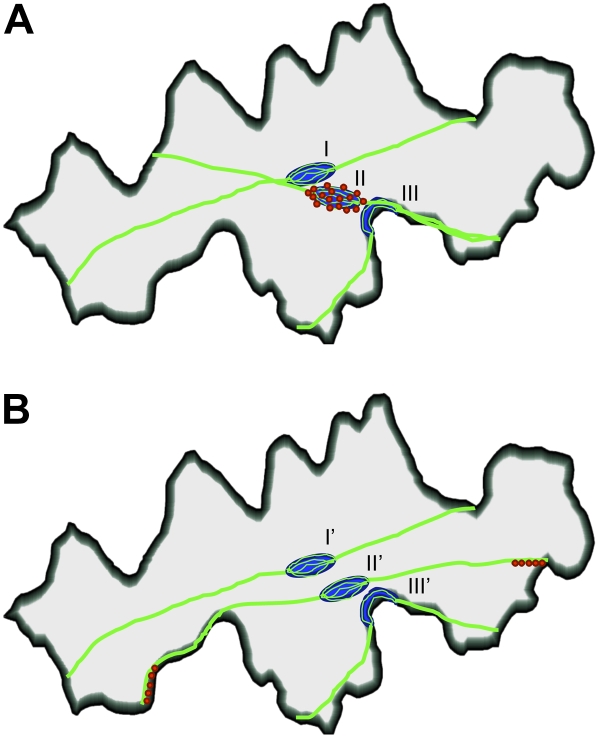
Here, we propose two models for light-dependent nuclear positioning (Fig. 8). One model is nuclear movement along a reorganized actin bundle (Fig. 8A). In darkness, the nucleus is located at the center of inner periclinal wall in association with thick actin bundles periclinally arranged roughly parallel to the longest axis of the cell. Upon blue light irradiation, the bundles associated with the nucleus rearrange: one end of the bundle connects to the nearest concave region of the anticlinal wall and then the nucleus moves along it, finally becoming anchored at the anticlinal wall. In this model, motility is driven by myosin fixed on the nuclear envelope. The other model is bundle-dependent movement (Fig. 8B). In this model, upon blue light irradiation, actin bundles associated with the nucleus move laterally from the cell center toward the periphery, thereby bringing the nucleus toward anticlinal wall. Here, nuclei are anchored statically to the actin bundles, while myosin is localized at or near the ends of the bundles, enabling them to translocate by changing the attachment point along the anticlinal wall. That myosin moves actin filament attachment points along an anticlinal wall has been suggested for BY-2 cells (Hoffmann and Nebenführ, 2004). On the other hand, we do not exclude the possibility that nuclear migration is simply the myosin-propelled translocation along an actin bundle toward the anticlinal wall, with the actin reorganization happening secondarily to anchor the nucleus at the new, anticlinal cell wall position.
Figure 8.
Models for light-dependent nuclear motility in epidermal cells. A, In darkness, the nucleus (blue) is located at the cell center (along the inner cell wall) in association with thick, longitudinally arranged actin bundles (green; I). Under blue light, the bundles associated with the nucleus become connected to the anticlinal wall nearest the nucleus (II). The nucleus moves along the actin bundles and becomes anchored at the anticlinal wall (III). B, In darkness, the nucleus is located as in A (I'). Under blue light, actin bundles associated with the nucleus laterally move toward the anticlinal wall (II'), thereby drawing nucleus with them, where it eventually becomes anchored at the anticlinal wall nearest the center of cell (III'). Note that myosin (red) is specifically localized on the nuclear periphery and around the attachment site of the actin bundle to the anticlinal wall in models A and B, respectively.
In both of these models, motility is driven by myosin. An actomyosin-based mechanism functions in various movements of plant cell organelles, including mitochondria (Van Gestel et al., 2002), peroxisomes (Jedd and Chua, 2002; Mathur et al., 2002), and nuclei (Chytilova et al., 2000). Plants contain two classes of myosin, type VIII and XI. The genome of Arabidopsis has 17 myosin sequences, with four type VIIIs and 13 type XIs (Berg et al., 2001; Reddy and Day, 2001). Myosin XI has been most clearly identified with organelle motility; the myosins XI-K, MYA1, and MYA2 have been suggested to play major roles in intracellular trafficking of Golgi stacks, peroxisomes, and mitochondria (Avisar et al., 2008, 2009; Peremyslov et al., 2008; Prokhnevsky et al., 2008), and myosin XI-I appears specifically localized to the nuclear envelope (Avisar et al., 2009). It will be worth testing whether myosin XI-I is involved in light-dependent nuclear positioning.
In contrast to the conspicuous actin bundles involved in nuclear motility, chloroplasts are thought to move using a somewhat cryptic, small, circular actin structure that forms on the outer envelope and without the involvement of cytoplasmic actin bundles (Kadota et al., 2009). Therefore, although there are similarities between the movement of chloroplasts and nuclei in strong blue light, the role of actin appears to differ. To further characterize the mechanism of these motile events, we are making transgenic plants that will allow both actin filaments and nuclei to be imaged in living cells, taking advantage of reporters that have been used successfully for actin filaments (Higaki et al., 2007; Staiger et al., 2009).
Light-Driven Plastid Motility in Leaf Epidermis
In epidermal cells, plastid-like structures and nuclei change their distributions from inner periclinal wall toward anticlinal wall in strong blue light (Fig. 6). The structures were stained with two different DNA stains, propidium iodide and Hoechst 33342. Similar structures in Arabidopsis leaf epidermis are labeled with a plastid-targeted GFP (Oikawa et al., 2003). Therefore, the structures are probably plastids.
Blue-light-dependent redistribution of these plastid-like structures might be regulated by phototropin2 (Fig. 7). Furthermore, the association of plastid-like structures with actin filaments suggests that their redistribution is actin dependent. Although movement of nongreen plastids in the epidermis of tobacco was reported to depend on actin (Kwok and Hanson, 2003), nearly all previous work on plastid motility has focused on chloroplast movement. It appears that there are notable similarities among the movements of nuclei, chloroplast, and nongreen plastids, particularly in leaves. The physiological significance and motile system for the light-dependent positioning of nongreen plastids are worth investigating.
MATERIALS AND METHODS
Plant Materials
Wild-type Arabidopsis (Arabidopsis thaliana), ecotype Columbia, phot1-5 (Huala et al., 1997), phot2-1 (Kagawa et al., 2001), phot1-5phot2-1, and gl1 mutants were used. Phototropin mutants are all in the gl1 background. Seeds were sown on compost and grown under a photoperiod of 16 h of white light (80 μ mol m−2 s−1) and 8 h of dark at 22°C. Rosette leaves were taken from 4- to 5-week-old plants.
Dark Adaptation and Light Irradiation
Leaves were detached from the plants at the petioles, floated on distilled water in a petri dish, and then kept in darkness for 16 h. The dark-adapted leaves were irradiated with blue light (470 nm) using a light-emitting diode light source system (MIL-C1000T for the light source controller, MIL-U200 for the light source frame, and MIL-B18 for the light-emitting diode; SMS). Light intensity was measured with a quantum sensor and data logger (LI-1400; LI-COR).
Nuclear Staining
Sample leaves were fixed with 2% (w/v) formaldehyde (FA) freshly prepared from paraformaldehyde in fixation buffer (50 mm PIPES, 10 mm EGTA, and 5 mm MgSO4, pH 7.0) for 2 h with evacuation for the first 5 min. The leaves were stained with 5 μ g/mL Hoechst 33342 (CalBiochem) with 0.03% (v/v) Triton X-100 in fixation buffer for 1.5 h and then with a Hoechst 33342 solution without Triton X-100 overnight when needed. Epidermal and mesophyll cells of the adaxial side were observed and photographed using a fluorescence microscope (BX-50; Olympus) equipped with a charge-coupled device camera (Coolsnap; RS Photometrics).
Parameters for Spatial Analyses of Nuclear Positioning
Digitized photographs of adaxial epidermal cells were processed using image-processing software (Adobe Photoshop; Adobe Systems). Nuclei and cells on each photograph were separately traced and binarized using the Photoshop and then opened in Image J (http://rsb.info.nih.gov/ij). The centers of gravity for the nucleus (GN) and cell (GC), as well as the shortest (MinR) and longest distances (MaxR) between GC and the anticlinal wall, were found using the Image J plug-in Particles8, written by Gabriel Landini (http://www.dentistry.bham.ac.uk/landinig/software/software.html). The circularity index of the epidermal cell was calculated as (4 πA)/P2 (where A is area and P is perimeter of the cell). The area and perimeter of cell were obtained from the binary images using Particles8. The distance between GC and GN (D) was calculated as the Pythagorean distance from the coordinates of GC and GN.
Phalloidin Staining
Sample leaves were fixed as for nuclear staining. They were cut into small pieces of approximately 1 mm × 1 mm and then stained with 200 nm Alexa Fluor 488 phalloidin (Invitrogen) in fixation buffer containing 0.03% (v/v) Triton X-100 for 1 h in darkness. Specimens were observed and photographed as described for nuclear staining.
Note that we manipulated Figure 6, A and B, by blackening neighboring cells using image-processing software (Adobe Photoshop) to highlight the representative cells.
Immunofluorescence Microscopy
Sample leaves were fixed with 2% (w/v) FA and 0.3% (w/v) glutaraldehyde in fixation buffer under a vacuum for 5 min, then with 2% (w/v) FA in fixation buffer for 30 min, and finally with 4% (w/v) FA in fixation buffer for 2 h. When investigating effects of actin-depolymerizing and microtubule-depolymerizing reagents, treated leaves were fixed in 2% (w/v) FA and 0.3% glutaraldehyde (w/v) in fixation buffer for 1 h. After fixation, the leaves were cut into small pieces of approximately 3 mm × 3 mm and digested with 1% (w/v) Cellulase Onozuka RS (Yakult) and 0.1% (w/v) Pectolyase Y-23 (Kyowa Chemical Products) in fixation buffer for 15 to 30 min at 37°C. To obtain better images, the duration of cell wall digestion was set 20 to 30 min for dark-adapted leaves and 15 to 20 min for blue-light-irradiated leaves. After removing the abaxial epidermis and vascular tissues using tweezers, samples were permeabilized with 0.5% (v/v) Triton X-100 in fixation buffer for 1 h with gentle agitation. This step was most important to achieve satisfactory staining. Samples were blocked in 2% (w/v) bovine serum albumin (Calbiochem) in fixation buffer for 1 h.
Samples were immunostained at 37°C overnight and for 3 h in primary and secondary antibody solutions, respectively, diluted in fixation buffer supplemented with 3% (w/v) bovine serum albumin. Primary antibodies were mouse monoclonal anti-actin (Clone C4; MP Biomedicals) diluted to 1:500 (v/v) or mouse monoclonal anti- α -tubulin (Clone DM 1A; Sigma-Aldrich) diluted to 1:500 (v/v). The secondary antibody was Alexa fluor 488-conjugated anti-mouse IgG (Invitrogen) diluted to 1:500 (v/v). Nuclei were stained with 5 μ g/mL propidium iodide (Calbiochem) in fixation buffer for 1 h in darkness. Each specimen was mounted on a glass slide with 0.1% (w/v) p-phenylenediamine (an antifade agent) in 50% (v/v) glycerol and phosphate buffered saline (130 mm NaCl, 5.1 mm Na2HPO4, 1.6 mm KH2PO4, pH 9.0–9.5, with KOH) and observed with a confocal microscope (FV300; Olympus). Washes were done carefully with fixation buffer several times in each step except for the step between blocking and primary antibody.
Note that we manipulated Figure 6, C to G, and Figure 7 by blackening neighboring cells using image-processing software (Adobe Photoshop) to highlight the representative cells.
Inhibitor Treatment
Latrunculin B (Calbiochem) and propyzamide (Wako) were used. To examine nuclear migration from the cell center to anticlinal wall, inhibitors were applied to sample leaves for 1 h before the end of the 16-h dark treatment, and subsequent light irradiation was performed in the presence of those inhibitors. To examine nuclear migration from the anticlinal wall to cell center, inhibitors were applied at the onset of transfer of sample leaves into darkness and remained present during the dark treatment for 5 h. To demonstrate inhibitor activity, inhibitors were applied to sample leaves for 1 h before the end of the 16-h dark treatment. In all the above experiments, the first 5 min of inhibitor treatments was performed under vacuum. Stock solutions of inhibitors were prepared in dimethyl sulfoxide (DMSO) and diluted to final concentration with deionized water for treatment. The same concentration of DMSO was applied to control leaves.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Distribution of nuclei in mesophyll cells under dark and light treatments.
Supplemental Figure S2. Nuclear positioning in mesophyll cells of phototropin mutants.
Supplemental Figure S3. Effects of cytoskeletal inhibitors in epidermal cells.
Acknowledgments
We thank Dr. T. Baskin (University of Massachusetts, Amherst) for critical reading of the manuscript, Dr. H. Tsukaya (University of Tokyo) for the kind gift of phototropin mutant seeds, and Dr. T. Shikanai (Kyoto University) for the kind gift of gl1 mutant seed.
References
- Avisar D, Abu-Abied M, Belausov E, Sadot E, Hawes C, Sparkes IA. (2009) A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol 150: 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avisar D, Prokhnevsky AI, Makarova KS, Koonin EV, Dolja VV. (2008) Myosin XI-K Is required for rapid trafficking of Golgi stacks, peroxisomes, and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol 146: 1098–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JS, Powell BC, Cheney RE. (2001) A millennial myosin census. Mol Biol Cell 12: 780–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A, et al. (2001a) The phototropin family of photoreceptors. Plant Cell 13: 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Christie JM, Salomon M. (2001b) Phototropins: a new family of flavin-binding blue light receptors in plants. Antioxid Redox Signal 3: 775–788 [DOI] [PubMed] [Google Scholar]
- Britz SJ. (1979) Chloroplast and nuclear migration. Encycl Plant Physiol 7: 170–205 [Google Scholar]
- Christie JM. (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Chytilova E, Macas J, Sliwinska E, Rafelski SM, Lambert GM, Galbraith DW. (2000) Nuclear dynamics in Arabidopsis thaliana. Mol Biol Cell 11: 2733–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z. (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolig F. (1998) Nuclear centering in Spirogyra: force integration by microfilaments along microtubules. Planta 204: 54–63 [DOI] [PubMed] [Google Scholar]
- Harada A, Sakai T, Okada K. (2003) phot1 and phot2 mediate blue light-induced transient increase in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100: 8583–8588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Shimazaki K. (2007) Phototropins and blue light-dependent calcium signaling in higher plants. Photochem Photobiol 83: 102–111 [DOI] [PubMed] [Google Scholar]
- Higaki T, Sano T, Hasezawa S. (2007) Actin microfilament dynamics and actin side-binding proteins in plants. Curr Opin Plant Biol 10: 549–556 [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Nebenführ A. (2004) Dynamic rearrangements of transvacuolar strands in BY-2 cells imply a role of myosin in remodeling the plant actin cytoskeleton. Protoplasma 224: 201–210 [DOI] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han I, Larsen E, Briggs WR. (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Sakai T, Takagi S. (2007) Blue light-dependent nuclear positioning in Arabidopsis thaliana leaf cells. Plant Cell Physiol 48: 1291–1298 [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Takagi S. (2008) How and why do plant nuclei move in response to light?. Plant Signal Behav 3: 266–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR. (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410: 952–954 [DOI] [PubMed] [Google Scholar]
- Jedd G, Chua NH. (2002) Visualization of peroxisomes in living plant cells reveals acto-myosin-dependent cytoplasmic streaming and peroxisome budding. Plant Cell Physiol 43: 384–392 [DOI] [PubMed] [Google Scholar]
- Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, Ichikawa S, Kagawa T, Nakano A, Wada M. (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci USA 106: 13106–13111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Wada M. (1993) Light-dependent nuclear positioning in prothallial cells of Adiantum capillus-veneris. Protoplasma 177: 82–85 [Google Scholar]
- Kagawa T, Wada M. (1995) Polarized light induces nuclear migration in prothallial cells of Adiantum capillus-veneris L. Planta 196: 775–780 [Google Scholar]
- Katsuta J, Hashiguchi Y, Shibaoka H. (1990) The role of the cytoskeleton in positioning of the nucleus in premitotic tobacco BY-2 cells. J Cell Sci 95: 413–422 [Google Scholar]
- Katsuta J, Shibaoka H. (1988) The roles of the cytoskeleton and the cell wall in nuclear positioning in tobacco BY-2 cells. Plant Cell Physiol 29: 403–413 [Google Scholar]
- Ketelaar T, Faivre-Moskalenko C, Esseling JJ, de Ruijter NCA, Grierson CS, Dogterom M, Emons AMC. (2002) Positioning of nuclei in Arabidopsis root hairs: an actin-regulated process of tip growth. Plant Cell 14: 2941–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kong SG, Suzuki T, Tamura K, Mochizuki N, Hara-Nishimura I, Nagatani A. (2006) Blue light-induced association of phototropin 2 with the Golgi apparatus. Plant J 45: 994–1005 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR. (2003) Microfilaments and microtubules control the morphology and movement of non-green plastids and stromules in Nicotiana tabacum. Plant J 35: 16–26 [DOI] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Hülskamp M. (2002) Simultaneous visualization of peroxisomes and cytoskeletal elements reveals actin and not microtubule-based peroxisome motility in plants. Plant Physiol 128: 1031–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai R. (1993) Regulation of intracellular movements in plant cells by environmental stimuli. Int Rev Cytol 145: 251–310 [Google Scholar]
- Oikawa K, Kasahara M, Kiyosue T, Kagawa T, Suetsugu N, Takahashi F, Kanegae T, Niwa Y, Kadota A, Wada M. (2003) Chloroplast unusual positioning1 is essential for proper chloroplast positioning. Plant Cell 15: 2805–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV. (2008) Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol 146: 1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhnevsky AI, Peremyslov VV, Dolja VV. (2008) Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc Natl Acad Sci USA 105: 19744–19749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, Day IS. (2001) Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol 2: RESEARCH0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch S, Gönczy P. (1998) Mechanisms of nuclear positioning. J Cell Sci 111: 2283–2295 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Briggs WR. (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG. (2003) Cytoskeletal control of plant cell shape: getting the fine points. Curr Opin Plant Biol 6: 63–73 [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Blanchoin L. (2006) Actin dynamics: old friends with new stories. Curr Opin Plant Biol 9: 554–562 [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Sheahan MB, Khurana P, Wang X, McCurdy DW, Blanchoin L. (2009) Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. J Cell Biol 184: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Kagawa T, Wada M. (2005) An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol 139: 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S. (2003) Actin-based photo-orientation movement of chloroplasts in plant cells. J Exp Biol 206: 1963–1969 [DOI] [PubMed] [Google Scholar]
- Takagi S, Takamatsu H, Sakurai-Ozato N. (2009) Chloroplast anchoring: its implications for the regulation of intracellular chloroplast distribution. J Exp Bot 60: 3301–3310 [DOI] [PubMed] [Google Scholar]
- Tsuboi H, Suetsugu N, Kawai-Toyooka H, Wada M. (2007) Phototropins and neochrome1 mediate nuclear movement in the fern Adiantum capillus-veneris. Plant Cell Physiol 48: 892–896 [DOI] [PubMed] [Google Scholar]
- Van Gestel K, Köhler RH, Verbelen JP. (2002) Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. J Exp Bot 53: 659–667 [DOI] [PubMed] [Google Scholar]
- Wada M, Kagawa T, Sato Y. (2003) Chloroplast movement. Annu Rev Plant Biol 54: 455–468 [DOI] [PubMed] [Google Scholar]
- Wada M, Suetsugu N. (2004) Plant organelle positioning. Curr Opin Plant Biol 7: 626–631 [DOI] [PubMed] [Google Scholar]