Abstract
Fluorescent and phosphorescent probes that have readily interpretable emission properties can be specifically inserted into biological macromolecules to reveal facets of their structure and dynamics: (1) Proximity. Singlet-singlet and triplet-singlet energy transfer can serve as spectroscopic rulers in the 10-65 Å range, whereas triplet-triplet transfer can be used to show that two groups are less than about 12 Å apart. (2) Rotational mobility. Nanosecond fluorescence polarization measurements can reveal whether a macromolecular system has any modes of flexibility in times of nanoseconds. (3) Polarity. The presence of mobile dipoles in the environment of certain chromophores is reflected in their fluorescence quantum yield and emission spectrum.
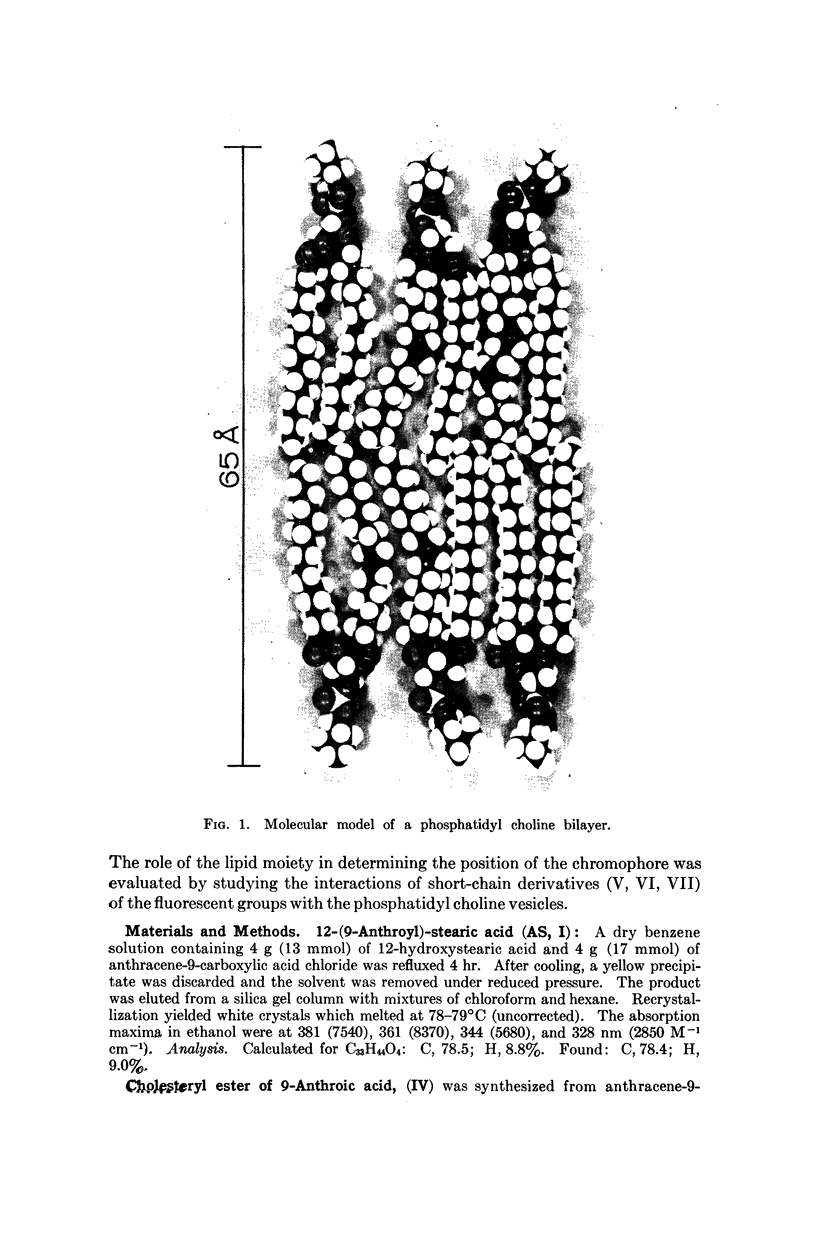
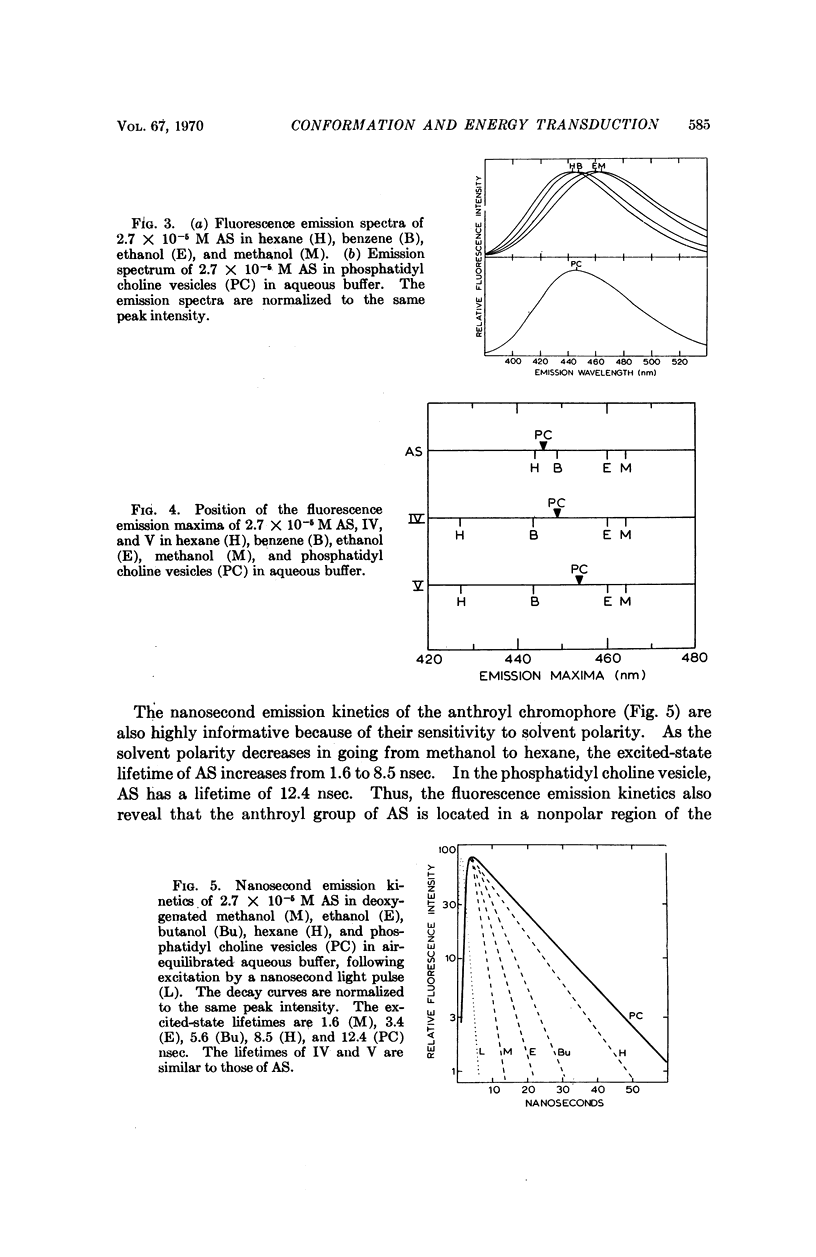
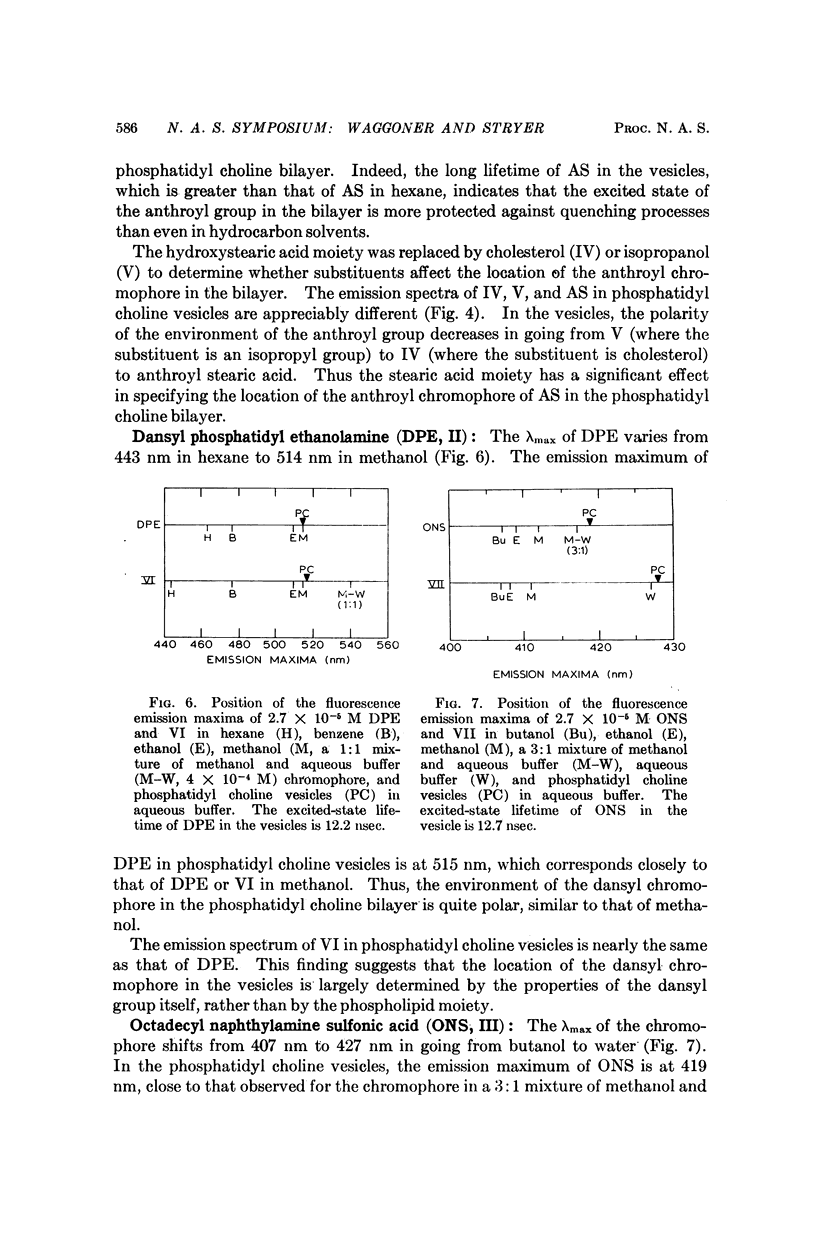
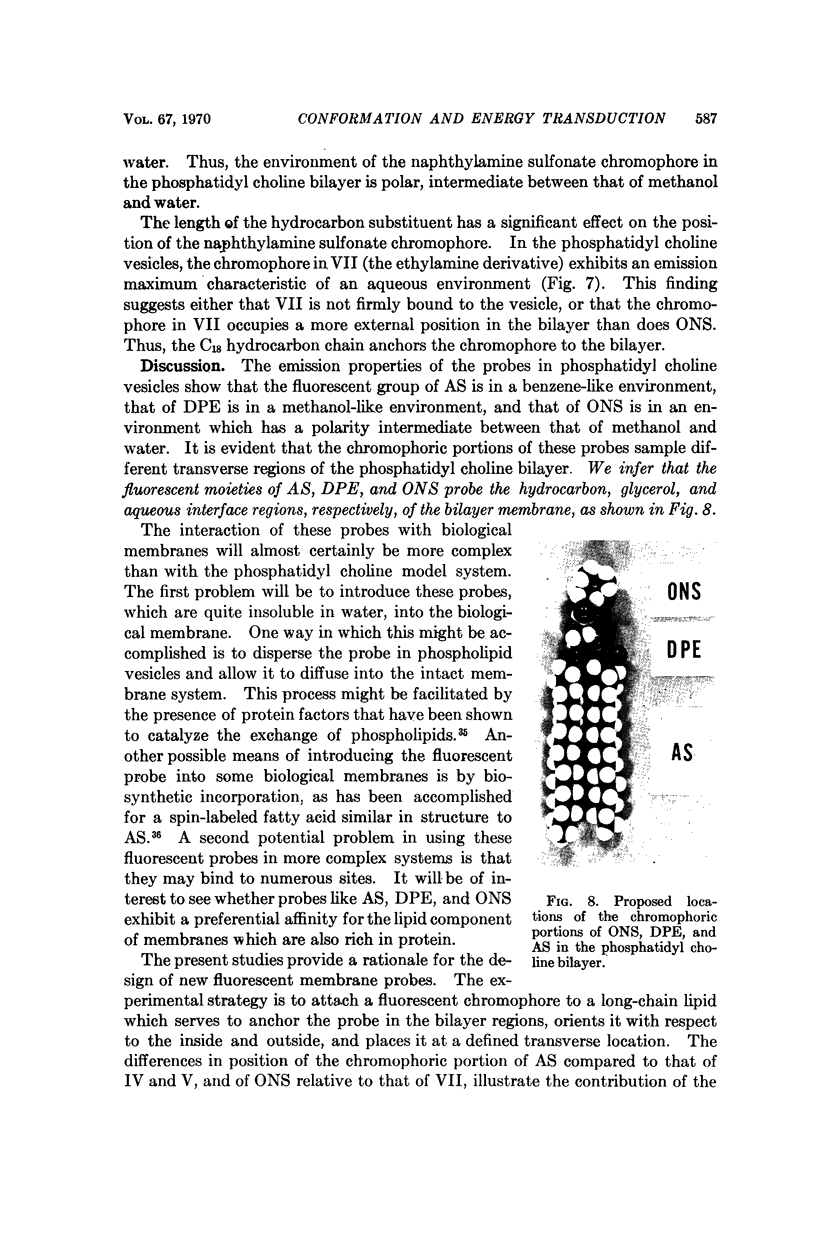
We have synthesized a number of new fluorescent probes for biological membranes. Anthroyl stearic acid (I), dansyl phosphatidyl ethanolamine (II), and octadecyl naphthylamine sulfonic acid (III) are readily incorporated into bilayer vesicles composed of phosphatidyl choline. The emission spectra of these probes in the vesicles indicate that the chromophore of I is located in the hydrocarbon region, that of II is located in the glycerol layer, and that of III is located at the aqueous interface of the bilayer. Thus, fluorescent chromophores can be selectively placed in different transverse regions of a model membrane system.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Chance B., Radda G. K., Lee C. P. A fluorescence probe of energy-dependent structure changes in fragmented membranes. Proc Natl Acad Sci U S A. 1969 Feb;62(2):612–619. doi: 10.1073/pnas.62.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley K., Cantor C. R. Studies of transfer RNA tertiary structure by singlet-singlet energy transfer. Proc Natl Acad Sci U S A. 1970 Jan;65(1):39–46. doi: 10.1073/pnas.65.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnay L. D., Barry W. H. Turbidity, birefringence, and fluorescence changes in skeletal muscle coincident with the action potential. Science. 1969 Aug 8;165(3893):608–609. doi: 10.1126/science.165.3893.608. [DOI] [PubMed] [Google Scholar]
- Chen R. F., Kernohan J. C. Combination of bovine carbonic anhydrase with a fluorescent sulfonamide. J Biol Chem. 1967 Dec 25;242(24):5813–5823. [PubMed] [Google Scholar]
- Galley W. C., Stryer L. Triplet-singlet energy transfer in proteins. Biochemistry. 1969 May;8(5):1831–1838. doi: 10.1021/bi00833a008. [DOI] [PubMed] [Google Scholar]
- Galley W. C., Stryer L. Triplet-triplet energy transfer in proteins as a criterion of proximity. Proc Natl Acad Sci U S A. 1968 May;60(1):108–114. doi: 10.1073/pnas.60.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler C., Rubalcava B., Caswell A. Fluorescence changes of ethidium bromide on binding to erythrocyte and mitochondrial membranes. Biochim Biophys Acta. 1969;193(2):479–481. doi: 10.1016/0005-2736(69)90208-9. [DOI] [PubMed] [Google Scholar]
- Haugland R. P., Yguerabide J., Stryer L. Dependence of the kinetics of singlet-singlet energy transfer on spectral overlap. Proc Natl Acad Sci U S A. 1969 May;63(1):23–30. doi: 10.1073/pnas.63.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Hundley L., Coburn T., Garwin E., Stryer L. Nanosecond fluorimeter. Rev Sci Instrum. 1967 Apr;38(4):488–492. doi: 10.1063/1.1720743. [DOI] [PubMed] [Google Scholar]
- Kasai M., Podleski T. R., Changeux J. -P. Some structural properties of excitable membranes labelled by fluorescent probes. FEBS Lett. 1970 Mar 16;7(1):13–19. doi: 10.1016/0014-5793(70)80605-6. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Waggoner A. S., Griffith O. H. Spin-labeled mitochondrial lipids in Neurospora crassa. Proc Natl Acad Sci U S A. 1968 Nov;61(3):819–826. doi: 10.1073/pnas.61.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- McClure W. O., Edelman G. M. Fluorescent probes for conformational states of proteins. I. Mechanism of fluorescence of 2-p-toluidinylnaphthalene-6-sulfonate, a hydrophobic probe. Biochemistry. 1966 Jun;5(6):1908–1919. doi: 10.1021/bi00870a018. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Yoo T. J., Johnson M. C., Godt S. M. Fluorescent probes for the study of the antibody-hapten reaction. I. Binding of the 5-dimethylaminonaphthalene-1-sulfonamido group by homologous rabbit antibody. Biochemistry. 1967 Nov;6(11):3408–3416. doi: 10.1021/bi00863a011. [DOI] [PubMed] [Google Scholar]
- Rubalcava B., Martínez de Muñoz D., Gitler C. Interaction of fluorescent probes with membranes. I. Effect of ions on erythrocyte membranes. Biochemistry. 1969 Jul;8(7):2742–2747. doi: 10.1021/bi00835a008. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence spectroscopy of proteins. Science. 1968 Nov 1;162(3853):526–533. doi: 10.1126/science.162.3853.526. [DOI] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Sandlin R., Carnay L. Changes in fluorescence, turbidity, and birefringence associated with nerve excitation. Proc Natl Acad Sci U S A. 1968 Nov;61(3):883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Sandlin R., Carnay L. Changes in fluorescence, turbidity, and birefringence associated with nerve excitation. Proc Natl Acad Sci U S A. 1968 Nov;61(3):883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. C., Brand L. Quantitative estimation of protein binding site polarity. Fluorescence of N-arylaminonaphthalenesulfonates. Biochemistry. 1968 Oct;7(10):3381–3390. doi: 10.1021/bi00850a011. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Ferber E., Selin D., Weidekamm E., Fischer H. The study of lipid-protein interactions in membranes by fluorescent probes. Biochim Biophys Acta. 1970 Mar 17;203(1):67–76. doi: 10.1016/0005-2736(70)90036-2. [DOI] [PubMed] [Google Scholar]
- Wirtz K. W., Zilversmit D. B. Participation of soluble liver proteins in the exchange of membrane phospholipids. Biochim Biophys Acta. 1969 Oct 14;193(1):105–116. doi: 10.1016/0005-2736(69)90063-7. [DOI] [PubMed] [Google Scholar]