During the past few years important breakthroughs have been made in the understanding of the 5q-syndrome. Scientists have struggled for decades to unravel the pathophysiology, and several candidate genes located on 5q have been proposed. Now, gene dosage and deletion of micro-RNA due to haploinsufficiency are recognized as key mediators of the disease. Moreover, the clinical management of patients with isolated 5q deletion has changed radically since lenalidomide was shown to have remarkable activity in this particular subgroup of myelodysplastic syndrome (MDS), although safety concerns have been raised recently. These issues will be discussed in detail.
Clinically, the 5q- syndrome is characterized by a macrocytic anemia, normal or slightly elevated platelet counts, hypolobated megakaryocytes, and a long-term risk of evolution into acute myeloid leukemia (AML) of around 10%.1 The World Health Organization classification of 2008 recognizes MDS associated with isolated del(5q) as a distinct entity, defined as MDS with less then 5% bone marrow blasts in addition to del(5q) as the sole karyotypic abnormality.2
Novel insights into the pathogenesis of myelodysplastic syndrome with isolated del(5q)
Haploinsufficiency of the ribosomal gene RPS14 impairs erythropoiesis
MDS with isolated del(5q) is a true stem cell disease, with more than 90% of the hematopoietic stem cells being part of the clone.3 The del(5q) abnormality has been demonstrated in B cells as well as in natural killer (NK) cells.3–5 The bone marrow is generally normo- or hypercellular.
It has been an enigma how an expanded clonal progenitor pool could result in anemia, but impaired erythroid development is strongly implied. The commonly deleted region (CDR) at 5q has been shown to contain around 40 genes expressed in hematopoietic progenitor cells.6 None of these has been found to be mutated, and understanding the role of haploinsufficiency of one or more genes within this region has proven to be a great challenge.
In 2008 Ebert et al. performed a series of experiments knocking-down each of the 40 implicated genes located within the CDR at 5q32-33. They elegantly demonstrated that decreased expression of RPS14 results in poor erythroid development and increased erythroid apoptosis. Moreover, forced expression of RPS14 in cells from MDS patients with del(5q) reduced the erythroid impairment.7
RPS14 is a component of the ribosomal subunit 40S. Intriguingly, germline mutations of other ribosomal protein genes, in particular RPS19, have been shown to cause Diamond-Blackfan anemia, which is also characterized by a macrocytic anemia. Furthermore, animal models have demonstrated that defects in ribosomal genes result in ineffective erythropoiesis and an increased risk of cancer.8 RPS14 deficiency is a plausible explanation of the anemia of 5q- syndrome. However, it does not explain the elevated platelet counts or the stem cell expansion.
Loss of micro-RNA leads to thrombocytosis and contributes to clonal expansion
Starczynowski, Karsan and others recently screened for micro-RNA close to the CDR at 5q and identified miR-145 and miR-146a as being significantly expressed in CD34+ bone marrow cells and significantly less expressed in del(5q) marrow cells compared to normal bone marrow cells. Knock-down of these two micro-RNA in mice resulted in a phenotype mimicking key features of the 5q- syndrome: hypolobated megakaryocytes and peripheral thrombocytosis. Moreover, the innate immune response pathway was shown to be modulated by predicted targets for miR-145 and miR-146a. Two genes in the Toll-like receptor signaling pathway were verified as true targets: TIRAP (miR-145) and TRAF6 (miR-146a). TIRAP interacts with TRAF6 and subsequently results in activation of nuclear factor-κ B. Next it was elegantly demonstrated that a proportion of mice transplanted with TRAF6-over-expressing marrow developed marrow failure or AML over time.9
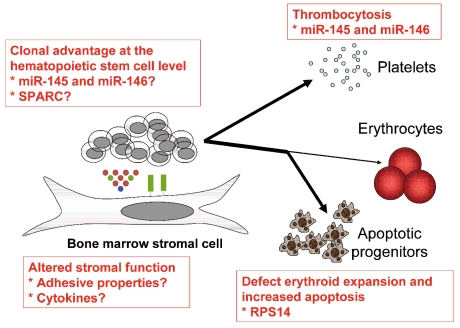
It is tempting to speculate that decreased expression of RPS14, miR-145, and miR-146a may co-operate to cause several of the key features of the 5q- syndrome. However, it remains to be demonstrated that these alterations are sufficient to give rise to the disease. Other haploinsufficient genes within the CDR at 5q, such as SPARC, may also be of importance.10 The potential tumor suppressor gene SPARC modulates cell adhesion and the surrounding matrix, induces apoptosis, and may inhibit angiogenesis.11 It is conceivable that increased adhesion of hematopoietic stem cells to the supporting niche due to the lack of SPARC expression may lead to a clonal advantage (Figure 1).
Figure 1.
Overview of the pathophysiology of the 5q- syndrome.
Somewhat centromeric of the 1.5 Mbp CDR on 5q there is a region containing several genes that have been implicated in high-risk MDS and AML with del(5q), such as EGR1 and CTNNA1. This region is included in the most frequent deletions in patients with classical low-risk 5q-syndrome. EGR1 is important for stem cell quiescence, and mice haploinsufficient for this gene are more prone to develop myeloid malignancies upon exposure to toxic substances. Data also indicate that decreased expression of the tumor suppressor gene CTNNA1 may increase the risk of transformation.8
A mouse model demonstrates a role for p53-mediated apoptosis in the pathogenesis of 5q- syndrome
Barlow et al. recently described a mouse model of 5q-syndrome. In mice, the genes incorporated within the CDR at 5q in humans are divided in two separate regions on chromosomes 11 and 18. Using chromosomal engineering, mice with variable deletions within the regions of interest were generated. The only deleted segment associated with a clear hematologic phenotype was the one harboring the Rps14 gene, with macrocytic anemia and impaired hematopoietic progenitor cell production being evident. These mice also had increased apoptosis in the bone marrow and over-expression of p53. Next, mice with the same deletion were bred with p53 knock-out mice. Complete knock-out of p53 reversed the phenotype completely, with normalized progenitor and red blood cell populations. Hence, p53-mediated apoptosis is likely to be of importance for the Rps14-induced anemia in the 5q-syndrome.12
None of the deletions was able to induce thrombocytosis, which is characteristic early in the clinical course of 5q- syndrome, and further studies are required to confirm whether any of these deleted regions in mice provide a clonal advantage necessary for neoplastic development.
Decreased erythroid cell expansion rather than block in differentiation
In this issue of the journal, Gardaret et al. add important new insights into the understanding of the impaired erythropoiesis in 5q-syndrome.13 It has been unclear whether there is a block in erythroid maturation or merely decreased expansion of the erythroid cells.
A three-step in vitro culture of CD34+ hematopoietic progenitor cells was used in order to generate mature erythrocytes. As expected, the red blood cell expansion was impaired compared to that in healthy controls. Next, single cell cultures were performed using limiting dilution assays. Interestingly, there was no block in terminal differentiation as determined by the frequency of enucleation of the del(5q) erythroid progenitors. However, during the 18-day cell expansion a single CD34+ progenitor cell with del(5q) generated on average 88 times fewer mature red blood cells compared to the number produced by a normal CD34+ progenitor cell. The deficit of RPS14 was highly pronounced in the del(5q) cultured cells, in particular during the first 11 days of culture, and this correlated with a lower proliferation rate.13 These results suggest that there is impaired erythroid cell expansion in 5q- syndrome rather than a deficiency in differentiation. It is attractive to hypothesize that this reduced proliferative ability is a result of the RPS14 deficiency.
Alterations of the bone marrow stroma in 5q- syndrome
In another study published in this issue of the journal, Ximeri et al. provide evidence that the bone marrow stroma in patients with 5q- syndrome is altered. Irradiated bone marrow stromal layers from patients with del(5q) were shown to have an impaired ability to support the growth of CD34+ progenitor cells from healthy donors. However, after achieving a complete response to lenalidomide, new stromal cells from the same patients were cultured. The progenitor supporting capacity was then increased and did not differ from that of stromal layers from healthy controls. Bone marrow cytokines were also analyzed, and it was found that stromal cell-derived factor 1 (SDF-1) and intracellular adhesion molecule 1 (ICAM-1), in particular, were up-regulated by lenalidomide.14 SDF-1 binds to the receptor CXCR4 on hematopoietic stem cells and is crucial for homing to the stem cell niche. This indicates that the stroma is significantly altered in 5q- syndrome and that lenalidomide directly or indirectly, through reduction of the malignant clone, may reverse this defect.
The role of lenalidomide in the management of myelodysplastic syndrome with isolated del(5q)
Standard of care
Most international guidelines propose erythropoietic growth factors as the first-line therapy of anemia in MDS with isolated del(5q), provided that the patients have a reasonable probability of response according to a predictive model based on transfusion requirements and serum erythropoietin level.15–18 Allogeneic stem cell transplantation is not recommended up front; it may, however, be considered in selected patients as signs of disease progression are manifested.
Lenalidomide has unparalleled efficacy
In studies pioneered by List et al., the immunomodulatory drug lenalidomide has shown remarkable efficacy in MDS with del(5q).19,20 The first large phase 2 trial (MDS-003) enrolled 148 transfusion-dependent MDS patients with del(5q) and low or intermediate-1 risk disease according to the International Prognostic Scoring System. Of these patients, 67% achieved transfusion independency and 45% entered complete cytogenetic remission.19 Important side effects included severe neutropenia and thrombocytopenia, which occurred in around half of the patients and were managed with dose interruptions and supportive granulocyte colony-stimulating factor. The median response duration was around 2 years.20 Long-term responders exist, although del(5q) cells may still be detected in the marrow with sensitive methods in these patients. Hence, there is no evidence of cure.
Controversial role of lenalidomide in the management of the disease
Lenalidomide was approved by the US Food and Drug Administration already in 2005. However, the European Medicines Agency (EMEA) requested extended studies. Despite its unquestionable efficacy, the EMEA later refrained from approval of lenalidomide in May 2008 due to safety concerns, in particular the observed rate of leukemic evolution.
In a long-term follow-up of the MDS-003 trial it was clearly demonstrated that cytogenetic response was inversely associated with AML evolution. Partial and complete cytogenetic responders had a 15% risk of their disease transforming into AML, while evolution into leukemia occurred in 67% of non-cytogenetic responders.20 In untreated patients with classical 5q- syndrome, the long-term risk of AML has previously been estimated to be no more than 10%.1 However, the patients included in the lenalidomide study were not treated at the time of diagnosis, all were transfused, and some had additional karyotypic abnormalities or a slight increase of blasts, making a direct comparison inappropriate.
Göhring et al. recently published a comprehensive analysis of the outcome of the 42 German patients enrolled in the MDS-003 trial.21 Of these 42 patients, 19 patients fulfilled the criteria for MDS associated with isolated del(5q). Leukemic transformation occurred in 15 of 42 patients (36%) overall. Evolution to AML occurred in seven of the 19 patients (37%) with MDS with isolated del(5q) and a normal blast count. Furthermore, karyotypic evolution was observed in 17 of 42 patients, and 11 of these had complex karyotypes.21 This high degree of disease progression was very unexpected based on the historical data referred to above. On the other hand, data from cohorts of patients suitable for comparisons have not been published. It cannot be excluded that this marked propensity for leukemic transformation actually reflects the natural course of the disease. The issue of potential negative effects of lenalidomide on long-term outcome is now under intense investigation.
Recent data suggest that there may be prognostic heterogeneity within classical low-risk del(5q) MDS. We recently described a patient with a small clone of p53 mutated cells in the marrow already at time of diagnosis. This subclone gradually expanded during treatment with lenalidomide, despite a complete erythroid and partial cytogenetic response. Eventually the clonal expansion resulted in leukemic transformation.22 The frequency of patients with p53 mutation is currently being assessed with next generation sequencing in a larger cohort of patients.
Mechanisms of action of lenalidomide
Lenalidomide has a broad mode of action, including inhibition of tumor necrosis factor-α and interleukin-6, induction of caspase-mediated apoptosis, cell adhesion, angiogenesis and stimulation of T cells and NK cells via induction of interleukin-2 and interferon-γ.23
Wei et al. showed that lenalidomide potently inhibits two phosphatases that regulate the cell cycle, Cdc25C and PP2Acα. The genes for both these phosphatases are located at 5q, and are deleted in the majority of patients with 5q- syndrome.24 Several studies have shown that del(5q) cells are significantly more sensitive to lenalidomide compared to non-(del5q) cells.10,24,25 It is, therefore, conceivable that the haploinsufficiency for the genes for Cdc25C and PP2Acα may induce this increased sensitivity.
As discussed above, the SPARC gene - located within the CDR at 5q and thus haploinsufficient - may be of importance for the clonal dominance in MDS. Intriguingly, lenalidomide restored SPARC expression to normal levels in del(5q) progenitors.10 This may further explain why del(5q) cells are particularly sensitive to this drug.
Immune effects such as stimulation of NK cells may also be of importance for the remarkable clinical effects of lenalidomide.23 As described above, lenalidomide potentially affects the stromal cells in patients with 5q- syndrome, although it cannot be ruled out that elimination of the del(5q) clone itself may alter the stroma so that it regains its normal functions.14
Conclusions and future perspectives
The recent identification of RPS14, miR-145, and miR-146a has delineated key aspects of the pathogenesis of MDS with isolated del(5q), and further understanding of the affected cellular pathways may lead to potential new targets for treatment. The emerging drug lenalidomide is highly efficient, although recent data have raised safety concerns regarding the risk of disease progression. Large cohort studies or, preferably, prospective randomized trials are necessary to clarify this issue. Until sufficient data exist, lenalidomide should be used with great caution in MDS with del(5q).
Footnotes
Conflicts of interest: Participation in Celgene advisory board meetings. Funding: Research funding include: Fellowship 2009/06 awarded by the European Hematology Association, The MDS Foundation Young Investigator’s Grant 2006, The Robert Lundberg Foundation, KI/SLL post-doctorate grant 2009.
(Related Original Articles on pages 398 and 406)
References
- 1.Giagounidis AA, Germing U, Haase S, Hildebrandt B, Schlegelberger B, Schoch C, et al. Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia. 2004;18(1):113–9. doi: 10.1038/sj.leu.2403189. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 3.Nilsson L, Astrand-Grundstrom I, Arvidsson I, Jacobsson B, Hellstrom-Lindberg E, Hast R, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96(6):2012–21. [PubMed] [Google Scholar]
- 4.Jaju RJ, Jones M, Boultwood J, Kelly S, Mason DY, Wainscoat JS, et al. Combined immunophenotyping and FISH identifies the involvement of B-cells in 5q- syndrome. Genes Chromosomes Cancer. 2000;29(3):276–80. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1035>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Kiladjian JJ, Bourgeois E, Lobe I, Braun T, Visentin G, Bourhis JH, et al. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia. 2006;20(3):463–70. doi: 10.1038/sj.leu.2404080. [DOI] [PubMed] [Google Scholar]
- 6.Boultwood J, Fidler C, Strickson AJ, Watkins F, Gama S, Kearney L, et al. Narrowing and genomic annotation of the commonly deleted region of the 5q- syndrome. Blood. 2002;99(12):4638–41. doi: 10.1182/blood.v99.12.4638. [DOI] [PubMed] [Google Scholar]
- 7.Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451(7176):335–9. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebert BL. Deletion 5q in myelodysplastic syndrome: a paradigm for the study of hemizygous deletions in cancer. Leukemia. 2009;23(7):1252–6. doi: 10.1038/leu.2009.53. [DOI] [PubMed] [Google Scholar]
- 9.Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, et al. Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med. 2010;16(1):49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 10.Pellagatti A, Jadersten M, Forsblom AM, Cattan H, Christensson B, Emanuelsson EK, et al. Lenalidomide inhibits the malignant clone and up-regulates the SPARC gene mapping to the commonly deleted region in 5q- syndrome patients. Proc Natl Acad Sci USA. 2007;104(27):11406–11. doi: 10.1073/pnas.0610477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92(4):679–90. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- 12.Barlow JL, Drynan LF, Hewett DR, Holmes LR, Lorenzo-Abalde S, Lane AL, et al. A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome. Nat Med. 2010;16(1):59–66. doi: 10.1038/nm.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garderet L, Kobari L, Mazurier C, De Witte C, Giarratana M-C, Pérot C, et al. Unimpaired terminal erythroid differentiation and preserved enucleation capacity in myelodysplastic 5q(del) clones: a single cell study. Haematologica. 2010;95(3):398–405. doi: 10.3324/haematol.2009.012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ximeri M, Galanopoulos A, Klaus M, Parcharidou A, Giannikou K, Psyllaki M, et al. on behalf of the Hellenic MDS Study Group. Effect of lenalidomide therapy on hematopoiesis of patients with myelodysplastic syndrome associated with chromosome 5q deletion. Haematologica. 2010;95(3):406–14. doi: 10.3324/haematol.2009.010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alessandrino EP, Amadori S, Barosi G, Cazzola M, Grossi A, Liberato LN, et al. Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica. 2002;87(12):1286–306. [PubMed] [Google Scholar]
- 16.Bowen D, Culligan D, Jowitt S, Kelsey S, Mufti G, Oscier D, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003;120(2):187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 17.NCCN Clinical Practice Guidelines in Oncology/Myelodysplastic Syndromes. 2008. [Available from: www.nccn.org]
- 18.The Nordic MDS Group Care Program. 2008. [Available from: www.nmds.org/ez4/index.php?/nmds/Nordic-Care-Programme]
- 19.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 20.Melchert M, List A. Targeted therapies in myelodysplastic syndrome. Semin Hematol. 2008;45(1):31–8. doi: 10.1053/j.seminhematol.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Gohring G, Giagounidis A, Busche G, Kreipe HH, Zimmermann M, Hellstrom-Lindberg E, et al. Patients with del(5q) MDS who fail to achieve sustained erythroid or cytogenetic remission after treatment with lenalidomide have an increased risk for clonal evolution and AML progression. Ann Hematol. 2009 Oct 24; doi: 10.1007/s00277-009-0846-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Jadersten M, Saft L, Pellagatti A, Gohring G, Wainscoat JS, Boultwood J, et al. Clonal heterogeneity in the 5q- syndrome: p53 expressing progenitors prevail during lenalidomide treatment and expand at disease progression. Haematologica. 2009;94(12):1762–6. doi: 10.3324/haematol.2009.011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 24.Wei S, Chen X, Rocha K, Epling-Burnette PK, Djeu JY, Liu Q, et al. A critical role for phosphatase haplodeficiency in the selective suppression of deletion 5q MDS by lenalidomide. Proc Natl Acad Sci USA. 2009;106(31):12974–9. doi: 10.1073/pnas.0811267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tehranchi R, Fadeel B, Schmidt-Mende J, Forsblom AM, Emanuelsson E, Jadersten M, et al. Antiapoptotic role of growth factors in the myelodysplastic syndromes: concordance between in vitro and in vivo observations. Clin Cancer Res. 2005;11(17):6291–9. doi: 10.1158/1078-0432.CCR-04-1850. [DOI] [PubMed] [Google Scholar]