Abstract
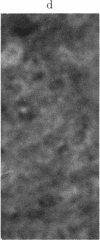
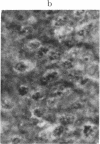
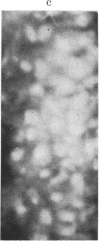
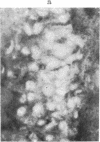
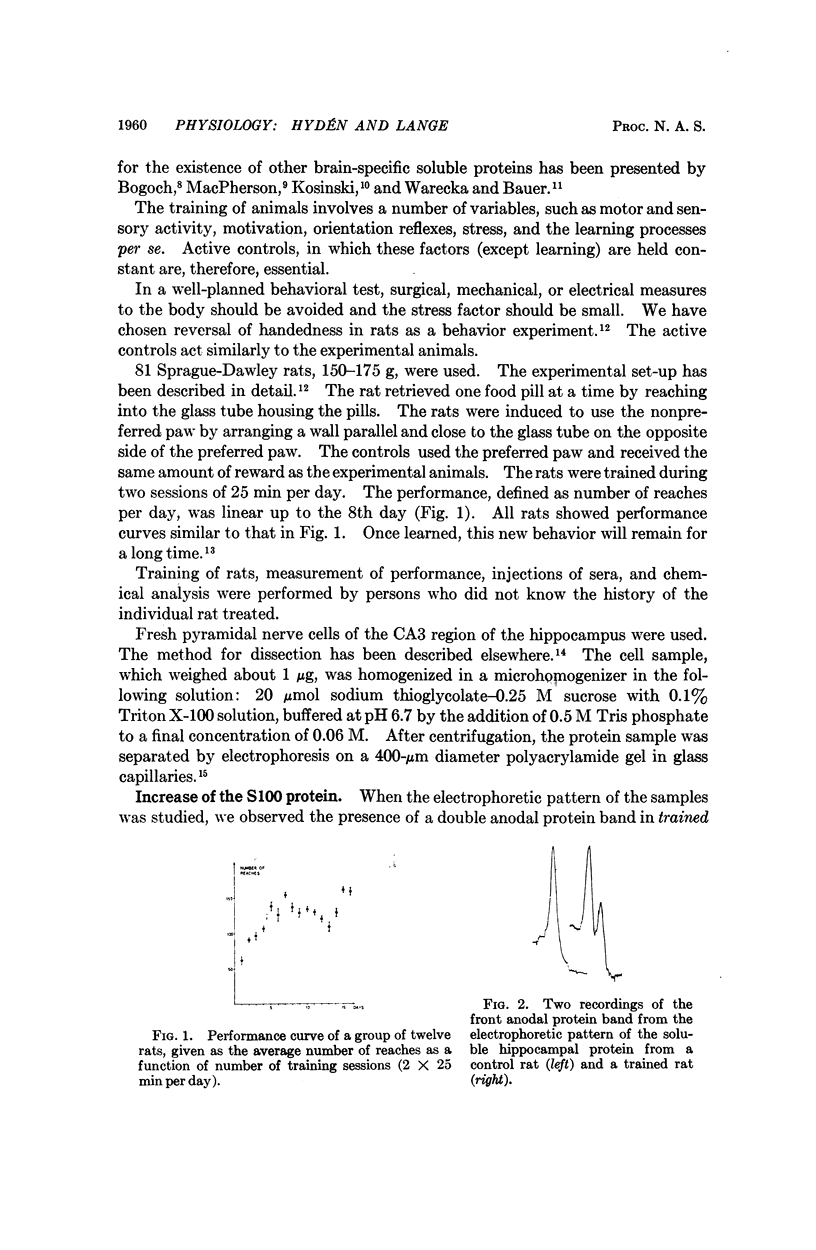
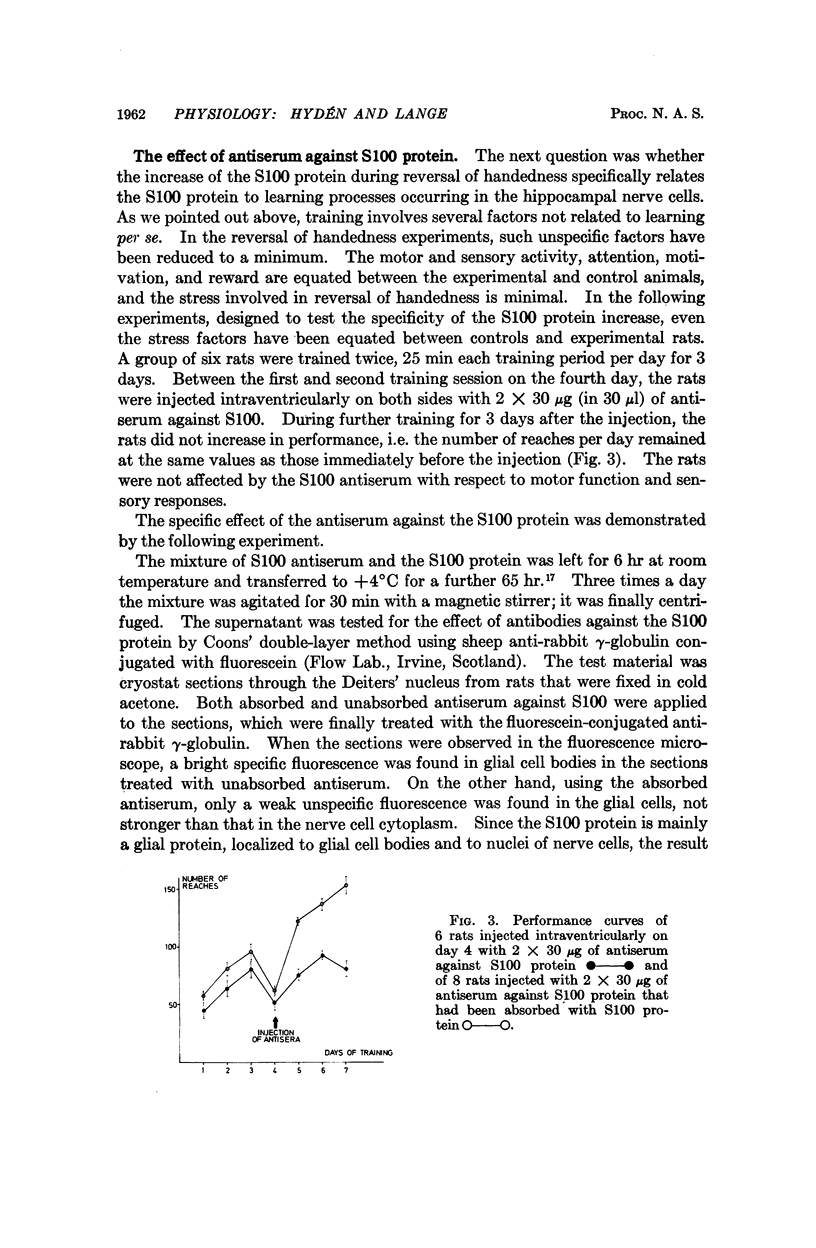
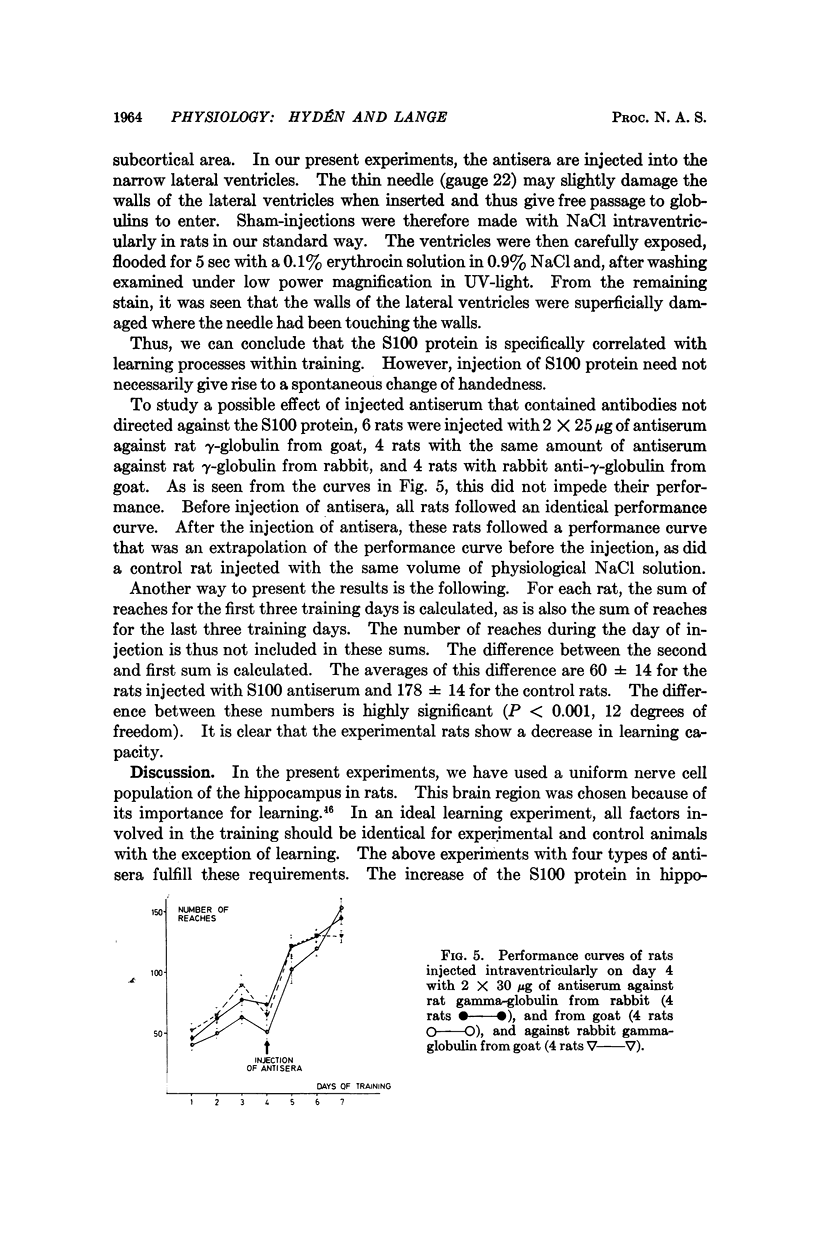
The brain-specific acidic protein, S100, in the pyramidal nerve cells of the hippocampus was investigated as a possible correlate to learning during transfer of handedness in rats. The amount of S100 increased during training. Intraventricular injection of antiserum against the S100 protein during the course of training prevented the rats from further increases in learned behavior but did not affect motor function in the animals. Antibodies against the S100 protein could be localized after injection by immunofluorescence, in hippocampal structures, penetrating presumably through slight ependymal lesions caused by the injection. By contrast, control animals subjected to the same training and injected with S100 antiserum that had been absorbed with S100 protein or with other antisera against γ-globulins showed no decrease in their ability to learn. The conclusion is that the brain-specific protein, S100, is linked to the learning process, at least within the training used.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. S., Edelman G. M. Isolation of an acidic protein from rat brain. J Biol Chem. 1968 Dec 10;243(23):6234–6241. [PubMed] [Google Scholar]
- Dannies P. S., Levine L. Demonstration of subunits in beef brain acidic protein (S-100). Biochem Biophys Res Commun. 1969 Nov 6;37(4):587–592. doi: 10.1016/0006-291x(69)90849-3. [DOI] [PubMed] [Google Scholar]
- HYDEN H., EGYHAZI E. CHANGES IN RNA CONTENT AND BASE COMPOSITION IN CORTICAL NEURONS OF RATS IN A LEARNING EXPERIMENT INVOLVING TRANSFER OF HANDEDNESS. Proc Natl Acad Sci U S A. 1964 Oct;52:1030–1035. doi: 10.1073/pnas.52.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyden H., Lange P. W. Protein synthesis in limbic structures during change in behavior. Brain Res. 1970 Sep 16;22(3):423–425. doi: 10.1016/0006-8993(70)90488-9. [DOI] [PubMed] [Google Scholar]
- Hydén H., Lange P. W. Micro-electrophoretic determination of protein and protein synthesis in the 10-9 to 10-7 gram range. J Chromatogr. 1968 Jun 18;35(3):336–351. doi: 10.1016/s0021-9673(01)82395-5. [DOI] [PubMed] [Google Scholar]
- Hydén H., McEwen B. A glial protein specific for the nervous system. Proc Natl Acad Sci U S A. 1966 Feb;55(2):354–358. doi: 10.1073/pnas.55.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janković B. D., Rakić L., Veskov R., Horvat J. Effect of intraventricular injection of anti-brain antibody on defensive conditioned reflexes. Nature. 1968 Apr 20;218(5138):270–271. doi: 10.1038/218270a0. [DOI] [PubMed] [Google Scholar]
- Kosinski E., Grabar P. Immunochemical studies of rat brain. J Neurochem. 1967 Mar;14(3):273–281. doi: 10.1111/j.1471-4159.1967.tb09524.x. [DOI] [PubMed] [Google Scholar]
- MOORE B. W., MCGREGOR D. CHROMATOGRAPHIC AND ELECTROPHORETIC FRACTIONATION OF SOLUBLE PROTEINS OF BRAIN AND LIVER. J Biol Chem. 1965 Apr;240:1647–1653. [PubMed] [Google Scholar]
- McEwen B. S., Hydén H. A study of specific brain proteins on the semi-micro scale. J Neurochem. 1966 Sep;13(9):823–833. doi: 10.1111/j.1471-4159.1966.tb05878.x. [DOI] [PubMed] [Google Scholar]
- Mihailović L. J., Hydén H. On antigenic differences between nerve cells and glia. Brain Res. 1969 Nov;16(1):243–256. doi: 10.1016/0006-8993(69)90097-3. [DOI] [PubMed] [Google Scholar]
- Mihailović Lj, Divac I., Mitrović K., Milosević D., Janković B. D. Effects of intraventricularly injected anti-brain antibodies on delayed alteration and visual discrimination tests performance in rhesus monkeys. Exp Neurol. 1969 Jun;24(2):325–336. doi: 10.1016/0014-4886(69)90025-9. [DOI] [PubMed] [Google Scholar]
- Walsh R. N., Budtz-Olsen O. E., Penny J. E., Cummins R. A. The effects of environmental complexity on the histology of the rat hippocampus. J Comp Neurol. 1969 Nov;137(3):361–366. doi: 10.1002/cne.901370309. [DOI] [PubMed] [Google Scholar]
- Warecka K., Bauer H. Studies on "brain-specific" proteins in aqueous extracts of brain tissue. J Neurochem. 1967 Jul;14(7):783–787. doi: 10.1111/j.1471-4159.1967.tb10313.x. [DOI] [PubMed] [Google Scholar]
- Zuckerman J. E., Herschman H. R., Levine L. Appearance of a brain specific antigen (th S-100 protein) during human foetal development. J Neurochem. 1970 Feb;17(2):247–251. doi: 10.1111/j.1471-4159.1970.tb02207.x. [DOI] [PubMed] [Google Scholar]