Abstract
Cells subjected to sustained high osmolarity almost universally respond by accumulating compatible organic osmolytes that, in contrast to inorganic ions, are not deleterious even at high intracellular concentrations. Their accumulation from the external environment by known organic osmolyte transporters, such as the four identified in mammals, occurs only slowly in response to sustained high osmolarity, by synthesis of new transporter proteins. Most cells, however, are not subject to high or varying osmolarity, and it is not clear whether organic osmolytes are generally required at normal osmolarities or how they are regulated. The fertilized egg of the mouse is protected in the oviduct from perturbations in osmolarity. However, deleterious effects of osmotic stress were evident in vitro even at normal oviductal osmolarity. Glycine was found to protect development, indicating that early mouse embryos may use glycine as an organic osmolyte at physiological osmolarity. We have now found that GLYT1, a glycine transporter of the neurotransmitter transporter gene family, functions as the organic osmolyte transporter that mediates the osmotically regulated accumulation of glycine and regulates cell volume in early embryos. Furthermore, osmotic stimulation of GLYT1 transport was immediate, without a requirement for protein synthesis, implying regulation different from known organic osmolyte transporters. Thus, GLYT1 appears to have a previously unidentified role as an organic osmolyte transporter that functions in acute organic osmolyte and volume homeostasis near normal osmolarity.
Animal cells precisely regulate their size by adjusting the cytoplasmic concentration of osmotically active substances. The acute response to decreased cell volume is a rapid stimulation of preexisting inorganic ion transporters in the cell membrane (1, 2). The consequent increase in uptake and cytoplasmic accumulation of inorganic ions raises internal osmotic pressure and thus swells the cell until its normal size is regained. Some cells are subject to sustained periods of high osmolarity (e.g., in kidney or marine organisms) and thus would require a very high intracellular concentration of inorganic ions for osmotic support. Such high intracellular ionic strength is extremely detrimental to cell biochemistry and metabolism, and most cells cannot survive for extended periods under these conditions (3). Consequently, cells subject to sustained high osmolarity have developed means to preferentially accumulate benign compatible osmolytes, a diverse array of small neutral organic compounds, which can provide intracellular osmotic support without perturbing cell function even at high concentrations (3, 4). While some organic osmolytes are synthesized within cells, most are imported by specialized transporters that have evolved in animals, plants, and microorganisms to mediate their accumulation from the external environment (3, 5, 6). These include the four organic osmolyte transporters identified in mammals: the Na+/myo-inositol transporter (SMIT), betaine transporter (BGT1), taurine/β-amino acid transporter (TAUT), and system A amino acid transporter (4, 5, 7).
In general, the rapid osmotic stimulation of inorganic ion uptake is regulated at least in part by signaling that is independent of transcription or protein synthesis, whereas, in a wide range of diverse organisms, the marked stimulation of organic osmolyte transport by sustained high osmolarity is mediated by a slow process requiring transcription and synthesis of new transporter proteins (2, 5, 6, 8). The up-regulation of organic osmolyte transporter synthesis by sustained increases in osmolarity has been extensively studied in mammals, particularly in kidney, and a tonicity-responsive transcription factor that regulates transcription of several organic osmolyte transporter genes has been identified (8–10).
Organic osmolytes have also been found in a range of mammalian cell types that are not normally exposed to high or varying osmolarity (11). This observation implies that, even without significant osmotic perturbations in their physiological environments, many cells may prefer a significant portion of intracellular osmotic support to be provided by organic osmolytes. Little is known, however, about the regulation of organic osmolytes or their transport in cells whose normal environment has nearly constant osmolarity.
The fertilized eggs and early embryonic stages of mammals develop in vivo in the protected environment of the oviduct, where osmolarity is ≈300 milliosmolar (mOsM) (12), resembling that of other mammalian extracellular fluids. In vitro, however, it was found that the fertilized egg of the mouse developed optimally only under conditions of low osmolarity (≈250–270 mOsM), with in vitro embryo development severely impaired at 300 mOsM or above (13–16). Interestingly, in vitro development at more normal osmolarities could be substantially rescued by the presence of any one of several putative organic osmolytes in the external medium (14, 15, 17), implying that early embryos might normally require a portion of intracellular osmolarity to be provided by organic osmolytes. Glycine was one of the most effective compounds found to be osmoprotective for mouse embryos (15, 17, 18), indicating that it might be transported into early mouse embryos to act as an organic osmolyte. However, an organic osmolyte transporter that might serve this function in early mouse embryos had not been identified.
Mouse eggs and embryos do express one of the known mammalian organic osmolyte transporters, the TAUT β-amino acid transporter (19), but TAUT cannot transport glycine. Furthermore, the mammalian organic osmolyte transporter known to transport glycine, system A, is not expressed before the blastocyst stage (20, 21) and thus could not account for osmoprotection by glycine in fertilized eggs and early cleavage-stage embryos. On the basis of kinetics and substrate specificity, essentially all glycine transport in mouse eggs and cleavage-stage embryos has been proposed (20) to be by means of the highaffinity GLYT1 glycine transporter (slc6a9 or glyt1 gene), a Na+- and Cl–-dependent, sarcosine-sensitive member of the neurotransmitter transporter (NTT) family (22, 23). GLYT1 mediates reuptake of glycine into glia at synapses in the CNS, but is also expressed outside the CNS, where its function is unknown. We therefore hypothesized that the GLYT1 transporter might have a previously undescribed function in early embryos as an organic osmolyte transporter.
Materials and Methods
Media. Media were based on KSOM embryo culture medium (24), with glutamine omitted and poly(vinyl alcohol) (1 mg/ml) substituted for BSA. Hepes-KSOM (pH 7.4) was used for embryo handling (24). Osmolarity was adjusted with d(+)-raffinose and confirmed by osmometer (Vapro 5520, Wescor, Logan, UT) as previously described (15).
Animals and Embryos. Female CF1 strain mice (Charles River, St-Constant, PQ, Canada), whose eggs are particularly sensitive to in vitro conditions including osmolarity (25), were superovulated by i.p. injection of 5 international units of equine chorionic gonadotropin followed 47.5 h later by 5 international units of human chorionic gonadotropin and mated overnight with B6D2F1 males. Embryos were removed from excised oviducts by flushing with Hepes-KSOM and were freed from residual cumulus matrix by brief exposure to 300 μg/ml hyaluronidase (26). Fertilized eggs (one-cell embryos) were identified by the presence of two pronuclei. Embryos were cultured in microdrops (15 per drop, constituting one replicate) under mineral oil at 37°C in 5% CO2/95% air (15, 24, 26). One-cell embryos were placed into culture (day 1) and the proportions reaching the four-cell or greater stage on day 3 [i.e., passing the stress-induced block at the two-cell stage (25)] and the blastocyst stage on day 6 are reported. Development is slower at higher osmolarities, and thus culture was extended 1 additional day beyond what is required in optimal media to allow maximal blastocyst formation (15). Development was assessed by standard morphological criteria (26): number of normal, equal-sized cells on day 3, and formation of an expanded, fluid-filled blastocyst on day 6. One-cell embryo volumes were calculated from the average of two measured perpendicular diameters, assuming a sphere.
3H-Labeled Compound Measurements in Embryos. [3H]Glycine ([2-3H]glycine; 10–60 Ci/mmol; 1 Ci = 37 GBq) and other 3H-labeled amino acids (Amersham Pharmacia or New England Nuclear) were added directly to media. Standard curves for conversion of cpm to concentration were generated weekly. 3H in embryos was measured with a liquid scintillation counter (2200CA TriCarb, Packard Instrument) as previously described (18), with 10–15 embryos pooled for each replicate. Labeled compounds were added to media at the concentrations specified, either as the stock 3H-labeled amino acid for low concentrations or as a proportional mixture of 3H-labeled and unlabeled to achieve higher total concentrations. Trichloroacetic acid (TCA) precipitation was performed as described previously (27). For experiments in which the acute rate of glycine transport by embryos was determined, a 10-min incubation in the presence of [3H]glycine was used. Uptake was linear with time over at least 20 min at a glycine concentration of 1 mM. For experiments in which inhibition of transport was assessed, a 10- or 45-min incubation with 1–5 μM 3H-labeled compound was used. For these concentrations, uptake was linear with time for at least 1 h. Rates were calculated by dividing total intracellular labeled compound by the period of incubation. In one set of experiments, unlabeled glycine was measured instead by amino acid microanalysis (BioLC with AminoPac PA10 column, Dionex), with each replicate being the TCA-soluble fraction from a pool of 90 embryos, calibrated with glycine standards. The column was prewashed and samples were run with 8% TCA.
ORG23798. The GLYT1 transport inhibitor ORG23798 [bis(4-fluorophenyl)methylenepiperidineacetic acid, lithium salt] was synthesized at Organon Laboratories. Inhibition of glycine transporters was determined from uptake of [3H]glycine into Chinese hamster ovary (CHO) cells stably expressing either hGLYT1b or hGLYT2 by methods previously described (28). Interaction with other NTT systems was assessed by using rat brain synaptosomal preparations and, for a range of receptors, by using membranes derived from NIH 3T3 (mouse) or CHO cells stably transfected with human cloned receptors (29). For use with embryos, ORG23798 stocks (5 mM) were prepared in DMSO from dry powder and stored at –20°C until used.
Data Analysis. Curves were fit by linear or nonlinear regression by using sigmaplot 8.2 (SPSS, Chicago). Comparisons between independent means were made by ANOVA and Tukey–Kramer Multiple Comparisons test, or Student's two-tailed t test (instat, GraphPad, San Diego). Proportional data were arcsine-transformed. Multivariate data were analyzed by using linear models with ANOVA (Vista Visual Statistics System, F. W. Young and L. L. Thurstone Psychometric Laboratory, University of North Carolina, Chapel Hill).
Results
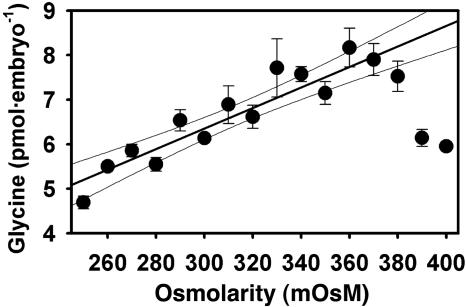
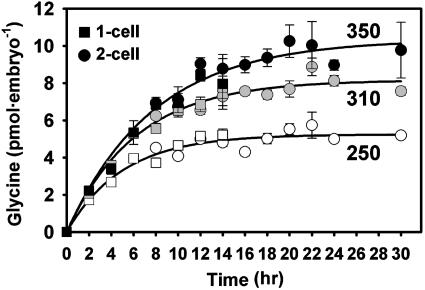
Osmosensitive Glycine Accumulation in Mouse Embryos. Mouse embryos were cultured for 24 h from the one- to two-cell stage at different external osmolarities in the presence of 1 mM glycine (3H-labeled), a concentration previously shown to confer maximal osmoprotection on embryos (15) and approximating the concentration in oviductal fluid (30). The amount of intracellular glycine accumulated by embryos after 24 h increased linearly as osmolarity was increased from 250 to about 380 mOsM (Fig. 1), as predicted for counterbalancing external osmolarity. The time course of glycine accumulation was determined at 250, 310, and 350 mOsM. Accumulation of glycine began immediately with no measurable lag period and reached a plateau whose level depended on osmolarity (Fig. 2). The data could be fitted well by a function describing a constant rate of accumulation and a passive efflux, having the form G(t) = Gmax[1 – e–at], where G(t) is total glycine accumulated, Gmax is the asymptotically approached maximum (steady-state) concentration, and t is time (Fig. 2). This curve-fitting yielded Gmax of 5.3, 8.2, and 10.3 pmol per embryo at 250, 310, and 350 mOsM, respectively. Thus, the total amount of glycine accumulated by mouse one- to two-cell embryos is regulated as a function of osmolarity, approximately doubling as osmolarity is increased from 250 to 350 mOsM.
Fig. 1.
Total glycine in mouse embryos after 24-h culture from the one-cell stage in 1 mM glycine as a function of osmolarity. The line shown was obtained by linear regression (excluding data >380 mOsM where decrease likely reflects loss of viability; r2 = 0.85; 95% confidence intervals indicated). Each point represents mean ± SEM of three replicates.
Fig. 2.
Total glycine accumulated by one-cell embryos as a function of time at various osmolarities. Embryos were cultured from the one-cell stage in the presence of 1 mM glycine in 250, 310, or 350 mOsM medium, as indicated. One-cell embryos cleaved to the two-cell stage during this period (see key); where both were present, glycine was determined in each separately. Curves were fit by nonlinear least-squares (see text). Each point represents mean ± SEM of three or four replicates, except one or two replicates for points for two-cell embryos at 8–10 h (where few had yet cleaved) and six or seven at 24 h.
Glycine that serves as an organic osmolyte must be free rather than incorporated into macromolecules. After 24 h at 250 or 350 mOsM in the presence of 1 mM [3H]glycine, 90% and 95%, respectively, of total 3H in embryo lysates was TCA-soluble. Precipitable 3H (0.44 and 0.36 pmol per embryo) did not change with osmolarity, but soluble glycine increased from 4.1 ± 0.3 to 7.0 ± 0.6 pmol per embryo as osmolarity increased from 250 to 350 mOsM (n = 5, P = 0.002). We also separately measured total TCA-soluble glycine by amino acid microanalysis after culture for 24 h with 1 mM unlabeled glycine. Total soluble glycine in embryos was found to be 4.2 ± 0.6 vs. 8.0 ± 0.4 pmol per embryo at 250 and 350 mOsM, respectively (n = 5, P = 0.0009). This finding also validated that measurements with [3H]glycine are representative of the entire intracellular glycine pool. Thus, almost all glycine accumulated by embryos was soluble, and total soluble glycine increased with osmolarity, as expected for an organic osmolyte.
Selectivity and Potency of ORG23798 Against GLYT1 Transport. To elucidate the mechanism of osmotically regulated glycine transport in mouse embryos, we used ORG23798, a specific inhibitor of GLYT1. ORG23798 inhibited uptake of [3H]glycine into GLYT1-expressing Chinese hamster ovary cells with an IC50 of 1.17 μM, nearly 100-fold more potent than sarcosine (IC50 ≈ 100 μM). In contrast, IC50 was >100 μM in GLYT2-expressing cells, indicating high selectivity for GLYT1 over GLYT2. Selectivity in rat brain synaptosomal preparations for inhibition of glycine transport over that of other NTT gene family members was high (>100-fold) for serotonin (5HT) and γ-aminobutyric acid and moderate for dopamine (IC50 = 17 μM) and norepinephrine (IC50 = 19 μM). ORG23798 exhibited moderate affinity for 5HT2A and 5HT2C neurotransmitter receptors (Ki ≈ 12 μM), with little or no significant affinity for >40 other neurotransmitter (including glycine) receptors. Thus, ORG23798 was found to be a specific and potent inhibitor for GLYT1 at the concentrations used here.
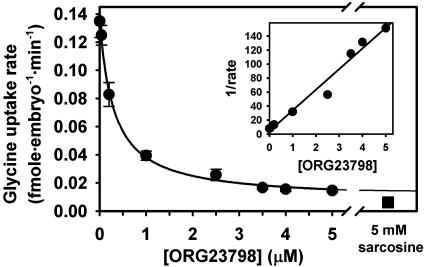
We next tested the effectiveness of ORG23798 at blocking short-term (45-min) glycine transport into one-cell mouse embryos, and found that ORG23798 inhibited sarcosine-sensitive [3H]glycine (1 μM) uptake with an IC50 of 0.3 μM (Fig. 3). ORG23798 was used in subsequent experiments at 5 μM, a concentration that eliminated virtually all transport by GLYT1 in embryos. Inhibition was reversible by a 30-min washout (not shown).
Fig. 3.
Inhibition of glycine uptake into one-cell embryos by ORG23798. Rate of uptake was determined from the total glycine accumulated during 45 min in the presence of 1 μM [3H]glycine with ORG23798 (0–5 μM) or 5 mM sarcosine, as indicated. The curve is of the form rate = r0 + a/(b + [ORG23798]), fit by nonlinear least-squares to determine IC50 (b) and residual rate at infinite ORG23798 (r0). A reciprocal plot (after subtraction of residual rate) is shown in the Inset, fit by linear regression (r2 = 0.97). Each point represents mean ± SEM of four replicates.
To confirm its specificity in mouse embryos, we determined whether ORG23798 at 5 μM could affect other amino acid transport systems expressed by one-cell embryos. For this determination, we measured the rates of uptake (over 10 min) of 1–5 μM 3H-labeled substrates, with each substrate chosen because the large majority of its transport is solely by means of one of the known transport systems in embryos [determined on the basis of published work (19, 31–34)]. No significant differences between transport rates in the presence of 5 μM ORG23798 vs. DMSO vehicle alone (by t tests, n = 3 replicates each) were found for the following substrates: β-alanine (transported by TAUT), glutamic acid (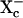 ), leucine (L), lysine (b0,+), tryptophan in the presence of 5 mM unlabeled leucine (T), or proline (unidentified Na+-dependent transporter; unpublished data), whereas glycine transport was inhibited by 93% under the same conditions. In addition, transport of glycine was unaffected by ORG23798 in mouse blastocysts, where virtually all glycine transport is by B0,+ and GLYT1 activity is no longer detectable (20). Thus, ORG23798 had no measurable effect on the activities of any of the known amino acid transporters expressed by early preimplantation mouse embryos, except for GLYT1.
), leucine (L), lysine (b0,+), tryptophan in the presence of 5 mM unlabeled leucine (T), or proline (unidentified Na+-dependent transporter; unpublished data), whereas glycine transport was inhibited by 93% under the same conditions. In addition, transport of glycine was unaffected by ORG23798 in mouse blastocysts, where virtually all glycine transport is by B0,+ and GLYT1 activity is no longer detectable (20). Thus, ORG23798 had no measurable effect on the activities of any of the known amino acid transporters expressed by early preimplantation mouse embryos, except for GLYT1.
Function of GLYT1 in Osmosensitive Glycine Accumulation. The molecular identity of the mechanism mediating the osmotically regulated accumulation of glycine had not been established. We thus used ORG23798 to help determine whether the GLYT1 transporter is responsible for osmoregulated accumulation of glycine and osmoprotection by glycine in embryos.
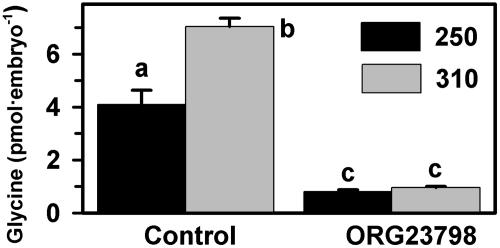
We first investigated whether specifically blocking glycine transport by GLYT1 with ORG23798 prevented the osmotically regulated accumulation of glycine. As previously shown, more glycine was accumulated by one-cell embryos when they were cultured for 24 h at 310 than at 250 mOsM (Fig. 4). ORG23798 (5 μM) eliminated >80% of glycine accumulation by embryos, and the residual glycine accumulation in the presence of ORG23798 was not different between 250 and 310 mOsM (Fig. 4). This finding indicated that all osmotically regulated accumulation of glycine in embryos occurs by means of GLYT1.
Fig. 4.
Effect of ORG23798 on accumulation of glycine by one-cell embryos cultured for 24 h. Embryos were cultured at 250 or 310 mOsM (see key) in the presence or absence of ORG23798 (5 μM; DMSO vehicle alone in control). Different letters over bars indicate significant differences (P < 0.001 by ANOVA and Tukey–Kramer test). Each bar represents mean ± SEM of five replicates.
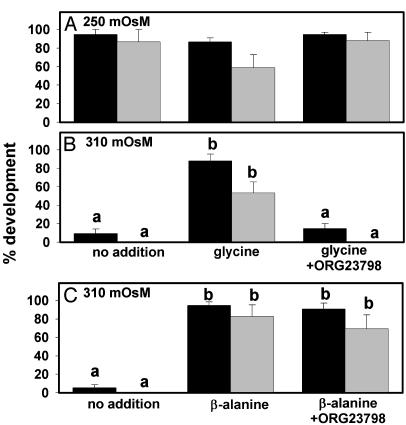
We next determined whether inhibiting GLYT1 would affect the ability of glycine to protect embryo development against the detrimental effects of increased osmolarity. At 250 mOsM, ≈90% of one-cell embryos developed to the blastocyst stage (Fig. 5A), and the presence of ORG23798 did not decrease development either with glycine (Fig. 5A) or in its absence (data not shown). As found for several other putative osmolytes in embryos (13, 14), the presence of glycine at 250 mOsM somewhat depressed development to the blastocyst stage (not quite significant, P = 0.08), an effect that appeared to be reversed by ORG23798. Thus, ORG23798 exhibited no toxicity toward embryos under these conditions.
Fig. 5.
Effect of ORG23798 on embryo development. Development of one-cell embryos is shown to the four-cell or greater stage on day 3 (black bars) and to blastocysts by day 6 (gray bars). (A) Development in 250 mOsM medium with no addition, glycine (1 mM), or glycine plus ORG23798 (5 μM), as indicated by labels below B. (B) Development in 310 mOsM medium under the same conditions as in A. (C) Development in 310 mOsM medium with no addition, β-alanine (5 mM), or β-alanine plus ORG23798 (5 μM). β-Alanine was at 5 mM because this is the minimum concentration providing optimal osmoprotection for embryos (15). ANOVAs were done on four-cell and blastocyst data separately in all three panels. There were no significant differences in A (P = 0.61 for four-cell or greater; P = 0.08 for blastocysts). In B and C, different letters over bars indicate significant differences within each developmental stage and each panel (P < 0.001 for four-cell data, P < 0.01 in B and P < 0.05 in C for blastocyst data; by ANOVA with Tukey–Kramer test after inverse sine transform). Each bar represents mean ± SEM of five replicates.
As previously shown (15), embryo development was poor at 310 mOsM, with very few embryos developing to the four-cell stage (most were arrested at the two-cell stage) and none to blastocysts (Fig. 5B), but their development could be substantially rescued by either 1 mM glycine or 5 mM β-alanine (Fig. 5 B and C). Rescue by either was incomplete, as development is slower than at 250 mOsM (e.g., on day 3, mean cell number was about 4.0 at 310 mOsM with either osmolyte vs. 5.5 at 250; data not shown), but the rescue effect is highly significant nonetheless (Fig. 5). ORG23798 (5 μM) completely eliminated the ability of glycine to support embryo development to the four-cell stage or beyond at 310 mOsM (Fig. 5B). In contrast, ORG23798 had no effect on osmoprotection of embryos by β-alanine (Fig. 5C). Thus, osmoprotection by glycine was eliminated by inhibition of GLYT1 with ORG23798, whereas osmoprotection by β-alanine, which is transported by the β-amino acid transporter TAUT, remained unaffected.
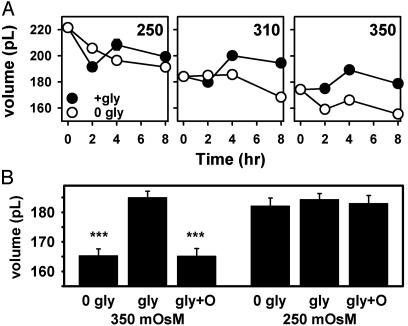
An organic osmolyte may also confer an improved ability to maintain cell volume in the face of increased osmolarity. Consistent with this prediction, one-cell embryos maintained larger volumes in the presence of glycine than in its absence at 310 and 350 mOsM for up to 8 h (Fig. 6A). We then determined whether ORG23798 eliminated this ability of glycine to maintain one-cell embryo volume. Embryos cultured for 8 h at 350 mOsM had an ≈12% greater volume in the presence of glycine vs. its absence (Fig. 6B). When ORG23798 was present in addition to glycine, however, cell volume was not maintained and was virtually identical to that in the absence of glycine, differing by only 0.04% (Fig. 6B). Thus, GLYT1 activity is required for glycine to maintain embryo cell volume. At 250 mOsM, where glycine does not affect embryo volume (Fig. 6A), ORG23798 also had no effect on the volume of one-cell embryos (Fig. 6B).
Fig. 6.
Effect of glycine and ORG23798 on embryo cell volume. (A) Volume of one-cell embryos as a function of time in the presence or absence of glycine (1 mM; see key) at 250, 310, or 350 mOsM, as labeled. The effect of glycine was significant at 310 and 350 mOsM (P < 0.0001; by ANOVA) but not at 250 mOsM (P > 0.05). (B) Volume of one-cell embryos at 350 or 250 mOsM, without glycine (0 gly), with 1 mM glycine (gly), or with glycine plus ORG23798 (gly+O). ***, P < 0.001. Each point or bar represents the mean ± SEM of 30 embryos.
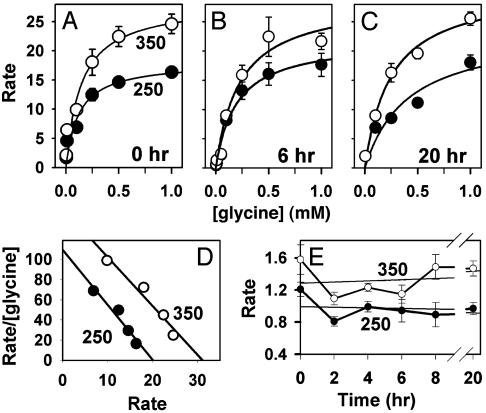
Osmotic Regulation of Intracellular Glycine Concentration in Embryos. If GLYT1 functions as an organic osmolyte transporter in embryos, its activity should be regulated in response to changes in osmolarity. Therefore, we examined the effect of osmolarity on the kinetics of GLYT1-mediated glycine transport in embryos. The rate of acute [3H]glycine uptake (over 10 min) was measured as a function of external glycine concentration immediately after embryos were removed from the oviduct, washed, and transferred into [3H]glycine-containing media at either 250 or 350 mOsM. After subtraction of the small amount of glycine influx not due to GLYT1, glycine transport by GLYT1 was found to exhibit simple saturation kinetics at both 250 and 350 mOsM (Fig. 7A), but the rate of transport was higher overall at 350 than at 250 mOsM, showing that GLYT1 transport was stimulated by increased osmolarity. The kinetic parameters of GLYT1 transport at each osmolarity were determined by nonlinear least-squares fits to the Michaelis–Menten equation (Fig. 7A), which indicated that Km did not appear to change appreciably while Vmax increased by ≈50% (from 18 fmol per embryo per min at 250 mOsM to 28 fmol per embryo per min at 350 mOsM). Analysis by Eadie–Hofstee plot (Fig. 7D) found a similar increase in Vmax (from 20 to 31 fmol per embryo per min).
Fig. 7.
Kinetics of GLYT1 transport in embryos. (A–C) The acute rate of uptake of glycine was measured immediately after (A), 6 h after (B), or 20 h after (C) transfer of embryos to 250 mOsM (○) or 350 mOsM (•) medium. Rate units are fmol per embryo per min. Data are presented after subtraction of the nonspecific linear component determined in the presence of 5 μM ORG23798 plus 5 mM sarcosine (≤15% of total at 1 mM; not significantly different from rate in ORG23798 or sarcosine alone; not shown). Curves represent the Michaelis–Menten relation fit by nonlinear least squares. Each point represents the mean ± SEM of five replicates. (D) Eadie–Hofstee plot of data shown in A, excluding concentrations <0.1 mM, where rates were low and hence data too variable. (E) Acute rate or uptake of 10 μM glycine measured as a function of time at 250 or 350 mOsM. There was no trend toward an increase over time (slopes by linear regression yield changes in rate of –0.004 and +0.007 fmol per embryo per min for each hour at 250 and 350 mOsM, respectively). Each point represents the mean ± SEM of five replicates.
We also assessed whether glycine transport kinetics changed with time during osmotically regulated glycine accumulation (after 6 h at 250 or 350 mOsM) or after accumulation had reached steady state (after 20 h), compared with those measured immediately after transfer to media of different osmolarities. Embryos were thus cultured for these periods in the presence of 1 mM unlabeled glycine at the same osmolarity at which kinetic measurements were subsequently made (Fig. 7 B and C). Although there appeared to be increased variability in the data, particularly during active accumulation at 6 h, the rate of glycine transport appeared higher at 350 mOsM than at 250 mOsM after both periods of incubation.
It seemed from these data that the kinetics of transport did not change appreciably with time, with longer periods (6 or 20 h) at higher osmolarity not appearing to further increase Vmax of glycine transport by GLYT1 over that seen when measurements were made immediately after placing embryos into 250 and 350 mOsM media. To confirm that transport rates did not change over time, we determined the rate of transport of 10 μM glycine (below Km and thus sensitive to changes in either Km or Vmax) at 0–20 h, and found no systematic increase in rate with time (Fig. 7E). However, the increase in transport rate at 350 over 250 mOsM was again highly significant (P < 0.0001 by ANOVA).
This time-independence allowed the kinetic measurements that had been performed after each of the three durations of culture (0, 6, and 20 h; Fig. 7 A–C) to be combined for analysis. t tests then confirmed that there was no significant difference in Km at 250 vs. 350 mOsM, with means of 0.24 ± 0.08 mM and 0.21 ± 0.03 mM, respectively (P = 0.71). However, the increase in Vmax that occurred when osmolarity increased from 250 to 350 mOsM was highly significant (P = 0.015), increasing from a mean of 21.3 ± 1.7 to 29.3 ± 0.9 fmol per embryo per min, respectively. The Vmax values yielded by Eadie–Hofstee plots were similar, increasing from a mean of 20.7 ± 0.3 to 30.7 ± 0.3 fmol per embryo per min as osmolarity increased from 250 to 350 mOsM (P < 0.0001). Increased osmolarity thus stimulated GLYT1 transport in embryos by an increase in Vmax without changing its apparent affinity for glycine, and this increase occurred as quickly as measurements could be made.
Because both the stimulation of the rate of glycine transport and the onset of osmotically stimulated glycine accumulation (Fig. 2) occurred in embryos without an apparent lag time, it seemed unlikely that the up-regulation of GLYT1 relied on transcription and translation like that required for the hypertonic stimulation of the other known mammalian organic osmolyte transporters. We therefore tested whether the stimulation of glycine accumulation in embryos would still occur in the absence of protein synthesis. Protein synthesis was blocked by using cycloheximide (50 μg/ml) continually present beginning 1 h before [3H]glycine was introduced. After 8-h exposure to glycine, embryos had accumulated 3.8 ± 0.2 and 5.8 ± 0.6 pmol of glycine per embryo at 250 and 350 mOsM, respectively, in the absence of cycloheximide, compared with 3.9 ± 0.2 and 6.5 ± 0.4 pmol per embryo at 250 and 350 mOsM, respectively, in its presence. At each osmolarity, glycine accumulation was not significantly different in the presence vs. absence of cycloheximide (P > 0.05 by ANOVA and Tukey–Kramer test, n = 5), and the amount of glycine accumulated at 350 mOsM in the presence of cycloheximide was significantly greater than that at 250 mOsM (P < 0.01). Thus, inhibition of protein synthesis did not affect the ability of increased osmolarity to stimulate glycine accumulation by one-cell embryos.
Discussion
We have shown here that the GLYT1 glycine transporter of the NTT family functions as an organic osmolyte transporter in early preimplantation mouse embryos. First, its substrate, glycine, acts as an organic osmolyte in embryos, because it protected against increased osmolarity, it supported the maintenance of normal cell volume, and its intracellular accumulation was regulated by osmolarity. Second, GLYT1 activity is required for osmoprotection by glycine in embryos, because a GLYT1-specific inhibitor, ORG23798, eliminated osmoprotection by glycine, reversed its ability to maintain cell volume, and blocked the osmotically regulated intracellular accumulation of glycine. Third, the rate of glycine transport by GLYT1 in early preimplantation embryos was regulated in response to changes in osmolarity, with Vmax increasing by ≈50% as osmolarity increased from 250 to 350 mOsM.
The NTT family also includes two of the established organic osmolyte transporters, BGT1 and TAUT (23). Similar to these, GLYT1 cotransports its substrate with both Na+ and Cl–, permitting the accumulation of its substrate to very high intracellular levels. Thus, GLYT1, like these other NTT family members, is ideally suited to function as an organic osmolyte transporter because concentrations of at least tens of millimolar must be achieved to be osmotically significant.
The regulation of GLYT1 by osmolarity apparently differs from that of previously described mammalian organic osmolyte transporters. The four that are known (as well as aldose reductase, which mediates synthesis of the organic osmolyte sorbitol) respond to increased osmolarity by increasing Vmax only after a lag of hours to days, and stimulation by hypertonicity requires mRNA and protein synthesis (4, 5, 7). In contrast, osmosensitive glycine accumulation in embryos began immediately in response to higher osmolarity, and the rate of glycine transport by GLYT1 was rapidly increased at higher osmolarity and persisted at a nearly constant rate with no substantial change in kinetics over at least 20 h. In addition, osmotic stimulation of intracellular glycine accumulation was undiminished when protein synthesis was suppressed.
The role GLYT1 plays in cell volume regulation and osmoprotection may also differ from that of other established organic osmolyte transporters. While the latter appear designed to respond to long-term, sustained changes in osmolarity (as evidenced by substantial delays before transport rate increases), GLYT1 in embryos appears better suited for maintaining steady-state intracellular organic osmolyte concentration by responding quickly to deviations from preferred cell volume or the desired intracellular organic osmolyte concentration. Its rapid, synthesis-independent response, which more closely resembles that of volume-regulatory inorganic ion transporters than known organic osmolyte transporters (1), is consistent with such a role.
We do not yet know whether GLYT1 functions as an organic osmolyte transporter in cells other than those in the early embryo. The earliest stages of development, starting with the large fertilized egg, are characterized by unique reductive cleavages without intervening growth phases. These produce several generations of progressively smaller totipotent cells before cell lineages begin to diverge just before blastocyst formation. During the preimplantation period, GLYT1 activity is present only during these cleavage stages (20, 35). GLYT1 is then expressed again in a tissue-specific manner during postimplantation development and organogenesis (36), including the CNS, where it functions in neurotransmitter reuptake. Further work is needed to determine whether GLYT1's role in volume regulation is unique to the brief period when it is expressed in the very early cleavage-stage embryo, or whether GLYT1 has a more general role as an organic osmolyte transporter in differentiated tissues as well as in the early embryo.
Acknowledgments
We thank Dr. Richard Armer for the gift of ORG23798 and Dr. Lon Van Winkle and Dr. Ajoy Basak for helpful discussions. This work was supported by Canadian Institutes of Health Research Grants MOP12040 and IHD61218/MOP62730.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NTT, neurotransmitter transporter; mOsM, milliosmolar; TAUT, taurine/β-amino acid transporter; TCA, trichloroacetic acid; ORG23798, GLYT1 inhibitor bis(4-fluorophenyl)methylenepiperidineacetic acid, lithium salt.
References
- 1.Hallows, K. R. & Knauf, P. A. (1994) in Cellular and Molecular Physiology of Cell Volume Regulation, ed. Strange, K. (CRC, Boca Raton, FL), pp. 3–29.
- 2.Wehner, F., Olsen, H., Tinel, H., Kinne-Saffran, E. & Kinne, R. K. (2003) Rev. Physiol Biochem. Pharmacol. 148, 1–80. [DOI] [PubMed] [Google Scholar]
- 3.Yancey, P. H., Clark, M. E., Hand, S. C., Bowlus, R. D. & Somero, G. N. (1982) Science 217, 1214–1222. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Perez, A. & Burg, M. B. (1991) J. Membr. Biol. 119, 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Kwon, H. M. & Handler, J. S. (1995) Curr. Opin. Cell Biol. 7, 465–471. [DOI] [PubMed] [Google Scholar]
- 6.Kempf, B. & Bremer, E. (1998) Arch. Microbiol. 170, 319–330. [DOI] [PubMed] [Google Scholar]
- 7.Pastor-Anglada, M., Felipe, A., Casado, F. J., Ferrer-Martinez, A. & Gomez-Angelats, M. (1996) Amino Acids 11, 135–151. [DOI] [PubMed] [Google Scholar]
- 8.Miyakawa, H., Woo, S. K., Dahl, S. C., Handler, J. S. & Kwon, H. M. (1999) Proc. Natl. Acad. Sci. USA 96, 2538–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takenaka, M., Preston, A. S., Kwon, H. M. & Handler, J. S. (1994) J. Biol. Chem. 269, 29379–29381. [PubMed] [Google Scholar]
- 10.Ferraris, J. D., Williams, C. K., Persaud, P., Zhang, Z., Chen, Y. & Burg, M. B. (2002) Proc. Natl. Acad. Sci. USA 99, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang, F., Busch, G. L. & Volkl, H. (1998) Cell Physiol. Biochem. 8, 1–45. [DOI] [PubMed] [Google Scholar]
- 12.Collins, J. L. & Baltz, J. M. (1999) Biol. Reprod. 60, 1188–1193. [DOI] [PubMed] [Google Scholar]
- 13.Biggers, J. D., Lawitts, J. A. & Lechene, C. P. (1993) Mol. Reprod. Dev. 34, 380–390. [DOI] [PubMed] [Google Scholar]
- 14.Lawitts, J. A. & Biggers, J. D. (1992) Mol. Reprod. Dev. 31, 189–194. [DOI] [PubMed] [Google Scholar]
- 15.Dawson, K. M. & Baltz, J. M. (1997) Biol. Reprod. 56, 1550–1558. [DOI] [PubMed] [Google Scholar]
- 16.Baltz, J. M. (2001) in Current Topics in Developmental Biology, ed. Schatten, G. P. (Academic, San Diego), pp. 55–106.11529430
- 17.Van Winkle, L. J., Haghighat, N. & Campione, A. L. (1990) J. Exp. Zool. 253, 215–219. [DOI] [PubMed] [Google Scholar]
- 18.Dawson, K. M., Collins, J. L. & Baltz, J. M. (1998) Biol. Reprod. 59, 225–232. [DOI] [PubMed] [Google Scholar]
- 19.Van Winkle, L. J., Patel, M., Wasserlauf, H. G., Dickinson, H. R. & Campione, A. L. (1994) Biochim. Biophys. Acta 1191, 244–255. [DOI] [PubMed] [Google Scholar]
- 20.Van Winkle, L. J., Haghighat, N., Campione, A. L. & Gorman, J. M. (1988) Biochim. Biophys. Acta 941, 241–256. [DOI] [PubMed] [Google Scholar]
- 21.Jamshidi, M. B. & Kaye, P. L. (1995) J. Reprod. Fertil. 104, 91–97. [DOI] [PubMed] [Google Scholar]
- 22.Guastella, J., Brecha, N., Weigmann, C., Lester, H. A. & Davidson, N. (1992) Proc. Natl. Acad. Sci. USA 89, 7189–7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson, N. (1998) J. Neurochem. 71, 1785–1803. [DOI] [PubMed] [Google Scholar]
- 24.Lawitts, J. A. & Biggers, J. D. (1993) Methods Enzymol. 225, 153–164. [DOI] [PubMed] [Google Scholar]
- 25.Biggers, J. D. (1998) Int. J. Dev. Biol. 42, 879–884. [PubMed] [Google Scholar]
- 26.Hogan, B., Beddington, R., Constantini, F. & Lacy, E. (1994) Manipulating the Mouse Embryo: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 27.Borland, R. M. & Tasca, R. J. (1974) Dev. Biol. 36, 169–182. [DOI] [PubMed] [Google Scholar]
- 28.Morrow, J. A., Collie, I. T., Dunbar, D. R., Walker, G. B., Shahid, M. & Hill, D. R. (1998) FEBS Lett. 439, 334–340. [DOI] [PubMed] [Google Scholar]
- 29.Brown, A., Carlyle, I., Clark, J., Hamilton, W., Gibson, S., McGarry, G., McEachen, S., Rae, D., Thorn, S. & Walker, G. (2001) Bioorg. Med. Chem. Lett. 11, 2007–2009. [DOI] [PubMed] [Google Scholar]
- 30.Guerin, J. F., Gallois, E., Croteau, S., Revol, N., Maurin, F., Guillaud, J. & Menezo, Y. (1995) Revue Med. Vet. 146, 805–814. [Google Scholar]
- 31.Van Winkle, L. J., Mann, D. F., Wasserlauf, H. G. & Patel, M. (1992) Biochim. Biophys. Acta 1107, 299–304. [DOI] [PubMed] [Google Scholar]
- 32.Van Winkle, L. J., Campione, A. L., Gorman, J. M. & Weimer, B. D. (1990) Biochim. Biophys. Acta 1021, 77–84. [DOI] [PubMed] [Google Scholar]
- 33.Van Winkle, L. J. & Campione, A. L. (1990) Biochim. Biophys. Acta 1028, 165–173. [DOI] [PubMed] [Google Scholar]
- 34.Van Winkle, L. J., Mann, D. F., Campione, A. L. & Farrington, B. H. (1990) Biochim. Biophys. Acta 1027, 268–277. [DOI] [PubMed] [Google Scholar]
- 35.Hobbs, J. G. & Kaye, P. L. (1990) Reprod. Fertil. Dev. 2, 651–660. [DOI] [PubMed] [Google Scholar]
- 36.Adams, R. H., Sato, K., Shimada, S., Tohyama, M., Puschel, A. W. & Betz, H. (1995) J. Neurosci. 15, 2524–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]