Abstract
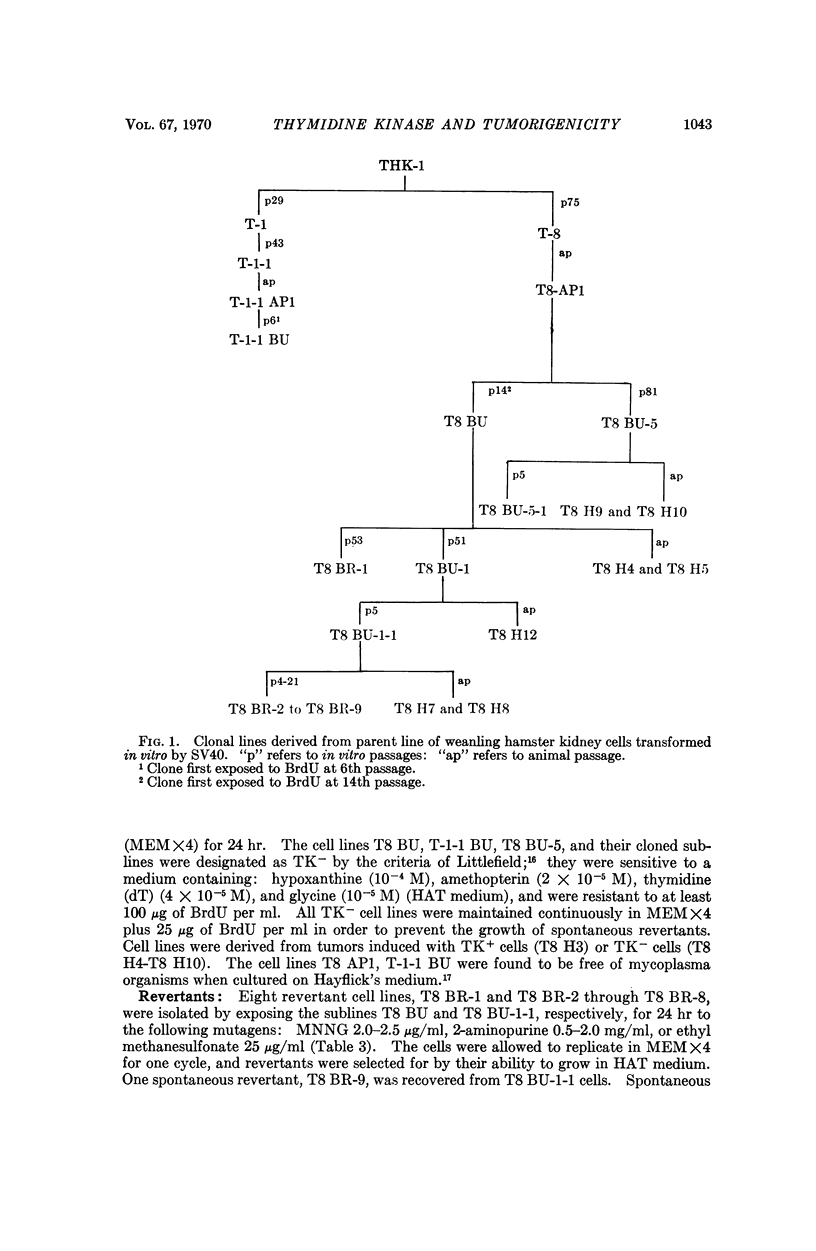
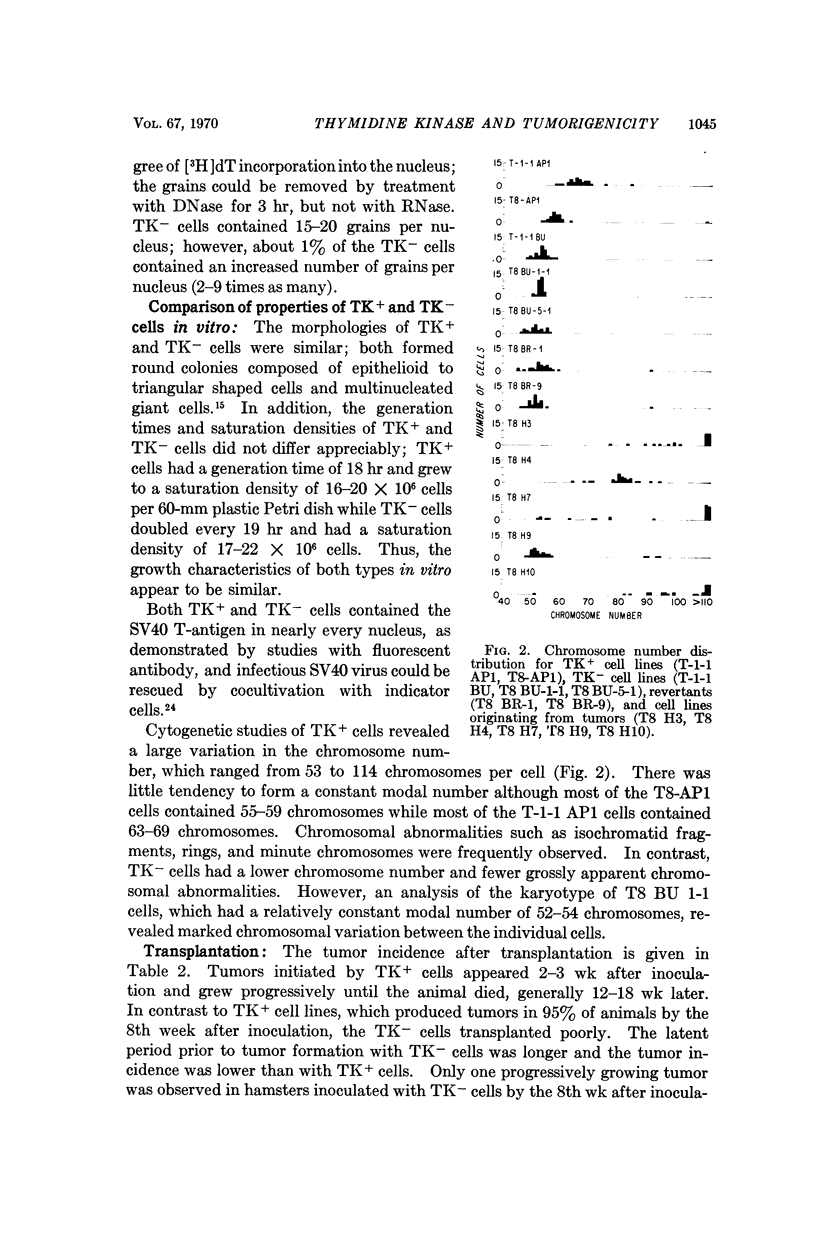
Cells deficient in the enzyme thymidine kinase were derived from transplantable SV40-transformed hamster cells. The resultant cell lines were less transplantable when inoculated into hamsters. Tumors which did arise from such cells had prolonged latent periods and were found to contain a mixture of enzyme-containing and enzyme-deficient cells. Revertant cell lines obtained either spontaneously or after mutagenesis in vitro contained intermediate levels of thymidine kinase activity and displayed an oncogenic potential which was intermediate between the wild type and enzyme-deficient cells. It is postulated that salvage pathway enzymes may play a rate-limiting role in tumorigenesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., ROWE W. P., COOPER H. L. AN ANALYSIS OF SV 40-INDUCED TRANSFORMATION OF HAMSTER KIDNEY TISSUE IN VITRO. II. STUDIES OF THREE CLONES DERIVED FROM A CONTINUOUS LINE OF TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Nov;50:847–854. doi: 10.1073/pnas.50.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P. VIRAL STUDIES OF SV40 TUMORIGENESIS IN HAMSTERS. J Natl Cancer Inst. 1964 Jan;32:253–265. [PubMed] [Google Scholar]
- BUKOVSKY J., ROTH J. S. SOME FACTORS AFFECTING THE PHOSPHORYLATION OF THYMIDINE BY TRANSPLANTABLE RAT HEPATOMAS. Cancer Res. 1965 Apr;25:358–364. [PubMed] [Google Scholar]
- Basilico C., Matsuya Y., Green H. Origin of the thymidine kinase induced by polyoma virus in productively infected cells. J Virol. 1969 Feb;3(2):140–145. doi: 10.1128/jvi.3.2.140-145.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. H. An analysis of SV40-induced transformation of hamster kidney tissue in vitro. 3. Persistence of SV40 viral genome in clones of transformed hamster cells. J Natl Cancer Inst. 1966 Oct;37(4):487–493. [PubMed] [Google Scholar]
- Black P. H., Berman L. D., Maloof R. An analysis of SV40-induced transformation of hamster kidney tissue in vitro. IV. Studies of the pathology of hamster tumors induced with SV40-transformed hamster cell clones. J Natl Cancer Inst. 1966 Oct;37(4):495–504. [PubMed] [Google Scholar]
- Blakley R. L., Vitols E. The control of nucleotide biosynthesis. Annu Rev Biochem. 1968;37:201–224. doi: 10.1146/annurev.bi.37.070168.001221. [DOI] [PubMed] [Google Scholar]
- Bresnick E., Mainigi K. D., Mayfield E. D., Jr, Morris H. P. Activities of enzymes of pyrimidine nucleotide synthesis in slowly growing kidney tumors. Cancer Res. 1969 Nov;29(11):1932–1936. [PubMed] [Google Scholar]
- Bürk R. R., Pitts J. D., Subak-Sharpe J. H. Exchange between hamster cells in culture. Exp Cell Res. 1968 Oct;53(1):297–301. doi: 10.1016/0014-4827(68)90380-7. [DOI] [PubMed] [Google Scholar]
- Chan P. C., Goldman A., Wynder E. L. Hydroxyurea: suppression of two-stage carcinogensis in mouse skin. Science. 1970 Apr 3;168(3927):130–132. doi: 10.1126/science.168.3927.130. [DOI] [PubMed] [Google Scholar]
- Domon M., Rauth A. M. Ultraviolet irradiation of mouse L cells: effects on DNA synthesis and progression through the cell cycle. Radiat Res. 1968 Aug;35(2):350–368. [PubMed] [Google Scholar]
- Eker P. Studies on thymidine kinase of human liver cells in culture. J Biol Chem. 1966 Feb 10;241(3):659–662. [PubMed] [Google Scholar]
- Ephrussi B., Davidson R. L., Weiss M. C., Harris H., Klein G. Malignancy of somatic cell hybrids. Nature. 1969 Dec 27;224(5226):1314–1316. doi: 10.1038/2241314a0. [DOI] [PubMed] [Google Scholar]
- Feinendegen L. E., Bond V. P., Hughes W. L. Physiological thymidine reutilization in rat bone marrow. Proc Soc Exp Biol Med. 1966 Jun;122(2):448–455. doi: 10.3181/00379727-122-31159. [DOI] [PubMed] [Google Scholar]
- Gordon H. L., Bardos T. J., Chmielewicz Z. F., Ambrus J. L. Comparative study of the thymidine kinase and thymidylate kinase activities and of the feedbach inhibition of thymidine kinase in normal and neoplastic human tissue. Cancer Res. 1968 Oct;28(10):2068–2077. [PubMed] [Google Scholar]
- Harris H., Miller O. J., Klein G., Worst P., Tachibana T. Suppression of malignancy by cell fusion. Nature. 1969 Jul 26;223(5204):363–368. doi: 10.1038/223363a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963 Jan;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M. HeLa cells resistant to bromodeoxyuridine and deficient in thymidine kinase activity. Int J Cancer. 1966 Jan;1(1):19–30. doi: 10.1002/ijc.2910010105. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. STUDIES ON THYMIDINE KINASE IN CULTURED MOUSE FIBROBLASTS. Biochim Biophys Acta. 1965 Jan 11;95:14–22. doi: 10.1016/0005-2787(65)90206-6. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THE INOSINIC ACID PYROPHOSPHORYLASE ACTIVITY OF MOUSE FIBROBLASTS PARTIALLY RESISTANT TO 8-AZAGUANINE. Proc Natl Acad Sci U S A. 1963 Sep;50:568–573. doi: 10.1073/pnas.50.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALEY G. F., LORENSON M. G., MALEY F. INHIBITORS OF PROTEIN SYNTHESIS: EFFECT ON THE LEVELS OF DEOXYCYTIDYLATE DEAMINASE, THYMIDYLATE SYNTHETASE, AND THYMIDINE KINASE IN REGENERATING RAT LIVER. Biochem Biophys Res Commun. 1965 Feb 3;18:364–370. doi: 10.1016/0006-291x(65)90715-1. [DOI] [PubMed] [Google Scholar]
- Rothschild H., Black P. H. Analysis of SV40-induced transformation of hamster kidney tissue in vitro. VII. Induction of SV40 virus from transformed hamster cell clones by various agents. Virology. 1970 Sep;42(1):251–256. doi: 10.1016/0042-6822(70)90264-3. [DOI] [PubMed] [Google Scholar]
- SALAMAN M. H., ROE F. J. COCARCINOGENESIS. Br Med Bull. 1964 May;20:139–144. doi: 10.1093/oxfordjournals.bmb.a070307. [DOI] [PubMed] [Google Scholar]


