Abstract
Vesicular stomatitis virus (VSV) has proven to be an effective vaccine vector for immunization against viral infection, but its potential to induce an immune response to a self-tumor antigen has not been investigated. We constructed a recombinant VSV expressing human dopachrome tautomerase (hDCT) and evaluated its immunogenicity in a murine melanoma model. Intranasal delivery of VSV-hDCT activated both CD4+ and CD8+ DCT-specific T-cell responses. The magnitude of these responses could be significantly increased by booster immunization with recombinant adenovirus (Ad)-hDCT, which led to enhanced efficacy against B16-F10 melanoma in both prophylactic and therapeutic settings. Notably, the interval of VSV/Ad heterologous vaccination could be shortened to as few as 4 days, making it a potential regimen to rapidly expand antigen-specific effector cells. Furthermore, VSV-hDCT could increase DCT-specific T-cell responses primed by Ad-hDCT, suggesting VSV is efficient for both priming and boosting of the immune response against a self-tumor antigen.
Introduction
The identification and molecular characterization of tumor-associated antigens (TAAs) have provided the basis for specific immunization against cancer.1,2 However, cancer cells are self-derived, so most endogenous TAAs are poorly immunogenic due to mechanisms governing self-tolerance.3 Potent immune responses to tumors can be induced by a strategy known as xenogeneic immunization, which utilizes homologous proteins derived from another species.4,5 Although the mechanism(s) through which xenoimmunization overcomes self-tolerance remain to be conclusively determined, this strategy may improve CD8+ T cells in a number of ways. For instance, heteroclitic CD8+ T-cell epitopes derived from xenoproteins can increase major histocompatibility complex (MHC)–binding affinity or T-cell receptor contact, leading to improved T-cell activities that are crossreactive to native proteins.4,5 Alternatively, our recent studies indicate that foreign epitopes present in the xenogeneic antigen can activate helper T cells leading to enhanced autoantigen-specific CD8+ T-cell immunity.6 Xenoantigens can be delivered as genetic materials using viral vectors that effectively target both MHC class I and class II processing pathways.7,8,9,10 Furthermore, by combining recombinant viral vectors in heterologous prime–boost regimens, the frequency of TAA-specific T cells can be increased.11,12,13 These studies have led to a continued effort to improve the immunogenicity of existing viral vectors and to identify new vectors and vector combinations.
Vesicular stomatitis virus (VSV) is a negative-strand RNA virus that primarily infects livestock and causes only a mild, biphasic infection in humans.14,15 Most humans are seronegative for VSV, so clinical application would not be impaired by pre-existing immunity. Moreover, the potential toxicity of the wild-type virus can be further attenuated through molecular manipulations.16,17 Together with its capacity to accommodate insertion of large foreign genes, VSV is an attractive vaccine vector candidate. Indeed, previous work has shown that vaccination with recombinant VSV vectors is highly effective in protecting against challenges with numerous viral pathogens.18,19,20,21,22 Furthermore, the potential of VSV as a recombinant cancer vaccine vector has recently been explored in animal models where antitumor T-cell responses can be induced when a foreign antigen is expressed by both the vector and the tumor.19,23 However, the potential of VSV expressing a native TAA, alone or in heterologous prime–boost strategies, for T-cell priming and cancer therapy has not yet been evaluated.
We have engineered a recombinant VSV to express human dopachrome tautomerase (VSV-hDCT) and evaluated the potential of this vector to induce T-cell responses against murine B16-F10 melanoma that expresses endogenous murine DCT. The VSV-hDCT induced T-cell responses to both MHC class I and II–associated DCT-specific epitopes, and these responses could be rapidly boosted with a previously characterized recombinant adenoviral vector expressing hDCT (Ad-hDCT) leading to improved prophylactic and therapeutic efficacy in an antimelanoma treatment regime.
Results
Construction of recombinant VSV vectors
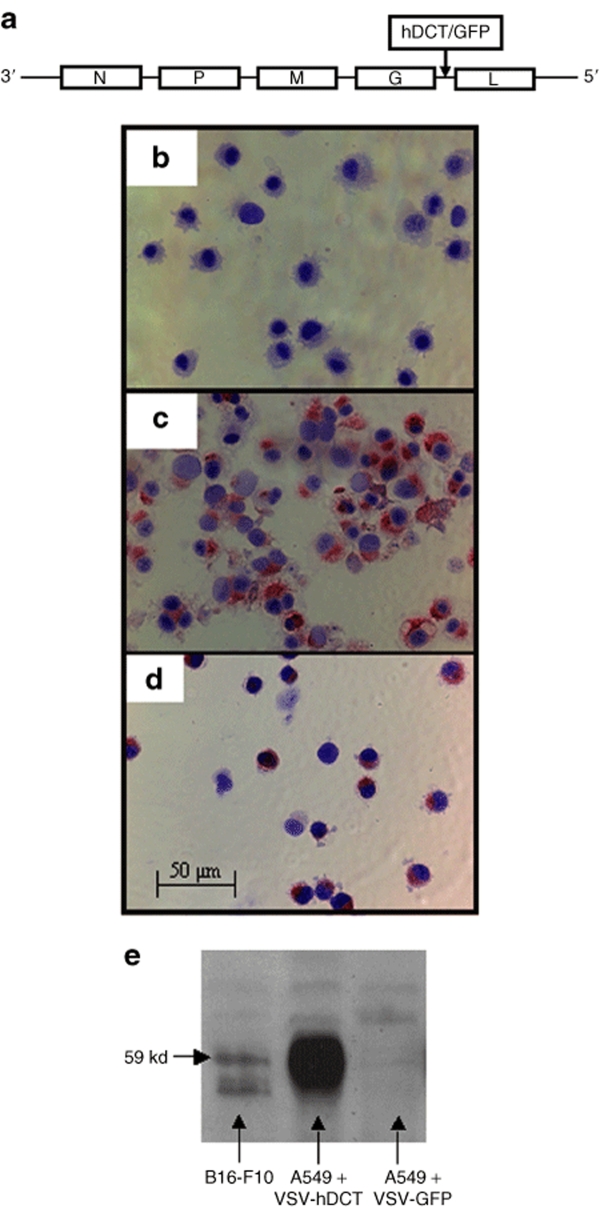
Recombinant VSV-hDCT and VSV carrying the transgene for green fluorescent protein (GFP) were constructed as outlined in Figure 1a. The characterization and application of VSV-GFP have been described previously.17 To confirm correct integration and expression of the hDCT gene in VSV-hDCT, MCA205 cells that do not express endogenous DCT were infected with VSV-GFP (Figure 1b) or VSV-hDCT (Figure 1c). Immunohistochemical staining using a DCT-specific antibody indicated robust expression of DCT protein in VSV-hDCT-infected MCA205 (Figure 1c) and endogenous DCT protein in murine B16-F10 cells (Figure 1d). Correct protein size was confirmed by western blotting (Figure 1e).
Figure 1.
Vector construction and expression of DCT in VSV-hDCT-infected tumor cells. (a) VSV expressing hDCT or GFP was made by inserting the full-length sequence for hDCT or GFP between the G and L genes of the recombinant VSV-XN genome (Indiana serotype). (b–d) The presence of DCT in cytospins was detected as red color by using the αPEP8h primary antibody in immunohistochemical staining. Cells were counterstained with Mayer's Hematoxylin, and images were captured at a magnification of ×200. (b) MCA205 fibrosarcoma cells that do not express DCT served as negative controls. (c) MCA205 cells infected with VSV-hDCT expressed DCT. (d) B16-F10 melanoma cells, a positive control, constitutively express endogenous murine DCT. (e) The αPEP8h antibody was also used for detection of DCT protein by western blotting of lysates from B16-F10 cells (positive control), A549 cells infected with VSV-hDCT, or A549 cells infected with VSV-GFP (negative control). GFP, green fluorescent protein; hDCT, human dopachrome tautomerase; VSV, vesicular stomatitis virus.
Tolerance of C57BL/6 mice to intranasal delivery of recombinant VSV
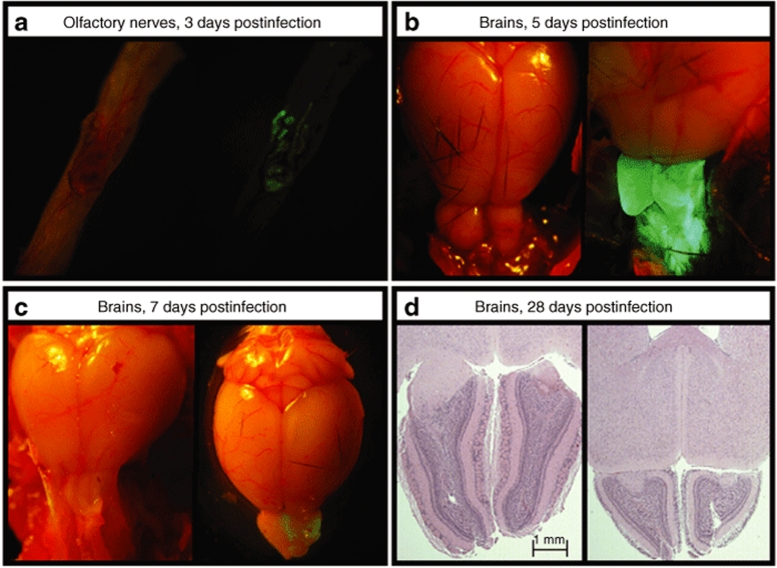
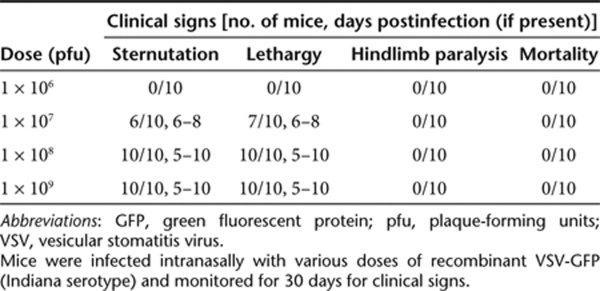
Intranasal administration of VSV has proven to be a well-tolerated and efficacious vaccination method in mice.24,25,26 Previous reports of the sensitivity of C57BL/6 mice to VSV infection have varied widely;27,28 therefore, we set out to evaluate the potential neurotoxicity of our recombinant VSV. We delivered various doses of recombinant VSV-GFP [106–109 plaque-forming units (pfu)] to C57BL/6 mice by intranasal instillation and assessed both systemic and local reactions for at least 30 days. Expression of GFP observed by fluorescence microscopy was limited to olfactory nerves at day 3 (Figure 2a) but extended throughout the olfactory bulbs by day 5 (Figure 2b). GFP expression decreased on day 7 (Figure 2c) and disappeared on day 10 (data not shown). The expression of GFP was not visualized beyond the olfactory bulb at any time point (Figure 2b) and was not observed at the lowest dose (1 × 106 pfu). Viral titration of infected brains with the olfactory bulbs removed did not yield any plaques at any infection dose, confirming that little or no VSV migrated into the brain hemispheres (data not shown). As summarized in Table 1, there were no mortalities or onset of hindlimb paralysis in mice at any dose. At doses ≥1 × 107 pfu, mice displayed mild flu-like signs (i.e., sternutation and lethargy) between 5 and 8 days postinfection. Histological analysis of mice receiving ≥1 × 108 pfu of VSV-GFP revealed evidence of olfactory bulb atrophy 28 days postinfection but not at days 7 or 14 (Figure 2d). Atrophy was not observed at lower doses nor was any lesion apparent in other parts of the brain at any dose (data not shown). On the basis of these findings, 1 × 107 pfu was deemed a safe dose for vaccination.
Figure 2.
Vesicular stomatitis virus (VSV) infection is limited to the olfactory bulbs following intranasal delivery. Mice received intranasal phosphate-buffered saline (PBS) or VSV-GFP in 10 µl PBS. Doses ranged from 1 × 106 to 1 × 109 plaque-forming units (pfu). Intact brains from mice euthanized 2, 5, 7, and 10 days postinfection were evaluated by fluorescence microscopy for evidence of GFP expression. Shown are representative images from mice treated with PBS (left panel) and 1 × 108 pfu VSV-GFP (right panel). (a) Olfactory nerves, 3 days postinfection. (b) Brains, 5 days postinfection. (c) Brains, 7 days postinfection. (d) Brains were harvested at 7, 14, and 28 days postinfection, fixed in formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin to look for signs of neurotoxicity. Representative sections from mice 28 days after receiving PBS (left panel) and 1 × 108 pfu VSV-GFP (right panel) are shown. GFP, green fluorescent protein.
Table 1.
Biosafety of recombinant VSV in C57BL/6 mice
Induction of both CD4+ and CD8+ T-cell responses by VSV-hDCT immunization
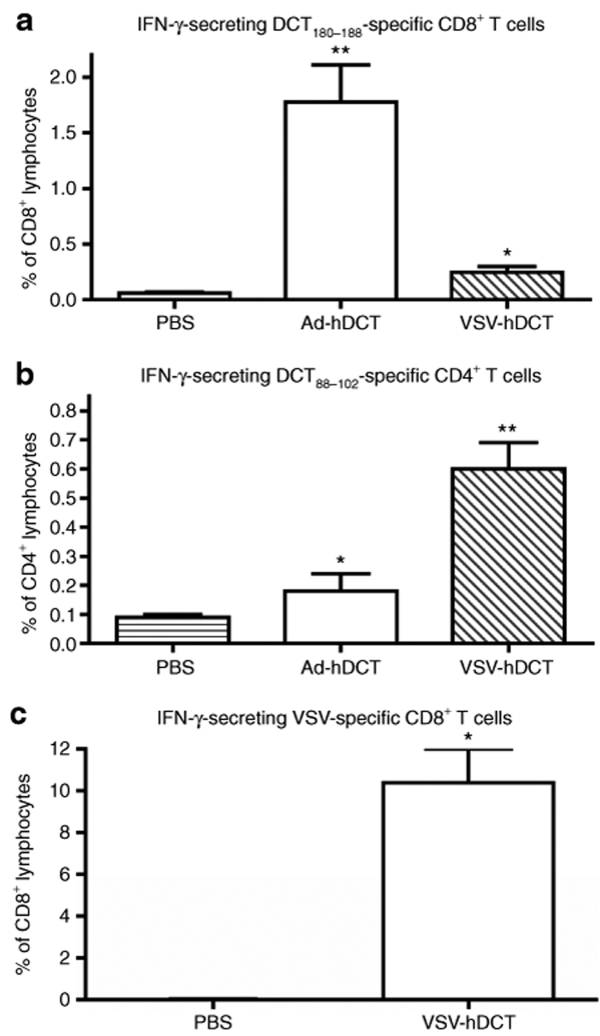
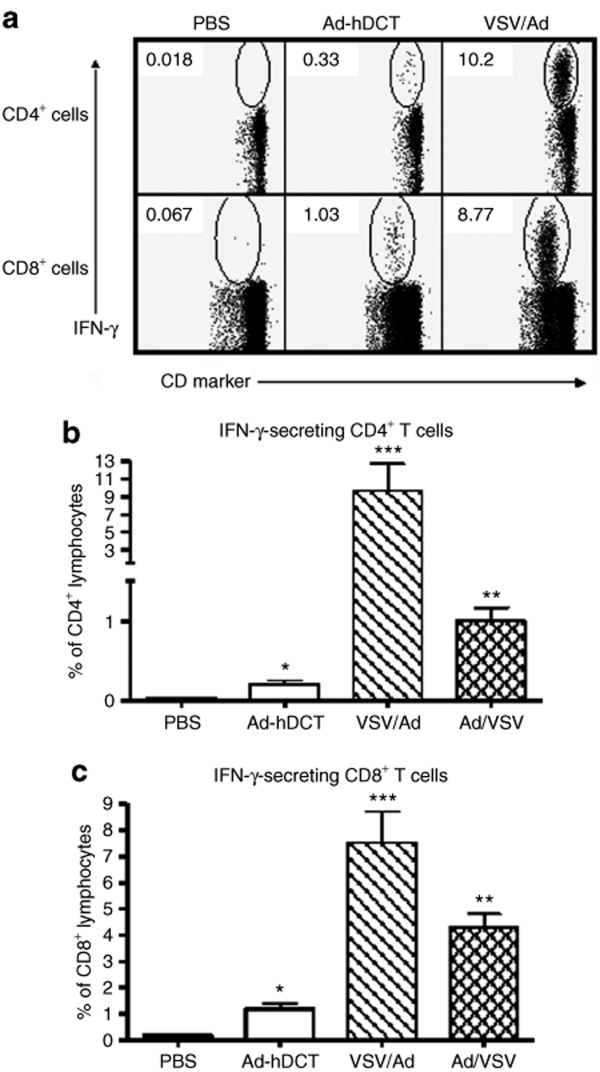
Using Ad-hDCT as a xenogeneic vaccine, we and others have shown that activated CD8+ T cells can recognize an immunodominant epitope, DCT180–188, shared between human and mouse that leads to protection against murine B16-F10 tumor challenge.6,29 Furthermore, our recent studies demonstrated that the potency of Ad-hDCT is due to a heteroclitic helper epitope (DCT88–102) that greatly enhances CD8+ T-cell responses.6 Using these two defined epitopes, we quantified the frequency of antigen-specific CD4+ and CD8+ T cells following vaccination with VSV-hDCT and compared its potency with the Ad-hDCT vaccine. Compared to phosphate-buffered saline (PBS) controls, significant antigen-specific interferon-γ-producing CD4+ and CD8+ T-cell responses were detected 7 days after VSV-hDCT immunization (Figure 3). Higher doses of VSV-hDCT did not enhance these T-cell responses (data not shown). Interestingly, Ad-hDCT induced a CD8+ T-cell response of 3.6 times greater magnitude than that of VSV-hDCT (P = 0.008; Figure 3a), whereas the CD4+ T-cell response to VSV-hDCT was 2.4 times greater than that following Ad-hDCT vaccination (P = 0.003; Figure 3b). Because there is a Kb-restricted immunodominant epitope from the N protein of VSV that may influence DCT-specific cytotoxic T lymphocyte (CTL) immunity, we also measured the VSV-specific CD8+ T-cell response. Indeed, the response to the immunodominant epitope of VSV was of much higher magnitude than the DCT-specific response 7 days following vaccination with VSV-hDCT (means of 10.0% versus 0.2%, respectively; Figure 3c versus 3a).
Figure 3.
VSV-hDCT immunization elicits antigen-specific CD4+ and CD8+ T-cell responses. C57BL/6 mice (n = 5/treatment) were immunized with 1 × 107 plaque-forming units (pfu) of VSV-hDCT. Negative and positive controls received PBS and 1 × 108 pfu Ad-hDCT, respectively. Seven days later DCT-specific, blood-derived (a) CD4+ and (b) CD8+ T cells and (c) VSV-specific CD8+ T cells were quantified by flow cytometry after in vitro peptide restimulation and intracellular cytokine staining. Data shown are means plus standard error bars. Asterisks denote significant differences compared to PBS control (P ≤ 0.05, by one-way analysis of variance). Ad, adenovirus; hDCT, human dopachrome tautomerase; IFN-γ, interferon-γ; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
Heterologous prime–boost vaccine dramatically increased the magnitude of the DCT-specific T-cell response
Because VSV-hDCT induced a robust CD4+ helper T-cell response and only a moderate CD8+ T-cell response against the transgene, we wondered whether priming the host with VSV-hDCT would allow a potential boost by Ad-hDCT. To test this possibility, we first evaluated sequential immunization with VSV-hDCT followed 14 days later by Ad-hDCT (VSV/Ad), a time point beyond the peak of primary CTL responses induced by VSV and other vectors. Indeed, examples of cytokine staining for interferon-γ shown in Figure 4a indicate that this heterologous vaccination approach induced a 46 times greater CD4+ and a 6 times higher CD8+ T-cell responses against DCT than the mean responses by Ad-hDCT immunization alone (Figure 4b,c). To determine whether enhanced T-cell responses were dependent on the order of immunization, we reversed the priming and boosting inoculation. Immunization with Ad-hDCT followed by VSV-hDCT (Ad/VSV) also increased the CD4+ and CD8+ T-cell responses to 5 and 4 times higher, respectively, than Ad-hDCT alone (P = 0.001 and 0.0002; Figure 4b,c). Our results suggest these two vectors in either order can achieve the prime–boost effect, though the VSV/Ad combination was superior (P = 0.02 for CD4 and P = 0.04 for CD8).
Figure 4.
Sequential immunization with VSV-hDCT and Ad-hDCT generates a strong prime–boost effect. C57BL/6 mice (n = 5/group) were treated as follows: (i) PBS on days 0 and 14 (PBS), (ii) PBS on day 0 and Ad-hDCT on day 14 (Ad-hDCT), (iii) VSV-hDCT on day 0 and Ad-hDCT on day 14 (VSV/Ad), and (iv) Ad-hDCT on day 0 and VSV-hDCT on day 14 (Ad/VSV). Blood sampled on day 21 was used to quantify T-cell responses by flow cytometry after in vitro peptide restimulation and intracellular cytokine staining. (a) Representative dot plots showing DCT-specific CD4+ (top row) and CD8+ (bottom row) T-cell responses in the PBS controls (first column), mice immunized with Ad-hDCT only (second column), and mice immunized with VSV-hDCT and subsequently boosted with Ad-hDCT (third column). A summary of CD4+ and CD8+ T-cell responses to DCT is shown in (b) and (c), respectively. Data shown are means plus standard error bars. Asterisks denote significant differences compared to PBS control (P ≤ 0.05, by one-way analysis of variance). Ad, adenovirus; hDCT, human dopachrome tautomerase; IFN-γ, interferon-γ; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
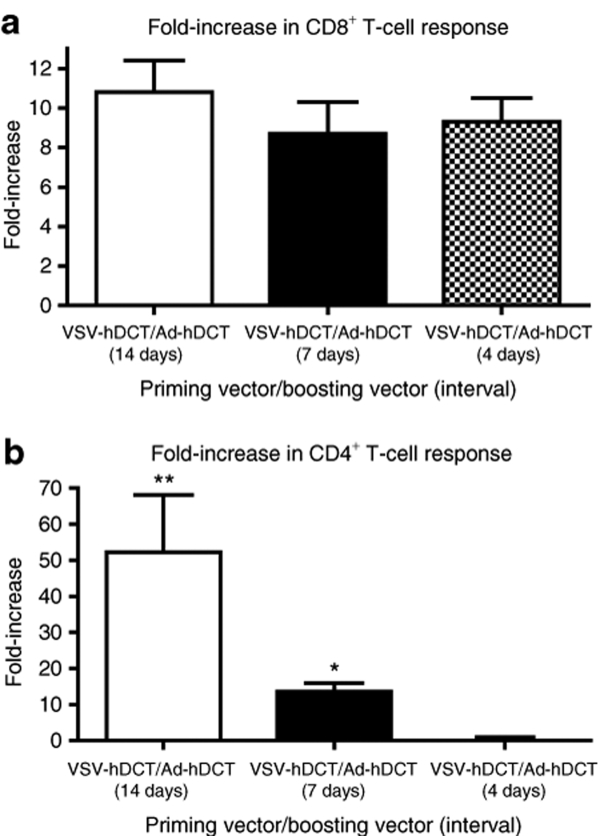
Rapid priming and boosting could be achieved without compromising the magnitude of the CD8+ T-cell response
Many viral vector–induced primary CTL responses peak at 7–12 days. This kinetic and the level of primary CTL response could affect the efficacy of an early boosting immunization.30,31 Considering the VSV-hDCT-primed CTL response was moderate and the success in boosting the response at day 14, we hypothesized that CD8+ T cells might be boosted even earlier by secondary immunization with Ad-hDCT. Consistent with our hypothesis, data in Figure 5 indicate that levels of CD8+ T-cell responses similar to those achieved with a 14-day boosting interval (Figure 4) could be obtained when this interval was shortened to 7 or even as few as 4 days (Figure 5a,b). However, enhancement of the CD4+ T-cell response was reduced at an interval of 7 days and completely diminished with a 4-day interval.
Figure 5.
Heterologous vaccination with VSV-hDCT, followed by Ad-hDCT allowed rapid priming and boosting of the CD8+ T-cell response. C57BL/6 mice (n = 5/group) received 1 × 107 plaque-forming units (pfu) of VSV-hDCT, followed 4, 7, or 14 days later by 1 × 108 pfu Ad-hDCT. Single-immunized controls received phosphate-buffered saline on day 0 and 1 × 108 pfu Ad-hDCT on day 14. On day 21, antigen-specific, blood-derived T cells were quantified by flow cytometry after in vitro peptide restimulation and intracellular cytokine staining. The mean fold-increase in percentage of (a) CD4+ and (b) CD8+ T cells relative to the single-immunized controls are shown. Asterisks denote significant differences (P ≤ 0.05, one-way analysis of variance). Ad, adenovirus; hDCT, human dopachrome tautomerase; VSV, vesicular stomatitis virus.
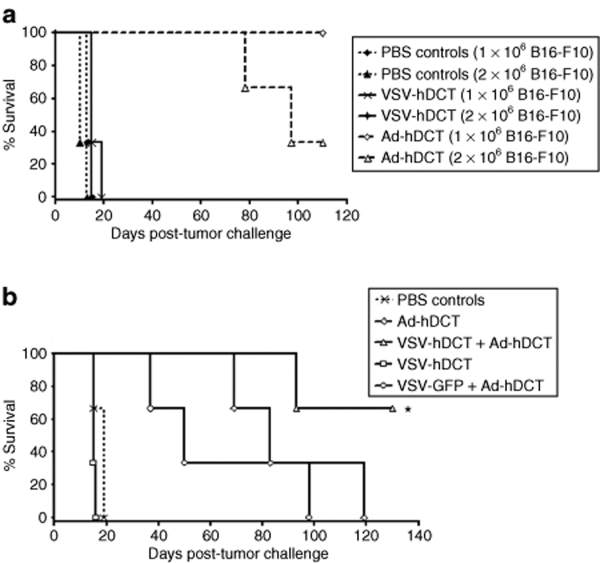
Heterologous prime–boost vaccination provided greater protection against tumor challenge than Ad-hDCT alone
We hypothesized that higher magnitude T-cell responses induced by heterologous prime–boost vaccination would confer better protection against tumor challenge than either vector alone. To test this, mice were immunized with VSV-hDCT, followed by Ad-hDCT, the sequence that yielded the highest DCT-specific T-cell responses. A 7-day interval was chosen because it represented the shortest window that maximized the CD8+ T-cell response without excessively compromising induction of CD4+ T cells. A shorter interval is also better to accommodate therapeutic models where the time frame for the treatment is often limited. We immunized mice with Ad-hDCT alone and then challenged with various numbers of B16-F10 cells (results for 1 × 104 and 1 × 105 cells are not shown) to determine the dose at which protection was lost. As shown in Figure 6a, Ad-hDCT vaccination can protect against a subcutaneous tumor challenge of up to 1 × 106 B16-F10 cells, confirming our previous results;8 sterile protection was lost at a dose of 2 × 106. Therefore, 2 × 106 cells were used to evaluate our prime–boost regime (Figure 6b). Consistent with their ability to activate CD8+ T cells, Ad-hDCT was more effective than VSV-hDCT as a single vaccine to confer protection against tumor growth (Figure 6a). When used together in a prime–boost strategy, however, VSV-hDCT priming enabled a booster immunization with Ad-hDCT, leading to complete protection in 70% of the mice. Such an extended survival was not seen in the control group that received VSV-GFP followed by Ad-hDCT, confirming that the efficacy of the VSV-hDCT/Ad-hDCT prime–boost regime was due to enhanced antigen-specific immunity.
Figure 6.
Enhanced survival of mice following heterologous vaccination and tumor challenge. (a) Single vaccination: C57BL/6 mice received PBS, 1 × 107 plaque-forming units (pfu) of VSV-hDCT or 1 × 108 pfu of Ad-hDCT on day 0. On day 7, they were challenged subcutaneously with 1 × 106 or 2 × 106 B16-F10 cells. (b) Prime–boost: mice received the following treatments on days 0 and 7, respectively. (i) PBS/PBS (PBS controls), (ii) PBS/Ad-hDCT (Ad-hDCT), (iii) PBS/VSV-hDCT (VSV-hDCT), (iv) VSV-hDCT/Ad-hDCT (VSV-hDCT + Ad-hDCT), and (v) VSV-GFP/Ad-hDCT (VSV-GFP + Ad-hDCT). On day 16, mice were challenged subcutaneously with 2 × 106 B16-F10 cells. End point was defined as a tumor volume >1,000 mm3. Data are representative of three independent experiments. Asterisks denote significant differences compared to Ad-hDCT alone or VSV-GFP + Ad-hDCT (P ≤ 0.0013). Ad, adenovirus; GFP, green fluorescent protein; hDCT, human dopachrome tautomerase; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
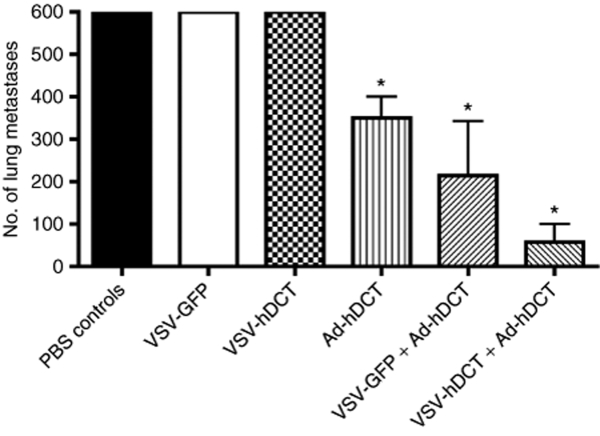
Enhanced therapeutic efficacy of heterologous prime–boost vaccination in a multifocal metastatic lung cancer model
To evaluate the potential therapeutic efficacy of the heterologous prime–boost vaccine, we employed a lung metastasis model. In this model, lung surface tumor nodules can be counted, giving a quantitative assessment of tumor burden. Mice were challenged by intravenous injection of 1 × 106 B16-F10 cells. After three days, mice were treated with VSV-GFP (a control for priming immunization) or VSV-hDCT. Seven days after VSV priming, mice were boosted with Ad-hDCT. Additional controls received PBS, Ad-hDCT, or VSV-hDCT alone. A comparable outgrowth of lung metastases (>600 tumor nodules per mouse) was observed in PBS-treated mice and those receiving a single VSV inoculation (Figure 7). In contrast, a significant reduction of metastatic nodules (<400, P < 0.05) was achieved by Ad-hDCT immunization. This therapeutic efficacy could be further enhanced by preimmunization with VSV-hDCT but not VSV-GFP (Figure 7, P < 0.01 versus PBS controls, P < 0.05 versus Ad-hDCT).
Figure 7.
Increased therapeutic efficacy of heterologous vaccination. To establish multifocal lung tumors, 1 × 106 B16-F10 cells were injected intravenously into C57BL/6 mice (n = 5/group) on day 0. Mice then received the following treatments on days 3 and 10, respectively. (i) PBS/PBS (PBS controls), (ii) PBS/VSV-GFP (VSV-GFP), (iii) PBS/VSV-hDCT (VSV-hDCT), (iv) PBS/Ad-hDCT (Ad-hDCT), (v) VSV-GFP/Ad-hDCT (VSV-GFP + Ad-hDCT), and (vi) VSV-hDCT/Ad-hDCT (VSV-hDCT + Ad-hDCT). On day 21, lungs were harvested and surface metastases enumerated. Data are representative of two independent experiments. Asterisks denote a significant difference compared to PBS control or VSV alone (P < 0.05). Ad, adenovirus; GFP, green fluorescent protein; hDCT, human dopachrome tautomerase; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
Discussion
We have evaluated the potency of VSV as a novel vaccine vector, on its own or in combination with a recombinant Ad vaccine, against a self-TAA. In vitro and in vivo infection with recombinant VSV resulted in high-level transgene expression. Intranasal immunization with VSV-hDCT elicited antigen-specific CD8+ and CD4+ T-cell responses that could be significantly increased by a booster immunization with Ad-hDCT. Mice immunized with VSV-hDCT, followed by Ad-hDCT boosting were effectively protected against subsequent tumor challenge or early established tumors. Interestingly, the interval of the VSV/Ad heterologous vaccination could be shortened to 4–7 days making it a potential regimen to rapidly expand antigen-specific effector cells. Furthermore, VSV-hDCT could increase CD4+ and CD8+ T-cell responses primed by Ad-hDCT suggesting the VSV vector is suitable for both priming and boosting of the immune response against a self-TAA.
Different from other reports,16,26 vaccination with VSV-hDCT as a single vector did not induce a robust CD8+ T-cell response against the transgene in our hands. It is likely that the coincidence of immunodominant epitopes from both the transgene (DCT180–188) and the virus (RGYVYQGL) that share the same Kb allele resulted in clonal competition of antitransgene and antiviral CD8+ T cells. The fact that VSV-hDCT-induced CD8+ T-cell responses to the RGYVYQGL peptide were almost two orders of magnitude higher than those observed against DCT, confirms VSV as a vaccine vector does not have an inherent defect for MHC class I peptide presentation. Interestingly, compared to Ad-hDCT, immunization with VSV-hDCT produced a higher magnitude CD4+ T-cell response, which may be explained by its cytopathic property. The VSV used in this study is replication competent and cytolytic; it is possible that the transgene protein released by VSV-infected cells could be reprocessed by host antigen-presenting cells leading to enhancement of MHC class II presentation.
The potency of heterologous prime–boost vaccines has been demonstrated in different disease models, though the underlying mechanisms remain to be fully understood.32 Both the magnitude and phenotype of activated primary CD8+ T cells have been suggested to affect the efficacy and interval of booster immunization, possibly because preactivated effector T cells can impair robust antigen presentation.33,34,35 This phenomenon has been interpreted as a negative feedback mechanism where recently activated CTL rapidly eliminate antigen-bearing dendritic cells and prevent naive or memory T cells from accessing the boosting antigen.36 Because the CTL response activated by VSV was of low magnitude, similar to that observed following priming with DNA vaccines, a rapid boosting effect could be achieved due to the relative lack of antigen competition. Although the early development of memory T cells by certain vaccines can be an alternative mechanism to facilitate amplification of T-cell responses by booster immunization, we were unable to address this possibility due to the low frequency of DCT-specific CTL induced by VSV.37,38 Another possible explanation is that VSV induced a robust hDCT-specific CD4+ T-cell response that may have provided help for the CD8+ T-cell expansion. Indeed, we have shown that CD4+ T cells specific for the heteroclitic helper epitope (DCT88–102) greatly enhance DCT180–188-specific CD8+ T-cell responses.6
It is interesting that the immunogenicity of the prime–boost regime in a reversed order (Ad/VSV) could also be achieved at a relatively short interval (14 days), though the underlying mechanism is unclear. Previous studies, including ours, demonstrated that primary effector CD8+ T cells could eliminate antigen-carrying cells before they reached the lymph nodes and prevent a boosting immunization if it is delivered too soon.35,39 However, other studies have shown that VSV infection can result in a rapid distribution in secondary lymphoid organs that may bypass the elimination by circulating CTL and lead to de novo expression of the transgene in lymphoid organs.40,41 In that scenario, the antigen will be presented in the presence of a minimal number of effector CD8+ T cells, allowing engagement of memory or naive T cells for expansion. This possibility is currently under active investigation in our lab.
Our data confirmed that the recombinant version of VSV is relatively safe in C57BL/6 mice, especially at the dose we tested that enables a rapid and robust booster immunization without evidence of apparent local or significant systemic pathology. Our data extend the potential of VSV for anticancer treatment, as a vaccine vector for priming antitumor immunity against a self-antigen, in addition to its traditional role as an oncolytic agent.42,43,44,45 Future studies focusing on a combination of the immunotherapeutic and oncolytic properties may unleash the full anticancer potential of this virus. The fact that VSV-primed CTL responses can be boosted quickly is of potential benefit in the treatment of diseases with short therapeutic windows, including cancer and antiviral therapy where both the magnitude and rapid generation of effector cells are critical.
Materials and Methods
Cells and culture conditions. Cells used included Vero (derived from African green monkey kidney), MCA205 (murine fibrosarcoma), B16-F10 (murine melanoma), and L929 (murine fibrosarcoma) (American Type Culture Collection, Manassas, VA). Vero cells were grown in α-minimum essential medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mmol/l L-glutamine, and antibiotics (all cell culture reagents from Invitrogen, Grand Island, NY). MCA205 and L929 cells were cultured in Dulbecco's Modified Eagle's Medium containing 10% fetal bovine serum, 2 mmol/l L-glutamine, and antibiotics. B16-F10 cells were grown in F11-minimum essential medium with 10% fetal bovine serum, 2 mmol/l L-glutamine, 5 ml sodium pyruvate, 5 ml minimum essential medium nonessential amino acids, 5 ml vitamin solution, 55 µmol/l 2-mercaptoethanol, and antibiotics. All cultures were grown at 37 °C in a humidified atmosphere with 5% CO2.
Recombinant VSV. A recombinant VSV of the Indiana serotype was engineered to express the hDCT gene by subcloning a full-length hDCT PCR fragment into the XhoI and NheI sites between the G and L genes (Figure 1a) of the plasmid pVSV-XN (provided by John Rose, Yale University School of Medicine, New Haven, CT). Recombinant genomes were rescued using standard techniques46 to generate replication-competent VSV-hDCT. VSV was propagated and titered in Vero cell cultures. A VSV carrying the same pVSV-XN plasmid but with the full-length GFP gene inserted into the genome was previously described and used as a control vector in this study.17
Characterization of DCT expression. To confirm successful construction of VSV-hDCT, MCA205 cells, which lack endogenous DCT, were incubated with the virus for 18 hours at a multiplicity of infection of 10. VSV-GFP-infected MCA205 and B16-F10 cells, which constitutively express endogenous murine DCT, provided negative and positive controls for DCT expression, respectively. DCT was detected in harvested cells by immunohistochemical staining and western blotting using a rabbit antiserum specific for DCT (αPEP8h; provided by Vincent Herring, National Cancer Institute, Bethesda, MD).
Mice. Age-matched (8–10 weeks old at initiation of each experiment) female C57BL/6 mice (H-2b) were purchased from Charles River Laboratories (Wilmington, MA) and housed in specific pathogen-free conditions. Animal studies complied with Canadian Council on Animal Care guidelines and were approved by McMaster University's Animal Research Ethics Board.
Determination of the appropriate VSV dose for intranasal administration. To determine the appropriate dose for in vivo immunization, anesthetized, supine mice received intranasal doses of VSV-GFP ranging from 1 × 106 to 1 × 109 pfu in 10 µl of PBS and were monitored daily for 30 days for signs of illness. Using additional mice, hematoxylin and eosin staining of brain tissues harvested 7, 14, and 28 days postinfection was performed to evaluate neurotoxicity. To visualize the location and kinetics of transgene expression following intranasal delivery of VSV-GFP, intact brains and lungs harvested from mice 2, 5, 7, and 10 days postinfection were viewed under a fluorescent microscope to determine the extent of GFP expression.
Recombinant Ad. The Ad-hDCT vector was an E1/E3-deleted human type 5 Ad that expressed the full-length hDCT gene and was propagated in 293 cells and purified on a CsCl gradient as described previously.47
Peptides. Immunodominant peptides from DCT that bind to H-2Kb (Trp2180–188, SVYDFFVWL; shared by hDCT and murine DCT)48 and I-Ab (hDCT88–102, RKFFHRTCKCTGNFA)6 were synthesized by PepScan Systems (Lelystad, the Netherlands). The H-2Kb-restricted epitope from the N protein of VSV (RGYVYQGL) was purchased from Biomer Technologies (Hayward, CA).
Antibodies. The following monoclonal antibodies used for flow cytometry assays were purchased from BD Biosciences (San Diego, CA): anti-CD4-PE-Cy5 (clone RM4-5), anti-CD8-PE-Cy7 (clone 53-6.7), and anti-interferon-γ-antigen-presenting cells (clone XMG1.2).
Detection of DCT-specific T-cell responses. C57BL/6 mice were immunized with VSV vectors in 10 µl of PBS by intranasal administration or Ad vaccines in 100 µl of PBS by intramuscular injection (50 µl/hamstring). For heterologous VSV/Ad prime–boost vaccination, intervals of 14, 7, and 4 days between immunizations were evaluated. Antigen-specific T-cell responses were quantified by flow cytometric analysis 7 days after primary or secondary vaccination using blood samples. Mononuclear cells were stimulated with peptides (1 µg/ml) and brefeldin A (BD Biosciences; 1 µg/ml) was added after 2 hours of incubation. After 6 hours of total incubation time, cells were treated with Fc Block (BD Biosciences) and stained for surface expression of CD4 and CD8. Cells were subsequently permeabilized and stained for intracellular interferon-γ. Data were acquired using a FACSCanto with FACSDiva 5.0.2 software (BD Biosciences) and analyzed with FlowJo Mac Version 6.3.4 (Tree Star, Ashland, OR).
Prophylactic treatment of subcutaneous melanomas. To evaluate heterologous prime–boost vaccine efficacy, mice were immunized intranasally with VSV-hDCT, followed 7 days later with intramuscular Ad-hDCT. Single immunization with either vector alone, VSV-GFP, or PBS was included as controls. Nine days after the single or second vaccination, 1–2 × 106 B16-F10 cells in 100 µl of PBS were injected subcutaneously between the scapulae. Tumor volume (height × length × width) was measured using digital vernier calipers (Mitutoyo Canada, Toronto, Ontario, Canada) and deemed to be at end point when >1,000 mm3.
Therapeutic treatment of metastatic lung melanomas. Lung metastases were established by tail vein injection with 1 × 106 B16-F10 cells. Mice were treated intranasally with VSV-hDCT 3 days after tumor inoculation and boosted with intramuscular Ad-hDCT 10 days later. Control mice received one of the following inoculation pairs on days 3 and 10, respectively: VSV-GFP and Ad-hDCT; PBS and Ad-hDCT; or two injections of PBS. Twenty-one days after tumor challenge, lungs were harvested and visible metastases were enumerated using a dissection microscope (Zeiss TLB3000 series; Diagnostic Instruments, Sterling Heights, MI).
Statistical analyses. T-cell response, tumor volume, and lung metastasis data were graphed and analyzed by Student's two-tailed t-test, one- or two-way analysis of variance using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). Differences between means were considered significant at P ≤ 0.05. Means plus standard error bars are shown.
Acknowledgments
We thank Mary-Jo Smith and Mary Bruni for assistance with immunohistochemical staining; Xueya Feng, Duncan Chong, and Natasha Kazdhan for technical assistance with virus preparation. This work was supported by grants to Y.W. from the Canadian Institutes of Health Research [MOP-67066] and the Ontario Cancer Research Network. J.E.B. is supported by studentships from the Natural Sciences and Engineering Research Council. We have no financial conflicts of interest related to this research.
REFERENCES
- Rosenberg SA. Development of cancer immunotherapies based on identification of the genes encoding cancer regression antigens. J Natl Cancer Inst. 1996;88:1635–1644. doi: 10.1093/jnci/88.22.1635. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- Naftzger C, Takechi Y, Kohda H, Hara I, Vijayasaradhi S., and , Houghton AN. Immune response to a differentiation antigen induced by altered antigen: a study of tumor rejection and autoimmunity. Proc Natl Acad Sci USA. 1996;93:14809–14814. doi: 10.1073/pnas.93.25.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall R, Bowne WB, Weber LW, LeMaoult J, Szabo P, Moroi Y, et al. Heteroclitic immunization induces tumor immunity. J Exp Med. 1998;188:1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianizad K, Marshall LA, Grinshtein N, Bernard D, Margl R, Cheng S, et al. Elevated frequencies of self-reactive CD8+ T cells following immunization with a xenoantigen are due to the presence of a heteroclitic CD4+ T-cell helper epitope. Cancer Res. 2007;67:6459–6467. doi: 10.1158/0008-5472.CAN-06-4336. [DOI] [PubMed] [Google Scholar]
- Leitch J, Fraser K, Lane C, Putzu K, Adema GJ, Zhang QJ, et al. CTL-dependent and -independent antitumor immunity is determined by the tumor not the vaccine. J Immunol. 2004;172:5200–5205. doi: 10.4049/jimmunol.172.9.5200. [DOI] [PubMed] [Google Scholar]
- Lane C, Leitch J, Tan X, Hadjati J, Bramson JL., and , Wan Y. Vaccination-induced autoimmune vitiligo is a consequence of secondary trauma to the skin. Cancer Res. 2004;64:1509–1514. doi: 10.1158/0008-5472.can-03-3227. [DOI] [PubMed] [Google Scholar]
- Fu TM, Mylin LM, Schell TD, Bacik I, Russ G, Yewdell JW, et al. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J Virol. 1998;72:1469–1481. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Guarnieri FG, Staveley-O'Carroll KF, Viscidi RP, Levitsky HI, Hedrick L, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey KR, Gritz L, Sherry R, Abati A, Fetsch PA, Goldfeder LC, et al. Evaluation of prime/boost regimens using recombinant poxvirus/tyrosinase vaccines for the treatment of patients with metastatic melanoma. Clin Cancer Res. 2006;12:2526–2537. doi: 10.1158/1078-0432.CCR-05-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng WS, Butterfield LH, Ribas A, Dissette VB, Heller JB, Miranda GA, et al. alpha-Fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res. 2001;61:8782–8786. [PubMed] [Google Scholar]
- Näslund TI, Uyttenhove C, Nordström EK, Colau D, Warnier G, Jondal M, et al. Comparative prime-boost vaccinations using Semliki Forest virus, adenovirus, and ALVAC vectors demonstrate differences in the generation of a protective central memory CTL response against the P815 tumor. J Immunol. 2007;178:6761–6769. doi: 10.4049/jimmunol.178.11.6761. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Porosnicu M, Markovic D., and , Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson RP, Rasmussen AF, Jr, Brandly CA., and , Brown JW. Human infection with the virus of vesicular stomatitis. J Lab Clin Med. 1950;36:754–758. [PubMed] [Google Scholar]
- Egan MA, Chong SY, Rose NF, Megati S, Lopez KJ, Schadeck EB, et al. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res Hum Retroviruses. 2004;20:989–1004. doi: 10.1089/aid.2004.20.989. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106:539–549. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Brandsma JL, Shylankevich M, Su Y, Roberts A, Rose JK, Zelterman D, et al. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J Virol. 2007;81:5749–5758. doi: 10.1128/JVI.02835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddario-DiCaprio KM, Geisbert TW, Ströher U, Geisbert JB, Grolla A, Fritz EA, et al. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet. 2006;367:1399–1404. doi: 10.1016/S0140-6736(06)68546-2. [DOI] [PubMed] [Google Scholar]
- Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K., and , Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340:174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, et al. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72:4704–4711. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, et al. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J Virol. 2005;79:15043–15053. doi: 10.1128/JVI.79.24.15043-15053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter JD, Vivas-Gonzalez BE, Gomez D, Wilson JH, Brandsma JL, Greenstone HL, et al. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J Virol. 2002;76:8900–8909. doi: 10.1128/JVI.76.17.8900-8909.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Wilson JH, Buonocore L, Palin A, Rose JK., and , Reuter JD. Intranasal immunization with recombinant vesicular stomatitis virus expressing murine cytomegalovirus glycoprotein B induces humoral and cellular immunity. Comp Med. 2008;58:129–139. [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Jing Q, Georgel P, New L, Chen J, Mols J, et al. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity. 2007;27:123–134. doi: 10.1016/j.immuni.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Ireland DD, Palian BM., and , Reiss CS. Interleukin (IL)-12 receptor beta1 or IL-12 receptor beta 2 deficiency in mice indicates that IL-12 and IL-23 are not essential for host recovery from viral encephalitis. Viral Immunol. 2005;18:397–402. doi: 10.1089/vim.2005.18.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E., and , Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Wong P, Lara-Tejero M, Ploss A, Leiner I., and , Pamer EG. Rapid development of T cell memory. J Immunol. 2004;172:7239–7245. doi: 10.4049/jimmunol.172.12.7239. [DOI] [PubMed] [Google Scholar]
- Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hermans IF, Ritchie DS, Yang J, Roberts JM., and , Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Bonilla WV, Dumrese T, Odermatt B, Zinkernagel RM., and , Hengartner H. Perforin-independent regulation of dendritic cell homeostasis by CD8(+) T cells in vivo: implications for adaptive immunotherapy. Eur J Immunol. 2001;31:1772–1779. doi: 10.1002/1521-4141(200106)31:6<1772::aid-immu1772>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, et al. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J Immunol. 2007;179:5024–5032. doi: 10.4049/jimmunol.179.8.5024. [DOI] [PubMed] [Google Scholar]
- Belz GT, Zhang L, Lay MD, Kupresanin F., and , Davenport MP. Killer T cells regulate antigen presentation for early expansion of memory, but not naive, CD8+ T cell. Proc Natl Acad Sci USA. 2007;104:6341–6346. doi: 10.1073/pnas.0609990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS., and , Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- McWilliams JA, McGurran SM, Dow SW, Slansky JE., and , Kedl RM. A modified tyrosinase-related protein 2 epitope generates high-affinity tumor-specific T cells but does not mediate therapeutic efficacy in an intradermal tumor model. J Immunol. 2006;177:155–161. doi: 10.4049/jimmunol.177.1.155. [DOI] [PubMed] [Google Scholar]
- Yang J, Huck SP, McHugh RS, Hermans IF., and , Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci USA. 2006;103:147–152. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CW, Stephenson KB, Hanson S, Kucharczyk M, Duncan R, Bell JC, et al. The p14 FAST protein of reptilian reovirus increases vesicular stomatitis virus neuropathogenesis. J Virol. 2009;83:552–561. doi: 10.1128/JVI.01921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, et al. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF., and , Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Ebert O, Shinozaki K, Huang TG, Savontaus MJ, García-Sastre A., and , Woo SL. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 2003;63:3605–3611. [PubMed] [Google Scholar]
- Gaddy DF., and , Lyles DS. Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J Virol. 2007;81:2792–2804. doi: 10.1128/JVI.01760-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Stillman EA, Whitt MA., and , Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P, Parks RJ, Cummings DT, Evelegh CM., and , Graham FL. An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum Gene Ther. 2000;11:693–699. doi: 10.1089/10430340050015590. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA., and , Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895–4901. [PubMed] [Google Scholar]