Abstract
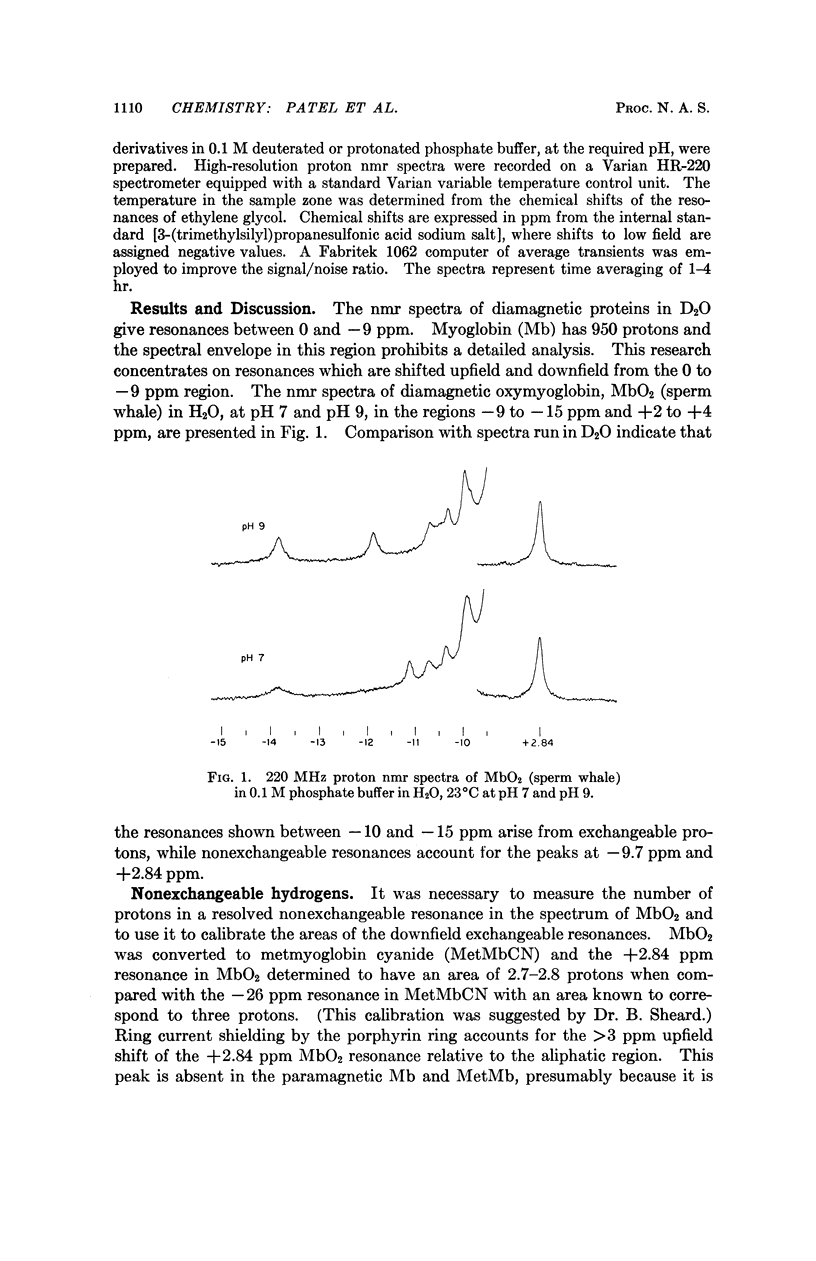
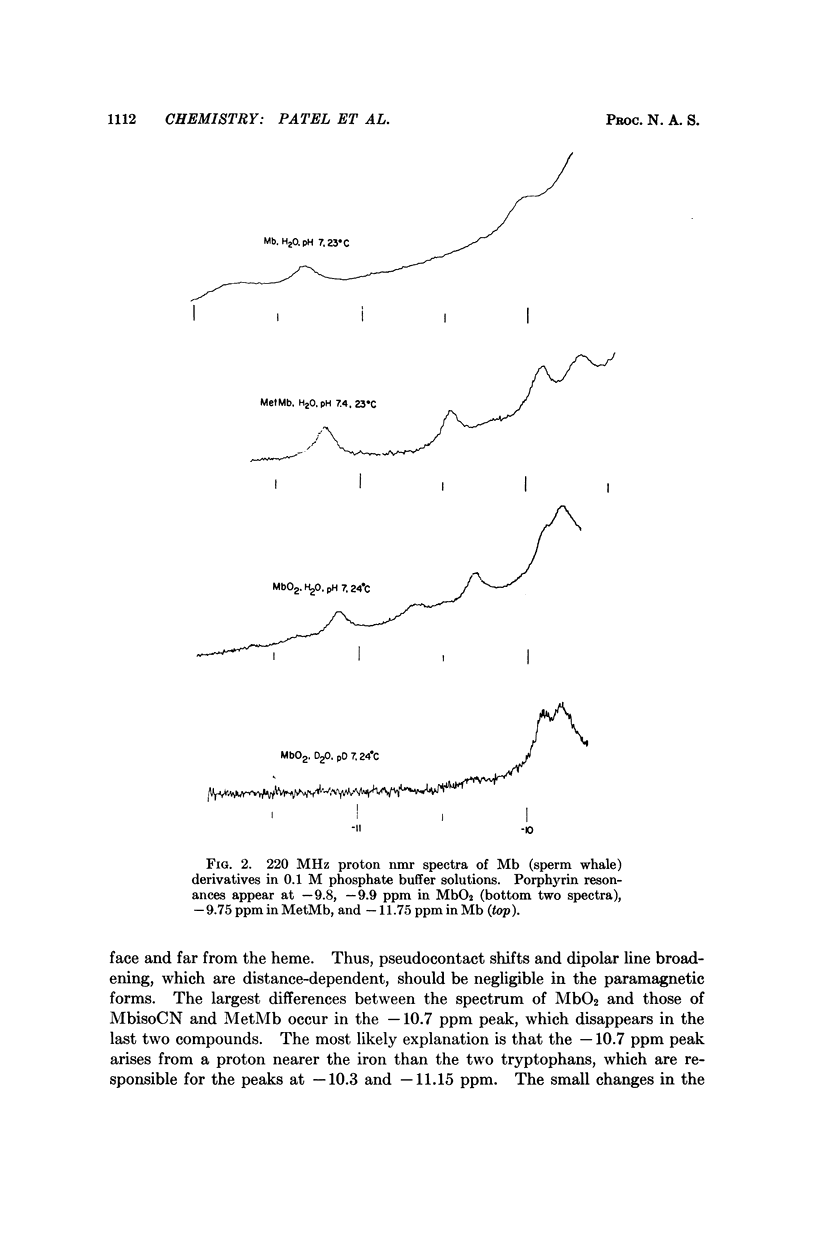
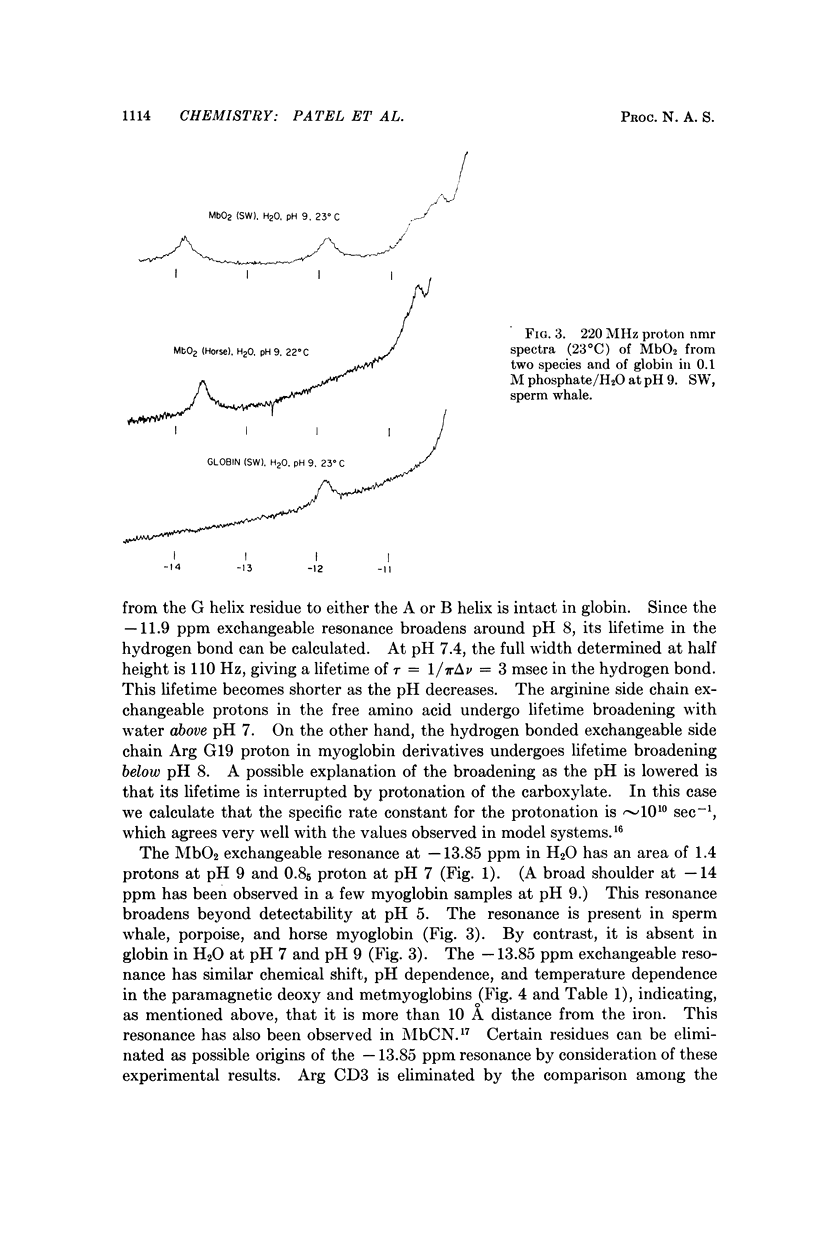
Exchangeable hydrogens in proteins can be identified by comparison of nuclear magnetic resonance spectra obtained in H2O and in D2O. In oxymyoglobin and myoglobin we have been able to observe resonances of the NH protons of the two tryptophans, as well as one resonance from arginine and one from histidine in the range -10 to -15 ppm downfield from 3-(trimethylsilyl)propanesulfonic acid (sodium salt). These resonances have been identified by chemical modifications coupled with considerations of crystallographic structure and the dependence of the resonances on the species (sperm whale, porpoise, horse) from which the myglobin was obtained and on spin, pH, and temperature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradshaw R. A., Gurd F. R. Comparison of myoglobins from harbor seal, porpoise, and sperm whale. V. The complete amino acid sequences of harbon seal and porpoise myoglobins. J Biol Chem. 1969 Apr 25;244(8):2167–2181. [PubMed] [Google Scholar]
- Dautrevaux M., Boulanger Y., Han K. K., Moschetto Y., Biserte G. Séquence partielle des aminoacides de la globine de myoglobine de cheval. Bull Soc Chim Biol (Paris) 1967 Nov 10;49(10):1409–1410. [PubMed] [Google Scholar]
- Glickson J. D., McDonald C. C., Phillips W. D. Assignment of tryptophan indole NH proton resonances of lysozyme. Biochem Biophys Res Commun. 1969 May 22;35(4):492–498. doi: 10.1016/0006-291x(69)90373-8. [DOI] [PubMed] [Google Scholar]
- HILL R. J., KONIGSBERG W., GUIDOTTI G., CRAIG L. C. The structure of human hemoglobin. I. The separation of the alpha and beta chains and their amino acid composition. J Biol Chem. 1962 May;237:1549–1554. [PubMed] [Google Scholar]
- Hapner K. D., Bradshaw R. A., Hartzell C. R., Gurd F. R. Comparison of myoglobins from harbor seal, porpoise, and sperm whale. I. Preparation and characterization. J Biol Chem. 1968 Feb 25;243(4):683–689. [PubMed] [Google Scholar]
- Hugli T. E., Gurd F. R. Carboxymethylation of sperm whale myoglobin in the crystalline state. J Biol Chem. 1970 Apr 25;245(8):1930–1938. [PubMed] [Google Scholar]
- Katz L., Penman S. Association by hydrogen bonding of free nucleosides in non-aqueous solution. J Mol Biol. 1966 Jan;15(1):220–231. doi: 10.1016/s0022-2836(66)80222-x. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Shulman R. G., Peisach J. High-resolution proton magnetic resonance spectra of sperm whale cyanometmyoglobin. Proc Natl Acad Sci U S A. 1968 Jun;60(2):373–380. doi: 10.1073/pnas.60.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich K., Shulman R. G., Yamane T., Wyluda B. J., Hugli T. E., Gurd F. R. High resolution proton magnetic resonance studies of cyanoferrimyoglobins and alkylated derivatives from different species. J Biol Chem. 1970 Apr 25;245(8):1947–1953. [PubMed] [Google Scholar]


