Abstract
By releasing neuroactive agents, including proinflammatory cytokines, prostaglandins and neurotrophins, microglia and astrocytes are proposed to be involved in nociceptive transmission, especially in conditions of persistent, pathological pain. The specific action on dorsal horn neurons of agents released from astrocytes, such as glutamate, has been, however, poorly investigated. By using patch-clamp and confocal microscope calcium imaging techniques in rat spinal cord slices, we monitored the activity of dorsal horn lamina II neurons following astrocyte activation. Results obtained revealed that stimuli that triggered Ca2+ elevations in astrocytes, such as the purinergic receptor agonist BzATP and low extracellular Ca2+, induce in lamina II neurons slow inward currents (SICs). Similarly to SICs triggered by astrocytic glutamate in neurons from other central nervous system regions, these currents (i) are insensitive to tetrodotoxin (TTX), (ii) are blocked by the NMDA receptor (NMDAR) antagonist d-AP5, (iii) lack an AMPA component, and (iv) have slow rise and decay times. Ca2+ imaging also revealed that astrocytic glutamate evokes NMDAR-mediated episodes of synchronous activity in groups of substantia gelatinosa neurons. Importantly, in a model of peripheral inflammation, the development of thermal hyperalgesia and mechanical allodynia was accompanied by a significant increase of spontaneous SICs in dorsal horn neurons. The NMDAR-mediated astrocyte-to-neuron signalling thus represents a novel pathway that may contribute to the control of central sensitization in pathological pain.
Introduction
Over the last decade, studies investigating the function of microglia and astrocytes in the spinal cord have suggested an important contribution of these cells to the generation and maintenance of pathological pain. A wide array of conditions that induce hyperalgesia, such as subcutaneous inflammation, peripheral nerve trauma, bone cancer, and spinal cord trauma, increase expression of the astrocytic marker glial acidic fibrillary protein (GFAP) in the spinal cord (see for example Garrison et al. 1991; Raghavendra et al. 2004; Zhang et al. 2005; Gwak & Hulsebosch, 2009). The observations that dorsal spinal cord astrocytes respond with Ca2+ oscillations to neurotransmitters such as glutamate (Ahmed et al. 1990) and ATP (Salter & Hicks, 1994; Fam et al. 2000; Werry et al. 2006), and can release pro-inflammatory cytokines (Martin et al. 1992; Pineau et al. 2010) and prostaglandins (Palma et al. 1998) hint at a participation of these cells in the mechanism of hyperalgesia (Milligan & Watkins, 2009).
Ca2+ elevations in astrocytes can trigger the release also of various neuron-active agents, now termed gliotransmitters. Among these, the excitatory amino acid glutamate is the most widely studied. Glutamate can be released by astrocytes through various mechanisms (Malarkey & Parpura, 2008), including a Ca2+-dependent exocytosis of glutamate containing vesicles (Montana et al. 2004). Elevations of the cytosolic Ca2+ have been reported to be sufficient and necessary to cause glutamate release from cultured astrocytes and astrocytes from acute brain slices (Parpura et al. 1994; Bezzi et al. 1998; however, see also Shigetomi et al. 2008). The effects of glutamate-mediated astrocyte-to-neuron signalling systems have been studied both in cultures and in acute slice preparations from different brain regions, including hippocampus (Araque et al. 1998; Fellin et al. 2004), cerebellum (Kulik et al. 1999), thalamus (Parri et al. 2001), retina (Newman & Zahs, 1998) and nucleus accumbens (D’Ascenzo et al. 2007). Astrocytic glutamate has been shown to act on metabotropic (mGlu) and N-methyl-d-aspartate receptors (NMDARs) located at presynaptic terminals to change the probability of neurotransmitter release and to induce a long-lasting enhancement of synaptic transmission (Fiacco & McCarthy, 2004; Jourdain et al. 2007), as well as on extrasynaptic NMDARs at the postsynaptic membrane to promote synchronous neuronal firing (Fellin et al. 2004).
Although these glial cells appear to be widely involved in the dynamic control of neurotransmission in the brain, the role of astrocyte-to-neuron signalling, in general, and the effects of astrocyte glutamate release, in particular, have been poorly investigated in the spinal cord. By patch-clamp recordings from acute spinal cord slice neurons, we here demonstrate that stimulation of Ca2+ elevations in astrocytes triggers NMDAR-mediated slow inward currents (SICs) in lamina II neurons. As revealed by confocal microscope Ca2+ imaging, a striking feature of this NMDAR response is that it occurs synchronously in multiple dorsal horn neurons. In a model of peripheral inflammation, we also found that a SIC frequency increase accompanies the development of thermal hyperalgesia and mechanical allodynia.
By revealing a glutamate-mediated astrocyte-to-neuron signalling in the dorsal horn and its amplification associated with the development of a peripheral inflammation, our study provides a mechanistic hypothesis for a role of astrocytes in the modulation of central pain transmission.
Methods
Ethical approval
All experimental procedures were in strict accordance with the Italian and EU regulation on animal welfare and had prior authorization from the Italian Ministry of Health. The experiments included in these studies comply with the polices and regulations detailed by Drummond (2009).
Slice preparation for electrophysiology
Sprague–Dawley rats from postnatal day (P)8 to P14 were anaesthetized with halothane and decapitated. Older animals (P21–P28) were used in one group of experiments. The spinal cord and vertebrae were rapidly removed and placed in ice-cold extracellular solution (composition in mm: 125 NaCl, 2.5 KCl, 25 NaHCO3, 1 NaH2PO4·H2O, 25 glucose, 6 MgCl2, 1.5 CaCl2, 1 kynurenic acid), bubbled with carboxygen (95% O2, 5% CO2). The lumbar part of the spinal cord was isolated, laid down on an agarose block, and transverse slices (500 μm thick) were obtained using a vibrating microtome (Campden Instruments, Loughborough, UK). Slices were incubated in oxygenated extracellular solution (composition: as above, without kynurenic acid) at 35°C for 30 min and used for recording within 6–7 h.
Patch clamping
Patch-clamp recording in the whole-cell configuration was performed on lamina II neurons at room temperature. Neurons were visualized by using a Zeiss Axioskop microscope (Zeiss, Oberkochen, Germany), fitted with Nomarski optics and connected to a camera (Dage-MTI, Michigan City, IN, USA). Slices were perfused at 1–2 ml min−1. Thick-walled borosilicate pipettes, having a resistance of 3–5 MΩ, were filled with intracellular solution (composition in mm: 130 caesium gluconate, 10 CsCl, 11 EGTA, 1 CaCl2, 10 Hepes, 2 Mg2+-ATP; pH adjusted to 7.2 with NaOH, osmolarity adjusted to 310 mosmol l−1 with sucrose). Junction potentials were corrected after recording.
Data were recorded and acquired using a Multiclamp 700A amplifier and pCLAMP 9 software (Molecular Devices, Sunnyvale, CA, USA). Sampling rate was 10 kHz; data were filtered at 2 kHz. Neurons were voltage clamped at −60 mV. Neuronal spontaneous activity was recorded in Mg2+-free extracellular solution (composition in mm: 125 NaCl, 2.5 KCl, 25 NaHCO3, 1 NaH2PO4·H2O, 25 glucose, 2 CaCl2) in the constant presence of 1 μm tetrodotoxin (TTX). Slices were incubated 20 min before recording in Mg2+-free extracellular solution. Ca2+ free solution was obtained by replacing CaCl2 with EGTA (0.25 mm). We refer to this condition as ‘low extracellular Ca2+’ since it is not possible to obtain a complete wash-out of calcium ions in slices.
Transient inward currents with 20–80% rise time slower than 10 ms and peak amplitude greater than −20 pA were classified as SICs. Cells displaying an increased number of SICs during the period of chemical stimulation (BzATP or low extracellular calcium) with respect to an equal time period before stimulation (5 min) were considered responsive. The frequency of SICs was measured in these responsive neurons. Amplitude threshold for detection of miniature EPSCs was 10 pA, since recordings obtained in zero extracellular Mg2+ were usually quite noisy. Number of cells is indicated as ‘n’, while the analysis of individual SICs is expressed as ‘n of SICs’.
Dye loading in spinal cord slices and calcium imaging
Transverse spinal cord slices were prepared from P10–P14 Wistar rats and loaded with Oregon Green BAPTA 1-AM (excited at 488 nm) as previously described (Aimar et al. 1998; Fellin et al. 2004). A Leica TCS SP2 RS laser scanner microscope (Leica, Mannheim, Germany) was used for calcium imaging in neurons and astrocytes as described previously (Fellin et al. 2006a). Slices were continuously perfused with the same solution that was used in electrophysiological recording with sulfinpyrazone (0.2 mm) that by inhibiting organic anion transporters limits the secretion of the fluorescence indicator in its free acid form. Laser emission at 488 nm was used for excitation of Oregon Green BAPTA. Experiments were performed at room temperature. Cells in the focal plane 10–30 μm beneath the surface of the slice were monitored. Time frame acquisitions from 0.5 to 2 s (with 2–8 line averaging) were used. No background subtraction or other manipulations were applied to digitized Ca2+ signal images that are reported as raw data, with the exception of the difference image in Fig. 4B, which was obtained by subtracting the prestimulation image from the poststimulation image. To activate Ca2+ elevations in astrocytes, we used the same stimuli that in patch-clamp experiments triggered SICs. The maximal extension of the neuronal domain that displayed a synchronized response was estimated as the distance between the centres of the somata of the two neurons positioned at the borders of the responsive domain. Neurons and astrocytes were distinguished on the basis of the distinct kinetics of their Ca2+ response to stimulation with a high K+ extracellular solution (40 mm) obtained by isosmotic replacement of Na+ with K+ (Pasti et al. 1997), applied at the end of the recording session. While cultured astrocytes were found to express voltage-dependent Ca2+ channels (MacVicar, 1984; Corvalan et al. 1990), no unambiguous evidence exists for their functional presence in astrocytes under in situ physiological conditions in either the hippocampus or spinal cord (Carmignoto et al. 1998). Accordingly, as in previous studies in hippocampal slices (Pasti et al. 1997; Fellin et al. 2006a), in spinal cord slices the response of neurons to a high K+ extracellular stimulation protocol is characterized by an early Ca2+ rise with fast kinetics (mean rise time, 2.80 ± 0.29 s) due to voltage-dependent Ca2+ channel opening, while that of astrocytes is characterized by a delayed Ca2+ response with slower kinetics (mean rise time, 12.28 ± 0.81 s). The rise time was calculated using the 20–80% criterion.
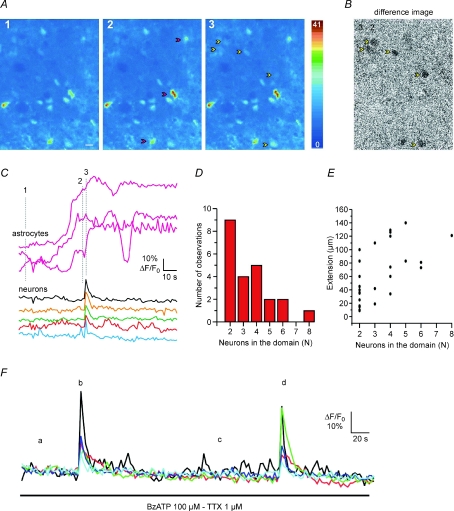
Figure 4. Ca2+ elevation in astrocytes triggers domain Ca2+ responses in lamina II neurons of spinal cord slices.
A, sequence of dorsal horn pseudocolour images after incubation with Oregon Green BAPTA 1-AM illustrating the Ca2+ signal at rest (1) and during BzATP application (2 and 3) in constant presence of TTX. The BzATP-induced Ca2+ increase in astrocytes (2, red arrowheads) is followed by a domain response in five neurons (3, yellow arrowheads). B, difference image obtained by subtracting the image of the neuronal domain Ca2+ response (3) from the image captured before the domain response (2). C, time course of the BzATP-mediated Ca2+ signal change in three astrocytes (labelled in panel A2) and the following highly synchronous Ca2+ elevation in five neurons belonging to the domain response. Numbers 1, 2 and 3 refer to the corresponding panels in A. D, histogram showing the number of neurons composing a domain response following stimulation with BzATP, PGE2 or low Ca2+ (n= 14). E, maximal spatial extension of domains in relation to the number of neurons in the domain. F, time course of the BzATP-mediated Ca2+ signal change in five neurons: BzATP evokes two successive, similar domain responses from the same neurons (b and d).
The astrocyte response is consistent with metabotropic receptor activation by neurotrasmitters released by depolarized neuronal terminals.
Behavioural nociceptive and inflammation tests on inflamed animals
Postnatal rats (P13–P14, weight: 30–35 g) were randomly divided into groups of five to six animals. Zymosan A (from Saccharomyces cerevisiae, 30 μl of a suspension of 35–40 mg ml−1) or saline (same volume) were subcutaneously injected into the plantar surface of the right paw. Six hours after the injection the animals were subjected to behavioural tests (thermal plantar or von Frey filament tests) or to electrophysiology experiments (as described above).
In particular, paw volume was determined by plethysmometry (Ugo Basile, Comerio, Italy) before the injection of zymosan or saline and 6 h afterwards, immediately before the behavioural tests. The plethysmometer allows measurement of the volume displacement (expressed in μl) produced by dipping the rat paw in a water-filled cell. The extent of oedema was expressed as the percentage increase of the right paw volume in the saline-injected and zymosan-treated groups.
Thermal hyperalgesia was assessed using the method of Hargreaves et al. (1988). The Paw Withdrawal Algesimeter (Plantar test, Ugo Basile, Comerio, Italy) was used to measure thermal nociceptive threshold. Radiant heat was applied to the plantar surface of each hind paw and the withdrawal latency was measured (mean paw withdrawal latency was determined by performing three measurements on each hind paw at 5 min interval, after a 15 min habituation period). The intensity of the light beam was calibrated so that baseline latencies were 6–8 s in naive rats. The cut-off value was determined as 20 s in order to avoid tissue damage. Thresholds of each rat were defined as the mean of the values from the last three stable threshold responses.
The von Frey filament test was performed by placing the animals in a transparent plastic chamber upon a perforated metal sheet allowing the application of mechanical stimuli to the hind paw from below. Testing began after an adaptation period of 15 min. A mechanical stimulus measured with a series of calibrated von Frey hairs of ascending forces (0.08 to 8 g) was applied to the fat part of the heel. Von Frey hairs were indented on the plantar surface until they bent 5 times at a frequency of about 2 s−1. At threshold, the rat responded by withdrawal. The pain threshold was determined as the lower force that evoked a withdrawal response to three of the five stimuli. Animals were tested sequentially on the inflamed and control paw until threshold was reached.
Data analysis and statistics
Patch-clamp data were analyzed by using the pClamp software (Clampfit, Molecular devices, Sunnyvale, CA, USA). Miniature EPSCs were analyzed with Mini Analysis Program (Synaptosoft, Decatur, GA, USA). Statistic analysis was performed by using Sigmaplot 11 (SSI, San Jose, CA, USA) or Graphpad software (GraphPad Software, La Jolla, CA, USA). The specific statistic tests used are indicated in the text or in the figure legends. Data are expressed as means ±s.e.m.
Drugs
2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP), kynurenic acid and Zymosan A were from Sigma (Milan, Italy); tetrodotoxin (TTX) and d-AP5 (or D-APV) were from Ascent Scientific (Weston-Super-Mare, UK); 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX) and (RS)-3,5-dihydroxyphenylglycine (DHPG) were from Tocris Bioscience (Bristol, UK).
Results
Astrocyte stimulation triggers NMDAR-mediated slow inward currents in lamina II neurons
Patch-clamp recordings from spinal cord acute slice neurons, in the virtual absence of extracellular Mg2+ and in the presence of TTX (1 μm), revealed slow inward currents (SICs) occurring both spontaneously, in a low percentage of neurons (14/112 cells, 12.5%), and in the presence of astrocyte activating stimuli (see below). Compared to miniature excitatory postsynaptic currents (mEPSCs), spontaneous SICs exhibited a larger peak amplitude (−80.3 ± 12.8 pA versus 19.6 ± 2.04 pA) and a slower rise time (83.5 ± 16.1 ms vs. 0.7 ± 0.1 ms) (see Table 1 for number of analysed SICs). The SIC decay phase was usually fitted by a single exponential function (mean τdecay= 423.1 ± 65.9 ms), while mEPSC decay was usually interpolated by a double exponential function (τ1= 4.7 ± 0.5 ms; τ2= 89.3 ± 19.5 ms; A1/A1+A2= 0.67 ± 0.01, number of recorded cells = 9, Fig. 1A).
Table 1.
Analysis of peak amplitude and kinetics of SICs recorded under different experimental conditions
| Condition | Number of cells exhibiting SICs | Amplitude (pA) | Rise time 20–80% (ms) | Decay τ (ms) |
|---|---|---|---|---|
| Spontaneous (control animals) | 14/112 (12.5%) | −80.3 ± 12.8 | 83.5 ± 16.1 | 423.1 ± 65.9 |
| (n= 22) | (n= 21) | (n= 18) | ||
| −20 to −244.3 | 11 to 330 | 67–1059 | ||
| Spontaneous (inflamed animals) | 21/47 (44.7%) | −214.2 ± 113.6 | 54.4 ± 7 | 334.4 ± 64.7 |
| (n= 30) | (n= 27) | (n= 21) | ||
| −19 to −3470 | 8.6–160 | 65–1095 | ||
| BzATP | 15/37 (40.5%) | −78.1 ± 10.1 | 89.6 ± 23.3 | 341.9 ± 75.7 |
| (n= 21) | (n= 19) | (n= 19) | ||
| −25 to −191 | 10–362 | 78–1225 | ||
| Low Ca2+ | 12/20 (60%) | −179.7 ± 42.9 | 47.9 ± 6.6 | 364.7 ± 127 |
| (n= 32) | (n= 31) | (n= 28) | ||
| −31 to −1283 | 18.2–116.9 | 115–731.7 | ||
| Low Ca2++ NBQX | 15/27 (55.5%) | −126.3 ± 23.3 | 74.4 ± 13.4 | 394 ± 79.6 |
| (n= 28) | (n= 28) | (n= 22) | ||
| −30 to −500 | 12–288.4 | 85–1400 | ||
| Low Ca2++ 1 Mg2+ | 14/32 (43.7%) | −45.3 ± 7.5 | 91.4 ± 21.7 | 416.9 ± 94.6 |
| (n= 26) | (n= 23) | (n= 19) | ||
| −19 to −201 | 10–348 | 45–1261 |
SIC recordings were obtained from postnatal rats (P8–P14), in basal conditions (both in control and zymosan-treated animals) or in the presence of a stimulus. Data obtained in the first 5 conditions were acquired in the virtual absence of extracellular Mg2+ and in the presence of 1 μm TTX. Numbers and percentages of cells exhibiting at least one SIC during 5 min recording are reported for each experimental situation. The mean value ±s.e.m., the number of analysed SICs (n) and the range of values are indicated for each parameter (amplitude, rise time and decay τ). Decay τ values were determined by fitting the current decay phase with a single exponential function, as shown in Fig. 1. Note that the high value of SIC peak amplitude in inflamed animals is due to the presence of a single very large SIC (−3470 pA). The mean peak value of SICs in zymosan, obtained excluding this current, would be −102 ± 17.6 pA.
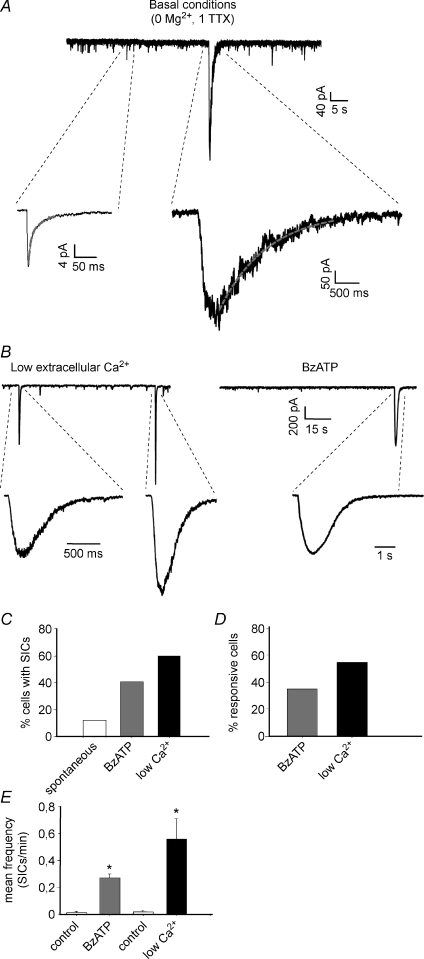
Figure 1. Slow inward currents (SICs) recorded from lamina II neurons in basal conditions and after astrocyte stimulation.
A, representative patch-clamp recordings showing several miniature EPSCs (mEPSCs), mediated by both AMPA and NMDA receptors, and a single slow inward current. Insets: expanded mEPSC (left) and SIC (right). The SIC exhibits slower kinetics and larger amplitude than the miniature synaptic current. The decay phase of the mEPSC is interpolated by a double exponential function (red line), while the SIC is fitted by a single exponential. B, recordings of SICs from two different lamina II neurons, in ‘low extracellular Ca2+’ (left) and in the presence of the purinergic receptor agonist benzoylbenzoyl-ATP (BzATP, 100 μm, right). C, percentage of cells exhibiting at least one SIC in the different conditions, during 5 min recording. D, percentage of responsive cells, i.e. cells where the number of SICs during the stimulus is higher than in control. E, the application of BzATP or low extracellular Ca2+ causes a significant increase of SIC mean frequency in the responsive neurons (Wilcoxon's signed rank test, P < 0.01 for both groups; low Ca2+, n= 11; BzATP, n= 13).
These spontaneous SICs recorded from lamina II neurons are reminiscent of similar currents previously recorded in neurons from different brain regions that are mediated by glutamate released from astrocytes (Parri et al. 2001; Fellin et al. 2004; Angulo et al. 2004; D’Ascenzo et al. 2007). To confirm the involvement of glial cells in the generation of the slow currents in dorsal horn, we tested whether SICs could be triggered by stimuli that induce Ca2+ elevations in astrocytes (see below). Stimulation of Ca2+ signals in astrocytes can be achieved by activation of purinergic receptors that in the dorsal horn are widely expressed in these cells (Ho et al. 1995; Kobayashi et al. 2006). We thus first used BzATP, an agonist of purinergic ionotropic receptors, such as the P2X7, and metabotropic receptors, such as the P2Y1, that in spinal cord cultured astrocytes mediate Ca2+ elevations, propagating Ca2+ waves (Fam et al. 2000; Gallagher & Salter, 2003; Suadicani et al. 2006) and glutamate release (Zeng et al. 2009). In hippocampal slice preparations, BzATP application was also reported to induce Ca2+-dependent glutamate release in astrocytes and SICs in neurons (Fellin et al. 2006b).
We found that BzATP is an effective stimulus for evoking robust Ca2+ elevations in dorsal horn astrocytes. Following stimulation with 100 μm BzATP, we also detected SICs in 40.5% of the lamina II neurons, during 5 min recording (15/37; mean frequency of responsive cells, 0.27 ± 0.03SIC min−1 in BzATP vs. 0.01 ± 0.01 in control, n= 13, Fig. 1B–E).
As an additional stimulus for astrocytes we next used slice perfusion with a ‘low Ca2+’ extracellular solution. Low Ca2+ has been previously reported to evoke robust Ca2+ oscillations in astrocytes not only from the hippocampus and cortex (Zanotti & Charles, 1997; Parri et al. 2001), but also from the spinal cord (Suadicani et al. 2006). In our experiments, we found that exposure to low Ca2+ evoked Ca2+ elevations in dorsal horn astrocytes and SICs in neurons. The percentage of neurons exhibiting at least one SIC during the first 5 min of exposure to low Ca2+ increased from 10% (2/20) in control to 60% (12/20) in low Ca2+ and the mean SIC frequency increased significantly in the responsive cells (control: 0.02 ± 0.01 SIC min−1; low Ca2+: 0.56 ± 0.15, n= 11, Fig. 1B–E).
Peak amplitude, 20–80% rise time and the decay time constant were determined for the SICs recorded in basal conditions (spontaneous SICs), in low extracellular Ca2+ and during application of BzATP (Table 1). Kinetic parameters did not differ significantly in the three experimental conditions (Kruskal–Wallis test, P > 0.05). Peak amplitudes of SICs recorded in low Ca2+ were significantly larger than those obtained in basal conditions or in BzATP (Kruskal–Wallis test, P < 0.01, followed by Dunn's method of pairwise multiple comparison).
BzATP and low Ca2+ triggered SICs also in neurons located in deeper laminae (III–V) (data not shown). In older rats (P21–P28), stimulation of astrocytes by low Ca2+ induced SICs in 61.5% of the recorded lamina II neurons (n= 13) and the mean SIC frequency increased from 0 to 0.40 ± 0.07 in the responsive cells (n= 8). Similarly, application of 100 μm BzATP was effective in generating SICs also in older animals (percentage of neurons with SICs: 46.7%vs. 6.7% in control, n= 15). In the responsive neurons, mean SIC frequency increased from 0 in control to 0.30 ± 0.04 in BzATP (n= 7).
We then tested the effect of mGluR activation on SIC generation. Application of 15 μm DHPG in low Mg2+ and in the presence of TTX failed to evoke SICs in lamina II neurons from postnatal rats (n= 9).
SICs recorded from lamina II neurons are mediated by NMDARs
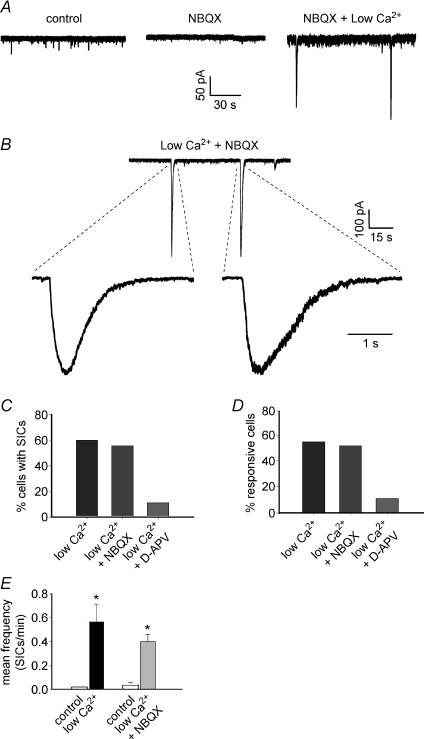
Studies performed in several areas of the brain showed that SICs are due to activation of NMDA receptors, mainly expressed at extrasynaptic sites (Fellin et al. 2004; D’Ascenzo et al. 2007). AMPA receptors do not appear to contribute significantly to the generation of SICs (Fellin et al. 2004), which is likely to be due to their rapid desensitization caused by the slowly increasing concentration of astrocytic glutamate in their vicinity. Consistent with these results, we observed that SICs from lamina II neurons were inhibited by the NMDA receptor antagonist d-AP5. The NMDA receptor antagonist was able to completely block SICs during BzATP application in all tested cells (n= 11), while only one SIC was observed during recording from nine cells in low Ca2+ in the presence of d-AP5 (Fig. 2C and D). The AMPA receptor antagonist NBQX had no effect on SICs (n= 27; Fig. 2). SICs recorded in low Ca2+ and in the presence of NBQX exhibited similar amplitudes and kinetics compared to the currents obtained in low Ca2+ only (Mann–Whitney U test, P > 0.05; Table 1).
Figure 2. SICs are mediated by NMDA receptors, while AMPA receptors are not involved.
A, effects of NBQX application (10 μm) on synaptic activity (mEPSCs) and appearance of SICs in a lamina II neuron. Control conditions are represented by zero extracellular Mg2+ and 1 μm TTX. Application of NBQX blocks the AMPA mediated mEPSCs, while two SICs are still apparent in low extracellular Ca2++ NBQX. B, examples of SICs recorded in low Ca2+ and NBQX from another lamina II neuron. Again, blocking non-NMDA receptors does not prevent the appearance of SICs. C and D, application of NBQX in low Ca2+ does not alter the percentage of cells with SICs or the percentage of responsive neurons, while addition of 100 μm d-AP5 inhibits almost completely the appearance of SICs (only 1 SIC observed in 9 recorded neurons). E, a significant increase of SIC frequency during low Ca2+ can be observed also in the presence of NBQX (paired t test, P < 0.01 and n= 14). Frequencies of SICs in low Ca2+ and low Ca2++ NBQX are not significantly different (Mann–Whitney U test, P > 0.05).
SICs recorded from older animals (P20–P28), both in basal conditions and in low extracellular Ca2+, were also mediated by NMDA receptors since they were completely abolished by d-AP5 (n= 8).
We next investigated in voltage-clamped neurons whether BzATP may directly affect neuronal membrane excitability. Experiments were performed in the presence of d-AP5 and TTX. Results obtained revealed that BzATP failed to affect the holding current and also failed to trigger transient currents that could resemble SICs. Analysis of spontaneous neuronal activity only revealed that in six of eleven neurons BzATP resulted in a significant decrease in the frequency, and not in the amplitude of mEPSCs (Supplementary Fig. 1).
In other CNS regions SICs appear to be mediated by NMDA receptors expressing the NR2B subunit since they are inhibited by the specific NR2 antagonist ifenprodil. In dorsal horn, this subunit is expressed during development and in the adult animals, both at the synaptic and extrasynaptic site (Monyer et al. 1994; Momiyama, 2000; Nie & Weng, 2009.) We thus tested whether the NR2B subunit is responsible for SICs in spinal cord dorsal horn. Ifenprodil (10 μm) applied to slices from young rats at postnatal day P21 to P28, differently from what was observed in the brain, failed to affect SICs. The frequency of SICs was 0.40 ± 0.07 in control (n= 8) and 0.46 ± 0.12 in ifenprodil (n= 6, Student's t test, P > 0.05), while SIC mean amplitude was −169 ± 53 pA in control (n of SICs = 17) and −106 ± 22.6 in ifenprodil (n of SICs = 14, Mann–Whitney U test, P > 0.05).
SICs are still present at physiological Mg2+ concentrations
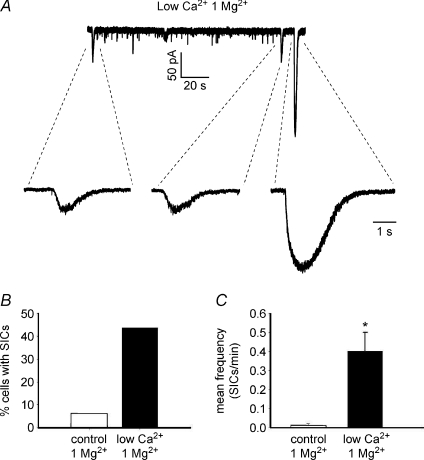
As previously observed in different brain regions (Parri et al. 2001; Fellin et al. 2004), the ability of astrocytic glutamate to activate NMDAR-mediated SICs in neurons is preserved at physiological concentrations of extracellular Mg2+. In 1 mm Mg2+ and 1 μm TTX, only two out of 32 cells exhibited SICs during 5 min recordings, but upon low Ca2+ stimulation both the number of neurons exhibiting SICs (14 of 32) and SIC frequency (control, 0.01 ± 0.01; low Ca2+, 0.40 ± 0.10, n= 14) were significantly increased (Fig. 3). However, by contrast with 0 Mg2+ conditions that favour the activation of the NMDA receptor, SICs were of similar kinetics, but significantly lower amplitude (Mann–Whitney U test, P > 0.05 for rise and decay times, P < 0.01 for peak amplitudes; see also Table 1) and they were observed in a reduced percentage of neurons (43.7%, n= 32 vs. 60%, n= 20, Fig. 3).
Figure 3. SICs can be observed also in the presence of extracellular magnesium.
A, recording of SICs from a lamina II neuron, during low extracellular Ca2+ and in the presence of 1 mm Mg2+. Three SICs can be observed in these conditions. B and C, lowering Ca2+ concentration in 1 mm Mg2+ still causes an increase in the percentage of cells exhibiting at least one SIC in 5 min recording (B) and a significant enhancement of SIC frequency (C, Wilcoxon's signed rank test, P < 0.01, n= 13).
Astrocytic glutamate triggers synchronized Ca2+ elevations in multiple neurons
We previously found that Ca2+ elevations in astrocytes triggered by different stimuli, including low Ca2+, were followed by simultaneous Ca2+ elevations in small groups of contiguous neurons, which we termed a domain response (Fellin et al. 2004). This domain response reflects a synchronous activation by astrocytic glutamate of the NMDARs in these neurons (Fellin et al. 2004). We asked whether astrocytic glutamate evokes similar domain responses in dorsal horn neurons. As in hippocampal slices (Pasti et al. 1997), cells loaded with a Ca2+ dye in spinal cord slices could be classified as neurons or astrocytes according to the different delay of the Ca2+ change triggered in these two cells by high K+ stimulation (see Methods). As illustrated in Fig. 4, the Ca2+ elevation evoked by BzATP in three astrocytes (Fig. 4A2, red arrows and Fig. 4C) was followed by a synchronous Ca2+ elevation from five spinal neurons (Fig. 4A3, yellow arrows and Fig. 4C). This domain response is evident in Movie 1 (see Supplementary Movie 1) and it is also clearly illustrated by the difference image generated by subtracting the image captured immediately before from that in which a synchronous Ca2+ elevation was detected in neurons (Fig. 4B). Ca2+ elevations triggered in astrocytes by different stimuli, such as BzATP (n= 5), PGE2 (n= 3) and low Ca2+ (n= 6), could evoke domain responses. The same individual neurons within the same domain show repetitive responses. As illustrated in Fig. 4F, astrocyte stimulation with BzATP evoked two successive domain responses from the same five neurons. Results from 14 experiments revealed that the majority of domains are composed of two to four neurons, although domains comprising a higher number of neurons were also present (Fig. 4D). The determination of the maximal spatial extent of the domain in relation to the number of neurons composing the domain revealed that two or three non-contiguous neurons located up to a distance of about 100 μm can display synchronized responses (Fig. 4E). The domain responses activated by astrocyte stimulation with BzATP were abolished by slice perfusion with d-AP5 and recovered after d-AP5 washout (Supplementary Fig. 2), confirming the distinct role of the NMDAR in astrocyte-mediated neuronal synchronies (n= 3). In the presence of d-AP5, Ca2+ elevations evoked by BzATP or low Ca2+ in astrocytes were unchanged.
Similarly to what was observed in the patch-clamp experiments, DHPG (15–50 μm) failed to induce neuronal domains in lamina II (n= 7), although it induced Ca2+ elevations in 60.61% of dorsal horn astrocytes (40 of 66, 7 experiments).
Peripheral inflammation increases the frequency of spontaneous SICs in lamina II neurons
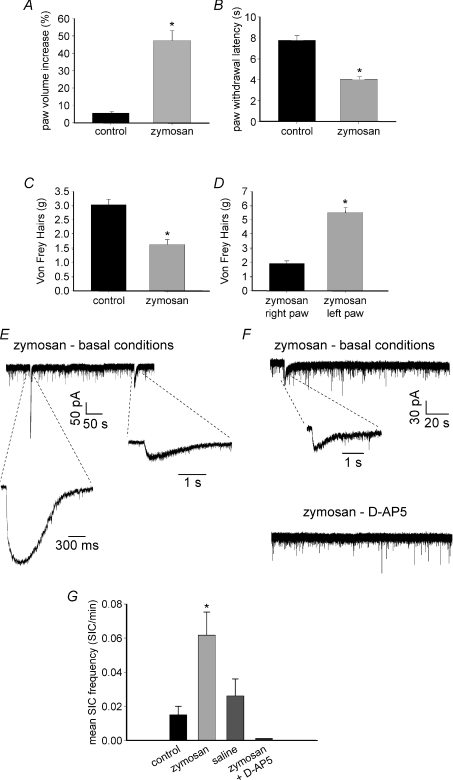
We next investigated possible changes in the astrocyte-to-neuron signalling during the development of peripheral inflammation. As an experimental model we used the intraplantar injection of an inflammatory agent, zymosan. Our behavioural tests indicated a full development of thermal hyperalgesia and mechanical allodynia 6–8 h after zymosan application (Fig. 5A–D). Patch-clamp recordings from lamina II neurons revealed that 6–8 h after zymosan application the frequency of spontaneous SICs was drastically increased. SICs were detected, during 10 min recording, in 14% of cells from non-treated animals, in 44.7% of neurons obtained from inflamed rats and in 19.5% of cells from rats injected with saline (n= 50, 47 and 46, respectively). All recordings were obtained in low Mg2+ and in the presence of 1 μm TTX. Slice perfusion with the NMDA receptor antagonist d-AP5 abolished SICs also in slices from inflamed animals (Fig. 5F and G). SICs recorded in zymosan-treated rats exhibited peak amplitudes and kinetic parameters that were not significantly different from those observed in control animals (Mann–Whitney U test, P > 0.05; Table 1).
Figure 5. Effects of intraplantar injection of zymosan on nociceptive behaviour and induction of SICs.
A–D, behavioural tests. A, zymosan (30 μl, 35–40 mg ml−1) causes a significant increase of paw volume (oedema), compared with control animals injected with the same volume of saline (Mann–Whitney U test, P < 0.05; mean baseline paw volume ranged from 281 to 301 μl in both groups). B, zymosan induces thermal hyperalgesia evaluated as a significant decrease of paw withdrawal latency to a thermal stimulus during the plantar test (Mann–Whitney U test, P < 0.01 for both sets of data). C, mechanical allodynia assessed using von Frey filaments on the right paw of animals receiving zymosan or saline (Mann–Whitney U test, P < 0.01). Zymosan causes a significant decrease of paw withdrawal threshold. D, mechanical allodynia tested by performing the von Frey test on the right (injected with zymosan) and left paw (non treated) of each animal. The injected paw exhibits a significant decrease of withdrawal threshold compared to the untreated one (Mann–Whitney U test, P < 0.01). Data are expressed as means ±s.e.m. of 5–6 rats for each group. E–G, patch-clamp recordings of SICs in lamina II neurons, obtained from non-treated rats (control) and animals injected with zymosan or saline. Recordings were performed at −60 mV in basal conditions (i.e. zero extracellular Mg2+ and 1 μm TTX). E, representative traces of SICs recorded from an inflamed animal, in basal conditions. F, traces recorded in a different lamina II neuron, from a treated animal, showing the presence of a spontaneous SIC in control, abolished by the subsequent application of 100 μm d-AP5. G, mean frequency of SICs determined from all cells obtained from the 3 animal groups. Zymosan causes a significant increase of SIC frequency compared to control and saline (P < 0.001 and P < 0.05, respectively), while no significant difference is observed between control and saline (Kruskal–Wallis test followed by Dunn's method for multiple comparisons). The application of 100 μm d-APV during recording from treated animals completely abolishes SICs (control: n= 50; zymosan: n= 47; saline: n= 46, zymosan +d-APV: n= 10).
Discussion
We here demonstrate that stimuli that evoke Ca2+ oscillations in astrocytes trigger NMDAR-mediated SICs and synchronous Ca2+ elevations in superficial dorsal horn neurons.
Properties of SICs recorded in spinal cord dorsal horn
The SICs that we recorded from lamina II neurons following Ca2+ elevations in astrocytes exhibit many properties that distinguish them from glutamate-dependent synaptic currents such as slow kinetics, resistance to TTX and sensitivity to NMDA receptor antagonists (Araque et al. 1998; Parri et al. 2001; Angulo et al. 2004; Fellin et al. 2004; Fellin et al. 2006b; Xu et al. 2007). SICs similar to the events that we described in lamina II neurons were evoked in different brain regions, such as the hippocampus (Fellin et al. 2004), the neocortex (Ding et al. 2007) and the nucleus accumbens (D’Ascenzo et al. 2007) by a selective stimulation of single astrocytes through photolysis of Ca2+-caged compounds. This observation, together with the sensitivity of these SICs to d-AP5 (Fellin et al. 2004; D’Ascenzo et al. 2007), provides evidence for a causal role of astrocytic glutamate in SIC generation.
Similarly to hippocampus, the kinetic parameters (rise and decay time constant) of dorsal horn SICs are much slower than those of synaptic currents. The slow kinetics suggests that SICs are mediated by glutamate slowly diffusing from astrocytes, while the variability is likely to depend on the distance and the geometrical arrangement of astrocytes around the recorded neurons.
The insensitivity of SICs to the non-NMDA receptor antagonist NBQX suggests that the AMPA receptor is not activated by astrocytic glutamate, probably due to the slow diffusion of astrocytic glutamate that may cause the desensitization of AMPA receptors on lamina II neurons. Consistent with this hypothesis, we previously found that in the presence of inhibitors of AMPA receptor desensitization, TTX and d-AP5, the stimulation of glutamate release in astrocytes evoked in hippocampal neurons AMPA receptor-mediated currents (Fellin et al. 2004).
The sensitivity to d-AP5 demonstrates that SICs in dorsal horn are mediated by NMDA receptors. In the brain, SICs are significantly inhibited also by the specific NR2B antagonist ifenprodil and are therefore mediated by the extrasynaptic, NR2B-containing NMDA receptor (Fellin et al. 2004; Jourdain et al. 2007). In spinal cord dorsal horn, ifenprodil was unable to produce a significant block of SICs, suggesting that NR subunits other than the NR2B contribute to these currents (Cull-Candy et al. 2001). Indeed, the NMDA receptor expressed by dorsal horn neurons is enriched by the NR2D subunit (Monyer et al. 1994; Tong et al. 2008), which could be preferentially located at the extrasynaptic site (Momiyama, 2000). The NR2D-containing NMDAR has a low Mg2+ sensitivity (Monyer et al. 1994) and has been suggested to play a specific role in the development of allodynia or hyperalgesia in different models (Hizue et al. 2005). The release of d-serine may also contribute to the low Mg2+ sensitivity of SICs, and it has been shown to be triggered in astrocytes by Ca2+ elevations (Mothet et al. 2005), and to control NMDA receptor activity (Panatier et al. 2006). Notably, the application of d-serine has been reported to potentiate NMDA mediated EPSCs in dorsal horn neurons from spinal cord slices (Siarey et al. 1991).
Stimuli for Ca2+-dependent gliotransmission in dorsal horn astrocytes
While considerable differences exist in the functional and morphological organization of the dorsal horn with respect to that of hippocampus, where the astrocytic origin of SICs has been first revealed, our results indicate that Ca2+ elevations in dorsal horn astrocytes can mediate a release of glutamate that activates preferentially NMDA receptors. Our study of dorsal horn neurons suggests that glutamate-dependent gliotransmission, although it may be differently regulated in different areas, may occur in many CNS regions.
Not all the stimuli that triggered Ca2+ elevations in astrocytes were effective. In our experiments, we found that BzATP, low Ca2+ and DHPG triggered similar Ca2+ elevations in dorsal horn astrocytes. However, while the first two stimuli were similarly effective, DHPG (which in astrocytes from different brain regions triggers glutamate release) failed to trigger glutamate release in these cells. Notably, in hippocampal astrocytes agonists of P2Y and PAR-1 receptors induced similar Ca2+ elevations in astrocytes, but only activation of the PAR-1 receptor leads to glutamate release and NMDA receptor-mediated SICs in neurons (Shigetomi et al. 2008). Apparently, the release of glutamate is evoked only when astrocytes are appropriately excited and this may occur upon activation of the specific signalling pathways that in a distinct region characterize neuron-to-astrocyte signalling. Ca2+ elevations may thus be necessary but not sufficient for activating glutamate release in astrocytes.
To stimulate dorsal horn astrocytes we first used BzATP, an unspecific purinergic receptor agonist that activates mainly the P2X7R, but also other P2XRs and P2YRs. Ca2+ elevations and Ca2+ waves can be, indeed, triggered in spinal cord astrocytes by ATP, mainly through activation of P2Y1 and -2 receptors (Salter & Hicks, 1994; Idestrup and Salter, 1998; Fam et al. 2000; Gallagher & Salter, 2003). In dorsal horn cultured cells, activation of astrocyte P2Y1 receptor has been recently shown to induce glutamate release from these cells and glutamate-mediated slow inward currents in neurons (Zeng et al. 2009). P2X7 receptors may also play an important role in propagating Ca2+ waves, particularly when Ca2+ elevations are evoked by a lowering of extracellular divalent cations (Suadicani et al. 2006).
In our study, BzATP triggered Ca2+ elevations in astrocytes and the release of glutamate that in neurons activated synchronous domain responses and SICs. In the presence of d-AP5 and TTX, BzATP still triggered Ca2+ elevations in astrocytes, but it failed to activate SICs and domain responses. Voltage-clamp experiments aiming to investigate possible direct effects of BzATP on the neuronal membrane also revealed that BzATP failed to affect the holding current in dorsal horn neurons, while it decreased spontaneous EPSC frequency in some neurons. These observations provide evidence that SICs and domain responses in neurons are not due to direct effects of BzATP on postsynaptic neuronal membrane. The BzATP effect on the frequency, but not the amplitude, of spontaneous events suggests that it may be mediated by activation of presynaptic receptors. P2X7 receptors have been, indeed, reported to be expressed at presynaptic levels in the dorsal horn (Deuchars et al. 2001; Nakatsuka & Gu, 2006).
To activate astrocytes we also used slice perfusion with a low Ca2+ solution. The reduction in external Ca2+ is recognized as a powerful stimulus that triggers massive Ca2+ signal transmission within the astrocytic network by promoting ATP release (Zanotti & Charles, 1997; Stout & Charles, 2003; Suadicani et al. 2006). An increase of connexin 43 hemichannel conductance and an enhancement of purinergic P2X7 receptor sensitivity to its endogenous ligand are mechanisms proposed to form the basis of the large pore formation in the astrocyte membrane that allows the efflux into the extracellular space of small molecules, such as ATP and also glutamate (Duan et al. 2003).
SICs recorded in different brain regions upon activation of astrocytes by different stimuli have similar mean amplitude. In contrast, SICs evoked in dorsal horn neurons by low Ca2+ are of larger amplitude than spontaneous and BzATP-evoked SICs. Given the high expression of the NR2D-containing NMDARs in the spinal cord, this difference might rely on the different NR2 subunit composition of the receptor that crucially determines distinct properties of the channel opening. Additional experiments are, however, required to analyse whether the NR2D-containing NMDARs is specifically affected by the reduction in the screening effect on the membrane surface charge caused by low Ca2+ (Frankenhaeuser & Hodgkin, 1957).
Functional effects of glutamate released from astrocytes
In the spinal cord, pathological pain is determined by elevated responsiveness and activity of dorsal horn neurons, a phenomenon termed ‘central sensitization’. High intensity and repetitive stimulation of nociceptive primary afferent fibres activates NMDA receptors on dorsal horn neurons, causing the increase of neuronal excitability and triggering Ca2+-dependent plasticity mechanisms (reviewed in D’Mello & Dickenson, 2008). Based on these considerations, the NMDA-mediated SICs and Ca2+ elevations triggered in dorsal horn neurons by glutamate released from astrocytes could play an important role in the induction and maintenance of pain sensitization at the spinal cord level. It is worth emphasizing that in neuropathic pain, groups of dorsal horn neurons exhibit spontaneous synchronous discharges. This type of activity is believed to be responsible for the shooting pain sensation typical of this condition and for causing the spreading of the pain sensation beyond the innervation patterns of peripheral nerves (Maleki et al. 2000; Schoffnegger et al. 2008). Our observation that glutamate released from dorsal horn astrocytes induces synchronous Ca2+ elevations in domains of neurons hints at a possible contribution of astrocyte-to-neuron signalling in these events. In support of such a role for astrocytes, gap junctional communication inhibitors, which deeply affect excitability in the astrocyte network, blocked the spread of pain in animal models (Watkins & Maier, 2003).
Gliotransmission may thus form the basis of the proposed role of astrocytes in inflammatory and neuropathic pain. However, to fully characterize this role, in vivo conditional studies deriving from modern genetic approaches are probably required. Although in their infancy, these studies have already revealed pivotal roles of astrocytes in the regulation of neuronal excitability and behaviour, such as sleep homeostasis (Halassa et al. 2009).
Our results are consistent with a large number of studies from different brain regions showing that gliotransmission, mediated by glutamate, d-serine or ATP/adenosine, affects important neuronal functions (Haydon & Carmignoto, 2006). The physiological role and even the existence of gliotransmission have been, however, questioned following results obtained in transgenic mice that express selectively in astrocytes an exogenous G protein-coupled receptor (MrgA1) linked to intracellular Ca2+ mobilization. In hippocampal slices from this mouse, Ca2+ elevations evoked in astrocytes by a specific MrgA1 receptor ligand failed to evoke SICs in CA1 neurons (Fiacco et al. 2007). The possibility that Ca2+ elevations are necessary but not sufficient to trigger glutamate release and the absence in this exogenous receptor of the appropriate coupling to the intracellular signalling cascades that in astrocytes mediate this release are plausible explanations for this negative result.
In the present study, we also reported that a peripheral inflammation developing following an intraplantar injection of zymosan is accompanied by an increased frequency of spontaneous SICs in lamina II neurons. As previously reported, and confirmed by our behavioural tests, this agent is able to generate, on a rapid time scale, peripheral inflammation and oedema. At the spinal cord level, zymosan has a number of effects, including the increase of COX-2 expression, the release of PGE2 and the alteration of neuronal firing thresholds (Meller & Gebhart, 1997; Randich et al. 1997; Maihöfner et al. 2000). More relevant to this study, zymosan induces a significant activation of both microglia and astrocytes in dorsal horn, as revealed by an increase of immunoreactivity to the microglia and astrocyte markers OX-42 and GFAP (Sweitzer et al. 1999; Clark et al. 2007). Our finding is consistent with these observations and suggests that peripheral inflammation results in a potentiation of glutamate release from astrocytes, as revealed by the increased number of SICs in lamina II neurons. Several mediators could be involved in the potentiation of astrocyte-to-neuron signalling, such as ATP, glutamate, substance P, cytokines and PGE2.
It is important to note that most experiments described in this paper, including those regarding inflammation, have been performed on postnatal rats, while studies investigating the role of glia in inflammatory pain have been predominantly obtained from adult animals. It is known, however, that hyperalgesia and dorsal horn sensitization can be induced by several inflammatory agents also in neonates, although with different patterns and magnitude than in adults (Fitzgerald, 2005). Our data indicate that SICs and Ca2+ elevations triggered in dorsal horn neurons by astrocytic glutamate could contribute to pain sensitization starting from an early age. Furthermore, the observation that SICs are still inducible in older animals suggests that this mechanism could play an important role also in the mature spinal cord.
In conclusion, our work describes a novel form of interaction between astrocytes and neurons in the spinal cord dorsal horn mediated by glutamate. While further studies are required to define the conditions and modalities that activate this astrocyte-to-neuron signalling, our results hint at a contribution of this signalling pathway in the control of pain transmission in the spinal cord.
Acknowledgments
This study was supported by funding from the Italian MIUR, the Human Frontiers of Science Project Organization (Grant team RGP0013/2004), the European Community 7th Framework Program (NeuroGlia, HEALTH-F2-2007-202167) and Fondazione Cariparo, Progetti di Eccellenza.
Glossary
Abbreviations
- GFAP
glial acidic fibrillary protein
- mEPSC
miniature excitatory postsynaptic current
- SIC
slow inward current
Author contributions
R.B. and G.C. contributed to the design of the study, performed experiments, analysed and interpreted data, and wrote the manuscript. A.G, M.Z and C.B. performed experiments and analysed data. G.V., V.R. and M.S. contributed to the design of behavioural experiments and to the interpretation of the data. All authors contributed to the drafting and revision of the manuscript content and gave their final approval of the version to be published. Experiments were carried out at the University of Modena and Reggio Emilia (Department of Biomedical Sciences) and at the University of Padova (Department of Experimental Biomedical Sciences), Italy.
Supplemental material
SUPPLEMENTARY FIG.1
SUPPLEMENTARY FIG.2
Supplementary Movie 1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Ahmed Z, Lewis CA, Faber DS. Glutamate stimulates release of Ca2+ from internal stores in astroglia. Brain Research. 1990;516:165–169. doi: 10.1016/0006-8993(90)90914-w. [DOI] [PubMed] [Google Scholar]
- Aimar P, Pasti L, Carmignoto G, Merighi A. Nitric oxide-producing islet cells modulate the release of sensory neuropeptides in the rat substantia gelatinosa. J Neurosci. 1998;18:10375–10388. doi: 10.1523/JNEUROSCI.18-24-10375.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Pasti L, Pozzan T. On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci. 1998;18:4637–4645. doi: 10.1523/JNEUROSCI.18-12-04637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Corvalan V, Cole R, De Vellis J, Hagiwara S. Neuronal modulation of calcium channel activity in cultured rat astrocytes. Proc Natl Acad Sci U S A. 1990;87:4345– 4348. doi: 10.1073/pnas.87.11.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J Neurosci. 2007;27:10674–10684. doi: 10.1523/JNEUROSCI.2001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Salter MW. P2Y1 purinoceptor-mediated Ca2+ signalling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J Neurosci. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G, Haydon PG. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J Neurosci. 2006a;26:9312–9322. doi: 10.1523/JNEUROSCI.2836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem. 2006b;281:4274–4284. doi: 10.1074/jbc.M510679200. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24:722–732. doi: 10.1523/JNEUROSCI.2859-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B, Hodgkin AL. The action of calcium on the electrical properties of squid axons. J Physiol. 1957;137:218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CJ, Salter MW. Differential properties of astrocyte calcium waves mediated by P2Y1 and P2Y2 receptors. J Neurosci. 2003;23:6728–6739. doi: 10.1523/JNEUROSCI.23-17-06728.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565:1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hizue M, Pang CH, Yokoyama M. Involvement of N-methyl-D-aspartate-type glutamate receptor epsilon1 and epsilon4 subunits in tonic inflammatory pain and neuropathic pain. Neuroreport. 2005;16:1667–1670. doi: 10.1097/01.wnr.0000183328.05994.9e. [DOI] [PubMed] [Google Scholar]
- Ho C, Hicks J, Salter MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idestrup CP, Salter MW. P2Y and P2U receptors differentially release intracellular Ca2+ via the phospholipase c/inositol 1,4,5-triphosphate pathway in astrocytes from the dorsal spinal cord. Neuroscience. 1998;86:913–923. doi: 10.1016/s0306-4522(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Kulik A, Haentzsch A, Luckermann M, Reichelt W, Ballanyi K. Neuron-glia signalling via α1 adrenoceptor-mediated Ca2+ release in Bergmann glial cells in situ. J Neurosci. 1999;19:8401–8408. doi: 10.1523/JNEUROSCI.19-19-08401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA. Voltage-dependent calcium channels in glial cells. Science. 1984;226:1345–1347. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Tegeder I, Euchenhofer C, deWitt D, Brune K, Bang R, Neuhuber W, Geisslinger G. Localization and regulation of cyclo-oxygenase-1 and -2 and neuronal nitric oxide synthase in mouse spinal cord. Neuroscience. 2000;101:1093–1108. doi: 10.1016/s0306-4522(00)00361-4. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy) Pain. 2000;88:259–266. doi: 10.1016/S0304-3959(00)00332-8. [DOI] [PubMed] [Google Scholar]
- Martin FC, Charles AC, Sanderson MJ, Merrill JE. Substance P stimulates IL-1 production by astrocytes via intracellular calcium. Brain Res. 1992;599:13–18. doi: 10.1016/0006-8993(92)90846-2. [DOI] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur J Pain. 1997;1:43–52. doi: 10.1016/s1090-3801(97)90052-5. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J Physiol. 2000;523:621–628. doi: 10.1111/j.1469-7793.2000.t01-1-00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Gu JG. P2X purinoceptors and sensory transmission. Pflugers Arch. 2006;452:598–607. doi: 10.1007/s00424-006-0057-6. [DOI] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Weng HR. Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn. J Neurophysiol. 2009;101:2041–2051. doi: 10.1152/jn.91138.2008. [DOI] [PubMed] [Google Scholar]
- Palma C, Minghetti L, Astolfi M, Ambrosini E, Silberstein FC, Manzini S, Levi G, Aloisi F. Functional characterization of substance P receptors on cultured human spinal cord astrocytes: synergism of substance P with cytokines in inducing interleukin-6 and prostaglandin E2 production. Glia. 1998;21:183–193. doi: 10.1002/(sici)1098-1136(199710)21:2<183::aid-glia2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet J-P, Toquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2009.11.007. in press. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Randich A, Meller ST, Gebhart GF. Responses of primary afferents and spinal dorsal horn neurons to thermal and mechanical stimuli before and during zymosan-induced inflammation of the rat hindpaw. Brain Res. 1997;772:135–148. doi: 10.1016/s0006-8993(97)00883-4. [DOI] [PubMed] [Google Scholar]
- Salter MW, Hicks JL. ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. J Neurosci. 1994;14:1563–1575. doi: 10.1523/JNEUROSCI.14-03-01563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffnegger D, Ruscheweyh R, Sandkuhler J. Spread of excitation across modality borders in spinal dorsal horn of neuropathic rats. Pain. 2008;135:300–310. doi: 10.1016/j.pain.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siarey RJ, Long SK, Evans RH. Potentiation of synaptic reflexes by D-serine in the rat spinal cord in vitro. Eur J Pharmacol. 1991;195:241–244. doi: 10.1016/0014-2999(91)90541-w. [DOI] [PubMed] [Google Scholar]
- Stout C, Charles A. Modulation of intercellular calcium signalling in astrocytes by extracellular calcium and magnesium. Glia. 2003;43:265–273. doi: 10.1002/glia.10257. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signalling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1β expression that correlates with pain behaviour in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Tong CK, Kaftan EJ, MacDermott AB. Functional identification of NR2 subunits contributing to NMDA receptors on substance P receptor-expressing dorsal horn neurons. Mol Pain. 2008;4:44. doi: 10.1186/1744-8069-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Werry EL, Liu GJ, Bennett MR. Glutamate-stimulated ATP release from spinal cord astrocytes is potentiated by substance P. J Neurochem. 2006;99:924–936. doi: 10.1111/j.1471-4159.2006.04133.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J. Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem. 2007;282:24185–24197. doi: 10.1074/jbc.M700452200. [DOI] [PubMed] [Google Scholar]
- Zanotti S, Charles A. Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signalling. J Neurochem. 1997;69:594–602. doi: 10.1046/j.1471-4159.1997.69020594.x. [DOI] [PubMed] [Google Scholar]
- Zeng JW, Liu XH, Zhao YD, Xiao Z, He WJ, Hu ZA, Ruan HZ. Role of P2Y1 receptor in astroglia-to-neuron signalling at dorsal spinal cord. J Neurosci Res. 2009;87:2667–2676. doi: 10.1002/jnr.22108. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–136. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.