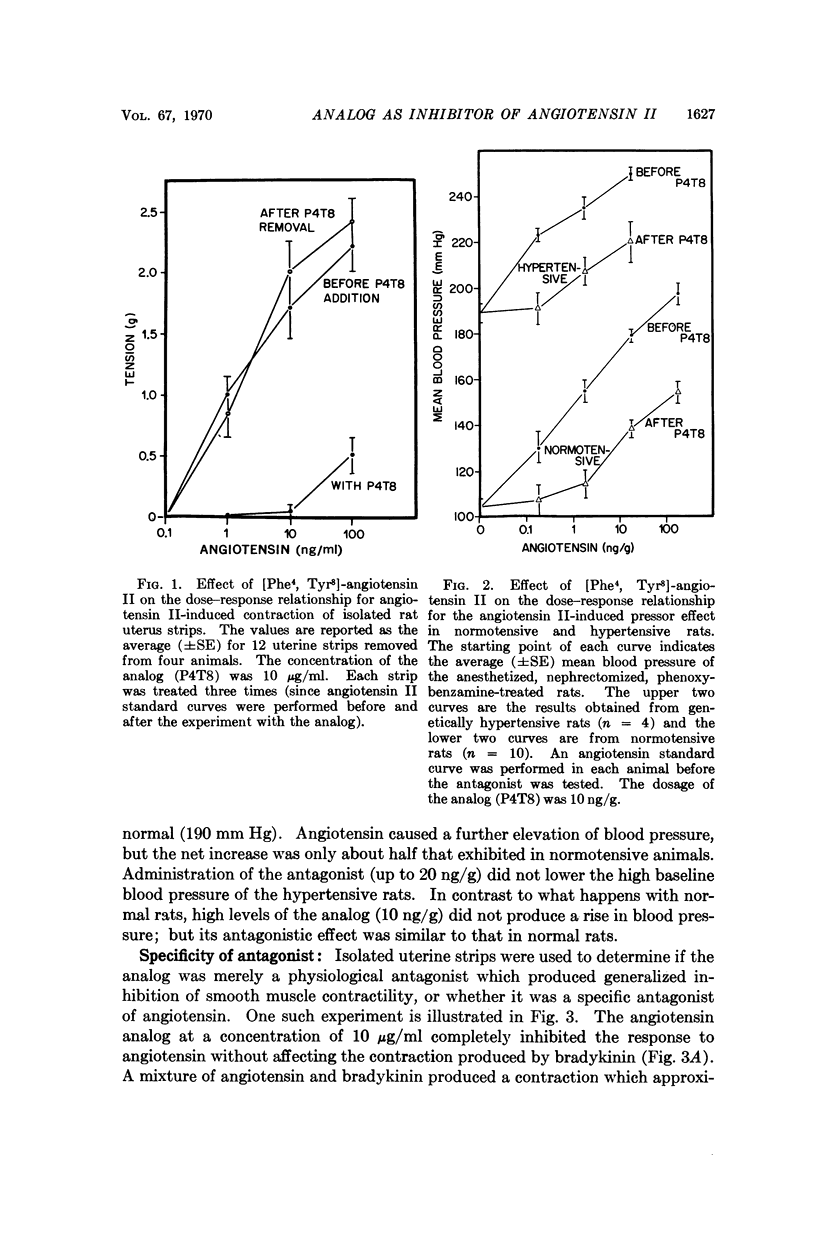
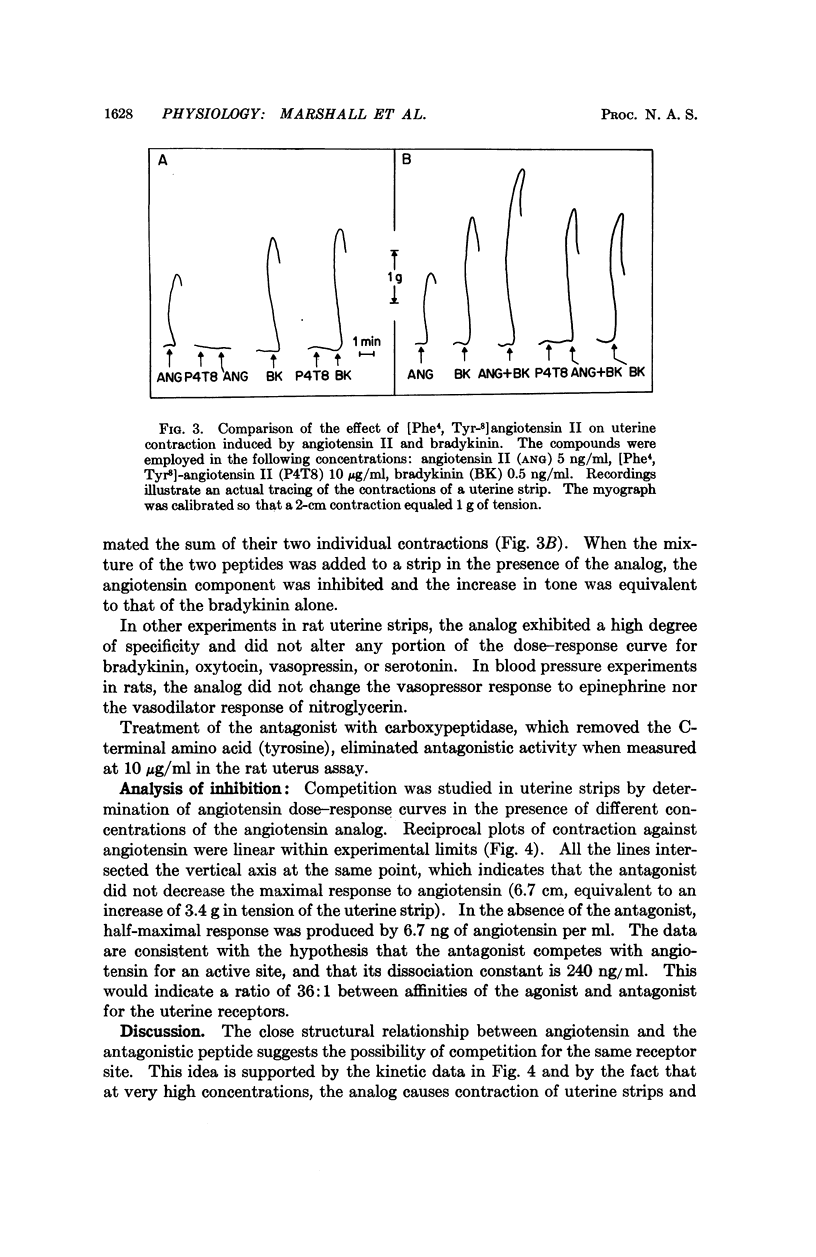
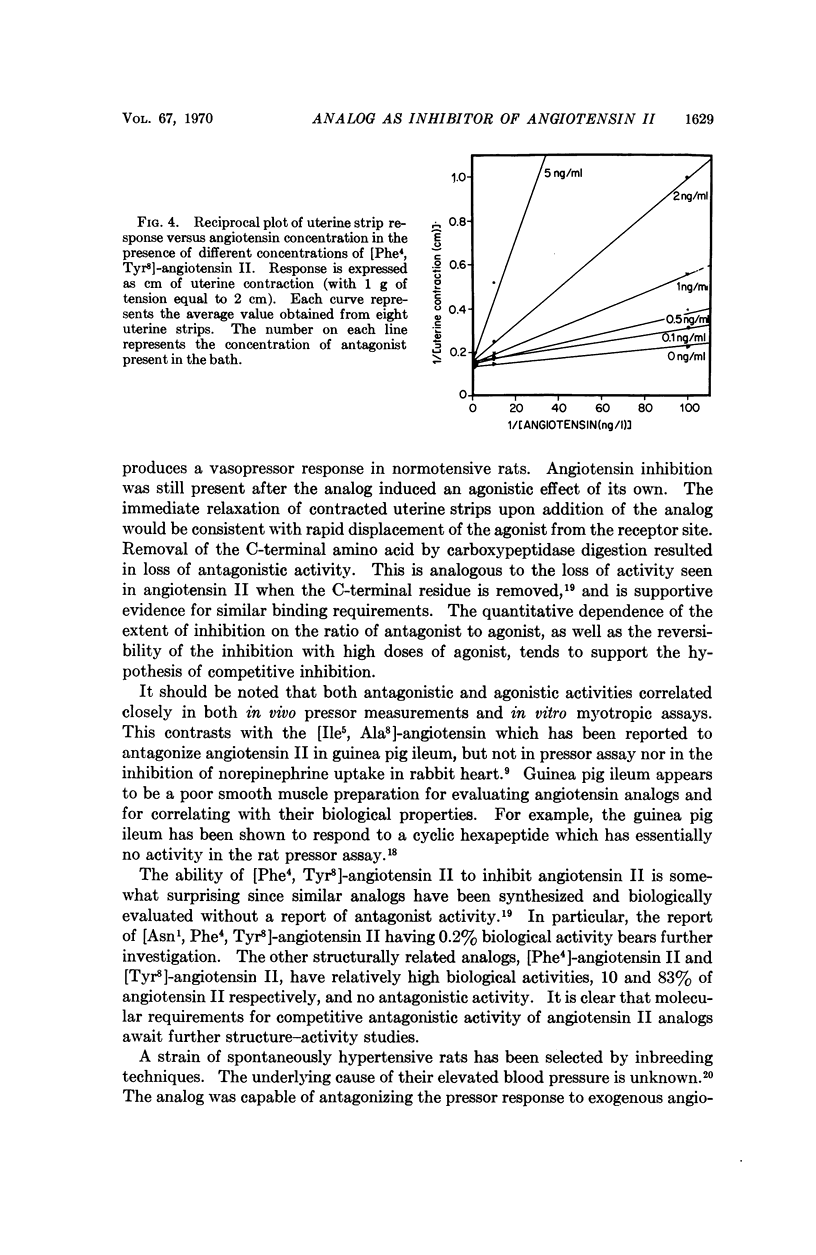
Abstract
A peptide analog, [4-phenylalanine, 8-tyrosine]-angiotensin II, was prepared by solid phase peptide synthesis and its structure confirmed. The compound was found to be a potent inhibitor of angiotensin II in vitro (isolated rat uterus strips) and in vivo (rat blood pressure). The analog was found to be highly specific and did not inhibit the action of a number of other peptides and spasmogenic compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clineschmidt B. V., Geller R. G., Govier W. C., Sjoerdsma A. Reactivity to norepinephrine and nature of the alpha adrenergic receptor in vascular smooth muscle of a genetically hypertensive rat. Eur J Pharmacol. 1970 Apr;10(1):45–50. doi: 10.1016/0014-2999(70)90155-x. [DOI] [PubMed] [Google Scholar]
- Dousa T., Hechter O., Walter R., Schwartz I. L. [8-Arginine]-vasopressinoic acid: an inhibitor of rabbit kidney adenyl cyclase. Science. 1970 Feb 20;167(3921):1134–1135. doi: 10.1126/science.167.3921.1134. [DOI] [PubMed] [Google Scholar]
- FLOURET G., DUVIGNEAUD V. THE SYNTHESIS OF D-OXYTOCIN, THE ENANTIOMER OF THE POSTERIOR PITUITARY HORMONE, OXYTOCIN. J Am Chem Soc. 1965 Aug 20;87:3775–3776. doi: 10.1021/ja01094a045. [DOI] [PubMed] [Google Scholar]
- Goodman J. W., Nitecki D. E. Immunochemical studies on the poly-gamma-D-glutamyl capsule of Bacillus anthracis. I. Characterization of the polypeptide and of the specificity of its reaction with rabbit antisera. Biochemistry. 1966 Feb;5(2):657–665. doi: 10.1021/bi00866a036. [DOI] [PubMed] [Google Scholar]
- Jorgensen E. C., Patton W. Angiotensin II analogs. II. Cyclo-(-Val-Tyr-Ile-His-Pro-Phe-). J Med Chem. 1969 Sep;12(5):935–936. doi: 10.1021/jm00305a061. [DOI] [PubMed] [Google Scholar]
- Khairallah P. A., Toth A., Bumpus F. M. Analogs of angiotensin II. II. Mechanism of receptor interaction. J Med Chem. 1970 Mar;13(2):181–184. doi: 10.1021/jm00296a003. [DOI] [PubMed] [Google Scholar]
- Lenard J., Robinson A. B. Use of hydrogen fluoride in Merrifield solid-phase peptide synthesis. J Am Chem Soc. 1967 Jan 4;89(1):181–182. doi: 10.1021/ja00977a057. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Bumpus F. M., Khairallah P. A. Inhibition of norepinephrine uptake in hearts by angiotensin II and analogs. J Pharmacol Exp Ther. 1969 Jun;167(2):291–299. [PubMed] [Google Scholar]
- Scotchler J., Lozier R., Robinson A. B. Cleavage of single amino acid residues from Merrifield resin with hydrogen chloride and hydrogen fluoride. J Org Chem. 1970 Sep;35(9):3151–3152. doi: 10.1021/jo00834a067. [DOI] [PubMed] [Google Scholar]
- Smeby R. R., Khairallah P. A., Bumpus F. M. Tritiated angiotensin: preparation and purification. Nature. 1966 Sep 10;211(5054):1193–1194. doi: 10.1038/2111193a0. [DOI] [PubMed] [Google Scholar]
- Stewart J. M., Woolley D. W. All-D-bradykinin and the problem of peptide antimetabolites. Nature. 1965 May 8;206(984):619–620. doi: 10.1038/206619b0. [DOI] [PubMed] [Google Scholar]
- Vogler K., Studer R. O., Lergier W., Lanz P. Synthese von All-D-Val-5-Angiotensin II-Asp-1-beta-Amid. Helv Chim Acta. 1965 Sep 20;48(6):1407–1414. doi: 10.1002/hlca.19650480621. [DOI] [PubMed] [Google Scholar]
- YAJIMA H., KUBO K. STUDIES ON PEPTIDES. II. SYNTHESIS AND PHYSIOLOGICAL PROPERTIES OF D-HISTIDYL-D-PHENYLALANYL-D-ARGINYL-D-TRYPTOPHYLGLYCINE, AN OPTICAL ANTIPODE OF AN ACTIVE FRAGMENT OF ALPHA-MELANOCYTE-STIMULATING HORMONE. J Am Chem Soc. 1965 May 5;87:2039–2044. doi: 10.1021/ja01087a032. [DOI] [PubMed] [Google Scholar]


