Abstract
Certain glycolipid antigens (Ags) for Vα14i NKT cells can direct the overall cytokine balance of the immune response. TH2-biasing OCH has a lower TCR avidity than the most potent agonist known, αGalCer. Although the CD1d-exposed portions of OCH and αGalCer are identical, structural analysis indicates that there are subtle CD1d conformational differences due to differences in the buried lipid portion of these two Ags, likely accounting for the difference in antigenic potency. TH1 biasing C-glycoside/CD1d has even weaker TCR interactions than OCH/CD1d. Despite this, C-glycoside caused a greater downstream activation of NK cells to produce IFNγ, accounting for its promotion of TH1 responses. We found that this difference correlated with the finding that C-glycoside/CD1d complexes survive much longer in vivo. Therefore, we suggest that the pharmacokinetic properties of glycolipids are a major determinant of cytokine skewing, suggesting a pathway for designing therapeutic glycolipids for modulating iNKT cell responses.
Keywords: natural killer T cells, T lymphocyte, antigen receptor, glycolipid, affinity
Introduction
Natural Killer T cells with an invariant Vα14 TCR rearrangement (Vα14i NKT cells) are a distinct lineage of αβ T lymphocytes that have metaphorically been referred to as the ‘Swiss-Army knife’ of the immune system (1) due to their remarkable ability to regulate many different aspects of immunity. They have characteristics that classify them as innate lymphocytes, functioning to give an immediate effector response. For example, Vα14i NKT cells constitutively express high levels of CD69 and CD44, and engagement of glycolipid/CD1d complexes stimulates the production of effector cytokines, including IL-4 and IFNγ, within minutes (2). Vα14i NKT express a T cell Ag receptor (TCR) comprised of an invariant Vα14-Jα18 TCR α-chain paired with a limited subset of TCR Vβ chains. They also express receptors associated with NK cells, such as NK1.1, Ly49 molecules, and CD122. The TCRs expressed by Vα14i NKT cells are reactive to CD1d, a non-polymorphic class I-like antigen-presenting molecule that presents lipid Ags (3-6).
Vα14i NKT cells have been implicated both in the progression and resolution of diverse animal models of disease (7). This depends on the cytokines produced by Vα14i NKT cells, and cytokines produced by other cell types stimulated downstream of Vα14i NKT activation. Although there are exceptions (8), in general TH2 cytokines, such as IL-4 and IL-13, have a palliative effect on several autoimmune models, but they are essential for the pathogenesis of allergic disease. TH1 cytokines, such as IFNγ, aggravate autoimmunity and inflammatory disease models, but they are necessary for the clearance of infections and the prevention of tumor metastases. As a result, considerable research has been directed towards possible mechanisms by which cytokine production by Vα14i NKT cells, and/or their effects on other cell types, can be polarized to ensure the preferential production of those cytokines that are beneficial.
The signals that polarize conventional CD4+ T cells, including cytokines such as IL-12 and IL-4, Ag dose, and engagement of co-stimulatory molecules such as CD28 or CD40, did not affect in vivo IL-4 or IFNγ cytokine production by Vα14i NKT cells in the first few hours after stimulation with the potent glycolipid Ag α-galactosyl ceramide (αGalCer) (9). However, there have been reports that structural variants of αGalCer (Fig. 1) induce a systemic polarization of cytokine production initiated by Vα14i NKT cells (10-15). For example, OCH, an analog of αGalCer with a sphingosine base reduced from 18 to nine carbons and an acyl chain reduced by only two carbons (Fig. 1), has been reported to be TH2 polarizing (10, 14). It has beneficial effects on several autoimmune models including experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (14) and type I diabetes (16). The C-glycoside analog of αGalCer, by contrast, has been reported to have a TH1 polarizing effect, and therefore to enhance the clearance of parasites in a cerebral malaria model (15), to prevent melanoma metastases (17), and to ameliorate ocular autoimmunity in experimental autoimmune uveitis (18).
Figure 1. Serum cytokine responses to glycolipid Ags.
A. Glycolipid Ag structures. B. C57BL/6 mice were injected i.v. with 1μg of the indicated glycolipid and bled at the indicated times. Cytokines in the serum were measured by ELISA. Representative data from one of two experiments using three mice per group are shown as average ± standard deviation.
The current study was undertaken to determine the mechanisms for the altered cytokine bias induced by these glycolipid Ags. There have been a number of previous studies on the mechanism of TH2 cytokine polarization by OCH, and these suggest that the main cause is a reduced stability of binding of OCH to CD1d, as a result of the shortened sphingosine base, (10, 19), along with a reduced TCR affinity (19). According to this view, induction of IFNγ secretion by Vα14i NKT cells requires a longer period of stimulation (10) and or a higher Ag concentration (19). Vα14i NKT cells activated by OCH also had a reduced induction of CD40L expression (11). This in turn led to reduced production of IL-12 by APC, which causes decreased synthesis of IFNγ by NK cells, which are activated rapidly following Vα14i NKT cell stimulation. Therefore, the TH2 skewing effect of OCH was believed to be due to a combination of direct effects on Vα14i NKT cells, and indirect effects on cells stimulated downstream of the activated Vα14i NKT cells, all attributable to a decreased duration of glycolipid Ag stimulation or decreased TCR affinity. All TH2 cytokine skewing by Vα14i NKT cells cannot be attributed to decreased antigenic potency, however, because the C20:2 glycolipid, with a fatty acid having two unsaturated bonds, causes TH2 cytokine skewing without decreasing antigenic potency (12), as do some other compounds with alterations in the fatty acid moiety (20). In this instance, evidence was provided that these compounds were much less dependent upon Ag internalization and excluded from CD1d molecules in lipid rafts (20), suggesting that the cellular site of Ag loading is important for function.
An implication of these studies on OCH is that the TH1 skewing Ag C-glycoside might have a higher TCR affinity or more stable binding to CD1d. There have been no previous comparisons, however, of OCH and C-glycoside, which are the paradigmatic TH2 and TH1 skewing glycolipid Ags. Therefore, here we carried out a comprehensive biochemical, structural, cellular and in vivo comparison of these glycolipids. Our data are not consistent with the prevailing view that TH2 cytokine skewing relates in a simple, linear way to the stability of CD1d binding, or to the affinity of the TCR interaction. Instead, our data show that the stability of in vivo presentation by CD1d is likely to be important, suggesting that the pharmacokinetic properties of the glycolipid Ag, allowing it to give a prolonged stimulus, may in some cases be the critical factor.
Materials and Methods
Mice, reagents and hybridomas
C57BL/6J mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice genetically deficient for CD40 (CD40−/−) on the C57BL/6 background were the gift of Dr. Stephen Schoenberger (La Jolla Institute for Allergy & Immunology, La Jolla, CA). CD1d-deficient mice (CD1d−/−) were the kind gift of Dr. Luc Van Kaer (Vanderbilt University, Nashville TN). All mice were housed in accordance with the Institutional Animal Care Committee guidelines.
Mouse CD1d was produced in our laboratory as previously described (21). αGalCer and OCH were obtained from the Kirin pharmaceutical research corporation (Gunma, Japan). C-glycoside was synthesized as previously described (17, 22). For experiments in which glycolipids were pulsed onto cells or injected into mice each glycolipid was dissolved in DMSO at a concentration of 100 μg/mL, then further diluted in PBS prior to incubation or injection. For experiments in which glycolipids were conjugated to CD1d to form tetramers, αGalCer and OCH were dissolved in vehicle (0.5% polysorbate-20 and 0.9% NaCl) at a concentration of 200μg/mL and C-glycoside was dissolved in DMSO at a concentration of 1mg/mL and then further diluted with vehicle to 200μg/mL. In all experiments, glycolipids were heated to 56°C for 10 min and vortexed extensively prior to use. The DN3A4-1.2 (referred to as 1.2) and N38-2C12 (referred to as 2C12) Vα14i NKT hybridoma cells have been described previously (23, 24). A20-CD1d B lymphomas that stably over express CD1d have also been previously described (24).
Tetrameric glycolipid/CD1d complexes.
Tetramers of CD1d bound to αGalCer, OCH and C-glycoside were produced as described (21) with slight modifications. CD1d molecules were incubated at a 1:3 (CD1d:glycolipid) molar ratio with each glycolipid or vehicle. After 24 h glycolipid:CD1d complexes were tetramerized by the addition of premium grade streptavidin labeled PE (Molecular Probes/Invitrogen, Carlsbad, CA).
For equilibrium staining of hybridomas, 2 × 105 cells were stained in R10 medium (RPMI medium 1640 supplemented with 10% FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, and 0.292 mg/ml L-glutamine (GIBCO/Invitrogen, Grand Island, NY) with azide (0.05%), to prevent internalization, for 3 h at room temperature in the dark with glycolipid/CD1d tetramers and 2.5 μg/mL FITC labeled anti-TCRβ mAb. The cells were then washed twice in staining buffer and fixed in 1% paraformaldehyde (PFA, Sigma-Aldrich, St. Louis, MO) in PBS. The intensity of fluorescence of tetramer binding was determined by flow cytometry.
For tetramer dissociation experiments, Vα14i NKT hybridoma cells were incubated with a saturating dose of each glycolipid/CD1d tetramer for 1.5 h at room temperature. Excess tetramer was washed away, an anti-CD1d mAb (1B1; 100μg/mL) was added to prevent re-binding of tetramers, and samples were placed at 37°C for the decay period. After 15 min of equilibration, aliquots of the bulk tetramer-labeled population were removed and chilled on ice-cold R10 with azide. Following the decay period, hybridoma cells were incubated with anti-TCRβ on ice. Cells were washed again and fixed in 1% PFA in PBS. The intensity of fluorescence of tetramer binding was determined by flow cytometry. Mean tetramer fluorescence was calculated on hybridoma cells. Decay data are presented as time vs. log (tetramer fluorescence for each point / initial tetramer fluorescence). After linear regression, tetramer half-life was determined by t1/2 = log2/slope.
Surface Plasmon Resonance
All real-time binding experiments were performed at 25°C on a BIAcore 3000 biosensor system (GE Healthcare, Biacore, Piscataway, NJ). Kinetic parameters were obtained by fitting the specific sensorgrams with BIAEVAL 4 software.
Decay of glycolipids from CD1d
Analysis of the decay of glycolipids from soluble CD1d was performed with an αGalCer/CD1d specific antibody L317.11.9 that has been described previously (25). Hexa-His tagged CD1d proteins were pulsed with the indicated glycolipids overnight. The following day, glycolipid/CD1d complexes were bound to each Ni-pulsed channel of a NTA chip (Biacore, Piscataway, NJ). The glycolipid/CD1d complexes were allowed to decay for various times before addition of saturating doses of L317.11.9 and determination of the degree of binding.
Measurement of ERK1/2 phosphorylation
ERK1/2 phosphorylation was detected as previously described (26). Briefly, either 106/ml A20-CD1d cells or 106/ml splenic B cells positively selected with anti-CD19 conjugated MACS beads (Miltenyi Biotech, Auburn, CA) were pulsed with glycolipid Ags for 2 h, or 106 BMDC were pulsed with glycolipid Ags for 24 h at 37°C. The cells were washed once with cold media and Vα14i NKT hybridoma cells were mixed with pulsed APCs at a 4:1 ratio and placed on ice. At the indicated time points, cells were pelleted in a desktop centrifuge and placed at 37°C. Following the incubation, the supernatant was removed and the cell pellet was resuspended in ice-cold PBS-2.5mM EDTA followed immediately by the addition of PFA fixing buffer (4% PFA in PBS, pH7.4). The cells were incubated on ice for 30 min. The PFA was washed away and cells were incubated with anti-Thy1.2 FITC for 30 min on ice. After the incubation, cells were pelleted and resuspended in ice-cold permeabilization buffer (90% MeOH 10% water) and incubated on ice for 30 minutes. Cells were washed twice with room temperature staining buffer (4% FBS in PBS) and then incubated with anti-ppERK1/2 (E10 Alexa 647) for 30 min at room temperature. Cells were washed twice with staining buffer and resuspended in 1% PFA and analyzed by flow cytometry. Gates were set on Thy1.2 positive cells (Vα14i NKT cells) using t = 0 as a negative control. The data were plotted as time (min.) vs. (% ppERK1/2+glycolipid − % ppERK1/2+vehicle).
Crystal structure determination
Soluble and fully glycosylated mouse CD1d-β2-microglobulin heterodimeric protein was expressed and purified as described (27). Synthetic OCH was loaded by incubating mouse CD1d with a 4-fold molar excess of lipid. The CD1d-lipid mixture contained 100 mM Tris/HCl, 0.07% Tween 20, 10 μM mCD1d, 40 μM OCH, pH 7.0, and was incubated for 16 h at room temperature under gentle agitation. The OCH-CD1d complexes were separated from unbound lipid by size exclusion chromatography on Superdex S200 resin (GE Healthcare) and concentrated to 8.5 mg/ml in 10 mM Hepes, 30 mM NaCl, pH 7.5 for crystallization. Crystals were grown over a period of five months at 4°C in condition 35 of the commercial Peg/Ion 2 screen, containing 16% polyethylene glycol 3350, 0.1 M Tris/HCl pH 8.5 and 2% tacsimate pH 8.0. Diffraction data of a single crystal were collected at Beamline 11.1 of the Stanford Synchrotron Radiation Laboratory (SSRL) and processed to 1.6 Angstroms (Å) with the Denzo-Scalepack suite (28) in spacegroup P222 (unit cell dimensions: a = 42.2 Å; b = 107.1 Å; c = 106.7 Å). One OCH-CD1d complex occupies the asymmetric unit with an estimated solvent content of 50%, based on a Matthews' coefficient (Vm) of 2.46 Å3/Dalton. Molecular replacement in P212121 was carried out in CCP4 (29) using the program MOLREP (30) and the iGb3-CD1d structure (PDB code 2Q7Y) as the search model, with the glycolipid ligand and carbohydrates removed, and resulted in a crystallographic R factor (Rcryst) of 44.7% and a correlation coefficient (CC) of 0.53. TLS refinement in REFMAC (31) was essentially carried out as described for the iGb3-CD1d structure (32). The REFMAC library for OCH was created using the Dundee PRODRG2 server (33), after modification of the αGalCer coordinates from the αGalCer-human CD1d structure (1zt4). The OCH-CD1d structure has a final Rcryst = 20.2% and Rfree = 23.2%, and the quality of the model (Supplementary Table II) was excellent as assessed with the program Molprobity (34). The software program Pymol (DeLano Scientific, Palo Alto) was used to prepare figure 4. The PDB2PQR server (35) and the program APBS (36) were used to calculate the electrostatic surface potentials in figure 4. Structure factor and atomic coordinates for the OCH-mouse CD1d complex have been deposited in the Protein Data Bank (www.rcsb.org) under PDB ID code 3G08.
Figure 4. Potency of glycolipid Ags for Vα14i NKT cells.
A. C57BL/6 mice were injected i.v. with 1 μg glycolipid. After two hours, liver lymphocytes were isolated and analyzed for surface reactivity to αGalCer/CD1d tetramers and TCRβ; as well as intracellular production of IFNγ or IL-4. Cells were analyzed directly ex vivo in the absence of a reagent to block exit from the Golgi apparatus. Flow cytometry plots are shown as cytokine expression vs. tetramer binding and are gated on live lymphocytes; percentages of live lymphocytes are shown in each quadrant. B. Quantification of intracellular staining from experiments in A. C. C57BL/6 mice were injected intravenously with 1 μg glycolipid. After two hours, splenic Vα14i NKT cells were examined for expression of CD40L and CD69. Flow cytometry histograms are shown gated on live lymphocytes and TCRβ+ αGalCer/CD1d tetramer+ cells. All experiments were performed at least two times with similar results.
Cell Preparation
Single-cell suspensions were prepared from the liver, spleen, thymus and bone marrow. Prior to extraction, the liver was perfused with PBS via the portal vein until opaque and pressed through a 70 μm cell strainer (Becton Dickinson). Total liver cells were then resuspended in a 40% isotonic Percoll solution and underlaid with a 60% isotonic Percoll solution. After centrifugation for 20 min at 900 g, mononuclear cells were isolated from the 40/60% interface. The cells were washed once with R10 medium and 48.49 mM 2-mercaptoethanol (Fisher Scientific). For the spleen, lymphocytes were isolated by mechanical dissociation and filtered through a 70 μm cell strainer (Becton Dickinson). Red blood cells were lysed (Red Cell Lysis Buffer (RCLB) from Sigma Aldrich, St. Louis MO) and white blood cells were washed once in R10 medium. Bone marrow cells were isolated by flushing the femurs of C57BL/6 mice with PBS followed by lysis of red blood cells (RCLB). Incubation with mAbs was done simultaneously with the tetramer in staining buffer (PBS, 10% FCS) at 4°C for 45 min. For analysis of intracellular cytokines, cells were permeabilized using the Cytofix/Cytoperm reagents (BD Biosciences, San Diego CA) and incubated with mAbs according to the manufacturer's protocol.
MACS technology (Miltenyi Biotec, Auburn, CA) was used for the magnetic separation of cell populations. For enrichment of thymic Vα14i NKT cells, thymocytes were incubated with biotinylated anti-CD8α and anti-CD24 monoclonal antibodies (BD Biosciences, San Diego, CA) followed by incubation with anti-biotin magnetic beads.
Mouse DC were prepared by culturing bone marrow progenitors with recombinant mouse granulocyte macrophage colony stimulating factor at 20 ng/ml (PeproTech, Rocky Hill, NJ) for six days. For DC isolation from spleen, the organ was chopped and digested by collagenase type D (Roche,USA) at 37°C. Digested tissues were treated with 5 mM of EDTA for an additional 5 min and mashed through a 70μm cell strainer. CD11c+ cells from bone marrow cultures or spleen were enriched by incubation with anti-CD11c magnetic beads. Magnetically labeled cells were passed over MACS columns according to the manufacturer's protocols for positive or negative selection and analyzed for purity by flow cytometry. Glycolipid pulsed DC (105/well) were either cultured with hybridomas (2 × 105/well) or injected intravenously (105) into mice.
Monoclonal antibodies and flow cytometric analysis
The following monoclonal antibodies (mAbs) were used in this study: CD1d (1B1), CD8α (53-6.72), CD8β (53-5.8), TCRβ (H57-597), Thy1.2 (30-H12 and 53-2.1), CD24 (M1/69), NK1.1 (PK136), TNF (MP6-XT22), IL-4 (11B11), IFNγ (XMG1.2) and phospho-ERK1/2 (E10). Antibodies were purchased from BD Biosciences (San Diego, CA) or Cell Signaling Technology (Danvers, MA). Antibodies were biotinylated or conjugated to FITC, PE, PerCP-Cy5.5, Alexa Fluor 488, Alexa Fluor 647 or APC. Multicolor acquisition of fluorescently labeled cells was performed on a BD FACSCalibur or BD LSRII (BD Biosciences, San Diego, CA). Data analysis and production of figures was performed on FlowJo software (Tree Star Inc., Ashland, OR).
Analysis of cytokine secretion
Cytokines in the sera of mice immunized with glycolipids measured by a sandwich ELISA using paired anti-IL-4 and anti-IFNγ mAbs (BD PharMingen, San Diego, CA). Cytokines in the supernatant of hybridoma cultures were measured by a sandwich ELISA using paired anti-IL-2 mAbs (BD PharMingen, San Diego, CA).
Results
Glycolipid Ags induce skewed systemic cytokine production
To analyze systemic cytokine production induced by different glycolipid Ags (structures in Fig. 1A), C57BL/6J mice were injected intravenously (i.v.) with 1 μg of αGalCer, OCH, C-glycoside or vehicle and were bled at the indicated times after injection. Cytokine levels in the sera were measured by enzyme-linked immunosorbent assay (ELISA) (Fig. 1B). As reported previously (9, 15), IL-4 peaked within a few hours after Ag, while peak amounts of serum IFNγ required a longer time. OCH induced a systemic TH2 biased response, with high levels of IL-4, equivalent to αGalCer, and relatively low levels of IFNγ (Fig. 1B), in agreement with earlier studies (10, 14). Therefore, the overall TH2 bias resulting from OCH was due to reduced production of IFNγ, rather than increased IL-4. C-glycoside induced 3-4 fold less IL-4 in the serum than αGalCer, but it induced amounts of IFNγ that were decreased less dramatically, to approximately 50% of the amount induced by αGalCer (Fig. 1B), resulting in an overall TH1 profile of cytokine production. Therefore, for both OCH and C-glycoside, the cytokine bias is a relative one manifested most clearly as a difference in the proportion of the concentrations of IFNγ produced relative to IL-4. This proportion is approximately 1:1 for OCH but nearly 10:1 for C-glycoside.
OCH and C-glycoside display reduced TCR avidity
As has been proposed for peptide recognition by conventional CD4+ T cells (37, 38), the altered pattern of cytokine production initiated by Vα14i NKT cell activation could be due to differences in the binding kinetics or affinity of the glycolipid/CD1d complex for the Vα14i TCR. We therefore determined the affinity of Vα14i NKT cell TCRs for CD1d bound to each Ag, using tetramer-based binding assays to measure the avidity and dissociation rates of TCRs expressed by Vα14i NKT cell hybridomas and polyclonal Vα14i NKT cells. Tetramers of OCH/CD1d, C-glycoside/CD1d, and αGalCer/CD1d were used to determine the equilibrium dissociation constant (KD), a measure of TCR avidity (Fig. 2A), and the dissociation rates, to determine the half-life (t1/2) of the TCR interaction (Fig. 2B).
Figure 2. OCH/CD1d and C-glycoside/CD1d complexes exhibit reduced TCR binding and intracellular signaling.
A. Equilibrium tetramer staining of Vα14i NKT cells. 2C12 (top) and 1.2 (bottom) Vα14i NKT hybridoma cells were incubated with doses of tetrameric glycolipid/CD1d complexes for 3 h to reach equilibrium. Plots are shown as tetramer concentration vs. mean fluorescence intensity of tetramer staining. B. Tetramer dissociation. 2C12 (top) and 1.2 (bottom) hybridoma cells were incubated with saturating doses of glycolipid-CD1d complexes, the free complexes were washed away and dissociation was measured over time (see Methods for more details). Plots are shown as time (min) vs. log (MFI bound tetramer at time indicated/ MFI bound tetramer at time = 0). After linear regression, tetramer half-life was determined by t1/2 = log2/slope. C. Intracellular analysis of the phosphorylation state of ERK1/2 in Vα14i NKT cells. A20-CD1d cells were pulsed with the indicated glycolipid ligands, washed and incubated with the 1.2 Vα14i NKT hybridoma for the indicated time periods. Phosphorylation of ERK1/2 was assessed by flow cytometry. Gates on the histograms indicate the percent positive cells. D. Evaluation of Vα14i NKT cell ppERK1/2 staining using different APCs. Plots are shown gated on Vα14i NKT cells. Each experiment was performed at least two times with similar results.
Equilibrium tetramer binding measurements using the 2C12 Vα14i NKT cell hybridoma, with a Vα14i/Vβ8.2 TCR that has been analyzed extensively previously (39), showed that αGalCer/CD1d complexes bound with the highest avidity (KD = 0.171 nM +/− 0.024). OCH and C-glycoside complexed to CD1d each bound this TCR approximately equally, but with a much weaker avidity than αGalCer/CD1d complexes (Fig. 2A, top panel, KD = 6.843 nM +/− 3.001 and 3.012 nM +/− 1.921 respectively). The differences with αGalCer were statistically significant (αGalCer vs. OCH p = 0.056, αGalCer vs. C-glycoside p = 0.003) but not for OCH vs. C-glycoside (p = 0.38; Mann-Whitney test). The avidity of glycolipid/CD1d interaction with the TCR exhibited clonal variability, however, as evidenced by the results from binding Ag/CD1d complexes to 1.2, another Vα14i NKT cell hybridoma with a Vα14i/Vβ8.2 TCR. Although these two hybridomas differ only for the CDR3 region of the TCR β chain, OCH-CD1d complexes were barely able to bind the 1.2 hybridoma cells and the C-glycoside complexes did not achieve binding higher than the vehicle-loaded controls. Despite this, 1.2 binding αGalCer/CD1d complexes was similar to 2C12, although in agreement with previous results (39), the binding by 1.2 was slightly weaker (Fig. 2A, bottom panel, KD = 0.305 nM +/− 0.049).
The decay of tetramer binding can be used as a measure of the stability of the interaction between tetramers and TCRs (40). For peptide-reactive T cells, the half-life of the interaction between peptide-MHC complexes and the TCR, as determined by surface plasmon resonance measurements, can be more indicative of antigenic potency than the equilibrium binding constant (41). The decay of glycolipid-CD1d tetramer binding to 2C12 hybridoma cells was more rapid for OCH-CD1d tetramers than for αGalCer-CD1d tetramers (Fig. 2B), while C-glycoside-CD1d tetramers had an even shorter half-life of binding (Fig. 2B; t1/2 = 385 +/− 56 min, 114 +/− 22 min and 58 +/− 5 min minutes for αGalCer, OCH, and C-glycoside, respectively; αGalCer vs. OCH p = 0.011, αGalCer vs. C-glycoside p < 0.0001, OCH vs. C-glycoside p = 0.016, Mann-Whitney test). The decay of αGalCer-CD1d tetramer binding to 1.2 hybridoma cells also had a long half-life (Fig. 2B, t1/2 = 197 min), although consistent with the equilibrium measurement, slightly shorter than for 2C12. The low amount of staining of 1.2 hybridoma cells with tetramers loaded with the two other glycolipid Ags prevented this type of decay experiment.
Glycolipid Ags display a hierarchy in intracellular signaling
The reduced TCR half-life exhibited by OCH/CD1d and C-glycoside/CD1d in the tetramer decay experiments could be due either to a decreased TCR avidity or to a decreased stability of binding of these glycolipids to CD1d. Therefore, we adapted an assay to measure the phosphorylation of the extracellular signal-regulated kinases (ERK1 and ERK2) (26, 42), which requires only very brief periods to measure intracellular signaling events. As described by Altan-Bonnet et al. (42) for conventional T cells, we found that the Vα14i NKT cells exhibited an on-off or binary pp-ERK signal, with cells either ppERK+ or ppERK− (Fig. 2C). Thus, in Fig. 2D we display the data as percentage of ppERK+ cells over time. The percentage of Vα14i NKT cells that became pp-ERK1/2+ after incubation with glycolipid-pulsed APCs was dependent on the dose of Ag, it could be blocked by an anti-CD1d mAb in a dose-dependent manner, and the MEK inhibitor UO126 ablated the signal (Supplementary Fig. 1A-C). An unrelated peptide-reactive T cell hybridoma (B3Z) did not phosphorylate ERK1/2 after incubation with αGalCer-pulsed CD1d transfected cells (Supplementary Fig. 1D). The rapidity, peaking at 1-5 min, and extent of ERK1/2 phosphorylation over the 30 min assay in these cells (Fig. 2C-D) was comparable to naïve peptide-reactive T cells (26).
We measured the response to glycolipids presented by three APC, including CD1d transfected A20 B lymphoma cells, bone marrow-derived DC (BMDC), and splenic B cells (Figs. 2D). Using fluorescent beads to quantify the number of CD1d molecules on each cell type, A20-CD1d cells expressed ~700,000 surface CD1d molecules, BMDCs expressed ~30,000 molecules, and splenic CD19+ B cells expressed ~50,000 molecules (data not shown). The B cell population, however, is a mixture of more abundant follicular B cells, which expressed ~40,000 surface CD1d molecules, and marginal zone B cells, which expressed higher levels, ~130,000 (data not shown). When A20-CD1d cells were used as APCs, the extent of ERK1/2 phosphorylation induced by OCH and αGalCer were indistinguishable (Fig. 2D), despite the difference in equilibrium tetramer binding (Fig. 2A). This probably reflects the extraordinarily high expression of CD1d by the A20 cells, because when BMDCs or splenic B cells were used as APCs, OCH induced fewer 1.2 cells to phosphorylate ERK1/2 than αGalCer (Fig. 2D). These data are in keeping with the finding that OCH is a weaker Ag. C-glycoside induced very few 1.2 cells to phosphorylate ERK1/2 (Fig. 2D), in keeping with the finding that C-glycoside is a weaker Ag than either αGalCer or OCH. Therefore, the results from tetramer binding experiments and measurements of ERK phosphorylation are in agreement in showing that OCH and C-glycoside are both less potent Ags than αGalCer, with C-glycoside the weakest of the three Ags. Therefore, decreased TCR affinity for Ag does not correlate with TH2 cytokine skewing.
Binding off-rate of glycolipid Ags to CD1d
We used the recently described monoclonal antibody L317, which recognizes αGalCer/CD1d complexes (25), to measure the off-rate of glycolipid antigen binding to CD1d. Using surface plasmon resonance we observed that L317 cross-reacts with CD1d in complex with either OCH or C-glycoside, and that the affinity of L317 was highest for αGalCer bound to CD1d, weaker for OCH and significantly weaker for C-glycoside (Supplementary Table I). Because L317 was able to detect all three glycolipid Ags by surface plasmon resonance, we were able to measure the release of each of these glycolipids from CD1d, as described previously (13). After incubation for various times to induce decay of the glycolipid/CD1d complexes, the samples were analyzed by flowing L317 over the biosensor chip. Data were normalized to the maximum binding for each Ag. Note that the time needed for this antibody-based assay was too brief to be affected by the decay of antibody binding, even considering the shorter half-life of L317 binding to C-glycoside/CD1d complexes. The analysis indicated that αGalCer bound CD1d quite stably, with a half-life of 302.6 min (Table I and Supplementary Fig. 2). In keeping with previous reports (10, 19), OCH dissociated from CD1d more rapidly than αGalCer, with a half-life of 65.5 min. C-glycoside also dissociated more rapidly than αGalCer or OCH, with a half-life of 24.2 min. Thus, the off-rate for both OCH and C-glycoside for binding CD1d is faster than for αGalCer, with C-glycoside showing a trend toward being even less stable in binding to CD1d than OCH (Table I). These results indicated that reduced stability of glycolipid ligand binding to CD1d was not likely to be sufficient for the induction of TH2 cytokine skewing.
Table I.
Dissociation of Glycolipids from CD1d as measured by L317 binding
| Ligand | t1/2 (minutes) | n |
|---|---|---|
| CD1d/αGalCer | 302.6 +/− 132.4 | 7 |
| CD1d/OCH | 65.5 +/− 37.8 | 8 |
| CD1d/C-glycoside | 24.2 +/− 2.4 | 2 |
OCH-CD1d crystal structure
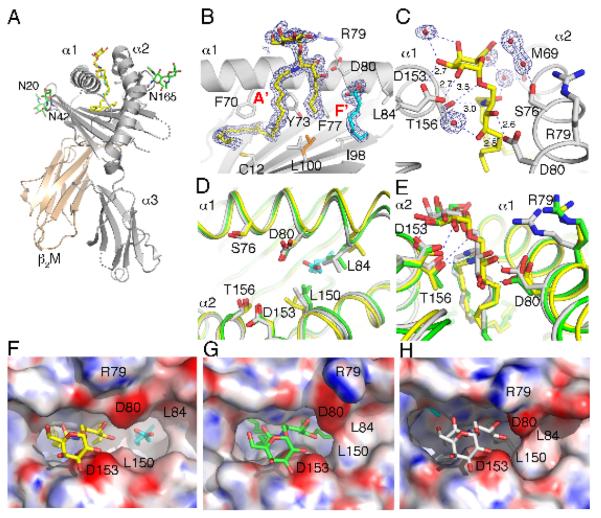
OCH and αGalCer differ only in their alkyl chains, which are buried deep in the CD1d antigen binding groove. Therefore, the antigenic moieties appearing above the CD1d groove should appear similar. To analyze the underlying basis for the reduced TCR avidity for OCH-CD1d complexes, and to determine if structural differences might account for the observed functional differences comparing OCH to αGalCer, we crystallized OCH bound to mouse CD1d and determined the structure to 1.6 Å resolution (Fig. 3 and Supplementary Table II). The general mode of glycolipid binding to mouse CD1d has been well documented (43, 44). More specifically, the OCH ligand binds with the C24-fatty acid in the A′ pocket and the C9-sphingosine moiety in the entrance to the F′ pocket, while the galactose is exposed at the CD1d surface for T cell recognition (Fig. 3B). Interestingly, as the sphingosine chain is truncated from the original C18 of αGalCer to C9, the F′ pocket is not completely filled. Instead a spacer lipid is recruited to stabilize the remainder of the F′ pocket. Although not formally identified as such, the shape and length of the electron density for the spacer lipid suggests a short chain fatty acid (C8, caprylic acid), rather than a more linear breakdown product of polyethylene glycol from the crystallization solution (Fig. 3B). However, both the weaker electron density and the higher b-values for the spacer ligand suggest that not all CD1d molecules of the crystal are occupied by the spacer lipid. The galactose headgroup is stabilized by several hydrogen bond interactions with CD1d residues and also through water mediated hydrogen bonds, involving the 6′OH of the galactose, the 4′-OH and the amide oxygen of the ceramide backbone of the ligand and residues Thr156, Ser76 and Met69 of CD1d (Fig. 3C). These interactions have not been observed in any of the previous mouse or human αGalCer-CD1d structures (45, 46).
Figure 3. Structural features of the mCD1d-OCH complex.
(A) The glycolipid OCH (yellow) is bound in the hydrophobic binding groove between the α1 and α2 helices of the CD1d heavy chain (grey) that non-covalently associates with β2-microblobulin (β2M, cream) to form a heterodimer. Three N-linked glycosylation sites (Asn20 (N20), Asn42 and Asn165) carry well-ordered carbohydrates (green sticks). A spacer lipid (cyan) is present in the binding groove to complement the short C9-sphingosine chain of the synthetic ligand OCH. (B) Electron density map (2Fo-Fc) for OCH and the spacer lipid bound in the A′ and F′ pockets of mCD1d is drawn as a blue mesh and contoured at 1σ. The entire OCH ligand is very well ordered and gives rise to excellent electron density. Note that Leu150 (orange) exists in two alternate conformations in the crystal structure, possibly because of the reduced length of the sphingosine chain. (C) Hydrogen-bond interactions between CD1d residues (grey) and the polar moieties of OCH (yellow) are drawn as blue dotted lines with distance given in Angstroms for the direct CD1d-OCH h-bonds. Water molecules (red balls with surrounding electron density) are bound to OCH and enhance the hydrogen bond network between CD1d and OCH. (D) Structure of CD1d with OCH bound (yellow) does not result in an induced fit, likely due to the presence of the short spacer lipid (cyan), which sterically interferes with reorientation of L84. The induced fit of mCD1d bound to short chain αGalCer (PDB code 1Z5L in green) as well as full length αGalCer (PDB code 3HE6 in grey) is shown superimposed. (E) Direct binding comparison of OCH (yellow) and αGalCer (PDB code 1Z5L, grey), as well as αGalCer from the recent mCD1d-Vα14Vβ8.2 TCR co-crystal structure (71) (PDB code 3HE6, green) (F-H) Surface representation with electrostatic potentials (red, electronegative and blue, electropositive contoured from −30 to +30 kT/e). (F) OCH (yellow) binding to mCD1d. (G) αGalCer (green) binding to mCD1d (PDB 3HE6). (H) αGalCer (grey) binding to mCD1d (PDB 1Z5L). Several residues that are involved in the shaping of the CD1d binding grooves or in polar interactions with OCH are depicted in the figure as one-letter code with residue number. Note the formation of the F′ roof in panel G and H, as a direct result of the induced fit, as illustrated in panel D.
Although the overall binding of OCH is very similar to that of αGalCer, there is one major difference (Fig. 3D-H). The previously reported induced fit of mCD1d upon αGalCer binding (PDB 1Z5L) is not observed in the OCH structure, while it is observed in the recently determined structure of the tri-molecular complex (PDB 3HE6). As a result, no F′ roof above the OCH ligand is formed, which in the αGalCer structure tightly tugs the sphingosine chain underneath by forming additional non-polar van der Waals interactions. This structural difference explains the shorter half-life of the OCH-CD1d complexes as compared to αGalCer-CD1d complexes. The induced fit is likely abrogated by the spacer lipid, which interferes with the re-orientation of Leu84. Reorientation of Leu84 and Leu150 are the key structural changes that lead to the induced fit (Fig. 3E), which in turn affects the position of amino acids such as Arg 79 (Fig. 3E), which are known to be important for TCR recognition (23, 39, 47, 48). The binding of the galactose headgroup appears to be more dynamic. In the OCH, as well as the αGalCer structure complexed with the TCR, the galactose is not as closely bound to the α2-helix, while the short chain αGalCer from the unliganded mCD1d structure makes more intimate contacts (Fig. 3E). These differences between two highly similar ligands clearly illustrate that although the TCR docking orientation onto CD1d likely will be conserved on both ligand structures, and likely will involve the same key residues of CD1d and the TCR, the interacting surfaces are not identical and, hence, likely to impact TCR avidity and signaling.
OCH and C-glycoside do not polarize Vα14i NKT cell cytokine production
As shown in Fig. 1, when the sera of immunized mice were analyzed, OCH induced a systemic TH2 biased response and C-glycoside induced a more TH1 biased response. We measured several features of Vα14i NKT cell activation to determine if these two Ags directly affect Vα14i NKT cells in different ways. Two hours after injection, αGalCer induced the highest percentage Vα14i NKT cells to produce IFNγ, IL-4 and TNF (Fig. 4A and data not shown), and the highest level of CD69 and CD40L expression (Fig. 4C). By these criteria, OCH was less potent than αGalCer, and C-glycoside the least potent of the three. However, we did not observe an enhanced decline in the percentage of cells producing only one of the cytokines, for example, comparing C-glycoside to αGalCer, a greater decrease in IL-4 producing cells than in IFNγ producing cells (Fig. 4B). We observed similar patterns of cytokine production both in the spleen at two hours and in the spleen and liver six hours after Ag injection (data not shown). Therefore, there was no immediate or early polarity in cytokine production from Vα14i NKT cells activated with the different Ags, only a difference in the number of cells activated. These findings suggest that the systemic cytokine polarity of the immune responses induced by OCH and C-glycoside is likely mediated by events downstream, which could include the length of time of Vα14i NKT cell activation beyond six hours, or effects on other cells. Moreover, reduced early CD40L expression cannot by itself be responsible for TH2 cytokine polarization of the systemic immune response, as has been proposed (Oki et al., 2005), because OCH induced more CD40L than C-glycoside at 2 h after injection (Fig. 4C).
Impaired NK cell trans-activation by OCH
Glycolipid Ag stimulation of Vα14i NKT cells in vivo causes the activation of several other cell types, a phenomenon sometimes called trans-activation (49). Prominent among these events is the activation of NK cells to secrete IFNγ (50), and in fact, most of the serum IFNγ following αGalCer injection is due to NK cells (9). We examined IFNγ production by NK cells 18 h after injection of Ags using intracellular cytokine staining (ICCS) (Fig. 5). αGalCer injection induced the highest percentage of IFNγ-expressing NK cells in both the liver and spleen, followed by C-glycoside and then OCH (Fig. 5). This corresponds to the levels of IFNγ observed in the serum at 18 h (Fig. 1B), and it agrees with earlier observations comparing αGalCer either to C-glycoside (15) or to OCH (11), although a direct comparison of OCH and C-glycoside has not been carried out previously. It is noteworthy, however, that the hierarchy of effectiveness for NK cell trans-activation is different from those defined previously for TCR avidity/half-life, TCR proximal signaling events, immediate cytokine production and cytolytic function.
Figure 5. NK cell trans-activation correlates with the cytokine bias of glycolipid Ags.
C57BL/6 mice were injected intravenously with 1 μg of the indicated Ags. After 18 h, splenic NK cells were examined for the production of IFNγ by intracellular cytokine staining. Flow cytometry histograms are shown gated on TCRβ− NK1.1+ live lymphocytes; the percentage of cells expressing each molecule is indicated. This experiment was performed three times with similar results.
Because C-glycoside's ability to trans-activate NK cells was discordant with its observed affinity for the TCR we sought to determine if CD40:CD40L interactions were responsible, as they are required for αGalCer-mediated trans-activation (9, 51). CD40L, expressed by Vα14i NKT cells after exposure to αGalCer, engages CD40 expressed by APCs. The APCs become activated and produce IL-12 (52), which contributes to NK cell production of IFNγ. IFNγ levels after either αGalCer or C-glycoside administration were strongly reduced in CD40-deficient mice (Supplementary Figure 3). Therefore, the relatively strong activation of NK cells following C-glycoside, compared to OCH, requires a CD40-dependent mechanism, similar to trans-activation following αGalCer, although as noted above, the amount of CD40L induced cannot explain the difference between OCH-induced and C-glycoside-induced responses.
Glycolipid Ags have a similar requirement for CD1d internalization
A possible mechanism to explain the ability of the weak agonist C-glycoside to effectively trans-activate NK cells could derive from an enhanced requirement for this glycolipid Ag to be internalized for CD1d loading. This possibility is suggested by earlier work on the TH2 polarizing C20:2 glycolipid Ag, which exhibited a decreased requirement for CD1d internalization (12). A requirement for internalization could translate into preferential uptake in vivo by cell types, such as DC, that might preferentially stimulate TH1 responses. Alternatively, as suggested previously (12), a requirement for internalization could be manifested at the single cell level, by causing a preferential loading into CD1d molecules that locate to lipid rafts. Lipid raft localization of MHC class II molecules can affect the polarization of cytokine production by conventional CD4+ T cells (53), and some CD1d molecules are found in lipid rafts (54).
To address these possibilities with OCH and C-glycoside, we used mutant A20-CD1d cells, where CD1d was impaired in entry to endocytic compartments due to a Y → A point mutation in the four-amino acid motif, Tyr-Gln-Asp-Ile (YQDI), of the cytoplasmic tail. Three amino acids of this motif, including the tyrosine, are required for proper CD1d intracellular trafficking and presentation of glycolipid Ags dependent upon intracellular loading (55). In keeping with previously published reports (56), the disaccharide glycolipid Gal(α1-2)αGalCer (Fig. 1A) required the intact YQDI motif. This compound requires lysosomal carbohydrate processing to produce the monosaccharide αGalCer, which is loaded into CD1d molecules in lysosomes. The A20-CD1d cells with Y → A point mutation (A20-CD1d YA) therefore could not present this compound. In this experiment, αGalCer stimulation had a partial requirement for normal CD1d internalization, as has been observed in some previous studies (12). By contrast, the presentation of OCH was not affected by the CD1d cytoplasmic tail point mutation (Fig. 6). C-glycoside was intermediate between OCH and αGalCer in terms of exhibiting sensitivity to the cytoplasmic tail mutation of CD1d. Our data therefore indicate that, unlike for the disaccharide, there is not an absolute requirement for normal endosomal localization of CD1d for Ag presentation. Furthermore, the data are not consistent with a strong requirement for normal CD1d internalization for TH1 cytokine production.
Figure 6. CD1d endosomal localization is not required for glycolipid Ag presentation.
A. A20-CD1d and A20-CD1d YA cells were pulsed with the indicated concentration of Ags, washed and incubated with the 1.2 Vα14i NKT cell hybridoma overnight before measurement of IL-2 in the supernatant by ELISA. This experiment was performed twice with similar results.
Enhanced stability of C-glycoside/CD1d/complexes in vivo
C-glycoside originally was synthesized because the replacement of the glycosidic oxygen with a CH2 group likely would make this glycosphingolipid more resistant to catabolism in vivo. We tested the functional stability of the glycolipid Ags in vivo by immunizing mice and harvesting APC from these mice at different times after immunization. These cells were then used in Ag presentation assays. We used anti-CD11c magnetic beads to enrich for DC from the spleens of immunized mice, providing enrichment to approximately 30% CD11c+ MHC class II+ cells in the positive fraction, and a depleted fraction that was essentially devoid of this population (Supplementary Fig. 4).
We analyzed the DC from these immunized mice to determine if DC were altered differently following administration of particular compounds. Immunization with C-glycoside and OCH induced increased expression of CD80, CD86 and CD1d in splenic DC to the same extent as αGalCer at 17.5 h and 44 h (Supplementary Fig. 5), suggesting all three compounds induced a similar degree of DC maturation. To verify that the enriched DC from the immunized mice remained capable of presenting glycolipids to Vα14i NKT cells, we incubated each enriched DC population with αGalCer in vitro, and the Ag-incubated cells then were cultured with the 1.2 hybridoma. DC from mice immunized with each of the three Ags were capable of presenting αGalCer when isolated either 1.5 h or 17.5 h after immunization (Supplementary Fig. 6). When obtained from any of the glycolipid-immunized mice, however, the DC induced less IL-2 than the DC from vehicle immunized mice. This suggests that Vα14i NKT cell activation reduced the ability of the DC to present αGalCer to Vα14i NKT cells or that a highly stimulatory subpopulation of the DC were eliminated by cytotoxicity.
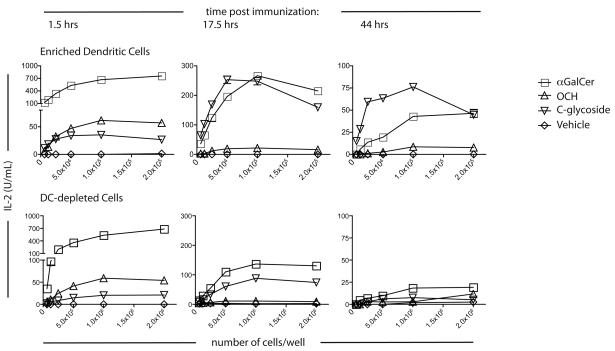
A dose range of both the DC enriched and DC depleted fractions from immunized mice were cultured with 1.2 hybridoma cells without exogenous Ag addition, and IL-2 release in the supernatant was used as a bioassay for the available glycolipid/CD1d complexes that remained. APC from either the DC enriched or DC depleted fractions stimulated IL-2 release when derived from mice immunized with αGalCer, OCH or C-glycoside 1.5 h earlier (Fig. 7). αGalCer gave the best response, and OCH led to a higher production of IL-2 than C-glycoside. At 17.5 h and 44 h, however, the amount of activation induced following OCH immunization was considerably reduced compared to αGalCer and C-glycoside (Fig. 7). This finding is particularly remarkable for C-glycoside, because it does not bind to CD1d in as stable a fashion as αGalCer. Furthermore, the number of available C-glycoside/CD1d complexes must be significantly higher than αGalCer/CD1d complexes in order to achieve the same IL-2 production, because the avidity of C-glycoside/CD1d for the invariant TCR is much lower. At 17.5 h, the DC depleted fraction was capable of presenting αGalCer and C-glycoside, to approximately one-third the amount as the enriched DCs (Fig. 7). By 44 h, only the enriched DCs induced detectable hybridoma IL-2 release, and this was decreased from the 17.5 h time point. Thus, from these data, we conclude that complexes of CD1d with αGalCer and C-glycoside are much more stable in vivo than OCH, with C-glycoside the most stable. This feature of C-glycoside allows for a sustained activation of Vα14i NKT cells that is correlated with its uniquely TH1-biasing properties.
Figure 7. Stability of glycolipid/CD1d complexes in vivo.
C57BL/6 mice were injected i.v. with 1 μg of the indicated glycolipids, and at the indicated times splenic CD11c+ DC were enriched by collagenase digestion and MACS bead isolation. The enriched DC or the DC-depleted cells were then cultured with the 1.2 Vα14i NKT hybridoma overnight, and IL-2 in the supernatant was assessed by ELISA. Results are plotted as the number of cells/well vs. IL-2 (Top panel: enriched DC. Bottom panel: DC-depleted cells). This experiment was performed twice with similar results.
Discussion
It is important to understand the rules by which Vα14i NKT cells can drive the overall immune response in either a TH1 or TH2 direction, not only for achieving a basic understanding of the role of this T cell subset in immunity, but also because clinical trials based on glycolipid Ag activation of the homologous population of human Vα24i NKT cells remain ongoing. It has been established that cytokines, such as the IL-12 elicited from APC following engagement of TLRs, can stimulate IFNγ secretion in the absence of IL-4 release by Vα14i NKT cells, and furthermore, that this does not require exogenous Ag (57-59). Similarly, IL-2 has been reported to cause the selective synthesis of IL-5 by Vα14i NKT cells (60). Functional subsets of Vα14i NKT cells that are pre-committed to the synthesis of different cytokines (61, 62) may also be selectively engaged under some circumstances. The most practical means, however, of inducing preferentially TH1 or TH2 cytokines from the broad population of iNKT cells may be the use of particular glycolipid Ags. Here, therefore, we have focused on the mechanism whereby different glycolipid Ags that are closely related to αGalCer initiate different immune responses.
The finding that αGalCer stimulates Vα14i NKT cells to produce both TH1 and TH2 cytokines (63) triggered the synthesis of chemical variants that would cause a purely TH1 or TH2 –biased immune response. Since that time, several variants have been produced (10-12, 14, 15, 64-70). Many of these variants bias towards a TH2 cytokine response, and of these, OCH, the first such Ag discovered, has been the most widely studied. It has been suggested that OCH acts to stimulate a more TH2 skewed response because of a reduced duration of TCR signaling, the result of a decreased half-life of binding to CD1d as well as a reduced TCR affinity (10, 19). Additional compounds have been reported to bias cytokines towards a TH1 response (65, 66). There have been only limited investigations, however, of the mechanism of action of TH1 biasing compounds. Furthermore, a side-by-side comparison of the mechanisms by which TH1- and TH2-biasing αGalCer variants skew the cytokine response had not been carried out previously.
We found that a shorter half-life of interaction with CD1d and a lower TCR affinity do not provide a simple explanation for TH2 cytokine skewing. OCH does indeed have a shorter half-life of binding to CD1d than αGalCer. This had been suggested previously using immune response assays, and was established herein directly using a CD1d binding assay. C-glycoside binding to CD1d, however, tended to be even shorter lived than OCH binding. In agreement with earlier studies (10, 19), complexes of OCH/CD1d also had a lower TCR avidity than αGalCer/CD1d complexes. Regardless, using a variety of biochemical and functional assays, C-glycoside/CD1d complexes exhibited equal or even weaker binding to the Vα14i TCR. If there were a simple, linear relationship between cytokine polarization and the strength of the glycolipid-CD1d interaction, or with the strength of the interaction of the glycolipid Ag-CD1d complex with the TCR, then C-glycoside should have stimulated a stronger TH2 response than OCH.
The decreased avidity of the invariant TCR for OCH complexes with mouse CD1d is surprising, as the chemical structures of the hydrophilic portions of OCH and αGalCer are identical. Previous surface plasmon resonance studies of the interaction of the human Vα24 invariant TCR with human CD1d, using different αGalCer variants, showed that the length of the sphingosine chain bound in the F′ pocket modulates TCR affinity (13). The authors proposed a model in which reduction of the sphingosine chain would result in partial collapse of the less than fully occupied F′ pocket, which in turn would change the CD1d T cell recognition surface. Although the mouse and human CD1d structures are very similar, the proposed F′ pocket collapse was not observed in the OCH-mouse CD1d structure, perhaps due to the recruitment of the spacer lipid. An additional difference comparing mouse and human is that binding of αGalCer does not lead to an induced fit in human CD1d, as there is no closing of the roof of the F′ pocket (13), although this occurs with mouse CD1d. It appears, however, that changes more subtle than the proposed F′ collapse are unique to αGalCer when bound to mouse CD1d. These changes, particularly those that close the roof over the F′ pocket, affect the CD1d surface and can account for the more stable CD1d binding exhibited by αGalCer. They also create a structure for TCR recognition of αGalCer that is different from OCH-mouse CD1d complexes, thereby explaining the TCR affinity difference. Interestingly, the recent crystal structure of the mouse CD1d-αGalCer-Vα14Vβ8.2 TCR (71) also reveals the reported induced fit upon αGalCer binding. As a result both α-helices could be more dynamic in interacting with the various glycolipids than previously assumed.
Two factors could contribute to a polarization of the cytokine response induced following Ag activation of Vα14i NKT cells: the cytokine polarity of the Vα14i NKT cell response itself, or the efficacy with which Vα14i NKT cells induce an indirect or trans-activation of other cell types. Our study examined both of these aspects. We have shown that while OCH induces amounts of serum IL-4 that are comparable to, but not greater than αGalCer, it does induce less serum IFNγ, while for C--glycoside the decrease in IL-4 synthesis is more profound. Therefore, for both OCH and C-glycoside, the cytokine bias is a relative one manifested most clearly as a difference in the proportion of the cytokines produced. Critically, while both glycolipids induced the predicted biased systemic cytokine responses, neither C-glycoside nor OCH caused a significant bias in the immediate cytokine production by Vα14i NKT cells, when measured by ICCS directly ex vivo. Instead, the immediate response to these compounds followed an affinity hierarchy, with αGalCer > OCH > C-glycoside, which related to the number of Vα14i NKT cells activated as opposed to a particular cytokine bias. This outcome agrees with results from some studies (15) but in other cases (11) a bias in the cytokines directly produced by Vα14i NKT cells was observed using different assays, such as cytokine production measured by ELISA. While the ex vivo ICCS method we used most closely reflects the in vivo activity of Vα14i NKT cells, because of the rapid TCR down regulation caused by some Ags, particularly αGalCer, it has the limitation of only detecting the early cytokine response. Therefore, it remains possible that at later times after activation the different glycolipid Ags induced a more polarized cytokine response directly from Vα14i NKT cells, although this is unlikely to provide a complete explanation for their effects.
In contrast to the TCR affinity hierarchy, C-glycoside induced a more effective trans-activation of NK cells to produce IFNγ than OCH, although it was less effective than αGalCer. Therefore, our data are most consistent with the idea that the bias in cytokine production after OCH and C-glycoside immunizations occurs at least in part systemically, rather than at the level of the initial response of the Vα14i NKT cell, and that it is the result of differences in the trans-activation of other cell types, particularly NK cells, which are the major source of serum IFNγ induced after stimulation with glycolipid Ags (9). Therefore, our data demonstrate that these Ags do not act as the glycolipid equivalents of altered peptide ligands, and rather than stimulating an altered pattern of cytokine production directly from Vα14i NKT cells, they act by altering the network of cellular interactions that occurs downstream follow the initial stimulation event. This suggests that some other feature of the compounds, such as the length of time they are able to stimulate Vα14i NKT cells, the preferential loading of Ag in certain subcellular compartments, or the presentation by certain types of APCs, could explain the different responses they elicit.
There is evidence that the TH2 bias elicited by the C20:2 analog of αGalCer is related to its increased ability to be loaded into CD1d at the cell surface (12). While this paper was in preparation, we published a study showing that several TH2 biasing analogs of αGalCer, all with alterations in the fatty acid, are characterized by rapid and direct loading onto CD1d on the cell surface, and exclusion form lipid rafts (20). Using cells that expressed a mutant version of CD1d with a reduced ability to enter endosomal compartments (55, 72), we observed that the responses to OCH and C-glycoside were not greatly different when compared to presentation by wild type CD1d, although C-glycoside presentation was somewhat more affected. Of note, the glycolipids were pulsed onto the APC for only two hours prior to washing, and therefore in this time the cells expressing CD1d with a cytoplasmic tail mutation must have been able to take up for later binding, or bind to CD1d directly, similar or nearly similar amounts of glycolipid Ag for presentation as cells expressing wild type CD1d. Our data suggest that factors other than exclusive or selective uptake of C-glycoside into deep intracellular compartments are likely responsible for some of the functional differences between OCH and C-glycoside and furthermore, that there is unlikely to be a single explanation for the TH2 biasing effects of different compounds.
A striking finding from these studies is that C-glycoside forms long-lived, functional complexes on the surface of APCs in vivo, while OCH does not. APCs from mice injected with C-glycoside more than 17 h earlier stimulated Vα14i NKT cell hybridomas as effectively as APCs from mice injected with αGalCer. Considering that C-glycoside/CD1d complexes have a weaker TCR avidity, we conclude that within hours after Ag injection there must be more complexes of C-glycoside/CD1d than αGalCer/CD1d complexes. Our results are in agreement with those from a previous study that showed C-glycoside formed more stable complexes than αGalCer on bone marrow DC cultured in vitro and subsequently loaded with compound in vitro, and then tested for their ability to activate iNKT cells in vivo (73). While this manuscript was in preparation, it was reported that a TH2 biasing glycolipid with an acyl-chain shortened to 8 carbons can be internalized efficiently to lysosomes, but that it is displaced from CD1d easily by other lipids in this acidic environment (74). As CD1d recycles from lysosomes to the cell surface, this internal displacement of the lipid Ag led to a shorter half-life of the glycolipid Ag-CD1d complexes on the cell surface. Several other TH2 biasing compounds behaved similarly. Therefore, the stability of glycolipid complexes with CD1d is likely to be a critical determinant of cytokine polarization, but what can account for the increased stability of C-glycoside? Certainly the stability of binding of C-glycoside to CD1d is not a factor, because C-glycoside binding is less stable than αGalCer binding to CD1d. Selective uptake by certain types of APC could be involved, and while this cannot be absolutely ruled out, we observed a similar functional stability when populations enriched for CD11c+ DC or depleted of DC were compared. All of the compounds tested can load into the CD1d groove effectively at the cell surface, but CD1d recycles through endosomes, and therefore it is possible that C-glycoside binding is more resistant to acid pH, or that it rebinds to CD1d more effectively in the low pH environment. Finally, it should be noted that the strategy of synthesizing a C-glycoside variant of αGalCer was undertaken based on the hypothesis that the C-analog would provide additional chemical stability, because O-glycosidase enzymes would not degrade it. We consider it likely that this is the case.
In conclusion, our studies illustrate the means by which subtle alterations in glycolipid Ag structure can alter the immune response initiated by Vα14i NKT cell activation. The analogy to altered peptide ligands is not an apt one, because much of the difference in the immune responses elicited by different Ags can be attributed to the cascade of effects on other cells that unfold rapidly following the activation of Vα14i NKT cells. We have established that OCH provides an antigenic stimulation that is intense, although weaker than αGalCer, but which is relatively short-lived due in part to a reduced stability of binding to CD1d. C-glycoside, by contrast, provides a weak TCR stimulus, but one which is prolonged, and which is maintained over 17 hours and increased relative to the other compounds, despite a reduced half life of CD1d binding. This functional stability of C-glycoside Ag presentation is likely due to resistance to catabolism. Therefore, an important parameter for a glycolipid Ag to influence the cytokine response is the length of stimulation, with a longer stimulation in vivo promoting IFNγ production due to a more effective trans-activation of NK cells. The length of stimulation is likely influenced by the pharmacokinetics of the Ag. OCH and C-glycoside are not potent activators of human Vα24i NKT cells, but efforts are underway in several laboratories to synthesize glycosphingolipid Ags that induce a highly polarized cytokine response from human cells, and some success is being achieved (75). Our findings suggest that the stability of the Ag in cells will be a critical parameter for the induction of such polarized cytokine responses.
Supplementary Material
Supplemental Figure 1. Detection of ERK1/2 phosphorylation is sensitive and specific.
αGalCer induces a dose-dependent increase in ppERK. A20-CD1d cells were pulsed with the indicated dose of αGalCer for 2 h, washed and incubated with the 1.2 hybridoma for 5 min at 37°C. Phosphorylation of ERK1/2 was assessed by flow cytometry. Gates on the histograms indicate the percent positive cells. B. The phosphorylation of ERK1/2 can be inhibited by an anti-CD1d monoclonal antibody (1B1) in a dose-dependent manner. A20-CD1d cells were pulsed with 200 ng/mL αGalCer for 2 h, washed, incubated with 1B1 for 1 h, washed and incubated with the 1.2 hybridoma for 5 min at 37°C. C. The phosphorylation of ERK1/2 can be inhibited by the MEK inhibitor UO126. A20-CD1d cells were pulsed with 200 ng/mL αGalCer for 2 h, washed and incubated with the 1.2 hybridoma for 5 min at 37°C in the presence of the MEK inhibitor UO126 (20μM) or vehicle (Veh.). PMA was used as a positive control. “Vehicle” indicates A20-CD1d cells pulsed with the vehicle without αGalCer. “αGalCer + Veh” indicates αGalCer with the vehicle for UO126. D. A peptide-reactive hybridoma (B3Z) does not phosphorylate ERK1/2 in response to αGalCer. A20-CD1d cells were pulsed with 200 ng/mL αGalCer for 2 h, washed and incubated with the B3Z or 1.2 hybridomas for 5 min at 37°C. PMA was used as a positive control.
Supplemental Figure 2. Decay of CD1d/glycolipid complexes
Decay data are presented as time vs. normalized L317 mAb binding. After linear regression, glycolipid/CD1d complex half-life was determined by t1/2 = log2/slope. The calculated rates of decay for these assays are shown in Table I.
Supplemental Figure 3. CD40-CD40L interactions are essential for C-glycoside induced IFNγ production.
C57BL/6 mice (open symbols) and CD40−/− mice (closed symbols) were injected intravenously with 1 μg of the indicated glycolipid. After 6 h and 24 h, serum was isolated from the immunized mice and assessed for the quantity of IFNγ by ELISA. This experiment was performed twice with similar results.
Supplemental Figure 4. Enrichment of dendritic cells from the spleens of immunized mice.
1.5 h following glycolipid immunization, splenic CD11c+ dendritic cells were enriched by collagenase digestion and MACS bead isolation (see Materials and Methods for details) and analyzed by flow cytometry. For each glycolipid, or vehicle, immunized mouse, the enriched DCs (top panel) are compared with DC-depleted splenyocytes (bottom panel), gating on IAb+ CD11c+ and IAb− CD11c+ cells. Of these two populations, only the IAb+ CD11c+ cells carried antigenic complexes ex vivo and presented to the 1.2 hybridoma (data not shown). This analysis was carried twice out for each time point (1.5, 17.5 and 44h), with similar results.
Supplemental Figure 5. Phenotypic assessment of enriched DCs from glycolipid immunized mice.
After 1.5, 17.5 or 44h, splenic CD11c+ dendritic cells were enriched by collagenase digestion and MACS bead isolation and assessed for CD80, CD86 and CD1d expression by flow cytometry. Plots are shown gated on IAb+ CD11c+ cells. This experiment was performed twice with similar results.
Supplemental Figure 6. Presentation of αGalCer is reduced in glycolipid immunized mice.
After 1.5 or 17.5 h, splenic CD11c+ DC were enriched by collagenase digestion and MACS bead isolation (see Materials and Methods for details). Enriched DC from mice immunized with the indicated glycolipid were tested for the capacity to present αGalCer to the 1.2 Vα14i NKT hybridoma. Cells were cultured with 200 ng/mL αGalCer and the 1.2 Vα14i NKT hybridoma overnight. The level of IL-2 in the supernatant was assessed by ELISA. The 1.2 Vα14i NKT hybridomas alone did not produce IL-2 (data not shown). This experiment was performed twice with similar results.
Acknowledgments
The authors would like to thank Archana Khurana, Ryan Severins, Donna M. Lien for outstanding technical assistance and advice with experiments, and the SSRL staff of beamline 11.1 for assistance with remote data collection.
Footnotes
This work was funded by NIH grants RO1 AI45053 and R37 AI71922 (M.K.), R56/RO1 AI074952 (D.M.Z.), RO1AI45889 (S.A.P.), F32 AI62015 (B.A.S.), from Cytheris, Inc. (R.W.F. and M.T.) and an Outgoing International Fellowship by the Marie Curie Actions (G.W.). D.M.Z. is recipient of a CRI Investigator award from the Cancer Research Institute.
Competing Interest
The authors have no competing interests regarding this work.
References
- 1.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 4.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 7.Yu KO, Porcelli SA. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Coppieters K, Van Beneden K, Jacques P, Dewint P, Vervloet A, Vander Cruyssen B, Van Calenbergh S, Chen G, Franck RW, Verbruggen G, Deforce D, Matthys P, Tsuji M, Rottiers P, Elewaut D. A single early activation of invariant NK T cells confers long-term protection against collagen-induced arthritis in a ligand-specific manner. J Immunol. 2007;179:2300–2309. doi: 10.4049/jimmunol.179.4.2300. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, Kronenberg M. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003;100:8395–8400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oki S, Tomi C, Yamamura T, Miyake S. Preferential T(h)2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int Immunol. 2005;17:1619–1629. doi: 10.1093/intimm/dxh342. [DOI] [PubMed] [Google Scholar]
- 12.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, Salio M, Denkberg G, Reddington F, Tarlton A, Reddy BG, Schmidt RR, Reiter Y, Griffiths GM, van der Merwe PA, Besra GS, Jones EY, Batista FD, Cerundolo V. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 15.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuno M, Masumura M, Tomi C, Chiba A, Oki S, Yamamura T, Miyake S. Synthetic glycolipid OCH prevents insulitis and diabetes in NOD mice. J Autoimmun. 2004;23:293–300. doi: 10.1016/j.jaut.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Schmieg J, Tsuji M, Franck RW. The C-glycoside analogue of the immunostimulant alpha-galactosylceramide (KRN7000): synthesis and striking enhancement of activity. Angew Chem Int Ed Engl. 2004;43:3818–3822. doi: 10.1002/anie.200454215. [DOI] [PubMed] [Google Scholar]
- 18.Grajewski RS, Hansen AM, Agarwal RK, Kronenberg M, Sidobre S, Su SB, Silver PB, Tsuji M, Franck RW, Lawton AP, Chan CC, Caspi RR. Activation of invariant NKT cells ameliorates experimental ocular autoimmunity by A mechanism involving innate IFN-gamma production and dampening of the adaptive Th1 and Th17 responses. J Immunol. 2008;181:4791–4797. doi: 10.4049/jimmunol.181.7.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanic AK, Shashidharamurthy R, Bezbradica JS, Matsuki N, Yoshimura Y, Miyake S, Choi EY, Schell TD, Van Kaer L, Tevethia SS, Roopenian DC, Yamamura T, Joyce S. Another view of T cell antigen recognition: cooperative engagement of glycolipid antigens by Va14Ja18 natural T(iNKT) cell receptor [corrected] J Immunol. 2003;171:4539–4551. doi: 10.4049/jimmunol.171.9.4539. [DOI] [PubMed] [Google Scholar]
- 20.Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, Jerud ES, Goldberg MF, Baena A, Yu KO, Ndonye RM, Howell AR, Yuan W, Cresswell P, Chang YT, Illarionov PA, Besra GS, Porcelli SA. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidobre S, Kronenberg M. CD1 tetramers: a powerful tool for the analysis of glycolipid-reactive T cells. J Immunol Methods. 2002;268:107–121. doi: 10.1016/s0022-1759(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Schmieg J, Tsuji M, Franck RW. Efficient synthesis of alpha-C-galactosyl ceramide immunostimulants: use of ethylene-promoted olefin cross-metathesis. Org Lett. 2004;6:4077–4080. doi: 10.1021/ol0482137. [DOI] [PubMed] [Google Scholar]
- 23.Burdin N, Brossay L, Degano M, Iijima H, Gui M, Wilson IA, Kronenberg M. Structural requirements for antigen presentation by mouse CD1. Proc Natl Acad Sci U S A. 2000;97:10156–10161. doi: 10.1073/pnas.97.18.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu KO, Im JS, Illarionov PA, Ndonye RM, Howell AR, Besra GS, Porcelli SA. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand alpha-galactosylceramide bound to mouse CD1d. J Immunol Methods. 2007;323:11–23. doi: 10.1016/j.jim.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 27.Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, Kumar V, Wilson IA. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. HKL: Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 29.CCP4 Collaborative Computational Project, Number 4. The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 30.Vagin AA, Teplyakov A. MOLREP:an automated programm for molecular replacement. J. Appl. Cryst. 1997;30:1022–1025. [Google Scholar]
- 31.Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. 2001;D57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 32.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Vα14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuettelkopf AW, van Aalten DM. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. 2004;D60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 34.Lovell SC, Davis IW, Arendall WB, 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Cα geometry: φ,ψ and Cβ deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 35.Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665–667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao X, Grant C, Constant S, Bottomly K. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J Immunol. 1997;158:4237–4244. [PubMed] [Google Scholar]
- 38.Lyons DS, Lieberman SA, Hampl J, Boniface JJ, Chien Y, Berg LJ, Davis MM. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 39.Sidobre S, Naidenko OV, Sim BC, Gascoigne NR, Garcia KC, Kronenberg M. The V alpha 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J Immunol. 2002;169:1340–1348. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]
- 40.Wang XL, Altman JD. Caveats in the design of MHC class I tetramer/antigen-specific T lymphocytes dissociation assays. J Immunol Methods. 2003;280:25–35. doi: 10.1016/s0022-1759(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 41.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 42.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zajonc DM, Kronenberg M. CD1 mediated T cell recognition of glycolipids. Curr Opin Struct Biol. 2007;17:521–529. doi: 10.1016/j.sbi.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zajonc DM, Wilson IA. Architecture of CD1 proteins. Curr Top Microbiol Immunol. 2007;314:27–50. doi: 10.1007/978-3-540-69511-0_2. [DOI] [PubMed] [Google Scholar]
- 45.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;8:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 46.Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamada N, Iijima H, Kimura K, Harada M, Shimizu E, Motohashi S, Kawano T, Shinkai H, Nakayama T, Sakai T, Brossay L, Kronenberg M, Taniguchi M. Crucial amino acid residues of mouse CD1d for glycolipid ligand presentation to V(alpha)14 NKT cells. Int Immunol. 2001;13:853–861. doi: 10.1093/intimm/13.7.853. [DOI] [PubMed] [Google Scholar]
- 48.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 49.Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, Ehara H, Sakai T, Serizawa I, Wu L, Wang CR, Joyce S, Van Kaer L. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J Immunol. 2004;173:3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 50.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 51.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 53.Buatois V, Baillet M, Becart S, Mooney N, Leserman L, Machy P. MHC class II-peptide complexes in dendritic cell lipid microdomains initiate the CD4 Th1 phenotype. J Immunol. 2003;171:5812–5819. doi: 10.4049/jimmunol.171.11.5812. [DOI] [PubMed] [Google Scholar]
- 54.Lang GA, Maltsev SD, Besra GS, Lang ML. Presentation of alpha-galactosylceramide by murine CD1d to natural killer T cells is facilitated by plasma membrane glycolipid rafts. Immunology. 2004;112:386–396. doi: 10.1111/j.1365-2567.2004.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawton AP, Prigozy TI, Brossay L, Pei B, Khurana A, Martin D, Zhu T, Spate K, Ozga M, Honing S, Bakke O, Kronenberg M. The mouse CD1d cytoplasmic tail mediates CD1d trafficking and antigen presentation by adaptor protein 3-dependent and -independent mechanisms. J Immunol. 2005;174:3179–3186. doi: 10.4049/jimmunol.174.6.3179. [DOI] [PubMed] [Google Scholar]
- 56.Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 57.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 58.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 59.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J Immunol. 2007;179:3452–3462. doi: 10.4049/jimmunol.179.6.3452. [DOI] [PubMed] [Google Scholar]
- 61.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K, Iwamura C, Nakajima H, Nakayama T, Taniguchi M. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 64.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci U S A. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 66.Leung L, Tomassi C, Van Beneden K, Decruy T, Elewaut D, Elliott T, Al-Shamkhani A, Ottensmeier C, Van Calenbergh S, Werner J, Williams T, Linclau B. Synthesis and in vivo evaluation of 4-deoxy-4,4-difluoro-KRN7000. Org Lett. 2008;10:4433–4436. doi: 10.1021/ol801663m. [DOI] [PubMed] [Google Scholar]
- 67.Sidobre S, Hammond KJ, Benazet-Sidobre L, Maltsev SD, Richardson SK, Ndonye RM, Howell AR, Sakai T, Besra GS, Porcelli SA, Kronenberg M. The T cell antigen receptor expressed by Valpha14i NKT cells has a unique mode of glycosphingolipid antigen recognition. Proc Natl Acad Sci U S A. 2004;101:12254–12259. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, Wong CH, Yu AL. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci U S A. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park JJ, Lee JH, Ghosh SC, Bricard G, Venkataswamy MM, Porcelli SA, Chung SK. Synthesis of all stereoisomers of KRN7000, the CD1d-binding NKT cell ligand. Bioorg Med Chem Lett. 2008;18:3906–3909. doi: 10.1016/j.bmcl.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, 3rd, Teyton L, Bendelac A, Savage PB. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 71.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elewaut D, Lawton AP, Nagarajan NA, Maverakis E, Khurana A, Honing S, Benedict CA, Sercarz E, Bakke O, Kronenberg M, Prigozy TI. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Valpha14i NKT cells. J Exp Med. 2003;198:1133–1146. doi: 10.1084/jem.20030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bai L, Sagiv Y, Liu Y, Freigang S, Yu KO, Teyton L, Porcelli SA, Savage PB, Bendelac A. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen alphaGalCer. Proc Natl Acad Sci U S A. 2009;106:10254–10259. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Chen G, Garcia-Navarro R, Franck RW, Tsuji M. Identification of C-glycoside analogues that display a potent biological activity against murine and human invariant natural killer T cells. Immunology. 2009;127:216–225. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Detection of ERK1/2 phosphorylation is sensitive and specific.
αGalCer induces a dose-dependent increase in ppERK. A20-CD1d cells were pulsed with the indicated dose of αGalCer for 2 h, washed and incubated with the 1.2 hybridoma for 5 min at 37°C. Phosphorylation of ERK1/2 was assessed by flow cytometry. Gates on the histograms indicate the percent positive cells. B. The phosphorylation of ERK1/2 can be inhibited by an anti-CD1d monoclonal antibody (1B1) in a dose-dependent manner. A20-CD1d cells were pulsed with 200 ng/mL αGalCer for 2 h, washed, incubated with 1B1 for 1 h, washed and incubated with the 1.2 hybridoma for 5 min at 37°C. C. The phosphorylation of ERK1/2 can be inhibited by the MEK inhibitor UO126. A20-CD1d cells were pulsed with 200 ng/mL αGalCer for 2 h, washed and incubated with the 1.2 hybridoma for 5 min at 37°C in the presence of the MEK inhibitor UO126 (20μM) or vehicle (Veh.). PMA was used as a positive control. “Vehicle” indicates A20-CD1d cells pulsed with the vehicle without αGalCer. “αGalCer + Veh” indicates αGalCer with the vehicle for UO126. D. A peptide-reactive hybridoma (B3Z) does not phosphorylate ERK1/2 in response to αGalCer. A20-CD1d cells were pulsed with 200 ng/mL αGalCer for 2 h, washed and incubated with the B3Z or 1.2 hybridomas for 5 min at 37°C. PMA was used as a positive control.
Supplemental Figure 2. Decay of CD1d/glycolipid complexes
Decay data are presented as time vs. normalized L317 mAb binding. After linear regression, glycolipid/CD1d complex half-life was determined by t1/2 = log2/slope. The calculated rates of decay for these assays are shown in Table I.
Supplemental Figure 3. CD40-CD40L interactions are essential for C-glycoside induced IFNγ production.
C57BL/6 mice (open symbols) and CD40−/− mice (closed symbols) were injected intravenously with 1 μg of the indicated glycolipid. After 6 h and 24 h, serum was isolated from the immunized mice and assessed for the quantity of IFNγ by ELISA. This experiment was performed twice with similar results.
Supplemental Figure 4. Enrichment of dendritic cells from the spleens of immunized mice.
1.5 h following glycolipid immunization, splenic CD11c+ dendritic cells were enriched by collagenase digestion and MACS bead isolation (see Materials and Methods for details) and analyzed by flow cytometry. For each glycolipid, or vehicle, immunized mouse, the enriched DCs (top panel) are compared with DC-depleted splenyocytes (bottom panel), gating on IAb+ CD11c+ and IAb− CD11c+ cells. Of these two populations, only the IAb+ CD11c+ cells carried antigenic complexes ex vivo and presented to the 1.2 hybridoma (data not shown). This analysis was carried twice out for each time point (1.5, 17.5 and 44h), with similar results.
Supplemental Figure 5. Phenotypic assessment of enriched DCs from glycolipid immunized mice.
After 1.5, 17.5 or 44h, splenic CD11c+ dendritic cells were enriched by collagenase digestion and MACS bead isolation and assessed for CD80, CD86 and CD1d expression by flow cytometry. Plots are shown gated on IAb+ CD11c+ cells. This experiment was performed twice with similar results.
Supplemental Figure 6. Presentation of αGalCer is reduced in glycolipid immunized mice.
After 1.5 or 17.5 h, splenic CD11c+ DC were enriched by collagenase digestion and MACS bead isolation (see Materials and Methods for details). Enriched DC from mice immunized with the indicated glycolipid were tested for the capacity to present αGalCer to the 1.2 Vα14i NKT hybridoma. Cells were cultured with 200 ng/mL αGalCer and the 1.2 Vα14i NKT hybridoma overnight. The level of IL-2 in the supernatant was assessed by ELISA. The 1.2 Vα14i NKT hybridomas alone did not produce IL-2 (data not shown). This experiment was performed twice with similar results.