Abstract
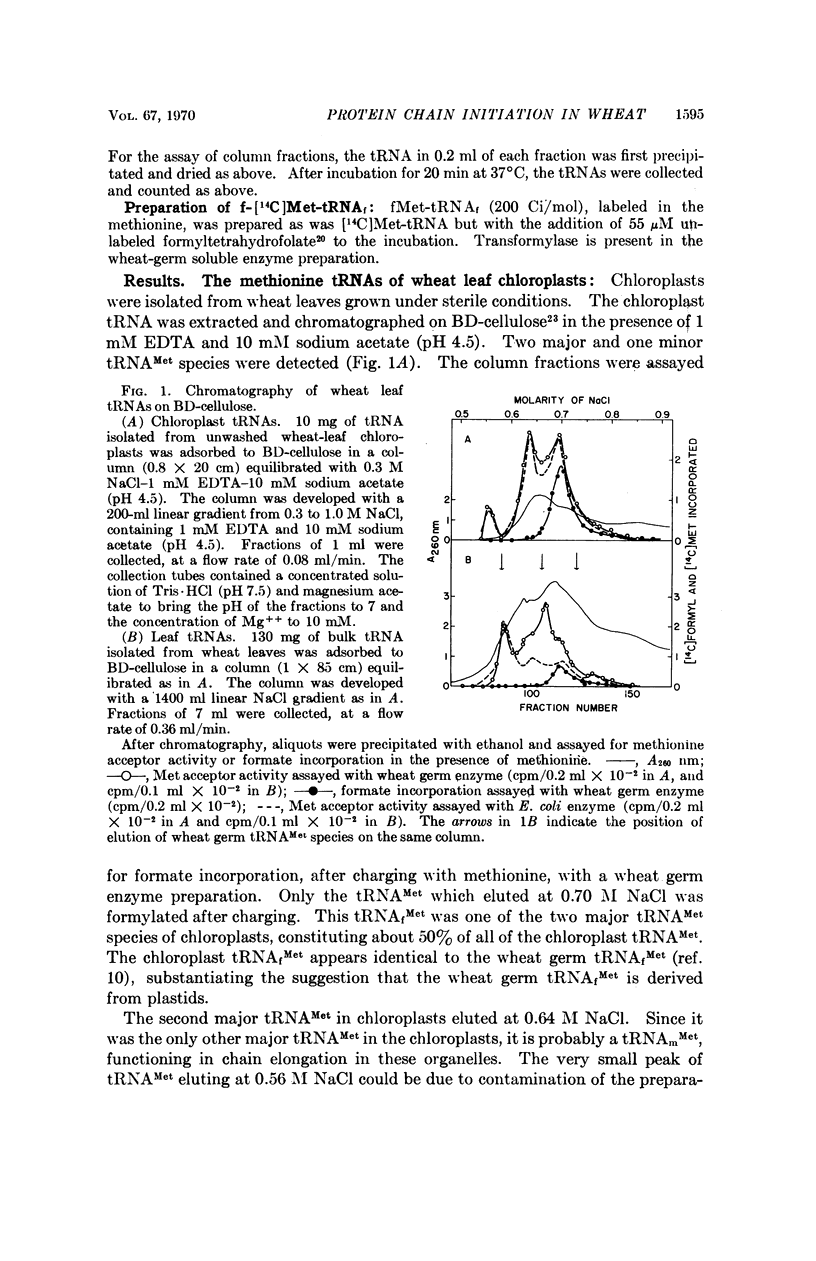
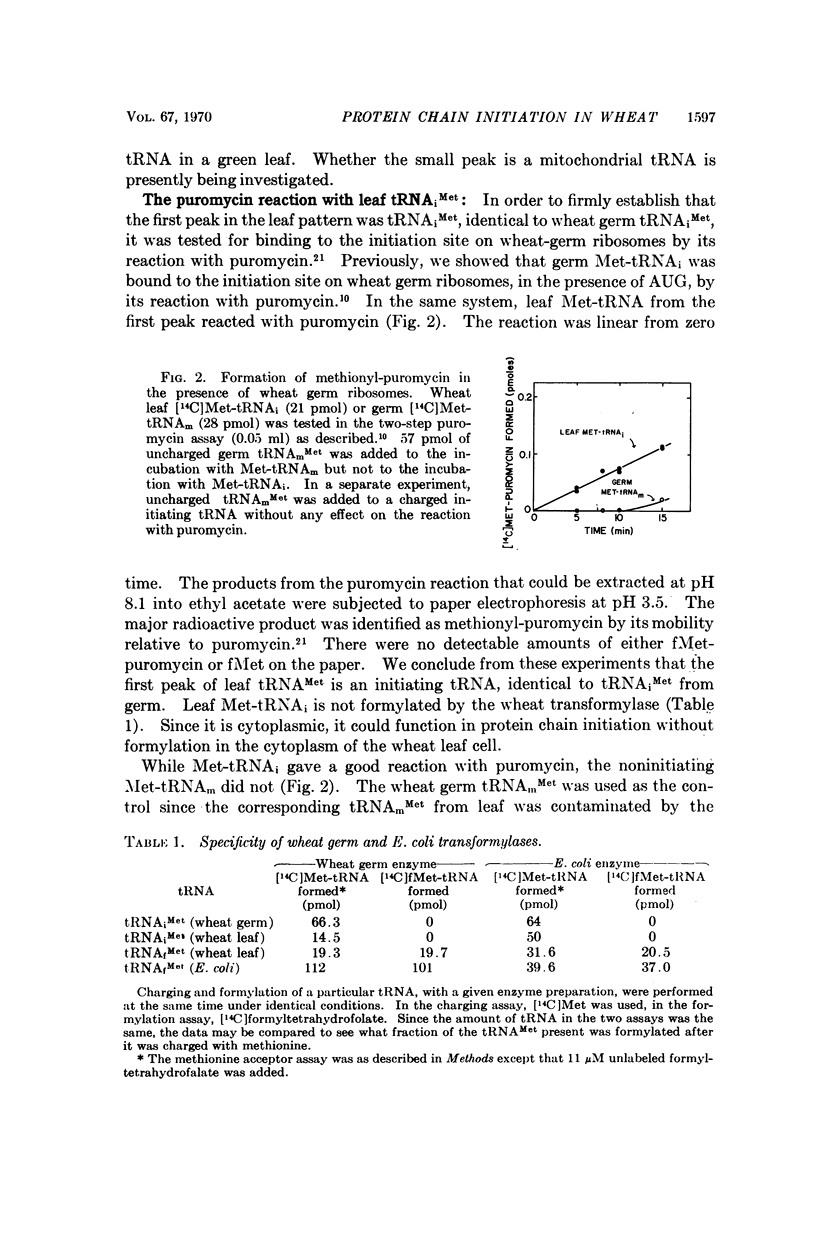
The role of methionine in protein chain initiation in wheat has been studied. Two chain-initiating methionine tRNAs have been found. One of these is located in the cytoplasm of the wheat cell. This methionyl-tRNA is not formylated by wheat extracts and appears to function in protein chain initiation in the cytoplasm without prior formylation. The other initiating tRNA is from chloroplasts. This methionyl-tRNA is formylated by a transformylase present in wheat extracts and functions in chain initiation in chloroplasts as formylmethionyl-tRNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaduri S., Chatterjee N. K., Bose K. K., Gupta N. K. Initiation of protein synthesis in rabbit reticulocytes. Biochem Biophys Res Commun. 1970 Jul 27;40(2):402–407. doi: 10.1016/0006-291x(70)91023-5. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Smith A. E. Initiator codons in eukaryotes. Nature. 1970 May 16;226(5246):610–612. doi: 10.1038/226610a0. [DOI] [PubMed] [Google Scholar]
- Burkard G., Eclancher B., Weil J. H. Presence of N-formyl-methionyl-transfer RNA in bean chloroplasts. FEBS Lett. 1969 Aug;4(4):285–287. doi: 10.1016/0014-5793(69)80257-7. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Redfield B., Weissbach H. Formylation of guinea pig liver methionyl-sRNA. Arch Biochem Biophys. 1967 Apr;120(1):119–123. doi: 10.1016/0003-9861(67)90605-4. [DOI] [PubMed] [Google Scholar]
- Galper J. B., Darnell J. E. The presence of N-formyl-methionyl-tRNA in HeLa cell mitochondria. Biochem Biophys Res Commun. 1969 Jan 27;34(2):205–214. doi: 10.1016/0006-291x(69)90633-0. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA. Biochem Biophys Res Commun. 1970 Jul 27;40(2):416–421. doi: 10.1016/0006-291x(70)91025-9. [DOI] [PubMed] [Google Scholar]
- Loehr J. S., Keller E. B. Dimers of alanine transfer RNA with acceptor activity. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1115–1122. doi: 10.1073/pnas.61.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCKER K., SANGER F. N-FORMYL-METHIONYL-S-RNA. J Mol Biol. 1964 Jun;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Pine M. J., Gordon B., Sarimo S. S. Protein initiation without folate in Streptococcus faecium. Biochim Biophys Acta. 1969 Apr 22;179(2):439–447. doi: 10.1016/0005-2787(69)90052-5. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Formyltetrahydrofolate synthetase. I. Isolation and crystallization of the enzyme. J Biol Chem. 1962 Sep;237:2898–2902. [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- Ranalletti M., Gnanam A., Jagendorf A. T. Amino acid incorporation by isolated chloroplasts. Biochim Biophys Acta. 1969 Jul 22;186(1):192–204. doi: 10.1016/0005-2787(69)90502-4. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Meyer R., Eisenstadt J. M., Brawerman G. Involvement of N-formylmethionine in initiation of protein synthesis in cell-free extracts of Euglena gracilis. J Mol Biol. 1967 May 14;25(3):571–574. doi: 10.1016/0022-2836(67)90210-0. [DOI] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Parker J. H., Putterman G. J., Agrawal B. B., Margoliash E. The relationship of gene structure and protein structure of iso-I-cytochrome c from yeast. Symp Soc Exp Biol. 1970;24:85–107. [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. N-formylmethionyl transfer RNA in mitochondria from yeast and rat liver. J Mol Biol. 1968 Dec 14;38(2):241–243. doi: 10.1016/0022-2836(68)90409-9. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Ukita T., Nishimura S. Characterization of two species of methionine transfer ribonucleic acid from bakers' yeast. J Biol Chem. 1968 Nov 10;243(21):5761–5768. [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D. In vitro protein synthesis: chain initiation. Proc Natl Acad Sci U S A. 1966 Jan;55(1):155–161. doi: 10.1073/pnas.55.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. M. Protein chain initiation in rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1282–1289. doi: 10.1073/pnas.66.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]


