Abstract
The constitutive androstane receptor (CAR) is known as a xeno-sensor that regulates genes involved in xenobiotic excretion and energy metabolism. This study tested a variety of polyphenols for their ability to modulate CAR activity. HepG2 cells were transfected with a CAR expression plasmid and a reporter plasmid containing the human CYP2B6 regulatory region and then treated with flavonoids, catechins and other bioactive polyphenols. Luciferase assays revealed that baicalein (5, 6, 7-OH flavone) was a potent activator of both human and mouse CAR. Catechin gallates also activated human and mouse CAR. Wild-type and CAR knockout mice were treated with baicalein and chrysin (5, 7-OH flavone), and their liver mRNA was analyzed by real-time PCR. A significant increase in cyp2b10 mRNA content was observed only in wild-type mice fed chrysin. These results suggest that dietary flavonoids regulate CAR activity and thereby accelerate both detoxification and energy metabolism.
Keywords: flavonoid, catechin, chrysin, constitutive androstane receptor, pregnane X receptor, cyp2b10, detoxification, energy metabolism
INTRODUCTION
Most foods contain phytochemicals such as flavones and catechins. Plants produce these polyphenols as the first line of defense against ultraviolet irradiation, radical oxygen production and/or invading organisms. From the perspective of human physiology, the radical scavenging activity of these compounds can benefit our health by preventing arterial sclerosis and modulating immunological responses (1, 2). In addition, some of these phytochemicals act on specific cellular proteins and thus exhibit so-called “food functionality.” For example, chrysin and baicalein can antagonistically bind to the aryl hydrocarbon receptor (AhR) in the presence of its activator 2,3,7,8-Tetrachlorodibenzodioxin. The intake of these flavonoids from food may also reduce the risk of malformation and carcinogenesis during development (3). In China, Yin Chin tea has long been recognized as a traditional treatment for neonatal jaundice caused by the accumulation of bilirubin. Dimethylsculetin is the effective component of Yin Chin tea, having a structure resembling A and C rings of flavonoids. It has been shown that this compound acts through the constitutive androstane receptor (CAR) to induce genes responsible for solubilizing and excreting bilirubin (4).
CAR was identified as a drug-responsive nuclear receptor that binds to the phenobarbital-responsive enhancer module (PBREM) located at positions 2339–2289 of the mouse cyp2b10 gene (5). CAR belongs to the nuclear receptor subfamily (NR1I) along with its relative the pregnane X receptor (PXR) (6). Both proteins share common ligands and target genes. In response to exposure to xenobiotics, CAR and PXR act to protect the body by up-regulating phase I, II and III detoxifying genes. These receptors are also known to accelerate energy consumption by regulating genes involved in lipid and sugar metabolism (7). This kind of integrative gene regulation is reasonable because the detoxification processes consist of oxidation, conjugation and membrane transport, which are coupled with the consumption of high energy phosphorous compounds such as NADPH and ATP. Another characteristic of CAR is its ability to recognize a broad range of compounds including barbiturates, polychlorinated biphenyls, bile acids and steroids (8). It is possible that this promiscuity is a result of selective pressure on CAR to be activated by structurally diverse toxic compounds in the natural world, although historically CAR has been identified as a drug-responsive receptor. Thus, it may be beneficial for our health to increase CAR activity by ingesting the dimethylsculetin in Yin Chin tea and other flavones and catechins in food. In this study, we screened several dietary polyphenols for their ability to activate CAR. We found that most flavones activated human and mouse CAR in a similar manner. The results provide useful information for evaluating the effectiveness of functional components of foods.
MATERIALS AND METHODS
Chemicals and reagents
Flavonoids and catechins were provided by the Institute for Health Care Science, Suntory Ltd (Osaka, Japan). The chemicals, 6-(4-chlorophenyl)-imidazo[2,1-b]thiazole-5-carbaldehyde (CITCO) and (3,5-dichloropyridyloxy)] benzene (TCPOBOP) were purchased from Sigma-Aldrich (St. Louis, MO). DMSO and curcumin were obtained from Kanto Chemical Co., Inc (Tokyo, Japan). Naringin was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). Hesperidin was a gift from the Hayashibara Biochemical Labs., Inc (Okayama, Japan). All other reagents were obtained from commercial sources. The Dual-Luciferase Reporter Assay System was from Promega (Madison, WI), cell culture reagents were from Invitrogen (Carlsbad, CA), primers and probes for real-time PCR analysis were from Applied Biosystems (Foster City, CA) and TransIT-LT1 transfection reagent was from Mirus Bio Corporation (Madison, WI).
Plasmids
The CAR coding region from mouse and humans was cloned into the pcDNA3.1 V5-His vector (Invitrogen), and the reporter plasmid containing CYP2B6 PBREM and the thymidine kinase promoter (PBREM-TK-pGL3) were constructed as described previously (9, 10). The phRL-TK Renilla luciferase control reporter was purchased from Promega.
Luciferase assay
HepG2 cells were seeded at 9×107 cells/well in 24-well plates in MEM (Minimum Essential Medium) supplemented with 10% fetus bovine serum and 1% penicillin and streptomycin. The cells were maintained for 24 h prior to transfection with PBREM-TK-pGL3 (0.1 μg/well), mCAR/hCAR expression vector (0.1μg/well) and pRL-TK plasmid (0.1μg/well). Four hours after transfection, cells were treated with solvent (0.1% DMSO) and test compounds (catechins, flavonoids, curcumin and naringin each at a concentration of 10 μM, hesperidin at 50 μM), CITCO at 2 μM or TCPOBOP at 250 nM) for 48 h in minimum essential medium supplemented with 2% fetal bovine serum and 1% penicillin and streptomycin.
Subsequently, cell lysates were assayed for luciferase activity normalized against Renilla luciferase activity using a Dual-Luciferase kit. Data are represented as means±SD of three individual transfection trials. The effect of each compound on CAR activity was calculated as a relative value compared to the response to the vehicle solution and then normalized by the response to positive control drugs (CITCO and TCPOBOP, which induced 2.5-fold and 5-fold increases, respectively). Negative control experiments were performed using cells transfected with the pcDNA3.1 V5-His vector, and the response to each of the polyphenols was subtracted from that of cells transfected with the CAR plasmid.
Animal experiments
All animals were housed and acclimated to cage temperature, light and humidity controls, and fed the MF Diet (Oriental Yeast Co., Ltd) and water ad libitum. Housing and experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals from the U.S. National Institutes of Health. Male C3H/HeNCrlCrlj mice aged 6 weeks were orally administered the following compounds in 3 ml corn oil/kg body weight for 3 days: chrysin (7.62 mg/kg), baicalein (8.10 mg/kg) or TCPOBOP (0.3 mg/kg). Corn oil alone was used as a control. Animals were sacrificed 24 hr after the last administration.
Real-time PCR
Total RNA was isolated from liver tissue (50 mg) using Trizol (Invitrogen) and reverse transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The Taqman mixture of cyp2b10 primers and probe (Assay ID: Mm00456591_m1) was purchased from Applied Biosystems (Foster City, CA). The mRNA content of cyp2b10 was normalized to the content of GAPDH (Applied Biosystems, Foster City, CA). Real-time PCR analyses were performed in optical 96-well reaction plates on an ABI PRIZM 7000 Sequence Detection System (Applied Biosystems).
RESULTS
Activation of CAR by dietary polyphenols
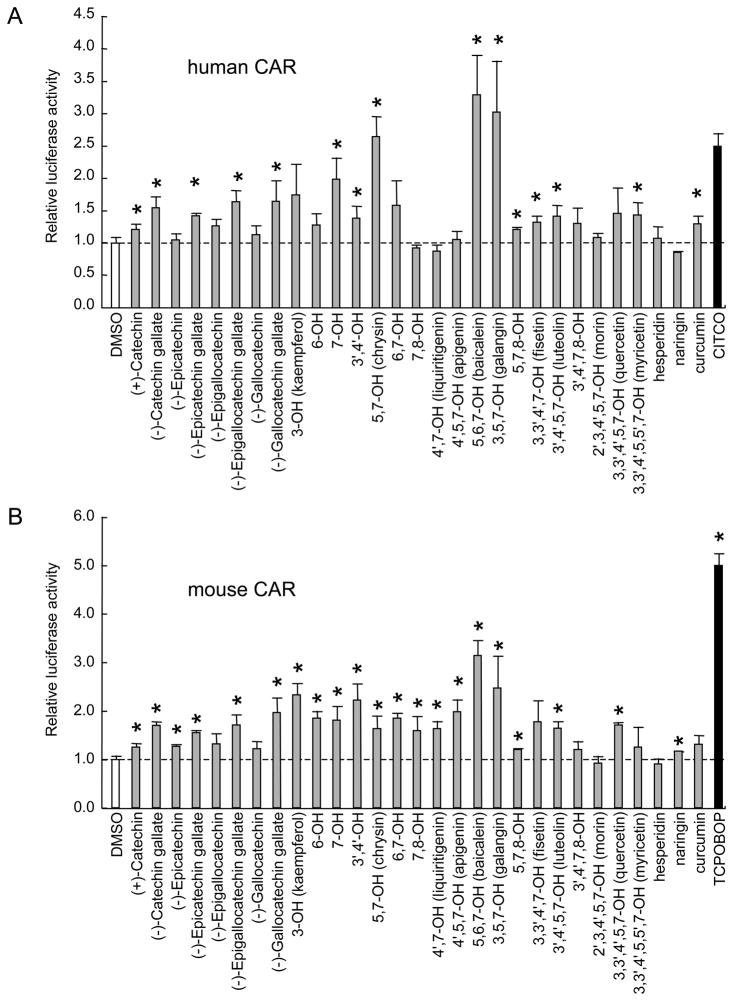
Food-derived phenolic compounds that are known to exhibit certain physiological activities were assayed in a cell-based system (Fig. 1). We used the CYP2B6 PBREM reporter (9, 10) to detect CAR activation. The activity of CAR has been known to be regulated in vivo by drug dependent translocation into nucleus, while it exhibits constitutive activity in cell based assay system (19). In our system, CAR expressed from the CAR expression plasmid has decreased its constitutive activity due to a V5 tag at activation function 2. When cells were co-transfected with this plasmid and the reporter gene and treated with a CAR activator, there was an increase of several fold in the reporter luciferase activity over the basal activity seen in the absence of the activator. Since preliminary experiments revealed that some of the compounds caused a change in cell morphology at concentrations higher than 20 μM, the following experiments were performed with compounds at concentrations lower than 10 μM. Figures 2A and 2B show the responses of human and mouse CAR to these compounds. There is an overall similarity in the response profile between human CAR and mouse CAR. For example, 5,6,7-OH flavone (baicalein) and 3,5,7-OH flavone (galangin) activated both human and mouse CAR, while a lower degree of activation was seen in both human and mouse CAR with 2′,3,4′,5,7-OH flavone (morin), hesperigin and naringin. It is notable that chrysin, baicalein and galangin are more effective activators of human CAR than CITCO, which has been identified as the most potent activator of human CAR.
Figure 1. Dietary polyphenols and CAR activators.
Catechins, catechin gallates and their epimers as well as flavonoids with a variety of hydroxyl moieties were assayed for their stimulatory effect on CAR. Other functional polyphenols such as hesperidin and curcumin were also used. CITCO and TCPOBOP, potent activators of human and mouse CAR, respectively, were used as positive controls. Structural formulae are shown in the right column.
Figure 2. Response profile of CAR to dietary polyphenols.
The responses of CAR to various dietary polyphenols were examined with a HepG2 cell based luciferase assay system. The cells were transfected with the CAR expression vector PBREM-TK-pGL3 as a reporter and phRL-TK as an internal control prior to treatment with chemicals for 48 hr. The assay was then conducted for luciferase activity. The effect of each compound on reporter activity was calculated as a relative value compared to the response to the vehicle solution and then normalized by the response to positive control drugs (CITCO and TCPOBOP, which induced 2.5-fold and 5-fold increases, respectively). Data are representative of three independent experiments, each in triplicate. Significant increases in luciferase activity versus DMSO controls are asterisked (Welch's t-test, P < 0.05, n=3). A, baicalein (3.29-fold) activated human CAR most efficiently, which was followed by galangin (3.02-fold) and chrysin (2.65-fold). B, baicalein (3.15-fold) activated mouse CAR almost similarly to galangin (2.37-fold) and kaempferol (2.33 fold). Catechins tended to cause greater activation when esterified by gallate (A).
Contribution of hydroxyl and gallic acid moieties to CAR activation
We then analyzed the positional effects of the hydroxyl groups in flavones on CAR activation (Tables 1 and 2). The tabulated values represent the fold induction of the reporter gene. The distribution of the significant values (shaded columns) indicates that the presence of hydroxyl groups at the 5 and 7 positions is indispensable for the activation of both human and mouse CAR. In addition, the presence of hydroxyl groups at the 3′ and 4′ positions has a positive effect on CAR activation. This does not contradict the finding that catechins, which have hydroxyl groups located at the 3′ and 4′ positions, activate CAR as described below. Catechins are major components of tea polyphenols, representing more than 10 % of green tea dry matter. Half of each catechin is esterified by gallic acid at the 3-OH. Our study revealed that catechins activate CAR depending on the composition of their gallic acid moiety. In both human and mouse CAR, catechin gallates tended to result in greater CAR activation than unesterified catechins. In addition, there was no significant difference in CAR activating ability among catechin derivatives and epicatechin derivatives (Fig. 2A and B).
Table 1.
Contribution of hydroxyl group in flavonoid to human CAR activation
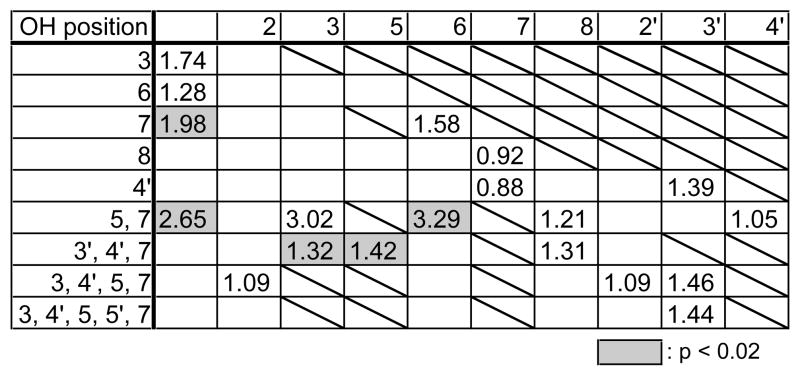 |
Table 2.
Contribution of hydroxyl group in flavonoid to mouse CAR activation
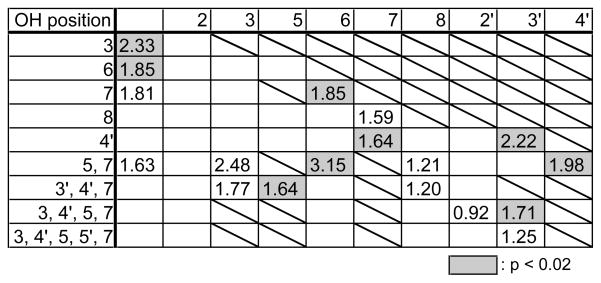 |
Dose-dependent activation of CAR by flavonoids
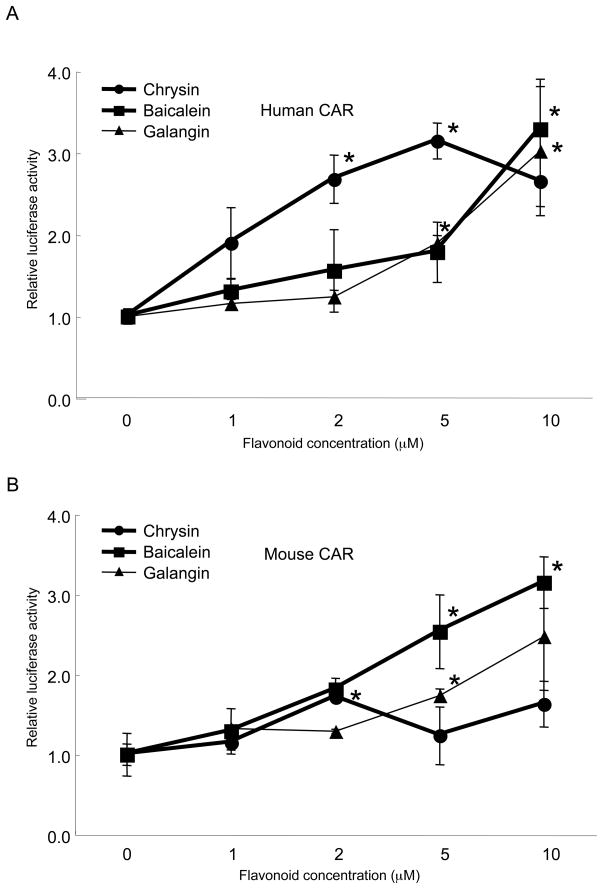
We next examined dose-dependent CAR activation by the common flavonoids chrysin, baicalein and galangin, which occur naturally in many plants at concentrations of 10 to 100 mg/kg by fresh weight (21). As shown in Fig. 3A, chrysin activated human CAR more than baicalein and galangin did at 1, 2 and 5 μM. However, this result was reversed at a higher flavonoid concentration such as 10 μM. Similar results were obtained in the case of mouse CAR (Fig. 3B). We also observed that the decrease of relative luciferase activity coincided with the decrease of control reporter activity. It is possible that actual activity of chrysin has been underestimated in this assay system because of the cell toxicity at higher concentrations.
Figure 3. Dose-dependent activation of CAR by flavonoids.
Chrysin, baicalein and galangin were examined for their ability to activate human (A) and mouse (B) CAR in a dose-dependent manner. Significant increase of realative luciferase activity compared to DMSO controls were marked by asterisks (Welch's t-test, P < 0.05, n=3). In contrast to chrysin, which showed maximum CAR activation at 5 μM, baicalein and galangin showed constant increases in CAR response in a dose-dependent manner at 10 μM and below.
In vivo activation of CAR by flavonoids
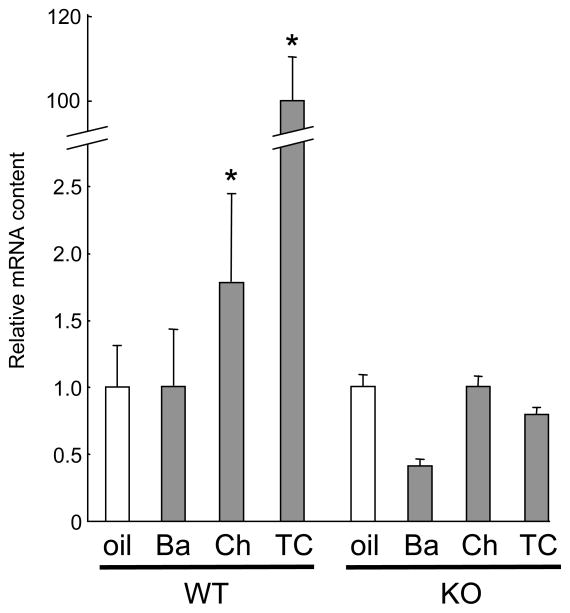
To determine whether these flavonoids can activate CAR in vivo, wild-type mice and CAR knockout (KO) mice were orally administered baicalein or chrysin suspended in corn oil, as described in MATERIALS AND METHODS, for three days and their liver cDNA transcripts were analyzed using real-time PCR. Compared to the oil-fed controls, wild-type mice fed TCPOBOP showed an approximately 100-fold increase in cyp2b10 mRNA content (Fig. 4A). In the mice fed flavonoids, a significant change of cyp2b10 mRNA content was seen only with chrysin ingestion (Fig. 4A, inset). Since the mouse cyp2b10 gene is coordinately regulated by CAR and PXR thorough PBREM and the xenobiotic-responsive enhancer module (11), the response of the cyp2b10 gene in a CAR KO background is indicative of PXR responsiveness to these flavonoids. As shown in Fig. 4B, CAR KO mice showed no change in cyp2b10 mRNA content when treated with flavonoids or TCPOBOP. These results suggest that chrysin is one of the food factors activating CAR in vivo.
Figure 4. In vivo induction of cyp2b10 by flavonoids.
Wild-type mice (WT) and CAR knockout mice (KO) were administered corn oil (oil), baicalein (Ba), chrysin (Ch) or TCPOBOP (TC) for 3 days, and the cyp2b10 mRNA content of their livers was quantified by real-time PCR analysis. A significant increase in cyp2b10 mRNA content versus the oil-treated control (Welch's t-test, P < 0.05, n=4) was detected only in wild-type mice fed chrysin or TCPOBOP and not in those fed baicalein (inset). No such change in cyp2b10 mRNA content was observed in CAR KO mice.
DISCUSSION
Several NR1 members have been reported to respond to naturally occurring bioactive polyphenols; PXR responds to 10 μM tangeretein (4′,5,6,7,8-OCH3 flavone), while kaempferol, chrysin, fisetin, luteolin, morin, quercetin and myricetin did not show significant PXR activation in the same transient assay system (12).
In our study, CAR exhibited a significant response to most of these flavonoids except morin (Fig. 2A and B). Similar results have been obtained by Li et al using PBREM of UDP-glucuronosyltransferase 1A1 gene (20). It has also been reported that PXR cannot be activated by catechin gallates at 100 μM. These results suggest that CAR has a broader spectrum of response to flavonoids than PXR does. Peroxisomal proliferator-activated receptors (PPARs) are known to be key lipid metabolism regulators. They are activated by free fatty acids as endogenous ligands and also by anti-diabetic drugs and dietary flavonoids as exogenous ligands (13, 14). PPARγ can be activated by apigenin, chrysin and kaempferol (15), the latter two of which activated CAR at 10 μM in our cell-based system. In addition, catechin and catechin gallate activate both PPARα and γ (16, 17). These results indicate that the ligand response profiles of CAR and PPARs partially overlap with each other. It is therefore possible that CAR and PPARs coordinately regulate energy metabolism in response to dietary flavonoids.
Interestingly, among the two flavonoids examined, only chrysin induced the cyp2b10 gene in vivo (Fig. 4A and B), although baicalein activated CAR more efficiently than chrysin in the cell-based system (Fig. 2A and B). One possible reason is that these flavonoids show differences in effective concentration in liver among one another. Chrysin is relatively resistant to microbial degradation in the human intestine (18). It is also possible that in vivo glycosylation affects their excretion rates (21). In the future, we will elucidate the bioavailability of CAR-activating flavonoids found in the present work.
From the data collected thus far, we conclude that structural characteristics of flavonoids determine the activation of CAR, raising the possibility that dietary flavonoids can activate CAR to stimulate detoxification and energy expenditure.
Acknowledgments
This research was supported by Japan Society of Promotion of Science (#19880024, #40373196, #20688015, #10151094) and Iijima kinen grant for research.
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
LITERATURE CITED
- 1.Garcia-Lafuente A, Guillamon E, Villares A, Rostagno MA, Martinez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 2.Shenouda SM, Vita JA. Effects of flavonoid-containing beverages and EGCG on endothelial function. J Americ College Nutri. 2007;26:366S–372S. doi: 10.1080/07315724.2007.10719625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Env Health Perspec. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113:137–143. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;10:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Burch PE, Cooney AJ, Lanz RB, Pereira FA, Wu J, Gibbs RA, Weinstock G, Wheeler DA. Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome. Genome Res. 2004;14:580–590. doi: 10.1101/gr.2160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konno Y, Negishi M, Kodama S. The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab Pharmacokinet. 2008;23:8–13. doi: 10.2133/dmpk.23.8. [DOI] [PubMed] [Google Scholar]
- 8.Reschly EJ, Krasowski MD. Evolution and function of the NR1i nuclear hormone receptor subfamily, VDR, PXR, and CAR, with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metabol. 2006;7:349–365. doi: 10.2174/138920006776873526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swales K, Kakizaki S, Yamamoto Y, Inoue K, Kobayashi K, Negishi M. Novel CAR-mediated mechanism for synergistic activation of two distinct elements within the human cytochrome P450 2B6 gene in HepG2 cells. J Biol Chem. 2005;280:3458–3466. doi: 10.1074/jbc.M411318200. [DOI] [PubMed] [Google Scholar]
- 10.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. A novel distal enhancer module regulated by Pregnane X receptor/Constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003;278:14146–14152. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- 12.Satsu H, Hiura Y, Mochizuki K, Hamada M, Shimizu M. Activation of pregnane X receptor and induction of MDR1 by dietary phytochemicals. J Agric Food Chem. 2008;56:5366–5373. doi: 10.1021/jf073350e. [DOI] [PubMed] [Google Scholar]
- 13.Martin PGP, Guillou H, Lasserre F, Dejean S, Lan A, Pascussi JM, SanCristobal M, Legrand P, Besse P, Pineau T. Novel aspects of PPAR-mediated regulation of lipid and xenobiotic metabolism revealed through a nutrigenomic study. Hepatology. 2007;45:767–777. doi: 10.1002/hep.21510. [DOI] [PubMed] [Google Scholar]
- 14.Salam NK, Huang TH, Kota BP, Kim MS, Li Y, Hibbs DE. Novel PPAR-gamma agonists identified from a natural product library: a virtual screening, induced-fit docking and biological assay study. Chem Biol Drug Des. 2008;71:57–70. doi: 10.1111/j.1747-0285.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- 15.Lianga Y, Tsai S, Tsai D, Lin-Shiau S, Lin J. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12–18. doi: 10.1016/s0014-5793(01)02393-6. [DOI] [PubMed] [Google Scholar]
- 16.Shin DW, Kim SN, Lee SM, Lee W, Song MJ, Park SM, Lee TR, Baik JH, Kim HK, Hong JH, Noh M. (-)-Catechin promotes adipocyte differentiation in human bone marrow mesenchymal stem cells through PPAR gamma transactivation. Biochem Pharmacol. 2009;77:125–33. doi: 10.1016/j.bcp.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Lee K. Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts. J Vet Sci. 2004;5:325–330. [PubMed] [Google Scholar]
- 18.Simons AL, Renouf M, Hendrich S, Murphy PA. Human gut microbial degradation of flavonoids: Structure-function relationships. J Agric Food Chem. 2005;53:4258–4263. doi: 10.1021/jf0500177. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B Gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Stanton JD, Tolson AH, Luo Y, Wang H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm Res. 2009;26:872–882. doi: 10.1007/s11095-008-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]