Abstract
The adenosine deaminases that act on RNA (ADARs) catalyze the site-specific conversion of adenosine to inosine (A to I) in primary mRNA transcripts, thereby affecting the splicing pattern or coding potential of mature mRNAs. Although the subnuclear localization of A-to-I editing has not been precisely defined, ADARs have been shown to act before splicing, suggesting that they function near nucleoplasmic sites of transcription. Here we demonstrate that ADAR2, a member of the vertebrate ADAR family, is concentrated in the nucleolus, a subnuclear domain disparate from the sites of mRNA transcription. Selective inhibition of ribosomal RNA synthesis or the introduction of mutations in the double-stranded RNA-binding domains within ADAR2 results in translocation of the protein to the nucleoplasm, suggesting that nucleolar association of ADAR2 depends on its ability to bind to ribosomal RNA. Fluorescence recovery after photobleaching reveals that ADAR2 can shuttle rapidly between subnuclear compartments. Enhanced translocation of endogenous ADAR2 from the nucleolus to the nucleoplasm results in increased editing of endogenous ADAR2 substrates. These observations indicate that the nucleolar localization of ADAR2 represents an important mechanism by which RNA editing can be modulated by the sequestration of enzymatic activity from potential RNA substrates in the nucleoplasm.
The conversion of adenosine to inosine (A to I) by RNA editing was first observed in yeast tRNAs (1) but has since been identified in numerous viral and cellular mRNA transcripts (2–4). A-to-I editing is most often identified as an adenosine-to-guanosine (A-to-G) discrepancy between genomic and cDNA sequences due to the similar base-pairing properties of inosine and guanosine during cDNA synthesis. A-to-I conversion is catalyzed via hydrolytic deamination at the C6 position of the adenine ring (5) and requires an extended region of double-stranded RNA (dsRNA) in potential RNA substrates formed by intramolecular base-pairing interactions between exon and intron sequences (2–4). A family of adenosine deaminases that act on RNA (ADARs) have been extensively characterized and are responsible for catalyzing the site-specific A-to-I conversion observed in cellular mRNA transcripts (6–8). ADAR-mediated RNA editing events can change the amino acid coding potential of genomically encoded transcripts to produce protein products with altered functional properties (2, 3). For example, the editing of RNAs encoding mammalian ionotropic glutamate receptor (GluR) subunits can lead to the production of heteromeric glutamate-gated channels with altered ion permeation and agonist-induced desensitization kinetics (9–11), whereas the editing of transcripts encoding the 2C-subtype of serotonin receptor (5-HT2CR) can generate receptor isoforms that couple to heterotrimeric G proteins with decreased efficiency (12–14). A-to-I editing events have also been described in nontranslated RNA species and noncoding regions of mRNA transcripts (15–17), suggesting that such modifications may also affect other aspects of RNA function including splicing, translation efficiency, and transcript stability.
The confinement of biomolecules within compartments is crucial for the formation and function of the cell. Ribosomal RNA (rRNA) transcription and processing occur in the nucleolus, a nonmembrane-bound organelle that is separated from sites of mRNA transcription. mRNA is transcribed and processed within the interchromatin space, whereas transcription and processing factors are often concentrated in nonchromatin domains such as speckles, Cajal bodies, and promyelocytic leukemia bodies (18). Although membranes play a major role in maintaining discrete subcellular divisions, compartmentalization of the nucleus is not restricted to membrane-bound organelles and may result from apparently stable subnuclear structures that can be generated by the steady-state localization of highly dynamic components (19). The major steps in precursor mRNA (pre-mRNA) processing are thought to be cotranscriptional events resulting either from recruitment of processing machinery to the C-terminal domain of the large subunit of RNA polymerase II (20, 21) or simply from the rapid kinetics of these processing reactions relative to the time that it takes to synthesize an entire pre-mRNA (22). Because A-to-I editing requires an RNA duplex that generally is formed by base-pairing between intron and exon sequences (3, 4, 23), it is presumed that editing takes place at sites of pre-mRNA transcription before splicing (15, 23, 24). We demonstrate that ADAR2 is concentrated in the nucleolus in a rRNA-dependent manner and can rapidly shuttle between subnuclear compartments. The translocation of endogenous ADAR2 from the nucleolus to the nucleoplasm correlates with increased editing of pre-mRNA substrates, indicating that the nucleolar localization of ADAR2 represents an important mechanism for sequestering editing activity from sites of pre-mRNA transcription.
Materials and Methods
Plasmids. The cloning of the rat ADAR2b (rADAR2b) cDNA was previously described (15). A DNA sequence encoding the hemagglutinin (HA) epitope tag (underlined) plus seven additional amino acids (MAYPYDVPDYASGRFT) was added to the 5′-end of the rADAR2b cDNA and subcloned into the pBABE-Puro retroviral vector (25). To generate the enhanced GFP (EGFP)-ADAR2 fusion constructs, cDNAs encoding wild-type rADAR2b, as well as the Δ76–281 and KA (127, 281) mutants, were initially inserted in-frame into the pEGFP-C1 plasmid (Clontech); the remaining polylinker sequence encoded a 32-aa linker (SGLRSRAQASNSADIHHTGGRFTMDYKDDDDK) between amino acid 239 of EGFP and the second amino acid of rADAR2b. The entire EGFP-rADAR2b coding sequence was then subcloned into the pBABE-puro retroviral vector.
Antibodies. Sheep polyclonal ADAR2 antiserum (Exalpha Biologicals, Watertown, MA) was affinity purified as described (26) by using a GST fusion protein containing amino acids 6–66 of rADAR2b. Affinity-purified anti-ADAR2 (1 ng/μl) and mouse monoclonal antinucleolin (2.5 ng/μl; Santa Cruz Biotechnology) antisera were used with Alexa-488-(1:1,000; Molecular Probes) or Cy3-conjugated (1:1,000; Jackson ImmunoResearch) secondary antibodies. Chromopure normal sheep IgG (Jackson ImmunoResearch) was used as a control for nonspecific IgG staining.
Cell Culture, Transduction, and Transfection. NIH 3T3 mouse fibroblasts and C6 rat glioma cells (American Type Culture Collection) were maintained in DMEM (Life Technologies, Grand Island, NY) supplemented with 10% (vol/vol) bovine calf serum or FCS (HyClone), respectively. Transfections of NIH 3T3 cells with pEGFP-derived constructs were performed by using FuGENE 6 according to the manufacturer's instructions (Boehringer Mannheim). To obtain cells with uniform EGFP expression levels, the cells were subjected to fluorescence-activated cell sorting 24 h after transfection; EGFP-positive cells were immediately plated onto coverslips and grown overnight before fixation and immunofluorescent staining of nucleolin. For transduction of NIH 3T3 cells with retroviral vectors, virus was collected from BOSC-23 packaging cells (27) 48 h after calcium phosphate transfection (28) with the pBABE-derived constructs. The day before retroviral transduction, 2.5 × 104 NIH 3T3 cells were plated on a 60-mm dish, and cells were subsequently incubated with virus containing polybrene (8 μg/ml; Sigma) for three successive 3-h intervals. Forty-eight hours after retroviral transduction, infected cells were selected (2 μg/ml; 2 days) and maintained (1 μg/ml) in puromycin (Sigma).
Immunofluorescence Microscopy. NIH 3T3 and C6 cells were grown on glass coverslips overnight before fixation and immunofluorescence analysis. Cells were fixed in freshly made 4% paraformaldehyde-PBS for 10 min at room temperature, permeabilized in 0.2% Triton X-100 in PBS for 5 min, and blocked with 8% normal donkey serum-PBS (Jackson ImmunoResearch). All primary antibodies were incubated with the cells for ≈1 h in 1.5% normal donkey serum-PBS. For RNase A studies, NIH 3T3 cells expressing EGFP-ADAR2 were grown on Matek dishes (Matek, Ashland, MA), and fluorescence was monitored immediately after the addition of permeabilization buffer containing 0.1% Triton X-100, 10 mM Pipes (pH 7.0), 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, and 1 mM EGTA with or without 100 μg/ml RNase A. For mutant localization studies, average EGFP fluorescence intensity (metamorph, Universal Imaging, Media, PA) was measured in all areas containing nucleolin staining and in the extranucleolar space within the nucleus. All images were acquired by using a Zeiss Axiovert S100 wide-field microscope with either a ×63/1.25 or a ×40/0.75 Plan Neofluar objective.
Western Blotting. For Western blot analysis, crude nuclear extracts were prepared from NIH 3T3 or C6 glioma cells (29) and immunoblotted as described (26) by using an anti-ADAR2 antibody (50 ng/μl), followed by a secondary antibody conjugated to horseradish-peroxidase (Jackson ImmunoResearch). The secondary antibody was detected by using the SuperSignal West Dura Extended chemiluminescence reaction kit (Pierce) in accordance with the manufacturer's instructions. Chemiluminescence was monitored by using the Bio-Rad image detection system, and quantitation was performed by using quantity one software (Bio-Rad) on serially diluted samples that fell within the linear range of detection.
Fluorescence Recovery After Photobleaching. NIH 3T3 cells were grown in media supplemented with 30 mM Hepes, pH 7.1, and maintained at 37°C by using a Delta T Controlled Culture Dish System (Bioptechs, Butler, PA) on the stage of a Zeiss LSM510 inverted microscope. The samples were illuminated with the 488-nm line of an argon laser and imaged with a ×63/1.40 Plan-Apochromat objective. Nucleolar areas of 30 × 30 pixels (14.5 μm2) were selectively bleached by 20 iterations of scanning; photobleaching intensity was 1,000-fold that of the imaging intensity. Images were collected before, immediately after, and at 1- to 15-sec intervals after bleaching. Fluorescence integrated intensity of the bleached nucleolus, of other nucleoli in bleached cells, and of nucleoli in adjacent nonbleached cells was measured by using metamorph (Universal Imaging) image analysis software.
Quantification of A-to-I Editing. Total RNA from NIH 3T3 or C6 glioma cells was prepared by the guanidinium isothiocyanate method (26). For measurements of ADAR2 (site -1) and GluR-B (Q/R site) pre-mRNA editing, total RNA was amplified by RT-PCR by using oligonucleotide primer pairs specific for intronic sequences within ADAR2 (intron 4, CTAGCCCCGAGCAGTTACATCCTT; intron 5, AGGCTGCTTAGAAATGT-TAT) and GluR-B (intron 10, GCATTGTGTTTGCCTACATTGGGG; and intron 11, GACTCTGTAGGAAAAAGC). Primer-extension analysis of the gel-purified PCR products was performed as described (15, 30).
Results
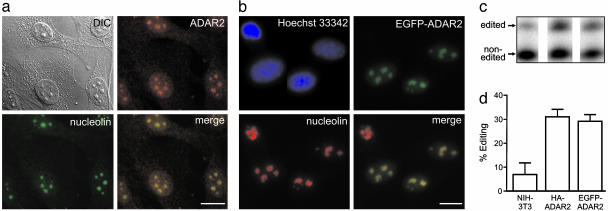
ADAR2 Is Concentrated in the Nucleolus. Immunofluorescent staining of NIH 3T3 fibroblasts revealed that ADAR2 was concentrated in subnuclear domains that were identified as nucleoli based on Nomarski differential interference contrast microscopy and colocalization with nucleolin (Fig. 1a), a well characterized nucleolar protein involved in multiple steps of ribosome biogenesis (31). Endogenous ADAR2 immunoreactivity was also localized to the nucleoli of rat C6 glioma and rat primary choroid plexus epithelial cells that express ADAR2 substrates encoding the B-subunit of the α-amino-3-hydroxy-5-methyl-isoxazole-4-propionate subtype of GluR (GluR-B) and the 2C-subtype of serotonin receptor (5-HT2CR), respectively (3, 32) (data not shown). To further examine the subcellular localization of ADAR2, the expression patterns of rat ADAR2 fused to an N-terminal human influenza HA epitope (HA-ADAR2) or EGFP (EGFP-ADAR2) were determined in stably expressing polyclonal NIH 3T3 cell lines. HA-ADAR2 (data not shown) and EGFP-ADAR2 were largely confined to nucleoli (Fig. 1b), demonstrating a subnuclear expression pattern identical to that of the endogenous mouse ADAR2 protein. Expression of the full-length fusion proteins was confirmed by Western blotting analyses of nuclear extracts from HA-ADAR2 and EGFP-ADAR2 cell lines (data not shown); both proteins were shown to be functional, based on their ability to edit endogenous ADAR2 pre-mRNA transcripts (site -1) (Fig. 1 c and d), thereby validating the use of these fusion proteins in further localization/function studies.
Fig. 1.
Nucleolar localization of endogenous ADAR2 and an EGFP-ADAR2 fusion protein. (a) The subcellular localization of endogenous mouse ADAR2 in NIH 3T3 cells was determined by using affinity-purified ADAR2 antiserum with a Cy3-conjugated secondary antibody. Nucleoli were identified by using differential interference contrast or antinucleolin immunofluorescence microscopy. (b) NIH 3T3 cells were transfected with an EGFP-ADAR2 fusion protein that colocalized with the nucleolar marker (nucleolin); nuclei were visualized with Hoechst 33342. (c) RNA editing of endogenous ADAR2 pre-mRNA transcripts (site -1) in wild-type NIH 3T3 cells or cell lines stably expressing either HA-ADAR2 or EGFP-ADAR2; the migration position, identity of primer-extension products (d), and quantitative PhosphorImager analysis of primer-extension products are indicated (mean ± SD; n = 3).
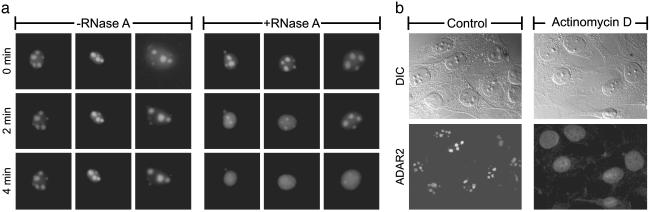
Subnuclear Localization of ADAR2 Depends on rRNA. The dsRNA-binding motifs (DRBMs) of ADAR2 are similar to the domains that mediate dsRNA interactions in a large variety of proteins, including dsRNA-dependent protein kinase, Drosophila staufen, and Escherichia coli RNase III (33). Binding of the DRBM appears to be independent of RNA sequence (33–35), because protein/RNA cocrystal analyses have revealed that this domain interacts with the sugar-phosphate backbone of RNA duplexes without directly contacting the functional groups of the bases (35). Because rRNA is predicted to contain regions with extensive duplex character, including giant and stable secondary structures in the 5′-external transcribed spacer (5′-ETS) and the second internal transcribed spacer (ITS-1) of precursor rRNA (36, 37), we tested the hypothesis that rRNA was required for the nucleolar localization of ADAR2. Initially, NIH 3T3 cells expressing EGFP-ADAR2 were permeabilized in the presence or absence of RNase A. Incubation of permeabilized cells with RNase A resulted in a rapid loss of EGFP-ADAR2 fluorescence from nucleoli within 4 min, whereas cells incubated with permeabilization buffer alone (–RNase A) retained their nucleolar fluorescence over the same time period (Fig. 2a), demonstrating that the nucleolar localization of ADAR2 depends on the presence of RNA. Next, to examine more specifically whether rRNA was required for the subnuclear localization of ADAR2, NIH 3T3 (Fig. 2b) and C6 glioma cells (data not shown) were incubated with a concentration of actinomycin D (0.05 μg/ml) that was selective for the inhibition of rRNA synthesis (38). Except for a previously described decrease in the mean diameter of the nucleoli (39) (Fig. 2b Upper), the overall morphology of NIH 3T3 cells, as assessed by differential interference contrast microscopy, was unaffected by the actinomycin D treatment. However, there was a gradual actinomycin d-dependent translocation of ADAR2 immunofluorescence from the nucleolus to the nucleoplasm and a nucleolar exclusion that appeared complete by 2 h (Fig. 2b Lower), a time course consistent with the processing and export of nascent rRNA from the nucleus (40). These results indicate that the localization of ADAR2 within nucleoli depends on the presence of rRNA.
Fig. 2.
rRNA is required for ADAR2 nucleolar localization. (a) NIH 3T3 cells stably expressing EGFP-ADAR2 were permeabilized with Triton X-100 in the presence or absence of RNase A, and the localization of EGFP-ADAR2 was monitored by epifluorescence microscopy over time. The time course (0–4 min) for three representative cells is presented for the control (–RNase A) and treated (+RNase A) groups. (b) NIH 3T3 cells were treated for 2 h with actinomycin D (0.05 μg/ml) to selectively inhibit rRNA synthesis and assessed for overall cellular morphology by differential interference contrast microscopy, where a decrease in the size of the nucleoli (35%, n = 20 nucleoli, P < 0.05) was observed. The subcellular localization of endogenous ADAR2 was determined by using fluorescence microscopy with an anti-ADAR2 antibody.
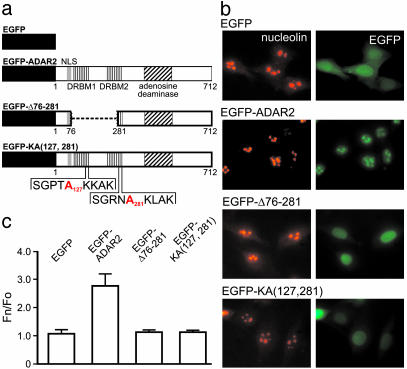
Nucleolar Localization of ADAR2 Depends on dsRNA Binding. The observation that rRNA was required for the nucleolar localization of ADAR2 (Fig. 2) suggested that the ability of ADAR2 to bind rRNA was necessary for concentration in the nucleolus. To test whether the DRBMs of ADAR2 affected its subcellular distribution, we generated a mutant EGFP-ADAR2 fusion protein in which the region encoding both DRBMs was deleted (EGFP-Δ76–281) (Fig. 3a). Although EGFP itself was distributed throughout the nucleus and cytoplasm of transiently transfected NIH 3T3 cells (Fig. 3 b and c), the full-length EGFP-ADAR2 fusion protein was predominantly localized to nucleoli as previously observed in Fig. 1b; however, the localization of EGFP-Δ76–281 was diffusely nuclear, suggesting that dsRNA binding was required for the localization of ADAR2 to nucleoli. Because the deletion of undefined domain(s) within and between the DRBMs or misfolding of the mutant protein could also result in ADAR2 mislocalization, we introduced point mutations in each DRBM to ablate dsRNA binding. A lysine residue, conserved in all DRBMs, has previously been shown to hydrogen-bond directly to the phosphodiester backbone of dsRNA, and mutations of this residue result in a loss of RNA binding by DRBMs from staufen, and dsRNA-dependent protein kinase, without affecting the overall structure of the domains (35, 41, 42). The corresponding lysine residue in each DRBM of ADAR2 was mutated to alanine, and the modified cDNA was fused to EGFP [EGFP-KA (127, 281)] (Fig. 3a). Like the EGFP-Δ76–281 mutant, replacement of the conserved lysine moieties resulted in a diffuse pattern of nuclear fluorescence in transfected NIH 3T3 cells (Fig. 3 b and c), indicating that the nucleolar localization of ADAR2 depends on its ability to bind dsRNA.
Fig. 3.
The DRBM domains of ADAR2 are required for nucleolar localization. (a) A schematic diagram indicating the structure of EGFP as well as wild-type (EGFP-ADAR2) and mutant fusion proteins is presented showing deletion (EGFP-Δ76–281) or mutation [EGFP-KA (127, 281)] of the DRBM domains. The sequences surrounding conserved lysine residues at positions 127 and 281 in DRBM1 and DRBM2, respectively, are indicated by the one-letter amino acid code, and introduced alanine residues at these positions are indicated in red. NLS, nuclear localization signal. (b) Subcellular localization of ADAR2 and nucleoli was determined by fluorescence microscopy for EGFP (green) or by using an antinucleolin antibody (red), respectively. (c) Average EGFP fluorescence overlapping with nucleolin localization was quantified by using metamorph image analysis software and defined as nucleolar localization. The ratio of average fluorescence intensity for all nucleoli in a cell (Fn) to the average nucleoplasmic (nonnucleolar) fluorescence intensity (Fo) is shown (n = 30 cells; mean ± SD).
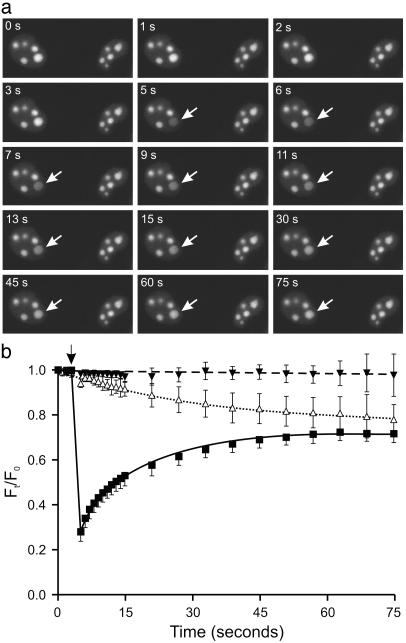
ADAR2 Shuttles Rapidly Between Subnuclear Compartments. The localization of ADAR2 to nucleoli could represent stable static protein aggregates or steady-state compartments resulting from rapid protein movement between subcellular domains. Previous studies using fluorescence recovery after photobleaching have demonstrated that a variety of nuclear proteins move freely throughout the nucleus, whereas proteins such as histone-H2B and nuclear lamin B are relatively stable in their localization (43, 44). To assess the mobility of EGFP-ADAR2 between subnuclear compartments, we irreversibly bleached single nucleoli in stably transfected NIH 3T3 cells by iteratively scanning the area with a high-power laser (Fig. 4a); alterations in fluorescence intensity for bleached and nonbleached nucleoli within the same nucleus were measured over time (Fig. 4b). We also monitored nucleoli in adjacent nonbleached cells to confirm that EGFP-ADAR2 was not bleached by the low-power monitoring laser during the course of the experiment (Fig. 4b). Recovery of fluorescence in the bleached nucleolus was essentially complete within 1 min, suggesting that the recovered nucleolar fluorescence primarily resulted from the influx of unbleached EGFP-ADAR2 fusion protein rather than de novo protein synthesis. Because examination of individual nuclei revealed a decreased fluorescence in unbleached nucleoli that approached the same steady-state level as the single bleached and recovered nucleolus (Fig. 4b), these results demonstrated that there was a rapid shuttling of the EGFP-ADAR2 fusion protein between the nucleolus and nucleoplasm, resulting in a complete redistribution of the EGFP-ADAR2 pool among nucleolar compartments.
Fig. 4.
ADAR2 can rapidly shuttle between nucleolar and nucleoplasmic compartments. (a) A single nucleolus was bleached (arrow) in NIH 3T3 cells stably expressing EGFP-ADAR2, and nuclei were monitored by using confocal microscopy; the time interval (seconds) after the start of fluorescence monitoring is indicated. (b) Quantification of fluorescence intensity by using metamorph image analysis software is presented as the ratio of the fluorescence at time t (Ft) and initial fluorescence (F0) for bleached nucleoli (▪), unbleached nucleoli within the same cell (▵), and the nucleoli in adjacent cells (▾) (mean ± SD; n = 9 pairs of cells). The time at which the single nucleolus was bleached (5 s) is indicated with an arrow.
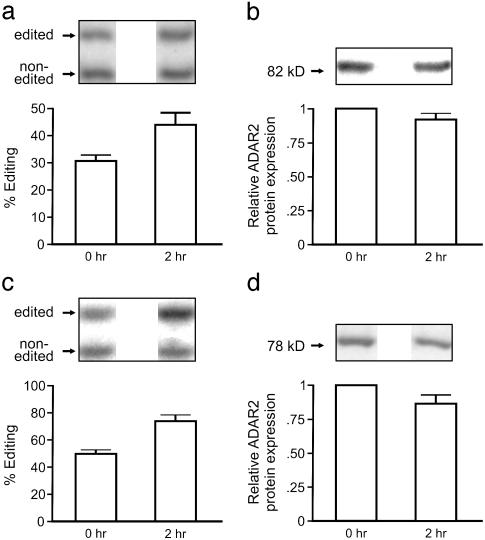
rRNA Limits ADAR2 Editing Activity. The known function of the nucleolus has recently expanded beyond a traditional role in ribosome biogenesis to include diverse cellular processes such as the regulation of telomerase activity, Arf-mediated stabilization of the tumor-suppressor protein p53, and a putative role in the assembly of signal recognition particle (45–47). The discrepancy between the steady-state concentration of ADAR2 in the nucleolus and the proposed site of its action during pre-mRNA transcription indicated that the nucleolus may play a pivotal role in the regulation of RNA editing by functionally sequestering ADAR2 from potential substrates in the nucleoplasm. To examine whether the extent of ADAR2-mediated editing is limited by nucleolar compartmentalization, NIH 3T3 cells stably expressing HA-ADAR2 were incubated with actinomycin D to induce the translocation of HA-ADAR2 to the nucleoplasm; the extent of editing for endogenous ADAR2 pre-mRNA (site -1) was then quantified by primer-extension analysis (Fig. 5a). A complete translocation of HA-ADAR2 from the nucleolus to the nucleoplasm on actinomycin D treatment (similar to Fig. 2b) correlated with a 30% increase in the editing of endogenous ADAR2 pre-mRNA (Fig. 5a). Because quantitative Western blotting of whole cell extracts revealed no significant alteration in HA-ADAR2 protein levels in response to actinomycin D treatment (Fig. 5b), these results suggest that the nucleolus serves as a site of functional sequestration for ADAR2 activity. Because the translocation of overexpressed HA-ADAR2 could be regulated in a manner distinct from endogenous ADAR2 protein, we also examined the effect of actinomycin D treatment on the editing of GluR-B transcripts by endogenous ADAR2 in rat C6 glioma cells (Fig. 5c); C6 cells were chosen for these studies because they express GluR-B transcripts that are efficiently edited at the Q/R site (≈50%) by ADAR2 protein. The editing status of the steady-state pool of mature GluR-B mRNAs in the cytoplasm would not be affected acutely by alterations in the subnuclear compartmentalization of ADAR2. Thus, we limited our analysis to short-lived intron-containing GluR-B pre-mRNAs that were largely transcribed during the period of actinomycin D treatment (2 h). Results from these studies revealed a complete translocation from the nucleolus to the nucleoplasm for endogenous ADAR2 immunoreactivity (data not shown), coinciding with a 46% increase in the editing of endogenous GluR-B pre-mRNAs. Once again, no significant alteration in ADAR2 protein levels was observed in response to actinomycin D treatment (Fig. 5d), further indicating that the nucleolus limits ADAR2 activity in the nucleoplasm.
Fig. 5.
Translocation of ADAR2 from the nucleolus to the nucleoplasm correlates with increased editing activity. (a) Editing of endogenous ADAR2 pre-mRNA transcripts (site –1) was analyzed by primer-extension analysis for RNA isolated from control and actinomycin D-treated NIH 3T3 cells stably expressing HA-ADAR2. The migration position, identity of primer-extension products, and quantitative analysis of editing efficiency are indicated (mean ± SD; n = 5, P < 0.05). (b) Quantitative Western blotting analysis of HA-ADAR2 expression was performed with an ADAR2-specific antiserum; the predicted molecular mass and expected migration position for HA-ADAR2 are indicated. (c) Editing of endogenous GluR-B transcripts (Q/R site), isolated from control and actinomycin D-treated rat C6 glioma cells, was analyzed by primerextension analysis. The migration position, identity of primer-extension products, and quantitative analysis of editing efficiency are indicated (mean ± SD; n = 3, P < 0.05). (d) Quantitative Western blotting analysis of endogenous mouse ADAR2 expression was performed with an ADAR2-specific antiserum; the predicted molecular mass and expected migration position for rADAR2b are indicated.
Discussion
Although the processing of pre-mRNA precursors can occur in isolation from transcription in vitro, recent studies have indicated that factors involved in splicing and 3′-end formation are tightly linked to the transcriptional machinery via the C-terminal domain of RNA polymerase II (20, 21). Because the conversion of adenosine to inosine by RNA editing requires an extended RNA duplex, generally formed by base-pairing interactions between intron and exon sequences (3, 4, 23), it has been presumed that the editing machinery (ADARs) would be localized to the nucleoplasm near sites of premRNA transcription (15, 23, 24). However, we have demonstrated that ADAR2, a limiting factor in A-to-I editing, is shuttled rapidly between the nucleoplasm and the nucleolus (Fig. 4), raising questions regarding the role of such dynamic movement as well as the subnuclear site(s) at which A-to-I editing takes place. Although it is possible that RNA transcripts destined for A-to-I modification are selectively targeted to nucleoli, recent studies have demonstrated that editing-competent GluR-B transcripts accumulate in the nucleoplasm of transfected HeLa cells yet are not detected in nucleoli (48), suggesting that editing is predominantly a nucleoplasmic event. Mislocalization of ADAR2 to the nucleoplasm results in a significant increase in the editing of pre-mRNA substrates (Fig. 5), further suggesting that A-to-I conversion takes place near nucleoplasmic sites of pre-mRNA transcription.
The nucleolus has no known physical barrier separating it from the nucleoplasm, and in principle, any soluble protein should be able to diffuse in and out of nucleoli. Although specific nucleolar localization signals have been identified for a number of nucleolar proteins, including coilin (49), WRN helicase (50), MDM2 (51), ING1 (52), and HIV Tat (53), it has been suggested that both nucleolar assembly and the targeting of many nucleolar proteins are related to direct or indirect interaction with the ribosomal DNA or rRNA (54, 55). The localization of ADAR2 to the nucleolus depends on both the presence of rRNA and the ability of ADAR2 to bind to RNA duplexes by means of its DRBM domains (Figs. 2 and 3). Although we cannot formally exclude the possibility that ADAR2 is interacting with other rRNA-dependent nucleolar proteins or RNAs, our observations suggest that ADAR2 is targeted to the nucleolus by directly interacting with rRNA transcripts. Because the binding of proteins containing DRBMs to dsRNA appears to be independent of RNA sequence (33–35), it is likely that any region of rRNA with sufficient duplex character could serve as an effective binding site and/or substrate for ADAR2.
In eukaryotes, rRNAs are cotranscribed in the nucleolus as a single large precursor RNA (pre-rRNA) that is processed into mature 18S, 5.8S, and 25S rRNAs by removal of the external transcribed spacers (5′-ETS and 3′-ETS) and internal transcribed spacers (ITS1 and ITS2) (36, 56). Although the biological role(s) of these spacer regions are not completely defined, they exist as evolutionarily conserved giant duplexes (37) that are required for interactions with small nucleolar ribonucleoprotein complexes to direct pre-rRNA endonucleolytic processing events (57, 58). The presence of these extended duplex regions within rRNA precursors suggests that they represent effective binding sites for ADAR2, yet whether such regions of pre-rRNA are also modified by ADAR2 is currently unknown. The relatively low adenosine content within the pre-rRNA spacers (<10%) (37, 56) suggests that the binding of ADAR2 to the ETS and ITS regions may not lead to productive deamination. Recent studies using scanning force microscopy have indicated that ADAR2 can transiently interact with specific duplex regions where no editing is found, even though significant binding is detected (59).
Although the nucleolus is a stable subcellular structure, the localization of ADAR2 is dynamic, with the concentration of ADAR2 in the nucleolus representing the steady-state during rapid shuttling between the nucleolus and nucleoplasm (Fig. 4). A similar dynamic organization for many nuclear processes (mRNA transcription, pre-mRNA splicing, and DNA replication and repair) has recently been elucidated, and in many instances, key proteins shuttle between their sites of storage and their sites of action (60, 61). Recent studies by Misteli (19) have suggested that the subnuclear localization of various proteins results from diffusion-based mobility in which proteins are continuously roaming the nuclear space for high-affinity binding sites. The localization of ADAR2 may be dictated by a simple binding equilibrium between transient associations with rRNA and functional interactions with pre-mRNA substrates in the nucleoplasm. Further support for this hypothesis was recently reported by Desterro et al. (48), in which overexpression of an ADAR substrate led to translocation of both ADAR1 and ADAR2 from the nucleolus to the nucleoplasm. Nucleolar sequestration of ADAR2 could represent a regulatory mechanism by which a pool of editing activity is readily available for responding to rapid changes in the expression of potential RNA substrates, yet simultaneously preventing aberrant editing activity by maintaining a low concentration of ADAR2 at its site of action in the nucleoplasm.
Acknowledgments
We thank Drs. Joey Barnett, Pat Levitt, and James Patton for critical reading of this manuscript. This work was supported by National Institutes of Health Grant NS33323 (to R.B.E.).
Abbreviations: ADAR, adenosine deaminase that acts on RNA; dsRNA, double-stranded RNA; GluR, glutamate receptor; rRNA, ribosomal RNA; pre-mRNA, precursor mRNA; rADAR2b, rat ADAR2b; EGFP, enhanced GFP; HA, hemagglutinin; DRBM, dsRNA-binding motif.
References
- 1.Holley, R. W. (1965) J. Am. Med. Assoc. 194, 868–871. [Google Scholar]
- 2.Rueter, S. & Emeson, R. (1998) in Modification and Editing of RNA, eds. Grosjean, H. & Benne, R. (Am. Soc. Microbiol. Press, Washington, DC), pp. 343–361.
- 3.Emeson, R. & Singh, M. (2000) in RNA Editing, ed. Bass, B. (Oxford Univ. Press, Oxford), pp. 109–138.
- 4.Bass, B. L. (2002) Annu. Rev. Biochem. 71, 817–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polson, A. G., Crain, P. F., Pomerantz, S. C., McCloskey, J. A. & Bass, B. L. (1991) Biochemistry 30, 11507–11514. [DOI] [PubMed] [Google Scholar]
- 6.Melcher, T., Maas, S., Herb, A., Sprengel, R., Seeburg, P. H. & Higuchi, M. (1996) Nature 379, 460–464. [DOI] [PubMed] [Google Scholar]
- 7.O'Connell, M. A., Krause, S., Higuchi, M., Hsuan, J. J., Totty, N. F., Jenny, A. & Keller, W. (1995) Mol. Cell. Biol. 15, 1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, U., Garner, T. L., Sanford, T., Speicher, D., Murray, J. M. & Nishikura, K. (1994) J. Biol. Chem. 269, 13480–13489. [PubMed] [Google Scholar]
- 9.Kohler, M., Burnashev, N., Sakmann, B. & Seeburg, P. H. (1993) Neuron 10, 491–500. [DOI] [PubMed] [Google Scholar]
- 10.Lomeli, H., Mosbacher, J., Melcher, T., Hoger, T., Geiger, J. R., Kuner, T., Monyer, H., Higuchi, M., Bach, A. & Seeburg, P. H. (1994) Science 266, 1709–1713. [DOI] [PubMed] [Google Scholar]
- 11.Burnashev, N., Monyer, H., Seeburg, P. H. & Sakmann, B. (1992) Neuron 8, 189–198. [DOI] [PubMed] [Google Scholar]
- 12.Wang, Q., O'Brien, P. J., Chen, C. X., Cho, D. S., Murray, J. M. & Nishikura, K. (2000) J. Neurochem. 74, 1290–1300. [DOI] [PubMed] [Google Scholar]
- 13.Price, R. D., Weiner, D. M., Chang, M. S. & Sanders-Bush, E. (2001) J. Biol. Chem. 276, 44663–44668. [DOI] [PubMed] [Google Scholar]
- 14.Burns, C. M., Chu, H., Rueter, S. M., Hutchinson, L. K., Canton, H., Sanders-Bush, E. & Emeson, R. B. (1997) Nature 387, 303–308. [DOI] [PubMed] [Google Scholar]
- 15.Rueter, S. M., Dawson, T. R. & Emeson, R. B. (1999) Nature 399, 75–80. [DOI] [PubMed] [Google Scholar]
- 16.Morse, D. P., Aruscavage, P. J. & Bass, B. L. (2002) Proc. Natl. Acad. Sci. USA 99, 7906–7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber, A., Grosjean, H., Melcher, T. & Keller, W. (1998) EMBO J. 17, 4780–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector, D. L. (2001) J. Cell Sci. 114, 2891–2893. [DOI] [PubMed] [Google Scholar]
- 19.Misteli, T. (2001) Science 291, 843–847. [DOI] [PubMed] [Google Scholar]
- 20.Minvielle-Sebastia, L. & Keller, W. (1999) Curr. Opin. Cell Biol. 11, 352–357. [DOI] [PubMed] [Google Scholar]
- 21.Corden, J. L. & Patturajan, M. (1997) Trends Biochem. Sci. 22, 413–416. [DOI] [PubMed] [Google Scholar]
- 22.Neugebauer, K. M. (2002) J. Cell Sci. 115, 3865–3871. [DOI] [PubMed] [Google Scholar]
- 23.Maas, S., Rich, A. & Nishikura, K. (2003) J. Biol. Chem. 278, 1391–1394. [DOI] [PubMed] [Google Scholar]
- 24.Raitskin, O., Cho, D. S., Sperling, J., Nishikura, K. & Sperling, R. (2001) Proc. Natl. Acad. Sci. USA 98, 6571–6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgenstern, J. P. & Land, H. (1990) Nucleic Acids Res. 18, 3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausubel, F., Kingston, R., Moore, D., Seidman, J., Smith, J. & Struhl, K., eds. (1989) Current Protocols in Molecular Biology (Wiley, New York).
- 27.Fassati, A., Takahara, Y., Walsh, F. S. & Dickson, G. (1994) Nucleic Acids Res. 22, 1117–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen, C. & Okayama, H. (1987) Mol. Cell. Biol. 7, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber, E., Matthias, P., Muller, M. M. & Schaffner, W. (1989) Nucleic Acids Res. 17, 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rueter, S. M., Burns, C. M., Coode, S. A., Mookherjee, P. & Emeson, R. B. (1995) Science 267, 1491–1494. [DOI] [PubMed] [Google Scholar]
- 31.Ginisty, H., Sicard, H., Roger, B. & Bouvet, P. (1999) J. Cell Sci. 112, 761–772. [DOI] [PubMed] [Google Scholar]
- 32.Schaub, M. & Keller, W. (2002) Biochimie 84, 791–803. [DOI] [PubMed] [Google Scholar]
- 33.St Johnston, D., Brown, N. H., Gall, J. G. & Jantsch, M. (1992) Proc. Natl. Acad. Sci. USA 89, 10979–10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckmann, C. R. & Jantsch, M. F. (1997) J. Cell Biol. 138, 239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryter, J. M. & Schultz, S. C. (1998) EMBO J. 17, 7505–7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michot, B., Joseph, N., Mazan, S. & Bachellerie, J. P. (1999) Nucleic Acids Res. 27, 2271–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourbon, H., Michot, B., Hassouna, N., Feliu, J. & Bachellerie, J. P. (1988) DNA 7, 181–191. [DOI] [PubMed] [Google Scholar]
- 38.Perry, R. P. & Kelley, D. E. (1970) J. Cell Physiol. 76, 127–139. [DOI] [PubMed] [Google Scholar]
- 39.Busch, H. & Smetana, K. (1970) The Nucleolus (Academic, New York).
- 40.Thiry, M., Cheutin, T., O'Donohue, M. F., Kaplan, H. & Ploton, D. (2000) RNA 6, 1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMillan, N. A., Carpick, B. W., Hollis, B., Toone, W. M., Zamanian-Daryoush, M. & Williams, B. R. (1995) J. Biol. Chem. 270, 2601–2606. [DOI] [PubMed] [Google Scholar]
- 42.Ramos, A., Grunert, S., Adams, J., Micklem, D. R., Proctor, M. R., Freund, S., Bycroft, M., St Johnston, D. & Varani, G. (2000) EMBO J. 19, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phair, R. D. & Misteli, T. (2000) Nature 404, 604–609. [DOI] [PubMed] [Google Scholar]
- 44.Moir, R. D., Yoon, M., Khuon, S. & Goldman, R. D. (2000) J. Cell Biol. 151, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong, J. M., Kusdra, L. & Collins, K. (2002) Nat. Cell Biol. 4, 731–736. [DOI] [PubMed] [Google Scholar]
- 46.Weber, J. D., Taylor, L. J., Roussel, M. F., Sherr, C. J. & Bar-Sagi, D. (1999) Nat. Cell Biol. 1, 20–26. [DOI] [PubMed] [Google Scholar]
- 47.Politz, J. C., Yarovoi, S., Kilroy, S. M., Gowda, K., Zwieb, C. & Pederson, T. (2000) Proc. Natl. Acad. Sci. USA 97, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desterro, J. M., Keegan, L. P., Lafarga, M., Berciano, M. T., O'Connell, M. & Carmo-Fonseca, M. (2003) J. Cell Sci. 116, 1805–1818. [DOI] [PubMed] [Google Scholar]
- 49.Bohmann, K., Ferreira, J. A. & Lamond, A. I. (1995) J. Cell Biol. 131, 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Kobbe, C. & Bohr, V. A. (2002) J. Cell Sci. 115, 3901–3907. [DOI] [PubMed] [Google Scholar]
- 51.Lohrum, M. A., Ashcroft, M., Kubbutat, M. H. & Vousden, K. H. (2000) Nat. Cell Biol. 2, 179–181. [DOI] [PubMed] [Google Scholar]
- 52.Scott, M., Boisvert, F. M., Vieyra, D., Johnston, R. N., Bazett-Jones, D. P. & Riabowol, K. (2001) Nucleic Acids Res. 29, 2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siomi, H., Shida, H., Maki, M. & Hatanaka, M. (1990) J. Virol. 64, 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oakes, M., Aris, J. P., Brockenbrough, J. S., Wai, H., Vu, L. & Nomura, M. (1998) J. Cell Biol. 143, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmo-Fonseca, M., Mendes-Soares, L. & Campos, I. (2000) Nat. Cell Biol. 2, E107–E112. [DOI] [PubMed] [Google Scholar]
- 56.Michot, B., Bachellerie, J. P. & Raynal, F. (1983) Nucleic Acids Res. 11, 3375–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma, K. & Tollervey, D. (1999) Mol. Cell. Biol. 19, 6012–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dragon, F., Gallagher, J. E., Compagnone-Post, P. A., Mitchell, B. M., Porwancher, K. A., Wehner, K. A., Wormsley, S., Settlage, R. E., Shabanowitz, J., Osheim, Y., et al. (2002) Nature 417, 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klaue, Y., Kallman, A. M., Bonin, M., Nellen, W. & Ohman, M. (2003) RNA 9, 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Misteli, T., Caceres, J. F. & Spector, D. L. (1997) Nature 387, 523–527. [DOI] [PubMed] [Google Scholar]
- 61.Gama-Carvalho, M. & Carmo-Fonseca, M. (2001) FEBS Lett. 498, 157–163. [DOI] [PubMed] [Google Scholar]