Abstract
Purpose of review
Diabetes results from inadequate functional mass of pancreatic β-cells, and so replenishing with new glucose-responsive β-cells is an important therapeutic option. In addition to replication of pre-existing β-cells, new β-cells can be produced from differentiated adult cells using in vitro or in vivo approaches. This review will summarize recent advances in in vivo generation of β-cells from cells that are not β-cells (neogenesis) and discuss ways to overcome current limitations of this process.
Recent findings
Multiple groups have shown that adult pancreatic ducts, acinar and even endocrine cells exhibit cellular plasticity and can differentiate into β-cells in vivo. Several different approaches, including misexpression of transcription factors and tissue injury, induced neogenesis of insulin-expressing cells in vivo and ameliorated diabetes.
Summary
Recent breakthroughs demonstrating cellular plasticity of adult pancreatic cells to form new β-cells are a positive first step towards developing in vivo regeneration based therapy for diabetes. Currently, neogenesis processes are inefficient and do not generate sufficient amounts of β-cells required to normalize hyperglycemia. However, improved understanding of mechanisms regulating neogenesis of β-cells from adult pancreatic cells and of their maturation into functional glucose-responsive β-cells can make therapies based on in vivo regeneration a reality.
Keywords: In vivo regeneration, neogenesis, transdifferentiation, β-cells, diabetes
Introduction
Replenishing β-cell mass by transplantation, or better yet by in vivo regeneration, is an important strategy to ameliorate diabetes. Recent demonstrations of pancreatic ducts, acinar and even endocrine cells to acquire new cell fates in vivo suggests in vivo regeneration could replenish inadequate β-cell mass (1-5**). Some of these cell types transdifferentiate [conversion of a differentiated cell of one developmental commitment into a differentiated cells of another lineage without first reverting to a more primitive stem cell or progenitor (6-8)] into β-cells, while others achieve this goal by dedifferentiation and redifferentiation (1-5, 9, 10). We will briefly review in vitro differentiation strategies and then focus on the limitations and strengths of recent studies demonstrating in vivo neogenesis of β-cells.
Cell sources for in vitro generation of β-cells
In vitro differentiation of stem/progenitor cells into β-cells is an important approach to generate a reliable and replenishable cell source of β-cells. A significant effort is underway to differentiate embryonic stem cells into glucose-responsive β-cells (11*-19*). Several reports showed successful isolation and differentiation of putative stem/progenitor cells from adult and fetal pancreatic tissues into insulin-expressing cells. These include clonal cells isolated from adult islet and ducts (20) and prospective isolation from adult and fetal pancreas using FACS (21, 22). Progenitors isolated from fetal tissue could be true progenitors/stem cells, but since the in vivo identity of progenitors isolated from adult pancreas is not known, they may represent dedifferentiated mature cells.
Interestingly, some fetal and differentiated adult cells from non-pancreatic sources, including hepatic cells, can differentiate into insulin-expressing cells. Fetal liver cells, adult hepatocytes, hepatic cell lines, and biliary epithelial cells were shown to induce insulin expression upon expression of key pancreatic transcription factors (23-30). Similarly, primary intestinal epithelial cells and cell lines expressing pancreatic transcription factors expressed insulin mRNA when treated with GLP-1 and betacellulin (31, 32). These observations suggest that expression of pancreatic transcription factors in some non-pancreatic cells in presence of a few signaling molecules can induce insulin expression. However, the amounts of insulin produced by these cells were often several orders of magnitude lower than mature β-cells.
Pancreatic ductal cell lines and primary ductal cells have been successfully differentiated into insulin-expressing cells by in vitro approaches, including treatment with growth factors (e.g., EGF, Gastrin, exendin), expression of pancreatic transcription factors, and aggregation (9, 10, 33-37). Neogenesis of insulin-producing cells from differentiated pancreatic ductal cells results from their dedifferentiation into progenitors, expressing markers like PDX1, which redifferentiate into insulin-producing and other pancreatic cells. Hence, “terminally” differentiated ductal cells can be considered facultative stem cells (34). Like ductal cells, lineage-marked acinar cells in response to EGF underwent in vitro differentiation into insulin-expressing cells (38). A role for acinar-to-ductal transdifferentiation has also been suggested in conversion of acinar cells into endocrine cells (39). These observations demonstrate that multiple cell sources can differentiate into insulin-producing cells under in vitro culture conditions.
Thus, we suggest that like ES cells, adult pancreatic and non-pancreatic cells should be considered as potential in vitro cell sources for generating insulin-producing cells.
Cell sources for in vivo generation of β-cells
Over the last decade significant advances have been made in developmental biology of the pancreas and other endoderm-derived organs (25, 40-43). We gained a greater understanding of transcription factors and signals involved in the formation of β-cells and determined the molecular events that regulate differentiation of endoderm into pancreas, liver, and intestine. Analyses of lineage-commitment in transgenic and knockout mice demonstrated that altering expression of key factors during pancreatic development can alter cell fate decisions (44-46), and in some cases, cells destined to be part of the pancreas become part of a different organ (47). These findings show plasticity of pancreatic progenitors in acquiring different cell fates during embryonic development. Recently in vivo neogenesis of β-cells from pancreatic and non-pancreatic cells has been reported in adults. We will discuss these results and factors that can improve the efficiency of in vivo regeneration of glucose-responsive β-cells.
Neogenesis of β-cells from non-pancreatic cells
Liver represents an attractive in vivo source for generating β-cells due to its related developmental origin to the pancreas and its ease of genetic and surgical manipulation. In vivo conversion of pancreatic cells into hepatocyte-like cells was shown in copper-deprived rats (48, 49), while that of hepatic cells into acinar cells was reported in rats treated with polychlorinated biphenyls (50). Expression of transcription factor Pdx1 in adult mouse liver triggered the formation of insulin-expressing cells (51), while in liver of Xenopus tadpoles, expression of Pdx1-VP16, but not Pdx1 alone, formed ectopic pancreas containing both exocrine and endocrine compartments (52). The VP16 activation domain was essential for transdifferentiation of liver cells by another key pancreatic transcription Ptf1a, but it only formed acinar cells. However, expression of Ptf1a alone in stomach and duodenum generated both pancreatic acinar and endocrine cells (53). Combination of Pdx1, NeuroD1 and MafA expression, or NeuroD1 expression with betacellulin transdifferentiated liver cells into insulin-expressing cells, which could ameliorate streptozotocin-induced diabetes (51, 54, 55). Insulin-producing cells generated upon Adenoviral-mediated-expression of Pdx1 in the liver improved the blood sugar levels in 43% of cyclophosphamide-accelerated diabetic NOD mice (56). These results support the feasibility of using non-pancreatic cells for in vivo generation of insulin-producing cells.
Plasticity of pancreatic cells and in vivo neogenesis of β-cells
Strong evidence to support the presence in adult pancreas of a population of undifferentiated stem/progenitor cells that can give rise to all pancreatic cell types is still lacking. It has been shown that replication of differentiated β-cells contribute new β-cells during normal adult life (57). However, neogenesis of islets also occurs during normal development (at least during the first month) and in response to physical and physiological stress (34, 58). In this review, we will limit our discussion to neogenesis of β-cells in adults.
Neogenesis of β-cells from pancreatic ductal cells
Morphological analysis of pancreas following various manipulations, as well as in vitro differentiation of pancreatic ductal cells, suggest that pancreatic “progenitors” reside in ducts. In an elegant study using lineage-tracing and pancreatic ductal ligation in adult mice, Xu et al. demonstrated that the neogenesis of β-cells accompanied the induction of Ngn3-expressing endocrine progenitors in the ductal lining in the regenerating portion but not in the non-injured pancreas (4**). However, a recent publication reports a low level expression of Ngn3 in adult endocrine cells (59*), raising concerns about using Ngn3 expression as a marker of endocrine progenitors and neogenesis in adult pancreas. Interestingly, the Ngn3-expressing cells seen in the Xu et al. study did not express hormones, yet the increase in β-cell mass after ductal ligation depended on Ngn3 expression (4**). These observations confirm that the neogenesis, i.e., formation of β-cells from non-islet cells, occurs in adult pancreas.
By a direct lineage-tracing approach using a duct-specific human CAII promoter driving Cre or CreERT, one adult pancreatic cell type that gives rise to multipotent progenitors was identified (5**). CAII expression begins late in gestation (embryonic day 18.5) and is seen throughout the ductal tree but not in β-cells. Bigenic (CAIICre;R26R) mice at birth had lineage marker expression restricted to ductal cells, but by four weeks marker was also seen in endocrine cells (both α- and β-cells) and in acinar cells; 38% of the islets examined showed expression of the reporter gene. In inducible CAIICreERT;R26R mice ductal ligation after marking the adult ductal cells resulted in lineage-marked islets and acinar cells. Impressively, two weeks after duct ligation, 42% of islets and 24% of β-cells had lineage marker. These results clearly demonstrate that a significant proportion of adult pancreatic ductal cells retain the potential to differentiate into other pancreatic cell types including β-cells.
Acinar cell transdifferentiation to β-cells
Acinar and endocrine cells probably have a similar epigenetic profile as they share a common multipotent progenitor (60), which should make transdifferentiation of acinar cells into β-cells easier than from non-pancreatic cells. Yet, adult lineage-marked acinar cells were not seen to transdifferentiate into other pancreatic cell type in several experimental paradigms (61). Acinar cells can regenerate, as shown in chemical induced pancreatitis (caerulein) model, predominantly through their dedifferentiation and redifferentiation (62). However, misexpression of pancreatic developmental regulators in adult acinar cells using adenoviruses encoding Ngn3, Pdx1, and MafA transduced nearly 20% of acinar cells expressing these factors into insulin-producing β-cells that were indistinguishable from endogenous islet β-cells in size, shape and structure (2**). These new β-cells did not organize into islets and remained isolated. They improved, but not normalized, glycemia in diabetic mice. Additional studies will be needed to determine why only a fraction of acinar cells transdifferentiate into β-cells.
Transdifferentiation from other endocrine cells
Can pancreatic endocrine cells, like pancreatic ductal and acinar cells, transdifferentiate or dedifferentiate/redifferentiate into other cell types? Analyses of pancreas from some pancreatic transcription factor (e.g., Nkx2.2, Pax4, and Arx) knockout mice demonstrate that lineage commitment of endocrine progenitors can be reprogrammed (44-46). Nkx2.2 knockout mice lack β-cells and have reduced α- and PP-cells but increased ghrelin-expressing cells (46). Similarly, the relative expression of transcription factors, Pax4 and Arx, in endocrine progenitors determined the lineage of a differentiated cell. In Pax4 knockout mice, loss of β- and δ-cells was compensated by a proportional increase of α-cells (44), while in Arx-deficient mice the loss of α-cells was compensated by a proportional increase in β- and δ-cells (45). Interestingly, committed endocrine progenitors can also differentiate into other pancreatic cell types. In Pax4:constitutively activated Notch mice, Pax4-expressing endocrine progenitors differentiated into ducts (63). Thus, during embryonic development, lineage commitment of endocrine progenitors can be regulated.
Altering expression of transcription factors in differentiated endocrine cells can also change their endocrine fate. Arx misexpression in β-cells, using either an InsCre or in adult β-cells using an inducible Pdx1CreERT (64), reduced insulin-expressing cells and increased α- and PP-cells. In both cases, the vast majority of α- and PP-cells contained lineage marker confirming their derivation from insulin/Pdx1-expressing β-cells. Interestingly, expression of Pax4 in mature α-cells using GcgCre, increased β-cell mass and dramatically reduced that of α-cells (3**). The increase could not result from proliferation of new β-cells nor from the normal number of α-cells in controls. Most β-cells contained the lineage marker indicating their derivation from α-cells. In spite of increased β-cell mass and initial hypoglycemia, these mice gradually became hyperglycemic. This system was also able to reconstitute β-cells in streptozotocin-treated mice of different ages. Younger mice showed preferential survival, possibly by inducing neogenesis of α-cells in response to reduced glucagon signaling, and reprogramming them in the presence of Pax4 to β-cells. Thus, these results demonstrate that adult α-cells, like pancreatic ductal and acinar cells, can serve as in vivo source for generating β-cells.
Steps to enhance in vivo regeneration of β-cells
As reviewed above, adult cells in response to appropriate in vitro and in vivo factors/signals can differentiate into new β-cells (Figure 1). Although one should be careful to equate results from animal studies with human situation, the reviewed literature provides rationale for developing in vivo regeneration-based treatments for diabetes. Acinar and ductal cells constitute over 95% of the pancreas, and their ability to proliferate in response to various stimuli makes them attractive targets for in vivo regeneration of β-cells. Developing efficient strategies to deliver regulatory factors to these cells, either on their own or when combined with short-term growth stimulus, should result in increasing β-cell mass. Transdifferentiation of α-cells into β-cells is an equally attractive approach for in vivo regeneration due to their location and the neogenic response of α-cells to impaired glucagon signaling. To realize in vivo regeneration based on this approach will require developing strategies to induce Pax4 expression in α-cells and combining it with approaches to inhibit glucagon signaling. However, before we make a significant investment in developing therapies based on acinar, ductal or α-cells, it will be important to determine the maximum extent of neogenesis that can be achieved using these cell types.
Figure 1.
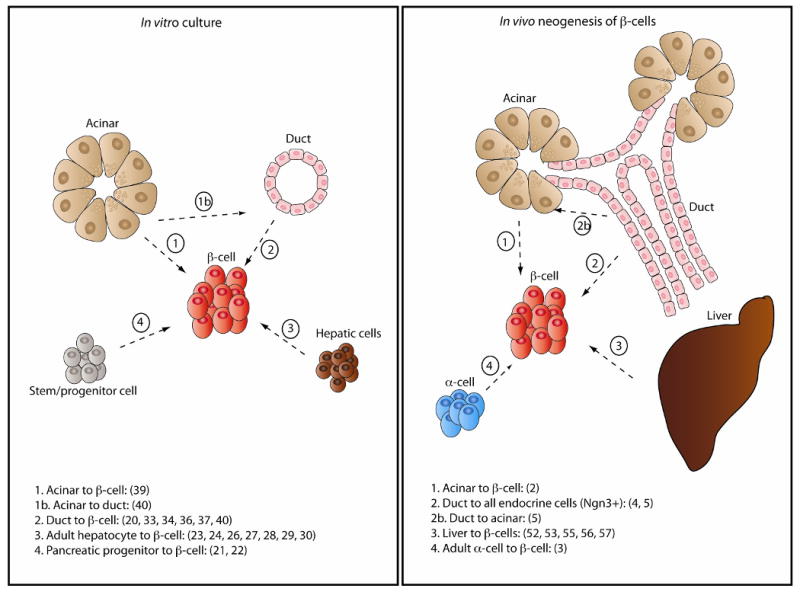
Schematic showing different cell sources that under in vitro or in vivo differentiation conditions transdifferentiate or dedifferentiate/redifferentiate into insulin-producing cells. Numbers in parenthesis refer to reference numbers.
At present these approaches cannot normalize hyperglycemia, and at least in some cases (3**), animals gradually redeveloped hyperglycemia, demonstrating the inability of these approaches to generate/maintain sufficient amounts of glucose-responsive β-cells. The limited success of current approaches could result from: 1) incomplete dedifferentiation of terminally differentiated cells to a progenitor state, 2) inefficient redifferentiation of dedifferentiated progenitors into β-cells, and/or 3) inability of insulin-producing cells to survive/proliferate and mature into glucose-responsive β-cells.
The yield of β-cell neogenesis from ducts is possibly limited by their first acquiring a dedifferentiated fate and then redifferentiating into multiple cell types (endocrine, acinar and ducts) (4*, 34, 58). In case of acinar reprogramming, 20% of infected acinar cells became β-cells, without significant proliferation or expression of markers of a common embryonic acinar-endocrine progenitor (2**). However, it is still likely that these cells partially dedifferentiated and that affected the efficiency of neogenesis. In contrast, the majority of α-cells that expressed Pax4 transdifferentiated into β-cells possibly without dedifferentiating into a progenitor stage (3**). Thus, in order to enhance the efficiency of in vivo neogenesis of β-cells, it will be essential to understand the process of dedifferentiation and redifferentiation and to determine which of these steps is rate-limiting. Identification of factors that can efficiently dedifferentiate adult pancreatic cells into progenitors having an epigenetic profile similar to that of pancreatic/endocrine progenitors will be important. Our ability to monitor at the single cell level the loss of differentiated phenotype, induction of progenitor and β-cell markers, and changes to the epigenetic profile will be key to enhancing the efficiency of neogenesis. Such analyses will be crucial for determining whether all, or only a few, cells can acquire “true” epigenetic makeup of pancreatic/endocrine progenitor and thus can differentiate into β-cells.
It is also possible that the newly generated β-cells are immature and functionally impaired, which would limit their ability to normalize hyperglycemia, leading to suppression of several key genes in β–cells and further exacerbating the impairment. Newly-formed insulin-producing cells undergo maturation steps that include reduction in the expression of transcription factors MafB and Pax4 and induction of MafA expression (65, 66). MafA knockout mice had impaired glucose-stimulated insulin secretion (GSIS) and gradually developed diabetes (67). Furthermore, MafB-expressing insulin-positive cells generated from differentiation of human ES cells were not glucose-responsive (18). These results suggest a need to examine the newly generated β-cells whether they underwent proper maturation. An increasing body of evidence suggests that β-cells in the pancreas are heterogeneous in nature and can be specified by more than one pathway (65, 66, 68*, 69*). It was recently shown that zebrafish β-cells derived from the dorsal pancreatic bud are different from those derived from the ventral bud (69*). Dorsal bud-derived β-cells were quiescent and had reduced expression of insulin, while those from ventral bud proliferated actively and expressed more insulin, Pdx1, NeuroD1, Pax6 and MafA mRNA at 12 days-post-fertilization than their dorsal counterpart (69*). Intriguingly, dorsal bud-derived β-cells expressed more MafB than the β-cells from ventral bud. These results suggest that the origin of β-cells may govern their ability to proliferate and function. However, a study examining the replication potential of adult β-cells showed that all β-cells contribute equally to islet growth and maintenance (70). This result needs to be reconciled with the zebrafish data unless the proliferation of newly-formed β-cells differs from that of adult β-cells. We suggest that understanding of pathways regulating specification and maturation of β-cells will help determine whether insulin-producing cells generated from differentiation of adult cells have the same capacity to proliferate and mature into glucose-responsive β-cells. This knowledge, combined with improved efficiency of dedifferentiation and redifferentiation of adult pancreatic cells into β-cells, should result in new ways to treat diabetes.
Conclusion
Significant efforts are underway to generate β-cells in vitro for cell-based therapies. Over the past few years, significant progress has been made that supports the inclusion of in vivo regeneration of β-cells as a potential therapy for diabetes. These studies successfully demonstrate that in vivo neogenesis of β-cells could ameliorate STZ-induced and autoimmune diabetes. Combining an efficient delivery system for regulatory factors with ways to enhance efficiency of neogenesis of glucose-responsive β-cell from in vivo differentiation of adult cells will be critical for developing novel therapies for diabetes.
Acknowledgments
fellowships #R7-A714-B576 from Lundbeckfonden, Denmark and 3-2009-683 from JDRF. Research in Sharma and Bonner-Weir laboratories was supported by grants from National Institutes of Health (NIH/NIDDK), JDRF, and ADA. We thank members of our laboratories for their contribution to this research.
Our research has been supported by grants from National Institutes of Health (NIH/NIDDK), JDRF, and ADA; KJ is a recipient of Post-doctoral fellowships #R7-A714-B576 from Lundbeckfonden, Denmark and 3-2009-683 from JDRF.
References and recommended reading
- 1.Bonner-Weir S, Inada A, Yatoh S, et al. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008 Jun;36(Pt 3):353–6. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- 2**.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008 Oct 2;455(7213):627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper provides evidence for reprogramming of adult pancreatic cells by demonstrating that in vivo misexpression of Ngn3, Pdx1 and MafA in adult acinar cells converts them to insulin-producing cells.
- 3**.Collombat P, Xu X, Ravassard P, et al. The Ectopic Expression of Pax4 in the Mouse Pancreas Converts Progenitor Cells into [alpha] and Subsequently [beta] Cells. Cell. 2009;138(3):449–62. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors provide genetic evidence that Pax4 induced in adult glucagon-expressing cells transdifferentiate them to β-cells. Furthermore, hypoglycemic condition triggers neogenesis of α-cells that upon expression of Pax4 transdifferentiate into β-cells.
- 4**.Xu X, D'Hoker J, Stange G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008 Jan 25;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]; Manuscript provides evidence for in vivo neognesis of β-cells. This study demonstrates induction of Ngn3-expressing cells in adults upon ductal ligation and that the neogenesis of b-cells depends on the expression of Ngn3.
- 5**.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19915–9. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper shows genetic lineage tracing evidence that newly formed endocrine cells are derived from CAII expressing duct cells both in neonatal stage and in adult pancreas following duct ligation.
- 6.Thowfeequ S, Myatt EJ, Tosh D. Transdifferentiation in developmental biology, disease, and in therapy. Developmental Dynamics. 2007;236(12):3208–17. doi: 10.1002/dvdy.21336. [DOI] [PubMed] [Google Scholar]
- 7.Li WC, Yu WY, Quinlan JM, et al. The molecular basis of transdifferentiation. J Cell Mol Med. 2005;9(3):569–82. doi: 10.1111/j.1582-4934.2005.tb00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slack JM, Tosh D. Transdifferentiation and metaplasia--switching cell types. Curr Opin Genet Dev. 2001;11(5):581–6. doi: 10.1016/s0959-437x(00)00236-7. [DOI] [PubMed] [Google Scholar]
- 9.Xia B, Zhan XR, Yi R, et al. Can pancreatic duct-derived progenitors be a source of islet regeneration? Biochem Biophys Res Commun. 2009 Jun 12;383(4):383–5. doi: 10.1016/j.bbrc.2009.03.114. [DOI] [PubMed] [Google Scholar]
- 10.Hanley NA, Hanley KP, Miettinen PJ, et al. Weighing up beta-cell mass in mice and humans: self-renewal, progenitors or stem cells? Mol Cell Endocrinol. 2008 Jun 25;288(12):79–85. doi: 10.1016/j.mce.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 11*.Evans-Molina C, Vestermark GL, Mirmira RG. Development of insulin-producing cells from primitive biologic precursors. Curr Opin Organ Transplant. 2009 Feb;14(1):56–63. doi: 10.1097/MOT.0b013e3283186fc1. [DOI] [PMC free article] [PubMed] [Google Scholar]; A current review and discussion about pancreatic transcription factors and embryonic stem cell differentiation to β-cells.
- 12*.Guo T, Hebrok M. Stem Cells to Pancreatic {beta}-Cells: New Sources for Diabetes Cell Therapy. Endocr Rev. 2009 May 1;30(3):214–27. doi: 10.1210/er.2009-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review about embryonic stem cell differentiation to insulin-producing cells based on pancreatic development, signaling pathways and transcription factors.
- 13.Chen S, Borowiak M, Fox JL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009 Apr;5(4):258–65. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Melton DA. Pathways to New {beta} Cells. Cold Spring Harb Symp Quant Biol. 2008 Nov 6; doi: 10.1101/sqb.2008.73.002. [DOI] [PubMed] [Google Scholar]
- 15.Serafimidis I, Rakatzi I, Episkopou V, et al. Novel effectors of directed and Ngn3-mediated differentiation of mouse embryonic stem cells into endocrine pancreas progenitors. Stem Cells. 2008 Jan;26(1):3–16. doi: 10.1634/stemcells.2007-0194. [DOI] [PubMed] [Google Scholar]
- 16.Murry CE, Keller G. Differentiation of Embryonic Stem Cells to†Clinically Relevant Populations: Lessons from Embryonic Development. Cell. 2008;132(4):661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Ku HT, Chai J, Kim YJ, et al. Insulin-expressing colonies developed from murine embryonic stem cell-derived progenitors. Diabetes. 2007 Apr;56(4):921–9. doi: 10.2337/db06-0468. [DOI] [PubMed] [Google Scholar]
- 18.D'Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006 Nov;24(11):1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 19*.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. doi: 10.1038/nbt1393. Epub 2008 Feb 20. [DOI] [PubMed] [Google Scholar]; The paper demonstrates the first successful protocol for differentiating hESCs into mature glucose-responsive insulin-producing cells.
- 20.Seaberg RM, Smukler SR, Kieffer TJ, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nature Biotechnology. 2004;22(9):1115–24. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama T, Rodriguez RT, McLean GW, et al. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci U S A. 2007 Jan 2;104(1):175–80. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004;53(8):2143–52. doi: 10.2337/diabetes.53.8.2143. [DOI] [PubMed] [Google Scholar]
- 23.Nagaya M, Katsuta H, Kaneto H, et al. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrinol. 2009 Apr;201(1):37–47. doi: 10.1677/JOE-08-0482. [DOI] [PubMed] [Google Scholar]
- 24.Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54(9):2568–75. doi: 10.2337/diabetes.54.9.2568. [DOI] [PubMed] [Google Scholar]
- 25.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008 Dec 5;322(5907):1490–4. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muniappan L, Ozcan S. Induction of insulin secretion in engineered liver cells by nitric oxide. BMC Physiol. 2007 Oct 17;7(1):11. doi: 10.1186/1472-6793-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li WC, Horb ME, Tosh D, et al. In vitro transdifferentiation of hepatoma cells into functional pancreatic cells. Mech Dev. 2005;122(6):835–47. doi: 10.1016/j.mod.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima-Nagata N, Sakurai T, Mitaka T, et al. In vitro induction of adult hepatic progenitor cells into insulin-producing cells. Biochem Biophys Res Commun. 2004;318(3):625–30. doi: 10.1016/j.bbrc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 29.Ber I, Shternhall K, Perl S, et al. Functional, persistent and extended liver to pancreas transdifferentiation. J Biol Chem. 2003 doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Li S, Hatch H, et al. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci U S A. 2002;99(12):8078–83. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5034–9. doi: 10.1073/pnas.0936260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojima H, Nakamura T, Fujita Y, et al. Combined expression of pancreatic duodenal homeobox 1 and islet factor 1 induces immature enterocytes to produce insulin. Diabetes. 2002 May;51(5):1398–408. doi: 10.2337/diabetes.51.5.1398. [DOI] [PubMed] [Google Scholar]
- 33.Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. ProcNatlAcadSciUSA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonner-Weir S, Sharma A. Pancreatic stem cells. J Pathol. 2002;197(4):519–26. doi: 10.1002/path.1158. [DOI] [PubMed] [Google Scholar]
- 35.Suarez-Pinzon WL, Lakey JR, Brand SJ, et al. Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet {beta}-cells from pancreatic duct cells and an increase in functional {beta}-cell mass. J Clin Endocrinol Metab. 2005;90(6):3401–9. doi: 10.1210/jc.2004-0761. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Pineyro MA, Wang X, et al. Exendin-4 differentiation of a human pancreatic duct cell line into endocrine cells: involvement of PDX-1 and HNF3beta transcription factors. J Cell Physiol. 2002;192(3):304–14. doi: 10.1002/jcp.10143. [DOI] [PubMed] [Google Scholar]
- 37.Bonner-Weir S, Sharma A. Generation of Beta Cells From Pancreatic Duct Cells and/or Stem Cells. In: Efrat S, editor. Stem Cell Therapy for Diabetes, Stem Cell biology and Regenerative Medicine. Humana Press a part of Springer Science+Buisness Media; 2009. [Google Scholar]
- 38.Minami K, Okuno M, Miyawaki K, et al. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci U S A. 2005;102(42):15116–21. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rooman I, Heremans Y, Heimberg H, et al. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia. 2000;43:907–14. doi: 10.1007/s001250051468. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen MC, Ahnfelt-Ronne J, Hald J, et al. An Illustrated Review of Early Pancreas Development in the Mouse. Endocrine Reviews. 2007;28(6):685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- 41*.Gittes GK. Developmental biology of the pancreas: A comprehensive review. Developmental Biology. 2009;326(1):4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]; A comprehensive review about the signaling cascades in the early pancreatic development.
- 42.Scoville DH, Sato T, He XC, et al. Current view: intestinal stem cells and signaling. Gastroenterology. 2008 Mar;134(3):849–64. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 43.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006 May;7(5):349–59. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 44.Sosa-Pineda B, Chowdhury K, Torres M, et al. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997 Mar 27;386(6623):399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 45.Collombat P, Mansouri A, Hecksher-Sorensen J, et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17(20):2591–603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prado CL, Pugh-Bernard AE, Elghazi L, et al. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A. 2004;101(9):2924–9. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawaguchi Y, Cooper B, Gannon M, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32(1):128–34. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 48.Rao MS, Subbarao V, Reddy JK. Induction of hepatocytes in the pancreas of copper-depleted rats following copper repletion. Cell Differ. 1986 Mar;18(2):109–17. doi: 10.1016/0045-6039(86)90005-9. [DOI] [PubMed] [Google Scholar]
- 49.Rao MS, Dwivedi RS, Subbarao V, et al. Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation. Biochem Biophys Res Commun. 1988 Oct 14;156(1):131–6. doi: 10.1016/s0006-291x(88)80814-3. [DOI] [PubMed] [Google Scholar]
- 50.Rao MS, Bendayan M, Kimbrough RD, et al. Characterization of pancreatic-type tissue in the liver of rat induced by polychlorinated biphenyls. J Histochem Cytochem. 1986 Feb;34(2):197–201. doi: 10.1177/34.2.2418098. [DOI] [PubMed] [Google Scholar]
- 51.Ferber S, Halkin A, Cohen H, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6(5):568–72. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 52.Horb ME, Shen CN, Tosh D, et al. Experimental conversion of liver to pancreas. Curr Biol. 2003;13(2):105–15. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 53.Jarikji ZH, Vanamala S, Beck CW, et al. Differential ability of Ptf1a and Ptf1a-VP16 to convert stomach, duodenum and liver to pancreas. Dev Biol. 2007 Apr 15;304(2):786–99. doi: 10.1016/j.ydbio.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaneto H, Matsuoka TA, Nakatani Y, et al. A crucial role of MafA as a novel therapeutic target for diabetes. J Biol Chem. 2005;280(15):15047–52. doi: 10.1074/jbc.M412013200. [DOI] [PubMed] [Google Scholar]
- 55.Kojima H, Fujimiya M, Matsumura K, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003 May;9(5):596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 56.Shternhall-Ron K, Quintana FJ, Perl S, et al. Ectopic PDX-1 expression in liver ameliorates type 1 diabetes. J Autoimmun. 2007 Mar-May;28(23):134–42. doi: 10.1016/j.jaut.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 57.Dor Y. beta-cell proliferation is the major source of new pancreatic beta cells. Nat Clin Pract Endocrinol Metab. 2006;2(5):242–3. doi: 10.1038/ncpendmet0187. [DOI] [PubMed] [Google Scholar]
- 58.Bonner-Weir S, Toschi E, Inada A, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5 2:16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 59*.Wang S, Jensen JN, Seymour PA, et al. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9715–20. doi: 10.1073/pnas.0904247106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper suggests not relying on only Ngn3 expression as an indication of neogenesis of beta cells in adult pancreas.
- 60.Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120(1):35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 61.Desai BM, Oliver-Krasinski J, De Leon DD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007 Apr;117(4):971–7. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen JN, Cameron E, Garay MV, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128(3):728–41. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Greenwood AL, Li S, Jones K, et al. Notch signaling reveals developmental plasticity of Pax4(+) pancreatic endocrine progenitors and shunts them to a duct fate. Mech Dev. 2007 Feb;124(2):97–107. doi: 10.1016/j.mod.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Collombat P, Hecksher-Sorensen J, Krull J, et al. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007 Apr;117(4):961–70. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishimura W, Kondo T, Salameh T, et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293(2):526–39. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Elghazi L, Parker SE, et al. The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic beta-cell differentiation. Dev Biol. 2004;266(1):178–89. doi: 10.1016/j.ydbio.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C, Moriguchi T, Kajihara M, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25(12):4969–76. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68*.Nishimura W, Rowan S, Salameh T, et al. Preferential reduction of [beta] cells derived from Pax6-MafB pathway in MafB deficient mice. Developmental Biology. 2008;314(2):443–56. doi: 10.1016/j.ydbio.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper suggests multiple pathways to specify insulin-expressing cells.
- 69*.Hesselson D, Anderson RM, Beinat M, et al. Proceedings of the National Academy of Sciences. 35. Vol. 106. 2009. Sep 1, Distinct populations of quiescent and proliferative pancreatic Œ≤-cells identified by HOTcre mediated labeling; pp. 14896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides evidence that dorsal pancreas-derived β-cells proliferate slower, express less insulin, more MafB than ventral-derived β-cells.
- 70.Brennand K, Huangfu D, Melton D. All beta Cells Contribute Equally to Islet Growth and Maintenance. PLoS Biol. 2007 May 29;5(7):e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]


