Abstract
Every individual experiences stressful life events. In some cases acute or chronic stress leads to depression and other psychiatric disorders, but most people are resilient to such effects. Recent research has begun to identify the environmental, genetic, epigenetic and neural mechanisms that underlie resilience, and has shown that resilience is mediated by adaptive changes in several neural circuits involving numerous neurotransmitter and molecular pathways. These changes shape the functioning of the neural circuits that regulate reward, fear, emotion reactivity and social behaviour, which together are thought to mediate successful coping with stress.
Resilience refers to a person's ability to adapt successfully to acute stress, trauma or more chronic forms of adversity. A resilient individual has thus been tested by adversity1 and continues to demonstrate adaptive psychological and physiological stress responses, or `psychobiological allostasis'2,3. The study of resilience, or stress-resistance, originated in the 1970s with a group of researchers who directed their attention to the investigation of children capable of progressing through normal development despite exposure to significant adversity4. For many years research focused on identifying the psychosocial determinants of stress resistance, such as positive emotions, the capacity for self-regulation, social competence with peers and a close bond with a primary caregiver, among other factors5,6. Later studies turned to understanding the psychosocial determinants of resilience in trauma-exposed adults7,8.
It is only in recent years that significant scientific and technological advances have made it possible to begin to understand the underlying biological processes associated with resilient phenotypes3,9. Findings from recent studies suggest that genetic influences on biological responses — such as neural responses to affective stimuli, measured with brain imaging — are larger than genetic influences on complex behavioural responses10,11. Examining stress responses at multiple phenotypic levels, including not only behavioural and psychological measurements, but also measurements of neurochemical, neuroendocrine and neural systems, could thus help to delineate an integrative model of resilience11. Animal studies are a key component in the search for biological determinants of resilience, and are beginning to identify neural circuits and molecular pathways that mediate resilient phenotypes1,12. This Review outlines and attempts to integrate recent developments in resilience research from psychosocial, developmental, genetic and neurobiological perspectives.
Psychosocial factors in resilience
Studies have identified a range of psychosocial factors that promote successful adaptation to stress and that might help to prevent the onset of psychiatric disorders such as post-traumatic stress disorder (PTSD), major depressive disorder and others. We are now beginning to identify the neurobiological mechanisms that underlie some of these traits (BOX 1). The use of active coping strategies such as problem solving and planning has been linked to improved well-being and a greater capacity to handle stressful situations in diverse populations, ranging from trauma-exposed to medically ill individuals13. Active coping with stress requires an individual to face their fears, and resilient individuals exhibit lower levels of denial, avoidant coping behaviour and behavioural disengagement8,14. Resilient individuals are also characterized by dispositional optimism and high positive emotionality15,16. Positive emotions promote adaptive coping and openness to social support15, and are associated with greater flexibility of thinking and exploration, a broadened focus of attention and decreased autonomic activity17. Resilience has been linked to being able to perceive stressful events in less threatening ways, promoting adaptive coping strategies13; such cognitive reappraisal allows individuals to re-evaluate or reframe adverse experiences in a more positive light. Social competence and the ability to harness social support have also been linked to better mental well-being and health. Increased social support has buffering effects on mental and physical illness and fosters adaptive coping strategies13. Other psychosocial characteristics associated with stress resilience include a sense of purpose in life, a moral compass, spirituality and the ability to find meaning in the midst of trauma8,13,18,19.
Although many of the above psychological characteristics cannot be measured in animals, some behavioural traits associated with resilience have been identified. In numerous animal models (see Supplementary information S1 (box)), rodents display a range of responses to stress: at one extreme are active or `fight-flight' responses (for example, attempts to escape and aggression), and at the other extreme are passive responses (for example, freezing and submission)27. Active-coping animals are often considered to be resilient, based on numerous functional end points, whereas their more passive counterparts are not; however, both types of responses can be seen as adaptive depending on the particular context. The availability of these animal models has made it possible to study the neurobiological and molecular mechanisms that underlie these behavioural traits, as is discussed below.
Resilient responses to stress
Numerous hormones, neurotransmitters and neuropeptides are involved in the acute psychobiological responses to stress. Differences in the function, balance and interaction of these factors underlie inter-individual variability in stress resilience.
Hypothalamus-pituitary-adrenal (HPA) axis
Corticotropin-releasing hormone (CRH) is released by the hypothalamus in response to stress, leading to activation of the HPA axis and the release of cortisol. Early life stress has been linked to chronically high levels of CRH in human and animal studies20 (BOX 2). Although the short-term actions of cortisol are protective and promote adaptation, sustained exposure to abnormally high levels of cortisol can be harmful, leading to hypertension, immunosuppression, cardiovascular disease and other health problems21. In the brain, excessive cortisol is associated with complex structural effects in the hippocampus and amygdala in humans and animals, including atrophic effects in certain types of neurons22,23. Thus, reduced CRH release and adaptive changes in CRH receptor activity might promote resilience.
In several animal models and in some human studies, resilience is associated with rapid activation of the stress response and its efficient termination24. Resilience is associated with the capacity to constrain stress-induced increases in CRH and cortisol through an elaborate negative feedback system, involving optimal function and balance of glucocorticoid and mineralocorticoid receptors3,24,25. Studies suggest that the HPA disturbances that are associated with PTSD are different from those that are associated with major depression24. Of note, animals that adopt active responses to environmental threat show lower glucocorticoid responses than those that adopt passive responses, although the relationship between coping strategies and HPA axis activity is probably complex (for example, see REF. 26). In humans these two personality types have been linked with risk for different sets of disorders, demonstrating that the relationship between health and the functioning of the stress response system is complex27. In addition, dehydroepiandrosterone (DHEA), which is also released in response to stress, has antiglucocorticoid effects in the brain. Higher DHEA sulphate/cortisol ratios in individuals undergoing rigorous military survival training were associated with lower dissociative symptoms and better military performance, possibly indicating higher resilience to stress28. In a study of male veterans with PTSD, higher DHEA levels were associated with symptom improvement29. DHEA has additional central effects in the brain, notably on the GABA (γ-aminobutyric acid)-ergic system, which could also play a part in resilience30.
Noradrenergic system
Stress also leads to release of noradrenaline from brainstem nuclei, most importantly the locus coeruleus. The result is increased noradrenergic stimulation of numerous forebrain areas implicated in emotional behaviour, such as the amygdala, the nucleus accumbens, the prefrontal cortex (PFC) and the hippocampus (see below). Unchecked, chronic hyper-responsiveness of the locus coeruleus noradrenergic system is associated with anxiety disorders and cardiovascular problems, and blockade of β-adrenergic receptors in the amygdala can oppose the development of aversive memories in animals and humans31,32. This suggests that reduced responsiveness of the locus coeruleus noradrenergic system could promote resilience.
Serotonergic and dopaminergic systems
Serotonin neurons project widely in the brain. Acute stress is associated with increased serotonin turnover in several brain regions, including the amygdala, the nucleus accumbens and the PFC. Serotonin modulates neural responses to stress, with both anxiogenic and anxiolytic effects (depending on the brain region and receptor subtype involved)3. Serotonin function is also closely linked to mood regulation. Dopamine neurons are activated in response to reward or the expectation of reward and generally are inhibited by aversive stimuli, as detailed below. Dopamine signalling facilitates fear extinction, but its role in resilience per se is unclear.
Neuropeptide Y (NPY)
NPY, a neuropeptide that is widely distributed in the brain, has anxiolytic-like effects in rodents and is thought to enhance cognition under stressful conditions. NPY also counteracts the anxiogenic effects of CRH in the amygdala, the hippocampus, the hypothalamus and the locus coeruleus, and resilience might involve maintaining a balance between NPY and CRH levels during stress33. In a study of special forces soldiers, who are considered to be highly stress resilient, higher NPY levels during rigorous military training were associated with better performance34. Another study found higher plasma NPY levels in combat-exposed veterans without PTSD than in those with PTSD35. These findings in humans are consistent with recent studies in rats: central administration of NPY in rats inhibits the development, and promotes the extinction, of fear conditioning, with NPY antagonists exerting the opposite actions. These effects are mediated at least in part by the amygdala36. Moreover, intra-amygdala NPY administration promotes resilient responses to stress, in the form of reduced anxiety-like behaviours in response to acute restraint37.
Brain-derived neurotrophic factor (BDNF)
BDNF, an important nerve growth factor that is expressed at high levels in the brain, is best known for its role, in rodent models, in promoting the functioning of the adult hippocampus, including the survival of newly born granule cell neurons throughout adult life. In rodents, stress decreases BDNF expression in the hippocampus, an effect that is reversed by chronic antidepressant treatment38. Similar findings have been observed in human hippocampus examined post-mortem. However, BDNF exerts very different effects in other brain regions. Chronic stress increases BDNF expression in the rodent nucleus accumbens, and this has been linked to prodepression-like effects in several behavioural assays39,40. Interestingly, this induction of BDNF is causally related to the degree to which rodents are vulnerable versus resilient to the deleterious effects of stress (for example, passive coping, social avoidance and anhedonia), with resilient individuals showing no increase in BDNF levels41. Humans with depression also show increased BDNF levels in the nucleus accumbens41, which highlights the very different effects exerted by BDNF in different neural circuits. Indeed, stress also produces distinct effects on BDNF in the amygdala and the PFC, but BDNF in these regions has not yet been studied in resilience models.
Genetic influences on resilience
Complex interactions between an individual's genetic make-up and his or her particular history of exposure to environmental stressors determine the degree of adaptability of neurochemical stress response systems (reviewed above) to new adverse exposures, as well as the function of the neural circuitry involved in stress responses, discussed below.
HPA axis-related genes
Regulation of the HPA axis is affected by genetic factors. A recent study in two independent populations found that polymorphisms and haplotypes of the CRH type 1 receptor gene (CRHR1) (for example, a haplotype formed by three single-nucleotide polymorphisms (SNPs) in intron 1) moderate the influence of child abuse on depressive symptoms in adulthood, with certain alleles (rs7209436 and rs242940) and haplotypes exerting a protective effect42. Functional variants of the brain mineralocorticoid and glucocorticoid receptor (GR) genes, which are respectively involved in setting the threshold and regulating the termination of the HPA axis response to stress, have also been identified in humans25. For example, carriers of the N363S variant of the GR gene were shown to exhibit higher cortisol responses to the Trier Social Stress Test, a stress-inducing public speaking and mental arithmetic task43. Interestingly, four SNPs of FKBP5 (rs9296158, rs3800373, rs1360780 and rs9470080), a gene that codes for a `chaperone' protein that regulates GR sensitivity, were found to interact with the severity of childhood abuse in the prediction of PTSD symptoms in adults44. Another study demonstrated an association between genetic variation in FKBP5 and inefficient recovery of HPA axis activity after the Trier Social Stress Test in healthy participants, identifying a potential risk factor for chronically elevated cortisol levels and ultimately stress-related psychopathology45.
Serotonin transporter
The best-studied gene-environment interaction involves a naturally occurring variation in the promoter of the human serotonin transporter gene (5-HTTLPR; also known as SLC6A4). The short allele of 5-HTTLPR is associated with decreased serotonin transporter availability and a resulting lower reuptake of serotonin from synaptic clefts. Carriers of the short allele show elevated risk for depression on exposure to stressful life events, including childhood maltreatment, compared with long-allele homozygotes in some but not all studies46–48. A recent meta-analysis has called into question whether this reported gene-environment interaction truly modifies risk for major depression given the limited sample sizes used in the studies49. Functional brain imaging studies have demonstrated increased amygdala reactivity to environmental threat50 and decreased coupling between the amygdala and the regulatory perigenual cingulate region51 in short-allele carriers, representing likely biological markers of increased susceptibility to stress in these individuals. These findings were confirmed in another recent meta-analysis, with this polymorphism accounting for up to 10% of phenotypic variance52. A recent study found an association between the long allele of 5-HTTLPR and emotional resilience in college students53.
Catechol-O-methyltransferase (COMT)
Another polymorphism that is relevant to resilience (Val158Met) is found in the gene that codes for COMT, an enzyme that degrades dopamine and noradrenaline. Individuals with the low-functioning Met158 allele have higher circulating levels of these neurotransmitters. Possibly as a result, they tend to exhibit higher anxiety levels, increased plasma adrenaline levels in response to stress, lower resilience to negative mood states, and increased limbic reactivity to unpleasant stimuli54. Recent functional MRI (fMRI) studies have extended the link between the COMT Met158 allele and increased limbic reactivity to other emotion-processing and reward tasks, and have shown differential cortico-limbic connectivity in Met158 allele carriers, which is further discussed below55.
NPY
A recent study showed greater amygdala reactivity to threat-related facial expressions in individuals with a low-expression diplotype of the gene that encodes NPY than in individuals with other diplotypes11. The level of NPY mRNA expression showed an inverse correlation with trait anxiety, as well as a direct correlation with levels of stress-induced endogenous opioid release, which is implicated in the suppression of pain and stress responses11.
BDNF
An SNP (G196A, Val66Met) in the gene that encodes BDNF in humans significantly impairs BDNF's intracellular trafficking and activity-dependent release56. It is also associated with reduced hippocampal volume57. Mice that express the Met66 BDNF allele, which is associated with reduced BDNF function, show greater anxiety-like behaviour and impaired hippocampus-dependent learning, but are more resilient to chronic stress41,56. These data underscore the complex effects of BDNF on the brain, which are highly region specific.
Gene-gene and gene-environment interactions
Recent studies have begun to yield evidence of gene-gene and gene-environment interactions underlying inter-individual variability in stress responses. Findings include a monoamine oxidase A (MAOA)-COMT interaction that affects endocrine responses to a psychological challenge task58, a 5-HTTLPR-COMT-stressful life events interaction that affects the risk for depression59, and a COMT-5-HTTLPR interaction that affects limbic reactivity to unpleasant stimuli in healthy subjects60. Kaufman and collaborators reported gene-environment interactions that influence the risk for depression in maltreated children. Social support seems to mitigate the effects of the short allele of 5-HTTLPR61, and the 5-HTTLPR and BDNF Val66Met genotypes interact with stressful life events to predict risk for depression62,63.
Epigenetic mechanisms of resilience
Epigenetics refers to stable changes in chromatin structure that underlie long-lasting alterations in gene expression and that are not associated with changes in DnA sequence64. Epigenetic mechanisms involve altered acetylation or methylation of histones, or methylation of DnA itself, among many other modifications. When these changes occur in germ cells they can be heritable.
Direct evidence for epigenetic changes that occur during brain development and underlie sensitivity to stress comes from work by Meaney and colleagues65. Some rat mothers naturally display high levels of nurturing behaviours, such as licking, grooming and arched-back nursing, whereas others display low levels of such behaviours. Offspring of high-nurturing mothers are less anxious and display more nurturing maternal behaviour towards their own pups. They also have attenuated corticosterone responses to stress and express higher levels of GR in the hippocampus. This enhanced GR expression is mediated in part by the transcription factor nerve growth factor-inducible protein A (NGFI-A; also known as EGR1) (FIG. 1). Interestingly, pups that received little nurturing show increased methylation of the GR gene promoter at the NGFI-A binding site in the hippocampus, an epigenetic change that is associated with reduced GR expression66. This difference in methylation emerges in the first week of life and persists into adulthood. As a result, adult offspring of low-nurturing mothers have reduced hippocampal GR expression, which contributes to the behavioural deficits that these animals exhibit and pass on to their offspring. The finding that there are epigenetic changes that underlie life-long differences in behaviour suggests that drugs that influence DNA methylation and related epigenetic mechanisms might promote resilience in humans.
Figure 1. Epigenetic mechanisms of stress responsiveness.
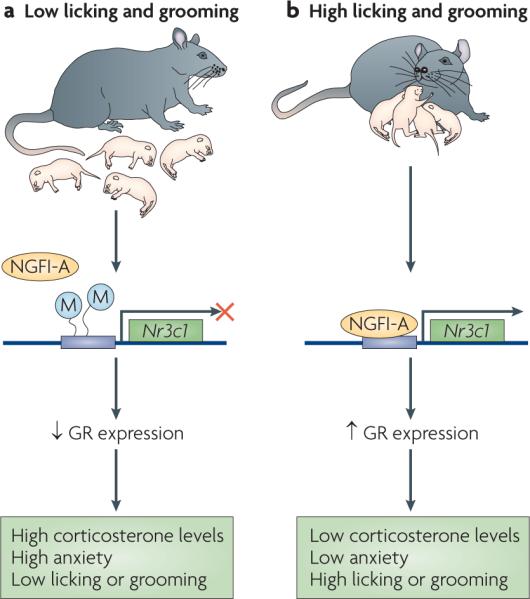
Female rats show a range of maternal behaviours, from low levels of licking and other types of grooming of their pups to high levels. These differences during early life can give rise to life-long differences in stress responsiveness65,66. a | Receiving low levels of grooming results in low levels of the transcription factor nerve growth factor-inducible protein A (NGFI-A; also known as EGR1) in the hippocampus, which permits increased methylation and repression of the glucocorticoid receptor (GR) gene in this brain region. Lower levels of GR expression in the hippocampus contribute to several traits in adulthood: higher levels of baseline and post-stress glucocorticoid (corticosterone) secretion, higher levels of anxiety-like behaviour and, in females, lower levels of grooming behaviour towards their own offspring. b | The offspring of high-grooming mothers have higher levels of hippocampal NGFI-A, resulting in less methylation of the GR gene and higher GR expression in the hippocampus. In adulthood this is associated with lower levels of baseline and post-stress corticosterone secretion, low anxiety-like behaviour and, in females, high levels of grooming of offspring.
The demonstration that resilient and vulnerable responses to stress are dramatically distinct in populations of genetically identical mice with highly controlled environmental histories41 (see Supplementary information S1 (box)) raises the possibility that a third type of mechanism — epigenetics — could be involved. The hypothesis is that stochastic, epigenetic changes that occur during brain development are an additional means by which behavioural variability is generated in individuals, better preparing the species for a host of possible environmental challenges. According to this scheme, random epigenetic changes that drive resilience would promote survival during periods of extreme duress, whereas those associated with vulnerability would generate animals that cope better in times of plenty.
Transcriptional mechanisms of resilience
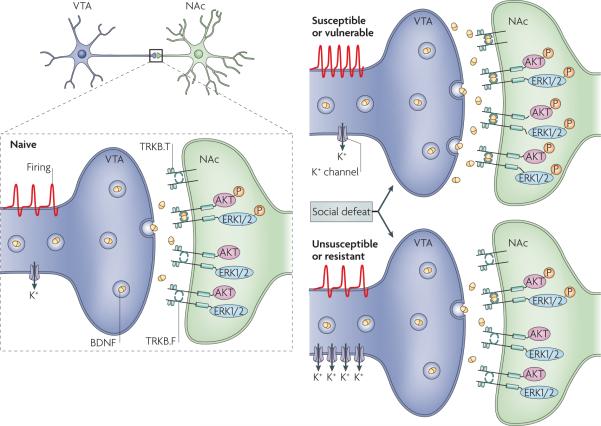
Animal research has begun to provide insight into the molecular mechanisms that underlie resilience. One series of studies has focused on the ventral tegmental area (VTA)-nucleus accumbens reward circuit. In the social defeat stress model40,41 (see Supplementary information S1 (box)) resilience, among a population of inbred (genetically identical) mice, is associated not only with the absence of many of the changes in gene expression that are seen in the VTA-nucleus accumbens of vulnerable mice, but also with the induction of distinct changes in gene expression that occur in resilient mice only41. These findings underscore the view that resilience is not simply the absence of maladaptive changes that occur in vulnerable individuals; rather, it is mediated by a unique set of adaptive changes. As just one example, chronic defeat stress induces in resilient mice the expression of several K+ channel subunits in VTA dopamine neurons, which prevents the stress-induced increase in VTA excitability and the consequent release of BDNF onto the nucleus accumbens — maladaptations that contribute to vulnerability41,67 (FIG. 2). The transcriptional regulation of dozens of additional genes in the VTA and nucleus accumbens of resilient mice now provides novel pathways towards understanding the molecular basis of resilience as well as towards developing new treatments for depression and other stress-related disorders.
Figure 2. Neurobiological mechanisms of resilience in a mouse model.
In a chronic social defeat paradigm, vulnerable mice show increased firing of ventral tegmental area (VTA) dopamine neurons, which subsequently gives rise to heightened brain-derived neurotrophic factor (BDNF) protein levels in the nucleus accumbens (NAc) and to a range of depression-like behaviours40,41. Vulnerable mice also showed activation of signalling molecules downstream of the BDNF receptor TRKB, including phosphorylated AKT and extracellular signal regulated kinase 1 and 2 (ERK1/2), indicating increased BDNF signalling. Unsusceptible or resilient mice resist this adverse cascade of events by upregulating several K+ channels in the VTA. TRKB.T, truncated TRKB receptor; TRKB.F, full-length TRKB receptor. Figure is modified, with permission, from REF. 41 © (2007) Cell Press.
In a related study, individual variations in the development of learned helplessness (see Supplementary information S1 (box)) among inbred mice were related to the expression of a transcription factor, FoSB, in the ventrolateral region of the periaqueductal grey (vlPAG) in the midbrain68. This brain area has long been associated with passive responses to stress69, which can predict vulnerability over resilience (see above). Berton et al.68 showed that the induction of FoSB in vlPAG neurons in resilient animals suppressed the expression of the neuropeptide substance P in these cells, which in turn reduced substance P transmission to target regions such as the nucleus accumbens and perhaps the amygdala. Inhibition of substance P in the nucleus accumbens specifically was then shown to be sufficient to mimic resilience. These observations suggest the use of substance P antagonists — that is, neurokinin 1 receptor antagonists — as a way to promote resilience in humans.
Neural circuitry of resilience
A growing number of brain imaging studies in humans, along with work in rodents and non-human primates, are beginning to define the brain circuits that mediate distinct aspects of mood and emotion under normal circumstances and in various pathological conditions that are indicative of low resilience. The field has identified several limbic regions in the forebrain, which are highly inter-connected and function as a series of integrated parallel circuits that regulate emotional states (FIG. 3). In the sections that follow, we review how these various regions interact to mediate distinct emotional behaviours that are related to resilience. The neural regulation of endocrine and autonomic responses to stress, described in detail in REF. 70, can be studied in humans by monitoring stress responses during functional imaging studies71.
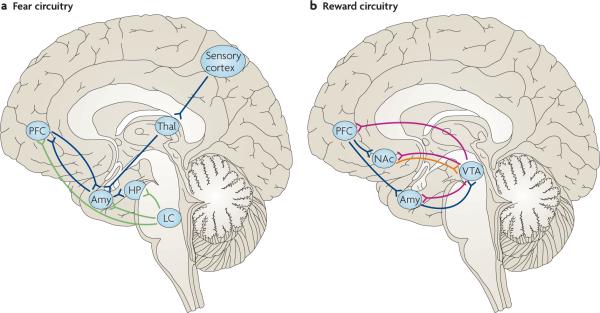
Figure 3. Neural circuitries of fear and reward.
A simple schematic of the key limbic regions in the fear and reward circuitries. These regions are highly interconnected and function as a series of integrated parallel circuits that regulate emotional states. Each is heavily innervated by the brain's monoaminergic systems — noradrenaline (from the locus coeruleus (LC)), dopamine (from the ventral tegmental area (VTA)) and serotonin (from the raphe nuclei (not shown)) — which are thought to modulate the activity of these areas. a | Fear-inducing sensory information is relayed through the thalamus (Thal) to the amygdala (Amy). The amygdala is particularly important for conditioned aspects of learning and memory, as is best studied in fear models. The hippocampus (HP) has a crucial role in declarative memory, but it probably functions more broadly in regulating emotional, including fear, behaviour82. b | The nucleus accumbens (NAc) is a key reward region that regulates an individual's responses to natural rewards and mediates the addicting actions of drugs of abuse. The prefrontal cortex (PFC) — which is composed of multiple regions (for example, the dorsolateral PFC, the medial PFC, the orbitofrontal cortex and the anterior cingulate cortex, among others) with distinct but overlapping functions — is sometimes also included in the limbic system and is essential to emotion regulation. PFC regions provide top-down control of emotional responses by acting on both the amygdala and the NAc (a and b). Several regions that are important for fear and reward learning are not shown in the respective circuits; for example, the NAc also regulates responses to fearful stimuli and the hippocampus also regulates responses to rewarding stimuli. The limbic regions depicted are also part of integrated circuits that mediate social responses and behaviour. The functional status of all of these circuits has important implications for resilience to stressful life events. Notably, alterations in one neurotransmitter, neuropeptide or hormone system will affect more than one circuit. Blue lines represent glutamatergic connections; green lines represent noradrenergic connections; red lines represent dopaminergic connections; the orange line represents a GABA (γ-aminobutyric acid)-ergic connection.
Neural circuitry of fear
Current models of the patho-physiology of PTSD, an example of conditions that are characterized by diminished resilience on exposure to a traumatic stressor, involve abnormal fear learning and an underlying dysfunction in the neural circuitry of fear, comprising the amygdala, the hippocampus and the ventromedial PFC (vmPFC)72,73 (FIG. 3). Brain imaging studies in healthy participants have shown that acquisition of fear conditioning is centred in the amygdala74, whereas extinction of fear memory involves both the vmPFC and the amygdala; activation in these structures, as well as the thickness of the vmPFC, has been associated with extinction success74,75. A recent fmRI study examined the ability of physiological and neural fear responses to adapt flexibly to stimuli that changed from threatening to safe, and from safe to threatening76. Both the initial fear response and the subsequent flexible shift were associated with activation of a network that includes the amygdala, the striatum and the vmPFC76. In particular, the vmPFC seemed to mediate the shifting of fear to a different stimulus under stressful conditions76. Findings from a recent study suggest that emotion regulation — a more advanced cognitive function — of conditioned fear might act through connections with more basic mechanisms of fear extinction, which humans share with other species77.
The neural circuitry of fear is clearly important in resilience, but it has not been studied carefully in resilient individuals. It is possible that a well-functioning system in resilient individuals can prevent over-generalizing from specific conditioned stimuli, induce differential functioning of reconsolidation and extinction processes, or lead to an increased capacity for enhanced inhibition of amygdala responses by the medial PFC (mPFC) in stressful situations78. Of note, preliminary findings suggest that treating patients with PTSD using cognitive behaviour therapy might have beneficial effects, by reducing amygdala activation and increasing rostral anterior cingulate cortex (ACC) activation during fear processing79.
Much of our knowledge about the neural circuitry of fear comes from animal studies. The amygdala mediates the ability of cues that were associated with a fearful stimulus (for example, a footshock or a predator odour) to become aversive in their own right, as established in fear conditioning and related paradigms80,81. The hippocampus mediates contextual and temporal aspects of fear conditioning82. Reactivation of memories (that is, re-exposure to the cue or context) can lead to either reconsolidation (further strengthening of the memory) or extinction, with extinction generally requiring more intensive training. Conversely, conditioned inhibition of fear, in which animals are trained to feel protected from a threat in a certain environment, induces several antidepressant-like effects in mice83. Animal studies have also made it possible to examine distinct functions of central, basolateral and medial amygdala nuclei84.
Both amygdala- and hippocampus-dependent fear conditioning in animals have been related to long-term potentiation and other forms of synaptic plasticity. Accordingly, blockade of NMDA (N-methyl-D-aspartate) glutamate receptors in the amygdala blocks cue-associated fear conditioning, and NMDA receptor blockade in the hippocampus blocks context-dependent fear conditioning85. Consistent with the notion that extinction represents the formation of a new memory rather than the erasure of an existing memory, administration of D-cycloserine, an NMDA receptor partial agonist, can enhance extinction of fear conditioning in animal models and in patients with PTSD undergoing prolonged exposure therapy86. Blockade of β-adrenergic receptors in the amygdala can also block cue-dependent fear conditioning, and β-adrenergic antagonists have been tested in patients exposed to trauma, with mixed results32,87. In addition, fear conditioning and its extinction are regulated by activation of GRs in the hippocampus and perhaps in the amygdala, which suggests the possible use of glucocorticoids in the treatment of trauma88. However, whether β-adrenergic and GR levels or functioning are different in resilient and non-resilient individuals has not yet been studied.
Neural circuitry of reward
Patients with major depressive disorder and PTSD have shown evidence of reward system dysfunction in fmRI studies, with reduced striatal activation during the performance of reward-related tasks89–91. Altered activation of reward circuits in depressed adolescents was associated with self-reports of reduced positive affect in naturalistic settings92. Differential reward system function has also been demonstrated in children of depressed versus never-depressed parents93. Of note, there is evidence that inter-individual variability in neural responses to reward anticipation in healthy individuals is associated with the Val158met COMT polymorphism55.
Trait optimism, which is linked to resilience (as discussed above), might relate to reward circuit function. Sharot et al.94 scanned participants who were imagining positive and negative future events. Optimism bias — the tendency to expect future events to be positive — was associated with higher activation in the amygdala and the rostral ACC when imagining positive events than when imagining negative events. The level of activation in the rostral ACC was positively correlated with dispositional optimism94. Research on special forces soldiers showed that their reward-processing regions had higher reactivity than those of healthy civilian controls95. Conversely, susceptibility to social-reward frustration in healthy males was associated with increased activation in prefrontal (top-down control) areas during performance of a monetary task96.
Animal studies have greatly informed our understanding of the brain's reward circuitry and its possible importance for resilience. The best-established reward circuit is the mesolimbic dopamine system, which involves dopaminergic neurons of the VTA and their innervation of the nucleus accumbens and many other forebrain limbic regions (FIG. 3). VTA dopamine neurons can be viewed as gauges of reward: they are activated in response to a reward (for example, food, sex or social interaction) or even the expectation of a reward, and are inhibited by an aversive stimulus or the absence of an expected reward97. However, certain dopaminergic neurons are also activated by aversive stimuli, suggesting that they are more generally involved in mood regulation98. Indeed, an increasing number of studies report the involvement of the VTA–nucleus accumbens circuit in depression and antidepressant responses in humans and rodents, although there is not yet a clear consensus on the role of dopamine function per se in resilience and vulnerability. A recent study in the social-defeat paradigm in mice (see Supplementary information S1 (box)) has shown that increased activity of VTA dopamine neurons mediates vulnerability by increasing the activity-dependent release of BDNF onto nucleus accumbens neurons, and that resilient animals escape vulnerability in part by upregulating K+ channels in the VTA to prevent this increase in neuronal excitability and BDNF release41,67 (FIG. 2).
Neural circuitry of emotion regulation
A greater capacity for emotion regulation has also been related to stress resilience, and studies of individuals with psychiatric disorders suggest that they have abnormalities in their emotion regulation systems5,99. A neural model of emotion regulation consisting of ventral and dorsal systems has been described, with various patterns of abnormalities associated with a range of psychiatric disorders100,101. Studies in mood and anxiety disorders have most consistently identified abnormalities in amygdala, hippocampus, subgenual ACC and PFC function102.
In healthy individuals, differential amygdala reactivity to negative stimuli could represent an intermediate phenotype associated with vulnerability to anxiety and depressive disorders60. Indeed, several studies have linked the short allele of 5-HTTLPR and the Met158 allele of COMT with higher anxiety levels, vulnerability to negative mood, increased amygdala reactivity to negative stimuli and altered functional coupling between the amygdala and the cortex50,51,103,104. Furthermore, individual differences in cortico-limbic connectivity suggest that some people might have a genetic predisposition to inflexible emotion processing103. As mentioned above, recent imaging studies have shown evidence that multiple gene interactions have an effect on limbic reactivity to unpleasant stimuli60. In addition, studies of healthy children and young adults at high familial risk for depression have yielded evidence that their neural responses to emotional stimuli differed from those of controls at low familial risk for depression93,105.
One mechanism of emotion regulation — cognitive reappraisal — has received particular attention. fMRI studies have shown increased activation in lateral and medial PFC regions and decreased amygdala activation during reappraisal, with increased activation in the lateral PFC associated with reappraisal success106,107. It has thus been suggested that the PFC regulates the intensity of emotional responses by modulating the activation of the amygdala.
A recent fMRI study108 using mediation analysis demonstrated that the vlPFC acts on both the amygdala and the nucleus accumbens, resulting in opposite behavioural responses: the pathways through the amygdala and the nucleus accumbens were associated with reduced and increased reappraisal success, respectively108. These findings are consistent with animal studies, which have established that the amygdala and the nucleus accumbens work in concert to regulate an individual's responses to both negative and positive emotional stimuli. Thus, variability in the functions of these two pathways might underlie individual differences in emotional response and emotion regulation in stressful contexts. Greater use of reappraisal in everyday life has also been linked to greater PFC and lower amygdala activation to negative stimuli, suggesting that there might be a central mechanism through which reappraisal could promote successful coping and reduce the risk of mood disorder onset109.
A recent fMRI study found that resilient women with a history of sexual trauma were more successful at cognitively enhancing emotional responses to aversive pictures than women with PTSD after sexual trauma and healthy, non-traumatized controls. This increased capacity to enhance emotional responses was associated with increased PFC activation110. These results highlight the complexity of emotion regulation systems and suggest that resilience could also be associated with the ability to sustain attention to unpleasant stimuli. Perhaps this increased attention is related to a more accurate or optimistic appraisal of the perceived threats.
Additional neural circuits relevant to social behaviour
The capacity for empathy enables individuals to generate appropriate emotional responses in social contexts and might be related to social competence, which is a characteristic of resilient individuals111,112. Recent years have seen a surge of interest in the study of the so-called mirror neuron system, which comprises cortical neurons that fire similarly when an animal performs a task or observes another animal of the same species performing that task113. It is proposed that this system, acting in conjunction with limbic brain regions, has a central role in understanding others' emotions and intentions112. In humans, the vmPFC is activated both when people think about their own mental states and when they think about those of other people, and patients with lesions of this region have deficits in social emotions such as shame, guilt and empathy. Much future work is needed to understand possible links between the capacity for empathy, mirror neuron system function and resilience. Preliminary findings of greater activation in presumed mirror neuron and associated limbic areas during imitation of emotional faces in children with higher levels of empathy and interpersonal competence are promising114. Of note, a recent study suggests that the neuropeptide oxytocin could improve a person's ability to infer the mental states of others115.
The role of oxytocin in promoting social attachment in humans has recently received increased attention. A study in healthy men participating in a laboratory-based economic trust game showed that intranasal administration of oxytocin increased trust, and suggested involvement of the amygdala116. Imaging studies have demonstrated that mutual cooperation induces activation in reward circuitry regions, which are modulated by oxytocin and vasopressin117. Conversely, oxytocin reduced amygdala activation in response to fear-inducing visual stimuli and reduced connectivity between the amygdala and brainstem areas that mediate autonomic and behavioural fear responses118. Oxytocin thus seems to facilitate social attachment by enhancing the reward value of social stimuli and reducing potential fear responses119. In laboratory animals, central release of oxytocin and vasopressin regulates anxiety and social behaviour120. In rodent species, oxytocin and vasopressin increase social recognition, pair bonding and affiliation121.
Social contact promotes health and well-being122,123. An fMRI study of married women demonstrated that holding hands with their husband attenuated neural responses to the threat of receiving a shock, a response that was proportional to the quality of their relationship124. As discussed above, social competence and openness to social support are core characteristics of resilient individuals, and these qualities might help to modulate central responses to stress in these individuals. The effects of social contact on neural responses to threat, and the potential involvement of neuropeptides that promote social attachment, warrants direct investigation in resilient individuals.
An integrated model of resilience
Stress resilience refers to an individual's capacity for successful adaptation to acute stress, trauma or more chronic forms of adversity. Although the range of complex mechanisms that lead to resilient phenotypes is far from being fully determined, a model of resilience has begun to emerge from the study of adaptive stress responses at multiple phenotypic levels. Beginning in development, an individual's genes and their inter action with environmental factors (and perhaps with stochastic epigenetic events) shape the neural circuitry and neuro chemical function that are expressed in an observable range of psychological strengths and behaviours characteristic of resilient individuals. Various genetic polymorphisms affect a person's limbic reactivity and prefrontal-limbic connectivity, influencing their initial responses to negative or traumatic events, as well their capacity for cognitive reappraisal of those events.
The functional capacity of the brain structures that are involved in the integrated circuits that mediate mood and emotion determines stress resilience, and is in turn reflected in an individual's psychological make-up. More adaptive functioning of fear, reward, emotion regulation or social-behaviour circuits is thought to underlie a resilient individual's capacity to face fears, experience positive emotions, search for positive ways to reframe stressful events and derive benefit from supportive friendships. Thus, resilience is an active process, not just the absence of pathology, and it can be promoted by enhancing protective factors. Much more study is required to achieve a deeper understanding of stress resistance — in particular, research in resilient individuals who have recovered from traumatic experiences.
Can individuals become more resilient? Studies have shown that certain forms of psychotherapy can enhance psychological attributes associated with resilience. For example, cognitive behavioural therapy can enhance optimism and facilitate reappraisal of traumatic events in a more positive light. Other forms of therapy can promote meaning making and help to preserve a person's sense of purpose in the face of trauma125. Interventions early in development are likely to maximize stress resistance. In addition, findings from animal and human studies might eventually yield new pharmacologic agents that can maximize the adaptive function of the HPA axis as well as monoamine, neuropeptide and other neurochemical stress response systems. Finally, new intervention modalities might become possible with increased understanding of the neural circuitry that underlies resilience, as illustrated by recent studies demonstrating that individuals can be trained to modulate their own brain activity using real-time fMRI-based neurofeedback126,127.
Box 1 Psychosocial factors and possible neurobiological underpinnings associated with resilience.
Facing fears and active coping
Facing fears promotes active coping strategies such as planning and problem solving. The ability to face one's fears might be facilitated by stress inoculation (exposure to tolerable levels of stress) during development, and might be linked to the optimal functioning of fear extinction mechanisms. Active, or `fight-flight', responses in animals have been linked to more transient activation of the hypothalamus-pituitary-adrenal (HPA) axis27, although the relationship between HPA axis activity and active or passive coping might not be straightforward, as positive associations have also been found26. Physical exercise, which can be viewed as a form of active coping, has positive effects on mood, attenuates stress responses and is thought to promote neurogenesis13.
Optimism and positive emotions
Positive emotions might contribute to healthier cognitive responses17,128 and decreased autonomic arousal128. Mesolimbic dopamine pathways might be more reward responsive and/or stress resistant in individuals who remain optimistic when faced with trauma3. Accordingly, resilience in animals has been related to specific molecular adaptations in the mesolimbic dopamine system41.
Cognitive reappraisal, positive reframing and acceptance
Cognitive reappraisal involves reinterpreting the meaning of negative stimuli, with a resulting reduction in emotional responses. Resilient individuals might be better at reappraisal or might use reappraisal more frequently. Neurobiological mechanisms that underlie some of these processes include memory suppression, memory consolidation and cognitive control of emotion106,107.
Social competence and social support
Social competence and openness to social support promote resilience in children and adults5,13. Mutual cooperation is associated with activation of brain reward circuits. Oxytocin enhances the reward value of social attachments and reduces fear responses. Future research might identify potential differences in these measures in resilient individuals.
Purpose in life, a moral compass, meaning and spirituality
A sense of purpose and an internal framework of beliefs about right and wrong are characteristic of resilient individuals8,13. Religious and spiritual beliefs and practices might also facilitate recovery and finding meaning after trauma13. Brain imaging studies are beginning to identify the neural correlates of human morality129.
Box 2 Development of resilience.
Early life stress and vulnerability
Early physical and sexual abuse is associated with long-lasting interrelated hormonal, neurotransmitter and CNS changes20,130,131 that are likely to mediate increased vulnerability to psychiatric disorders into adulthood.
Animal studies have shown that prolonged maternal separation in early life has enduring adverse effects on stress responsivity20.
Resilience to early life stress
Studies in children adopted away from institutional orphanages in Romania illustrate the capacity of adaptive systems to resist or recover from marked disturbances when they are healthy and functional4,132.
Rodent studies have demonstrated that a positive, or more enriched, environment during development makes animals less vulnerable to drugs of abuse and to stress later in life133, and can even reverse some of the behavioural impairments that are induced by early prolonged maternal separation134.
Resilience factors
During development a range of factors are potentially protective, as identified in studies of children. These include a close relationship with a caring adult, social competence and agreeableness, positive emotionality and the capacity for self-regulation135–137. In particular, studies have shown that proximity to the caregiver is an important modulator of a child's sense of safety when facing trauma, as recognized in the theory of attachment122,123. Rodent studies have shown that high levels of licking, grooming and arched-back nursing produce offspring that are less fearful as adults and that show attenuated hormonal responses to stress66. Cross-fostering experiments have demonstrated that these effects are transmitted behaviourally, and recent research has suggested an epigenetic basis for this phenomenon (see main text)66,138.
It is also likely that exposure to manageable stressors during development is associated with more adaptive coping with stress during adulthood139. In studies of squirrel monkeys and rodents, early exposure to manageable stressors (`stress inoculation') was found to be associated with reduced behavioural and hormonal responses to stress later in life65,140. Adaptive responses seem to be associated with the degree of behavioural control an animal has over stress141.
Finally, the theory of predictive adaptive responses posits that early life experience can programme a certain set of responses that may be adaptive given a particular early environment, but that may prove maladaptive later on if there is a mismatch between this set of responses and the environment in adulthood142. In a recent rodent study, the adult offspring of low- as opposed to high-licking and grooming mothers demonstrated greater experience-dependent structural plasticity and learning under some contexts, but lesser structural and behavioural plasticity in other contexts143.
Supplementary Material
Footnotes
DATABASES Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene 5-HTTLPR | BDNF | COMT | CRHR1 |FKBP5 | MAOA | NGFI-A | NPY
SUPPLEMENTARY INFORMATION See online article: S1 (box)
References
- 1.Rutter M. Implications of resilience concepts for scientific understanding. Ann. NY Acad. Sci. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Mood disorders and allostatic load. Biol. Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 3.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am. J. Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]; In this review, the author presents a psychobiological model of resilience and vulnerability to extreme stress and reviews neurochemical, neuropeptide, hormonal and neural mechanisms associated with resilience.
- 4.Masten AS. Ordinary magic. Resilience processes in development. Am. Psychol. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- 5.Masten AS, Coatsworth JD. The development of competence in favorable and unfavorable environments. Lessons from research on successful children. Am. Psychol. 1998;53:205–220. doi: 10.1037//0003-066x.53.2.205. [DOI] [PubMed] [Google Scholar]
- 6.Rutter M. Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. Br. J. Psychiatry. 1985;147:598–611. doi: 10.1192/bjp.147.6.598. [DOI] [PubMed] [Google Scholar]
- 7.Bonanno GA. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? Am. Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- 8.Alim TN, et al. Trauma, resilience, and recovery in a high-risk African-American population. Am. J. Psychiatry. 2008;165:1566–1575. doi: 10.1176/appi.ajp.2008.07121939. [DOI] [PubMed] [Google Scholar]; This study identifies psychosocial factors associated with resilience and recovery from psychiatric disorders in a sample of African-American adults exposed to severe trauma.
- 9.Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: implications for the developing brain, neural plasticity, and preventive interventions. Ann. NY Acad. Sci. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- 10.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu. Rev. Clin. Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 14.Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int. J. Behav. Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 15.Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. J. Pers. Soc. Psychol. 2006;91:730–749. doi: 10.1037/0022-3514.91.4.730. [DOI] [PubMed] [Google Scholar]
- 16.Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. J. Pers. Soc. Psychol. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am. Psychol. 2001;56:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryff CD, Keyes CL. The structure of psychological well-being revisited. J. Pers. Soc. Psychol. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- 19.Pargament KI, Smith BW, Koenig HG, Perez L. Patterns of positive and negative religious coping with major life stressors. J. Sci. Study Relig. 1998;37:710–724. [Google Scholar]
- 20.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 21.Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J. Clin. Epidemiol. 2002;55:696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown ES, Woolston DJ, Frol AB. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol. Psychiatry. 2008;63:705–709. doi: 10.1016/j.biopsych.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]; This excellent review summarizes reciprocal interactions between limbic networks and the HPA axis and describes how imbalances in mineralocorticoid and GR signalling can increase vulnerability for mental illness.
- 25.de Kloet ER, Derijk RH, Meijer OC. Therapy insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nature Clin. Pract. Endocrinol. Metab. 2007;3:168–179. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- 26.Lu A, et al. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol. Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- 27.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Morgan CA, et al. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch. Gen. Psychiatry. 2004;61:819–825. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]; Results from this study of soldiers enrolled in military survival training indicate that the DHEA sulfate/cortisol ratio might correlate with an individual's degree of stress resilience.
- 29.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr. Scand. 2006;114:187–193. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 30.Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:169–192. doi: 10.1016/j.pnpbp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr. Scand. Suppl. 2003:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 32.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 33.Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Morgan CA, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol. Psychiatry. 2000;47:902–909. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- 35.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol. Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]; The authors report higher plasma NPY levels in trauma-exposed veterans without PTSD and in veterans showing recovery from PTSD than in veterans with PTSD, suggesting a relationship between NPY and resistance to or recovery after trauma exposure.
- 36.Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J. Neurosci. 2008;28:12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sajdyk TJ, et al. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J. Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Eisch AJ, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol. Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]; Using the social defeat paradigm in mice, the authors demonstrate that a subset of inbred C57Bl/56J mice are resilient to many of the deleterious effects of the stress and identify some of the underlying molecular mechanisms.
- 42.Bradley RG, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study in two independent populations finds that polymorphisms and haplotypes of the CRH gene moderate the influence of child abuse on depressive symptoms in adulthood.
- 43.Derijk RH, de Kloet ER. Corticosteroid receptor polymorphisms: determinants of vulnerability and resilience. Eur. J. Pharmacol. 2008;583:303–311. doi: 10.1016/j.ejphar.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 44.Binder EB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report a gene–environment interaction between four SNPs of the stress-related FKBP5 gene and severity of child abuse that predicts adult PTSD symptoms.
- 45.Ising M, et al. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur. J. Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- 46.Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 47.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch. Gen. Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 48.Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol. Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- 49.Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol. Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Hariri AR, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch. Gen. Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 51.Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 52.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol. Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stein MB, Campbell-Sills L, Gelernter J. Genetic variation in 5HTTLPR is associated with emotional resilience. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009 Jan 16; doi: 10.1002/ajmg.b.30916. doi:10.1002/ajmg.b.30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev. Neurosci. 2006;17:359–367. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- 55.Schmack K, et al. Catechol-O-methyltransferase val158met genotype influences neural processing of reward anticipation. Neuroimage. 2008;42:1631–1638. doi: 10.1016/j.neuroimage.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors generated mice that duplicate the Val66Met polymorphism in the BDNF gene seen in humans, and demonstrate differences in emotional behaviour and other behavioural domains.
- 57.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 58.Jabbi M, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol. Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- 59.Mandelli L, et al. Interaction between serotonin transporter gene, catechol-O-methyltransferase gene and stressful life events in mood disorders. Int. J. Neuropsychopharmacol. 2007;10:437–447. doi: 10.1017/S1461145706006882. [DOI] [PubMed] [Google Scholar]
- 60.Smolka MN, et al. Gene-gene effects on central processing of aversive stimuli. Mol. Psychiatry. 2007;12:307–317. doi: 10.1038/sj.mp.4001946. [DOI] [PubMed] [Google Scholar]
- 61.Kaufman J, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc. Natl Acad. Sci. USA. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JM, et al. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol. Psychiatry. 2007;62:423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 63.Kaufman J, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol. Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 64.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nature Rev. Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 65.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review summarizes the authors' elegant work in rats, which has demonstrated a role for methylation of the GR gene in the hippocampus in mediating life-long effects of maternal care on an individual's behaviour.
- 66.Weaver IC, et al. Epigenetic programming by maternal behavior. Nature Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 67.Krishnan V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol. Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berton O, et al. Induction of ΔFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]; The authors demonstrate that induction of the transcription factor FOSB in the periaqueductal grey is a mechanism of resilience: such induction, partly through the regulation of substance P neurotransmission, promotes adaptive responses to stress.
- 69.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 70.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Rev. Neurosci. 2009 May 13; doi: 10.1038/nrn2647. doi:10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dedovic K, et al. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005;30:319–325. [PMC free article] [PubMed] [Google Scholar]
- 72.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol. Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol. Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Milad MR, et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc. Natl Acad. Sci. USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J. Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 79.Felmingham K, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol. Sci. 2007;18:127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 80.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 81.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann. NY Acad. Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 82.Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev. Neurosci. 2007;18:253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- 83.Pollak DD, et al. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the β-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. J. Neurosci. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis M, Myers KM. The role of glutamate and γ-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol. Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- 86.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]; This is an elegant example of applying basic research in animals to early clinical trials, in which the authors demonstrate the ability of an NMDA receptor partial allosteric agonist to promote extinction.
- 87.Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: results from a randomized controlled proof-of-concept trial in physically injured patients. J. Trauma. Stress. 2007;20:923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 88.Cai WH, Blundell J, Han J, Greene RW, Powell CM. Postreactivation glucocorticoids impair recall of established fear memory. J. Neurosci. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pizzagalli DA, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with major depressive disorder. Am. J. Psychiatry. 2009 May 1; doi: 10.1176/appi.ajp.2008.08081201. doi:10.1176/appi. ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sailer U, et al. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46:2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 91.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forbes EE, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]; This fMRI study links altered striatal response to monetary reward in depressed adolescents with reports of lower subjective positive affect in natural environments.
- 93.Monk CS, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 94.Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- 95.Vythilingam M, et al. Reward circuitry in resilience to severe trauma: an fMRI investigation of resilient special forces soldiers. Psychiatry Res. 2009;172:75–77. doi: 10.1016/j.pscychresns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Siegrist J, et al. Differential brain activation according to chronic social reward frustration. Neuroreport. 2005;16:1899–1903. doi: 10.1097/01.wnr.0000186601.50996.f7. [DOI] [PubMed] [Google Scholar]
- 97.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 98.Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontalsubcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this fMRI study, the authors demonstrate abnormalities in top-down regulation of the amygdala by the PFC in depressed individuals.
- 100.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 101.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 102.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Drabant EM, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch. Gen. Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- 104.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 105.van der Veen FM, Evers EA, Deutz NE, Schmitt JA. Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacology. 2007;32:216–224. doi: 10.1038/sj.npp.1301212. [DOI] [PubMed] [Google Scholar]
- 106.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ochsner KN, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 108.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol. Psychiatry. 2009;65:367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.New AS, et al. An fMRI study of deliberate emotion regulation in PTSD and resilience. Biol. Psychiatry. doi: 10.1016/j.biopsych.2009.05.020. in the press. [DOI] [PubMed] [Google Scholar]
- 111.Iarocci G, Yager J, Elfers T. What gene-environment interactions can tell us about social competence in typical and atypical populations. Brain Cogn. 2007;65:112–127. doi: 10.1016/j.bandc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 112.Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J. Cogn. Neurosci. 2007;19:1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- 113.Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 114.Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others' emotions relates to empathy and interpersonal competence in children. Neuroimage. 2008;39:2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 116.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 117.Rilling J, et al. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 118.Kirsch P, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skuse DH, Gallagher L. Dopaminergicneuropeptide interactions in the social brain. Trends Cogn. Sci. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 120.Storm EE, Tecott LH. Social circuits: peptidergic regulation of mammalian social behavior. Neuron. 2005;47:483–486. doi: 10.1016/j.neuron.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Insel TR. A neurobiological basis of social attachment. Am. J. Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 122.Bowlby J. Attachment and loss. Basic Books; New York: 1982. [Google Scholar]
- 123.Charuvastra A, Cloitre M. Social bonds and posttraumatic stress disorder. Annu. Rev. Psychol. 2008;59:301–328. doi: 10.1146/annurev.psych.58.110405.085650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychol. Sci. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 125.Lee V, Cohen SR, Edgar L, Laizner AM, Gagnon AJ. Clarifying “meaning” in the context of cancer research: a systematic literature review. Palliat. Support. 2004;2:291–303. doi: 10.1017/s1478951504040386. [DOI] [PubMed] [Google Scholar]
- 126.Caria A, et al. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage. 2007;35:1238–1246. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 127.Weiskopf N, et al. Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI) J. Physiol. Paris. 2004;98:357–373. doi: 10.1016/j.jphysparis.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 128.Folkman S, Moskowitz JT. Positive affect and the other side of coping. Am. Psychol. 2000;55:647–654. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- 129.Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Soc. Cogn. Affect. Neurosci. 2006;1:203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol. Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 131.Vythilingam M, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. English and Romanian Adoptees (ERA) Study Team. J. Child Psychol. Psychiatry. 1998;39:465–476. [PubMed] [Google Scholar]
- 133.Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl.) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 134.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Masten AS, Best KM, Garmezy N. Resilience and development: contributions from the study of children who overcome adversity. Dev. Psychopathol. 1991;2:425–444. [Google Scholar]
- 136.Luthar SS, Sawyer JA, Brown PJ. Conceptual issues in studies of resilience: past, present, and future research. Ann. NY Acad. Sci. 2006;1094:105–115. doi: 10.1196/annals.1376.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parker G. Parental `affectionless control' as an antecedent to adult depression. A risk factor delineated. Arch. Gen. Psychiatry. 1983;40:956–960. doi: 10.1001/archpsyc.1983.01790080038005. [DOI] [PubMed] [Google Scholar]
- 138.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. J. Trauma. Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- 140.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch. Gen. Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 141.Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin. Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this article the authors review their findings that demonstrate, in animals, that control over stressful events limits the deleterious consequences on the individual.
- 142.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 143.Champagne DL, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.