Summary
Bone remodeling is regulated by various neuronal inputs, one of the best understood being the sympathetic tone which inhibits bone mass accrual. This function of the sympathetic nervous system raises the prospect that the other arm of the autonomic nervous system, the parasympathetic nervous system, may also affect bone remodeling. Using various mutant mouse strains, each lacking one of the muscarinic receptors that mediate parasympathetic activity, we show here that the parasympathetic nervous system acting through the M3 muscarinic receptor is a positive regulator of bone mass accrual by increasing bone formation and decreasing bone resorption. Expression studies, cell-specific gene deletion experiments and analysis of compound mutant mice showed that the parasympathetic nervous system favors bone mass accrual by acting centrally and by decreasing the sympathetic tone. By showing that both arms of the autonomic nervous system affect bone remodeling, this study further underscores the importance of its neuronal regulation.
Introduction
Bone remodeling, the physiological function assuring renewal of bone tissue, consists of a perpetual alternance of a resorption phase followed by de novo bone formation (Rodan and Martin, 2000). One of the many significant advances that took place in recent years in our understanding of the regulation of bone remodeling has been the identification of its neuronal control which was first revealed when deciphering the mechanisms whereby the adipocyte-derived hormone leptin regulates bone mass (Ducy et al., 2000).
Intracerebroventricular infusion of leptin in wildtype (WT) and leptin-deficient (ob/ob) mice before or after chemical lesioning of hypothalamic neuronal populations, together with the analysis of multiple cell-specific gene deletion mouse models, have established that leptin inhibits bone mass accrual by acting solely through neuronal means (Shi et al., 2008). First, after binding to its receptor on neurons of the brainstem, leptin inhibits synthesis and release of serotonin (Yadav et al., 2009). Brain-derived serotonin then signals through the Htr2c receptor present on ventromedial hypothalamic (VMH) neurons. This signaling event triggers a decrease of the activity of the sympathetic nervous system (SNS), a known negative regulator of bone mass accrual acting through the ß2 adrenergic receptor (Adrb2) present on osteoblasts (Elefteriou et al., 2005; Fu et al., 2005; Takeda et al., 2002). The crucial role of the SNS in the regulation of bone remodeling begs the question of the possible role that the other arm of the autonomic nervous system, the parasympathetic nervous system (PNS), may have in this context.
The PNS originates from medial medullary sites. Vagal efferent innervations extend from the medulla to postganglionic nerves in various locations in the body (Agostoni et al., 1957; Brodal, 1981). The main neurotransmitter used by PNS endings is acetylcholine, which binds to a small family of G-protein coupled receptors, the muscarinic acetylcholine receptors (Caulfield, 1993; Wess, 1996). Molecular cloning identified five different muscarinic receptors (MRs), which can be subdivided into two major classes. The M1R, M3R, and M5R selectively couple to G-proteins of the Gq family, while the M2R and M4R preferentially activate Gi/Go-type of G-proteins (Caulfield, 1993; Caulfield and Birdsall, 1998; Wess, 1996).
In an effort to determine whether or not the PNS regulates bone remodeling, we studied the bone phenotype of mice lacking muscarinic receptors. Here we show that among the four receptors tested, M3 receptor (M3R) is the only muscarinic receptor subtype influencing bone remodeling. Furthermore, studies of mutant mouse strains harboring cell-specific gene deletion establish that the M3R fulfills this function through its neuronal expression and by decreasing sympathetic activity. Thus, these experiments reveal that the PNS is a positive regulator of bone mass accrual and that, unlike the SNS, it acts through a neuronal, not an osteoblast relay, to fulfill this function.
Results and Discussion
Signaling through M3R favors bone mass accrual
To determine whether signaling through the PNS affects bone remodeling in vivo, we studied various strains of mutant mice, each of them lacking, in all cells, one of the muscarinic receptors that mediate acetylcholine signaling (Wess, 2004; Wess et al., 2007).
For that purpose, histological and microcomputed tomography (μCT) analyses of vertebrae and long bones were performed in 6-, 12-, and 24-week-old WT and mutant mice. As shown in Figure 1C-E, even when studied in relatively old mice (24 weeks of age), the absence of either M1R, M2R or M4R did not affect significantly bone mass as measured histologically by the ratio of bone volume over tissue volume (BV/TV). None of these three mutations affected either bone formation or bone resorption parameters (Figure 1C-1E). In contrast, female M3R-/- mice, that are slightly shorter than WT littermates (Figure S1A-S1C) as previously reported (Matsui et al., 2000), displayed at 6-, 12-, and 24-week of age a low bone mass phenotype affecting both the axial (vertebrae) and appendicular (long bones) skeleton (Figure 1A, 1B and S1F), while cortical thickness was unaffected (Figure S1G). The same low bone mass phenotype was observed in male M3R-/- mice (data not shown). The rest of the analysis was done in female WT and mutant mice.
Figure 1. The parasympathetic nervous system, acting through M3 receptor, favors bone mass accrual by increasing bone formation and decreasing bone resorption.
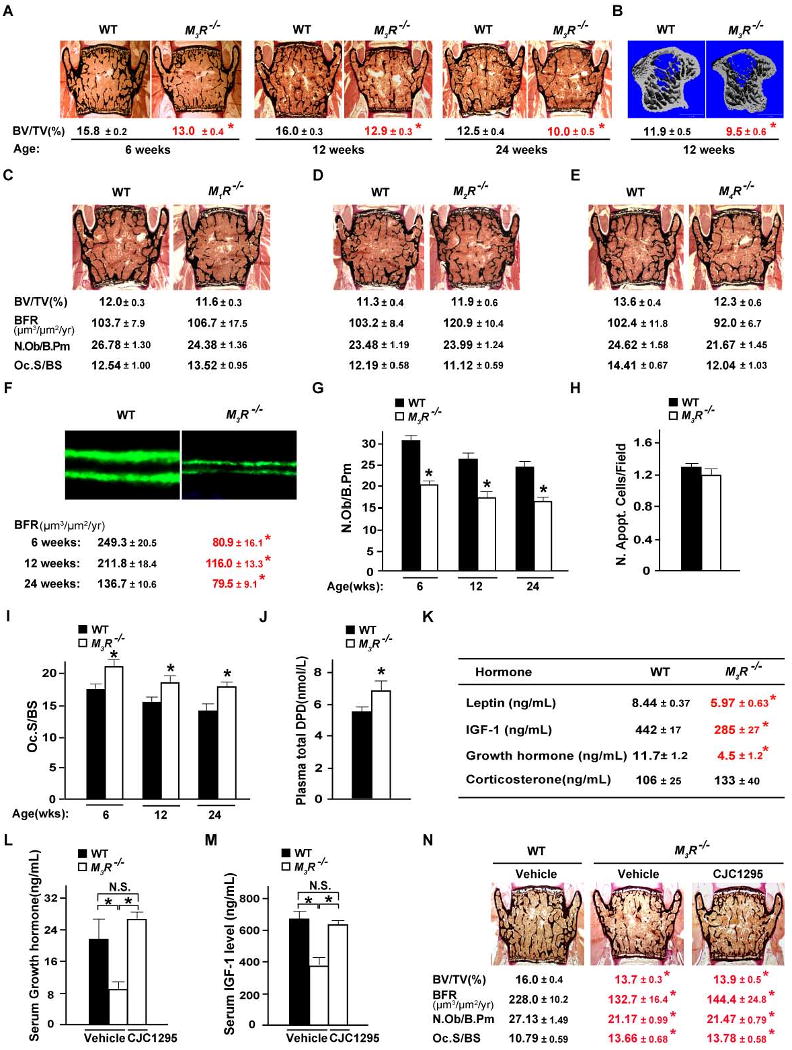
(A) Histological analyses of WT and M3R-/- vertebrae. Bone mass is measured as the ratio of mineralized trabecular bone volume (black) over total tissue volume (BV/TV, %) after Von Kossa/Van Gieson staining. (B) μCT analyses of WT and M3R-/- proximal tibiae. (C-E) Histomorphometric analyses of vertebrae of 24-week-old M1R-/- (C), M2R-/- (D), M4R-/- (E) and corresponding WT littermates. Histomorphometric parameters: BFR, bone formation rate; N.Ob/B.Pm: number of osteoblasts per bone perimeter; Oc.S/BS: osteoclast surface per bone surface. (F, G and I) Histomorphometric analyses of WT and M3R-/- vertebrae. (H) TUNEL assay of calvarial sections. The number of apoptotic cells per 0.16 mm2 field was comparable between 3-week-old WT and M3R-/-mice. (J) ELISA analysis of plasma levels of total deoxypyridinoline (DPD), a biomarker of bone resorption, in M3R-/- mice. (K) Serum levels of hormones or growth factors in WT and M3R-/- mice. (L-N) ELISA analysis of serum growth hormone (L) and IGF-1 (M) levels and histomorphometric analysis of vertebrae (N) of CJC1295- and vehicle-treated M3R-/- and WT mice. N.S., statistically non-significant. n = 6-10 for all other experiments; *P < 0.05 comparing to WT group; results are represented as mean ± SEM; error bars represent SEM.
To better understand the pathogenesis of this low bone mass phenotype, we first asked whether it could be explained by abnormalities in the levels of circulating hormones or growth factors. As shown in Figure 1K, leptin serum levels were significantly lower in M3R-/- than in WT littermates, an abnormality that should increase bone mass and therefore could not explain the low bone mass phenotype of the M3R-/- mice (Karsenty, 2006). In our hands, corticosterone serum levels were within the normal range in M3R-/- mice (Figure 1K) and therefore could not account for their bone phenotype either. In contrast, serum levels of growth hormone and of insulin-like growth factor 1 (IGF-1), a cytokine that may promote bone formation (Kawai and Rosen, 2008), were decreased in M3R-/- mice (Figure 1K). One experimental evidence indicated, however, that the low bone mass phenotype observed in M3R-/- mice was not due primarily to this decrease in circulating levels of growth hormone and IGF-1. Indeed, when M3R-/- mice were treated (once daily s.c. injection of 2 μg for 8 weeks) with CJC1295, a synthetic long-acting growth hormone-releasing hormone analog that enhances growth hormone secretion and as a result increases circulating levels of IGF-1 (Jette et al., 2005), we observed, as expected, a normalization of their growth hormone and IGF-1 serum levels (Figure 1L and 1M). However, these CJC1295-treated M3R-/- mice remained osteopenic (Figure 1N). These observations indicate that parasympathetic signaling through the M3R affects bone mass accrual and does it, for the most part, in a growth hormone and IGF-1-independent manner.
Decreased bone formation and increased bone resorption in M3R-/- mice
To define the cellular bases of the low bone mass phenotype observed in M3R-/- mice, we relied on histological and biochemical studies. Histomorphometry analyses of vertebrae showed that the low bone mass phenotype of M3R-/- mice was secondary to a decrease in bone formation parameters, such as bone formation rate and osteoblast numbers (Figure 1F and 1G). Importantly, we did not observe any increase in osteoblast apoptosis in M3R-/- bones (Figure 1H), a feature usually observed when bone formation is hampered by corticosterone (Weinstein et al., 1998). In addition, there was also an increase in bone resorption parameters, such as the surface covered by osteoclasts and an increase in the circulating levels of total deoxypyridinoline, a degradation product of collagen and a biomarker of bone resorption (Eyre et al., 1988), in M3R-/- mice (Figure 1I and 1J). Taken together, results of these analyses showed that the PNS is a positive regulator of bone mass accrual affecting both the formation and resorption arms of bone remodeling and identified M3R as a muscarinic receptor involved in this process.
M3R is expressed in neurons involved in the control of bone mass
Given the direct effect of sympathetic signaling on osteoblasts, we next asked whether the PNS regulates bone mass accrual by acting directly on bone cells or through a different mechanism. As a first approach, we studied M3R expression.
As shown in Figure 2A and B, M3R expression in osteoblasts was two to three orders of magnitude lower than in various regions of the brain, or than in other peripheral tissues where it is known to be expressed and functional (Matsui et al., 2000; Yamada et al., 2001). Moreover, M3R expression in osteoblasts was also three orders of magnitude lower than the expression of Adrb2, the adrenergic receptor mediating sympathetic signaling in osteoblasts (Takeda et al., 2002) (Figure 2C). In the face of this rather weak expression of M3R in osteoblasts and in an effort to explain the bone phenotype of the M3R-/- mice, we then asked where in the nervous system M3R was expressed.
Figure 2. Expression pattern of M3R.
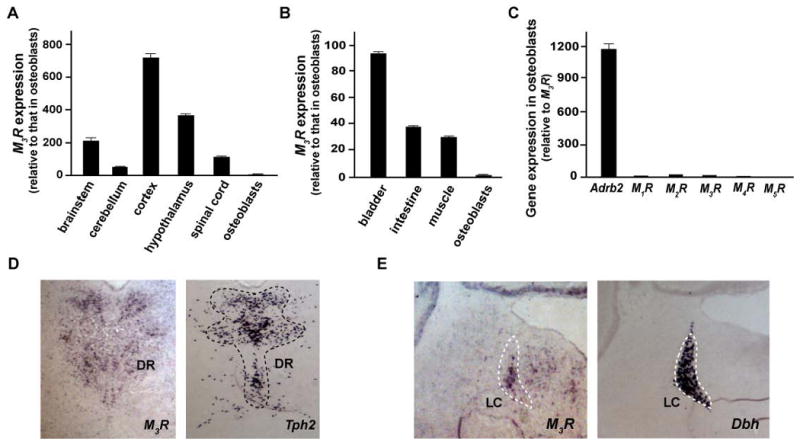
(A-C) Gene expression by real-time quantitative PCR (qPCR) analysis. Comparison of M3R expression between osteoblasts, various regions of the brain (A) and several peripheral tissues (B). (C) Gene expression of M1R, M2R, M3R, M4R and M5R in osteoblasts compared to that of Adrb2. The osteoblasts used were primary osteoblast cultures with 5-day induction for differentiation. (D and E) In situ mRNA hybridization analysis of M3R expression in brain. Tph2 and Dbh are the molecular markers of the Dorsal Raphe (DR) and Locus Coeruleus (LC) nuclei respectively.
In situ hybridization studies revealed that, in addition to its previously described expression in the hippocampus, visual cortex and lateral hypothalamus (data not shown) (Buckley et al., 1988), M3R is also expressed in neurons of the Dorsal Raphe (DR) and Locus Coeruleus (LC) nuclei in brainstem (Figure 2D and 2E). The DR and LC cluster serotonergic neurons and noradrenergic neurons respectively, two pathways affecting bone mass accrual (Takeda et al., 2002; Yadav et al., 2009). In contrast, we failed to detect any M3R expression in neurons of VMH, a hypothalamic structure whose integrity is required for the regulation of bone mass accrual (Takeda et al., 2002) (Figure S2A). We also failed to detect M3R expression in sympathetic chain ganglia (Figure S2B). In summary, these various studies of M3R expression indicated that M3R expression in osteoblasts is barely above the limit of detection of quantitative PCR, while it is expressed in the brain structures regulating bone mass accrual. As a result, they suggested that the PNS favors bone mass accrual through an indirect mechanism, i.e. its expression in neurons, rather than by acting directly on osteoblasts.
Neuronal-specific but not osteoblast-specific inactivation of M3R results in a low bone mass
To determine formally the cell type in which signaling through M3R must occur to favor bone mass accrual, we relied on cell-specific gene inactivation experiments in the mouse.
Crossing mice harboring a floxed allele of M3R (Gautam et al., 2006) with α1(I)Collagen-Cre mice, a transgenic mouse line expressing the Cre recombinase specifically in osteoblasts (Dacquin et al., 2002), allowed us to generate M3Rosb-/- mice. Southern blot analysis demonstrated that we had successfully deleted M3R in osteoblasts of M3Rosb-/- mice but not in other tissues (Figure 3A). Study of M3Rosb-/- mice at 12 weeks of age, a time point when M3R-/- mice are profoundly osteopenic, failed to detect any change in bone mass, bone formation or bone resorption parameters (Figure 3B), indicating that, in the conditions of this experiment, M3R does not regulate bone mass through its expression in osteoblasts. This result is consistent with the very weak expression of M3R in these cells, and also consistent with the fact that muscarinic receptor agonist treatment of WT or M3R-/- osteoblasts does not affect their ability to form nodules and to produce alkaline phosphatase (Figure S3A and S3B).
Figure 3. Neuronal mediation of the parasympathetic regulation of bone mass accrual by M3R.
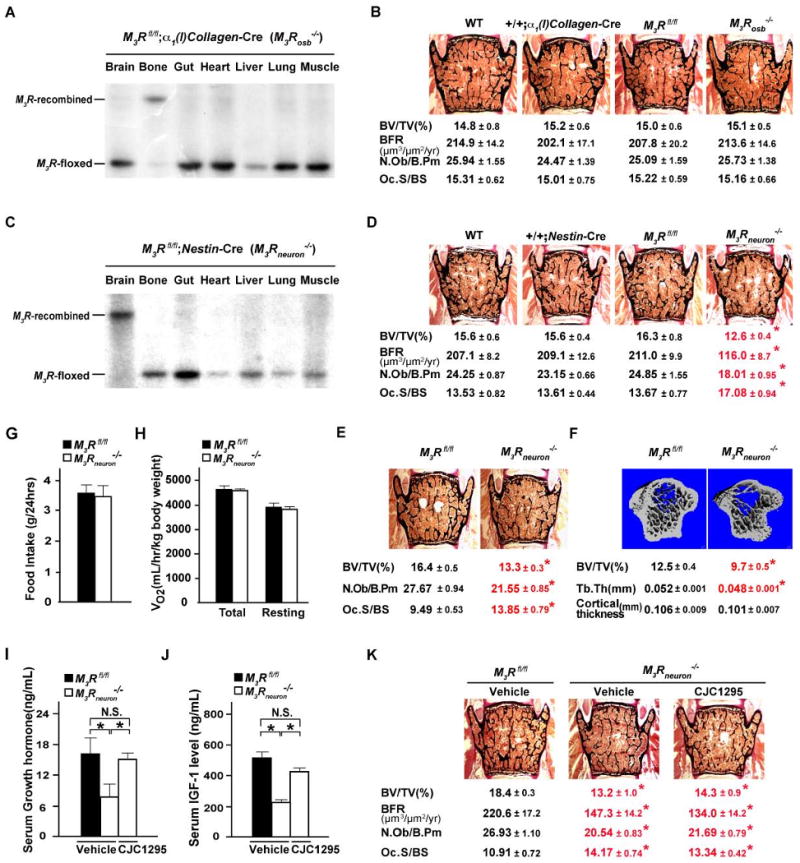
(A and C) Recombination efficiency of the M3R locus in various tissues/organs of osteoblast-specific (A) and neuron-specific M3R-deficient mice (C) by southern blot. (B, D and E) Histomorphometric analysis of female 12-week-old M3Rosb-/- (B), 12-week-old M3Rneuron-/- (D), 6-week-old M3Rneuron-/- (E) and corresponding control vertebrae. (F) μCT analyses of 12-week-old female M3Rfl/fl and M3Rneuron-/- proximal tibiae. Tb.Th, trabecular thickness. (G and H) 24-hour food intake and energy expenditure assays of M3Rneuron-/- and M3Rfl/fl control mice. (I-K) ELISA analysis of serum growth hormone (I) and IGF-1 (J) levels and histomorphometric analysis of vertebrae (K) of CJC1295- and vehicle-treated M3Rneuron-/- and M3Rfl/fl control mice. N.S., statistically non-significant. n = 6-10 per group; *P < 0.05 comparing to WT group; results are represented as mean ± SEM.
Next, we asked whether a deletion of M3R in all neurons would affect bone mass by crossing Nestin-Cre transgenic mice (Tronche et al., 1999) with floxed M3R mice. Southern blot analysis verified that we had effectively deleted M3R in neurons but not in other cell types (Figure 3C). M3Rneuron-/- mice of either sex showed, at 6 and 12 weeks of age, a low bone mass phenotype of similar severity as the one observed in mice lacking M3R in all cells (Figure 3D and 3E). These histological findings were confirmed by μCT analysis (Figure 3F). That the phenotype of M3R-/- and M3Rneuron-/- mice was of equal severity indicates that the bulk of the influence of the PNS on bone remodeling occurs through a neuronal relay. As it was the case in M3R-/- mice, this osteopenia was due to a decrease in bone formation and an increase in bone resorption parameters. These results established that it is through its neuronal not through its osteoblastic expression that the M3R regulates bone mass. Remarkably, appetite, which is low in M3R-/- mice [Figure S1D and S1E and (Yamada et al., 2001)], was unaffected in M3Rneuron-/- mice (Figure 3G and 3H). This may have at least two explanations. The first one is that the parasympathetic regulation of bone mass accrual occurs independently of its regulation of energy metabolism. A second one is that the deletion of M3R in neurons is not complete but large enough to affect bone mass but not appetite. Consistent with this latter hypothesis, we should point out that we observed the same discrepancy between the effect on bone mass and appetite in a mouse model of gain of function of leptin signaling (Shi et al., 2008).
M3Rneuron-/- mice displayed, like the M3R-/- mice, a decrease in growth hormone and IGF-1 circulating levels as previously reported (Gautam et al., 2009). To determine whether this abnormality explained their low bone mass phenotype, we treated them with CJC-1295. Although this treatment normalized the serum levels of growth hormone and IGF-1, the low bone mass phenotype of M3Rneuron-/- mice was not rescued, indicating that this phenotype develops independently of the growth hormone and IGF-1 abnormalities (Figure 3I-3K).
Parasympathetic signaling favors bone mass accrual by inhibiting sympathetic activity
The neuronal mediation of the PNS regulation of bone mass accrual along with the expression pattern of M3R in the DR nuclei were two reasons that led us to ask whether the PNS regulates bone mass by affecting synthesis and release of serotonin by brainstem neurons. However, Tph2 expression was normal in M3R-/- mice as was the serotonin brainstem content (Figure 4C and 4D). These two lines of experimental evidence argue strongly against a model whereby the PNS would regulate bone mass by affecting serotonergic output.
Figure 4. Increased sympathetic activity in M3R-/- mice leads to low bone mass.
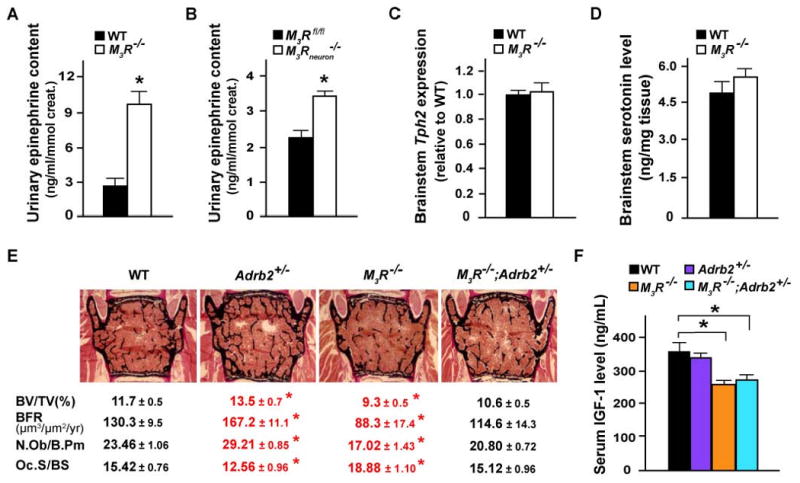
(A and B) ELISA assays of epinephrine content in urine of M3R-/- (A) and M3Rneuron-/- (B) as well as WT control mice. (C and D) qPCR analysis of Tph2 mRNA expression (C) and HPLC measurement of serotonin level in brainstem of WT and M3R-/- mice. (E and F) Histomorphometric analysis of vertebrae (E) and ELISA analysis of serum IGF-1 levels (F) of 24-week-old WT, Adrb2+/-, M3R-/-, and Adrb2+/-;M3R-/- mice. n = 6-10 per group; *P < 0.05 comparing to WT group; results are represented as mean ± SEM.
M3R is also expressed in the noradrenergic neurons of the LC nuclei in brainstem (Figure 2E) and activation of these neurons is correlated with an increase of sympathetic activity, as measured by an increase in plasma levels of epinephrine (Crawley et al., 1980). Thus, we tested whether signaling through M3R regulates bone mass accrual by affecting the sympathetic tone. A first experimental argument supporting this mode of action was that urinary levels of epinephrine were elevated in both M3R-/- and M3Rneuron-/- mice (Figure 4A and 4B). A second experimental evidence relies on genetic epistasis (Cordell, 2002). Indeed, if parasympathetic signaling through M3R and sympathetic signaling through Adrb2 lie in the same genetic pathway, one would expect that removing one allele of Adrb2 from M3R-/- background should rescue the bone phenotype of the latter mutant mice. Consistent with this hypothesis, M3R-/-;Adrb2+/- mice had normal bone mass, bone formation and bone resorption parameters (Figure 4E). That the low serum levels of growth hormone and IGF-1 characterizing M3R-/- mice were not corrected in M3R-/-;Adrb2+/- mice (Figure 4F) added further support to the notion that the low bone mass phenotype of the M3R-/- mice is not secondary to their low serum levels of growth hormone and IGF-1. Thus, our results suggest that the PNS favors bone mass accrual by inhibiting the activity of the sympathetic nervous system.
In summary, we present here in vivo evidence that the PNS, signaling through the M3R is, unlike the SNS, a positive regulator of bone mass. Although they highlight the importance of M3R in the context of the PNS regulation of bone mass, our results do not rule out that M5R, which was not tested here, may contribute to the regulation of bone mass by the PNS. An unexpected feature of the PNS regulation of bone mass accrual is that it does not occur on osteoblasts but rather on neurons, where it decreases sympathetic activity. The expression of M3R in the LC, a brain structure necessary for sympathetic output, and the decrease of epinephrine urinary contents in both M3R-/- and M3Rneuron-/- mice all argue for a model whereby the PNS would favor bone mass accrual by inhibiting sympathetic activity.
More generally, these observations broaden the importance of the autonomic regulation of bone mass and add further credence to the existence of the neuronal regulation of bone remodeling. The PNS is here to dampen the negative influence of the sympathetic tone on bone mass; such a function is also observed in other physiological systems, such as cardiac and digestive activity among others (Goldstein, 2001). Moreover and although it is difficult to compare their influence, it is striking that both the LRP5/serotonin-dependent and the leptin/sympathetic regulation of bone mass accrual inhibits bone formation (Ducy et al., 2000; Yadav et al., 2008). This observation raises the prospect that other regulators whose role would be to enhance bone formation in physiological situations must exist.
Experimental Procedures
Animals
M1R-/-, M2R-/-, M3R-/-, M4R-/-, M3Rfl/fl, α1(I)Collagen-Cre and Adrb2+/-mice used in these studies have been reported previously (Chruscinski et al., 1999; Dacquin et al., 2002; Gautam et al., 2006; Gomeza et al., 1999a; Gomeza et al., 1999b; Miyakawa et al., 2001; Yamada et al., 2001) and all are on C57BL/6J background. Nestin-Cre transgenic mice (C57BL/6J background) were from the Jackson Laboratory (Bar Harbor, MA).
Bone Histology, μCT Analysis and TUNEL Assays
Static and dynamic histomorphometric analyses were performed on undecalcified vertebral column specimens (See Supplemental Experimental Procedures). μCT analyses were performed on proximal tibiae using a micro computed tomography system (See Supplemental Experimental Procedures). TUNEL assay was performed on 3-week-old mice to evaluate in vivo osteoblast apoptosis as per manufacturer's instructions (In Situ Cell Death Detection Kit, Roche Applied Science, Indianapolis, IN). Four pups per genotype were analyzed and ten calvarial sections were counted per animal.
Food intake, energy expenditure, and CJC-1295 treatment
24-hr food intake was measured using metabolic cages (Nalge) and energy expenditure was assessed by an indirect calorimetry method using a 6-chamber Oxymax system (Columbus Instruments) as previously described (Shi et al., 2008). One-week-old M3R-/-, M3Rneuron-/- and their WT littermates were treated with once daily s.c. injection of 2 μg of CJC-1295 for 8 weeks.
Bioassays
Serum levels of total DPD (Quidel), leptin (R&D System), growth hormone (DSL), IGF-1 (DSL), corticosterone (MP Biomedicals) were measured using commercially available kits. Urinary epinephrine contents were measured in acidified spot urine samples by EIA (Bi-CAT kit, Alpco) and creatinine (Quidel) was used to standardize between urine samples.
Molecular studies
RNA isolation (Trizol, Invitrogen) and cDNA synthesis (Superscript III, Invitrogen) were performed as per manufacturer's instructions. Real-time quantitative PCR (qPCR) was performed on an MX3000 instrument (Stratagene, La Jolla, CA) using primers from SABiosciences (Frederick, MD) and the Taq SYBR Green supermix with ROX (Biorad, Hercules, CA). Expression levels of the studied genes were normalized by β-actin levels. Southern blot were performed using probe 3 as previously described (Gautam et al., 2006). In situ mRNA hybridization was performed on 20 μm cryosections using DIG-labeled riboprobe hybridizing at 68°C (See Supplemental Experimental Procedures).
Statistical analyses
Statistical significance was assessed by unpaired Student's two-tailed t test. Values were considered statistically significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Patricia Ducy for critical reading of the manuscript and constructive comments. This work was supported by grant DK58883 from National Institutes of Health (G.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal A. Neurological Anatomy in Relation to Clinical Medicine. Oxford, UK: Oxford University Press; 1981. [Google Scholar]
- Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Cordell HJ. Epistasis: what it means, what it doesn't mean, and statistical methods to detect it in humans. Hum Mol Genet. 2002;11:2463–2468. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Maas JW, Roth RH. Evidence against specificity of electrical stimulation of the nucleus locus coeruleus in activating the sympathetic nervous system in the rat. Brain Res. 1980;183:301–311. doi: 10.1016/0006-8993(80)90466-7. [DOI] [PubMed] [Google Scholar]
- Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224:245–251. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. The Biochemical journal. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Gautam D, Jeon J, Starost MF, Han SJ, Hamdan FF, Cui Y, Parlow AF, Gavrilova O, Szalayova I, Mezey E, Wess J. Neuronal M3 muscarinic acetylcholine receptors are essential for somatotroph proliferation and normal somatic growth. Proc Natl Acad Sci U S A. 2009;106:6398–6403. doi: 10.1073/pnas.0900977106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. The autonomic nervous system in health and disease. New York: Marcel Dekker; 2001. [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999a;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999b;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette L, Leger R, Thibaudeau K, Benquet C, Robitaille M, Pellerin I, Paradis V, van Wyk P, Pham K, Bridon DP. Human growth hormone-releasing factor (hGRF)1-29-albumin bioconjugates activate the GRF receptor on the anterior pituitary in rats: identification of CJC-1295 as a long-lasting GRF analog. Endocrinology. 2005;146:3052–3058. doi: 10.1210/en.2004-1286. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Kawai M, Rosen CJ. Insulin-like growth factor-I and bone: lessons from mice and men. Pediatr Nephrol. 2008 doi: 10.1007/s00467-008-1040-6. [DOI] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers M, Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


