Abstract
Background
Apart from regional lymph node metastases, systemic metastases occur sporadically in papillary thyroid carcinomas (PTC). The lung and bones are the most frequent localizations. Additionally known but extremely rare locations are metastases of the skeletal muscles, ovaries, submandibular gland, sphenoidal sinus, brain, adrenals, and, as shown in only two previously published cases to date, the pancreas.
Summary
In this article we report about two additional patients with pancreatic metastases from PTC. There is almost no prior experience about therapeutic approaches to this type of metastases. In both patients distant metastases within the pancreas were successfully removed. Postoperative histology confirmed the diagnoses. Supplemental genetic analysis did not demonstrate a BRAF V600E mutation or expression of a RET/PTC1 rearrangement in one case, but revealed a BRAF V600E mutation in the second case. Surgery avoided impending complications maintaining quality of life. One patient had a tumor-specific survival of 42 months. The other patient has occult disease.
Conclusions
Our two patients benefited of a calculated aggressive surgical action. Thus, if low perioperative mortality and morbidity can be warranted, surgical measures are justifiable in selected cases.
Introduction
Papillary thyroid carcinoma (PTC) is the most frequent form of well-differentiated thyroid cancer (1). Apart from the classical PTC, which is associated with an excellent prognosis, 14 different histological variants have been described, among which the follicular variant PTC (FVPTC) is the most common (2,3).
The main route of metastasis for PTC is locoregional spread to the lymph nodes (LN) of the neck (4,5). In about 5% of patients there are systemic metastases, most commonly to lung and bone (6–10). Additional rare metastatic locations for PTC are the skeletal muscles (11,12), ovaries (13), submandibular gland (14), sphenoidal sinus (15), brain (16,17), and adrenals (18). Metastasis of PTC to the pancreas is extremely rare, as there have been only two published cases (19,20). Here we report two additional patients with pancreatic metastases from PTC and illustrate the apparent value of surgical treatment.
Patients
Case 1
A 34-year-old woman underwent total thyroidectomy in 1998 for a right-sided encapsulated, 6.0 cm PTC, which was a follicular subtype (FVPTC). The TNM classification (5th ed.) was pT3N1a(0/2)V1, cM0. During follow-up visits in 2000 rising thyroglobulin (TG) levels were noted due to locoregional LN metastases in the right cervical and submandibular region and the left side of the neck. Further, LN metastases were subsequently removed in 2001 and 2002 by LN dissection. Three cycles of radioiodine (RAI) therapy (cumulated activity of 22.2 GBq) were administered in December 2000, April 2001, and September 2001. In 2003 a cervical exploration was performed for suspected recurrence in the right paratracheal region. Pathologic examination revealed FVPTC tissue with venous infiltration (V1) but no lymphoid structures. Due to a lack of RAI uptake of the lesion, 54 Gy external radiation was administered to the right supraclavicular region.
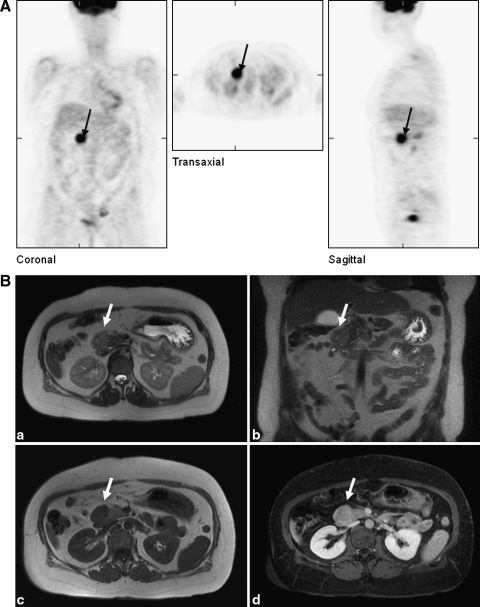
During the years until 2007 the serum TG rose to 2879 ng/mL. Using positron emission tomography (PET) with 18-fluor-fluordeoxyglucose (18F-FDG) under exogenic thyrotropin (TSH) stimulation, a light pathological enhancement (SUV max: 4.23) in the right upper abdominal quadrant was found. However, neither an iodine-131 whole-body scintigraphy nor ultrasound investigations, computed tomography (CT), or magnetic resonance imaging (MRI) identified a lesion in this region. Finally, in 2008, an 18F-FGD-PET was performed under continued levothyroxine therapy with 150 μg/day (TG level: 5931 ng/mL), but exogenous recombinant human-TSH stimulation resulted in a sufficient rise of the TG to 8470 ng/mL. It demonstrated clear enhancement in the right upper abdominal quadrant (SUV max: 11.6), the right cervical region (SUV max: 2.5), and multifocal uptake in the ventrocranial region of the bladder (Fig. 1). Using CT investigation of the abdomen, thorax, and neck and an MRI of the abdomen, possibly correlating structures with condensed soft tissue were found in the head of the pancreas, and in the right cervical and left ovary/uterus regions (Fig. 1). There were no gynecological abnormalities apart from myomas and ovarial cysts, indicating that the latter observation was not likely due to metastasis. Although metastatic PTC to the pancreas was known to be very rare, it could not be ruled out despite the lack of RAI uptake in the pancreatic region.
FIG. 1.
Metastasis (black arrows and white arrows) of the PTC of patient #1 within the head of pancreas as observed (A) in a positron emission tomography with 18-fluor-fluordeoxyglucose and (B) upon magnetic resonance imaging investigation. PTC, papillary thyroid carcinoma.
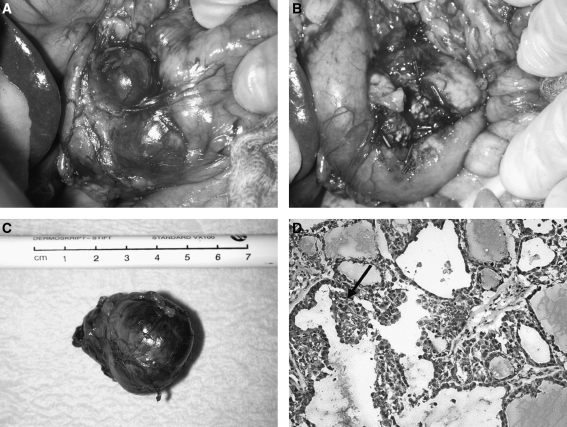
As a prognosis-determining metastasis to the pancreas needed to be ruled out before cervical reexploration, an abdominal exploration was performed. At surgery a mass was noted in the pancreatic head on palpation and ultrasound, but the abdomen was otherwise negative. A 4 cm encapsulated tumor was identified within the pancreas head, which could clearly be demarcated from the remaining pancreatic tissue by intraoperative ultrasound. The tumor was enucleated (Fig. 2). Upon histology, the tentative diagnosis was confirmed as systemic metastasis of a PTC rich in follicles (Fig. 2). It appeared that the tumor had been completely removed (pR0). Immunohistological staining revealed strong expression of TG by the tumor cells and confirmed pancreatic metastasis of a PTC (Fig. 3). There were no LN metastases in the specimen. Two months postoperatively, the serum TG concentrations had decreased to 811 ng/mL. Seven months later, a resection of the cervical metastases, confirmed by histology, was performed. At that time, the serum TG concentration was 1752 ng/mL despite the fact that she was under continuous TSH suppression therapy.
FIG. 2.
Pancreatic metastasis of the PTC of patient #1 (A) before and (B, C) after enucleation. In (D), a tissue section showing papillary clusters of a PTC (arrow) is depicted.
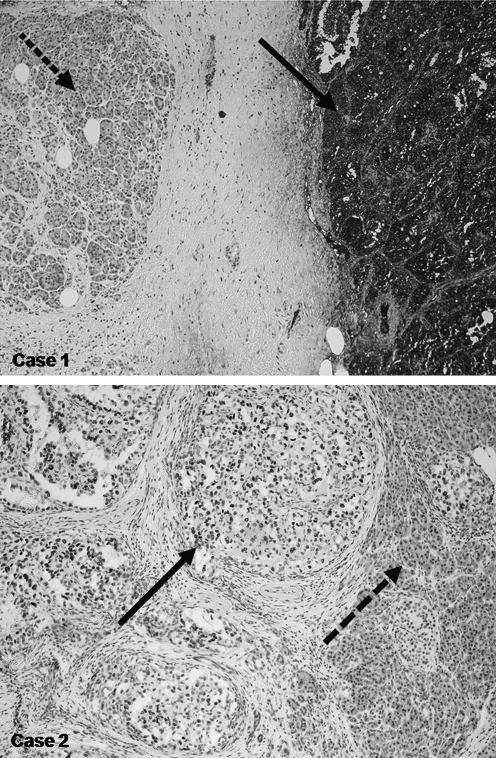
FIG. 3.
Immunoperoxidase staining. The dashed arrow indicates pancreatic parenchyma negative for thyroglobulin expression (antithyroglobulin); the black arrow highlights positive areas of tumor tissue.
To complete the assessment of the PTC in this case, a retrospective molecular genetic analysis of the local recurrence as well as of the metastases from the head of the pancreas was undertaken. The supplemental analysis did not detect a BRAF V600E mutation or the RET rearrangement product RET/PTC1. The mutation analysis to detect the BRAF V600E mutation was carried out according to Sapio et al. and Frasca et al. (21,22). Positive results were verified by direct sequencing. The RET/PTC1 rearrangement was analyzed by polymerase chain reaction with hybrid-specific oligonucleotides.
Case 2
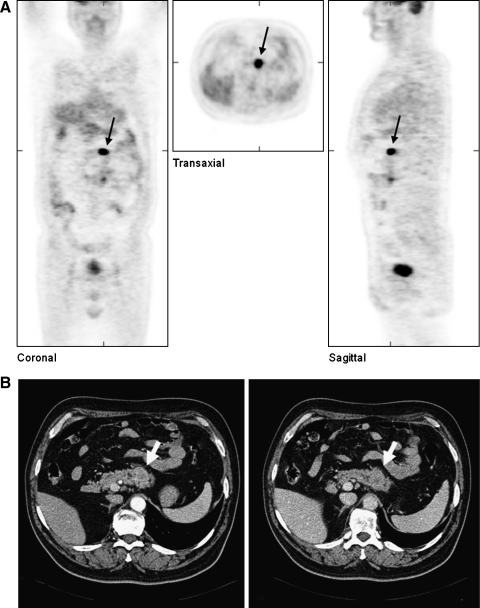
A 46-year-old man underwent a total thyroidectomy in 1987 for right-sided multifocal PTC (TNM classification [4th ed.]: pT3N1, cM0). Postoperatively, he received two treatments with RAI (cumulated activity 7.4 GBq) and adjuvant radiation therapy with a cumulated dose of 50.4 Gy. In 2000, after a 13-year recurrence-free interval during which he received TSH suppressive therapy, multiple LN metastases were detected in the right cervical region. These were resected, but new metastases in the right dorsal cervical triangle again required removal 1 year later. After each operation he received 7.4 GBq RAI therapies. In 2002, serum TG levels were noted to be rising, and new metastases were detected by 18F-FDG-PET in four different regions: the right cervical area, the supraclavicular region, segment 10 of the left lobe of the lung, and the pancreas body (Fig. 4). For further assessment of these suspicious areas, CT investigations of the head, neck, thorax, and abdomen were performed, and the investigations confirmed abnormalities in these areas but not elsewhere. A cervical and supraclavicular LN dissection was performed 4 weeks later by resection of the inferior lobe of the left lung. Two months later the left pancreas was resected without complications. Specimens of both the lung and the pancreas showed expression of TG by immunohistochemical studies (Fig. 3).
FIG. 4.
Metastasis (black and white arrows) of the PTC of patient #2 within the pancreas body as observed (A) in a positron emission tomography with 18-fluor-fluordeoxyglucose and (B) in a computed tomography investigation.
Three months after the last surgery the serum TG was <2 ng/mL. Nine months after this, however, the serum TG had increased slightly to 30 ng/mL. A follow-up 18F-FDG-PET showed new lesions in the lungs, the right neck, and the right proximal tibia. MRI and CT confirmed lesions in these areas, but iodine-131 whole-body scintigraphy was negative. Because of the risk of tibial fracture, palliative radiotherapy was administered to the involved region of the tibia. After redifferentiation therapy with isoretinoin (13-cis-retinoic acid), another RAI treatment (7.4 GBq) was performed without success. The tumor continued to progress with new metastases and a tibia fracture. The fracture was treated by osteosynthesis, and a skin metastasis was removed for palliation. The patient died from the consequences of progressive cancer in 2005. The retrospective gene analysis showed a heterozygous somatic BRAF V6000E mutation.
Discussion
Apart from the frequently observed regional LN metastases and distant lung and bone metastases, other locations of distant spread are only sporadically reported in PTC. To our knowledge, based on a careful search in www.pubmed.gov, only two cases of PTC with pancreatic metastasis have been published (19,20). Certainly, there have probably been other patients with pancreatic metastases due to PTC that were not detected; this may be because in some cases they were thought to be due to primary pancreatic cancer but did not have this diagnosis confirmed as the chances of cure were deemed to be very low.
Metastases to the pancreas are most frequently observed in patients with renal or lung carcinomas (23). When a pancreatic mass is identified in a patient with history of a primary cancer in other regions, choosing to resect the mass may be difficult as was the case in the first patient described. This is particularly true if the patient's cancer is recurrent but radiological assessments do not clearly reveal a tumor progress. To select patients at risk for distant metastases, immunohistochemical studies (HBME-1, cytokeratin 19, and galectin-3) or molecular biological studies (RET/PTC rearrangement, TP53 mutation, and BRAF mutation) to determine the PTC subtype may be helpful (24–29), but not definitive.
In both our patients with advanced disease, iodine was not taken up by the tumor in the course of the disease; therefore, an 18F-FDG PET was helpful to the diagnosis (30). The shift from iodine uptake to 18F-FDG uptake depicting an inverse relation seems to be associated with increasingly aggressive behavior. In this clinical setting, the indication to resect distant metastases is difficult. On the other hand, distant metastases can be observed after a long latency of several years and can continue to grow very slowly (6–8,31). A 5- and 10-year disease-specific survival of patients with distant metastases of a PTC were reported with rates of 65% and 45%, respectively (31). This indolent course sometimes motivates physicians against further surgical measures. However, especially patients with slow-growing metastases of PTC may achieve even more benefit if their malignant lesions are removed surgically (6,31).
In our first patient, a primary malignancy of the exocrine pancreas was excluded by explorative laparotomy, the preoperative diagnosis of PTC metastases was confirmed, and the tumor was excised. Especially in light of the young age of the patient, this surgery seemed to be justified by likely improved prognosis and avoidance of local complications such as infiltration of the duodenum. In our second patient, the first recurrence of PTC was noted 13 years after primary surgery, but he then had significant distant metastases. In our opinion, the multiple operations performed achieved a prolongation of the patient's life of further 5 years.
As was the case for one of our patients, both previously reported patients with pancreatic metastases of PTC had lung metastases. Despite the small but slowly growing pulmonary metastases in one of these patients, a 62-year-old man with a tall cell variant of a PTC, there was benefit from a pylorus-preserving pancreaticoduodenectomy. At follow-up, his disease was stable after 2 years. In contrast, the second previously reported patient, a 53-year-old man, had progressive growth of pulmonary metastases for which he received chemotherapy. Due to bleeding complications from adjuvant measures, he died within a year after pancreaticoduodenectomy.
The management of patients with PTC who have distant metastases must be individualized and alternative approaches must be considered. The lack of RAI uptake almost invariably indicates progression of the tumor to an undifferentiated more aggressive phenotype. If the tumor appears to be slow growing, however, we favor surgical measures to prevent local complications and perhaps increase longevity. We took this approach in the two patients reported here with apparent variable degrees of success.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Albores-Saaverda J. Gould E. Vardaman C. Vuitch F. The macrofollicular variant of papillary thyroid carcinoma: a study of 17 cases. Hum Pathol. 1991;22:1195–1205. doi: 10.1016/0046-8177(91)90101-t. [DOI] [PubMed] [Google Scholar]
- 2.LiVolsi VA. Albores-Saavedra J. Asa SL. Baloch ZW. Sobrinho-Simoes M. Wenig B. DeLellis RA. Cady B. Papillary carcinoma (World Health Organization classification of tumors) In: De Lellis RA, editor; Lloyd RV, editor; Heitz PU, editor; Eng C, editor. Pathology and Genetics of Tumours of Endocrine Organs. IARC Press; Lyon, France: 2004. pp. 57–66. [Google Scholar]
- 3.Yuksel O. Kurukahvecioglu O. Ege B. Ekinci O. Aydin A. Poyraz A. Tezel E. Taneri F. The relation between pure papillary and follicular variant in papillary thyroid carcinoma. Endocr Regul. 2008;42:29–33. [PubMed] [Google Scholar]
- 4.Toniato A. Boschin I. Casara D. Mazzarotto R. Rubello D. Pelizzo M. Papillary thyroid carcinoma: factors influencing recurrence and survival. Ann Surg Oncol. 2008;15:1518–1522. doi: 10.1245/s10434-008-9859-4. [DOI] [PubMed] [Google Scholar]
- 5.Bardet S. Malville E. Rame JP. Babin E. Samama G. De Raucourt D. Michels JJ. Reznik Y. Henry-Amar M. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol. 2008;158:551–560. doi: 10.1530/EJE-07-0603. [DOI] [PubMed] [Google Scholar]
- 6.Lin JD. Huang MJ. Juang JH. Chao TC. Huang BY. Chen KW. Chen JY. Li KL. Chen JF. Ho YS. Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid. 1999;9:1227–1235. doi: 10.1089/thy.1999.9.1227. [DOI] [PubMed] [Google Scholar]
- 7.Haigh PI. Urbach DR. Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol. 2005;12:81–89. doi: 10.1007/s10434-004-1165-1. [DOI] [PubMed] [Google Scholar]
- 8.Kim S. Wei JP. Braveman JM. Brams DM. Predicting outcome and directing therapy for papillary thyroid carcinoma. Arch Surg. 2004;139:390–394. doi: 10.1001/archsurg.139.4.390. [DOI] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, End Results Program. www.seer.cancer.gov. www.seer.cancer.gov SEER*Stat Database: Incidence-SEER 12 Regs Public-Use, November 2002 Sub (1973–2000), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2003, based on the November 2002 submission.
- 10.Fritz A. Ries L. SEER extent of disease, codes, coding instructions (No., T-899; Pub. No. 98-4354) In: Fritz A, editor; Ries L, editor. The SEER Program Code Manual. 3. National Cancer Institute; Bethesda, MD: 1998. p. 7. [Google Scholar]
- 11.Panoussopoulos D. Theodoropoulos G. Vlahos K. Lazaris ACh. Papadimitriou K. Distant solitary skeletal muscle metastasis from papillary thyroid carcinoma. Int Surg. 2007;92:226–229. [PubMed] [Google Scholar]
- 12.Pucci A. Suppo M. Lucchesi G. Celeste A. Viberti L. Pellerito R. Papotti M. Papillary thyroid carcinoma presenting as a solitary soft tissue arm metastasis in an elderly hyperthyroid patient. Case report and review of the literature. Virchows Arch. 2006;448:857–861. doi: 10.1007/s00428-006-0187-4. [DOI] [PubMed] [Google Scholar]
- 13.Brogioni S. Viacava P. Tomisti L. Martino E. Macchia E. A special case of bilateral ovarian metastases in a woman with papillary carcinoma of the thyroid. Exp Clin Endocrinol Diabetes. 2007;115:397–400. doi: 10.1055/s-2007-973853. [DOI] [PubMed] [Google Scholar]
- 14.Sarda AK. Pandey D. Bhalla SA. Goyal A. Isolated submandibular gland metastasis from an occult papillary thyroid cancer. Indian J Cancer. 2004;41:89–91. [PubMed] [Google Scholar]
- 15.Argibay Vázquez S. Lancha Hernández C. Martínez Muñiz A. Metastases in the sphenoidal sinus in a patient with papillary thyroid cancer. Clin Transl Oncol. 2005;7:324–327. doi: 10.1007/BF02710273. [DOI] [PubMed] [Google Scholar]
- 16.Pazaitou-Panayiotou K. Kaprara A. Chrisoulidou A. Boudina M. Georgiou E. Patakiouta F. Drimonitis A. Vainas I. Cerebellar metastasis as first metastasis from papillary thyroid carcinoma. Endocr J. 2005;52:653–657. doi: 10.1507/endocrj.52.653. [DOI] [PubMed] [Google Scholar]
- 17.Imamura Y. Kasahara Y. Fukuda M. Multiple brain metastases from a diffuse sclerosing variant of papillary carcinoma of the thyroid. Endocr Pathol. 2000;11:97–108. doi: 10.1385/ep:11:1:97. [DOI] [PubMed] [Google Scholar]
- 18.Wagenaar N. Oosterhuis JW. Rozendaal L. Comans E. Simsek S. Adrenal metastasis from a primary papillary thyroid carcinoma. Intern Med. 2008;47:2165–2168. doi: 10.2169/internalmedicine.47.1582. [DOI] [PubMed] [Google Scholar]
- 19.Jobran R. Baloch ZW. Aviles V. Rosato EF. Schwartz S. LiVolsi VA. Tall cell papillary carcinoma of the thyroid: metastatic to the pancreas. Thyroid. 2000;10:185–187. doi: 10.1089/thy.2000.10.185. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui AA. Olansky L. Sawh RN. Tierney WM. Pancreatic metastasis of tall cell variant of papillary thyroid carcinoma: diagnosis by endoscopic ultrasound-guided fine needle aspiration. JOP. 2006;7:417–422. [PubMed] [Google Scholar]
- 21.Sapio MR. Posca D. Troncone G. Pettinato G. Palombini L. Rossi G. Fenzi G. Vitale M. Detection of BRAF mutation in thyroid papillary carcinomas by mutant allele-specific PCR amplification (MASA) Eur J Endocrinol. 2006;154:341–348. doi: 10.1530/eje.1.02072. [DOI] [PubMed] [Google Scholar]
- 22.Frasca F. Nucera C. Pellegriti G. Gangemi P. Attard M. Stella M. Loda M. Vella V. Giordano C. Trimarchi F. Mazzon E. Belfiore A. Vigneri R. BRAF (V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 23.Zgraggen K. Fernandez-del Castillo C. Rattner DW. Sigala H. Warshaw AL. Metastases to the pancreas and their surgical extirpation. Arch Surg. 1998;133:413–417. doi: 10.1001/archsurg.133.4.413. [DOI] [PubMed] [Google Scholar]
- 24.Quiros RM. Ding HG. Gattuso P. Prinz RA. Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268. doi: 10.1002/cncr.21073. [DOI] [PubMed] [Google Scholar]
- 25.Imkamp F. von Wasielewski R. Musholt TJ. Musholt PB. Rearrangement analysis in archival thyroid tissues: punching microdissection and artificial RET/PTC 1–12 transcripts. J Surg Res. 2007;143:350–363. doi: 10.1016/j.jss.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Al-Brahim N. Asa SL. Papillary thyroid carcinoma–an overview. Arch Pathol Lab Med. 2006;130:1057–1062. doi: 10.5858/2006-130-1057-PTCAO. [DOI] [PubMed] [Google Scholar]
- 27.Baloch ZW. Livolsi VA. Follicular-patterned lesions of the thyroid: the bane of the pathologist. Am J Clin Pathol. 2002;117:143–150. doi: 10.1309/8VL9-ECXY-NVMX-2RQF. [DOI] [PubMed] [Google Scholar]
- 28.Ruter A. Dreifus J. Jones M. Nishiyama R. Lennquist S. Overexpression of p53 in tall cell variants of papillary thyroid carcinoma. Surgery. 1996;120:1046–1050. doi: 10.1016/s0039-6060(96)80053-5. [DOI] [PubMed] [Google Scholar]
- 29.Xing M. BRAF Mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical Implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 30.Palmedo H. Bucerius J. Joe A. Strunk H. Hortling N. Meyka S. Roedel R. Wolff M. Wardelmann E. Biersack HJ. Jaeger U. Integrated PET/CT in differentiated thyroid cancer: diagnostic accuracy and impact on patient management. J Nucl Med. 2006;47:616–624. [PubMed] [Google Scholar]
- 31.Sugitani I. Fujimoto Y. Yamamoto N. Papillary thyroid carcinoma with distant metastases: survival predictors and the importance of local control. Surgery. 2008;143:35–42. doi: 10.1016/j.surg.2007.06.011. [DOI] [PubMed] [Google Scholar]