Abstract
In recent years, there have been huge advances in the use of genetically modified mice to study pathophysiological mechanisms involved in schizophrenia. This has allowed rapid progress in our understanding of the role of several proposed gene mechanisms in schizophrenia, and yet this research has also revealed how much still remains unresolved. Behavioral studies in genetically modified mice are reviewed with special emphasis on modeling psychotic-like behavior. I will particularly focus on observations on locomotor hyperactivity and disruptions of prepulse inhibition (PPI). Recommendations are included to address pharmacological and methodological aspects in future studies. Mouse models of dopaminergic and glutamatergic dysfunction are then discussed, reflecting the most important and widely studied neurotransmitter systems in schizophrenia. Subsequently, psychosis-like behavior in mice with modifications in the most widely studied schizophrenia susceptibility genes is reviewed. Taken together, the available studies reveal a wealth of available data which have already provided crucial new insight and mechanistic clues which could lead to new treatments or even prevention strategies for schizophrenia.
Keywords: schizophrenia risk gene, dopamine, glutamate, neuregulin-1, DISC-1, dysbindin, locomotor hyperactivity, prepulse inhibition
Introduction
In the last 10 years or so, there have been huge advances in the use of genetically modified mice to study pathophysiological mechanisms involved in schizophrenia. Much of that progress has been driven by rapid developments in molecular biology techniques, allowing both better identification of neurogenetic mechanisms putatively involved in this illness, as well as generating more and more sophisticated gene modifications in mice. Thus, there has been rapid progress in our understanding of the role of several proposed gene mechanisms in schizophrenia, and yet this research has also revealed how much still remains unresolved.
This review article addresses pharmacological and methodological aspects of behavioral studies in genetically modified mice with special emphasis on modeling psychotic-like behavior. This article is not about the validity or strength of the evidence for an association of various genetic factors with schizophrenia; the reader is referred to several excellent other review articles which address those aspects. 1–14
There have been many discussion papers on the need for more comprehensive phenotyping and the use of standard behavioral test “batteries” to reveal behavioral effects of genetic modifications in mice,3,13–19 but pharmacological characterization of behavioral changes in a particular mouse model is not always part of that. This is an unfortunate shortcoming of some studies because including relevant pharmacological challenges in the behavioral phenotyping battery may reveal changes in behavioral reactivity which may go unnoticed if only baseline behaviors are assessed. Here, I will particularly focus on psychotropic drug-induced locomotor hyperactivity and disruptions of prepulse inhibition (PPI) for reasons which will be outlined below. If unavailable, I will also include observations on baseline locomotor activity, baseline PPI, and some other relevant behaviors and neurochemical measures. I will focus on detailing the results of behavioral testing of mutant mice of 2 main groups of factors, including the most important neurotransmitter systems and candidate/risk genes in schizophrenia, respectively.2–7,9,11–14 Other symptom domains, including behavioral methods with relevance to negative symptoms of schizophrenia, or cognitive deficits, will be addressed in other reviews in this series.
Animal Models of Psychosis: General Features and Methodological Considerations
While the positive symptoms of schizophrenia, such as auditory hallucinations and delusions, are uniquely human, the literature on genetically modified mouse models of this symptom cluster has focused on 2 main categories of behavior: locomotor hyperactivity and disruptions of PPI.
Locomotor Hyperactivity
Some insight is available into brain neurotransmitter mechanisms involved in psychotic symptoms, and these neurotransmitter changes can potentially be “recreated” in rodents with drug treatments. Historically, subcortical hyperdopaminergia was postulated in schizophrenia mostly on the basis of the neuropharmacological action of antipsychotic drugs, which are virtually all dopamine receptor antagonists (with varying affinity for other neurotransmitter receptors).20,21 The other line of evidence comes from imaging studies which have shown enhanced dopaminergic reactivity and enhanced effects of amphetamine in the forebrain of subjects with schizophrenia (eg, Drevets et al22 and Laruelle et al23,24). It is reasonable to state that changes in dopaminergic activity are unlikely to be the primary cause of schizophrenia—or even the positive symptoms of schizophrenia—but there may be a final common dopaminergic pathway through which genetic, environmental, and developmental factors combine to result in varying degrees of symptomatology.25,26 With respect to animal models, it then makes sense to assess for changes in dopamine-related behaviors, an approach which has construct validity rather than face validity, as between humans and mice the underlying neuropharmacological mechanisms may be the same, but the behavioral consequences are quite different. It has been suggested that locomotor hyperactivity may have some face validity for certain components of the positive symptoms of schizophrenia, such as psychotic agitation.12 However, here I will focus mainly on the use of locomotor hyperactivity to reveal underlying neurotransmitter changes.
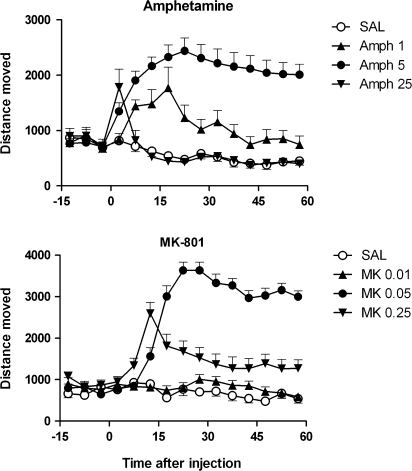
Because of its relative ease of quantification, locomotor activity testing has been widely used in modeling the positive symptoms of schizophrenia. Although complex and multifactorial, the role of dopamine in movement control is reasonably well defined, with a predominant involvement of the mesolimbic and nigrostriatal dopamine systems and a relatively clear pharmacology. In its simplest form, the concept of testing for locomotor hyperactivity is based upon the premises that enhanced dopaminergic activity in rodents leads to enhanced motor activity, be it horizontal locomotor activity, rearing, or, at higher doses, stereotyped behaviors (see figure 1). Most of these behaviors can be measured by automated photocell cages or scored by observation. More sophisticated methodology includes ethological assessment of a range of natural behaviors, including motor activity, or qualitative analysis of patterns and perseverative aspects of behavior.27,28
Fig. 1.
The Effect of Different Doses of Amphetamine (amph, Top Panel) and MK-801 (MK, Bottom Panel) on Locomotor Activity of C57BL/6 Mice (M. van den Buuse, unpublished data). Both drugs showed little effect at the lowest dose, markedly increase locomotor distance moved at the middle dose, but induced low scores at the highest dose, presumably because of the induction of stereotyped responses which disrupted ambulatory activity. Depending on the dose of the drug, an increase of locomotor distance moved displayed by a genetically modified mouse model could either mean hypersensitivity or hyposensitivity to the treatment. Locomotor distance moved was assessed using automated photocell cages (see van den Buuse et al175 for details). Doses indicated are in milligram per kilogram. There were 8–20 mice per group, and data are expressed as mean ± standard error of the mean.
Classic experiments have shown the role of different parts of the dopamine system in locomotor hyperactivity in rats.29 For example, 6-hydroxydopamine (6-OHDA)–induced lesions of the nucleus accumbens abolished amphetamine-induced hyperactivity in this species.30,31 Thus, altered amphetamine-induced locomotor hyperactivity can then be used as a measure of altered dopaminergic neurotransmission in the mesolimbic system and has construct validity for enhanced dopaminergic activity in schizophrenia. Importantly, it should be noted that amphetamine also stimulates extracellular levels of other neurotransmitters, such as noradrenaline and serotonin, and the possible involvement of those systems should therefore not be ignored.32–34 Similar, but not identical anatomical and pharmacological substrates are involved in the action of cocaine on locomotor activity.32,35 In all this, it is important to note that the vast majority of the evidence for the involvement of mesolimbic dopaminergic activity in amphetamine-induced locomotor hyperactivity has been obtained in rats and that this same relationship has not yet been thoroughly studied in mice.36
The second main line of research using locomotor hyperactivity as an in vivo measure of central neurotransmitter system activity has been to assess the effect of N-methyl-D-aspartate (NMDA) receptor antagonists, such as phencyclidine and MK-801.37,38 Construct validity for this approach stems from the effect of dissociative anesthetics, such as ketamine and phencyclidine, to induce hallucinations with similarities to the positive symptoms of schizophrenia.39,40 In rats and mice, moderate doses of these drugs induce locomotor hyperactivity, while at higher doses, sedative and anesthetic effects or the emergence of stereotyped behaviors lead to a apparent reduction of locomotor counts, similar to that seen with high doses of amphetamine (see Yates et al41 and figure 1). While these compounds indirectly activate dopaminergic activity in humans42 and rats,43 locomotor hyperactivity induced by these drugs in rodents is largely independent of dopaminergic activation. For example, phencyclidine-induced locomotor hyperactivity is temporally dissociated from its effect on dopamine release.43 Moreover, phencyclidine effects on locomotor activity are not readily attenuated by pretreatment with dopaminergic antagonists, such as haloperidol, unless these drugs are administered at high doses which may nonspecifically cause general motor inhibition and catatonia.37,38
PPI of Startle
As reviewed previously, loss of normal PPI is widely accepted as an endophenotype of schizophrenia44 and considered indicative of disrupted sensorimotor gating, a precognitive process to prevent sensory overload and cognitive fragmentation (for references, see Powell et al,12 van den Buuse et al,14 Geyer et al,17 and Geyer45). It should not be considered as a straightforward model of positive symptoms of schizophrenia but is more likely to represent the “interface of psychosis and cognition.”4 Moreover, disruptions of PPI have been described in other neurological and psychiatric diseases.12,46
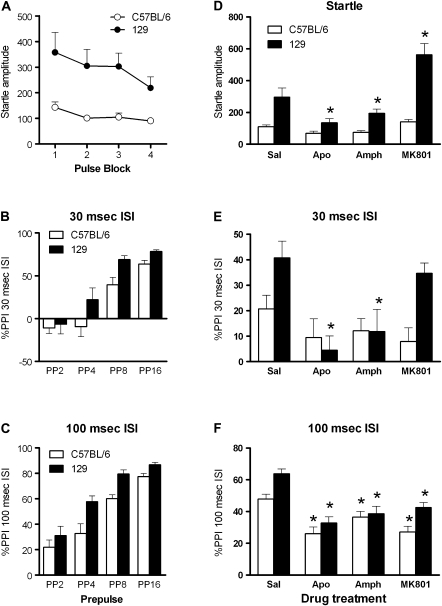
Several studies have suggested overlapping neural substrates and pharmacological mechanisms between PPI in rodents and in humans, and PPI is therefore usually referred to as a “cross-species” measure of sensory gating. At the same time, it should be recognized that the pharmacology of PPI appears to be different at a number of levels between rodents and humans47 and even between mice and rats. Administration of direct dopamine D1 and D2 receptor agonists, such as apomorphine, indirect dopamine agonists, such as amphetamine, and NMDA receptors antagonist generally results in disruption of PPI in mice12,47 (also see figure 2). Thus, PPI can be used to model both a hyperdopaminergic and a hypoglutamatergic state. However, the role of the nucleus accumbens in the effects of dopamine D2 receptor agonists on PPI appears to be opposite in rats and mice.48,49 Similarly, some drugs, such as serotonin-1A receptor agonists, disrupt PPI in rats but increase PPI in mice.47,50 In addition, baseline PPI is different between mouse strains51 which likely reflects differences in the inherent activity of dopaminergic or other neurotransmitter systems in these animals (see figure 2). Indeed, in a mouse strain with particularly low baseline PPI, the DBA/2J line, antipsychotic treatment increased PPI to a much greater extent than in C57BL/6 where baseline PPI was already higher.52 Also the extent of other drug-induced changes in PPI appear to be strain specific in mice.17,47
Fig. 2.
Comparison of PPI of C57BL/6 and 129Sv Mice (M. van den Buuse, unpublished data). Startle amplitude was much greater in 129Sv mice than in C57BL/6 although startle habituation was not different (panel A). PPI tended to be higher in 129Sv mice than in C57BL/6 at both the 30-ms ISI (panel B) or 100 ms ISI (panel C). There were differential as well as similar effects between the strains, depending on the parameter measured and drug tested. Genetic modifications on either a C57BL/6 or 129Sv genetic background may have profoundly different effects on these baseline levels. Experiments included the effect of 5 mg/kg of apomorphine, 5 mg/kg of amphetamine, and 0.25 mg/kg of MK-801 on average startle (panel D), PPI at the 30-ms ISI (panel E), and PPI at the 100-ms ISI (panel E). For methodological details, see van den Buuse et al.56,175 Data are expressed as mean ± SEM of n = 8–12. *P < .05 compared with the saline condition in the same strain (analyzed by analysis of variance).
PPI of acoustic startle can be assessed using automated startle boxes, and there is reasonable agreement in the literature as to the protocols and equipment used for these kinds of experiments.45,53 However, some seemingly minor differences in the details of PPI protocols can impact on the results, including technical details such as prepulse-pulse interval and stimulus modalities, and experimental details such as mouse background strain,50,54 sex of the animals, and drug doses used.17,45,50,55,56 Overall, while several studies on genetically modified mouse models of schizophrenia have included PPI testing,12,17 many others have not and few include pharmacological testing. The latter is important as it is very well conceivable that mice with a given gene mutation show apparently normal PPI and startle because of compensatory mechanisms in the brain such as receptor upregulation or altered activity in parallel neurotransmitter systems.47,57 In that situation, the animal may respond more or less to specific drug challenges (figure 2), similar to locomotor hyperactivity. Conversely, it could be argued that mice with altered baseline PPI represent only the more severe perturbations of regulatory mechanisms which cannot readily be compensated for. In either case, whether there is altered baseline PPI or not, pharmacological studies can identify subtle shifts in the relative activity of its neurotransmitter control.
Methodological Considerations
As will become obvious from the available literature on genetically modified mouse models of aspect of psychosis (see below), some methodological recommendations may be useful. Many excellent reviews for other behavioral domains and psychiatric illnesses have discussed discrepancies between behavioral phenotyping studies caused by methodological factors.10,15,18,19,51,53,58 It is therefore important that details are reported on housing conditions, age, and male/female ratio of the mice. Solitary housing has profound effects on locomotor activity and PPI in rats,46 and comparable effects are found in mice.59 As outlined below, housing conditions can in fact become a second neurodevelopmental “hit,” and then the experimental model becomes a gene/environment model instead of just the effect of the gene. In order to be able to replicate results between laboratories, it is also important that experimental details, such as open field dimensions and testing conditions (eg, light), are reported. For PPI, where possible, different interstimulus intervals (ISIs) or stimulus modalities should be used, and effects on startle should always be analyzed and included.
Another factor of importance in studying genetically modified mouse models of psychiatric illnesses is background strain. The majority of studies report the background strain that a given genetic modification is on. However, this information also reveals variability of genetic backgrounds used, and in some cases, this can strongly influence the result.51,56 We compared 2 of the most commonly used inbred mouse lines, C57BL/6 and 129Sv, for baseline PPI and the effect of apomorphine, amphetamine, and MK-801, and found marked differences in behavioral responses (M. van den Buuse, unpublished data, see figure 2) which could influence the impact of genetic modifications on these genetic backgrounds. In the literature, mouse strains used range from “pure” 129Sv, 129/C57BL/6 mixed to “pure” C57BL/6, while some studies include FVB or BALB/c background. Although backcrossing onto a C57BL/6 background is commonly recommended, it should be noted that even after prolonged backcrossing, there is still a chance of flanking genes influencing the behavioral outcomes.60 In addition, C57BL/6 are clearly different in several behavioral tests from other strains (eg, figure 2 and Kalueff et al19 and Paylor and Crawley51), and this could mask a relatively subtle effect of a genetic modification.
In several otherwise excellent studies, only baseline behaviors are reported. Indeed, a major caveat in the literature is the common use of baseline locomotor activity in the same way as drug-induced locomotor hyperactivity. Thus, if genetically modified mice display locomotor hyperactivity at baseline, it is often interpreted to indicate enhanced dopaminergic activity or “psychotic-like” behavior. This is clearly a simplification as the pharmacology of baseline (ie non-drug induced) locomotor activity, and amphetamine-induced locomotor hyperactivity is likely to be different. While the effect of amphetamine on locomotor activity may involve neurotransmitters other than just dopamine, baseline activity may involve many other transmitter systems again. For example, glutamatergic pathways are involved in non-dopaminergic modulation of motor activity (see above). Furthermore, manipulation of the activity of several other neurotransmitter systems, such as noradrenaline and serotonin, can induce changes in locomotor activity. These may involve dopaminergic activation, but the primary cause for the altered behavior then lies outside the dopamine system itself.
In general, it is fair to say that the pharmacology of behavior in mice is less well studied than that in rats. It is important to note that mice are not simply “small rats” and do not necessarily respond to the same doses of drugs. Indeed, there are large differences in dose ranges between rats and mice for some drugs, eg, amphetamine needs to be administered at milligram per kilogram doses about 10× in mice than in rats to elicit a significant hyperactivity response; however, clozapine doses are generally lower in mice than in rats. These species variations may be explained by differences in the neural substrates and neuropsychopharmacological mechanisms involved in the regulation of locomotor hyperactivity and PPI in mice vs rats. In addition, species differences in mechanisms outside the central nervous system, such as drug metabolism and pharmacokinetics, always need to be considered as well.
Ideally, genetically modified mice should be tested for changes in both baseline activity and locomotor hyperactivity induced by a predominantly dopaminergic stimulus, such as amphetamine, and NMDA receptor antagonists, such as phencyclidine or MK-801. Pharmacological mechanisms can be further identified by the use of appropriate antagonists drugs, including, eg, antipsychotics such as haloperidol. Where this is done, the effect of the antipsychotics on baseline behavior should also be assessed to ascertain that any inhibition of psychotropic drug-induced hyperactivity is specific. Unfortunately, with some excellent exceptions, not many studies adopt such an approach. The result of such drug testing can be that it is found that a mouse model does not display changes in baseline behaviors, but responses to certain drugs are enhanced or reduced. It is important to test multiple doses of drugs, which can cause locomotor hyperactivity as well as reduced activity due to stereotyped responding. Thus, with drug such as amphetamine or MK-801 (see figure 1), it is important to ascertain whether enhanced locomotor activity scores in fact reflect increased or decreased sensitivity to the treatment. Antagonist treatment, including antipsychotic drugs, can also be used as tools to probe for pharmacological specificity; however, it is important to assess the effect of these drugs on baseline behavior to exclude nonspecific effects, such as sedation, catatonia, or stereotyped responses. If automated photocell cages are to be used, such responses may easily be missed and interpreted as a reduced locomotor hyperactivity response, where it is in fact enhanced responding. In PPI experiments, some of these effects could be reflected by reduced startle responses.
Neurotransmitter Models of Psychosis
Dopamine
In accordance with the widespread historical interest in the “hyperdopaminergic hypothesis” of schizophrenia, many mouse models have been developed which address virtually all components of dopaminergic activity in the brain, from its synthesis by tyrosine hydroxylase, to release and the regulation of extracellular levels, including by dopamine transporters and catabolic enzymes, and the role of the 5 dopamine receptors.
Dopamine D1 Receptors.
Dopamine D1 receptor knockouts were generated as early as 1994 by 2 separate laboratories.61,62 One of these mouse lines showed reduced horizontal locomotion61 and the other baseline locomotor hyperactivity.62–64 Both D1 knockout lines showed a reduced hyperactivity response to acute and chronic treatment with cocaine63,65 or amphetamine66,67 (table 1).
Table 1.
Summary of the Effect of Dopaminergic Drugs on Locomotor Activity and PPI in Dopamine Receptor Mutant Mice
| Wild type | D1 KO | D2 KO | D3 KO | D4 KO | D5 KO | |
| Locomotor activity | ||||||
| Baseline (vs wild type) | ↓/↑61,62 | ↓↓/↓71,73 | ↑84,86 | ↓91 | 0 | |
| Amphetamine (vs saline) | ↑↑ | ↑66,67 | ↑/073 | ↑↑↑86 | ↑↑↑91,93 | ND |
| Cocaine (vs saline) | ↑↑ | 063,65 | ↑74 | ↑↑↑86,89 | ↑↑↑91,92 | ↑/096,97 |
| PCP/MK-801 (vs saline) | ↑↑ | ND | ND | ↑/090 | ND | ND |
| PPI | ||||||
| Baseline (vs wild type) | 0 | ↓68 | 0 | 0 | 0 | |
| Apomorphine (vs saline) | ↓↓ | 068 | ↓↓68 | ND | ND | ND |
| Amphetamine (vs saline) | ↓↓ | ↓↓68 | 068,83 | ↓↓83 | ↓↓83 | ND |
| Cocaine (vs saline) | ↓↓ | 069 | ↓69 | ↓↓↓69 | ND | ND |
| PCP/MK-801 (vs saline) | ↓↓ | ↓↓68 | ↓↓68 | ND | ND | ND |
Note: KO, knockout; ND, not determined. Drug effects in wild-type mice have been schematically “normalized” to 2 arrows. A “0” then indicates virtual or complete abolition of the locomotor stimulation or PPI disruption, with single arrows indicating partial changes.
PPI was similar in D1 receptor knockout mice as in wild-type controls.68 In contrast to the loss of its effect on locomotor activity in D1 receptor knockouts,66,67 the effect of amphetamine to disrupt PPI was not altered in these animals.68 The effect of MK-801 to disrupt PPI was also not altered; however, in contrast, the effect of the dopamine D1/D2 receptor agonist, apomorphine, was absent in D1 receptor knockouts.68 Surprisingly, and in contrast to amphetamine, the effect of cocaine to disrupt PPI was completely absent in D1 receptor knockouts.69 These results (table 1) suggest that the dopamine receptors involved in amphetamine-induced locomotor hyperactivity and disruption of PPI are not the same in mice, with the D1 receptor being involved in the former but not the latter. The effect of cocaine was equally absent for locomotor hyperactivity or PPI disruption in D1 knockouts. Finally, the results confirm studies with antipsychotic drugs and receptor antagonists that dopamine D1 receptors are not involved in the effect of MK-801 on PPI.
In addition to D1 receptor knockouts, a mouse line overexpressing D1 receptors has also been developed.70 These transgenic animals had 2- to 5-fold increases in D1 receptor levels in a variety of brain regions but not the caudate nucleus, olfactory tubercle, or nucleus accumbens. Paradoxically, while the dopamine D1 receptor agonist, SKF81297, caused a dose-dependent increase in locomotor activity in wild-type mice, it had no effect or actually decreased activity in D1 transgenics.70 The effect of amphetamine and cocaine was not altered in the transgenic mice. These results were interpreted as indicating the presence of 2 types of D1 receptors, one inhibiting activity and overexpressed in the transgenic mice and the other decreasing activity. Alternatively, behavior in the transgenic mice could have been altered by an imbalance between D1 and D2 receptor–mediated effects.70
Dopamine D2 Receptors
Initial reports on the behavioral phenotype of dopamine D2 receptor knockouts described these animals as severely bradykinetic.71 Later studies found several aspects of behavior of these animals to be essentially normal, but there was a profound loss of responsiveness to the behavioral activation by treatment with a D2 receptor agonist.72 A parallel D2 receptor knockout was developed which did not display severe bradykinesia73 and showed a diminished locomotor hyperactivity response to acute treatment with methamphetamine, cocaine, or MDMA.74–76 These data (table 1) and other findings77 are another example of how compensatory changes in the density or coupling of other receptors may mask some of the phenotypic changes in a particular receptor mutant and emphasize that appropriate pharmacological challenges should be part of behavioral phenotyping.77,78 A dopamine D2 receptor transgenic line has also been developed,79 but as yet, these mice have not been tested for drug-induced locomotor hyperactivity or PPI disruptions.
Further studies focused on selective deletion of either the “long” or “short” form of the D2 receptor. Thus, D2long knockouts showed normal baseline and novelty-induced locomotor activity compared with wild-type controls.80–82 Dopamine D2 expression in the brain of these mice was normal, suggesting an upregulation of D2short expression in the absence of the D2long isoform.82 However, these animals showed no cataleptic effect of haloperidol treatment, similar to the knockout of both isoforms.80,82 Similarly, these animals had absent or markedly reduced responses to treatment with apomorphine or D1 receptor agonists. Thus, it was concluded that the D2long isoform synergistically interacts with D1 receptors in locomotor activity regulation, whereas the D2short isoform may functionally oppose the D1 receptor.80
PPI and startle were normal in D2 receptor knockout mice. However, the effect of amphetamine to disrupt PPI was completely abolished in these animals.68,83 The effect of cocaine on PPI was partially attenuated,69 whereas the effects of apomorphine, SKF81297, or MK-801 on PPI were not significantly altered in D2 receptor knockouts.68 In D2long knockouts, baseline PPI and the effects of amphetamine and apomorphine were not altered.81 These results suggest a critical role of the D2short form in mediating the action of amphetamine on PPI.
Dopamine D3, D4, and D5 Receptors.
Compared with the D1 and D2 receptors, less is known about the effect of mutation of the D3, D4, and D5 receptor on locomotor behavior and PPI. Several laboratories have generated D3 receptor knockout lines.84–87 Generally, the mice displayed mild-to-moderate baseline locomotor hyperactivity.84–86 The effect of amphetamine or cocaine to induce locomotor hyperactivity was also greater in these mice compared with wild-type controls, particularly at moderate doses.86,88,89 In contrast, the effect of the NMDA receptor antagonist, MK-801, was markedly reduced in D3 knockout mice.90 These results are consistent with a role of the D3 receptor as a postsynaptic inhibitory dopamine receptor, but the importance of this role appears to differ depending on the pharmacological stimulus used.
Dopamine D4 receptor knockout mice were moderately hypoactive in the open field but showed hyperresponsiveness to the effects of cocaine,91,92 methamphetamine,91 and amphetamine.93 However, a more recent report suggested that D4 knockouts show dose-dependent changes in the locomotor hyperactivity response to amphetamine and no difference in the acute effect of cocaine.94 These discrepancies with earlier studies were explained by the use of lower doses of amphetamine and cocaine. It was suggested that the higher doses of these psychostimulants in the earlier studies in fact revealed serotonergic compensatory changes in D4 knockouts.94
Dopamine D5 receptor knockout mice showed reduced effects of the dopamine D1/D5 receptor agonist, SKF81297, to increase behavioral activity. However, there was no genotype difference in the effect of the D1/D5 antagonist, SCH23390, to decrease activity.95 The acute effect of cocaine to induce locomotor hyperactivity was reduced in D5 knockouts in one study96 but unchanged in another.97 Several other psychotropic drugs have not been tested for their effects on locomotor activity in D3, D4, and D5 mutant mice (table 1).
There were no changes in baseline PPI in either D3 or D4 knockouts83 or D5 knockouts.95 The effect of amphetamine on PPI was not altered in D3 or D4 receptor knockouts.83 The effect of the dopamine D1/D5 receptor agonist, SKF81297, to disrupt PPI tended to be reduced in D5 knockouts.95 Taken together with the locomotor activity observations (see above), these findings led the authors to conclude that “the results support the interpretation that the D5 receptor subtype plays a minor functional role, complementary to the D1 receptor, in dopaminergic pathways mediating behavior.”95 However, further studies are needed to assess the effect of other drugs, particularly the mixed D1/D2 agonist, apomorphine, and NMDA receptor antagonists, such as MK-801 or phencyclidine, on PPI in these mutant mice (see table 1).
Dopamine Transporter.
Knockout of the dopamine transporter has been shown to severely disrupt regulation of extracellular dopamine levels,98,99 an effect which was more pronounced with the mutation on a DBA/2 genetic background compared with a C57BL/6 background.100 Dopamine transporter mice were highly hyperactive in a novel environment and showed no effect of either amphetamine or cocaine on locomotor activity.99 Treatment with dopamine D1 and D2 antagonists attenuated the locomotor hyperactivity in dopamine transporter knockout mice,101 but the NMDA receptor antagonist, MK-801, increased locomotor activity to a greater extent than in controls.102 Furthermore, PPI was significantly disrupted, an effect which could also be reversed by treatment with dopamine D2 receptor antagonists.101,103 A multitude of other behavioral, neurochemical, and molecular changes have been described in these animals (for references, see Gainetdinov98 and Gainetdinov and Caron104), and it was suggested that these hyperdopaminergic animals could be a novel model for psychotic symptoms in schizophrenia.99 It should be noted, that the reduced activity of amphetamine in this mouse model is in contrast to enhanced effects of this drug in schizophrenia.23 Furthermore, the chronic nature of the profoundly elevated extracellular dopamine levels in the dopamine transporter knockout mouse, different from the more phasic upregulation of dopaminergic activity in schizophrenia, induced considerable compensatory responses in other transmitter systems.98,99,104
Instead of a complete knockout, a “knockdown” mutant displayed a 90% reduction of dopamine transporter expression.105 These animals were hyperactive in a novel environment,105,106 and this hyperactivity could paradoxically be reduced by amphetamine and apomorphine, which induced hyperactivity in wild-type control mice.105 In contrast, the effect of cocaine to induce locomotor hyperactivity was enhanced in these mice.107 These differential effects of the dopamine transporter knockdown on the action amphetamine vs cocaine once again reveal fundamental differences in the neuropsychopharmacological mechanism of action of these drugs and the compensatory mechanisms they recruit in the brains of genetically modified mice. Baseline PPI was normal in these mice.106
Glutamate
Similar to hyperdopaminergia as a contributing mechanism to psychosis, in the literature, there has been extensive focus on the “hypoglutamatergic hypothesis” of schizophrenia. It is therefore not surprising that there are a large number of studies on mutant models of glutamate function, including glutamate receptor subtype mutants.108 Of the ion channel subfamily of glutamate receptors, the NMDA receptor has received most attention. Of the G-protein–coupled metabotropic subfamily of glutamate receptors, most work has been done with mGluR5 mutants. Studies have furthermore addressed other components of glutamate function in the brain, such as glycine or excitatory amino acid transporters (EEATs).
Ion Channel Glutamate Receptors.
Based on the “hypoglutamatergic hypothesis” of schizophrenia, an NMDA receptor hypomorph was generated which expressed only 5%–10% of the NR1 subunit common to all NMDA receptor isoforms.109 In contrast to complete knockout of this subunit, which is lethal,110 these mice were viable and markedly hyperactive at baseline. Acute treatment with phencyclidine, at a dose which induced marked hyperactivity in wild-type controls, had no effect in the NR1 hypomorphs. Studies with antipsychotic drug pretreatment or microdialysis109,111 revealed that reduced NMDA receptor function in these mice was not associated with enhanced dopaminergic activity.
These NR1 hypomorphic mice also showed disrupted PPI at rest7–9 and other sensory gating deficits,112,113 again similar to the effect of NMDA receptor antagonists in this species.17 In contrast to the locomotor hyperactivity, however, treatment with both typical (haloperidol) and atypical antipsychotic drugs (olanzapine, risperidone, quetiapine, or clozapine) had similar effects in NR1 hypomorphs and wild-type controls.111,114,115 Treatment with amphetamine reduced the already lower PPI in NR1 hypomorphs even further and at doses lower than those effective in wild-type mice, suggesting a hypersensitivity to dopaminergic disruption of PPI in these mice.116
In addition to the NR1 hypomorphic model, which had already generated substantial insight into the role of NMDA receptors in locomotor hyperactivity and PPI, other, more region-specific approaches were also used. Mice with specific ablation of the NR1 subunit in striatum by CreLox conditional gene targeting developed normally until postnatal day (pnd) 12.117 After that, the mice developed abnormal gait and failed to grow, eventually dying at around pnd 21. At pnd 16, but not pnd 9, the pups showed marked locomotor hyperactivity. Surprisingly, treatment with both a dopamine D1 receptor agonist (SKF81297) or a D2 receptor agonist (bromocriptine) at this age reduced the hyperactive phenotype of these mice, with the opposite occurring in wild-type controls, ie, an expected increase in locomotor activity.117 The authors discussed these paradoxical results in terms of a possible interaction of dopamine D1 and D2 receptors with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in these mutant mice.117 These almost complete but striatum-specific knockouts of NMDA receptor function contrast with other regionally specific models where little effect on motor function was observed. For example, using a complex combination of 4 genetic modifications, a region-specific (cortex, hippocampus and striatum), inducible and reversible NR1 knockout could be produced.118 These animals showed normal locomotor activity in the open field.118 A more regionally specific, but not inducible knockout similarly showed approximately 65% reduction of NR1 expression in the striatum but similarly no changes in locomotor activity in the open field.119 Further cellular specificity was achieved by restricting the expression of mutated NR1 to dopamine D1 receptor–expressing cells only.120 These mice showed no change in baseline locomotor activity or the locomotor hyperactivity induced by treatment with amphetamine or cocaine.120 The relative lack of overt phenotype in these mice,120 when compared with more complete striatum knockout mice117 could be due to a lower extent of NMDA receptor hypofunction or its restriction to D1 receptor–expressing neurons.
Mice with mutations in members of the NR2 subunit group showed varying levels of altered behavior. For example, mice lacking the ϵ1 subunit (NR2A) were hyperactive in an open field, and this effect could be attenuated by treatment with the typical antipsychotic, haloperidol, or the atypical antipsychotic, risperidone.121 In contrast, a mutation in the ϵ4 member of the NR2 subunit (NR2D) resulted in hypoactivity and reduced rearing in the mice.122 Mice lacking the ϵ3 subunit (NR2C) appeared to have no behavioral phenotype.123 However, compensatory upregulation of the expression of other subunits could play a role in this apparent lack of effect. For example, combined deletion of both the NR2A and NR2C subunit resulted in motor incoordination, where the single mutations had no such effect.124
A comprehensive comparison of NR2 mutant mice revealed changes in startle amplitudes and PPI depending on which subunit was targeted.125 Startle was markedly increased in NR2B (ϵ2) heterozygous mice, but only a moderate increase was observed in NR2A (ϵ1) and NR2D (ϵ4) knockout mice, whereas NR2C (ϵ3) mutants showed no change in startle.125 PPI was moderately increased in NR2B (ϵ2) heterozygous mice, with no change observed in the other NR2 subunit mutants.125
Compared with the NR1 and NR2 subunits of the NMDA receptor, much less is known about the NR3 subunit which is able to form a glycine-sensitive binding site in combination with the NR1 subunit (for references, see Brody et al126). There were no differences in PPI between double-transgenic, inducible NR3A overexpressing mice and wild-type controls PPI.126 In NR3A knockouts, baseline PPI was significantly increased in males, but not females, at 3 weeks of age, when PPI is low in wild-type and heterozygous mice. With age, PPI in the controls and heterozygotes increased and a genotype difference disappeared.126 The sex specificity of the effect in NR3A knockouts may be related to an interaction of estrogen with PPI regulation and NMDA receptor function.126
Another way to indirectly affect NMDA receptor function is to specifically target glycine-mediated binding mechanisms on the NR1 subunit. Two different point mutations in the glycine site on the NMDA receptor resulted in a 5-fold and 86-fold reduction of glycine binding.127 Mutant mice with the modest reduction showed no overt changes in locomotor activity, stereotypy or PPI, but increased startle. In contrast, mice with the large reduction of glycine binding died within 48 h after birth.127 Compound heterozygous mice, which carried one allele of each mutation and showed an approximately 90% reduction of glycine affinity, were behaviorally hyperactive.128 MK-801 treatment caused no further increase in these double mutants, but the effect of amphetamine tended to be reduced. The antipsychotics, haloperidol and clozapine, had no effect in the hyperactive double mutants.128 These mice may represent a model of severe NMDA receptor hypofunction which is resistant to pharmacological inhibition.128
Extracellular glutamate concentrations are tightly regulated by glutamate transporters on glial cells. Therefore, in the context of enhanced glutamatergic tone associated with the hypoglutamatergic model of schizophrenia, mice with disrupted glutamate transporter function may be a promising experimental and drug development tool. Specifically, mice deficient in the glial glutamate and aspartate transporter (GLAST, also known as EEAT1) showed locomotor hyperactivity in a novel open field but not in the home cage.129,130 This genotype effect could be blocked by haloperidol and the mGlu2/3 receptor agonist, LY379268, at doses which had no effect in wild-type controls.129 MK-801 treatment induced enhanced locomotor hyperactivity in GLAST knockout mice.129 Startle amplitudes were reduced, but PPI was not significantly different to controls.130
Compared with the NMDA receptor, far less is known about “psychosis-like” behavioral changes in mice with genetic modification of AMPA function. One extensive study characterized AMPA knockout mice in a battery of tests131 and found the animals to be hyperactive in a novel environment but not in the familiar home cage. MK-801 treatment had no effect on locomotor activity in AMPA knockouts. Finally, these mice showed normal startle amplitudes but moderately reduced PPI.131 These data extended previous observations in these animals (for references, see Wiedholz et al131) and confirmed a range of functions of the AMPA receptor.
Metabotropic Glutamate Receptors.
The group I metabotropic glutamate receptors includes mGluR1 and mGluR5 and has received considerable interest because of their possible role in schizophrenia.132 The first study on mGluR1 knockout mice revealed a severe motor coordination deficit.133,134 Despite this, spontaneous locomotor activity was normal, but the mice showed significantly increased responding to amphetamine treatment.135 These mice showed disrupted baseline PPI, but no significant changes in startle amplitude or habituation.136 The PPI disruption was not affected by pretreatment with the dopamine D2 receptor antagonist, raclopride, but the mood stabilizer, lamotrigine, tended to increase PPI more in mGluR1 knockouts than in wild-type controls.136 This was interpreted as indicating a potential role of the mGluR1 in bipolar disorder rather than schizophrenia.
Mice with mutations in the mGluR5 receptor did not show the same motor coordination deficits as mGluR1 knockouts and were normally active at baseline.137 However, cocaine at doses which induced locomotor hyperactivity in controls had no effect in mGluR5 knockouts.137 PPI showed a modest disruption in mGluR5 knockout mice in one study138 but a more severe deficit in another study.139 Parametric studies investigated the influence of different experimental conditions on the severity of the PPI deficit in these mice.139–141 Thus, a similar extent of disruption was observed in mGluR5 knockouts on either a C57BL/6 or 129Sv genetic background, and startle amplitude was significantly increased in both cases, despite the C57BL/6 mice showing higher baseline PPI and lower startle than 129Sv mice.139 The earlier PPI study in mGluR5 knockout mice was done with animals on a CD-1 outbred genetic background, which may explain the more moderate disruption observed.138 Indeed, a more robust disruption of PPI was attained when the mutation on a CD-1 background was backcrossed onto a C57BL/6 background.142 In this line of mGluR5 knockouts, MK-801 had no effect on PPI at doses which reduced PPI in wild-type controls to levels even lower than those seen in the knockouts.142 Further parametric analysis revealed that the genotype difference in PPI was similarly seen using different PPI modalities (eg, light or tactile stimuli) and was not caused by altered responses of the mutants to the prepulse nor by changes in the prepulse-pulse temporal relationship or deficits in hearing.139
Surprisingly, when mGluR5 knockout mice were tested after acute pretreatment with the typical antipsychotic, raclopride, the atypical antipsychotic, clozapine, the mood stabilizer, lamotrigine, or the 5-HT2A receptor antagonist, M100,907, none of the treatments appeared to have any effect, despite being used at doses which reversed pharmacological disruptions of PPI in control experiments.140 However, in a later study, it was shown that chronic treatment with clozapine could ameliorate the PPI deficit.143 In that study, the mGluR5 knockouts showed a modest locomotor hyperactivity at baseline and were hypersensitive to the effect of MK-801. Chronic clozapine treatment also reversed the baseline locomotor hyperactivity.143
Compared with mGluR1 and mGluR5 mutants, much less is known on behavioral changes in mice with modifications of other metabotropic glutamate receptors. mGluR2 receptor knockout mice showed normal startle and PPI144 but were slightly hyperactive in a novel open field.144 The acute effect of cocaine on locomotor activity was approximately doubled in mGluR2 knockouts compared with controls.144 Much of the other available work on mGluR2 and mGluR3 receptor knockouts has been done in the context of development of mGluR2/3 receptor agonists which may be beneficial in schizophrenia by counteracting hyperglutamatergia caused by reduced NMDA receptor function.145 For example, amphetamine and phencyclidine (PCP) dose dependently induced locomotor hyperactivity with little difference between mGluR2 knockouts and C57BL/6 wild-type controls.146 The novel mGluR2/3 receptor agonist, LY379268, dose dependently reduced spontaneous locomotor activity only in wild-type mice but not mGluR2 knockouts.146 In addition, LY379268 reduced both amphetamine- and PCP-induced locomotor hyperactivity in wild-type mice but not in mGluR2 knockouts.146 The possibility was discussed that mGluR2/3 agonist drugs exert their effects essentially by a generalized reduction of locomotor activity, both at the level of baseline activity and PCP-induced hyperactivity and, thus, have the potential of significant side effects.146,147
Comparison of mGluR2148 and mGluR3 receptor knockout mice149 showed that the latter receptor was not involved in the action of the mGluR2/3 receptor agonist drugs.146 Interestingly, however, the mGluR3 knockout mice showed a number of behavioral changes. For example, unlike mGluR2 knockouts, mGluR3 knockouts were slightly hyperactive at baseline and had a reduced response to treatment with PCP but not amphetamine.146 In contrast, the direct effect of the mGluR2/3 agonist was greater in mGluR3 knockout mice than in wild-type controls. These differences in the pharmacology of behavior between mGluR2 and mGluR3 knockout mice could be related to the cellular localization of the 2 receptors, with mGluR2 receptors being directly involved in presynaptic modulation and dampening of excessive glutamate release but mGluR3 receptors being localized more distinctly and potentially not involved with negative feedback regulation.146
A comprehensive parallel study on the action of another novel mGluR2/3 receptor agonist, LY404039, compared mGluR2, mGluR3, and mixed mGluR2/mGluR3 knockouts.150 There was no significant difference in baseline activity or the effect of a high 7.5-mg/kg dose of PCP between wild types and any of the knockouts. The increase in ambulation induced by this dose of PCP was attenuated by the mGluR2/3 agonist in wild-type mice and mGluR3 knockouts but not in mGluR2 knockouts and combined mGluR2/mGluR3 knockouts.150 Treatment with clozapine or risperidone also blocked the effect of PCP, but this effect was similar in wild-type and combined double knockouts, suggesting that these antipsychotics acted independently from mGluR2 or mGluR3 receptors.150 It should be noted that the effect of these high doses of antipsychotics on baseline behavior was not reported, and therefore, a generalized sedative effect in this experiment cannot be excluded. LY404039 also inhibited the hyperlocomotion induced by amphetamine.150 Interestingly, in mGluR2 and particularly mGluR2/mGluR3 double knockouts, the effect of amphetamine appeared to be reduced.
In addition to providing clues about the pharmacological mechanism of novel antipsychotic compounds, these studies have also described behavioral changes in mGluR2 and mGluR3 receptor knockouts. A recent report showed that dopamine D2 receptor signalling was upregulated in these mice by 67-fold and 17-fold, respectively, as measured by a GTPγS assay. This was associated with a marked elevation of dopamine D2 receptors in the high-affinity state in both mutant lines and could explain some of the altered responses to psychotropic drug stimuli, such as amphetamine and PCP.151
Taken together, studies on behavioral changes in mice with disruption of specific components of dopaminergic and glutamatergic neurotransmission, have shown the complexity of seemingly simple behaviors such as baseline locomotor activity levels or PPI and provided important clues to begin to unravel these regulatory pathways.
Schizophrenia “Risk” Gene Models of Psychosis
A large number of previous studies have suggested many risk genes in schizophrenia. However, recent meta-analyses have produced a more limited list, including 24 variants in 16 genes (APOE, COMT, DAO, DRD1, DRD2, DRD4, DTNBP1, GABRB2, GRIN2B, HP, IL1B, MTHFR, PLXNA2, SLC6A4, TP53, and TPH1).1,152 Of these, 4 were proposed as having a “strong degree of epidemiological credibility (DRD1, DTNBP1, MTHFR, and TPH1).”1 Dopamine D1 receptors and other dopamine receptors (DRD1, DRD2, and DRD4) have been discussed in previous sections of this review. Dysbindin (DTNBP1) is described below (the “Dysbindin” section). MTHFR encodes for 5,10-methylenetetrahydrofolate reductase which is indirectly involved in homocysteine metabolism and methylation processes and may be involved in negative symptoms of schizophrenia.153 However, as yet, mice with mutations in this pathway have not been tested for locomotor hyperactivity or PPI disruption. Finally, with respect to tryptophan hydroxylase 1 (TPH1), the identified polymorphism does not appear to have a functional effect.1 Moreover, while this isoform of the enzyme may play a role in PPI regulation in early neurodevelopment,154 several studies have shown it to be nonneuronal in adulthood (eg, 155,156) and Tph1 knockout mice do not show changes in serotonin levels in the brain.157
Several other genes have been identified by genetic association studies as potentially involved in the development of schizophrenia, but it is beyond the scope of this article to review the evidence for the strength of these associations. Even if these proposed candidate genes were not included in a recent meta-analysis,1 the often large amount of literature on mutant mouse models for these genes justified inclusion in this review.
Dysbindin
The dysbindin gene (DTNBP1) has been proposed as one of the most promising candidate genes in schizophrenia.1,11,158 This protein is the receptor for dystobrevins, a family of proteins originally characterized as required for maintenance of muscle integrity and function.159 The sandy (sdy) mouse has a deletion of 2 of the exons of the dysbindin gene160 and has therefore emerged as a naturally occurring dysbindin knockout.158
Sdy mice were originally compared with DBA/2J mice for behavioral characterization and were found to be less active and with lower levels of dopamine, but not glutamate, in the cerebral cortex, hippocampus, and hypothalamus.161 Other studies failed to find clear differences between the genotypes in psychosis-like behaviors,162,163 including PPI (although no data were shown160) or reported that locomotor hyperactivity induced by acute treatment with a single 2.5-mg/kg dose of amphetamine was about 50% less than in DBA wild-type controls.164 Altered dopaminergic and glutamatergic activity was suggested in sdy mice by observations on dopamine/metabolite ratios or kinetics of transmitter release.165,166 The significance of these neurochemical findings remains to be established with more detailed drug testing in sdy mice, eg, the effect of NMDA receptor antagonists. Recently, the sdy mutation was studied after backcrossing on a C57BL/6 genetic background.167 These animals were hyperactive in the open field, but no drug studies or PPI were reported as yet.167
Taken together, the sdy mice represent some behavioral phenotypes with relevance to schizophrenia, but in general, the data on psychotic-like behaviors are too limited to draw firm conclusions.
Neuregulin 1
Because a genome-wide scan study first identified neuregulin 1 as a candidate study for schizophrenia,168 several mutant mouse models have been generated to investigate its role in behavior.169,170 Neuregulins are a family of growth factor proteins which are involved in neurodevelopment at a number of levels and interact with specific tyrosine kinase receptors, the predominant one being ErbB4.171,172 There are 4 neuregulin genes, and alternative splicing is able to generate several neuregulin 1 isoforms which all contain a transmembrane domain and share a common epidermal growth factor (EGF)-like signalling domain.170–172 In addition, types I and II neuregulin 1 share an immunoglobulin domain which is not present in type III.
Although homozygous knockout of neuregulin 1 is developmentally lethal, heterozygous mice are viable and develop normally.168 Several studies showed that mice heterozygous for a mutation in the transmembrane domain are mildly hyperactive when placed in a novel open field.168,173–175 Pretreatment with a moderate dose of clozapine reduced this hyperactivity to the level of wild-type mice, where the treatment had no effect. The result could therefore not be explained by nonspecific sedative effects of clozapine.168 Despite the baseline locomotor hyperactivity, the additional hyperactivity induced by amphetamine, phencyclidine, or MK-801 was not different between transmembrane domain heterozygotes and wild-type controls.175 On the other hand, treatment with tetra-hydrocanabinol (THC), the main psychoactive component of cannabis, suppressed locomotor hyperactivity more in mutants than in controls176 although the difference in baseline activity may be a complicating factor in the interpretation of this result. Thus, the neuregulin 1 mutants “started” from a higher baseline, but after THC treatment reached levels of locomotor activity, no different from controls.176 Such methodological considerations do not negate an enhanced sensitivity of neuregulin 1 mutants to cannabis-like compounds, but illustrate how baseline behavioral differences between genetically modified mice and wild-type controls could be an important factor to influence the extent of drug effects observed.
Measurement of PPI in neuregulin 1 transmembrane heterozygous mutant mice showed a moderate disruption in one study168 with only a nonsignificant tendency found in another study176 and no difference with wild-type controls in a third, recent study.175 Close inspection of the data reveals that the baseline PPI level in wild-type mice could play a role in the differences between these studies with a significant PPI disruption in neuregulin 1 hypomorphs (average around 48%) occurring when baseline PPI in wild-type mice was 60%–65%. In contrast, a trend for a disruption of PPI in mutants (50%–55% PPI) was found where baseline PPI was about 60% in controls and no change in hypomorphs was found where baseline PPI was 45%–50%.168,175,176 The reasons for these baseline effects are unclear but could be due to differences in housing or handling procedures or the degree of backcrossing onto a congenic C57BL/6 background. Where observed, the disruption of PPI was not reversed by clozapine treatment.168 Treatment of the mice with THC elicited significant increases in PPI in neuregulin 1 hypomorphs but not in wild-type controls although the level of PPI in both genotypes was similar after treatment.176 Thus, neuregulin 1 hypomorphs may be more sensitive to the effects of cannabis-like compounds but not to a level where they reach PPI values significantly greater than controls. Treatment with apomorphine, amphetamine, or MK-801 reduced PPI to a similar extent in neuregulin 1 hypomorphs and controls.175 Treatment with the 5-HT1A receptor agonist, 8-OH-DPAT, induced a disruption of PPI in neuregulin 1 hypomorphs, but not in wild-type controls.175 However, this treatment also elicited a significant reduction of startle amplitude in the mutants only, and the PPI result should therefore be interpreted with caution as sedation may have occurred. Interestingly, also THC elicited a reduction of startle in neuregulin 1 hypomorphs but not wild-type controls,176 perhaps, pointing toward an increased sensitivity of these mice to the sedative actions of cannabinoid and/or serotonergic compounds.
Mice with mutations in the EGF-like domain replicate the hyperactive phenotype of those with deletion of the transmembrane domain.168,177 These animals showed reduced PPI although this difference with the controls was not significant.177 The effect of MK-801 and amphetamine was included in these studies, but the drugs did not induce a clear disruption of PPI nor had differential effects between the genotypes.177
Mice heterozygous for mutations in the immunoglobulin domain were not hyperactive in an open field.178 However, locomotor activity was reduced in the mutants, but not in wild-type mice, after treatment with 1 mg/kg of clozapine178 (the same dose used in transmembrane domain and EGF domain mutants168). This result was interpreted as a greater sensitivity to the “sedative effects” of clozapine in the mutant mice but was also seen as evidence for the “schizophrenia-like phenotype” of these animals.178 It would be interesting to test if neuregulin 1 mutants display enhanced sensitivity to other antipsychotic drugs with less sedative effects in mice than clozapine.
Mice heterozygous for deletion of type III neuregulin 1 showed normal motor activity in the open field. There were also no differences with wild-type controls in startle amplitude, but PPI was profoundly disrupted in these mice.179 Because of the high rate of smoking in schizophrenia and because type III neuregulin 1 has been shown to be important for cholinergic function, the mice were treated chronically with nicotine. This 6-week treatment increased PPI in mutants and normalized their PPI deficit to control levels.179
Mutant models for the neuregulin 1 receptors, ErbB2, ErbB3, and ErbB4 are also available. Mice heterozygous for ErbB2 or ErbB3 showed no changes in spontaneous locomotor activity in the open field.180 Just like neuregulin 1 transmembrane hypomorphs, the ErbB4 heterozygous null mice tended to be hyperactive in an open field, although the difference with wild-type controls in this case was small. These mice did not have significant PPI disruption.168 To avoid the problem of lethality due to disrupted cardiac development in homozygous mutants, a brain-specific knockout of ErbB4 was generated by using a Nestin-Cre/Lox approach on a C57BL/6 background.181 These mice showed spontaneous locomotor hyperactivity at 3 weeks of age but were hypoactive in the home cage and in an open field at 9–10 weeks of age.181 However, a similar mutation on an FVB background showed no change in open field locomotor activity,182 again showing how important methodological factors, such as background strain, may influence the results.
Proteolytic processing of neuregulin 1 can be mediated by β-site amyloid precursor protein cleaving enzyme 1 (BACE1).169,183 Therefore, BACE1 mutant mice may potentially display psychotic-like behavior mediated by altered levels or activity of neuregulin 1. A comprehensive behavioral phenotyping study, indeed, showed that homozygous BACE1 knockouts, but not heterozygotes, display marked disruption of PPI, enhanced novelty-induced spontaneous locomotor hyperactivity, and markedly increased MK-801-induced locomotor hyperactivity.184 Pretreatment with a moderate dose of clozapine had no effect on startle or PPI in wild-type controls and caused only a mild reduction of locomotor activity. In contrast, in BACE1 knockouts, clozapine attenuated the PPI deficit and blocked the enhanced novelty-induced locomotor hyperactivity.184
Another enzyme involved in neuregulin 1 cleavage is Aph1B/C-γ-secretase (Aph1BC).185 This proteolytic enzyme was found to be expressed in high levels in frontal cortex, hippocampus, and cerebellum in adult mouse brain. Aph1BC knockouts showed normal baseline motor activity and startle responses but disrupted PPI.185 Treatment with MK-801 induced an even further disruption of PPI in the knockouts, compared with much more modest effects in the wild-type controls. The disruption of baseline PPI could be blocked by treatment of the mice with either 1 mg/kg of clozapine or 1 mg/kg of haloperidol.185 These antipsychotic results suggested altered dopaminergic activity in these mice. Indeed, amphetamine-induced locomotor hyperactivity was significantly greater in Aph1BC knockouts whereas dopamine turnover, as measured by HVA+DOPAC/dopamine ratio, was enhanced in the ventral striatum of these mice.185
The large number of neuregulin 1 isoforms and the variety of changes seen in the available mutant mice so far illustrate the wide range of brain mechanisms that neuregulin is involved in and the many different possible ways altered neuregulin expression could be contributing (or not) to the development of psychotic features. Clearly, further characterization of existing mutant models and the generation of further, more sophisticated models of altered neuregulin function, such as inducible knockouts, are needed to resolve these issues.
DISC1
After the discovery of DISC1 as a potential schizophrenia candidate gene,186,187 some of the earliest animal studies on its potential role included the detection of natural mutations in different mouse strains. Thus, it was shown that 129S6/SvEv mice carry a 26-bp deletion in the mDISC1 gene, leading to reduced levels of one isoform of the protein.188 Other commonly used strains, such as the C57BL/6J, BALB/c or DBA/2J, did not show this mutation. Congenic C57BL/6, into which the mutation was backcrossed, showed deficits in a working memory task but no locomotor hyperactivity or disruption of PPI.188 Further studies confirmed the presence of the deletion in all 129 mouse substrains,189 an important finding as virtually all embryonic stem cell lines used for gene-targeting studies are derived from substrains of the 129 strain. Therefore, if such newly generated mouse lines are studied on their original 129 genetic background or if not backcrossed for a sufficient number of generations on a congenic background, such as C57BL/6, any possible behavioral effect would have to be interpreted taking the DISC1 mutation into account. Indeed, some of these mice could be considered models of epistatis, ie, where multiple mutations are present at the same time.
Two further mouse lines with DISC1 mutations were generated by ENU mutagenesis and backcrossing onto a C57BL/6 background.190 Both these new lines, 100P and 31L, showed disruption of PPI, although more so in 100P mice.190 These more severely affected mice also responded to treatment with the antipsychotics, haloperidol and clozapine. The 100P mice furthermore also showed higher spontaneous locomotion and rearing in an open field.190
In order to study the role of reduced DISC1 function on behavior, straightforward knockout or knockdown models were considered less useful because of the multiexon nature of the DISC1 gene and the resulting complex pattern of multiple isoforms.191 Therefore, a transgenic approach has been used by a number of groups to generate more subtle genetic modifications similar to those observed in humans. For example, forebrain-specific expression of dominant-negative truncated DISC1 under the control of the calcium-calmodulin–dependent kinase II promoter in C57BL/6 mice resulted in hyperactivity in the open field and slower habituation but only minor differences in PPI.191 A tamoxifen-inducible and reversible mutant line on a C57BL/6 background expressing only the C-terminal portion of DISC1 showed no changes in open field activity, but no specific psychosis testing was done.192 Constitutive expression of the well-defined DISC1 schizophrenia risk allele resulted in truncation of DISC1 transcription and reduced levels of the protein in the hippocampus and prefrontal cortex.193 These mice displayed deficits in working memory and executive functioning, but again specific psychosis-like behavioral models were not reported.193 Another elegant inducible transgenic approach coupled to the CAMKII promoter introduced mutant human DISC1 predominantly into forebrain neurons.194 In these mice, expression of the mutant transgene did not result in either changes in spontaneous hyperlocomotion in the open field, in startle or in PPI. On the other hand, spontaneous locomotor activity measured over a 22-h period was markedly higher in male but not female mutants.194 It was concluded that “Increased motor activity in male transgenic mice is an important feature of current pharmacological models of schizophrenia because hyperlocomotion has been correlated with the positive symptoms of schizophrenia.”194 However, as discussed in the “Locomotor Hyperactivity” section, in the absence of pharmacological characterization, the mechanism underlying the spontaneous hyperlocomotion in these male mutants, and the relevance of this behavioral change for psychosis, remains unclear.
Behavioral changes in DISC1 mutant mice have been suggested to be related to altered interaction in these animals of DISC1 and phosphodiesterase-4B (PDE-4B).190,195 Indeed, PDE-4B knockout mice showed significantly reduced PPI although startle was enhanced in these mice196 which could have influenced the result. With respect to open-field locomotor hyperactivity, PDE-4B knockouts showed a modest hypersensitivity to a high dose, but not a low dose, of amphetamine.196 Other studies have suggested that the effects of DISC1 deficiency are mediated by the GSK3β and β-catenin signalling pathways.197 It is then interesting to note that mice with altered expression of GSK3β showed little changes in psychosis models (see “Akt-GSK3β signaling” section). Similarly, forebrain-specific β-catenin knockout mice, which showed 60%–70% depletion in the forebrain, showed little change in spontaneous open-field locomotor activity or the effect of amphetamine.198 β-catenin transgenic mice displayed hypolocomotion but no difference in the acute or chronic effects of amphetamine.199
These mutant lines generated by different genetic modification mechanisms, thus, produced a wide variety of rather subtle psychosis-like phenotypes. Unfortunately, the behavioral test battery used in the studies was dissimilar and at times incomplete, and therefore, substantial further characterization work remains to be done to determine sufficient details of behavioral alterations in these animals.
Akt-GSK3β Signaling
In addition to being a possible target for lithium treatment,200 Akt-GSK3β signaling has been implicated in the development of schizophrenia.3,4 PPI levels in a range of different mouse strains (C57BL/6J, C57BL/10J, C3H/HeJ, BALB/cByJ, FVB/NJ, DBA/2J, 129/J, A/J, 129/SvJ, and AKR/J) correlated positively with GSK3 activity but not protein levels.201 Gsk3β+/− haploinsufficient mice showed reduced exploration but normal spontaneous locomotor activity and PPI.202–204 However, in one study, these mice showed a blunted hyperactivity response to a 1- or 2-mg/kg dose of amphetamine,205 suggesting Akt-GSK3β as a second major signaling pathway of dopamine receptor activation in addition to adenylate cyclase/protein kinase A mechanisms.206 However, a subsequent detailed behavioral characterization could not replicate this genotype difference.204 These authors found no difference between Gsk3β+/− mice and wild-type controls in amphetamine-induced locomotor hyperactivity, apomorphine-induced stereotyped climbing, baseline PPI, or startle habituation.204 The authors concluded that their results did not support a role of GSK3β as an important factor in schizophrenia or a major target for lithium action. Also other behavioral observations in the Gsk3β+/− model were not consistent between the different research groups.202,204,205 The reason for these marked discrepancies is unclear and highlights again that small differences between experimental conditions between laboratories may result in marked variations in experimental outcome.
In contrast to Gsk3β+/− mice with reduced Akt-GSK3β signaling, transgenic mice constitutively overexpressing an active mutated form of GSK3β were hyperactive in the open field and habituated slightly slower.207 These animals also showed higher startle responses and reduced startle habituation, although PPI was not reported.207 Other factors involved in Akt-GSK3β signaling and lithium effects have also been studied in genetically modified mice. Mice deficient in Akt1, for which GSK3β is a substrate, did not display changes in baseline PPI or in the effect of MK-801. By contrast, treatment with amphetamine at a dose which did not affect PPI in wild-type controls, significantly disrupted PPI in Akt1−/− mice. There were no genotype effects on startle in any of the conditions.208 These authors discussed these behavioral results in the context of a possible link of changes in Akt-GSK3β signaling with reelin (see “Other Genetically Modified Mice With Relevance to Psychotic-Like Behaviours” section) and neuregulin-1 (see “Neuregulin 1” section) in schizophrenia. Such links suggest that epistatic interactions could be important and future studies may be able to address this using double-mutant models.
Overall, the available studies show some behavioral and neuropharmacological changes in mice with altered activity of the Akt-GSK3β signaling pathway. However, the studies were sometimes inconsistent and mostly focused on lithium effects and Bipolar Disorder.209,210 Therefore, no conclusions as to major deficits in behavioral models related to psychosis can be drawn as yet.
The 22q11.2 Deletion Syndrome and Its Associated Genes
Several studies have shown that deletion of the 22q11.2 chromosomal region (22q11.2 deletion syndrome, 22q11.2DS), which includes at least 40 genes, is associated with schizophrenia.211–213 Df1/+ mice are hemizygous for deletion of a region syntenic with the human deleted region.214 These animals show normal exploratory activity in an open field and normal startle habituation. However, average startle amplitudes were increased, whereas PPI was moderately disrupted.214,215 The 22q11.2 chromosomal region contains several genes of interest for schizophrenia, such as catechol-O-methyltransferase (Comt) and proline dehydrogenase (Prodh). In a way, Df1/+ mice are a model of epistatic interaction because multiple genes which were subsequently associated with schizophrenia are deleted in these animals.214
The Pro/Re strain of mice display altered proline metabolism and enhanced plasma proline levels. These mice were shown to carry a missense mutation in Prodh which resulted in the production of a mutant Prodh protein with reduced enzymatic activity. These animals showed normal spontaneous locomotor activity and startle, but PPI was moderately disrupted.216 However, it was argued214 that the PPI disruption in these homozygous mice was less severe than that seen in heterozygous Df1/+ mice, indicating that Prodh was unlikely to be solely responsible for the effect of 22q11.2DS on sensorimotor gating. The same is true for mice with deletion of the transmembrane palmitoyltransferase, ZDHHC8, which showed a small reduction of PPI in females but not males.217 Interestingly, these animals also showed locomotor hypoactivity and a virtual absence of an effect of a high, 0.4-mg/kg dose of MK-801, which induced locomotor hyperactivity in the wild-type controls.217 The latter marked phenotypic change would need to be further investigated by scoring associated behaviors, such as stereotypy, and testing lower doses of MK-801 as well (see figure 1). Potentially, these results point at a novel regulatory mechanism for NMDA receptor–mediated locomotor hyperactivity.
Following from the early studies with Prodh knockdown mice,216 the same mutation was introduced into the 129/SvEv strain through backcrossing.218 Similar to ZDHHC8 mutant mice, these animals were hypoactive in the open field and showed reduced responding to acute treatment with a high but not a moderate dose of MK-801. In marked contrast to the result with MK-801, locomotor hyperactivity induced by a moderate or a high dose of amphetamine was significantly greater in Prodh-deficient mice.218 It will be interesting to assess the effect of lower doses of MK-801 in these animals to confirm that the apparent hyposensitivity is not caused by the emergence of stereotyped behaviors (see figure 1).
Prodh-deficient mice showed marked upregulation of Comt in the cortex but not striatum.218 Amphetamine treatment increased dopamine release in both brain regions, but this effect was significantly potentiated in Prodh-deficient mice in the cortex but not striatum.218 To further analyze the possible epistatic interaction between Prodh and Comt, the effect of the Comt inhibitor, tolcapone, was studied in the Prodh mutants. Tolcapone pretreatment potentiated the effect of a low dose of amphetamine on locomotor activity and induced a disruption of PPI in the mutants but not wild-type mice.218 These results explain why in a number of behavioral testing paradigms, the changes in Prodh-deficient animals have been mild or inconsistent. Only if the upregulated Comt activity is also targeted, done here with tolcapone treatment, is a clear phenotype unmasked. These elegant experiments are therefore also a good example of how assessing only baseline behaviors may miss effects of genetic modifications.
In parallel to the studies on Prodh/Comt interactions, several studies have assessed the effect of mutants with altered activity of Comt, the gene for which is also located in the 22q11.2 region213 and which was identified in the recent schizophrenia risk gene meta-analysis.1 Normal frontal cortical dopaminergic activity relies strongly on intact activity of COMT, and molecular genetic studies have suggested an association of aberrant COMT activity and psychiatric illnesses.1 Mice with deletion of Comt displayed a 2- to 3-fold increase in endogenous dopamine levels in the frontal cortex, but not the striatum,219 and changes in the locomotor hyperactivity response to amphetamine dependent on the dose and time after injection.220 The relatively small extent of behavioral changes in these mice was attributed to the presence of dopamine transporter activity which constitutes the most important mechanism to maintain extracellular dopaminergic tone. Therefore, the effects of cocaine and the dopamine transporter inhibitor, GBR12909, were investigated.221 Both drugs induced the expected locomotor hyperactivity, but these effects were attenuated in male, but not female COMT knockout mice.221
To specifically study the common Val/Met single-nucleotide polymorphism found in several human studies, recently a transgenic mouse was generated which carries the human COMT-Val variant as opposed to the wild-type COMT-Leu mice.222 In humans, the Val form leads to increased COMT activity and the COMT-Val transgenic mice can therefore be considered as a model of enhanced COMT function, as opposed to COMT knockout. As part of a large behavioral phenotyping study, the authors found no changes in baseline locomotor activity or in amphetamine-induced locomotor hyperactivity.222 Startle responses were reduced, but PPI was not significantly different between transgenic animals and controls. By contrast, in COMT knockouts, startle reactivity was increased, again, however, without changes in PPI.222
In order to further identify the critical genes involved in 22q11.2DS and the behavioral changes in Df1/+ mice, a series of mutants with overlapping deletions were generated.223 Single-gene mutants were then generated to assess more specific gene modifications. It was concluded from this approach that the previously reported PPI deficits in Df1/+ mice214,215 could be explained by haploinsufficiency of 2 genes, Tbx1 and Gnb1l,223 with the disruption being greatest in Tbx1 heterozygous mice.223 However, others suggested that Tbx1 deletion was more important for the heart abnormalities and craniofacial development defects in 22q11.2DS.224 These latter authors found no disruption of PPI in Tbx1+/− mice. Instead, mice haploinsuffient for Lgdel, a large deletion which overlaps with the 22q11.2 deletion, showed significant disruption of PPI.224 The reasons for these discrepancies are unclear but could be related to the different background strains used in these studies.223,224
Another gene which maps to the 22q11.2 region is the Nogo receptor 1 (RTN4R) which regulates axonal growth.225 Similar to ZDHHC8 mutants, Rtn4r mutant mice were hypoactive in an open field225,226 but showed little change in PPI.225 Despite the significant reduction of open field locomotor activity, the authors concluded that RTN4R was unlikely to contribute in a major way to schizophrenia.225 Interestingly, mice deficient in Nogo-A (Rtn4), one of the ligands for Nogo receptor 1, were hyperactive in the dark phase, but not light phase, of the circadian cycle and showed a much greater amphetamine-induced locomotor hyperactivity response than wild-type controls.227 PPI was not reported, and the relevance of these data for 22q11.2DS remains to be established.
Other Genetically Modified Mice With Relevance to Psychotic-Like Behaviors
Several other genes have been implicated in schizophrenia, related to pathophysiological aspects of the disease, neurotransmitter systems involved in antipsychotic drug action, as novel risk/susceptibility genes, or found serendipitously through random mutagenesis or chance and subsequent behavioral phenotyping, ie, a “bottom-up” approach.12 This review article cannot include full details of all these additional genetic mechanisms potentially involved in schizophrenia, even though the available studies may contain important clues for future investigation.
In addition to dopaminergic involvement, a “classical” neuropathological mechanism in schizophrenia is represented by altered activity of gamma-aminobutyric acid (GABA) neurons in the brain.228 Mice with genetically modified GABAergic activity show various psychosis-like behaviors,229,230 and GABAergic drugs may be beneficial against sensorimotor gating231 and cognitive deficits in schizophrenia.232 Other work has focused on the serotonin system.233,234 Of the 14 serotonin receptors, several mutant models have shown changes in behavior with relevance to psychosis, such as altered motor activity or disruption of PPI.50,235–240 Also the cholinergic system, particularly muscarinic receptors, has been the focus of many studies.241,242 Knockout mice for all 5 muscarinic receptors have been investigated and have suggested important drug development targets for cognitive and psychotic symptoms of schizophrenia.243,244
Molecular genetic and postmortem studies have identified several other genes which could be associated with the development of schizophrenia. For example, several studies have shown reduced levels of brain-derived neurotrophic factor (BDNF) in the frontal cortex of patients with schizophrenia (for references, see Angelucci et al245). Consequently, studies have addressed psychosis-like behavior of BDNF mutant mice. Cocaine-induced hyperlocomotion was markedly reduced in BDNF heterozygotes compared with wild-type controls,246,247 but the effect of amphetamine was increased248 or prolonged.249 In addition to constitutive changes in BDNF expression, more regionally and temporally specific modifications have been studied. In conditional BDNF knockout mice, BDNF deletion was restricted to forebrain areas, such as the frontal cortex, amygdala, and hippocampus.250 These Emx-BDNF knockout mice showed normal startle and PPI but reduced spontaneous locomotor activity.250 Further studies used a trigenic approach to generate inducible and conditional BDNF knockouts which were found to be hyperactive.251–253 Two additional conditional BDNF mutants, generated by crossing floxed BDNF mice with 2 different tetracyclin transactivator–induced Cre-producing lines, showed moderate or near-complete BDNF depletion in hippocampus and cortical regions.252 Similar to previous studies with other BDNF mutants,254,255 both the conditional mutants in this study were hyperactive in a novel environment; however, it was also shown that this phenotype was only present in male mice and not in female mice.252 The latter is an interesting observation not only because of its potential importance for gender differences in psychiatric illness but also because many behavioral studies in mutant mouse lines appear to test only male mice, a mix of male and female mice, or not indicate the sex of the animals. In further studies, rather than full or partial genetic deletion of BDNF gene expression, a particularly elegant model of altered BDNF regulation was aimed at reproducing the Val66Met single-nucleotide polymorphism observed in clinical populations.256 BdnfMetMet animals showed normal BDNF levels in the brain but dysregulated BDNF release. Baseline locomotor activity was normal, but drug-induced locomotor hyperactivity or PPI were not reported.256 Overall, this large variety of BDNF mutant models supports a role for this neurotrophin in behavioral models with relevance to schizophrenia. However, the information is still limited and much further characterization of these mice is needed.
Reelin (RELN) is a protein involved in axon guidance, dendritic spine morphology, and neural plasticity.257,258 In postmortem brain of patients with schizophrenia, levels of RELN and RELN mRNA were decreased by about 50% in cortex and hippocampus.259 The rl/+ heterozygous mouse has a reduction of reelin levels similar to that seen in schizophrenia260,261 and displays reduced sensitivity to the locomotor hyperactivity-inducing effects of metamphetamine.262 The rl/+ heterozygotes also displayed reduced baseline locomotor activity, an effect which could be reversed with chronic treatment with the atypical antipsychotic, olanzapine.263 Only rl/rl-null mutants, but not rl/+ heterozygotes, showed disrupted PPI and startle habituation, and the authors questioned the validity of the rl/+ mice as a potential animal model of schizophrenia.264 However, another study showed an age-dependent decrease of PPI,265 but the genotype effect appears to be sensitive to environmental modulation. For example, the significant difference in PPI between rl/+ mice and wild-type controls was not observed when the animals were single housed (see Tueting et al258). Single housing leads to disruption of PPI in mice,266 and indeed, PPI in wild-type controls was decreased by isolation housing to the level of rl/+ mice where there was no further decrease (unpublished, see Tueting et al258). In other studies, a PPI deficit could only be demonstrated in these mice with a cross-modal PPI protocol combining acoustic startle stimuli with tactile prepulses.267 Furthermore, where wild-type mice showed the expected inverted U-shaped relationship between ISI and levels of PPI, with optimal intervals between 40 and 80 ms, in rl/+ mice, this relationship was skewed toward shorter intervals of 20–60 ms.267 Considering the large variety of PPI protocols employed in the literature, this could have marked consequences for the interpretation of a particular genetically modified mouse line like rl/+ mice as a valid model for sensory gating deficits in schizophrenia. Clearly, these heterozygous mice display a subtle behavioral phenotype which appears to vary between research laboratories258,268 and is dependent on behavioral test conditions. These findings add to the growing knowledge that external conditions, such as stress, housing, and handling, may have major effects of the results of behavioral genotyping and emphasize the importance of standardization of these factors between laboratories (see “Methodological Considerations” section and van der Staay and Steckler18).
Another neurodevelopmental factor is the nucleus receptor Nurr1, which is important for development of dopaminergic neurons. Nurr1 heterozygous mutant mice show reduced brain dopamine levels and enhanced spontaneous and stress-induced locomotor hyperactivity.269 The effect of amphetamine or MK-801 to induce locomotor hyperactivity, over and above the already existing increased activity, was not different between Nurr1 heterozygous mice and controls,269 a result similar to our recent findings in neuregulin 1 heterozygous mice.175 PPI was not different at baseline between the genotypes when the animals were group housed, but post-weaning isolation induced a disruption of PPI in the Nurr1 heterozygous mice, but not the wild-type controls.270 This is an interesting example of gene-environment interaction, similar to discussed above for reelin heterozygous mice. It again emphasizes that housing conditions can significantly impact on the results and need to be reported in detail. Parallel studies on a similar Nurr1 heterozygous model confirmed these results.271
The phosphatase, calcineurin, has also been implicated in schizophrenia, and forebrain-specific knockouts of calcineurin have been generated.272 These animals showed marked spontaneous locomotor hyperactivity and disruption of PPI and were more sensitive to low doses of MK-801, but not amphetamine.273
Finally, genetically modified mice for a variety of other factors which have been implicated in schizophrenia, such as Regulator of G-protein Signalling-4(RGS-4), neural adhesion molecule, and the LPA1 receptor, have shown behavioral changes in the locomotor hyperactivity or PPI domain.274–278 In addition, several genetically modified mice have been generated in the course of basic studies into neural plasticity mechanisms, addiction, neurotransmitter regulation, stress, and hormonal modulation of behavior, and in many of these animals, changes in drug-induced locomotor hyperactivity or PPI disruptions have been found. These studies include a variety of factors, eg, angiotensin-converting enzyme,279 oxytocin,280 estrogen,55,281 certain G-proteins,57,282 pituitary adenylate cyclase–activating polypeptide(PACAP),283 and Homer1.284 However, in the absence of consistent molecular genetic evidence, the immediate relevance of these results for schizophrenia remains to be established.
Conclusions
The studies reviewed above suggest that a multitude of genetic mechanisms are directly or indirectly involved in (drug induced) locomotor hyperactivity and PPI disruption. This complexity not only reflects the exquisite intricacy of behavioral regulation but, similarly, and perhaps consequently, the multifactorial nature of psychotic features in schizophrenia. Clearly, further studies are needed to resolve these complex mechanisms.
Genetically modified mice, ranging from straightforward knockouts to sophisticated conditional modifications, have become a standard tool in neuropsychopharmacological research into the mechanisms involved in psychosis-like behavior. Locomotor hyperactivity, either at baseline or after treatment with psychoactive drugs, such as amphetamine or MK-801, and disruption of PPI, have become widely used behavioral tools to investigate the consequences of these genetic modifications in the domain of psychosis-like behaviors. The available literature reveals variability between laboratories in terms of pharmacological and methodological details of behavioral phenotyping strategies. It will therefore be important to aim for more comprehensive testing of mouse mutant models and better standardize methods and conditions. Nevertheless, the wealth of available data has provided essential new insight and mechanistic clues which could lead to new treatments or even prevention strategies for schizophrenia.
Funding
Dr van den Buuse is a Senior Research Fellow of the National Health and Medical Research Council of Australia (grant 435500).
References
- 1.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nature. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 2.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: from hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Desbonnet L, Waddington JL, O'Tuathaigh CM. Mutant models for genes associated with schizophrenia. Biochem Soc Trans. 2009;37:308–312. doi: 10.1042/BST0370308. [DOI] [PubMed] [Google Scholar]
- 5.Desbonnet L, Waddington JL, O'Tuathaigh CMP. Mice mutant for genes associated with schizophrenia: common phenotype or distinct endophenotypes? Behav Brain Res. 2009;204:258–273. doi: 10.1016/j.bbr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Gainetdinov RR, Mohn AR, Caron MG. Genetic animal models: focus on schizophrenia. Trends Neurosci. 2001;24:527–533. doi: 10.1016/s0166-2236(00)01886-5. [DOI] [PubMed] [Google Scholar]
- 7.Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: emergence of positional candidates and future directions. Trends Pharmacol Sci. 2006;27:226–233. doi: 10.1016/j.tips.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 9.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression. neuropathology: on the matter of their convergence. Mol Psychiatry. 2004;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 10.Hunter AJ, Nolan PM, Brown SD. Towards new models of disease and physiology in the neurosciences: the role of induced and naturally occurring mutations. Hum Mol Genet. 2000;9:893–900. doi: 10.1093/hmg/9.6.893. [DOI] [PubMed] [Google Scholar]
- 11.O'Tuathaigh CM, Babovic D, O'Meara G, Clifford JJ, Croke DT, Waddington JL. Susceptibility genes for schizophrenia: characterisation of mutant mouse models at the level of phenotypic behaviour. Neurosci Biobehav Rev. 2007;31:60–78. doi: 10.1016/j.neubiorev.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res. 2009;204:282–294. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takao K, Yamasaki N, Miyakawa T. Impact of brain-behavior phenotypying of genetically-engineered mice on research of neuropsychiatric disorders. Neurosci Res. 2007;58:124–132. doi: 10.1016/j.neures.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 14.van den Buuse M, Garner B, Gogos A, Kusljic S. Importance of animal models in schizophrenia research. Aust N Z J Psychiatry. 2005;39:550–557. doi: 10.1080/j.1440-1614.2005.01626.x. [DOI] [PubMed] [Google Scholar]
- 15.Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 16.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 17.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 18.van der Staay FJ, Steckler T. The fallacy of behavioral phenotyping without standardisation. Genes Brain Behav. 2002;1:9–13. doi: 10.1046/j.1601-1848.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalueff AV, Wheaton M, Murphy DL. What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter WT, Koenig JI. The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology. 2008;33:2061–2079. doi: 10.1038/sj.npp.1301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leucht S, Wahlbeck K, Hamann J, Kissling W. New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet. 2003;361:1581–1589. doi: 10.1016/S0140-6736(03)13306-5. [DOI] [PubMed] [Google Scholar]
- 22.Drevets WC, Gautier C, Price JC, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 23.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 24.Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Forti M, Lappin JM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17:S101–S107. doi: 10.1016/j.euroneuro.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2004;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Clifford JJ, Tighe O, Croke DT, Sibley DR, Drago J, Waddington JL. Topographical evaluation of the phenotype of spontaneous behaviour in mice with targeted gene deletion of the D1A dopamine receptor: paradoxical elevation of grooming syntax. Neuropharmacology. 1998;37:1595–1602. doi: 10.1016/s0028-3908(98)00116-6. [DOI] [PubMed] [Google Scholar]
- 28.Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology. 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- 29.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- 31.Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- 32.Kuczenski R, Segal DS. In vivo measures of monoamines during amphetamine-induced behaviors in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1991;14:S37–S50. doi: 10.1016/0278-5846(90)90085-u. [DOI] [PubMed] [Google Scholar]
- 33.Moore KE. The actions of amphetamine on neurotransmitters: a brief review. Biol Psychiatry. 1977;12:451–462. [PubMed] [Google Scholar]
- 34.Rothman RB, Baumann MH, Dersch CM, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F. Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr. 1994;145:1–18. [PubMed] [Google Scholar]
- 36.Zocchi A, Orsini C, Cabib S, Puglisi-Allegra S. Parallel strain-dependent effect of amphetamine on locomotor activity and dopamine release in the nucleus accumbens: an in vivo study in mice. Neuroscience. 1998;82:521–528. doi: 10.1016/s0306-4522(97)00276-5. [DOI] [PubMed] [Google Scholar]
- 37.Gunduz-Bruce H. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev. 2009;60:279–286. doi: 10.1016/j.brainresrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 39.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 40.Tamminga CA, Lahti AC, Medoff DR, Gao XM, Holcomb HH. Evaluating glutamatergic transmission in schizophrenia. Ann N Y Acad Sci. 2003;1003:113–118. doi: 10.1196/annals.1300.062. [DOI] [PubMed] [Google Scholar]
- 41.Yates JW, Meij JTA, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci Lett. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kegeles LS, Abi-Dargham A, Zea-Ponce Y, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 43.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Res. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geyer MA. Assessing prepulse inhibition of startle in wild-type and knockout mice. Psychopharmacology (Berl) 1999;147:11–13. doi: 10.1007/s002130051130. [DOI] [PubMed] [Google Scholar]
- 46.Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 787–798. [Google Scholar]
- 47.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 48.Mohr D, Pilz PK, Plappert CF, Fendt M. Accumbal dopamine D2 receptors are important for sensorimotor gating in C3H mice. Neuroreport. 2007;18:1493–1497. doi: 10.1097/WNR.0b013e3282e9a863. [DOI] [PubMed] [Google Scholar]
- 49.Wan FJ, Geyer MA, Swerdlow NR. Accumbens D2 modulation of sensorimotor gating in rats: assessing anatomical localization. Pharmacol Biochem Behav. 1994;49:155–163. doi: 10.1016/0091-3057(94)90470-7. [DOI] [PubMed] [Google Scholar]
- 50.Gogos A, Bogeski M, Van den Buuse M. Role of serotonin-1A receptors in the action of antipsychotic drugs: comparison of prepulse inhibition studies in mice and rats and relevance for human pharmacology. Behav Pharmacol. 2008;19:548–561. doi: 10.1097/FBP.0b013e32830cd822. [DOI] [PubMed] [Google Scholar]
- 51.Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 52.Olivier B, Leahy C, Mullen T, et al. The DBA/2J strain and prepulse inhibition of startle: a model system to test antipsychotics? Psychopharmacology (Berl) 2001;156:284–290. doi: 10.1007/s002130100828. [DOI] [PubMed] [Google Scholar]
- 53.Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2003 doi: 10.1002/0471142301.ns0817s24. Chap. 8:Unit 8 17. [DOI] [PubMed] [Google Scholar]
- 54.Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000;39:2170–2179. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 55.Van den Buuse M, Simpson ER, Jones ME. Prepulse inhibition of acoustic startle in aromatase knock-out mice: effects of age and gender. Genes Brain Behav. 2003;2:93–102. doi: 10.1034/j.1601-183x.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- 56.van den Buuse M, Martin S, Holgate J, Matthaei K, Hendry I. Mice deficient in the alpha subunit of Gz show changes in pre-pulse inhibition, anxiety and responses to 5-HT1A receptor stimulation, which are strongly dependent on the genetic background. Psychopharmacology. 2007;195:273–283. doi: 10.1007/s00213-007-0888-7. [DOI] [PubMed] [Google Scholar]
- 57.van den Buuse M, Martin S, Brosda J, Leck KJ, Matthaei KI, Hendry I. Enhanced effect of dopaminergic stimulation on prepulse inhibition in mice deficient in the alpha subunit of Gz. Psychopharmacology. 2005;183:358–367. doi: 10.1007/s00213-005-0181-6. [DOI] [PubMed] [Google Scholar]
- 58.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 59.Pietropaolo S, Singer P, Feldon J, Yee BK. The postweaning social isolation in C57BL/6 mice: preferential vulnerability in the male sex. Psychopharmacology. 2008;197:613–628. doi: 10.1007/s00213-008-1081-3. [DOI] [PubMed] [Google Scholar]
- 60.Matthaei K. Genetically manipulated mice: a powerful tool with unsuspected caveats. J Physiol. 2007;582:481–488. doi: 10.1113/jphysiol.2007.134908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drago J, Gerfen CR, Lachowicz JE, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu M, Moratalla R, Gold LH, et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- 63.Xu M, Hu XT, Cooper DC, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 64.Centonze D, Grande C, Saulle E, et al. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74:813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- 66.Crawford CA, Drago J, Watson JB, Levine MS. Effects of repeated amphetamine treatment on the locomotor activity of the dopamine D1A-deficient mouse. Neuroreport. 1997;8:2523–2527. doi: 10.1097/00001756-199707280-00021. [DOI] [PubMed] [Google Scholar]
- 67.Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- 68.Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doherty JM, Masten VL, Powell SB, et al. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–2656. doi: 10.1038/sj.npp.1301657. [DOI] [PubMed] [Google Scholar]
- 70.Dracheva S, Xu M, Kelley KA, et al. Paradoxical locomotor behavior of dopamine D1 receptor transgenic mice. Exp Neurol. 1999;157:169–179. doi: 10.1006/exnr.1999.7037. [DOI] [PubMed] [Google Scholar]
- 71.Baik JH, Picetti R, Saiardi A, et al. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 72.Clifford JJ, Usiello A, Vallone D, Kinsella A, Borrelli E, Waddington JL. Topographical evaluation of behavioural phenotype in a line of mice with targeted gene deletion of the D2 dopamine receptor. Neuropharmacology. 2000;39:382–390. doi: 10.1016/s0028-3908(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 73.Kelly MA, Rubinstein M, Phillips TJ, et al. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL. Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology (Berl) 2002;163:54–61. doi: 10.1007/s00213-002-1142-y. [DOI] [PubMed] [Google Scholar]
- 75.Kelly MA, Low MJ, Rubinstein M, Phillips TJ. Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes Brain Behav. 2008;7:568–577. doi: 10.1111/j.1601-183X.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- 77.Clifford JJ, Kinsella A, Tighe O, et al. Comparative, topographically-based evaluation of behavioural phenotype and specification of D(1)-like:D(2) interactions in a line of incipient congenic mice with D(2) dopamine receptor ‘knockout’. Neuropsychopharmacology. 2001;25:527–536. doi: 10.1016/S0893-133X(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 78.Waddington JL, Clifford JJ, McNamara FN, Tomiyama K, Koshikawa N, Croke DT. The psychopharmacology-molecular biology interface: exploring the behavioural roles of dopamine receptor subtypes using targeted gene deletion (‘knockout’) Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:925–964. doi: 10.1016/s0278-5846(01)00152-x. [DOI] [PubMed] [Google Scholar]
- 79.Kellendonk C, Simpson EH, Polan HJ, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 80.Usiello A, Baik JH, Rouge-Pont F, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 81.Xu R, Hranilovic D, Fetsko LA, Bucan M, Wang Y. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7:1075–1082. doi: 10.1038/sj.mp.4001145. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ralph RJ, Varty GB, Kelly MA, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Accili D, Fishburn CS, Drago J, et al. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joseph JD, Wang YM, Miles PR, et al. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience. 2002;112:39–49. doi: 10.1016/s0306-4522(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 86.Xu M, Koeltzow TE, Santiago GT, et al. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 87.Jung MY, Skryabin BV, Arai M, et al. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- 88.McNamara RK, Logue A, Stanford K, Xu M, Zhang J, Richtand NM. Dose-response analysis of locomotor activity and stereotypy in dopamine D3 receptor mutant mice following acute amphetamine. Synapse. 2006;60:399–405. doi: 10.1002/syn.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- 90.Leriche L, Schwartz JC, Sokoloff P. The dopamine D3 receptor mediates locomotor hyperactivity induced by NMDA receptor blockade. Neuropharmacology. 2003;45:174–181. doi: 10.1016/s0028-3908(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 91.Rubinstein M, Phillips TJ, Bunzow JR, et al. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 92.Katz JL, Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK. Cocaine-induced locomotor activity and cocaine discrimination in dopamine D4 receptor mutant mice. Psychopharmacology (Berl) 2003;170:108–114. doi: 10.1007/s00213-003-1513-z. [DOI] [PubMed] [Google Scholar]
- 93.Kruzich PJ, Suchland KL, Grandy DK. Dopamine D4 receptor-deficient mice, congenic on the C57BL/6J background, are hypersensitive to amphetamine. Synapse. 2004;53:131–139. doi: 10.1002/syn.20043. [DOI] [PubMed] [Google Scholar]
- 94.Thanos P, Bermeo C, Rubinstein M, et al. Conditioned place preference and locomotor activity in response to methylphenidate, amphetamine and cocaine in mice lacking dopamine D4 receptors. J Psychopharmacol. 2009 doi: 10.1177/0269881109102613. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holmes A, Hollon TR, Gleason TC, et al. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115:1129–1144. [PubMed] [Google Scholar]
- 96.Elliot EE, Sibley DR, Katz JL. Locomotor and discriminative-stimulus effects of cocaine in dopamine D5 receptor knockout mice. Psychopharmacology (Berl) 2003;169:161–168. doi: 10.1007/s00213-003-1494-y. [DOI] [PubMed] [Google Scholar]
- 97.Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 2008;200:117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gainetdinov RR. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:301–313. doi: 10.1007/s00210-007-0216-0. [DOI] [PubMed] [Google Scholar]
- 99.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 100.Morice E, Denis C, Giros B, Nosten-Bertrand M. Phenotypic expression of the targeted null-mutation in the dopamine transporter gene varies as a function of the genetic background. Eur J Neurosci. 2004;20:120–126. doi: 10.1111/j.1460-9568.2004.03465.x. [DOI] [PubMed] [Google Scholar]
- 101.Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci USA. 2001;98:11047–11054. doi: 10.1073/pnas.191353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 104.Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 105.Zhuang X, Oosting RS, Jones SR, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci USA. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, Geyer MA. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biol Psychiatry. 2003;53:352–359. doi: 10.1016/s0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- 107.Tilley MR, Cagniard B, Zhuang X, Han DD, Tiao N, Gu HH. Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression. BMC Neurosci. 2007;8:42. doi: 10.1186/1471-2202-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Enomoto T, Noda Y, Nabeshima T. Phencyclidine and genetic animal models of schizophrenia developed in relation to the glutamate hypothesis. Methods Find Exp Clin Pharmacol. 2007;29:291–301. doi: 10.1358/mf.2007.29.4.1075358. [DOI] [PubMed] [Google Scholar]
- 109.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 110.Forrest D, Yuzaki M, Soares HD, et al. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 111.Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav. 2006;85:481–491. doi: 10.1016/j.pbb.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bickel S, Lipp HP, Umbricht D. Impaired attentional modulation of auditory evoked potentials in N-methyl-D-aspartate NR1 hypomorphic mice. Genes Brain Behav. 2007;6:558–568. doi: 10.1111/j.1601-183X.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 113.Bickel S, Lipp HP, Umbricht D. Early auditory sensory processing deficits in mouse mutants with reduced NMDA receptor function. Neuropsychopharmacology. 2008;33:1680–1689. doi: 10.1038/sj.npp.1301536. [DOI] [PubMed] [Google Scholar]
- 114.Fradley RL, O'Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res. 2005;163:257–264. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 115.Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology (Berl) 2006;184:190–200. doi: 10.1007/s00213-005-0214-1. [DOI] [PubMed] [Google Scholar]
- 116.Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Res. 2006;1089:186–194. doi: 10.1016/j.brainres.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 117.Ohtsuka N, Tansky MF, Kuang H, et al. Functional disturbances in the striatum by region-specific ablation of NMDA receptors. Proc Natl Acad Sci USA. 2008;105:12961–12966. doi: 10.1073/pnas.0806180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cui Z, Wang H, Tan Y, Zaia KA, Zhang S, Tsien JZ. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron. 2004;41:781–793. doi: 10.1016/s0896-6273(04)00072-8. [DOI] [PubMed] [Google Scholar]
- 119.Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–6657. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, Nabeshima T. Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor epsilon1 subunit. J Neurosci. 2001;21:750–757. doi: 10.1523/JNEUROSCI.21-02-00750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ikeda K, Araki K, Takayama C, et al. Reduced spontaneous activity of mice defective in the epsilon 4 subunit of the NMDA receptor channel. Brain Res Mol Brain Res. 1995;33:61–71. doi: 10.1016/0169-328x(95)00107-4. [DOI] [PubMed] [Google Scholar]
- 123.Ebralidze AK, Rossi DJ, Tonegawa S, Slater NT. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J Neurosci. 1996;16:5014–5025. doi: 10.1523/JNEUROSCI.16-16-05014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kadotani H, Hirano T, Masugi M, et al. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takeuchi T, Kiyama Y, Nakamura K, et al. Roles of the glutamate receptor epsilon2 and delta2 subunits in the potentiation and prepulse inhibition of the acoustic startle reflex. Eur J Neurosci. 2001;14:153–160. doi: 10.1046/j.0953-816x.2001.01620.x. [DOI] [PubMed] [Google Scholar]
- 126.Brody SA, Nakanishi N, Tu S, Lipton SA, Geyer MA. A developmental influence of the N-methyl-D-aspartate receptor NR3A subunit on prepulse inhibition of startle. Biol Psychiatry. 2005;57:1147–1152. doi: 10.1016/j.biopsych.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 127.Kew JN, Koester A, Moreau JL, et al. Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci. 2000;20:4037–4049. doi: 10.1523/JNEUROSCI.20-11-04037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ballard TM, Pauly-Evers M, Higgins GA, et al. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neurosci. 2002;22:6713–6723. doi: 10.1523/JNEUROSCI.22-15-06713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karlsson RM, Tanaka K, Heilig M, Holmes A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol Psychiatry. 2008;64:810–814. doi: 10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karlsson R-M, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wiedholz LM, Owens WA, Horton RE, et al. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry. 2008;13:631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- 132.Pietraszek M, Nagel J, Gravius A, Schafer D, Danysz W. The role of group I metabotropic glutamate receptors in schizophrenia. Amino Acids. 2007;32:173–178. doi: 10.1007/s00726-006-0319-9. [DOI] [PubMed] [Google Scholar]
- 133.Conquet F, Bashir ZI, Davies CH, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 134.Aiba A, Kano M, Chen C, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 135.Mao L, Conquet F, Wang JQ. Augmented motor activity and reduced striatal preprodynorphin mRNA induction in response to acute amphetamine administration in metabotropic glutamate receptor 1 knockout mice. Neuroscience. 2001;106:303–312. doi: 10.1016/s0306-4522(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 136.Brody SA, Conquet F, Geyer MA. Disruption of prepulse inhibition in mice lacking mGluR1. Eur J Neurosci. 2003;18:3361–3366. doi: 10.1111/j.1460-9568.2003.03073.x. [DOI] [PubMed] [Google Scholar]
- 137.Chiamulera C, Epping-Jordan MP, Zocchi A, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 138.Kinney GG, Burno M, Campbell UC, et al. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- 139.Brody SA, Dulawa SC, Conquet F, Geyer MA. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry. 2004;9:35–41. doi: 10.1038/sj.mp.4001404. [DOI] [PubMed] [Google Scholar]
- 140.Brody SA, Conquet F, Geyer MA. Effect of antipsychotic treatment on the prepulse inhibition deficit of mGluR5 knockout mice. Psychopharmacology (Berl) 2004;172:187–195. doi: 10.1007/s00213-003-1635-3. [DOI] [PubMed] [Google Scholar]
- 141.Brody SA, Geyer MA. Interactions of the mGluR5 gene with breeding and maternal factors on startle and prepulse inhibition in mice. Neurotox Res. 2004;6:79–90. doi: 10.1007/BF03033300. [DOI] [PubMed] [Google Scholar]
- 142.Lipina T, Weiss K, Roder J. The ampakine CX546 restores the prepulse inhibition and latent inhibition deficits in mGluR5-deficient mice. Neuropsychopharmacology. 2007;32:745–756. doi: 10.1038/sj.npp.1301191. [DOI] [PubMed] [Google Scholar]
- 143.Gray L, van den Buuse M, Scarr E, Dean B, Hannan AJ. Clozapine reverses schizophrenia-related behaviours in the metabotropic glutamate receptor 5 knockout mouse: association with N-methyl-D-aspartic acid receptor up-regulation. Int J Neuropsychopharmacol. 2009;12:45–60. doi: 10.1017/S1461145708009085. [DOI] [PubMed] [Google Scholar]
- 144.Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci USA. 2005;102:4170–4175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22:308–322. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- 146.Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- 147.Spooren WP, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R. Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. Eur J Pharmacol. 2000;397:R1–R2. doi: 10.1016/s0014-2999(00)00269-7. [DOI] [PubMed] [Google Scholar]
- 148.Yokoi M, Kobayashi K, Manabe T, et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 149.Corti C, Battaglia G, Molinaro G, et al. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 2007;27:8297–8308. doi: 10.1523/JNEUROSCI.1889-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039) J Pharmacol Exp Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- 151.Seeman P, Battaglia G, Corti C, Corsi M, Bruno V. Glutamate receptor mGlu2 and mGlu3 knockout striata are dopamine supersensitive, with elevated D2(High) receptors and marked supersensitivity to the dopamine agonist (+)PHNO. Synapse. 2009;63:247–251. doi: 10.1002/syn.20607. [DOI] [PubMed] [Google Scholar]
- 152.Bertram L. Genetic research in schizophrenia: new tools and future perspectives. Schizophr Bull. 2008;34:806–812. doi: 10.1093/schbul/sbn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roffman JL, Weiss AP, Purcell S, et al. Contribution of methylenetetrahydrofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008;63:42–48. doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 154.Nakamura K, Koyama Y, Takahashi K, et al. Requirement of tryptophan hydroxylase during development for maturation of sensorimotor gating. J Mol Biol. 2006;363:345–354. doi: 10.1016/j.jmb.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 155.Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 156.Zill P, Buttner A, Eisenmenger W, Moller HJ, Ackenheil M, Bondy B. Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: a post-mortem study. J Psychiatr Res. 2007;41:168–173. doi: 10.1016/j.jpsychires.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 157.Savelieva KV, Zhao S, Pogorelov VM, et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Williams NM, O'Donovan MC, Owen MJ. Is the dysbindin gene (DTNBP1) a susceptibility gene for schizophrenia? Schizophr Bull. 2005;31:800–805. doi: 10.1093/schbul/sbi061. [DOI] [PubMed] [Google Scholar]
- 159.Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–24241. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- 160.Li W, Zhang Q, Oiso N, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hattori S, Murotani T, Matsuzaki S, et al. Behavioral abnormalities and dopamine reductions in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Biochem Biophys Res Commun. 2008;373:298–302. doi: 10.1016/j.bbrc.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 162.Feng YQ, Zhou ZY, He X, et al. Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophr Res. 2008;106:218–228. doi: 10.1016/j.schres.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 163.Takao K, Toyama K, Nakanishi K, et al. Impaired long-term memory retention and working memory in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Mol Brain. 2008;1:11. doi: 10.1186/1756-6606-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bhardwaj SK, Baharnoori M, Sharif-Askari B, Kamath A, Williams S, Srivastava LK. Behavioral characterization of dysbindin-1 deficient sandy mice. Behav Brain Res. 2009;197:435–441. doi: 10.1016/j.bbr.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 165.Murotani T, Ishizuka T, Hattori S, Hashimoto R, Matsuzaki S, Yamatodani A. High dopamine turnover in the brains of Sandy mice. Neurosci Lett. 2007;421:47–51. doi: 10.1016/j.neulet.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 166.Chen XW, Feng YQ, Hao CJ, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181:791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cox MM, Tucker AM, Tang J, et al. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav. 2009;8:390–397. doi: 10.1111/j.1601-183X.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stefansson H, Sigurdsson E, Steinthorsdottir V, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 170.O'Tuathaigh CM, Desbonnet L, Waddington JL. Neuregulin-1 signaling in schizophrenia: ‘Jack of all trades’ or master of some? Expert Rev Neurother. 2009;9:1–3. doi: 10.1586/14737175.9.1.1. [DOI] [PubMed] [Google Scholar]
- 171.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 172.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.O'Tuathaigh CM, O'Sullivan GJ, Kinsella A, et al. Sexually dimorphic changes in the exploratory and habituation profiles of heterozygous neuregulin-1 knockout mice. Neuroreport. 2006;17:79–83. doi: 10.1097/01.wnr.0000192738.31029.0a. [DOI] [PubMed] [Google Scholar]
- 174.Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: implications for understanding schizophrenia. Genes Brain Behav. 2007;6:677–687. doi: 10.1111/j.1601-183X.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 175.van den Buuse M, Wischhof L, Xi Lee R, Martin S, Karl T. Neuregulin 1 hypomorphic mutant mice: enhanced baseline locomotor activity but normal psychotropic drug-induced hyperlocomotion and prepulse inhibition regulation. Int J Neuropsychopharmacol. 2009;12:1383–1393. doi: 10.1017/S1461145709000388. [DOI] [PubMed] [Google Scholar]
- 176.Boucher AA, Arnold JC, Duffy L, Schofield PR, Micheau J, Karl T. Heterozygous neuregulin 1 mice are more sensitive to the behavioural effects of Δ9-tetrahydrocannabinol. Psychopharmacology (Berl) 2007;192:325–336. doi: 10.1007/s00213-007-0721-3. [DOI] [PubMed] [Google Scholar]
- 177.Duffy L, Cappas E, Scimone A, Schofield PR, Karl T. Behavioral profile of a heterozygous mutant mouse model for EGF-like domain neuregulin 1. Behav Neurosci. 2008;122:748–759. doi: 10.1037/0735-7044.122.4.748. [DOI] [PubMed] [Google Scholar]
- 178.Rimer M, Barrett DW, Maldonado MA, Vock VM, Gonzalez-Lima F. Neuregulin-1 immunoglobulin-like domain mutant mice: clozapine sensitivity and impaired latent inhibition. Neuroreport. 2005;16:271–275. doi: 10.1097/00001756-200502280-00014. [DOI] [PubMed] [Google Scholar]
- 179.Chen YJ, Johnson MA, Lieberman MD, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- 181.Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 182.Thuret S, Alavian KN, Gassmann M, et al. The neuregulin receptor, ErbB4, is not required for normal development and adult maintenance of the substantia nigra pars compacta. J Neurochem. 2004;91:1302–1311. doi: 10.1111/j.1471-4159.2004.02809.x. [DOI] [PubMed] [Google Scholar]
- 183.Hu X, Hicks CW, He W, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 184.Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dejaegere T, Serneels L, Schafer MK, et al. Deficiency of Aph1B/C-gamma-secretase disturbs Nrg1 cleavage and sensorimotor gating that can be reversed with antipsychotic treatment. Proc Natl Acad Sci USA. 2008;105:9775–9780. doi: 10.1073/pnas.0800507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roberts RC. Schizophrenia in translation: disrupted in schizophrenia (DISC1): integrating clinical and basic findings. Schizophr Bull. 2007;33:11–15. doi: 10.1093/schbul/sbl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Muir WJ, Pickard BS, Blackwood DH. Disrupted-in-schizophrenia-1. Curr Psychiatry Rep. 2008;10:140–147. doi: 10.1007/s11920-008-0025-2. [DOI] [PubMed] [Google Scholar]
- 188.Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci USA. 2006;103:3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Clapcote SJ, Roder JC. Deletion polymorphism of Disc1 is common to all 129 mouse substrains: implications for gene-targeting studies of brain function. Genetics. 2006;173:2407–2410. doi: 10.1534/genetics.106.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Clapcote SJ, Lipina TV, Millar JK, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 191.Hikida T, Jaaro-Peled H, Seshadri S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li W, Zhou Y, Jentsch JD, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci USA. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kvajo M, McKellar H, Arguello PA, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pletnikov MV, Ayhan Y, Nikolskaia O, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. 115. [DOI] [PubMed] [Google Scholar]
- 195.Millar JK, Mackie S, Clapcote SJ, et al. Disrupted in schizophrenia 1 and phosphodiesterase 4B: towards an understanding of psychiatric illness. J Physiol. 2007;584:401–405. doi: 10.1113/jphysiol.2007.140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Siuciak JA, McCarthy SA, Chapin DS, Martin AN. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2008;197:115–126. doi: 10.1007/s00213-007-1014-6. [DOI] [PubMed] [Google Scholar]
- 197.Mao Y, Ge X, Frank CL, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gould TD, O'Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 200.Beaulieu J-M, Caron MG. Looking at lithium: molecular moods and complex behaviour. Mol Interv. 2008;8:230–241. doi: 10.1124/mi.8.5.8. [DOI] [PubMed] [Google Scholar]
- 201.Amar S, Jones BC, Nadri C, Kozlovsky N, Belmaker RH, Agam G. Genetic correlational analysis of glycogen synthase kinase-3 beta and prepulse inhibition in inbred mice. Genes Brain Behav. 2004;3:178–180. doi: 10.1111/j.1601-183X.2004.00065.x. [DOI] [PubMed] [Google Scholar]
- 202.O'Brien WT, Harper AD, Jove F, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Beaulieu JM, Zhang X, Rodriguiz RM, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bersudsky Y, Shaldubina A, Kozlovsky N, Woodgett JR, Agam G, Belmaker RH. Glycogen synthase kinase-3beta heterozygote knockout mice as a model of findings in postmortem schizophrenia brain or as a model of behaviors mimicking lithium action: negative results. Behav Pharmacol. 2008;19:217–224. doi: 10.1097/FBP.0b013e3282feb099. [DOI] [PubMed] [Google Scholar]
- 205.Beaulieu JM, Sotnikova TD, Yao WD, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Beaulieu J-M, Gainetdinov RR, Caron MG. The Akt–GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 207.Prickaerts J, Moechars D, Cryns K, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 209.Koros E, Dorner-Ciossek C. The role of glycogen synthase kinase-3beta in schizophrenia. Drug News Perspect. 2007;20:437–445. doi: 10.1358/dnp.2007.20.7.1149632. [DOI] [PubMed] [Google Scholar]
- 210.Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 211.Arinami T. Analyses of the associations between the genes of 22q11 deletion syndrome and schizophrenia. J Hum Genet. 2006;51:1037–1045. doi: 10.1007/s10038-006-0058-5. [DOI] [PubMed] [Google Scholar]
- 212.Prasad SE, Howley S, Murphy KC. Candidate genes and the behavioral phenotype in 22q11.2 deletion syndrome. Dev Disabil Res Rev. 2008;14:26–34. doi: 10.1002/ddrr.5. [DOI] [PubMed] [Google Scholar]
- 213.Karayiorgou M, Gogos JA. The molecular genetics of the 22q11-associated schizophrenia. Brain Res Mol Brain Res. 2004;132:95–104. doi: 10.1016/j.molbrainres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 214.Paylor R, Lindsay E. Mouse models of 22q11 deletion syndrome. Biol Psychiatry. 2006;59:1172–1179. doi: 10.1016/j.biopsych.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 215.Paylor R, McIlwain KL, McAninch R, et al. Mice deleted for the DiGeorge/velocardiofacial syndrome region show abnormal sensorimotor gating and learning and memory impairments. Hum Mol Genet. 2001;10:2645–2650. doi: 10.1093/hmg/10.23.2645. [DOI] [PubMed] [Google Scholar]
- 216.Gogos JA, Santha M, Takacs Z, et al. The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat Genet. 1999;21:434–439. doi: 10.1038/7777. [DOI] [PubMed] [Google Scholar]
- 217.Mukai J, Liu H, Burt RA, et al. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet. 2004;36:725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- 218.Paterlini M, Zakharenko SS, Lai WS, et al. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- 219.Gogos JA, Morgan M, Luine V, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huotari M, Garcia-Horsman JA, Karayiorgou M, Gogos JA, Mannisto PT. D-amphetamine responses in catechol-O-methyltransferase (COMT) disrupted mice. Psychopharmacology (Berl) 2004;172:1–10. doi: 10.1007/s00213-003-1627-3. [DOI] [PubMed] [Google Scholar]
- 221.Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Mannisto PT. Effect of dopamine uptake inhibition on brain catecholamine levels and locomotion in catechol-O-methyltransferase-disrupted mice. J Pharmacol Exp Ther. 2002;303:1309–1316. doi: 10.1124/jpet.102.043042. [DOI] [PubMed] [Google Scholar]
- 222.Papaleo F, Crawley JN, Song J, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Paylor R, Glaser B, Mupo A, et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci USA. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Long JM, LaPorte P, Merscher S, et al. Behavior of mice with mutations in the conserved region deleted in velocardiofacial/DiGeorge syndrome. Neurogenetics. 2006;7:247–257. doi: 10.1007/s10048-006-0054-0. [DOI] [PubMed] [Google Scholar]
- 225.Hsu R, Woodroffe A, Lai WS, et al. Nogo receptor 1 (RTN4R) as a candidate gene for schizophrenia: analysis using human and mouse genetic approaches. PLoS ONE. 2007;2:e1234. doi: 10.1371/journal.pone.0001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 227.Willi R, Aloy EM, Yee BK, Feldon J, Schwab ME. Behavioral characterization of mice lacking the neurite outgrowth inhibitor Nogo-A. Genes Brain Behav. 2009;8:181–192. doi: 10.1111/j.1601-183X.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- 228.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 229.Heldt SA, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- 230.Yee BK, Keist R, von Boehmer L, et al. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 232.Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 233.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Progr Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 234.Roth BL, Meltzer HY. The role of serotonin in schizophrenia. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 1215–1227. [Google Scholar]
- 235.Dulawa SC, Gross C, Stark KL, Hen R, Geyer MA. Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology. 2000;22:650–659. doi: 10.1016/S0893-133X(99)00164-5. [DOI] [PubMed] [Google Scholar]
- 236.Dulawa SC, Scearce-Levie KA, Hen R, Geyer MA. Serotonin releasers increase prepulse inhibition in serotonin 1B knockout mice. Psychopharmacology (Berl) 2000;149:306–312. doi: 10.1007/s002130000373. [DOI] [PubMed] [Google Scholar]
- 237.Gonzalez-Maeso J, Weisstaub NV, Zhou M, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 238.Grailhe R, Waeber C, Dulawa SC, et al. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron. 1999;22:581–591. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 239.Halberstadt AL, van der Heijden I, Ruderman MA, et al. 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Semenova S, Geyer MA, Sutcliffe JG, Markou A, Hedlund PB. Inactivation of the 5-HT7 receptor partially blocks phencyclidine-induced disruption of prepulse inhibition. Biol Psychiatry. 2008;63:98–105. doi: 10.1016/j.biopsych.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Felder CC, Porter AC, Skillman TL, et al. Elucidating the role of muscarinic receptors in psychosis. Life Sci. 2001;68:2605–2613. doi: 10.1016/s0024-3205(01)01059-1. [DOI] [PubMed] [Google Scholar]
- 242.Scarr E, Dean B. Muscarinic receptors: do they have a role in the pathology and treatment of schizophrenia? J Neurochem. 2008;107:1188–1195. doi: 10.1111/j.1471-4159.2008.05711.x. [DOI] [PubMed] [Google Scholar]
- 243.Wess J, Duttaroy A, Zhang W, et al. M1-M5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Recept Channels. 2003;9:279–290. [PubMed] [Google Scholar]
- 244.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 245.Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 246.Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- 248.Dluzen DE, Gao X, Story GM, Anderson LI, Kucera J, Walro JM. Evaluation of nigrostriatal dopaminergic function in adult +/+ and +/− BDNF mutant mice. Exp Neurol. 2001;170:121–128. doi: 10.1006/exnr.2001.7698. [DOI] [PubMed] [Google Scholar]
- 249.Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes Brain Behav. 2008;7:906–914. doi: 10.1111/j.1601-183X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- 251.Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact of brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Psychiatry. 2009;66:84–90. doi: 10.1016/j.biopsych.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Monteggia LM, Luikart B, Barrot M, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 253.Monteggia LM, Barrot M, Powell CM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rios M, Fan G, Fekete C, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 256.Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Costa E, Chen Y, Davis J, et al. REELIN and schizophrenia: a disease at the interface of the genome and the epigenome. Mol Interv. 2002;2:47–57. doi: 10.1124/mi.2.1.47. [DOI] [PubMed] [Google Scholar]
- 258.Tueting P, Doueiri MS, Guidotti A, Davis JM, Costa E. Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev. 2006;30:1065–1077. doi: 10.1016/j.neubiorev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 259.Impagnatiello F, Guidotti AR, Pesold C, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis. 2001;8:723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- 261.Pappas GD, Kriho V, Pesold C. Reelin in the extracellular matrix and dendritic spines of the cortex and hippocampus: a comparison between wild type and heterozygous reeler mice by immunoelectron microscopy. J Neurocytol. 2001;30:413–425. doi: 10.1023/a:1015017710332. [DOI] [PubMed] [Google Scholar]
- 262.Matsuzaki H, Minabe Y, Nakamura K, et al. Disruption of reelin signaling attenuates methamphetamine-induced hyperlocomotion. Eur J Neurosci. 2007;25:3376–3384. doi: 10.1111/j.1460-9568.2007.05564.x. [DOI] [PubMed] [Google Scholar]
- 263.Ognibene E, Adriani W, Caprioli A, et al. The effect of early maternal separation on brain derived neurotrophic factor and monoamine levels in adult heterozygous reeler mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1269–1276. doi: 10.1016/j.pnpbp.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 264.Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of RELN gene dosage and social isolation. Behav Neurosci. 2003;117:1257–1275. doi: 10.1037/0735-7044.117.6.1257. [DOI] [PubMed] [Google Scholar]
- 265.Tueting P, Costa E, Dwivedi Y, et al. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- 266.Varty GB, Powell SB, Lehmann-Masten V, Buell MR, Geyer MA. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behav Brain Res. 2006;169:162–167. doi: 10.1016/j.bbr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 267.Barr AM, Fish KN, Markou A, Honer WG. Heterozygous reeler mice exhibit alterations in sensorimotor gating but not presynaptic proteins. Eur J Neurosci. 2008;27:2568–2574. doi: 10.1111/j.1460-9568.2008.06233.x. [DOI] [PubMed] [Google Scholar]
- 268.Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res. 2004;153:43–54. doi: 10.1016/j.bbr.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 269.Eells JB, Lipska BK, Yeung SK, Misler JA, Nikodem VM. Nurr1-null heterozygous mice have reduced mesolimbic and mesocortical dopamine levels and increased stress-induced locomotor activity. Behav Brain Res. 2002;136:267–275. doi: 10.1016/s0166-4328(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 270.Eells JB, Misler JA, Nikodem VM. Early postnatal isolation reduces dopamine levels, elevates dopamine turnover and specifically disrupts prepulse inhibition in Nurr1-null heterozygous mice. Neuroscience. 2006;140:1117–1126. doi: 10.1016/j.neuroscience.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 271.Rojas P, Joodmardi E, Hong Y, Perlmann T, Ogren SO. Adult mice with reduced Nurr1 expression: an animal model for schizophrenia. Mol Psychiatry. 2007;12:756–766. doi: 10.1038/sj.mp.4001993. [DOI] [PubMed] [Google Scholar]
- 272.Zeng H, Chattarji S, Barbarosie M, et al. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 273.Miyakawa T, Leiter LM, Gerber DJ, et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci USA. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Grillet N, Pattyn A, Contet C, Kieffer BL, Goridis C, Brunet JF. Generation and characterization of Rgs4 mutant mice. Mol Cell Biol. 2005;25:4221–4228. doi: 10.1128/MCB.25.10.4221-4228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Harrison SM, Reavill C, Brown G, et al. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol Cell Neurosci. 2003;24:1170–1179. doi: 10.1016/j.mcn.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 276.Irintchev A, Koch M, Needham LK, Maness P, Schachner M. Impairment of sensorimotor gating in mice deficient in the cell adhesion molecule L1 or its close homologue, CHL1. Brain Res. 2004;1029:131–134. doi: 10.1016/j.brainres.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 277.Pillai-Nair N, Panicker AK, Rodriguiz RM, et al. Neural cell adhesion molecule-secreting transgenic mice display abnormalities in GABAergic interneurons and alterations in behavior. J Neurosci. 2005;25:4659–4671. doi: 10.1523/JNEUROSCI.0565-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Roberts C, Winter P, Shilliam CS, et al. Neurochemical changes in LPA1 receptor deficient mice—a putative model of schizophrenia. Neurochem Res. 2005;30:371–377. doi: 10.1007/s11064-005-2611-6. [DOI] [PubMed] [Google Scholar]
- 279.van den Buuse M, Zheng TW, Walker LL, Denton DA. Angiotensin-converting enzyme (ACE) interacts with dopaminergic mechanisms in the brain to modulate prepulse inhibition in mice. Neurosci Lett. 2005;380:6–11. doi: 10.1016/j.neulet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 280.Caldwell HK, Stephens SL, Young WS., 3rd Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14:190–196. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- 281.Bertrand PP, Paranavitane UT, Chavez C, Gogos A, Jones M, van den Buuse M. The effect of low estrogen state on serotonin transporter function in mouse hippocampus: a behavioral and electrochemical study. Brain Res. 2005;1064:10–20. doi: 10.1016/j.brainres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 282.Kelly MP, Stein JM, Vecsey CG, et al. Developmental etiology for neuroanatomical and cognitive deficits in mice overexpressing Galphas, a G-protein subunit genetically linked to schizophrenia. Mol Psychiatry. 2009;14:398–415. doi: 10.1038/mp.2008.124. 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hashimoto R, Hashimoto H, Shintani N, et al. Pituitary adenylate cyclase-activating polypeptide is associated with schizophrenia. Mol Psychiatry. 2007;12:1026–1032. doi: 10.1038/sj.mp.4001982. [DOI] [PubMed] [Google Scholar]
- 284.Szumlinski KK, Lominac KD, Kleschen MJ, et al. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]