Abstract
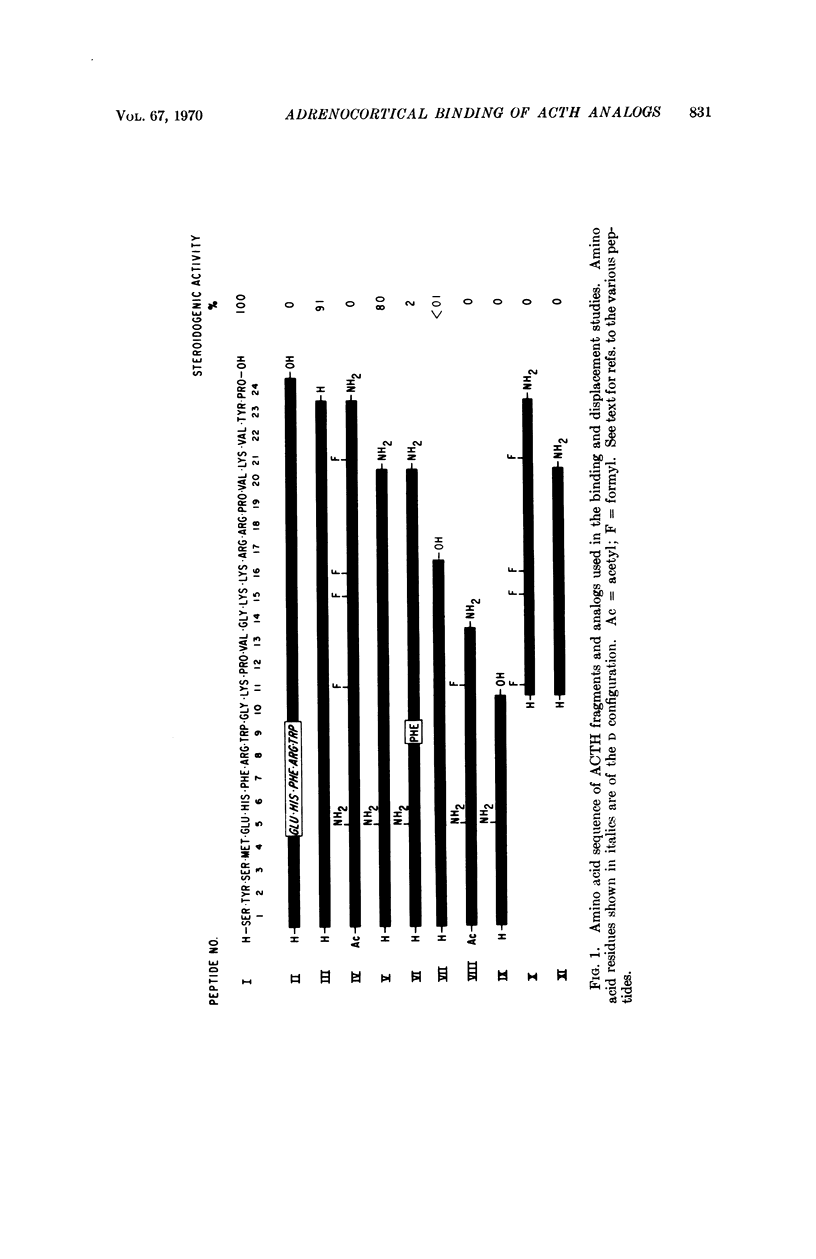
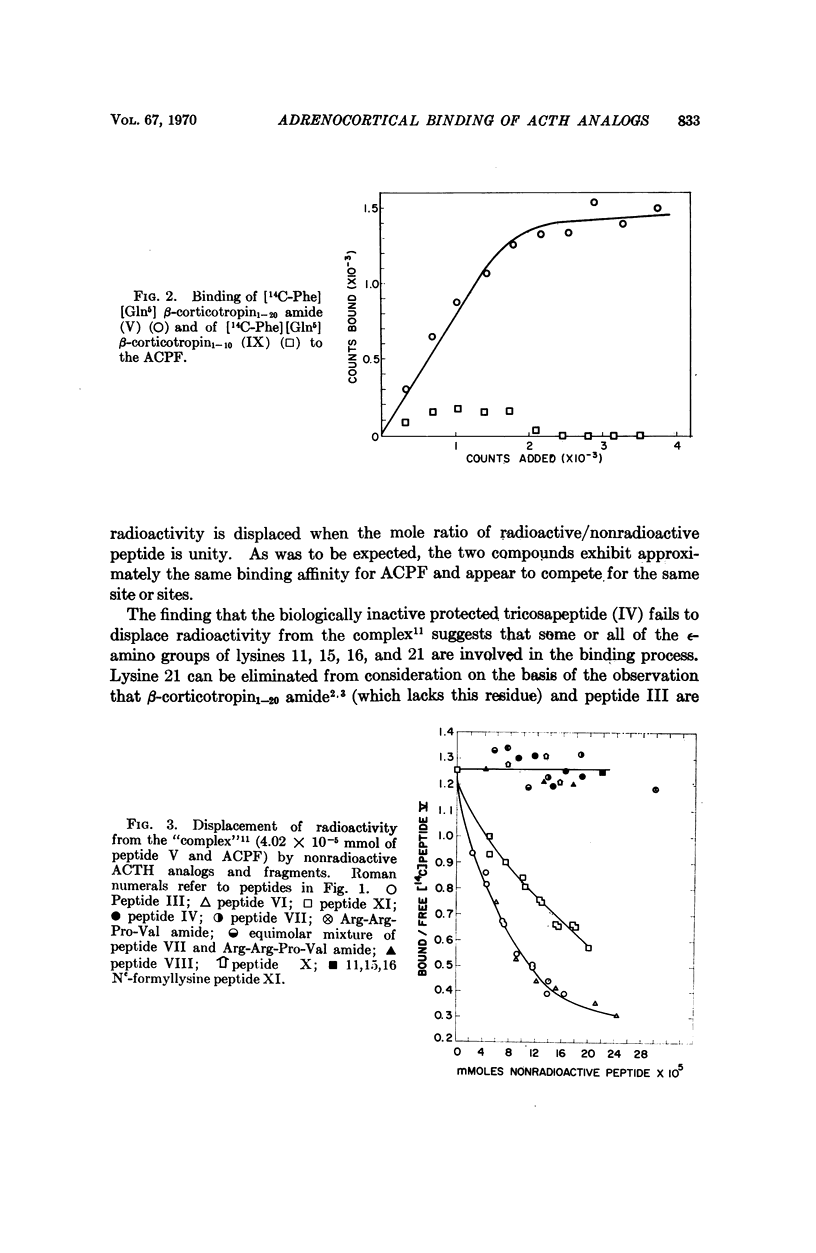
This study deals with the interaction of polypeptide hormones with their receptors. Specifically it involves the binding of synthetic ACTH analogs and fragments to a particulate fraction from beef adrenal cortical tissue. This fraction was found to bind synthetic [14C-Phe] [Gln5]β-corticotropin1-20 amide but failed to bind significant amounts of [14C-Phe] [Gln5]β-corticotropin1-10. The former peptide exhibits a high degree in vivo adrenocorticotropic activity in the rat; the latter is inactive. [14C-Phe] [Gln5]β-corticotropin1-20 amide exhibited little affinity for similarly prepared particulates from beef kidney, liver, or adrenal medulla. Using a series of synthetic homogeneous nonradioactive analogs or fragments of β-corticotropin1-20 amide to displace [14C-Phe] [Gln5]β-corticotropin1-20 amide from the particulate fraction a significant correlation between binding and in vivo adrenocorticotropic activity was established. Major „active” and „binding” sites were shown to reside in different sections of the ACTH molecule. Charged groups play a major role in the attachment of ACTH to the particulate fraction and the sequence Lys-Lys-Arg-Arg proved to be particularly significant. The high degree of binding specificity for ACTH peptides which is exhibited by the particulate suggests that it contains ACTH receptor(s). Moreover, the striking similarity between observations with the S-peptide-S-protein model system and the system described would seem to indicate that the ACTH receptor is a protein.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- DEDMAN M. L., FARMER T. H., MORRIS C. J. Studies on pituitary adrenocorticotrophin. 3. Identification of the oxidation-reduction centre. Biochem J. 1961 Feb;78:348–352. doi: 10.1042/bj0780348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- FINN F. M., HOFMANN K. STUDIES ON POLYPEPTIDES. 33. ENZYMIC PROPERTIES OF PARTIALLY SYNTHETIC RIBONUCLEASES. J Am Chem Soc. 1965 Feb 5;87:645–651. doi: 10.1021/ja01081a043. [DOI] [PubMed] [Google Scholar]
- Geiger R., Schröder H. G., Siedel W. Synthetische Analoga des Corticotropins. Veränderung der N-terminalen Amino-Gruppe des Corticotropin (1-23)-trikosipeptid-amids. Justus Liebigs Ann Chem. 1969;726:177–187. doi: 10.1002/jlac.19697260125. [DOI] [PubMed] [Google Scholar]
- HOFMANN K. Preliminary observations relating structure and function in some pituitary hormones. Brookhaven Symp Biol. 1960 Nov;13:184–202. [PubMed] [Google Scholar]
- Hofmann K., Andreatta R., Bohn H., Moroder L. Studies on polypeptides. XLV. Structure-function studies in the beta-corticotropin series. J Med Chem. 1970 May;13(3):339–345. doi: 10.1021/jm00297a001. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Finn F. M., Limetti M., Montibeller J., Zanetti G. Studies on polypeptides. XXXIV. Enzymic properties of partially synthetic De(16-20)- and De(15-20)-ribonucleases S'1-3. J Am Chem Soc. 1966 Aug 5;88(15):3633–3639. doi: 10.1021/ja00967a030. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Visser J. P., Finn F. M. Studies on polypepides. XLIV. Potent synthetic S-peptide antagonists. J Am Chem Soc. 1970 May 6;92(9):2900–2909. doi: 10.1021/ja00712a049. [DOI] [PubMed] [Google Scholar]
- Jenny M., Muller A. F., Mach R. S. The adrenocorticotropic action of a new synthetic pentacosapeptide. Experientia. 1966 Aug 15;22(8):528–530. doi: 10.1007/BF01898672. [DOI] [PubMed] [Google Scholar]
- LEBOVITZ H. E., ENGEL F. L. RELATIONSHIPS BETWEEN THE STRUCTURE AND BIOLOGICAL ACTIVITIES OF CORTICOTROPIN AND RELATED PEPTIDES: BIOLOGICAL ACTIVITIES OF A SYNTHETIC EICOSAPEPTIDE AMIDE. Endocrinology. 1964 Dec;75:831–837. doi: 10.1210/endo-75-6-831. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pricer W., Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970 Mar;65(3):745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M. ON THE ENZYMIC ACTIVITY OF SUBTILISIN-MODIFIED RIBONUCLEASE. Proc Natl Acad Sci U S A. 1958 Feb;44(2):162–166. doi: 10.1073/pnas.44.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer B. P., Ueda K., Sato G. H. Site of action of adrenocorticotropic hormone (ACTH) in adrenal cell cultures. Biochem Biophys Res Commun. 1968 Sep 6;32(5):806–810. doi: 10.1016/0006-291x(68)90312-4. [DOI] [PubMed] [Google Scholar]
- Tesser G. I., Schwyzer R. Synthese des 17,18-Diornithin-beta-corticotropin-(1-24)-tetracosapeptides, eines biologisch aktiven Analogons des adrenocorticotropen Hormons. Helv Chim Acta. 1966 Apr 20;49(3):1013–1022. doi: 10.1002/hlca.19660490302. [DOI] [PubMed] [Google Scholar]


