Abstract
OBJECTIVE:
To determine the effect of maternal body mass index (BMI) on the incidence of neonatal prematurity morbidities in those who receive corticosteroids.
STUDY DESIGN:
Secondary analysis of a trial of corticosteroids in women at risk for preterm birth. Women receiving a single course of corticosteroids were classified by their pre-pregnancy BMI (<25 and ≥ 25) and compared on a composite outcome comprised of several neonatal morbidities, and on each individual outcome.
RESULTS:
Of 183 eligible women, 96 (52.5%) had a BMI < 25 and 87 (47.5%) had a BMI ≥ 25. The composite outcome occurred more frequently in the BMI ≥ 25 group (28.7%), compared with those with a BMI < 25 (18.8%), although this was not statistically significant (odds ratio 1.75; 95% confidence interval 0.83-3.72). BMI was not associated with outcomes after adjusting for confounding.
CONCLUSION:
Maternal BMI did not affect neonatal prematurity morbidities in those receiving corticosteroids.
Keywords: antenatal corticosteroids, BMI, neonatal morbidity, obesity, prematurity
Introduction
Obesity is associated with an increased risk of adverse maternal and neonatal outcomes including gestational diabetes, hypertensive disorders of pregnancy, and intrauterine fetal death1. As a result of these complications, it is not surprising that the rate of induction of labor and preterm delivery is also increased in those who are obese2,3,4. With nearly two-thirds of the adult United States population now being overweight or obese5, the complications of prematurity including neonatal morbidity and mortality are of growing concern.
Antenatal corticosteroids have been shown to be effective in reducing the complications associated with prematurity, including respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), and neonatal death6. The intramuscular administration of antenatal corticosteroids utilized by Liggins and Howie7, was felt to be the optimal mode of delivery given its ability to cross the human placenta.
The absorption of intramuscular medications depends on a number of factors, including obesity. One study found that the standard needles used to give intramuscular injections were often too short for proper administration of medications, making them less effective8. In a study examining the bioavailability of hCG, in obese versus non-obese patients given intramuscular injections undergoing in-vitro fertilization, it was demonstrated that the levels of available hCG were significantly lower for those patients that were obese9.
It is the goal of this study to determine the effect of maternal pre-pregnancy BMI on the incidence of neonatal morbidity and mortality in women receiving a single course of antenatal corticosteroids. Given the rising rates of obesity, we feel that this is an important question that will give us a better understanding of how obesity alters the benefits of antenatal corticosteroid in reducing neonatal complications and whether a weight-based dosing of antenatal corticosteroids should be considered.
Materials and Methods
Design
This study was a secondary analysis of data collected in a study by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, where 495 women at risk for preterm delivery were randomized to a single course versus repeat weekly courses of antenatal corticosteroids10.
Inclusion criteria for the primary study included having an entry gestational age between 23 0/7 and 31 6/7 weeks, intact amniotic membranes, being at risk for preterm delivery, and having received a single full course of antenatal corticosteroids between 6 and 10 days prior to enrollment (i.e. two intramuscular injections of 12mg of Betamethasone 24 hours apart or four intramuscular injections of Dexamethasone 12 hours apart). The primary study exclusion criteria included documented fetal lung maturity, chorioamnionitis, non-reassuring fetal monitoring, active preterm labor (cervical dilation ≥ 5 cm or ≥ 6 contractions per hour), insulin requiring diabetes at study enrollment, and concurrent use of systemic corticosteroids. In addition to these criteria, this analysis focused on women with a singleton pregnancy who received only one course of antenatal corticosteroids (i.e. the placebo arm of the study).
Given that this study was utilizing an existing de-identified database, it was considered exempt from full-review by the Institutional Review Board at Oregon Health & Science University.
Data
Maternal data regarding age (less than 35 years old or 35 years and older), race (white or other), pre-pregnancy weight, and height were extracted from the original dataset. Maternal pre-pregnancy body mass index (BMI) was calculated using the formula, BMI = pre-pregnancy weight in kilograms / (height in meters)2. Patients were initially categorized into the BMI categories of underweight (BMI <18.5), normal weight (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30), obese (30 ≤ BMI < 40), and morbidly obese (BMI ≥ 40), but secondary to limited numbers of subjects in certain BMI categories (14, 82, 48, 33, and 6, respectively), the decision was made to categorize the groups into BMI < 25 and BMI ≥ 25.
Data regarding gestational age at delivery and birth weight were collected in addition to neonatal morbidity and mortality data, which were used as outcomes of interest. The primary outcome was a composite outcome including neonatal RDS, bronchopulmonary dysplasia (BPD), chronic lung disease (CLD), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), sepsis, and stillbirth or neonatal death. These individual neonatal outcomes, which were defined previously in reports of the study10, were considered as secondary outcomes.
Statistical analysis
Analyses were completed using SAS (SAS Institute, Cary, NC). Statistical analysis for continuous variables utilized descriptive statistics as well as the Wilcoxon rank sum test. Categorical variables were analyzed with Fisher's exact test; exact confidence intervals were also constructed for odds ratios. Multivariable logistic regression was then used to estimate the effect of pre-pregnancy maternal BMI on the primary outcome, while adjusting for maternal age, maternal race, gestational age at delivery, birth weight, and gestational diabetes. The analysis was performed with pre-pregnancy maternal BMI as a categorical and continuous variable.
Results were presented as odds ratios (OR), 95% confidence intervals (CI), and p-values. A result was considered to have statistical significance if the 95% CI did not include 1.0, or with a p-value < 0.05. No adjustments for multiple comparisons were made.
Results
Two hundred and forty-three of the original 495 study subjects were randomized to the placebo arm of the primary study, where they only received a single full course of antenatal corticosteroids. The 183 women (75.3%) that were considered eligible for inclusion in this study (53 were excluded for having a multiple gestation and 7 were excluded for missing BMI data), were categorized into two groups of BMI < 25 (n = 96, 52.5%) and BMI ≥ 25 (n = 87, 47.5%). The two groups were similar to each other with respect to maternal, fetal, and obstetric parameters, with the exception of maternal pre-pregnancy weight and gestational age at randomization (see Table 1).
Table 1.
Patient characteristics by maternal pre-pregnancy BMI
| Characteristic | BMI < 25 (n= 96) |
BMI ≥ 25 (n = 87) |
p-value |
|---|---|---|---|
| Maternal age (years old, SD) | 25.5 (5.9) | 26.7 (5.4) | 0.09 |
| Maternal race – Caucasian (n, %) | 34 (35.4) | 20 (23.0) | 0.08 |
| Pre-pregnancy weight (kilograms, SD) | 56.3 (7.7) | 81.1 (17.6) | <0.001 |
| Gestational age at randomization (weeks, SD) | 28.5 (2.3) | 27.6 (2.3) | 0.01 |
| Gestational age at delivery (weeks, SD) | 35.7 (3.4) | 34.4 (4.4) | 0.06 |
| Latency from randomization to delivery (days, SD) | 50.0 (25.2) | 47.7 (29.7) | 0.52 |
| Birthweight (grams, SD) | 2549.3 (734.3) | 2366.2 (905.5) | 0.14 |
| Chorioamniotis (n, %) | 4 (4.2) | 1 (1.2) | 0.37 |
| Gestational diabetes (n, %) | 0 (0.0) | 3 (3.5) | 0.11 |
| Preeclampsia/Gestational hypertension (n, %) | 1 (1.0) | 3 (3.5) | 0.35 |
| PPROM (n, %) | 11 (11.5) | 15 (17.2) | 0.29 |
| Cesarean delivery (n, %) | 25 (26.0) | 27 (31.0) | 0.51 |
SD = standard deviation
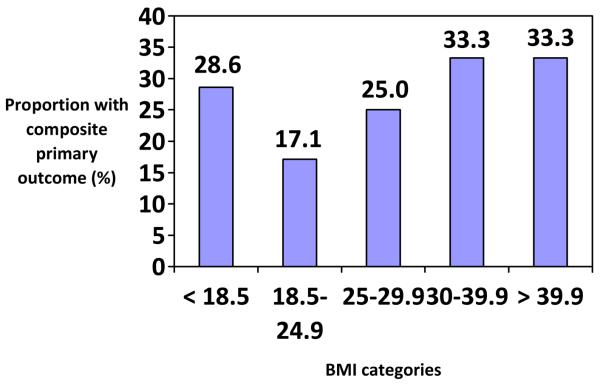
The primary composite outcome occurred more often in the BMI ≥ 25 group (28.7%), compared with those with a BMI < 25 (18.8%), although this was not statistically significant (p=0.120). There were also no visual differences in the proportion of individuals with the primary composite outcome by more specific BMI categories (see Figure 1). The frequencies of the secondary outcomes were also similar between the two BMI groups (see Table 2). After adjusting for the confounding variables, there continued to be no effect of maternal pre-pregnancy BMI on the primary composite outcome (OR 0.70, 95% CI 0.24-2.06). Secondary outcomes were too rare to allow for the adjusted analysis. When considering maternal pre-pregnancy BMI as a continuous variable, we found no significant associations with the primary outcome whether or not the confounding variables were adjusted. Although non-significant (without adjustment of the confounding variables), a five-unit increase in BMI was found to be associated with a 22.8% increase in the primary outcome (OR 1.228, 95% CI 0.959-1.573).
Figure 1.
The proportion of women with the composite primary outcome by BMI category
Table 2.
Frequency of outcomes and univariate analyses
| Outcome | Frequency – n (%) | p-value | Odds Ratio |
95% Confidence Interval (CI) |
|
|---|---|---|---|---|---|
| BMI < 25 (n = 96) |
BMI ≥ 25 (n = 87) |
||||
| PRIMARY | |||||
| Composite | 18 (18.8) | 25 (28.7) | 0.12 | 1.75 | 0.83-3.72 |
| SECONDARY | |||||
| RDS | 12 (12.5) | 10 (11.5) | 1.00 | 0.91 | 0.33-2.45 |
| BPD | 6 (6.3) | 11 (12.6) | 0.20 | 2.17 | 0.69-7.47 |
| CLD | 3 (3.1) | 6 (6.9) | 0.31 | 2.30 | 0.47-14.58 |
| IVH* | 4 (4.5) | 9 (10.7) | 0.15 | 2.55 | 0.67-11.74 |
| PVL* | 0 | 0 | - | - | - |
| NEC | 4 (4.2) | 2 (2.3) | 0.69 | 0.54 | 0.05-3.90 |
| ROP | 3 (3.1) | 9 (10.3) | 0.07 | 3.58 | 0.85-21.11 |
| Sepsis | 1 (1.0) | 2 (2.3) | 0.61 | 2.24 | 0.11-133.2 |
| Stillbirth or Neonatal death |
1 (1.0) | 0 (0.0) | 1.00 | - | - |
RDS = respiratory distress syndrome; BPD = bronchopulmonary dysplasia; CLD = chronic lung disease; IVH = intraventricular hemorrhage; PVL = periventricular leukomalacia; NEC = necrotizing enterocolitis; ROP = retinopathy of prematurity.
Results are based on 89 patients with BMI < 25 and 84 patients with BMI ≥ 25.
Comment
As the prevalence of obesity continues to increase in our society, health care providers have to be concerned about the potential medical ramifications. It has been demonstrated in obstetrics that adverse outcomes, including both indicated and spontaneous birth, are more frequent in those considered to be overweight or obese. We currently use antenatal corticosteroids to reduce the risk of neonatal morbidities of prematurity, however the current intramuscular dose was established decades ago, when obesity was not as prevalent.
In this study, maternal pre-pregnancy BMI was not associated with either the primary composite or secondary outcomes. Given this finding, it appears that maternal BMI does not affect the incidence of neonatal morbidity or mortality in those receiving a single course of antenatal corticosteroids.
While this study has the strength of being a novel topic with potential clinical implications, it also has limitations that must be considered in interpreting the results. First, being a secondary analysis, this study was restricted to the sample size that was available, which affected the power to detect a true difference. Although lacking statistical significance, the frequency of the composite outcome, BPD, CLD, IVH, ROP, and sepsis were higher in the BMI ≥ 25 group, with corresponding OR's ranging from 1.75 to 3.58. Also, when BMI was considered as a continuous variable, we found a non-significant increase of 22.8% in the primary outcome with each five-unit increase in maternal pre-pregnancy BMI. Thus, the lack of power must be considered when interpreting these results. Second, our limited sample size precluded our ability to analyze the effect of BMI by the common categories of underweight, normal weight, overweight, obese, and morbidly obese. Even though the limited sample sizes restricted our ability to accurately evaluate for any trends in outcomes by increasing or decreasing BMI categories, Figure 1 showed the lack of a significant trend or association by more narrow BMI categories. Third, as with any other secondary analysis, this study was limited to the data that were collected in the primary study. Lastly, it is necessary to consider the possibility of selection bias introduced by the selection criteria of the original study. In the study, women were enrolled and randomized to antenatal corticosteroids or placebo, one week after receiving their initial course of antenatal corticosteroids. If women with a BMI < 25 had a higher rate of delivery within that initial week after antenatal corticosteroids (i.e. before enrollment and randomization), this would exclude those outcomes from our analyses and skew our results to show that more women in the BMI ≥ 25 group had the primary and secondary outcomes. Unfortunately, information about delivery rates after initial antenatal corticosteroid administration was not available.
Based on the findings of this study, it appears that a BMI-based dosing of antenatal corticosteroids may not be necessary. While this finding supports the current use of a standard dosing regimen for antenatal corticosteroids, the lack of power to detect a true difference in this study, suggests that additional data regarding the effect of maternal BMI on the benefit of antenatal corticosteroids are necessary to preclude any possible differences. With the continued world-wide rise of obesity and its associated risk for adverse perinatal outcome, the need for maximizing preventive measures such as the use of antenatal corticosteroids has never been more important.
Acknowledgments
Supported by grants (HD21410, HD21414, HD27869, HD27917, HD27905, HD27860, HD27861, HD27915, HD34122, HD34116, HD34208, HD34136, HD40500, HD40485, HD40544, HD40545, HD40560, HD40512, HD40485, HD36801) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and M01-RR-000080 from the National Center for Research Resources
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation:
Society of Maternal-Fetal Medicine – 2009
San Diego, California
January 25 – January 31, 2009
Disclaimers:
None
References
- 1.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obesity. 2001;25:1175–82. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 2.Cedergren MI. Maternal Morbid Obesity and the Risk of Adverse Pregnancy Outcome. Obstet Gynecol. 2004;103:219–24. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 3.Smith GCS, Shah I, Pell JP, et al. Maternal Obesity in Early Pregnancy and Risk of Spontaneous and elective Preterm Deliveries: A Retrospective Cohort Study. Am J Pub Health. 2007;97:157–162. doi: 10.2105/AJPH.2005.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Bukus EA, Lambe M. Pregnancy Complications and Outcomes Among Overweight and Obese Nulliparous Women. Am J Pub Health. 2001;91(3):436–440. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among U.S. adults, 1999-2000. JAMA. 2002;288:1723–27. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Development Panel Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–18. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 7.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25. [PubMed] [Google Scholar]
- 8.Nisbet AC. Intramuscular gluteal injections in the increasingly obese population: retrospective study. Br Med Jr. 2006;332:637–38. doi: 10.1136/bmj.38706.742731.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CC, Ng EH, Chan MM, et al. Bioavailability of hCG after intramuscular or subcutaneous injection in obese and non-obese women. Hum Reprod. 2003;18(11):2294–97. doi: 10.1093/humrep/deg446. [DOI] [PubMed] [Google Scholar]
- 10.Wapner RJ, Sorokin Y, Thom EA, et al. Single versus weekly courses of antenatal corticosteroids: Evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–42. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]