Abstract
Summary: Outer membrane (OM) vesicles are ubiquitously produced by Gram-negative bacteria during all stages of bacterial growth. OM vesicles are naturally secreted by both pathogenic and nonpathogenic bacteria. Strong experimental evidence exists to categorize OM vesicle production as a type of Gram-negative bacterial virulence factor. A growing body of data demonstrates an association of active virulence factors and toxins with vesicles, suggesting that they play a role in pathogenesis. One of the most popular and best-studied pathogenic functions for membrane vesicles is to serve as natural vehicles for the intercellular transport of virulence factors and other materials directly into host cells. The production of OM vesicles has been identified as an independent bacterial stress response pathway that is activated when bacteria encounter environmental stress, such as what might be experienced during the colonization of host tissues. Their detection in infected human tissues reinforces this theory. Various other virulence factors are also associated with OM vesicles, including adhesins and degradative enzymes. As a result, OM vesicles are heavily laden with pathogen-associated molecular patterns (PAMPs), virulence factors, and other OM components that can impact the course of infection by having toxigenic effects or by the activation of the innate immune response. However, infected hosts can also benefit from OM vesicle production by stimulating their ability to mount an effective defense. Vesicles display antigens and can elicit potent inflammatory and immune responses. In sum, OM vesicles are likely to play a significant role in the virulence of Gram-negative bacterial pathogens.
INTRODUCTION
Outer membrane (OM) vesicles are a naturally secreted product of Gram-negative bacteria. Vesicles form when a portion of the outer membrane with periplasmic content is selectively “blebbed” off to form round vesicles (11, 94, 95). The production of OM vesicles has been observed for a wide variety of gram-negative bacteria in all stages of growth as well as in a variety of growth environments, such as infected tissues. The production of vesicles was further demonstrated to be linked to the bacterial stress response (97). Vesiculation levels increase during periods of bacterial stress, such as what might be experienced during the colonization of host tissues.
Analyses of OM vesicle components have demonstrated that vesicles contain a wide variety of virulence factors (see references in Table 1). These virulence factors include protein adhesins, toxins, and enzymes as well as nonprotein antigens such as lipopolysaccharide (LPS). Purified vesicles have displayed the ability to act as a delivery system for virulence factors by interacting with both prokaryotic and eukaryotic cells. Furthermore, vesicles are laden with pathogen-associated molecular patterns (PAMPs) and other OM components that can impact the course of infection and host responses to infection. This review will focus on examining the roles that OM vesicles and their specific components play as virulence factors and their ability to interact with and trigger responses from target cells. The diverse abilities of OM vesicles to modulate immune responses, deliver toxins and other virulence factors to host cells, and aid in biofilm formation all attest to the importance that these secreted elements can have in bacterial pathogenesis.
TABLE 1.
Virulence factors and activities associated with native OM vesiclesb
| Species | Vesicle-associated virulence factor(s) (reference[s]) | Virulence-associated activity (reference[s])a |
|---|---|---|
| Actinobacillus actinomycetemcomitans | Leukotoxin, GroEL (46, 73) | Bone-resorbing activity, chicken embryo lethality, cytotoxic (22, 73, 105) |
| Actinobacillus pleuropneumoniae | Apx toxin, proteases (102) | Proteolytic (102) |
| Bacteroides fragilis | Hemagglutinin, alkaline phosphatase, esterase lipase, acid phosphatase, phosphohydrolase, α- and β-galactosidases, α-glucosidase, glucosaminidase, β-glucuronidase (109) | Hemagglutinating and enzymatic activities (109) |
| Bacteroides succinogenes | Cellulase, xylanase (35) | Aryl-β-glucosidase, aryl-β-xylosidase, endoglucanase, xylanase activities (35) |
| Bordetella pertussis | AC-Hly, FHA, pertussis toxin (Ptx) (62) | ND |
| Borrelia burgdorferi | OspA, OspB, OspD (26, 123) | HUVEC adherence (123) |
| Brucella melitensis | Omp25, Omp31 (42) | ND |
| Burkholderia cepacia | PLC-N, lipase, PSCP, 40-kDa protease (2) | Enzyme activities (2) |
| EHEC O111:H- | ClyA (136) | Pore forming (136) |
| ETEC | LT (61, 137, 138) | Enterotoxic and vacuolating activities (61, 78) |
| ExPEC | Alpha-hemolysin, CDT, iron and hemin binding OMPs (6, 10) | Hemolytic, causing detachment of cells from monolayer (6) |
| STEC O157:H7 | Shiga toxin 2 (81, 143) | Cytotoxic (143) |
| UPEC | CNF1 (83) | Cytotoxic (83) |
| Helicobacter pylori | VacA, Lewis antigen LPS (34, 63, 75) | Vacuolating activity (75), cytotoxic, stimulating proliferation, IL-8 secretion (66, 76) |
| Legionella pneumophila | Mip (lpg0791), IcmK/IcmX, flagellin, phospholipase C, LaiE/LaiF, phospholipase, chitinase, acid phosphatases, Hsp60, proteases, diphosphohydrolase (40) | Inhibition of phagolysosome fusion, proteolytic and lipase activity (33, 40) |
| Moraxella catarrhalis | UspA1/UspA2 (127) | Binds C3 complement in serum (127) |
| Neisseria meningitidis | PorA, NlpB, NarE (putative) (93, 134) | TNF-α, IL-6, activation of tissue factor (procoaggulant), profibrinolytic and antifibrinolytic factors (12, 119) |
| Photorhabdus luminescens | Toxin AB, GroEL (49) | Insecticidal (79) |
| Porphyromonas (Bacteroides) gingivalis | Arg- and Lys-gingipain cysteine proteinases (27, 48, 72) | Cleavage and loss of CD14 from macrophage, cleavage of IgG, C3, IgM (47) |
| Pseudomonas aeruginosa | Phospholipase C, hemolysin, alkaline phosphatase, Cif, PQS, quinolines, protease, β-lactamase (20, 21, 70, 89, 91, 92, 119) | Decrease of apical CFTR expression, in vitro enzyme activities, bactericidal quinolines, IL-8 stimulation (8, 20, 21, 70, 91, 92) |
| Salmonella enterica serovar Typhimurium | Protective antigens (9) | ND |
| Shigella dysenteriae serotype 1 | Shiga toxin 1 (30) | Toxicity (30) |
| Shigella flexneri | IpaB, IpaC, IpaD (68) | Invasion (68) |
| Treponema denticola | Dentilysin, adhesins, proteases (19, 116) | Chymotryptic activity, disruption of tight junctions (19, 116) |
| Vibrio anguillarum | Metalloprotease, hemolysin, phospholipase (58) | Protease, metalloprotease, hemolytic activities (58) |
| Vibrio cholerae | RTX toxin (14) | Cell rounding, depolymerizing actin (14) |
| Xanthomonas campestris | Cellulase, β-glucosidase, xylosidase, avirulence proteins, type 3 secretion system proteins (124) | ND |
| Xenorhabdus nematophilus | Bacteriocin, fimbrial adhesin, pore-forming protein, chitinase (79) | Chitinase activity, insecticidal (79) |
Abbreviations not defined in the text: AC-Hly, adenylate cyclase-hemolysin; FHA, filamentous hemagglutinin; CDT cytolethal distending toxin; OMP, outer membrane protein; PLC-N, phospholipase, nonhemolytic; UPEC, uropathogenic E. coli; CNF1, cytotoxic necrotizing factor 1; ND, not determined.
OUTER MEMBRANE VESICLE FORMATION BY PATHOGENS
An ever-growing number of pathogens have been documented to produce and secrete natural OM vesicles (Table 1). Morphological and biochemical evidence for infected host tissues and fluids supports the idea that the production of vesicles by pathogens occurs during infection and, in fact, may be induced during infection.
What Are OM Vesicles?
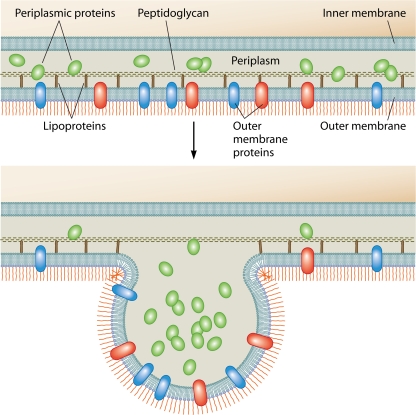
OM vesicles are closed spheroid particles of a heterogeneous size (∼10 to 300 nm in diameter) released from Gram-negative bacteria during all phases of growth (11, 94, 95). Electron microscopy (EM) studies reveal that OM vesicles are formed from OM bulges and the subsequent fission of vesicles containing electron-dense material (11, 18, 48, 70, 81, 88, 94, 109). In general, OM vesicles reflect the OM composition, containing LPS, glycerophospholipids, and OM proteins as well as enclosed periplasmic components (43, 56, 61, 70, 95) (Fig. 1). Importantly, OM vesicles are not a product of cell death since they contain newly synthesized proteins and are produced without concomitant bacterial lysis (96, 99, 144). Several OM vesicle proteomes have been evaluated recently, and all were determined to be enriched in envelope components, although some cytosolic and inner membrane proteins were also present in these preparations (10, 40, 84, 86, 87, 124, 141).
FIG. 1.
Model of OM vesicle production. Shown is the budding of the Gram-negative bacterial envelope. Released OM vesicles contain periplasmic material and OM proteins and lipids, including PAMPs and other virulence factors, as described in the text. Although details of the mechanism remain unclear, budding is thought to occur in places where lipoprotein links between the OM and the peptidoglycan are broken or missing.
In addition to naturally shed vesicles, proteoliposomes can be artificially generated by sonication or detergent treatment of bacteria or the bacterial OM. These proteoliposomes are often referred to as bacterial vesicles. In some cases, it was claimed that brief treatments of detergent or sonication simply release preformed OM vesicles. However, in most cases, these treatments have been proven to differ in composition and activity from naturally produced OM vesicles (12, 23). We have focused this review of the literature on naturally produced OM vesicles.
Natural OM Vesicles
All Gram-negative bacteria investigated to date naturally release OM vesicles. Calculations of native OM vesicle production show that the vesicles represent a significant fraction of cellular material. For instance, vesicles produced by typical laboratory cultures of growing and dividing Pseudomonas aeruginosa and Escherichia coli cells account for ∼1% of the OM material in the culture (8, 43, 139). In contrast, Neisseria meningitidis produces abundant numbers of vesicles, constituting 8 to 12% of radiolabeled protein and endotoxin in log-phase cultures (24). Not only are OM vesicles produced by free-living cells, they are also abundant in naturally occurring biofilms (120). In addition, intracellular pathogens such as Legionella pneumophila, Salmonella spp., and Francisella spp. produce OM vesicles in both intraphagosomal and extraphagosomal compartments (3, 33, 44, 45). In the case of Flavobacterium, vesicles are made late in the growth phase (82). The mechanism of vesicle production is complex (95), and progress in this field will be updated in detail in a separate review (A. Kulp and M. J. Kuehn, unpublished data).
Modulation of Vesiculation In Situ
Rates of OM vesicle production are not uniform, even for a particular strain, as production has long been seen to be influenced by environmental factors and by sources of cellular stress (74, 103, 129). In studies of both nonpathogenic and pathogenic species, vesiculation was found to be upregulated by conditions that activate the σE envelope stress response (97). In fact, vesiculation appears to be critical to surviving stress. When vesiculation mutants of E. coli were challenged with lethal envelope stressors, the vesicle-underproducing mutant succumbed, but the overproducing mutants survived better than the wild type (97). Considering the harsh antimicrobial environments encountered in a host during infection, the capacity to modulate vesicle production is likely critical for pathogens.
EM evidence has shown that vesiculation can be induced by exposure to host components and tissue. In a model that simulates meat spoilage, Pseudomonas fragi was inoculated into pig muscle (29). Unlike counterparts grown in a protein-free medium, the surfaces of bacteria that contacted the meat were covered with vesicles. These vesicles are thought to contain proteolytic enzymes that disrupt myofibrils seen in the bacterial spoilage of meat. In addition, using a mouse model of enterotoxigenic E. coli (ETEC) infection, vesicles were highly evident on ETEC cells recovered from the intestine 2 h after intragastric inoculation (Fig. 2).
FIG. 2.
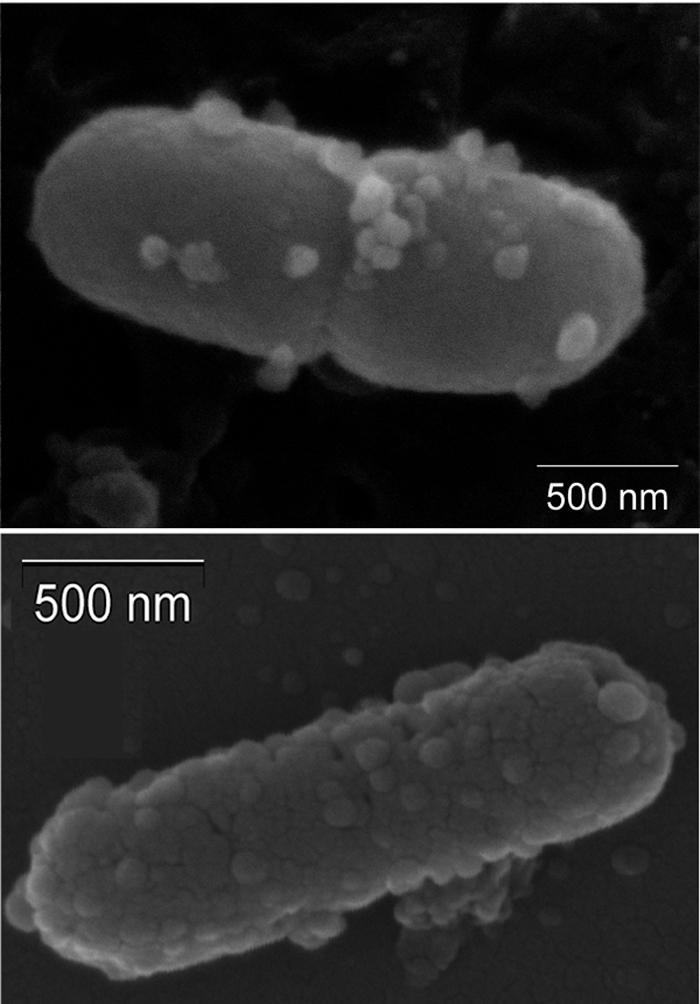
Enterotoxigenic E. coli recovered postinfection. Shown are data from scanning electron microscopy of a representative ETEC strain recovered from mouse small intestines 2 h after intragastric inoculation. (Courtesy of Amanda McBroom.)
Antibiotic treatment has been demonstrated to influence several aspects of vesiculation. The level of Shiga toxin-associated OM vesicle production by Shigella dysenteriae increased with mitomycin C treatment but not upon treatment with other tested antibiotics (30). Mitomycin C induces toxin production (135), and it is tempting to consider that one consequence of increased toxin production is hypervesiculation; however, this has not been proven. Gentamicin treatment also increased the vesiculation of P. aeruginosa; however, when the composition of these vesicles was inspected, they were not identical to native OM vesicles, as they also contained inner membrane and cytoplasmic material (70). Nevertheless, it is important to consider the tendency for particular antibacterials to elicit the release of proinflammatory and toxic materials.
A careful biochemical analysis by Fernandez-Moreira et al. highlighted how naturally produced vesicles can mediate the OM surface remodeling that is critical for virulence (33). Their data demonstrated significant differences in the composition, LPS, and activity of OM vesicles shed by Legionella during developmentally distinct periods of growth. This work supports a model by which transmissive Legionella pneumophila sheds OM vesicles into the phagosome not only to inhibit fusion with lysosomes but also to promote the remodeling of the bacterial surface to the intracellular replicative form (33).
Vesiculation Observed during Infection
Examination of animal and human biopsy specimens, tissues, and host fluids provides evidence that bacterial vesicles are formed during infection. EM has provided the most direct observation of vesicle production by pathogens in host tissues. Some of the oldest evidence of bacterial OM vesicles comes from EM of gingival plaque, which demonstrates abundant membrane vesicles interspersed between Gram-negative and Gram-positive bacteria (50). This material, termed biofilms, has been the subject of much further study and is discussed in more detail below.
Several biopsy studies have identified OM vesicles within human tissues. EM examination of Helicobacter pylori-infected human biopsy specimens revealed that bacterially derived vesicles contact intestinal epithelial cells (34, 51, 63, 75). Hynes et al. further noted that these vesicles could adsorb antibodies in sera from infected patients (63). EM examination revealed a highly vesiculating Neisseria meningitidis serogroup B strain associated with a fatal septic infection in a 20-year-old patient. The abundant “blebs” were thought to be the contributor to the high endotoxin level of 1,700 endotoxin units/ml (equivalent to the activity of 170 ng/ml pure E. coli LPS) that led to this fatal septicemia (101). Additionally, OM vesicles were observed in the cerebrospinal fluid of an infant infected with N. meningitidis and in the sera of three patients with lethal meningococcal endotoxemia (16, 125).
In several cases, microscopic examination of infected tissues revealed that OM vesicles are produced (or at least are more apparent) near host cells. A nasal sample from a child with Moraxella catarrhalis sinusitis was examined by transmission EM, and it was determined that, when in close association with leukocytes, M. catarrhalis secretes OM vesicles. Immunogold labeling of the C3 complement binding factor UspA1/UspA2 showed that these proteins were located near or on OM vesicles in the biopsy specimen (127). In another study, a Salmonella strain isolated from a human food-poisoning infection was inoculated into the ileum of chickens. A subsequent EM analysis determined that a majority of the Salmonella cells proximal to the ileal epithelial cells could be seen producing OM vesicles (142).
OM vesicles have also been observed at sites more disseminated from the direct site of bacterial colonization. OM vesicle antigens were found in urine, blood, and several organs of mice, dogs, and humans infected with Borrelia burgdorferi (26). Vesicles were observed on the surfaces of these spirochetes recovered from infected ticks and from infected mouse urinary bladder, spleen, liver, heart, and brain tissues, indicating that these vesicles are formed by B. burgdorferi in vivo.
Even without direct observation by EM, OM vesicles have been implicated in the pathogenic process. In a series of studies of E. coli infection, LPS and OM protein-containing bacterial fragments were isolated from the serum of septic rats (52-54). E. coli cells grown in culture with serum were found to shed OM material with the same composition as the material shed in vivo (55). Several factors indicate that these OM components are actually OM vesicles. They are composed of LPS, OM proteins, and lipoproteins; are larger than 0.1 μm in size; and are stable (55).
These studies leave no doubt that OM vesicles or blebs are found surrounding and attached to the surface of pathogenic Gram-negative organisms during infection. Subsequent studies have been done to examine what roles they may play in this niche and to address whether the vesicles are instruments of virulence and inflammation. It remains to be determined whether OM vesicles contribute to the benefit of the bacterium (as a mediator of toxin transfer or inflammatory damage), to the benefit of the host (as a mobile, potent indicator of infection), or to both.
ROLES OF OM VESICLES IN INTERBACTERIAL INTERACTIONS
The interaction of bacteria with cocolonizing and commensal bacteria can be hostile or neighborly. As discussed below, bacteria destroy coinfectors to prevent competition for limited nutrients or to provide nutrients for themselves. In other cases, vesicles facilitate bacterial communities where interspecies interactions are not only tolerated but are promoted in order to combat otherwise lethal environmental conditions. In yet other cases, OM vesicles from one species have further been observed to aid in the survival of an entire mixed bacterial infective population by actively destroying host defenses.
Vesiculating bacteria may have a survival advantage in mixed-population infections by their capacity to eliminate competing bacterial strains. As fuel for competition, OM vesicles package periplasmic peptidoglycan hydrolases (70, 88). Their activity in OM vesicles has been attributed to the killing of cocultured Gram-negative as well as Gram-positive bacteria (89). Thus, OM vesicle production could be an advantage for growth in a mixed bacterial population where nutrition is limiting. Further, β-lactamase was found to be packaged into P. aeruginosa vesicles produced by strains that expressed β-lactamase (20). OM vesicles containing β-lactamase can protect the vesicle-producing strain from a cocolonizing, β-lactam-producing species that could otherwise eliminate them. Additionally, some OM vesicles have been found to contain scavenging proteases (29, 129), xylanase, and cellulase (35), which can aid in nutrient acquisition and thereby provide a survival advantage.
Vesicles can act as bridging factors in biofilms that produce an environment that is resistant to antibiotics and antibacterials. Vesicles from oral bacteria, in particular, promote biofilm formation and colonization (48, 71, 72, 94). Vesicles are abundant in natural biofilms, and OM vesicle surface-bound DNA appears to be an electrostatic, bridging component that is central to membrane vesicle-bacterium-biofilm matrix interactions (120, 121). Porphyromonas gingivalis vesicles mediate the coaggregation of the periodontopathogen Tannerella forsythia, and vesicles mediate the interaction/aggregation of staphylococci and Prevotella intermedia (65, 71, 72). By participating in quorum sensing, OM vesicles can also aid survival by contributing to communication within mixed populations of pathogens. P. aeruginosa OM vesicles contain quorum-sensing molecules, and vesicle production is also stimulated by the addition of the Pseudomonas quinolone-signaling molecule, PQS (92).
Not only can OM vesicles provide interbacterial glue to generate a nearly impervious multicellular structure, they can also promote the growth of a cocolonizing pathogen. For instance, in a mixed population of bacteria, secreted vesicles that titrate an antimicrobial agent or degrade β-lactams (described above) could significantly aid in the survival of any neighboring bacteria. Furthermore, OM vesicles released by one strain could cause increased inflammation, resulting in the exposure of host extracellular matrix proteins and the upregulation of epithelial cell surface receptors that are beneficial to colonization by another strain. Moraxella catarrhalis is frequently found in mixed infections with pathogens such as Haemophilus influenzae. As such, the ability of M. catarrhalis OM vesicles to bind complement and allow H. influenzae survival is a relationship that might also benefit M. catarrhalis (127). Thus, it was proposed that proinflammatory vesicle-mediated changes in host tissue can pave the way for the adherence and survival of cocolonizing pathogens.
Vesicles can also enable the exchange of bacterial products. Biochemical analyses revealed that DNase-resistant DNA and a periplasmic antibiotic could be transferred between bacteria by OM vesicles, suggesting a fusion event (25, 67). In EM studies where nonhydrolytic OM vesicles were used, P. aeruginosa and Shigella flexneri vesicles were found to confer stably integrated LPS onto the surfaces of other Gram-negative bacteria (69). These results are particularly intriguing considering that they show a potential role for vesicles in natural transformation and the acquisition of drug resistance in infections.
VESICLE-ASSOCIATED VIRULENCE FACTORS
Extracellular products of pathogens are often associated with acute infection and are essential for maximal virulence. As secreted extracellular entities, OM vesicles can actively modify the bacterial environment. OM vesicles have been found to be capable of delivering active toxins and other virulence-associated bacterial proteins to host cells. In some cases, toxins are enriched in OM vesicles, suggesting that their entry into vesicles is somehow regulated.
Vesicle-Associated Toxins
Toxic materials are associated with native OM vesicles from a variety of Gram-negative pathogens. In many cases, vesicle-associated toxins have indeed been found to deliver active toxins to host cells (Table 1). Enterotoxigenic E. coli (ETEC) produces heat-labile enterotoxin (LT) that associates with LPS in the particulate fraction of the cell culture supernatant (43, 61, 137, 138). Purified ETEC vesicles specifically bind, enter, and deliver active toxin into epithelial and Y1 adrenal cells (61, 78). OM vesicles from Actinobacillus actinomycetemcomitans exhibit cytotoxicity (22, 73, 105), and Xenorhabdus nematophilus and Photorhabdus luminescens OM vesicles were cytotoxic for insect larvae as well as in tissue culture (79). Kolling and Matthews demonstrated, by immunoblotting, that Shiga toxin is present inside OM vesicles produced by O157:H7 cells (81), and this was corroborated for many strains of Shiga toxin-producing E. coli (STEC) under both aerobic and anaerobic conditions (143). NarE is a putatively vesicle-associated protein of Neisseria meningitidis vesicles that exhibits ADP-ribosyltransferase and NAD-glycohydrolase activities typically associated with toxicity (93). Toxic vesicles produced by extraintestinal E. coli (ExPEC) include a hemolysin; an RTX toxin, which becomes surface bound; as well as cytolethal distending toxin, which has a lipid binding domain to bind the OM (6, 10).
In some cases, vesicle-associated toxins have been observed directly in biopsy specimens of infected tissues. For instance, OM vesicles containing the vacuolating cytotoxin VacA were observed in H. pylori-colonized human gastric epithelium biopsy specimens and were similar in appearance and composition to H. pylori vesicles made in vitro (34). The VacA-related toxicity of H. pylori vesicles has been studied in some detail. OM vesicle-associated VacA causes vacuolization in HEP-2 cells (75), although the relative contribution of vesicle-associated VacA and free-soluble VacA is debated (114). In contrast, Galka et al. found that extracellular Mip was found exclusively in the OM vesicle fraction of Legionella pneumophila cultures (40).
H. pylori vesicles with either full-length VacA or a natural carboxyl-terminal truncate of VacA (from a VacA− phenotype strain) induce cytochrome c-independent apoptosis in gastric epithelial cells (5). The truncated VacA contains the active domain but not the type I secretion system signal; thus, the results with the truncate were surprising because this was classified as a “nonsecreted” version of VacA. It was proposed that the OM vesicles mediate the toxicity of the truncate by enabling its presentation to host cells. This type of datum suggests that care should be taken when bacteria are assigned as having a toxin-deficient phenotype—they could be secreting toxins via vesicles.
Investigations of several pathogens demonstrate directly or support that toxins are often localized to the surface of the vesicles. Extensive studies have detailed how LT is secreted, becomes associated with LPS, and eventually is shed via OM vesicles, which have LT tightly bound to LPS on their surface as well as soluble periplasmic LT in their lumen (59-61, 98) (Fig. 3). Although Kolling and Matthews demonstrated that Shiga toxin (Shiga toxin 1 and/or 2) is protease resistant in OM vesicles produced by O157:H7 (81), the ability of LPS to bind and neutralize Shiga toxin 2 (41) suggests that at least some of the Shiga toxin may be found on the OM vesicle surface in a protease-resistant form. Immunogold EM was used to detect a high-molecular-mass protease on the surface of Actinobacillus pleuropneumoniae vesicles, which also package Apx toxin (102). The vacuolating toxin VacA was also seen to be closely associated with the membrane of H. pylori vesicles (75). VacA appears to be in a mature, monomeric form when associated with vesicles, as it is when it is associated with cells (64, 114). Studies of Campylobacter coli, C. fetus, and C. jejuni OM vesicles showed that although present in both whole cells and OM vesicles, some proteins were not exposed to labeling in whole cells but were labeled in OM vesicles (90). This suggests that either the topology changed (which is considered unlikely) or the proteins internal in whole cells are externally associated with OM vesicles.
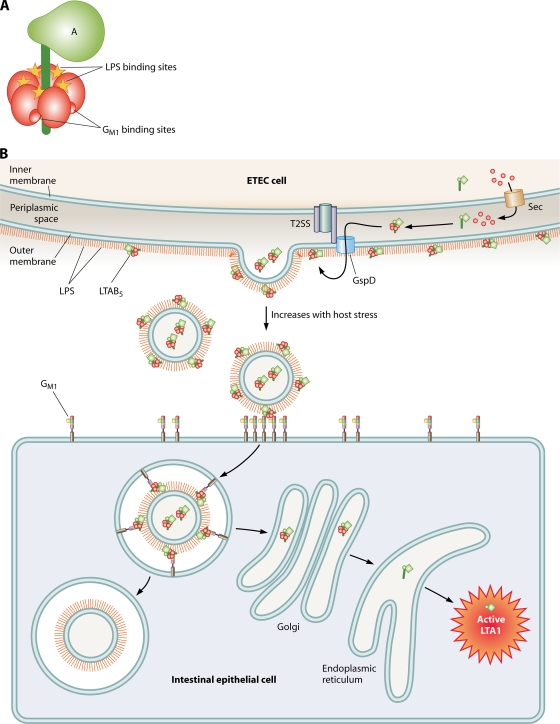
FIG. 3.
Models of binding, secretion, and vesicle-mediated LT transport into host cells. (A) LT consists of an LTA subunit in a complex with 5 LTB subunits (LTAB5). LPS and GM1 both bind in interfaces between subunits of the LTAB5 complex, but the binding sites are distinct from each other. (B) LT is secreted through the inner membrane by the Sec machinery, folds into the periplasm, is secreted through the outer membrane via GspD of the type 2 secretory system, and binds to LPS on the cell surface via LTB5. Consequently, vesicles released from the cell have LT on their surface that can act as a tether between GM1 on the host cell and LPS on the vesicle. Vesicles are internalized by GM1- and cholesterol-rich microdomains (lipid rafts). Vesicle-associated LT traffics through the Golgi apparatus and ER and is retrograde translocated into the cytosol, where the A1 fragment of LTA is catalytically active. The remaining vesicle material is maintained intracellularly in a nonacidified compartment.
Toxins associated with the OM vesicle surface are typically very protease resistant, although they are demonstrably on the exterior surface of vesicles. Leukotoxin detected on the surface of A. actinomycetemcomitans cells is found in a tight association with the membrane of its vesicles (73, 106). Similarly, the cytotoxins found to be associated with X. nematophilus OM vesicles were not strippable from OM vesicles except with sodium dodecyl sulfate, which destroyed its activity as well (79). LT and alpha-hemolysin (HlyA) were also tightly bound to the membrane of ETEC and ExPEC vesicles, respectively (6, 61). Whether the ability of toxins to tightly associate with the vesicle surface is due to the high degree of curvature of the OM vesicle or to their interaction with a specific component of the vesicles can be tested experimentally.
Nontoxin Virulence Factors in OM Vesicles
OM vesicles are a mechanism by which bacteria can secrete many periplasmic and membrane components associated with the virulence of the bacterium. Often, OM components of vesicles include adhesins that allow vesicles to interact with host cells (Table 1) (discussed in more detail below). In addition, OM vesicles can include proteases and signaling molecules. For example, Pseudomonas OM vesicles include virulence-associated enzymes, antimicrobial quinolines, and quorum-sensing molecules (70, 89, 91, 92, 113). Also, the P. aeruginosa CiF protein is preferentially secreted into vesicles, and CiF-containing vesicles decrease levels of apical lung epithelial expression of the cystic fibrosis transmembrane conductance regulator protein (CFTR), the central protein in the genetic disease of cystic fibrosis (15, 91).
OM vesicle-associated proteins include active proteases that can degrade host cells. Vesicles from Treponema denticola, a periodontopathogen, have active proteases that confer host cell-damaging characteristics similar to those of the whole bacterium (19, 116, 119). In addition, Neisseria meningitidis culture supernatant endotoxic filtrates have procoagulant and fibrinolytic factors for human monocytes (119).
OM vesicles can directly bind, titrate, and even destroy host bactericidal factors. Indeed, Neisseria vesicles can bind and remove cell-targeted bactericidal factors in serum (110). H. pylori expresses Lewis antigens on its OM LPS, and this “molecular mimicry” contributes to H. pylori virulence. Since OM vesicles produced by H. pylori contain those Lewis antigens on their LPS, it was proposed that these vesicles could contribute to the chronic stimulation of the host immune system (63). These vesicles can absorb anti-Lewis antigen autoantibodies from the serum, and it was suggested that they may play a role in the putative autoimmune aspects of H. pylori pathogenesis. M. catarrhalis OM vesicles carry UspA1 and UspA2, which bind to C3 in the complement cascade (127). As a result, M. catarrhalis vesicles inhibit the bactericidal effects of complement in normal human sera that are also directed against nontypeable strains of H. influenzae. Thus, vesicles from one species can also contribute indirectly to the pathogenicity of another by binding and depleting complement in their immediate environment.
Additionally, OM vesicles can influence critical bacterial cell-host cell associations in a variety of tissues. P. gingivalis vesicles enhance the attachment and invasion of T. forsythia in periodontal epithelial cells (65). Likewise, the addition of adherent-invasive E. coli (AIEC) vesicles to intestinal epithelial cells significantly increased the internalization of a vesicle-underproducing mutant of AIEC (115). The vesicle components that contribute to bacterial entry have yet to be determined.
Enrichment of Vesicle-Associated Virulence Factors
Particular OM vesicle components may be enriched compared to their abundance in the originating bacterial cell. While vesicle OM protein profiles are typically very similar to those of the OM, they are not identical. Enrichment and depletion of the OM and periplasmic cargo were found when the contents of vesicles and bacterial OM fractions were compared (95). Most relevant to this discussion is the enrichment of virulence factors in OM vesicles. Proteins identified as being enriched in vesicles include LT of ETEC and the P. aeruginosa aminopeptidase, both of which increase the association of vesicles with cultured epithelial cells (7, 78). Leukotoxin and leukotoxic activities are 4- to 5-fold enriched in OM vesicles compared with OM preparations for some strains of A. actinomycetemcomitans (73). Similarly, a subfraction of the vesicles secreted by ExPEC cells expressing HlyA is enriched in that virulence factor (6). In contrast, CagA, an H. pylori virulence factor, is notable for its absence from VacA-containing OM vesicles from Cag pathogenicity island-positive strains (66, 75).
Quantitative proteomic techniques are useful tools to determine exactly what is enriched in the OM vesicle population. When investigating the Xanthomonas campestris OM vesicle proteome, surprisingly, less than half of the most abundant OM proteins could also be identified in the vesicle fraction (124). Further analysis revealed that nearly half of the proteins associated with the OM vesicle fraction are involved or are putatively involved in virulence, and of the 21 proteins enriched in the OM vesicle fraction, most of them are virulence associated. It was also evident that the composition of the OM vesicle proteins was partially determined by the growth medium. This indicates that in addition to their abundance, OM vesicle proteomes, including their associated virulence factors and toxins, are highly mutable depending on the physiological surroundings.
VESICLE INTERACTIONS WITH HOST CELLS
Protease- and toxin-containing OM vesicles interact with host cells and thereby act as virulence factor delivery vehicles. As discussed below, their interaction can occur via a membrane fusion event or via adhesin-receptor-mediated attachment. In some cases, adherence is followed by vesicle uptake, even by nonphagocytic cell types. Data from diverse pathogens support the theory that vesicle binding contributes to infection by enabling the delivery of toxic bacterial cargo to host cells, often by the internalization of the entire content of the vesicle. Thus, it is not surprising that, in addition to toxicity, the ramifications of such a delivery process include signaling and innate and adaptive immune responses in the host cells.
Adherence of Vesicles to Host Cells
Binding to cultured host cells has been observed for OM vesicles produced by a variety of pathogens. Protease- and toxin-containing OM vesicles from E. coli, Shigella, Actinobacillus, and Borrelia strains interact with bacterial as well as mammalian cells (43, 68, 73, 117, 123). Vesicle-associated aminopeptidase increases the ability of P. aeruginosa vesicles to associate with both primary and cultured human lung epithelial cells, although whether this is due to a receptor binding or receptor-uncovering property of this enzyme is not yet clear (7).
OM vesicles adhere to host cells not only in tissue culture but also in the complex environment of an infected host tissue. For instance, H. pylori OM vesicles were bound to intestinal cells in biopsy specimens from infected patients (51, 75). Bacteroides fragilis OM vesicles have hemagglutinating activity, which indicates that they are able to act as adhesive bridging entities between mammalian cells (109).
Host cell adherence tropism depends on bacterial strain-specific factors. Not surprisingly, therefore, vesicles and bacteria can use identical host cell receptors. B. burgdorferi vesicles adhere to human umbilical vein endothelial cells (HUVECs) in a manner that competes with whole Borrelia cells (140). Tropism in host cell binding was demonstrated for OM vesicles from A. actinomycetemcomitans (22) and was specifically engineered for vesicles formed by E. coli that expressed the Yersinia enterocolitica adhesin/invasin, Ail (77).
Vesicle Entry into Cells
In many cases, after OM vesicles adhere, they can be internalized into host cells. Toxins can act as adhesins for the OM vesicles and allow vesicles to enter using the receptor-mediated endocytic pathway also used by the soluble toxin. Topologically, toxin-mediated vesicle adherence and uptake can occur only if the toxin-vesicle interaction does not interfere with the toxin-receptor interaction. This has been carefully mapped in the case of LT, the toxin/adhesin associated with ETEC vesicles. Although the LT-B subunit forms a pentamer, and thus both LPS and GM1 binding sites do not need to be simultaneously occupied to confer vesicle binding, LPS (vesicle) and GM1 (host cell) binding sites on LT-B are entirely distinct (60, 98) (Fig. 3A). As a result, ETEC vesicles bind to and enter epithelial cells via lipid raft-mediated endocytosis governed by GM1 (78) (Fig. 3B). Once inside the cell, the LT-A subunit is trafficked to the endoplasmic reticulum (ER) and the cytosol, where it catalyzes the ADP-ribosylation of Gsα, leading to enterotoxicity. The remaining vesicle components are stably maintained in a nonacidified intracellular compartment.
Other examples of toxin-mediated vesicle adherence have been investigated. H. pylori VacA is taken up by gastric epithelial cells both as a free toxin and as a vesicle-associated toxin (34, 114). As yet, it is not known if VacA is the vesicle adhesin/entry ligand. Common components of vesicles such as OmpA could also contribute to the host cell entry of vesicles. OmpA mediates invasion for E. coli K1, causing neonatal meningitis, by interacting with a surface receptor, Ecgp, on brain microvascular endothelial cells (111, 112). Not all vesicle toxins are required for effective vesicle adhesion. A. actinomycetemcomitans-derived OM vesicles, while highly enriched in leukotoxin (73), do not require leukotoxin for rapid vesicle-cell interactions (22).
The destination of some vesicle-associated material is transepithelial. Dentilysin-carrying OM vesicles from Treponema denticola can disrupt the epithelial cell monolayer in culture and proceed to penetrate through the monolayer and emerge on the basolateral side, as measured by the appearance of dentilysin activity in that compartment (19).
Host factors that contribute to vesicle uptake by specific host cell types have been identified. For instance, bactericidal/permeability-increasing protein (BPI), but not LPS binding protein (LBP), enhanced the uptake of N. meningitidis vesicles into dendritic cells, and confocal imaging visualized BPI colocalized with internalized OM vesicles (122). These interactions were surprisingly selective, since the same did not occur for macrophages, and BPI did not promote similar interactions of free lipooligosaccharide aggregates with the dendritic cells.
Vesicle Fusion with Cell Membrane
Experimental evidence from several studies supports the hypothesis that certain OM vesicles can fuse with host cell plasma membranes. Salmonella cells growing within host cell vacuoles were found to release LPS that could be detected within host epithelial cell vesicle membranes. This process was determined to be dependent on the presence of the O antigen in the LPS structure, as detected by antibodies to the O antigen itself (44). According to another report, A. actinomycetemcomitans vesicles conferred a lipid-tracking dye to host cell plasma membranes within 2 min of coincubation (22). Likewise, L. pneumophila OM vesicles tracked with a fluorescent tag were observed on the surface of alveolar epithelial cells, suggesting that they are lengthily adherent or have fused (40).
While these biochemical and visual data are compelling, abundant and definitive biophysical evidence of OM vesicle-membrane fusion is lacking. Meanwhile, it is intriguing to consider how the phospholipid bilayer of mammalian cells could possibly accommodate the foreign architecture of LPS, much less bacterial OM protein β-barrel structures (if these are also transferred). The incorporation of such bacterial structures into a eukaryotic membrane could also influence the sensing of the ligands by innate immune receptors and overall host responses.
IMMUNOMODULATORY ACTIVITIES
The composition of OM vesicles makes them significant activators of host innate and acquired immune response pathways. In addition to the potent immunomodulatory molecule LPS, vesicles contain OM porins and other important innate immune-activating ligands. Together, vesicle components appear to act synergistically to modulate the host response in ways that can either stimulate the clearance of the pathogen, enhance the virulence of the infection, or both. In addition, the immunogenic properties of OM vesicles lead to protective mucosal and systemic bactericidal antibody responses that have been exploited for vaccine purposes.
Vesicle-Mediated Activation of Inflammation and Innate Immunity
Vesicles are likely a key factor in effecting an inflammatory response to pathogens. OM vesicles produced by colonizing bacteria encounter and may be taken up by epithelial cells and macrophages to trigger an immediate innate host response. The response of epithelial cells and macrophages to secreted bacterial products is a well-established trigger of the inflammatory cascade.
The ability of OM vesicles to trigger inflammatory responses was thoroughly investigated by Alaniz et al. (1). Their analysis demonstrated that OM vesicles from Salmonella enterica serovar Typhimurium are potent stimulators of proinflammatory cytokine secretion and immune cell activation. Salmonella OM vesicles activate macrophages and dendritic cells to increase levels of surface major histocompatibility complex class II (MHC-II) expression as well as the production of the proinflammatory mediators tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12). OM vesicles also activated CD4+ T cells, indicating that protein antigen components of vesicles were effectively processed and presented by the activated antigen-presenting cells.
A proinflammatory response to OM vesicles was also observed for several other pathogens. Studies of epithelial cell responses found that H. pylori OM vesicles potently elicit an IL-8 response (66), as do P. aeruginosa vesicles (8). Detergent-generated vesicles from N. meningitidis, while not naturally produced, have well-characterized host responses due to their development into a protective vaccine. These manufactured vesicles have been shown to trigger the production of numerous proinflammatory cytokines from neutrophils, including TNF-α, IL-1β, IL-8, macrophage inflammatory protein 1β (MIP-1β), and IP-10. (85) Naturally produced Neisseria OM vesicles were further found to stimulate IL-8, RANTES, and IP-10 while activating dendritic cell MHC-II expression (28). Likewise, OM vesicles purified from the fish pathogen Vibrio anguillarum stimulated the production of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 when inoculated into flounder (58).
It should be considered that stimulation by OM vesicles is more likely to have a pathogen- and site-specific “signature” than is stimulation by individual purified components such as LPS or purified OM proteins. The protein composition of vesicles can vary substantially with the bacterial strain of origin and with the environment (87). Prominent OM proteins such as porins are known to promote proinflammatory activities in a variety of Gram-negative pathogens, including H. pylori, Salmonella, Fusobacterium, and Yersinia species (38, 39, 126, 130, 131). Recently, the OM adhesin of N. meningitidis, NadA, was shown to be required for the optimal activation of macrophage responses. This adhesin fully activated macrophage cytokine production when presented in the conformation of the OM vesicle membrane rather than as a mixture of purified vesicle components (128). Furthermore, the immunoglobulin responses to the Neisseria antigen PorB delivered as a recombinant purified protein, as a virus-like particle, and in outer membrane vesicles were compared. Zhu et al. observed that only the OM vesicle delivery system generated a bactericidal serum response (145).
These data, as well as data from numerous vaccine studies (17, 104), demonstrate that the three-dimensional conformation in which immune ligands are presented can greatly impact the type and potency of the host response generated. Numerous studies have demonstrated that the composition of OM vesicles differs from that of the bacterial OM by the enrichment or exclusion of specific outer membrane proteins and LPS modifications (see above for a discussion of enrichment) (8, 70, 95). Given these differences in composition, it is hypothesized that the responses to these two bacterial membranes would be significantly different. Naturally produced OM vesicles and whole-cell OM preparations have not yet been compared in activation assays. Such experiments would help elucidate the specific roles that OM vesicles play in diverting host responses to prevent bacterial clearance.
LPS and Other PAMPs
All experimental evidence to date indicates that the innate immune response to OM vesicles results from the combination of vesicle pathogen-associated molecular patterns (PAMPs) and LPS recognized in their natural context. Lipoproteins and OM proteins present in vesicles are biologically active molecules known to activate immune cells and induce leukocyte migration (140). The identity of PAMPs and other components of OM vesicles capable of modulating immune responses can perhaps best be investigated by studying the effects of OM vesicles from genetic mutants lacking specific envelope components.
The single most abundant, and typically considered most potent, immune-stimulating component of OM vesicles is LPS. LPS content can exceed the total protein content of vesicles by ratios as high as 10:1 (our unpublished observations). Numerous studies have characterized how LPS is sensed by the Toll-like receptor 4 (TLR4) complex, triggering a proinflammatory response common to the majority of Gram-negative bacterial infections. High levels of LPS (endotoxin) and TLR4 activation can lead to LPS toxicity and play a role in septic shock (107). Given the high LPS content, all investigations into immune responses to OM vesicles must define the contribution of LPS to the host response. OM vesicles, as LPS delivery vehicles, have the capacity to either enhance bacterial clearance or cause host tissue damage by activating an inflammatory response.
Much of the research into the TLR4-mediated response to LPS has utilized pure, chemically extracted LPS. However, it is more likely that LPS is naturally shed from bacteria in the form of OM vesicles. OM vesicles are heterogeneous proteoliposomes that have a larger dimension than liposomes composed solely of LPS (11), and these properties likely impact the type and potency of the innate immune response. The predicted endotoxic capacity of vesicles has been established by numerous studies, but a more interesting question that has consequently arisen is whether vesicle-associated LPS is equally as endotoxic as cellular LPS.
To address this issue, Munford et al. compared the abilities of phenol-extracted vesicle LPS and whole-cell LPS to bind to high-density lipoproteins (HDLs) (100). Those authors determined that purified and vesicle LPSs were similarly active in binding HDL, while cell-associated LPS was less active. As a result, they concluded that vesicle-associated LPS is the native presentation of LPS that has the highest biological activity. In a similar comparison, endotoxin secreted into the culture supernatant from N. meningitidis cells was found to more effectively stimulate monocyte expression of plasminogen activator and the procoagulant factor TF than phenol-extracted, pure LPS (119).
Studies of LPS structure during the course of infection further demonstrated that LPS can be modified in response to environmental stimuli. LPS from P. aeruginosa has been well documented to exhibit an altered lipid A acylation pattern when growing in the lungs of cystic fibrosis patients (32). Variations of O-antigen composition have also been shown to vary with environmental conditions, as Sabra et al. were able to induce P. aeruginosa to increase B-band LPS production under low O2 conditions (116a). OM vesicles may also exhibit a distinctive population of LPS molecules, as an analysis of P. aeruginosa vesicles revealed vesicles enriched in B-band LPS (70). It is unclear if vesicles aid in the turnover of LPS molecules during colonization; however, the impact of these chemical alterations due to environmental conditions should be factored into the experimental design to determine host responses to these LPS species.
The sensing of LPS by the TLR4 complex requires the extraction of LPS from the membrane by accessory proteins such as CD14, LPS binding protein (LBP), or bactericidal/permeability-increasing protein (BPI). While the mechanism by which these factors remove LPS from the bacterial membrane for presentation to the TLR4 complex is not known, studies have established OM vesicles both as key sources of LPS to activate inflammation and as decoys, binding these soluble factors to impede inflammation.
Vesy et al. found that the LPS in Salmonella OM vesicles was extracted by LBP but not by other factors such as CD14. The depletion of LBP from culture supernatants inhibited LPS binding to cell surface TLR4-CD14 complexes (133). In a similar LPS binding study, Schultz et al. investigated the role of BPI in binding Neisseria OM vesicle-derived LPS. Those authors observed that BPI binding of LPS was required for the OM vesicle interaction with dendritic cells, leading to CD14-mediated signaling and the expression of proinflammatory cytokines (122).
Vesicle-mediated components other than LPS have also been shown to modulate the sensing of LPS by the host. Vesicles from the dental pathogen P. gingivalis have been shown to decrease the level of membrane-bound expression of CD14 on macrophage surfaces, leading to a decreased ability of the macrophages to trigger LPS-stimulated cytokine production. A loss of CD14 is prevalent in cases of chronic periodontitis. Additionally, P. gingivalis OM vesicles were shown to bind and actively degrade soluble CD14 with vesicle-derived enzymes. P. gingivalis vesicles were also shown to degrade IgG, IgM, and complement factor C3 (47). These studies demonstrate that LPS from OM vesicles both is directly sensed by the innate immune system and directly interacts with host factors to modulate that same response.
Purified LPS and vesicle-bound LPS not only differ in their potencies of stimulating an innate immune response but also may differ in their ability to distribute, and thus be cleared, in host tissues. This is of particular importance for in vivo studies that instill pure LPS into animal models. The biophysical properties of vesicles, as heterogeneous, proteinaceous, amphipathic structures, may allow greater movement through tissues. In contrast, pure LPS would likely exhibit more hydrophobic qualities, resulting in more rapid binding to available host membranes. As a result, vesicles could travel deeper into tissues where resident phagocytes are located. These same vesicle properties may also result in vesicles being more readily recognized and cleared by tissue phagocytes. Studies investigating the role of aggregate size in LPS binding by host cells support this hypothesis. Kitchens and Munford found that the size of LPS complexes directly impacts the CD14-dependent internalization of LPS (80). Additionally, Elsbach noted that the inflammatory responses to LPS are directly impacted by the presence of other membrane components as well as the membrane conformation (31).
OM vesicles have also been shown to contain other PAMPs in addition to LPS. P. aeruginosa vesicles were shown to contain both flagellin monomers and CpG DNA (8, 113). Studies utilizing LPS-deficient OM vesicles from Neisseria demonstrated the ability of non-LPS PAMPs to enhance immune responses to vesicles (36, 108). However, to date, no studies have demonstrated either flagellin or CpG DNA directly impacting the host responses to intact vesicles containing LPS.
Vaccines, Antigenicity, and Adjuvanticity of OM Vesicles
The capacity of OM vesicles to stimulate an adaptive memory immune response has already been exploited in the development of effective OM vesicle-based acellular vaccines. The most successful use of OM vesicles as a vaccine has been in the development of a vaccine against serogroup B N. meningitidis. The development and properties of these serogroup B vaccines have recently been comprehensively reviewed by Holst and colleagues (57). As detailed in that review, vesicle-based vaccines have proven to be the only protective formulation against serogroup B infections, with over 55 million doses administered to date. Several different formulations of the vaccine exist, targeted to strains and antigens specific to a given geographic region. All preparations of Neisseria vesicle vaccines stimulate protective mucosal and systemic bactericidal antibody responses, with the antibody response being generated predominantly to the outer membrane porins PorA and PorB (37). Research is currently focused on engineering bacterial strains to produce OM vesicles containing multiple PorA proteins derived from different strains in the hope of developing a global N. meningitidis serogroup B vaccine (132).
The concept of developing OM vesicle-based vaccines has also been investigated for cholera infections. Schild et al. used the neonatal mouse model of passive antibody transfer to demonstrate that OM vesicles from Vibrio cholerae generate a protective antibody response (118). B-cell responses to OM vesicles from S. Typhimurium (1), B. burgdorferi (140), and Flavobacterium (4) have also been documented, indicating that OM vesicles can easily be used as antigen delivery systems to generate effective antibody responses.
The presence of LPS in OM vesicle-based vaccines has emphasized the ability of LPS to act as a natural adjuvant to the immune system. Neisseria is unusual in that it is a Gram-negative pathogen that can tolerate the deletion of the biosynthetic genes for LPS. However, immune responses to LPS-free OM vesicle preparations are poor, and the addition of exogenous PAMP adjuvants was shown to be necessary to generate an optimal host response (28, 36, 108). The most commonly used commercial OM vesicle vaccine against N. meningitidis utilizes manufactured vesicles from detergent-treated bacteria, which greatly reduces, but does not eliminate, the endotoxic content of vesicles to prevent toxicity. Data generated by utilizing LPS-deficient OM vesicles as well as the observation that the Neisseria adhesin NadA generates an effective immune response only when presented with LPS indicate that the adjuvant activity of LPS may be critical to the engineering of an effective vaccine. Recent investigations into how TLR ligand sensing impacts other phagocyte functions such as phagosomal maturation, the selection of antigens for MHC presentation, and dendritic cell maturation indicate that the immune system may be best adapted to sensing a “mixture” of LPS and other OM bacterial components to generate an effective host response (13).
FUTURE DIRECTIONS
Since every pathogen expresses different virulence characteristics and utilizes these characteristics for distinct purposes, the quest to uncover the roles that OM vesicles play for every pathogen is daunting. Nevertheless, this substantial body of research has generated a great deal of excitement, since it is revealing both common and pathogen-specific roles that OM vesicles can play in bacterial virulence. Future work in this field is likely to take advantage of this knowledge in order to reduce pathogenicity and to exploit the capacities of this complex natural delivery mechanism.
Targeting Vesicles To Reduce Virulence
Disrupting the interactions between host cells and vesicle-associated virulence factors and between cocolonizing bacteria and vesicles is likely to be a good strategy to reduce virulence. Preventing the generation of vesicles by pathogens and reducing the proinflammatory effects of OM vesicles in host tissues are also likely to reduce pathogenicity. As these processes become sufficiently understood, we can proceed to finding therapeutics that can disarm vesicle effects and block vesiculation (if vesiculation is not an essential bacterial function) to make the bacteria more susceptible to host defenses.
Taking Advantage of OM Vesicles
The utilization of OM vesicles as a complex of antigens in their native context with a natural adjuvant has already proven successful for human vaccines. Future efforts will likely result in OM vesicle vaccines engineered to reduce endotoxicity and to include multispecies-specific antigens. Additionally, synthetic proteoliposomes have been developed to deliver drugs to tumors and specific tissues, although off-target effects are a continuing problem. Making use of adherence and entry mechanisms that target native OM vesicles to host cells could improve the specific targeting of engineered therapeutic liposomes.
Acknowledgments
We are grateful to Amanda McBroom for the postinfection image of ETEC obtained with the scanning electron microscope expertise of Leslie Eibest in the Duke Biology Department.
This work was supported by a Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease award, NIH/NIAID grants R01AI064464 and R01AI079068 (to M.J.K.), and a postdoctoral fellowship from The Hartwell Foundation (to T.N.E.).
REFERENCES
- 1.Alaniz, R. C., B. L. Deatherage, J. C. Lara, and B. T. Cookson. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 179:7692-7701. [DOI] [PubMed] [Google Scholar]
- 2.Allan, N. D., C. Kooi, P. A. Sokol, and T. J. Beveridge. 2003. Putative virulence factors are released in association with membrane vesicles from Burkholderia cepacia. Can. J. Microbiol. 49:613-624. [DOI] [PubMed] [Google Scholar]
- 3.Anthony, L. D., R. D. Burke, and F. E. Nano. 1991. Growth of Francisella spp. in rodent macrophages. Infect. Immun. 59:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki, M., M. Kondo, Y. Nakatsuka, K. Kawai, and S. Oshima. 2007. Stationary phase culture supernatant containing membrane vesicles induced immunity to rainbow trout Oncorhynchus mykiss fry syndrome. Vaccine 25:561-569. [DOI] [PubMed] [Google Scholar]
- 5.Ayala, G., L. Torres, M. Espinosa, G. Fierros-Zarate, V. Maldonado, and J. Melendez-Zajgla. 2006. External membrane vesicles from Helicobacter pylori induce apoptosis in gastric epithelial cells. FEMS Microbiol. Lett. 260:178-185. [DOI] [PubMed] [Google Scholar]
- 6.Balsalobre, C., J. M. Silvan, S. Berglund, Y. Mizunoe, B. E. Uhlin, and S. N. Wai. 2006. Release of the type I secreted alpha-haemolysin via outer membrane vesicles from Escherichia coli. Mol. Microbiol. 59:99-112. [DOI] [PubMed] [Google Scholar]
- 7.Bauman, S. J., and M. J. Kuehn. 2009. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauman, S. J., and M. J. Kuehn. 2006. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 8:2400-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergman, M. A., L. A. Cummings, S. L. Barrett, K. D. Smith, J. C. Lara, A. Aderem, and B. T. Cookson. 2005. CD4+ T cells and Toll-like receptors recognize Salmonella antigens expressed in bacterial surface organelles. Infect. Immun. 73:1350-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlanda Scorza, F., F. Doro, M. J. Rodriguez-Ortega, M. Stella, S. Liberatori, A. R. Taddei, L. Serino, D. Gomes Moriel, B. Nesta, M. R. Fontana, A. Spagnuolo, M. Pizza, N. Norais, and G. Grandi. 2008. Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli deltatolR IHE3034 mutant. Mol. Cell. Proteomics 7:473-485. [DOI] [PubMed] [Google Scholar]
- 11.Beveridge, T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjerre, A., B. Brusletto, E. Rosenqvist, E. Namork, P. Kierulf, R. Ovstebo, G. B. Joo, and P. Brandtzaeg. 2000. Cellular activating properties and morphology of membrane-bound and purified meningococcal lipopolysaccharide. J. Endotoxin Res. 6:437-445. [PubMed] [Google Scholar]
- 13.Blander, J. M., and R. Medzhitov. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808-812. [DOI] [PubMed] [Google Scholar]
- 14.Boardman, B. K., B. M. Meehan, and K. J. F. Satchell. 2007. Growth phase regulation of Vibrio cholerae RTX toxin export. J. Bacteriol. 189:1827-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bomberger, J. M., D. P. Maceachran, B. A. Coutermarsh, S. Ye, G. A. O'Toole, and B. A. Stanton. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandtzaeg, P., K. Bryn, P. Kierulf, R. Ovstebo, E. Namork, B. Aase, and E. Jantzen. 1992. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Invest. 89:816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buonaguro, F. M., M. L. Tornesello, and L. Buonaguro. 2009. Virus-like particle vaccines and adjuvants: the HPV paradigm. Expert Rev. Vaccines 8:1379-1398. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee, S. N., and J. Das. 1967. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J. Gen. Microbiol. 49:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Chi, B., M. Qi, and H. K. Kuramitsu. 2003. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res. Microbiol. 154:637-643. [DOI] [PubMed] [Google Scholar]
- 20.Ciofu, O., T. J. Beveridge, J. Kadurugamuwa, J. Walther-Rasmussen, and N. Hoiby. 2000. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45:9-13. [DOI] [PubMed] [Google Scholar]
- 21.Cota-Gomez, A., A. I. Vasil, J. Kadurugamuwa, T. J. Beveridge, H. P. Schweizer, and M. L. Vasil. 1997. PlcR1 and PlcR2 are putative calcium-binding proteins required for secretion of the hemolytic phospholipase C of Pseudomonas aeruginosa. Infect. Immun. 65:2904-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demuth, D. R., D. James, Y. Kowashi, and S. Kato. 2003. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell. Microbiol. 5:111-121. [DOI] [PubMed] [Google Scholar]
- 23.Deslauriers, M., D. ni Eidhin, L. Lamonde, and C. Mouton. 1990. SDS-PAGE analysis of protein and lipopolysaccharide of extracellular vesicules and Sarkosyl-insoluble membranes from Bacteroides gingivalis. Oral Microbiol. Immunol. 5:1-7. [DOI] [PubMed] [Google Scholar]
- 24.Devoe, I. W., and J. E. Gilchrist. 1973. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J. Exp. Med. 138:1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorward, D. W., C. F. Garon, and R. C. Judd. 1989. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171:2499-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorward, D. W., T. G. Schwan, and C. F. Garon. 1991. Immune capture and detection of Borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J. Clin. Microbiol. 29:1162-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan, L., M. Yoshioka, F. Chandad, and D. Grenier. 2004. Loss of lipopolysaccharide receptor CD14 from the surface of human macrophage-like cells mediated by Porphyromonas gingivalis outer membrane vesicles. Microb. Pathog. 36:319-325. [DOI] [PubMed] [Google Scholar]
- 28.Durand, V., J. Mackenzie, J. de Leon, C. Mesa, V. Quesniaux, M. Montoya, A. Le Bon, and S. Y. Wong. 2009. Role of lipopolysaccharide in the induction of type I interferon-dependent cross-priming and IL-10 production in mice by meningococcal outer membrane vesicles. Vaccine 27:1912-1922. [DOI] [PubMed] [Google Scholar]
- 29.Dutson, T. R., A. M. Pearson, J. F. Price, G. C. Spink, and P. J. Tarrant. 1971. Observations by electron microscopy on pig muscle inoculated and incubated with Pseudomonas fragi. Appl. Microbiol. 22:1152-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta, S., K. Iida, A. Takade, Y. Meno, G. B. Nair, and S. Yoshida. 2004. Release of Shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release. Microbiol. Immunol. 48:965-969. [DOI] [PubMed] [Google Scholar]
- 31.Elsbach, P. 2000. Mechanisms of disposal of bacterial lipopolysaccharides by animal hosts. Microbes Infect. 2:1171-1180. [DOI] [PubMed] [Google Scholar]
- 32.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Moreira, E., J. H. Helbig, and M. S. Swanson. 2006. Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes. Infect. Immun. 74:3285-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiocca, R., V. Necchi, P. Sommi, V. Ricci, J. Telford, T. L. Cover, and E. Solcia. 1999. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188:220-226. [DOI] [PubMed] [Google Scholar]
- 35.Forsberg, C. W., T. J. Beveridge, and A. Hellstrom. 1981. Cellulase and xylanase release from Bacteroides succinogenes and its importance in the rumen environment. Appl. Environ. Microbiol. 42:886-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fransen, F., C. J. Boog, J. P. van Putten, and P. van der Ley. 2007. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infect. Immun. 75:5939-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frasch, C. E. 1990. Production and control of Neisseria meningitidis vaccines. Adv. Biotechnol. Processes 13:123-145. [PubMed] [Google Scholar]
- 38.Galdiero, F., G. C. de L'ero, N. Benedetto, M. Galdiero, and M. A. Tufano. 1993. Release of cytokines induced by Salmonella typhimurium porins. Infect. Immun. 61:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galdiero, M., A. Folgore, M. Molitierno, and R. Greco. 1999. Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leucocyte transmigration through human endothelial cells in vitro. Clin. Exp. Immunol. 116:453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galka, F., S. N. Wai, H. Kusch, S. Engelmann, M. Hecker, B. Schmeck, S. Hippenstiel, B. E. Uhlin, and M. Steinert. 2008. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect. Immun. 76:1825-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamage, S. D., C. M. McGannon, and A. A. Weiss. 2004. Escherichia coli serogroup O107/O117 lipopolysaccharide binds and neutralizes Shiga toxin 2. J. Bacteriol. 186:5506-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamazo, C., and I. Moriyon. 1987. Release of outer membrane fragments by exponentially growing Brucella melitensis cells. Infect. Immun. 55:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gankema, H., J. Wensink, P. A. Guinee, W. H. Jansen, and B. Witholt. 1980. Some characteristics of the outer membrane material released by growing enterotoxigenic Escherichia coli. Infect. Immun. 29:704-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-del Portillo, F., M. A. Stein, and B. B. Finlay. 1997. Release of lipopolysaccharide from intracellular compartments containing Salmonella typhimurium to vesicles of the host epithelial cell. Infect. Immun. 65:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golovliov, I., V. Baranov, Z. Krocova, H. Kovarova, and A. Sjostedt. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goulhen, F., A. Hafezi, V. J. Uitto, D. Hinode, R. Nakamura, D. Grenier, and D. Mayrand. 1998. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect. Immun. 66:5307-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grenier, D. 1992. Inactivation of human serum bactericidal activity by a trypsinlike protease isolated from Porphyromonas gingivalis. Infect. Immun. 60:1854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grenier, D., and D. Mayrand. 1987. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect. Immun. 55:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo, L., R. O. Fatig III, G. L. Orr, B. W. Schafer, J. A. Strickland, K. Sukhapinda, A. T. Woodsworth, and J. K. Petell. 1999. Photorhabdus luminescens W-14 insecticidal activity consists of at least two similar but distinct proteins. Purification and characterization of toxin A and toxin B. J. Biol. Chem. 274:9836-9842. [DOI] [PubMed] [Google Scholar]
- 50.Halhoul, N., and J. R. Colvin. 1975. The ultrastructure of bacterial plaque attached to the gingiva of man. Arch. Oral Biol. 20:115-118. [DOI] [PubMed] [Google Scholar]
- 51.Heczko, U., V. C. Smith, R. M. Meloche, A. M. Buchan, and B. B. Finlay. 2000. Characteristics of Helicobacter pylori attachment to human primary antral epithelial cells. Microbes Infect. 2:1669-1676. [DOI] [PubMed] [Google Scholar]
- 52.Hellman, J., P. M. Loiselle, M. M. Tehan, J. E. Allaire, L. A. Boyle, J. T. Kurnick, D. M. Andrews, K. S. Kim, and H. S. Warren. 2000. Outer membrane protein A, peptidoglycan-associated lipoprotein, and murein lipoprotein are released by Escherichia coli bacteria into serum. Infect. Immun. 68:2566-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellman, J., P. M. Loiselle, E. M. Zanzot, J. E. Allaire, M. M. Tehan, L. A. Boyle, J. T. Kurnick, and H. S. Warren. 2000. Release of gram-negative outer-membrane proteins into human serum and septic rat blood and their interactions with immunoglobulin in antiserum to Escherichia coli J5. J. Infect. Dis. 181:1034-1043. [DOI] [PubMed] [Google Scholar]
- 54.Hellman, J., and H. S. Warren. 2001. Outer membrane protein A (OmpA), peptidoglycan-associated lipoprotein (PAL), and murein lipoprotein (MLP) are released in experimental Gram-negative sepsis. J. Endotoxin Res. 7:69-72. [PubMed] [Google Scholar]
- 55.Hellman, J., E. M. Zanzot, P. M. Loiselle, S. F. Amato, K. M. Black, Y. Ge, J. T. Kurnick, and H. S. Warren. 1997. Antiserum against Escherichia coli J5 contains antibodies reactive with outer membrane proteins of heterologous gram-negative bacteria. J. Infect. Dis. 176:1260-1268. [DOI] [PubMed] [Google Scholar]
- 56.Hoekstra, D., J. W. van der Laan, L. de Leij, and B. Witholt. 1976. Release of outer membrane fragments from normally growing Escherichia coli. Biochim. Biophys. Acta 455:889-899. [DOI] [PubMed] [Google Scholar]
- 57.Holst, J., D. Martin, R. Arnold, C. C. Huergo, P. Oster, J. O'Hallahan, and E. Rosenqvist. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl. 2):B3-B12. [DOI] [PubMed] [Google Scholar]
- 58.Hong, G. E., D. G. Kim, E. M. Park, B. H. Nam, Y. O. Kim, and I. S. Kong. 2009. Identification of Vibrio anguillarum outer membrane vesicles related to immunostimulation in the Japanese flounder, Paralichthys olivaceus. Biosci. Biotechnol. Biochem. 73:437-439. [DOI] [PubMed] [Google Scholar]
- 59.Horstman, A. L., S. J. Bauman, and M. J. Kuehn. 2004. Lipopolysaccharide 3-deoxy-D-manno-octulosonic acid (Kdo) core determines bacterial association of secreted toxins. J. Biol. Chem. 279:8070-8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horstman, A. L., and M. J. Kuehn. 2002. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J. Biol. Chem. 277:32538-32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horstman, A. L., and M. J. Kuehn. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 275:12489-12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hozbor, D., M. E. Rodriguez, J. Fernandez, A. Lagares, N. Guiso, and O. Yantorno. 1999. Release of outer membrane vesicles from Bordetella pertussis. Curr. Microbiol. 38:273-278. [DOI] [PubMed] [Google Scholar]
- 63.Hynes, S. O., J. I. Keenan, J. A. Ferris, H. Annuk, and A. P. Moran. 2005. Lewis epitopes on outer membrane vesicles of relevance to Helicobacter pylori pathogenesis. Helicobacter 10:146-156. [DOI] [PubMed] [Google Scholar]
- 64.Ilver, D., S. Barone, D. Mercati, P. Lupetti, and J. L. Telford. 2004. Helicobacter pylori toxin VacA is transferred to host cells via a novel contact-dependent mechanism. Cell. Microbiol. 6:167-174. [DOI] [PubMed] [Google Scholar]
- 65.Inagaki, S., S. Onishi, H. K. Kuramitsu, and A. Sharma. 2006. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by “Tannerella forsythia.” Infect. Immun. 74:5023-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ismail, S., M. B. Hampton, and J. I. Keenan. 2003. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect. Immun. 71:5670-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadurugamuwa, J. L., and T. J. Beveridge. 1996. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178:2767-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kadurugamuwa, J. L., and T. J. Beveridge. 1998. Delivery of the non-membrane-permeative antibiotic gentamicin into mammalian cells by using Shigella flexneri membrane vesicles. Antimicrob. Agents Chemother. 42:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kadurugamuwa, J. L., and T. J. Beveridge. 1999. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology 145:2051-2060. [DOI] [PubMed] [Google Scholar]
- 70.Kadurugamuwa, J. L., and T. J. Beveridge. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177:3998-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamaguchi, A., K. Nakayama, S. Ichiyama, R. Nakamura, T. Watanabe, M. Ohta, H. Baba, and T. Ohyama. 2003. Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr. Microbiol. 47:485-491. [DOI] [PubMed] [Google Scholar]
- 72.Kamaguchi, A., T. Ohyama, E. Sakai, R. Nakamura, T. Watanabe, H. Baba, and K. Nakayama. 2003. Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for co-aggregation with Prevotella intermedia. Microbiology 149:1257-1264. [DOI] [PubMed] [Google Scholar]
- 73.Kato, S., Y. Kowashi, and D. R. Demuth. 2002. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 32:1-13. [DOI] [PubMed] [Google Scholar]
- 74.Katsui, N., T. Tsuchido, R. Hiramatsu, S. Fujikawa, M. Takano, and I. Shibasaki. 1982. Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli. J. Bacteriol. 151:1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keenan, J., T. Day, S. Neal, B. Cook, G. Perez-Perez, R. Allardyce, and P. Bagshaw. 2000. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol. Lett. 182:259-264. [DOI] [PubMed] [Google Scholar]
- 76.Keenan, J. I., and R. A. Allardyce. 2000. Iron influences the expression of Helicobacter pylori outer membrane vesicle-associated virulence factors. Eur. J. Gastroenterol. Hepatol. 12:1267-1273. [DOI] [PubMed] [Google Scholar]
- 77.Kesty, N. C., and M. J. Kuehn. 2004. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 279:2069-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kesty, N. C., K. M. Mason, M. Reedy, S. E. Miller, and M. J. Kuehn. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23:4538-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khandelwal, P., and N. Banerjee-Bhatnagar. 2003. Insecticidal activity associated with the outer membrane vesicles of Xenorhabdus nematophilus. Appl. Environ. Microbiol. 69:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitchens, R. L., and R. S. Munford. 1998. CD14-dependent internalization of bacterial lipopolysaccharide (LPS) is strongly influenced by LPS aggregation but not by cellular responses to LPS. J. Immunol. 160:1920-1928. [PubMed] [Google Scholar]
- 81.Kolling, G. L., and K. R. Matthews. 1999. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 65:1843-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kondo, M., K. Kawai, K. Yagyu, K. Nakayama, K. Kurohara, and S. Oshima. 2001. Changes in the cell structure of Flavobacterium psychrophilum with length of culture. Microbiol. Immunol. 45:813-818. [DOI] [PubMed] [Google Scholar]
- 83.Kouokam, J. C., S. N. Wai, M. Fällman, U. Dobrindt, J. Hacker, and B. E. Uhlin. 2006. Active cytotoxic necrotizing factor 1 associated with outer membrane vesicles from uropathogenic Escherichia coli. Infect. Immun. 74:2022-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwon, S. O., Y. S. Gho, J. C. Lee, and S. I. Kim. 2009. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol. Lett. 297:150-156. [DOI] [PubMed] [Google Scholar]
- 85.Lapinet, J. A., P. Scapini, F. Calzetti, O. Perez, and M. A. Cassatella. 2000. Gene expression and production of tumor necrosis factor alpha, interleukin-1beta (IL-1beta), IL-8, macrophage inflammatory protein 1alpha (MIP-1alpha), MIP-1beta, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect. Immun. 68:6917-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee, E. Y., J. Y. Bang, G. W. Park, D. S. Choi, J. S. Kang, H. J. Kim, K. S. Park, J. O. Lee, Y. K. Kim, K. H. Kwon, K. P. Kim, and Y. S. Gho. 2007. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7:3143-3153. [DOI] [PubMed] [Google Scholar]
- 87.Lee, E. Y., D. S. Choi, K. P. Kim, and Y. S. Gho. 2008. Proteomics in Gram-negative bacterial outer membrane vesicles. Mass Spectrom. Rev. 27:535-555. [DOI] [PubMed] [Google Scholar]
- 88.Li, Z., A. J. Clarke, and T. J. Beveridge. 1996. A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division and secretion in surface membrane vesicles. J. Bacteriol. 178:2479-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li, Z., A. J. Clarke, and T. J. Beveridge. 1998. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180:5478-5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Logan, S. M., and T. J. Trust. 1982. Outer membrane characteristics of Campylobacter jejuni. Infect. Immun. 38:898-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.MacEachran, D. P., S. Ye, J. M. Bomberger, D. A. Hogan, A. Swiatecka-Urban, B. A. Stanton, and G. A. O'Toole. 2007. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect. Immun. 75:3902-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mashburn, L. M., and M. Whiteley. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422-425. [DOI] [PubMed] [Google Scholar]
- 93.Masignani, V., E. Balducci, F. Di Marcello, S. Savino, D. Serruto, D. Veggi, S. Bambini, M. Scarselli, B. Arico, M. Comanducci, J. Adu-Bobie, M. M. Giuliani, R. Rappuoli, and M. Pizza. 2003. NarE: a novel ADP-ribosyltransferase from Neisseria meningitidis. Mol. Microbiol. 50:1055-1067. [DOI] [PubMed] [Google Scholar]
- 94.Mayrand, D., and D. Grenier. 1989. Biological activities of outer membrane vesicles. Can. J. Microbiol. 35:607-613. [DOI] [PubMed] [Google Scholar]
- 95.McBroom, A., and M. J. Kuehn. 12 May 2005, posting date. Chapter 2.2.4, Outer membrane vesicles. In R. Curtiss III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC.
- 96.McBroom, A. J., A. P. Johnson, S. Vemulapalli, and M. J. Kuehn. 2006. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 188:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McBroom, A. J., and M. J. Kuehn. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63:545-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mudrak, B., D. L. Rodriguez, and M. J. Kuehn. 2009. Residues of heat-labile enterotoxin involved in bacterial cell surface binding. J. Bacteriol. 191:2917-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mug-Opstelten, D., and B. Witholt. 1978. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim. Biophys. Acta 508:287-295. [DOI] [PubMed] [Google Scholar]
- 100.Munford, R. S., C. L. Hall, J. M. Lipton, and J. M. Dietschy. 1982. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J. Clin. Invest. 70:877-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Namork, E., and P. Brandtzaeg. 2002. Fatal meningococcal septicaemia with “blebbing” meningococcus. Lancet 360:1741. [DOI] [PubMed] [Google Scholar]
- 102.Negrete-Abascal, E., R. M. Garcia, M. E. Reyes, D. Godinez, and M. de la Garza. 2000. Membrane vesicles released by Actinobacillus pleuropneumoniae contain proteases and Apx toxins. FEMS Microbiol. Lett. 191:109-113. [DOI] [PubMed] [Google Scholar]
- 103.Nikaido, H. 1994. Isolation of outer membranes. Methods Enzymol. 235:225-234. [DOI] [PubMed] [Google Scholar]
- 104.Nordly, P., H. B. Madsen, H. M. Nielsen, and C. Foged. 2009. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin. Drug Deliv. 6:657-672. [DOI] [PubMed] [Google Scholar]
- 105.Nowotny, A., U. H. Behling, B. Hammond, C. H. Lai, M. Listgarten, P. H. Pham, and F. Sanavi. 1982. Release of toxic microvesicles by Actinobacillus actinomycetemcomitans. Infect. Immun. 37:151-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohta, H., H. Hara, K. Fukui, H. Kurihara, Y. Murayama, and K. Kato. 1993. Association of Actinobacillus actinomycetemcomitans leukotoxin with nucleic acids on the bacterial cell surface. Infect. Immun. 61:4878-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Opal, S. M. 2007. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int. J. Med. Microbiol. 297:365-377. [DOI] [PubMed] [Google Scholar]
- 108.Opal, S. M., J. E. Palardy, W. H. Chen, N. A. Parejo, A. K. Bhattacharjee, and A. S. Cross. 2005. Active immunization with a detoxified endotoxin vaccine protects against lethal polymicrobial sepsis: its use with CpG adjuvant and potential mechanisms. J. Infect. Dis. 192:2074-2078. [DOI] [PubMed] [Google Scholar]
- 109.Patrick, S., J. P. McKenna, S. O'Hagan, and E. Dermott. 1996. A comparison of the haemagglutinating and enzymic activities of Bacteroides fragilis whole cells and outer membrane vesicles. Microb. Pathog. 20:191-202. [DOI] [PubMed] [Google Scholar]
- 110.Pettit, R. K., and R. C. Judd. 1992. The interaction of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae with normal human serum. Mol. Microbiol. 6:729-734. [DOI] [PubMed] [Google Scholar]
- 111.Prasadarao, N. V. 2002. Identification of Escherichia coli outer membrane protein A receptor on human brain microvascular endothelial cells. Infect. Immun. 70:4556-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prasadarao, N. V., C. A. Wass, J. N. Weiser, M. F. Stins, S. H. Huang, and K. S. Kim. 1996. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect. Immun. 64:146-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Renelli, M., V. Matias, R. Y. Lo, and T. J. Beveridge. 2004. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 150:2161-2169. [DOI] [PubMed] [Google Scholar]
- 114.Ricci, V., V. Chiozzi, V. Necchi, A. Oldani, M. Romano, E. Solcia, and U. Ventura. 2005. Free-soluble and outer membrane vesicle-associated VacA from Helicobacter pylori: two forms of release, a different activity. Biochem. Biophys. Res. Commun. 337:173-178. [DOI] [PubMed] [Google Scholar]
- 115.Rolhion, N., N. Barnich, L. Claret, and A. Darfeuille-Michaud. 2005. Strong decrease in invasive ability and outer membrane vesicle release in Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 with the yfgL gene deleted. J. Bacteriol. 187:2286-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosen, G., R. Naor, E. Rahamim, R. Yishai, and M. N. Sela. 1995. Proteases of Treponema denticola outer sheath and extracellular vesicles. Infect. Immun. 63:3973-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116a.Sabra, W., H. Lundsdorf, and A. P. Zeng. 2003. Alterations in the formation of lipopolysaccharide and membrane vesicles on the surface of Pseudomonas aeruginosa PA01 under oxygen stress conditions. Microbiology 149:2789-2795. [DOI] [PubMed] [Google Scholar]
- 117.Saunders, N. B., D. R. Shoemaker, B. L. Brandt, E. E. Moran, T. Larsen, and W. D. Zollinger. 1999. Immunogenicity of intranasally administered meningococcal native outer membrane vesicles in mice. Infect. Immun. 67:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schild, S., E. J. Nelson, and A. Camilli. 2008. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect. Immun. 76:4554-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schlichting, E., T. Lyberg, O. Solberg, and B. M. Andersen. 1993. Endotoxin liberation from Neisseria meningitidis correlates to their ability to induce procoagulant and fibrinolytic factors in human monocytes. Scand. J. Infect. Dis. 25:585-594. [DOI] [PubMed] [Google Scholar]
- 120.Schooling, S. R., and T. J. Beveridge. 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188:5945-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schooling, S. R., A. Hubley, and T. J. Beveridge. 2009. Interactions of DNA with biofilm-derived membrane vesicles. J. Bacteriol. 191:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schultz, H., J. Hume, D. S. Zhang, T. L. Gioannini, and J. P. Weiss. 2007. A novel role for the bactericidal/permeability increasing protein in interactions of Gram-negative bacterial outer membrane blebs with dendritic cells. J. Immunol. 179:2477-2484. [DOI] [PubMed] [Google Scholar]
- 123.Shoberg, R. J., and D. D. Thomas. 1993. Specific adherence of Borrelia burgdorferi extracellular vesicles to human endothelial cells in culture. Infect. Immun. 61:3892-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sidhu, V. K., F. J. Vorholter, K. Niehaus, and S. A. Watt. 2008. Analysis of outer membrane vesicle associated proteins isolated from the plant pathogenic bacterium Xanthomonas campestris pv. campestris. BMC Microbiol. 8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stephens, D. S., K. M. Edwards, F. Morris, and Z. A. McGee. 1982. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J. Infect. Dis. 146:568. [DOI] [PubMed] [Google Scholar]
- 126.Takada, H., T. Ogawa, F. Yoshimura, K. Otsuka, S. Kokeguchi, K. Kato, T. Umemoto, and S. Kotani. 1988. Immunobiological activities of a porin fraction isolated from Fusobacterium nucleatum ATCC 10953. Infect. Immun. 56:855-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tan, T. T., M. Morgelin, A. Forsgren, and K. Riesbeck. 2007. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J. Infect. Dis. 195:1661-1670. [DOI] [PubMed] [Google Scholar]
- 128.Tavano, R., S. Franzoso, P. Cecchini, E. Cartocci, F. Oriente, B. Arico, and E. Papini. 2009. The membrane expression of Neisseria meningitidis adhesin A (NadA) increases the proimmune effects of MenB OMVs on human macrophages, compared with NadA-OMVs, without further stimulating their proinflammatory activity on circulating monocytes. J. Leukoc. Biol. 86:143-153. [DOI] [PubMed] [Google Scholar]
- 129.Thompson, S. S., Y. M. Naidu, and J. J. Pestka. 1985. Ultrastructural localization of an extracellular protease in Pseudomonas fragi by using the peroxidase-antiperoxidase reaction. Appl. Environ. Microbiol. 50:1038-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tufano, M. A., F. Rossano, P. Catalanotti, G. Liguori, C. Capasso, M. T. Ceccarelli, and P. Marinelli. 1994. Immunobiological activities of Helicobacter pylori porins. Infect. Immun. 62:1392-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tufano, M. A., F. Rossano, P. Catalanotti, G. Liguori, A. Marinelli, A. Baroni, and P. Marinelli. 1994. Properties of Yersinia enterocolitica porins: interference with biological functions of phagocytes, nitric oxide production and selective cytokine release. Res. Microbiol. 145:297-307. [DOI] [PubMed] [Google Scholar]
- 132.van den Dobbelsteen, G. P., H. H. van Dijken, S. Pillai, and L. van Alphen. 2007. Immunogenicity of a combination vaccine containing pneumococcal conjugates and meningococcal PorA OMVs. Vaccine 25:2491-2496. [DOI] [PubMed] [Google Scholar]
- 133.Vesy, C. J., R. L. Kitchens, G. Wolfbauer, J. J. Albers, and R. S. Munford. 2000. Lipopolysaccharide-binding protein and phospholipid transfer protein release lipopolysaccharides from Gram-negative bacterial membranes. Infect. Immun. 68:2410-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vipond, C., J. Suker, C. Jones, C. Tang, I. M. Feavers, and J. X. Wheeler. 2006. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics 6:3400-3413. [DOI] [PubMed] [Google Scholar]
- 135.Wagner, P. L., J. Livny, M. N. Neely, D. W. Acheson, D. I. Friedman, and M. K. Waldor. 2002. Bacteriophage control of Shiga toxin 1 production and release by Escherichia coli. Mol. Microbiol. 44:957-970. [DOI] [PubMed] [Google Scholar]
- 136.Wai, S. N., B. Lindmark, T. Soderblom, A. Takade, M. Westermark, J. Oscarsson, J. Jass, A. Richter-Dahlfors, Y. Mizunoe, and B. E. Uhlin. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25-35. [DOI] [PubMed] [Google Scholar]
- 137.Wai, S. N., A. Takade, and K. Amako. 1995. The release of outer membrane vesicles from the strains of enterotoxigenic Escherichia coli. Microbiol. Immunol. 39:451-456. [DOI] [PubMed] [Google Scholar]
- 138.Wensink, J., H. Gankema, W. H. Jansen, P. A. Guinee, and B. Witholt. 1978. Isolation of the membranes of an enterotoxigenic strain of Escherichia coli and distribution of enterotoxin activity in different subcellular fractions. Biochim. Biophys. Acta 514:128-136. [DOI] [PubMed] [Google Scholar]
- 139.Wensink, J., and B. Witholt. 1981. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur. J. Biochem. 116:331-335. [DOI] [PubMed] [Google Scholar]
- 140.Whitmire, W. M., and C. F. Garon. 1993. Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdorferi. Infect. Immun. 61:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xia, X. X., M. J. Han, S. Y. Lee, and J. S. Yoo. 2008. Comparison of the extracellular proteomes of Escherichia coli B and K-12 strains during high cell density cultivation. Proteomics 8:2089-2103. [DOI] [PubMed] [Google Scholar]
- 142.Yashroy, R. C. 2007. Mechanism of infection of a human isolate Salmonella (3,10:r:-) in chicken ileum: ultrastructural study. Indian J. Med. Res. 126:558-566. [PubMed] [Google Scholar]
- 143.Yokoyama, K., T. Horii, T. Yamashino, S. Hashikawa, S. Barua, T. Hasegawa, H. Watanabe, and M. Ohta. 2000. Production of Shiga toxin by Escherichia coli measured with reference to the membrane vesicle-associated toxins. FEMS Microbiol. Lett. 192:139-144. [DOI] [PubMed] [Google Scholar]
- 144.Zhou, L., R. Srisatjaluk, D. E. Justus, and R. J. Doyle. 1998. On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol. Lett. 163:223-228. [DOI] [PubMed] [Google Scholar]
- 145.Zhu, W., C. E. Thomas, C. J. Chen, C. N. Van Dam, R. E. Johnston, N. L. Davis, and P. F. Sparling. 2005. Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan equine encephalitis virus replicon particles. Infect. Immun. 73:7558-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]