Abstract
Gas2-like proteins harbour putative binding sites for both the actin and the microtubule cytoskeleton and could thus mediate crosstalk between these cytoskeletal systems. Family members are highly conserved in all metazoans but their in vivo role is not clear. The sole Drosophila Gas2-like gene, CG3973 (pigs), was recently identified as a transcriptional target of Notch signalling and might therefore link cell fate decisions through Notch activation directly to morphogenetic changes. We have generated a null mutant in CG3973 (pigs): pigs1 mutants are semi-viable but adult flies are flightless, showing indirect flight muscle degeneration, and females are sterile, showing disrupted oogenesis and severe defects in follicle cell differentiation, similar to phenotypes seen when levels of Notch/Delta signalling are perturbed in these tissues. Loss of Pigs leads to an increase in Notch signalling activity in several tissues. These results indicate that Gas2-like proteins are essential for development and suggest that Pigs acts downstream of Notch as a morphogenetic read-out, and also as part of a regulatory feedback loop to relay back information about the morphogenetic state of cells to restrict Notch activation to appropriate levels in certain target tissues.
Keywords: Cytolinker, Gas2-like, Notch signalling, Cytoskeleton, Follicle cell differentiation, Drosophila
INTRODUCTION
During development, signalling pathways are repeatedly used to assign different cell fates among identical precursors. Differentiating cells then undergo regulated changes in gene expression and cell morphology appropriate for their function in a tissue. Cell shape changes are largely mediated by the cytoskeleton. Downstream effectors of developmental signalling pathways thus have to impinge on the cytoskeleton to exert the desired changes.
Spatial and temporal coordination of cytoskeletal elements can be performed by ‘cytolinker’ proteins that have the ability to interact with more than one cytoskeletal system at a time (Leung et al., 2002; Leung et al., 2001; Röper et al., 2002). Only two proteins in Drosophila contain a combination of an actin-binding Calponin homology (CH) domain and a microtubule-binding domain of the Gas2 family. One is the fly spectraplakin Short stop (Gregory and Brown, 1998), and spectraplakins serve many important functions during tissue morphogenesis in both flies and mammals (Leung et al., 2002; Röper and Brown, 2003; Röper and Brown, 2004). The other protein is encoded by the gene CG3973, which we have named pigs (‘pickled eggs’, referring to the mutant phenotype, see Results). There are four close paralogues of Pigs in mammals, and these are the only proteins in mammals to have Gas2 domains apart from the two spectraplakins MACF1 and BPAG1 (DST — Human Gene Nomenclature Database). The first relative of Pigs identified in mammals was called Growth arrest specific 2 (Gas2), which also gave the domain its name (Brancolini et al., 1992). Although subsequent studies did not confirm a role for Gas2 in growth arrest induction, three further relatives were found and named Gas2-like 1 (Gas2L1), Gas2L2 and Gas2L3. All have CH and Gas2 domains (Fig. 1A) and can associate with both the actin and the microtubule cytoskeleton in tissue culture (Goryunov et al., 2007). However, the in vivo role of Gas2 or the Gas2-like proteins is unclear, and to date, no loss-of-function analysis has been reported in any species. As these proteins share with the spectraplakins the presence of both a Gas2 domain and an actin-binding CH domain, we suspect that they serve a similarly important, albeit non-overlapping, function in the regulation of the cytoskeleton. Thus, Drosophila, with only one Gas2-like homologue, seems an excellent system to analyse the function of this class of proteins, avoiding the possibility of redundant functions between paralogues masking phenotypes.
Fig. 1.
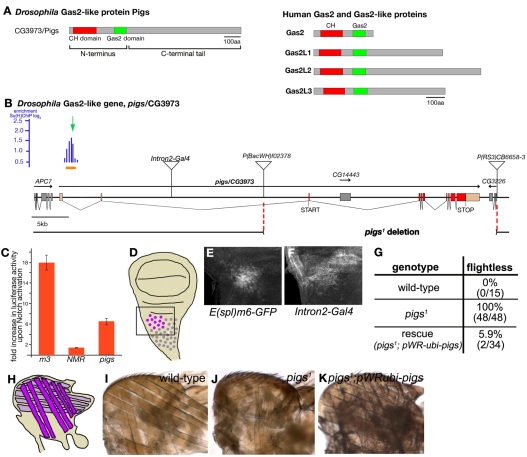
CG3973 (pigs) encodes the Drosophila orthologue of the Gas2-like protein family and is a downstream target of Notch signalling. (A) Protein domain structure of the fly Gas-like protein CG3973 (Pigs) in comparison with the four human Gas2-like proteins. (B) Gene locus of pigs. P-element insertions are indicated by triangles. The deletion in the pigs1 allele is shown below the sequence. Also shown is the region identified to contain binding sites for Su(H) (Krejci et al., 2009): blue bars indicate oligonucleotides hybridizing to enriched fragments in ChIP (height of bars indicate enrichment; AvgM log2, 0.3 to 2.3). (C) In S2 cells the pigs intron 1 region (‘pigs’), indicated by the orange bar in B, is sufficient to drive luciferase expression from a fusion construct in the presence of the Notch intracellular domain (NICD). Expression is compared to a known target of Notch, E(spl)m3 (‘m3’), and a mutated Notch responsive element (‘NMR’). Values are fold-activation relative to enhancer expression in the absence of the NICD. (D) Diagram depicting the region of myoblast precursors in third instar wing disc. The box indicates the areas shown in E and F. (E) Expression of a Notch activity reporter, E(spl)m6-GFP in the myoblast region of a wing disc. (F) A Gal4 trap P-element insertion into the second intron of pigs, Intron2-Gal4, reveals that pigs is expressed in the myoblast precursor region. (G) pigs1 mutant adults are flightless, but flight is restored when a ubiquitous pigs cDNA is expressed. (H) Schematic of the adult indirect flight muscles (both D and H modified from Sudarsan et al., 2001). (I-K) Indirect flight muscles severely degenerate in a pigs1 mutant (J) compared with wild-type (I) and this degeneration can be rescued by ubiquitous overexpression of a pigs cDNA (K).
The pigs (CG3973) gene has recently been identified as containing sites occupied by the transcription factor Supressor of Hairless [Su(H)] upon activation of Notch signalling (Krejci et al., 2009), suggesting that pigs could act as a direct downstream target of Notch. The Notch receptor is activated through one of its membrane-bound ligands, such as Delta, on neighbouring cells. The intracellular domain of Notch (NICD) is then cleaved and released, free to act as a transcriptional co-activator together with Su(H) (Bray, 2006). Notch signalling is required for many cell fate decisions during development, but relatively few genes directly activated by Notch signalling have been identified over the years. A recent study has identified what could be a comprehensive set of direct targets of Notch activation in Drosophila myogenic cells (Krejci et al., 2009). This study used genome-wide chromatin immunoprecipitation (ChIP) analysis with Su(H) antibodies to identify genomic regions occupied by Su(H). The first intron of pigs contained a peak of Su(H)-bound sites and thus might be a new target (Fig. 1B).
As most previously described targets of Notch are themselves transcription factors (Portin, 2002), we were intrigued to find a potential cytolinker protein as a possible direct target, and thus effector, of Notch signalling. Here we set out to analyse the phenotypes observed in the absence of Pigs function and to elucidate its potential role downstream of Notch. This study shows that, in addition to modifying the cytoskeleton and cell shape, Pigs appears to act as part of a feedback loop that negatively regulates the activity of Notch during morphogenesis in certain tissues.
MATERIALS AND METHODS
Fly stocks
The following fly stocks were used in this study: pWR-ubi-pigs and UASp-GFP-Pigs (see below); UASt-RNAi-Pigs p{GD11177}v34772 (VDRC); p{GawB}NP1005 (DGRC, Kyoto, Japan); p{GawB}NP2478 (DGRC); A101-lacZ (Bier et al., 1989); E(Spl)m6-GFP; E(Spl)m7-lacZ; E(Spl)mβ1.5-lacZ; and [Gbe+Su(H)]-lacZ (Cooper et al., 2000). The bab1-Gal4 line was a gift from Acaimo Gonzalez-Reyes (Bolivar et al., 2006).
Generation of the pigs1 allele
Two FRT-containing P-elements were used to generate the pigs1 allele by FLP-mediated FRT recombination: p{RS3}6658-3 (Szeged Drosophila Stock Centre) and pBac{WH}CG3973f02378 (Exelixis Drosophila Stock Collection). Males carrying pBac{WH}CG3973f02378 were mated with females carrying a FLP recombinase transgene. Progeny males carrying both the element and FLP recombinase were mated to females carrying p{RS3}6658-3. After 2 days of egg-laying, the progeny were heat-shocked for 1 hour at 37°C, followed by further heat shocks for 4 days. Virgin female progeny were selected to generate stocks. Candidate lines were screened by PCR and the pigs1 deletion was verified by sequencing the product of a PCR spanning the deletion (data not shown).
Cloning of pigs for rescue and overexpression experiments
The full-length cDNA construct RE60364 was used to generate all pigs constructs [Drosophila Gene Collection (DGC)]. Sequences were cloned into a modified P-element vector, pWhiteRabbit, containing the Ubiquitin-63E promoter pWRpAUbiqP (gift of Dr Nick Brown, University of Cambridge, UK) or into pUASp-GFP (Röper and Brown, 2003). Primers used were CG33973-NT (ATGGCCATGTTAGAGGAGA) and CG3973-CT (CCACTACTGTACGAATCCAAGGAG).
Luciferase assays
The enhancer fragment was amplified from Drosophila genomic DNA using primers CG3973-up (CTGGGGTAAAGGAGTGAAAGG) and CG3973-down (CTCGCAGTTAAAGCAAACAGC) and cloned into a luciferase vector containing a minimal Hsp70 gene promoter (pGL3::Min). Cell transfections were performed using Fugene transfection reagent (Roche), as described previously (Krejci et al., 2009). Three biological replicates were performed.
Ovary fixation and immunostaining
Ovaries were dissected from well-fed 2- to 4-day-old females unless stated otherwise. Freshly dissected ovaries were fixed at room temperature for 8 minutes in 8% ultrapure formaldehyde (Polyscience), rinsed twice in PBS and incubated for more than 1 hour in PBT (PBS + 0.3% Triton X-100 and 0.5% BSA) at room temperature. Primary and secondary antibodies in PBT were incubated overnight at 4°C.
Antibodies used were: anti-Shot-Spectrin Repeats (Strumpf and Volk, 1998) and anti-α-tubulin-FITC (DM1A; Sigma F2168). Additional antibodies from the Developmental Studies Hybridoma Bank at the University of Iowa used were: Armadillo, β-galactosidase, Engrailed, Eya, HtsF-1B1, Fasciclin III and NICD. Actin was labelled using Rhodamine-Phalloidin (Molecular Probes R-415); nuclei were labelled with DAPI. Secondary antibodies used were coupled to Alexa 488, Cy2, Cy3 and Cy5 (Molecular Probes and Jackson ImmunoResearch).
Confocal images were acquired using an Olympus FluoView 1000. Confocal laser, iris and amplification settings in experiments comparing intensities of labelling were set to identical values. Brightfield images were acquired on a Zeiss Axioskop microscope using a Jenoptik C14 camera and Openlab software (Improvision). Images were assembled in Adobe Photoshop and z-stacks and projections were assembled using ImageJ.
β-galactosidase staining
Ovaries were dissected in PBS and fixed in 15 mM KH2PO4-K2HPO4 (pH 6.8), 0.5% glutaraldehyde for 8 minutes with agitation and washed in PBS + 0.1% Triton X-100. Staining was carried out at 37°C in 10 mM NaH2PO4-Na2HPO4 (pH 7.2), 150 mM NaCl, 1 mM MgCl2, 3 mM K4[FeII(CN)6], 3 mM K3[FeIII(CN)6], 0.5% Triton X-100 and 0.2% X-gal, and ovaries mounted in 70% glycerol.
RESULTS
pigs (CG3973) encodes the fly orthologue of the Gas2-like protein family and is a direct target of Notch signalling
The assigned open reading frame pigs (CG3973) encodes the sole Drosophila orthologue of the human Gas2-like protein family. Like the human proteins, Pigs (CG3973) harbours a combination of a potentially actin-binding Calponin homology (CH) domain and a microtubule-binding Gas2-domain (Fig. 1A). The single predicted transcript contains several large introns, the first of which contains a region occupied by the Notch-dependent transcriptional regulator Su(H) in Drosophila myogenic cells, based on results from genome-wide ChIP experiments (Krejci et al., 2009). In those experiments, pigs (CG3973) expression was also upregulated upon Notch activation, hence its assignment as a direct Notch target.
To confirm that the identified region indeed responds to Notch stimulation, the corresponding fragment was cloned into a luciferase reporter vector containing a minimal promoter from the Hsp70 gene and transfected into S2 cells in the presence or absence of activated Notch. The intron 1 fragment of pigs consistently conferred a greater than 6-fold response to Notch (Fig. 1C), indicating that the pigs gene is indeed a direct target of Notch activation. Like the effects detected on the endogenous gene (Krejci et al., 2009), the magnitude of the response was less than that for a characterized E(spl) target but was significantly greater than a control enhancer (Krejci et al., 2009; Krejci and Bray, 2007).
The cell line used for the ChIP experiments, DmD8, appears closely related to wing disc muscle precursors. We thus investigated whether pigs was expressed in these cells in vivo. mRNA expression analysis of pigs [public databases (Arbeitman et al., 2002; Chintapalli et al., 2007)] and our own in situ data (data not shown) suggested that pigs mRNA was expressed at low levels in most tissues. To analyse pigs expression in wing discs we used two enhancer-trap Gal4 lines (Bellen et al., 1989; O'Kane and Gehring, 1987; Wilson et al., 1989). The enhancer traps were located in the first and second intron of pigs, termed Intron1-Gal4 and Intron2-Gal4 (Fig. 1B; only Intron2-Gal4 is shown). Both lines, when crossed to a UAS-GFP reporter line, showed expression of pigs in the notal region of the wing disc, where the adult flight muscle precursors, the myoblasts, reside, and colocalised with Cut labelling that marks the myoblasts (Fig. 1D-F; data not shown; for expression analysis in ovaries using Intron2-Gal4, see Fig. S1 in the supplementary material). Thus, in larval myoblasts where Notch is required to prevent premature differentiation (Anant et al., 1998), Pigs could be a direct effector of Notch activation.
Pigs is essential for development
To assess the in vivo function of Gas2-like proteins in general, and of Pigs (CG3973) in particular, we generated a null mutant in flies. Site-specific recombination between FRT sites in two P-elements flanking the open reading frame of CG3973 (Fig. 1B) was used to delete the intervening sequence. The deletion was confirmed by PCR and sequencing across the deletion site (data not shown). The allele generated, termed pigs1 (see below), also eliminated two additional small open reading frames, the gene CG3226, located 3′ of CG3973, and the gene CG14443, located in intron 3 of CG3973 (Fig. 1B). CG14443 encodes a predicted RNA helicase, whereas CG3226 encodes a predicted Calcyclin-binding protein. Neither has been studied in flies so far. Because pigs1 also eliminated these two genes, we tested whether any phenotypes observed were due to the absence of Pigs and could be rescued through ubiquitous expression of a full-length pigs cDNA in the pigs1 mutant background (Table 1; see also Fig. S3 in the supplementary material). Only those phenotypes that were rescued were analysed further.
Table 1.
pigs1 phenotypic rescue data of ovarian phenotypes
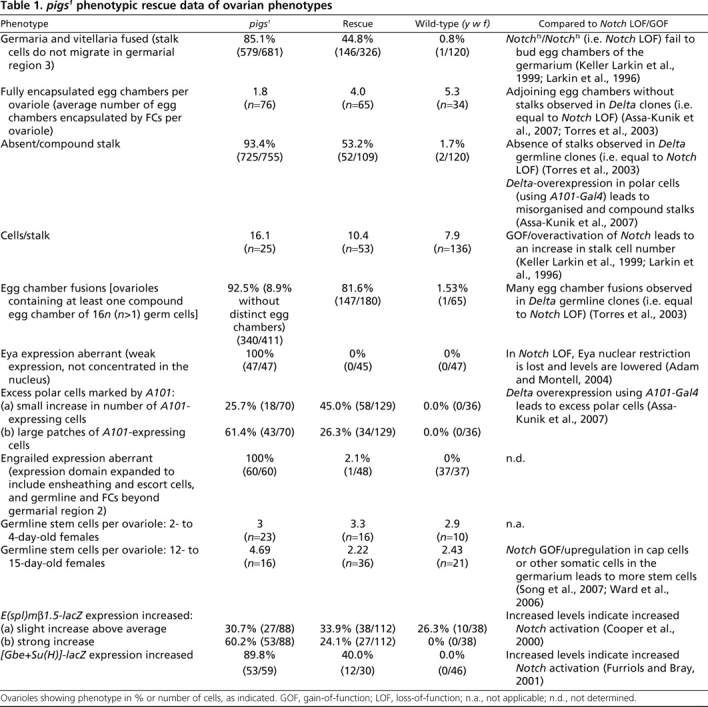
pigs1 mutant flies were semi-viable: 17% died before reaching adulthood and the surviving adults were flightless (Fig. 1G), showing strong degeneration of the indirect flight muscles (IFMs; Fig. 1I versus J), similar to that seen when Notch signalling during IFM maturation is affected (Bernard et al., 2006). The second very prominent adult phenotype observed was female sterility. Ovarian morphology was strongly disrupted in pigs1 mutants (Fig. 2; see also Fig. S2 in the supplementary material). Reflecting this ovarian phenotype, we named the gene pickled eggs (pigs). Like flight muscle development, differentiation of somatic and germline tissues during oogenesis crucially depends on precisely balanced Notch/Delta signalling (Assa-Kunik et al., 2007; Lopez-Schier and St Johnston, 2001; Torres et al., 2003). We thus decided to analyse the role of Pigs in Notch signalling further in this tissue.
Fig. 2.
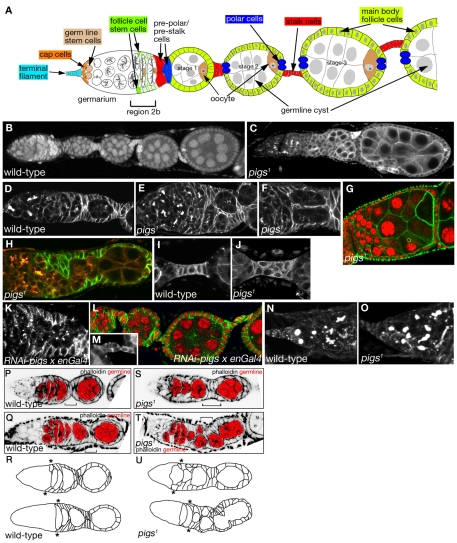
Ovarian and follicle cell phenotypes observed in the absence of Pigs. (A) Scheme depicting a single ovariole of a fly ovary. All cells arise from germline stem cells (GSCs) and follicle cell stem cells (FCSCs) in the germarium. A Delta signal from the germline induces pre-polar fate in a subset of FCs contacting the germline in the germarium. These pre-polar cells send a weaker Delta signal to the neighbouring pre-stalk cells (for details see Assa-Kunik et al., 2007). FCs in the germarium invade between the forming cysts to encapsulate each egg chamber. (B) Wild-type ovariole (Propidium Iodide labelling nuclei in red). Stalks of 6-8 cells separate each egg chamber. (C) A pigs1 mutant ovariole, showing the typical abutting egg chambers with missing or malformed stalks, labelled for the spectraplakin Shot. (D-F) In pigs1 mutant germaria, cysts often appear to accumulate in region 2b compared with the fairly linear arrangement in the wild-type. Labelling shows the adducin-like protein HtsF. (G) Fused egg chambers with nuclei of different sizes are often found in pigs1 mutant ovarioles. Phalloidin in green and DAPI for nuclei in red. (H) Early FCs fail to invaginate between germline cysts to encapsulate them in pigs1 mutant ovarioles (Shot in red and HtsF in green). (I,J) In contrast to orderly arranged wild-type stalks (I), stalks in pigs1 mutants (J), when present, often have multiple layers of cells and contain supernumerous cells (Table 1). Labelling is for Shot. (K-M) Expression of a UAS-pigs-RNAi construct in the FCs using en-Gal4 leads to similar phenotypes to those seen in the pigs1 mutant: encapsulation problems (K) and aberrant stalks (L). K shows HtsF labelling, L shows Phalloidin in green, DAPI for nuclei in red, and the inset M shows Shot labelling. (N,O) In aged pigs1 mutant ovaries (12-14 days after eclosion), germaria contain supernumerous GSCs compared with wild-type. Labelling is for HtsF. (P-R) Examples of wild-type germaria. (S-U) Examples of pigs1 mutant germaria, with S showing a mildly and T showing a strongly affected one. The germline cysts from region 2a onwards are indicated in red in P, Q, S and T. R and U trace FC outlines. Note the highly aberrant FC shapes even in the overtly mildly affected germarium. Asterisks in R and U indicate the approximate position of the two FCSCs.
pigs1 mutants show aberrant follicle cell shapes and morphogenesis
The most prominent defects in pigs1 mutant ovaries were malformed or missing stalks (Fig. 2C,E,J), egg chamber fusions (Fig. 2C,G), abnormal germarial structure (Fig. 2E,F,H), increased number of germline stem cells (GSCs) in aged females (Fig. 2N,O) and aberrant cell sizes and morphologies (Fig. 2S-U; see also Fig. S2A,B in the supplementary material). Additional ovarian phenotypes observed included abnormal ring canal morphology (see Fig. S2C-E in the supplementary material), semi-dumplessness (deficient transfer of nurse cell contents to the oocyte; see Fig. S2F in the supplementary material) and disorganized actin cages that compromised dumping (see Fig. S2G-J in the supplementary material). Each phenotype showed variable penetrance, thus all phenotypes were quantified as ‘observed per ovariole’ (Table 1). Phenotypes increased in severity with age: GSCs accumulated in germaria and encapsulation defects and aberrant follicle cell (FC) migration increased dramatically over time.
Similar phenotypes to those observed in pigs1 mutant ovaries were also observed when an RNA hairpin construct targeting pigs RNA was expressed using the UAS/Gal4 system (Brand and Perrimon, 1993). Expression of this construct under the control of the engrailed or bab1 promoter (Bolivar et al., 2006) led to encapsulation defects and abnormal stalk morphology (Fig. 2K-M). As both manipulations will result in knockdown in the early somatic cells in the ovary, these phenotypes suggest that Pigs is required in these cells.
We used mosaic analysis to further determine the requirement for Pigs in the somatic and germline cells of the ovary. In ovarioles with complete somatic FC clones (i.e. all FCs were pigs1 mutant, whereas the germline was wild-type), phenotypes were highly similar to those observed in ovarioles completely mutant for pigs (see Fig. S2M in the supplementary material). In ovarioles in which only germline cells were mutant, stalks largely formed normally between egg chambers and encapsulation occurred normally (data not shown), thus confirming that the phenotypes are primarily due to loss of Pigs in somatic cells rather than the germline.
pigs1 mutants show defects in follicle cell differentiation
The aberrant FC morphologies and migration behaviours described above strongly suggested that somatic FC differentiation was disrupted in pigs1 ovaries. Differentiation of FCs into main body FCs, stalk cells and polar cells depends on a number of signalling events, including Notch/Delta signalling (Assa-Kunik et al., 2007; Lopez-Schier and St Johnston, 2001; Torres et al., 2003). Several of the morphological phenotypes of pigs1 mutant ovarioles, such as the absence of stalks, encapsulation problems and egg chamber fusions, resembled those seen with Notch loss-of-function, in agreement with the suggestion that Pigs functions as an effector of Notch (Table 1). By contrast, some defects, such as the increase in cell number in the stalks and increase in GSCs, appeared more similar to increased Notch activity (Table 1). The diverse effects prompted us to analyse further the phenotypes in pigs1 mutant ovarioles using several markers of FC fate.
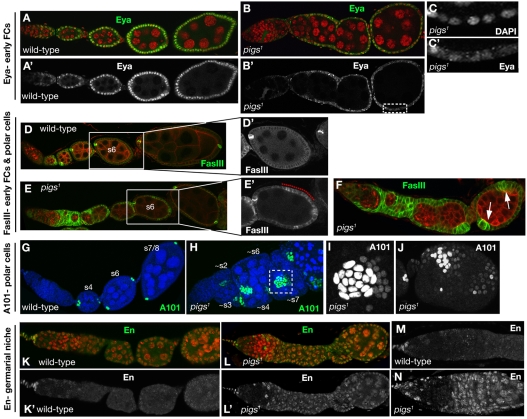
Eyes absent (Eya), a nuclear protein phosphatase, is the earliest known FC fate marker and is a key regulator of FC fate in that it represses polar and stalk cell fate (Bai and Montell, 2002). It is normally expressed in all precursor and main body FCs but not in differentiated polar and stalk cells. Notch signalling appears to aid in restricting Eya expression to the main body FCs, and Eya expression is reduced and loses its nuclear localisation when Notch function is compromised (Adam and Montell, 2004). Similar defects in Eya were detected in pigs1 mutant ovarioles: Eya expression was strongly reduced and it was no longer concentrated in FC nuclei (Fig. 3B-C′). This phenotype was fully penetrant and completely rescued by the expression of a ubiquitous pigs transgene (Table 1).
Fig. 3.
Follicle cell fate markers are aberrant in pigs1 mutant ovarioles. (A,A′) Eya is the earliest described marker of FC fate and is localised to the nucleus. Eya is maintained in all main body FCs, but is lost from polar and stalk cells once these differentiate in the vitellarium. (B-C′)In pigs1 mutant egg chambers, eya expression was not properly upregulated, and Eya did not accumulate in FC nuclei (C,C′ show boxed region in B′). (D,D′) In wild-type egg chambers, FasIII marks immature FCs in the germarium and young egg chambers until ~stage 4, when FasIII is only maintained in the polar cells. (E-F) pigs1 mutant egg chambers frequently contain more than two polar cells per cluster (arrows in F) and show persisting FasIII expression in the main body FCs beyond stage 4 (E and higher magnification in E′), when main body FCs in the wild-type have lost FasIII (D,D′). FasIII is in green, Shot is in red. (G) A101-lacZ is expressed in two pairs of mature polar cells at the anterior and posterior end of each wild-type egg chamber. (H-J) pigs1 mutant egg chambers contain elevated numbers of polar cells, as many as 18 polar cells at a single pole (box in H that is also shown in I). Extra polar cells were not always located at the poles (J). (K-N) Engrailed (En) is misexpressed in pigs1 mutant ovaries. En labels somatic cap and terminal filament cells in wild-type ovaries (green in K and as a single channel in K′ and M). In pigs1 mutant ovarioles, En was expressed in almost all somatic and germline cells (L,L′,N). Nuclei are red in K and L.
Strong expression of the adhesion protein Fasiclin III (FasIII) (Snow et al., 1989) is detected from stage 2b and is usually only maintained in the polar cells beyond stage 3, when it is downregulated in the stalk and main body FCs (Fig. 3D,D′). In pigs1 mutant ovarioles, FasIII expression remained very strong in large clusters of FCs in egg chambers that just left the germarium (Fig. 3E-F, arrows). This phenotype is similar to that seen in Notch or extra macrochaetae (emc) loss-of-function clones (Adam and Montell, 2004). In addition, FasIII expression was often maintained at abnormally high levels in main body FCs up to stage 6 (Fig. 3E′, dotted line), when wild-type main body FCs showed no remaining FasIII expression (Fig. 3D′).
Both the ectopic FasIII and the abnormally low levels of Eya have been associated with the formation of ectopic polar cells (Bai and Montell, 2002). To investigate polar cell number, we used the enhancer trap line A101-lacZ that is normally expressed only in the polar cells. Wild-type egg chambers at stages 2 and 3 contain up to five cells expressing A101; by stage 4, expression is mostly restricted to two cells and from stage 5 onward, no more than two cells at each pole show A101 expression (Besse and Pret, 2003). pigs1 mutant ovarioles contained a significant proportion of stage 4 and older egg chambers with more than two polar cells. Some egg chambers had up to eighteen polar cells that strongly expressed A101, often with adjacent clusters of cells that expressed A101 at lower levels but that were still above the expected level (Fig. 3H-J). In the analysis of Notch and emc mutant clones, the mutant cells did not express the polar cell marker PZ80 despite the elevated FasIII and reduced Eya. In this respect, the phenotype of pigs1 appears more similar to that produced by overexpressing the ligand Delta, which results in ectopic polar cells (Assa-Kunik et al., 2007). These complex outcomes reflect the fact that Notch signalling is required both in the main body FCs, where it activates Eya and suppresses polar cell fate, and in the polar cell precursors, where it induces polar fate. The main differing factor appears to be the level of Notch activation that is very strong in the future polar cells and relatively low in the main body FCs (Assa-Kunik et al., 2007).
The defects in FC morphogenesis observed in the pigs1 mutant ovarioles could be due to Pigs altering the process of cell fate determination directly or to it having an indirect effect by causing aberrant morphogenesis. For example, the positioning of cells could affect the levels of Delta signal received and thus alter the levels of Notch activation and resulting FC fates. The fact that the earliest markers of FC fate were affected in pigs1 mutant ovarioles supports the first scenario. The majority of phenotypes are also consistent with Pigs functioning downstream of Notch as an effector. However, in some cases, the outcome more closely resembles that of Notch gain-of-function, suggesting a more complex relationship.
Signalling in the stem cell niche of the germarium is perturbed in pigs1 mutant ovarioles
Perturbation of early FC fate markers such as Eya indicated that early FC differentiation was aberrant in pigs1 ovaries. Key determinants of early FC differentiation are provided by the FC stem cell (FCSC) niche, which in turn is regulated by Notch, Hedgehog, Wingless (Wnt) and JAK/STAT signals from the GSC niche, constituted by the terminal filament and the cap cells, so that the proliferation and differentiation of germline and somatic tissues are coordinated (Nystul and Spradling, 2007; Xie and Li, 2007; Li and Xie, 2005; Song and Xie, 2003). We thus analysed two key GSC niche signalling components in pigs1 mutant ovarioles. Armadillo (β-catenin) is expressed strongly in the cap cells where it localises to specialised adherens junctions that mediate signals between GSCs and the niche (Song et al., 2002). Its expression and localisation appeared normal in pigs1 mutant ovarioles (data not shown). The transcription factor Engrailed (En) is normally strongly expressed in only the terminal filament and cap cells (Fig. 3K,K′,M), but in pigs1 mutant ovarioles, En was ectopically expressed in all somatic and germline cells beyond region 2 in the germarium and also in a subset of escort and ensheathing cells (Fig. 3L,L′,N). This phenotype was completely penetrant and nearly fully rescued by ubiquitous pigs expression (Table 1; see also Fig. S3E-J in the supplementary material). The function of En within the niche is currently not understood, but its striking disruption in pigs1 mutant germaria suggests a disruption of niche function.
The stem cell niche in the germarium is maintained through reciprocal signalling between the GSCs and the cap cells, mediated in part by a Delta signal from the GSCs that activates Notch in the cap cells. Upregulation of Notch signalling in the cap or other somatic cells in the germarium leads to an increase in GSC number (Song et al., 2007; Ward et al., 2006). In aged pigs1 mutant germaria (12-15 days after eclosion), GSCs accumulated (Table 1), a phenotype resembling that when Notch is over-activated. The increase in GSC number in aged pigs1 mutant germaria was accompanied by an increase in cap cell number (data not shown), in agreement with the reciprocal signalling between stem cells and niche to regulate niche size. Thus, in addition to a potential function downstream of Notch in the differentiation of FCs, Pigs also operates in another Notch-dependent process, the germarial stem cell niche, where its effects more closely resemble those of a negative regulator of Notch, rather than an effector.
Does Pigs work as a cytoskeletal regulator?
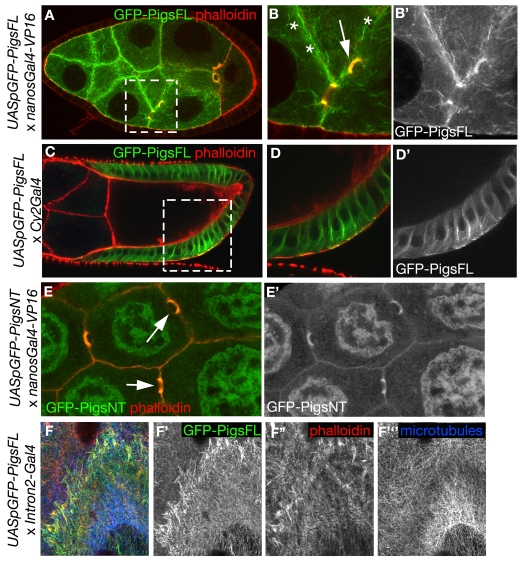
To test whether Pigs can associate with the cytoskeleton and exert all or part of its function through regulation of the cytoskeleton, we analysed its subcellular localisation. We expressed full-length Pigs fused to GFP (GFP-PigsFL) in both the germline and the FCs during oogenesis using the UAS/Gal4 system (Brand and Perrimon, 1993). When GFP-PigsFL was overexpressed in the germline it associated with microtubules, cell cortices and actin-based ring canals (Fig. 4A-B′), and when expressed in the FCs, it labelled microtubules (Fig. 4C-D′). In the giant epithelial cells of late larval salivary glands, GFP-PigsFL colocalised with both actin and microtubules (Fig. 4F-F‴), suggesting that it might have the ability to act as a cytoskeletal crosslinker. We also expressed only the N-terminal half of Pigs, comprising the CH and Gas2 domains, fused to GFP (GFP-PigsNT) in the germline. This fusion protein appeared to associate with cell cortices and ring canals but did not label microtubules (Fig. 4E,E′), suggesting the Gas2 domain might only function as a microtubule-binding domain in the context of the whole protein. Thus, at least when expressed at high levels, Pigs appeared to be a cytoskeletal protein. The association with ring canals and actin-rich cell cortices could explain the aberrant ring canal morphology (see Fig. S2C-E in the supplementary material) and disorganised and kinked actin ‘cages’ (see Fig. S2H-J in the supplementary material) in pigs1 mutant egg chambers.
Fig. 4.
Pigs can associate with both actin and microtubules. (A-B′) GFP-tagged full-length Pigs (GFP-PigsFL) expressed in the germline using nanosGal4-VP16 associates with microtubules near cell cortices (asterisks in B) and actin-based ring canals (arrow in B). (C-D′) GFP-PigsFL expressed in the FCs using Cy2Gal4 associates with microtubules. (E,E′) A GFP-tagged N-terminal version of Pigs containing the CH and Gas2 domains (GFP-PigsNT) expressed in the germline using nanosGal4-VP16 associates with actin-based ring canals (arrows in E) but not microtubules. (F-F‴) GFP-PigsFL expressed in late larval salivary glands using Intron2-Gal4 associates with both actin and microtubules at the basal surface of these huge epithelial cells, suggesting that it might have the ability to act as a cytoskeletal crosslinker.
We also analysed whether the cytoskeleton and cell shapes were affected early in the germarium. At the level of resolution obtainable by light microscopic analysis, filamentous actin appeared normal in pigs1 mutant germaria (Fig. 2P,Q versus S,T). In the wild-type germarium, FC morphology is highly ordered (Fig. 2R). From region 2b onwards, FCs stretch and migrate between the germline cysts to begin encapsulating them. Between regions 2 and 3, the pre-stalk cells begin to intercalate. In pigs1 mutant germaria, FC shapes were irregular and often appeared uncoordinated between neighbouring cells (Fig. 2U). Pre-stalk cells were less efficient in extending processes towards the other side, potentially contributing to, or even causing, the encapsulation defects.
Thus, Pigs had the ability to associate with both the actin and microtubule cytoskeleton, and several of the defects in the mutant, such as aberrant cell shapes in the germarium and late phenotypes observed during dumping, indicate that at least part of its function is via the cytoskeleton.
Notch signalling is upregulated in pigs1 mutants
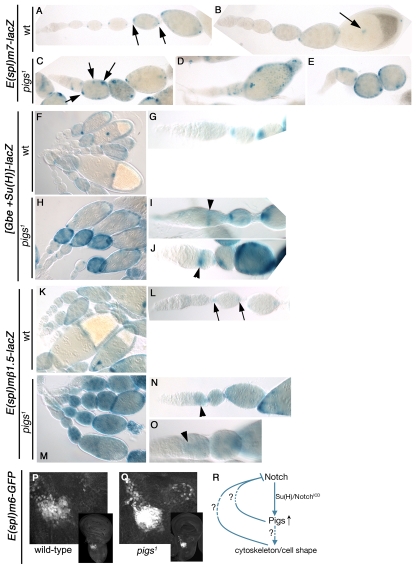
To test whether Pigs itself impinges on the signalling pathways that regulate FC fate, we analysed genetic reporters of the Notch/Delta signalling pathway. To assess Notch target activation, several reporter genes were used: E(spl)m7-lacZ, E(spl)mβ1.5-lacZ and [Gbe+Su(H)]-lacZ (Cooper et al., 2000; Furriols and Bray, 2001). In wild-type ovaries, E(Spl)m7-lacZ was strongly expressed in polar cells, weakly in stalk cells and upregulated slightly in all FCs at stage 5-6, concomitant with the Notch-dependent switch from mitosis to endoreduplication (Assa-Kunik et al., 2007). In pigs1 mutant ovarioles, E(spl)m7-lacZ expression was elevated in the main body FCs in all ovarioles prior to stage 6 (Fig. 5C-E), with particularly strong expression in small clusters of cells (Fig. 5C, arrows), and this elevation could be rescued by ubiquitous expression of pigs (data not shown). The same increase of reporter expression was observed with [Gbe+Su(H)]-lacZ and E(spl)mβ1.5-lacZ in the pigs1 mutant background (Fig. 5H-J,M-O) and both phenotypes were significantly rescued by ubiquitous expression of pigs (Table 1). For these latter two reporters, an increase in Notch activity was already apparent in region 2b in the germarium, when germline cyst encapsulation normally commences (Fig. 5I,J,N,O, arrowheads). Elevated Notch signalling in these early pre-stalk FCs could be contributing to the encapsulation defects observed, as at this stage, pre-stalk and pre-polar cell fate is induced through Notch signalling from both the germline and the just-established pre-polar FCs themselves (Assa-Kunik et al., 2007). An increase in Notch activity in the absence of Pigs could also be observed in the myoblast precursors in the wing disc. Expression of the Notch activity marker E(spl)m6-GFP was strongly increased in the pigs1 mutant, both in level and in the number of cells showing it (Fig. 5Q compared with Fig. 1E and Fig. 5P), and was rescued by ubiquitous expression of pigs (data not shown).
Fig. 5.
Loss of Pigs leads to upregulation of Notch signalling in different tissues. Analysis of Notch signalling reporters in different tissues. (A-E) E(spl)m7-lacZ is expressed in the polar cells from ~stage 4 onwards in wild-type ovaries (A,B, arrows). In pigs1 mutant ovarioles (C-E), expression is upregulated and also found in ectopic cells (arrows). (F,G) [Gbe+Su(H)]-lacZ labels anterior polar and border cells in the wild-type and main body FCs from stage 6 onwards. (H-J) In pigs1 mutant ovarioles, levels of [Gbe+Su(H)]-lacZ are strongly increased in polar cells and main body FCs from stage 6 onwards. Also, ectopic activation in the invading and encapsulating FCs in the germarium is observed (I,J, arrowheads). (K-O) E(spl)mβ1.5-lacZ is expressed in the polar cells from ~stage 2-3 onwards in wild-type ovaries (K,L, arrows in L) but is strongly upregulated in all cells in pigs1 mutant ovarioles (M-O, arrowheads in N,O). (P,Q) In the developing wing disc, E(Spl)m6-GFP labels the myoblast precursors. Insets show the whole disc and the large panel is a magnification of the myoblast region. In pigs1 mutant discs, this labelling is strongly increased in level and number of cells labelled. (R) Schematic of Pigs as a direct target of Notch and possible feedback loops to Notch.
The increase in Notch reporter activity suggests that part of the function of Pigs might be to work in a negative regulatory feedback loop and assist in terminating Notch signalling activity in certain target tissues such as muscle precursors and ovaries.
DISCUSSION
The data presented above demonstrate that not only is the Gas2-like protein Pigs essential for development in the fly and is a direct target of Notch signalling, but also that Pigs is probably part of a negative regulatory feedback loop that restricts Notch signalling to appropriate levels in certain tissues. These findings are intriguing, as the domain composition of Pigs would suggest it to function as a cytoskeletal crosslinker protein, helping to coordinate actin and microtubules and aiding tissue morphogenesis. Although we never found a strong disruption of either actin or microtubule cytoskeleton in early stages of oogenesis in pigs1 mutant ovaries, at late stages during the dumping phase, actin cages appeared highly disrupted. Also, already at early stages of oogenesis in the germarium, cell shapes of FCs were very irregular. As cell shape is largely determined by the cytoskeleton, this suggests that, although not visible at the level of light microscopic analysis, cytoskeletal function is impaired in pigs1 mutant cells.
The ChIP data and reporter assays indicate that Pigs is directly regulated by Notch in at least some tissues, and in both the muscles and the ovaries, the phenotypes are compatible with Pigs being an effector of Notch. However, assays of Notch pathway activity in pigs mutants indicate that Pigs is a negative regulator of Notch activity. How can we resolve this paradox? We can envisage three alternate models for Pigs function at a molecular level. Pigs might facilitate cytoskeletal rearrangements induced by Notch signalling by stabilising the cytoskeleton at certain subcellular sites, and the execution of necessary changes could induce further signalling factors to terminate Notch signalling. Alternatively, Pigs could directly link Notch signalling to the cytoskeleton through sequestering Notch at a particular subcellular localisation (i.e. through linkage to the cytoskeleton), and this could bring Notch in proximity to factors that switch off the signalling appropriately. In a third scenario, the morphological changes downstream of Notch could be independent of Pigs, but Pigs could act as a molecular ‘sensor’ to determine if the actin and the microtubule cytoskeleton have rearranged in an appropriate fashion. In support of the second scenario, the localisation of Notch (based on detection of the intracellular domain), is changed in pigs1 mutant FCs that are attempting to interdigitate and encapsulate a germline cyst (see Fig. S4F-G″ in the supplementary material).
Although Pigs is a cytoskeleton-associated protein, the phenotypes observed in pigs1 mutant ovaries are not those generally seen in mutants for structural cytoskeletal proteins. FCs mutant for actin regulators such as CAP, Cofilin (Twinstar — FlyBase), Profillin (Chickadee — FlyBase), Ena or Abl show alterations in the actin cytoskeleton and, in some cases, multi-layering of the follicular epithelium, but do not lead to phenotypes resembling Notch-misregulation (Baum and Perrimon, 2001; Conder et al., 2007). This supports the notion that the function of Pigs confers more than just cytoskeletal changes in the FCs.
Pigs is likely to be only one of several downstream effectors of Notch in the tissues where it is directly regulated, and it remains to be proven that it is a target in the ovary. emc is a previously characterised effector of Notch in the ovary and has overlapping phenotypes with pigs1 (Adam and Montell, 2004), supporting the model that pigs is one of several Notch targets in the ovary. This also explains the observation that the defects in pigs1 mutant ovaries are milder than those of Notch alleles as the overall pigs loss-of-function phenotype is expected to represent just a subset of the defects caused by Notch loss-of-function. Furthermore, pigs function is only needed in a subset of tissues that depend on Notch signalling for their differentiation. For example, we do not observe any sensory organ defects in the pigs1 deletion and would therefore conclude that Pigs does not function downstream of Notch in the differentiation of this tissue. Consistent with this, a recent genome-wide analysis of genes involved in Notch signalling that focused on phenotypes in the external sensory organs of the notum did not identify pigs as a candidate (Mummery-Widmer et al., 2009).
The data presented here suggest that Pigs could serve a dual function in specific Notch-dependent processes: aiding morphological changes downstream of a differentiation signal combined with a regulatory role that allows that signal to be terminated when appropriate. Thus, the Notch—Pigs interaction described above provides an opportunity to further dissect the link between Notch-induced differentiation, cell shape and control of the cytoskeleton.
Supplementary Material
Acknowledgements
The authors thank Acaimo Gonzalez-Reyes for fly stocks, the Developmental Studies Hybridoma Bank at the University of Iowa for antibodies, Nick Brown for the use of his confocal microscope, John Overton for embryo injections and Sean Munro for helpful comments on the manuscript. This work was supported by the BBSRC (Grant No. BB/B501798/1) to K.R., MRC Programme and project grants to S.J.B., a Long Term Fellowship from EMBO to F.B, and a grant from the Association for International Cancer Research to B.E.H. Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.043224/-/DC1
References
- Adam J. C., Montell D. J. (2004). A role for extra macrochaetae downstream of Notch in follicle cell differentiation. Development 131, 5971-5980 [DOI] [PubMed] [Google Scholar]
- Anant S., Roy S., VijayRaghavan K. (1998). Twist and Notch negatively regulate adult muscle differentiation in Drosophila. Development 125, 1361-1369 [DOI] [PubMed] [Google Scholar]
- Arbeitman M. N., Furlong E. E., Imam F., Johnson E., Null B. H., Baker B. S., Krasnow M. A., Scott M. P., Davis R. W., White K. P. (2002). Gene expression during the life cycle of Drosophila melanogaster. Science 297, 2270-2275 [DOI] [PubMed] [Google Scholar]
- Assa-Kunik E., Torres I. L., Schejter E. D., Johnston D. S., Shilo B. Z. (2007). Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development 134, 1161-1169 [DOI] [PubMed] [Google Scholar]
- Bai J., Montell D. (2002). Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development 129, 5377-5388 [DOI] [PubMed] [Google Scholar]
- Baum B., Perrimon N. (2001). Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat. Cell Biol. 3, 883-890 [DOI] [PubMed] [Google Scholar]
- Bellen H. J., O'Kane C. J., Wilson C., Grossniklaus U., Pearson R. K., Gehring W. J. (1989). P-element-mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev. 3, 1288-1300 [DOI] [PubMed] [Google Scholar]
- Bernard F., Dutriaux A., Silber J., Lalouette A. (2006). Notch pathway repression by vestigial is required to promote indirect flight muscle differentiation in Drosophila melanogaster. Dev. Biol. 295, 164-177 [DOI] [PubMed] [Google Scholar]
- Besse F., Pret A. M. (2003). Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster. Development 130, 1017-1027 [DOI] [PubMed] [Google Scholar]
- Bier E., Vaessin H., Shepherd S., Lee K., McCall K., Barbel S., Ackerman L., Carretto R., Uemura T., Grell E., et al. (1989). Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 3, 1273-1287 [DOI] [PubMed] [Google Scholar]
- Bolivar J., Pearson J., Lopez-Onieva L., Gonzalez-Reyes A. (2006). Genetic dissection of a stem cell niche: the case of the Drosophila ovary. Dev. Dyn. 235, 2969-2979 [DOI] [PubMed] [Google Scholar]
- Brancolini C., Bottega S., Schneider C. (1992). Gas2, a growth arrest-specific protein, is a component of the microfilament network system. J. Cell Biol. 117, 1251-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415 [DOI] [PubMed] [Google Scholar]
- Bray S. J. (2006). Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678-689 [DOI] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A. (2007). Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39, 715-720 [DOI] [PubMed] [Google Scholar]
- Conder R., Yu H., Zahedi B., Harden N. (2007). The serine/threonine kinase dPak is required for polarized assembly of F-actin bundles and apical-basal polarity in the Drosophila follicular epithelium. Dev. Biol. 305, 470-482 [DOI] [PubMed] [Google Scholar]
- Cooper M. T., Tyler D. M., Furriols M., Chalkiadaki A., Delidakis C., Bray S. (2000). Spatially restricted factors cooperate with notch in the regulation of Enhancer of split genes. Dev. Biol. 221, 390-403 [DOI] [PubMed] [Google Scholar]
- Furriols M., Bray S. (2001). A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr. Biol. 11, 60-64 [DOI] [PubMed] [Google Scholar]
- Goryunov D., Adebola A., Jefferson J. J., Leung C. L., Messer A., Liem R. K. (2007). Molecular characterization of the genetic lesion in Dystonia musculorum (dt-Alb) mice. Brain Res. 1140, 179-187 [DOI] [PubMed] [Google Scholar]
- Gregory S. L., Brown N. H. (1998). kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 143, 1271-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller Larkin M., Deng W. M., Holder K., Tworoger M., Clegg N., Ruohola-Baker H. (1999). Role of Notch pathway in terminal follicle cell differentiation during Drosophila oogenesis. Dev. Genes Evol. 209, 301-311 [DOI] [PubMed] [Google Scholar]
- Krejci A., Bray S. (2007). Notch activation stimulates transient and selective binding of Su(H)/CSL to target enhancers. Genes Dev. 21, 1322-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci A., Bernard F., Housden B. E., Collins S., Bray S. J. (2009). Direct response to Notch activation: signaling crosstalk and incoherent logic. Sci. Signal. 2, ra1 [DOI] [PubMed] [Google Scholar]
- Larkin M. K., Holder K., Yost C., Giniger E., Ruohola-Baker H. (1996). Expression of constitutively active Notch arrests follicle cells at a precursor stage during Drosophila oogenesis and disrupts the anterior-posterior axis of the oocyte. Development 122, 3639-3650 [DOI] [PubMed] [Google Scholar]
- Leung C. L., Liem R. K., Parry D. A., Green K. J. (2001). The plakin family. J. Cell Sci. 114, 3409-3410 [DOI] [PubMed] [Google Scholar]
- Leung C. L., Green K. J., Liem R. K. (2002). Plakins: a family of versatile cytolinker proteins. Trends Cell Biol. 12, 37-45 [DOI] [PubMed] [Google Scholar]
- Li L., Xie T. (2005). Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21, 605-631 [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H., St Johnston D. (2001). Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 15, 1393-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery-Widmer J. L., Yamazaki M., Stoeger T., Novatchkova M., Bhalerao S., Chen D., Dietzl G., Dickson B. J., Knoblich J. A. (2009). Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature 458, 987-992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul T., Spradling A. (2007). An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1, 277-285 [DOI] [PubMed] [Google Scholar]
- O'Kane C. J., Gehring W. J. (1987). Detection in situ of genomic regulatory elements in Drosophila. Proc. Natl. Acad. Sci. USA 84, 9123-9127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portin P. (2002). General outlines of the molecular genetics of the Notch signalling pathway in Drosophila melanogaster: a review. Hereditas 136, 89-96 [DOI] [PubMed] [Google Scholar]
- Röper K., Brown N. H. (2003). Maintaining epithelial integrity: a function for gigantic spectraplakin isoforms in adherens junctions. J. Cell Biol. 162, 1305-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K., Brown N. H. (2004). A spectraplakin is enriched on the fusome and organizes microtubules during oocyte specification in Drosophila. Curr. Biol. 14, 99-110 [PubMed] [Google Scholar]
- Röper K., Gregory S. L., Brown N. H. (2002). The ‘Spectraplakins’: cytoskeletal giants with characteristics of both spectrin and plakin families. J. Cell Sci. 115, 4215-4225 [DOI] [PubMed] [Google Scholar]
- Snow P. M., Bieber A. J., Goodman C. S. (1989). Fasciclin III: a novel homophilic adhesion molecule in Drosophila. Cell 59, 313-323 [DOI] [PubMed] [Google Scholar]
- Song X., Xie T. (2003). Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development 130, 3259-3268 [DOI] [PubMed] [Google Scholar]
- Song X., Zhu C. H., Doan C., Xie T. (2002). Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855-1857 [DOI] [PubMed] [Google Scholar]
- Song X., Call G. B., Kirilly D., Xie T. (2007). Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development 134, 1071-1080 [DOI] [PubMed] [Google Scholar]
- Strumpf D., Volk T. (1998). Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, vein, at the muscle-tendon junction site. J. Cell Biol. 143, 1259-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsan V., Anant S., Guptan P., VijayRaghavan K., Skaer H. (2001). Myoblast diversification and ectodermal signaling in Drosophila. Dev. Cell 1, 829-839 [DOI] [PubMed] [Google Scholar]
- Torres I. L., Lopez-Schier H., St Johnston D. (2003). A Notch/Delta-dependent relay mechanism establishes anterior-posterior polarity in Drosophila. Dev. Cell 5, 547-558 [DOI] [PubMed] [Google Scholar]
- Ward E. J., Shcherbata H. R., Reynolds S. H., Fischer K. A., Hatfield S. D., Ruohola-Baker H. (2006). Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr. Biol. 16, 2352-2358 [DOI] [PubMed] [Google Scholar]
- Wilson C., Pearson R. K., Bellen H. J., O'Kane C. J., Grossniklaus U., Gehring W. J. (1989). P-element-mediated enhancer detection: an efficient method for isolating and characterizing developmentally regulated genes in Drosophila. Genes Dev. 3, 1301-1313 [DOI] [PubMed] [Google Scholar]
- Xie T., Li L. (2007). Stem cells and their niche: an inseparable relationship. Development 134, 2001-2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.