Abstract
Regulatory T (Treg) cells can suppress a wide variety of immune responses, including antitumor and alloimmune responses. The mechanisms by which Treg cells mediate their suppressive effects depend on the context of their activation. We previously reported that granzyme B is important for Treg cell–mediated suppression of antitumor immune responses. We therefore hypothesized that granzyme B may likewise be important for suppression of graft-versus-host disease (GVHD). We found that allogeneic mismatch induces the expression of granzyme B in mixed lymphocyte reactions and in a model of graft-versus-host disease (GVHD). However, wild-type and granzyme B–deficient Treg cells were equally able to suppress effector T (Teff) cell proliferation driven by multiple stimuli, including allogeneicantigen-presenting cells. Surprisingly, adoptive transfer of granzyme B–deficient Treg cells prevented GVHD lethality, suppressed serum cytokine production in vivo, and prevented target organ damage. These data contrast strikingly with our previous study, which demonstrated that granzyme B plays a nonredundant role in Treg cell–mediated suppression of antitumor responses. Taken together, these findings suggest that targeting specific Treg cell–suppressive mechanisms, such as granzyme B, may be therapeutically beneficial for segregating GVHD and graft-versus-tumor immune responses.
Introduction
CD4+Foxp3+ regulatory T (Treg) cells play an indispensable role in maintaining peripheral tolerance to self-antigens by suppressing effector immune responses. Mice or humans with a deficiency of Treg cells, induced by antibody-mediated1,2 or toxin-mediated3,4 depletion or by mutations5 and deletions6,7 of the lineage specification factor Foxp3, manifest severe autoimmune disease. In addition to preventing autoimmunity, Treg cells can also suppress immune responses generated against tumor cells,8,9 alloantigens,10 allergens,11–13 and microbial antigens.14,15
Several mechanisms have been proposed to explain how Treg cell–mediated suppression of effector immune responses occurs. In certain model systems, Treg-cell secretion of anti-inflammatory cytokines, such as transforming growth factor-β and interleukin-10 (IL-10), has been shown to be required for suppressive function.16–18 In other experimental settings, contact-dependent mechanisms, such as interactions between CTLA-4 on Treg cells and CD80/CD86 on antigen-presenting cells (APCs), have also been reported.19–21 Because of the variety of animal models, in vitro activation methods, and readouts for suppression, rigorously defining nonredundant Treg-suppressive mechanisms has been challenging and controversial. It is probable that Treg cells use multiple mechanisms depending on the context in which they are activated in vivo.22
Our group previously demonstrated that human regulatory T cells can use the perforin/granzyme pathway to suppress effector T (Teff)–cell proliferation and kill autologous immune cells.23,24 These findings were subsequently extended to a murine tumor challenge model, where we showed that adoptively transferred granzyme B– and perforin-deficient Treg cells were defective in their ability to inhibit antitumor responses.25 In that study, we reported that Gzmb−/− mice in the 129/SvJ background have markedly improved survival (compared with strain-matched wild-type [WT], Prf1−/−, and other Gzm−/− mice) after intravenous challenge with a variety of tumor cell lines.25 RMAS lymphoma and B16 melanoma cells, both derived from C57Bl/6 mice, are mismatched with 129/SvJ mice across minor histocompatibility barriers; MB0 cells, an acute myeloid leukemia cell line generated via retroviral transduction of bone marrow cells, are syngeneic to 129/SvJ mice. These findings suggested an immunoregulatory role for granzyme B in tumor clearance, and we hypothesized that granzyme B plays a nonredundant role in Treg cell–mediated suppression of the antitumor immune response. Flow-cytometric studies confirmed that granzyme B was expressed in Treg cells harvested from the tumor microenvironment. Further, using bioluminescence imaging, we demonstrated that adoptive transfer of Treg cells into Gzmb−/− RMAS-tumor-bearing hosts restored tumor burden in a granzyme B– and perforin-dependent manner. Taken together, we attributed the improved survival of Gzmb−/− mice after tumor challenge to defective Treg-cell function in these hosts, and we concluded that granzyme B is important for Treg cell–mediated suppression of antitumor responses.
In this study, we examined the role of granzyme B in Treg-cell function within the context of another mouse model of alloimmunity, graft-versus-host disease (GVHD). We initially hypothesized that granzyme B would be important for the suppression of GVHD. However, using several readouts of suppressive function in vitro and in vivo, we unexpectedly found that granzyme B was not required for suppression of GVHD, even though Treg cells up-regulate granzyme B in this model. Taken together with previously reported findings from our tumor challenge studies (as well as other allograft models), these data suggest that the use of granzyme B as a Treg-suppressive mechanism is context-dependent and could potentially be exploited to segregate GVHD and graft-versus-tumor effects.
Methods
Mice
WT 129/SvJ (H-2b) and Balb/c mice (H-2d) were obtained from The Jackson Laboratory. Foxp3-ires-GFP (FIG) reporter mice have been previously described.26 Targeted FIG 129/SvJ ES clones were a generous gift from Talal Chatila (University of California–Los Angeles). FIG mice were rederived in the 129/SvJ background and were bred with Gzmb−/− mice to generate granzyme B–deficient FIG mice. All mice were maintained in specific pathogen–free housing, and all experiments were conducted in accordance with institutional animal care and use guidelines under appropriate protocols approved by the Washington University School of Medicine.
Antibodies and reagents
Antibodies used include CD4 (RM4-5), CD8 (53-6.7), CD25 (7D4), CD16/32 (2.4G2; BD Biosciences), Foxp3 (FJK-16s; eBioscience), H-2Kb (CTKb), and granzyme B (GB12; Invitrogen). Serum cytokines were analyzed using the Bio-Plex Pro Th1/Th2 Mouse Panel Kit, according to the manufacturer's instructions (Bio-Rad). Samples were analyzed on a Bio-Plex 200 Workstation. CD3/CD28 Dynabeads were obtained from Invitrogen. Endotoxin-free recombinant human IL-2 was obtained from Chiron and was stored at −80°C after reconstitution.
Cell isolation and stimulation
All cells were cultured in K10 media (RPMI 1640, 10% fetal calf serum, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1% nonessential amino acid, 1% sodium pyruvate, 1% l-glutamine, 1× penicillin/streptomycin, 0.57μM β-mercaptoethanol). One-way mixed lymphocyte reactions (MLRs) were prepared by culturing 4 × 106 129/SvJ responder splenocytes with 4 × 106 irradiated (2000 cGy) Balb/c stimulator cells in 6-well plates with 5 mL of media supplemented with 50 U/mL IL-2. At indicated time points, cells were harvested for analysis. CD4+ and CD8+ splenic T cells were purified using the Pan T cell isolation kit, followed by cell separation on the AutoMACS, according to the manufacturer's instructions (Miltenyi Biotec). CD4+CD25− Teff cells were negatively selected using the Pan T cell isolation kit supplemented with biotinylated anti-CD8 and anti-CD25 antibodies. Bone marrow cells and splenic APCs were depleted of T cells using CD90.2 Microbeads. Treg cells were purified using the CD4+CD25+ regulatory T-cell isolation kit. For purification of CD4+Foxp3+ Treg cells, splenocytes from FIG mice were surface stained with anti-CD4 and CD4+GFP+ cells were isolated on a Reflection (iCyt) cell sorter (routinely ≥ 95% pure).
Suppression assays
Concanavalin A (ConA)–based Treg suppression assays were performed as described.6 Briefly, 2 × 104 CD4+CD25− Teff cells were stimulated for 72 hours with 2 μg/mL ConA in the presence of 8 × 104 T cell–depleted, irradiated (2000 cGy) APCs and a 1:2 titration of WT or Gzmb−/− Treg cells in 96-well round-bottomed plates. Cocultures were pulsed with 1 μCi per well of [3H]thymidine for the final 8 to 16 hours. Allogeneic Treg suppression assays were performed as described.27 Briefly, 105 CD4+CD25− Teff cells were mixed with a 1:2 titration of WT or Gzmb−/− Treg cells from 129/SvJ mice and stimulated for 5 days with 105 irradiated (2000 cGy) Balb/c APCs in 96-well round-bottomed plates. Cocultures were pulsed with 1 μCi per well of [3H]thymidine for the final 8 to 16 hours. All data shown are mean [3H]thymidine incorporation in triplicate cultures. For ex vivo suppression assays, purified CD4+CD25− Teff cells were washed with phosphate-buffered saline, resuspended at 106 cells/mL, and then labeled at 37°C for 15 minutes with 300nM CellTrace Far Red DDAO-SE (Invitrogen). Labeling reactions were stopped with RPMI 1640 media containing 10% fetal bovine serum. A total of 4 × 104 labeled Teff cells were cultured in the presence or absence of 4 × 104 CD3/CD28 Dynabeads and indicated numbers of fluorescence-activated cell sorter (FACS)–purified WT or Gzmb−/− CD4+GFP+ GVHD-activated Treg cells. After 3 days of culture in 96-well round-bottomed plates, cells were harvested for flow cytometric analysis.
Intracellular staining and flow cytometry
A total of 106 cells were washed and resuspended in staining buffer (phosphate-buffered saline, 0.5% bovine serum albumin, 0.5mM ethylenediaminetetraacetic acid). Samples were labeled with primary-conjugated antibodies against cell-surface markers, fixed and permeabilized (Foxp3 staining kit; eBioscience), and stained with primary-conjugated anti–granzyme B antibody and anti-Foxp3 antibody. Sample data were acquired on a Cytek-modified FACScan (BD Biosciences) flow cytometer and analyzed with FlowJo (TreeStar) software.
GVHD model
For flow cytometric analyses and cell sorting of GVHD-activated Treg cells, Balb/c hosts were given total body irradiation (900 Gy) and injected with donor cells via the tail vein within 24 hours. All mice received 2 × 106 bone marrow cells from 129/SvJ WT mice and 2 × 106 T cells from 129/SvJ WT mice, WT FIG mice, or Gzmb−/− FIG mice. Splenocytes were harvested at indicated time points and stained for flow cytometric analysis or cell sorting. For GVHD survival studies and serum cytokine analyses, Balb/c hosts were given total body irradiation and injected intravenously with donor cells within 24 hours. Mice received 2 × 106 T cell–depleted bone marrow cells only, bone marrow cells with 4 × 105 CD25-depleted CD4+ and CD8+ Teff cells, or bone marrow and Teff cells with either WT or Gzmb−/− Treg cells at the indicated ratios. Survival and appearance of mice were monitored daily. Mice were bled for serum cytokine analysis 7 days after transplantation. Some mice were killed 7 days after transplantation for GVHD histopathology analysis.
GVHD histopathology
Seven days after transplantation, tissues (lung, liver, and gut) were harvested, fixed immediately in 10% buffered formalin, and then embedded in paraffin. Sections were cut and stained with hematoxylin and eosin for histologic evaluation of GVHD. As previously reported, a semiquantitative scoring system was used to assess abnormalities associated with GVHD or allograft rejection.28 The scoring system designated 0 as normal, 0.5 as focal and rare, 1.0 as focal and mild, 2.0 as diffuse and mild, 3.0 as diffuse and moderate, and 4.0 as diffuse and severe. Scores were added to provide a total score for each specimen.
Results
Granzyme B is expressed in alloactivated Treg cells in vitro and in vivo
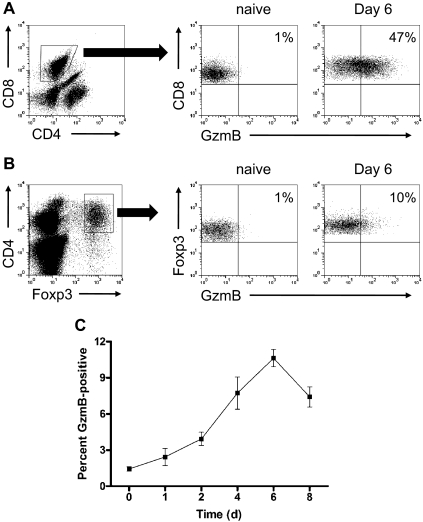
To determine whether Treg-cell expression of granzyme B is induced by allogeneic mismatch, we established 1-way MLRs and used flow-cytometric analysis to monitor the kinetics of granzyme B expression in alloactivated Treg cells in vitro. The 129/SvJ bulk splenocytes were cultured with irradiated Balb/c stimulator splenocytes in 6-well plates, and cells were harvested for analysis over an 8-day time course. Under these conditions, there was robust induction of granzyme B in effector CD8+ T cells on day 6, shown in Figure 1A, which is consistent with our previously reported observations.29 The proportion of granzyme B–expressing CD4+Foxp3+ Treg cells increased during alloactivation, peaking on day 6 at approximately 10%, and began to decline on day 8 (Figure 1B-C). This proportion is low relative to the percentage of granzyme B+ CD8+ T cells; however, it demonstrates that signals generated during allogeneic mismatch can result in Treg-cell expression of granzyme B independently of tumor-derived signals.
Figure 1.
Alloactivated Treg cells express granzyme B during in vitro MLRs. The 129/SvJ WT (H-2Kb) splenocytes were cultured with irradiated (2000 cGy) Balb/c WT (H-2Kd) splenocytes in complete medium supplemented with 50 U/mL IL-2. At various time points, splenocytes were harvested and analyzed by flow cytometry for granzyme B expression. (A) Representative flow plots of granzyme B expression, gated on naive and day 6 MLR-stimulated CD8+ T cells. (B) Representative flow plots of granzyme B expression, gated on naive and day 6 MLR-stimulated CD4+Foxp3+ Treg cells. (C) Summary graph of percentage granzyme B–expressing Treg cells (n = 3 independent MLR cultures per time point).
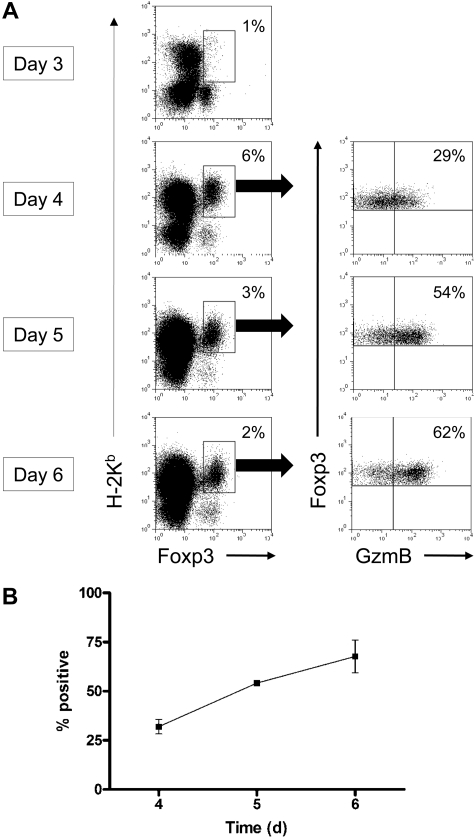
Next, we used a fully mismatched GVHD mouse model to determine whether Treg cells acquire granzyme B during alloactivation in vivo. In this model, Balb/c mice are lethally irradiated on day −1 and reconstituted with 2 × 106 bone marrow cells and 2 × 106 T cells from 129/SvJ WT mice on day 0. On days 3 to 6 after transplantation, mice were killed and splenocytes were analyzed for granzyme B expression within the donor Treg-cell (H-2Kb+Foxp3+) compartment. On day 3 after transplantation, there were very few donor-derived Treg cells in the spleen (Figure 2). By day 4, however, there was a substantial migration and/or expansion of donor-derived splenic Treg cells, and granzyme B protein was detected in approximately 30% of the gated cells. That proportion continued to rise throughout the 6 days of in vivo alloactivation, and by day 6, more than 60% of donor-derived Treg cells were granzyme B–positive. Therefore, Treg cells express granzyme B protein during in vitro and in vivo alloactivation across major histocompatibility barriers.
Figure 2.
Alloactivated Treg cells express granzyme B in a mouse model of GVHD in vivo. Lethally irradiated Balb/c mice were reconstituted with 2 × 106 bone marrow cells and 2 × 106 T cells derived from 129/SvJ WT mice. Splenocytes were harvested from recipient mice on days 3 to 6 for flow cytometric analysis of granzyme B expression in donor-derived Treg cells. (A) Representative flow plots, gated on H-2Kb+Foxp3+ Treg cells, are shown. (B) Summary graph of percentage granzyme B–expressing Treg cells is shown (n = 3 mice per time point).
Granzyme B is not required for Treg-cell function in classic suppression assays
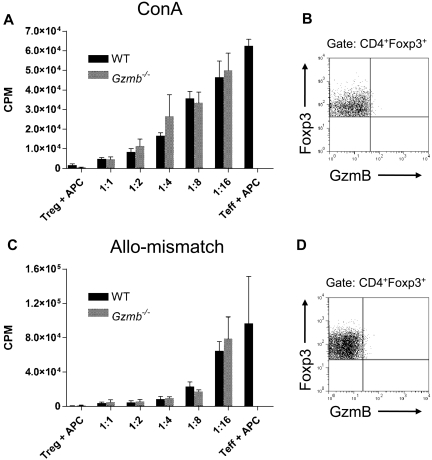
We performed in vitro Treg suppression assays to interrogate the role of granzyme B in Treg cell–mediated inhibition of CD4+CD25− Teff-cell proliferation. First, we used the lymphocyte mitogen ConA as a polyclonal stimulator of T-cell proliferation. Consistent with previously reported findings, ConA-stimulated WT Treg cells cultured with APCs are hypoproliferative and can suppress the proliferation of Teff cells in a dose-dependent manner (Figure 3A).6 Gzmb−/− Treg cells were also able to suppress ConA-driven Teff-cell proliferation.
Figure 3.
Granzyme B is not required for Treg cell–mediated suppression of ConA-activated and alloactivated Teff-cell proliferation in vitro. (A) Suppression of CD4+CD25− Teff-cell proliferation stimulated by ConA in the presence of syngeneic T cell–depleted, irradiated APCs. (B) Representative flow plot of granzyme B expression, gated on wild-type Treg cells cultured for 3 days with ConA-activated Teff cells under maximal suppression conditions (1:1). (C) Dose-dependent suppression of 129/SvJ CD4+CD25− Teff-cell proliferation stimulated by fully mismatched Balb/c T cell–depleted APCs. (D) Representative flow plot of granzyme B expression, gated on wild-type Treg cells cultured for 5 days with alloactivated Teff cells under maximal suppression conditions (1:1). All data shown are representative of 3 independent experiments.
Because we observed Treg cell expression of granzyme B in MLRs, we used allogeneic mismatch to drive Teff-cell proliferation in these suppression assays. Teff cells were cultured with irradiated Balb/c stimulator APCs and were assayed for thymidine incorporation after culturing for 5 days. In concordance with reported observations, WT Treg cells also suppressed Teff-cell proliferation in a dose-dependent manner, and Gzmb−/− Treg cells were equally capable of inhibiting this allogeneic effector function (Figure 3C).27,30
To determine whether granzyme B was expressed under experimental conditions where Treg cells are actively suppressing ConA- or allo-mismatch–driven Teff-cell proliferation, we examined these Treg cells for granzyme B expression. At a 1:1 Treg/Teff cell ratio, where proliferation is maximally suppressed after the 3 (ConA) or 5 (allo-mismatch) days of culture, cells were harvested and analyzed by flow cytometry. In both assays, granzyme B was not detected in the gated CD4+Foxp3+ Treg-cell population (Figure 3B,D). In particular, for the allo-mismatched Treg suppression assay, the absence of granzyme B–expressing Treg cells contrasts with our MLR expression studies, where we found a small proportion of granzyme B–positive Treg cells (Figure 1C). This is probably the result of differences in cell populations in culture. Whereas the MLRs contained unfractionated splenocytes in both the responder and stimulator populations, the allogeneic Treg suppression assay cocultures contained only purified responder Treg- and Teff-cell populations, along with mismatched T cell–depleted stimulator APCs. The absence of accessory cells in the Treg suppression assay cocultures, which could produce soluble factors and/or cell contact-mediated signals, may potentially account for the differential expression of granzyme B under the 2 allogeneic culture conditions. Together, these data demonstrate that granzyme B is not required for Treg cell–mediated suppression of Teff-cell proliferation in vitro.
In vivo–activated Treg cells purified from mice with GVHD suppress Teff-cell proliferation ex vivo
Because no granzyme B was detectable in functional Treg cells from classic suppression assay cocultures, it remained unclear whether alloactivated Treg cells require granzyme B for its function under the particular conditions when granzyme B is expressed. To address this experimentally, we rederived Foxp3 reporter mice in the 129/SvJ background from targeted embryonic stem cell clones that were engineered by Haribhai et al where a bicistronic FIG construct was targeted to the endogenous Foxp3 locus.26 Consistent with their published findings, GFP fluorescence accurately reported Foxp3 expression (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). FIG mice were bred with strain-matched Gzmb−/− mice to homozygosity. Analysis of these strains confirmed that Treg-cell numbers were not affected by granzyme B deficiency (supplemental Figure 2). These WT and Gzmb−/− FIG mice would allow for Treg cells to be alloactivated in vivo in mismatched hosts and subsequently FACS-purified for assays directed at interrogating the requirement for granzyme B in Treg-suppressive function.
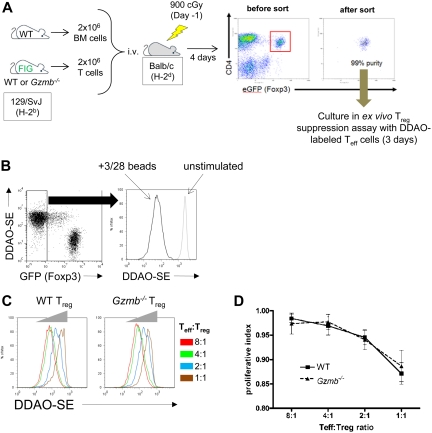
In Figure 2, we showed that more than 25% of donor-derived Treg cells express granzyme B 4 days after transplantation in a mouse model of GVHD. We used this same model but instead transferred 2 × 106 WT or Gzmb−/− FIG T cells into lethally irradiated Balb/c hosts, together with 2 × 106 WT bone marrow cells (Figure 4A). On day 4 after transplantation, Balb/c hosts were killed and WT or Gzmb−/− CD4+GFP+ donor-derived FIG Treg cells were FACS-purified. Notably, there was no difference in spleen cellularity or in the proportion of donor-derived WT or Gzmb−/− CD4+Foxp3+ Treg cells, suggesting that Gzmb−/− Treg cells have no intrinsic defect in survival or proliferative capacity compared with WT Treg cells (data not shown). These alloactivated Treg cells were then cocultured with DDAO-SE–stained CD4+CD25− Teff cells and stimulated ex vivo with anti-CD3/CD28 beads. After 72 hours, Teff-cell proliferation was analyzed by flow cytometric analysis of DDAO-SE fluorescence gated on GFP− effector cells. Teff cells cultured in the absence of anti-CD3/CD28 beads retained DDAO-SE staining, whereas Teff cells stimulated with the beads lost DDAO-SE fluorescence by orders of magnitude (Figure 4B). Coculturing Teff cells with a 1:2 serial titration of WT GVHD-activated Treg cells resulted in the dose-dependent suppression of Teff-cell proliferation (Figure 4C). Similarly, a 1:2 titration of Gzmb−/− GVHD-activated Treg cells inhibited Teff-cell proliferation. These data were summarized by deriving a proliferative index, calculated by normalizing the loss of DDAO-SE fluorescence in Treg/Teff cell cocultures to the maximal loss of fluorescence observed in Teff cells stimulated by anti-CD3/CD28 beads in the absence of Treg cells (Figure 4D). These data demonstrate that granzyme B is not required for Treg cell–mediated suppression of Teff-cell proliferation, even under conditions where Treg cells acquire granzyme B protein via endogenous signals generated during alloactivation in vivo.
Figure 4.
In vivo alloactivated Treg cells do not require granzyme B to suppress Teff-cell proliferation ex vivo. (A) Experimental protocol for generation of WT and Gzmb−/− GVHD-activated Treg cells: 2 × 106 T cells from 129/SvJ WT or Gzmb−/− FIG reporter mice were injected intravenously in lethally irradiated (900 cGy) Balb/c hosts, together with 2 × 106 bone marrow cells from WT (non-FIG) mice. Four days after transplantation, splenic WT or Gzmb−/− CD4+GFP+ Treg cells were sort-purified and cultured ex vivo with DDAO-SE–stained CD4+CD25− Teff cells for 3 days. T cells were either unstimulated or stimulated with CD3/CD28 beads. (B) Representative flow plot and histogram of Teff-cell proliferation (ie, loss of DDAO-SE staining) in the presence or absence of CD3/CD28 beads. (C) Dose-dependent inhibition of Teff-cell proliferation mediated by wild-type or Gzmb−/− GVHD-activated Treg cells. (D) Summary graph of normalized data from 3 independent experiments.
Adoptive transfer of Gzmb−/− Treg cells rescues reconstituted hosts from lethal GVHD equivalently to recipients of WT Treg cells
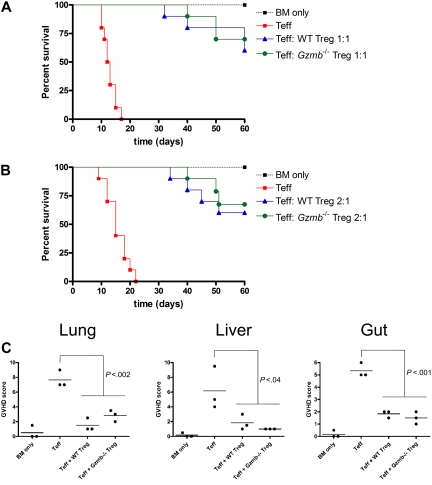
In addition to inhibiting Teff-cell proliferation, Treg cells have been reported to suppress a variety of effector functions, resulting in diverse physiologic outcomes depending on the model being studied. The ability of donor-type WT Treg cells to protect hosts from acute GVHD lethality has been demonstrated by multiple groups.27,30–32 To determine whether there is a granzyme B–dependent component to this survival phenotype, we adopted an acute GVHD mouse model in which lethally irradiated Balb/c mice were reconstituted with T cell–depleted bone marrow cells only, bone marrow cells with CD25-depleted CD8+ and CD4+ Teff cells, or bone marrow cells transferred with Teff cells and either WT or GzmB−/− CD4+CD25+ Treg cells at a 1:1 ratio (Figure 5A). Recipient mice were monitored for survival and GVHD morbidity over a 60-day period. All mice receiving only T cell–depleted bone marrow cells survived with no signs of acute GVHD during this period. Mice receiving bone marrow cells with Teff cells died with 100% penetrance within the first 3 weeks after transplantation. These mice exhibited classic signs of acute GVHD, such as weight loss, hunching, fur ruffling, and diarrhea. GVHD lethality and morbidity were significantly reduced by cotransfer of either WT or Gzmb−/− Treg cells, and the latency of lethality was prolonged by approximately 2 weeks. A lower Treg cell dose in this mouse model produced similar findings (Figure 5B). Furthermore, histopathologic scoring of GVHD severity in relevant target organs (eg, lung, liver, and gut) revealed that adoptive transfer of either WT or Gzmb−/− Treg cells was equally able to prevent target organ damage in this model (Figure 5C). These data suggest that granzyme B is not required within the donor Treg compartment for rescuing hosts from acute lethal GVHD.
Figure 5.
Treg cells do not require granzyme B to rescue hosts from GVHD lethality or to prevent GVHD target organ damage. Lethally irradiated (900 cGy) Balb/c mice received 2 × 106 129/SvJ TCD BM cells with or without 4 × 105 129/SvJ CD25− Teff cells (both CD4+ and CD8+) and either (A) 4 × 105 or (B) 2 × 105 wild-type or Gzmb−/− CD4+CD25+ Treg cells. Kaplan-Meier survival curves of recipient mice, pooled from 2 independent experiments (n = 10 mice per group), are shown. (C) Seven days after transplantation, 3 mice per experimental group (as outlined in panel A) were killed, and portions of lung, liver, and gut were prepared for histopathologic analysis. There was no statistically significant difference between groups receiving WT or Gzmb−/− Treg cells.
Inhibition of GVHD-associated serum cytokine production by adoptively transferred Treg cells is granzyme B–independent
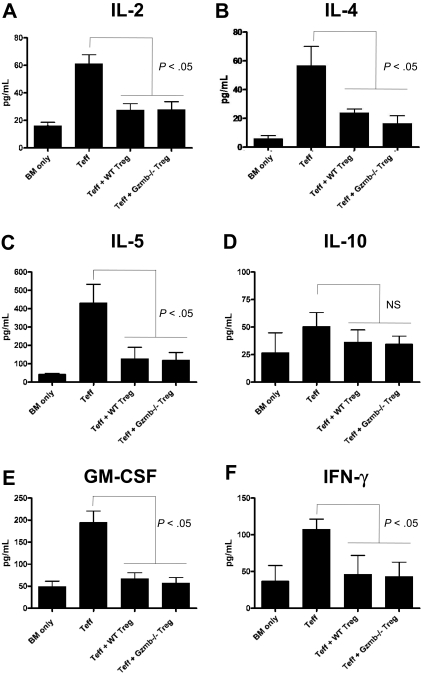
Aberrant cytokine production is a central component to the pathophysiology of GVHD and has previously been shown to be mitigated by the transfer of Treg cells.30 Thus, we measured serum levels of cytokines commonly associated with GVHD in our mouse model, where transfer of Teff and Treg cells at a 1:1 ratio suppressed GVHD lethality irrespective of whether the transferred Treg cells were WT or Gzmb−/−. Seven days after conditioned Balb/c hosts were transplanted with bone marrow and/or T cells as described in Figure 5, the mice were bled and sera were analyzed using cytokine bead arrays. Shown in Figure 6A, mice receiving CD25− Teff cells with no Treg cells had significantly higher amounts of serum IL-2 relative to recipients of T cell–depleted bone marrow cells alone, and cotransfer of WT or Gzmb−/− Treg cells restored IL-2 levels to near baseline levels. Suppression of IL-4 (Figure 6B), IL-5 (Figure 6C), granulocyte-macrophage colony-stimulating factor (Figure 6E), and interferon-γ production (Figure 6F) occurred in mice receiving Treg cells independently of granzyme B genotype. There was no statistically significant difference in IL-10 levels (Figure 6D) across the 4 experimental groups, which is expected given that IL-10 is an anti-inflammatory cytokine that has been shown to play a role in Treg cell–mediated suppression of GVHD lethality.27 In addition, there was also no difference in TNF-α levels across these groups (data not shown). Taken together with the GVHD survival data and proliferation assays, these findings demonstrate that Gzmb−/− Treg cells have no defect in suppressing lethality caused by GVHD, partly because their capacities to inhibit cytokine production and block Teff-cell proliferation remain intact.
Figure 6.
Treg cells do not require granzyme B to suppress production of GVHD-associated cytokines in vivo. Lethally irradiated (900 cGy) Balb/c mice received 2 × 106 129/SvJ TCD BM cells with or without 4 × 105 129/SvJ CD25− Teff cells (both CD4+ and CD8+) and 4 × 105 wild-type or Gzmb−/− CD4+CD25+ Treg cells. Seven days after transplantation, serum was harvested and analyzed via cytometric bead array for the production of (A) IL-2, (B) IL-4, (C) IL-5, (D) IL-10, (E) granulocyte-macrophage colony-stimulating factor, and (F) interferon-γ. There was no statistically significant difference between groups receiving WT or Gzmb−/− Treg cells.
Discussion
Based on our previous data, we hypothesized that granzyme B would be important for suppression of alloimmune responses in MLRs and in a mouse model of GVHD. In this report, we showed that Treg cells do indeed up-regulate granzyme B protein in both settings. However, granzyme B was not required for the suppression of Teff-cell proliferation driven by either ConA or mismatched APCs in vitro, and no granzyme B was detected in the Treg compartment even when Teff-cell proliferation was maximally suppressed. This contrasts with our finding that granzyme B protein was detected in a small proportion of MLR-activated Treg cells. The absence of accessory responder and stimulator cells in the suppression assay cocultures, which were present in the MLRs and could produce soluble molecules and/or cell contact-mediated signals, may be partially responsible for the differential expression of granzyme B in these experiments. These data also suggest that coligation of the T-cell receptor and traditional costimulatory molecules, although sufficient to activate other T-cell effector functions (ie, proliferation), is insufficient to induce expression of granzyme B in Treg cells.
Because the signals that are required for granzyme B induction in Treg cells have yet to be defined in vitro, we alloactivated Treg cells in vivo, using endogenous signals generated during GVHD to arm Treg cells with granzyme B. The use of FIG mice allowed for the purification of viable activated Treg cells based on expression of Foxp3, the most definitive marker that distinguishes between Treg and activated Teff cells. We found that these purified cell populations did not require granzyme B to suppress Teff-cell proliferation ex vivo. Because of technical limitations, we were unable to purify sufficient numbers of in vivo–activated Treg cells to determine whether Gzmb−/− Treg cells were defective in suppressing other aspects of Teff cell function, such as cytotoxicity, in ex vivo assays. We also attempted to measure suppression of Teff-cell expansion in vivo by measuring the loss of carboxyfluorescein succinimidyl ester in labeled Teff cells that were cotransferred with or without WT or Gzmb−/− Treg cells. However, because the injected cells were distributed systemically, we were unable to collect enough events to determine whether there was a significant difference between mice that received WT or Gzmb−/− Treg cells.
Although a reductionist approach for characterizing Treg-cell function in suppression assays has been helpful in elucidating candidate molecules and pathways that are important for suppressive function in vitro, it remains to be determined whether these same molecules and pathways are relevant in vivo.33 It is therefore important to functionally validate candidate molecules involved in suppression within the physiologic context of an appropriate disease model. To that end, we adopted an acute GVHD mouse model that was previously shown to be mitigated by the transfer of donor-type Treg cells.27 Using multiple physiologic readouts, including survival, cytokine production, and target organ damage, we unexpectedly found that Gzmb−/− Treg cells had no defect in their ability to ameliorate GVHD. Taken together, these results show that granzyme B is not required for Treg cell–mediated suppression of GVHD lethality and effector functions across major histocompatibility barriers.
These observations are different from our reported findings on Treg-cell function in the setting of tumor challenge, where granzyme B and perforin play nonredundant roles in Treg cell–mediated suppression of antitumor immune responses.25 Granzyme B–dependent Treg function has also been confirmed by others in different systems. Gondek et al reported that Treg cell–mediated inhibition of Teff-cell proliferation in vitro required granzyme B but not perforin,34 whereas Zhao et al demonstrated that activated Treg cells can suppress B-cell proliferation by killing antigen-presenting (but not bystander) B cells in a perforin- and granzyme B–dependent manner.35 Gondek et al later validated their in vitro findings in a skin allograft model by showing that hosts reconstituted with granzyme B–deficient Treg cells were unable to establish long-term tolerance to skin allografts.36
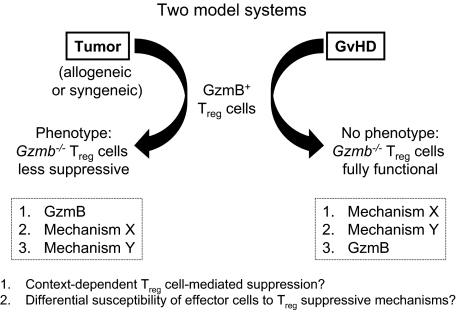
This presents a dichotomy in the mechanisms used by alloactivated Treg cells to suppress effector immune responses, even though allogeneic T-cell activation is a shared feature of all of these models. In the tumor model, the perforin/granzyme pathway is a nonredundant component of Treg-cell function, although others have shown that additional molecules, such as CTLA-4 and GITR, also play important roles.21,37 In the GVHD model, the contribution of granzyme B to Treg cell–suppressive function may potentially be masked by additional mechanisms that are activated under these specific conditions (Figure 7). A recent study of a fully mismatched pancreatic islet allograft model showed that Treg cell–mediated inhibition of dendritic cell migration to the graft occurred in a transforming growth factor-β– and IL-10–dependent manner, raising the possibility that these classic Treg-associated cytokines could be involved in other alloimmune settings such as GVHD.38 However, it is clear from multiple studies that not all allogeneic mouse models are equivalent; the experimental context in which Treg-cell activation occurs may play a critical role in defining the particular mechanism(s) operative in that context.33,39,40
Figure 7.
Model-dependent role of granzyme B in Treg cell–mediated suppressive function. Schematic illustrating the differential phenotypes observed with Gzmb−/− Treg cells in suppressing antitumor responses and GVHD.
Finally, the differential requirement of granzyme B for Treg-cell function in GVHD and tumor models also raises the potential of targeting specific Treg-suppressive mechanisms for therapeutic benefit. Allogeneic bone marrow transplantation is a major treatment modality for hematologic malignancies, but the graft-versus-tumor effect mediated by donor lymphocyte populations against mismatched host tumor cells often comes at the expense of GVHD.41,42 We previously demonstrated that disarming granzyme B in Treg cells results in enhanced tumor clearance, but our findings in this report demonstrate that Treg cells lacking granzyme B are still able to prevent GVHD lethality. Moreover, Edinger et al demonstrated that transfer of Treg cells rescues hosts from GVHD while preserving graft-versus-tumor activity.30 Based on our studies, we hypothesize that different suppressive mechanisms used by alloactivated and tumor-activated Treg cells may explain this phenotype. We attempted to test this hypothesis using bioluminescence imaging to serially monitor the clearance of allogeneic tumors in our GVHD model system, but we were limited by the current sensitivity and specificity of the tumor clearance assay. However, it is possible that the inhibition of Treg-suppressive molecules, such as granzyme B, may allow for the segregation of GVHD and graft-versus-tumor activity within the same host. Adoptive Treg-cell immunotherapy, along with modulation of Treg-cell function, holds therapeutic promise and should be an area of continued investigation.
Acknowledgments
The authors thank the Siteman Cancer Center High Speed Cell Sorter Core and the Washington University Biomedical Informatics Core for their invaluable support; Talal Chatila (University of California–Los Angeles), who generously provided FIG-targeted ES cells; Mieke Hoock, who provided expert animal husbandry; and Dan Link, Tim Graubert, Beatriz Carreno, Mike Rettig, Dan George, Rhonda Ries, Andy Bredemeyer, Maggie Young, and Nicole Grieselhuber for their helpful advice and technical assistance.
This work was supported by the National Institutes of Health (grant DK49786; T.J.L.). This work was also supported by the Barnes Jewish Foundation (T.J.L.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.F.C. and X.C. performed experiments; S.F.C., X.C., A.H., T.A.F., and T.J.L. contributed to experimental design and data analysis; and S.F.C. and T.J.L. wrote the paper.
Conflict-of-interest disclosure: T.J.L. holds the license for the mouse granzyme A monoclonal antibody clone 3G8.5 (Santa Cruz Biotechnology). The remaining authors declare no competing financial interests.
The current address of X.C. is Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY.
Correspondence: Timothy J. Ley, Division of Oncology, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: tley@dom.wustl.edu.
References
- 1.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset: I. Evidence for the active participation of T cells in natural self-tolerance: deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 3.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 4.Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 7.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 8.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 9.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Hess AD. Modulation of graft-versus-host disease: role of regulatory T lymphocytes. Biol Blood Marrow Transplant. 2006;12:13–21. doi: 10.1016/j.bbmt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Ostroukhova M, Ray A. CD25+ T cells and regulation of allergen-induced responses. Curr Allergy Asthma Rep. 2005;5:35–41. doi: 10.1007/s11882-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler B, Hufnagl K, Spittler A, et al. The role of Foxp3+ T cells in long-term efficacy of prophylactic and therapeutic mucosal tolerance induction in mice. Allergy. 2006;61:173–180. doi: 10.1111/j.1398-9995.2006.01014.x. [DOI] [PubMed] [Google Scholar]
- 14.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 15.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T-cell–mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 21.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 23.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 25.Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T-cell–mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nervi B, Rettig MP, Ritchey JK, et al. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp Hematol. 2007;35:1823–1838. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai SF, Fehniger TA, Cao X, et al. Differential expression of granzyme B and C in murine cytotoxic lymphocytes. J Immunol. 2009;182:6287–6297. doi: 10.4049/jimmunol.0804333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 31.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 33.Shevach EM. Mechanisms of foxp3(+) T regulatory-cell–mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 35.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gondek DC, Devries V, Nowak EC, et al. Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol. 2008;181:4752–4760. doi: 10.4049/jimmunol.181.7.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 40.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 42.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]