Abstract
A homozygous missense mutation, C566T, in the follicle stimulation hormone receptor (FSHR) gene has been linked to premature ovarian failure. The disease leads to infertility in a normal karyotype female with an elevated follicle stimulating hormone (FSH) and decreased serum estrogen level. Female mice carrying mutated FSHR gene, called follitropin receptor knockout (FORKO), display similar phenotype and are sterile because of a folliculogenesis block at a primary stage. We investigated the effects of bilateral intra-ovarian injection of an adenovirus expressing a normal copy of human FSHR on the reproductive system of 6–10 weeks female FORKO mice. Ad-LacZ was injected directly into each ovary of the control group. Animals were sacrificed at 2, 4, 8 and 12 weeks post-injection and tissues collected for evaluation. Treated mice showed estrogenic changes in daily vaginal smear whereas control animals remained fixated in the diestrus stage. Histological evaluation showed on average 26 ± 4 follicles/ovary in treated group with 8 ± 2 follicles at the antral stage compared with only 5 ± 2 with zero follicles at antral stage in Ad-LacZ control mice. There was no significant change in serum level of progesterone, however, estrogen level increased 2–3-fold (P < 0.02) and FSH decreased by up to 50% (P < 0.04) in treated animals. FSHR mRNA was detected in the ovaries of the treated group. In conclusion, intra-ovarian injection of an adenovirus expressing human FSHR gene is able to restore FSH responsiveness and reinitiate ovarian folliculogenesis as well as resume estrogen production in female FORKO mice. Ad-LacZ injections indicate the absence of systemic viral dissemination or germ line transmission of adenovirus DNA to offspring.
Keywords: FORKO mice, follicle-stimulating hormone receptor, gene therapy of ovarian failure, primary amenorrhea, primary ovarian failure (POF)
Introduction
Folliculogenesis and ovulation are arrested in the pre-pubertal ages, and they are induced by follicle stimulating hormone (FSH), through FSH receptor (FSHR) at puberty (Aittomaki et al., 1995). FSHR-mediated FSH signaling is considered to be essential for follicular development and maturation in females, and for initiation of spermatogenesis and maintenance of quantitatively normal sperm production in adult males (Tapanainen et al., 1997). FSH is a member of the glycoprotein hormone family, which also includes luteinizing hormone (LH), human chorionic gonadotrophin (hCG) and thyrotrophic hormone. FSH and LH are essential for gonadal development and maturation, as well as for gamete production during the pre-pubertal and fertile phases of life in both males and females (Simoni et al., 1997). These gonadotroph hormones are synthesized in the same cells of the pituitary gland (Pierce and Parsons, 1981; Bousfield et al., 1994), and act through their corresponding receptors. FSHRs are localized on the surface of sertoli cells in the testes and granulosa cells in the ovary (Pierce and Parsons, 1981; Bousfield et al., 1994; Simoni et al., 1997). Human FSHR consists of 10 exons spanning 85 kb of genomic DNA (Sprengel et al.,1990; Minegishi et al., 1991; Kelton et al., 1992), located on chromosome 2p21–p16 (Rousseau-Merck et al., 1993; Gromoll et al., 1994). Exons 1–9 encode the extracellular domain of the protein and exon 10 encodes the proximal portion of the extra cellular domain, the transmembrane domain and the intracellular domain (Simoni et al., 1997). The first mutation identified in the FSHR gene was an inactivating point mutation (C566T), resulting in an Ala189Val change (Aittomaki et al., 1995). This missense mutation occurs in a region encoding the extracellular ligand-binding domain of the receptor and is located in a highly conserved region of FSHR in humans and other species such as monkey, sheep and rat (Sprengel et al., 1990; Gromoll et al., 1993; Yarney et al., 1993), which suggests that it is of high functional importance. Transfection experiments with such mutated FSHR showed a dramatic decrease in the response to FSH by reduced cyclic adenosine monophosphate production and binding capacity (Aittomaki et al., 1995). Males homozygous for the C566T mutation have variable degrees of spermatogenic failure but do not show azoospermia or absolute infertility (Tapanainen et al., 1997). On the other hand, young women homozygous for the C566T mutation are diagnosed with premature ovarian failure due to resistant ovary syndrome (ROS). They typically present with hypergonadotrophic primary amenorrhea, variable degrees of development of secondary sex characterizations, normal internal and external genitalia and normal karyotype (Aittomaki et al., 1995). The disorder is heterogeneous with autosomal recessive mode of inheritance in most cases (Simpson et al., 1971). To date, similar clinical manifestations have been reported in patients with other mutations in different regions of the hFSHR gene (Beau et al., 1998; Touraine et al., 1999; Doherty et al., 2002; Allen et al., 2003). In these women ovarian follicular growth is arrested, and follicle atresia continues (Aittomaki et al., 1995). Affected females are infertile, and there is no effective treatment at the present time, other than symptomatic relief with hormone replacement therapy. This condition is devastating for women who are interested in becoming pregnant. There is little chance of these women achieving a spontaneous pregnancy. Ovulation stimulation with human menopausal gonadotrophin treatment has been unsuccessful (Talbert et al., 1984). The only method for women with ROS to become pregnant is through using donated oocytes followed by in vitro fertilization (Wentz, 1996). Oocyte donation, however, is prohibitively expensive, and many women are ethically opposed to this method. Additionally, the child will not be genetically related to at least one of the parents (Wentz, 1996).
Replication-incompetent adenoviral vectors (Ad) have emerged as very popular and safe vectors for human gene therapy and are the most commonly used vehicle in clinical trials (Hallenbeck and Stevenson, 2000). Our earlier laboratory studies demonstrated the optimal ability of Adenovirus to transfect both human and Eker rat derived uterine leiomyoma cells (ELT3), and thus pointed out the potential use of adenovirus (Ad)-mediated gene therapy for uterine leiomyoma (Al-Hendy et al., 2004). In Eker rat, an immunocompetent animal model of uterine leiomyoma; we demonstrated that the Ad-HSV1TK/GCV approach was very efficient in decreasing uterine leiomyoma volume (Hassan et al., 2009). Applying mutation compensation gene-therapy strategy via delivery of dominant negative estrogen receptor by Adenovirus vehicle to perturb the estrogen-signaling pathway was very encouraging (Al-Hendy et al., 2004; Hassan et al., 2007, 2009). Additionally, Ad-transfection was localized and safe to non-target tissues which confirmed the safety.
We have recently (Ghadami et al., 2008) constructed an adenovirus vector expressing full length normal human FSHR gene (Ad-hFSHR), and have demonstrated its ability to mediate FSH responsiveness to various cell lines that are lacking internal FSHR. Additionally, Ad-hFSHR restored FSH responsiveness to cells transfected with the inactivating C566T point mutation indicating that Ad-hFSHR responds to FSH and can functionally transcomplement C566T-mutated hFSHR in various cell lines (Ghadami et al., 2008).
In the current study, we investigated the effects of bilateral intra-ovarian injection of Ad-hFSHR on the reproductive system and ovarian function in female mice with deleted FSHR gene, so-called follitropin receptor knockout (FORKO) mouse. FORKO mouse is an appropriate animal model for studying human hypergonadotropic ovarian dysgenesis and infertility. The phenotype of FSHR(−/−) mouse is reminiscent of human ROS (Dierich et al., 1998; Danilovich et al., 2000). Female FORKO mice display thin uteri, small ovaries, elevated serum level of FSH, decrease in estrogen and are sterile due to a block in ovarian folliculogenesis at the primary stages of follicular development (Dierich et al., 1998; Danilovich et al., 2000).
Materials and Methods
Preparation of Ad-hFSHR
Ad-hFSHR was constructed and virus stocks were propagated and prepared as we have described earlier (Ghadami et al., 2008). Ad-hFSHR was amplified on QBI-HEK-293 and purified using ultra centrifuged by a double cesium chloride step as previously described (Salama et al., 2007). A typical Ad-hFSHR prep containing about 1011 pfu/ml was obtained. Ad-LacZ was prepared in a similar fashion to serve as a vehicle control.
Animals
FSHR deficient mice (FSHR−/−) were generated as described by Dierich et al. (1998). A FORKO breeding colony is established at Meharry Medical College and all animals used in this work were generated from that colony. Animals were housed in the Meharry Medical College Animal Care Facility and provided free access to food and water. The Meharry Medical College Institutional Animal Use and Care Committee approved all procedures involving animal care, euthanasia and tissue collection. Mice heterozygous for FSHR (FSHR+/−) were mated and offsprings were genotyped to determine the zygosity status (Danilovich et al., 2000). Six to ten weeks old female FORKO mice were used for both Ad-hFSHR treated and control Ad-LacZ injected in this experiment.
To assess the ability of the adenovirus to infect our target cells (granulosa cells) in vivo, we conducted animal experiments in both the athymic nude mice as well as in the normal immune-competent C57BL/6N mice (Jackson Laboratory, Bar Harbor, ME, USA). An adenoviral vector that carries a marker gene coding for bacterial β-galactosidase gene, Ad-LacZ (a kind gift from Dr Savio Woo, Mount Sinai School of Medicine, New York) was used. After laparotomy, Ad-LacZ (3 × 109 PFU) was delivered by direct intraovarian injection into the left ovary using a G30 Hamilton syringe mounted on a screw-actuated micrometer device (Harvard apparatus, Holliston, MA, USA).The dose used was based on preliminary safety and toxicity experiments assessing the ability of adenovirus to infect murine granulosa cells and calculated at 100 pfu/cell using the general rule of 108 cells/cm3 solid organ. The left ovary was then collected from mice at 1, 2, 3 and 4 week time points and subjected to X-gal staining as described previously (Al-Hendy et al., 2005).
Female FORKO mice were anesthetized using inhalation isoflurane induction. The abdominal area were shaved and cleaned with betadine disinfectant. A small 1–2 cm incision was made in the skin and fascia and abdomen entered sharply. The ovaries were identified under operating microscope delivered in the operative field. The ovaries were then injected with the therapeutic factor (Ad-hFSHR or Ad-LacZ) and returned in the pelvis. About 3 × 109 pfu of Ad-hFSHR, or Ad-LacZ were injected directly into the each ovary of treated and control animals, respectively, using a G30 Hamilton syringe mounted on a screw-actuated micrometer device (Harvard apparatus).
The fascia was closed using interrupted sutures and/or skin stapled. Sterile techniques were used all the time. After surgery, animals were allowed to recover for 1 week. Subsequently, all mice were weighed daily and tested for estrus cycle phases using vaginal smears as described previously (Maeda et al., 2000; Ohkura et al., 2000). All procedures were approved by animal care facilities at Meharry Medical College.
Ad-FSHR treated and Ad-LacZ control animals were mated with age related normal males at different time points of experiments and assessed for possible ovulation and pregnancy that may happened at any time during the experiment. Organ samples (brain, lung, heart, liver, spleen, femur, adipose, ovary, uterus, vagina and cervix), were collected at 2, 4, 8 and 12-week time points. Organ samples were weighed and stored at −70°C until further processing. Organ weight was considered as a percentage of total body weight. A portion of organ samples were fixed at 10% formalin for 16 h and used for hematoxylin/eosin staining and histological evaluations.
Injection of PMSG/hCG and searching oviducts for the release of oocytes and corpera lutea formation
Female mice in control and treated groups (N = six/group) were subjected to an ovulation induction protocol detailed by Serge-Alain et al. (1998). Briefly, folliculogenesis was induced in each mouse with an intra-peritoneal (IP) injection of 2.5 IU of pregnant mare serum gonadotrophin (PMSG). Forty-eight hours post-PMSG injection, ovulation was induced in each mouse with an IP injection of 2.5 IU of hCG. Subsequently, mice were sacrificed between 10 and 13 h post-hCG by CO2 asphyxiation, following which uterus/oviduct complexes were excised and trimmed of fat. Oviducts were separated from the uterine horns with the aid of a dissecting microscope and flushed with Dulbecco supplemented with 0.1% hyaluronidase (Sigma Chemical Co., St Louis, MO, USA) that was pre-incubated at 37°C, into a watch glass. Individual oviductal flushings were examined with Nikon TMD inverted microscope at 400× magnification for the presence of ovulated ova.
Safety and toxicity studies
To evaluate any potential toxicity or major adverse events secondary to direct intraovarian inoculation of adenovirus vectors, we conducted a series of safety studies.
We treated a group of mice by unilateral (left) intraovarian injection of adenovirus carrying LacZ as described above. Multiple organ samples were collected to test for possible dissemination of adenovirus. We stained all organs by X-gal and then examined multiple sections for β-galactosidase expression using an in situ β-galactosidase staining kit (Stratagene, La Jolla, CA, USA). This method consists of the following brief steps: tissues were fixed for 10 min at room temperature in 2% formaldehyde and 0.2% (v/v) glutaraldehyde. After washing twice in PBS, the tissues were stained for 4 h with the staining solution containing 1 mg/ml 5-Bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal). To detect the presence of adenovirus DNA, we amplified the essential E4 region of the virus genome (forward primer: TGTGACTGATTGAGCGGTG and reverse primer: CCCATTTAACACGCCATGCA) as we have described earlier (Al-Hendy et al., 2005). PCR performed for 30 cycles at 94°C for 30 s, 54°C for 30 s and 72°C for 30 s. The presence of adenovirus was documented by the presence of the expected fragment of 714 bp on 1% agarose gel. We also tested if intraovarian-delivered adenovirus might inadvertently transduce the ova, theoretically leading to transmission to the next generation. We treated a group of mice by bilateral intraovarian injection of adenovirus as described above. After recovering for 2 weeks, they were mated with age- and size-matched males. Fertilization was confirmed by screening for the presence of sperm plugs in the vagina as described previously (Maeda et al., 2000). We then collected E12 (gestational Day 12 embryos) for X-gal staining and adenovirus E4 PCR analysis. Another group of these mice was allowed to proceed with pregnancy and delivery with no intervention. All the resulting pups were collected and examined closely for number, weight or any grossly visible congenital anomalies.
Hormone assays
Blood samples were collected from treated and control animals before and after treatment. Serum FSH, LH, estrogen and progesterone levels where measured. FSH measurements were performed by radioimmunoassay (RIA) techniques using reagents provided by Dr A.F. Parlow and the National Hormone and Peptide Program and procedures validated earlier (Gay et al., 1970). Mouse FSH reference prep was used for assay standards and Mouse FSH antiserum (guinea pig) diluted to a final concentration of 1:400 000 was used as primary antibody. The secondary antibody purchased from Equitech-Bio, Inc. was diluted to a final concentration of 1:25. The assay has a sensitivity of 2.0 ng/ml and less than 0.5% cross-reactivity with other pituitary hormones. The intra-assay and inter-assay coefficients of variations (CVs) are 6.9 and 13.3%, respectively. LH was measured in serum by a modified supersensitive two-site sandwich immunoassay (Haavisto et al., 1993; Fallest et al., 1995) using monoclonal antibodies MAB1 against bovine LH and TMA against the human LH-beta subunit (Medix Kauniainen, Finland) as described previously (Haavisto et al., 1993). The tracer antibody (kindly provided by Dr Janet Roser, Department of Animal Science, University of California, Davis; Matteri et al., 1987) was iodinated by the chloramine T method and purified on Sephadex G-50 columns. The capture antibody was biotinylated and immobilized on avidin-coated polystyrene beads (7 mm; Nichols Institute, San Juan Capistrano, CA, USA). Mouse LH reference preparation provided by Dr A.F. Parlow and the National Hormone and Peptide program was used as standard. The assay has a sensitivity of 0.07 ng/ml, and the average intra-assay and inter-assay CVs for the Quality Controls are 3.6 and 9.2%, respectively. The concentrations of estradiol-17β (E2) and progesterone (P4) were measured in sera, with commercially available RIA kits according to the manufacturer's instructions (Diagnostic Laboratories Inc., Webster, TX, USA). The sensitivity for E2 assay was 4.7 pg/ml and the intra-assay CVs were 5.3, 5.3 and 3.2% for low, medium and high E2 containing samples, respectively. The inter-assay CVs for E2 assay for the aforementioned samples were 8.1, 9.3 and 8.1%, respectively. The sensitivity for P4 assay was 0.1 ng/ml and the intra-assay CVs were 6.4, 3.3, 5.6% for low, medium and high P4 containing samples and the inter-assay CVs for the above mentioned samples containing P4 were 2.4, 1.7 and 3.3%, respectively.
Ovarian histology
The ovaries were fixed in 10% formalin overnight and embedded in paraffin. Serial sections of 5 µm thick were stained with hematoxylin and eosin for light microscopic histology. In all samples the fifth cut was chosen to count the number of follicles and to evaluate follicular development. Follicles were classified as pre-antral if they contained an oocyte with a visible nucleolus, more than one layer and less than five layers of granulosa cells and lacked an antral space. Follicles were classified as antral if they contained an oocyte with a visible nucleolus, more than five layers of granulosa cells and/or an antral space as described previously (Britt et al., 2000).
FSHR RNA isolation and RT–PCR analysis
Tissues were collected from treated and control animals immediately after euthanasia, snap-frozen and stored at −70°C until further processing. Total RNA was extracted from ovarian tissue using the RNeasy Mini Kit (QIAGEN, Inc., Valencia, CA, USA) according to the manufacturer's protocol. cDNA was generated using 0.5–1 µg of total RNA using an Omniscript reverse transcriptase kit (QIAGEN) and Oligo-dT primer according to the manufacturer's protocols. Subsequent PCR analysis was carried out on 40 µl of the cDNA, and the products were analyzed by electrophoresis on a 1% agarose gel. Primer sequences for FSHR product were as follows: (F) 5′-TCTCCTTGCTGGCATTCTTG-3′ and (R) 5′-CAAGACATCATTCTGAGAGATCTCTA-3′. This primer set specifically amplifies a 230 bp region of FSHR gene which is deleted in FORKO animals.
Statistical analysis
Data are presented as the mean ± SEM and were analyzed by Student t-test with a Fischer least square difference post hoc test using P≤ 0.05 as the level of significance.
Results
Adenovirus vector is able to infect the mouse ovary in vivo
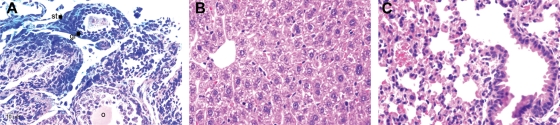
X-gal staining of the ovaries injected by Ad-LacZ revealed blue staining, which suggests viral transfection and β-galactosidase expression in granulosa cells as well as in stromal cells in ovaries (Fig. 1A). As shown in Fig. 1A, whole follicles have been transfected successfully. There was persistent blue staining of granulosa and stromal cells up to 4 weeks post-inoculation which is the last time point tested. There was never demonstrable blue staining of the germ cells (ova) in any of the ovaries treated with Ad-LacZ.
Figure 1.
(A) Ad-LacZ was effective in infecting both granulosa (gr) cells and stromal (st) cells when delivered by direct intra-ovarian injection in nude mice (108 PFU/ovary), (o) indicates ooplasm; (B) Section of liver and (C) lung from nude mice treated by direct intraovarian injection with Ad-LacZ (103 PFU/ovary). They appear healthy with no LacZ (blue) expression. These photos were taken 4 weeks post-inoculation, H&E, ×400.
In vivo transfection of the mice ovary does not lead to systemic viral dissemination
No blue staining was detected by X-gal staining in the liver (Fig. 1B) and lungs (Fig. 1C) of treated animals. We also detected no such staining in the uterus, vagina, contra lateral ovary, spleen, kidney or brain (data not shown). We were able to amplify adenovirus E4 DNA only from the injected left ovary and, to a lesser degree, from adjacent oviduct while all other organs including contralateral ovary were negative (Fig. 2). These results were reassuring that intraovarian-delivered adenovirus was not associated with systemic viral dissemination.
Figure 2.
For safety and toxicity evaluation of direct intraovarian inoculation of adenovirus vectors, a group of mice were treated by unilateral (left) intraovarian injection of adenovirus. Multiple organ samples were collected to detect adenovirus DNA 1 week post-intra-ovarian injection. The presence of adenovirus was documented by PCR amplification of 714 bp fragment from the essential E4 region of the virus genome documented on 1% agarose gel. Adenoviral specific DNA was detectable only in the injected ovary (OV) and adjacent oviduct (T). Positive controls are shown on the left side. The following organs were run in the middle of the figure and were negative for E4 DNA: uterus, vagina, contralateral ovary, spleen, liver, lung, kidney and brain.
No detectable germ line transmission of adenovirus DNA
After intra-ovarian injection of Ad-LacZ, a group of treated female mice were mated with size and age matched normal males as described in materials and methods. There was no statistically significant difference in number or weight of the resulting pups compared with a matched sham (laparotomy but no ovarian injection) mice. Additionally, using Adenovirus E4 PCR, we found no evidence of adenovirus transmission to any embryo in this experiment, and there were no detectable congenital anomalies. These results suggest that direct intra-ovarian injection of adenovirus is not associated with germ line virus DNA transmission.
PMSG/hCG injection
Ova were not detected in oviductal flushings of neither control nor treated FORKO mice, indicating that the observed gene therapy-induced resumption in folliculogenesis based on increases in serum estrogen concentrations was not accompanied by ovulation.
Mating with age related males
After recovery from surgery and PMSG-hCG injection of treated and control animals, mating with age related normal males did not result in pregnancy for either group within the 12 weeks trial period of this experiment. We also searched the oviducts (under the microscope) for possible oocyte release and no oocytes were found in either the treated or the control group. The ovary histology survey also did not reveal any corpera lutea in either group, indicating absence of ovulation.
Vaginal smear and body weight changes
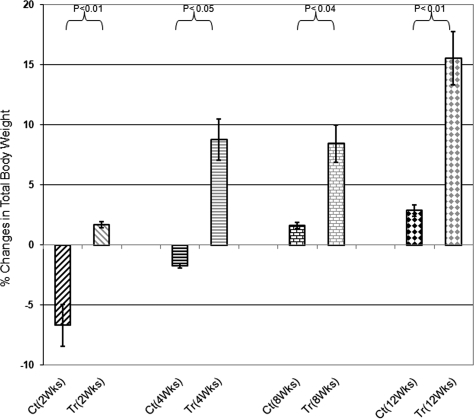
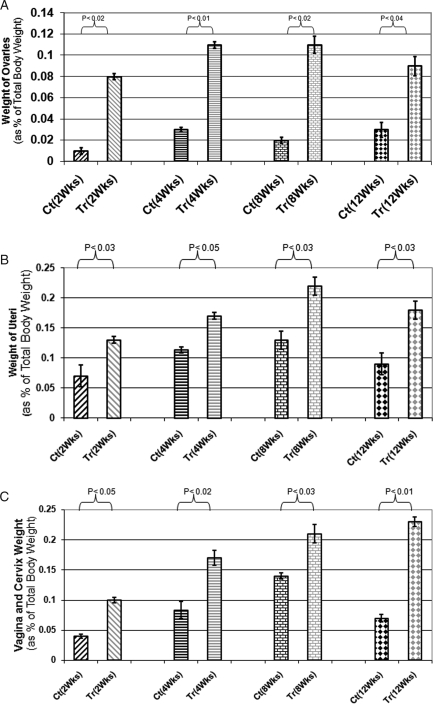
A week after intraovarian injection of Ad-FSHR, treated animals started to show changes in vaginal smears though the changes were not as regular as normal animals (i.e. longer or shorter than normal estrus cycle). However, all phases of diestrus, pro-estrus, estrus, metestrus were observed in animals treated with Ad-hFSHR although cyclicity in control animals remained unchanged through out the experiment and arrested at diestrus phase. As expected, both treated and control animals lost weight immediately after surgery (Fig. 3). However, the treated animals reached their pre-surgery weight faster and gained more weight by the end of the experiment (P < 0.03) at different time points of the experiment as shown in Fig. 3. Reproductive organs which are highly modulated by estrogen showed remarkable increase in weight at all time points of experiment, as shown in Fig. 4A–C. Statistical analysis revealed P < 0.02, 0.04 and 0.03, respectively, for ovary, uterus and vagina and cervix for treated versus control animals whereas changes in other organ weights were not significant (data not shown). These changes are consistent with increased estrogen levels in treated animals.
Figure 3.
Changes of total body weight in treated (Tr) versus Control (Ct) animals at different time points. Control groups at Weeks 2 and 4 after treatment lost weight although body weight in treated group increased after 2 weeks and continue to increase by 12 weeks of experiment (P < 0.02).
Figure 4.
(A) Ovaries weight of treated (Tr) versus control (Ct) animals. Weight considered as percentage of total body weight for each animal: as indicated weight of ovaries significantly increased at all time points of experiments (P < 0.02); (B) Uterus weight of treated (Tr) versus control (Ct) animals: weight considered as percentage of total body weight for each animal: uteri weight of treated animals increased at all time points of experiments up to 12 weeks (P < 0.04); (C) Vagina and Cervix weight of treated (Tr) versus control (Ct) animals: weight considered as percentage of total body weight for each animal. As indicated vagina and cervix weight increased in treated animals compared with control animals (P < 0.03).
Ad-hFSHR treatment restores folliculogenesis
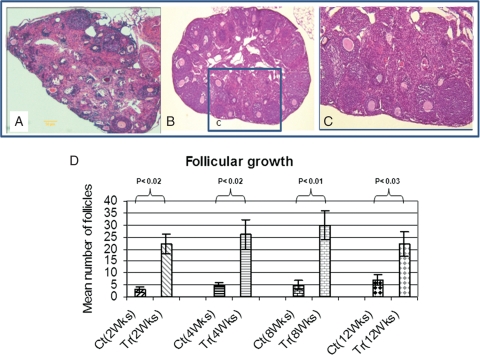
Females FORKO have an overall reduced size of the ovaries and typically fail to develop antral follicles (Balla et al., 2003). In the ovaries of Ad-FSHR treated animals (Fig. 5B), both the total number of follicles and the number of antral follicles were significantly higher than those from Ad-LacZ control group ovaries (Fig. 5A). On average 26 ± 4 follicles/ovary were observed in Ad-hFSHR treated group of which 8 ± 2 follicles were at the antral stage whereas only 5 ± 2 follicles observed in Ad-LacZ control group, with zero follicles at antral stage (P < 0.02, Fig. 5D). No corpus luteum was observed in any of the treated animals (Fig. 5C). No documented pregnancy was observed in any of the animals in either the treated or control groups.
Figure 5.
Development of the ovary in Ad-LacZ control animal (A) compared with Ad-FSHR treated animal (B). Both the total number of follicles and the number of antral follicles are significantly higher in Ad-FSHR treated than Ad-LacZ control group. Histological evaluation showed on average 26 ± 4 follicles/ovary in treated group with 8 ± 2 follicles at the antral stage compared with only 5 ± 2 with zero follicles at antral stage in Ad-LacZ control mice. Photos were taken at the same magnification (×600); (C) is an area from treated ovary (inset marked—c) shown at 10× higher magnification; (D) Mean number of follicles at different time points of experiments. As shown the total numbers of follicles are significantly increased in treated versus control animals (P < 0.02). The most number of follicle increase observed at 8 weeks of treatment.
Circulating hormone levels
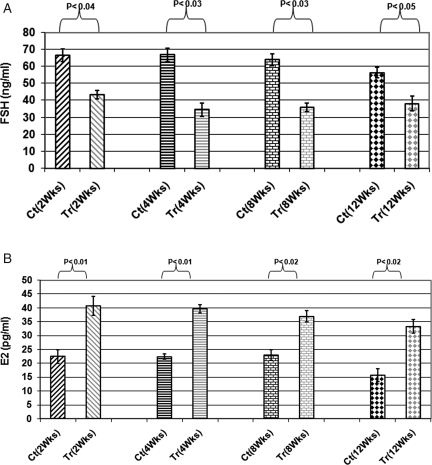
FORKO female mice have a high level of serum FSH, a drastic increase of at least 15-fold compare to normal (Dierich et al., 1998), because of the lack of the regulatory feedback at the level of FSHR-dependent ovarian follicles. As shown in Fig. 6A, the serum FSH level decreased 40–50% in Ad-hFSHR treated animals at different time points up to 12 weeks post-virus inoculation (P < 0.04). Female FORKO animals suffer sever hypoestrogenemia due to arrest of ovarian folliculogenesis (Dierich et al., 1998). Ad-hFSHR resulted in a significant increase in serum estrogen level by 2–3-fold compared with Ad-LacZ treatment (P < 0.02, Fig. 6B). Again, these improvement in serum estrogen levels persisted for up to 12 weeks Ad-hFSHR post-treatment. There were no statistically significant changes in serum progesterone and LH in the serum of treated or control group were not significant (data not shown).
Figure 6.
(A) Serum FSH of treated (Tr) versus Cotrol (Ct) animals at different time points. Ad-FSHR treated animals have shown 40–50% decrease in serum FSH compared with Ad-LacZ treated animals (P < 0.04); (B) Serum estrogen of treated (Tr) versus control (Ct) animals at different time points. Ad-FSHR treated animals have shown 2–3-fold increased in serum estrogen compared with Ad-LacZ treated at all time points of experiment (P < 0.02).
FSHR expression
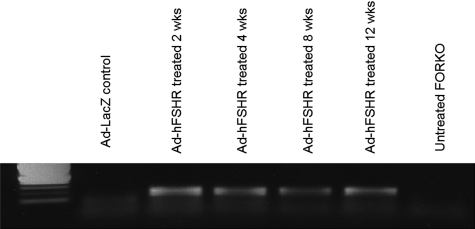
To confirm that the observed reproductive changes are due to Ad-hFSHR-mediated FSHR expression, we evaluated hFSHR expression in treated versus control mice. As shown in Fig. 7, RNA extracted from the ovaries of treated and control animals showed that FSHR is in fact expressed in the ovaries of treated mice at corresponding time point whereas no expression was observed in Ad-LacZ treated group. Additionally, no expression of FSHR was observed in any of the other tested organs of treated group (data not shown). This finding suggests that the expression of FSHR in treated animals is responsible for the increased serum estrogen levels and subsequently the reduction in serum level of FSH in Ad-FSHR treated group compared with the Ad-LacZ group.
Figure 7.
Expression of FSHR mRNA at different time points of experiment in treated animals and controls. As shown, there is no FSHR mRNA expression in Ad-LacZ treated and untreated controls. But FSHR mRNA was expressed in Ad-FSHR treated animals up to 12 weeks.
Discussion
Ovarian failure is the most common cause of primary amenorrhea. In the presence of normal karyotype, ovarian failure is usually due to ROS. Affected women typically present with primary amenorrhea, high serum level of FSH and arrested folliculogenesis in the ovary as judged by transvaginal ultrasound or ovarian histology. There is no treatment for this condition at this time, only symptom management. If pregnancy is desired, the only option available is in vitro fertilization with donor oocytes. These processes, besides being ethically unacceptable to some couples, are expensive, have a modest success rate, and expose both the donor and recipient to considerable medical risks. Additionally, the infant resulting from the oocyte donation will not be genetically related to the recipient mother. There is a need for improved management options for this devastating condition. Recent findings that mutations in FSHR gene can cause ROS indicate that gene therapy could be a potentially effective option in these patients who desire future fertility. The goal of our work is to evaluate the utility of FSHR gene therapy in an animal model for ROS. FSH has markedly different effects on follicular growth and maturation at various developmental stages of follicles. Initiation of follicular growth in mice and rats does not require FSH (Halpin et al., 1986). Also FSHR gene is not expressed in non-growing human follicles (Oktay et al., 1997) but FSH is needed for further follicular development between the primary and the antral stage. FSH is essential for gonadal maturation and development in both males and females and acts through its corresponding receptor: the FSHR. FSHRs are localized on the surface of sertoli cells in the testes and granulosa cells in the ovary (Pierce and Parsons, 1981; Bousfield et al., 1994; Simoni et al., 1997). An inactivating mutation in exon 7 of the FSHR gene causes ovarian failure due to ROS. The disorder is heterogeneous with autosomal recessive mode of inheritance in most cases (Simpson et al., 1971).
Adult FORKO female mice are infertile and estrogen deficient (Dierich et al., 1998). The phenotype of FSHR(−/−) mice, that is, anovulatory and infertile is reminiscent of human ROS (Dierich et al., 1998). In this study, we have shown the effects of Ad-hFSHR treatment on folliculogenesis and ovulation in female FORKO mice after a single intraovarian inoculation.
Our vector is able to reduce serum FSH levels in FORKO mice by 40–50% and increase serum estrogen levels by 2–3-fold. Treated animals have further developed follicles compared with controls. Significant increase in total body weight and the weight of reproductive organs (ovary, uterus and vagina), which are highly modulated by estrogen, were observed in treated animals. The expression of FSHR, which is deleted in FORKO mice, was demonstrated in the ovaries of FORKO mice after Ad-hFSHR treatment.
The fact that intra-ovarian injection of Ad-LacZ was not associated with systemic viral dissemination, and no effect of Ad-LacZ ovarian delivery on fecundity or pregnancy outcome in mice is encouraging and is in agreement with prior reports (Rhee et al., 2004; Al-Hendy et al., 2005). Another theoretical concern in this experimental approach is the possibility of germ line transmission of viral genes via the oocyte DNA. Again, when Ad-LacZ was delivered directly to the ovary, no adenoviral or LacZ gene or gene products were detected in resulting pups, which suggests no germ line transmission.
Our treatment with adenovirus carrying FSHR gene was able to induce hormonal changes in FORKO model. However, ovulation or pregnancy did not take place within 12 weeks trial period following treatment. We injected both treated and control animals with PMSG followed by hCG injection, but no oocytes were released in the oviducts and no corpera lutea were formed. Animals were mated with age matched normal males, but none of them got pregnant. There are potentially several explanations for this observation. The full follicular maturation and ovulation process is a very intricate and complicated process. There is intimate inverse regulatory loop between FSHR and LHR in the growing follicle to orchestrate a successful maturation and ovulation. One of the limitations of our vector is that the hFSHR is under control of the strong constitutive CMV5 promoter. Clearly such a design will not allow the expected down-regulation of hFSHR later on in the follicular cycle to support increased expression of LHR, which in turn will mediate cellular changes to facilitate ovulation. To overcome such limitation, we plan future experiments with an improved Ad-hFSHR vector using the authentic human FSHR promoter.
Our study showed that intraovarian injection of Ad-LacZ does not negatively affect the ability of our animal model to reproduce. The Ad-hFSHR recombinant viral construct was able to restore FSHR function, leading to follicular development and partial correction of hormonal (estrogen and FSH) abnormalities. These data indicate that intra-ovarian injection of Ad-hFSHR vector in FORKO mice restored ovarian folliculogenesis to antral stage but not to the ultimate ovulation. Further investigation is needed to fully develop gene and possibly cell therapy as a safe and effective treatment option for ovarian causes of female infertility.
Authors' Roles
A.A.-H. was the principal investigator in this study and all parts of the study was done under his leadership. M.G. was the main researcher in this study. He planned the study, expanded the FORKO colony, prepared adenovirus vectors, performed gene therapy and surgery, collected the data, evaluated the results and wrote the manuscript draft. E.E.-D. participated in colony expansion, performing gene therapy and surgery and evaluating histopathology results. S.A.S. participated in vector expansion. A.A.B. and B.R.B. participated in histological evaluation of the results. A.E.A. participated in hormones assay. The knock out FORKO mice was first established by X.C. and M.R.S. in 1999 and they have provided the appropriate expertise in maintaining our FORKO colony.
Funding
This study was supported by NIH R21HD046639 grant.
Acknowledgement
We appreciate Dr Veera Rajaratnam, Director of Scientific Publications at the Center for Women's Health Research, Meharry Medical College, for her excellent editing and expertise rendered for graphics of this manuscript.
References
- Aittomaki K, Lucerna JL, Pakarinen P, Sistonen P, Tapanainen JS, Gromoll J, Kaskikari R, Sankila EM, Lehvaslaiho H, Engel AR. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191:1621–1631. doi: 10.1016/j.ajog.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Wang H, Salama SA. Towards Gene Therapy of Ovarian Failure: Intraovarian injected adenovirus successfully transduced granulosa and stromal but not germ cells. J Soc Gynecol Investig. 2005;12 Ref Type: Abstract. [Google Scholar]
- Allen LA, Achermann JC, Pakarinen P, Kotlar TJ, Huhtaniemi IT, Jameson JL, Cheetham TD, Ball SG. A novel loss of function mutation in exon 10 of the FSH receptor gene causing hypergonadotrophic hypogonadism: clinical and molecular characteristics. Hum Reprod. 2003;18:251–256. doi: 10.1093/humrep/deg046. [DOI] [PubMed] [Google Scholar]
- Balla A, Danilovich N, Yang Y, Sairam MR. Dynamics of ovarian development in the FORKO immature mouse: structural and functional implications for ovarian reserve. Biol Reprod. 2003;69:1281–1293. doi: 10.1095/biolreprod.103.015552. [DOI] [PubMed] [Google Scholar]
- Beau I, Touraine P, Meduri G, Gougeon A, Desroches A, Matuchansky C, Milgrom E, Kuttenn F, Misrahi M. A novel phenotype related to partial loss of function mutations of the follicle stimulating hormone receptor. J Clin Invest. 1998;102:1352–1359. doi: 10.1172/JCI3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfield GR, Perry WM, Ward DN. Gonadotropins: chemistry and biosynthesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1749–1792. [Google Scholar]
- Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK. An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology. 2000;141:2614–2623. doi: 10.1210/endo.141.7.7578. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141:4295–4308. doi: 10.1210/endo.141.11.7765. [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty E, Pakarinen P, Tiitinen A, Kiilavuori A, Huhtaniemi I, Forrest S, Aittomaki K. A Novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metab. 2002;87:1151–1155. doi: 10.1210/jcem.87.3.8319. [DOI] [PubMed] [Google Scholar]
- Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone beta gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod. 1995;53:103–109. doi: 10.1095/biolreprod53.1.103. [DOI] [PubMed] [Google Scholar]
- Gay VL, Midgley AR, Jr, Niswender GD. Patterns of gonadotropin secretion associated with ovulation. Fed Proc. 1970;29:1880–1887. [PubMed] [Google Scholar]
- Ghadami M, Salama SA, Khatoon N, Chilvers R, Nagamani M, Chedrese PJ, Al-Hendy A. Toward gene therapy of primary ovarian failure: adenovirus expressing human FSH receptor corrects the Finnish C566T mutation. Mol Hum Reprod. 2008;14:9–15. doi: 10.1093/molehr/gam077. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Weinbauer GF, Simoni M, Nieschlag E. Effects of antiandrogens and ethane dimethane sulphonate (EDS) on gene expression, free subunits, bioactivity and secretion of pituitary gonadotrophins in male rats. Mol Cell Endocrinol. 1993;91:119–125. doi: 10.1016/0303-7207(93)90263-j. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Dankbar B, Gudermann T. Characterization of the 5' flanking region of the human follicle-stimulating hormone receptor gene. Mol Cell Endocrinol. 1994;102:93–102. doi: 10.1016/0303-7207(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132:1687–1691. doi: 10.1210/endo.132.4.8462469. [DOI] [PubMed] [Google Scholar]
- Hallenbeck PL, Stevenson SC. Targetable gene delivery vectors. Adv Exp Med Biol. 2000;465:37–46. doi: 10.1007/0-306-46817-4_4. [DOI] [PubMed] [Google Scholar]
- Halpin DM, Jones A, Fink G, Charlton HM. Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic-pituitary ovarian axis. J Reprod Fertil. 1986;77:287–296. doi: 10.1530/jrf.0.0770287. [DOI] [PubMed] [Google Scholar]
- Hassan MH, Salama SA, Arafa HM, Hamada FM, Al-Hendy A. Adenovirus-mediated delivery of a dominant negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. J Clin Endocrinol Metab. 2007;92:3949–3957. doi: 10.1210/jc.2007-0823. [DOI] [PubMed] [Google Scholar]
- Hassan M, Zhang D, Salama F, Hamada S, Arafa H, Fouad H, Walker C, Al-Hendy A. Towards fibroid gene therapy: adenovirus-mediated delivery of herpes simplex virus 1 thymidine kinase gene/ganciclovir shrinks uterine leiomyoma in the Eker rat model. Gynecol Obstet Invest. 2009;68:19–32. doi: 10.1159/000209675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelton CA, Cheng SV, Nugent NP, Schweickhardt RL, Rosenthal JL, Overton SA, Wands GD, Kuzeja JB, Luchette CA, Chappel SC. The cloning of the human follicle stimulating hormone receptor and its expression in COS-7, CHO, and Y-1 cells. Mol Cell Endocrinol. 1992;89:141–151. doi: 10.1016/0303-7207(92)90220-z. [DOI] [PubMed] [Google Scholar]
- Maeda K, Ohkura S, Tsukamura H. Physiology of Reproduction. In: Krinke GJ, editor. The Laboratory Rat. Stein, Switzerland: Academic Press; 2000. pp. 145–173. [Google Scholar]
- Matteri RL, Roser JF, Baldwin DM, Lipovetsky V, Papkoff H. Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domest Anim Endocrinol. 1987;4:157–165. doi: 10.1016/0739-7240(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Minegishi T, Nakamura K, Takakura Y, Ibuki Y, Igarashi M, Minegish T. Cloning and sequencing of human FSH receptor cDNA. Biochem Biophys Res Commun. 1991;175:1125–1130. doi: 10.1016/0006-291x(91)91682-3. [DOI] [PubMed] [Google Scholar]
- Ohkura S, Tsukamura H, Maeda K. Endocrinology. In: Krinke GJ, editor. The Laboratory Rat. Stein, Switzerland: Academic Press; 2000. pp. 401–415. [Google Scholar]
- Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82:3748–3751. doi: 10.1210/jcem.82.11.4346. [DOI] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Rhee GS, Lee HJ, Kim SS, Kwack SJ, Lee RD, Seok JH, et al. Reproductive safety evaluation and quantification of adenoviral vectors in the mouse. Mol Ther. 2004;9:S172. [Google Scholar]
- Rousseau-Merck MF, Atger M, Loosfelt H, Milgrom E, Berger R. The chromosomal localization of the human follicle-stimulating hormone receptor gene (FSHR) on 2p21-p16 is similar to that of the luteinizing hormone receptor gene. Genomics. 1993;15:222–224. doi: 10.1006/geno.1993.1041. [DOI] [PubMed] [Google Scholar]
- Salama S, Kamel M, Christman G, Wang H, Al-Hendy A. Gene therapy of uterine fibroids: Adenovirus-mediated Herpes Simplex Virus thymidine kinase/ganciclovir treatment inhibits growth of human and rat leiomyoma cells. Gynecol. Obstet. Invest. 2007;63:61–70. doi: 10.1159/000095627. Ref Type, Electronic Citation. [DOI] [PubMed] [Google Scholar]
- Serge-Alain W, Wood TL, Crawford J, Levison SW, Hammond JM. Expression of Mouse Ovarian Insulin Growth Factor System Components During Follicular Development and Atresia. Endocrinology. 1998;139:5205–5212. doi: 10.1210/endo.139.12.6367. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Christakos AC, Horwith M, Silverman FS. Gonadal dysgenesis in individuals with apparently normal chromosomal complements: tabulation of cases and compilation of genetic data. Birth Defects Orig Artic Ser. 1971;7:215–228. [PubMed] [Google Scholar]
- Sprengel R, Braun T, Nikolics K, Segaloff DL, Seeburg PH. The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol. 1990;4:525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- Talbert LM, Raj MH, Hammond MG, Greer T. Endocrine and immunologic studies in a patient with resistant ovary syndrome. Fertil Steril. 1984;42:741–744. doi: 10.1016/s0015-0282(16)48200-2. [DOI] [PubMed] [Google Scholar]
- Tapanainen JS, Aittomaki K, Min J, Vaskivuo T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205–206. doi: 10.1038/ng0297-205. [DOI] [PubMed] [Google Scholar]
- Touraine P, Beau I, Gougeon A, Meduri G, Desroches A, Pichard C, Detoeuf M, Paniel B, Prieur M, Zorn JR, et al. New natural inactivating mutations of the follicle-stimulating hormone receptor: correlations between receptor function and phenotype. Mol Endocrinol. 1999;13:1844–1854. doi: 10.1210/mend.13.11.0370. [DOI] [PubMed] [Google Scholar]
- Wentz AC. Resistant Ovary syndrome. In: Adachi A, Hancock JA, Rosenwaks Ze, editors. Reproductive Endocrinology, Surgery, and Technology. Philadelphia: Lippincott-Raven; 1996. pp. 13885–11392. [Google Scholar]
- Yarney TA, Sairam MR, Khan H, Ravindranath N, Payne S, Seidah NG. Molecular cloning and expression of the ovine testicular follicle stimulating hormone receptor. Mol Cell Endocrinol. 1993;93:219–226. doi: 10.1016/0303-7207(93)90127-6. [DOI] [PubMed] [Google Scholar]