Since the engineering and first successful application of recombinant adeno-associated virus (rAAV) serotype 2 as a gene transfer vehicle in 1984 (ref. 1), numerous additional rAAV serotypes have been isolated and characterized. Although many of these viruses have shown robust transduction efficiencies in various organ systems throughout the body by means of simple systemic delivery methods, widespread transduction of the central nervous system (CNS) via relatively noninvasive methods has been elusive. The mature blood-brain barrier (BBB) serves as a protective barrier that excludes potentially damaging molecules and microorganisms based on size, charge, and lipid solubility. Consequently, the BBB efficiently blocks rAAV diffusion into the CNS.2,3 Thus, to efficiently target the CNS using rAAV, researchers have had to resort to direct intraparenchymal injections.
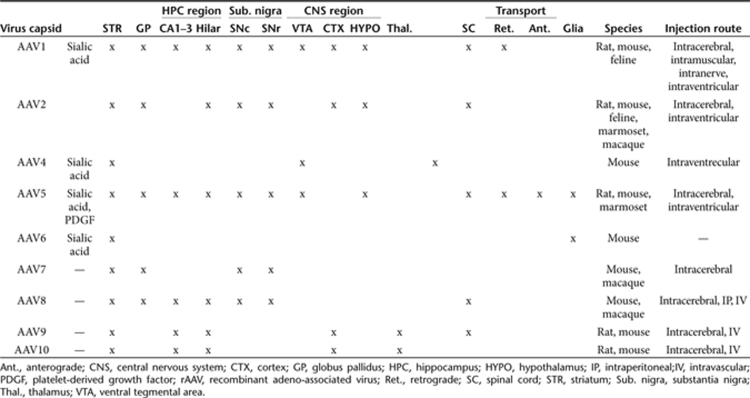
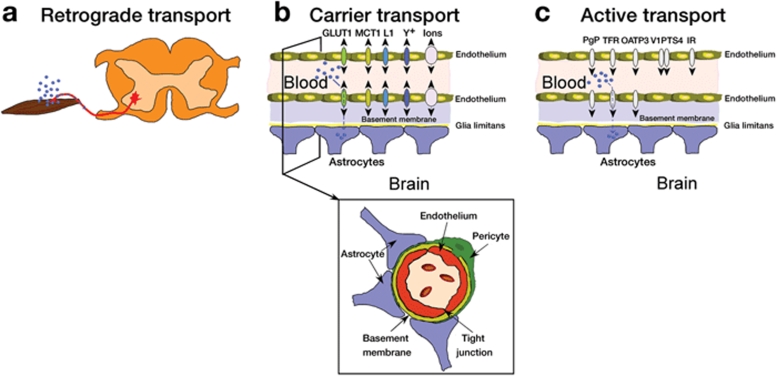
Intraparenchymal rAAV injections result in robust but relatively local transduction. Local delivery methods are advantageous when attempting gene therapy for neurological disorders that result from neuropathology that is localized to a specific anatomical region or anatomical circuitry such as in the case of Parkinson's disease. In contrast, treatment of neurological disorders that are due to single-gene defects or those that affect motor neurons of the spinal cord will probably require transduction of large proportions of the brain or spinal cord, respectively. In the case of widespread CNS transduction, intraparenchymal injections are impractical.4,5 Therefore, development of less invasive trans-BBB delivery methods for vectors is an extremely important endeavor. Numerous attempts to use molecules that are known to interact with various active transport mechanisms to convey proteins across the BBB have been reported with varying results.6 Currently, our understanding of rAAV transduction indicates that this process is a receptor-mediated event (e.g., see refs. 7,8,9,10). Given the large number of AAV serotypes availwwable (see Table 1), it is not inconceivable that one or more serotypes bind a cell-entry receptor that is capable of transporting the AAV capsid across the BBB in some manner (see Figure 1). Moreover, the crystal structures of the AAV1–AAV8 capsids have been solved,11,12,13,14,15,16 and mutational studies that change tropisms of these capsids are under way.17 Thus, it may be possible to engineer AAV capsids that target BBB-associated ligands to permit BBB access, but this approach is likely to be painstaking and time-consuming.
Table 1.
rAAV capsid types and their reported tropisms in various anatomical structures in the central nervous system
Figure 1.
Schematic of potential AAV access to the CNS from the periphery. (a) Retrograde transport. Infection of nerve endings or axons allows retrograde transport of rAAV and transduction of the mother soma. The schematic depicts a red motoneuron exiting the ventral spinal cord and viral particles infecting at the neuromuscular junction and being transported back to the soma. Although there has been some success with this method, the efficiency and replicability have been low. There are also several “weak spots” in the BBB that may allow better retrograde transport, such as the nasal epithelium leading to the olfactory bulb, the hypothalamus via intraventricular administration, and the area postrema in the medulla. (b) Schematic of carrier transport through the adult BBB. There are numerous channels that actively transport important molecules from the blood into the brain that might be candidate AAV9 receptors. Several candidates are depicted, but this is not a complete list of possible transporters. This schematic is based on Zlokovic.2 GLUT1, glucose transporter; L1, transporter for essential amino acids; MCT1, monocarboxylate transporter 1 for lactate; y+, transporter for cationic amino acids.2 There are also active transporters for various ions as indicated. (c) Schematic of active transport through the adult BBB. In addition to active transport, there are also proteins that bind molecules in the blood, thus permitting these complexes to cross into the brain. Again, the carrier systems depicted here are not a complete list, and the schematic is based on Zlokovic.2 IR, insulin receptor; OATP3, organic anion transporter 3; PgP, multidrug resistance P-glycoprotein; PTS4-V1, peptide transport system 4 and vasopressin receptor 1 transport arginine-vasopressin; TFR, transferrin receptor.2
However, a major step toward the goal of systemic gene delivery to the CNS using rAAV may have been taken by Foust and colleagues in their recent report.5 In this exciting study, peripheral intravascular (i.v.) administration of rAAV9 in neonatal or adult mice resulted in a widespread transduction of either the spinal cord or the brain. In addition, transduction of non-neuronal cells in the CNS by rAAV has historically proven to be extremely limited. However, in this study, depending on the age of the injected animal, a significant portion of non-neuronal cells in the brain and the spinal cord were infected. Although previous studies have shown that intraventricular injections of rAAV1 or rAAV2 in newborn mice resulted in widespread transduction of the brain,18,19 no indication of non-neuronal infection was seen in these earlier studies; thus, the findings by Foust et al.5 could be of great clinical importance, in that this would expand the repertoire of rAAV delivery to other cell types in the CNS that have been inaccessible thus far. For the purposes of clinical strategies, the ability to transduce CNS cells across the BBB in the adult is the most promising part of the Foust et al. study, in that closure of the BBB to large proteins occurs much earlier in development in the human as opposed to the rodent.3
Of the earlier characterized AAV capsid serotypes, rAAV 1, 2, and 5 display varying levels and specificity of transduction depending on injection site when administered directly to the brain20,21 (see Table 1). For instance, rAAV2, which is the most characterized rAAV serotype, displays a relatively low transduction efficiency of various brain areas. rAAV2 enters the cell via the heparan sulfate proteoglycan primary receptor and fibroblast growth factor receptor 1 co-receptors.22,23 In contrast, AAV5 typically results in a more widespread infection pattern, and identified receptors include several sialic acid receptors that have also been identified as important receptors for several infectious agents in the CNS.24 Thus, clearly, transduction of rAAV in any organ system is very much tied to the local population density of a specific receptor.
In the study by Foust et al.5 it seems that peripheral rAAV9 administration in the adult infects astrocytes through its interaction with astrocytic perivascular endfeet in direct contact with vascular endothelial cells that may contain a receptor population different from that exposed in direct intraparenchymal injections, thus resulting in the observed unique AAV9-mediated transduction properties. Foust et al.5 reasonably speculate that rAAV9 infection of astrocytic endfeet in the adult is a receptor-mediated event (see Figure 1). Indeed, the disparity of transduction pattern of i.v. rAAV9 between adults and neonates is highly suggestive of a specific receptor-mediated event in the adult, and this receptor expression is likely different in the neonate. It is likely that the resulting CNS transduction pattern from peripheral administration of rAAV9 in the neonate is due to the ability of this specific virus to bypass a not yet fully mature BBB. In contrast, even if the BBB were opened via pharmacological means in the adult, the 80-nm rAAV particle would still not be expected to cross the BBB to become freely accessible to potential AAV receptors in the parenchyma.
Direct CNS delivery of rAAV also yields different results depending on the targeted species, probably as a result of differential expression of receptors across species. For instance, direct administration of rAAV5 into the marmoset brain resulted in consistent detection of transduced astrocytes, albeit a minority compared with neurons, whereas injection of the same viral preparation into the rodent brain infected only neurons.25,26 In addition, variability in transduction pattern and efficiency could be due to the method of viral purification, which may lead to impurities in the vector preparation that may act as carrier proteins. However, in this study Foust et al.5 attempted to control for this issue.
The clinical relevance of the recent findings of Foust et al.5 is considerable. Efficiently targeting the spinal cord with rAAV to treat or study motor neuron diseases such as spinal muscular atrophy and amyotrophic lateral sclerosis is a direct implication of these findings. Thus far, the most widespread rAAV-mediated transduction of the spinal cord has been through direct injections of the red nucleus27 or peripheral injections into muscle,28 resulting in anterograde or retrograde transport, respectively (see Figure 1). Although the results in the current study indicate that the transduction of neurons in the spinal cord is limited in adult animals (which would be the target group of most clinical relevance), the transduction of astrocytes may serve to be of equal, or greater, clinical relevance.5 Thus far, a majority of therapeutic agents proposed for treatment of neurodegenerative disease are secreted trophic factors such as insulin-like growth factor 1 and glial cell line–derived neurotrophic factor.29 Because most trophic factors are typically secreted by glia, it is easy to envision that rAAV-mediated overexpression of trophic factors in these supporting cells would result in significant improvement of cell rescue, similar to or better than that seen when neurons themselves have been targeted.
Although the neuronal transduction in the adult brain was limited in scope in the Foust et al. study,5 the ubiquitous astrocytic transduction holds similar promise to that of astrocytic infection of the spinal cord. Trophic factors have been implicated as potential therapeutic agents aimed at slowing disease progression in neurodegenerative diseases such as Parkinson's disease. In the case of disorders that require more targeted delivery such as Parkinson's disease, the use of i.v. AAV9 might still be advantageous if tissue-specific promoters are included in the therapeutic construct, as pointed out correctly by Foust et al.5 Although brain neurosurgery is relatively safe, systemic delivery across the BBB remains an even safer alternative to direct CNS injections.
Going forward, widespread protein expression in the CNS via the use of rAAV9 needs a proof of principle in a rodent model of a neurodegenerative disorder that can be treated with ectopic gene expression in astrocytes. Moreover, replication of these results in other laboratories as well as in larger species will also constitute the next important hurdle for the method described by Foust et al.
REFERENCES
- Hermonat PL., and , Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Habgood MD., and , Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clin Exp Pharmacol Physiol. 1999;26:85–91. doi: 10.1046/j.1440-1681.1999.02987.x. [DOI] [PubMed] [Google Scholar]
- Foust KD, Poirier A, Pacak CA, Mandel RJ., and , Flotte TR. Neonatal intraperitoneal or intravenous injections of recombinant adeno-associated virus type 8 transduce dorsal root ganglia and lower motor neurons. Hum Gene Ther. 2008;19:61–70. doi: 10.1089/hum.2007.093. [DOI] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM., and , Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS., and , Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M., and , Samulski RJ. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J Virol. 2006;80:11393–11397. doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Miller E, Agbandje-McKenna M., and , Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M., and , Samulski RJ. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci USA. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMattia M, Govindasamy L, Levy HC, Gurda-Whitaker B, Kalina A, Kohlbrenner E, et al. Production, purification, crystallization and preliminary X-ray structural studies of adeno-associated virus serotype 5. Acta Crystallogr F Struct Biol Cryst Commun. 2005;61:917–921. doi: 10.1107/S1744309105028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron E, Bowman V, Kaludov N, Govindasamy L, Levy H, Nick P, et al. Structure of adeno-associated virus type 4. J Virol. 2005;79:5047–5058. doi: 10.1128/JVI.79.8.5047-5058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EB, Gurda-Whitaker B, Govindasamy L, McKenna R, Zolotukhin S, Muzyczka N, et al. Production, purification and preliminary X-ray crystallographic studies of adeno-associated virus serotype 1. Acta Crystallogr F Struct Biol Cryst Commun. 2006;62:1271–1274. doi: 10.1107/S1744309106048184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada O, Gurda B, Govindasamy L, McKenna R, Kohlbrenner E, Aslanidi G, et al. Production, purification and preliminary X-ray crystallographic studies of adeno-associated virus serotype 7. Acta Crystallogr F Struct Biol Cryst Commun. 2007;63:1073–1076. doi: 10.1107/S1744309107060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Lane MD, Padron E, Gurda B, McKenna R, Kohlbrenner E, et al. Structure of adeno-associated virus serotype 8, a gene therapy vector. J Virol. 2007;81:12260–12271. doi: 10.1128/JVI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Asokan A, Wu Z, Van Dyke T, DiPrimio N, Johnson JS, et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol Ther. 2008;16:1252–1260. doi: 10.1038/mt.2008.100. [DOI] [PubMed] [Google Scholar]
- Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL., and , Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levites Y, Jansen K, Smithson LA, Dakin R, Holloway VM, Das P, et al. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J Neurosci. 2006;26:11923–11928. doi: 10.1523/JNEUROSCI.2795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk O, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C., and , Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V., and , Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276:20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, et al. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain. 2007;130:799–815. doi: 10.1093/brain/awl382. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Flotte TR, Reier PJ., and , Mandel RJ. Recombinant adeno-associated virus-mediated global anterograde delivery of glial cell line–derived neurotrophic factor to the spinal cord: comparison of rubrospinal and corticospinal tracts in the rat. Hum Gene Ther. 2008;19:71–82. doi: 10.1089/hum.2007.104. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD., and , Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Suzuki M., and , Svendsen CN. Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends Neurosci. 2008;31:192–198. doi: 10.1016/j.tins.2008.01.006. [DOI] [PubMed] [Google Scholar]