Abstract
Pregnancy is dependent upon the endometrium acquiring a receptive phenotype that facilitates apposition, adhesion and invasion of a developmentally competent embryo. Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry of mid-secretory endometrial biopsies revealed a 28 kDa protein peak that discriminated highly between samples obtained from women with recurrent implantation failure and fertile controls. Subsequent tandem mass spectroscopy unambiguously identified this peak as apolipoprotein A-I (apoA-I), a potent anti-inflammatory molecule. Total endometrial apoA-I levels were, however, comparable between the study and control group. Moreover, endometrial apoA-I mRNA expression was not cycle-dependent although there was partial loss of apoA-I immunoreactivity in luminal and glandular epithelium in mid-secretory compared with proliferative endometrial samples. Because of its putative anti-implantation properties, we examined whether endometrial apoA-I expression is regulated by embryonic signals. Human chorionic gonadotrophin (hCG) strongly inhibited apoA-I expression in differentiating explant cultures but not when established from eutopic endometrium from patients with endometriosis. Pelvic endometriosis was associated with elevated apoA-I mRNA levels, increased secretion by differentiating eutopic endometrial explant cultures and lack of hCG-dependent down-regulation. To corroborate these observations, we examined endometrial apoA-I expression and its regulation by hCG in a non-human primate model of endometriosis. As in humans, hCG strongly inhibited endometrial apoA-I mRNA expression in disease-free baboons, but this response was entirely lost upon induction of pelvic endometriosis. Together, these observations indicate that perturbations in endometrial apoA-I expression, modification or regulation by paracrine embryonic signals play a major role in implantation failure and infertility.
Keywords: apolipoprotein A-I, endometrium, endometriosis implantation, infertility, proteomics
Introduction
In humans and other mammals with invasive placentae, implantation involves apposition of the blastocyst to the luminal epithelium of the endometrium, followed by adhesive contact and then invasion of maternal tissues (Fazleabas and Kim, 2003; Genbacev et al., 2003; Dey et al., 2004). The apical membranes of epithelial cells are not normally adhesive, and the endometrial luminal epithelium must transiently acquire a receptive phenotype to allow attachment of the blastocyst (Denker, 1993; Rahnama et al., 2009). Interestingly, endometrial receptivity is both species-specific and selective for embryonic trophoblast (Hohn et al., 2003). In humans, the period of endometrial receptivity, often referred to as the ‘implantation window’, starts 6 days (d) after ovulation and is thought to last between 2 and 4 d (Wilcox et al., 1999; Quinn and Casper, 2009). A shortened or absent receptive phase is widely believed to be a major cause of conception delay, which affects ∼10% of couples during the reproductive years, and to contribute to the relatively low success rates of assisted reproductive technologies, such as IVF (Boivin et al., 2007; Diedrich et al., 2007; Feroze-Zaidi et al., 2007). Conversely, implantation beyond the normal period of endometrial receptivity is strongly associated with early pregnancy loss (Wilcox et al., 1999), which is probably accounted for by the nidation of developmentally compromised embryos.
Endometrial receptivity is dependent upon regulation of evolutionarily conserved gene networks that control the expression of key transcription factors (e.g. HOXA10, STAT3, p53) (Catalano et al., 2005; Nakamura et al., 2006; Hu et al., 2007; Vitiello et al., 2007; Lynch et al., 2008), growth factors and cytokines [e.g. HB-EGF, leukaemia-inhibiting factor (LIF), prokineticin 1] (Stewart et al., 1992; Evans et al., 2009; Lim and Dey, 2009) and cell adhesion molecules and their ligands (e.g. αvβ3 integrin, trophinin, l-selectin ligand) (Aoki and Fukuda, 2000; Genbacev et al., 2003; Donaghay and Lessey, 2007), all of which are essential for coordinated cross-talk with the implanting embryo. Microarray analyses have been extensively used to identify the gene networks that underpin either the receptive and unreceptive states of the endometrium (Carson et al., 2002; Kao et al., 2002, 2003; Mirkin et al., 2005; Talbi et al., 2006; Feroze-Zaidi et al., 2007). Although each study yielded numerous candidate genes, the number of common ‘endometrial receptivity’ genes is relatively small (Horcajadas et al., 2007), probably due to differences in experimental approach, timing of endometrial sampling and array platforms used. Nevertheless, impaired expression of endometrial receptivity genes is increasingly linked to common reproductive disorders. A case in point is endometriosis, a prevalent disorder that affects 5–10% of women and a major cause of subfertility (Bulun, 2009). This disease is not only characterized by the presence of pelvic endometrial implants but also, especially during the early- and mid-secretory phase of the cycle, by gross perturbations in gene expression in the eutopic endometrium (Burney et al., 2007), including key regulators of implantation such as HOXA10 and αvβ3 integrin (Taylor et al., 1999; Donaghay and Lessey, 2007).
The extent to which endometrial receptivity is perturbed in subfertile patients without overt pathology is as yet not well defined (Dimitriadis et al., 2007). On the basis of microarray analysis, we reported that unexplained infertility is associated with enhanced expression and activation of serum and glucocorticoid regulated kinase 1, a serine/threonine kinase essential for uterine fluid handling, during the mid-secretory phase of the cycle (Feroze-Zaidi et al., 2007). More recently, various techniques have been employed to survey the endometrial proteome in proliferative and secretory samples (DeSouza et al., 2005) and to identify key proteins that may discriminate between the pre-receptive and receptive phase of the cycle (Dominguez et al., 2009). In the present study, we also used a proteomic approach to screen for abnormalities during the putative window of endometrial receptivity in patients with unexplained recurrent implantation failure (RIF), defined as a failure to achieve a positive pregnancy test after 3 or more consecutive IVF treatment cycles with transfer of good-quality embryos. Our data suggest that RIF is associated with a distinct endometrial protein fingerprint. Moreover, we identified apolipoprotein A-I (apoA-I) as an endometrial factor whose expression and regulation in response to embryonic signals are dysregulated in endometriosis.
Methods and Materials
Patient selection and sample collection
The Local Research and Ethics Committees of Hammersmith and Addenbrooke's Hospitals NHS Trusts, UK, approved the study. Written informed consent was obtained from 15 women with proven fertility (control group) and from 10 patients with three or more consecutive IVF treatment failures that could not be attributed to poor embryo quality (study group). All patients had regular cycles and their daily urinary luteinizing hormone (LH) levels were monitored using an ovulation prediction kit (Assure Ovulation Predictor, San Diego, CA, USA). Endometrial biopsies, timed between 5 and 10 d following the pre-ovulatory LH surge (LH+5 to LH+10), were taken using a pipelle catheter. The demographic details of the study and control groups used in the proteomic analysis are summarized in Table I. Each biopsy was divided and one portion snap-frozen in liquid nitrogen and stored at −80°C. The other portion was fixed in formalin for histological dating using standard criteria. A venopuncture was also performed and progesterone levels measured to ensure that ovulation had occurred. For validation studies, additional endometrial samples at the time of laparoscopy for pelvic pain, infertility or tubal sterilization were obtained. Eutopic endometrial samples from women without overt pelvic pathology and patients with histologically confirmed endometriosis were used for primary and explant cultures, and the demographic details of these patients are summarized in Table II.
Table I.
Patient characteristics and timing of endometrial sampling.
| Fertile (n = 15) | RIF (n = 10) | |
|---|---|---|
| Age (years) | 34.33 ± 5.21 | 36 ± 5.03 |
| Parity | 2.07 ± 1.33 | 0 |
| Cycle length (days) | 29.07 ± 1.44 | 30 ± 2.21 |
| Progesterone level (nmol/l) | 40.44 ± 19.01 | 37.42 ± 21.04 |
| Days from LH peak | 8.07 ± 0.80 | 8.5 ± 0.97 |
Values represent mean ± SD.
Table II.
Demographic details of endometriosis patients and control.
| Endometriosis (n = 19) | Control (n = 18) | |
|---|---|---|
| Age (mean ± SD) | 33.3 ± 4.6 years | 34.9 ± 5.1 years* |
| Parity | 0.5 ± 1 | 0.8 ± 0.9* |
| Proliferative samples | 11 | 11 |
| Secretory samples | 8 | 7 |
| Stage of disease | ||
| Severe | 7 | — |
| Moderate | 5 | — |
| Minimal/mild | 7 | — |
*P > 0.05.
Proteomic analysis
Endometrial protein profiles were determined by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS), using a strong anion-exchange (Q10) and a weak cation-exchange (CM10) ProteinChip Arrays (Ciphergen Biosystems, CA, USA). Prior to sample loading, Q10 and CM10 arrays were equilibrated with 75 µl of binding/wash buffer (50 mM TRIS, 0.1% v/v Triton X-100 at pH 5.0, 7.5 or 9.5 for Q10; 50 mM ammonium acetate, 0.01% v/v Triton X-100 at pH 4.0, 6.0 or 7.5 for CM10). Tissue samples were lysed in buffer containing dithiothreitol 1% w/v, urea 9.5 M, 2% CHAPS, centrifuged at 13 000 r.p.m. (16.4g) for 1 h at 15°C, and protein concentration in the supernatant was determined by Bradford analysis. Samples were adjusted to 60 µg protein in 100 µl total volume, and 50 µl was added to each spot in duplicate. Following 30-min incubation with agitation, the spots were washed first three times with 75 µl of binding/wash buffer and then briefly with water and air-dried. Saturated sinapinic acid (SPA) or α-cyano-4-hydroxycinnamic acid (CHCA) was applied and the dried ProteinChip Arrays transferred into the ProteinChip Biomarker System (Ciphergen Biosystems) in a randomized fashion to remove handling bias. Following optimization of the laser settings, data were collected from spots 20 to 80, delta 5 with 16 shots collected per position, preceded by two warming shots at the appropriate laser setting, the data from which were discarded. Using Biomarker Wizard software 3.0 (Ciphergen Biosystems), the spectra were normalized to total ion current over a mass range appropriate to the matrix used (2–10 kDa for CHCA and 10–50 kDa for SPA). The settings used to autodetect peaks to cluster in the first pass were a signal-to-noise ratio of 5, second-pass signal-to-noise ratio of 2 and a mass tolerance of 0.3%. All peaks detected were required to be present in at least 5% of the spectra to avoid the inclusion of peaks with poor discriminatory power. The number of peaks, dependent on ProteinChip and wash condition, varied 17–78.
We set out to characterize the ∼11 987 and ∼28 063 Da protein peaks, as we reasoned they could serve as the most informative biomarker of unreceptive endometrium. Briefly, protein lysates were subjected to reverse-phase fractionation, resolved on sodium dodecyl sulphate (SDS)–poly acrylamide gel (PAGE) followed by in-gel trypsin digestion of the proteins of interest and SELDI-TOF-MS analysis. However, the ∼11 987 protein peak was resistant in-gel trypsin digestion, thereby precluding further analysis. The analysis of the ∼28 063 Da protein yielded 23 peptide mass fingerprints, which were submitted to ProFound (http://prowl.rockefeller.edu/prowl-cgi/profound.exe), taking in account potential protein modifications such as methionine oxidation. The protein of interest was identified as apoA-I precursor (Z = 2.43, 47% coverage), and this was confirmed by Q-Star MS/MS analysis (data not shown).
Primary human endometrial stromal cell cultures and endometrial explants
Human endometrial stromal cells (HESCs) were separated from epithelial cells, passed into culture and decidualized in DMEM/F-12 containing 2% dextran-coated carbon-stripped fetal bovine serum with 0.5 mM 8-br-cAMP (Sigma) and 10−6 M medroxy-progesterone actetate (MPA) (Sigma) for 24 or 72 h (Brosens et al., 1999), in the presence or absence recombinant human chorionic gonadotrophin (hCG; Sigma). All experiments were carried out before the third cell passage in three or more biological replicates. Explants cultures were also prepared. Briefly, 24 endometrial fragments, measuring approximately 2–3 mm2 each, were placed in Millicell-CM culture inserts in six-well plates containing DMEM/F-12 medium supplemented l-glutamine (1%) and penicillin and streptomycin (1%). The explants were cultured for 24 h in the presence of 0.5 mM 8-br-cAMP (Sigma) and 10−6 M MPA with or without hCG.
Animals studies
Cycling female baboons (Papio anubis), ranging in age from 7 to 12 years and weighing between 12 and 18 kg, were housed in individual cages in the Biological Research Laboratories of the University of Illinois. All animal procedures were approved by the Animal Care Committee of the University of Illinois at Chicago. Endometriosis was experimentally induced in five female baboons with regular menstrual cycles by intraperitoneal inoculation of menstrual endometrium on two consecutive menstrual cycles, and endometrial tissues were obtained between 3 and 15 months post-inoculation following treatment with CG. Details of the inoculation procedure have been described previously (Fazleabas et al., 2002, 2003). Uterine tissue was obtained from six control baboons and five animals induced with disease by endometriectomy or hysterectomy, on d 10 post-ovulation (PO). Ovulation was detected in cycling female baboons by measuring peripheral serum levels of estradiol, beginning 7 d after the first day of menses. The day of the estradiol surge was designated as d − 1, with d 0 as the day of the ovulatory LH surge and d 1 as the day of ovulation. On d 5 PO, an oviductal cannula was attached to an Alzet osmotic minipump, and recombinant hCG was infused at the rate 1.25 IU/h for 5 d, as described previously (Fazleabas et al., 1999). For the enzyme-linked immunosorbent assay (ELISA), serum was collected from four control and four baboons with endometriosis during the later stages of the disease.
Immunohistochemistry, western blot and ELISA
Formalin-fixed, paraffin-embedded samples were stained for apoA-I expression. Briefly, after mounting, the samples were de-paraffinized and rehydrated in graded concentrations of ethanol, and endogenous peroxidase activity was blocked by immersion of the slides for 30 min in a freshly prepared solution of 2 ml of 30% hydrogen peroxide diluted in 200 ml of methanol. The slides were then washed in PBS, pre-incubated in 1.5% non-immune murine serum in PBS for 30 min at RT and incubated overnight at 4°C with a mouse monoclonal apoA-I antibody (1:50 dilution; Calbiochem, Nottingham, UK) and the staining visualized using biotinylated goat anti-mouse IgG (Vector Laboratories, Peterborough, UK). Liver tissue sections were included as positive control, and the primary antibody was omitted as a negative control. The tissue sections of proliferative and secretory endometrium have been described elsewhere (Goto et al., 2008). Additional tissue sections of mid-secretory endometrium from patients with and without endometriosis, proven by laparoscopy, were provided by S.L.Y. and B.A.L., respectively.
Total protein lysates from snap-frozen samples and primary cultures were subjected to western blot analysis as described previously (Pohnke et al., 2004). The mouse monoclonal antibodies against apoA-I and β-actin (Abcam, Cambridge, UK) were diluted at 1:5000 and 1:100 000, respectively. apoA-I levels in the supernatant of endometrial explants maintained in serum-free cultures were determined using ELISA (AlerCHEK, Portland, ME, USA), according to the manufacturer's instructions, and the data were normalized to the weight of the explants in each culture. Serum apoA-I and high-density lipoprotein (HDL) levels were measured by the Clinical Pathology Laboratory at Hammersmith Hospital, London, UK.
Real-time quantitative PCR
Real-time quantitative PCR (RTQ-PCR) analysis was performed as described previously (Feroze-Zaidi et al., 2007). All measurements were performed in triplicate. The following gene-specific primer pairs, designed using the ABI Primer Express software, were used: apoA-I-sense (5′-GGC AGA GAC TAT GTG TCC CAG TT-3′) and apoA-I-antisense (5′-GTC CCA GTT GTC AAG GAG CTTT-3′); L19-sense (5′-GCG GAA GGG TAC AGC CAA T-3′) and L19-antisense (5′-GCA GCC GGC GCA AA-3′). RTQ-PCR analysis for β-actin has been described previously (Cornet et al., 2005).
Statistical analysis
Differences in protein peaks were analysed with Ciphergen Express Software, which uses the Mann–Whitney non-parametric test to calculate statistically significant differences across the groups (P < 0.05). In addition, the two-tailed Student's t-test (demographic details of patients groups) and Wilcoxon signed-rank matched-pairs test (endometriosis data) were used, as appropriate, to test for significance between groups.
Results
Proteomic profiling of mid-secretory endometrium in RIF and controls
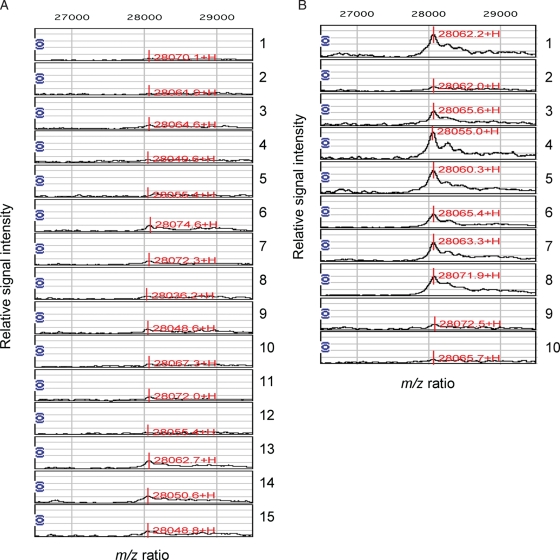
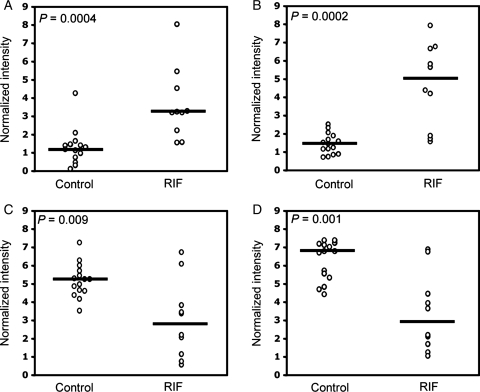
To provide insights into pathological mechanisms that interfere with embryo implantation, we used SELDI-TOF-MS to survey the endometrial proteome of 15 women with proven fertility (control group) and 10 patients with three or more consecutive IVF treatment failures that could not be attributed to poor embryo quality (RIF group). For practical reasons, samples were obtained during a 6-day period in the menstrual cycle (LH+5 to +10) that spans the putative window of implantation. The relative intensities of four protein peaks, all detected on the strong anionic exchange Q10 array (pH 5.0), were significantly different between the study and control groups. Figure 1 shows the normalized spectra of the single most discriminatory peak, corresponding to a protein of ∼28 063 Da, whereas Fig. 2 represents scatter plots of all four protein peaks. The relative intensities of two peaks, corresponding to proteins of ∼11 987 and ∼28 063 Da, were 2.7- and 4.2-fold higher in the study group compared with the control group, respectively (Fig. 2A and B). Conversely, the average intensities of the ∼15 867 and ∼16 075 Da peaks were significantly lower in samples from patients with multiple IVF treatment failure (Fig. 2C and D). The data show that protein fingerprints exist, capable of discriminating between RIF and control endometrium.
Figure 1.
Normalized SELDI-TOF-MS of mid-secretory endometrial biopsies from fertile women (A) and RIF patients (B) reveal a discriminatory 28 063 mass-to-charge (m/z) protein peak. Sample numbers are indicated on the right. The X-axis denotes the m/z values, whereas the Y-axis is a relative intensity scale. The mass spectra were obtained at pH 5.0 on Q10 chips, which capture positively charged proteins due to its negatively charged surface.
Figure 2.
Relative intensities of the four discriminatory protein peaks with m/z values of 11 987 (A), 28 063 (B), 15 867 (C) and 16 075 (D). Open circles represent the values of individual samples. Horizontal bars denote the median of normalized intensities.
Identification of apoA-I in receptive and unreceptive endometrium
As shown in Fig. 2B, the normalized intensities of the ∼28 kDa protein peak clustered together in all 15 control samples, whereas they were considerably higher in 7 out of 10 RIF samples. Therefore, we set out to characterize this ∼28 kDa protein peak, as we reasoned it could serve as the most informative biomarker of unreceptive endometrium. Briefly, protein lysates were subjected to reverse-phase fractionation, resolved by SDS–PAGE followed by in-gel trypsin digestion of the ∼28 kDa band and SELDI-TOF-MS analysis (data not shown). The resulting peptide mass fingerprints were submitted to ProFound (http://prowl.rockefeller.edu/prowl-cgi/profound.exe), taking in account potential protein modifications, such as methionine oxidation. The protein of interest was identified as apoA-I precursor, and this was unambiguously confirmed by Q-Star MS/MS analysis (data not shown). Notably, the apoA-I precursor encodes for a protein with a theoretical mass of 30 276 Da but, after cleavage of the peptide signal and pro-peptide, the molecular weight of mature apoA-I is 28 078 Da.
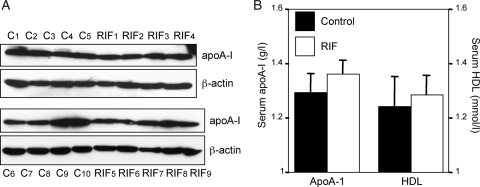
To validate these findings, we examined apoA-I expression levels by western blot analysis in 10 receptive and 9 unreceptive endometrial samples also used in the SELDI-TOF-MS screen. Although the abundance of apoA-I varied between samples, there was no apparent increase in endometrial biopsies obtained from RIF patients (Fig. 3A) or an obvious correlation with the SELDI-TOF-MS spectra (Fig. 1). The abundance of apoA-I transcripts in the endometrium was several magnitudes lower when compared with the liver (data not shown), raising the possibility that endometrial protein levels may, at least in part, reflect circulating levels. Although there was a trend towards higher apoA-I and HDL plasma levels in patients with RIF compared with fertile controls, this was not statistically significant (Fig. 3B). Thus, endometrial and circulating apoA-I levels were comparable between control and study patients, suggesting that the more efficient capture of apoA-I from RIF samples on the strong anionic exchange Q10 array may have been due to protein modifications, such as oxidation or sialylation, that enhance the negative charge of apoA-I.
Figure 3.
Endometrial apoA-I expression and plasma levels in fertile and infertile patients during the mid-luteal phase of the cycle. (A) Nineteen of the 25 endometrial samples used for SELDI-TOF-MS were resolved on SDS–PAGE and immunoblotted for apoA-I. Control samples from fertile patients are indicated as C1-10, whereas biopsies from patients with RIF are denoted RIF1-9. β-Actin served as loading control. (B) Circulating apoA-I and HDL levels at the time of biopsy were determined in serum samples of fertile women (controls, n = 15) and patients with RIF (n = 10); P > 0.05.
Expression and regulation of endometrial apoA-I is impaired in endometriosis
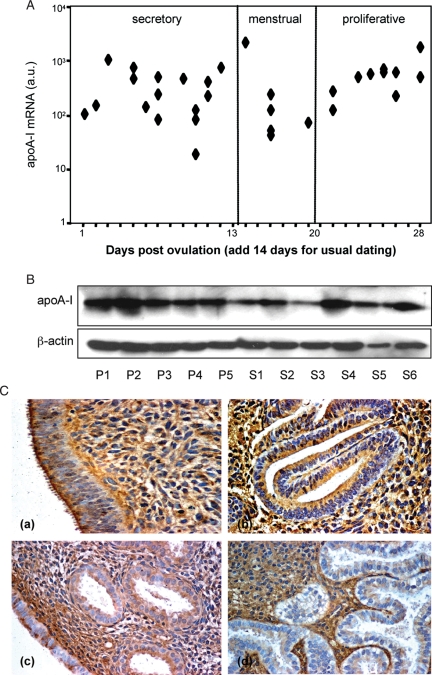
Although western blot analysis did not support a clear association of RIF with enhanced apoA-I levels, we set out to further characterize the expression and regulation of this lipoprotein in the endometrium for two reasons. First, apoA-I has potent anti-inflammatory properties (Hyka et al., 2001; Rohrer et al., 2006; Van Lenten et al., 2009), raising the possibility that the expression of this lipoprotein in the endometrium may be restricted to the peri-implantation window. Second, we recently identified apoA-I in the baboon uterus as a gene repressed in response to hCG infusion (Sherwin et al., 2007). We first examined whether apoA-I expression in the human endometrium is subject to cycle-dependent regulation. RTQ-PCR analysis revealed no significant differences in apoA-I mRNA levels between proliferative, secretory or menstrual endometrial samples (Fig. 4A; P > 0.05). Similarly, western blot analysis showed apoA-I expression throughout the cycle, although levels appeared more variable in secretory compared with proliferative endometrium (Fig. 4B). Immunohistochemistry of paraffin-embedded samples showed strong apoA-I staining in proliferative endometrium, which was equally prominent in the epithelial and stromal compartments (Fig. 4C). By the mid-secretory phase of the cycle, however, apoA-I immunoreactivity in the epithelial compartment was decreased and patchy, especially in the luminal epithelium, whereas staining in the stroma appeared largely unchanged. In view of the unaltered transcript levels, the lower and patchy immunostaining in epithelial cells suggests that apoA-I is actively secreted during the luteal phase of the cycle.
Figure 4.
apoA-I expression and tissue distribution in proliferative and secretory endometrium. (A) RTQ-PCR analysis of apoA-I mRNA expression throughout the cycle. The relative expression level of apoA-I transcripts, normalized to β-actin mRNA, was determined in 33 endometrial samples, spanning the entire menstrual cycle. The results are presented on a logarithmic scale. (B) Western blot analysis of apoA-I expression in proliferative (P1-5) and secretory (S1-6) endometrial samples. β-Actin served as loading control. (C) apoA-I immunolocalization in proliferative and secretory endometrium. apoA-I staining, performed on five proliferative endometrial samples (cycle days 8–12), was equally prominent in surface epithelium, glandular epithelium and the stromal compartment (a, b). In surface epithelium, apoA-I was expressed predominantly at the apical border of the cells (a). Immunostaining decreased markedly in the glandular but not stromal compartment in mid-secretory endometrium (cycle days 19–24; n = 6), whereas apoA-I expression in luminal epithelial cells appeared highly variable (c, d).
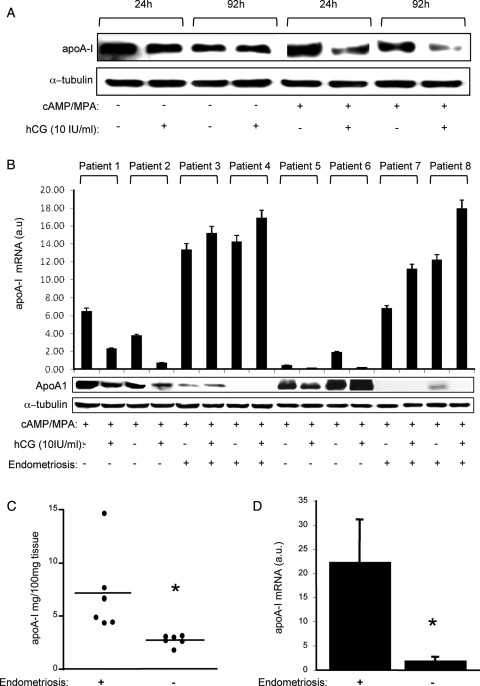
Next we examined whether embryonic signals, and more specifically hCG, antagonize apoA-I expression in the human endometrium, as has been reported for the baboon (Sherwin et al., 2007). To determine the optimal treatment conditions, the effect of hCG on apoA-I expression was first studied in undifferentiated primary endometrial stromal cells (ESCs) or cells differentiated for 24 or 72 h in response to 8-br-cAMP and MPA, a progestin (Jones et al., 2006). Intriguingly, hCG markedly reduced apoA-I levels in differentiating (decidualizing) but not in undifferentiated ESCs (Fig. 5A). Moreover, the effect of hCG on apoA-I expression in decidualizing cells was time- and dose-dependent and mimicked at transcript level (data not shown). To examine whether this hCG response is maintained within the context of a complex tissue, we established endometrial explant cultures from fresh biopsy samples. On the basis of the findings in primary ESCs, the explants were maintained in the presence of 8-br-cAMP and MPA and treated with or without hCG for 24 h. As shown in Fig. 5B, hCG was effective in inhibiting apoA-I mRNA and protein levels although, strikingly, not in explant cultures established from eutopic endometrium of patients with endometriosis. Moreover, endometrial explants from endometriosis patients were characterized by much higher apoA-I mRNA but lower protein levels, suggesting either a translational block or active secretion. To differentiate between these possibilities, fresh explant cultures were established and cultured in serum-free medium for 24 h. Analysis of the supernatant confirmed that apoA-I is more readily secreted by endometrial explants from patients with endometriosis compared with disease-free controls (Fig. 5C). Moreover, RTQ-PCR analysis of snap-frozen secretory endometrial samples confirmed that pelvic endometriosis is associated with increased expression of apoA-I mRNA transcripts in vivo (Fig. 5D).
Figure 5.
Regulation of endometrial apoA-I expression by hCG. (A) Undifferentiated human endometrial stroma cells (ESCs) and cultures decidualized with 8-Br-cAMP and MPA were co-treated with or without hCG for the indicated time-points. Total protein lysates were subjected to western blot analysis for apoA-I. The membranes were then probed for α-tubulin, a loading control. (B) Endometrial explants established from patients with and without endometriosis were cultured for 24 h in the presence of 8-Br-cAMP and MPA with or without hCG. Parallel cultures were harvested for western blot or RTQ-PCR analyses. (C) The supernatant of explant cultures established from eutopic endometrial samples from patients with and without endometriosis were analysed for apoA-I using ELISA. The levels were normalized to the weight of the explants. *P < 0.01. (D) apoA-I mRNA levels determined by RTQ-PCR in snap-frozen, luteal-phase, eutopic, endometrial samples from patients with (n = 5) and without pelvic endometriosis (n = 5); *P < 0.01.
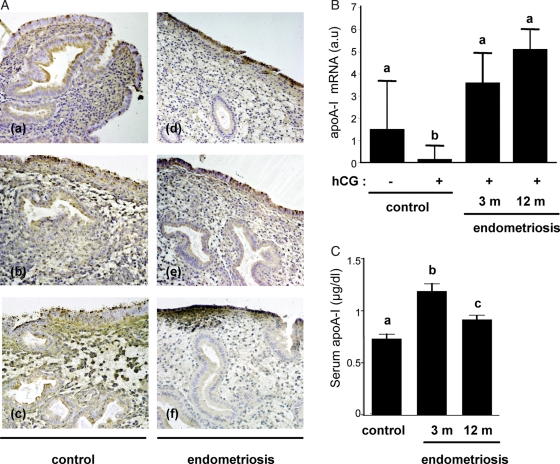
To corroborate these observations, the expression pattern of apoA-I in mid-secretory endometrial samples (LH+6 to +10) from patients with endometriosis (n = 8) was examined by immunohistochemistry and compared with disease-free controls (n = 8). As exemplified in Fig. 6A, apoA-I immunoreactivity in the luminal and glandular epithelial compartments was invariable patchy in control tissue sections. In contrast, six out of eight biopsies from endometriosis patients showed strong staining of the luminal epithelial cells, whereas the glands often showed little or no apoA-I expression.
Figure 6.
Endometriosis is associated with aberrant endometrial apoA-I expression in human and baboon endometria. (A) apoA-I staining in mid-secretory endometrium (cycle days 8–12) in disease-free controls (a–c; n = 8) and patients with endometriosis (d–f; n = 8). (B) RTQ-PCR analysis of endometrial RNA obtained from control animals (n = 4) and baboons with endometriosis (n = 5) following hCG infusion. Different letters above the error bars indicate that those groups are significantly different from each other at P< 0.05. (C) Sequential serum samples were obtained from baboons (n = 4) at 3 and 12 months following the induction of endometriosis and circulating apoA-I levels compared with those from control disease-free baboons (n = 4) at the same point during the menstrual cycle, i.e. between d 9 and 11 PO. Different letters above the error bars indicate that those groups are significantly different from each other at P< 0.05.
Next, we explored whether failure of hCG to antagonize endometrial apoA-I expression was a direct consequence of the presence of pelvic endometriosis. To this end, we determined endometrial apoA-I mRNA levels upon hCG infusion in the baboon uterus before and after the induction of pelvic endometriosis. As shown in Fig. 6B, endometrial apoA-I transcript levels remained elevated in response to in vivo uterine hCG infusion in baboons with endometriosis, whereas a significant decrease in mRNA expression was evident in disease-free animals. Interestingly, circulating serum levels of apoA-I also increased in baboons 3 and 12 months after induction of pelvic endometriosis (Fig. 6C). The data in Fig. 6B and C represent sampling of the same animals during the course of the disease process.
Discussion
apoA-I is best known as the major protein component of circulating HDL and the primary acceptor for cholesterol in extra-hepatic tissues. Yet, proteomic analyses of diverse tissue samples and body fluids other than serum have identified apoA-I as a protein dysregulated in a variety of diseases, ranging from pre-eclampsia, polycystic ovary syndrome and rheumatoid arthritis to a host of neurological disorders and cancers (Kozak et al., 2005; Yang et al., 2005; Ai et al., 2006; Corton et al., 2008; Giusti et al., 2008; Park et al., 2008; Trocme et al., 2008). The relative abundance of apoA-I captured on ProteinChip platforms often, but not always, correlates with in vivo expression levels, which in turn reflect either local synthesis or extravasation of serum proteins in a diseased tissue (Yang et al., 2005). Here, we investigated apoA-I as a putative biomarker of non-receptive endometrium, although its increased capture from RIF samples on a strong anionic exchange array did not correlate with either total endometrial levels by western blot or circulating apoA-I levels. It is conceivable that posttranslational modifications, such as phosphorylation, oxidation, sialylation and acylation (Hoeg et al., 1986; Holian et al., 1991; Millar, 2001; von Eckardstein et al., 2005), sufficiently impact on the isoelectric point of apoA-I to account for the differences in the SELDI-TOF spectra between control and RIF samples. The broad base of the 28-kDa apoA-I peak (Fig. 1) is indeed compatible with the presence of modified protein species.
Embryo implantation first involves binding of l-selectin expressed by the trophoblast to oligosaccharide ligands present on the endometrial surface followed by ligand-dependent interactions with other cell adhesion molecules, such as integrins and trophinin, that produce stable adherence of the blastocyst to the luminal epithelium (Fazleabas and Kim, 2003; Genbacev et al., 2003; Dey et al., 2004; Diedrich et al., 2007; Donaghay and Lessey, 2007). This mechanism is remarkably similar to that involved in rolling and subsequent adherence of leucocytes to vascular endothelium. It is well established that the serum apoA-I/HDL concentrations correlate inversely with the risk of cardiovascular disease and, conversely, that patients with lower levels of apoA-I are more likely to develop systemic inflammatory response syndrome after major trauma or surgery (Chenaud et al., 2004). These protective effects of apoA-I are at least partly due to its ability to inhibit the synthesis of major inflammatory mediators, such as tumour necrosis factor and interleukin-1β, and to block cytokine-induced expression of selectins and other cellular adhesion molecules on endothelium, thereby diminishing neutrophil adherence and subsequent tissue injury (Hyka et al., 2001; Chenaud et al., 2004; Liao et al., 2005; Shi and Wu, 2008; Van Lenten et al., 2009). On the basis of its role in vascular biology, it is tempting to speculate that apoA-I may also serve to restrict embryo implantation to a confined period in the cycle and, conversely, that aberrant endometrial secretion of this lipoprotein contributes to implantation failure. Several lines of evidence further support this notion. For example, female mice that lack the HDL receptor (scavenger receptor class B, type I) are infertile as a consequence of abnormal lipoprotein metabolism (Miettinen et al., 2001). Interestingly, fertility in these animals is restored not only upon administration of the cholesterol-lowering drug, probucol, but also upon simultaneous silencing of the Apoa1 gene. It is also striking that the postovulatory progesterone surge triggers in human endometrium the expression of several genes involved in lipid metabolism, including lipoproteins apoD, apoL2 and apoE (Talbi et al., 2006). Although we found no evidence that endometrial APOA1 expression is regulated by ovarian hormones, immunohistochemistry was compatible with secretion by differentiating endometrium during the luteal phase, an observation supported by the detection of apoA-I in the supernatant of endometrial explant culture. Finally, APOA1 was recently identified as one of relatively few peri-implantation genes repressed upon hCG infusion of the baboon uterus (Sherwin et al., 2007).
hCG is one of the earliest and most abundant glycoproteins secreted by embryonic trophoblast. Besides maintaining progesterone production by the ovarian corpus luteum during the first trimester of pregnancy, hCG exerts numerous direct effects on progesterone-primed endometrium that are essential for implantation (Licht et al., 2007). For example, hCG has been shown to enhance the expression of the pro-implantation cytokines prokeneticin 1 and LIF (Perrier d'Hauterive et al., 2004; Evans et al., 2009), to promote local angiogenesis (Berndt et al., 2006), to stimulate proliferation of specialized uterine natural killer cells (Kane et al., 2009) and to inhibit apoptosis in decidualizing ESCs (Jasinska et al., 2006). The observation that hCG inhibition of endometrial apoA-I expression is a conserved response in humans and other primates further underscores the importance of this lipoprotein as an anti-implantation factor.
Perhaps, the most compelling evidence for a causal role of apoA-I in implantation failure came from the observation that endometriosis is associated with elevated apoA-I mRNA levels, aberrant expression in mid-secretory endometrium, increased secretion by differentiating eutopic endometrial explant cultures and lack of hCG-dependent inhibition. Endometriosis is foremost a pelvic inflammatory disorder (Bulun, 2009) and a major cause of infertility. A meta-analysis of pregnancies after IVF showed that conception rate in women with endometriosis is approximately half that in patients with tubal-factor infertility (Barnhart et al., 2002). In addition to its aberrant expression in eutopic endometrium, characterized by enhanced and decreased apoA-I immunoreactivity in luminal and glandular epithelial compartments, respectively, apoA-I levels are reportedly also higher in the peritoneal fluid of patients with endometriosis (Ferrero et al., 2007), suggesting that enhanced secretion of this anti-inflammatory/anti-implantation lipoprotein in both eutopic and ectopic endometria is a hallmark of the disease.
Whether or not pelvic inflammation accounts for abnormal gene expression in the eutopic endometrium during the peri-implantation period has been a matter of considerable debate (Giudice and Kao, 2004). Here, we show that surgical induction of endometriosis in the baboon is sufficient to persistently enhance basal endometrial apoA-I mRNA expression and to abrogate its inhibition by hCG. Moreover, increased expression of apoA-I transcripts upon induction of endometriosis in the baboon coincided with an increase in circulating apoA-I levels. Interestingly, fasting serum apoA-I but not HDL levels are also significantly higher in women with endometriosis compared with healthy controls (Crook et al., 1997), which may reflect a hepatic response to elevated levels of circulating inflammatory mediators (Akoum et al., 1996; Bedaiwy et al., 2002).
This study has raised a number of important questions. For example, the mechanism of hCG inhibition of endometrial apoA-I expression, and its failure in endometriosis, is unknown and warrants further investigation. As alluded to, apoA-I is subject to numerous posttranslational modifications (Hoeg et al., 1986; Holian et al., 1991; Millar, 2001; von Eckardstein et al., 2005), which may or may not differ between fertile women and RIF patients. These modifications require further characterization, as they may profoundly impact on the anti-inflammatory/anti-implantation properties of uterine apoA-I (von Eckardstein et al., 2005).
In summary, proteomic analysis of timed endometrial samples from fertile women and patients with unexplained RIF yielded a distinct proteomic fingerprint that may discriminate between receptive and unreceptive endometrium. Our results complement other recent attempts at characterizing the receptive endometrial proteome (Dominguez et al., 2009). Whether or not proteomic fingerprinting can be used clinically to predict the likelihood of pregnancy after IVF treatment is under evaluation in a prospective study. We also identified apoA-I as a putative anti-implantation factor secreted by differentiating endometrium and inhibited by embryonic signals. Finally, we demonstrated that pelvic endometriosis increases apoA-I expression in secretory endometrium and perturbs its regulation by hCG, an observation that provides new insights into the mechanisms of implantation failure and subfertility associated with this debilitating condition. Together, the data suggest that dysregulation or altered modification of apoA-1 may be a point of conversion in various pathologies associated with infertility, such as endometriosis and RIF.
Funding
This project was supported by funds from Schering, CONRAD/CICCR (the Female AMPPA-Project) and NIHR Biomedical Research Centre funding scheme to J.J.B, F.F.-Z. and P.R.L; Grant-in-Aid 19591909 and 20591928 from the Ministry of Education, Science, and Culture, Japan, to T.K; and the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD 40093 (to A.T.F.) and U54 HD035041 (to S.L.Y. and B.A.L.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Authors' roles
The study was designed by J.J.B., who also wrote the manuscript. A.H., F.F.-Z. and P.R.L. performed the proteomic analysis. M.S.S, F.F.-Z. and T.K. performed the analysis of apoA-I in vitro. F.F.-Z., L.F., J.H., G.L.R, S.L.Y. and B.A.L. phenotyped the patients, obtained samples and interpreted the data. J.R.A.S. and A.T.F. were responsible for the animal experiments and data analysis. All authors contributed to the writing of the manuscript.
Acknowledgements
We thank Remi Oke, Anna Brown, Ewa Soltys, Jenny Steel, Gail Grossman, Angela Houwing and Julia Francis for their technical assistance and Professors Steven Smith, Anne Soutar and Anne Dell for their insightful suggestions. We are also grateful to the patients who kindly provided endometrial samples.
References
- Ai J, Tan Y, Ying W, Hong Y, Liu S, Wu M, Qian X, Wang H. Proteome analysis of hepatocellular carcinoma by laser capture microdissection. Proteomics. 2006;6:538–546. doi: 10.1002/pmic.200500257. [DOI] [PubMed] [Google Scholar]
- Akoum A, Lemay A, McColl SR, Paradis I, Maheux R. Increased monocyte chemotactic protein-1 level and activity in the peripheral blood of women with endometriosis. Le Groupe d'Investigation en Gynecologie. Am J Obstet Gynecol. 1996;175:1620–1625. doi: 10.1016/s0002-9378(96)70115-1. [DOI] [PubMed] [Google Scholar]
- Aoki R, Fukuda MN. Recent molecular approaches to elucidate the mechanism of embryo implantation: trophinin, bystin, and tastin as molecules involved in the initial attachment of blastocysts to the uterus in humans. Semin Reprod Med. 2000;18:265–271. doi: 10.1055/s-2000-12564. [DOI] [PubMed] [Google Scholar]
- Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–1155. doi: 10.1016/s0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17:426–431. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- Berndt S, Perrier d'Hauterive S, Blacher S, Pequeux C, Lorquet S, Munaut C, Applanat M, Herve MA, Lamande N, Corvol P, et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 2006;20:2630–2632. doi: 10.1096/fj.06-5885fje. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–4820. doi: 10.1210/endo.140.10.7070. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: a nonsteroidal approach to contraception. Proc Natl Acad Sci USA. 2005;102:8585–8590. doi: 10.1073/pnas.0502343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenaud C, Merlani PG, Roux-Lombard P, Burger D, Harbarth S, Luyasu S, Graf JD, Dayer JM, Ricou B. Low apolipoprotein A-I level at intensive care unit admission and systemic inflammatory response syndrome exacerbation. Crit Care Med. 2004;32:632–637. doi: 10.1097/01.ccm.0000114820.47460.0a. [DOI] [PubMed] [Google Scholar]
- Cornet PB, Galant C, Eeckhout Y, Courtoy PJ, Marbaix E, Henriet P. Regulation of matrix metalloproteinase-9/gelatinase B expression and activation by ovarian steroids and LEFTY-A/endometrial bleeding-associated factor in the human endometrium. J Clin Endocrinol Metab. 2005;90:1001–1011. doi: 10.1210/jc.2004-1277. [DOI] [PubMed] [Google Scholar]
- Corton M, Botella-Carretero JI, Lopez JA, Camafeita E, San Millan JL, Escobar-Morreale HF, Peral B. Proteomic analysis of human omental adipose tissue in the polycystic ovary syndrome using two-dimensional difference gel electrophoresis and mass spectrometry. Hum Reprod. 2008;23:651–661. doi: 10.1093/humrep/dem380. [DOI] [PubMed] [Google Scholar]
- Crook D, Howell R, Sidhu M, Edmonds DK, Stevenson JC. Elevated serum lipoprotein(a) levels in young women with endometriosis. Metabolism. 1997;46:735–739. doi: 10.1016/s0026-0495(97)90115-3. [DOI] [PubMed] [Google Scholar]
- Denker HW. Implantation: a cell biological paradox. J Exp Zool. 1993;266:541–558. doi: 10.1002/jez.1402660606. [DOI] [PubMed] [Google Scholar]
- DeSouza L, Diehl G, Yang EC, Guo J, Rodrigues MJ, Romaschin AD, Colgan TJ, Siu KW. Proteomic analysis of the proliferative and secretory phases of the human endometrium: protein identification and differential protein expression. Proteomics. 2005;5:270–281. doi: 10.1002/pmic.200400920. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Diedrich K, Fauser BC, Devroey P, Griesinger G. The role of the endometrium and embryo in human implantation. Hum Reprod Update. 2007;13:365–377. doi: 10.1093/humupd/dmm011. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Sharkey AM, Tan YL, Salamonsen LA, Sherwin JR. Immunolocalisation of phosphorylated STAT3, interleukin 11 and leukaemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window. Reprod Biol Endocrinol. 2007;5:44. doi: 10.1186/1477-7827-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez F, Garrido-Gomez T, Lopez JA, Camafeita E, Quinonero A, Pellicer A, Simon C. Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated. Hum Reprod. 2009;24:2607–2617. doi: 10.1093/humrep/dep230. [DOI] [PubMed] [Google Scholar]
- Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med. 2007;25:461–475. doi: 10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal–maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazleabas AT, Kim JJ. Development. What makes an embryo stick? Science. 2003;299:355–356. doi: 10.1126/science.1081277. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA. 1999;96:2543–2548. doi: 10.1073/pnas.96.5.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann NY Acad Sci. 2002;955:308–317. doi: 10.1111/j.1749-6632.2002.tb02791.x. Discussion 340–302, 396–406. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril. 2003;80(Suppl. 2):820–827. doi: 10.1016/s0015-0282(03)00982-8. [DOI] [PubMed] [Google Scholar]
- Feroze-Zaidi F, Fusi L, Takano M, Higham J, Salker MS, Goto T, Edassery S, Klingel K, Boini KM, Palmada M, et al. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology. 2007;148:5020–5029. doi: 10.1210/en.2007-0659. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Gillott DJ, Remorgida V, Anserini P, Leung KY, Ragni N, Grudzinskas JG. Proteomic analysis of peritoneal fluid in women with endometriosis. J Proteome Res. 2007;6:3402–3411. doi: 10.1021/pr060680q. [DOI] [PubMed] [Google Scholar]
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast l-selectin-mediated adhesion at the maternal–fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Giusti L, Iacconi P, Ciregia F, Giannaccini G, Donatini GL, Basolo F, Miccoli P, Pinchera A, Lucacchini A. Fine-needle aspiration of thyroid nodules: proteomic analysis to identify cancer biomarkers. J Proteome Res. 2008;7:4079–4088. doi: 10.1021/pr8000404. [DOI] [PubMed] [Google Scholar]
- Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, et al. Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene. 2008;27:9–19. doi: 10.1038/sj.onc.1210626. [DOI] [PubMed] [Google Scholar]
- Hoeg JM, Meng MS, Ronan R, Fairwell T, Brewer HB., Jr Human apolipoprotein A-I. Post-translational modification by fatty acid acylation. J Biol Chem. 1986;261:3911–3914. [PubMed] [Google Scholar]
- Hohn HP, Donner AJ, Denker HW. Endometrial receptivity: selective adhesion competence of rabbit uterine epithelium for trophoblast but not for various tumor cells. Cells Tissues Organs. 2003;173:204–216. doi: 10.1159/000070376. [DOI] [PubMed] [Google Scholar]
- Holian O, Kumar R, Attar B. Apoprotein A-1 is a cofactor independent substrate of protein kinase C. Biochem Biophys Res Commun. 1991;179:599–604. doi: 10.1016/0006-291x(91)91413-7. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Pellicer A, Simon C. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum Reprod Update. 2007;13:77–86. doi: 10.1093/humupd/dml046. [DOI] [PubMed] [Google Scholar]
- Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, III, Roux-Lombard P, Burger D. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- Jasinska A, Strakova Z, Szmidt M, Fazleabas AT. Human chorionic gonadotropin and decidualization in vitro inhibits cytochalasin-d-induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology. 2006;147:4112–4121. doi: 10.1210/en.2005-1577. [DOI] [PubMed] [Google Scholar]
- Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci USA. 2006;103:16272–16277. doi: 10.1073/pnas.0603002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by hCG and mediated via the mannose receptor. Endocrinology. 2009;150:2882–2888. doi: 10.1210/en.2008-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Su F, Whitelegge JP, Faull K, Reddy S, Farias-Eisner R. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–4596. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- Liao XL, Lou B, Ma J, Wu MP. Neutrophils activation can be diminished by apolipoprotein A-I. Life Sci. 2005;77:325–335. doi: 10.1016/j.lfs.2004.10.066. [DOI] [PubMed] [Google Scholar]
- Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocrinol. 2007;269:85–92. doi: 10.1016/j.mce.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Dey SK. HB-EGF: a unique mediator of embryo–uterine interactions during implantation. Exp Cell Res. 2009;315:619–626. doi: 10.1016/j.yexcr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch VJ, Tanzer A, Wang Y, Leung FC, Gellersen B, Emera D, Wagner GP. Adaptive changes in the transcription factor HoxA-11 are essential for the evolution of pregnancy in mammals. Proc Natl Acad Sci USA. 2008;105:14928–14933. doi: 10.1073/pnas.0802355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen HE, Rayburn H, Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest. 2001;108:1717–1722. doi: 10.1172/JCI13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JS. The sialylation of plasma lipoproteins. Atherosclerosis. 2001;154:1–13. doi: 10.1016/s0021-9150(00)00697-3. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod. 2005;20:2104–2117. doi: 10.1093/humrep/dei051. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kimura T, Koyama S, Ogita K, Tsutsui T, Shimoya K, Taniguchi T, Koyama M, Kaneda Y, Murata Y. Mouse model of human infertility: transient and local inhibition of endometrial STAT-3 activation results in implantation failure. FEBS Lett. 2006;580:2717–2722. doi: 10.1016/j.febslet.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Park JS, Oh KJ, Norwitz ER, Han JS, Choi HJ, Seong HS, Kang YD, Park CW, Kim BJ, Jun JK, et al. Identification of proteomic biomarkers of preeclampsia in amniotic fluid using SELDI-TOF mass spectrometry. Reprod Sci. 2008;15:457–468. doi: 10.1177/1933719108316909. [DOI] [PubMed] [Google Scholar]
- Perrier d'Hauterive S, Charlet-Renard C, Berndt S, Dubois M, Munaut C, Goffin F, Hagelstein MT, Noel A, Hazout A, Foidart JM, et al. Human chorionic gonadotropin and growth factors at the embryonic–endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum Reprod. 2004;19:2633–2643. doi: 10.1093/humrep/deh450. [DOI] [PubMed] [Google Scholar]
- Pohnke Y, Schneider-Merck T, Fahnenstich J, Kempf R, Christian M, Milde-Langosch K, Brosens JJ, Gellersen B. Wild-type p53 protein is up-regulated upon cyclic adenosine monophosphate-induced differentiation of human endometrial stromal cells. J Clin Endocrinol Metab. 2004;89:5233–5244. doi: 10.1210/jc.2004-0012. [DOI] [PubMed] [Google Scholar]
- Quinn CE, Casper RF. Pinopodes: a questionable role in endometrial receptivity. Hum Reprod Update. 2009;15:229–236. doi: 10.1093/humupd/dmn052. [DOI] [PubMed] [Google Scholar]
- Rahnama F, Thompson B, Steiner M, Shafiei F, Lobie PE, Mitchell MD. Epigenetic regulation of E-cadherin controls endometrial receptivity. Endocrinology. 2009;150:1466–1472. doi: 10.1210/en.2008-1142. [DOI] [PubMed] [Google Scholar]
- Rohrer L, Cavelier C, Fuchs S, Schluter MA, Volker W, von Eckardstein A. Binding, internalization and transport of apolipoprotein A-I by vascular endothelial cells. Biochim Biophys Acta. 2006;1761:186–194. doi: 10.1016/j.bbalip.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, Fazleabas AT. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology. 2007;148:618–626. doi: 10.1210/en.2006-0832. [DOI] [PubMed] [Google Scholar]
- Shi N, Wu MP. Apolipoprotein A-I attenuates renal ischemia/reperfusion injury in rats. J Biomed Sci. 2008;15:577–583. doi: 10.1007/s11373-008-9258-7. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–1331. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- Trocme C, Marotte H, Baillet A, Pallot-Prades B, Garin J, Grange L, Miossec P, Tebib J, Berger F, Nissen MJ, et al. Apolipoprotein A-I and platelet factor 4 are biomarkers for Infliximab response in rheumatoid arthritis. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten BJ, Wagner AC, Anantharamaiah GM, Navab M, Reddy ST, Buga GM, Fogelman AM. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11:52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello D, Kodaman PH, Taylor HS. HOX genes in implantation. Semin Reprod Med. 2007;25:431–436. doi: 10.1055/s-2007-991040. [DOI] [PubMed] [Google Scholar]
- von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–152. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- Yang JW, Czech T, Gelpi E, Lubec G. Extravasation of plasma proteins can confound interpretation of proteomic studies of brain: a lesson from apo A-I in mesial temporal lobe epilepsy. Brain Res Mol Brain Res. 2005;139:348–356. doi: 10.1016/j.molbrainres.2005.06.010. [DOI] [PubMed] [Google Scholar]