Abstract
To elucidate the role of juvenile hormone (JH) in metamorphosis of Drosophila melanogaster, the corpora allata cells, which produce JH, were killed using the cell death gene grim. These allatectomized (CAX) larvae were smaller at pupariation and died at head eversion. They showed premature ecdysone receptor B1 (EcR-B1) in the photoreceptors and in the optic lobe, downregulation of proliferation in the optic lobe, and separation of R7 from R8 in the medulla during the prepupal period. All of these effects of allatectomy were reversed by feeding third instar larvae on a diet containing the JH mimic (JHM) pyriproxifen or by application of JH III or JHM at the onset of wandering. Eye and optic lobe development in the Methoprene-tolerant (Met)-null mutant mimicked that of CAX prepupae, but the mutant formed viable adults, which had marked abnormalities in the organization of their optic lobe neuropils. Feeding Met27 larvae on the JHM diet did not rescue the premature EcR-B1 expression or the downregulation of proliferation but did partially rescue the premature separation of R7, suggesting that other pathways besides Met might be involved in mediating the response to JH. Selective expression of Met RNAi in the photoreceptors caused their premature expression of EcR-B1 and the separation of R7 and R8, but driving Met RNAi in lamina neurons led only to the precocious appearance of EcR-B1 in the lamina. Thus, the lack of JH and its receptor Met causes a heterochronic shift in the development of the visual system that is likely to result from some cells ‘misinterpreting’ the ecdysteroid peaks that drive metamorphosis.
Keywords: Juvenile hormone, Optic lobe, Methoprene-tolerant, Ecdysone receptor-B1
INTRODUCTION
Insect molting and metamorphosis are governed primarily by ecdysone (used in the generic sense) and juvenile hormone (JH), with ecdysone causing molting and JH preventing metamorphosis. Juvenile hormone has a classic ‘status quo’ action in preventing the program-switching action of ecdysone during larval molts (Riddiford, 1994; Riddiford et al., 2001) and in maintaining the developmental arrest of imaginal primordia during the intermolt periods (Truman et al., 2006). Its effects at the outset of metamorphosis, though, are more complex. Studies mainly on Lepidoptera show that for selected tissues JH needs to be present to allow them to undergo pupal differentiation, rather than undertaking a precocious adult differentiation (Williams, 1961; Kiguchi and Riddiford, 1978; Champlin and Truman, 1998a; Champlin et al., 1999).
The mechanism through which JH maintains the status quo and directs early development at metamorphosis is still poorly understood. Whether JH has one or multiple receptors (Wheeler and Nijhout, 2003; Riddiford, 2008), and the nature of these receptors, is still controversial. The best candidate for a receptor is the product of the Methoprene-tolerant (Met) gene, a PAS domain protein that was originally isolated in Drosophila melanogaster (Ashok et al., 1998). In vitro transcribed and translated Met protein has been shown to bind JH with high affinity (Miura et al., 2005), and RNAi knock-down experiments in Tribolium castaneum show that Met is essential for mediating the status quo action of JH in this beetle (Konopka and Jindra, 2007; Parthasarathy et al., 2008).
In D. melanogaster, JH is thought to have no role in the onset of metamorphosis, as exogenous JH only delays but does not prevent pupariation (Riddiford and Ashburner, 1991; Riddiford et al., 2003). Although it has no apparent effect on the development of the imaginal discs, JH prevents normal adult development of the abdominal integument when given at pupariation (for a review, see Riddiford, 1993) (see also Zhou and Riddiford, 2002). Internally, JH at this time affects normal reorganization of the central nervous system and development of the thoracic musculature (Restifo and Wilson, 1998). These effects of JH on metamorphosis do not occur in Met mutants, unless at least 100 times the dose is given (Wilson and Fabian, 1986; Restifo and Wilson, 1998). The Met27-null mutants proceed through larval development and metamorphosis apparently normally (Wilson and Ashok, 1998). However, if in addition, RNAi is used to suppress expression of Germ-Cell Expressed (Gce), a related bHLH protein with a high similarity to Met that heterodimerizes with it (Godlewski et al., 2006), Met-null mutants die as pharate adults (Liu et al., 2009). In the Met-deficient mutant, the adult eye shows a few (<12) defective ommatidia in the posterior region (Wilson et al., 2006). Also, the females mature fewer eggs at a slower rate than do wild-type females (Wilson and Ashok, 1998), indicating that Met is also important for JH effects in egg maturation (Soller et al., 1999). While this paper was under review, Liu et al. (Liu et al., 2009) showed that the fat body underwent precocious cell death beginning in the third larval instar in larvae that were allatectomized using the same protocol as we describe below.
Here, we have genetically allatectomized Drosophila larvae by targeting expression of a cell death gene to the corpora allata (CA), the gland that produces JH. These larvae formed smaller puparia and showed precocious maturation of the visual system, but died around head eversion.
MATERIALS AND METHODS
Animals
Aug21-GAL4 [a stock obtained by T. Siegmund and G. Korge but not reported in the screen for neurosecretory neurons (Siegmund and Korge, 2001)], UAS-grim (Wing et al., 1998), and the Met27-null mutant (Wilson and Ashok, 1998) stocks were used. The GAL4 lines R15E10, R17A12, R23C06, R25B08 and R27G05 were constructed as described by Pfeiffer et al. (Pfeiffer et al., 2008). The UAS-EcR-B1 (Lee et al., 2000) and UAS-MetRNAi (45852 and 45854 from the Vienna Drosophila RNAi Center) lines were used. The Aug21-GAL4/CyO, actin-GFP, the R27G05; UAS-mCD8::GFP and the Met27; Aug21-GAL4, UAS-GFP/CyO stock were constructed at the Janelia Farm Fly Core facility.
The Met27 fly stocks and the GAL4>UAS-EcR-B1 and the GAL4>UAS-MetRNAi crosses were maintained at 25°C. The Aug21-GAL4/CyO, actin-GFP×UAS-grim crosses were maintained at 29°C until the late third instar, then transferred to 25°C. The Aug21>grim larvae were separated from the CyO, UAS-grim larvae by the expression of a GFP transgene on the CyO chromosome.
Juvenile hormone treatments
Larvae or white puparia were given the JH mimic (JHM) pyriproxifen (Sumitomo Chemical Company, Osaka, Japan) or 7R-JH III (Toong et al., 1988) (gift of Dr Y. Toong) topically or in the diet. For topical application, 0.2 μl cyclohexane containing a specified amount of JH III or JHM was applied to wandering larvae under CO2 anesthesia or to white puparia. For the feeding experiments, 5 μg JHM in 50-100 μl ethanol was mixed with 5 ml of standard diet to give a diet containing 1 part per million (ppm) JHM.
Immunocytochemistry and imaging
Brains and other tissues were dissected and fixed in 3.9% formaldehyde (Fisher Scientific, Fairlawn, NJ, USA) in phosphate-buffered saline (PBS; Mediatech, Manassas, VA, USA) for 30 minutes, then rinsed and incubated in PBS-1% Triton X-100 (TX) with 2% normal donkey serum for 30 minutes. They were then incubated in PBS-1% TX with primary antibody for 1-2 days at 4°C, rinsed, then incubated in secondary antibody overnight at 4°C. Primary antibodies used were: ecdysone receptor, B1 isoform (EcR-B1; mouse ascites fluid AD 4.4; 1:10,000; from C. S. Thummel, University of Utah, Salt Lake City, UT, USA); Fasciclin II [mouse monoclonal antibody (MAb) 1D4; 1:50; from C. Goodman, University of California, Berkeley, Berkeley, CA, USA]; Deadpan (rat MAb; 1:1; from C. Q. Doe; Boone and Doe, 2008); phosphohistone H3 (PH3; 1:1000; Upstate, Millipore, Billerica, MD, USA); and chaoptin (mouse MAb 24B10; 1:50), N-cadherin (rat MAb MNCD2; 1:50) and neuroglian (mouse MAb BP104; 1:40; all from the Developmental Studies Hybridoma Bank, Iowa City, IA, USA). Secondary antibodies were fluorescein, Texas Red or Cy5 conjugated and were used at 1:500 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Oregon Green 488 phalloidin (Molecular Probes, Invitrogen, Carlsbad, CA, USA) was sometimes used as a counterstain.
Immunostained preparations were typically dehydrated through a graded ethanol series, cleared in xylene and mounted in DPX (Fluka BioChemika, Sigma-Aldrich, St Louis, MO, USA). Eye discs that were counterstained with phalloidin were mounted directly into Vectashield (Vector Laboratories, Burlingame, CA, USA) to preserve the phallodin immunofluorescence. The preparations were imaged on a Zeiss 510 confocal microsocope and processed with Image J. Counts of the number of dividing cells, as indicated by PH3 labeling, were performed using V3D software developed by H. Peng (Janelia Farm Research Campus, HHMI, Ashlam, VA, USA; http://penglab.janelia.org/proj/v3d/v3d2.html).
RESULTS
Effects of genetic allatectomy on larval development and metamorphosis
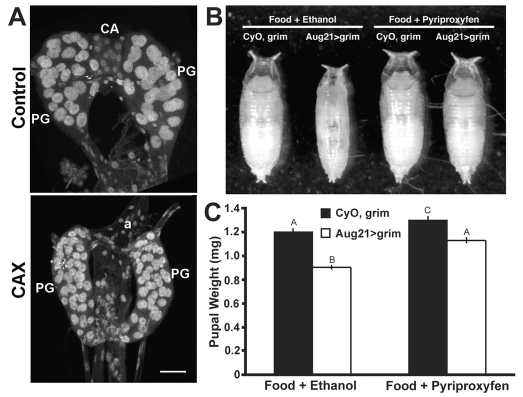
We confirmed that Aug21-GAL4 drove UAS-GFP expression in the CA (T. Siegmund and G. Korge, personal communication) and found that it was also expressed strongly in the salivary glands. When Aug21-GAL4 drove the cell death gene grim (Wing et al., 1998) in these tissues, both sets of glands were either missing or in a state of advanced degeneration by the onset of the third instar (data not shown), and all remnants of the CA had disappeared by the onset of wandering (Fig. 1A). Therefore, the larvae had been allatectomized (CAX).
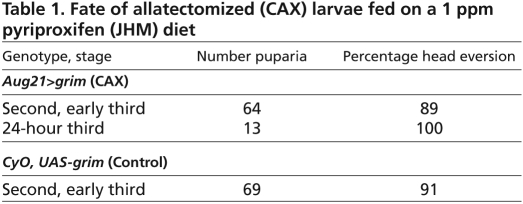
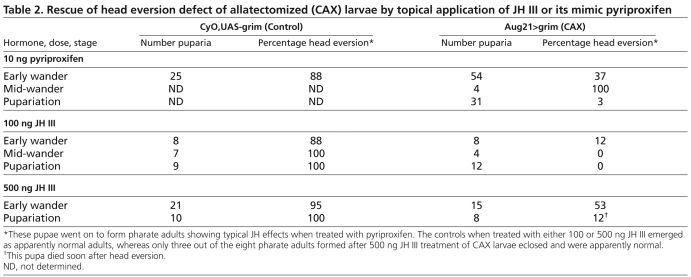
Fig. 1.
Genetic allatectomy and its effect on puparial size. (A)The ring gland of control CyO, UAS-grim (above) and allatectomized (CAX) Aug21>grim (below) wandering larvae showing the loss of the corpora allata (CA) in the latter. a, aorta; PG, prothoracic gland. Scale bar: 25 μm. (B,C) Sample puparia (B) and the pupal weights (C) of CyO, UAS-grim (control) and Aug21>grim (allatectomized) larvae raised on normal diet containing 1% ethanol or 1 ppm pyriproxyfen (JHM) in 1% ethanol. Bars represent the average ± s.d. for 50-60 puparia (one day after wandering). Different letters denote average weights that are significantly different (P≤0.0001, using one-way ANOVA and Tukey-Kramer HSD comparison of means).
The Aug21>grim (CAX) larvae developed slightly slower than did their CyO, UAS-grim counterparts, and pupariated about 8-12 hours later. They were also about 75% of the weight of their CyO, UAS-grim siblings (Fig. 1B,C). Survival to pupariation was similar for both the CAX and the control (CyO, UAS-grim) larvae; of a total of 1300 puparia scored, 51% were from CAX larvae and 49% from CyO, UAS-grim larvae. When fed a diet containing 1 ppm JHM during the third instar, the CAX larvae grew to the normal size (Fig. 1B,C), showing that the effects on growth were due to the lack of the CA and not to the degeneration of the salivary glands. Both the control CyO, UAS-grim and the CAX larvae on the JHM diet delayed pupariation by 1-2 days.
Although the CAX larvae showed normal viability up to pupariation, they typically died around the time of head eversion. Video imaging showed that they initiated head eversion at the normal time but were unable to eject the mouth hooks completely and evert the pupal head (six out of seven animals). One of these did complete head eversion. Such ‘escapers’ were not found at 29°C, but occurred 20% of the time when the white puparia were placed at 18°C (n=20). When fed a 1 ppm JHM diet during the third instar, 89% (n=64) continued beyond head eversion and arrested as pharate adults just before eclosion (Table 1). These showed truncated and missing abdominal bristles, the typical effects of JH application at pupariation (Postlethwait, 1974; Riddiford and Ashburner, 1991). When 10 ng JHM was applied to early wandering larvae, 37% (n=54) formed pharate adults, but only one pharate adult was formed after the same treatment of white puparia (Table 2). A similar rescue was obtained after treatment with 500 ng JH III during early wandering (Table 2).
Table 1.
Fate of allatectomized (CAX) larvae fed on a 1 ppm pyriproxifen (JHM) diet
Table 2.
Rescue of head eversion defect of allatectomized (CAX) larvae by topical application of JH III or its mimic pyriproxifen
Effect of allatectomy on development of the visual system
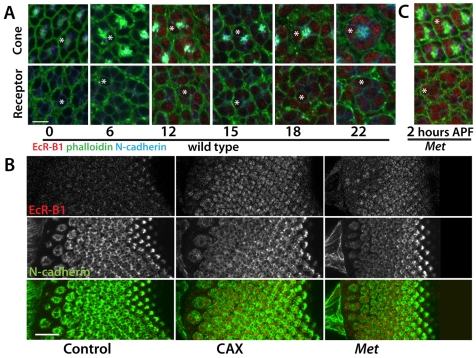
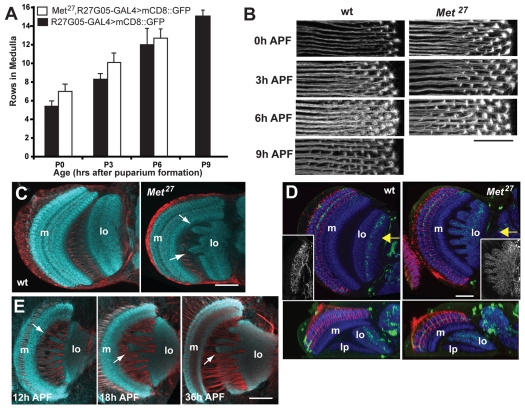
In the tobacco hornworm, Manduca sexta, lack of JH during the prepupal molt leads to premature adult differentiation of the eye (Kiguchi and Riddiford, 1978; Champlin and Truman, 1998a). To determine whether JH has a similar effect in Drosophila, we looked at the early development of the visual system in the CAX animals. We used the appearance of the B1 isoform of the Ecdysone Receptor (EcR-B1) as a molecular marker for developmental timing in the retina and the optic lobes. In controls EcR-B1 is absent from the eye disc at pupariation (Fig. 2A). It becomes evident in the crystalline cone cells and underlying photoreceptors by 12 hours after puparium formation (APF). Its expression then transiently fades in these cells but returns at 18 to 22 hours APF. Although expression is prominent in the cones, it is absent from most of the surrounding pigment cells. In the optic lobes, the only neurons to express EcR-B1 at pupariation are the three larval visual interneurons (Truman et al., 1994). The first traces of EcR-B1 expression appear in neurons in the inner proliferation zone at about 6 hours APF (data not shown). By 8 hours, EcR-B1 expression is evident in the lamina, medulla and lobula; it is missing from the inner and outer proliferation zone stem cells, which are Deadpan positive (Egger et al., 2007), and the strongest EcR-B1 expression is in the neurons that are the farthest from the proliferation zones, suggesting that neurons need to be a certain time after their birth before they are competent to express EcR-B1 (Fig. 3A,D). EcR-B1 expression is maintained in optic lobe neurons through about 50 hours APF (Truman et al., 1994) (see also Fig. 6C).
Fig. 2.
Developmental appearance of the ecdysone receptor EcR-B1 in the photoreceptors in CyO, UAS-grim (control), Aug21>grim (allatectomized, CAX), and Met27 prepupae and pupae. (A) Confocal sections of developing ommatidia at the levels of the crystalline cone cells (top) and the photoreceptors (bottom) showing the time course of appearance of EcR-B1 (red) in wild-type prepupae and pupae. Cellular outlines are delineated by phalloidin staining (green). Times shown are hours after puparium formation (APF). Note that EcR-B1 is first evident at 12 hours APF. Scale bar: 5 μm. (B) Confocal sections showing the precocious presence of EcR-B1 in the photoreceptors of allatectomized (CAX) and Met27 eye discs at the time of pupariation. N-cadherin (green) stains developing photoreceptors. Scale bar: 20 μm. (C) Ommatidia from the eye disc of a Met27 prepupa at 2 hours APF showing the precocious appearance of EcR-B1. In A and C, the asterisks indicate an example of a cone cell (top) or a photoreceptor (bottom).
Fig. 3.
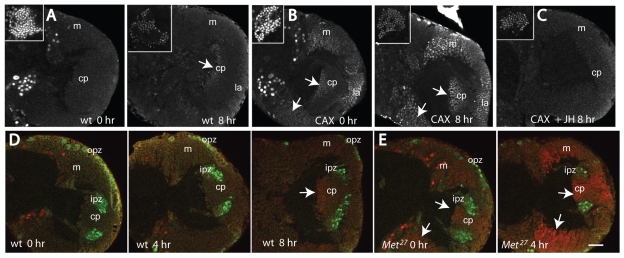
Precocious appearance of the ecdysone receptor EcR-B1 in the optic lobes (OL) of Aug21>grim allatectomized (CAX) prepupae and its suppression by feeding larvae on a diet containing 1 ppm pyriproxyfen (JHM). (A-E) OL confocal sections at different times (X hours; hr) APF of (A,D) CyO, UAS-grim (control) prepupae, (B) CAX prepupae after feeding on normal diet, (C) CAX prepupa after feeding on the JHM diet, and (E) Met27 prepupae. Arrows indicate EcR-B1-positive neurons. Insets in A-C show endogenous EcR-B1 in the mushroom body neurons. (D,E) Deadpan-positive neuroblasts (green); EcR-B1 neurons (red). cp, ‘central plug’ neurons produced by the neuroblasts of the inner proliferation zone (ipz); la, lamina; m, medulla; opz, outer proliferation zone. Scale bar: 25 μm.
Fig. 6.
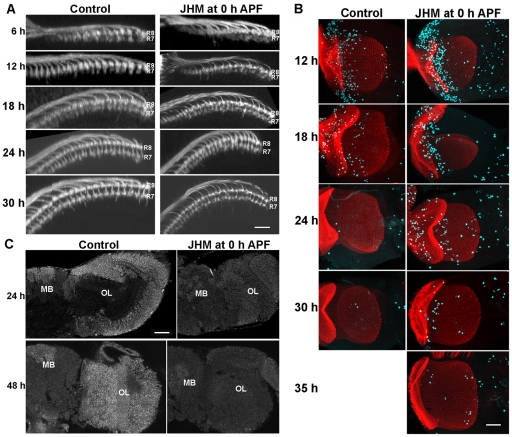
The effect of 100 ng pyriproxifen (JHM) applied at the time of pupariation on OL development. (A) Dorsal view of the medulla showing that JHM treatment retards the separation of the R7 and R8 growth cones. (B) Frontal projections of the optic lobe showing the prolongation of proliferation in the JHM-treated animals: red, chaoptin; aqua, phosphohistone H3. (C) JHM treatment suppresses the appearance of EcR-B1. h, hours APF; MB, mushroom body. Scale bars: 10 μm in A; 25 μm in B,C.
The expression of EcR-B1 was significantly advanced in the CAX prepupae, such that it was evident in both the eye disc and the optic lobes at the time of pupariation. In the eye disc, it was expressed in the cone cells and photoreceptors in the posterior region of the disc (Fig. 2B). It was absent in cells anterior to the furrow (data not shown) and in the youngest ommatidia that were organizing just posterior to the furrow (Fig. 2B). In the optic lobes, EcR-B1 expression was seen in neurons of the medulla and the ‘central plug’ (Fig. 3B), but not in the neuroblasts of the inner or outer proliferation zones (Fig. 3B). When third instar CAX larvae were fed on a diet containing 1 ppm JHM, no EcR-B1 was detectable throughout the prepupal period (Fig. 3C; data not shown). Therefore, the early appearance of EcR-B1 in the photoreceptors and optic lobe neurons in CAX larvae is due to the lack of JH during the late third larval instar.
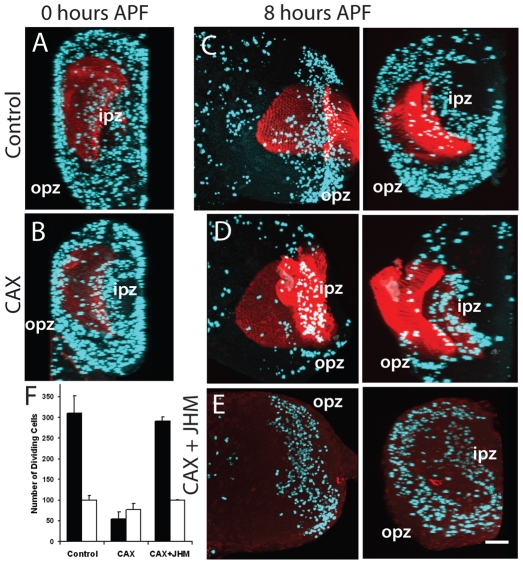
We assessed the cellular effects of the lack of JH by examining the patterns of proliferation in the visual system and also the morphogenesis of the growth cones of the photoreceptors that project into the medulla. Proliferation was monitored by immunostaining for PH3 to detect mitotic cells. We saw no obvious effect of removal of JH on the proliferation pattern in the imaginal discs (data not shown). We did, however, see a strong effect on proliferation in the optic lobes. At pupariation both intact and CAX animals had comparable amounts of proliferation in both the inner and outer proliferation zones (Fig. 4A,B), although the brains of the CAX larvae were somewhat smaller because of the overall reduction in body size. In control animals, proliferation continued at a high level in the outer proliferation zone through the next 8 hours (Fig. 4C), but had markedly declined by 12 hours APF (data not shown). In the CAX prepupae, by contrast, proliferation in the outer zone had dropped down to about 15% of control values by 8 hours APF (Fig. 4D,F). When the CAX larvae were fed on a JHM-containing diet, the number of mitotic cells in the outer proliferation zone was elevated to levels seen in intact prepupae (Fig. 4E,F). Proliferation in the inner proliferation zone in the CAX prepupae showed only a slight, but significant (P=0.003), reduction, which was also restored by feeding on a JHM-containing diet (Fig. 4C-F).
Fig. 4.
Precocious decline in proliferation in the OL in Aug21>grim allatectomized (CAX) prepupae and its prevention by feeding larvae on diet containing 1 ppm pyriproxyfen (JHM). (A-E) The OL at different times (hours) APF of (A,C) CyO, UAS-grim (control) prepupae, (B,D) CAX prepupae after feeding on normal diet, and (E) a CAX prepupa after feeding on the JHM diet. (C-E) Frontal z-projection of the optic lobe (left) and a 90° projection along the x-axis (right), showing an end-on view to discriminate the inner (ipz) and outer (opz) proliferation zones; (A,B) x-axis projections. Aqua represents PH3 staining of mitotic cells; red is chaoptin staining of the incoming photoreceptors, except in E where it was omitted. Scale bar: 50 μm. (F) Mean number (±s.d.) of proliferating cells in the opz (black bars) and the ipz (white bars) of 3-5 animals at 8 hours APF.
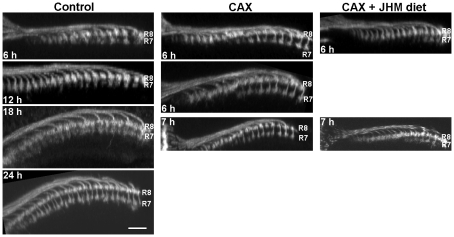
The adult optic lobe depends on incoming photoreceptors from the eye to direct its development (Meinertzhagen and Hanson, 1993; Ting and Lee, 2007). At mid-third instar, the morphogenetic furrow begins to move across the eye disc, behind which the photoreceptors in each ommatidium begin to differentiate (Wolff and Ready, 1993). This process continues through the prepupal period until head eversion, when all the ommatidia have formed. We examined the effects of JH on neuronal morphogenesis by focusing on the outgrowth of photoreceptors R7 and R8, which extend axons into the medulla. For a given ommatidium, the R8 growth cone is the first to arrive, followed many hours later by the R7 growth cone, which extends slightly beyond it (e.g. Fig. 5, Control). The growth cones start to separate from each other at 18 hours APF and are clearly separated by 24 hours (Ting et al., 2005). Ingrowing lamina interneurons fill much of the space in between. In the CAX prepupae, we already see the distinct separation between R7 and R8 soon after pupariation (Fig. 5). When CAX larvae were fed on a 1 ppm JHM-containing diet in the third instar, the premature separation did not occur (Fig. 5).
Fig. 5.
Dorsal views of the medulla showing the effects of JH manipulations on the timing of separation of the R7 and R8 growth cones. (Left) Control prepupae showing that growth cones become completely separated by 18 to 24 hours (h) APF. (Middle) Allatectomized (CAX) prepupae show complete separation by 6 hours APF. (Right) Feeding CAX larvae on a JHM diet blocks the precocious separation. Scale bar: 10 μm.
Processes that occur prematurely in CAX animals are delayed or suppressed in wild-type larvae that are treated with JHM. As seen in Fig. 6A, puparia treated with JHM show only a partial separation of R7 from R8 at 24 hours, although separation looks normal by 30 hours APF. Also, in the JHM-treated animals, proliferation was extended by about 12 hours (Fig. 6B) and EcR-B1 expression in the optic lobe was severely suppressed (Fig. 6C).
The role of the Methoprene-tolerant (Met) gene in JH regulation of optic lobe development
The Met gene encodes a likely JH receptor. The Met27 null mutants did not show the small size or prepupal lethality characteristic of the CAX larvae, but they did show similar effects on the development of the optic lobe. As in the CAX prepupae, EcR-B1 appeared precociously in the photoreceptors (Fig. 2B,C) and in the optic lobe (Fig. 3E, Fig. 7A) of Met27 prepupae. Also, proliferation in both the outer and the inner proliferation zones showed an early suppression (Fig. 7B,G), and R7 showed precocious separation from R8 (Fig. 7C).
Fig. 7.
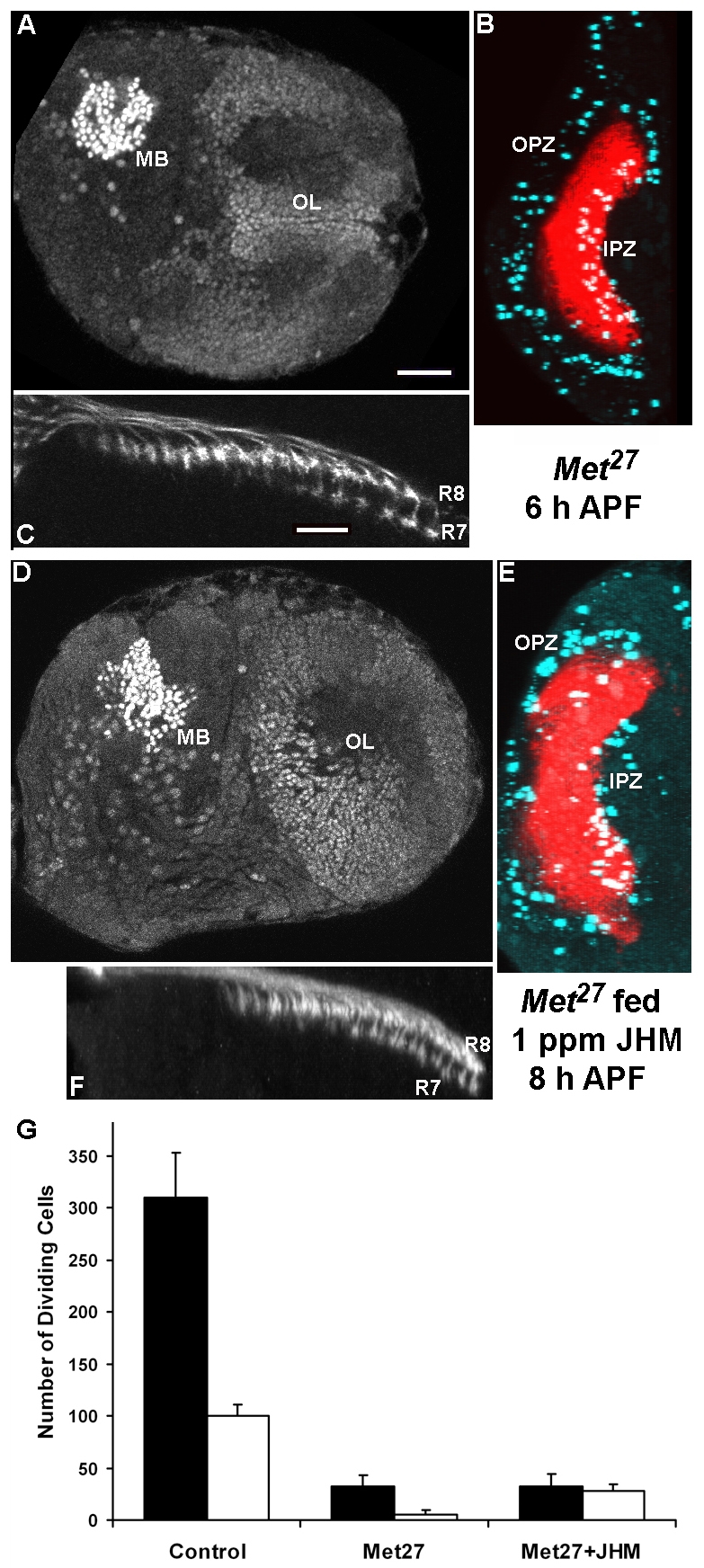
The Met27 mutant shows precocious OL development and reduced sensitivity to a JHM (1 ppm pyriproxifen) diet. (A,D) Frontal sections through the brain and OL showing precocious EcR-B1 immunostaining in the OL of both Met27 prepupae (A) and those fed a JHM diet (D). (B,E) An x-projection of the OL showing that activity in the outer proliferation zone (OPZ) is also precociously reduced in both groups. red, chaoptin; aqua, phosphohistone H3; IPZ, inner proliferation zone. (C,F) Dorsal view of the medulla showing that the prominent separation of the R7 and R8 growth cones is partially suppressed by feeding the mutant JHM. In the above series, the Met27 examples were at 6 hours APF and the Met27 fed JHM were at 8 hours APF. (G) Mean number (±s.d.) of proliferating cells in the OPZ (black bars) and IPZ (white bars) of 4-5 animals at 8 hours APF. The reduced proliferation in the OPZ is not rescued in the mutants that were fed on the JHM diet, whereas the number of dividing cells in the IPZ is partially restored. Scale bars: in A, 25 μm for A,B,D,E; in C, 10 μm for C,F.
When third instar Met27 larvae were fed a diet containing 1 ppm JHM, the hormone mimic was unable to block most of the precocious changes. EcR-B1 still appeared precociously in the prepupal optic lobe (Fig. 7D) and proliferation in the outer proliferation zone was reduced (Fig. 7E,G), just as in prepupae from Met27 larvae fed on a normal diet (control; Fig. 7A,B). By contrast, the proliferation in the inner proliferation zone was significantly increased (P=0.003), although not back to the levels seen in the wild-type prepupae (Fig. 7G). Furthermore, the R7-R8 separation was not as pronounced (Fig. 7F) as in the Met27 prepupae (Fig. 7C), but was more extended than in control prepupae (Fig. 5). Therefore, JH apparently is acting via the Met pathway in controlling EcR-B1 expression and proliferation in the outer proliferation zone, but only partially so to prevent premature shutdown of proliferation in the inner proliferation zone and growth cone separation.
In the Met27 mutant, the separation of the R7 and R8 terminals is evident by 6 hours APF, about 12 hours earlier than normal (Ting et al., 2005) (Fig. 7). To determine if Met was causing an advancement of the development of the entire visual system, relative to pupariation, we used a GAL4 driver line from the Rubin collection of enhancer tester lines (Pfeiffer et al., 2008). R27G05-GAL4 drives expression in lamina neurons that project into the medulla. Using a mouse CD8 (mCD8)::GFP reporter, we counted the number of rows of lamina axon terminals that had grown into the medulla at various times after pupariation. Over the 6 to 9 hours of the prepupal period, both wild-type and Met27 prepupae added approximately one row of terminals per hour, but the Met27 individuals in the early stages were two rows ahead of the controls (Fig. 8A,B). Hence, the loss of Met results in a two-hour advancement in the ingrowth of lamina neurons, relative to the time of puparium formation. However, this difference in the timing of ingrowth is much less than the 12-hour advancement seen for the separation of R7 from R8.
Fig. 8.
Comparison of OL development in wild-type and Met27 mutants. (A,B) R27G05-GAL4 driving mCD8::GFP revealed lamina axon ingrowth into the medulla in wild-type and Met27 backgrounds. (A) The mean number (±s.d.) of rows of lamina neuron terminals seen in the medulla of wild-type (black bars) and Met27 (white) individuals at various times after pupariation; 3-5 samples per bar. (B) Confocal projections of rows of lamina neuron terminals in the medulla at various times APF. Scale bar: 10 μm. (C,D) Frontal sections of the adult OL. (C) The lobula (lo) of the Met27 mutant has abnormal lobes (arrows) that project towards the medulla (m); (D) expression of GFP in the R23C06 line reveals lobula interneurons whose dendrites are normally confined to defined layers of the lobula, but in the mutant they extend throughout this neuropil: red, chaoptin; blue, N-cadherin. Insets show a z-projections of the entire dendritic tree. Below are horizontal sections at the level of the arrow; lp, lobula plate. (E) Sections through the developing optic lobe of Met27 mutants show that lobula irregularities are evident as early as 12 hours APF. Staining in C and E: N-cadherin, aqua; neuroglian, red. Scale bars: 20 μm in B; 50 μm in C-E.
As the Met27 mutants became viable adults, we were able to see the final outcome of the lack of JH signaling on the development of the optic lobes. The gross anatomy of the lamina and medulla appeared normal and we did not see a mistargeting of photoreceptor axons to inappropriate layers (Fig. 8D). The lobula, however, showed gross abnormalities with irregular lobes that projected towards, or sometimes penetrated, the medulla neuropil (Fig. 8C). These projections severely disrupted the layering of the lobula and the lobula plate, such that neurons that normally had their dendrites confined to a single layer, now projected in a disorganized fashion throughout the lobula (Fig. 8D). The beginnings of these irregular projections were evident at 12 hours APF, the same time that cellular changes were occurring in the more superficial neuropils (Fig. 8E).
Cell-cell interactions and the action of JH
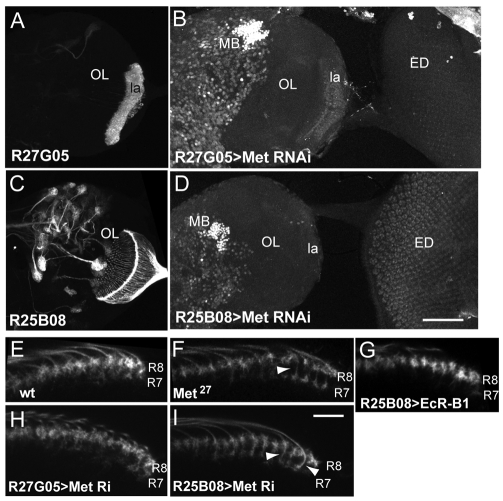
Possible interactions between different neuronal populations in generating some of the Met27 mutant phenotypes were tested using R27G05-GAL4 (Fig. 9A), which expresses in the lamina, and R25B08-GAL4, which expresses strongly in photoreceptors R1 through R7 (Fig. 9C). We used UAS-MetRNAi constructs to reduce Met levels in the photoreceptors or the lamina neurons. We lacked a reliable antibody to measure the level of Met reduction in response to the RNAi, but immunostaining for EcR-B1 at 3 hours APF showed that driving UAS-MetRNAi by R25B08-GAL4 or by R27G05-GAL4 resulted in the precocious appearance of EcR-B1 in the photoreceptors or the lamina neurons, respectively (Fig. 9B,D). Similar results were seen when UAS-MetRNAi was driven in the photoreceptors by two other GAL4 lines (data not shown). The levels of EcR-B1 expression in these cells was similar to that seen in comparably aged, Met27 null mutants, and both RNAi constructs gave similar results. Therefore, the RNAi constructs give sufficient Met reduction to mimic at least one aspect of the Met null phenotype.
Fig. 9.
Effects of targeted expression of genes involved in the response to JH. (A,C) Images of wandering larval brains showing that (A) R27G05 drives expression in lamina neurons (la), and (C) R25B08 drives expression in the photoreceptors, but not in any intrinsic neurons in the lamina or medulla. (B,D) Confocal projection showing EcR-B1 expression in the brain hemisphere and attached eye imaginal disc (ED) at 3 hours APF. Expression of Met RNAi under the control of R27G05 and R25B08 results in precocious appearance of EcR-B1 in lamina neurons (la) and photoreceptors, respectively. Mushroom bodies (MB) show strong endogenous expression. (E-I) Transverse slices through the medulla at 6 hours APF, showing the relative position of the terminals of photoreceptors R7 and R8. (E) Control showing the overlap of R7 and R8. F) Met27 mutant in which R7 appears to have a distinct stalk (arrowhead) that separates it from R8. (G) Expression of EcR-B1 in the photoreceptors does not produce an early separation of the two terminals. (H) Knockdown of Met in lamina neurons by RNAi does not produce a separation. (I) Knockdown of Met in the photoreceptors results in early, stalked (arrowheads) terminals for R7. Scale bars: in D, 100 μm for A-D; in I, 10 μm for E-I.
Examination of the terminal projections of R7 and R8 at 6 hours APF showed that UAS-MetRNAi driven in the photoreceptors caused many of the R7 photoreceptors to separate from the R8 photoreceptors (Fig. 9I), just as in the Met27 mutant (Fig. 9F). By contrast, when R27G05-GAL4 was used to knock down Met levels in the lamina neurons, the photoreceptor terminals stayed in their pre-18 hour APF positions (Fig. 9H). To see if the precocious expression of EcR-B1 in the photoreceptors could also cause early separation of R7 and R8, we used R25B08-GAL4 to drive UAS-EcR-B1 in the photoreceptors. As seen in Fig. 9G, the two receptor terminals remained associated despite the expression of EcR-B1 in R7. Therefore, the loss of Met function must be producing other effects that are needed for the precocious separation of the growth cones.
DISCUSSION
Although a number of studies have reported the effects of applying exogenous JH or JH mimics to Drosophila (for a review, see Riddiford, 1993; Riddiford and Ashburner, 1991; Restifo and Wilson, 1998), there are only two very recent studies of the effects of manipulating endogenous JH on larval growth and metamorphosis, both of which appeared while this paper was under review. JH is normally present in the early larval instars, declines substantially during the last (third) larval stage and then returns transiently around the time of pupariation (Bownes and Rembold, 1987; Sliter et al., 1987). The CAX larvae undergo the expected two larval molts, but because we sometimes see remains of degenerating CA cells at the start of the last larval stage, we cannot conclude anything about the requirements of JH for these larval molts. Recently, Jones et al., using 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR) RNAi to depress the level of JH and its farnesoid precursors in early larvae, showed that the larvae mainly die during the molt to the third instar (Jones et al., 2010), indicating that JH may be required for that molt.
The destruction of the CA by the third instar allowed us to examine the role of JH during the last instar and early metamorphosis. Our finding that these larvae were smaller than their CyO, UAS-grim siblings at pupariation could be explained by either the loss of JH or by the loss of the salivary glands, as these glands are also destroyed. Because dietary JH in the final instar rescued these larvae to normal size, the lack of the CA, rather than the lack of the salivary glands, is the cause of their reduced growth. Preliminary studies show that CAX larvae grow more slowly in the third instar (C.K.M. and L.M.R., unpublished), but the underlying basis for this retardation is not yet understood. Similarly, allatectomized third instar larvae displayed premature apoptosis of the fat body and downregulation of several enzymes involved in energy metabolism at the onset of wandering (Liu et al., 2009). These fat body effects could underlie the reduced larval growth seen in CAX larvae.
A major effect of the removal of JH was on the timing of events during the prepupal period. Studies on the wild silkmoth Hyalophora cecropia first showed that removal of the CA in the last larval stage resulted in the formation of a pupa with adult characteristics (Williams, 1961). Other moths, like Manduca sexta, showed more subtle responses to allatectomy, with premature adult differentiation most evident in the patterned region of the compound eye, posterior to the morphogenetic furrow (Kiguchi and Riddiford, 1978; Champlin and Truman, 1998a). Subsequent studies on a variety of tissues in Manduca showed that the eye, the optic lobe and the ventral diaphragm each had a prolonged period of proliferation that extended from the prepupal period through early adult differentiation (Champlin and Truman, 1998a; Champlin and Truman, 1998b; Champlin et al., 1999). This proliferation was maintained by α-ecdysone or low levels of 20-hydroxyecdysone (20E), but was terminated by high levels of 20E, which induced differentiation. These tissues are exposed to differentiation-inducing titers of 20E that occur during the larval-pupal transition early in their growth, but studies on the ventral diaphragm showed that JH ‘protects’ them from these high 20E levels, allowing them to continue proliferating (Champlin et al., 1999). Removal of JH resulted in these tissues undergoing premature termination of tissue growth and precocious adult differentiation.
The response of Drosophila larvae to the loss of JH is in line with the effects seen in Manduca, and is also most evident in the developing visual system. In normal individuals, the appearance of EcR-B1 in the optic lobe (Truman et al., 1994) and the termination of proliferation in the outer proliferation zone (T. A. Awad, PhD thesis, University of Washington, 1995; Fig. 6B) coincide with the ecdysteroid peak at head eversion (Handler, 1982) and become more pronounced at 18 hours APF with the rise of ecdysteroid for adult differentiation (Handler, 1982). The separation of the R7 and R8 growth cones also begins about this latter time (Ting et al., 2005) (Fig. 5). The only one of these tissues that has been directly tested in vitro for 20E sensitivity is the optic lobe (T. A. Awad, PhD thesis, University of Washington, 1995) and in this case high levels of 20E do indeed suppress proliferation. We assume that the other processes also respond to the changing ecdysteroid levels. The lack of JH results in a heterochronic advance of these events by 10 to 12 hours, consistent with the tissues now responding to the earlier ecdysteroid peak that causes pupariation. Although the removal of JH advances these processes, we find that the application of JH mimics delays them (Fig. 6). Consequently, in selective tissues in Drosophila, JH acts to direct the nature of tissue responses to ecdysone.
The removal of JH or of one its receptors, Met, has a mixed effect on the developing visual system. We saw no effect on proliferation or inductive events in the eye disc itself, in that in CAX animals the morphogenetic furrow continues to move and similar rows of ommatidia have sent R8 axons into the medulla by 6 hours APF, as compared with controls (Fig. 5). Likewise, in Met27 individuals there is only a slight advance (about 2 hours) in the schedule of lamina interneuron ingrowth into the medulla (Fig. 8). However, for some of the cellular and molecular events, like the appearance of EcR-B1 and the separation of R7 from R8, there is a 10- to 18-hour advance in their occurrence. Hence, the lack of JH or of its receptor Met causes a heterochronic shift within the developing visual system with some differentiation responses being advanced relative to the normal schedule of neuronal birth and axon ingrowth. At least in the case of the photoreceptors, the effect of Met removal is largely cell autonomous, with the reduction of Met function in just those cells being sufficient to cause the precocious appearance of EcR-B1 and the early separation of R7 from R8 (Fig. 9D). By contrast, the reduction of Met in lamina interneurons allowed these cells to precociously express EcR-B1 but did not affect the behavior of the R7 and R8 growth cones. This suggests that the separation of R7 and R8 is an active response of the photoreceptors, which is likely to be caused by the rising ecdysteroid titer driving adult differentiation. Although the lack of JH or Met function at the outset of metamorphosis results in the cell-autonomous expression of EcR-B1 in the photoreceptors, misexpression experiments (Fig. 9F) show that the appearance of this receptor alone is not sufficient to bring about the early separation of R7 and R8. Therefore, although the upregulation of EcR-B1 is a prominent response to rising ecdysteroid titers, it is not the key change responsible for the repositioning of the receptor terminals.
As it is viable, the Met mutant allowed us to see the final results of the mistiming of development in the optic lobes. We could not detect any permanent effect of the early separation of the R7 and R8 growth cones on the final anatomy of these projections in the medulla, or on the structure of the later neuropil. However, the lobula was grossly distorted and the normal layering of dendritic arbors disrupted (Fig. 8C-E). This aberrant morphogenesis also starts early, being already evident by 12 hours APF. The cellular basis for the lobula distortion, however, is not yet known.
Heterochronic shifts in the timing of development that extend beyond the visual system are likely to be the cause of the lethality seen in the CAX puparia. Puparia appear normal through the first 6 to 7 hours after pupariation but then abruptly undergo tissue collapse. In normal flies, the early part of metamorphosis is accomplished by a complicated replacement of histolyzing larval tissues by the growing adult tissues. Diverse tissues show individualized times of histolysis that are tied to the ecdysteroid titer. For instance, the larval midgut cells degenerate in response to the pupariation peak of ecdysone, whereas the larval salivary gland degeneration is triggered by the small rise of ecdysteroid at the end of the prepupal period (Jiang et al., 1997). We suspect that without JH, some of the histolysis events are mistimed, leading to the rapid death of the prepupa. Liu et al. have recently shown in CAX larvae that the fat body undergoes precocious programmed cell death beginning in the third larval instar (Liu et al., 2009). Interestingly, this lethal effect was not seen in animals in which Aug21-GAL4 drove RNAi for JH acid O-methyltransferase, the enzyme that converts JH acid to JH, in the CA (Niwa et al., 2008). Whether this indicates that JH acid plays a role in prepupal development or merely reflects the incomplete loss of JH in these animals is unknown.
All these effects of allatectomy can be rescued by JH either fed during the third instar or applied at the time of early wandering, but not at pupariation. Both Bownes and Rembold (Bownes and Rembold, 1987) and Sliter et al. (Sliter et al., 1987) found a decline of JH III in the third instar; this was followed by a peak of JH during late wandering (Sliter et al., 1987). When JH begins to rise is unknown, as their measurements were made every 24 hours. Presumably it is the lack of this JH during wandering when the ecdysteroid titer is rising and peaking that leads to the optic lobe anomalies and the premature histolysis.
The finding that the Met27 null mutant has the same defects in optic lobe development as are found in CAX prepupae strongly suggests that JH is acting via the Met pathway in controlling the timing of some events in the optic lobe. Accordingly, JHM treatment cannot suppress most of the premature development seen in prepupae lacking Met (Fig. 7). However, a major difference between the CAX animals and the Met27 mutants is that the CAX prepupae died before head eversion, whereas the Met27 animals were viable. This difference is also seen in the precocious cell death of the fat body caused by allatectomy, which does not occur in the Met null mutant even in the presence of gce RNAi (Liu et al., 2009). Instead precocious cell death of the fat body was seen when Met was overexpressed in that tissue and the death could be suppressed by exogenous methoprene (a JH mimic). This latter finding suggests that JH would act in this case to suppress Met-mediated cell death. We tested this idea by seeing whether the removal of Met would protect the prepupa from the death caused by early allatectomy. When Met27; Aug21-GAL4>UAS-GFP/CyO females were crossed with UAS-grim males, 44% (n=88; 18 males and 22 females) eclosed, all showing the CyO phenotype. The remainder died at head eversion, and should have been half CAX, Met-heterozygous females and half CAX, Met-null males. We then separated another group by sex prior to pupariation. Forty-nine percent of the females (n=57) and 48% of the males (n=52) died at head eversion. All of the adults that emerged were CyO, showing that all the CAX prepupae died regardless of whether or not they were lacking Met function.
These results together with our findings that JHM treatment of the Met27 mutant gave a partial rescue of the premature separation of R7 and R8, and of the decreased proliferation in the inner proliferation zone (Fig. 7), indicate that there may be more than one receptor for JH. Thus, we support the idea summarized by Wheeler and Nijhout (Wheeler and Nijhout, 2003) that JH might act through multiple pathways. A major pathway involves Met, but Gce or some other mediator may serve as an alternate pathway in some tissues. A similar protective role of JH at pupation mediated by Met is found in Tribolium, as injection of Met RNAi into either fourth instar larvae (Konopova and Jindra, 2007) or final instar larvae (Parthasarathy et al., 2008) caused the precocious appearance of adult eyes, adult antennae and other features in the resulting pupae.
These studies show that JH has an endogenous function in regulating Drosophila metamorphosis, a specific example being in orchestrating the timing of differentiation events in the developing visual system. These effects of JH are primarily mediated through the Met pathway. JH also is necessary for normal larval growth and has another, as yet undefined, crucial role in prepupal development that prevents death at head eversion. The latter effect is not mediated through Met, indicating that JH might act through multiple pathways.
Acknowledgements
We thank Drs G. Korge, J. R. Nambu, T. G. Wilson and A. Shingleton for gifts of the Aug21-GAL4, UAS-grim, Met27 and UAS-MetRNAi stocks, respectively; Dr G. M. Rubin for the R15E10, R17A12, R23C06, R25B08,and R27G05 lines; Drs C. S. Thummel, C. Goodman and C. Q. Doe for the EcR-B1, Fasciclin II and Deadpan antibodies, respectively; and Karen Hibbard and James McMahon of the Janelia Farm Fly Core facility for stock construction. The study was supported by the Howard Hughes Medical Institute. Early preliminary experiments were supported by NSF IBN0344933 to L.M.R. Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Ashok M., Turner C., Wilson T. G. (1998). Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc. Natl. Acad. Sci. USA 95, 2761-2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone J. Q., Doe C. Q. (2008). Identification of Drosophila Type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev. Neurobiol. 68, 1185-1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownes M., Rembold H. (1987). The titre of juvenile hormone during the pupal and adult stages of the life cycle of Drosophila melanogaster. Eur. J. Biochem. 164, 709-712 [DOI] [PubMed] [Google Scholar]
- Champlin D. T., Truman J. W. (1998a). Ecdysteroids govern two phases of eye development during metamorphosis of the moth, Manduca sexta. Development 125, 2009-2018 [DOI] [PubMed] [Google Scholar]
- Champlin D. T., Truman J. W. (1998b). Ecdysteroid control of cell proliferation during optic lobe neurogenesis in the moth, Manduca sexta. Development 125, 269-277 [DOI] [PubMed] [Google Scholar]
- Champlin D. T., Reiss S. E., Truman J. W. (1999). Hormonal control of ventral diaphragm myogenesis during metamorphosis of the moth, Manduca sexta. Dev. Genes Evol. 209, 265-274 [DOI] [PubMed] [Google Scholar]
- Egger B., Boone J. Q., Stevens N. R., Brand A., Doe C. Q. (2007). Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Dev. 2, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski J., Wang S., Wilson T. G. (2006). Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem. Biophys. Res. Commun. 342, 1305-1311 [DOI] [PubMed] [Google Scholar]
- Handler A. M. (1982). Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev. Biol. 93, 73-82 [DOI] [PubMed] [Google Scholar]
- Jiang C., Baehrecke E. H., Thummel C. S. (1997). Steroid regulated programmed cell death during Drosophila metamorphosis. Development 124, 4673-4683 [DOI] [PubMed] [Google Scholar]
- Jones D., Jones G., Teal P., Hammac C., Messmer L., Osborne K., Belgacem Y. H., Martin J.-R. (2010). Suppressed production of methyl farnesoid hormones yields developmental defects and lethality in Drosophila larvae. Gen. Comp. Endocrin. 165, 244-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi K., Riddiford L. M. (1978). The role of juvenile hormone in pupal development of the tobacco hornworm, Manduca sexta. J. Insect Physiol. 24, 673-680 [Google Scholar]
- Konopova B., Jindra M. (2007). Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc. Natl. Acad. Sci. USA 104, 10488-10493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Marticke S., Sung C., Robinow S., Luo L. (2000). Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 28, 807-818 [DOI] [PubMed] [Google Scholar]
- Liu Y., Sheng Z., Liu H., Wen D., He Q., Wang S., Shao W., Jiang R.-J., An S., Sun Y., et al. (2009). Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development 136, 2015-2025 [DOI] [PubMed] [Google Scholar]
- Meinertzhagen I. A., Hanson T. E. (1993). The development of the optic lobe. In The Development of Drosophila (ed. Bate M., Martinez-Arias A.), pp. 1363-1491 Plainview: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Miura K., Oda M., Makita S., Chinzei Y. (2005). Characterization of the Drosophila Methoprene-tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 272, 1169-1178 [DOI] [PubMed] [Google Scholar]
- Niwa R., Niimi T., Honda N., Yoshiyama M., Itoyama K., Kataoka H., Shinoda T. (2008). Juvenile hormone acid O-methyltransferase in Drosophila melanogaster. Insect Biochem. Mol. Biol. 38, 714-720 [DOI] [PubMed] [Google Scholar]
- Parthasarathy R., Tan A., Palli S. R. (2008). bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval-pupal metamorphosis. Mech. Dev. 125, 601-616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer B. D., Jenett A., Hammonds A. S., Ngo T. B., Misra S., Murphy C., Scully A., Carlson J. W., Wan K. H., Laverty T. R., et al. (2008). Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 105, 9715-9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait J. H. (1974). Juvenile hormone and the adult development of Drosophila. Biol. Bull. 147, 119-135 [DOI] [PubMed] [Google Scholar]
- Restifo L. L., Wilson T. G. (1998). A juvenile hormone agonist reveals distinct developmental pathways mediated by ecdysone-inducible Broad Complex transcription factors. Dev. Genet. 22, 141-159 [DOI] [PubMed] [Google Scholar]
- Riddiford L. M. (1993). Hormones and Drosophila development. In The Development of Drosophila (ed. Bate M., Martinez-Arias A.), pp. 899-939 Plainview: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Riddiford L. M. (1994). Cellular and molecular actions of juvenile hormone. I. General considerations and premetamorphic actions. Adv. Insect Physiol. 24, 213-227 [Google Scholar]
- Riddiford L. M. (2008). Juvenile hormone action: a 2007 perspective. J. Insect Physiol. 54, 895-901 [DOI] [PubMed] [Google Scholar]
- Riddiford L. M., Ashburner M. (1991). Role of juvenile hormone in larval development and metamorphosis in Drosophila melanogaster. Gen. Comp. Endocrin. 82, 172-183 [DOI] [PubMed] [Google Scholar]
- Riddiford L. M., Cherbas P. T., Truman J. W. (2001). Ecdysone receptors and their biological actions. Vit. Horm. 60, 1-73 [DOI] [PubMed] [Google Scholar]
- Riddiford L. M., Hiruma K., Zhou X., Nelson C. A. (2003). Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem. Mol. Biol. 33, 1327-1338 [DOI] [PubMed] [Google Scholar]
- Siegmund T., Korge G. (2001). Innervation of the ring gland of Drosophila melanogaster. J. Comp. Neurol. 431, 481-491 [DOI] [PubMed] [Google Scholar]
- Sliter T. J., Sedlak B. J., Baker F. C., Schooley D. A. (1987). Juvenile hormone in Drosophila melanogaster: identification and titer determination during development. Insect Biochem. 17, 161-165 [Google Scholar]
- Soller M., Bownes M., Kubli E. (1999). Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 208, 337-351 [DOI] [PubMed] [Google Scholar]
- Ting C. Y., Lee C. H. (2007). Visual circuit development in Drosophila. Curr. Opin. Neurobiol. 17, 65-72 [DOI] [PubMed] [Google Scholar]
- Ting C. Y., Yonekura S., Chung P., Hsu S. N., Robertson H. M., Chiba A., Lee C. H. (2005). Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents. Development 132, 953-963 [DOI] [PubMed] [Google Scholar]
- Toong Y. C., Schooley D. A., Baker F. C. (1988). Isolation of insect juvenile hormone III from a plant. Nature 333, 170-171 [Google Scholar]
- Truman J. W., Talbot W. S., Fahrbach S. E., Hogness D. S. (1994). Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development 120, 219-234 [DOI] [PubMed] [Google Scholar]
- Truman J. W., Hiruma K., Allee J. P., MacWhinnie S. G. B., Champlin D. T., Riddiford L. M. (2006). Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science 312, 1385-1388 [DOI] [PubMed] [Google Scholar]
- Wheeler D. E., Nijhout H. F. (2003). A perspective for understanding the modes of juvenile hormone action as a lipid signaling system. BioEssays 25, 994-1001 [DOI] [PubMed] [Google Scholar]
- Williams C. M. (1961). The juvenile hormone. II. Its role in the endocrine control of molting, pupation, and adult development of the cecropia silkworm. Biol. Bull. 116, 323-338 [Google Scholar]
- Wilson T. G., Fabian J. (1986). A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev. Biol. 118, 190-201 [DOI] [PubMed] [Google Scholar]
- Wilson T. G., Ashok M. (1998). Insecticide resistance resulting from an absence of target-site gene product. Proc. Natl. Acad. Sci. USA 95, 14040-14044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. G., Wang S., Beno M., Farkas R. (2006). Wide mutational spectrum of a gene involved in hormone action and insecticide resistance in Drosophila melanogaster. Mol. Genet. Genomics 276, 294-303 [DOI] [PubMed] [Google Scholar]
- Wing J. P., Zhou L., Schwartz L. M., Nambu J. R. (1998). Distinct cell killing properties of the Drosophila reaper, head involution defective, and grim genes. Cell Death Differ. 5, 930-939 [DOI] [PubMed] [Google Scholar]
- Wolff T., Ready D. F. (1993). Pattern formation in the Drosophila retina. In The Development of Drosophila (ed. Bate M., Martinez-Arias A.), pp. 1277-1325 Plainview: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Zhou X., Riddiford L. M. (2002). Broad-Complex specifies pupal development and mediates the prevention of the pupal-adult transformation by juvenile hormone in Drosophila and Manduca. Development 129, 2259-2269 [DOI] [PubMed] [Google Scholar]