Abstract
Background & Aims
The APC tumor suppressor is well known for its ability to regulate Wnt signaling through mediation of β-catenin levels in the cell. Transient over-expression of the tumor suppressor gene APC in colon cancer cells prevents entry into S-phase of the cell cycle, a phenotype only partially restored by co-transfection of a transcriptionally active form of β-catenin. In an attempt to define its transcription-independent tumor suppressor functions, we tested whether APC directly affects DNA replication.
Methods
A transcriptionally quiescent in vitro DNA replication system, the polymerase chain reaction, DNA binding assays, and transient transfections in colon cancer cell lines were used to determine the effects of APC on DNA replication and the mechanism by which it works.
Results
We report that exogenous full-length APC inhibits replication of template DNA through a function that maps to amino acids 2140–2421, a region of the protein commonly lost by somatic or germline mutation. This segment of APC directly interacts with DNA, while mutation of the DNA-binding S(T)PXX motifs within it abolishes DNA binding and reduces inhibition of DNA replication. Phosphorylation of this segment by cyclin-dependent kinases (CDKs) also reduces inhibition of DNA replication. Furthermore, transient transfection of an APC segment encoding amino acids 2140–2421 into a colon cancer cell line with mutant APC prevents cell cycle progression into or through S-phase.
Conclusions
Our results suggest that APC can negatively regulate cell cycle progression through inhibition of DNA replication by direct interaction with DNA.
Keywords: APC, Wnt signaling, DNA replication, DNA binding, CDK, cell cycle
Introduction
Mutation of APC is a rate-limiting event in the development of most colorectal tumors, both inherited and sporadic.1 The APC tumor suppressor gene encodes a large protein of 2843 amino acids, normally expressed in non-proliferating colorectal epithelium, and essential for maintaining normal growth control and differentiation. Loss of APC function is associated with deregulation of several physiologic processes that govern intestinal epithelial cell homeostasis, including cell cycle control, migration, differentiation, and apoptosis.2
APC is a component of the Wnt signaling pathway and is best known for its ability to down-regulate β-catenin and its consequent effects on transcriptional regulation. Mutation of APC alters the transcriptional profile of cells through changes in the expression of genes that encode proteins such as c-myc and cyclin D1 that promote cell growth,3,4 survivin that inhibits apoptosis,5 and matrilysin (MMP-7) that mediates differentiation.6 In addition to its role in regulating β-catenin in Wnt signaling, APC controls cell growth and differentiation through several transcription-independent mechanisms, including effects on cell adhesion, microtubules and chromosome dynamics.7–11
Previous publications have shown that introduction of the APC gene into colon carcinoma cells prevents entry into and/or progression through S-phase of the cell cycle.12–14 Cellular arrest induced by APC is only partially recovered by cotransfection of oncogenic β-catenin.13 These data argue that loss of APC confers broader consequences than deregulation of Wnt signaling and suggest that APC may also function in the transcription-independent inhibition of cellular proliferation. APC is primarily a cytoplasmic protein, but can shuttle between the nucleus and cytoplasm;15 nuclear APC and its export may affect β-catenin localization, turnover, and transcription. For example, nuclear APC counteracts the activation of Wnt target genes by β-catenin.16 Interestingly, APC directly interacts with genomic DNA. Using fragments of recombinant APC protein, Deka et al.17 mapped three DNA binding domains within APC: one within the β-catenin binding and regulatory domain, and two within the carboxyl-terminal third of the APC protein. These domains contain clusters of repetitive S(T)PXX sequence motifs; similar to other S(T)PXX-containing proteins, APC binds preferentially to A/T-rich DNA sequences rather than to a specific DNA sequence.17 Furthermore, the tubulin-binding region of APC shares high similarity and several common features with the microtubule-associated protein Tau.18 Tau, as well as other microtubule-associated proteins, interact with DNA and repress DNA replication.19 These observations led us to investigate the effects of APC on DNA replication.
As the separation of transcriptional effects of APC from its non-transcriptional effects is difficult in cell culture experiments, we used an in vitro system that includes Xenopus laevis egg extract and an exogenously added DNA template to measure the direct effects of APC on DNA replication.20–23 This cell-free in vitro system is transcriptionally and translationally quiescent, as the component eggs are collected during maturation when transcription and translation are not ongoing. Exogenous proteins can be added or endogenous protein depleted to identify those proteins important for DNA replication. Here, we demonstrate that full-length APC protein and a specifically defined carboxyl-terminal segment including amino acids 2140–2421 inhibit DNA replication. This occurs by nonspecifically binding of APC to DNA through S(T)PXX motifs regulated by CDK-dependent phosphorylation. Introduction of this carboxyl-terminal segment into a colon cancer cell line prevents DNA replication.
Material and Methods
DNA constructs
APC cDNA was excised from the pEGFP-APC expression vector13 and subcloned into the yeast expression vector pYES2/NT (Clontech). Segments of APC previously cloned into pEGFP (Clontech) were also excised and subcloned directionally into the pYES2/NT using BamHI and NotI to generate smaller protein-coding segments of APC. Smaller regions of the carboxyl-terminus of APC (APC4) were generated by PCR using the Pfu thermostable DNA polymerase (Stratagene) with primers that inserted BamHI and NotI sites at the 5’ and 3’ ends respectively of each segment, and by subcloning into pYESB. Mutant isoforms of APC4-2 were generated using the PCR-based QuickChange site-directed mutagenesis kit (Stratagene). Primer sequences are listed in supplementary table1.
Protein expression and purification
Recombinant proteins were purified after overexpression in S. cerevisiae strain JEL1 using a procedure described by Qian et al.24 Protein was quantified using Bradford analysis; concentrations of protein samples were equalized using dialysis buffer.
Cell-free in vitro replication assay
X. laevis egg extract for the replication assay was prepared as described in Crowe and Barton.23 X. laevis egg extracts support DNA replication and progression into S-phase upon addition of exogenous DNA template and formation of functional, synthetic nuclear organelles.20, 21, 23 DNA replication assays were carried out in 125 µl reaction volumes containing the following: 500 ng of pBluescript DNA, 18 µg creatine kinase, 1.5 µM creatine phosphate, 1.25 µCi α 32P dCTP, 1.2 µM DTT, 0.5 µM MgCl2, and 12.5 nM EGTA pH 7.2. Replication reactions were carried out at room temperature for 20 minutes, stopped using 30 µl of 10% SDS and 7.5 µl of 20 mg/mL proteinase K, and incubated overnight at 37°C. After phenol/chloroform extractions, reactions were subjected to electrophoresis.
PCR and agarose gel electrophoresis
PCR mixtures (25 µl) contained: 4 mM dNTP, 100ng 5’ and 3’ primer, 1 U of Pfu DNA polymerase, 50 ng of DNA template and APC segments at different concentrations. A 2000 bp DNA fragment encoding human APC 4-1 in the pYESN/T2 vector was used as template. PCR conditions were as follows: one cycle of 95°C for 2 min, 25 cycles of 95°C for 40s, 56°C for 30s, 68°C for 2 min and one final cycle at 68°C for 2 min. Reactions were analyzed by agarose gel electrophoresis.
Electrophoretic mobility shift assays
Gel shift assays were performed as described.17 For agarose gel-shift experiments, 1 µg of plasmid DNA (pBluescript) was incubated with purified proteins for 30 min, electrophoresed in 1.5% agarose and visualized by ethidium bromide-staining. For polyacrylamide gel-shift experiments, an oligonucleotide was 5' end-labeled using 32P-ATP polynucleotide kinase. Varying APC protein concentrations were incubated with 2 nM labeled oligonucleotide (see supplementary table 1) for 30 min on ice. Reaction products were separated on non-denaturing 8% polyacrylamide gels and analysed using a Typhoon 9400 phosphorimager and ImageQuant software.
Kinase assays
In vitro kinase assays used recombinant CDK1/cyclin B, CDK2/cyclinA (New England Biolabs) in 30 µl of manufacturer’s kinase buffer plus 100 µM ATP. Reactions were incubated at 30°C for 30 minutes and terminated. Protein phosphorylation was determined using anti-phospho-threonine-proline mouse mAb (Cell Signaling Technology).
Cell culture, transfections and bromodeoxyuridine labeling
The colorectal cancer cell line SW480 was cultured at 37°C using Dulbecco’s modified Eagle’s medium and 10% FBS (Hyclone). Transfections were performed using Lipofectamine 2000 (Invitrogen) as per manufacturer’s recommendations. BrdU labeling was performed as described.13
Results
APC inhibits DNA replication in vitro in a dose-dependent manner
Previous work has demonstrated the strong inhibitory effects of full-length APC and the shorter β-catenin-binding regions of APC on entry into S-phase of the cell cycle in cancer cell lines.12–14 To identify transcription-independent functions of APC in tumor suppression, we tested whether APC could inhibit DNA replication in vitro. X. laevis egg extracts support DNA replication and progression into and through S-phase upon addition of exogenous template, such as pBluescript, and formation of functional, synthetic nuclear organelles.20,21,23 DNA replication reaches a plateau at approximately two hours and can be measured by incorporation of [32P]--dCTP into newly synthesized DNA. Replicated, labeled DNA is separated by electrophoresis and visualized by autoradiography. This assay has been used successfully by others to define other DNA replication effectors.25, 26, 27
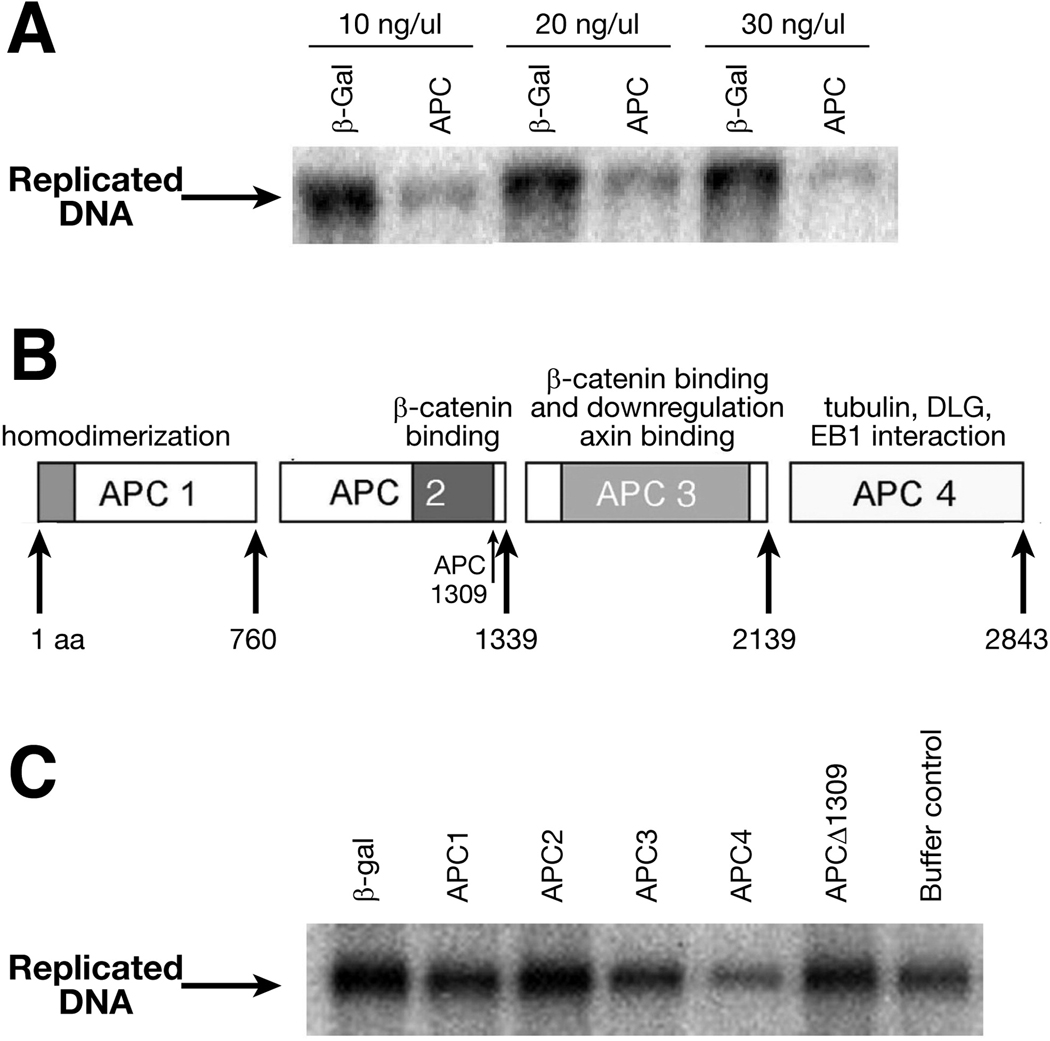
His-tagged full-length APC (2843 amino acids) or β-galactosidase was expressed and purified from a yeast expression system using a nickel column.24 The addition of recombinant full length APC to the X. laevis egg extracts inhibits replication of pBluescript in comparison to recombinant β-galactosidase (Figure 1A). The inhibitory effects of 100 ng, 150 ng and 200 ng of APC were measured by densitometry and found to reduce DNA replication by 57%, 66% and 73%, respectively, to control protein, demonstrating a dose-dependent inhibition of DNA replication. The effect of APC on replication is independent of gene expression, as cyclohexamide is added during the preparation of extracts23.
Figure 1. APC inhibits DNA replication in vitro in a dose-dependent manner.
A. APC inhibits DNA replication in vitro in a dose-dependent manner. Representative results from an in vitro DNA replication assay. Replicated, labeled DNA is separated by electrophoresis and visualized by autoradiography. The final concentration of protein in each assay is indicated above the autoradiogram.
B. Schematic diagram of four nonoverlapping APC segments containing unique amino acids and functional domains.
C. The carboxyl-terminus of APC inhibits DNA replication in vitro. Representative results from an in vitro DNA replication assay. Replicated, labeled DNA is separated by electrophoresis and visualized by autoradiography. The final concentration of proteins in each assay was 200 nM.
To localize the region of APC responsible for this inhibition, we divided APC into four non-overlapping segments that roughly encompass putative functional domains (Figure 1B). Each protein segment, APC1 (1–760), APC2 (761–1339), APC3 (1340–2139) and APC4 (2140–2843), was expressed and purified as previously described.24 A truncated APC protein, designed to mimic a common germline APC mutation in familial adenomatous polyposis coli (FAP) families, was also expressed and purified. This truncated protein (APC1309) lacks the carboxyl-terminal of APC from amino acids 1310 to 2845. Using the cell-free replication assay, only APC4, the carboxyl-terminal segment of APC, inhibited DNA replication, while other segments and truncated APC had no effect (Figure 1 C and D). These results suggest that the region within APC that confers the ability of the tumor suppressor to inhibit DNA replication resides within the carboxyl-terminus. This region of APC is almost uniformly deleted by truncating mutations of APC in most colorectal tumors, sporadic or familial.
Inhibition of DNA replication is determined by amino acids 2140–2421 within the carboxyl-terminus of APC
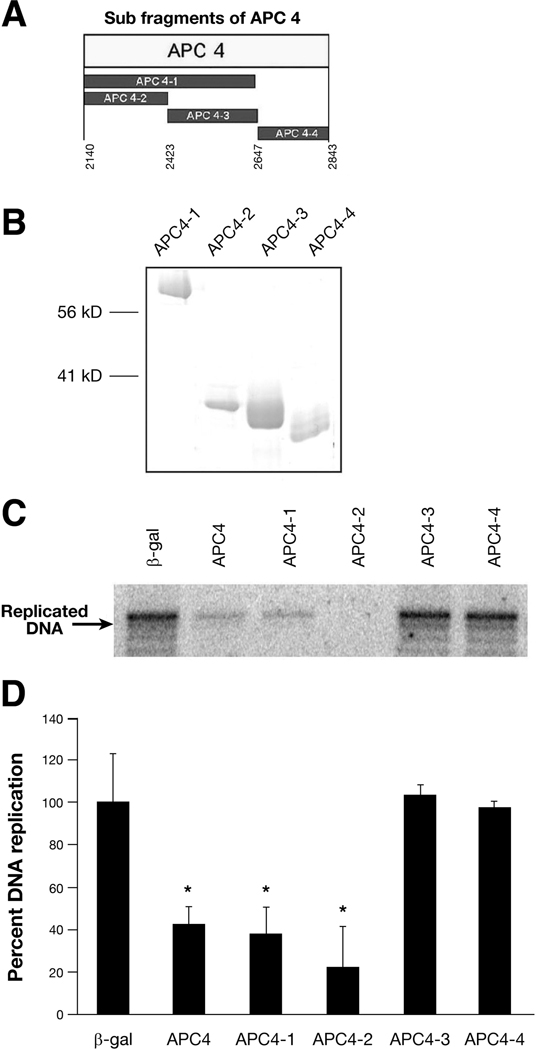
To define further the domain responsible for replication inhibition, the carboxyl-terminal segment of APC was divided into smaller overlapping and non-overlapping segments named APC4-1, 4-2, 4-3, and 4-4 (Figure 2A). These His-tagged proteins were expressed, purified (Figure 2B) and tested for their ability to inhibit DNA replication in vitro (Figure 2C). APC4-1 and APC4-2 inhibited DNA replication as effectively as APC4 (Figure 2 C and D). APC 4-2 produced an average 78% reduction in replication compared with β-galactosidase, as measured by densitometry (22% +/− 18% vs 100% +/−23%) (P<0.05) (Figure 2C and D). The other segments of APC4, APC4-3 and APC4-4, had no effect on DNA replication. Thus, the ability of APC to suppress DNA replication maps to amino acids 2140–2421. Interesting, this domain of APC has similarity to the microtubule-associated protein Tau, known to repress DNA replication.17
Figure 2. Inhibition of DNA replication is conferred by amino acids 2140–2421 within the carboxyl-terminus of APC.
A. Schematic diagram of four APC carboxyl segments containing amino acids are shown below the protein segments.
B. Coomassie blue staining of purified APC segments. Purified proteins were separated by 10% SDS-PAGE and stained with Coomassie bright blue.
C. Representive in vitro cell free DNA replication assay of purified APC segments. Replicated, labeled DNA is separated by electrophoresis and visualized by autoradiography. The final concentration of protein in each assay was 200 nM.
D. Quantitation of the replicated DNAs from three independent experiments, as shown in C. Asterisks denote significant difference (P<0.05 verses β-gal.).
APC amino acids 2140–2421 inhibit DNA replication by directly binding to DNA
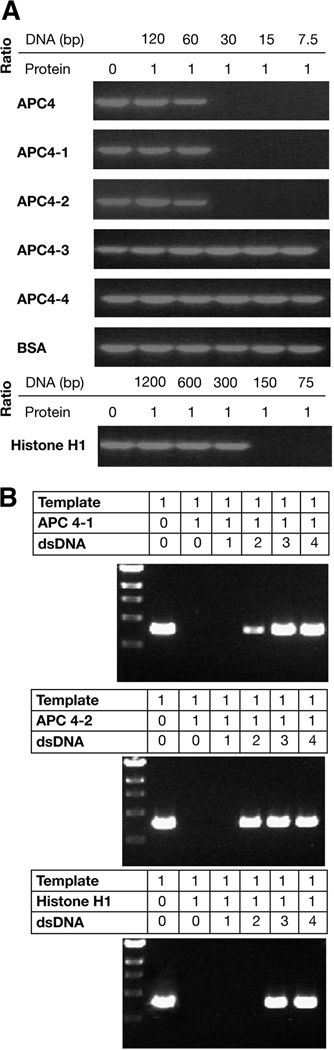
The conservation of mechanism between DNA amplification by the polymerase chain reaction (PCR) and semi-conservative DNA replication allows PCR amplification to be used as a simple method of determining the role of a given protein in replication.19 Therefore, we used PCR to simulate DNA replication to validate and extend our previous experiments. Purified APC segments were added to PCR mixtures before initiation by enzyme addition. Histone H1, a DNA-binding protein that binds to DNA nonspecifically to represses both DNA replication and transcription, was used as a positive control. Separation of PCR products by agarose gel electrophoresis shows that the yields of synthesized DNA decrease as the quantitative ratio ([Protein]/[DNA]) of APC 4, APC 4-1 and APC 4-2 to template DNA increase (Figure 3A). In contrast, APC 4-3 and APC 4-4 have no effect on DNA synthesis in PCR (Figure 3A).
Figure 3. The carboxyl-terminus of APC (amino acids 2140–2421) inhibits the polymerase chain reaction (PCR) by directly affecting the DNA template.
A. Agarose gel electrophoregsis of PCR products generated in the presence of APC carboxyl terminal segments at different quantitative ratios of [protein]/[DNA (bp)] (ratios between the number of protein molecules and number of DNA base pairs). Histone H1 and BSA were used as positive and negative controls, respectively. PCR products were separated by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
B. Generation of PCR products is restored by addition of competitive dsDNA. The generation of PCR products was gradually restored when competitive dsDNA was added to the PCR mixture and bound to the repressing protein. The quantitative ratios of [DNA template], [protein] and [competitive DNA] are shown above each panel. The quantitative ratios of [DNA template]:[protein]:[competitive DNA]=1:1:1 are equivalent to 20 (DNA) bps: 1 protein molecule: 20 (DNA) bps for APC 4-1 and APC 4-2, and to 75 (DNA) bps: 1 protein molecule: 75 (DNA) bps for histone H1.
To investigate the mechanism by which the APC carboxyl-terminus inhibits DNA duplication in vitro, we tested whether DNA replication is inhibited by the interaction of APC and DNA or by the interaction of APC and DNA polymerase. In competitive PCR, using threshold conditions in which DNA replication is completely repressed, the addition of competitor dsDNA gradually rescues repression and permits the generation of PCR products (Figure 3B). Competitor dsDNA, interacting with APC, releases the DNA template from associated APC fragments. Under similar conditions, histone H1 is bound to competitor dsDNA and dissociated from the DNA template to permit PCR reaction (Figure 3B). These results suggest that the repression of DNA replication is due to direct DNA-binding of APC protein fragments, but not to interactions between APC and polymerase, either directly or indirectly.
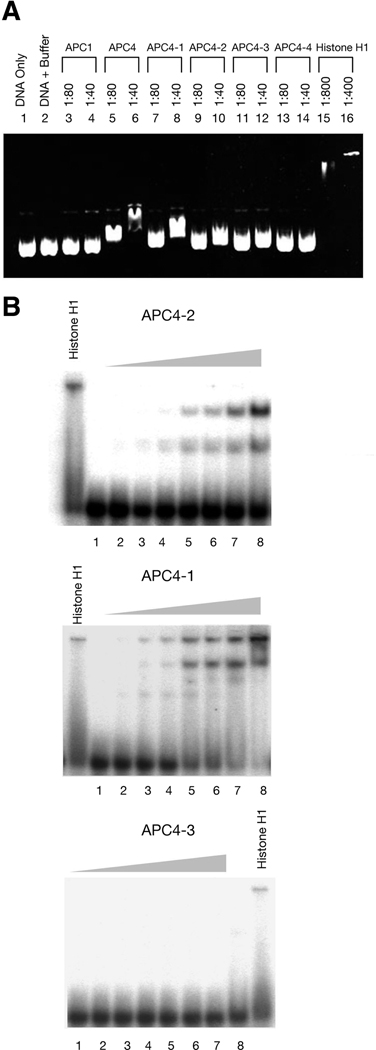
Considering that APC can bind to nonspecific DNA sequences,17 we hypothesized that APC inhibits DNA replication via its carboxyl-terminus by directly binding to DNA. To confirm this, we evaluated the ability of APC to alter the electrophoretic mobility of plasmid DNA through an agarose gel in the presence of purified APC protein fragments. In this assay, DNA mobility was retarded in the presence of three APC fragments: APC4, APC4-1, and APC4-2, but not in the presence of APC4-3 or 4-4 (Figure 4A). We also tested the binding of APC segments to different radiolabelled oligonucleotides using polyacrylamide gel electrophoresis. The electrophoretic mobility gel-shift assay clearly revealed the binding of APC4-1 or APC4-2 but not APC4-3 to single-strand (Figure 4B) and double-strand DNA (data not shown).
Figure 4. APC binds directly to DNA.
A. Agarose gel electrophoresis of pBluescript plasmids with recombinant proteins. Plasmid DNA was incubated in the absence (control) and presence of the corresponding proteins at different quantitative ratios, separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining. The quantitative ratios of [DNA]/[protein] shown above each panel represent the ratios between the number of DNA base pairs and the number of protein molecules.
B. Gel mobility shifts of radioactively labeled oligonucleotides. A radiolabelled 68-base single-stranded oligonucleotide (2 nM) was incubated with protein as noted, separated by 8% PAGE, and analyzed by phosphorimager. The protein concentrations from lane 1 to 8 are: 0, 18.75, 37.5, 75, 150, 200, 250, 300 nM, respectively.
Mutation of the S(T)PXX binding motifs located within amino acids 2140–2421 reduces the inhibition of DNA replication
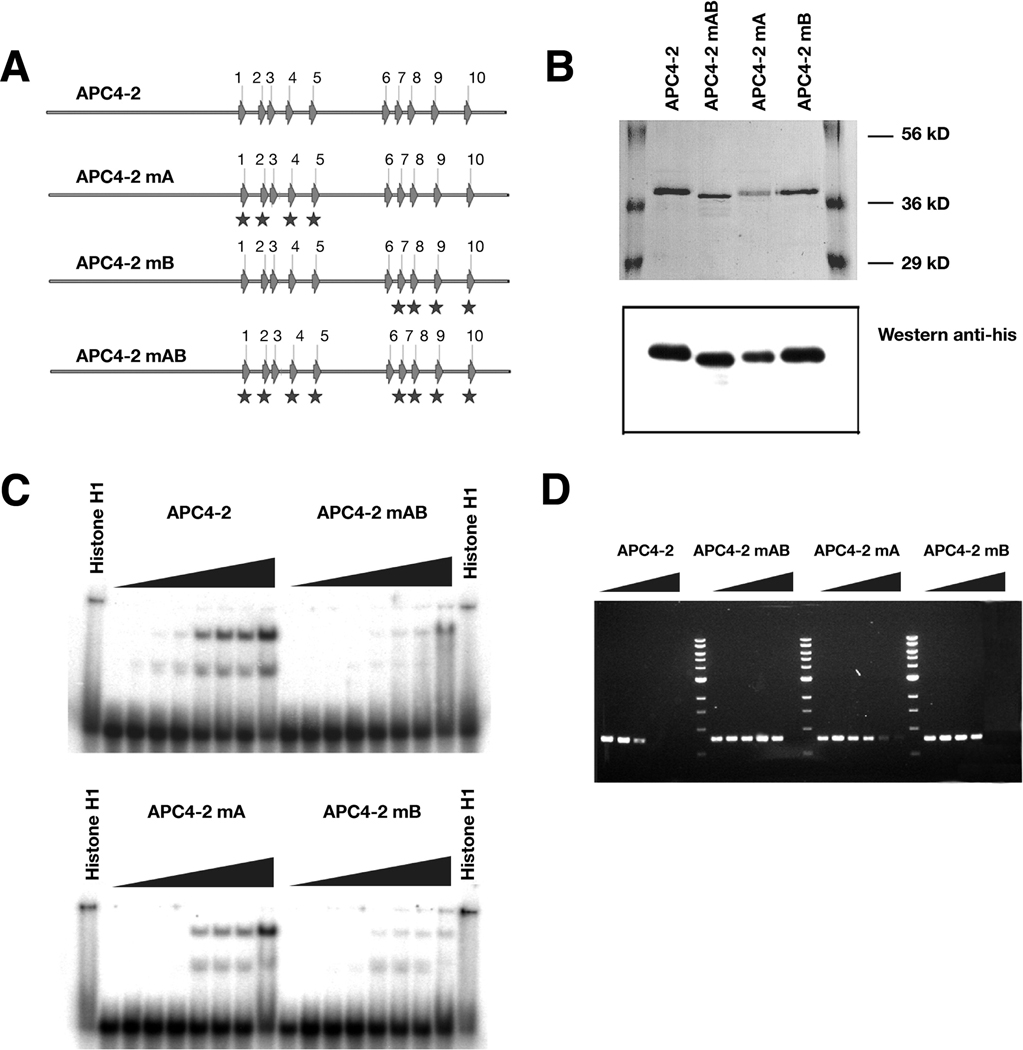
Clusters of so-called S(T)PXX motifs are often found in DNA-binding proteins like histone H1, RNA polymerase, and gene regulatory proteins.17 Full-length APC contains a total of 51 S(T)PXX motifs (43 SPXX and 8 TPXX, with at least one X as K, R, Y, S, T, A, L or P) that comprise five DNA binding clusters of S(T)PXX motifs; two proximate repetitive motifs are necessary for DNA binding.17 These five S(T)PXX clusters are located within amino acids 1360–1383 (4 S(T)PXX motifs), 2244–2286 (5 S(T)PXX motifs), 2323–2371 (5 S(T)PXX motifs), 2449–2488 (5 S(T)PXX motifs), and 2760–2792 (3 S(T)PXX motifs). There are two clusters of five S(T)PXX motifs located within the DNA replication-inhibitory domain of amino acids 2140–2421 (Figure 5A).
Figure 5. Mutation of DNA-binding motifs within APC impede DNA binding and inhibition of replication.
A. Schematic diagram of S(T)PXX binding motifs within APC 4-2 and the sites altered by mutations. Mutation sites are indicated with stars.
B. Coomassie blue staining and western blotting of purified mutant APC segments. Purified proteins were separated by 10% SDS-PAGE, stained with Coomassie bright blue (upper panel) and probed with a monoclonal anti-His antibody (lower panel).
C. Gel mobility shifts of radioactively labeled oligonucleotides in the presence of wild-type or mutant APC4-2. A radiolabelled 68-base single-stranded oligonucleotide (2 nM) was incubated with the corresponding proteins, separated by 8% PAGE and analyzed by phosphorimager. The protein concentrations from low to high are: 0, 18.75, 37.5, 75, 150, 200, 250, and 300 nM, respectively.
D. Agarose gel electrophoresis of PCR products prepared in the presence of wild-type or mutant APC4-2 at different quantitative ratio of [protein]/[DNA (bp)] as Figure 3A. PCR products were separated using 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
To determine whether these motifs affect the direct interaction of APC with DNA and are required for its inhibitory effect on replication, we tested the ability of mutated APC4-2 to alter DNA replication. APC4-2 segments with S(T)PXX motifs in either or both clusters were changed to AAXX motifs by site-direct mutagenesis as shown in Figure 5A (APC4-2mA, APC4-2mB and APC4-2mAB). His-tagged APC4-2 mutants were expressed and purified from yeast. The proteins were shown by Coommasie blue staining to be >90% purified and validated by western blotting (Figure 5B). Compared with wild-type APC4-2, the mutated APC4-2 proteins had significantly reduced binding affinities for DNA and decreased inhibitory effects on DNA replication (Figure 5C and D). Interestingly, the reduction of binding affinity and effects on DNA replication are proportional to the number of sites mutated within the clusters of S(T)PXX motifs.
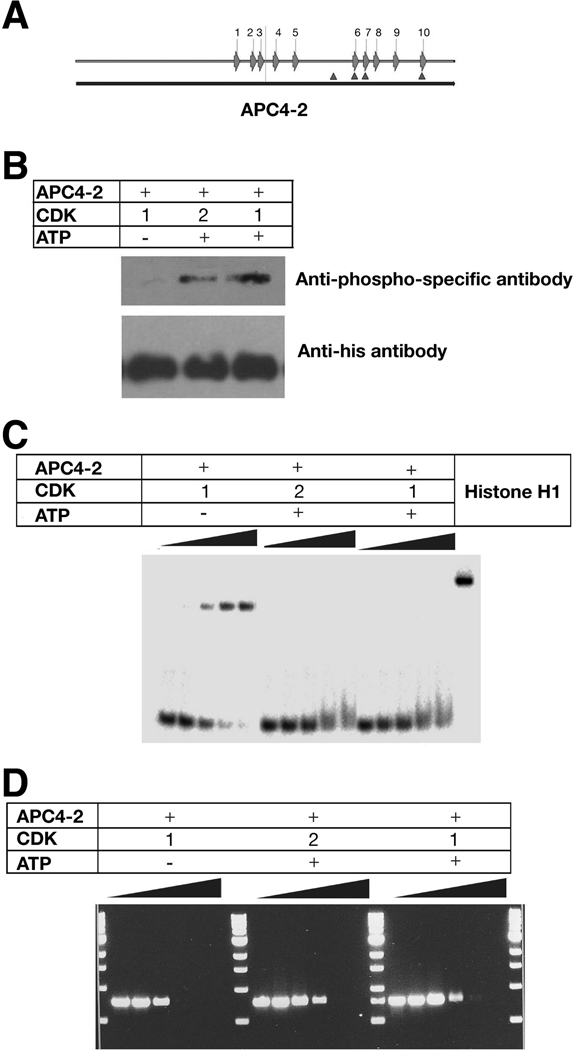
Phosphorylation of APC 4-2 by cyclin dependent kinase (CDK) reduces the inhibition of DNA replication
APC is a phosphoprotein that can be phosphorylated by many kinases, including GSK3β, MAPK, cyclin-dependent kinases (CDK), protein kinase A, casein kinase I and II, and calmodulin kinase.2 Eleven cyclin-dependent kinase (CDK) consensus phosphorylation sites (S/T)PX(R/K) are present in full length APC,28 four of which within amino acids 2140–2421, and three of which overlapping the secondary cluster of S(T)PXX motifs (Figure 6A and Supplementary Figure 2). As proteins with S(T)PXX motifs exhibit reduced binding to DNA when modified by phosphorylation of Ser or Thr residues,29 we speculated that phosphorylation of APC by CDKs may regulate the ability of APC to bind DNA and subsequently alter DNA replication. To test this, APC4-2 was phosphorylated in vitro by recombinant CDK, and tested for its ability to bind DNA and affect replication. In the presence of ATP, purified APC4-2 was phosphorylated by both CDK1 or CDK2 in vitro (Figure 6B). Phosphorylated APC4-2 reduced its binding to DNA and its inhibition of PCR (Figure 6C and D). The inhibition of DNA replication by APC can be repressed by CDK phosphorylation.
Figure 6. CDK phosphorylation of APC reduces DNA binding and inhibition of replication.
A. Schematic diagram of S(T)PXX binding motifs and CDK consensus phosphorylation sites within APC4-2. Arrows represent S(T)PXX binding motifs and triangles represent CDK consensus phosphorylation sites.
B. Western results of APC 4-2 phosphorylation by CDKs. Purified APC4-2 proteins were incubated with CDK1 or 2 in the absence (control) and presence of ATP, separated by 10% SDS-PAGE, and probed with a anti-phospho-threonine-proline monoclonal antibody (upper panel) or a monoclonal anti-His antibody (lower panel).
C. Gel mobility shifts of radioactively labeled oligonucleotides in the presence of phosphorylated or nonphosphorylated APC4-2. A radiolabelled 60-base single-stranded oligonucleotide (2 nM) was incubated with the corresponding proteins, separated by 8% PAGE and analyzed by phosphorimager. The protein concentrations for each sample are: 0, 50, 75, 100, and 125 nM, respectively.
D. Agarose gel electrophoresis of PCR products prepared in the presence of phosphorylated or nonphosphorylated APC4-2 at different quantitative ratio of [protein]/[DNA] as in Figure 3A. PCR products were separated using 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
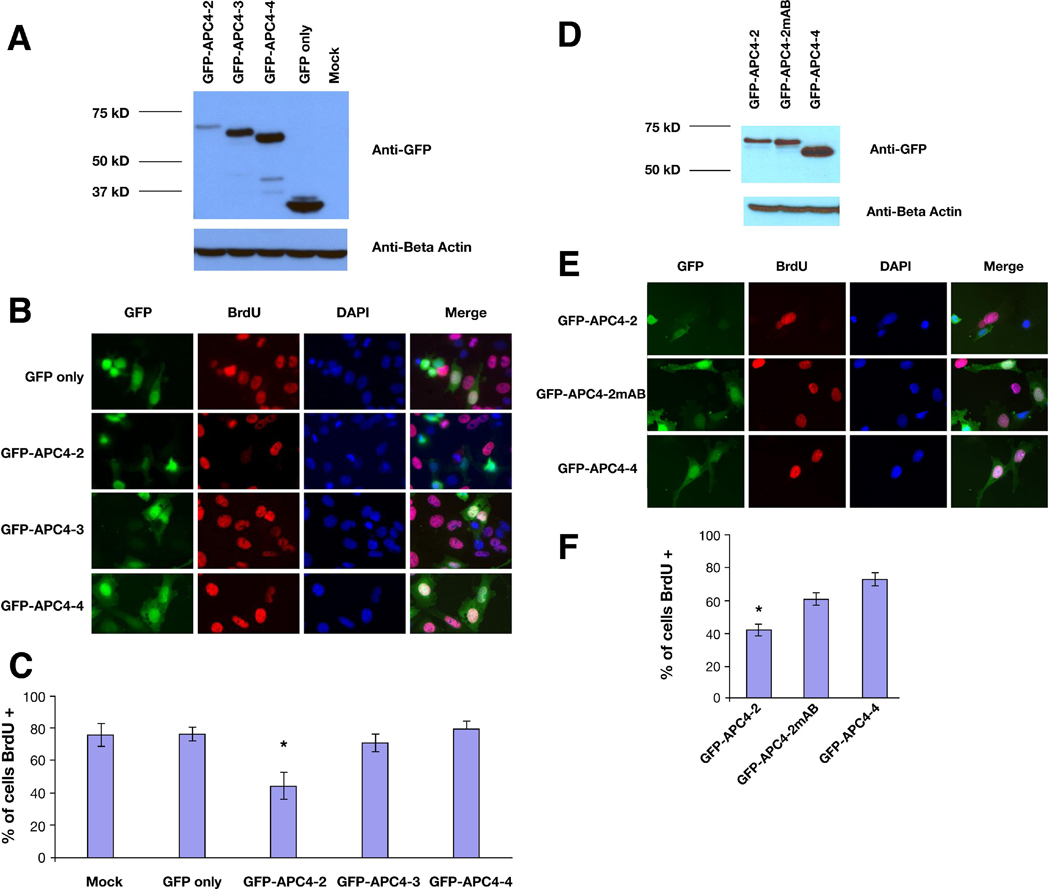
Overexpression of APC 4-2 negatively regulates cell cycle progression from the G1 to S-phase
To test the effects of the carboxyl-terminus of APC on DNA replication and cell cycle progression in cultured cells, a colon cancer cell line (SW480) expressing truncated APC (1–1309) was transfected with GFP-tagged carboxyl-terminal segments of APC or GFP alone, and cultured in medium including bromodeoxyuridine (BrdU) as a marker for DNA replication and S-phase entry and progression. All GFP-tagged carboxyl-terminal segments were expressed after transfection, although GFP-tagged APC4-2 expressed less than GFP-tagged APC4-3 or APC4-4 (Figure 7A and D). BrdU was added 24 hours after transfection and cells were grown another 16 hours before fixation. Cells were stained with an antibody specific for BrdU and evaluated by fluorescence microscopy (Figure7B). While the majority of cells transfected with GFP, GFP-APC4-3 or GFP-APC4-4 displayed clear BrdU staining indicative of DNA synthesis (75.7% +/− 7.1%, 71.0% +/− 5.6%, 79.7% +/− 4.9%, respective), a significantly lower percentage of cells transfected with GFP-APC4-2 (44.3% +/− 8.5%) stained positive for BrdU (P <0.05 compared with GFP control) (Figure 7C). In contrast, cells transfected with GFP-APC4-2mAB, encoding the APC4-2 segment with mutated S(T)PXX motifs in both clusters (Figure 5A), displayed a higher percentage of BrdU staining than those transfected with GFP-APC4-2 (60.9% +/− 3.4% verses 42.2% +/−3.4%, P<0.05). These results demonstrate that APC4-2 can alter cell cycle progression by inhibiting DNA replication via DNA binding.
Figure 7. Overexpression of APC 4-2 negatively regulates cell cycle progression from G1- to S-phase.
A and D. Lysates from SW480 cells transfected with GFP, GFP-APC4-2, GFP-APC4-2mAB, GFP-APC4-3 and GFP-APC4-4 were examined by western blotting using an anti-GFP antibody.
B and E. SW480 cells transfected with GFP, GFP-APC4-2, GFP-APC4-2mAB, GFP-APC4-3 or GFP-APC4-4 were cultured in medium containing BrdU for 16 hours. The cells were stained with an anti-BrdU antibody and examined by fluorescent microscopy. Magnification, 400X.
C and F. The percentage of BrdU-positive cells was calculated by counting at least 100 cells for each of three experiments. Values are the mean+/−SE of these three experiments. Each bar represents BrdU incorporation for the cells transfected as indicated on the X-axis. The asterisks denotes a significant difference (P<0.05 verses GFP only, P<0.05 verses GFP-APC4-2mAB).
Conclusions
The APC tumor suppressor is remarkably versatile, with multiple functions and distinct subcellular destinations. The relationship between the subcellular localization of APC and its role as a tumor suppressor is not well understood but is of significant interest. Although the majority of APC resides in the cytoplasm, normal but not mutant APC has been detected in the nuclei of some epithelial cell types.30 Consistently, two functional nuclear localization signals (NLS) and four nuclear export signals (NES) have been identified within APC.31, 32 APC shuttling between the nucleus and cytoplasm may actively transport β-catenin and therefore provide a more finely tuned mechanism to regulate β-catenin degradation or control of transcription.33 32, 34 In addition, nuclear APC associates with DNA; the consequences of this association remain elusive, although specific APC binding to the promoter of the C-Myc proto-oncogene has been observed.16 Our data demonstrate that APC inhibits DNA replication in a transcription-independent manner by directly binding to DNA. This activity maps to a region of the protein distinct from that responsible for β-catenin binding and down-regulation, separating this activity from its role in Wnt signaling and suggesting a novel tumor suppressor function of APC.
Mutation of the APC tumor suppressor gene occurs in the development of most colorectal tumors.2 The majority of mutations in APC are chain-terminating and confined to its central region called the mutation cluster region (MCR); these mutations result in the expression of truncated proteins lacking the normal carboxy-terminus.2 These truncated proteins lack the domains of APC responsible for interactions with axin, β-catenin, microtubules, the microtubule-binding protein EB1, SIAH-1, and DLG-1.35,36,9,37,38–40 Our results map the DNA replication-inhibiting effect of APC to the carboxyl-terminus, suggesting that the loss of this ability contributes to tumorigenesis associated with APC mutation by truncation. Although a small percentage of colorectal tumors are characterized by activating mutations of β-catenin rather than loss-of-function of APC, these tumors may carry other gene mutations or signaling pathway alterations that affect DNA replication.
Our studies further delineate the domain responsible for DNA replication inhibition to amino acids 2140–2421 in the carboxyl-terminus. This region of APC is enriched in basic amino acids, with a high content of arginine, lysine and proline residues in a stretch of approximately 200 amino acids between residues 2200 and 2400. This region also mediates the direct interaction of APC with microtubules.41, 42 In addition, amino residues from 2244 to 2312 show high similarity to Tau,18 a microtubule-associated protein capable of repressing DNA replication by binding to DNA.19 Therefore, APC may inhibit DNA replication in a manner similar to Tau. The overlap of the DNA-binding and microtubule-binding domain of APC raises intriguing questions. Is the binding competitive or synergistic? Does the interaction with microtubules or DNA regulate the shuttling of APC between the nucleus and the cytoplasm? If so, what is the regulation mechanism? All these questions require further investigation.
S(T)PXX motifs bind preferentially to A/T-rich DNA sequences and require at least two proximal repetitive motifs for DNA binding.29,43 S(T)PXX motifs may neutralize the negative charges on the inside of a DNA superhelix and thus stabilize superhelical bending. Consistent with this, mutation of the S(T)PXX motifs within APC4-2 may diminish the ability of the motifs to neutralize negative charges of DNA, as DNA binding and DNA replication inhibition are dramatically reduced. Another advantage of S(T)PXX motifs for DNA binding is that the strength of this interaction can be modified by serine or threonine phosphorylation.29 Four CDK consensus phosphorylation sites (S/T)PX(R/K) are located within amino acids 2140–2421 of APC; three sites overlap the S(T)PXX motifs. Phosphorylation of APC4-2 by CDKs weakens DNA binding and replication inhibition, suggesting that the APC-mediated DNA replication repression may be regulated by CDKs during the cell cycle. Consistant with this, cyclin-CDK inhibits the cell-cycle blocking activity of APC following co-microinjection into NIH 3T3 cells.29
Our data show that the APC tumor suppressor inhibits DNA replication via its carboxyl-terminus and by direct binding to DNA, although we cannot completely eliminate the possibility that APC may also interact with proteins critical to replication or that regulate DNA replication. Previous work by our group and others has shown that APC inhibits cell growth by blocking entry into and/or progression through S-phase of the cell cycle.12, 13 APC controls entry into S-phase partially through its ability to regulate the cyclin D/RB pathway by disrupting β-catenin-mediated transcription.3, 4, 13, 14 Other lines of evidence suggest that APC also affects proliferation in a β-catenin-independent manner. First, cell cycle arrest caused by overexpression of APC in the colorectal cancer cell line SW480 cannot be completely counteracted by oncogenic β-catenin or relieved by a dominant negative TCF transcription factor.13 Second, β-catenin activity is presumably not affected by APC in the colorectal cancer cell line HCT116 that contains wild-type APC and oncogenic β-catenin, yet the addition of exogenous APC affects cell growth in these cells.13 Third, the ability of APC to block cell cycle progression from G0/G1 to S-phase in NIH3T3 cells, presumably wild-type at the mAPC locus, is independent of RB and, by extension, β-catenin12. Fourth, disruption of the APC/hDLG (the human homologue of the Drosophila tumor suppressor Dlg) complex, independent of β-catenin, interferes with the ability of APC to block cell cycle progression in NIH3T3 cells,44 although it remains unknown whether hDLG functions downstream of APC. Additionally, dominant negative mutant hDLGs only partially block APC-induced cell cycle arrest, arguing for the existence of other β-catenin-and hDLG-independent effect(s) of APC on cell cycle progression.44 Our cell culture assay using BrdU labeling shows that reintroduction of amino acids 2140–2421 of APC into SW480 cells partially restores the negative regulation of full length APC on cell cycle progression from the G1- through S-phase. These results support the idea that another tumor suppressor function of APC may be to regulate DNA replication directly.
Supplementary Material
Acknowledgments
This work was supported by NIH Awards CA-89403 (AML), CA-63507 (JG), and CA53683 (MCB), and a grant from Ohio Cancer Research Associates (AML).
Abbreviations
- CDK
cyclin-dependent kinase
- DLG
discs large protein
- EB1
end binding protein1
- FAP
familial adenomatous polyposis coli
- GSK3β
glycogen synthetase kinase 3β
- Ni-NTA
nickel-nitrilotriacetic acid
- CDK
cyclin-dependent kinase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist.
References
- 1.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 2.Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol. 2000;18:1967–1979. doi: 10.1200/JCO.2000.18.9.1967. [DOI] [PubMed] [Google Scholar]
- 3.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 4.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 5.Zhang T, Otevrel T, Gao Z, et al. Evidence that APC regulates survivin expression: A possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664–8667. [PubMed] [Google Scholar]
- 6.Crawford HC, Fingleton BM, Rudolph-Owen LA, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 7.Nathke IS, Adams CL, Polakis P, et al. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan KB, Burds AA, Swedlow JR, et al. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen MM, Tucker JB, Mackie JB, et al. The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells. J Cell Biol. 2002;157:1041–1048. doi: 10.1083/jcb.200203001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamada F, Bienz M. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear β-catenin from TCF. Dev Cell. 2004;7:677–685. doi: 10.1016/j.devcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Dikovskaya D, Newton IP, Nathke IS. The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts. Mol Biol Cell. 2004;15:2978–2991. doi: 10.1091/mbc.E03-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeg GH, Matsumine A, Kuroda T, et al. The tumour suppressor gene product APC blocks cell cycle progression from G0/G1 to S phase. EMBO J. 1995;14:5618–5625. doi: 10.1002/j.1460-2075.1995.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinen CD, Goss KH, Cornelius JR, et al. The APC tumor suppressor controls entry into S-phase through its ability to regulate the cyclin D/RB pathway. Gastroenterology. 2002;123:751–763. doi: 10.1053/gast.2002.35382. [DOI] [PubMed] [Google Scholar]
- 14.Carson DJ, Santoro IM, Groden J. Isoforms of the APC tumor suppressor and their ability to inhibit cell growth and tumorigenicity. Oncogene. 2004;23:7144–7148. doi: 10.1038/sj.onc.1207954. [DOI] [PubMed] [Google Scholar]
- 15.Brocardo M, Nathke IS, Henderson BR. Redefining the subcellular location and transport of APC: New insights using a panel of antibodies. EMBO Report. 2005;2:184–190. doi: 10.1038/sj.embor.7400329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierra J, Yoshida T, Joazeiro CA, et al. The APC tumor suppressor counteracts β-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20:586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deka J, Herter P, Sprenger-Haussels M, et al. The APC protein binds to A/T rich DNA sequence. Oncogene. 1999;18:5654–5661. doi: 10.1038/sj.onc.1202944. [DOI] [PubMed] [Google Scholar]
- 18.Deka J, Kuhlmann J, Muller O. A domain within the tumor suppressor protein APC shows very similar biochemical properties as the microtubule-associated protein Tau. Eur J Biochem. 1998;253:591–597. doi: 10.1046/j.1432-1327.1998.2530591.x. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Wang XS, Qu MH, et al. Human protein Tau represses DNA replication in vitro. Biochem Biophy Acta. 2005;1726:280–286. doi: 10.1016/j.bbagen.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Blow JJ, Laskey RA. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986;47:577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- 21.Newport J. Nuclear reconstitution in vitro: Stages of assembly around protein-free DNA. Cell. 1987;48:205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- 22.Newmeyer DD, Wilson KL. Egg extracts for nuclear import and nuclear assembly reactions. Meth Cell Biol. 1991;36:607–634. doi: 10.1016/s0091-679x(08)60299-x. [DOI] [PubMed] [Google Scholar]
- 23.Crowe AJ, Barton MC. In vitro reconstitution of nuclei for replication and transcription. Methods in Enzymology. 1999;304:63–76. doi: 10.1016/s0076-6879(99)04007-0. [DOI] [PubMed] [Google Scholar]
- 24.Qian J, Steigerwald K, Combs KA, et al. Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis. Oncogene. 2007;26:4872–4876. doi: 10.1038/sj.onc.1210265. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter PB, Mueller PR, Dunphy WG. Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature. 1996;379:357–360. doi: 10.1038/379357a0. [DOI] [PubMed] [Google Scholar]
- 26.Coleman TR, Carpenter PB, Dunphy WG. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 27.McGarry TJ, Kirschner MW. Germinin, a inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 28.Trzepacz C, Lowy AM, Kordich JJ, et al. Phosphorylation of the tumor suppressor adenomatous polyposis coli (APC) by the cyclin-dependent kinase p34. J Biol Chem. 1997;272:21681–21684. doi: 10.1074/jbc.272.35.21681. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M. SPKK, a new nucleic acid-binding unit of protein found in histone. EMBO J. 1989;8:797–804. doi: 10.1002/j.1460-2075.1989.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neufeld KL, White RL. Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc Natl Acad Sci USA. 1997;94:3034–3039. doi: 10.1073/pnas.94.7.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, White RL, Neufeld KL. Phosphorylation near nuclear localization signal regulates nuclear important of adenmoatous polyposis coli protein. Proc Natl Acad Sci USA. 2000;97:12577–12582. doi: 10.1073/pnas.230435597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosin-Arbesfeld, Townsley RF, Bienz M. The APC tumor suppressor has a nuclear export function. Nature. 2000;406:1009–1012. doi: 10.1038/35023016. [DOI] [PubMed] [Google Scholar]
- 33.Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nature Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- 34.Neufeld KL, Nix DA, Bogerd H, et al. Adenomatous polyposis coli tumor suppressor coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc Natl Acad Sci USA. 2000;97:12085–12090. doi: 10.1073/pnas.220401797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison EE, Wardleworth BN, Askham JM, et al. EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- 36.Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- 37.Su LK, Burrell M, Hill DE, et al. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- 38.Tirnauer JS, Bierer BE. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol. 2000;149:761–766. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Stenens J, Rote CA, et al. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell Biol. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 40.Matsumine A, Ogai A, Senda T, et al. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- 41.Munemitsu S, Souza B, Muller O, et al. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- 42.Smith KJ, Levy DB, Maupin P, et al. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–3675. [PubMed] [Google Scholar]
- 43.Churchill ME, Suzuki M. ‘SPKK’ motifs prefer to bind to DNA at A/T-rich sites. EMBO J. 1989;8:4189–4195. doi: 10.1002/j.1460-2075.1989.tb08604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishidate T, Matsumine A, Toyoshima K, et al. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene. 2000;19:365–372. doi: 10.1038/sj.onc.1203309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.