Abstract
Desensitization of the μ-opioid receptor (MOR) has been implicated as an important regulatory process in the development of tolerance to opiates. Monitoring the release of intracellular Ca2+ ([Ca2+]i), we reported that [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO)-induced receptor desensitization requires receptor phosphorylation and recruitment of β-arrestins (βArrs), while morphine-induced receptor desensitization does not. In current studies, we established that morphine-induced MOR desensitization is protein kinase C (PKC)-dependent. By using RNA interference techniques and subtype specific inhibitors, PKCε was shown to be the PKC subtype activated by morphine and the subtype responsible for morphine-induced desensitization. In contrast, DAMGO did not increase PKCε activity and DAMGO-induced MOR desensitization was not affected by modulating PKCε activity. Among the various proteins within the receptor signaling complex, Gαi2 was phosphorylated by morphine-activated PKCε. Moreover, mutating three putative PKC phosphorylation sites, Ser44, Ser144 and Ser302 on Gαi2 to Ala attenuated morphine-induced, but not DAMGO-induced desensitization. In addition, pretreatment with morphine desensitized cannabinoid receptor CB1 agonist WIN 55212-2-induced [Ca2+]i release, and this desensitization could be reversed by pretreating the cells with PKCε inhibitor or overexpressing Gαi2 with the putative PKC phosphorylation sites mutated. Thus, depending on the agonist, activation of MOR could lead to heterologous desensitization and probable crosstalk between MOR and other Gαi-coupled receptors, such as the CB1.
1. Introduction
Desensitization, a common phenomenon observed with G-protein-coupled receptor (GPCR) signaling, reflects the gradual decrease in GPCR activity during chronic agonist treatment. Opioids are considered as the most potent analgesic, but the tolerance developed after repetitive or chronic administration limits their clinical utility. Although the mechanisms involved have not been elucidated completely, desensitization of the μ-opioid receptor (MOR) has been implicated in contributing to tolerance development [1]. A common pathway for GPCR desensitization is mediated by G-protein-coupled receptor kinases (GRKs) and the β-arrestins (βArrs) [2]. In this model, agonist binding to GPCR leads to GRK-mediated receptor phosphorylation that subsequently increases the affinity of the agonist–receptor complex for cytosolic βArrs. Translocation of the βArrs to the receptor disrupts receptor–G-protein coupling and dampens signal transduction processes. However, this model has been challenged by the existence of agonists whose receptor complexes have low affinities for βArrs [3]. Such agonists might desensitize the receptor via pathways other than that involving GRK and βArrs. Consistent with this hypothesis, protein kinases such as protein kinase A, protein kinase C (PKC) and mitogen-activated protein kinase have also been reported to mediate GPCR desensitization [4].
As a prototypic Gi/o-coupled receptor, MOR undergoes extensive receptor desensitization [1], and the mechanism appears to be agonist-dependent [5, 6]. By monitoring the MOR-mediated release of intracellular Ca2+ ([Ca2+]i) or the activation of the G-protein-coupled inwardly rectifying potassium channel, the MOR peptide agonist [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO) was shown to induce receptor desensitization via MOR phosphorylation and βArrs translocation, whereas morphine does not. This is consistent with the lower efficacy of morphine to induce receptor phosphorylation and βArr recruitment relative to agonists such as DAMGO and etorphine [7]. Interestingly, PKC has been implicated in morphine-induced MOR desensitization [5, 6]. The preference of morphine to use PKC-dependent pathways for signal transduction in vitro and tolerance development in vivo further suggest the participation of PKC activity in morphine functions [8–12].
As an important kinase family, PKCs participate in numerous cellular signaling pathways, from short-term neurotransmitter release to long-term cellular adaptation responses [13]. Depending on their structure and activation mechanism, the PKC family has been divided into several subtypes: conventional, novel and atypical. In vivo studies revealed that morphine function is related to several PKC subtypes. PKCα, PKCγ and PKCε appear to contribute to morphine tolerance [14], and mice lacking PKCε show an increased response to morphine [15]. However, the exact PKC subtype(s) involved in MOR signal transduction, and the mechanisms by which they act (e.g., the kinase targets), remain unknown.
PKC translocates to the membrane upon activation [13]. Subsequent phosphorylation of membrane proteins could lead to PKC-mediated regulation of GPCR signaling. There are many potential PKC targets that might contribute to receptor desensitization, e.g. MOR and G-protein. Although GRK-mediated MOR phosphorylation does not modulate morphine-induced desensitization, PKC-mediated MOR phosphorylation might; and G-protein phosphorylation has also been implicated in GPCR desensitization [16].
Increased PKC activity can cause heterologous desensitization [17], and morphine pretreatment heterologously desensitizes other Gi/o-coupled receptors [18]. Thus, PKC might mediate the crosstalk between MOR and other GPCRs. MOR and the cannabinoid receptor CB1 colocalize in the central nervous system and these receptors might interact in vivo [19, 20]. In addition, CB1 shares most of signal transduction pathways with MOR in vitro and produces analgesia in vivo [21]. CB1 therefore provides a good model to study crosstalk between MOR and other signaling pathways during PKC activation. In this study, the mechanism by which PKC participates in morphine-induced heterologous desensitization of CB1 receptor was examined in vitro. The model derived from these in vitro studies for the interaction between these two Gi/o-coupled receptors might be the basis for the observed MOR-CB1 interaction in vivo.
2. Materials and Methods
2.1. Cell culture
Human embryonic kidney cells stably expressing hemagglutinin (HA)-tagged MOR (HEK293-MOR) were maintained in Eagle’s minimal essential medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA) and 200 μg/ml G418 sulfate in a 5% CO2 incubator. When PKC activities were monitored, cells were cultured in a serum-free medium overnight before treatment.
2.2. RNA interference
In order to down-regulate cellular levels of specific PKC subtypes, BLOCK-iT™ lentiviral Pol II miR RNAi Expression System with EmGFP (Invitrogen, Carlsbad, CA) was used to decrease the cellular level of specific PKC subtype. Generally, the kit enables the expression of miRNA-based knockdown cassettes driven by RNA Polymerase II promoters in mammalian cells. The sequences targeted by the expressed microRNAs or microRNA-like short RNAs were designed specifically to PKCα, γ and ε mRNAs using the software provided by Invitrogen (www.invitrogen.com/rnai). The sequences of oligonucletides used were listed as follow: PKCα: 5′-TGCTGTGAATTTGTGGTCTTTCACCTGTTTTGGCCACTGACTGACAGGTGAAACCACAAATTCA-3′ (PKCαRNAi); PKCγ:5′-TGCTGATAGGTGACAGTTTGTTCCATGTTTTGGC CACT GACTGACATGGAACACTGTCACCTAT-3′ (PKCγRNAi); and PKCε:5′-TGCTGAAAGACAGCCAGCTCGATCTTGTTTTGGCCACTGACTGACAAGAT CGATGGCTGTCTTT-3′ (PKCεRNAi). These sequences were inserted into the V5-DEST as described in the manufacturer’s protocol (Invitrogen, Carlsbad, CA). After transfecting into the cells, these constructs induce the decrease of cellular levels of their targeting PKC subtypes.
2.3. Transfection with Effectene® or Nucleofector®
Effectene (QIAGEN, Valencia, CA) was used to transfect plasmids into HEK293-MOR cells according to the manufacturer’s protocol. After transfection, cells were incubated at 37°C for 48 h before assays were performed. To overcome the coupling of endogenous Gαi to the [Ca2+]i signaling pathway [22], Nucleofector® (Lonza, Basel, Switzerland) was used to transfect pertussis toxin (PTX)-resistant Gαi constructs into HEK293-MOR cells to achieve higher transfection efficiency. Briefly, cells were cultured for 2 days to their logarithmic growth phase. Then cell were harvested and centrifuged at 900 × g for 10 min at room temperature. The supernatant was removed and 1 × 106 cells were mixed with 100 μl of Nucleofector® solution and 5 μg of plasmid. Plasmid DNA was transfected into cells by the manufacturer’s designated Nucleofector® program, and cells were immediately seeded into 96-well plates and incubated in a humidified atmosphere of 5% CO2 at 37°C for 24 h before performing [Ca2+]i assays. Gα subunits cloned into the pcDNA3 vector were mutated using a site-direction mutagenesis kit (Stratagene, La Jolla, CA): Cys352 on Gαi2 and Gαo or Cys351 on Gαi3 were mutated to Leu to render the Gα proteins resistant to ADP-ribosylation by PTX; mutations of individual or multiple putative PKC phosphorylation sites were generated using similar procedures.
2.4. Intracellular Ca2+ measurement
Cells were plated into black poly-L-lysine-coated 96-well black plates with clear, flat bottoms and maintained at 37°C in 5% CO2 for 24 h before assays. Release of [Ca2+]i was determined by measuring the change in fluorescence, as described previously [5]. The FLIPR® calcium assay reagent (Molecular Devices, Sunnyvale, CA) and FLEXstation® (Molecular Devices) were used to measure increased fluorescence in cells after agonist challenges. The FLIPR® reagent was dissolved in HBSS buffer (20 mM HEPES, 5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHCO3, 0.3 mM Na2HPO4, 5.6 mM D-glucose, 2.5 mM probenecid, 13 mM CaCl2). For PKC inhibition studies, cells were preincubated with the following subtype-specific inhibitors (Biomatik, Cambridge, Canada) for 3h before the assays: myristoylated PKCα pseudosubstrate (Myr-FARKGALRQ-OH), PKCγ antagonist (Myr-CRLVLASC-OH), and PKCε antagonist (Myr-EAVSLKPT-OH). The general PKC inhibitor Ro-31-8425 was purchased from LC Laboratories (Woburn, MA).
2.5. Immunoblotting and immunoprecipitation
After agonist treatment, cells were lysed with lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 50 mM NaF, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 50 mM sodium pyrophosphate, 10 mM sodium vanadate and 1X protease inhibitor cocktail [Roche, Indianapolis, IN]). After centrifugation at 12,000g for 5 min, SDS–PAGE sample buffer was added to the supernatants for immunoblotting analyses. For immunoprecipitation assays, mouse anti-HA (1:1,000) (Covance, Princeton, NJ) or rabbit anti-Gαi2 (1:500) [23] antibodies and rProtein-G–agarose (Invitrogen, Carlsbad, CA) were added to the supernatants and rotated overnight. Antibodies specific for phosphor-Ser PKC substrate (Cell Signaling Technology, Boston, MA), phosphor-Ser (Sigma, St. Louis, MO), PKCε (Cell Signaling Technology), HA and Gαi2 were used for immunoblotting. A Storm 860 system and ImageQuant analysis software (Amasham, Piscataway, NJ) were used to detect and quantify fluorescence intensities on immunoblots.
2.6. PKC subtype activity assay
The activities of PKC subtypes were determined after agonist treatment using HTScan® PKC subtype kinase assay kits (Cell Signaling Technology). After agonist treatment, cells were lysed and immunoprecipitated with antibodies against the PKC α, γ or ε subtypes (Cell Signaling Technology). PKC activities were determined by adding biotinylated PKA substrate peptides containing the peptide core sequence residues surrounding Ser133 of CREB (i.e., RRPS*YRK) (Cell Signaling Technology). The reacted substrates were collected with streptavidin beads; rabbit anti-phos-PKA substrate and anti-rabbit-488 antibodies were used to mark reacted substrates. α-Fusion plate reader (PerkinElmer Life and Analytical Sciences, Boston, MA) was used to determine the fluorescence intensities of the samples.
2.7. Continuous sucrose gradient
After pretreatment, cells were collected in 700 μl of 500 mM sodium carbonate (pH 11) and homogenized by passing them ten times through a 22-gauge needle. Crude homogenates were disrupted further using three 10-s bursts of a microprobe-equipped sonicator (Heat Systems-Ultrasonic, Inc., NY) at setting 4. The homogenates were mixed with an equal volume of 80% sucrose in morpholinoethanesulfonic acid-buffered saline, pH 6.8, and placed at the bottom of ultracentrifugation tubes. A continuous gradient of 5–30% sucrose formed using a Gradient Station (BioComp, Fredericton, Canada) was layered on top. Gradients were centrifuged at 32,000 rpm for 16 h in a SW41 rotor, and twelve 1-ml fractions were collected from each sample.
2.8. Statistical analyses
At least three independent experiments were conducted to obtain statistical results, presented as means ± standard deviations. Data were analyzed by unpaired Student’s t-tests to determine significance.
3. Results
3.1. Agonists induce rapid desensitization of MOR-dependent [Ca2+]i release
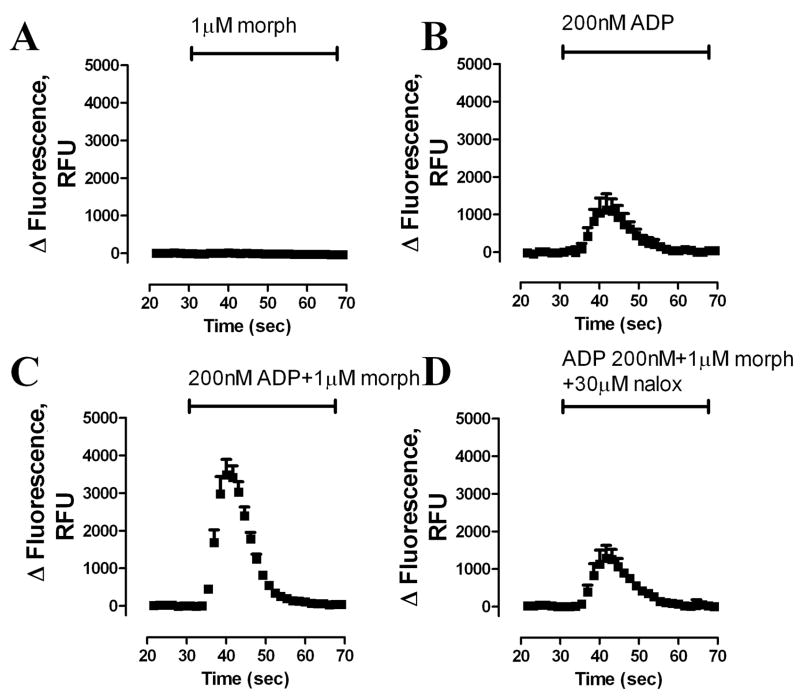
In order to study the agonist-induced MOR desensitization, a protocol monitoring [Ca2+]i release was used as reported previously [5]. By itself, morphine did not evoke [Ca2+]i release in HEK293-MOR cells [24] (Fig. 1A). In contrast, 200 nM of ADP, an agonist of the Gq-coupled purinergic P2Y receptor, induced transient but robust [Ca2+]i release (Fig. 1B). Furthermore, this ADP-induced release was potentiated significantly by treating the cells with 1 μM morphine together with ADP (Fig. 1C); while the MOR antagonist naloxone blocked the morphine-induced potentiation (Fig. 1D). The potentiation of morphine on ADP-induced [Ca2+]i release was used as an indicator of MOR activity in subsequent experiments.
Fig. 1. MOR-potentiates Gq-coupled receptor-induced [Ca2+]i release.
Real-time changes in intracellular fluorescence expressed in raw fluorescence units (RFU) were used to assess [Ca2+]i release from HA-tagged HEK293-MOR cells. Fluorescence changes were recorded using a 485-nm excitation wavelength and a 525-nm emission wavelength. After a 30-s baseline reading, agonists in HBSS buffer were added. (A) 1 μM morphine (morph); (B) 200 nM ADP; (C) 200 nM ADP + 1 μM morphine; (D) 200 nM ADP + 1 μM morphine + 30 μM naloxone (nalox) (n = 3).
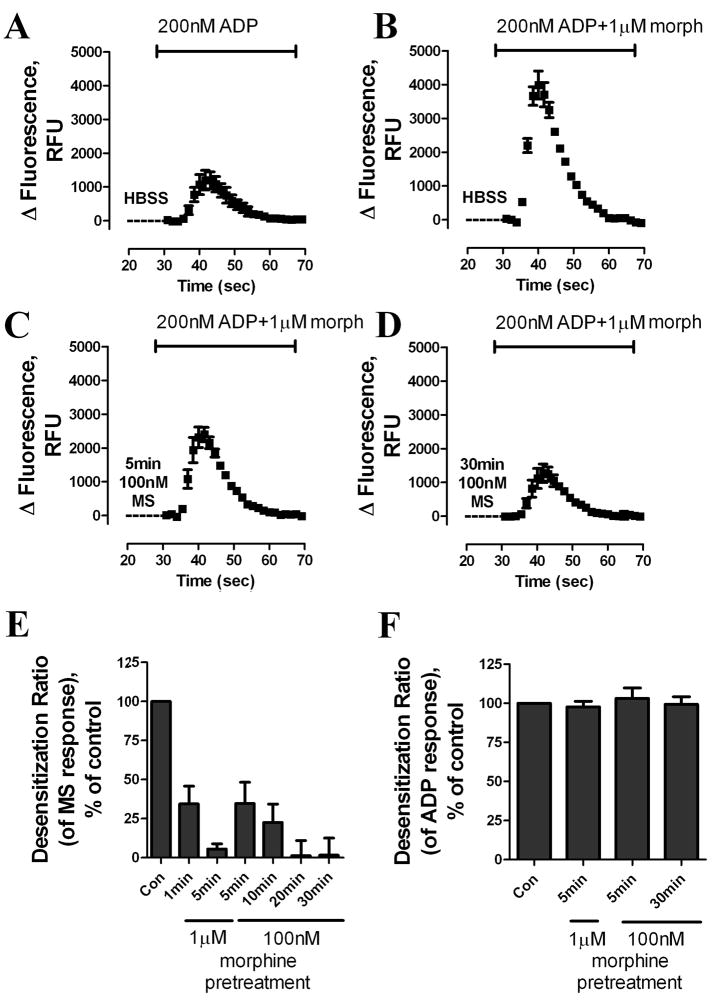
Pretreatment of cells with HBSS buffer for 5 min before addition of ADP and morphine had no effect on morphine-potentiated [Ca2+]i release (Fig. 1B, C and Fig. 2A, B). In contrast, pretreatment with 100 nM morphine for 5 min significantly decreased the release of [Ca2+]i by morphine and ADP (Fig. 2C): morphine-induced [Ca2+]i release after 100 nM morphine pretreatment for 5 min was only 35% ± 14% of the agonist response after HBSS pretreatment (Fig. 2C, E and Fig. 3A). If morphine pretreatment was extended to 30 min, the morphine-induced response decreased further to 2% ± 13% that of HBSS-treated samples (Fig. 2D, E and Fig. 3A). Furthermore, increasing the concentration of the morphine used in pretreatment accelerated the rate of decrease in the agonist response. Loss of response was observed as quickly as 1 min after a 1 μM morphine pretreatment; a 5-min pretreatment 1 μM morphine decreased [Ca2+]i release to 6% ± 4% that of controls (Fig. 2E). The decrease in morphine responsiveness appeared to be homologous, because the response to ADP alone was not affected by either a 5-min pretreatment with 1 μM morphine or a 30-min pretreatment with 100 nM morphine (Fig. 2F). Because pretreatment with morphine neither influenced [Ca2+]i reserves (i.e., morphine did not evoke calcium release by itself) nor desensitized the P2Y receptor, the decreased ability of morphine to potentiate ADP-induced [Ca2+]i release reflects the desensitization of MOR.
Fig. 2. Determining MOR desensitization by monitoring MOR-induced [Ca2+]i release.
(A–D) Real-time changes in intracellular fluorescence are expressed in RFU. HEK293-MOR cells were pretreated with HBSS or 100 nM morphine for (C) 5 min or (D) 30 min, followed by ADP and additional morphine to achieve final agonists’ concentrations as indicated in the figure. (E) Dose response and time course of morphine-induced receptor desensitization. HEK293-MOR cells were treated as described in (A–D). Total [Ca2+]i release after pretreatment was quantified by calculating the area under curves with a Prism program. Response to ADP was subtracted from total response to obtain the response to MOR. MOR response after morphine pretreatment was compared to MOR response after HBSS pretreatment to obtain the desensitization ratio. (F) Effect of morphine pretreatment on response to 1 μM ADP. After morphine pretreatment, ADP was added together with 10μM CTOP to block MOR response. Response to ADP was analyzed as described in Fig. 2E. (n≥3)
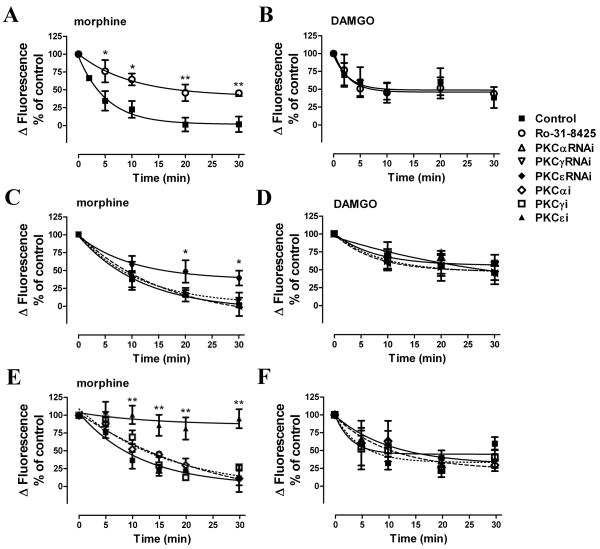
Fig. 3. Morphine-induced, but not DAMGO-induced, MOR desensitization is PKC-dependent.
Morphine-induced desensitization as measured by assessing agonist potentiation of ADP-mediated [Ca2+]i release. 100 nM morphine or DAMGO were used to pretreat HEK293-MOR cells for various time indicated in the X-axis. Desensitization ratios were determined as described in Fig. 2. HEK293-MOR cells were incubated for 3 h with DMSO (Control) (■), 5 μM general PKC inhibitor Ro-31-8425 (○), transfected for 48 h with PKCαRNAi (△), PKCγRNAi (▽), PKCεRNAi (◆) or incubated for 3 h 50 μM subtype-specific inhibitors of PKCα (PKCαi) (◇), γ (PKCγi) (□), or ε (PKCεi) (▲) before assays (*: p < 0.05; **: p < 0.01; n≥3).
3.2. Morphine-induced desensitization of MOR is PKCε-dependent
Although previous reports indicated that DAMGO uses GRK- and βArr-dependent pathways to desensitize MOR, other protein kinases have been implicated in morphine-induced MOR desensitization [25, 26]. To characterize the mechanism by which morphine induced MOR desensitization, HEK293-MOR cells were incubated with a Src kinase inhibitor (PP2), an extracellular signal-regulated kinase inhibitor (PD98059), or a PKC inhibitor (Ro-31-8425) before inducing receptor desensitization by pretreatment with 100 nM morphine. Among these inhibitors, only 5 μM of Ro-31-8425 attenuated morphine-induced, but not DAMGO-induced, MOR desensitization (Fig. 3A, B and data not shown). In the presence of the PKC inhibitor, the MOR response decreased by 55% ± 6% after a 30-min morphine pretreatment, which was significantly attenuated from control group (p <0.01, n≥3).
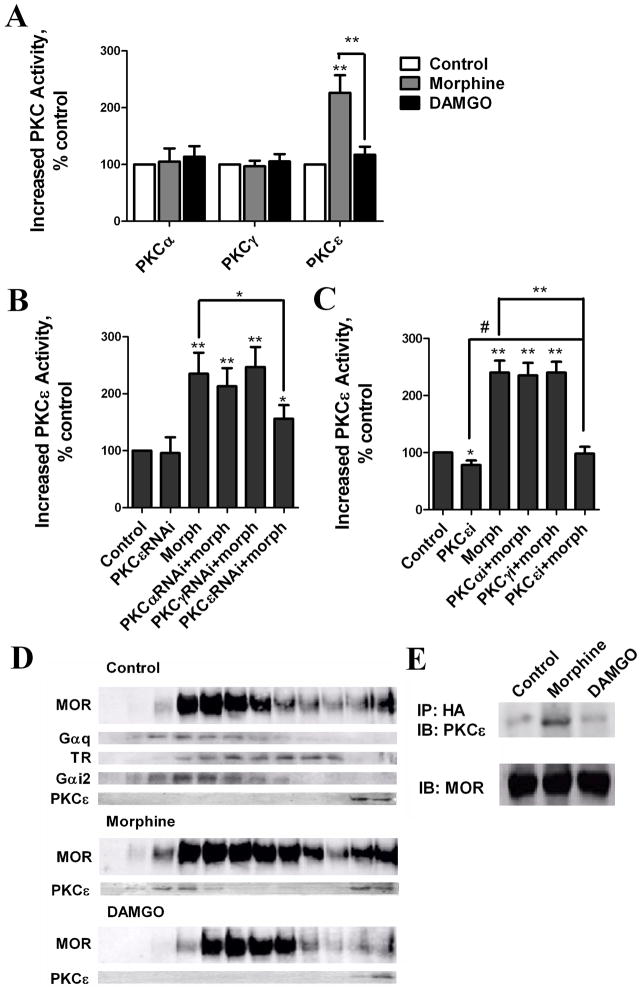
The identity of the PKC subtype responsible for morphine-induced desensitization was determined using RNA interference technique to down-regulate the cellular level of mRNAs of specific PKC subtype, as described in the Materials and Methods. Although all eleven PKC subtypes are present in HEK293 cells [27], we focused on the PKC subtypes α, γ and ε, which have been implicated in the development of morphine-induced tolerance in vivo [14]. Introducing interfering RNA (RNAi) against PKCα (PKCαRNAi), PKCγ (PKCγRNAi) or PKCε (PKCεRNAi) specifically down-regulated the endogenous levels of PKCα, PKCγ or PKCε, respectively (Suppl. Fig. 1). Morphine-induced MOR desensitization was attenuated significantly by overexpression of PKCεRNAi (p <0.05, n≥3), 30-min morphine pretreatment decreased MOR response by 39% ± 10%, correlated to the level of endogenous PKCε being down-regulated (Fig. 3C, Suppl. Fig. 1). In contrast, overexpression of PKCαRNAi or PKCγRNAi did not affect morphine-induced MOR desensitization; and DAMGO-induced MOR desensitization was not affected by any of these PKC subtypes RNAis (Fig. 3D).
Since morphine-induced MOR desensitization was only partially inhibited by Ro-31-8425 or PKCεRNAi. To further investigate whether PKCε plays the exclusive role in morphine-induced MOR desensitization, membrane-permeable, subtype-specific peptide inhibitors of PKC were used. These peptides inhibit PKC activity by binding to either the subtype’s catalytic sites or their corresponding anchoring protein RACK [28, 29]. Morphine-induced MOR desensitization was completely blocked by the PKCε inhibitor, but not by the inhibitors of PKCα or PKCγ (Fig. 3E). In contrast, DAMGO-induced MOR desensitization was unaffected by any of these inhibitors (Fig. 3F).
3.3. Morphine induces PKCε activation and translocation
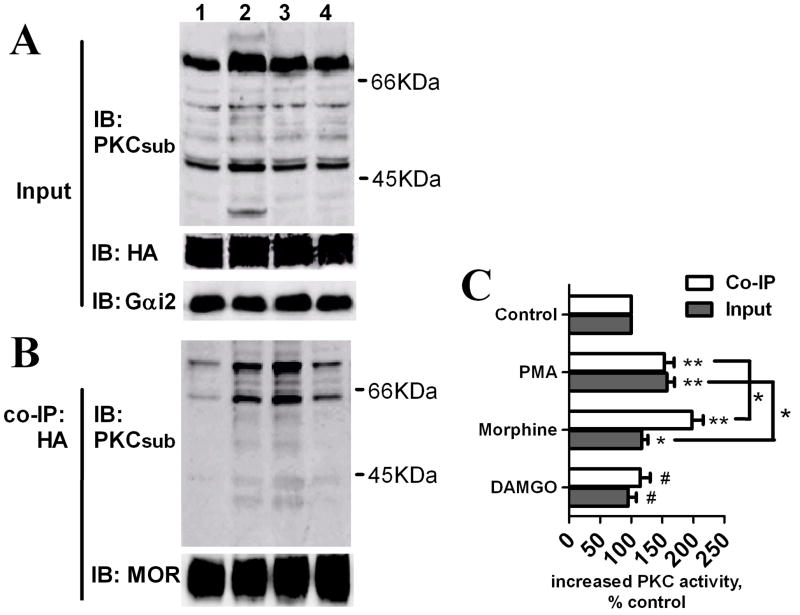
Morphine-induced receptor desensitization is mediated by PKC whereas DAMGO-induced desensitization is not, whether PKC is activated by MOR in an agonist-dependent manner was investigated. An antibody that recognizes phosphorylated PKC substrates was used to determine PKC activity after morphine pretreatment. When the immunoreactivities of phosphorylated PKC substrates were determined in whole cell lysates, a 5-min pretreatment with 1 μM morphine increased PKC activity to 120 ± 10% that of basal levels, whereas the general PKC activator phorbol 12-myristate 13-acetate (PMA) induced an increase to 160 ± 12% of control activity (Fig. 4A and C). MOR signaling complexes were specifically co-immunoprecipitated with MOR by using HA antibody (Suppl. Fig. 2A). Morphine pretreatment increased the amount of phosphorylated PKC substrates present in MOR signaling complexes by 2.0 ± 0.18 fold relative to controls, and this morphine-induced increase was greater than the 1.5 ± 0.16 fold increase induced by treatment with PMA (Fig. 4B and C). Thus, morphine activated PKC and the phosphorylated PKC substrates enriched in the receptor signaling complex. In contrast, DAMGO pretreatment did not significantly increase PKC activity, indicating that PKC activation was agonist-dependent (Fig. 4).
Fig. 4. Activation of PKC by morphine treatment.
HEK293-MOR cells were pretreated with Buffer (lane 1), 1μM PMA (lane 2), 1μM morphine (lane 3) or 1μM DAMGO (lane 4) for 5 mins. (A) PKC activity in whole cell lysates was determined by immunoblotting (IB) with an antibody against phosphorylated PKC substrates (PKCsub), and Gαi2 and MOR in whole cell lysates were used as input controls. (B) MOR signaling complexes were co-immumoprecipitated (co-IP) with HA antibody, and PKC activity was determined by IB with PKCsub; MOR within immumoprecipitates was determined with an antibody against MOR C-terminal. (C) Quantitative analysis of immunoreactivities of PKC phosphorylated substrates as determined from IBs. (#: no significant difference; *: p < 0.05; **: p < 0.01; n=3).
The antibody used against the phosphorylated PKC substrate provides indirect evidence for PKC activation, and cannot distinguish the activities of PKC subtypes. Therefore, the PKC subtypes were immunoprecipitated with their specific antibodies respectively from whole cell lysates and the enzymatic activities within the immunoprecipitates were determined. Among the three PKC subtypes examined, only the activity of PKCε was increased by morphine (226 ± 31%, relative to basal levels). In contrast, the PKCε activity in DAMGO-treated cells was only 117 ± 14% of the basal activity. Neither morphine nor DAMGO increase the activity of PKCα or PKCγ (Fig. 5A).
Fig. 5. Morphine increases PKCε activity and translocates PKCε to the MOR signaling complex.
(A) Determination of agonist-induced PKC subtype activity in cell lysates. HEK293-MOR cells were pretreated for 5 min with 1 μM morphine and DAMGO. Activities of individual PKC subtypes were determined (*: p < 0.05; **: p < 0.01; n>3). (B) Determination of morphine-induced PKCε activity in cell lysates after overexpression of PKC subtypes RNAis. HEK293-MOR cells were transfected with PKCαRNAi, PKCγRNAi or PKCεRNAi for 48 h before 5 min 1μM morphine pretreatment. Activities of PKCε subtype were determined (*: p < 0.05; **: p < 0.01; n≥3) (C) Determination of morphine-induced PKCε activity in cell lysates after. HEK293-MOR cells were incubated for 3 h 50 μM PKCε subtype-specific inhibitor (PKCεi) before 5 min 1μM morphine pretreatment. Activities of PKCε subtype were determined (*: p < 0.05; **: p < 0.01; n≥3) (D) Morphine-induced translocation of PKCε to the MOR signaling complex. Cells were pretreated with 1 μM morphine or DAMGO for 5 mins. Homogenates were fractionated on continuous sucrose gradients. Gαi2 and Gq were used as lipid raft markers; transferrin receptor (TR) as a non-raft marker Lanes. Left to right: sucrose gradient fractions 1–12. (E) Morphine-induced PKCε association with the MOR signaling complex. Cells were pretreated with 1 μM morphine or DAMGO for 5 min. MOR signaling complexes were immunoprecipitated with HA antibody and immunoblotted with PKCε antibody; MOR was used as loading control.
The specific activation of PKCε was further demonstrated by using the RNA interference. Although the down-regulation of PKC subtypes was incomplete, the increase in PKCε activity was attenuated significantly (Fig. 5B). This result was not due to the decreased basal activity of PKCε, because PKCεRNAi did not suppress the basal activity of PKCε significantly (Fig. 5B). The increased PKCε activities after morphine pretreatment was inhibited by PKCεRNAi but not PKCαRNAi or PKCγRNAi overexpression further indicating the specificity of RNAis (Fig. 5B). Overexpression of PKCεRNAi did not completely block PKCε activation. Hence PKC subtype-specific inhibitors were used. PKCε inhibitor decreased the basal activity of PKCε by 22 ± 8% (Fig. 5C), and prevent the morphine-induced PKCε activation (Fig. 5C).
Activated PKCs translocate to the plasma membrane [13] and phosphorylate substrates within the signaling complex. We therefore determined if PKCε translocated to the receptor signaling complex during agonist pretreatment. Lipid rafts in which MOR signaling complexes are located were separated from other membrane domains using a continuous sucrose gradient [30]. In control and DAMGO-treated cells, PKCε localized in the last two high-density fractions of the gradient; these fractions correspond to the cytosolic fraction of HEK293 cells. In contrast, morphine treatment induced translocation of PKCε from the last two fractions to the lipid raft fractions (i.e., fractions 3, 4), where MOR and Gαi2 are located (Fig. 5D). In addition, the amount of PKCε associated with the MOR signaling complex as detected by immunoprecipitating of MOR using anti-HA antibodies increased after morphine, but not DAMGO, treatment (Fig. 5D). Therefore, these results indicate that morphine induces the activation and translocation of PKCε into the MOR signaling complex.
3.4. Gαi2 is an essential component of MOR signal transduction
Morphine induces receptor phosphorylation more slowly and to a much lesser extent than does DAMGO [7]. Furthermore, morphine can induce MOR desensitization even after all the putative phosphorylation residues (i.e., Ser/Thr) on the MOR carboxyl tail are mutated [5]. These data suggest that MOR is not the target of PKCε within the signaling complex. Indeed, no PKC-mediated phosphorylation of the receptor was detected in MOR immunoprecipitates probed on immunoblots with antibody against phosphorylated PKC substrates (data not shown).
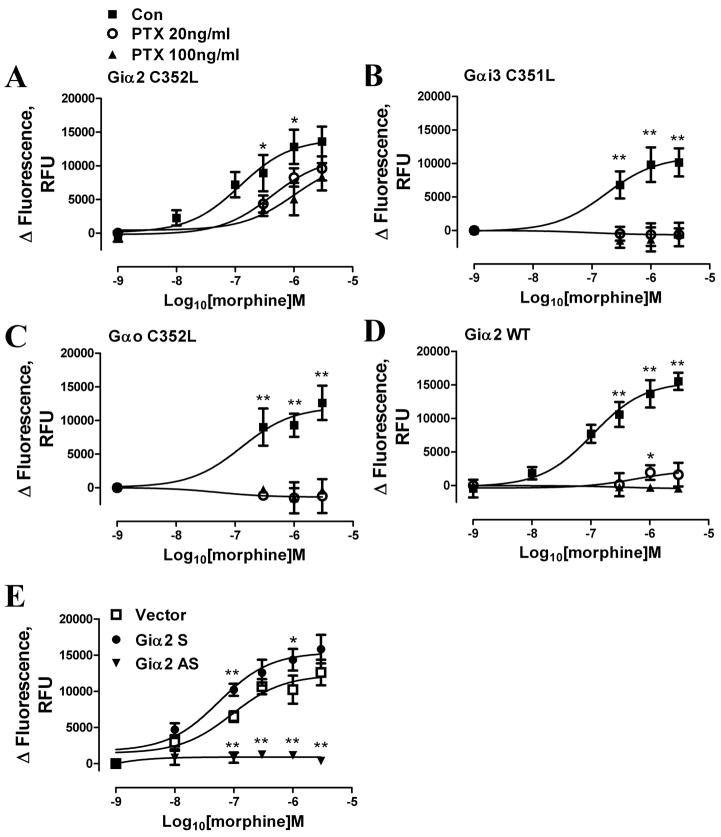
However, another critical molecule within the MOR signaling complex, Gα, has been proposed as the target of PKC activation and GPCR desensitization [31–33]. To test this hypothesis, the subtype of Gα used by MOR to invoke [Ca2+]i release was identified. PTX-resistant mutants of Gαi2 (Gαi2C352L), Gαi3 (Gαi3C351L) or Gαo (GαoC352L) [23] were overexpressed and verified by immunoblots, and [Ca2+]i release was monitored after PTX pretreatment. Only the Gαi2 mutant restored the morphine-mediated [Ca2+]i release in the PTX-treated cells (Fig. 6A–C). The inability of overexpressed wild type Gαi2 to restore morphine-mediated [Ca2+]i release after PTX pretreatment (Fig. 6D) excluded the possibility that the restoration of MOR activity was the result of relative insensitivity of Gαi2 toward PTX treatment and comparatively higher level of Gαi2 within the HEK293 cells. Moreover, decreases in the responsiveness in the morphine-induced [Ca2+]i release when the Gαi2 level was attenuated with Gαi2 antisense construct further confirmed the involvement of Gαi2 in this MOR signaling process(Fig. 6E).
Fig. 6. Gαi2 is essential for MOR signaling transduction.
(A–D) PTX-resistant Gαi2 mutants Gαi2C352L (A), Gαi3C351L (B), GαoC352L (C) or wild type Gαi2 (Gαi2WT; D) were overexpressed in HEK293-MOR cells for 48 h. Cells were pretreated with HBSS (Con) (■), PTX 20ng/ml (○) or PTX 100ng/ml (▲) for 16 h before morphine-induced [Ca2+]i release dose-response assays. (E) Vector (□), Gαi2 sense (S) (●) or antisense (AS) (▼;) constructs were overexpressed for 48 h before morphine-induced [Ca2+]i release dose-response assays. Different concentrations of morphine were added with 200nM ADP. Total [Ca2+]i release after pretreatment was quantified by calculating the area under curves with a Prism program. Response to ADP was subtracted from total response to obtain the response to morphine (*: p < 0.05; **: p < 0.01; n=3).
3.5. Gαi2 is the PKC substrate mediating morphine-induced desensitization
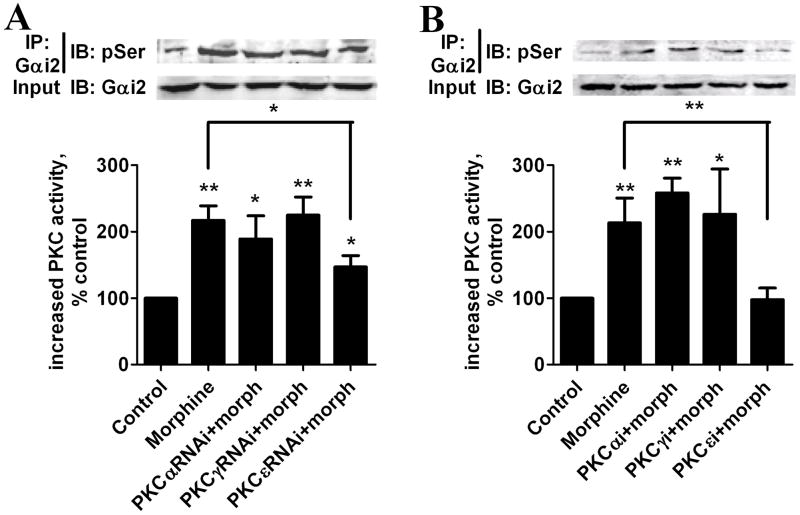
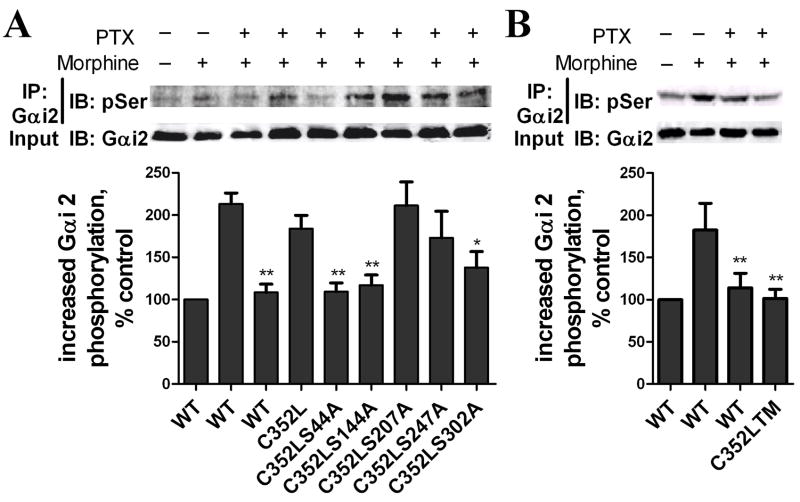
Increase of Gαi2 phosphorylation was determined after Gαi2 was specifically immunoprecipitated with Gαi2 antibody (Suppl. Fig. 2B). Phosphorylation of Gαi2 more than doubled after a 5-min pretreatment with 1 μM morphine (Fig. 7A and B). The increase in Gαi2 phosphorylation was attenuated by PKCεRNAi but not by PKCαRNAi or PKCγRNAi, indicating that the Gαi2 phosphorylation was PKCε-dependent (Fig. 7A). Moreover, complete eliminating of morphine-increased Gαi2 phosphorylation by PKCε specific inhibitor further demonstrated PKCε is absolutely required in morphine-increased Gαi2 phosphorylation (Fig. 7B). The Gαi2 sequence contains five Ser residues predicted to be putative PKCε phosphorylation sites [34, 35]. These sites were then mutated individually and in combination on the Gαi2C352L mutant. HEK293-MOR cells were transfected with these mutants and pretreated with PTX in order to eliminate the contribution of endogenous Gαi2 on MOR activity. Relative to the amount of PKC-mediated phosphorylation of Gαi2C352L, decreases in Gαi2 phosphorylation after PTX and morphine pretreatment were seen in the Gαi2S44A, Gαi2S144A and Gαi2S302A mutants, but not in the other two mutants (Fig. 8A). When these three residues were mutated in combination (Gαi2C352LTM), the increase in Gαi2 phosphorylation after PTX and morphine pretreatment was blocked completely (Fig. 8B).
Fig. 7. Gαi2 is phosphorylated by PKCε after morphine treatment.
HEK293-MOR cells were transfected with PKCαRNAi, PKCγRANi or PKCεRNAi for 48 h (A) or preincubated with specific inhibitors of PKC subtypes α (PKCαi), γ (PKCγi)or ε (PKCεi)for 3 h (B) before assays. Cells were pretreated with 1 μM morphine. Gαi2 was immunoprecipitated, and Gαi2 phosphorylation was determined using pSer antibody. Gαi2 in whole cell lysates was used as a loading control. (top): immunoblots; (bottom): quantitative analysis of immunoblots (#: no significant difference; *: p < 0.05; **: p < 0.01; n=3).
Fig. 8. Identifying morphine-induced Gαi2 phosphorylation sites.
(A) HEK293-MOR cells were transfected for 48 h with wild type Gαi2 plasmid (WT) PTX-resistant (C352L) Gαi2 plasmid or C352L constructs mutated at serine residues 44, 144, 207, 247 or 302 to alanine. Cells were pretreated with 100 ng/ml PTX for 16 h and with 1 μM morphine for 5 min before determination of Gαi2 phosphorylation as described in legend of Fig. 7. (B) Cells transfected with wild type Gαi2 plasmid (WT) or C352L mutated at serine residues 44, 144, and 302 (triple mutation; C352LTM) Phosphorylation of the Gαi2 was determined as described previously (*: p < 0.05; **: p < 0.01; n=3).
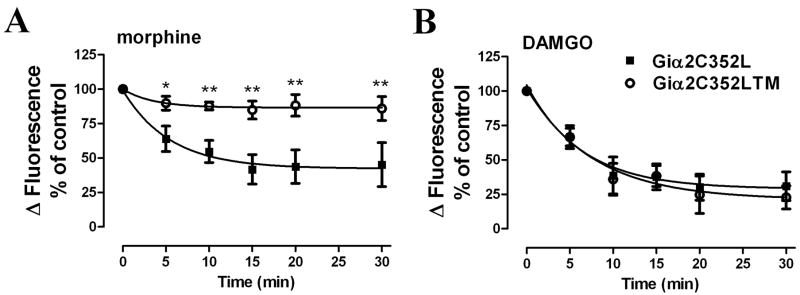
HEK293-MOR cells were transfected with either Gαi2C352L or the Gαi2C352LTM mutant. Then these cells were pretreated with PTX to eliminate endogenous Gαi2 activities. The ability of morphine to induce MOR desensitization in these cells was measured. As shown in Fig 9A, morphine induced receptor desensitization in HEK293-MOR cells overexpressing the Gαi2C352L after PTX pretreatment. However, in cells expressing the Gαi2C352LTM, morphine-induced MOR desensitization was attenuated significantly (Fig. 9A). In contrast, overexpression of the triple mutant did not affect DAMGO-induced MOR desensitization observed with the Gαi2C352L overexpression after PTX pretreatment (Fig. 9B). These data indicate that PKCε-induced phosphorylation of Gαi2 could be the basis for observed morphine-induced receptor desensitization.
Fig. 9. Morphine-induced MOR desensitization is mediated by PKC-induced Gαi2 phosphorylation.
MOR desensitization was determined by pretreating cells with (A) 100 nM morphine or (B) 100 nM DAMGO for various time as indicated in the X-axis. HEK293-MOR cells were transfected with Gαi2C352L (■) or Gαi2C352LTM (○) for 48 h and pretreated with 100 ng/ml PTX for 16 h before the desensitization assays (*: p < 0.05; **: p < 0.01; n=3).
3.6. Morphine pretreatment heterologously desensitizes CB1 receptor
PKC regulates various cellular responses [13], and GPCR desensitization mediated by PKC usually results in heterologous desensitization [4]. After morphine pretreatment, P2Y receptor activity was not altered (Fig. 2F). However, P2Y receptor mediates its function via Gq. Whether morphine-induced MOR desensitization is homologous when comparing the activities of other Gi/o receptors is unknown. Because MOR-activated PKCε activity concentrated within the MOR signaling complex (Fig. 5C and D), it is reasonable to hypothesize that morphine treatment might alter the activities of other GPCRs with cellular distributions similar to that of MOR. Previous studies reported possible interactions in vivo and in vitro between MOR and the cannabinoid CB1 receptor [19, 20], and like MOR, CB1 is also a Gi/Go-coupled receptor. As with morphine, treatment with the CB1 agonist WIN55,212-2 (Win-2) alone did not elicit [Ca2+]i release in HEK293-MOR cells transiently expressing the CB1 receptor, but Win-2 did potentiate P2Y receptor-induced [Ca2+]i release (Suppl. Fig. 3). Although possible allosteric effects between MOR and CB1 receptor in vitro have been suggested [36, 37], neither synergistic nor inhibitory effects were observed when morphine and Win-2 were added together (Suppl. Fig. 4). The absence of an additive response suggests that these receptors might share a common signaling pathway.
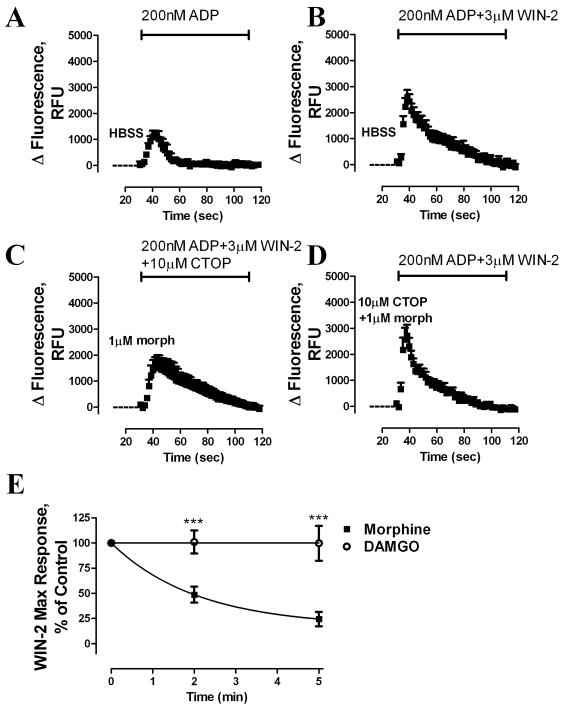
Whether morphine pretreatment could modulate subsequent CB1 response was examined. After a 5-min pretreatment with 1 μM morphine, Win-2 was added with ADP and the CB1 response was monitored; and 10 μM of the MOR-selective antagonist CTOP was added at the same time as the Win-2 and ADP to block MOR activity (Fig. 10C). Morphine pretreatment significantly reduced Win-2 potentiation of ADP-mediated [Ca2+]i release (Fig. 10B, C). This attenuation of CB1 activity was prevented by inclusion of 10 μM CTOP during the morphine pretreatment period (Fig. 10D), indicating that CB1 desensitization resulted heterologously from MOR activation. In contrast, DAMGO pretreatment did not alter the CB1 response (Fig. 10E).
Fig. 10. Morphine, but not DAMGO, induces heterologous desensitization of CB1 receptor.
Time-dependent changes in intracellular fluorescence (RFU) were used to assess [Ca2+]i release. HEK293-MOR cells transiently expressing the CB1 receptor were pretreated with (A–B) HBSS, (C) 1 μM morphine, or (D) 1 μM morphine + 10 μM CTOP for 5 min, followed addition of (A) 200 nM ADP, (B, D) 200 nM ADP + 3 μM Win-2, or (C) 200 nM ADP + 3 μM Win-2 + 10 μM CTOP. (E) CB1 desensitization after pretreatment with 1 μM morphine or DAMGO was measured at the time indicated in the X-axis (***: p < 0.001; n=3).
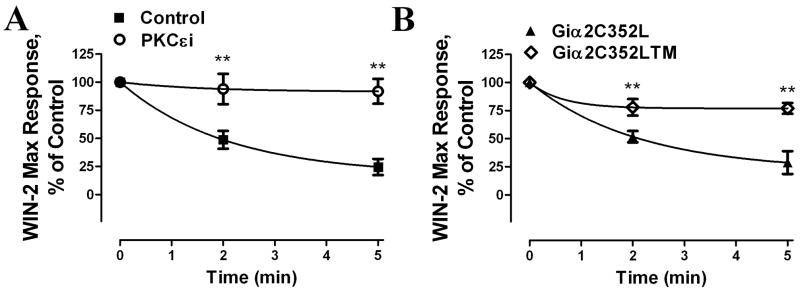
HEK293-MOR cells transiently transfected with CB1 were then preincubated with PKCε inhibitor; the ability of morphine pretreatment to blunt the Win-2 response was blocked by this inhibitor (Fig. 11A). Furthermore, overexpression of the Gαi2 triple mutant Gαi2C352LTM reversed the CB1 desensitization induced by morphine pretreatment while the overexpression of the Gαi2C352L did not (Fig. 11B). Taken together, these data suggest that phosphorylation of Gαi2 by morphine-activated PKCε heterologously desensitized CB1 receptor in HEK293 cells.
Fig. 11. Morphine-induced CB1 heterologous desensitization is mediated by PKCε-induced Gαi2 phosphorylation.
(A) Morphine-induced heterologous CB1 desensitization is PKCε dependent. Heterologous desensitization assays were performed as described in Fig. 10 After morphine pretreatment, the Win-2 induced maximum response was compared to the control. Cells were pretreated with PKCε inhibitor (PKCεi) (○) or DMSO (Control) (■) for 3 h before assays. (B) Morphine-induced heterologous CB1 desensitization is Gαi2-phosphorylation-dependent. HEK293-MOR cells transiently expressing the CB1 receptor overexpressing Gαi2C352L or Gαi2C352LTM was pretreated with 100 ng/ml PTX for 16 h before the desensitzation assays (**: p < 0.01; n=3).
4. Discussion
Morphine induces a higher degree of tolerance than other opioids such as fentanyl or etorphine administered at equivalent doses [38]. One hypothesis suggests that morphine inefficiently induce MOR phosphorylation and subsequent recruitment of βArrs, leading to prolonged MOR signaling and an profound adaptive cellular response that eventually results in tolerance development [39]. Another hypothesis, also based on the inability of morphine to recruit βArrs and induce MOR internalization, suggested that morphine-activated MOR cannot be resensitized by the receptor recycling process [40]. The morphine–MOR complexes stay on the cell membrane and remain inactive, leading to tolerance development in vivo. Both hypotheses are based on the low efficacy with which morphine recruits βArrs that play a major role in desensitization.
However, other reports indicate that the pathways leading to tolerance development are agonist-dependent, most notably the blunting of fentanyl-induced, but not morphine-induced, tolerance development in GRK3−/− mice [41]. The present study, as well as previous studies, indicates that MOR desensitization is also agonist-dependent: Morphine-induced receptor desensitization was PKC-dependent, whereas DAMGO-induced desensitization was not. This is reflected in the ability of morphine to activate PKCε to a much greater extend than DAMGO could (Fig. 3A). The contribution of PKC to morphine tolerance has been reported extensively [8–11, 42–44], and specific PKC subtypes (α, γ, and ε) have been proposed to play a role in morphine function in vivo [14, 15, 45–48]. Due to partially down-regulating endogenous PKC subtypes, miRNAs are not able to completely prevent morphine-induced MOR desensitization. However, the specificity of miRNA in down-regulating its target protein excludes the possibility of non-specific inhibition by the PKC subtypes peptide inhibitors. It is worth noting that in present studies, general PKC inhibitor Ro-31-8425 did not fully rescue morphine-induced desensitization while subtype-specific peptide PKCε inhibitor did. The different efficacies between the chemical and subtype-specific peptide PKC inhibitors were also observed in different assays [49]. It might due to the selectivity of PKC inhibitors or result from compensatory response by generally inhibiting PKCs. Verifying the subtypes of PKC involved with the miRNA constructs need to be accomplished if PKC inhibitors are to be used in blunting morphine tolerance in vivo. In present study, PKCε is confirmed to be specifically activated by morphine and is responsible for morphine-induced MOR desensitization. One could argue that morphine–induced activation of PKCε is limited to HEK293 cell model used in current studies. However, in the primary cultures of hippocampal neurons where MOR is expressed endogenously, morphine was observed to activate PKCε, but not PKCα and PKCγ, in these cultures (data not shown). Further, our observations on the role of PKCε on morphine-induced receptor desensitization parallel those reported for PKCε−/− mice [15]. Probably, some of chronic morphine in vivo actions could be mediated by the PKCε phosphorylation of cellular proteins.
Nevertheless, it is possible that different PKC subtypes might be involved in acute or chronic morphine actions in neurons other than those from hippocampus. Indeed, in neurons from the rat locus coeruleus, morphine-activated receptor is desensitized by muscarinic-activated PKCα; although whether morphine activates the PKCα in these neurons remains to be demonstrated [50]. In vivo studies also have indicated the involvement of PKCα and PKCγ in morphine analgesia and tolerance [14, 15, 45–48]. These discrepancies in the role of PKC subtypes in morphine action could be the result of differential expression of PKC subtypes in neurons expressing MOR. Also, PKCα and PKCγ both belong to the conventional PKC subfamily and are activated by Ca2+. In contrast, PKCε is a member of a novel PKC subfamily that is not sensitive to Ca2+ [13]. The differential requirement of Ca2+ for activation might also account for the different PKC subtype activities in different tissues. Mice knocked-out for the δ-opioid receptor (DOR) or the endogenous DOR agonist preproenkephalin fail to develop morphine tolerance, suggesting a possible role for DOR in MOR tolerance development [51, 52]. Although morphine activation of MOR cannot induce [Ca2+]i release, DOR induced [Ca2+]i release in various cell types, including neurons [53–55]. The distribution of MOR and DOR in the central nervous system [56] could explain the involvement of different PKC subtypes in the development of morphine tolerance in vivo.
Morphine-, but not DAMGO-induced MOR desensitization via PKC pathway has been reported in both HEK293 cells and locus coeruleus neurons [6, 57]. However, those studies failed to show activation of PKC by morphine; and purposed morphine-activated MOR is more vulnerable for PKC-mediated phosphorylation. Whether this hypothesis is correct or different mechanisms for morphine- and DAMGO-induced desensitization due to agonist-dependent signaling or the preference for PKC signaling pathway needs to be addressed further in the future.
The exact PKC targets involved in morphine-induced receptor desensitization and tolerance development requires further investigation, although the opioid receptor has been suggested as a possible target [58]. However, mutation of morphine-induced phosphorylation sites on MOR were unable to attenuate morphine-induced MOR desensitization [5]. Also, antibodies specific for PKC substrates failed to recognize MOR in the present study (data not shown). Clearly, these results do not support the hypothesis that MOR is a PKC target during chronic morphine treatment. The observed reduction in morphine-induced, but not DAMGO-induced, receptor phosphorylation by PKC inhibitors [58] could be attributed to PKC-mediated phosphorylation and activation of other protein kinases such as GRK or Src [59, 60] that are known to phosphorylate MOR. Whether morphine-induced MOR phosphorylation participates in either acute or chronic receptor signal transduction remains to be addressed.
Other molecules, such as Gβ, are phosphorylated by PKC and might be related to MOR signaling [61]. A role for Gαi in regulating GPCR desensitization has also been suggested previously [31, 32], and PKC-mediated Gαi phosphorylation inhibits Gαi activity [31]. Furthermore, phosphorylation of the Gα subunit inhibits its ability to reassociate with Gβγ, thereby impeded G-protein signaling [62]. The current study demonstrates that Gαi2 is a target for morphine-activated PKCε importance of PKC-induced phosphorylation of Gαi2 is demonstrated further by the attenuated desensitization observed when the putative PKC sites on the Gαi2 are mutated.
Inactivation of Gαi2 by PKC-mediated phosphorylation could reduce the activities of other GPCRs if they share a pool of Gα-subunits with MOR. Again, the current study indicates that morphine pretreatment attenuates the ability of CB1 to induce [Ca2+]i release. Functional cross-tolerance between MOR and CB1 in vivo has been reported [19, 63], and in vitro studies indicated that MOR and CB1 directly antagonize each other’s activity [36, 37, 64], which has been suggested to result from the heterodimerization of the two receptors [36]. The current study provides an alternative explanation for the crosstalk between MOR and CB1, i.e., PKC-mediated Gαi2 phosphorylation. Our observations are in accord with a recent study in which only activated MOR and CB1 antagonized each other’s activity [37]. In mice with decreased Gz protein, cross-tolerance between these receptors was attenuated [65]. The Gz-associated protein HINT1/RGSZ translocates PKCγ to MOR in mouse neurons [66]; it is reasonable to suggest that decreasing Gz levels also reduce the amount of translocated PKCγ. These observations support the possible involvement of PKC in cross-desensitization and cross-tolerance between MOR and CB1. That is, sharing the same pool of G-proteins is necessary and sufficient for morphine-activated PKC to cross-desensitize other GPCRs, and might indeed be the cellular mechanism for some of the interactions observed in vivo between MOR and other GPCRs.
The sharing of the same pool of Gαi2 between MOR and other GPCRs is critical for heterologous desensitization via PKC phosphorylation of Gα subunits. Colocalization within the same microdomain is a prerequisite. This is best illustrated by the inability of morphine-activated MOR to cross-desensitize α2-adrenergic receptor [67], which unlike MOR is located outside of the lipid rafts [30, 68]. In contrast, CB1 locates within the lipid rafts [69], and can be heterologously desensitized by morphine-mediated activation of MOR.
5. Conclusion
Present studies demonstrate agonist-dependent activation of PKCε by MOR; and such agonist-dependent MOR signaling leads to the different mechanisms of MOR desensitization. Instead of the classical βArr- and GRK-dependent pathways, morphine induces MOR desensitization via a PKCε-dependent mechanism. In addition, by using phosphorylation sites triple mutant Gαi2C352LTM, it is the first time to directly establish the involvement of Gαi proteins’ phosphorylation in MOR desensitization. Moreover, based on the mechanism used to desensitize MOR, agonists differ in their ability on affecting the other receptors cellular signaling transduction pathways. Because there is a clear distinction among MOR agonists as to whether the βArrs/GRK or PKC pathway is used, future studies on the functional interactions between MOR and other GPCRs will need to consider which signaling and regulatory pathways are involved. The microdomains in which the GPCRs are located relative to MOR also must be taken into account. Such functional interactions between GPCRs will have to be considered in drug design; specifically, whether homologous or heterologous desensitization among receptors occurs based on the GPCRs and subsequent pathways to be activated.
Supplementary Material
HEK293-MOR cells were transfected with PKCαRNAi, PKCγRNAi or PKCεRNAi for 48 h. PKCα, γ or ε expression in whole cell lysates were determined by IB with PKC subtypes antibodies. (*: p < 0.05; **: p < 0.01; n=3).
(A) MOR signaling complex was co-immunoprecipitated with or without HA antibody. PKC activity within immunoprecipitates was determined by PKCsub antibody; and MOR within immunoprecipitates was determined by MOR antibody. (B) Gαi2 was immunoprecipitated with or without Gαi2 antibody. Phosphorylation of Gαi2 was determined using pSer antibody; and Gαi2 within immunoprecipitates was determined by Gαi2 antibody.
Time-dependent changes in intracellular fluorescence (RFU) were used to assess [Ca2+]i release. HEK293-MOR cells transiently transfected with CB1 receptor were treated with (A) 3 μM Win-2, (B) 200 nM ADP, or (C) 200 nM ADP + 3 μM Win-2.
HEK293-MOR cells transiently transfected with CB1 receptor were treated with agonists individually or in combination as indicated. ADP: 200 nM ADP; morph: 1 μM morphine; DA: 1 μM DAMGO; Win-2: 3 μM WIN55,212-2; CTOP: 10 μM CTOP.
Acknowledgments
This research was supported in part by National Institutes of Health Grants DA007339, DA016674, DA000564, and DA011806. PYL and HHL are recipients of K05-DA00513 and K05-DA70544, respectively.
Abbreviations
- MOR
μ-opioid receptor
- GPCR
G-protein-coupled-receptor
- GRKs
G-protein coupled receptor kinases
- βArrs
β-arrestins
- [Ca2+]i
intracellular Ca2+
- DAMGO
[D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin
- HEK
human embryonic kidney
- HA
hemagglutinin
- PTX
pertussis toxin
- PMA
phorbol 12-myristate 13-acetate
- Win-2
WIN55,212-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borgland SL. Clin Exp Pharmacol Physiol. 2001;28(3):147–154. doi: 10.1046/j.1440-1681.2001.03418.x. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ. J Biol Chem. 1998;273(30):18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 3.Violin JD, Lefkowitz RJ. Trends Pharmacol Sci. 2007;28(8):416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 5.Chu J, Zheng H, Loh HH, Law PY. Cell Signal. 2008;20(9):1616–1624. doi: 10.1016/j.cellsig.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Mol Pharmacol. 2006;70(2):676–685. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- 7.Law PY, Wong YH, Loh HH. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 8.Bohn LM, Lefkowitz RJ, Caron MG. J Neurosci. 2002;22(23):10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith FL, Javed R, Elzey MJ, Welch SP, Selley D, Sim-Selley L, Dewey WL. Brain Res. 2002;958(1):28–35. doi: 10.1016/s0006-8993(02)03394-2. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Bilsky EJ, Wang D, Porreca F, Sadee W. Eur J Pharmacol. 1999;371(1):1–9. doi: 10.1016/s0014-2999(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Narita M, Makimura M, Hoskins B, Ho IK. Brain Res. 1994;667(1):133–137. doi: 10.1016/0006-8993(94)91724-8. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Loh HH, Law PY. Mol Pharmacol. 2008;73(1):178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton AC. Chem Rev. 2001;101(8):2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 14.Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. Pain. 2007;127(1–2):129–139. doi: 10.1016/j.pain.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ, Roberts AJ, Hodge CW, Messing RO. Genes Brain Behav. 2007;6(4):329–338. doi: 10.1111/j.1601-183X.2006.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, Zemaitaitis B, Muma NA. Mol Pharmacol. 2007;71(1):303–313. doi: 10.1124/mol.106.028241. [DOI] [PubMed] [Google Scholar]
- 17.Hosey MM. Am J Physiol. 1999;277(5 Pt 1):C856–858. doi: 10.1152/ajpcell.1999.277.5.C856. [DOI] [PubMed] [Google Scholar]
- 18.Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Ann N Y Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- 19.Gaveriaux-Ruff C, Kieffer BL. Neuropeptides. 2002;36(2–3):62–71. doi: 10.1054/npep.2002.0900. [DOI] [PubMed] [Google Scholar]
- 20.Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Neuroscience. 2004;127(1):101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Kerr B, Hill H, Coda B, Calogero M, Chapman CR, Hunt E, Buffington V, Mackie A. Neuropsychopharmacology. 1991;5(3):157–166. [PubMed] [Google Scholar]
- 22.Chieng BC, Lee DJ, Du YP, Osborne PB, Christie MJ, Massotte D. Neuroreport. 2008;19(18):1793–1796. doi: 10.1097/WNR.0b013e3283196b55. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Tetrault J, Wang W, Loh HH, Law PY. Mol Pharmacol. 2006;69(6):1810–1819. doi: 10.1124/mol.105.021352. [DOI] [PubMed] [Google Scholar]
- 24.Werry TD, Wilkinson GF, Willars GB. Biochem J. 2003;374(Pt 2):281–296. doi: 10.1042/BJ20030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polakiewicz RD, Schieferl SM, Dorner LF, Kansra V, Comb MJ. J Biol Chem. 1998;273(20):12402–12406. doi: 10.1074/jbc.273.20.12402. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Zhao H, Qiu Y, Loh HH, Law PY. J Biol Chem. 2009;284(4):1990–2000. doi: 10.1074/jbc.M807971200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanno T, Yamamoto H, Yaguchi T, Hi R, Mukasa T, Fujikawa H, Nagata T, Yamamoto S, Tanaka A, Nishizaki T. J Lipid Res. 2006;47(6):1146–1156. doi: 10.1194/jlr.M500329-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.House C, Kemp BE. Science. 1987;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 29.Mochly-Rosen D, Gordon AS. Faseb J. 1998;12(1):35–42. [PubMed] [Google Scholar]
- 30.Zheng H, Chu J, Qiu Y, Loh HH, Law PY. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katada T, Gilman AG, Watanabe Y, Bauer S, Jakobs KH. Eur J Biochem. 1985;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- 32.Bushfield M, Murphy GJ, Lavan BE, Parker PJ, Hruby VJ, Milligan G, Houslay MD. Biochem J. 1990;268(2):449–457. doi: 10.1042/bj2680449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murthy KS, Grider JR, Makhlouf GM. Am J Physiol Cell Physiol. 2000;279(4):C925–934. doi: 10.1152/ajpcell.2000.279.4.C925. [DOI] [PubMed] [Google Scholar]
- 34.Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X. Mol Cell Proteomics. 2008;7(9):1598–1608. doi: 10.1074/mcp.M700574-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blom N, Gammeltoft S, Brunak S. J Mol Biol. 1999;294(5):1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 36.Rios C, Gomes I, Devi LA. Br J Pharmacol. 2006;148(4):387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canals M, Milligan G. J Biol Chem. 2008;283(17):11424–11434. doi: 10.1074/jbc.M710300200. [DOI] [PubMed] [Google Scholar]
- 38.Duttaroy A, Yoburn BC. Anesthesiology. 1995;82(5):1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Whistler JL, von Zastrow M. Proc Natl Acad Sci U S A. 1998;95(17):9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finn AK, Whistler JL. Neuron. 2001;32(5):829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 41.Terman GW, Jin W, Cheong YP, Lowe J, Caron MG, Lefkowitz RJ, Chavkin C. Br J Pharmacol. 2004;141(1):55–64. doi: 10.1038/sj.bjp.0705595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabra BH, Bailey CP, Kelly E, Smith FL, Henderson G, Dewey WL. Brain Res. 2008;1217:70–77. doi: 10.1016/j.brainres.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olianas MC, Onali P. Br J Pharmacol. 1999;126(3):657–664. doi: 10.1038/sj.bjp.0702349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith FL, Javed RR, Elzey MJ, Dewey WL. Brain Res. 2003;985(1):78–88. doi: 10.1016/s0006-8993(03)03170-6. [DOI] [PubMed] [Google Scholar]
- 45.Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. Pain. 2001;94(3):245–253. doi: 10.1016/S0304-3959(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 46.Hua XY, Moore A, Malkmus S, Murray SF, Dean N, Yaksh TL, Butler M. Neuroscience. 2002;113(1):99–107. doi: 10.1016/s0306-4522(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 47.Matsushita Y, Ishikawa M, Abe K, Utsunomiya I, Chikuma T, Hojo H, Hoshi K, Quock RM, Taguchi K. Neuroscience. 2007;148(2):541–547. doi: 10.1016/j.neuroscience.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Fanjun M, Junfa L, Bingxi Z, Fang J. J Neurosurg Anesthesiol. 2006;18(2):119–124. doi: 10.1097/00008506-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. J Biol Chem. 2003;278(15):12729–12736. doi: 10.1074/jbc.M300430200. [DOI] [PubMed] [Google Scholar]
- 50.Bailey CP, Llorente J, Gabra BH, Smith FL, Dewey WL, Kelly E, Henderson G. Eur J Neurosci. 2009;29(2):307–318. doi: 10.1111/j.1460-9568.2008.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Neuron. 1999;24(1):243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 52.Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. J Neurosci. 2002;22(24):10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorlin T, Eriksson PS, Persson PA, Aberg ND, Hansson E, Ronnback L. Neuropharmacology. 1998;37(3):299–311. doi: 10.1016/s0028-3908(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 54.Okajima F, Tomura H, Kondo Y. Biochem J. 1993;290(Pt 1):241–247. doi: 10.1042/bj2900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin W, Lee NM, Loh HH, Thayer SA. J Neurosci. 1994;14(4):1920–1929. doi: 10.1523/JNEUROSCI.14-04-01920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. J Comp Neurol. 1994;350(3):412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 57.Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, Henderson G. Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Yu Y, Mackin S, Weight FF, Uhl GR, Wang JB. J Biol Chem. 1996;271(19):11449–11454. doi: 10.1074/jbc.271.19.11449. [DOI] [PubMed] [Google Scholar]
- 59.Krasel C, Dammeier S, Winstel R, Brockmann J, Mischak H, Lohse MJ. J Biol Chem. 2001;276(3):1911–1915. doi: 10.1074/jbc.M008773200. [DOI] [PubMed] [Google Scholar]
- 60.Moyers JS, Bouton AH, Parsons SJ. Mol Cell Biol. 1993;13(4):2391–2400. doi: 10.1128/mcb.13.4.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakrabarti S, Regec A, Gintzler AR. Brain Res Mol Brain Res. 2005;138(1):94–103. doi: 10.1016/j.molbrainres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Kozasa T, Gilman AG. J Biol Chem. 1996;271(21):12562–12567. doi: 10.1074/jbc.271.21.12562. [DOI] [PubMed] [Google Scholar]
- 63.Vigano D, Rubino T, Parolaro D. Pharmacol Biochem Behav. 2005;81(2):360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 64.Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ. Neuropharmacology. 2006;51(4):773–781. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Garzon J, de la Torre-Madrid E, Rodriguez-Munoz M, Vicente-Sanchez A, Sanchez-Blazquez P. Mol Pain. 2009;5:11. doi: 10.1186/1744-8069-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez-Munoz M, de la Torre-Madrid E, Sanchez-Blazquez P, Wang JB, Garzon J. Cell Signal. 2008;20(10):1855–1864. doi: 10.1016/j.cellsig.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 67.Prather PL, Tsai AW, Law PY. J Pharmacol Exp Ther. 1994;270(1):177–184. [PubMed] [Google Scholar]
- 68.Chini B, Parenti M. J Mol Endocrinol. 2004;32(2):325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- 69.Rimmerman N, Hughes HV, Bradshaw HB, Pazos MX, Mackie K, Prieto AL, Walker JM. Br J Pharmacol. 2008;153(2):380–389. doi: 10.1038/sj.bjp.0707561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HEK293-MOR cells were transfected with PKCαRNAi, PKCγRNAi or PKCεRNAi for 48 h. PKCα, γ or ε expression in whole cell lysates were determined by IB with PKC subtypes antibodies. (*: p < 0.05; **: p < 0.01; n=3).
(A) MOR signaling complex was co-immunoprecipitated with or without HA antibody. PKC activity within immunoprecipitates was determined by PKCsub antibody; and MOR within immunoprecipitates was determined by MOR antibody. (B) Gαi2 was immunoprecipitated with or without Gαi2 antibody. Phosphorylation of Gαi2 was determined using pSer antibody; and Gαi2 within immunoprecipitates was determined by Gαi2 antibody.
Time-dependent changes in intracellular fluorescence (RFU) were used to assess [Ca2+]i release. HEK293-MOR cells transiently transfected with CB1 receptor were treated with (A) 3 μM Win-2, (B) 200 nM ADP, or (C) 200 nM ADP + 3 μM Win-2.
HEK293-MOR cells transiently transfected with CB1 receptor were treated with agonists individually or in combination as indicated. ADP: 200 nM ADP; morph: 1 μM morphine; DA: 1 μM DAMGO; Win-2: 3 μM WIN55,212-2; CTOP: 10 μM CTOP.