Abstract
Abnormal neuronal calcium (Ca2+) homeostasis has been implicated in numerous diseases of the nervous system. The pathogenesis of two increasingly common disorders of the peripheral nervous system, namely neuropathic pain and diabetic polyneuropathy, has been associated with aberrant Ca2+ channel expression and function. Here we review the current state of knowledge regarding the role of Ca2+ dyshomeostasis and associated mitochondrial dysfunction in painful and diabetic neuropathies. The central impact of both alterations of Ca2+ signalling at the plasma membrane and also intracellular Ca2+ handling on sensory neuron function is discussed and related to abnormal endoplasmic reticulum performance. We also present new data highlighting sub-optimal axonal Ca 2+ signalling in diabetic neuropathy and discuss the putative role for this abnormality in the induction of axonal degeneration in peripheral neuropathies. The accumulating evidence implicating Ca2+ dysregulation with both painful and degenerative neuropathies, along with recent advances in understanding of regional variations in Ca2+ channel and pump structures, makes modulation of neuronal Ca2+ handling an increasingly viable approach for therapeutic interventions against the painful and degenerative aspects of many peripheral neuropathies.
Keywords: axon, axon degeneration, calcium channel, diabetes, dorsal root ganglia, insulin, mitochondria, neuropathic pain, neuropathy, neurotrophic factors, sensory neurone
INTRODUCTION
This review will present the critical role played by calcium (Ca2+) signalling and homeostasis in two key areas of neurological disease in the peripheral nervous system. Neuropathic pain has a huge impact on quality of life and aberrant Ca2+ channel physiology and expression has been implicated in a number of pain states. The review will also highlight the most common form of peripheral neuropathy, namely diabetic polyneuropathy. This disorder can include pain as one of its symptoms and ultimately progresses to marked degeneration of peripheral nerve fibres with attendant sensory loss. Abnormalities in Ca2+ signalling and homeostasis have been linked to the aetiology of both the painful and degenerative aspects of diabetic neuropathy.
Calcium dyshomeostasis in diabetic sensory neuropathy
Background
Diabetes mellitus in humans leads to development of complications that affect various tissues and organ systems, including heart muscle, retina, secretory glands, kidneys and peripheral nerves. Chronic metabolic stress induced by hyperglycaemia resulting from either low insulin production in type 1 diabetes or decreased peripheral sensitivity to insulin in type 2 diabetes affects cellular homeostasis in virtually all cell types. Hyperglycaemia is widely regarded to be the major process that triggers cellular pathology, and a variety of downstream mechanisms, including metabolic stress, with subsequent generation of reactive oxygen species (ROS) that damage membranes and other cellular systems have been identified [1, 2]. Recent studies have revealed a very early impairment of two connected intracellular signalling/integrative systems, namely Ca2+ homeostasis/signalling and mitochondrial physiology. These changes are similar in very different cell types, and may be regarded as a common pathologically relevant pathway. Here, we present our own data and data produced by other groups, which show related changes in Ca2+ homeostasis and mitochondrial dysfunction in peripheral neurones, and propose a major role for these alterations in cellular pathophysiology of neuropathy associated with diabetes mellitus.
Clinical impact of diabetes and diabetic sensory neuropathy
The World Health Organization (WHO) predicts that by 2025 there will be 300 million people with diabetes. In Europe and North America it is estimated that 60 and 20 million people, respectively, currently have diabetes which has an incidence of 6% and rising (see ADA, IDF and CDA web sites of International Diabetes Federation; http://www.idf.org/webdata/docs/StakeholderPresentation.pdf; American Diabetes Association; http://www.diabetes.org/diabetes-statistics.jsp or Canadian Diabetes Association; http://www.diabetes.ca/Section_About/prevalence.asp). Of these, about 90 – 95% have non-insulin-dependent diabetes mellitus (type 2 diabetes) and 5 – 10% have insulin-dependent diabetes mellitus (type 1 diabetes). In Europe and North America approximately 5–10% of all health care costs are devoted to treatment of diabetic complications [3–5]. Palliative treatment of diabetic sensory neuropathy can account for 20–30% of these costs [5]. For example, in North America, approximately $30 billion per annum of health service costs are spent on treatment of diabetic complications that include retinopathy, nephropathy, heart disease and neuropathy. In 1998 approximately $15 billion of heath service expenditure was associated with the neurological complications (sensory and autonomic neuropathy and blindness). Incidence of polyneuropathy in diabetic patients can be as high as 50% and leads to incapacitating pain, sensory loss, foot ulceration, infection, gangrene and poor wound healing. Up to 2 and 6 million North Americans and Europeans, respectively, with diabetes exhibit foot ulceration and associated downstream sequalae. The end result is often lower extremity amputation which accounts for approximately 90,000 cases each year in North America. There is no effective therapy and only palliative treatment is available at the present time [6]. These alarming figures are estimated to rise by approximately 5-fold over the next 10 years due to the epidemic in obesity and the associated increase in incidence and earlier time of onset of type 2 diabetes [7].
Nerve damage in diabetic sensory neuropathy
Diabetic neuropathy in type 1 and 2 diabetes in both humans and animal models is associated with reduction of motor and sensory nerve conduction velocity and structural changes in peripheral nerve including endoneurial microangiopathy, axonal degeneration, Schwann cell pathology, paranodal demyelination and loss of myelinated and unmyelinated fibers - the latter due to a dying-back of distal axons that presents clinically as reduced epidermal nerve fibre density [8–10]. All components, (sensory, motor and autonomic) of the peripheral nervous system are affected but neurodegeneration is most prominent in the longest axons of sensory neurons, while defective axon regeneration impedes tissue re-innervation [11]. There is no apoptosis-dependent loss of DRG sensory neurone perikarya in diabetic humans or animals [12, 13] and the distal dying-back of axons is the critical pathological feature [11, 14], mimicking axonal pruning observed in the CNS and PNS during other pathological states [15].
It is widely believed that oxidative stress is a key pathological process that induces nerve damage in diabetes [16]. The following pathways, triggered by hyperglycaemia and/or lack of insulin, have been proposed as contributing to oxidative stress in diabetic neuropathy: (i) Polyol pathway – excess glucose results in elevated flux through the polyol pathway and build-up of damaging levels of polyols and associated pro-oxidants [17]; (ii) High intracellular glucose concentrations elevate ROS through raised mitochondrial activity [1]; (iii) Protein glycation – hyperglycaemia induces glycation of proteins leading to their abnormal function and/or activation of the receptor for advanced glycation end-products (RAGE) which induces cellular stress, in part, through activation of inflammatory pathways [18, 19], and (iv) Reduced neurotrophic support – maintenance of normal phenotype of neurons is impaired due to diabetes-induced loss of neurotrophic support by insulin, insulin-like growth factors (IGF-I, IGF-II), nerve growth factor (NGF) and neurotrophin-3 (NT-3) [20–22].
Calcium signalling in cells
Several evolutionary conserved families of Ca2+ channels, transporters and Ca2+ buffers provide spatially and temporally organised fluctuations of intracellular Ca2+ concentration (Ca2+ signals). A number of Ca2+-sensors (represented in essence by Ca2+-regulated enzymes) act as effectors, which translate Ca2+ signals into physiological responses [23–31]. Importantly, resting free Ca2+ concentrations within the cell can vary considerably, being within the 50 – 100 nM range in the cytosol and approaching 0.5 – 1.0 mM in the lumen of endoplasmic reticulum (ER). Any long lasting shifts from these levels are detrimental and cytosolic Ca2+ overload and depletion of ER Ca2+ can have pathological consequences, including the triggering of various types of cell death [32–36].
Calcium dyshomeostasis in neurones from diabetic animals
Altered Ca2+ handling and Ca2+ signalling have been detected in a variety of preparations isolated from animals with experimentally-induced diabetes as well as from diabetic patients. Abnormalities of Ca2+ homeostasis have been found in most tissues studied, including skeletal, cardiac and smooth muscle, secretory cells, blood cells, kidney and osteoblasts, (see [37, 38] and references therein). These abnormalities generally manifest as an increased resting intracellular Ca2+ concentration ([Ca2+]i), decreased activity of Ca2+ transporters (although not always) and decreased stimulus-evoked Ca2+ signalling. Impaired Ca2+ handling has also been found in sensory neurones from animals with experimental diabetes.
Elevation in resting [Ca2+]i
A significant increase in resting [Ca2+]i in diabetic sensory neurones has been a common finding, although there are some differences between neuronal types. Kostyuk and colleagues published work showing elevated resting [Ca2+]i in small neurons of the dorsal root ganglia (DRG) of type 1 and type 2 diabetic mice. They demonstrated that resting [Ca2+]i was ~ 30% higher in small DRG neurones isolated from streptozotocin (STZ)-treated C57Bl/6 mice, (a model of type 1 diabetes) than in control (205 ± 16 nM vs. 156 ± 16 nM). The elevation in [Ca2+]i in small neurons was even greater in db/db mice (a model of type 2 diabetes). There were no differences in [Ca2+]i levels in large neurones in either mouse model [39]. It has to be noted that the same group also reported the absence of any changes in resting [Ca2+]i in DRG neurones isolated from type 1 STZ-diabetic rodents [40]. The reasons for this discrepancy may be differences in severity and/or length of STZ-diabetes within these studies. For example, in the later study the animals (both rats and mice) were only maintained in a diabetic state for 4 – 5 weeks [40] whereas it appears that the db/db mice (type 2 diabetes) may have been maintained for 2 – 3 months [39].
The finding of elevated [Ca2+]i in neurons from diabetic rodents has been confirmed by other groups. In STZ-diabetic Wistar rats of 8 – 14 weeks duration resting [Ca2+]i was substantially increased by 2–2.5-fold in both large and small DRG neurones isolated from lumbar L4–L6 DRG and the [Ca2+]i increase correlated with progression of the disease [41]. Interestingly, resting [Ca2+]i was not affected in neurons from ganglia located at higher levels of the spinal cord (C3 – L3). These differences correlated with higher susceptibility of lumbar DRG sensory neurones with the longest axons to diabetic neuropathy [42]. Some of the discrepancies with respect to measurement of [Ca2+]i between studies in different models and duration of diabetes may therefore also be a consequence of researchers assessing [Ca2+]i in different sub-populations of neurones from the DRG and at different levels of the spinal column.
Altered plasmalemmal Ca2+entry
Influx of Ca2+ to the cytoplasm through voltage-gated plasmalemmal Ca2+ channels represents an important component of Ca2+ signalling in excitable cells. Sensory neurones are endowed with several types of voltage-gated Ca2+ channels including low-threshold (T-type) and high-threshold (N and L-types) channels [43], which differ in conductance, voltage-dependence and pharmacology. The density of both low- and high-threshold Ca2+ currents was reported to increase in diabetic animals by 40 – 100% [44]. However, the amplitude of depolarisation-induced Ca2+ transients in isolated DRG neurones from diabetic and healthy animals is not generally affected [41, 45] and Ca2+ transients were depressed only in small neurones isolated from L4 – L6 DRG [41]. These apparent discrepancies may be explained in terms of more prominent Ca2+-dependent inactivation of Ca2+ currents in diabetic neurones possibly due to increased resting [Ca2+]i, while an increase in Ca2+ channel density may have a compensatory character.
ER Ca2+ homeostasis and Ca2+ release
The ER serves as a dynamic Ca2+ store, capable of accumulation, distribution and regulated release of Ca2+ ions [30]. ER Ca2+ homeostasis is accomplished by a complement of Ca2+ pumps represented by sarco(endo)plasmic reticulum Ca2+ ATP-ases (SERCAs) and Ca2+ release channels. The latter includes ryanodine receptors (RyR), inositol triphosphate receptors (InsP3R) and possibly nicotinic acid adenine dinucleotide phosphate (NAADP) receptors residing in the endomembrane [24–26, 46]. Free Ca2+ concentration within the ER lumen ([Ca2+]L) is high, approximately 0.5 – 1.0 mM [30, 47–51]. The [Ca2+]L level is functionally important as it controls the velocity of SERCA-dependent Ca2+ uptake, availability of Ca2+ release channels for activation, Ca2+ driving force and provides tight control over numerous ER-resident chaperones that are responsible for post-translational protein folding [27, 30, 34, 36, 52, 53]. Thus, any long-lasting changes in [Ca2+]L may have important signalling, functional and adaptive consequences.
The ER participates in rapid shaping of neuronal Ca2+ responses through their initiation (through metabotropically controlled InsP3-induced Ca2+ release), amplification (through Ca2+-induced Ca2+ release), propagation (by both regenerative activation of Ca2+-release channels and ER Ca2+ tunnelling) and termination (by SERCA-mediated Ca2+ uptake into the ER lumen); see [30, 45, 54–61]. All these processes are regulated though the [Ca2+]i and [Ca2+]L levels and metabolism of second messengers including InsP3, cyclic-ADP-ribose and NAADP.
Diabetes disrupts ER Ca2+ homeostasis in sensory neurones by lowering the ER Ca2+ content, thus reducing the amplitude of Ca2+ release from the store. Indeed, the amount of Ca2+ released from the ER is significantly decreased in diabetic DRG neurones, regardless of the interventions used to initiate Ca2+ efflux. Ca2+ release induced by low doses of ionomycin, by caffeine (activation of RyRs) or by ATP (metabotropic activation of InsP3Rs) were all significantly decreased in sensory neurones isolated from animals with STZ-diabetes [40, 41, 62]. Once more, the decrease in ER Ca2+ content was most pronounced in neurones from L1–L6 lumbar DRG compared with cervical and thoracic DRG [41]. Direct measurements of [Ca2+]L (Solovyova, Huang, Verkhratsky & Fernyhough, unpublished) and [Ca2+]i showed a significant decrease in caffeine-induced cytosolic Ca2+ transients. Reduction of [Ca2+]L and the velocity of Ca2+ uptake in diabetic neurones were associated with decreased expression of SERCA in homogenates of L4–L5 DRG from diabetic animals [63].
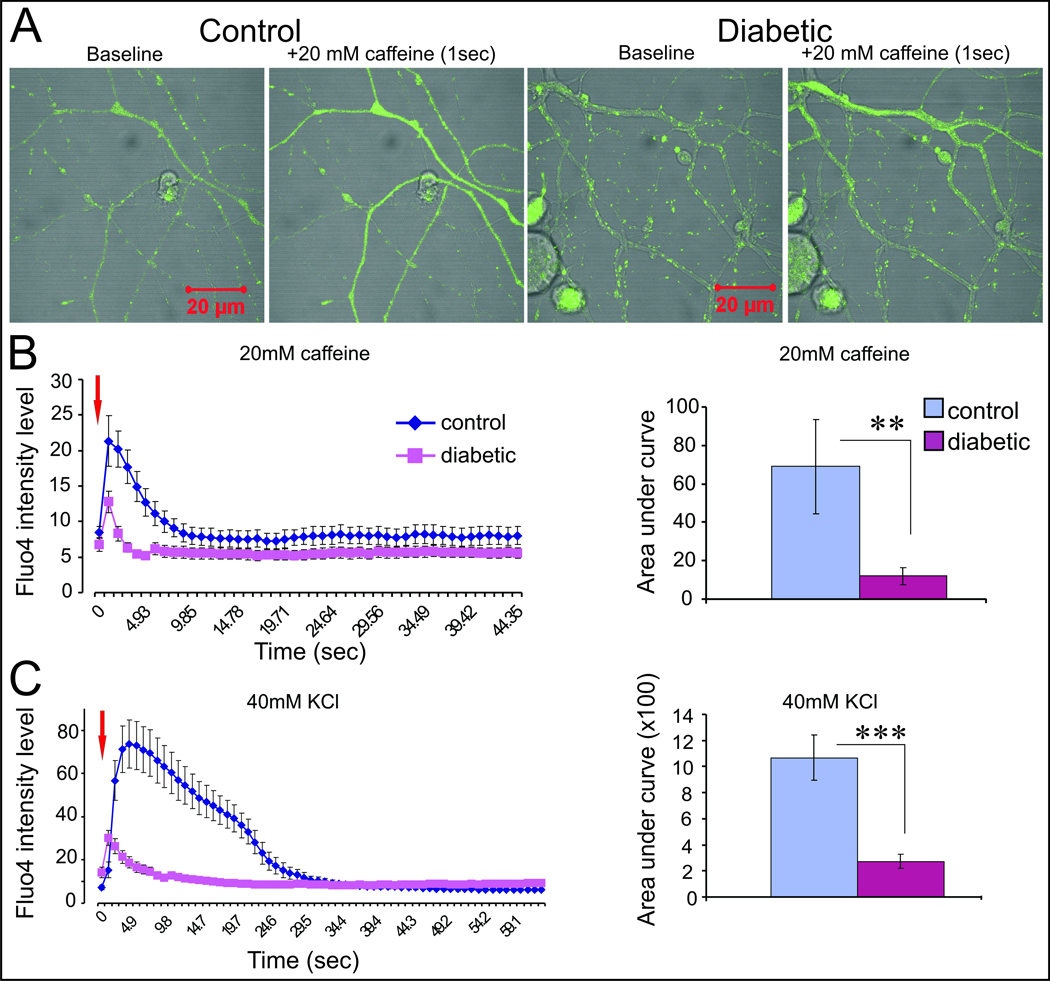
Our recent studies have shifted the focus of regulation of Ca2+ homeostasis from the perikarya to the axon. Recently published work demonstrates that, in cultured sensory neurons from diabetic rats, axons appear to be far more prone to high [glucose]-induced neurodegeneration. Adult sensory neurones were isolated from 3–5 month STZ-diabetic rats and grown in vitro for 1–4 days. Treatment with high [glucose] triggered oxidative stress leading to increased staining for adducts of 4-hydroxy-2-nonenal (a measure of ongoing lipid peroxidation), sub-optimal axonal outgrowth and the appearance of structural abnormalities in the axons akin to axonal dystrophy [13] that mimicked the axon degeneration seen in both animal models and humans with diabetes [64]. In stark contrast, the perikarya of these cultured neurones did not exhibit any overt signs of oxidative stress or degeneration. Interestingly, such glucose-induced toxicity of axons was only observed in neurones from diabetic animals and neurones grown from age matched control rats had no sensitivity to high [glucose]. We have now initiated studies focused on Ca2+ homeostasis in axons and have used real time confocal imaging with Fluo4-AM under high magnification (X100) to analyse Ca2+ transients in adult sensory neurones isolated from age matched control or 4–5 month STZ-diabetic rats. Fig.1A shows representative fluorescent images of the Fluo4-AM signal at baseline and following 1 second of caffeine perfusion. The response to caffeine, which prompts ER Ca2+ release, or KCl which initiates membrane depolarization-induced Ca2+ influx followed by Ca2+ mobilization from internal stores, was rapid and axonal in origin. There was no evidence of any waves of Ca2+ derived from the perikarya under the experimental conditions utilised. Fig.1B and C clearly reveal that axons from neurones with a diabetic background have severely impaired responses to caffeine and KCl. The results suggest reduced loading of Ca2+ within the ER store but also possibly impaired membrane polarisation since the fast Ca2+ peak in response to KCl-induced depolarisation was significantly reduced.
Figure 1. Axonal Ca2+ signals are abnormal in neurons cultured from STZ-diabetic rats compared with age-matched control.
Transient ER Ca2+ release and extracellular Ca2+ influx were induced by treatment with 20 mM caffeine or 40 mM KCl, respectively, in axons of adult lumbar DRG neurons cultured for 3 days from 4–5 month STZ-diabetic and age-matched control rats using Fluo4-AM Ca2+ ion detector. A: Real-time confocal images at X100 of the free Ca2+ ion at baseline and ER Ca2+ release subsequent to 1 sec of 20 mM caffeine treatment using a perfusion system. Cells were grown in Hams F12 defined medium with N2 additives (without insulin). Control neurons were treated with 10 mM D-glucose and 10 nM insulin and diabetic neurons exposed to 25 mM D-glucose and no insulin. Bar = 20 µm. B: Traces of Fluo4-AM fluorescence intensity level and bar chart of the area under the curve reflect the quantification of the ER Ca2+ release in response to 20 mM caffeine. Red arrow indicates point of caffeine addition. **P<0.01. C: Results with 40 mM KCl treatment. Values are means ± SEM, ***P<0.001, n=6–13 axons.
Ca2+ signal termination
The termination of Ca2+ signals triggered during periods of cellular activity is accomplished by either Ca2+ extrusion by plasmalemmal systems (Ca2+ pumps of plasmalemmal Ca2+ ATP-ase, PMCA, family and Na+/Ca2+ exchangers) or by Ca2+ uptake into ER and/or mitochondria. In neurones isolated from diabetic animals the functional capacity of extrusion systems seems to be impaired as indicated by the deceleration of [Ca2+]i recovery after stimulation towards resting levels [39–41, 45]. This may, at least in part, reflect the decrease in expression of PMCA pumps which has been observed in diabetic animals and patients [65, 66]. At the same time, the prolongation of Ca2+ signals is also associated with reduced Ca2+ uptake by the intracellular organelles, the ER and mitochondria. Decreased SERCA expression was found in the heart of STZ-diabetic animals [67], while over-expression of SERCA's rescued Ca2+ homeostasis abnormalities [68]. In neurones, decreased SERCA activity is manifested by a decrease in the velocity of Ca2+ uptake as judged by direct [Ca2+]L measurements (see above). Furthermore, mitochondrial Ca2+ buffering is also weakened in diabetic neurones as discussed below.
Mitochondrial dysfunction in diabetes
Mitochondrial depolarisation in diabetes
In cultured endothelial cells it has been shown that high intracellular glucose concentration drives excessive electron donation to the electron transport chain in mitochondria resulting in mitochondrial hyperpolarisation and elevated production of ROS [1, 2]. This mitochondrial-dependent process has been proposed as a central mediator of oxidative stress in complications of diabetes [1]. The theory suggests that high concentrations of glucose in tissue targets for diabetic complications leads to increased supply of NADH in the mitochondria and that this increased electron availability and/or saturation may cause partial reduction of oxygen to superoxide radicals in the proximal part of the electron transport chain. Subsequent large elevations in ROS then induce degeneration of tissue. However, our work and studies by others show that in adult sensory neurones from STZ-diabetic rats the mitochondrial inner membrane potential is depolarized, not hyperpolarised [69–72]. Our experiments on perikarya of acutely isolated sensory neurones also show that mitochondrial depolarisation in STZ-diabetes could be prevented by systemic treatment with low dose insulin or NT-3, further questioning a central role for high glucose concentration in mitochondrial dysfunction in diabetes [70, 71].
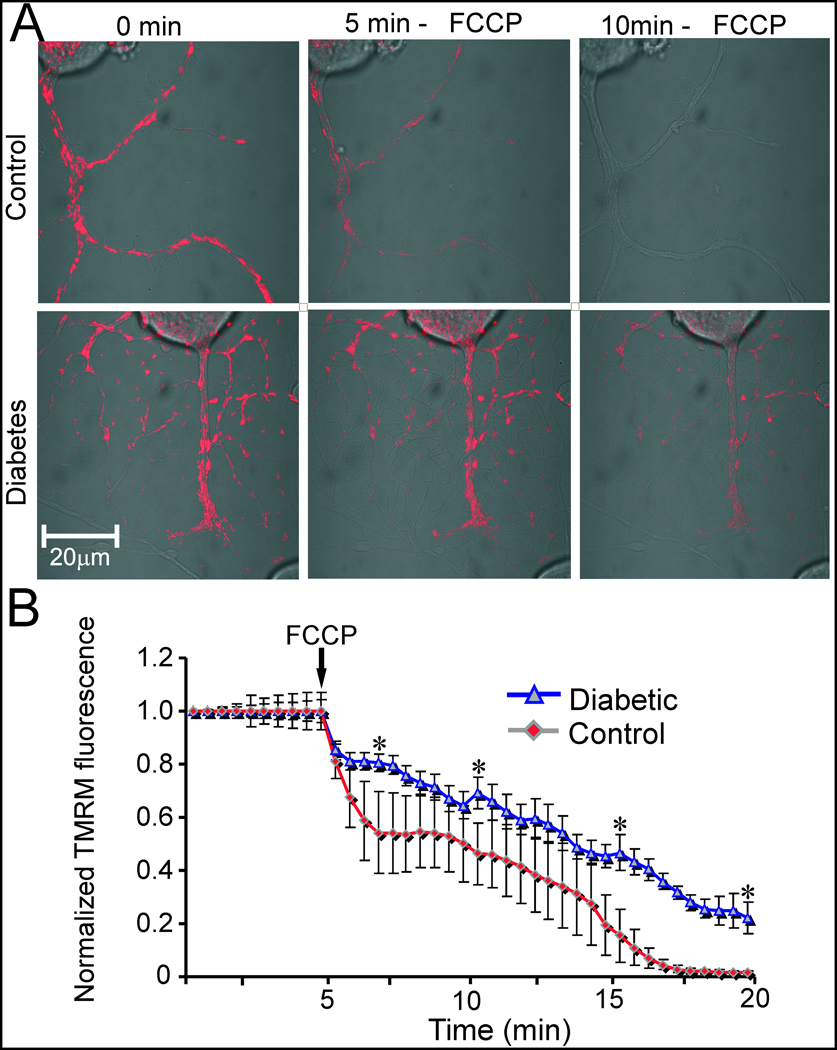
Fig. 2 presents preliminary data demonstrating that depolarization of the mitochondrial inner membrane is also occurring in the axons of sensory neurons from diabetic animals. Adult sensory neurones were cultured for 1 day from age matched and 3–5 month STZ-diabetic rats and then loaded with tetramethylrhodamine methyl ester (TMRM) and the dye used to detect mitochondrial membrane depolarisation in sub-quench mode [73]. The results show that axons of normal neurons underwent a much more rapid rate of mitochondrial depolarisation subsequent to addition of the uncoupler carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP). This suggests that prior to addition of FCCP the axonal mitochondria were more highly polarized than their diabetic neuron counterparts.
Figure 2. The mitochondrial membrane in the axons of normal neurons show greater levels of FCCP-induced depolarization compared with neurons from diabetic rats.
A: Fluorescence video confocal microscopy of mitochondria in live cultures of sensory neurons of dorsal root ganglion (DRG) isolated from adult age matched or 4–5 month STZ-diabetic rats. DRG neurons were cultured in defined Hams F12 media supplemented with N2 additives (no insulin) and 10 mM D-glucose (control; with 10 nM insulin) or 25 mM D-glucose (diabetic) for 24hr. Relative levels of mitochondrial inner membrane potential were determined by staining neurons with 3 nM tetramethyl rhodamine methyl ester (TMRM; Molecular Probes, Eugene, OR) for 1 hr and detecting the fluorescence signal with a Carl Zeiss LSM510 confocal microscope. FCCP was injected into the culture media to a final concentration of 2 µM at 5 min following baseline fluorescence measurements. B: Quantification of TMRM fluorescence levels in sub-quench mode (where decreased fluorescence intensity indicates reduced mitochondrial inner membrane potential) in the axons of cultured DRG neurons. The black arrow shows FCCP injection. Values are means ± SEM, n= 65–85 axons, *P < 0.001, Students t-test.
Studies in cultured embryonic sensory neurones demonstrate that high glucose concentration causes a chronic mitochondrial depolarization followed by apoptosis [74]. It should be noted that high glucose concentrations do not kill embryonic neurones permitted to mature in vitro or adult sensory neurones (even when exposed for 4 weeks at 50 mM glucose in vitro) [64, 75, 76]. In addition, there is no evidence of major mitochondrial structural abnormalities in neurones/axons or neuronal cell death in ganglia in human diabetic sensory or autonomic neuropathy [13, 77]. In rodent models of type 1 diabetes there is evidence of small unmyelinated neurone cell loss but no positive information supporting the presence of apoptosis or abnormal mitochondrial structure within the DRG [78–81]. However, recent studies on neurones isolated from diabetic rats demonstrate that axonal growth and morphology can be impaired by high [glucose] due to an elevation in oxidative stress that results in axonal degeneration but not cell death [64]. Whether, the glucose-induced pathology is the result of a mitochondrial-related pathway triggering oxidative stress remains unknown.
Impaired mitochondrial Ca2+ buffering
The high membrane potential of the mitochondrial membrane is also responsible for maintaining a continuous and important link between cytosolic Ca2+ and mitochondrial function. The electronegativity versus cytosol build up on the mitochondrial membrane provides an electro-driving force which pushes Ca2+ ions through the mitochondrial Ca2+ channel, generally referred to as the Ca2+ uniporter [82, 83]. Increases in [Ca2+]i, and specifically stimulus-driven generation of localised [Ca2+]i microdomains, which can be formed in the vicinity of mitochondria either by plasmalemmal Ca2+ entry or Ca2+ release from the ER lead to Ca2+ influx into the mitochondrial matrix [84–86]. This Ca2+ influx generates mitochondrial Ca2+ signals, which control activity of enzymes of the tricarboxylic acid (TCA) cycle, thus regulating ATP synthesis ([87, 88] for reviews). Conceptually, these mitochondrial Ca2+ signals couple cellular activity with energy generation. Termination of mitochondrial Ca2+ signals are associated with slow Ca2+ release through the Na+/Ca2+ exchanger.
Sensory neurones of STZ-diabetic rats simultaneously develop depolarisation of the mitochondrial inner membrane in the perikarya and axon. Importantly, a rise in resting [Ca2+]i to 200 nM (which is seen in diabetes) can trigger elevated mitochondrial Ca2+ buffering (a rise in [Ca2+]m) by entry of Ca2+ through the Ca2+ uniporter and other routes [83, 89–92], which causes partial or complete inner mitochondrial membrane depolarisation [93]. Plasma membrane depolarisation-induced Ca2+ transients are prolonged in diabetic neurones and blockade of mitochondrial uptake of Ca2+ using the mitochondrial uncoupler, carbonyl cyanide m-chlorophenylhydrazone (CCCP), prevented these abnormalities [39]. This implies, indirectly, that mitochondrial buffering of Ca2+ plays a role in shaping Ca2+ transients in diabetic neurones.
Mitochondrial dysfunction and Ca2+ dyshomeostasis can be prevented by insulin and neurotrophic factors
Both alterations of Ca2+ homeostasis and mitochondrial membrane depolarisation in adult sensory neurones occur early (3 – 14 weeks) in experimental type 1 (STZ) and type 2 (db/db) diabetes and therefore can be identified as potential key steps in the development of sensory neuropathy. The critical factor determining mitochondrial dysfunction is not hyperglycaemia but absence of insulin-dependent neurotrophic support. This was shown in both in vivo and in vitro experiments [70, 72]. Treatment of healthy cultured DRG neurones with 1 nM of insulin for 6 – 24 hours significantly raised the mitochondrial membrane potential compared with insulin-free cultures and increased the levels of ATP production. At the same time, treatment of cultures with 50 mM glucose in the presence of insulin caused no effect on the mitochondrial inner membrane potential [72]. Similar results were obtained in the in vivo system, where STZ-diabetic rats were treated with very low insulin concentrations that provided background insulin at a dose that did not affect hyperglycaemia [70]. Insulin was administered as a slow release implant for the final 7 weeks of the study and did not alter blood glucose, blood glycated haemoglobin or nerve sugar and polyol levels. At the same time, insulin therapy completely normalised mitochondrial membrane polarisation and levels of resting [Ca2+]i. Importantly, this treatment also normalised sensory and motor nerve conductance velocities. Mitochondrial polarisation and Ca2+ homeostasis in sensory neurones from STZ-diabetic animals can also be normalised by treatment with the neurotrophic factor, NT-3 [41, 71]. Support for a primary role for insulin deficiency in the pathogenesis of type 1 diabetic neuropathy also comes from experiments in which STZ-diabetic animals have been treated locally or systemically with low doses of insulin that do not alter the hyperglycaemic state. Local delivery of insulin to the spinal cord at the lumbar level (by the intrathecal route) or peripheral nerve (by local mini-osmotic pump) or by intranasal delivery improves SNCV and MNCV, and epidermal nerve fiber density in STZ-diabetic rodents [94–97].
Further experiments have identified the key role of phosphoinositide 3-kinase (PI 3-kinase) and protein kinase B (or AKT - see [98]) in regulation of mitochondrial membrane potential [72, 99]. This pathway is regulated, in part, by plasmalemmal receptors to insulin (comprising insulin receptor subunits α and β expressed in DRG neurones) and neurotrophin receptors. The involvement of PI-3/AKT system was directly demonstrated in DRG neurones treated with a specific inhibitor of PI 3-kinase (LY294002), which significantly inhibited insulin- and neurotrophin-dependent up-regulation of mitochondrial membrane potential and the insulin-dependent increase in ATP levels [72, 99].
Role of calcium in painful peripheral neuropathies
Background
Neuropathic pain, defined as pain arising from nerve injury, is a widespread condition that impedes quality of life and can be present in 5% or more of the general population [100]. The initiating nerve injury can have diverse origins and may not always be obvious. Direct lesions to nerves, such as those incurred during amputation, during compression by adjacent cancers or other injuries and by infection of nerves by viruses can induce long lasting pain states that persist beyond the initiating injury. Neuropathic pain is also associated with more insidious systemic disorders that cause peripheral neuropathies, such as are seen in diabetes, nutritional disorders, exposure to neurotoxins (either accidentally or as a side-effect of therapeutics) and malfunctions of the immune system. The pain syndrome produced may be intermittent or persistent, evoked by external stimuli or apparently spontaneous and can be experienced as a variety of different sensations. This lack of specificity may suggest that the mechanisms that promote neuropathic pain represent fundamental responses to nerve injury, irrespective of how the injury was incurred. Unfortunately, potential mechanistic considerations are further complicated by the unpredictability of neuropathic pain in a given population. For example, the prevalence of neuropathic pain in diabetic patients is only 10–20% and pain is not always associated with the presence of degenerative neuropathy or any other aspect of the diabetic condition [101]. Consequently, the mechanisms that underlie neuropathic pain remain poorly understood while current therapeutic approaches mostly treat symptoms rather than the underlying pathogenesis.
Attempts to understand how nerve injury may promote neuropathic pain have largely relied on a number of animal models in which some form of traumatic injury, such as crush, transection or compression, is applied to peripheral nerves followed by behavioral evaluation of limb responses to normally non-painful or painful (nociceptive) sensory stimuli [102]. Cleary, these lesions most accurately model neuropathic pain arising from acute physical nerve injury and such studies have identified fundamental mechanisms of how sensory nerves respond to trauma and also certain aspects of the injury response that can promote neuropathic pain. There is still an evolving appreciation of the specific mechanistic features of each nerve lesion model, while the extent to which they also model the pain of systemic diseases that cause peripheral neuropathy and which represent the majority of clinical cases of neuropathic pain, is less certain. Nevertheless, in the majority of both traumatic and disease-specific injury models, damage to peripheral sensory nerves and subsequent primary afferent activity is widely considered as the initiating event. In many cases, aberrant primary afferent activity may also contribute to persistence of the pain state. Given the established role of neuronal Ca2+ fluxes in axonal potential formation and neurotransmitter release by primary sensory neurons, the potential for misfunction of Ca2+ regulation to contribute to neuropathic pain has become a focus of attention in the search for targets for therapeutic interventions that prevent or alleviate neuropathic pain [103].
Calcium dysregulation and primary afferent conduction
Many of the mechanisms that have been proposed to explain neuropathic pain involve increased or enhanced activity of primary afferents as a means of initiating and sustaining the pain state. This peripheral drive may derive from increased excitability of peripheral terminals and either spontaneous activity or inappropriate local cross-excitation between adjacent axons in nerve trunks or cell bodies in the DRG. Voltage activated sodium channels are a primary determinant of axonal firing and blockade of voltage gated sodium channels is a well-established mechanism of action of many local anesthetics. Consequently, delivery of low doses of the local anesthetic lidocaine to depress excessive primary afferent activity without blocking sensory function has become an accepted approach to treating some forms of neuropathic pain [104].
Excitability of sensory neurons is also modulated by rapid changes of intracellular Ca2+ levels that are mediated by a variety of pumps and channels [105]. Low voltage activated T-type Ca2+ channels (designated as the Cav3.2 sub-type) are located on primary afferent terminals and cell bodies and their role in shaping the action potential and regulating neuronal firing patterns offers a particularly appealing target for modulating neuronal excitability. Inhibiting T-type Ca2+ channel activity, initially using non-selective antagonists [106] and more recently by depressing Cav3.2 expression [107, 108], has been shown to alleviate indices of neuropathic pain in a variety of nerve injury models. Reducing T type Ca2+ channel activity is also effective in animal models of painful peripheral neuropathy induced by chemotherapeutic agents [109] and diabetes [110]. Moreover, enhanced T -type Ca2+ channel mRNA and currents have been reported in small and medium sized sensory neuron cell bodies of diabetic rats and mice [110, 111], suggesting that over expression of these channels may contribute to the underlying mechanism of painful diabetic neuropathy.
Calcium dysregulation and neurotransmitter release
Primary afferent input to the spinal cord involves release of excitatory neurotransmitters from central terminals that project into the dorsal horn. Many models of neuropathic pain incorporate spinal sensitization in response to increased primary afferent activity as a mechanism of maintaining a persistent pain state after peripheral input to the spinal cord has subsided [112]. This is a particularly appealing hypothesis when trying to model the pain of degenerative peripheral neuropathies, such as those caused by diabetes or chemotherapies, that often progress towards of loss of peripheral sensory function while retaining a painful component. The trigger for excitatory neurotransmitter release from primary afferents is arrival of the action potential, local membrane depolarization and subsequent activation of high voltage activated Ca2+ channels. Influx of Ca2+ initiates docking of vesicles containing excitatory neurotransmitters and modulators such as glutamate, substance P and CGRP with the presynaptic membrane and release of these molecules into the synapse. Blocking Ca2+ influx to the presynaptic terminal is therefore a logical approach to preventing peripheral input to the spinal cord. Indeed, part of the mechanism by which opiates block pain perception is by inhibiting the pre-synaptic influx of Ca2+into small sensory neurons via activation of the G protein coupled opioid receptors [113]. However, use of opiates to manage neuropathic pain requires delicate dose titration in order to minimize loss of normal pain perception mechanisms and is also fraught with problems such as tolerance and the unwanted side effects that arise from the widespread distribution of opioid receptors.
The high density of the neuron-specific high voltage activated N type Ca2+ channel (Cav2.2 sub-type) on the central terminals of primary afferents [114] suggests an alternative target for blocking neurotransmitter release from these neurons that also offers a degree of selectivity. Selective small peptide inhibitors of N type channels have been identified in cone snail venoms [115] and a number of these conotoxins were shown to block pain perception in rats, albeit with dose-dependent induction of other neurological side effects that presumably reflects the distribution of the N type Ca2+ channel beyond primary afferent terminals [116]. The lack of tolerance to repeated conotoxin-induced block of N type Ca2+ channels [116] confers a potential clinical advantage over opioids and both conotoxins and small molecule mimetics have been developed for use in chronic pain states [117]. There have been relatively few studies of the therapeutic potential of blocking N type Ca2+ channels in animal models of neuropathic pain induced by systemic disease. Intrathecal delivery of a conotoxin alleviated hyperalgesia in a mouse model of vincristine-induced hyperalgesia, [118] and indices of painful neuropathy in diabetic rats were ameliorated by spinal delivery of a conotoxin, as well as by other agents that impede Ca2+-mediated neurotransmitter release such as opioid and α2 adrenoceptor agonists [119]. N type Ca2+ currents have been reported as being exaggerated in primary sensory neuron cell bodies of diabetic rats, possibly as a result of a loss of G-protein coupled receptor-mediated restraint [120, 121]. This may indicate a pathogenic mechanism for painful diabetic neuropathy, although subsequent studies suggest that diabetes does not increase expression of the α1B sub-unit of the N type Ca2+ channel in primary afferent cell bodies [122, 123]. Interestingly, the same studies reported that diabetes did up-regulate mRNA for sub-units associated with other high voltage activated Ca2+channels (L and P/Q types), changes that if translated to altered Ca2+ currents, could exaggerate release of excitatory neurotransmitters [124]
Calcium dysregulation and signal transduction
Aside from direct effects on membrane excitability and neurotransmitter release, altered intracellular Ca2+ levels have the potential to disrupt many cellular events that could indirectly contribute to neuropathic pain states, including regulation of the voltage activated Ca2+ channels themselves [125]. Steady state cytoplasmic Ca2+ levels are elevated in peripheral neurons of rodent models of diabetic neuropathy [41, 126, 127], most likely due to a impaired regulation of Ca2+ homeostasis by pumps in the cell membrane, ER and mitochondria that are discussed above in the context of degenerative diabetic neuropathy. Systemic delivery of agents intended to restore neuronal Ca2+ homeostasis have been reported to ameliorate indices of hyperalgesia in models of chemotherapeutic-induced neuropathy [128] and painful diabetic neuropathy [129–131], although the location and precise mechanisms of action remain to be determined.
Chronic dysregulation of Ca2+ homeostasis leading to elevated cytoplasmic Ca2+ concentrations has the potential to impact many aspects of sensory neuron function that promote aberrant pain sensations. Impaired Ca2+ homeostatic mechanisms at peripheral terminals of primary afferents could contribute to peripheral sensitization in which ligand binding at the terminal promotes intracellular signaling cascades [132]. For example, a neuropathic pain state induced by antiretroviral drugs used to treat HIV may be ameliorated by delivery to either the peripheral or central terminals of drugs that diminish the raised [Ca2+]i [133]. Beyond the central terminals of primary afferent, there is also evidence of elevated cytoplasmic calcium and impaired intracellular Ca2+ responses to external stimuli in post-synaptic neurons of the dorsal horn [134, 135]. As many of the consequences of synaptic activity, including modulation of synaptic plasticity and central sensitization mechanisms, are mediated by Ca2+ [112], there are many as yet largely unexplored potential mechanisms by which Ca2+ dysregulation may contribute to neuropathic pain at the PNS, spinal cord and higher CNS. Moreover, it is becoming clear that glial cells of the nervous system also play roles in central maintenance of neuropathic pain, including in models of diabetes [136, 137]. The potential of dysfunctional Ca2+ signaling in glial cells to promote neuropathic pain awaits detailed study.
Use of calcium modulators to treat neuropathic pain
The complexity and variability of neuropathic pain syndromes in patients makes treatment a largely phenomenological march through the pharmacopeia of approved drugs that balances efficacy with side effect profile [138]. That a number of the current and emerging drugs for neuropathic pain target aspects of neuronal Ca2+ regulation [139] emphasizes the potential importance of dysfunctional cell Ca2+ regulation in the genesis of neuropathic pain. Zirconotide (Prialt), an N type Ca2+ channel blocker, is approved to treat chronic pain [140], but unfortunately has restricted use as it is a conotoxin peptide that has to be delivered spinally. As yet, no selective inhibitor of T type Ca2+ channels has yet been developed for clinical use against neuropathic pain. However, recent experimental studies suggest that lipoic acid, which is used to treat painful diabetic neuropathy [141] and lipoamino acid, which shows anti-pain properties in animals [142], may act by blockade of the T type Ca2+ channel [143, 144]. Finally, the widespread clinical use of gabapentin (Neurontin) and the gabapentin derivative pregabalin (Lyrica) to treat neuropathic pain [103, 139] provides further illustration of the potential of modulating Ca2+ currents. Gabapentin was originally developed as an antiepileptic, but was also noted to produce pain relief in diabetic patients with peripheral neuropathy [145]. Efficacy was subsequently demonstrated in many animal models of neuropathic pain, including diabetes [146, 147] but only in those where nerve injury was associated with upregulation of the α2δ1 sub-unit of voltage activated Ca2+ channels [148]. The α2δ1 sub-unit binds a range of α1 sub-units, with binding causing an increase in current density and acceleration of activation/inactivation kinetics. It has become accepted that the anti-neuropathic pain effects of gabapentin are related to its ability to prevent an increase in α2δ1 sub-units in the plasma membrane following nerve injury [149] and recent studies indicate that the gabapentin derivative pregabalin prevents transport and membrane insertion of the α2δ1 sub-unit to neuronal terminals, thereby likely impeding any injury-induced increase in local Ca2+ currents that could promote neuronal sensitization [150].
The widespread use of gabapentin to treat many forms of neuropathic pain appears to validate the targeting of Ca2+ channels and pumps as therapeutic approach. Current challenges for drug development programs include more precise targeting of agents to reduce side effect profiles and the separation of anti-neuropathic pain versus anti-nociceptive actions, perhaps by producing agents with use-dependent inhibitory properties.
Summary and proposal of a calcium-centric mechanism of diabetic neuropathy
In the periphery, diabetes almost invariably affects sensory pathways resulting in symmetrical sensory neuropathy. Initially, diabetes diminishes nerve conduction velocity and diabetic patients may also experience various symptoms ranging from pain, altered sensation and impairment of reflexes. The cellular and molecular pathophysiology of diabetic polyneuropathy remains controversial and several pathways associated with hyperglycaemia, including polyol pathway flux [151, 152], oxidative stress [153], protein glycosylation [154] have been suggested. In addition the role of impaired neurotrophic support has also been considered [20, 22].
We now propose an alternative mechanism for the initiation of diabetic neuropathy, which is associated with early alterations of mitochondrial function and cellular Ca2+ homeostasis that are not directly driven by hyperglycaemia, but rather produced by impaired signalling cascades controlled by insulin receptors and neutrotrophic factors. Based on the results discussed above, we suggest that the initial pathogenesis of diabetic neuropathy is centred on reduced stimulation of insulin receptors. This triggers mitochondrial dysfunction and decreased ATP production. In turn, diminished ATP support affects Ca2+ homeostatic mechanisms, most profoundly altering plasmalemmal and ER Ca2+ pumps. Indeed, expression of the latter is also reduced by a so far unidentified mechanism [63]. A decrease in ER Ca2+ uptake lowers the intra-ER Ca2+ concentration, thus inducing mild but not chronic conditions of ER stress. The ER stress in turn affects protein synthesis, posttranslational modification and trafficking, which in turn diminishes the supply of voltage-gated channels to the axons, thus resulting in the decrease of nerve conduction velocity. These pathophysiological processes may occur more readily in the axon. This initial step is further exacerbated during progression of the disease, when other mechanisms associated with chronic hyperglycaemia come to the fore, resulting in severe sensory dysfunction and degenerative neuropathy.
ACKNOWLEDGEMENTS
Dr. Fernyhough would like thank the Juvenile Diabetes Research Foundation, St Boniface Hospital and Research Foundation and Canadian Institutes for Health Research for grant support for these studies. We thank our colleagues, Dr. Elena Zherebitskaya and Eli Kwaku Akude, for providing data that contributed towards this article. Dr. Calcutt was supported by NIH grant DK057629.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 3.Dawson KG, Gomes D, Gerstein H, Blanchard JF, Kahler KH. The economic cost of diabetes in Canada, 1998. Diabetes Care. 2002;25:1303–1307. doi: 10.2337/diacare.25.8.1303. [DOI] [PubMed] [Google Scholar]
- 4.Caro JJ, Ward AJ, O'Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–481. doi: 10.2337/diacare.25.3.476. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien JA, Patrick AR, Caro JJ. Cost of managing complications resulting from type 2 diabetes mellitus in Canada. BMC Health Serv Res. 2003;3:7. doi: 10.1186/1472-6963-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulton AJ. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47:1343–1353. doi: 10.1007/s00125-004-1463-y. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard JF, Wajda A, Green C. Diabetes and its Complications: Forecasting the coming storm. Winnipeg: Manitoba Health; 1998. Diabetes: A Manitoba strategy. [Google Scholar]
- 8.Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I, Sharma AK, Boulton AJ, King RH, Thomas PK, Ward JD. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48:578–585. doi: 10.1007/s00125-004-1663-5. [DOI] [PubMed] [Google Scholar]
- 9.Sima AA. Diabetic neuropathy in type 1 and type 2 diabetes and the effects of C-peptide. J Neurol Sci. 2004;220:133–136. doi: 10.1016/j.jns.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Yagihashi S. Pathogenetic mechanisms of diabetic neuropathy: lessons from animal models. J Peripher Nerv Syst. 1997;2:113–132. [PubMed] [Google Scholar]
- 11.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 12.Sidenius P, Jakobsen J. Reduced perikaryal volume of lower motor and primary sensory neurons in early experimental diabetes. Diabetes. 1980;29:182–186. doi: 10.2337/diab.29.3.182. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt RE, Dorsey D, Parvin CA, Beaudet LN, Plurad SB, Roth KA. Dystrophic axonal swellings develop as a function of age and diabetes in human dorsal root ganglia. J Neuropathol Exp Neurol. 1997;56:1028–1043. doi: 10.1097/00005072-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- 15.Nja A, Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 17.Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol. 2002;50:325–392. doi: 10.1016/s0074-7742(02)50082-9. [DOI] [PubMed] [Google Scholar]
- 18.Thornalley PJ. Glycation in diabetic neuropathy: characteristics, consequences, causes, and therapeutic options. Int Rev Neurobiol. 2002;50:37–57. doi: 10.1016/s0074-7742(02)50072-6. [DOI] [PubMed] [Google Scholar]
- 19.Yan SF, Barile GR, D'Agati V, Du Yan S, Ramasamy R, Schmidt AM. The biology of RAGE and its ligands: uncovering mechanisms at the heart of diabetes and its complications. Curr Diab Rep. 2007;7:146–153. doi: 10.1007/s11892-007-0024-4. [DOI] [PubMed] [Google Scholar]
- 20.Fernyhough P, Tomlinson DR. The therapeutic potential of neurotrophins for the treatment of diabetic neuropathy. Diabetes Reviews. 1999;7:300–311. [Google Scholar]
- 21.Ishii DN. Implication of insulin-like growth factors in the pathogenesis of diabetic neuropathy. Brain Res Brain Res Rev. 1995;20:47–67. doi: 10.1016/0165-0173(94)00005-a. [DOI] [PubMed] [Google Scholar]
- 22.Calcutt NA, Jolivalt CG, Fernyhough P. Growth factors as therapeutics for diabetic neuropathy. Curr Drug Targets. 2008;9:47–59. doi: 10.2174/138945008783431727. [DOI] [PubMed] [Google Scholar]
- 23.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton SL. Ryanodine receptors. Cell Calcium. 2005;38:253–260. doi: 10.1016/j.ceca.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Galione A, Ruas M. NAADP receptors. Cell Calcium. 2005;38:273–280. doi: 10.1016/j.ceca.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 28.Petersen OH. Ca2+ signalling and Ca2+-activated ion channels in exocrine acinar cells. Cell Calcium. 2005;38:171–200. doi: 10.1016/j.ceca.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium. 2005;38:161–169. doi: 10.1016/j.ceca.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 31.Verkhratsky A. Calcium ions and integration in neural circuits. Acta Physiol (Oxf) 2006;187:357–369. doi: 10.1111/j.1748-1716.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- 32.Leist M, Nicotera P. Calcium and neuronal death. Rev Physiol Biochem Pharmacol. 1998;132:79–125. doi: 10.1007/BFb0004986. [DOI] [PubMed] [Google Scholar]
- 33.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 34.Verkhratsky A, Petersen OH. The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur J Pharmacol. 2002;447:141–154. doi: 10.1016/s0014-2999(02)01838-1. [DOI] [PubMed] [Google Scholar]
- 35.Verkhratsky A, Toescu EC. Endoplasmic reticulum Ca2+ homeostasis and neuronal death. J Cell Mol Med. 2003;7:351–361. doi: 10.1111/j.1582-4934.2003.tb00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005;38:409–415. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Levy J, Gavin JR, 3rd, Sowers JR. Diabetes mellitus: a disease of abnormal cellular calcium metabolism? Am J Med. 1994;96:260–273. doi: 10.1016/0002-9343(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 38.Fedirko NV, Kruglikov IA, Kopach OV, Vats JA, Kostyuk PG, Voitenko NV. Changes in functioning of rat submandibular salivary gland under streptozotocin-induced diabetes are associated with alterations of Ca2+ signaling and Ca2+ transporting pumps. Biochim Biophys Acta. 2006;1762:294–303. doi: 10.1016/j.bbadis.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Kostyuk E, Svichar N, Shishkin V, Kostyuk P. Role of mitochondrial dysfunction in calcium signalling alterations in dorsal root ganglion neurons of mice with experimentally-induced diabetes. Neuroscience. 1999;90:535–541. doi: 10.1016/s0306-4522(98)00471-0. [DOI] [PubMed] [Google Scholar]
- 40.Kostyuk E, Voitenko N, Kruglikov I, Shmigol A, Shishkin V, Efimov A, Kostyuk P. Diabetes-induced changes in calcium homeostasis and the effects of calcium channel blockers in rat and mice nociceptive neurons. Diabetologia. 2001;44:1302–1309. doi: 10.1007/s001250100642. [DOI] [PubMed] [Google Scholar]
- 41.Huang TJ, Sayers NM, Fernyhough P, Verkhratsky A. Diabetes-induced alterations in calcium homeostasis in sensory neurones of streptozotocin-diabetic rats are restricted to lumbar ganglia and are prevented by neurotrophin-3. Diabetologia. 2002;45:560–570. doi: 10.1007/s00125-002-0785-x. [DOI] [PubMed] [Google Scholar]
- 42.Thomas PK, Tomlinson DR. Diabetic and hypoglycaemic neuropathy. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, editors. Peripheral Neuropathy. Philadelphia: W.B.Saunders Co; 1992. pp. 1219–1250. [Google Scholar]
- 43.Nilius B, Talavera K, Verkhratsky A. T-type calcium channels: the never ending story. Cell Calcium. 2006;40:81–88. doi: 10.1016/j.ceca.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Hall KE, Sima AA, Wiley JW. Voltage-dependent calcium currents are enhanced in dorsal root ganglion neurones from the Bio Bred/Worchester diabetic rat. J Physiol. 1995;486(Pt 2):313–322. doi: 10.1113/jphysiol.1995.sp020814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kostyuk E, Pronchuk N, Shmigol A. Calcium signal prolongation in sensory neurones of mice with experimental diabetes. Neuroreport. 1995;6:1010–1012. doi: 10.1097/00001756-199505090-00015. [DOI] [PubMed] [Google Scholar]
- 46.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium. 2005;38:291–302. doi: 10.1016/j.ceca.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 47.Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo I, Garcia AG, Garcia-Sancho J, Montero M, Alvarez J. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J. Cell Biol. 1999;144:241–254. doi: 10.1083/jcb.144.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez J, Montero M. Measuring [Ca2+] in the endoplasmic reticulum with aequorin. Cell Calcium. 2002;32:251–260. doi: 10.1016/s0143416002001860. [DOI] [PubMed] [Google Scholar]
- 49.Solovyova N, Verkhratsky A. Monitoring of free calcium in the neuronal endoplasmic reticulum: an overview of modern approaches. J Neurosci Methods. 2002;122:1–12. doi: 10.1016/s0165-0270(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 50.Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A. Ca2+ dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca2+-induced Ca2+ release triggered by physiological Ca2+ entry. Embo J. 2002;21:622–630. doi: 10.1093/emboj/21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tse FW, Tse A, Hille B. Cyclic Ca2+ changes in intracellular stores of gonadotropes during gonadotropin-releasing hormone-stimulated Ca2+ oscillations. Proc Natl Acad Sci U S A. 1994;91:9750–9754. doi: 10.1073/pnas.91.21.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Burdakov D, Verkhratsky A. Biophysical re-equilibration of Ca2+ fluxes as a simple biologically plausible explanation for complex intracellular Ca2+ release patterns. FEBS Lett. 2006;580:463–468. doi: 10.1016/j.febslet.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 54.Usachev Y, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience. 1993;57:845–859. doi: 10.1016/0306-4522(93)90029-f. [DOI] [PubMed] [Google Scholar]
- 55.Kano M, Garaschuk O, Verkhratsky A, Konnerth A. Ryanodine receptor-mediated intracellular calcium release in rat cerebellar Purkinje neurones. J Physiol. 1995;487(Pt 1):1–16. doi: 10.1113/jphysiol.1995.sp020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shmigol A, Svichar N, Kostyuk P, Verkhratsky A. Gradual caffeine-induced Ca2+ release in mouse dorsal root ganglion neurons is controlled by cytoplasmic and luminal Ca2+ Neuroscience. 1996;73:1061–1067. doi: 10.1016/0306-4522(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 57.Solovyova N, Verkhratsky A. Neuronal endoplasmic reticulum acts as a single functional Ca2+ store shared by ryanodine and inositol-1,4,5-trisphosphate receptors as revealed by intra-ER [Ca2+] recordings in single rat sensory neurones. Pflugers Arch. 2003;446:447–454. doi: 10.1007/s00424-003-1094-z. [DOI] [PubMed] [Google Scholar]
- 58.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 59.Verkhratsky A. The endoplasmic reticulum and neuronal calcium signalling. Cell Calcium. 2002;32:393–404. doi: 10.1016/s0143416002001896. [DOI] [PubMed] [Google Scholar]
- 60.Shmigol A, Kostyuk P, Verkhratsky A. Role of caffeine-sensitive Ca2+ stores in Ca2+ signal termination in adult mouse DRG neurones. Neuroreport. 1994;5:2073–2076. doi: 10.1097/00001756-199410270-00021. [DOI] [PubMed] [Google Scholar]
- 61.Petersen OH, Verkhratsky A. Endoplasmic reticulum calcium tunnels integrate signalling in polarised cells. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N. Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons. Pflugers Arch. 2004;448:395–401. doi: 10.1007/s00424-004-1263-8. [DOI] [PubMed] [Google Scholar]
- 63.Verkhratsky A, Fernyhough P. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium. 2008;44:112–122. doi: 10.1016/j.ceca.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Zherebitskaya E, Akude E, Smith DR, Fernyhough P. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes. 2009;58:1356–1364. doi: 10.2337/db09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janicki PK, Horn JL, Singh G, Franks WT, Franks JJ. Diminished brain synaptic plasma membrane Ca2+-ATPase activity in rats with streptozocin-induced diabetes: association with reduced anesthetic requirements. Life Sci. 1994;55:PL359–PL364. doi: 10.1016/0024-3205(94)00761-6. [DOI] [PubMed] [Google Scholar]
- 66.Migdalis IN, Xenos K, Chairopoulos K, Varvarigos N, Leontiades E, Karmaniolas K. Ca2+-Mg2+-ATPase activity and ionized calcium in Type 2 diabetic patients with neuropathy. Diabetes Res Clin Pract. 2000;49:113–118. doi: 10.1016/s0168-8227(00)00150-9. [DOI] [PubMed] [Google Scholar]
- 67.Teshima Y, Takahashi N, Saikawa T, Hara M, Yasunaga S, Hidaka S, Sakata T. Diminished expression of sarcoplasmic reticulum Ca2+-ATPase and ryanodine sensitive Ca2+ channel mRNA in streptozotocin-induced diabetic rat heart. J Mol Cell Cardiol. 2000;32:655–664. doi: 10.1006/jmcc.2000.1107. [DOI] [PubMed] [Google Scholar]
- 68.Vetter R, Rehfeld U, Reissfelder C, Weiss W, Wagner KD, Gunther J, Hammes A, Tschope C, Dillmann W, Paul M. Transgenic overexpression of the sarcoplasmic reticulum Ca2+ATPase improves reticular Ca2+ handling in normal and diabetic rat hearts. Faseb J. 2002;16:1657–1659. doi: 10.1096/fj.01-1019fje. [DOI] [PubMed] [Google Scholar]
- 69.Srinivasan S, Stevens M, Wiley JW. Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49:1932–1938. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 70.Huang TJ, Price SA, Chilton L, Calcutt NA, Tomlinson DR, Verkhratsky A, Fernyhough P. Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes. 2003;52:2129–2136. doi: 10.2337/diabetes.52.8.2129. [DOI] [PubMed] [Google Scholar]
- 71.Huang TJ, Sayers NM, Verkhratsky A, Fernyhough P. Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol. 2005;194:279–283. doi: 10.1016/j.expneurol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Huang TJ, Verkhratsky A, Fernyhough P. Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons. Mol Cell Neurosci. 2005;28:42–54. doi: 10.1016/j.mcn.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Nicholls DG, Ward MW. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci. 2000;23:166–174. doi: 10.1016/s0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- 74.Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. Faseb J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 75.Gumy LF, Bampton ET, Tolkovsky AM. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci. 2008;37:298–311. doi: 10.1016/j.mcn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Yu C, Rouen S, Dobrowsky RT. Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination. Glia. 2008;56:877–887. doi: 10.1002/glia.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalichman MW, Powell HC, Mizisin AP. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol (Berl) 1998;95:47–56. doi: 10.1007/s004010050764. [DOI] [PubMed] [Google Scholar]
- 78.Kamiya H, Zhang W, Sima AA. Degeneration of the Golgi and neuronal loss in dorsal root ganglia in diabetic BioBreeding/Worcester rats. Diabetologia. 2006;49:2763–2774. doi: 10.1007/s00125-006-0379-0. [DOI] [PubMed] [Google Scholar]
- 79.Kamiya H, Zhangm W, Sima AA. Apoptotic stress is counterbalanced by survival elements preventing programmed cell death of dorsal root ganglions in subacute type 1 diabetic BB/Wor rats. Diabetes. 2005;54:3288–3295. doi: 10.2337/diabetes.54.11.3288. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Y, Nyengaard JR, Zhang JS, Jakobsen J. Selective loss of calcitonin gene-related Peptide-expressing primary sensory neurons of the a-cell phenotype in early experimental diabetes. Diabetes. 2004;53:2669–2675. doi: 10.2337/diabetes.53.10.2669. [DOI] [PubMed] [Google Scholar]
- 81.Kennedy JM, Zochodne DW. Experimental diabetic neuropathy with spontaneous recovery: is there irreparable damage? Diabetes. 2005;54:830–837. doi: 10.2337/diabetes.54.3.830. [DOI] [PubMed] [Google Scholar]
- 82.Toescu EC. Mitochondria and Ca2+ signaling. J Cell Mol Med. 2000;4:164–175. doi: 10.1111/j.1582-4934.2000.tb00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 84.Svichar N, Kostyuk P, Verkhratsky A. Mitochondria buffer Ca2+ entry but not intracellular Ca2+ release in mouse DRG neurones. Neuroreport. 1997;8:3929–3932. doi: 10.1097/00001756-199712220-00017. [DOI] [PubMed] [Google Scholar]
- 85.Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- 86.Kopach O, Kruglikov I, Pivneva T, Voitenko N, Fedirko N. Functional coupling between ryanodine receptors, mitochondria and Ca2+ ATP-ases in rat submandibular acinar cells. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.08.001. in press. [DOI] [PubMed] [Google Scholar]
- 87.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 88.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 89.David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol. 1998;509:59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca2+] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20:7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 92.Coatesworth W, Bolsover S. Spatially organised mitochondrial calcium uptake through a novel pathway in chick neurones. Cell Calcium. 2006;39:217–225. doi: 10.1016/j.ceca.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 93.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 94.Singhal A, Cheng C, Sun H, Zochodne DW. Near nerve local insulin prevents conduction slowing in experimental diabetes. Brain Res. 1997;763:209–214. doi: 10.1016/s0006-8993(97)00412-5. [DOI] [PubMed] [Google Scholar]
- 95.Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53:1824–1830. doi: 10.2337/diabetes.53.7.1824. [DOI] [PubMed] [Google Scholar]
- 96.Toth C, Brussee V, Zochodne DW. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia. 2006;49:1081–1088. doi: 10.1007/s00125-006-0169-8. [DOI] [PubMed] [Google Scholar]
- 97.Francis G, Martinez J, Liu W, Nguyen T, Ayer A, Fine J, Zochodne D, Hanson LR, Frey WH, 2nd, Toth C. Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes. 2009;58:934–945. doi: 10.2337/db08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and - independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 99.Fernyhough P, Huang TJ, Verkhratsky A. Mechanism of mitochondrial dysfunction in diabetic sensory neuropathy. J Peripher Nerv Syst. 2003;8:227–235. doi: 10.1111/j.1085-9489.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- 100.Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain medicine (Malden, Mass ) 2008;9:660–674. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 102.Sorkin LS, Yaksh TL. Behavioral models of pain States evoked by physical injury to the peripheral nerve. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2009;6:609–619. doi: 10.1016/j.nurt.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. The journal of pain : official journal of the American Pain Society. 2006;7:S13–S30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 104.Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. Journal of clinical pharmacology. 2003;43:111–117. doi: 10.1177/0091270002239817. [DOI] [PubMed] [Google Scholar]
- 105.Gover TD, Moreira TH, Weinreich D. Role of calcium in regulating primary sensory neuronal excitability. Handbook of experimental pharmacology. 2009:563–587. doi: 10.1007/978-3-540-79090-7_16. [DOI] [PubMed] [Google Scholar]
- 106.Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porreca F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain. 2003;105:159–168. doi: 10.1016/s0304-3959(03)00177-5. [DOI] [PubMed] [Google Scholar]
- 107.Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. The EMBO journal. 2005;24:315–324. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wen X-j, Li Z-j, Chen Z-x, Fang Z-y, Yang C-x, Li H, Zeng Y-m. Intrathecal administration of Cav3.2 and Cav3.3 antisense oligonucleotide reverses tactile allodynia and thermal hyperalgesia in rats following chronic compression of dorsal root of ganglion. Acta pharmacologica Sinica. 2006;27:1547–1552. doi: 10.1111/j.1745-7254.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 109.Flatters SJL, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 110.Messinger RB, Naik AK, Jagodic MM, Nelson MT, Lee WY, Choe WJ, Orestes P, Latham JR, Todorovic SM, Jevtovic-Todorovic V. In vivo silencing of the Ca(V)3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain. 2009;145:184–195. doi: 10.1016/j.pain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jagodic MM, Pathirathna S, Nelson MT, Mancuso S, Joksovic PM, Rosenberg ER, Bayliss DA, Jevtovic-Todorovic V, Todorovic SM. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3305–3316. doi: 10.1523/JNEUROSCI.4866-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Willis WD. Role of neurotransmitters in sensitization of pain responses. Annals of the New York Academy of Sciences. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 113.Schroeder JE, Fischbach PS, Zheng D, McCleskey EW. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991;6:13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- 114.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Olivera BM, Cruz LJ, de Santos V, LeCheminant GW, Griffin D, Zeikus R, McIntosh JM, Galyean R, Varga J, Gray WR. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry. 1987;26:2086–2090. doi: 10.1021/bi00382a004. [DOI] [PubMed] [Google Scholar]
- 116.Malmberg AB, Yaksh TL. Effect of continuous intrathecal infusion of omega-conopeptides, N-type calcium-channel blockers, on behavior and antinociception in the formalin and hot-plate tests in rats. Pain. 1995;60:83–90. doi: 10.1016/0304-3959(94)00094-U. [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto T, Takahara A. Recent updates of N-type calcium channel blockers with therapeutic potential for neuropathic pain and stroke. Current topics in medicinal chemistry. 2009;9:377–395. doi: 10.2174/156802609788317838. [DOI] [PubMed] [Google Scholar]
- 118.Fukuizumi T, Ohkubo T, Kitamura K. Spinal sensitization mechanism in vincristine-induced hyperalgesia in mice. Neuroscience letters. 2003;343:89–92. doi: 10.1016/s0304-3940(03)00332-x. [DOI] [PubMed] [Google Scholar]
- 119.Calcutt NA, Chaplan SR. Spinal pharmacology of tactile allodynia in diabetic rats. British journal of pharmacology. 1997;122:1478–1482. doi: 10.1038/sj.bjp.0701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hall KE, Liu J, Sima AA, Wiley JW. Impaired inhibitory G-protein function contributes to increased calcium currents in rats with diabetic neuropathy. Journal of neurophysiology. 2001;86:760–770. doi: 10.1152/jn.2001.86.2.760. [DOI] [PubMed] [Google Scholar]
- 121.Hall KE, Sima AA, Wiley JW. Voltage-dependent calcium currents are enhanced in dorsal root ganglion neurones from the Bio Bred/Worchester diabetic rat. The Journal of physiology. 1995;486(Pt 2):313–322. doi: 10.1113/jphysiol.1995.sp020814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Umeda M, Ohkubo T, Ono J, Fukuizumi T, Kitamura K. Molecular and immunohistochemical studies in expression of voltage-dependent Ca2+ channels in dorsal root ganglia from streptozotocin-induced diabetic mice. Life sciences. 2006;79:1995–2000. doi: 10.1016/j.lfs.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 123.Yusaf SP, Goodman J, Gonzalez IM, Bramwell S, Pinnock RD, Dixon AK, Lee K. Streptozocin-induced neuropathy is associated with altered expression of voltage-gated calcium channel subunit mRNAs in rat dorsal root ganglion neurones. Biochemical and biophysical research communications. 2001;289:402–406. doi: 10.1006/bbrc.2001.5943. [DOI] [PubMed] [Google Scholar]
- 124.Satoh E, Takahashi A. Experimental diabetes enhances Ca2+ mobilization and glutamate exocytosis in cerebral synaptosomes from mice. Diabetes research and clinical practice. 2008;81:e14–e17. doi: 10.1016/j.diabres.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 125.Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends in pharmacological sciences. 2009;30:32–40. doi: 10.1016/j.tips.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 126.Lowery JM, Eichberg J, Saubermann AJ, LoPachin RM. Distribution of elements and water in peripheral nerve of streptozocin-induced diabetic rats. Diabetes. 1990;39:1498–1503. doi: 10.2337/diab.39.12.1498. [DOI] [PubMed] [Google Scholar]
- 127.Takigawa T, Yasuda H, Terada M, Maeda K, Haneda M, Kashiwagi A, Kitasato H, Kikkawa R. Increases in K+ conductance and Ca2+ influx under high glucose with suppressed Na+/K+-pump activity in rat myelinated nerve fibers. Neuroreport. 2000;11:2547–2551. doi: 10.1097/00001756-200008030-00040. [DOI] [PubMed] [Google Scholar]
- 128.Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesthesia and analgesia. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li F, Obrosova IG, Abatan O, Tian D, Larkin D, Stuenkel EL, Stevens MJ. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. American journal of physiology Endocrinology and metabolism. 2005;288:E29–E36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- 130.Ohsawa M, Kamei J. Role of intracellular calcium in thermal allodynia and hyperalgesia in diabetic mice. Brain research. 1999;833:278–281. doi: 10.1016/s0006-8993(99)01506-1. [DOI] [PubMed] [Google Scholar]
- 131.Shutov L, Kruglikov I, Gryshchenko O, Khomula E, Viatchenko-Karpinski V, Belan P, Voitenko N. The effect of nimodipine on calcium homeostasis and pain sensitivity in diabetic rats. Cellular and molecular neurobiology. 2006;26:1541–1557. doi: 10.1007/s10571-006-9107-z. [DOI] [PubMed] [Google Scholar]
- 132.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 133.Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 134.Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N. Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons. Pflugers Archiv : European journal of physiology. 2004;448:395–401. doi: 10.1007/s00424-004-1263-8. [DOI] [PubMed] [Google Scholar]
- 135.Voitenko NV, Kostyuk EP, Kruglikov IA, Kostyuk PG. Changes in calcium signalling in dorsal horn neurons in rats with streptozotocin-induced diabetes. Neuroscience. 1999;94:887–890. doi: 10.1016/s0306-4522(99)00330-9. [DOI] [PubMed] [Google Scholar]
- 136.Milligan EWL. Pathological and protective roles of glia in chronic pain. Nature REviews Neuroscience. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia. 2008;56:378–386. doi: 10.1002/glia.20623. [DOI] [PubMed] [Google Scholar]
- 138.O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. The American journal of medicine. 2009;122:S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 139.Dray A. Neuropathic pain: emerging treatments. British journal of anaesthesia. 2008;101:48–58. doi: 10.1093/bja/aen107. [DOI] [PubMed] [Google Scholar]
- 140.Snutch TP. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2:662–670. doi: 10.1602/neurorx.2.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabetic medicine : a journal of the British Diabetic Association. 2004;21:114–121. doi: 10.1111/j.1464-5491.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- 142.Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, Burstein S, Di Marzo V, Walker JM. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. The Journal of biological chemistry. 2001;276:42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- 143.Barbara G, Alloui A, Nargeot J, Lory P, Eschalier A, Bourinet E, Chemin J. T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13106–13114. doi: 10.1523/JNEUROSCI.2919-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee WY, Orestes P, Latham J, Naik AK, Nelson MT, Vitko I, Perez-Reyes E, Jevtovic-Todorovic V, Todorovic SM. Molecular mechanisms of lipoic acid modulation of T-type calcium channels in pain pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9500–9509. doi: 10.1523/JNEUROSCI.5803-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA : the journal of the American Medical Association. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 146.Cesena RM, Calcutt NA. Gabapentin prevents hyperalgesia during the formalin test in diabetic rats. Neurosci Lett. 1999;262:101–104. doi: 10.1016/s0304-3940(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 147.Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- 148.Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. The Journal of pharmacology and experimental therapeutics. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 149.Maneuf YP, Luo ZD, Lee K. alpha2delta and the mechanism of action of gabapentin in the treatment of pain. Seminars in cell & developmental biology. 2006;17:565–570. doi: 10.1016/j.semcdb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 150.Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tomlinson DR, Willars GB, Carrington AL. Aldose reductase inhibitors and diabetic complications. Pharmacol Ther. 1992;54:151–194. doi: 10.1016/0163-7258(92)90031-t. [DOI] [PubMed] [Google Scholar]
- 152.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 153.Van Dam PS, Van Asbeck BS, Erkelens DW, Marx JJ, Gispen WH, Bravenboer B. The role of oxidative stress in neuropathy and other diabetic complications. Diabetes Metab Rev. 1995;11:181–192. doi: 10.1002/dmr.5610110303. [DOI] [PubMed] [Google Scholar]
- 154.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43:836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]