Abstract
Transporters influence the disposition of chemicals within the body by participating in absorption, distribution, and elimination. Transporters of the solute carrier family (SLC) comprise a variety of proteins, including organic cation transporters (OCT) 1 to 3, organic cation/carnitine transporters (OCTN) 1 to 3, organic anion transporters (OAT) 1 to 7, various organic anion transporting polypeptide isoforms, sodium taurocholate cotransporting polypeptide, apical sodium-dependent bile acid transporter, peptide transporters (PEPT) 1 and 2, concentrative nucleoside transporters (CNT) 1 to 3, equilibrative nucleoside transporter (ENT) 1 to 3, and multidrug and toxin extrusion transporters (MATE) 1 and 2, which mediate the uptake (except MATEs) of organic anions and cations as well as peptides and nucleosides. Efflux transporters of the ATP-binding cassette superfamily, such as ATP-binding cassette transporter A1 (ABCA1), multidrug resistance proteins (MDR) 1 and 2, bile salt export pump, multidrug resistance-associated proteins (MRP) 1 to 9, breast cancer resistance protein, and ATP-binding cassette subfamily G members 5 and 8, are responsible for the unidirectional export of endogenous and exogenous substances. Other efflux transporters [ATPase copper-transporting β polypeptide (ATP7B) and ATPase class I type 8B member 1 (ATP8B1) as well as organic solute transporters (OST) α and β] also play major roles in the transport of some endogenous chemicals across biological membranes. This review article provides a comprehensive overview of these transporters (both rodent and human) with regard to tissue distribution, subcellular localization, and substrate preferences. Because uptake and efflux transporters are expressed in multiple cell types, the roles of transporters in a variety of tissues, including the liver, kidneys, intestine, brain, heart, placenta, mammary glands, immune cells, and testes are discussed. Attention is also placed upon a variety of regulatory factors that influence transporter expression and function, including transcriptional activation and post-translational modifications as well as subcellular trafficking. Sex differences, ontogeny, and pharmacological and toxicological regulation of transporters are also addressed. Transporters are important transmembrane proteins that mediate the cellular entry and exit of a wide range of substrates throughout the body and thereby play important roles in human physiology, pharmacology, pathology, and toxicology.
I. Introduction
Pharmacokinetics is determined by the absorption, distribution, metabolism, and excretion of a chemical from the body. These processes dictate the circulating and cellular levels of endogenous and exogenous compounds and, in turn, their physiological and pharmacological activity. Movement of chemicals across lipid bilayers is necessary for their function and elimination. In some cases, simple diffusion is sufficient for chemicals to enter as well as to exit cells. In other instances, physical and chemical properties such as size, charge, and hydrophilicity necessitate assistance for chemicals to cross membranes.
Transporters are specialized proteins that span cell membrane bilayers and mediate translocation of chemicals into and out of cells using active and passive mechanisms. Active transport occurs when solutes are transported across biological membranes against a concentration gradient and/or an electrochemical potential. Because of counter forces, active transport requires energy. In primary active transport, substrates pass unidirectionally through transport pumps using energy generated from the hydrolysis of ATP. During this process, substrates bind on one surface, leading to a conformational change in the transporter protein that allows release on the other side of the membrane. Secondary active transport occurs when uphill transport of a chemical by a carrier protein is coupled to the transport of a cosubstrate (typically, an ion). Coupling of the transport to solutes across a membrane is called cotransport. Cotransport can occur in the same direction (symport) or in opposite directions (antiport). Antiport transport will often create an electrochemical gradient in and of itself that can be used for tertiary active transport.
There are endogenous and exogenous substances that are substrates for transporters. Transporters are expressed in many tissues within the body for the circulation of physiological chemicals and nutrients, elimination of metabolic waste, and detoxification and removal of environmental chemicals and drugs. Transporters in the intestines are important for the absorption of some substrates and excretion of other substrates. Transporters on the surface of hepatocytes enable entry of some chemicals into the liver. Subsequent distribution of some chemicals to other tissues also involves transporters. Finally, certain transporters such as those in the kidneys participate in the excretion of chemicals from the body.
The disposition of drugs and endogenous chemicals such as bile acids and cholesterol is most often associated with two superfamilies of transporters: the solute carrier (SLC1) and ATP-binding cassette transporters (ABC) families. The SLC family is part of the major facilitator superfamily. The SLC transporters discussed in this review are typically considered to be uptake transporters, although there are examples of bidirectional transport. SLC transporters typically use secondary and tertiary active transport to move chemicals across biological membranes. The ABC are members of a superfamily of transporters and are found on extracellular and intracellular membranes. ABC transporters function as efflux pumps that remove chemicals from the cell or organelle using primary active transport. ABC transporters can exist as full and half transporters. In the case of half transporters, these proteins require homo- or heterodimerization for functional activity.
For a number of years, it has been difficult to dissect the biochemical and molecular events involved in transport without knowing the protein structure of transporters. An inability to determine the structure of many mammalian SLC and ABC transporters is due largely to difficulties associated with obtaining stable crystals of amphipathic membrane-associated proteins. Early attempts to determine the structure of one member of the ABCB subfamily, Abcb1 or Mdr1, also known as P-glycoprotein (Pgp), yielded low- to medium-resolution electron microscopy structural information (Rosenberg et al., 1997, 2001, 2003). Theories regarding the steps of transport (including substrate extraction from the bilayer, ATP binding, ATP hydrolysis, and conformational changes) have been postulated based on the crystal structures of evolutionarily related transporters. Aller et al. (2009) have published the X-ray crystal protein structure of mouse Abcb1 (Mdr1) with and without bound substrates. Although it has taken approximately 33 years from the first report of Pgp in drug-resistant cell lines (Juliano and Ling, 1976) to the elucidation of its protein structure by X-ray crystallography (Aller et al., 2009), a tremendous amount of information regarding this transporter has been obtained. Functional studies suggest that MDR1 is a “polyspecific” transporter that could accept compounds of varying sizes and structures with binding at multiple sites. The inward-facing crystal structure of Pgp confirmed distinct drug-binding sites in the internal cavity in which different and multiple substrates could associate. These findings will assist researchers in rational drug design and provide a better understanding of substrate cooperativity during transport.
This review article describes members of the SLC and ABC families (Table 1). Within the SLC family, a number of families [10, 15, 21 (SLCO), 22, 28, 29, and 47] will be discussed. With regard to ABC transporters, we will focus upon the A, B, C, and G subfamilies. Other efflux transporters that will be discussed include ATPase copper-transporting β polypeptide (ATP7B), ATPase class I type 8B member 1 (ATP8B1), and the organic solute transporters (OST). For each of these transporters, the tissue distribution, subcellular localization, and substrate preferences in humans and mice will be included. Transporter isoforms are denoted as rodent (lowercase) and/or human (uppercase). The function of uptake and efflux transporters in a variety of tissues will be highlighted. There are a variety of regulatory mechanisms that dictate transporter expression and function including post-translational processing and protein-protein interactions as well as sex, ontogeny, and pharmacological activation. Finally, the regulation of transporters during toxicological and pathological conditions of the liver will be discussed.
TABLE 1.
Human uptake and efflux transporter gene, mRNA, and protein nomenclature
The gene names and the chromosomal locations were obtained from Entrez Gene cytogenic band. The mRNA and splice variant information were obtained from NCBI Entrez Nucleotide. Protein names (including alternative names) were obtained from UniProt/Swiss-Prot and GeneCards.
| Gene |
mRNA |
Protein |
|||
|---|---|---|---|---|---|
| Name | Locus | Accession No | Splice Variants | Name | Other Names |
| SLC Transporters | |||||
| SLCO1A2 | 12p12 | NM_021094 | Yes | OATP1A2 | OATP, OATP-A |
| SLCO1B1 | 12p12 | NM_006446 | OATP1B1 | OATP2, LST-1, OATP-C | |
| SLCO1B3 | 12p12 | NM_019844 | OATP1B3 | OATP8, LST-2 | |
| SLCO1C1 | 12p12 | NM_001145946 | Yes | OATP1C1 | OATP-F |
| SLCO2A1 | 3q21 | NM_005630 | Yes | OATP2A1 | PGT |
| SLCO2B1 | 11q13 | NM_007256 | Yes | OATP2B1 | OATP-B |
| SLCO3A1 | 15q26 | NM_013272 | Yes | OATP3A1 | OATP-D |
| SLCO4A1 | 20q13 | NM_016354 | Yes | OATP4A1 | OATP-E |
| SLCO4C1 | 5q21 | NM_180991 | OATP4C1 | OATP-H | |
| SLCO5A1 | 8q13 | NM_030958 | Yes | OATP5A1 | OATP-J |
| SLCO6A1 | 5q21 | NM_173488 | OATP6A1 | OATP-I, GST | |
| SLC22A1 | 6q26 | NM_003057 | Yes | OCT1 | |
| SLC22A2 | 6q26 | NM_003058 | Yes | OCT2 | |
| SLC22A3 | 6q26–27 | NM_021977 | OCT3 | EMT, Orct3 | |
| SLC22A4 | 5q31.1 | NM_003059 | OCTN1 | ET | |
| SLC22A5 | 5q31 | NM_003060 | OCTN2 | ||
| SLC22A21 | 5q31 | N.D. | OCTN3 | ||
| SLC22A6 | 11q13.1–2 | NM_004790 | Yes | OAT1 | NKT |
| SLC22A7 | 6q21.1–2 | NM_153320 | Yes | OAT2 | NLT |
| SLC22A8 | 11q11 | NM_004254 | Yes | OAT3 | Roct |
| SLC22A11 | 11q13.1 | NM_018484 | Yes | OAT4 | |
| SLC22A10/19 | 11q12.3 | NM_001039752 | OAT5 | ||
| SLC22A20 | 11q13.1 | NM_001004326 | Yes | OAT6 | |
| SLC22A9 | 11q13.1 | NM_080866 | OAT7 | hUST3 | |
| SLC22A12 | 11q13.1 | NM_144585 | Yes | URAT | RST |
| SLC10A1 | 14q24.1 | NM_003049 | NTCP | ||
| SLC10A2 | 13q33 | NM_000452 | ASBT | IBAT, ISBT | |
| SLC15A1 | 13q33-q34 | NM_005073 | Yes | PEPT1 | HPEPT1 |
| SLC15A2 | 3q13.33 | NM_021082 | Yes | PEPT2 | |
| SLC28A1 | 15q25-q26 | NM_004213 | Yes | CNT1 | hCNT1 |
| SLC28A2 | 15q15 | NM_004212 | CNT2 | SPNT, hCNT2 | |
| SLC28A3 | 9q22.2 | NM_001532 | CNT3 | hCNT3 | |
| SLC29A1 | 6p21.2-p21.1 | NM_001078177 | Yes | ENT1 | |
| SLC29A2 | 11q13 | NM_022127 | Yes | ENT2 | DER12, HNP36 |
| SLC29A3 | 10q22.1 | NM_018344 | Yes | ENT3 | |
| SLC47A1 | 17p11.2 | NM_018242 | Yes | MATE1 | |
| SLC47A2 | 17p11.2 | NM_152908 | Yes | MATE2-K | H+/cation antiporter |
| ABC Transporters | |||||
| ABCA1 | 9q31.1 | NM_005502 | ABCA1 | ||
| ABCB1 | 7q21.1 | NM_000927 | MDR1 | Pgp | |
| ABCB4 | 7q21.1 | NM_000443 | Yes | MDR3 | PFIC3, PGY3 |
| ABCB11 | 2q24 | NM_003742 | BSEP | SPGP, PFIC2 | |
| ABCC1 | 16p13.1 | NM_004996 | Yes | MRP1 | MRP, GS-X |
| ABCC2 | 10q24 | NM_000392 | MRP2 | cMOAT, DJS | |
| ABCC3 | 17q22 | NM_003786 | Yes | MRP3 | MOAT-D, cMOAT2 |
| ABCC4 | 13q32 | NM_005845 | Yes | MRP4 | MOAT-B |
| ABCC5 | 3q27 | NM_005688 | Yes | MRP5 | MOAT-C, ABC11 |
| ABCC6 | 16p13.1 | NM_001171 | Yes | MRP6 | MOAT-E, PXE, ARA |
| ABCC10 | 6p21.1 | NM_033450 | Yes | MRP7 | |
| ABCC11 | 16q12.1 | NM_032583 | Yes | MRP8 | |
| ABCC12 | 16q12.1 | NM_033226 | Yes | MRP9 | |
| ABCG2 | 4q22 | NM_004827 | Yes | BCRP | MXR |
| ABCG5 | 2p21 | NM_022436 | ABCG5 | Sterolin-1 | |
| ABCG8 | 2p21 | NM_022437 | ABCG8 | Sterolin-2 | |
| ATP7B | 13q14.3 | NM_000053 | Yes | ATP7B | WD |
| ATP8B1 | 18q21-q22 | NM_005603 | ATP8B1 | PFIC1, FIC1, BRIC | |
| OSTα | 3q29 | NM_152672 | OSTα | ||
| OSTβ | 15q22.31 | NM_178859 | OSTβ | ||
ET, ergothioneine transporter; NKT, novel kidney transporter; NLT, novel liver transporter; Roct, reduced in osteosclerosis transporter; IBAT/ISBT, ileal sodium-dependent bile acid cotransporter; RST, renal-specific transporter; SPNT, sodium-dependent purine nucleoside transporter.
II. Transporter Families: Tissue Distribution, Subcellular Localization, and Substrates
Tables and figures in this section provide important details for each transporter discussed. Table 1 lists the gene, mRNA, and protein information for human transporter isoforms. Figures 1 through 5 illustrate the distribution of transporter mRNA in a number of tissues from humans and mice. Tables 2, 4 to 6, 8, and 9 document subcellular localization of transporter isoforms, in particular tissues or cell types. Tables 3 and 7 list a number of identified substrates for rodent and/or human transporter isoforms. Figures 1 through 5 and Tables 1 through 9 should be cross-referenced with the text of this section.
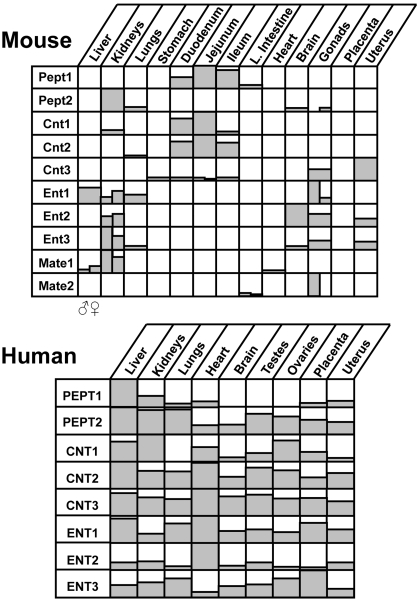
Fig. 1.
Tissue distribution of Oatp mRNA in mice and humans. Top, relative mRNA levels of transporters in mouse liver, kidneys, lung, stomach, duodenum, jejunum, ileum, large intestine, brain, gonads (testes and ovaries), and placenta are shown. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. References for mouse mRNA expression are included (Cheng et al., 2005a). Bottom, relative mRNA levels of transporters in human liver, kidneys, lung, heart, brain, testes, ovaries, placenta, and uterus are shown. Data for humans were obtained from GNF SymAtlas (http://symatlas.gnf.org/; now located at http://biogps.gnf.org). The GNF1H/MAS5 data set was accessed during September 2008.
Fig. 5.
Tissue distribution of Ntcp, Asbt, Bsep, Ost, Abca, and Abcg mRNA in mice and humans. Top, relative mRNA levels of transporters in mouse liver, kidneys, lung, stomach, duodenum, jejunum, ileum, large intestine, brain, gonads (testes and ovaries), and placenta are shown. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. References for mouse mRNA expression are included (Dieter et al., 2004; Cheng et al., 2007). Bottom, relative mRNA levels of transporters in human liver, kidneys, lung, heart, brain, testes, ovaries, placenta, and uterus are shown. Data for humans were obtained from GNF SymAtlas (http://symatlas.gnf.org/; now located at http://biogps.gnf.org). The GNF1H/MAS5 data set was accessed during September 2008.
TABLE 2.
Subcellular localization of uptake OATP/Oatp transporters in various species
For each transporter, the apical or basolateral localization in a particular tissue and/or species is provided. Species included rat (R), mouse (M), human (H), and bovine (B). Detailed information regarding particular cellular populations or regions of the tissue are provided for some transporter isoforms.
| Cellular Localization | Tissue | Species | Cell Types | References |
|---|---|---|---|---|
| Oatp1a1 | ||||
| Basolateral | Liver | R | Hepatocytes | Eckhardt et al., 1999 |
| Apical | Kidney | R | Proximal tubule cells | Bergwerk et al., 1996 |
| Apical | Choroid plexus | R | Epithelial cells | Angeletti et al., 1997 |
| Oatp1a4 | ||||
| Basolateral | Liver | R | Hepatocytes (midzonal to centrilobular) | Kakyo et al., 1999; Reichel et al., 1999 |
| Basolateral | Choroid plexus | R | Epithelial cells | Gao et al., 1999 |
| Basolateral | Brain | R | Capillary endothelial cells | Roberts et al., 2008 |
| Apical | Eye | R | Retinal pigment epithelium | Gao et al., 2002; Ito et al., 2002 |
| Oatp1a5 | ||||
| Apical | Choroid plexus | M | Epithelial cells | Ohtsuki et al., 2004b |
| Apical | Brain | M | Capillary endothelial cells | Ohtsuki et al., 2004b |
| Apical | Jejunum | R | Enterocytes | Walters et al., 2000 |
| N.D. | Eye | R | Nerve fiber, ganglion cells | Ito et al., 2002 |
| Oatp1b2 | ||||
| Basolateral | Liver | R | Hepatocytes | Cattori et al., 2001 |
| Oatp2a1 | ||||
| N.D. | Kidney | R | Glomeruli, endothelial cells, collecting ducts | Bao et al., 2002 |
| Oatp2b1 | ||||
| Basolateral | Brain | R | Capillary endothelial cells | Roberts et al., 2008 |
| Apical | Choroid Plexus | R | Epithelial cells | Roberts et al., 2008 |
| Oatp3a1 | ||||
| N.D. | Heart | R | Aorta endothelial cells | Adachi et al., 2003 |
| N.D. | Lung | R | Alveolar epithelial cells | Adachi et al., 2003 |
| N.D. | Trachea | R | Mucosal epithelium | Adachi et al., 2003 |
| N.D. | Testes | R | Spermatozoa tails | Adachi et al., 2003 |
| N.D. | Ovary | R | Oocytes and smooth muscle cells | Adachi et al., 2003 |
| N.D. | Uterus | R | Glandula uterine epithelium, smooth muscle cells of myometrium, surface epithelium of endometrium | Adachi et al., 2003 |
| N.D. | Kidney | R | Distal tubules and collecting ducts | Adachi et al., 2003 |
| Oatp4a1 | ||||
| N.D. | Eye | R | Corneal epithelium, ciliary body, iris, retina | Ito et al., 2003 |
| Oatp4c1 | ||||
| Basolateral | Kidney | R | Proximal tubule cells | Mikkaichi et al., 2004 |
| OATP1A2 | ||||
| Apical | Liver | H | Cholangiocytes | Lee et al., 2005a |
| Apical | Small Intestine | H | Enterocytes at the villus tip | Glaeser et al., 2007 |
| Apical | Kidney | H | Distal tubules | Lee et al., 2005a |
| N.D. | Brain | H | Capillary endothelial cells | Lee et al., 2005a |
| OATP1B1 | ||||
| Basolateral | Liver | H | Hepatocytes | Cui et al., 2003 |
| OATP1B3 | ||||
| Basolateral | Liver | H | Hepatocytes (centrilobular) | Cui et al., 2003 |
| OATP1C1 | ||||
| N.D. | Testes | H | Leydig cells | Pizzagalli et al., 2002 |
| OATP2B1 | ||||
| Basolateral | Liver | H | Hepatocytes | Grube et al., 2006a |
| Basolateral | Placenta | H | Syncytiotrophoblasts | St-Pierre et al., 2002; Grube et al., 2007 |
| Apical | Small Intestine | H | Enterocytes | Kobayashi et al., 2003 |
| N.D. | Heart | H | Vascular endothelium | Grube et al., 2006a |
| OATP4A1 | ||||
| Apical | Placenta | H | Syncytiotrophoblasts | Sato et al., 2003 |
N.D., not determined.
TABLE 4.
Subcellular localization of uptake Oct, Octn, Oat, and Urat transporters in various species
For each transporter, the apical or basolateral localization in a particular tissue and/or species is provided. Species included rat (R), mouse (M), human (H), and bovine (B). Detailed information regarding particular cellular populations or regions of the tissue are provided for some transporter isoforms.
| Cellular Localization | Tissue | Species | Cell Types | References |
|---|---|---|---|---|
| Oct1 | ||||
| Basolateral | Liver | R | Hepatocytes (centrilobular) | Meyer-Wentrup et al., 1998 |
| Basolateral | Kidney | R | Proximal tubule cells (S1/S2) | Karbach et al., 2000 |
| Basolateral | Jejunum | H | Enterocytes | Muller et al., 2005 |
| Basolateral | Testes | R | Sertoli cells | Maeda et al., 2007a |
| Apical | Lung | R, H | Ciliated epithelial cells | Lips et al., 2005 |
| Oct2 | ||||
| Basolateral | Kidney | R, H | Proximal tubule cells (S2/S3) | Karbach et al., 2000; Motohashi et al., 2002 |
| Apical | Lung | R, H | Ciliated epithelial cells | Lips et al., 2005 |
| Apical | Choroid plexus | R | Epithelial cells | Sweet et al., 2001 |
| Apical | Olfactory mucosa | B | Epithelial cells | Chemuturi and Donovan, 2007 |
| Oct3 | ||||
| Basolateral | Kidney | H | Proximal tubule cell line | Glube and Langguth, 2008 |
| Basolateral | Placenta | H | Syncytiotrophoblasts | Sata et al., 2005 |
| Apical | Jejunum | H | Enterocytes | Muller et al., 2005 |
| Apical | Testes | R | Sertoli cells | Maeda et al., 2007a |
| Apical | Lung | R, H | Ciliated epithelial cells | Lips et al., 2005 |
| Octn1 | ||||
| Apical | Kidney | M | Proximal tubule cells | Tamai et al., 2004 |
| Apical | Eye | H | Corneal-limbal and conjunctival epithelium | Garrett et al., 2008 |
| N.D. | Heart | M | Endothelial cells in blood vessels | Iwata et al., 2008 |
| Octn2 | ||||
| Apical | Kidney | M, R, H | Proximal tubule cells | Tamai et al., 2001a; Masuda et al., 2006 |
| Apical | Placenta | H | Syncytiotrophoblasts | Lahjouji et al., 2004; Grube et al., 2005 |
| Apical | Small intestine | R | Enterocytes | Duran et al., 2005 |
| Apical | Eye | H | Corneal-limbal and conjunctival epithelium | Garrett et al., 2008 |
| Apical | Epididymis | M | Principal cells, spermatozoa | Yakushiji et al., 2006; Kobayashi et al., 2007 |
| Basolateral | Epididymis | R | Epithelia of distal caput and corpus | Rodriguez et al., 2002 |
| Basolateral | Testes | R | Sertoli cells | Kobayashi et al., 2005a |
| Basolateral | Brain | R | Capillary endothelial cells | Miecz et al., 2008 |
| N.D. | Brain | M, R | Olfactory bulb and nerve, cortex, cerebellum, spinal cord, hippocampus, hypothalamus, choroid plexus, astrocytes | Inazu et al., 2006; Lamhonwah et al., 2008 |
| N.D. | Pancreas | M | α Cells | Kai et al., 2005 |
| N.D. | Heart | M, H | Cardiac muscle cells, endothelial cells | Grube et al., 2006b; Iwata et al., 2008 |
| Octn3 | ||||
| Basolateral | Small intestine | R | Enterocytes | Duran et al., 2005 |
| N.D. | Epididymis | M | Spermatozoa | Kobayashi et al., 2007 |
| N.D. | Brain | M | Olfactory bulb and nerve, cortex, cerebellum, grey matter, hippocampus, hypothalamus, choroid plexus | Lamhonwah et al., 2008 |
| Oat1 | ||||
| Basolateral | Kidney | M, R, H | Proximal tubule cells | Hosoyamada et al., 1999; Tojo et al., 1999; Eraly et al., 2006 |
| Basolateral | Choroid plexus | M | Ependymal cells | Bahn et al., 2005 |
| N.D. | Muscle | H | Skeletal muscle cells | Takeda et al., 2004 |
| N.D. | Adrenal gland | R | Outer zona fasciculate | Beery et al., 2003 |
| Oat2 | ||||
| Basolateral | Liver | R | Hepatocytes | Simonson et al., 1994 |
| Basolateral | Kidney | H | Proximal tubule cells | Enomoto et al., 2002b |
| Apical | Kidney | M, R | Proximal tubule cells | Kojima et al., 2002; Ljubojevic et al., 2007 |
| Oat3 | ||||
| Basolateral | Kidney | M, R, H | Proximal tubule cells | Cha et al., 2001; Kojima et al., 2002; Bahn et al., 2005 |
| Basolateral | Eye | R | Retinal vascular endothelial cells | Hosoya et al., 2008 |
| Basolateral | Brain | R | Capillary endothelial cells | Kikuchi et al., 2003; Roberts et al., 2008 |
| Apical | Choroid plexus | M | Epithelial cells | Sweet et al., 2002; Sykes et al., 2004 |
| N.D. | Muscle | H | Skeletal muscle cells | Takeda et al., 2004 |
| Oat4 | ||||
| Apical | Kidney | H | Proximal tubule cells | Babu et al., 2002a; Ekaratanawong et al., 2004 |
| Basolateral | Placenta | H | Syncytiotrophoblasts | Ugele et al., 2008 |
| Oat5 | ||||
| Apical | Kidney | R | Proximal tubule cells | Anzai et al., 2005 |
| Oat6 | ||||
| N.D. | Olfactory mucosa | M | Olfactory cells | Monte et al., 2004 |
| Oat7 | ||||
| Basolateral | Liver | H | Hepatocytes | Shin et al., 2007 |
| Urat1 | ||||
| Apical | Kidney | M, H | Proximal tubule cells | Enomoto et al., 2002a; Hosoyamada et al., 2004 |
N.D., not determined.
TABLE 6.
Subcellular localization of efflux Mdr transporters in various species
For each transporter, the apical or basolateral localization in a particular tissue and/or species is provided. Species included rat (R), mouse (M), human (H), and bovine (B). Detailed information regarding particular cellular populations or regions of the tissue are provided for some transporter isoforms.
| Cellular Localization | Tissue | Species | Cell Types | References |
|---|---|---|---|---|
| Mdr1a/1b/MDR1 | ||||
| Apical | Liver | H, R | Hepatocytes, cholangiocytes | Gigliozzi et al., 2000; Scheffer et al., 2002c |
| Apical | Brain | M, R | Endothelial cells, choroid plexus | Schinkel et al., 1994; Rao et al., 1999; Miller et al., 2000 |
| Apical | Placenta | M, H | Syncytiotrophoblasts | Lankas et al., 1998; St-Pierre et al., 2000 |
| Apical | Fetal membranes | M | Visceral yolk sac | Lankas et al., 1998 |
| Apical | Colon | M | Epithelial cells | Panwala et al., 1998 |
| Apical | Pancreas | H | Small epithelial ducts | Scheffer et al., 2002b |
| Apical | Small intestine | R | Enterocytes (jejunum/ileum) | Ujhazy et al., 2001; Rost et al., 2002 |
| Apical | Lung | H | Bronchial and bronchiolar epithelium | Scheffer et al., 2002c |
| Apical | Kidney | R, H | Proximal tubule cells | Jette et al., 1996; Ernest et al., 1997 |
| N.D. | Testes | H | Myoid, Leydig, capillary endothelial cells | Bart et al., 2004 |
| N.D. | Inner ear | M | Capillary endothelial cells | Zhang et al., 2000 |
| N.D. | Adrenal gland | H | Cortex | Scheffer et al., 2002b |
| Mdr2/MDR3 | ||||
| Apical | Liver | M, H | Hepatocytes | Buschman et al., 1992; de Vree et al., 1998 |
N.D., not determined.
TABLE 8.
Subcellular localization of efflux Mrp and Bcrp transporters in various species
For each transporter, the apical or basolateral localization in a particular tissue and/or species is provided. Species included rat (R), mouse (M), human (H), and bovine (B). Detailed information regarding particular cellular populations or regions of the tissue are provided for some transporter isoforms.
| Cellular Localization | Tissue | Species | Cell Types | References |
|---|---|---|---|---|
| Mrp1 | ||||
| Apical | Placenta | H | Syncytiotrophoblasts, endothelial cells | St-Pierre et al., 2000 |
| Apical | Brain | H, B | Capillary endothelial cells | Nies et al., 2004; Zhang et al., 2004d |
| Basolateral | Choroid plexus | M, R | Epithelial cells | Rao et al., 1999; Wijnholds et al., 2000a |
| Basolateral | Fetal membranes | H | Amnion, chorionic, decidual membranes | Aye et al., 2007 |
| Basolateral | Lung | M, H | Mucosal layer, bronchial epithelium | Wijnholds et al., 1998; Scheffer et al., 2002c |
| Basal | Testes | M, H | Leydig and Sertoli cells | Wijnholds et al., 1998; Bart et al., 2004 |
| Basolateral | Intestine | M | Crypt cells (Paneth cells) | Peng et al., 1999 |
| Basolateral | Heart | M | Sarcolemma | Jungsuwadee et al., 2006 |
| N.D. | Kidney | M | Limb of Henle and collecting ducts, glomeruli | Wijnholds et al., 1998; Peng et al., 1999 |
| Mrp2 | ||||
| Apical/Basolateral | Brain | M, R | Endothelial cells | Miller et al., 2000; Soontornmalai et al., 2006 |
| Apical | Placenta | H | Syncytiotrophoblasts | St-Pierre et al., 2000 |
| Apical | Fetal membranes | M, H | Visceral yolk sac, amnion | Aye et al., 2007; Aleksunes et al., 2008b |
| Apical | Kidney | R, H | Proximal tubule cells | Schaub et al., 1997; Scheffer et al., 2000 |
| Apical | Liver | R, H | Hepatocytes | Paulusma et al., 1996; Scheffer et al., 2000 |
| Apical | Gallbladder | H | Epithelial cells | Rost et al., 2001 |
| Apical | Small intestine | R | Enterocytes (jejunum) | Mottino et al., 2000; Rost et al., 2002 |
| Mrp3 | ||||
| Tight junction | Brain | M | Choroid plexus | Soontornmalai et al., 2006 |
| Basolateral | Liver | H | Hepatocytes (periportal) | Konig et al., 1999; Scheffer et al., 2000; Nies et al., 2004 |
| Basolateral | Liver | M, R | Hepatocytes (centrilobular) | Donner and Keppler, 2001; Soroka et al., 2001; Zelcer et al., 2006 |
| Basolateral | Liver | R, H | Cholangiocytes | Soroka et al., 2001; Scheffer et al., 2002b |
| Basolateral | Pancreas | M, H | Ductal cells | Scheffer et al., 2002b; Zelcer et al., 2006 |
| Basolateral | Kidney | H | Distal convoluted tubule cells, loop of Henle | Scheffer et al., 2002b |
| Basolateral | Gallbladder | H | Epithelial cells | Scheffer et al., 2002b |
| Basolateral | Small intestine/colon | M, R, H | Enterocytes (ileum), crypt cells | Rost et al., 2002; Scheffer et al., 2002b; Mutch et al., 2004 |
| N.D. | Adrenal gland | H | Zona reticularis, fasciculate | Scheffer et al., 2002b |
| Apical | Placenta | H | Syncytiotrophoblasts, endothelium | St-Pierre et al., 2000 |
| Mrp4 | ||||
| Apical | Brain | R, H | Capillary endothelial cells | Nies et al., 2004; Roberts et al., 2008 |
| Apical/Basolateral | Choroid plexus | R | Epithelial cells | Roberts et al., 2008 |
| Apical | Kidney | H | Proximal tubule cells | van Aubel et al., 2002 |
| Basolateral | Fetal membranes | M | Visceral yolk sac | Aleksunes et al., 2008b |
| Basolateral | Liver | M | Hepatocytes | Assem et al., 2004 |
| Basolateral | Prostate | H | Glandular epithelial cells | Lee et al., 2000b; Rius et al., 2005 |
| Mrp5 | ||||
| Basolateral | Brain | R | Ependymal cells | Roberts et al., 2008 |
| Apical | Brain | M, B, H | Endothelial cells, pyramidal neurons | Nies et al., 2004; Zhang et al., 2004d; Soontornmalai et al., 2006 |
| Basolateral | Placenta | H | Syncytiotrophoblasts | Meyer Zu Schwabedissen et al., 2005 |
| Basolateral/Apical | Fetal membranes | M, H | Visceral yolk sac, amnion | Aye et al., 2007; Aleksunes et al., 2008b |
| N.D. | Genitourinary | H | Corpus cavernosum, ureter, urethra, bladder | Nies et al., 2002b |
| N.D. | Heart | H | Auricular & ventricular cardiomyocytes, capillary endothelial cells, smooth muscle cells | Dazert et al., 2003 |
| Mrp6 | ||||
| Basolateral | Fetal membranes | M | Visceral yolk sac | Aleksunes et al., 2008b |
| Basolateral | Liver | M, R, H | Hepatocytes | Madon et al., 2000; Scheffer et al., 2002a; Gorgels et al., 2005 |
| Basolateral | Kidney | M, H | Proximal tubule cells | Scheffer et al., 2002a; Beck et al., 2003; Gorgels et al., 2005 |
| Apical | Tongue | M | Squamous epithelial cells | Beck et al., 2003 |
| N.D. | Eye | M | Neuron layer | Beck et al., 2003 |
| N.D. | Brain | M | Cerebrum neurons, Purkinje, ependymal cells | Beck et al., 2003 |
| N.D. | Intestine | M | Mucosal cells | Beck et al., 2003 |
| Bcrp | ||||
| Apical | Liver | M, H | Hepatocytes | Maliepaard et al., 2001; Jonker et al., 2002 |
| Apical | Gallbladder | H | Epithelium | Aust et al., 2004 |
| Apical | Kidney | M | Proximal tubule cells | Jonker et al., 2002 |
| Apical | Small Intestine | M, H | Enterocytes | Maliepaard et al., 2001; Jonker et al., 2002 |
| Apical | Brain | M, R, H | Brain capillaries, choroid plexus | Cooray et al., 2002; Hori et al., 2004; Lee et al., 2005b |
| Apical | Fetal membranes | M, H | Visceral yolk sac, amnion | Aleksunes et al., 2008b; Yeboah et al., 2008 |
| Apical/Basolateral | Placenta | M, H | Syncytiotrophoblasts | Maliepaard et al., 2001; Jonker et al., 2002 |
| Apical | Testes | M, H | Endothelial cells | Bart et al., 2004; Enokizono et al., 2007 |
| Apical | Epididymis | M | Body, head | Enokizono et al., 2007 |
| Apical | Mammary gland | H, R, B | Epithelia from lactating gland | Maliepaard et al., 2001; Pulido et al., 2006; Wang et al., 2008b |
| Apical | Eye | M | Retinal capillary endothelial cells | Asashima et al., 2006 |
| Apical | Lung | H | Epithelium, glands, endothelial cells | Scheffer et al., 2002c |
| N.D. | Heart | H | Capillary endothelial cells and arterioles | Meissner et al., 2006 |
N.D, not determined.
TABLE 9.
Subcellular localization of bile acid, cholesterol, aminophospholipid, and copper transporters in various species
For each transporter, the apical or basolateral localization in a particular tissue and/or species is provided. Species included rat (R), mouse (M), human (H), and bovine (B). Detailed information regarding particular cellular populations or regions of the tissue are provided for some transporter isoforms. N.D., not determined.
| Cellular Localization | Tissue | Species | Cell Types | References |
|---|---|---|---|---|
| Ntcp | ||||
| Basolateral | Liver | M, R, H | Hepatocytes | Stieger et al., 1994; Keitel et al., 2005; Aleksunes et al., 2006 |
| Apical | Pancreas | R | Acinar cells | Kim et al., 2002a |
| Asbt | ||||
| Apical | Ileum | M | Enterocytes | Dawson et al., 2005 |
| Apical | Kidney | R | Proximal convoluted tubules | Christie et al., 1996 |
| Apical | Liver | R | Cholangiocytes | Lazaridis et al., 1997 |
| Bsep | ||||
| Apical | Liver | M, R, H | Hepatocytes | Childs et al., 1998; Jansen et al., 1999; Green et al., 2000 |
| Ostα | ||||
| Basolateral | Ileum | M, R, H | Enterocytes | Ballatori et al., 2005; Dawson et al., 2005 |
| Basolateral | Liver | M, H | Hepatocytes, cholangiocytes | Ballatori et al., 2005 |
| Basolateral | Kidney | M, R, H | Proximal tubule cells | Ballatori et al., 2005 |
| Ostβ | ||||
| Basolateral | Ileum | M, H | Enterocytes | Ballatori et al., 2005; Dawson et al., 2005 |
| Abcg5 | ||||
| Apical | Liver | M, H | Hepatocytes, cholangiocytes | Graf et al., 2003; Klett et al., 2004a |
| Apical | Small intestine | M, H | Enterocytes | Graf et al., 2003; Klett et al., 2004a |
| Intracellular | Gallbladder | H | Mucosal epithelial cells | Klett et al., 2004a |
| Abcg8 | ||||
| Apical/Intracellular | Liver | H | Hepatocytes, cholangiocytes | Klett et al., 2004a |
| Apical | Small intestine | H | Enterocytes | Klett et al., 2004a |
| Intracellular | Gallbladder | H | Mucosal epithelial cells | Klett et al., 2004a |
| ATP7B | ||||
| Apical | Liver | R | Hepatocytes | Hernandez et al., 2008 |
| Apical | Placenta | H | Syncytiotrophoblasts | Hardman et al., 2004; Hardman et al., 2007 |
| Intracellular | Small intestine | M | Enterocytes | Weiss et al., 2008 |
| Intracellular | Mammary gland | M | Ductal epithelial cells | Michalczyk et al., 2000 |
| ATP8b1 | ||||
| Apical | Liver | M, R, H | Hepatocytes | Eppens et al., 2001; Ujhazy et al., 2001 |
| Apical | Small Intestine | R | Enterocytes | Ujhazy et al., 2001 |
TABLE 3.
Substrates for SLC transporters
Substrates of the various transporter isoforms were identified using in vitro transport studies of human or rodent isoforms or from in vivo studies using knockout mice or mutant rats. A number of substrates are provided. Not all substrates are included in this list.
| OATP1A2 | Sulfobromophthalein, BQ-123, cholic acid, dehydroepiandrosterone sulfate, deltophorin II, DPDPE, estrone-3-sulfate, fexofenadine, glycocholate, levofloxacin, methotrexate, microcystin-LR, ouabain, prostaglandin E2, rosuvastatin, saquinavir, taurocholate, thyroxine, triiodothyronine |
| OATP1B1 | Benzylpenicillin, bilirubin and its conjugates, bosentan, BQ-123, bromosulfophthalein, caspofungin, cerivastatin, cholic acid, dehydroepiandrosterone sulfate, DPDPE, estradiol 17β-glucuronide, estrone-3-sulfate, fluvastatin, glycocholate, irinotecan (SN38 metabolite), leukotriene C4, microcystin-LR, olmesartan, phalloidin, pravastatin, prostaglandin E2, rifampicin, rifampin, rosuvastatin, taurocholate, thromboxane B2, thyroxine, triiodothyronine, troglitazone sulfate, valsartan |
| OATP1B3 | Bilirubin conjugates, bosentan, sulfobromophthalein, BQ-123, cholecystokinin-8, dehydroepiandrosterone sulfate, deltorphin II, digoxin, DPDPE, docetaxel, estradiol 17β-glucuronide, fexofenadine, fluvastatin, glycocholate, irinotecan (SN38 metabolite), leukotriene C4, microcystin-LR, olmesartan, ouabain, paclitaxel, phalloidin, rifampicin, rifampin, rosuvastatin, taurocholate, telmisartan, thyroxine, triiodothyronine, valsartan |
| OCT1 | Acetylcholine, acyclovir, cimetidine, choline, dopamine, famotidine, ganciclovir, lamivudine, metformin, N-methylnicotinamide, 1-methyl-4-phenylpyridinium, quinine, ranitidine, serotonin, spermine, spermidine, tetraethylammonium, zalcitabine |
| OCT2 | Acetylcholine, amantadine, cimetidine, cisplatin, choline, dopamine, epinephrine, histamine, lamivudine, memantine, metformin, 1-methyl-4-phenylpyridinium, N-methylnicotinamide, norepinephrine, paraquat, prostaglandin E2, prostaglandin F2, quinine, ranitidine, serotonin, tetraethylammonium, zalcitabine |
| OCT3 | Atropine, dopamine, epinephrine, etilefrine, guanidine, histamine, 1-methyl-4-phenylpyridinium, tetraethylammonium |
| OCTN1 | l-Carnitine, ergothioneine, pyrilamine, quinidine, quinine, tetraethylammonium, verapamil |
| OCTN2 | l-Carnitine, cephaloridine, mildronate, pyrilamine, quinidine, spironolactone, tetraethylammonium, valproic acid, verapamil |
| OAT1 | Acetylsalicylate, acyclovir, adefovir, p-aminohippurate, cephaloridine, cidofovir, cimetidine, cyclic AMP and GMP, didanosine, edaravone sulfate, furosemide, ganciclovir, indoxyl sulfate, indomethacin, α-ketoglutarate, lamivudine, methotrexate, ochratoxin A, penicillin G, prostaglandins E2 and F2α, salicylate, stavudine, tetracycline, trifluridine, urate, zidovudine, zalcitabine |
| OAT2 | p-Aminohippurate, acetylsalicylate, allopurinol, bumetanide, cyclic AMP, dehydroepiandrosterone sulfate, estrone-3-sulfate, 5-fluorouracil, glutarate, α-ketoglutarate, methotrexate, paclitaxel, prostaglandins E2 and F2α, ochratoxin A, salicylate, tetracycline, valproic acid, zidovudine |
| OAT3 | Allopurinol, p-aminohippurate, benzylpenicillin, l-carnitine, cefazolin, cephaloridine, cholic acid, cimetidine, cortisol, dehydroepiandrosterone sulfate, edaravone sulfate, estrone-3-sulfate, famotidine, 5-fluorouracil, glutarate, glutathione, glycocholate, indoxyl sulfate, methotrexate, 6-mercaptopurine, ochratoxin A, pravastatin, prostaglandins E2 and F2α, rosuvastatin, taurocholate, tetracycline, urate, valacyclovir, zidovudine |
| OAT4 | p-Aminohippurate, dehydroepiandrosterone sulfate, estrone-3-sulfate, glutarate, indoxyl sulfate, ochratoxin A, tetracycline, zidovudine |
| PEPT1 | 5-Aminolevulinic acid, bestatin, cefadroxil, ceftibuten, cefixime, cephradine, cephalexin, glycylsarcosine |
| PEPT2 | 5-Aminolevulinic acid, bestatin, cefadroxil, glycylsarcosine, l-kyotorphin |
| CNT1 | Adenosine, cladribine, cytarabine, fialuridine, 5-fluorouridine, gemcitabine, stavudine, thymidine, uridine, zalcitabine, zidovudine |
| CNT2 | Adenosine, cladribine, clofarabine, cytidine, didanosine, fialuridine, 5-fluorouridine, formycin B, inosine, guanosine, ribavirin, tiazofurin, uridine |
| CNT3 | Adenosine, benzamide riboside, cladribine, clofarabine, cytarabine, cytidine, didanosine, fludarabine, 5-fluorouridine, gemcitabine, guanosine, inosine, 6-mercaptopurine, ribavirin, uridine, 6-thioguanine, tiazofurin, thymidine, zalcitabine, zebularine, zidovudine |
| ENT1 | Adenosine, cladribine, clofarabine, cytidine, fialuridine, gemcitabine, guanosine, ribavirin, thymidine, tiazofurin, uridine |
| ENT2 | Adenine, adenosine, clofarabine, cytidine, fialuridine, gemcitabine, guanine, guanosine, hypoxanthine, inosine, thymidine, tiazofurin, uridine |
| ENT3 | Adenine, adenosine, cladribine, fludarabine, guanosine, inosine, thymidine, uridine, zebularine, zidovudine |
| MATE1 | Acyclovir, cephalexin, cephradine, cimetidine, creatinine, estrone sulfate, ganciclovir, guanidine, 1-methyl-4-phenylpyridinium, metformin, oxaliplatin, paraquat, procainamide, tenofovir, tetraethylammonium, thiamine, topotecan |
| MATE2-K | Acyclovir, cimetidine, creatinine, estrone sulfate, ganciclovir, guanidine, metformin, 1-methyl-4-phenylpyridinium, N1-methylnicotinamide, oxaliplatin, procainamide, tetraethylammonium, thiamine, topotecan |
TABLE 7.
Substrates for ABC transporters
Substrates of the various transporter isoforms were identified using in vitro transport studies of human or rodent isoforms or from in vivo studies using knockout mice or mutant rats. A number of substrates are provided. Not all substrates are included in this list.
| MDR1 | Actinomycin D, amitriptyline, cerivastatin, colchicine, cyclosporine A, daunorubicin, digoxin, diltiazem, docetaxel, domperidone, doxorubicin, erlotinib, erythromycin, etoposide, fexofenadine, imatinib, indinavir, ivermectin, lapatinib, loperamide, losartan, lovastatin, nelfinavir, ondansetron, oseltamivir, paclitaxel, phenytoin, prazosin, quinidine, ritonavir, saquinavir, sparfloxacin, terfenadine, tetracycline, (99m)Tc-tetrofosmin, topotecan, vecuronium, verapamil, vinblastine, vincristine |
| MRP1 | Aflatoxin B1, daunorubicin, S-(2,4-dinitrophenyl)-glutathione, doxorubicin, epirubicin, estradiol-17β-glucuronide, estrone-3-sulfate, etoposide glucuronide, folate, fluo-3, oxidized glutathione, glutathione-conjugated aflatoxin B1, glutathione-conjugated chlorambucil, glutathione-conjugated ethacrynic acid, glutathione-conjugated 4-hydroxynonenal, glutathione-conjugated prostaglandin A, grepafloxacin, leukotrienes C4, D4, and E4 (glutathione-conjugated leukotriene C4), methotrexate, methoxychlor, vincristine |
| MRP2 | Acetaminophen-glucuronide, acetaminophen-sulfate, p-aminohippurate, arsenic-glutathione, bilirubin-glucuronide, BQ-123, diclofenac-glucuronide, S-(2,4-dinitrophenyl)-glutathione, estradiol-17β-glucuronide, ethinylestradiol glucuronide, glutathione-conjugated ethacrynic acid, glutathione-conjugated 4-hydroxynonenal, indinavir, leukotriene C4, methotrexate, morphine-3-glucuronide, ochratoxin A, oxidized and reduced glutathione, PhIP, ritonavir, saquinavir, sulfotaurolithocholic acid, taurine-conjugated cholic acid, taurolithocholate sulfate, vinblastine |
| MRP3 | Acetaminophen-glucuronide, bilirubin, estradiol-17β-glucuronide, ethinylestradiol glucuronide, etoposide, etoposide-glucuronide, folate, glycocholate, leucovorin, methotrexate, morphine-3-glucuronide, morphine-6-glucuronide, resveratrol-glucuronide, taurochenodeoxycholate-3-sulfate, taurocholate, taurolithocholate-3-sulfate |
| MRP4 | Adefovir, p-aminohippurate, bimane-glutathione, cefazolin, ceftizoxime, cholic acid, cyclic AMP and GMP, dehydroepiandrosterone sulfate, edaravone sulfate, estradiol-17β-glucuronide, folate, furosemide, glycine- and taurine-conjugated bile acids, hydrochlorothiazide, irinotecan and its active metabolite, leucovorin, leukotriene B4 and C4, 6-mercaptopurine, methotrexate, prostaglandins E1, E2, and F2α, taurocholate, tenofovir, thromboxane B2, topotecan, urate, zidovudine |
| MRP5 | Adefovir, cadmium chloride, cyclic AMP and GMP, 5-fluorouracil, folate, hyaluronan, 6-mercaptopurine, methotrexate, potassium antimonyl, 6-thioguanine |
| MRP6 | BQ-123, S-(2,4-dinitrophenyl)-glutathione, N-ethylmaleimide glutathione, etoposide, leukotriene C4, teniposide |
| MRP7 | Docetaxel, estradiol-17β-glucuronide, leukotriene C4, paclitaxel, vinblastine, vincristine |
| MRP8 | Cyclic AMP and GMP, dehydroepiandrosterone sulfate, estradiol-17β-glucuronide, estrone-3-sulfate, 5-fluorouracil, folate, glycocholate, leukotriene C4, methotrexate, taurocholate, zalcitabine |
| BCRP | Abacavir, aflatoxin B, albendazole sulfoxide, ciprofloxacin, coumestrol, daidzein, dantrolene, dehydroepiandrosterone sulfate, dipyridamole, edaravone sulfate, enrofloxacin, erlotinib, estradiol-17β-glucuronide, estrone-3-sulfate, etoposide, furosemide, gefitinib, genistein, glyburide, grepafloxacin, hematoporphyrin, Hoechst, hydrochlorothiazide, imatinib, lamivudine, lapatinib, methotrexate, mitoxantrone, nitrofurantoin, norfloxacin, ofloxacin, oxfendazole, pheophorbide a, PhIP, prazosin, resveratrol 3-sulfate, resveratrol di-sulfate, riboflavin, rosuvastatin, triamterene, ulifloxacin, zidovudine |
PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine.
A. Solute Carrier Transporters
1. Organic Anion Transporting Polypeptides.
Oatps/OATPs are members of the SLCO family (Table 1) and are responsible for the uptake of a wide range of substrates. Rat Oatp1a1 was the first member of the OATP family identified (Jacquemin et al., 1994) followed by rat Oatp2a1 (Kanai et al., 1995). OATP1A2 was the first human OATP to be cloned (Kullak-Ublick et al., 1995). The rapid and independent classification of multiple Oatp/OATP isoforms led to confusion regarding protein nomenclature. New nomenclature and classification of OATP isoforms according to evolutionary relationships and amino acid sequence identity were established in 2004 and approved by the HUGO Gene Nomenclature Committee (Hagenbuch and Meier, 2004). Oatps/OATPs with more than 40% amino acid sequence identity are members of the same family (OATP1, OATP2, OATP3…). Designation of isoforms to a particular subfamily (OATP1A, -1B, and -1C) requires more than 60% amino acid sequence identity. More than 15 rodent and 10 human isoforms have been described (Hagenbuch and Meier, 2004; Hagenbuch and Gui, 2008). In addition, an Oatp ortholog (Oatp1d1) was identified in skate liver and has been proposed to be an evolutionarily ancient precursor of mammalian OATP1B1/OATP1B3/Oatp1b2 responsible for uptake of cyclic peptides (Meier-Abt et al., 2007).
OATPs are integral membrane proteins predicted to contain 12 transmembrane helices with amino and carboxyl termini oriented to the cytoplasmic face (Noe et al., 1997; Hagenbuch et al., 2000; Mikkaichi et al., 2004). A large extracellular domain is thought to be located between transmembrane domains 9 and 10, with N-glycosylation sites present in extracellular loops 2 and 5 (Hagenbuch and Meier, 2003).
Expression of mouse Oatp isoforms varies among tissues (Fig. 1). Oatp1a1, -1a4, -1b2, and -2b1 are expressed in liver, whereas Oatp1a6, -3a1, and -4c1 are expressed in kidneys (Choudhuri et al., 2001; Cheng et al., 2005a). Levels of Oatp1a4 and -1c1 mRNA are high in the brain (Cheng et al., 2005a). Oatp1a5, -6b1, -6c1, and -6d1 mRNA are predominantly expressed in mouse testes. Within the testes, rat Oatp6b1 and -6c1 are expressed in Sertoli cells, spermatogonia, and Leydig cells (Suzuki et al., 2003). Oatp2a1, -4a1, and -5a1 are highest in the placenta (Cheng et al., 2005a).
The tissue distribution of the various OATP isoforms in humans also ranges from a single tissue to ubiquitous expression. For example, human OATP1B1 and OATP1B3 are primarily expressed in liver (Fig. 1) (Abe et al., 1999; Hsiang et al., 1999; König et al., 2000a,b). In contrast, OATP1C1, OATP2A1, OATP2B1, OATP3A1, and OATP4A1 mRNA can be detected in multiple tissues (Fig. 1) (Tamai et al., 2000a; Kullak-Ublick et al., 2001; Pizzagalli et al., 2002; Grube et al., 2006a).
There are similarities and differences in the tissue distribution of Oatp/OATP transporters between mice and humans (Fig. 1). For example, mouse Oatp1c1 and human OATP1C1 are highly expressed in brain (Pizzagalli et al., 2002; Cheng et al., 2005a). Likewise, both rat and mouse Oatp1a4 are most abundant in brain and liver (Noe et al., 1997; Cheng et al., 2005a). In contrast, Oatp4a1 is specifically expressed in mouse placenta, yet OATP4A1 is widely expressed in multiple human tissues (Tamai et al., 2000a; Cheng et al., 2005a). Therefore, differences in the tissue distribution of some Oatp/OATP isoforms are important when extrapolating from rodents to humans.
Cellular localization of Oatp/OATP isoforms can be apical or basolateral depending on the tissue and cell type (Table 2). In liver, Oatp/OATP isoforms are typically on the basolateral (also called sinusoidal) membrane of hepatocytes (Oatp1a1, -1a4, -1b2 and OATP1B1, -1B3, -2B1), although human OATP1A2 is localized to the apical surface of cholangiocytes (Bergwerk et al., 1996; Kakyo et al., 1999; Reichel et al., 1999; König et al., 2000a,b; Cattori et al., 2001; Lee et al., 2005a; Grube et al., 2006a). Within the liver, human OATP1B3 and rat Oatp1a4 are mostly confined to centrilobular hepatocytes, whereas human OATP1B1 is expressed uniformly throughout the lobule (Kakyo et al., 1999; Reichel et al., 1999; Ho et al., 2006b). Likewise, Oatps/OATPs are detected on both the apical and basolateral surfaces of the kidneys (apical OATP1A2 and Oatp1a1; basolateral Oatp4c1) and placenta (apical OATP4A1; basolateral OATP2B1) (Table 2) (Bergwerk et al., 1996; St-Pierre et al., 2002; Sato et al., 2003; Mikkaichi et al., 2004; Lee et al., 2005a; Grube et al., 2007).
Oatp/OATPs transport solutes with diverse characteristics. In general, Oatp/OATP substrates contain steroidal or peptide structural backbones and/or are anionic or cationic chemicals. Classes of pharmaceuticals transported by Oatp/OATPs include HMG-CoA reductase inhibitors (statins), angiotensin-converting enzyme inhibitors, angiotensin receptor II antagonists, and cardiac glycosides (Table 3). A number of endogenous chemicals, including thyroxine, steroid conjugates, bile acids, bilirubin, and prostaglandins are also substrates of Oatp/OATPs. It has long been recognized that chemicals secreted into bile are structurally larger than those excreted by the kidneys and may be due to selective extraction of bulky chemicals from the circulation by hepatic Oatp/OATPs. More recent evidence points to the influence of pH in the transport kinetics of Oatp/OATPs (Leuthold et al., 2009). Using Oatp/OATP-expressing oocytes and cultured cells, it was demonstrated that the transport activity of number of isoforms (with the exception of OATP1C1) is enhanced by low extracellular pH and that this flux is countered by bicarbonate efflux (Leuthold et al., 2009).
Although OATPs are typically considered uptake transporters, there are examples of bidirectional transport for various isoforms (Li et al., 2000; Mahagita et al., 2007). Transport of taurocholate and leukotriene C4 by rat Oatp1a1 in oocytes is cis inhibited and trans stimulated by glutathione, suggesting that glutathione efflux provides a driving force for uptake (Li et al., 1998). Additional research demonstrates that Oatp1a4-mediated transport of taurocholate is bidirectional and stimulated by glutathione and its conjugates (Li et al., 2000). More recent research demonstrates that human OATP1B1 and -1B3 are similarly bidirectional facilitated diffusion transporters, but that glutathione is not a substrate or activator of their transport activity (Mahagita et al., 2007).
Human OATP1A2 transports and is inhibited by a large number of endogenous compounds as well as pharmaceuticals in in vitro systems (Table 3). Because of the promiscuity of this transporter, a number of drug-drug interactions have been proposed for OATP1A2. OATP1A2-mediated transport of fexofenadine is inhibited by antivirals, antifungals, antibiotics, and anticholesterol drugs (Cvetkovic et al., 1999). OATP1A2 can also transport the fluoroquinolone antibiotic levofloxacin and is inhibited by other quinolones (Maeda et al., 2007b). In addition to pharmaceutical inhibition, dietary constituents may also modulate drug transport by inhibiting OATP1A2 function. The grapefruit flavonoid naringin inhibits OATP1A2 uptake of fexofenadine in vitro, and thus grapefruit juice alters clinical fexofenadine pharmacokinetics (Dresser et al., 2002; Bailey et al., 2007; Glaeser et al., 2007). These data suggest that naringin probably interferes with the intestinal OATP1A2-mediated absorption of oral fexofenadine (Glaeser et al., 2007). Similar reports demonstrate in vitro inhibition of OATP2B1 transport by grapefruit and orange juices as well as other herbal extracts (Satoh et al., 2005; Fuchikami et al., 2006).
Rodent Oatp1a1 and -1a4 share similar substrates with OATP1A2, including unconjugated and conjugated bile acids, bromosulfophthalein, sulfated steroids, thyroid hormones, ouabain, β-lactam antibiotics, and fexofenadine (Cvetkovic et al., 1999; Reichel et al., 1999; Hagenbuch et al., 2000; Meng et al., 2002; van Montfoort et al., 2002; Nakakariya et al., 2008). Rat Oatp1a1 and Oatp1a4 can transport opioid peptides [d-Pen2,d-Pen5]-enkephalin and deltorphin II (Oatp1a1 only), which may be important in their transport across the blood-brain and blood-cerebrospinal fluid barriers (Kakyo et al., 1999; Gao et al., 2000). There are notable differences in transport by mouse and rat Oatp1a4; digoxin is a high-affinity substrate for rat Oatp1a4 but a low-affinity substrate for mouse Oatp1a4 (Noe et al., 1997; van Montfoort et al., 2002). Likewise, bromosulfophthalein is transported by mouse Oatp1a4 but not by the rat isoform (van Montfoort et al., 2002).
OATP1B1 and -1B3 are the primary OATP1B isoforms in human livers. Oatp1b2 is the rodent ortholog of OATP1B1 and -1B3. Because of the prominent expression of these transporters on the basolateral membrane of hepatocytes, they represent a critical mechanism for chemical uptake into liver. OATP1B1 and -1B3 exhibit overlapping and specific substrates (Table 3). Human OATP1B1 transports various statin drugs as well as thyroxine, taurocholate, and dehydroepiandrosterone sulfate (Hsiang et al., 1999). Both OATP1B1 and -1B3 can transport conjugated bilirubin; however, 1B1 appears to be more important for unconjugated bilirubin uptake (Cui et al., 2001). Using overexpressing oocytes, Oatp1b2 and OATP1B3 transport cholecystokinin, a gastrointestinal peptide that is released postprandially and stimulates gallbladder contraction, release of pancreatic enzymes, and intestinal motility (Ismair et al., 2001).
More recently, attention has been brought to the uptake mechanisms for hepatotoxic drugs, including bosentan and troglitazone. Bosentan and its active metabolite are substrates of OATP1B1 and -1B3 (Treiber et al., 2007). Likewise, OATP1B1 can transport and be inhibited by troglitazone sulfate (Nozawa et al., 2004b). It is hypothesized that inhibition of OATP1B1 by troglitazone sulfate may be a novel mechanism underlying idiosyncratic hepatotoxicity associated with this pharmaceutical (Nozawa et al., 2004b).
Clinical drug interactions may also occur at the level of OATP transporters. OATP1B1 transports pravastatin (Nakai et al., 2001). OATP1B1 transport is inhibited by fibric acid derivatives and may contribute to known drug-drug interactions, such as gemfibrozil-cerivastatin (Shitara et al., 2004; Yamazaki et al., 2005) and rifampin-atorvastatin (Lau et al., 2007). In contrast, rosuvastatin can be transported by a number of OATP isoforms, including OATP1B1, -1B3, -2B1, and -1A2 as well as rat Oatp1a1, -1a4, -1a5, and -1b2, probably reducing the chance of drug-drug interactions (Ho et al., 2006b). OATP1B1 also transports the active metabolite of the anticancer drug irinotecan (Nozawa et al., 2005). OATP1B1 and -1B3 transport the angiotensin-II blocker olmesartan (Nakagomi-Hagihara et al., 2006). Further work is necessary to better characterize clinical-relevant drug-drug interactions of these and other OATP1B1 and -1B3 substrates.
OATP1B1, -1B3, and rat Oatp1b2 participate in the uptake of rifampin (Tirona et al., 2003). Overexpression of OATP1B1 not only enhances rifampin transport but also its function as evidenced by enhancement of rifampin-stimulated pregnane X receptor gene transactivation (Tirona et al., 2003). Rifampicin can inhibit OATP1B1 and -1B3 transport (and be transported by them), whereas rifamycin SV can also inhibit OATP1A2 and -2B1 (Vavricka et al., 2002).
OATP2B1 is expressed in human placenta and, along with the breast cancer resistance protein, (BCRP; ABCG2) is probably responsible for transepithelial transport of sulfated steroids from the fetus to the mother during pregnancy (St-Pierre et al., 2002; Grube et al., 2007). Likewise, OATP2B1 is also expressed in ductal epithelial cells of the mammary gland (Pizzagalli et al., 2003). OATP2B1 prefers sulfate conjugates (estrone sulfate) rather than glucuronide conjugates (i.e., estradiol-17β-glucuronide) (Tamai et al., 2001b; Nozawa et al., 2004a). OATP2B1 can also transport dehydroepiandrosterone sulfate, the antihistamine fexofenadine, and the antidiabetic drug glibenclamide (Nozawa et al., 2004a; Satoh et al., 2005).
2. Organic Cation Transporters.
OCTs are polyspecific cationic transporters of the SLC22 family (SLC22A1–3) (Table 1). In 1994, Oct1 was the first member of the organic cation transporter family cloned from a rat kidney cDNA library (Gründemann et al., 1994). Human and mouse orthologs were soon cloned thereafter (Schweifer and Barlow, 1996; Gorboulev et al., 1997; Zhang et al., 1997). Subsequently, Oct2 and Oct3, two organic cation transporters with high homology to Oct1, were cloned and characterized in humans, rats, mice, and rabbits (Okuda et al., 1996; Gorboulev et al., 1997; Zhang et al., 1997; Kekuda et al., 1998; Urakami et al., 1998; Karbach et al., 2000). Oct3 is also called the extraneuronal monoamine transporter and participates in the uptake of extraneuronal monoamines in peripheral tissues and glia cells (also known as the uptake-2 system) (Gründemann et al., 1998; Wu et al., 1998). The membrane topology of OCT isoforms is predicted to be similar with 12 α-helical transmembrane domains with intracellular amino and carboxy termini (Burckhardt and Wolff, 2000). An extracellular loop between transmembrane domains 1 and 2 contains potential N-glycosylation sites (Burckhardt and Wolff, 2000). A large intracellular loop resides between transmembrane domains 6 and 7 and possesses predicted phosphorylation sites.
In mice, Oct1 mRNA expression is highest in kidneys and liver (Fig. 2) (Alnouti et al., 2006). Human OCT1 is primarily expressed in liver and to a lesser extent in other organs (Fig. 2) (Gorboulev et al., 1997; Zhang et al., 1997). Oct1/OCT1 proteins are localized to the basolateral membrane of centrilobular hepatocytes, proximal tubule cells, Sertoli cells, enterocytes, and in serotoninergic neurons of the small intestine (Table 4) (Meyer-Wentrup et al., 1998; Karbach et al., 2000; Muller et al., 2005; Maeda et al., 2007a). Prominent expression of OCT1 on the sinusoidal membrane of hepatocytes suggests that this transporter mediates the first step in hepatic excretion of cationic drugs.
Fig. 2.
Tissue distribution of Oct, Octn, Oat, and Urat mRNA in mice and humans. Top, relative mRNA levels of transporters in mouse liver, kidneys, lung, stomach, duodenum, jejunum, ileum, large intestine, heart, brain, gonads (testes and ovaries), placenta, and uterus are shown. Male (♂) mRNA is shown on the left whereas female (♀) mRNA is shown on the right side of each box. References for mouse mRNA expression are included (Buist and Klaassen, 2004; Alnouti et al., 2006). Bottom, relative mRNA levels of transporters in human liver, kidneys, lung, heart, brain, testes, ovaries, placenta, and uterus are shown. Data for humans were obtained from GNF SymAtlas (http://symatlas.gnf.org/; now located at http://biogps.gnf.org). The GNF1H/MAS5 data set was accessed during September 2008.
Rodent Oct2 and human OCT2 mRNA are highest within the kidneys (Fig. 2) (Gorboulev et al., 1997; Slitt et al., 2002; Alnouti et al., 2006). Within renal proximal tubule cells, Oct2/OCT2 proteins are present on the basolateral membrane, which makes this transporter a key entry site for renally excreted cationic drugs (Table 4) (Karbach et al., 2000; Motohashi et al., 2002). Neurons of the human central nervous system have detectable OCT2 protein (Busch et al., 1998). Similar to Oct1, apical expression of Oct2 protein is seen in bovine olfactory mucosa and ciliated epithelial cells of rodent and human lungs (Lips et al., 2005; Kummer et al., 2006; Chemuturi and Donovan, 2007) (Table 4).
The tissue distribution of mouse Oct3 and human OCT3 is broader than Oct1/OCT1 and Oct2/OCT2. Oct3/OCT3 are expressed in many tissues with high levels in placenta, ovaries, and uterus (Fig. 2) (Kekuda et al., 1998; Wu et al., 1998, 2000b; Verhaagh et al., 1999; Slitt et al., 2002; Alnouti et al., 2006). Subcellular localization patterns for human OCT3 are cell-type specific. OCT3 protein is observed on basolateral (trophoblasts, renal tubule cells) and apical (enterocytes, Sertoli cells, ciliated lung epithelia) membranes (Table 4) (Lips et al., 2005; Muller et al., 2005; Sata et al., 2005; Kummer et al., 2006; Maeda et al., 2007a; Glube and Langguth, 2008) as well as in numerous regions of the rat brain (Vialou et al., 2004).
Oct/OCT transporters mediate the uptake of organic cations that are positively charged at physiological pH. OCTs are classified as uniporters and enhance cellular entry of chemicals by facilitated diffusion. OCT-mediated transport is electrogenic and independent from sodium (Koepsell and Endou, 2004). The primary driving force that determines the direction of translocation is the electrochemical gradient of the transported organic cation, typically an inside-negative membrane potential.
Substrates of Oct/OCT transporters have relatively low molecular weights and are hydrophilic organic cations with widely diverse molecular structures (Table 3). There is extensive overlap of substrate and inhibitor specificities among OCT1–3 from different species. Oct1/OCT1 orthologs from four species (rat, mouse, rabbit, and human) all transport tetraethylammonium. However, there are some differences in affinity and transport rates. In contrast to rabbit and human, rat and mouse Oct1 do not transport larger structural analogs (i.e., tetrapropylammonium and tetrabutylammonium) (Dresser et al., 2000).
Model compounds for Oct/OCT-mediated transport include tetraethylammonium, the neurotoxin 1-methyl-4-phenylpyridinium, and N1-methyl-nicotinamide (Busch et al., 1996b; Gorboulev et al., 1997; Zhang et al., 1997; Kekuda et al., 1998; Urakami et al., 1998; Wu et al., 2000b). Pharmaceuticals have also been identified as Oct/OCT substrates and consist of the antidiabetic drug metformin (Kimura et al., 2005), the antiviral drugs acyclovir and zalcitabine (Takeda et al., 2002; Jung et al., 2008), the antineoplastic agent cisplatin (Ciarimboli et al., 2005b; Yokoo et al., 2007), the N-methyl-d-aspartate-receptor antagonist memantine (Busch et al., 1998), and the histamine H2-receptor antagonist ranitidine (Bourdet et al., 2005). Biogenic amine neurotransmitters including dopamine, epinephrine, norepinephrine, and histamine are also transported by OCTs (especially OCT3 as the extraneuronal monoamine transporter) (Busch et al., 1996a, 1998; Amphoux et al., 2006). Substrates of the various isoforms are shown in Table 3.
3. Organic Cation/Carnitine Transporters.
Like OCT transporters, OCTNs are members of the SLC22 family. Although they can transport cationic chemicals, OCTNs are most notably known for their ability to influx carnitine (Table 4). OCTN1 was cloned from a human fetal liver cDNA library in 1997, and rat and mouse isoforms were subsequently isolated (Tamai et al., 1997, 2000b; Wu et al., 2000a). OCTN2 was cloned from a human kidney cDNA library (Tamai et al., 1998). Whereas OCTN1 protein is predicted to contain 11 transmembrane domains and one-nucleotide binding domain (Tamai et al., 1997), OCTN2 probably has 12 transmembrane domains (Tamai et al., 1998). Octn3 was first found in mice, and although OCTN3 protein has been detected in a human cell line, the human gene has not been described (Tamai et al., 2000b; Lamhonwah et al., 2003).
Mouse and rat Octn1 are most prominently expressed in kidneys, with detectable mRNA in small intestine, stomach, heart, etc. (Fig. 2) (Tamai et al., 2000b; Slitt et al., 2002; Alnouti et al., 2006). In situ hybridization localizes Octn1 transcript to rat brain, kidney (cortex and medulla), heart (myocardium and valves), and placenta (labyrinth zone) (Wu et al., 2000a). Human OCTN1 is expressed in kidneys, skeletal muscle, placenta, prostate, heart, fetal liver, eyes, and lungs (Fig. 2) (Tamai et al., 1997; Garrett et al., 2008). There is also prominent expression of human OCTN1 in spleen, bone marrow, and whole blood, with particularly high levels in CD14+ cells (Tokuhiro et al., 2003). Likewise, immunohistochemical findings demonstrate Octn1 in different regions of the mouse brain and on the apical membrane of mouse proximal tubule cells (Table 4) (Tamai et al., 2004; Lamhonwah et al., 2008). Although OCTN1 is typically localized to the plasma membrane, intracellular localization in mitochondria has been reported and may be responsible for carnitine accumulation in this organelle (Lamhonwah and Tein, 2006).
Mouse and rat Octn2 mRNA are primarily expressed in kidneys (Fig. 2) (Kido et al., 2001; Rodríguez et al., 2002; Slitt et al., 2002; Alnouti et al., 2006). Messenger RNA and/or protein staining also localize Octn2 to heart (myocardium, valves, and arterioles), epididymis, pancreas (α-cells), and brain (cortex, hippocampus, choroid plexus, cerebellum) (Table 4) (Wu et al., 1999; Rodríguez et al., 2002; Kai et al., 2005; Lamhonwah et al., 2008). Human OCTN2 is most notably detected in kidneys and placenta and to a lesser degree in other tissues (Fig. 2) (Tamai et al., 1998; Tokuhiro et al., 2003; Lahjouji et al., 2004; Garrett et al., 2008). Octn2 and OCTN2 proteins are present on the brush border membrane vesicles from kidneys (Tamai et al., 2001a), placental syncytiotrophoblasts (Grube et al., 2005), and small intestine enterocytes (Durán et al., 2005), as well as the basolateral surface of epididymal cells (Rodríguez et al., 2002) (Table 4).
Mouse testes and epididymal spermatozoa (middle piece of sperm tail) express the highest levels of Octn3 mRNA and/or protein (Fig. 2) (Tamai et al., 2000b; Alnouti et al., 2006; Kobayashi et al., 2007). Octn3 is also detected in mouse ovaries (Alnouti et al., 2006), along the basolateral membrane of rat enterocytes (Durán et al., 2005), and within multiple mouse brain regions (Lamhonwah et al., 2008). Although Octn3 and OCTN3 proteins are found on the plasma membrane of various cell types, localization of these proteins to the peroxisome has also been reported and may be important in supplying carnitine for peroxisomal lipid metabolism (Lamhonwah et al., 2005).
As implied by their name, Octn/OCTN proteins transport carnitine (Table 3). During the generation of metabolic energy, carnitine is required for the transport of fatty acids from the cytosol into the mitochondria during the breakdown of lipids. Carnitine transports long-chain acyl groups generated from fatty acids into the mitochondrial matrix, where they can be broken down through β-oxidation. Octn1/OCTN1 is an organic cation uniporter or H+/organic cation antiporter that can transport in both directions. Octn2/OCTN2 can act as organic cation uniporters or sodium-carnitine cotransporters (Tamai et al., 1998). Mouse Octn3 is the most selective transporter for carnitine, whereas Octn1 is the least (Tamai et al., 2000b). Mouse Octn1 and Octn2 transport carnitine in a sodium-dependent manner, and Octn3 transports carnitine in a sodium-independent manner (Tamai et al., 2000b).
Octn1/OCTN1 and Octn2/OCTN2 also transport organic cations (Table 3). Both OCTN1 and OCTN2 transport tetraethylammonium, verapamil, quinidine, ergothioneine, and pyrilamine (Tamai et al., 1997; Ohashi et al., 1999; Yabuuchi et al., 1999; Ganapathy et al., 2000; Grube et al., 2006b). OCTN2 also transports the antiseizure drug valproic acid, the antibiotic cephaloridine, and the diuretic spironolactone (Ohashi et al., 1999; Ganapathy et al., 2000; Grube et al., 2006b). In contrast to Octn1 and Octn2, Octn3 has little or no affinity for organic cation model compounds (i.e., tetraethylammonium) and seems to function only as a carnitine transporter (Tamai et al., 2000b).
4. Organic Anion Transporters.
OATs are members of the solute carrier family SLC22A along with OCTs and OCTNs (Table 1). OAT transporters have 12 predicted transmembrane domains arranged in two sets of six helical domains (Simonson et al., 1994; Hosoyamada et al., 1999; Race et al., 1999; Cha et al., 2000). OATs are thought to have two large loop structures between transmembrane domains 1 and 2 and domains 6 and 7 (Hosoyamada et al., 1999). The first loop is extracellular and contains glycosylation sites (Hosoyamada et al., 1999). Glycosylation at multiple sites often results in a range of molecular weights reported for OAT transporters. The second loop occurs intracellularly and contains phosphorylation sites (Hosoyamada et al., 1999). Based on hydropathy analysis, OAT1–3 probably contain cytoplasmic amino and carboxyl termini (Simonson et al., 1994; Hosoyamada et al., 1999). Individual OAT transporters are often linked as phylogenic pairs based upon closely related sequence alignment: OAT1 and OAT3; OAT4 and the urate transporter 1 (URAT1). For example, both OAT4 and URAT1 are found sequentially on chromosome 11q13.1.
Oat1 was first cloned from a rat kidney cDNA library in 1997 (Sekine et al., 1997; Sweet et al., 1997). During the same period, mouse Oat1 was cloned and called the novel kidney transporter (Lopez-Nieto et al., 1997). Human OAT1 was subsequently identified (Reid et al., 1998; Hosoyamada et al., 1999; Race et al., 1999). Rodent Oat1 and human OAT1 mRNA are highest in kidneys (Fig. 2) (Hosoyamada et al., 1999; Buist and Klaassen, 2004), and their proteins are abundantly expressed on the basolateral membranes of renal proximal tubules (Hosoyamada et al., 1999; Tojo et al., 1999). Specifically, OAT1 is strongly expressed on the basolateral membrane of proximal tubules in the S2 segment (Table 4) (Ljubojevic et al., 2004).
Oat2 was first identified in 1994 using a rat liver cDNA library and named the “novel liver-specific transporter” (Simonson et al., 1994). Oat2 was later recloned and renamed (Sekine et al., 1998; Sun et al., 2001b; Kobayashi et al., 2002b). Oat2/OAT2 show species differences in tissue distribution. Mouse Oat2 is found almost exclusively in kidneys (Fig. 2) (Kobayashi et al., 2002b; Buist and Klaassen, 2004). In contrast, rat Oat2 and human OAT2 are expressed primarily in liver with lower levels in kidneys (Fig. 2) (Sekine et al., 1998; Sun et al., 2001b). Furthermore, localization of Oat2/OAT2 proteins in kidney is species-dependent. Rodent Oat2 protein is expressed on the apical membrane of S3 proximal tubules (Table 4) (Kojima et al., 2002; Ljubojević et al., 2007), whereas human OAT2 protein is basolateral (Enomoto et al., 2002b). It is noteworthy that in liver, rat Oat2 protein traffics to the basolateral membrane of hepatocytes (Simonson et al., 1994).
Rat and mouse Oat3 and human OAT3 were identified simultaneously in 1999 (Brady et al., 1999; Kusuhara et al., 1999; Race et al., 1999). Mouse Oat3 was isolated from an animal model of osteosclerosis and termed reduced in osteosclerosis transporter (Brady et al., 1999). Expression of Oat3/OAT3 in mice and humans is confined primarily to the kidneys, where it is localized to the basolateral membrane of proximal tubule cells (Fig. 2, Table 4) (Cha et al., 2001; Kojima et al., 2002; Buist and Klaassen, 2004). Within the kidneys, rat Oat3 is observed in proximal tubule S1 and S2 segments as well as thick ascending limb, distal tubules, and collecting ducts (Ljubojevic et al., 2004). In brain, Oat3 mRNA is expressed in choroid plexus in rats (Choudhuri et al., 2003), and Oat3 protein localizes to the basolateral membrane of brain capillary endothelial cells in rodents (Kikuchi et al., 2003; Ohtsuki et al., 2004a). Mouse Oat3 protein is also expressed on the apical membrane of choroid plexus epithelial cells (Sweet et al., 2002) as well as in developing bone (Brady et al., 1999).
In 2000, OAT4 was identified and functionally characterized (Cha et al., 2000). OAT4 mRNA is expressed largely in kidneys and placenta (Fig. 2) (Cha et al., 2000). Within the kidneys, OAT4 is found on the apical membrane of renal proximal tubule cells (Table 4) (Babu et al., 2002a; Ekaratanawong et al., 2004). In contrast, OAT4 protein is expressed on the basolateral membrane of placental syncytiotrophoblasts (Ugele et al., 2008). No mouse or rat Oat4 ortholog has been identified.
Much less is known about OAT5–7. OAT5 was first identified in humans in 2001 (Sun et al., 2001b) and subsequently in mice (Youngblood and Sweet, 2004) and rats (Anzai et al., 2005). Mouse and rat Oat5 are primarily expressed in kidneys and localize to the apical membrane of proximal tubules in the outer medullary and juxtamedullary cortex in the S2 and S3 segments (Table 4) (Youngblood and Sweet, 2004; Anzai et al., 2005; Kwak et al., 2005). Oat6 has been described only in mice and is uniquely localized to the olfactory mucosa (Monte et al., 2004). OAT7 is the most recently described OAT and was cloned from a human liver cDNA library (Shin et al., 2007). OAT7 protein is localized to the basolateral membrane of human hepatocytes (Shin et al., 2007).
Like Oats, Urat1 is a member of the SLC22A family. The Urat1 transporter was first cloned from a mouse kidney cDNA library and named renal-specific transporter (Table 1) (Mori et al., 1997). The human URAT1 ortholog was later identified (Enomoto et al., 2002a). Urat1/URAT1 transporters are expressed predominantly in the kidneys along the apical border (Fig. 2, Table 4) (Enomoto et al., 2002a; Hosoyamada et al., 2004). Mouse Urat1 protein is also detected in brain capillaries and along the choroid plexus (Imaoka et al., 2004).
The function of Oat/OATs as organic anion exchangers (antiporters) is enabled by sodium and dicarboxylate gradients generated by the sodium-dicarboxylate cotransporter and the sodium-potassium ATPase. In the cases of OAT1 and -3, uptake of substrates across the basolateral membrane is coupled to an outwardly directed concentration gradient of dicarboxylates (i.e., α-ketoglutarate and glutarate) (Wolff et al., 1992; Sekine et al., 1997; Sweet et al., 1997; Bakhiya et al., 2003; Koepsell and Endou, 2004). The concentration gradient of the dicarboxylate provides the driving force for entry of organic anions against an opposing force (inside-negative membrane potential). Because the concentration gradient of the dicarboxylate is maintained by a sodium-potassium-ATPase pump, this mechanism of transport is typically referred to as tertiary transport (Srimaroeng et al., 2008). Coexpression of mouse Oat3 and a sodium-dicarboxylate transporter stimulates Oat3-mediated transport (Ohtsuki et al., 2004a). Rat Oat2 is not thought to be an organic anion-dicarboxylate exchanger (Sekine et al., 1998).
Oat1/OAT1 and Oat3/OAT3 display wide substrate selectivity including endogenous substrates (cyclic nucleotides, urate, indoxyl sulfate) and pharmaceuticals (antibiotics, nonsteroidal anti-inflammatory drugs, diuretics, anticancer drugs, uricosuric agents) (Table 3) (Sekine et al., 1997; Sweet et al., 1997; Apiwattanakul et al., 1999; Enomoto et al., 2003). p-Aminohippurate and estrone sulfate are the prototypical substrates of OAT1 and OAT3, respectively. Oat1/OAT1 transport antibiotics (penicillin G, cephaloridine, tetracycline) and antivirals (such as cidofovir, adefovir, zidovudine, acyclovir, etc.) (Cihlar et al., 1999; Jariyawat et al., 1999; Wada et al., 2000; Babu et al., 2002b). Mercapturic acids are N-acetyl-l-cysteine S-conjugates that are transported by Oat1 and thus eliminated by the kidneys. For example, rat Oat1 can transport S-(2,4-dinitrophenyl)-N-acetyl-l-cysteine (Pombrio et al., 2001). Oat1/OAT1 also transports the chelator 2,3-dimercapto-1-propanesulfonic acid and the mercury thiol conjugates of N-acetylcysteine, homocysteine, and cysteine, probably representing a mechanism for clearance of the environmental neurotoxin methyl mercury (Islinger et al., 2001; Pombrio et al., 2001; Koh et al., 2002; Zalups and Ahmad, 2005a,c).
Oat2/OAT2 mediates the sodium-independent uptake of not only p-aminohippurate but also endogenous (prostaglandins, glutarate) and other exogenous (methotrexate, valproic acid, allopurinol) chemicals (Sun et al., 2001b; Kobayashi et al., 2002b). Species-specific transport of salicylate has been noted: it is transported by rat Oat2, but not by mouse Oat2 (Sekine et al., 1998; Kobayashi et al., 2002b). OAT4 mediates sodium-independent transport of sulfate conjugates (estrone sulfate, indoxyl sulfate, dehydroepiandrosterone sulfate) (Cha et al., 2000; Babu et al., 2002a; Enomoto et al., 2003; Zhou et al., 2006). In general, Oat/OAT5–7 transport dehydroepiandrosterone sulfate and estrone sulfate (Anzai et al., 2005; Schnabolk et al., 2006; Shin et al., 2007). The mycotoxin ochratoxin A is also a substrate for mouse Oat5 (Youngblood and Sweet, 2004). Finally, human OAT7 transports butyrate (Shin et al., 2007).
Urat1/URAT1 seem to be urate-organic anion exchangers (Enomoto et al., 2002a) and are responsible for urate reabsorption in exchange for anions (Hosoyamada et al., 2004). Other organic anions transported by mouse Urat1 include ochratoxin, dehydroepiandrosterone sulfate, and benzylpenicillin (Imaoka et al., 2004).
5. Peptide Transporters.
PEPT1 and PEPT2 are members of the solute carrier family (SLC15A) that transports di- and tripeptides into cells (Table 1). Pept/PEPT1 and -2 were first identified as key peptide carriers in the small intestine and kidneys, respectively (Fei et al., 1994; Liu et al., 1995). PEPT transporters are predicted to have 12-α-helical transmembrane domains with a large extracellular loop between domains 9 and 10 and intracellular carboxyl and amino termini (Fei et al., 1994).
The tissue distribution of Pept/PEPT1 and -2 in mice and humans is shown in Fig. 3 (Saito et al., 1995; Rubio-Aliaga et al., 2000; Herrera-Ruiz et al., 2001; Lu and Klaassen, 2006). Pept/PEPT1 is most prominently expressed in the small intestine of rodents and humans, where it localizes to the apical membrane of enterocytes (Fig. 3, Table 5) (Fei et al., 1994; Ogihara et al., 1999; Shen et al., 1999; Terada et al., 2005; Lu and Klaassen, 2006). Both peptide transporters are detected in the kidneys with Pept1 along the brush border membrane of S1 proximal tubules in the rat and Pept2 expressed in the S2 and S3 segments (Shen et al., 1999). It is noteworthy that Pept1 mRNA is detected in rat but not mouse kidneys (Lu and Klaassen, 2006). In addition to plasma membrane localization, Pept1 is also found in lysosomes (Bockman et al., 1997; Zhou et al., 2000; Sun et al., 2001a). PEPT1 mRNA is also detected in human kidneys, lungs, colon, pancreas, and liver (Liang et al., 1995; Zhang et al., 2004a).
Fig. 3.
Tissue distribution of Pept, Cnt, Ent, and Mate mRNA in mice and humans. Top, relative mRNA levels of transporters in mouse liver, kidneys, lung, stomach, duodenum, jejunum, ileum, large intestine, heart, brain, gonads (testes and ovaries), placenta, and uterus are shown. Male (♂) mRNA is shown on the left whereas female (♀) mRNA is shown on the right side of each box. References for mouse mRNA expression are included (Lu et al., 2004; Lu and Klaassen, 2006; Lickteig et al., 2008). Bottom, relative mRNA levels of transporters in human liver, kidneys, lung, heart, brain, testes, ovaries, placenta, and uterus are shown. Data for humans were obtained from GNF SymAtlas (http://symatlas.gnf.org/; now located at http://biogps.gnf.org). The GNF1H/MAS5 data set was accessed during September 2008.
TABLE 5.
Subcellular localization of uptake Pept, Cnt, Ent, and efflux Mate transporters in various species
For each transporter, the apical or basolateral localization in a particular tissue and/or species is provided. Species included rat (R), mouse (M), human (H), and bovine (B). Detailed information regarding particular cellular populations or regions of the tissue are provided for some transporter isoforms.
| Cellular Localization | Tissue | Species | Cell Types | References |
|---|---|---|---|---|
| Pept1 | ||||
| Apical | Small intestine | R | Enterocytes | Ogihara et al., 1999 |
| Apical | Kidney | R | Proximal tubule cells | Shen et al., 1999 |
| Apical | Liver | M | Cholangiocytes | Knutter et al., 2002 |
| Intracellular | Pancreas | R | Acinar cells (lysosomes) | Bockman et al., 1997 |
| Pept2 | ||||
| Apical | Kidney | M, R | Proximal tubule cells | Shen et al., 1999; Rubio-Aliaga et al., 2000 |
| Apical | Brain | R | Choroid plexus | Shu et al., 2002; Shen et al., 2004 |
| Apical | Mammary gland | R | Glandular and ductal epithelial cells | Groneberg et al., 2002 |
| Apical | Lung | M, R, H | Tracheal and bronchial epithelial cells, Alveolar Type 2 pneumocytes | Groneberg et al., 2001; Bahadduri et al., 2005 |
| N.D. | Nasal mucosa | R | Epithelial cells | Quarcoo et al., 2008 |
| N.D. | Intestine | M | Glial cells and macrophages | Ruhl et al., 2005 |
| Cnt1 | ||||
| Apical | Small intestine | R, H | Enterocytes | Hamilton et al., 2001; Govindarajan et al., 2007 |
| Apical | Kidney | R, H | Proximal tubule cells | Hamilton et al., 2001; Govindarajan et al., 2007 |
| Apical | Liver | R | Hepatocytes | Hamilton et al., 2001; Duflot et al., 2002 |
| Cnt2 | ||||
| Apical | Kidney | H | Proximal tubule cells | Govindarajan et al., 2007 |
| Apical | Small intestine | H | Enterocytes | Govindarajan et al., 2007 |
| Basolateral | Liver | R, H | Hepatocytes | Duflot et al., 2002; Govindarajan et al., 2008 |
| Cnt3 | ||||
| Apical/Intracellular | Kidney | H | Proximal tubule cells, loop of Henle | Damaraju et al., 2007; Errasti-Murugarren et al., 2007 |
| Ent1 | ||||
| Apical | Placenta | H | Syncytiotrophoblasts, endothelial cells | Govindarajan et al., 2007 |
| Apical/Basolateral | Kidney | H | Proximal/distal tubule cells, Loop of Henle, collecting duct, corticomedullary junction | Mangravite et al., 2003; Damaraju et al., 2007; Govindarajan et al., 2007 |
| Basolateral | Liver | H | Hepatocytes | Govindarajan et al., 2008 |
| Lateral | Small intestine | H | Enterocytes, crypt cells | Govindarajan et al., 2007 |
| Ent2 | ||||
| Basolateral | Liver | H | Hepatocytes | Govindarajan et al., 2008 |
| Basolateral | Kidney | H | Epithelial cells | Mangravite et al., 2003 |
| Lateral | Small intestine | H | Enterocytes, crypt cells | Govindarajan et al., 2007 |
| N.D. | Heart | R | Sinoatrial node, atrial and ventricular cells | Musa et al., 2002 |
| MATE1 | ||||
| Apical | Liver | M, H | Hepatocytes, cholangiocytes | Otsuka et al., 2005 |
| Apical | Kidney | R, H | Proximal and distal tubule cells | Otsuka et al., 2005; Masuda et al., 2006; Nishihara et al., 2007 |
| Apical | Kidney | M | Cortical collecting ducts, proximal tubules, Thin limb of loop of Henle | Otsuka et al., 2005 |
| Mate2 | ||||
| N.D. | Testes | M | Leydig cells | Hiasa et al., 2007 |
| MATE2-K | ||||
| Apical | Kidney | H | Proximal tubule cells | Masuda et al., 2006; Tanihara et al., 2007 |
N.D., not determined.
Pept2 mRNA is expressed primarily in mouse kidneys (Fig. 3) (Lu and Klaassen, 2006). Pept2 mRNA is expressed in specific cell types of the brain, including astrocytes, subependymal cells, and ependymal cells, and Pept2 protein is detected along the apical membrane of epithelial cells of the choroid plexus (Table 5) (Berger and Hediger, 1999; Shu et al., 2002). Pept2/PEPT2 mRNA and/or protein are also expressed in the enteric nervous system, colon, liver, pancreas, lungs, nasal mucosa, and mammary glands (Groneberg et al., 2001, 2002; Zhang et al., 2004a; Bahadduri et al., 2005; Ruhl et al., 2005; Lu and Klaassen, 2006; Quarcoo et al., 2009).
Pept/PEPT1 and -2 have broad substrate and inhibitor specificity, including di- and tripeptides but not amino acids or tetrapeptides (Table 3) (Daniel and Herget, 1997; Terada et al., 2000; Daniel and Kottra, 2004). Peptide transport by PEPT1 and -2 is coupled with the inward translocation of protons leading to electrogenic transport. Key structural features of PEPT1 and -2 substrates have been described elsewhere (Rubio-Aliaga and Daniel, 2008). Glycylsarcosine (Gly-Sar) is a prototypical substrate for Pept/PEPT1 and -2 transport (Liang et al., 1995; Liu et al., 1995). A number of pharmaceuticals are substrates of Pept/PEPTs, including β-lactam antibiotics (cefadroxil, cefixime, ceftibuten), the photosensitizing agent 5-aminolevulinic acid, and the investigational anticancer drug bestatin (Saito et al., 1995, 1996; Wenzel et al., 1996; Döring et al., 1998; Ocheltree et al., 2004a,b; Xiang et al., 2006; Hu et al., 2007).
6. Concentrative Nucleoside Transporters.
Nucleosides are glycosylamines consisting of a sugar moiety and a purine or pyrimidine base; they include cytidine, uridine, adenosine, guanosine, thymidine, and inosine. Nucleosides are precursors for nucleotides used in DNA and RNA synthesis and are necessary for cell growth. Uptake of nucleosides by hematopoietic and other cell types is a prerequisite for nucleotide synthesis by salvage pathways because these cells lack de novo synthetic ability. Furthermore, adenosine is an important signaling molecule for neurotransmission, platelet aggregation, and other physiological events. Nucleoside analogs have been developed as drugs to treat viral infections and cancers. Nucleoside uptake transporters have been classified according to their transport properties: concentrative (high-affinity sodium-dependent transport using a physiologic sodium gradient) (SLC28A) and equilibrative (low-affinity facilitated carrier transport) (SLC29A) (for review, see Pastor-Anglada et al., 2008; Young et al., 2008) (Table 1).
CNT1–2 transporters have been cloned from multiple species and are predicted to contain 13 transmembrane helices with cytoplasmic amino termini and extracellular carboxyl termini (Huang et al., 1994; Ritzel et al., 1997, 1998; Wang et al., 1997; Baldwin et al., 1999; Patel et al., 2000; Hamilton et al., 2001; Shin et al., 2003). CNT2 was originally named the sodium-dependent purine nucleoside transporter (Wang et al., 1997; Ritzel et al., 1998). In mice and rats, Cnt1 and Cnt2 mRNA are expressed primarily in all three segments of the small intestine as well as kidneys (Cnt1) (Fig. 3) (Huang et al., 1994; Che et al., 1995; Hamilton et al., 2001; Shin et al., 2003; Lu et al., 2004). In humans, CNT1 and CNT2 mRNA are high in liver and kidneys and CNT2 mRNA is also detected in heart, brain, placenta, skeletal muscle, small intestine, and pancreas (Fig. 3) (Wang et al., 1997; Ritzel et al., 1998; Shin et al., 2003; Damaraju et al., 2007; Govindarajan et al., 2007). Immunohistochemical studies localize rat Cnt1 protein to the apical surface of multiple cell types including cortical renal tubules, hepatocytes, and enterocytes (Table 5) (Hamilton et al., 2001; Duflot et al., 2002). Cnt3 mRNA is expressed in the uterus, testes, and ovaries of mice and the lungs of rats (Fig. 3) (Lu et al., 2004). CNT3 mRNA and/or protein are expressed in multiple tissues (Fig. 3) (Ritzel et al., 2001; Damaraju et al., 2007). Within the kidneys, CNT3 localizes to the apical surface of proximal tubules and thick ascending loops of Henle along with some intracellular staining (Table 5) (Damaraju et al., 2007).
Within the concentrative transporters, CNT1 transports pyrimidines (but also adenosine), CNT2 transports purines (but also uridine), and CNT3 transports both purines and pyrimidines (Table 3) (Huang et al., 1994; Fang et al., 1996; Ritzel et al., 1997, 1998, 2001; Wang et al., 1997; Schaner et al., 1999). The chemotherapeutic drug gemcitabine and the antiviral drugs stavudine, zalcitabine, and zidovudine are also substrates of CNT1 (Huang et al., 1994; Ritzel et al., 1997; Mackey et al., 1999; Graham et al., 2000; Cano-Soldado et al., 2004). Substrates of CNT2 include nucleoside analog drugs such as the hepatitis drug ribavirin (Patil et al., 1998). As the broadest nucleoside transporter, CNT3 substrates are more numerous (5-fluorouridine, zebularine, gemcitabine, cladribine, fludarabine, etc.) (Ritzel et al., 2001; Toan et al., 2003).
7. Equilibrative Nucleoside Transporters.
As low-affinity, facilitated carriers, ENTs transport chemicals down concentration gradients. Intracellular levels of nucleosides are typically low because they are converted to nucleotides. Although ENT transporters are most often considered uptake carriers, they can function bidirectionally. ENT1–4 transporters have been detected and cloned from different species (Table 1) (Griffiths et al., 1997; Yao et al., 1997; Crawford et al., 1998; Kiss et al., 2000; Baldwin et al., 2005; Zhou et al., 2007b). Hydropathy analysis suggests that Ent1–2 proteins are composed of 11 transmembrane domains with an internal amino terminus and extracellular carboxyl tail (Yao et al., 1997; Crawford et al., 1998).
The mRNA expression of Ent1 is primarily in mouse and rat liver, kidneys, lung, brain, and testes (Fig. 3) (Choi et al., 2000; Lu et al., 2004; Redzic et al., 2005). Human ENT1 expression is wide ranging, including liver, lungs, heart, ovaries, brain, kidneys, erythrocytes, fetal liver, and placenta (Fig. 3) (Griffiths et al., 1997; Anderson et al., 1999b; Pennycooke et al., 2001; Damaraju et al., 2007; Govindarajan et al., 2007). Within the kidneys, ENT1 staining is observed on the apical surface of proximal tubules and on both the apical and basal membranes of the thick ascending loops of Henle and collecting ducts (Table 5) (Damaraju et al., 2007). Similar apical localization of ENT1 is noted in human placental syncytiotrophoblasts (Govindarajan et al., 2007). Ent2 and Ent3 mRNA share similar tissue distributions with highest levels observed in kidneys, brain, gonads, and uteri of mice and rats (Fig. 3) (Anderson et al., 1999a; Lu et al., 2004; Redzic et al., 2005). The profile of ENT2 mRNA demonstrates high levels in skeletal muscle and heart and detectable amounts in other organs (Fig. 3) (Pennycooke et al., 2001). Likewise, ENT3 exhibits broad tissue distribution with prominent expression in placenta, lung, ovaries, spleen, and bone marrow (Baldwin et al., 2005). Mutations in ENT3 have been linked to H syndrome, which is characterized by skin, auditory, heart, and spleen abnormalities (Table 10) (Molho-Pessach et al., 2008). A fourth ENT isoform, ENT4, has also been cloned, although there is less information available about its tissue distribution and transport properties (Barnes et al., 2006; Xia et al., 2007; Zhou et al., 2007b).
TABLE 10.
Genetic disorders due to transporter mutations
| Transporter | Disorder | Features and/or Symptoms |
|---|---|---|
| SLC22A5 (OCTN2) | Primary systemic carnitine deficiency syndrome | Cardiomyopathy, hypoglycemia, skeletal muscle myopathy |
| SLC10A2 (ASBT) | Primary bile acid malabsorption | Diarrhea, steatorrhea, low plasma cholesterol levels |
| SLC29A3 (ENT3) | H syndrome | Cutaneous hyperpigmentation, hearing loss, hepatosplenomegaly, heart anomalies, hypogonadism, short stature, hypertrichosis |
| ABCA1 | Tangier's disease | Mild hypertriglyceridemia, neuropathy, enlarged tonsils, premature atherosclerosis, splenomegaly, hepatomegaly |
| ABCB4 (MDR3) | PFIC-III | Elevated serum γ-glutamyltranspeptidase, activity, high serum bile acid levels, hepatosplenomegaly, portal hypertension, pruritus, jaundice |
| ABCB11 (BSEP) | PFIC-II | Reduced bile acid secretion, progressive hepatic dysfunction at early age, normal serum γ-glutamyltranspeptidase activity |
| ABCC2 (MRP2) | Dubin-Johnson syndrome | Conjugated hyperbilirubinemia, chronic jaundice, relatively benign clinical course |
| ABCC6 (MRP6) | Pseudoxanthoma elasticum | Calcifications of elastic fibers in arteries, and retina leading to arterial skin, insufficiency and macular degeneration |
| ABCG5/8 | Sitosterolemia | Atherosclerosis at a young age, tendon xanthomas, arthralgias |
| ATP7B | Wilson's disease | Liver disease due to copper accumulation, which requires transplantation, tremors, neurological and behavioral problems, brown pigment in cornea |
| ATP8B1 | PFIC-I/Byler's disease | Elevated serum bile acids, normal serum γ-glutamyltranspeptidase activity, decreased bile acid secretion, fat malabsorption, vitamin (lipid-soluble) deficiency, diarrhea, pancreatitis, pruritus, jaundice, hearing loss |
Ent/ENT transport is sodium-independent and, like Cnt/CNT transporters, endogenous nucleosides as well as cancer and antiviral nucleoside analogs are common substrates (Table 3) (Griffiths et al., 1997; Mackey et al., 1999; Kiss et al., 2000; Baldwin et al., 2005; Damaraju et al., 2005; Nagai et al., 2007; Govindarajan et al., 2008). It is noteworthy that ENT1 is also expressed in the mitochondria, where it may be involved in the cellular toxicity of antiviral nucleoside drugs (Lai et al., 2004; Govindarajan et al., 2009).
8. Multidrug and Toxin Extrusion Transporters.
For a number of years, it was understood that organic cations entered the cells via OCT transporters; however, how they exited was not clear. Transporters were first identified in bacteria and were called NorM and YdhE. They were later named the multidrug and toxin extrusion (MATE) transporters (Morita et al., 1998; Brown et al., 1999; Otsuka et al., 2005; Terada and Inui, 2008). Although MATE transporters are Slc transporters, they function as efflux proteins. Hiasa and colleagues reported that there are three subgroups of mammalian MATE transporters: class I includes rodent Mate1 and human MATE1; class II includes human MATE2 (no rodent ortholog of this subgroup); class III includes mouse and rat Mate2 (Hiasa et al., 2007; Moriyama et al., 2008). In an attempt to avoid confusion, it has been proposed that mouse and rat Mate2 be renamed Mate3 (Terada and Inui, 2008). However, for the purpose of this review, we will continue to use the existing Mate2 designation. Another MATE transporter, multidrug and toxin extrusion 2-K (MATE2-K), shows 94% amino acid similarity with MATE2 (Masuda et al., 2006). MATE2-K was first reported to be a splice variant of MATE2; however, this is currently under reconsideration, because a second attempt to clone human MATE2 has not been successful (Masuda et al., 2006; Terada and Inui, 2008). Mate1 (SLC47A1) and Mate2-K (SLC47A2) have also been cloned from rabbits (Zhang et al., 2007a) (Table 1). Recent work suggests that MATE1 and MATE2-K are composed of 13 putative transmembrane domains with amino and carboxyl termini on the intracellular and extracellular faces of the plasma membrane, respectively (Masuda et al., 2006; Zhang et al., 2007a; Terada and Inui, 2008).
Mouse Mate1 mRNA is most abundant in kidneys and is detected at lower levels in the liver and heart (Fig. 3) (Otsuka et al., 2005; Lickteig et al., 2008). Human MATE1 is expressed in heart, liver, adrenal gland, testes, skeletal muscle, and kidneys (Otsuka et al., 2005; Masuda et al., 2006). Mouse Mate2 mRNA is strongly detected in testes, whereas human MATE2-K is expressed in kidneys (but not in the testes) (Fig. 3) (Otsuka et al., 2005; Masuda et al., 2006; Hiasa et al., 2007; Lickteig et al., 2008). Differences in the tissue distributions of mouse Mate2 and human MATE2-K may be due to their classification in class II and III subgroups, respectively (Hiasa et al., 2007).
MATE1 effluxes organic cations such as tetraethylammonium, 1-methyl-4-phenylpyridinium, oxaliplatin, and paraquat using a proton-coupled electroneutral exchange (Table 3) (Otsuka et al., 2005; Terada et al., 2006; Chen et al., 2007b; Yokoo et al., 2007). Rat Mate1 also transports the histamine H2-receptor antagonist cimetidine, the antidiabetic drug metformin, and the antibiotic cephalexin (Ohta et al., 2006; Terada et al., 2006). Mouse Mate1 prefers N1-methylnicotinamide and guanidine as substrates, whereas mouse Mate2 prefers tetraethylammonium (Hiasa et al., 2007). MATE2-K transports organic cations including tetraethylammonium, 1-methyl-4-phenylpyridinium, cimetidine, procainamide, and metformin (Table 3) (Masuda et al., 2006).
B. ATP-Binding Cassette Transporters
1. Multidrug Resistance Proteins.
ABC transporters contain ATP-binding domains that possess ATPase activity (hydrolysis of ATP to ADP) to provide energy for translocating substrates across membranes, most often against concentration gradients. Pgp was the first drug transporter described (Juliano and Ling, 1976). Pgp is encoded by multiple MDR genes, including MDR1 (ABCB1) and MDR3 (ABCB4) in humans, although Pgp most often indicates the ABCB1 gene product (Table 1) (van der Bliek et al., 1988; Dhir et al., 1990; Lincke et al., 1991). The rodent orthologs of MDR1 and MDR3 are Mdr1a/1b and Mdr2, respectively. To make things more confusing, the Mdr1a gene was also referred to as Mdr3 within the older literature (Devault and Gros, 1990; Dhir et al., 1990). MDR1, Mdr1a, and Mdr1b are drug transporters (Dhir et al., 1990), whereas MDR3 and Mdr2 translocate phospholipids such as phosphatidylcholine from the inner to the outer canalicular membrane (Schinkel et al., 1991; Ruetz and Gros, 1994; van Helvoort et al., 1996). The structural topology of Pgp consists of two distinct regions containing six putative transmembrane domains and one nucleotide binding domain (van der Bliek et al., 1988; Devault and Gros, 1990; Aller et al., 2009). The amino and carboxyl termini of Pgp are located intracellularly.
The tissue distribution of Mdr1a/1b and MDR1 is broad with their mRNA detected in many tissues (Fig. 4) (Chin et al., 1989; Melaine et al., 2002; Hitzl et al., 2004). Mdr1a mRNA is most prominent in the large intestine followed by the small intestine, kidneys, and brain (Fig. 4) (Cui et al., 2009c). Meanwhile, Mdr1b expression is highest in the kidneys, lungs, brain, ovaries, and placenta (Fig. 4) (Cui et al., 2009c). Within these tissues, MDR1/Mdr1a/1b proteins are detected on the apical/luminal surface (Table 6) (Schinkel et al., 1994; Lankas et al., 1998; Panwala et al., 1998; Rao et al., 1999; Miller et al., 2000; St-Pierre et al., 2000; Ushigome et al., 2003; Soontornmalai et al., 2006; Sun et al., 2006). Expression of MDR3 and Mdr2 is primarily restricted to the liver, where they localize to the canalicular membrane (Table 6) (Buschman et al., 1992; Smit et al., 1994; de Vree et al., 1998; Scheffer et al., 2000; Cui et al., 2009c). Although MDR3 and Mdr2 mRNA have been detected in additional tissues, functional protein expression has not been shown (Smit et al., 1994; Cui et al., 2009c).
Fig. 4.
Tissue distribution of Mdr, Mrp, and Bcrp mRNA in mice and humans. Top, relative mRNA levels of transporters in mouse liver, kidneys, lung, stomach, duodenum, jejunum, ileum, large intestine, brain, gonads (testes and ovaries), and placenta are shown. Male (♂) mRNA is shown on the left whereas female (♀) mRNA is shown on the right side of each box. References for mouse mRNA expression are included (Maher et al., 2005b; Cui et al., 2009c). Bottom, relative mRNA levels of transporters in human liver, kidneys, lung, heart, brain, testes, ovaries, placenta, and uterus are shown. Data for humans were obtained from GNF SymAtlas (http://symatlas.gnf.org/; now located at http://biogps.gnf.org). The GNF1H/MAS5 data set was accessed during September 2008.
Overexpression of Mdr1a and -1b confers resistance to multiple drugs by enhancing cellular extrusion (Dhir et al., 1990; Raymond et al., 1990). Early work demonstrated that cells transfected with Mdr1b were resistant to colchicine and doxorubicin whereas cells overexpressing Mdr1a were resistant to actinomycin D (Table 7) (Devault and Gros, 1990; Tang-Wai et al., 1995). In addition, MDR1-transfected cells were resistant to vincristine, colchicine, daunorubicin, doxorubicin, and actinomycin D (Schinkel et al., 1991; Tang-Wai et al., 1995).
Mdr2 translocates a fluorescent phosphatidylcholine analog in overexpressing yeast cells (Ruetz and Gros, 1994). In addition, MDR3 overexpression in fibroblasts promotes the transfer of phosphatidylcholine from the inner to outer leaflet of the plasma membrane (Smith et al., 1994). Translocation from the inner to the outer leaflet of the canalicular membrane by MDR3 enhances the availability of phospholipids for extraction into the bile canaliculi by bile acids. Phospholipids form micelles with bile acids, thereby reducing the likelihood of injury to the biliary tree (Elferink et al., 1997).
2. Multidrug Resistance-Associated Proteins.
Mrp/MRP transporters constitute nine members of the ATP-binding cassette C subfamily (ABCC1–6, 10–12) (Table 1). Other transporters in the ABCC subfamily are the cystic fibrosis transmembrane conductance regulator (ABCC7) and two sulfonylurea receptor isoforms (ABCC8 and -9). The following nine Mrp/MRP isoforms have been cloned from various species: MRP1 (Cole et al., 1992), MRP2 (Ito et al., 1997; Keppler et al., 1997; Paulusma et al., 1997; Fritz et al., 2000), MRP3 (Kiuchi et al., 1998; Uchiumi et al., 1998), MRP4 (Kool et al., 1997; Lee et al., 1998; Chen and Klaassen, 2004), MRP5 (McAleer et al., 1999; Jedlitschky et al., 2000; Wijnholds et al., 2000b), MRP6 (Kool et al., 1999a), MRP7 (Hopper et al., 2001), MRP8, and MRP9 (Bera et al., 2001; Tammur et al., 2001; Shimizu et al., 2003). ABCC13 is likely to be a pseudogene encoding a truncated protein of fetal origin (Yabuuchi et al., 2002; Annilo and Dean, 2004).
MRP transporters contain consensus regions named the Walker A, Walker B, and Signature C motifs that are required for ATP binding. MRP1, -2, -3, -6, and -7 contain three membrane spanning domains with a total of 17 hydrophobic transmembrane regions. For these five MRP proteins, computational analysis suggests extracellular and intracellular amino and carboxyl termini, respectively. MRP4, -5, -8, and -9 are smaller proteins with only two domains that span the plasma membrane (12 total transmembrane regions). The amino and carboxyl termini are both predicted to be intracellular for MRP4, -5, -8, and -9. MRP proteins have two intracellular nucleotide binding domains. For details of MRP transporters, including historical highlights, the reader is referred to recent reviews (Jedlitschky et al., 2006; Nies et al., 2008; Toyoda et al., 2008; Zhou et al., 2008c).
Mouse Mrp1 mRNA is observed in testes, ovaries, brain, placenta, and stomach (Fig. 4) (Maher et al., 2005b). Human MRP1 mRNA and protein is most highly expressed in testes, lungs, heart, bladder, spleen, adrenal glands, placenta, kidneys, peripheral blood mononuclear cells, and skeletal muscle (Fig. 4) (Cole et al., 1992; Flens et al., 1996; Kool et al., 1997). Within the intestine, the highest MRP1 mRNA levels are found in the ascending and transverse colon (Zimmermann et al., 2005). Overexpression of MRP1 in MDCK cells traffics this protein to the basolateral membrane similar to its localization in the choroid plexus, bronchial epithelia, and intestinal crypt cells (Table 8) (Wright et al., 1998; Peng et al., 1999; Rao et al., 1999; Zhang et al., 2004d; Roberts et al., 2008). Likewise, MRP1 is expressed on the basolateral membrane of the amnion as well as the chorionic and decidua membranes, but on the apical membrane of placental syncytiotrophoblasts and brain capillary endothelial cells (St-Pierre et al., 2000; Pascolo et al., 2003; Zhang et al., 2004d; Aye et al., 2007).
Mouse Mrp2 mRNA is detected in the small intestine, liver, and kidneys (Fig. 4) (Maher et al., 2005b). The initial identification of Mrp2/MRP2 was associated with genetic disorders in rats and humans (Table 10) (Paulusma et al., 1996). Human MRP2 mRNA is most highly expressed in liver, followed by the duodenum and kidneys (Fig. 4) (Kool et al., 1997; Uchiumi et al., 1998). The expression of Mrp2/MRP2 decreases along the intestinal tract with lower detection in the colon compared with the duodenum and ileum (Mottino et al., 2000; Maher et al., 2005b; Zimmermann et al., 2005). It is noteworthy that little difference in Mrp2 mRNA expression was observed between the proximal and distal rat intestine, whereas protein levels declined from jejunum to ileum (Mottino et al., 2000). Overexpression of the ABCC2 gene in MDCK cells targets the MRP2 protein to the apical surface (Zhang et al., 2004d). Mrp2/MRP2 is expressed on the apical surface of hepatocytes, the amnion epithelial membrane, and proximal tubule cells (Fig. 4, Table 8) (Paulusma et al., 1996; Kinoshita et al., 1998; Scheffer et al., 2000, 2002a; van Aubel et al., 2002; Aye et al., 2007).
Mouse Mrp3 mRNA is expressed in the small and large intestine, liver, stomach, and retinal vascular endothelium (Fig. 4) (Maher et al., 2005b; Tachikawa et al., 2008). Levels of human MRP3 mRNA are highest in liver and are also detectable in duodenum, colon, pancreas, adrenal glands, kidneys, and lungs (Fig. 4) (Kool et al., 1997; Belinsky et al., 1998; Kiuchi et al., 1998; Uchiumi et al., 1998; König et al., 1999; Zimmermann et al., 2005). Typically, Mrp3/MRP3 protein localizes to the basolateral membrane of epithelial cells, including hepatocytes, cholangiocytes, distal convoluted tubules, gallbladder, pancreatic ductal cells, and enterocytes of the ileum and colon (Table 8) (Kool et al., 1999b; Scheffer et al., 2000, 2002b; Soroka et al., 2001; Rost et al., 2002; Zelcer et al., 2006). Two exceptions are localization to the lateral and apical membranes of the choroid plexus epithelial cells and placental syncytiotrophoblasts, respectively (St-Pierre et al., 2000; Soontornmalai et al., 2006).
Rodent Mrp4 mRNA is high in kidneys, prostate, and stomach (Fig. 4) (Chen and Klaassen, 2004; Maher et al., 2005b). Likewise, human MRP4 mRNA is most prominently expressed in the kidneys, followed by lungs, skeletal muscle, prostate, testes, ovaries, small intestine, bladder, platelets, and tonsil (Fig. 4) (Kool et al., 1997; Lee et al., 1998; Jedlitschky et al., 2004). Plasma membrane localization of MRP4 is dependent upon cell type (Table 8). For example, Mrp4/MRP4 localizes to the apical membrane in proximal tubule cells (van Aubel et al., 2002) and brain capillary endothelial cells (Roberts et al., 2008). In contrast, Mrp4/MRP4 protein is detected on the basolateral surface of hepatocytes (Assem et al., 2004), prostate glandular epithelial cells (Lee et al., 2000b; Rius et al., 2005), choroid plexus epithelia (Roberts et al., 2008), and visceral yolk sac epithelium (Aleksunes et al., 2008b).
Mouse Mrp5 and human MRP5 mRNA are widely expressed (Fig. 4) (Kool et al., 1997; Belinsky et al., 1998; McAleer et al., 1999; Dazert et al., 2003; Maher et al., 2005b). Overexpression of the ABCC5 gene in MDCK cells causes MRP5 protein localization to the basolateral membrane (Wijnholds et al., 2000b). In brain, MRP5 is expressed in astrocytes, pyramidal neurons, and along the blood-brain barrier (Nies et al., 2004). MRP5 is detected on the epithelial cells of the urethra and the urogenital tract (Nies et al., 2002b). Within the placenta, MRP5 is present on the basolateral membrane of syncytiotrophoblasts as well as near fetal blood vessels (Table 8) (Pascolo et al., 2003; Meyer Zu Schwabedissen et al., 2005). It is noteworthy that placental MRP5 mRNA decreases during human gestation (Meyer Zu Schwabedissen et al., 2005). In addition, the amniotic membrane from term pregnancies expresses MRP5 on its apical and basolateral epithelial cells (Aye et al., 2007).
MRP6 was first considered the anthracycline resistance-associated gene in resistant leukemia cell lines (Longhurst et al., 1996; O'Neill et al., 1998). Subsequent cloning demonstrated that the anthracycline resistance-associated gene is a partial protein product of ABCC6 (Belinsky and Kruh, 1999; Kool et al., 1999a). Despite expression of MRP6 in chemotherapy-resistant cell lines, it is not thought to play a role in conferring selective growth advantage to malignant cells (Kool et al., 1999a). The profiles of rodent Mrp6 and human MRP6 mRNA expression are largely similar (Fig. 4) (Kool et al., 1999a; Madon et al., 2000; Maher et al., 2005b, 2006b). In mice, rats, and humans, Mrp6/MRP6 mRNA is expressed in liver and kidneys, where their proteins are detected on the basolateral membranes of hepatocytes and proximal tubules (Table 6) (Kool et al., 1997; Belinsky and Kruh, 1999; Scheffer et al., 2002a; Maher et al., 2005b, 2006b). Likewise, overexpression of MRP6 in MDCK cells results in basolateral trafficking (Sinkó et al., 2003). MRP6 protein has also been found in enteroendocrine G cells of the stomach (Beck et al., 2005).
Mrp7/MRP7 is ubiquitously expressed (Fig. 4). Mouse Mrp7 is detected highly in testes, placenta, small intestine, kidneys, heart, and lungs (Kao et al., 2002; Maher et al., 2005b). Within the testes, Mrp7 is expressed in Sertoli cells (Augustine et al., 2005). Human MRP7 is expressed in skin, testes, stomach, spleen, colon, kidneys, brain, heart, and liver (Hopper et al., 2001). Human MRP8 is ubiquitously expressed in ovaries, heart, mammary glands, lungs, muscle, pancreas, testes, and intestine (Fig. 4) (Bera et al., 2001; Tammur et al., 2001). In the brain, MRP8 protein is located on axons in the white matter (Bortfeld et al., 2006). No mouse Mrp8 ortholog has been reported. In mice and rats, Mrp9 mRNA is only detected in testes (Fig. 4) (Maher et al., 2005b; Ono et al., 2007). Within the testes, mouse and boar sperm strongly express Mrp9 (Ono et al., 2007). Expression of human MRP9 is more ubiquitous than rodent counterparts (Tammur et al., 2001; Ono et al., 2007).
Although identification of MDR1 drew attention to the existence of efflux pumps in chemotherapy-resistant tumors, a number of cancers did not overexpress this gene. As a result, researchers proposed the likelihood of additional efflux pumps. MRP1 was first reported in 1992 (Cole et al., 1992) and subsequently linked to anticancer drug resistance (Barrand et al., 1994; Grant et al., 1994; Stride et al., 1997). Early studies demonstrated the ability of MRP1 to transport glutathione conjugates (including oxidized glutathione), prostaglandins, and leukotrienes (Table 7) (Leier et al., 1994, 1996; Jedlitschky et al., 1996; Pulaski et al., 1996; Zaman et al., 1996; Evers et al., 1997).
Functional transport analysis demonstrates the importance of Mrp2 in the apical excretion of substrates (such as β-lactam antibiotics, methotrexate, estradiol-17β-glucuronide) from the liver and gastrointestinal tract (Table 7) (Masuda et al., 1997; Gotoh et al., 2000; Morikawa et al., 2000; Kato et al., 2008). Overexpression of MRP2 in vitro confers resistance to a number of cytotoxic drugs, including etoposide, cisplatin, doxorubicin, and epirubicin (Cui et al., 1999; Kawabe et al., 1999). The excretion of glucuronide conjugates across the canalicular surface of hepatocytes is mediated by Mrp2/MRP2 and across the sinusoidal membrane by Mrp3/MRP3. Mrp3/MRP3 transports glucuronide conjugates in addition to chemotherapeutic drugs and bile acids (Hirohashi et al., 1999; Kool et al., 1999b; Zeng et al., 1999, 2000, 2001; Li et al., 2003). Similar substrate profiles have been observed between rat and human Mrp3/MRP3 proteins (Akita et al., 2002). Although both proteins can transport bile acids, rat Mrp3 has a higher affinity for bile acids such as taurine- and glycine-conjugated cholic acid compared with human MRP3 (Zelcer et al., 2003b). Instead, it is proposed that human MRP3 may be more important in bile acid handling during cholestasis (Zelcer et al., 2003b).
Initial studies of Mrp4/MRP4 function pointed to a role for this transporter in conferring resistance to nucleoside analog antiviral drugs such as 9-(2-phosphonylmethoxyethyl)adenine as well as the anticancer drugs 6-mercaptopurine, topotecan, and methotrexate (Table 7) (Schuetz et al., 1999; Lee et al., 2000b; Chen et al., 2001; Reid et al., 2003a; Tian et al., 2005; El-Sheikh et al., 2007). In addition, Mrp4/MRP4 transports endogenous molecules such as leukotrienes, prostaglandins, folate, bile acids, urate, and cyclic nucleotides (Chen et al., 2001, 2002; Lai and Tan, 2002; Reid et al., 2003b; Zelcer et al., 2003a; Jedlitschky et al., 2004; Van Aubel et al., 2005; Rius et al., 2006, 2008; Bataille et al., 2008; Lin et al., 2008). Mrp5/MRP5 transports endogenous (cAMP, cGMP, folate, hyaluronan) and exogenous chemicals [methotrexate, 6-mercaptopurine, 6-thioguanine, 9-(2-phosphonylmethoxyethyl)adenine, 5-fluorouracil] (McAleer et al., 1999; Jedlitschky et al., 2000; Wijnholds et al., 2000b; Wielinga et al., 2002, 2003, 2005; Reid et al., 2003a; Pratt et al., 2005; Schulz et al., 2007). To date, only a limited number of MRP6 substrates have been identified, including leukotriene C4, etoposide, and the endothelin receptor antagonist BQ-123 (Belinsky et al., 2002; Iliás et al., 2002). Likewise, MRP7 transports leukotriene C4 and estradiol-17β-glucuronide as well as a number of chemotherapeutic drugs (Chen et al., 2003; Hopper-Borge et al., 2004). Overexpression of MRP7 confers resistance to docetaxel, paclitaxel, and vincristine (Hopper-Borge et al., 2009). Consistent with these data, high levels of MRP7 correlate with the chemotherapeutic resistance of a number of cell lines (Naramoto et al., 2007; Oguri et al., 2008; Bessho et al., 2009). MRP8 transports cyclic nucleotides, sulfated steroids, antiviral drugs, and chemotherapeutic drugs (Guo et al., 2003; Chen et al., 2005b).
3. Breast Cancer Resistance Protein.
Despite the chemotherapeutic resistance conferred by MDR and MRP isoforms, resistant cancer cell lines lacking MDR/MRP over-expression suggested an additional ABC subfamily might be involved. A candidate gene named mitoxantrone resistance (MXR) was discovered in a resistant breast cancer cell line (Doyle et al., 1998; Miyake et al., 1999; Ross et al., 1999). At the same time, this transporter was also reported as the “ABC transporter highly expressed in placenta (ABCP)” (Allikmets et al., 1998). MXR/ABCP was later renamed the second member of the G subfamily of ABC transporters (ABCG2) or BCRP (Table 1). The BCRP protein is considered a “half-transporter” consisting of two domains: amino-terminal ATP-binding domain and carboxyl-terminal transmembrane domain (six transmembrane segments) (Wang et al., 2008a). To function, BCRP must form oligomers. Formation of a homodimer via extracellular loops between transmembrane helices 5 and 6 has been proposed (Henriksen et al., 2005a,b). Additional reports suggest that it is more likely that BCRP associates into higher order oligomers, specifically homotetramers (Xu et al., 2004).
Despite the original identification of BCRP in a breast cancer cell line, the expression of this transporter is quite variable among primary breast carcinomas, and there is no relationship between BCRP and the chemotherapeutic response to anthracyclines and/or survival of patients with breast cancer (Faneyte et al., 2002). Expression of BCRP in other tumor types is variable; detection is more frequent in adenocarcinomas of the digestive tract, endometrium, and lungs (Diestra et al., 2002). The relationship between BCRP expression and clinical outcomes in these other tumor types remains elusive.
Similar to other transporters (MDR and MRP) first associated with cancer cell resistance, BCRP is expressed not only in tumors but also in a number of organs associated with drug absorption, metabolism, and excretion. A similar distribution of mouse and rat Bcrp expression has been reported with high Bcrp mRNA in rodent kidneys, liver, small intestine, placenta, and testes (Fig. 4) (Tanaka et al., 2005). High expression of human BCRP mRNA is detected in the placenta as well as in the brain, liver, kidneys, small intestine, colon, prostate, spinal cord, adrenal gland, uterus, and testes (Fig. 4) (Doyle et al., 1998; Fetsch et al., 2006). Within the human gastrointestinal tract, BCRP mRNA is highest in the duodenum and decreases down to the rectum (Gutmann et al., 2005). Bcrp/BCRP is almost exclusively expressed on the apical surface of epithelial cells, including hepatocytes, proximal tubules, enterocytes, trophoblasts, yolk sac, and mammary glands as well as brain and retinal capillary endothelial cells (Table 8) (Maliepaard et al., 2001; Cooray et al., 2002; Jonker et al., 2002; Aronica et al., 2005; Tachikawa et al., 2005; Asashima et al., 2006; Fetsch et al., 2006; Pulido et al., 2006; Aleksunes et al., 2008b; Roberts et al., 2008). Expression of Bcrp/BCRP on lactating mammary glands in a number of species contributes to the excretion of chemicals into breast milk (Merino et al., 2005b; van Herwaarden et al., 2006, 2007; Pérez et al., 2009).
Because BCRP was first identified from chemotherapy-resistant cancer cells, early functional analysis focused upon anticancer drugs. Overexpression of Bcrp/BCRP reduces accumulation and confers resistance to mitoxantrone, daunorubicin, doxorubicin, topotecan, and rhodamine 123 (Doyle et al., 1998; Allen et al., 1999; Litman et al., 2000; Wang et al., 2003c). Bcrp/BCRP transports a wide range of substrates, including photosensitizers, antibiotics, antivirals, natural products, statins, and carcinogens (Table 7) (Merino et al., 2005a, 2006; Robey et al., 2005; Huang et al., 2006a; Ando et al., 2007; Enokizono et al., 2007; Pan et al., 2007; Myllynen et al., 2008). BCRP also transports the fluorescent dye Hoechst 33342, which is used to label stem cell populations (Kim et al., 2002b).
C. Bile Acid, Cholesterol, Aminophospholipid, and Copper Transporters
1. Sodium Taurocholate Cotransporting Polypeptide.
NTCP belongs to the SLC10A transporter family (Table 1). Ntcp was first cloned from rat liver (Hagenbuch et al., 1990). NTCP has seven putative transmembrane domains that are glycosylated with the carboxyl terminus oriented into the cytoplasm (Hagenbuch et al., 1991; Ananthanarayanan et al., 1994; Hagenbuch and Meier, 1994; Mareninova et al., 2005).
As part of the enterohepatic recirculation, Ntcp/NTCP is responsible for the basolateral uptake of bile acids from the portal blood into hepatocytes. Ntcp/NTCP transports bile acids such as taurocholate as well as conjugated di- and trihydroxy bile acids in a sodium-dependent manner (Hagenbuch et al., 1991; Boyer et al., 1994; Hagenbuch and Meier, 1994; Meier et al., 1997; Saeki et al., 2002). Although Ntcp was first identified in rat liver, this gene is expressed in livers of multiple species including human, mouse, rabbit, and guinea pig (Fig. 5) (Hagenbuch et al., 1991). Ntcp/NTCP proteins localize to the basolateral surface of hepatocytes in humans, rats, and mice (Table 9) (Ananthanarayanan et al., 1994; Stieger et al., 1994; Keitel et al., 2005; Aleksunes et al., 2006). It is noteworthy that Ntcp is also expressed in rat pancreas, where it traffics to the apical plasma membrane of acinar cells (Kim et al., 2002a). In addition to transporting bile acids and estrone sulfate, human NTCP (but not rat Ntcp) transports rosuvastatin (Craddock et al., 1998; Ho et al., 2006b).
2. Apical Sodium-Dependent Bile Acid Transporter.
It had been known for some time that there was active transport of bile acids across the apical membrane of ileal enterocytes, but it was not until 1994, when Asbt was cloned, that this process was better understood (Wong et al., 1994). ASBT is the second member of the SLC10A family and shares 35% amino acid identity to NTCP (Table 1) (Wong et al., 1994). Asbt was first cloned from a hamster ileal cDNA library and named the ileal sodium-dependent bile acid cotransporter (Wong et al., 1994). Similar to NTCP, hydropathy analysis predicts that the ASBT protein has seven putative transmembrane domains with an extracellular amino terminus and a cytoplasmic carboxyl terminus (Banerjee and Swaan, 2006).
Asbt-mediated uptake of bile acids represents the first step in bile acid reabsorption in intestine. Contrary to Ntcp/NTCP, which localizes to the basolateral membrane of hepatocytes, Asbt is found on the apical surface of cholangiocytes, where it participates in cholehepatic recirculation (Fig. 5, Table 9) (Alpini et al., 1997; Lazaridis et al., 1997). Shuttling or localization of ASBT to the apical surface is due to its cytoplasmic tail (Sun et al., 1998). In addition to ileal enterocytes and cholangiocytes, Asbt is also an apical protein in the kidneys (Christie et al., 1996).
Like Ntcp, Asbt/ASBT transports unconjugated and conjugated bile acids in a sodium-dependent manner (Wong et al., 1994; Craddock et al., 1998). The substrate specificity of Asbt is narrower than Ntcp (Craddock et al., 1998). Human ASBT prefers taurine- and glycine-conjugated bile acids, rather than the unconjugated forms (Craddock et al., 1998). In addition, the affinity of ASBT to dihydroxy bile acids is higher than that for trihydroxy bile acids (Craddock et al., 1998).
3. Bile Salt Export Pump.
Secretion of conjugated bile acids from hepatocytes into bile suggested the existence of an active transport mechanism across the canalicular membrane. In 1995, the sister of Pgp (SPGP, ABCB11) was cloned from a pig cDNA library (Childs et al., 1995) and later from additional species (Table 1) (Childs et al., 1998; Green et al., 2000; Lecureur et al., 2000; Byrne et al., 2002; Noe et al., 2002). SPGP was subsequently renamed Bsep (Gerloff et al., 1998). A 12-membrane-spanning domain protein containing putative glycosylation sites, nucleotide binding domains, and typical structures of ABC-transporters has been described for BSEP (Gerloff et al., 1998).
Bsep/BSEP is exclusively expressed in liver on the canalicular membrane of multiple species (Fig. 5) (Childs et al., 1995, 1998; Gerloff et al., 1998). As the primary canalicular bile acid transporter, Bsep/BSEP primarily transports conjugated bile acids (including taurochenodeoxycholate, taurocholate, tauroursodeoxycholate, glycochenodeoxycholate, and glycocholate) in an ATP-dependent manner (Gerloff et al., 1998; Green et al., 2000; Byrne et al., 2002). In contrast, Bsep does not transport cholic acid (Noe et al., 2002). Although BSEP primarily transports bile acids, it can also transport pharmaceuticals such as pravastatin (Hirano et al., 2005). A number of BSEP inhibitors have been identified (cyclosporine A, rifampicin, glibenclamide) (Byrne et al., 2002).
4. Organic Solute Transporters.
Bile acids are absorbed in the small intestine as part of the enterohepatic recirculation. Uptake of bile acids from the intestinal lumen is mediated by Asbt. Once inside the enterocyte, bile acids are translocated to the basolateral membrane by the intestinal bile acid binding protein and subsequently transported across the basolateral membrane by heterodimerized OSTα/β transporters. Ostα and -β were first identified in the liver of the marine skate (Wang et al., 2001d) and subsequently in human and mouse livers (Table 1) (Seward et al., 2003). OSTα is larger than OSTβ (Wang et al., 2001d). Whereas OSTα contains seven putative membrane-spanning domains, OSTβ contains only one (Seward et al., 2003).
Mouse Ostα and -β mRNA are highest in the ileum with detectable levels also in the kidneys, duodenum, jejunum, cecum, and the proximal colon (Fig. 5) (Ballatori et al., 2005; Dawson et al., 2005; Li et al., 2007b) Likewise, human OSTα and -β are expressed to varying levels in the testes, colon, liver, small intestine, adrenal glands, kidneys, and ovaries (Seward et al., 2003). Whereas OSTα is expressed in the human liver (hepatocytes and cholangiocytes), levels are very low in mouse liver and limited only to cholangiocytes (Ballatori et al., 2005). OSTα and -β localize to the basolateral membrane of ileal enterocytes, hepatocytes, cholangiocytes, and proximal renal tubules (Table 9) (Ballatori et al., 2005). Ostα is required for the delivery of Ostβ protein to the plasma membrane; in turn, OSTα and -β need to be coexpressed to function properly (Li et al., 2007b). Ostα and -β function as a heterodimer to transport not only bile acids but also estrone sulfate, digoxin, and prostaglandin E2 (Wang et al., 2001d; Seward et al., 2003; Dawson et al., 2005).
5. ATP-Binding Cassette Transporter A1.
ABCA1 is a member of the ABC subfamily A and was cloned in 1999 (Langmann et al., 1999) at the same time that it was determined that defects in ABCA1 cause Tangier's disease, a disorder of impaired cholesterol transport (Bodzioch et al., 1999; Brooks-Wilson et al., 1999; Marcil et al., 1999; Remaley et al., 1999; Rust et al., 1999; Schippling et al., 2008) (Tables 1 and 10). As suggested by the clinical presentation of patients with Tangier's disease, ABCA1 effluxes cholesterol and apolipoprotein A1 in vitro (Neufeld et al., 2001; Wang et al., 2001b). The absence of ABCA1 results in premature atherosclerosis, splenomegaly, and hepatomegaly in patients with Tangier's disease.
Abca1/ABCA1 is highly expressed in placenta, uterus, liver, adrenal glands, small intestine, lungs, and heart (Fig. 5) (Langmann et al., 1999). Within these tissues, ABCA1 is often expressed on macrophages in addition to epithelium (Langmann et al., 1999; Schmitz et al., 1999). In the transfected polarized hepatocyte-like WIF-B cell line, ABCA1 immunostaining is observed along the basolateral surface (Neufeld et al., 2002). These findings suggest ABCA1 participates in the regulation of intracellular cholesterol accumulation in hepatocytes. In addition to cell surface expression, ABCA1 is observed in early and late endosomes, which may participate in protein trafficking as well as shuttling cholesterol to the cell surface for efflux (Neufeld et al., 2001).
6. ATP-Binding Cassette Subfamily G Members 5 and 8.
ABCG5 and ABCG8 are efflux transporters that work in concert as a heterodimer to prevent the absorption of plant sterols (Graf et al., 2003) (Table 1). Both isoforms are necessary for trafficking from the endoplasmic reticulum to the canalicular membrane and, in turn, the excretion of plant sterols and cholesterol into bile (Graf et al., 2003). ABCG5 is predicted to contain six putative transmembrane domains with cytosole-facing amino and carboxyl termini (Lee et al., 2001b). Mouse Abcg5 and -g8 mRNA are detected within the liver and small intestine (Fig. 5) (Lu et al., 2002). Within the mouse small intestine, Abcg5 and -g8 are similarly expressed among the three segments (Dieter et al., 2004). ABCG5 and -G8 mRNA are expressed in human liver, small intestine, and colon (Fig. 5) (Berge et al., 2000; Lee et al., 2001b). Abcg/ABCG5 and -g8 proteins are localized to the apical membrane of enterocytes, cholangiocytes, and hepatocytes (Table 9) (Graf et al., 2003; Klett et al., 2004a).
7. ATPase Copper-Transporting β Polypeptide.
ATP7B is an ATP-dependent copper efflux transporter that is primarily expressed in liver (Petrukhin et al., 1994) (Table 1). Messenger RNA expression of ATP7B is widely expressed in multiple tissues (Fig. 5), and mice lacking Atp7b exhibit copper accumulation in kidney, brain, placenta, and lactating mammary glands (Buiakova et al., 1999). ATP7B protein is localized on the apical membrane of placenta syncytiotrophoblasts (Table 9) (Hardman et al., 2004, 2007). Likewise, ATP7B is responsible for the canalicular excretion of copper into bile (Hernandez et al., 2008). ATP7B localization changes depending upon copper concentrations. At low concentrations of copper, ATP7B is present in the trans-Golgi network. As copper accumulates, ATP7B shifts toward the apical membrane of polarized cells (Roelofsen et al., 2000; Guo et al., 2005; Lutsenko et al., 2007; Weiss et al., 2008).
8. ATPase Class I Type 8B Member 1.
ATP8B1 is an ATP-dependent aminophospholipid transporter, also called the familial intrahepatic cholestasis 1 protein (Ujhazy et al., 2001) (Table 1). It is in the type 4 subfamily of P-type ATPases that are termed flippases (Paulusma and Oude Elferink, 2005). The ATP8B1 protein is predicted to contain 10 transmembrane domains (Paulusma and Oude Elferink, 2005). ATP8B1 mRNA is expressed predominantly in the small intestine, uterus, and pancreas, with moderate expression in the bladder, stomach, prostate, liver, and heart (Fig. 5) (Bull et al., 1998). ATP8B1 is expressed on the canalicular membrane of mouse, rat, and human hepatocytes, where phosphatidylserine and phosphatidylethanolamine are translocated from the outer to the inner leaflet bilayer (Table 9) (Eppens et al., 2001; Ujhazy et al., 2001). Because of ATP8B1 activity, the proportion of sphingomyelin and cholesterol in the outer leaflet is increased and is likely to enhance membrane resistance to bile acid toxicity. ATP8B1 is also expressed along the apical surface of rat enterocytes (Ujhazy et al., 2001).
There are a variety of transporters with similarities and differences in their tissue distribution and substrate affinities. In some cases, certain transporter isoforms are restricted to one or two tissues, whereas other isoforms are broadly expressed in multiple tissues. Additional insight into the function of transporters in various tissue and cell types will be addressed in the next section of this review article.
III. Transporter Function in Various Tissues
Traditional in vitro overexpression systems are used to identify substrates of individual drug transporters. The in vivo function of transport proteins can be assessed using multiple approaches. First, transporter gene knockout mice have been genetically engineered and are useful for pharmacokinetic and toxicologic studies. Because of transporter functional redundancy, double- and triple-knockout mice have also been developed. Second, defects in some transport proteins result in genetic disorders with distinct phenotypic changes that provide clues to the functional activities of the transporters (Table 10). Third, single-nucleotide polymorphisms (SNPs) in the coding region of a transporter gene may introduce amino acid substitutions, leading to altered transporter intrinsic activity by changing the protein's affinity to substrates (Km) and/or translocation ability (Vmax) (Tables 11–20) (Evans and Relling, 1999). Nonsynonymous SNPs may also interfere with protein folding, post-translational modifications, and/or trafficking to the cellular membrane and subsequently influence pharmacokinetics and drug response of a particular person. Findings from in vitro overexpression of nonsynonymous transporter SNPs complement clinical observations in patients expressing the SNP on one or both alleles. Collectively, genetic disorders, genetically engineered mice, and human polymorphic variants provide valuable insights into transporter function in various tissues.
TABLE 11.
In vitro characterization of genetic polymorphisms in NTCP
In vitro function was assessed using prototypical substrates for NTCP (taurocholate and cholate). Data are from Ho et al. (2004).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| SLC10A1 | NTCP | ||
| T668C | I223T | ↓ | Intracellular |
| C800T | S267F | ↓ | Normal |
| T836C | I279T | ↓ | Normal |
| A940G | K314E | ↓ | Normal |
↓, reduced function.
TABLE 12.
In vitro characterization of genetic polymorphisms in OATP1A2, -1B1, and -1B3
In vitro function was assessed using prototypical substrates for OATP1A2 (estrone-3-sulfate), OATP1B1 (estrone-3-sulfate, estradiol 17β-glucuronide, rifampin), and OATP1B3 (estrone-3-sulfate, estradiol 17β-glucuronide). Some nucleotide positions were confirmed by PharmGKB (Hewett et al., 2002). OATP1A2 data from Lee et al. (2005a) and Badagnani et al. (2006). OATP1B1 data from Tirona et al. (2001); Michalski et al. (2002); Nozawa et al. (2002); Nishizato et al. (2003); Tirona et al. (2003); Iwai et al. (2004a); Morimoto et al. (2004); Kameyama et al. (2005). OATP1B3 data from Letschert et al. (2004).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| SLCO1A2 | OATP1A2 | ||
| T38C | I13T | ↑↔ | Normal |
| A382T | N128Y | ↔ | N.D. |
| A404T | N135I | ↓↔ | N.D. |
| C502T | R168C | ↓ | N.D. |
| A516C | E172D | ↓ | Intracellular |
| G559A | A187T | ↓ | Normal |
| A833- | Asn278STOP | ↓ | N.D. |
| C2003G | T668S | ↔ | Intracellular |
| SLCO1B1 | OATP1B1 | ||
| T217C | F73L | ↓ | Intracellular |
| T245C | V82A | ↓ | Intracellular |
| A388G | N130D | ↓↔ | Normal |
| A452G | N151S | N.D. | N.D. |
| C463A | P155T | ↔ | Normal |
| A467G | E156G | ↓ | Normal |
| T521C | V174A | ↓ | Intracellular/normal |
| T578G | L193R | ↓ | Intracellular |
| C1007G | P336R | N.D. | N.D. |
| T1058C | I353T | ↓ | Intracellular |
| A1294G | N432D | ↓↔ | Normal |
| A1385G | D462G | ↔ | Normal |
| G1454T | C485F | N.D. | N.D. |
| G1463C | G488A | ↓ | Intracellular |
| T1628G | L543W | N.D. | N.D. |
| A1964G | D655G | ↓↔ | Normal |
| A2000G | E667G | ↓↔ | Normal |
| SLCO1B3 | OATP1B3 | ||
| T334G | S112A | ↑↔ | Normal |
| G699A | M233I | ↔ | Normal |
| G1564T | G522C | ↓↔ | Reduced |
| G1748A | G583E | ↓↔ | Reduced |
↓, reduced function; ↑, increased function; ↔, no change in function; N.D. not determined.
TABLE 13.
In vitro characterization of genetic polymorphisms in human OCT1 and -2
In vitro function was assessed using prototypical substrates for OCT1 and 2 (1-methyl-4-phenylpyridinium, tetraethylammonium, metformin). Nucleotide position was confirmed by PharmGKB (Hewett et al., 2002). OCT1 data from Kerb et al. (2002); Shu et al. (2003); Takeuchi et al. (2003); Sakata et al. (2004); Kang et al. (2007); Shu et al., 2007). OCT2 data from Leabman et al. (2002); Fukushima-Uesaka et al. (2004); Fujita et al. (2006); Lazar et al. (2006); Kang et al. (2007); Song et al. (2008); Wang et al., (2008e).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| SLC22A1 | OCT1 | ||
| C41T | S14F | ↑ | N.D. |
| C181T | R61C | ↓ | Reduced |
| T262C | C88R | ↓ | N.D. |
| C480G | F160L | ↔ | Normal |
| C566T | S189L | ↔ | N.D. |
| G659T | G220V | ↓ | N.D. |
| C848T | P283L | ↓ | Normal |
| C859G | R287G | ↓ | Normal |
| C1022T | P341L | ↓↔ | Normal |
| G1201A | G401S | ↓ | N.D. |
| A1222G | M408V | ↔ | N.D. |
| 1258del | Met420STOP | ↓↔ | N.D. |
| G1393A | G465R | ↓ | Reduced |
| SLC22A2 | OCT2 | ||
| C160T | P54S | ↔ | N.D. |
| T481C | F161L | ↔ | N.D. |
| A493G | M165V | ↓↔ | N.D. |
| G495A | M165I | ↓↔ | N.D. |
| C596T | T199I | ↓ | Normal |
| C602T | T201M | ↓ | Normal |
| G808T | A270S | ↓ | Normal |
| C890G | A297G | ↔ | N.D. |
| C1198T | R400C | ↓ | N.D. |
| A1294C | K432Q | ↓↔ | N.D. |
↓, reduced function; ↑; increased function; ↔, no change in function; N.D. not determined.
TABLE 14.
In vitro characterization of genetic polymorphisms/mutations in BSEP
In vitro function was assessed using the prototypical substrate for BSEP (taurocholate). Some nucleotide positions were confirmed by PharmGKB (Hewett et al., 2002). Data from Strautnieks et al. (1998); Wang et al. (2002b); Hayashi et al. (2005); Noe et al. (2005); Lang et al. (2007).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| ABCB11 | BSEP | ||
| N.D. | G238V | N.D. | Intracellular |
| A890G | E297G | ↓ | Intracellular |
| N.D. | C336S | ↔ | Normal |
| G1296C | R432T | ↓ | Reduced |
| T1331C | V444A | ↔ | Normal/Reduced |
| A1445G | D482G | ↓ | Normal/Reduced |
| G2026T | D676Y | ↓ | Reduced |
| G2563A | G855R | ↓ | Reduced |
| G2944A | G982R | ↓ | Intracellular |
| C3457T | R1153C | ↓ | Intracellular |
| G3803A | R1268Q | ↓ | Intracellular |
TABLE 15.
In vitro characterization of genetic polymorphisms in BCRP
In vitro function was assessed using prototypical substrates BCRP (mitoxantrone, estrone-3-sulfate, dehydroepiandrosterone). Nucleotide position was confirmed by PharmGKB (Hewett et al., 2002). Data are from Honjo et al. (2002); Kondo et al. (2004); Mizuarai et al. (2004); Morisaki et al. (2005); Vethanayagam et al. (2005); Tamura et al., 2006a,b, 2007b).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| ABCG2 | BCRP | ||
| G34A | V12M | ↔ | Normal/intracellular |
| C376T | Gln126STOP | N.D. | Absent |
| C421A | Q141K | ↓ | Normal/reduced |
| G445C | A149P | ↔ | Normal |
| G448A | R163K | ↔ | Normal |
| C496G | Q166E | ↔ | Normal/reduced |
| A616C | I206L | ↓↔ | Normal |
| T623C | F208S | N.D. | Reduced |
| T742C | S248P | N.D. | Normal |
| C805T | P269S | ↓↔ | Normal |
| T1291C | F431L | ↓ | Normal/reduced |
| G1322A | S441N | ↓ | Reduced |
| T1465C | F489L | ↓↔ | Normal/reduced |
| A1768T | N590Y | ↓↔ | Increased |
| G1858A | D620N | ↓↔ | Normal |
↓, reduced function; ↔, no change in function; N.D. not determined.
TABLE 16.
In vitro characterization of genetic polymorphisms in OAT1 and -3
In vitro function was assessed using prototypical substrates for OAT1 (methotrexate, ochratoxin A) and OAT3 (estrone sulfate, cimetidine). Nucleotide position was confirmed by PharmGKB (Hewett et al., 2002). OAT1 data from Bleasby et al. (2005) and Fujita et al. (2005). OAT3 data from Erdman et al. (2006).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| SLC22A6 | OAT1 | ||
| G149A | R50H | ↔ | N.D. |
| C311T | P104L | ↔ | N.D. |
| T677C | I226T | ↔ | N.D. |
| C767T | A256V | ↔ | N.D. |
| C877T | R293W | ↔ | N.D. |
| G1361A | R454Q | ↓ | N.D. |
| SLC22A8 | OAT3 | ||
| C387A | F129L | ↔ | N.D. |
| C445A | R149S | ↓ | N.D. |
| C715T | Gln239STOP | ↓ | N.D. |
| T779G | I260R | ↓ | N.D. |
| C829T | R277W | ↓↔ | N.D. |
| T842C | V281A | ↔ | N.D. |
| A913T | I305F | ↓ | Normal |
| C929T | A310V | ↔ | N.D. |
| G1195T | A399S | ↔ | N.D. |
| G1342A | V448I | ↔ | N.D. |
↓, reduced function; ↔, no change in function; N.D. not determined.
TABLE 17.
In vitro characterization of genetic polymorphisms in PEPT1 and -2
In vitro function was assessed using prototypical substrates for PEPT1 (glycylsarcosine, cephalexin) and PEPT2 (glycylsarcosine). Nucleotide position was confirmed by PharmGKB (Hewett et al., 2002). PEPT1 data from Zhang et al. (2004b) and Anderle et al. (2006). PEPT2 data from Pinsonneault et al. (2004) and Terada et al. (2004).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| SLC15A1 | PEPT1 | ||
| G61A | V21I | ↔ | N.D. |
| T83A | F28Y | ↓ | Normal |
| G350A | S117N | ↔ | Normal |
| G364A | V122M | ↔ | Normal |
| G1256C | G419A | ↔ | Normal |
| G1348A | V450I | ↔ | Normal |
| C1352A | T451N | ↔ | Normal |
| C1609T | P537S | ↔ | N.D. |
| C1757T | P586L | ↓ | Reduced |
| SLC15A2 | PEPT2 | ||
| G207A | R57H | ↓ | Normal |
| C1048T | L350F | N.D. | N.D. |
| C1225T | P409S | ↔ | Normal |
| G1526A | R509K | N.D. | N.D. |
↓, reduced function; ↔, no change in function; N.D. not determined.
TABLE 18.
In vitro characterization of genetic polymorphisms in human OCTN1 and -2
In vitro function was assessed using prototypical substrates for OCTN1 (tetraethylammonium) and OCTN2 (tetraethylammonium, carnitine). Nucleotide position was confirmed by PharmGKB (Hewett et al. (2002). OCTN1 data from Kawasaki et al. (2004); Gazouli et al. (2005); Babusukumar et al. (2006); Russell et al. (2006); Urban et al. (2007, 2008). OCTN2 data from Urban et al., 2006).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| SLC22A4 | OCTN1 | ||
| G475A | V159M | ↔ | N.D. |
| A494G | D165G | ↓ | Normal |
| G615A | M205I | ↓ | Normal |
| C844T | Arg282STOP | ↓ | N.D. |
| C917T | T306I | ↔ | Normal |
| G1385A | G462E | ↓ | Normal |
| C1507T | L503F | ↑ | Normal |
| SLC22A5 | OCTN2 | ||
| C51G | F17L | ↓ | Intracellular |
| C430T | L144F | ↔ | Normal |
| T1345G | Y449D | ↓ | N.D. |
| G1441T | V481F | ↓ | N.D. |
| G1441A | V481I | ↔ | N.D. |
| T1522C | F508L | ↔ | N.D. |
| A1588G | M530V | ↔ | N.D. |
| C1645T | P549S | ↔ | Normal |
↓, reduced function; ↑, increased function; ↔, no change in function; N.D. not determined.
TABLE 19.
In vitro characterization of genetic polymorphisms in MDR1
In vitro function was assessed using prototypical substrates for MDR1 (vinblastine, verapamil, paclitaxel). Nucleotide position was confirmed by PharmGKB (Hewett et al., 2002). Data from Kimchi-Sarfaty et al. (2002); Morita et al. (2003a); Crouthamel et al. (2006); Salama et al. (2006); Schaefer et al. (2006); Gow et al. (2008); Woodahl et al. (2009).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| ABCB1 | MDR1 | ||
| A61G | N21D | ↔ | N.D. |
| T307C | F103L | N.D. | N.D. |
| G1199A | S400N | ↑↔ | Normal |
| C2005T | R669C | ↔ | N.D. |
| G2677T | A893S | ↓↑↔ | Normal |
| G2677A | A893T | ↑↔ | Notmal |
| T3421A | S1141T | ↓↔ | N.D. |
| C3435T | I1145I | ↓↔ | N.D. |
| G3751A | V1251I | ↓ | N.D. |
↓, reduced function; ↑, increased function; ↔, no change in function; N.D. not determined.
TABLE 20.
In vitro characterization of genetic polymorphisms in MRP1–4
In vitro function was assessed using prototypical substrates for MRP1 (leukotriene C4, estradiol 17β-glucuronide, methotrexate), MRP2 (leukotriene C4, estradiol 17β-glucuronide), MRP3 (estradiol 17β-glucuronide), and MRP4 (zidovudine, adefovir). Nucleotide position was confirmed by PharmGKB (Hewett et al., 2002). MRP1 data from Conrad et al. (2001); Conrad et al. (2002); Leslie et al. (2003); LŽtourneau et al. (2005). MRP2 data from Itoda et al. (2002); Moriya et al. (2002); Hirouchi et al. (2004); Izzedine et al. (2006); Rau et al. (2006); Haenisch et al. (2007); Sai et al. (2008). MRP3 data from Lang et al. (2004); Lee et al., 2004b; Fukushima-Uesaka et al. (2007); Kobayashi et al. (2008). MRP4 data from Abla et al. (2008) and Krishnamurthy et al. (2008).
| Nucleotide Change | Amino Acid Change | In Vitro Function | Protein Expression/Localization |
|---|---|---|---|
| ABCC1 | MRP1 | ||
| G128C | C43S | ↑↔ | Intracellular |
| C218T | T73I | ↑↔ | Normal |
| C257T | S92F | ↓↔ | Normal |
| C350T | T117M | ↓↔ | Normal |
| G689A | R230Q | ↔ | Normal |
| G1057A | V353M | N.D. | N.D. |
| G1299T | R433S | ↓↔ | Normal |
| G1898A | R633Q | ↓↔ | Normal |
| G2012T | G671V | ↔ | Normal |
| G2168A | R723Q | ↓ | Normal |
| G2965A | A989T | ↓↔ | Normal |
| G3140C | C1047S | ↑↔ | Normal |
| G3173A | R1058Q | ↔ | Normal |
| C4535T | S1512L | ↔ | Normal |
| ABCC2 | MRP2 | ||
| C-24T | N.D. | N.D. | |
| G1058A | R353H | N.D. | N.D. |
| G1249A | V417I | ↔ | Normal |
| C2366T | S789F | ↑↓ | Intracellular |
| T2780G | L927R | N.D. | N.D. |
| C3298T | R1100C | N.D. | N.D. |
| G3299A | R1100H | N.D. | N.D. |
| T3563A | V1188E | N.D. | N.D. |
| G4348A | A1450T | ↔ | Normal/Intracellular |
| G4544A | C1515Y | N.D. | N.D. |
| ABCC3 | MRP3 | ||
| G32A | G11D | ↔ | Normal |
| C202T | H68Y | N.D. | N.D. |
| G296A | R99Q | N.D. | Normal |
| C1037T | S346F | ↓ | Normal |
| C1537A | Q513K | N.D. | N.D. |
| T1643A | L548Q | N.D. | N.D. |
| G1820A | S607N | ↓ | Normal |
| C2221T | Gln741STOP | N.D. | N.D. |
| G2293C | V765L | ↔ | Normal |
| G2395A | V799M | N.D. | N.D. |
| C2758T | P920S | ↑ | Normal |
| G2768A | R923Q | ↑ | Normal |
| C3657A | S1219R | N.D. | N.D. |
| C3856G | R1286G | ↔ | Normal |
| G3890A | R1297H | N.D. | N.D. |
| C4042T | R1348C | ↑ | Normal |
| A4094G | Q1365R | ↔ | Normal |
| C4141A | R1381S | ↔ | Intracellular |
| C4217T | T1406M | N.D. | N.D. |
| G4267A | G1423R | N.D. | N.D. |
| ABCC4 | MRP4 | ||
| C52A | L18I | N.D. | N.D. |
| C232G | P78A | ↓↔ | Normal |
| T551C | M184T | N.D. | N.D. |
| G559T | G187W | ↓ | Reduced |
| A877G | K293E | ↔ | Normal |
| G912T | K304N | ↔ | Normal |
| C1067T | T356M | N.D. | N.D. |
| C1208T | P403L | ↓↔ | Normal |
| G1460A | G487E | ↓ | Normal |
| A1492G | K498E | ↔ | Normal |
| A1875G | I625M | N.D. | N.D. |
| C2000T | P667L | N.D. | N.D. |
| A2230G | M744V | ↔ | Normal |
| G2269A | E757K | N.D. | Intracellular |
| G2459T | R820I | N.D. | N.D. |
| G2560T | V854F | N.D. | N.D. |
| G2698T | V900L | N.D. | N.D. |
| G2867C | C956S | ↑↔ | Normal |
| G3211A | V1071I | ↔ | Normal |
| C3425T | T1142M | N.D. | N.D. |
| G3659A | R1220Q | N.D. | N.D. |
| A3941G | Q1314R | N.D. | N.D. |
↓, reduced function; ↑, increased function; ↔, no change in function; N.D. not determined.
This portion of the review focuses on the biological functions of uptake and efflux transporters with regard to genetic disorders, knockout mice, and human SNP variants. For the most part, only nonsynonmous coding region polymorphisms are discussed, although recent work has highlighted important roles for intronic and synonymous polymorphisms in regulating transporter expression and/or function. Likewise, the allele frequencies for the various polymorphisms are not included but can be ascertained from the Hapmap project or PharmGKB databases. For additional in depth information regarding genetic disorders of transport (such as cystic fibrosis, surfactant deficiency, adrenoleukodystrophy, macular degeneration), knockout mice, and SNPs from specific transporter classes, the reader is referred to recent reviews (Dean, 2005; Kubitz et al., 2005; Chinn and Kroetz, 2007; Kruh et al., 2007; Gradhand and Kim, 2008; Klaassen and Lu, 2008; Vlaming et al., 2009a).
A. Liver
A variety of uptake and efflux transporters are localized to the apical and basolateral membranes of hepatocytes and cholangiocytes. Figure 6 denotes the subcellular localization of these proteins.
Fig. 6.
Subcellular localization of uptake and efflux transport proteins in hepatocytes and cholangiocytes. The localization and orientation of uptake and efflux transporters in liver cells (primarily rodents) are shown.
1. Basolateral Uptake Transporters in Liver.
Ntcp (Slc10a1) is a bile acid uptake transporter that localizes to the basolateral membrane of hepatocytes (Stieger et al., 1994; Keitel et al., 2005). Its function is to take up bile acids (especially taurine-conjugated) into hepatocytes using a sodium gradient (Hagenbuch et al., 1991; Boyer et al., 1994; Hagenbuch and Meier, 1994; Saeki et al., 2002). Down-regulation of rat Ntcp using antisense oligonucleotides almost completely abolishes taurocholate transport (95% reduction) in Xenopus laevis oocytes (Hagenbuch et al., 1996). Four nonsynonymous SLC10A1 (NTCP) polymorphisms exhibit reduced taurocholate, cholate, and estrone sulfate transport in vitro (Table 11) (Ho et al., 2004). Lower transport activity in only one of these variants could be explained by impaired cell surface expression (Ho et al., 2004). NTCP has a limited ability to transport pharmaceuticals (Ho et al., 2006b). In addition to SLC10A1, SLCO1B1 (OATP1B1) may also contribute to bile acid uptake into the liver. SLCO1B1 loss-of-function variants are associated with elevated serum bile acids in vivo (Xiang et al., 2009).
Basolateral uptake transporters are important determinants of liver injury induced by drugs or toxins. Rat Oatp1b2, human OATP1B1, and OATP1B3 transport the phalloidin analog demethylphalloin (Fehrenbach et al., 2003; Meier-Abt et al., 2004). Oatp1b2-null mice are resistant to hepatotoxicity caused by the mushroom toxin phalloidin and the blue-green algae toxin microcystin-LR (Lu et al., 2008). In both instances, resistance to toxicity is a result of reduced hepatic uptake of the toxins. Conversely, inhibition of NTCP-mediated bile acid uptake (as well as BSEP efflux) has also been proposed as a mechanism for hepatotoxicity induced by certain xenobiotics. It is noteworthy that some cholestatic chemicals (such as rifampicin, rifamycin SV, glibenclamide, and cyclosporin) inhibit the transport of taurocholate in NTCP- and BSEP-overexpressing polarized cells (Mita et al., 2006). Likewise, a reduced ability of the hepatotoxin bosentan to inhibit NTCP compared with rat Ntcp may explain differences in hepatotoxicity sensitivity between these two species (human > rat) by causing hepatocellular bile acid accumulation (Leslie et al., 2007).
Because of the high expression of Oatps/OATPs and the critical synthesis of cholesterol in the liver, the ability of OATP1B1 and OATP1B3 to transport anticholesterol drugs (including the statins) is an area of active research. Rifampicin and pravastatin are prototypical substrates of OATP1B1 and -1B3, respectively (Spears et al., 2005; Niemi et al., 2006b; Seithel et al., 2007). Hepatic uptake of both drugs is reduced in Oatp1b2-null mice with dramatic reductions in their liver-to-plasma ratios (Zaher et al., 2008). A conflicting report showed little difference in pravastatin pharmacokinetics between wild-type and Oatp1b2-null mice, but did observe reduced hepatic concentrations of another statin, lovastatin (Chen et al., 2008). In contrast, simvastatin uptake into liver is unchanged in Oatp1b2-null mice, demonstrating distinct differences in hepatic extraction by Oatp1b2 for this class of drugs (Chen et al., 2008). Altered drug disposition in Oatp1b2-null mice demonstrates the utility of this in vivo model for investigating the possible contributions of OATP1B1/1B3 to hepatic transport.
The pharmacokinetics of statins have been investigated in patients with SLCO1B1 (OATP1B1) gene SNPs N130D and V174A. In vitro studies demonstrate normal and reduced pravastatin (as well as estrone sulfate) uptake in N130D- and V174A-overexpressing cells, respectively (Table 12) (Kameyama et al., 2005). The combination of N130D and V174A SLCO1B1 SNPs leads to reduced pravastatin and pitavastatin clearance (Nishizato et al., 2003; Chung et al., 2005). The V174A SNP alone is sufficient to increase plasma concentrations of pravastatin (single dose), primarily in subjects of European American descent, suggesting delayed uptake of pravastatin into liver (Mwinyi et al., 2004; Ho et al., 2007). Furthermore, statins exhibit attenuated efficacy in lowering total cholesterol in patients with the V174A allele (Tachibana-Iimori et al., 2004). It is noteworthy that other SLCO1B1 SNPs have opposite effects. The N130D SNP reduces the area under the curve of pravastatin after a single dose in white subjects, and seems to accelerate OATP1B1-mediated uptake of pravastatin (Mwinyi et al., 2004). Likewise, the P155T variant is associated with greater reduction in low-density lipoprotein cholesterol levels by fluvastatin than in patients with the reference allele, suggesting a gain of function for certain SLCO1B1 alleles (Couvert et al., 2008).
The L543W SLCO1B1 SNP has been detected only in the Japanese population. Although this variant is rare, it has been associated with pravastatin-induced myopathy (Morimoto et al., 2004). More recently, an intronic SNP in SLCO1B1 was also identified as a strong risk factor in patients with statin-induced myopathy, using a genomewide screen (SEARCH Collaborative Group et al., 2008). In that study, more than 60% of myopathy cases in patients treated with a statin could be strongly associated with the variant SLCO1B1 allele (SEARCH Collaborative Group et al., 2008).
Ezetimibe is a cholesterol-lowering drug that inhibits intestinal absorption of cholesterol via the Niemann-Pick C1 like 1 protein. Ezetimibe undergoes glucuronidation and extensive enterohepatic circulation. Ezetimibe-glucuronide inhibits transport of sulfobromophthalein mediated by OATP1B1 and -2B1 (Oswald et al., 2008). Uptake of ezetimibe-glucuronide by OATP1B1 is reduced in cells transfected with the SLCO1B1 V174A variant compared with the wild-type transporter (Oswald et al., 2008). When evaluating ezetimibe single oral dose pharmacokinetics, subjects who are homozygous for the N130D allele exhibit lower bioavailability of ezetimibe, whereas subjects who are heterozygous for the V174A allele have reduced fecal excretion of ezetimibe (Oswald et al., 2008).
Whereas OATPs mediate organic anion uptake into liver, OCT1 is responsible for the influx of organic cations. Using Oct1-null mice, the roles for this transporter in hepatic uptake have been shown. Oct1-null mice have reduced hepatic accumulation and/or biliary excretion of organic cations, such as the model substrate tetraethylammonium, the neurotoxin 1-methyl-4-phenylpyridinium, the anticancer drug metaiodobenzylguanidine, and the antidiabetic drug metformin (Jonker et al., 2001; Wang et al., 2002a; Shu et al., 2007). In addition to pharmacokinetic implications, impaired intestinal absorption and hepatic uptake of metformin have pharmacodynamic consequences (Wang et al., 2002a). Oct1-null hepatocytes are resistant to the glucose-lowering effects of metformin after glucagon challenge (Shu et al., 2007). It is noteworthy that reduced hepatic metformin uptake in Oct1-null mice is associated with lower blood lactate levels compared with wild-type mice, demonstrating that the liver is central to metformin-induced lactic acidosis (Wang et al., 2003a).
Similar to Oct1 in mice, metformin is transported by human OCT1 and OCT2 (Kimura et al., 2005; Song et al., 2008). In vitro analysis of SLC22A1 (OCT1) variants has identified 1 deletion (Met420STOP) and six nonsynonymous polymorphisms (S14F, R61C, S189L, G220V, G401S, G465R) that exhibit reduced metformin uptake (Shu et al., 2003, 2007) (Table 13). These SLC22A1 variants lead to impaired metformin efficacy in lowering blood glucose after an oral glucose challenge (Shu et al., 2007) and increased renal clearance (Tzvetkov et al., 2009). An intronic variant is also associated with the glucose-lowering effect of metformin (Becker et al., 2009). In addition, a study of 24 responders and 9 nonresponders to metformin (as determined by glycosylated hemoglobin A1c levels) demonstrated that the frequency of the SLC22A1 M408V allele is higher in nonresponders compared with responders (Shikata et al., 2007). Likewise, hepatic OCT1 mRNA levels are lower in livers of M408V carriers (Shikata et al., 2007). Patients with type 2 diabetes who carry the common variant M408V (allelic frequency higher than 10% in the general population) may have an insufficient therapeutic response to metformin therapy because of reduced uptake into liver, which is the major target for reducing circulating blood glucose.
2. Apical Efflux Transporters in Liver.
There are a large number of transporters on the apical surface of hepatocytes that are responsible for the biliary excretion of endobiotics and xenobiotics. Mutations and polymorphisms in canalicular transporters result in genetic disorders with distinct clinical phenotypes and/or marked alterations in chemical disposition.
The ability of Mdr1a/1b, also known as Pgp, to influence the disposition and hepatotoxicity of the environmental toxicant arsenic has been investigated in Mdr1a/1b-null mice (Liu et al., 2002). Administration of sodium arsenite to Mdr1a/1b-null mice yielded interesting findings, the null mice being more susceptible to hepatic injury and mortality than the wild-type mice (Liu et al., 2002). Enhanced susceptibility is probably due to elevated arsenic tissue concentrations in liver, kidneys, small intestine, and brain (Liu et al., 2002). Similar studies in Mdr1a/1b-null mice have been conducted for the mycotoxin fumonisin, but Pgp does not seem to be important for the disposition or toxicity of this chemical (Sharma et al., 2000).
The ability of Mdr2 to transport phospholipids as well as its localization in the canalicular membrane suggested a role for this transporter in protecting the biliary tree from bile acid toxicity by forming mixed phospholipid-bile acid micelles (Elferink et al., 1997). Results from Mdr2-null mice confirmed this hypothesis (Smit et al., 1993; Leveille-Webster and Arias, 1994). Livers from mice lacking Mdr2 exhibit focal hepatocyte necrosis, bile duct proliferation and inflammation, and elevated serum biomarkers of liver injury that are similar to nonsuppurative inflammatory cholangitis (Smit et al., 1993; Mauad et al., 1994). Pathology is more severe in female than male mice, which is thought to be due to the higher levels of hydrophobic bile acids in the bile of female Mdr2-null mice (van Nieuwerk et al., 1997). By 4 to 6 months of age, Mdr2-null mice develop preneoplastic nodules that progress to liver tumors (Mauad et al., 1994). Bile acid excretion into bile is similar in wild-type and Mdr2-null mice, whereas biliary excretion of phospholipids is absent in Mdr2-null mice (Smit et al., 1993; Oude Elferink et al., 1995). It is thought that bile duct proliferation contributes to the enhanced bile acid-independent bile flow in Mdr2-null mice (Oude Elferink et al., 1995; Elamiri et al., 2003). Mdr2-null mice are gaining utility as a rodent model of primary sclerosing cholangitis for identifying the interplay of phospholipids, sterols, and bile acids, as well as testing compounds as novel therapeutics (van Nieuwerk et al., 1997; Voshol et al., 1998; Elamiri et al., 2003; Fickert et al., 2006).
Cholestasis can be caused by genetic defects or as a secondary consequence of hepatobiliary obstruction or destruction. Progressive familial intrahepatic cholestasis (PFIC) represents a group of inherited, autosomal recessive disorders characterized by progressive liver disease with impaired bile flow but without irregularity of the hepatobiliary structure (Table 10). PFIC-III arises from mutations in the human ABCB4 (MDR3) gene, the human ortholog of mouse Mdr2 (de Vree et al., 1998). Patients with PFIC-III display elevated serum γ-glutamyltranspeptidase levels and marked bile duct proliferation. In addition, variants of ABCB4 are associated with the severe form of cholestasis of pregnancy, rare cases of juvenile cholesterol gallstones, and drug-induced hepatocellular and cholestatic injury (Lang et al., 2007; Wasmuth et al., 2007; Nakken et al., 2009; Bacq et al., 2009).
As the “sister” of Pgp, Bsep represents the primary bile acid exporter on hepatocyte canaliculi (Gerloff et al., 1998). Mutations in ABCB11 (BSEP) are responsible for PFIC-II in humans (Table 10) (Strautnieks et al., 1998; Jansen et al., 1999). A recent report identifies more than 10 mutations in the ABCB11 gene, although the functional relevance of these mutations has not been confirmed (Strautnieks et al., 2008). As expected from BSEP dysfunction, patients with PFIC-II present with high serum bile acid concentrations, normal serum γ-glutamyltranspeptidase activity and cholesterol, and low biliary bile acid concentrations. PFIC-II patients are at an increased risk of hepatobiliary malignancy (Knisely et al., 2006). Researchers have identified a number of mutations in ABCB11 that impair BSEP insertion into the apical membrane and therefore reduce taurocholate transport in vitro (Wang et al., 2002b). In addition, autoantibodies against BSEP have been implicated in recurrent graft failure after liver transplantation in a patient with PFIC-II (Keitel et al., 2009).
In an attempt to identify individuals with a genetic predisposition to drug-induced cholestasis or intrahepatic cholestasis of pregnancy, patients with acquired cholestasis have been genotyped for ABCB11 variants. Three highly conserved mutants/variants (V444A, D676Y, G855R) strongly associate with susceptibility to drug-induced cholestasis (Table 14) (Lang et al., 2007). Likewise, the V444A polymorphism is a risk factor for intrahepatic cholestasis of pregnancy in European patients as well as patients with contraceptive-induced cholestasis (Keitel et al., 2006; Dixon et al., 2008; Meier et al., 2008). More recent efforts have identified novel ABCB11 variants in the Japanese population (Kim et al., 2009).
In a surprising turn of events, Bsep-null mice exhibit a relatively mild cholestasis compared with humans lacking functional BSEP (Wang et al., 2001c). Bsep-null mice are viable and fertile but display growth retardation and lower liver weights compared with wild type (Wang et al., 2001c). Canaliculi from Bsep-null mice have dilated lumens, loss of microvilli, and retained biliary material (Wang et al., 2001c). Although the secretion of cholic acid is reduced in Bsep-null mice, total bile acid excretion is not abolished (∼30% of wild-type mice) (Wang et al., 2001c). Feeding a cholic acid-supplemented diet to Bsep-null mice does, however, precipitate a more pronounced PFIC-II-like phenotype (Wang et al., 2003b). A less severe phenotype in Bsep-null mice compared with patients with PFIC-II suggests that mice possess an alternate canalicular bile acid transport system or further hydroxylation of bile acids in the 6 position to increase their hydrophilicity and decrease their toxicity, all of which would compensate for the loss of Bsep. Subsequent research demonstrated the overexpression of Mdr1a and Mdr2 proteins in Bsep-null mice and led to the proposition that Mdr1a transports bile acids, albeit with a lower affinity (Lam et al., 2005). Indeed, in vitro Pgp overexpression is associated with taurocholate transport (Lam et al., 2005). Furthermore, triple-null mice lacking Bsep, Mdr1a, and Mdr1b exhibit a severe degree of cholestasis as evidenced by impaired bile formation, jaundice, and increased mortality (Wang et al., 2009). Mrp2 and Mrp3 proteins are also elevated in Bsep-null mice (to a lesser degree than Mdr1a) and may compensate for bile acid excretion.
Dubin-Johnson syndrome results from mutations in the ABCC2 (MRP2) gene (Kartenbeck et al., 1996; Paulusma et al., 1997; Tsujii et al., 1999; Keitel et al., 2000). These patients experience a benign clinical course and most notably exhibit chronic hyperbilirubinemia (Table 10). Before the development of Mrp2-null mice, researchers were able to identify functional roles for Mrp2 using rats lacking this transporter (Eisai hyperbilirubinemic rats on a Sprague-Dawley background and transport-deficient (TR−) on a Wistar background) (Paulusma et al., 1996; Ito et al., 1997). Mrp2-null mice are healthy and viable (Chu et al., 2006; Vlaming et al., 2006). The only notable phenotypic characteristic of Mrp2-null mice is chronic hyperbilirubinemia, which is similar to that in the mutant rat lines (Elferink et al., 1989; Chu et al., 2006; Vlaming et al., 2006). In addition, bile flow and biliary excretion of glutathione is markedly reduced in Mrp2-null mice (Chu et al., 2006; Vlaming et al., 2006). Biliary excretion of the organic anion dibromosulfophthalein is reduced in Mrp2-null mice (Chu et al., 2006). The biliary excretion of spiramycin is lower in single-pass perfused livers from Mrp2-null mice, compared with wild type (Tian et al., 2007). Likewise, the biliary excretion of rosuvastatin is lower in Eisai hyperbilirubinemic rats compared with Sprague-Dawley rats (Kitamura et al., 2008).
The role of Mrp2 in the hepatobiliary transport of the antihistamine fexofenadine differs between mice and rats (Tian et al., 2008). The biliary excretion rate of fexofenadine is reduced 85% in Mrp2-null mice compared with wild type (Tian et al., 2008). In contrast, TR− and Eisai hyperbilirubinemic rats lacking Mrp2 function demonstrate biliary elimination of fexofenadine similar to that of their Wistar and Sprague-Dawley rat counterparts, respectively (Tahara et al., 2005; Tian et al., 2007, 2008). In addition, the biliary excretion of fexofenadine is unchanged in Mdr1a/1b-null and Bcrp-null mice, suggesting that Mrp2 is the predominant canalicular transporter responsible for fexofenadine efflux in mice (Tian et al., 2007).
An important role for Mrp2 in the biliary clearance of morphine-3-glucuronide is evident in Mrp2-null mice (van de Wetering et al., 2007). The appearance of morphine-3-glucuronide in bile is markedly reduced in Mrp2-null mice (van de Wetering et al., 2007). Instead, morphine-3-glucuronide excretion shifts from biliary to urinary (van de Wetering et al., 2007). It is likely that Mrp3 compensates for the loss of Mrp2 and enables the hepatic efflux of morphine-3-glucuronide to the sinusoidal blood.
The biliary excretion of the cholesterol-lowering drug pravastatin is lower in TR− rats lacking functional Mrp2 protein (Yamazaki et al., 1997; Fukumura et al., 1998; Kivistö et al., 2005). Increased systemic exposure to oral pravastatin in TR− rats is associated with enhanced inhibition of HMG-CoA reductase as reflected by shifts in cholesterol levels (i.e., lower lathosterol to cholesterol concentration ratios) (Kivistö et al., 2005). Pravastatin plasma concentrations are reduced in healthy volunteers carrying the C1446G synonymous ABCC2 (MRP2) variant (Niemi et al., 2006a). This polymorphism increased hepatic MRP2 mRNA levels by 95% and, in turn, supports the link between MRP2 expression and pravastatin disposition (Niemi et al., 2006a).
Paclitaxel pharmacokinetics is altered in Mrp2-null and Mdr1a/1b-null mice as well as the triple null mice (Mrp2/Mdr1a/1b-null mice) (Lagas et al., 2006). The exact role(s) of Mrp2 and Mdr1a/1b in regulating paclitaxel pharmacokinetics depends upon the route of administration. Mdr1a/1b reduces plasma concentrations of paclitaxel when given orally (Lagas et al., 2006). It is noteworthy that intravenous administration of paclitaxel results in elevated plasma drug levels in Mrp2-null mice as well as Mdr1a/1b-null mice (Lagas et al., 2006). The biliary excretion of paclitaxel was absent in Mrp2-null mice, suggesting that Mrp2 is the principal canalicular pump for this anticancer drug (Lagas et al., 2006). These studies suggest that intestinal Mdr1a/1b and hepatic Mrp2 are important determinants of paclitaxel disposition. Docetaxel is an anticancer drug related to paclitaxel. Docetaxel-induced leucopenia/neutropenia is associated with a variant in the ABCC2 gene (Kiyotani et al., 2008). Similar to paclitaxel, the pharmacokinetics of other anticancer drugs are influenced by the expression of Mrp2 and/or Mdr1a/1b. The biliary excretion of doxorubicin is lower in Mrp2-null mice and is almost abolished in Mrp2/Mdr1a/1b-null mice (Vlaming et al., 2006).
In addition to pumping pharmaceuticals into bile, canalicular Mrp2 also seems to influence the biliary excretion of toxins. For example, transport of the Amanita spp. mushroom toxin demethylphalloin is reduced in TR− rats (Gavrilova et al., 2007). Demethylphalloin levels in the bile of Mdr1a/1b-null mice and Bcrp-null mice were similar to wild type, suggesting that Mrp2 is the primary canalicular pump for this toxin (Gavrilova et al., 2007). Likewise, TR− rats are protected from α-naphthylisothiocyanate-induced biliary damage, probably by preventing exposure of cholangiocytes to this chemical (Dietrich et al., 2001b). Finally, the plasma concentrations of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine are elevated in Mrp2-null mice (Vlaming et al., 2006) and the intestinal absorption of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine is limited by Mrp2 in the rat intestince (Dietrich et al., 2001a). Collectively, these findings suggest that Mrp2 functions as an intestinal and biliary transporter to protect the liver from injury. This is supported by translational studies that have associated ABCC2 variants with liver disease including intrahepatic cholestasis of pregnancy as well as herbal and drug-induced toxicity (Choi et al., 2007; Daly et al., 2007; Sookoian et al., 2008).
Similar to Mrp2, canalicular Bcrp is important for excretion of chemicals into bile. The biliary excretion of antibiotics (nitrofurantoin, ciprofloxacin, grepafloxacin, ofloxacin, ulifloxacin) and the anticholesterol drug rosuvastatin is lower in Bcrp-null mice (Merino et al., 2005c; Ando et al., 2007; Kitamura et al., 2008). Chinese men with one mutant Q141K ABCG2 (BCRP) allele demonstrate higher plasma concentrations of rosuvastatin compared with those with both wild-type alleles (Table 15) (Zhang et al., 2006b). Similar findings have been reported in Finnish volunteers with the Q141K ABCG2 (BCRP) SNP after atorvastatin or rosuvastatin administration (Keskitalo et al., 2009). Mrp2 and Bcrp both contribute to the biliary elimination of methotrexate and its toxic metabolite 7-hydroxymethotrexate after intravenous administration (Vlaming et al., 2009b). Plasma 7-hydroxymethotrexate levels are elevated in Mrp2-null mice but not Bcrp-null mice (Vlaming et al., 2009b). Double-null mice lacking both Mrp2 and Bcrp have greater than 20-fold reductions in the biliary excretion of methotrexate and its metabolite (Vlaming et al., 2009b). Therefore, it has been concluded that Mrp2 can compensate for loss of Bcrp function, whereas Bcrp can only partially compensate for loss of Mrp2 function in dictating methotrexate disposition.
The existence of a dietary cholesterol transport system was suspected before the identification of the ABCG5 and ABCG8 transporters. Sitosterolemia is a disorder of impaired clearance of plant-derived sterols that presents as tendon xanthomas, atherosclerosis at a young age, and premature coronary artery disease (Table 10) (Salen et al., 1992). This rare, autosomal recessive disorder results from mutations in ABCG5 and ABCG8 that consequently impair the biliary excretion of sterols (Berge et al., 2000; Lee et al., 2001b; Lu et al., 2001). Not surprisingly, disruption of both the Abcg5 and Abcg8 genes in mice also produces the sitosterolemia phenotype. Abcg5/g8-null mice have an increased fractional absorption of dietary plant sterols as well as plasma sitosterol levels. In addition, biliary cholesterol levels are very low in Abcg5/g8-null mice (Yu et al., 2002). Although the total sterol levels are similar in livers of wild-type and Abcg5/8-null mice, the proportion of sterols that are plant-based (as opposed to cholesterol) is much larger in Abcg5/g8-null mice (Yu et al., 2004). Single-null mice (Abcg5-null and Abcg8-null mice) have also been developed (Klett et al., 2004b; Plösch et al., 2004). Pharmacological inhibition of sterol absorption using ezetimibe ameliorates metabolic perturbations observed in Abcg5/g8-null mice (Yu et al., 2005). It is noteworthy that Mdr2-null mice also exhibit low biliary excretion of cholesterol, suggesting that all three transporters contribute to cholesterol excretion (Langheim et al., 2005). Collectively, disruption of ABCG5 and G8 in mice and humans demonstrates that these two transporters are required for efficient secretion of cholesterol into bile.
ATP7B is a critical transporter for the efflux of copper from liver to bile. Mutations in the ATP7B gene cause the autosomal recessive disorder Wilson's disease (Table 10) (Bull et al., 1993; Tanzi et al., 1993). This disorder typically presents in young patients, although adult onset has been reported. Patients with Wilson's disease demonstrate the sequelae of disrupted copper elimination (Lutsenko, 2008). As a result of impaired biliary excretion, copper accumulates in the liver, resulting in pathological conditions including cirrhosis, hepatitis, steatosis, and fulminant liver failure. ATP7B-null mice have been engineered; as expected, they exhibit neurological symptoms (tremor, ataxia, abnormal locomotor activity) and marked copper accumulation in the liver by 4 to 6 weeks of age (Buiakova et al., 1999). Pathological liver conditions are evident in adult ATP7B-null mice and entail swollen hepatocytes with enlarged nuclei, inflammation, necrosis, and proliferation of bile ducts. Enlarged nuclei are probably a result of copper accumulation and enhanced DNA synthesis (Huster et al., 2006). By 28 weeks, liver fibrosis and regenerating nodules are apparent (Huster et al., 2006). In addition, liver cholesterol and serum very-low-density lipoprotein levels are lower in ATP7B-null mice (Huster et al., 2007). Extrahepatic copper accumulation in ATP7B-null mice occurs in the kidneys, brain, placenta, and mammary glands (Buiakova et al., 1999).
ATP8B1 is a phosphatidylserine flippase in the canalicular membrane that transports phosphatidylserine. ATP8B1 is also called familial intrahepatic cholestasis 1 gene and is responsible for PFIC-I as well as the milder functional defect benign recurrent intrahepatic cholestasis (Table 10). PFIC-I is also known as Byler's disease, and patients develop end-stage liver disease during the second decade of life. Other symptoms include elevated serum bile acids, fat malabsorption, diarrhea, and jaundice. Atp8b1-deficient mice carry a mutation orthologous to a human ATP8B1 variant; surprisingly, these mice demonstrate only a mild phenotype (reduced weight at weaning, elevated serum bile acid levels, reduced bile acid excretion in bile) (Pawlikowska et al., 2004). As expected, phosphatidylserine levels are elevated in the bile of Atp8b1-null mice after taurocholate infusion (Paulusma et al., 2006). Increased bile acid levels in Atp8b1-deficient mice are not due to changes in intestinal bile acid absorption (Groen et al., 2006, 2007). It has been hypothesized that more bile acid hydroxylation in mice accounts for the milder phenotype in Atp8b1-deficient mice compared with patients with PFIC-I (Pawlikowska et al., 2004). Furthermore, biliary cholesterol concentrations are higher in Atp8b1-null mice, not because of Abcg5/8-mediated transport, but because of nonspecific extraction of cholesterol molecules from the canalicular membrane (Paulusma et al., 2006; Groen et al., 2008).
3. Basolateral Efflux Transporters in Liver.
Removal of chemicals from the hepatocyte to the sinusoidal blood is accomplished by transporters on the basolateral membrane including Mrp3, Mrp4, Mrp6, and Abca1. Ostα and Mrp3 are also expressed in the basolateral membrane of cholangiocytes.
Glucuronidation is important for the detoxification and excretion of polar chemicals. Glucuronide conjugates of morphine are substrates of MRP3 (Zelcer et al., 2005). In vivo evidence demonstrates that Mrp3-null mice are unable to efflux morphine-3-glucuronide from the liver to the blood (Zelcer et al., 2005; van de Wetering et al., 2007). Instead, morphine-3-glucuronide accumulates in liver and bile of Mrp3-null mice (Zelcer et al., 2005). A shift from urinary to fecal excretion of morphine conjugates in Mrp3-null mice corresponds with a decreased antinociceptive efficacy of morphine-6-glucuronide, probably because of reduced circulating levels (Zelcer et al., 2005). There is a similar shift in the excretion of acetaminophen-glucuronide, 4-methylumbelliferyl-glucuronide, and harmol-glucuronide from the plasma to the bile of Mrp3-null mice (Manautou et al., 2005; Zamek-Gliszczynski et al., 2006). Resveratrol is a dietary phytoestrogen that is under investigation for beneficial health effects. Mrp3 transports the glucuronide conjugate of resveratrol in vitro, and knockout mice lacking Mrp3 have reduced urinary and renal concentrations of resveratrol and its glucuronide conjugate (van de Wetering et al., 2009a). Likewise, Mrp3-null mice have been used in metabolomic experiments to identify glucuronide conjugates of dietary phytoestrogens (van de Wetering et al., 2009b).
Because of the in vitro ability of Mrp3 to transport bile acids (Hirohashi et al., 2000) and the induction of Mrp3 mRNA and protein in cholestatic livers (Scheffer et al., 2002b; Barnes et al., 2007; Slitt et al., 2007), it was hypothesized that hepatic injury would be enhanced in Mrp3-null mice. However, Mrp3-null and wild-type mice exhibit a similar extent of liver damage after bile-duct ligation (Zelcer et al., 2006). Despite similar injury, Mrp3-null mice do exhibit elevated hepatic bile acid content and reduced serum bilirubin glucuronide, which is consistent with the in vitro analysis of these substrates (Belinsky et al., 2005; Zelcer et al., 2006). Instead, Mrp4 seems to be important during extrahepatic cholestasis, as evidenced by more severe liver injury in Mrp4-null mice after bile-duct ligation (Mennone et al., 2006). Similar to MRP3, MRP4 mRNA and protein are up-regulated in cholestatic livers (Gradhand et al., 2008).
Mrp4 transports sulfate conjugates from the liver. Basolateral excretion of sulfate conjugates (including acetaminophen, harmol, and 4-methylumbelliferyl) into sinusoidal blood is reduced in Mrp4-null mice (Zamek-Gliszczynski et al., 2006). It is noteworthy that hepatic Sult2a1 mRNA is reduced in Mrp4-null mice, suggesting a conserved excretion pathway for sulfate conjugates from the liver (Assem et al., 2004).
Defects in the ABCA1 gene cause Tangier's disease, an autosomal recessive disorder of impaired cholesterol transport (Table 10) (Bodzioch et al., 1999; Brooks-Wilson et al., 1999; Marcil et al., 1999; Remaley et al., 1999; Rust et al., 1999; Schippling et al., 2008). Patients with Tangier's disease lack high-density lipoproteins that bind cholesterol. As a result, cholesterol builds up in various tissues. Like persons with Tangier's disease, mice lacking Abca1 have reduced plasma cholesterol, phospholipids, and almost no high density lipoproteins (McNeish et al., 2000). In addition, cholesterol absorption from the intestine is diminished in Abca1-null mice (Drobnik et al., 2001). The liver attempts to compensate for these perturbations by up-regulating cholesterol synthesis (Drobnik et al., 2001). As with the whole-body knockout, plasma cholesterol and high density lipoproteins are decreased in liver-specific Abca1-null mice (Timmins et al., 2005). It is important to note that the biliary secretion of cholesterol, bile acids, and phospholipids is unaffected by the absence of Abca1 (Groen et al., 2001). Cholesterol accumulates in a variety of Abca1-null tissues, including the lungs (intraaveolar macrophages and type II pneumocytes), intestine, and feces (McNeish et al., 2000; Drobnik et al., 2001). It has also been noted that body weight is reduced in Abca1-null mice (Orsó et al., 2000). In addition, the mutant mice develop splenomegaly, enlarged adrenal glands, and deficiencies in fat-soluble vitamins (Orsó et al., 2000).
B. Kidneys
Kidney transporters (primarily in proximal tubules) participate in the secretion and reabsorption of endogenous and exogenous chemicals. Basolateral influx of organic anions and cations by Oat1, Oat3, Oct1–3, and Oatp4c1 is the first step in renal secretion (Fig. 7). After extraction of chemicals from blood, efflux transporters on the apical brush-border membrane, including Mrp2, Mrp4, Bcrp, Mate1, and Mdr1b, secrete the chemicals into urine. Reabsorption of chemicals within the kidneys can be accomplished by apical transporters Asbt, Cnt1–3, Oat2, Oat4, Oat5, Urat1, Oatp1a1, Pept1–2, and Octn1–2 and subsequently across the basolateral surface by Abca1, Ent1, Ent2, Mrp6, Ostα, and Ostβ.
Fig. 7.
Subcellular localization of uptake and efflux transport proteins in renal proximal tubules. The localization and orientation of uptake and efflux transporters in the kidneys (primarily rodents) are shown.
1. Basolateral Uptake Transporters in Kidneys.
On the basolateral membrane of the kidneys, Oct1–3 are the primary cation transporters, whereas Oat1, Oat3, and Oatp4c1 are the primary anion transporters. Generation of null mice points to roles for these transporters in the active uptake of chemicals. Renal secretion of tetraethylammonium is reduced markedly in Oct1/2 double-null mice (Jonker et al., 2003). As a result, plasma tetraethylammonium levels are elevated 6-fold in Oct1/2-null mice compared with single knockouts and wild-type mice, suggesting functional overlap of these two isoforms in the kidneys (Jonker et al., 2003). As expected, Oat1-null and Oat3-null mice have deficits in renal organic anion secretion. P-aminohippurate uptake is absent in renal slices from Oat1-null mice (Eraly et al., 2006). Conflicting results demonstrate unchanged and reduced p-aminohippurate uptake in renal slices from Oat3-null mice (Sweet et al., 2002; Vanwert et al., 2007; Vallon et al., 2008b). Instead, Oat3-null mice (but not Oat1-null mice) display reduced estrone sulfate excretion (Sweet et al., 2002; Eraly et al., 2006). The plasma clearance and urinary excretion of ciprofloxacin, penicillin G, and methotrexate are reduced in Oat3-null mice (Vanwert et al., 2007, 2008; VanWert and Sweet, 2008). Urinary excretion of the diuretic furosemide and its naturetic response are lower in Oat1-null and Oat3-null mice (Eraly et al., 2006; Vallon et al., 2008b). It is noteworthy that Oat3-null mice exhibit 10 to 15% lower blood pressure than wild-type mice (Vallon et al., 2008a). Additional work is necessary to determine the mechanism of Oat3-mediated blood pressure regulation. Initial indications suggest altered blood pressure regulation may be due to accumulation of circulating endogenous organic anions (Vallon et al., 2008a).
A number of nephrotoxicants, including antivirals, antibiotics, and mercury-thiol conjugates, are transported into the kidneys by OAT transporters (Cihlar et al., 1999; Jariyawat et al., 1999; Tsuda et al., 1999; Jung et al., 2002b; Koh et al., 2002; Aslamkhan et al., 2003; Khamdang et al., 2003; Zalups and Ahmad, 2005a,b,c). As a result, OAT transporters are an important initial step in acute renal injury by accumulating toxicants within the nephron. Overexpression of OAT1–4 sensitizes cells to the cytotoxicity of the β-lactam antibiotic cephaloridine (Jariyawat et al., 1999; Jung et al., 2002b; Khamdang et al., 2003). Decreased viability of cephaloridine-exposed cells expressing OAT1, OAT3, and OAT4, but not OAT2, is reversible by the anion transporter inhibitor probenecid (Jung et al., 2002b; Khamdang et al., 2003). Like cephaloridine, OAT1-expressing kidney cells have enhanced susceptibility to cytotoxicity by the mycotoxin ochratoxin A as well as thiol-containing conjugates of inorganic and methylmercury (Tsuda et al., 1999; Koh et al., 2002; Aslamkhan et al., 2003; Zalups and Ahmad, 2005a,b,c). Additional work is necessary to confirm these findings in null mice lacking the various Oat isoforms as well as the in vivo relevance of SNPs (Table 16).
The antineoplastic drug cisplatin is both a substrate and inhibitor of OCT2 activity (Ciarimboli et al., 2005b; Yokoo et al., 2007; Filipski et al., 2008). Cisplatin accumulation and cytotoxicity are enhanced by overexpression of rat and human OCT2 and, to a lesser degree, OCT1 (Yonezawa et al., 2006; Yokoo et al., 2007). Nephrotoxicity in patients undergoing cisplatin-containing chemotherapeutic regimens often limits its therapeutic efficacy and has been demonstrated to be dependent upon basolateral organic cation transport in cultured kidney cells (Ludwig et al., 2004). Because of the prominent expression of OCT2 in kidneys, studies have started to address whether this transporter can alter cisplatin disposition and/or renal toxicity. Compared with wild-type mice, Oct1/2-null mice have reduced platinum excretion and are protected from cisplatin nephrotoxicity (Filipski et al., 2009). Likewise, the A270S SLC22A2 variant was associated with protection against renal damage in a small cohort of white patients receiving cisplatin-containing chemotherapy regimens (Table 13) (Filipski et al., 2008, 2009). Coadministration of the anticancer drug imatinib prevents renal accumulation and toxicity of cisplatin, probably by interfering with OCT2-mediated transport (Tanihara et al., 2009). Cytotoxicity of another platinum-based anticancer drug, oxaliplatin, is reduced by cimetidine (general OCT inhibitor) in colon cancer cell lines, suggesting that OCT expression may contribute to the antitumor specificity of oxaliplatin (Zhang et al., 2006a). Overexpression of rat and human OCT2 and OCT3 enhances oxaliplatin accumulation and cytotoxicity (Yonezawa et al., 2006; Yokoo et al., 2007). In fact, expression of OCT3 in a human colorectal cancer-derived cell line correlates with sensitivity to oxaliplatin cytotoxicity (Yokoo et al., 2008).
2. Apical Uptake Transporters in Kidneys.
Although a large number of transporters are expressed on the apical surface of the kidneys, only the abilities of Urat1, Pept2, and Octn2 to reabsorb chemicals from the urine have been demonstrated in vivo. The functional significance of Asbt, Cnts, Oats, and Oatps on the brush border membrane is hypothetical and warrants further investigation.
Urate is a product of purine metabolism and is eliminated by the kidneys. Accumulation of urate in the body leads to crystallization in the joints, resulting in gout. As the major transporter, altered URAT1 function or expression may influence the reabsorption and body burden of urate. Urat1-null mice were generated in 2008 (Eraly et al., 2008). Although Urat1-null mice have a 2-fold increase in urine urate concentration, there is no change in plasma urate levels (Eraly et al., 2008). These findings suggest that additional transporters contribute to the reabsorption of urate in mice. Messenger RNA analysis of alternate renal urate transporters (i.e., Oat and Mrp) in Urat1-null mice did not identify compensatory gene changes (Eraly et al., 2008).
It had been theorized that loss of URAT1 function in humans would reduce urate reabsorption from glomerular filtrate and decrease the likelihood of urate accumulation and crystallization (Komoda et al., 2004). For example, three SLC22A12 (URAT1) SNPs (R90H, R477H, Trp258STOP) are associated with idiopathic hypouricemia in Japanese patients (Enomoto et al., 2002a; Iwai et al., 2004b; Komoda et al., 2004). Even heterozygous carriers of the Trp258STOP mutation exhibit low levels of serum urate (Komoda et al., 2004). In addition, the Trp258STOP SNP reduces the incidence of gout in Japanese patients (Taniguchi et al., 2005). Oocytes injected with Trp258STOP SLC22A12 mRNA demonstrate no plasma membrane protein expression, suggesting alterations in subcellular trafficking and/or degradation and probably explaining a lack of urate transport (Enomoto et al., 2002a). Two additional SLC22A12 SNPs (T217M and E298D) exhibit reduced urate uptake despite normal protein localization (Enomoto et al., 2002a). It is surprising that Urat1-null mice demonstrate little phenotype with regard to urate reabsorption, whereas humans with SLC22A12 SNPs exhibit pronounced hypouricemia (Eraly et al., 2008). Additional research is needed to elucidate divergent findings between species. A recent genome-wide association study identified a common single-nucleotide polymorphism in ABCG2 (BCRP) as a determinant of serum urate levels and gout (Woodward et al., 2009). Subsequent functional analysis in vitro demonstrated that urate is indeed a substrate of BCRP and that this apical efflux transporter is important in the renal secretion of urate. After reabsorption from urine, urate seems to be transported back to the circulation via the facilitative glucose transporter 9 (Anzai et al., 2008). Similar to BCRP and URAT1, polymorphisms in facilitative glucose transporter 9 have also been linked to alterations in serum urate levels in humans (Li et al., 2007d; Matsuo et al., 2008; McArdle et al., 2008; Preitner et al., 2009).
Apical expression of Pept2 in renal tubules points to a role for this transporter in the reabsorption of di- and tripeptides (Ganapathy et al., 1997; Shen et al., 1999). In line with this hypothesis, renal accumulation of the fluorophore-conjugated dipeptide d-Ala-Lys-AMCA is markedly reduced in kidneys of Pept2-null mice (Rubio-Aliaga et al., 2003). The reabsorption of carnosine (β-alanyl-l-histidine) is impaired in Pept2-null mice, resulting in an 18-fold increase in the urinary excretion of this chemical (Kamal et al., 2009). Likewise, the total and renal clearance of glycylsarcosine is higher in Pept2-null mice, leading to reduced systemic concentrations and elevated urinary levels (Ocheltree et al., 2005; Frey et al., 2007). Pept2 accounts for 86% of glycylsarcosine reabsorption, whereas Pept1 is responsible for 14% of the reabsorbed substrate (Ocheltree et al., 2005). Additional efforts have been undertaken to identify Pept2 substrates as in vivo tracer compounds to evaluate chemical reabsorption (Nabulsi et al., 2005). Using microPET imaging, [11C]glycylsarcosine clearance from the kidneys is rapid in Pept2-null mice because of impaired reabsorption (Nabulsi et al., 2005). In vitro findings for the PEPT1 and -2 SNPs in overexpression systems are shown in Table 17.
3. Apical Efflux Transporters in Kidneys.
Brush-border efflux transporters Mrp2, Mrp4, Bcrp, Mate1, MATE2-K, and Mdr1b accomplish renal secretion of chemicals into urine. Most functional studies of these transporters in kidneys have focused upon Mrp4- and Bcrp-mediated transport. Diuretics (such as hydrochlorothiazide and furosemide) are in vitro substrates and inhibitors for MRP4 and BCRP (Hasegawa et al., 2007). Use of Mrp4-null mice and Bcrp-null mice suggests that Mrp4 is likely to play a more significant role than Bcrp in the renal clearance of hydrochlorothiazide and furosemide (Hasegawa et al., 2007). Mrp4-null mice exhibit reduced renal clearance of both diuretics and higher kidney retention of hydrochlorothiazide during urinary cannulation studies (Hasegawa et al., 2007). The disposition and excretion of hydrochlorothiazide and furosemide are similar in wild-type and Bcrp-null mice (Hasegawa et al., 2007). An important function for Mrp4 in the renal secretion of antiviral drugs (adefovir and tenofovir) has also been demonstrated using Mrp4-null mice (Imaoka et al., 2007). Likewise, the A3463G variant of ABCC4 (MRP4) is associated with higher tenofovir-diphosphate concentrations in the peripheral blood mononuclear cells of patients infected with HIV (Kiser et al., 2008).
Edaravone is a free radical scavenger used in Japan to treat patients with acute cerebral infarction. Urinary elimination of edaravone seems to involve OAT1- and OAT3-mediated basolateral uptake into the kidneys (Mizuno et al., 2007a). Once inside the proximal tubule cell, edaravone is conjugated with sulfate or glucuronic acid. The ability of Bcrp, as well as Mrp4, to mediate the urinary excretion of edaravone conjugates was investigated in vitro and in vivo (Mizuno et al., 2007b). Bcrp mediates the transport of edaravone-sulfate in membrane vesicles from BCRP-expressing cells, and the renal clearance of this conjugate is reduced in Bcrp-null mice (Mizuno et al., 2007b). Likewise, the glucuronide conjugate of edaravone displayed preference for MRP4 in vitro, and its urinary elimination is diminished in Mrp4-null mice (Mizuno et al., 2007b). In addition, the renal clearance of another xenobiotic conjugate, 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole sulfate, is lower in Bcrp-null mice, leading to higher concentrations of this conjugate in the kidneys (Mizuno et al., 2004).
Mate1-null mice have recently been developed (Tsuda et al., 2009). The functional significance of Mate1 in the kidneys is illustrated by altered metformin pharmacokinetics (Tsuda et al., 2009). Compared with wild-type mice, Mate1-null mice have increased plasma concentrations and reduced renal clearance of metformin (Tsuda et al., 2009). Further studies in these mice should be useful for investigating the renal elimination of organic cations.
4. Basolateral Efflux Transporters in Kidneys.
Efflux pumps on the basolateral membrane of the renal proximal tubule are an area of research that has largely remained unexplored. It is hypothesized that Ostα/β is responsible for the reabsorption of bile acids. In addition, Ent2 and Mrp6 may participate in the reabsorption of nucleosides and organic anions back to the blood. Initial in vitro characterization of transepithelial flux in human renal proximal tubule cells suggests that apical CNT3 and basolateral ENT2 are responsible for the reabsorption of adenosine (Elwi et al., 2009).
C. Intestine
Oral administration of drugs most often requires absorption in the small intestine. Although many drugs are thought to be absorbed by passive diffusion, extraction of chemicals from the intestinal lumen is also accomplished by a number of transporters, including Oct3, Octn1–2, Pept1, Cnt1–2, Oatps, and Asbt (Fig. 8). Likewise, there are apical efflux transporters on enterocytes (Mdr1a, Mrp2, Bcrp) that prevent the entry of chemicals into the systemic circulation and are often responsible for the poor oral bioavailability of pharmaceuticals. Additional apical efflux transporters Abcg5/8 and Atp8b1 are more important for the luminal secretion of sterols and phospholipids, respectively. Subsequent absorption of chemicals across the enterocyte basolateral surface into the systemic circulation is accomplished via Mrp3, Ostα/β, Ent1–2, and Abca1.
Fig. 8.
Subcellular localization of uptake and efflux transport proteins in enterocytes. The localization and orientation of uptake and efflux transporters in the intestine (primarily rodents) are shown.
1. Apical Uptake Transporters in the Intestine.
Caco-2 cells express OCTN2 and transport carnitine in a sodium-dependent manner (Elimrani et al., 2003; Hirano et al., 2006). Likewise, carnitine uptake across the apical enterocyte membrane is saturable with kinetics akin to OCTN2-overexpressing cells and can be inhibited by Octn2 inhibitors (Kato et al., 2006). Consistent with these findings, carnitine uptake across the apical surface of the small intestine is almost absent in Octn2-null mice (Kato et al., 2006). By 4 weeks of age, Octn2-null mice demonstrate atrophic intestinal villi, inflammation, ulcer formation, and gut perforation (Shekhawat et al., 2007).
Inflammatory bowel disease has been linked to mutations on a locus on chromosome 5 that localizes to the region where the SLC22A4 (OCTN1) and SLC22A5 (OCTN2) genes exist (5q31). The L503F variant of SLC22A4 is more frequent in white persons from New Zealand who have Crohn's disease (Leung et al., 2006) (Table 18). Moreover, the L503F variant of SLC22A4 and the −207G<C variant of SLC22A5 are found in adult Greek, British, and German patients with Crohn's disease, but not Italian or Hungarian patients (Gazouli et al., 2005; Török et al., 2005; Ferraris et al., 2006; Waller et al., 2006; Magyari et al., 2007; Taubert et al., 2009). Likewise, the genotype and haplotype frequencies of both of these variants are also increased in patients with ulcerative colitis (Palmieri et al., 2006). Childhood onset inflammatory bowel disease and Crohn's disease are similarly linked to variants in SLC22A4 (L503F) and SLC22A5 (−207G<C) in several pediatric populations (Babusukumar et al., 2006; Bene et al., 2006; Russell et al., 2006; Cucchiara et al., 2007). Polymorphisms in OCTN transporters are associated not only with the incidence of inflammatory bowel disease, but also with the pharmacological response to therapy. The −368 T>G polymorphism in the SLC22A5 promoter corresponds to steroid resistance and inadequate response of Japanese patients with inflammatory bowel disease (Nakahara et al., 2008). These data suggest linkage between inflammatory bowel disease and SLC22A4 and SLC22A5 variants among different ethnic groups.
Asbt is expressed on the apical surface of ileal enterocytes and transports conjugated and unconjugated bile acids in the intestine. Loss-of-function mutations in the ASBT gene cause primary bile acid malabsorption that is characterized by diarrhea, fat malabsorption, and malnutrition (Table 10). The first known molecular defect of human ASBT was a P290S substitution that abolished taurocholate transport without interfering with protein expression or subcellular distribution (Wong et al., 1995). Additional ASBT mutations were subsequently linked to primary bile acid malabsorption (Oelkers et al., 1997). Asbt-null mice seem healthy and physically similar to wild-type mice (Dawson et al., 2003). Fecal bile acid excretion is increased in Asbt-null mice, leading to a reduced total bile acid pool (Dawson et al., 2003). The residual bile acid pool is enriched in cholic acid. Contrary to human primary bile acid malabsorption, Asbt-null mice do not experience steatorrhea (Dawson et al., 2003). This mouse model is useful for investigating only some clinical features of primary bile acid malabsorption. Asbt-null mice exhibit reduced ileal mRNA expression of farnesoid X receptor and fibroblast growth factor 15. It is noteworthy that treatment of Asbt-null mice with either a farnesoid X receptor agonist or with fibroblast growth factor 15 reduces fecal bile acid elimination (Jung et al., 2007).
Glycylsarcosine is a dipeptide that is transported by PEPT1 (Ganapathy et al., 1995). A key role for Pept1 in the intestinal uptake of peptides was confirmed in Pept1-null mice after oral and intravenous glycylsarcosine dosing (Hu et al., 2008). Plasma concentrations of glycylsarcosine are similar in wild-type and Pept1-null mice after intravenous administration but reduced in Pept1-null mice after oral administration (Hu et al., 2008). Likewise, intestinal uptake of glycylsarcosine is lower in everted jejunal rings from Pept1-null mice than in those from wild-type mice (Hu et al., 2008).
2. Apical Efflux Transporters in Intestine.
Mdr1a/Pgp is expressed at the apical surface of enterocytes (Panwala et al., 1998), where it effluxes drugs from enterocytes back into the intestinal lumen and thereby decreases the absorption of orally administered drugs. Mdr1a-null mice and/or Mdr1a/1b-null mice have increased oral absorption of vinblastine (Ogihara et al., 2006), fexofenadine (Tahara et al., 2005), and paclitaxel (Bardelmeijer et al., 2000). However, these findings do not extend to all known Pgp substrates. For example, the well studied Pgp substrate verapamil is not absorbed in Mdr1a/1b-null mice after oral dosing (Ogihara et al., 2006). Further work is needed to classify Pgp substrates that are relevant to the disposition of orally administered drugs. In addition to using Mdr1a/1b-null mice, the human colon cancer cell line Caco-2 is routinely used to assess intestinal transporter function using general and specific inhibitors of transporter isoforms (Balimane et al., 2006).
Recent work demonstrates that regional-specific expression of intestinal drug transporters is important for dictating the intestinal permeability of the gout drug colchicine. In the rat intestine, Pgp protein expression increases from the proximal to the distal intestine, whereas Mrp2 protein expression decreases. Using Mrp2 and Pgp inhibitors in in situ single-pass intestinal perfusion of the rat proximal jejunum and distal ileum, it was shown that Pgp and Mrp2 are responsible for the proximal and distal intestinal transepithelial transport of colchicine, respectively (Dahan et al., 2009).
Maintenance of Mdr1a-null mice under specific pathogen-free conditions initiates spontaneous intestinal inflammation (including dysregulated epithelial cell growth and leukocyte infiltration in the mucosa) similar to human inflammatory bowel disease (Panwala et al., 1998). Colitis is prevented in Mdr1a-null mice after oral treatment with broad-spectrum antibiotics, suggesting intestinal microflora are necessary for this phenotype (Panwala et al., 1998). Work by another group demonstrates that Mdr1a influences the relative abundance of a subset of intraepithelial T lymphocytes in the intestines of mice (Eisenbraun et al., 2000). The consequence of a shift in T lymphocyte population with regard to colitis is not currently understood (Eisenbraun et al., 2000). The link between defective Mdr1a expression and colitis is unclear but may be important in unraveling the mechanism(s) of inflammatory bowel disease. Initial evaluation of ABCB1 (MDR1) polymorphisms and inflammatory bowel disease has yielded mixed results (Ho et al., 2006a; Oostenbrug et al., 2006; Fiedler et al., 2007). A meta-analysis of the available findings suggests that the I1145I SNP in ABCB1 (MDR1) is significantly associated with ulcerative colitis (Table 19) (Annese et al., 2006).
A functional role for Bcrp in the efflux of drug conjugates into the intestinal lumen has been illustrated using in situ perfusion of intestinal segments from Bcrp-null mice (Adachi et al., 2005). Similar to biliary excretion patterns, the efflux of the glucuronide and sulfate conjugates of 4-methylumbelliferone is lower in Bcrp-null small intestine segments compared with wild-type counterparts (Adachi et al., 2005). Oral bioavailability of xenobiotics such as the novel kinase inhibitor JNJ-7706621, as well as the inflammatory bowel disease drug sulfasalazine, is limited by Bcrp in the intestine (Seamon et al., 2006; Zaher et al., 2006). Oral absorption of these drugs is increased in Bcrp-null mice or wild-type mice treated with Bcrp inhibitors (Seamon et al., 2006; Zaher et al., 2006). In support of these findings, humans with the Q141K ABCG2 (BCRP) polymorphism display increased oral bioavailability of sulfasalazine (Table 15) (Urquhart et al., 2008; Yamasaki et al., 2008). Design and development of novel therapeutics that require high oral bioavailability should be screened as potential Bcrp and Mdr1 substrates and modified to prevent intestinal efflux. There is an additional approach to bypass intestinal efflux by incorporating into oral drug formulations excipients that inhibit Bcrp function. For example, the excipients Pluronic P85 and Tween 20 improve intestinal absorption of the Bcrp substrate topotecan in wild-type but not Bcrp-null mice (Yamagata et al., 2007).
3. Basolateral Efflux Transporters in Intestine.
Ostα and Ostβ are heterodimeric organic solute transporters that efflux bile acids and conjugated steroids across the basolateral membrane of epithelial cells in a variety of tissues. Despite stable expression of Ostβ mRNA, the protein expression of Ostβ in the kidneys and ileum is absent in mice lacking Ostα (Li et al., 2007b). Therefore, Ostα-null mice are essentially a “double-null” model for Ostα and -β. Ostα-null mice are viable and fertile but display growth retardation (Ballatori et al., 2008). There are a variety of pathological findings in Ostα-null mice, including hypertrophy of the small intestine and reduced bile acid pool, serum bile acid, cholesterol, and triglyceride levels (Ballatori et al., 2008). Ostα-null mice exhibit a number of compensatory gene changes, including higher intestinal, hepatic, and renal Mrp3 and lower hepatic Cyp7a1 (rate-limiting bile acid synthetic enzyme) levels. Combined loss of Ostα and Mrp3 in female Ostα/Mrp3-null mice results in nearly absent transileal transport of taurocholate (Rao et al., 2008). Ostα-null mice will continue to be a useful model for investigating enterohepatic recirculation of bile acids.
Mrp1 is expressed in intestinal proliferative crypt cells (Peng et al., 1999). Mrp1-null mice exhibit enhanced sensitivity to the gastrointestinal toxicity of methotrexate, as exhibited by nearly complete loss of small intestinal villi (Kato et al., 2009). Although the plasma concentration and biliary excretion of methotrexate are unchanged, immature proliferative cells from Mrp1-null mice have higher methotrexate accumulation compared with wild type (Kato et al., 2009).
The antiviral drug ribavirin is used to treat hepatitis C infection. Using Ent1-null mice, it was demonstrated that Ent1 participates in the intestinal absorption of ribavirin as well as its distribution to erythrocytes (Endres et al., 2009a,b). Therefore, Ent1 permits not only the oral absorption of ribavirin but may also enable the hemolytic anemia observed in some patients prescribed this therapy. The functional in vivo significance of the remaining apical (Oct3, Cnt1–2, Oatps) and basolateral (Oct1 and Octn3) transporters in enterocytes is not fully understood.
D. Brain
A number of uptake and efflux transporters have been identified in the brain. Some of these transporters have been localized to the apical (Mdr1a/1b, Mrp1–5, Bcrp, Oatp1a5) and basolateral (Octn2, Oatp1a4, Oatp2b1, Oat3) membranes of capillary endothelial cells (Fig. 9). The choroid plexus is also rich with drug transporters including Oatps (1a1, 1a4, 1a5, 2b1), Oat3, Pept2, Cnt2, and Oct2 as well as the efflux transporters Mrp1, -3, -4, and Mdr1b (Fig. 9). In addition, transporters have been identified in other regions of the brain.
Fig. 9.
Subcellular localization of uptake and efflux transport proteins in brain capillary endothelial cells and choroid plexus epithelial cells. The localization and orientation of uptake and efflux transporters in the brain (primarily rodents) are shown.
1. Uptake Transporters in Brain.
Oct3 protein staining is observed in multiple regions of the brain, including neurons in the subfornical organ of mice (Vialou et al., 2004). As part of the central nervous system, the subfornical organ participates in fluid and electrolyte exchange. Oct3-null mice exhibit increased ingestion of hypertonic saline under dehydrated and salt appetite conditions (Vialou et al., 2004). In addition, the cellular response of the subfornical organ to salt deprivation (as detected by Fos immunoreactivity) is attenuated in Oct3-null mice (Vialou et al., 2004). Collectively, these findings suggest that Oct3 participates in the response of the central nervous system to sodium depletion and water deprivation.
Treatment of mice with methamphetamine stimulates locomotor activity. Reduced Oct3 protein expression after antisense oligonucleotide administration to mice is associated with markedly higher methamphetamine-induced locomotor activity. Enhanced locomotor activity is probably due to reduced clearance of monoaminergic neurotransmitters, and thus more neuronal stimulation (Kitaichi et al., 2005). Likewise, brains from Oct3-null mice exhibit higher extracellular dopamine concentrations and greater loss of striatal nerve terminals after methamphetamine administration compared with wild type (Cui et al., 2009a). Based on the observed altered methamphetamine handling in mice with reduced expression of Oct3 (Kitaichi et al., 2005), the allelic frequencies of SLC22A3 (OCT3) polymorphisms were determined in 213 Japanese subjects with methamphetamine dependence (Aoyama et al., 2006). Two intronic variants were associated with the development of polysubstance abuse in this population (Aoyama et al., 2006). Collectively, these data suggest a role for Oct3/OCT3 in the “uptake-2 system” of neurotransmitters in brain.
Oat3 and Pept2 are expressed on the apical membrane of the choroid plexus and participate in the extraction of chemicals from the cerebrospinal fluid into the choroid plexus epithelium (Sweet et al., 2002). Cellular uptake of the Oat3 substrate fluorescein is reduced in the choroid plexus of Oat3-null mice (Sweet et al., 2002). Likewise, levels of Pept2 substrates (cefadroxil, glycylsarcosine, 5-aminolevulinic acid, l-kyotorphin) are reduced in the choroid plexus of Pept2-null mice (Ocheltree et al., 2004b, 2005; Hu et al., 2007; Shen et al., 2007; Jiang et al., 2009). Moreover, the heme precursor 5-aminolevulinic acid is markedly elevated in the cerebrospinal fluid of Pept2-null mice, leading to enhanced neurotoxicity and mortality (Hu et al., 2007). These data indicate roles for Oat3 and Pept2 in limiting the accumulation of xenobiotics in the cerebrospinal fluid.
Adenosine is an inhibitor of mouse Ent1 function and is thought to be a substrate (Kiss et al., 2000). ENT1 protein is localized to multiple regions of the human brain, including the frontal and parietal lobes of the cerebral cortex, thalamus, midbrain, and the basal ganglia and colocalized to regions rich with the adenosine A1 receptor (Jennings et al., 2001). Because adenosine is important in ethanol intoxication, Ent1-null mice were generated and have been shown to consume greater amounts of ethanol than wild-type mice (Choi et al., 2004). Ent1-null mice exhibit reduced hypnotic and ataxic responses in response to ethanol (Choi et al., 2004). Similar to modulating ethanol consumption, activation of adenosine receptors reduces anxiety-like behavior; therefore, the behavior of mice lacking Ent1 was tested in an open field and an elevated plus maze (Chen et al., 2007a). It is noteworthy that mice lacking Ent1 exhibit reduced anxiety-like behavior (Chen et al., 2007a). Microinjection of an ENT1 antagonist into the brains of wild-type mice also decreases anxiety-like behavior (Chen et al., 2007a). Likewise, Oct3-null mice are significantly less anxious than wild-type mice, possibly by altering serotonin signaling in the brain (Wultsch et al., 2009). Together, ENT1 and OCT3 may be important in stress and anxiety by modulating endogenous neurotransmitters.
2. Efflux Transporters in Brain.
Expression of Mdr1a/1b as well as other transporters (such as Bcrp) in the blood-brain barrier restricts the accumulation of chemicals in the brain. These transporters actively efflux chemicals across the luminal surface of blood vessels (Schinkel et al., 1994; Lee et al., 2005b). Mdr1a-null mice were first developed in 1994 (Schinkel et al., 1994). Early studies highlighted the critical role of Pgp in protecting the brain from xenobiotics. Brain concentrations of the pesticide ivermectin and the anticancer drug vinblastine are elevated in Mdr1a-null mice (Schinkel et al., 1994; van Asperen et al., 1996). In turn, Mdr1a-null mice are highly susceptible to the toxicity of ivermectin as well as vinblastine (Schinkel et al., 1994).
Because of the redundancy of Mdr1a and Mdr1b transporters, mice lacking both transporters simultaneously were developed to investigate their collective transport of substrates identified in in vitro systems (such as the antiviral drug oseltamivir, the anticholesterol drug cerivastatin, and the anticancer drug paclitaxel) (Ose et al., 2008). The concentrations of oseltamivir, cerivastatin, and paclitaxel are elevated in the brains of Mdr1a/1b-null mice (Gallo et al., 2003; Kivistö et al., 2004; Ose et al., 2008). Likewise, long-term oxycodone administration to rats increases brain Pgp protein expression, thereby reducing accumulation of paclitaxel in the brain (Hassan et al., 2007). These findings suggest that opiate-mediated induction of brain efflux transporters such as Pgp may be a source of drug-drug interactions, leading to reduced chemotherapeutic drug penetration into the brain (and possibly other tissues). Likewise, accumulation of the cardiac glycoside digoxin is markedly enhanced in the brains of Mdr1a-null and Mdr1a/1b-null mice compared with wild-type mice (Schinkel et al., 1997; Kawahara et al., 1999). Despite these intriguing digoxin findings in null mice, clinical pharmacokinetic analysis of the I1145I ABCB1 (MDR1) variant has yielded conflicting findings with regard to the disposition of a single oral digoxin dose (Table 19) (Hoffmeyer et al., 2000; Sakaeda et al., 2001; Morita et al., 2003b; Verstuyft et al., 2003; Chowbay et al., 2005).
The antihistamine drug fexofenadine is transported by MDR1 (Cvetkovic et al., 1999). When administered orally, plasma levels of fexofenadine are 6-fold higher in Mdr1a/1b-null mice compared with wild type (Tahara et al., 2005). The brain:plasma ratio of fexofenadine is elevated 3-fold in Mdr1a/1b-null mice (Tahara et al., 2005). Fexofenadine disposition is likewise altered in individuals with MDR haplotypes. Consistent with enhanced in vitro transport activity, the Ala893Ser ABCB1 (MDR1) variant is associated with reduced plasma fexofenadine concentrations (Table 19) (Kim et al., 2001). Other investigations have observed disparate results regarding ABCB1 genotype and fexofenadine disposition (Drescher et al., 2002; Yi et al., 2004).
The pharmacokinetics of corticosteroids such as cortisol, corticosterone, aldosterone, and progesterone are altered when administered exogenously to Mdr1a/1b-null mice (Uhr et al., 2002). In the absence of Mdr1a/1b, the four corticosteroids accumulate in mouse brains (Uhr et al., 2002). Moreover, it is hypothesized that Pgp influences the hypothalamic-pituitary-adrenal axis signaling by preventing access of glucocorticoids to the brain (Pariante et al., 2001, 2003). It is thought that antidepressants inhibit Pgp activity and in turn enhance glucocorticoid receptor function (Pariante et al., 2001, 2003). Administration of the antidepressant desipramine for 7 days induces mRNA expression of the glucocorticoid receptor in the brains of wild-type, but not Mdr1a-null mice (Yau et al., 2007). Induction of the glucocorticoid receptor corresponds with a decrease in corticosterone levels in desipramine-treated wild-type mice (Yau et al., 2007). From these findings, the authors suggest that Pgp is a molecular target of antidepressant regulation of the hypothalamic-pituitary-adrenal axis (Yau et al., 2007).
Pharmacological inhibition studies and transporter-null mice demonstrate that Mdr1a/1b and Bcrp play distinct and overlapping functions in pumping chemicals from the brain. One of the first pieces of evidence suggesting a link between these transporters in the brain is that the mRNA expression of Bcrp is elevated 3-fold in the brain microvessels of Mdr1a-null mice compared with wild type (Cisternino et al., 2004). Uptake of vinblastine is higher in Mdr1a-null mice compared with wild-type mice, with little change in the accumulation of prazosin and mitoxantrone (Cisternino et al., 2004). Likewise, Bcrp-null mice do not exhibit altered mitoxantrone uptake into the brain (Lee et al., 2005b). Inhibition of Pgp and Bcrp using the dual inhibitor GF120918 in Mdr1a-null mice increases uptake of prazosin and mitoxantrone, suggesting that Bcrp partially mediates transport of these two compounds from the brain capillary endothelial cells (Cisternino et al., 2004). Overlapping functions for Bcrp and Mdr1a/1b have also been demonstrated for the anticancer drug topotecan. Topotecan plasma concentrations are higher in Bcrp-null mice administered topotecan orally (Jonker et al., 2000). There is a slight increase in the brain concentrations of topotecan in Mdr1a/1b-null and Bcrp-null mice over wild-type mice, whereas triple null Bcrp/Mdr1a/1b-null mice exhibit a 12-fold increase in topotecan in the brain (de Vries et al., 2007).
Studies on tyrosine kinase inhibitors are an active area of research as anticancer drugs. Access of tyrosine kinase inhibitors to the brain is quite limited; therefore, these compounds do not seem to be viable therapeutic options for brain tumors. A better characterization of the transporters responsible for extrusion of tyrosine kinase inhibitors from the brain may lead to the development of transporter inhibitors that permit accumulation of anticancer drugs in the brain. Uptake of imatinib into the brain is enhanced in mice lacking Bcrp or Mdr1a/1b (Dai et al., 2003; Breedveld et al., 2005). The oral bioavailability and plasma concentrations of the tyrosine kinase inhibitor, erlotinib are higher in Mdr1a/1b/Bcrp-null mice (Marchetti et al., 2008). Likewise, another tyrosine kinase inhibitor, lapatinib, is also a substrate of MDR1 and BCRP (Polli et al., 2008). Mdr1a/1b-null mice exhibit a 3- to 4-fold increase in the brain-to-plasma ratio of lapatinib after intravenous infusion, with little change in Bcrp-null mice (Polli et al., 2009). Remarkably, triple-null mice lacking Mdr1a/1b and Bcrp have a 40-fold elevation in the brain-to-plasma ratio of lapatinib, suggesting a synergistic role of these transporters in limiting entry into the brain (Polli et al., 2009). Similar findings have been observed for imatinib and dasatinib (Lagas et al., 2009; Oostendorp et al., 2009). A polymorphism in ABCG2 (BCRP) (Q141K) is clinically associated with diarrhea as well as elevated concentrations in heterozygous patients receiving another tyrosine kinase inhibitor, gefitinib (Table 15) (Cusatis et al., 2006; Li et al., 2007a).
Initial evidence suggests novel roles for Pgp and Bcrp in the removal of amyloid-β, a hallmark of Alzheimer disease, from the brains of mice. The clearance of exogenously administered amyloid-β is reduced in the brains of Mdr1a/1b-null mice (Cirrito et al., 2005) as well as Bcrp-null mice (Xiong et al., 2009). Furthermore, administration of a Pgp inhibitor to transgenic mice that overexpress amyloid precursor protein stimulates accumulation of amyloid beta in the brain (Cirrito et al., 2005). Crossbreeding of Mdr1a/1b-null mice and amyloid precursor protein transgenic mice yields mice with higher levels of brain amyloid beta (Cirrito et al., 2005). In addition, not only do Alzheimer's patients with cerebral amyloid angiopathy overexpress BCRP mRNA and protein, but it has been shown that BCRP protein coprecipitates with amyloid-β in diseased tissue samples compared with age-matched controls (Xiong et al., 2009). These initial observations are very exciting and may reveal novel risk factors for accumulation of amyloid beta and the development of Alzheimer's disease.
The protective role of MDR1 at the blood-brain barrier led to the hypothesis that individuals with defective MDR1 might be at higher risk for the development of Parkinson's disease as a result of an impaired ability to remove environmental contaminants from the brain (Furuno et al., 2002). Although there is not a statistically significant association between screened ABCB1 (MDR1) polymorphisms and Parkinson's disease, there was a consistent trend for early-onset Parkinson's disease in patients with the I1145I ABCB1 variant (Table 19) (Furuno et al., 2002; Droździk et al., 2003). Conversely, there are two studies that suggest the I1145I ABCB1 variant protects against the development of Parkinson's disease (Lee et al., 2004a; Tan et al., 2005). Further studies are clearly needed to better delineate the role of MDR1 in Parkinson's disease.
Imaging techniques for transporter function and/or inhibition at the blood-brain barrier is an area of active research. Using positron emission tomography, it has been shown that administration of the Pgp inhibitor cyclosporine A to pregnant macaques increases the penetration of radiolabeled verapamil (Pgp substrate) into brain (Eyal et al., 2009). This imaging modality may be important in screening chemicals for Mdr1a/1b inhibition. An in vivo method to analyze MRP1 function using positron emission tomography and the probe 6-bromo-7-[11C]methylpurine was also recently developed (Okamura et al., 2009). Upon uptake into the brain, 6-bromo-7-[11C]methylpurine is conjugated with glutathione. Efflux of the glutathione conjugate is then monitored as an indicator of MRP1 function. Specificity of this biotracker probe was confirmed using Mrp1-null mice. The efflux rate of 6-bromo-7-[11C]methylpurine-glutathione from the brain is reduced markedly in Mrp1-null mice (Okamura et al., 2009).
E. Ears
Pgp protein is expressed in capillary endothelial cells of not only the brain but also the ear (Zhang et al., 2000). Doxorubicin and vinblastine drug levels are elevated in brains, inner ears, and small intestines of Mdr1a/1b-null mice (Zhang et al., 2000). It is noteworthy that doxorubicin- and vinblastine-treated Mdr1a/1b-null mice showed hearing impairment, whereas wild-type mice did not (Zhang et al., 2000). Likewise, enhanced ototoxicity could be achieved in wild-type mice using pharmacological inhibitors of Pgp transport (Zhang et al., 2000).
ATP8b1 is expressed in the stereocilia of cochlear hair cells (Stapelbroek et al., 2009). Loss of ATP8b1/ATP8B1 expression in null mice as well as in patients with PFIC-I causes hearing loss (Stapelbroek et al., 2009). Progressive degeneration of cochlear hair cells was responsible for hearing loss in ATP8b1-null mice (Stapelbroek et al., 2009). It is thought that ATP8B1 deficiency might alter the phospholipid concentration in the inner membrane leaflet of the hair structure and thereby interfere with mechanotransduction.
There are two types of human earwax: dry and wet. The frequency of dry earwax is highest among East Asians, whereas wet earwax is observed in other populations. It is noteworthy that dry earwax has been linked to a polymorphism (G180R) in ABCC11 (MRP8) that is more prevalent in persons of Chinese and Korean descent (Yoshiura et al., 2006; Kitano et al., 2008). An additional 27-base-pair deletion in the ABCC11 gene is also associated with dry earwax (Yoshiura et al., 2006; Kitano et al., 2008). Subsequently, the G180R variant in ABCC11 was associated with not only dry earwax but also lower colostrum production in Japanese women (Miura et al., 2007).
F. Lungs
Acetylcholine is a substrate of OCT1 and OCT2 but not OCT3 (Lips et al., 2005). As expected, airway epithelial acetylcholine content is higher in Oct1/2-null mice (Kummer et al., 2006). The functional consequence of this phenotype is not yet understood.
Corticosterone treatment reduces serotonin-induced bronchoconstriction. It is noteworthy that this pharmacological property is impaired in Oct3-null mice, providing novel information about the mechanism of action of corticosteroids (Kummer et al., 2006). Furthermore, OCT3 has also been implicated in the disposition of the long-acting β2-agonist formoterol in human lung tissue (Horvath et al., 2007). Taken together, combination therapy of formoterol and corticosteroids probably elicits synergistic activity with steroids inhibiting OCT3-mediated formoterol transport, thereby causing formoterol accumulation and prolonged bronchodilator action (Horvath et al., 2007).
Recent work suggests that OATP2B1 is responsible for uptake of amiodarone into lung alveolar epithelial type II cells (Seki et al., 2009). Knockdown of OATP2B1, using small interfering RNA, reduces amiodarone influx in vitro (Seki et al., 2009). Further studies are needed to determine whether OATP2B1-mediated transport permits accumulation of amiodarone in the lungs in vivo and subsequent pulmonary toxicity.
G. Heart
mRNA profiling of human heart samples demonstrate expression of transporters including BCRP, MDR1, MRP2, -4, -5, and -7, OATP2A1 and -2B1, OCT3, OCTN1 and -2 as well as OCTN2 in the atria and ventricles (Grube et al., 2006b; McBride et al., 2009). However, the exact localization of most of these transporters is unclear. Initial in vivo studies using Octn2-null and Mrp1-null mice and in vitro studies of OCTN1 suggest roles for these transporters in the heart.
Mouse Octn1 and Octn2 proteins are present on blood vessel endothelial cells and cardiac muscle cells, respectively (Iwata et al., 2008). Human OCTN1 protein is expressed in cardiomyocytes (McBride et al., 2009). The rare ventricular arrhythmia known as torsades de pointes is associated with a prolonged QT interval on an electrocardiogram that results from antagonism of the HERG potassium channel. QT prolongation is associated with the use of drugs such as quinidine (OCTN1 substrate). Coexpression of OCTN1 and the HERG channel facilitates the HERG blockade of a number of antiarrhythmic drugs pointing to a potential role for drug transporters as a contributing factor for torsades de pointes (McBride et al., 2009).
Mutations in SLC22A5 (OCTN2) were shown to be responsible for primary carnitine deficiency (Nezu et al., 1999; Wang et al., 1999). Primary carnitine deficiency is a disorder of fatty acid oxidation and is inherited through autosomal recessive transmission (Table 10). Patients with carnitine deficiency exhibit encephalopathy, progressive cardiomyopathy, hypoglycemia, and skeletal myopathy. More than 15 mutations in SLC22A5 have been associated with primary carnitine deficiency (Koizumi et al., 1999; Seth et al., 1999; Wang et al., 1999, 2000a,b; Mayatepek et al., 2000; Cederbaum et al., 2002; Spiekerkoetter et al., 2003; Amat di San Filippo and Longo, 2004; Makhseed et al., 2004; Melegh et al., 2004). One of these mutations (P478L) causes complete loss of carnitine uptake but still retains transport activity of organic cations (Seth et al., 1999). Therefore, patients with primary carnitine deficiency may have normal organic cation transport function (Seth et al., 1999).
Octn2 mutant mice are also known as juvenile visceral steatosis (JVS) mice. JVS mice are deficient in carnitine, which leads to defects in fatty acid oxidation. As a result, fat accumulates in the internal organs (viscera) of JVS mice. DNA sequencing of JVS mice has revealed a missense mutation from leucine to arginine at codon 352 in the sixth transmembrane domain of Octn2 (Lu et al., 1998). The Octn2 mutation is associated with impaired l-carnitine uptake into isolated JVS mouse hepatocytes (Yokogawa et al., 1999). Heterozygous Octn2 mice are viable and fertile, but Octn2 mutant mice survive for only approximately 3 to 4 weeks without carnitine supplementation (Shekhawat et al., 2004). At 3 weeks of age, the body weight of Octn2 mutant mice is 50% of that of age-matched wild-type mice (Shekhawat et al., 2004). Deprivation of carnitine supplements from Octn2 mutant mice between 6 and 8 weeks of age markedly reduces plasma β-hydroxybutyrate levels and skeletal muscle glycogen stores (Knapp et al., 2008). Consistent with carnitine deprivation and altered fatty acid oxidation, Octn2 mutant mice develop enlarged fatty liver with steatosis (Shekhawat et al., 2004; Knapp et al., 2008).
Carnitine uptake into heart slices is blocked by Octn2 inhibitors and is absent in slices from JVS mice (Iwata et al., 2008). Over time, Octn2 mutant mice develop dilated cardiomyopathy (Shekhawat et al., 2004). Because of the severe pathological condition in Octn2 mutant mice, Octn2 heterozygous mice have been used to investigate whether Octn2 mutations might alter the cardiomyopathy risk of individual subjects (Takahashi et al., 2007). In one study, Octn2(+/−) mice were subjected to ascending aortic constriction and evaluated 4 weeks later (Takahashi et al., 2007). Compared with wild-type mice, Octn2(+/−) mice had more pronounced cardiac hypertrophy and pulmonary congestion, deterioration of left ventricular fractional shortening, and higher mortality (Takahashi et al., 2007). It is noteworthy that l-carnitine supplementation prevented these changes in Octn(2+/−) mice (Takahashi et al., 2007). Long-term studies of Octn2(+/−) mice demonstrate age-associated cardiomyopathy after 2 years of age similar to that of Octn2 mutant mice at a younger age (Xiaofei et al., 2002).
Mrp1 is expressed in sarcolemmal membranes and the mitochondria of the mouse heart (Jungsuwadee et al., 2006, 2009). Early work by Ishikawa et al. (1986, 1989) demonstrated the ATP-dependent transport of leukotriene C4 and the glutathione conjugate of 4-hydroxynonenal in the heart via a glutathione S-conjugate transporter later identified as Mrp1. Hypertension induced by angiotensin II leads to an imbalance in glutathione homeostasis with elevated transport of oxidized glutathione and lower vascular levels of reduced glutathione (Widder et al., 2007). It is noteworthy that Mrp1-null mice demonstrate more favorable cardiovascular parameters after angiotensin II administration compared with wild type (Widder et al., 2007). Angiotensin II-induced increases in superoxide production and blood pressure are blunted in Mrp1-null mice (Widder et al., 2007). A critical role for Mrp1 in angiotensin II-induced hypertension may be via regulation of intracellular thiol concentrations (Leier et al., 1996). More recently, it has been suggested that Mrp1 protects the murine heart from doxorubicin-induced cardiotoxicity (Jungsuwadee et al., 2006, 2009).
H. Placenta
In an effort to understand the protection of the fetus from xenobiotic accumulation and toxicity, researchers are actively characterizing the expression of transporters in the placenta as well as supportive tissues, including the fetal membranes. Within the placenta, transporters are most often expressed in syncytiotrophoblasts. Basolateral uptake transporters Oat4, Oct3, and OATP2B1 and apical efflux transporters Mdr1a/1b, Mrp1–3, Bcrp, and ATP7B translocate chemicals from the fetus to the maternal blood (Fig. 10). Octn2, Ent1, and OATP4A1 seem to be more important in the transport of substrates from the maternal to the fetal blood. Recent attention has been placed on the presence of xenobiotic transporters in the human amnion membrane and the rodent yolk sac. For the most part, only efflux transporters (Mdr1a, Mrp1–2, Mrp4–6, Bcrp) seem to be expressed in the fetal membranes (Aye et al., 2007; Aleksunes et al., 2008b; Yeboah et al., 2008). Although the exact contribution of these transporters to fetal protection is not known, it can be hypothesized that Mdr1a, Mrp2, and Bcrp would be most important in fetal protection by transferring chemicals from fetal to maternal blood.
Fig. 10.
Subcellular localization of uptake and efflux transport proteins in syncytiotrophoblasts and fetal membranes. The localization and orientation of uptake and efflux transporters in placenta and fetal membranes (primarily rodents) are shown.
Because milk is rich in lipids, it is presumed that carnitine is important in preparing the fetus for a postnatal milk diet. Human OCTN2 protein is localized to the apical membrane of syncytiotrophoblasts (Lahjouji et al., 2004; Grube et al., 2005). Uptake of carnitine into apical placenta membrane vesicles is sodium-dependent and is inhibited by verapamil (Lahjouji et al., 2004; Grube et al., 2005). Therefore, it is thought that OCTN2 is responsible for delivery of carnitine to the fetus during development. The placentas and fetuses of Octn2 mutant mice contain reduced carnitine levels (Shekhawat et al., 2004). In an attempt to compensate for the loss of Octn2-mediated uptake of carnitine, β-oxidation enzymes are up-regulated in Octn2 mutant placenta (Shekhawat et al., 2004). This compensatory response may be an attempt to maintain the energy status of the placenta.
Use of certain anticonvulsants during pregnancy carries a risk of fetal malformations, including congenital heart disease as well as lip and palatal deformity. Using apical membrane vesicles from human placenta, carnitine uptake is inhibited by a number of antiseizure drugs (Wu et al., 2004). It is hypothesized that interference of OCTN1 and/or OCTN2-mediated carnitine uptake across the placenta by antiseizure drugs contributes to fetal anticonvulsant syndrome. In support of this hypothesis, cells transfected with the SLC22A4 (OCTN1) L503F variant have reduced sodium-independent uptake of the antiseizure drug gabapentin compared with wild-type OCTN1 (Table 18) (Urban et al., 2008).
Mdr1a/1b and MDR1 mRNA and protein are prominently expressed in mouse and human placenta and their expression decreases during gestation (Lankas et al., 1998; Ushigome et al., 2003; Aleksunes et al., 2008b; Cui et al., 2009c). Studies using brush border membrane vesicles from human placentas demonstrated functional Pgp activity that was blocked by Pgp inhibitors (Ushigome et al., 2003). Likewise, several ABCB1 (MDR1) polymorphisms are associated with reduced placental Pgp expression (Tanabe et al., 2001; Hitzl et al., 2004). Use of genetically engineered Mdr1a/1b-null mice and naturally occurring Mdr1a mutant (CF-1) mice has highlighted the protective role of Mdr transporters in the placenta (Umbenhauer et al., 1997; Smit et al., 1999; Pippert and Umbenhauer, 2001). CF-1 mice lacking Mdr1a develop cleft palate after fetal exposure to the teratogenic pesticide avermectin (Lankas et al., 1998). Likewise, fetal penetration of the cardiac glycoside digoxin, the antiviral drug saquinavir, and the chemotherapeutic agent paclitaxel is enhanced in Mdr1a/1b-null mice (Smit et al., 1999). Similar to knockout mice, when pregnant macaques are administered the Pgp inhibitor cyclosporine A, the penetration of radiolabeled verapamil (Pgp substrate) into maternal brain and fetal livers increases (Eyal et al., 2009). Active efflux of potentially toxic chemicals from the fetus to the mother via Pgp may represent a protective mechanism to limit drug-induced birth defects. The consequences of reduced Pgp expression in ABCB1 variants in the fetal to maternal transfer of xenobiotics need to be determined.
Bcrp mRNA and protein is abundantly expressed in the placenta and fetal membranes (visceral yolk sac in mice) in humans and rodents (Maliepaard et al., 2001; Grube et al., 2007; Aleksunes et al., 2008b; Cygalova et al., 2008; Yeboah et al., 2008). In both tissues, Bcrp faces the mother, suggesting that this transporter pumps chemicals from the fetus to the mother. This was first documented after topotecan accumulated in Bcrp-null fetuses to a greater extent than in wild-type fetuses (Jonker et al., 2000). It is noteworthy that a variant ABCG2 (BCRP) gene (Q141K) is associated with reduced BCRP protein accumulation in placenta, although the functional relevance of this polymorphism has not yet been determined (Table 15) (Kobayashi et al., 2005b).
The use of glyburide to treat gestational diabetes has led investigators to question how the fetus maintains very low concentrations of glyburide. In vitro transport studies demonstrate that inhibitors of MRP1–3 and Pgp are ineffective in preventing glyburide transport across the placental membrane (Gedeon et al., 2008a,b). Instead, inhibition of BCRP disrupts glyburide efflux (Gedeon et al., 2008b). In support of this, glyburide concentrations are higher in Bcrp-null fetuses compared with wild type (Zhou et al., 2008b). Likewise, the concentration of the antibiotic nitrofurantoin is elevated 5-fold in fetuses from Bcrp-null mice (Zhang et al., 2007c). These findings are of interest because glyburide and nitrofurantoin are routinely prescribed for pregnant patients for the treatment of gestational diabetes and urinary tract infections, respectively. So far, only one BCRP variant has been screened in healthy subjects administered nitrofurantoin, and it was not associated with altered pharmacokinetics (Adkison et al., 2008). Additional work is imperative to decipher the roles of Mrp as well as Oat/Oatp transporters in the placenta as well as delineate the contribution of efflux transporters in the fetal membranes.
I. Mammary Glands
The ability of the mammary gland to concentrate pharmaceuticals such as nitrofurantoin and cimetidine in human breast milk has been recognized (Oo et al., 1995; Gerk et al., 2001). More recently, it was hypothesized that drug transporters may be important in the active transport of chemicals into breast milk. Studies have shown that Bcrp is localized to the apical epithelial surface of the mammary glands of humans, sheep, cows, and rats (Maliepaard et al., 2001; Pulido et al., 2006; Wang et al., 2008b). Pharmacological inhibition of Bcrp using GF120918 blocks excretion of nitrofurantoin into milk of lactating rats (Wang et al., 2008b). It should be noted that Pgp is not expressed in lactating mammary glands; in turn, GF120918-mediated inhibition probably occurs via Bcrp (Wang et al., 2008b). The bioavailability of nitrofurantoin is elevated in Bcrp-null mice largely as a result of markedly reduced hepatobiliary excretion and milk secretion (Merino et al., 2005b). The milk-to-plasma ratio of nitrofurantoin is 80-fold higher in wild-type mice than in Bcrp-null mice (Merino et al., 2005b). Likewise, Bcrp transports fluoroquinolone antibiotics such as ciprofloxacin (Merino et al., 2006). Bcrp-null mice exhibit higher plasma concentrations of ciprofloxacin and a reduced milk-to-plasma ratio compared with wild-type mice (Merino et al., 2006). Endogenous molecules such as riboflavin are also actively transported into breast milk by Bcrp in the mammary gland (van Herwaarden et al., 2007). In addition to excreting pharmaceuticals and nutrients, Bcrp also transfers dietary carcinogens (aflatoxin B1 and other heterocyclic amines) into breast milk (Jonker et al., 2005b; van Herwaarden et al., 2006). Milk concentrations of aflatoxin are lower in Bcrp-null mice; instead, aflatoxin accumulates in the maternal plasma, lungs, kidneys, and brain of Bcrp-null mice (van Herwaarden et al., 2006). Bcrp function in the mammary glands is somewhat of a double-edged sword; it prevents xenobiotic accumulation in the mother and simultaneously delivers potentially toxic chemicals to newborns.
J. Testes
Transporters in the testes have been implicated in the physiological maintenance of sperm as well as the protection against xenobiotic accumulation and toxicity (Fig. 11). Carnitine is required for sperm formation, sperm maturation, and maintenance of sperm quality. Uptake of carnitine and acetylcarnitine into epididymal spermatozoa over time can be inhibited by OCTN2 substrates (Kobayashi et al., 2007). Immunohistochemical analysis demonstrates Octn2 in the distal portion of the sperm tail and Octn3 in the proximal portion of the sperm tail (near the nucleus) (Kobayashi et al., 2007). Octn2 is expressed in rat whole testes, epididymal epithelia, and primary-cultured Sertoli cells (Kobayashi et al., 2005a,c, 2007). Furthermore, Octn2 mutant mice develop obstructive azoospermia in the distal portion of the epididymis (Yakushiji et al., 2006). There are currently no reports on the relationship between OCTN2 genotype and male infertility.
Fig. 11.
Subcellular localization of uptake and efflux transport proteins in Sertoli and epididymal cells. The localization and orientation of uptake and efflux transporters in testes (primarily rodents) are shown.
Efflux transporters in the testes are probably important in restricting xenobiotic entry across the blood-testes barrier. Bcrp limits the in vivo accumulation of various pharmaceuticals (including dantrolene, triamterene, and prazosin) within the testes (Enokizono et al., 2008). Using Mrp1-null mice, it was demonstrated that methoxychlor-induced testicular damage is precipitated by the absence of Mrp1 in the testes (Tribull et al., 2003). Mrp1-null mice exhibit a reduced number of developing spermatocytes at a dose of methoxychlor that has little effect on the testes of wild-type mice (Tribull et al., 2003).
Additional xenobiotic transporters (Mrp5, Mrp7, Mrp8, Ent1–2, Oatp1a5, Oat2) have been identified by mRNA profiling in rodent testes (Augustine et al., 2005). It is anticipated that we will learn more about their function in testicular homeostasis and protection in upcoming years.
K. Immune and Stem Cells
Roles for transporters in a variety of immune and stem cells have been shown. Antiviral drug transport seems to be dictated by Mrp4, Bcrp, Oct1, and Oct2 in different cell types. In addition, Mrp1 expression alters susceptibility to infectious agents. With regard to inflammation, Octn1–2 and Mrp1 play unique roles. Transporters such as Mrp2 and Mrp4 are important in the disposition of immunosuppressing drugs. Finally, efflux transporters including Bcrp and Mdr1a/1b are expressed in stem cells and may confer chemoresistance to this cell population.
1. Infections.
Early studies demonstrated that MRP4 overexpression strongly correlated with drug resistance against the purine nucleotide analog antiviral 9-(2-(phosphomethoxy)ethyl)-adenine (PMEA, also known as adefovir) in a human T-lymphoid cell line (Schuetz et al., 1999). Two ABCC4 variants (G187W, G487E) demonstrate reduced in vitro MRP4 function as shown by higher intracellular accumulation of zidovudine and PMEA (Table 20) (Abla et al., 2008). In the case of the G187W variant, lower MRP4 function was due to reduced protein (Abla et al., 2008).
Evaluation of single- and double-null mice demonstrates that Mrp4 and Bcrp work in concert to transport PMEA in vivo. Pathological injury to the bone marrow, spleen, thymus, and gastrointestinal tract as well as lethality are increased in Mrp4-null mice treated with PMEA (Belinsky et al., 2007). PMEA markedly accumulates in the spleens and brains of Mrp4-null mice (Belinsky et al., 2007; Takenaka et al., 2007). It is noteworthy that Bcrp mRNA expression was enhanced in the spleens as well as the brains of Mrp4-null mice (Takenaka et al., 2007). Because of these findings, the role of Bcrp in dictating PMEA accumulation was investigated. Neither Mrp4-null nor Bcrp-null mice exhibited altered PMEA concentrations in livers, kidneys, or lungs (Takenaka et al., 2007). However, PMEA accumulated in the livers, kidneys, and lungs of Mrp4/Bcrp-null mice, suggesting that Mrp4 and Bcrp can each compensate for the other to enable transport of PMEA in single-null mice (Takenaka et al., 2007). These studies demonstrate how multiple organic anion transporters protect against purine analog toxicity and resistance.
OCT1- and OCT2-mediated transport of 1-methyl-4-phenylpyridinium is inhibited by the protease inhibitors nelfinavir, ritonavir, saquinavir, indinavir, and reverse transcriptase inhibitors lamivudine and zalcitabine (Jung et al., 2008). Lamivudine and zalcitabine are also transported by OCT1 and OCT2 (Jung et al., 2008). Expression of OCT1 and OCT2 mRNA is low in human lymph nodes from persons not infected with HIV but markedly increased in persons who are infected with HIV (Jung et al., 2008). It is hypothesized that OCT1 and OCT2 up-regulation may enhance delivery of antiviral drugs to infected cells.
Intranasal infection of Mrp1-null mice with Mycobacterium tuberculosis at 5 weeks of age results in an early increase in outgrowth of M. tuberculosis in the lungs and liver compared with wild-type mice (Verbon et al., 2002). However, by 4 months, the extent of mycobacterial outgrowth and animal survival is similar between genotypes, suggesting only an early role for Mrp1 in the immune response to infection (Verbon et al., 2002). In contrast, Mrp1-null mice are resistant to infection by Streptococcus pneumoniae, as demonstrated by diminished outgrowth of pneumococci in lungs and dramatically reduced mortality (Schultz et al., 2001). Whereas leukotriene C4 levels are reduced in the bronchial alveolar lavage fluid of pneumococcal-infected Mrp1-null mice, the concentrations of leukotriene B4 are elevated to levels seen in wild-type mice (Schultz et al., 2001). Given the divergent findings between mycobacterial and streptococcal exposure in Mrp1-null mice, further research is needed to understand the mechanisms of Mrp1 function during infection.
2. Inflammation.
Prior work has demonstrated a strong genetic component to the inflammatory disease rheumatoid arthritis. OCTN1 mRNA is detected in human spleen, bone marrow, and blood cells with levels higher than those observed in the kidneys (Tokuhiro et al., 2003). OCTN1 and 2 mRNA are detected in fibroblast-like synoviocytes from persons with rheumatoid arthritis. It is noteworthy that stimulation of these synoviocytes with tumor necrosis factor-α increases OCTN1 mRNA (Tokuhiro et al., 2003). Likewise, Octn1 mRNA is detected in the joints of mice with collagen-induced arthritis but not in normal mice (Tokuhiro et al., 2003). In the Japanese population, there is a significant association between rheumatoid arthritis and SLC22A4 (OCTN1) polymorphisms (Tokuhiro et al., 2003). Intron (slc2F1, slc2F2) variants of SLC22A4 are associated with rheumatoid arthritis in Japanese patients but not British, Spanish, or Canadian patients (Tokuhiro et al., 2003; Barton et al., 2005; Newman et al., 2005; Martínez et al., 2006; Takata et al., 2008).
Mrp1 has been implicated in inflammatory and infectious diseases because of its expression on activated Th1 lymphocytes (Prechtl et al., 2000) and its ability to transport signaling mediators (such as leukotrienes and glutathione) (Leier et al., 1996; Wijnholds et al., 1997). Although Mrp1 is expressed on activated Th1 lymphocytes, it is not necessary for activation of this immune cell population (Kleemann et al., 2006). Mrp1-null mice have an impaired response to inflammatory stimuli as a result of defective leukotriene secretion (Wijnholds et al., 1997). Topical administration of arachidonic acid to mice causes ear edema and increases vascular permeability. It is noteworthy that Mrp1-null mice demonstrate attenuated responses to arachidonic acid application (Wijnholds et al., 1997). Likewise, triple-null mice lacking Mrp1 as well as Mdr1a and Mdr1b are resistant to chronic obstructive pulmonary disease-like features after exposure to cigarette smoke (van der Deen et al., 2007). Pulmonary cytokine levels as well as inflammatory cell infiltration are elevated in smoke-exposed wild-type mice with little to no change in Mrp1/Mdr1a/1b-null mice (van der Deen et al., 2007). Collectively, these models suggest a role for Mrp1 in inflammation in rodents.
3. Immunosuppression.
Mycophenolic acid and its prodrug mycophenolate mofetil are immunosuppressant drugs prescribed for transplant recipients. The biliary excretion of mycophenolic acid glucuronide is reduced in TR− and Eisai hyperbilirubinemic rats, which lack Mrp2 function (Kobayashi et al., 2004; Westley et al., 2006). In addition, inhibition of Mrp2 function using cyclosporine A similarly reduces mycophenolic acid-glucuronide clearance into bile (Kobayashi et al., 2004). Patients who have undergone renal allografting who are receiving a mycophenolic acid-containing rejection regimen exhibit altered drug pharmacokinetics and experience more diarrhea if they possess the ABCC2 C-24T variant (Table 20) (Naesens et al., 2006). The V417I ABCC2 variant is also associated with higher plasma concentrations of mycophenolic acid-acyl glucuronide in Chinese recipients of renal transplants (Zhang et al., 2008b). Mycophenolic acid-acyl glucuronide pharmacokinetics are similarly altered in healthy volunteers with the C-24T ABCC2 variant (Lévesque et al., 2008). In addition to mediating the disposition of antirejection drugs, Mrp2 may also influence the redox status of graft kidneys (Grisk et al., 2009). In this same study, ABCC2 variants were associated with a delay in clinical graft function (Grisk et al., 2009).
Thiopurine drugs such as 6-mercaptopurine are used as immunosuppressants and anticancer drugs. However, the efficacy of 6-mercaptopurine is often limited by gastrointestinal and hematopoietic toxicity. MRP4 transports 6-mercaptopurine in vitro (Chen et al., 2001). Mrp4 mRNA is highly expressed in mouse monocyte and erythroid progenitors (Krishnamurthy et al., 2008). Mrp4-null mice are more sensitive to 6-mercaptopurine toxicity as evidenced by reduced survival compared with wild-type mice (Krishnamurthy et al., 2008). Enhanced mortality of Mrp4-null mice after 6-mercaptopurine is associated with reduced bone marrow cellularity and cell number as well as elevated levels of the 6-mercaptopurine active metabolite, 6-thioguanine nucleotides (Krishnamurthy et al., 2008). E757K is a frequent ABCC4 variant in the Japanese population (Table 20) (Krishnamurthy et al., 2008). This nonsynonmous substitution reduces membrane localization of MRP4 without altering total protein levels (Krishnamurthy et al., 2008). Cells overexpressing E757K are more susceptible to 6-mercaptopurine cytotoxicity (Krishnamurthy et al., 2008). These data may explain why some Japanese patients have enhanced thiopurine sensitivity as an adverse event (Ando et al., 2001).
4. Stem Cells.
Stem cells are identified by a “side population” phenotype by exhibiting low fluorescence after staining with fluorescent dyes. Efflux of these dyes (such as Hoechst and rhodamine) via Bcrp and Mdr1a/1b transporters has been implicated in the side population phenotype (Kim et al., 2002b; Uchida et al., 2002; Morisaki et al., 2005). Hepatic side population cells from Mdr1a/1b-null retain a normal ability to efflux rhodamine, suggesting involvement of other transporters in the liver side population phenotype (Uchida et al., 2002). Bcrp is prominently expressed in stem cells from various sources, including bone marrow and skeletal muscle (Zhou et al., 2001). Using single- and double-null mice, it was demonstrated that Bcrp and Mdr1a/1b account for the side population phenotype of stem cells of mammary glands (Jonker et al., 2005a). Only Bcrp conferred the side population phenotype in bone marrow and skeletal muscle (Zhou et al., 2002; Jonker et al., 2005a). In addition, Bcrp-null hematopoietic cells are more sensitive to mitoxantrone in drug-treated transplanted mice (Zhou et al., 2002). Taken together, these findings suggest that Bcrp is a cytoprotective phenotype of stem cells to protect against xenobiotic toxicity.
L. Connective Tissue and Skin
Pseudoxanthoma elasticum is a disease of the connective tissue that results from loss-of-function mutations of MRP6 on chromosome 16p13.1 (Table 10) (Le Saux et al., 2000; Cai et al., 2001; Pulkkinen et al., 2001). Patients with pseudoxanthoma elasticum demonstrate mineralized elastic fibers with pathological findings in the skin, eyes, and cardiovascular system (Li et al., 2009a). The recent generation of Mrp6-null mice confirms the critical nature of Mrp6 in preventing calcification of blood vessel walls (primarily in the kidney, aorta, and vena cava) and in Bruch's membrane in the eyes (Gorgels et al., 2005). Pathological findings in the eyes of Mrp6-null mice are intriguing because Mrp6 protein cannot be detected in wild-type eyes, suggesting that pseudoxanthoma elasticum is a systemic disorder (Gorgels et al., 2005). Furthermore, Mrp6-null mice have reduced high-density lipoprotein cholesterol plasma levels and elevated plasma creatinine levels. These findings are consistent with the R1268Q variant of ABCC6 in pseudoxanthoma elasticum patients that is associated with type IV hyperlipoproteinemia with hypoalphalipoproteinemia (Wang et al., 2001a).
Subsequent studies have attempted to identify additional Abcc transporters that might contribute to the pathological state of Mrp6-null mice (Li et al., 2007c). In a cohort of pseudoxanthoma elasticum patients, a number of ABCC1 (MRP1) polymorphisms have been identified, suggesting that additional MRP transporters might contribute to this disorder (Le Saux et al., 2000). Connective tissue mineralization in Mrp1/Mrp6-null and Mrp3/Mrp6-null mice is consistent with pathological changes in Mrp6-null mice, suggesting that loss of Mrp1 and Mrp3 does not modulate the effects of Mrp6 loss in this rodent model (Li et al., 2007c). Likewise, oxidative stress does not explain the connective tissue mineralization observed in Mrp6-null mice (Li et al., 2008). It is noteworthy that recent research suggests that dietary mineral modifications may influence the phenotypic abnormalities in Mrp6-null mice (LaRusso et al., 2009). The Mrp6-null mouse model will be useful in identifying strategies to treat pseudoxanthoma elasticum.
Bcrp-null mice are generally healthy but can develop phototoxic lesions on light-exposed skin after consuming diets with a high percentage of alfalfa (Jonker et al., 2002). A similar phototoxicity can be observed in Bcrp-null mice administered the chlorophyll-breakdown product, pheophorbide-a (Jonker et al., 2002). Because of the ability of BCRP to transport porphyrins and heme, the role of Bcrp in protecting against hypoxic injury has been investigated in Bcrp-null mice (Krishnamurthy et al., 2004). Bcrp is up-regulated in progenitor cells under hypoxic conditions in an attempt to lower heme and/or porphyrin levels and ensure cell survival (Krishnamurthy et al., 2004). Moreover, in vitro expression of human BCRP variants demonstrate impaired porphyrin and pheophorbide-a transport; as such, these mutant BCRP-expressing cells are more susceptible to phototoxicity (Tamura et al., 2006b, 2007a). More research is needed to elucidate Bcrp's role in heme homeostasis and skin photosensitivity reactions.
IV. Post-Translational Regulation and Subcellular Trafficking of Transporters
There are multiple levels of transporter regulation including transcriptional (activators and repressors), post-transcriptional (splice variants), chromosomal (epigenetic modifications), translational (mRNA stability), and post-translational (alteration of proteins) modifications. A number of post-translational modifications of transport proteins, including phosphorylation, glycosylation, ubiquitination, and SUMOylation, are described in this section. These biochemical events are central to protein folding, stability, subcellular trafficking, targeted degradation, and functional activity of transporters. The second half of this section focuses on the interaction of transporters with cytosolic scaffold proteins (Table 21) and membrane rafts as well as subcellular localization of transporters to distinct regions of the plasma membrane.
TABLE 21.
Transporter and scaffold protein interactions
| Transporter | Adaptor Protein | Cells | Observation | References |
|---|---|---|---|---|
| hOAT4 | PDZK1/NHERF1 | HEK293 | ↑ Transport activity/surface expression | Miyazaki et al., 2005 |
| hOAT4 | PDZK1/NHERF1 | LLC-PK1 | ↑ Transport activity/surface expression | Zhou et al., 2008a |
| hOAT4 | PDZK1/NHERF1 | BeWo | ↔ Transport activity/surface expression | Zhou et al., 2008a |
| OCTN1 | PDZK1 | HEK293 | ↑ Transport activity | Sugiura et al., 2006 |
| OCTN2 | PDZK1 | HEK293 | ↑ Transport activity | Kato et al., 2005; Sugiura et al., 2006 |
| OCTN2 | PDZK2 | HEK293 | ↑ Transport activity/surface expression | Watanabe et al., 2006 |
| PEPT1 | PDZK1 | HEK293 | ↑ Transport activity | Sugiura et al., 2008 |
| PEPT2 | PDZK1 | HEK293 | ↑ Transport activity | Kato et al., 2004; Sugiura et al., 2006 |
| PEPT2 | PDZK1 | HEK293 | ↑ Transport activity/surface expression | Noshiro et al., 2006 |
| PEPT2 | NHERF2 | Oocytes | ↑ Transport activity/surface expression | Boehmer et al., 2008 |
| URAT1 | PDZK1 | HEK293 | ↑ Transport activity/surface expression | Anzai et al., 2004 |
| MRP4 | NHERF1 | MDCK | ↑ Surface expression | Hoque et al., 2009 |
↑, increase; ↔, no change.
A. Phosphorylation
Phosphorylation is the addition of phosphates to hydroxyl side chains on amino acids including serine, threonine, and tyrosine. Phosphorylation is accomplished by protein kinases using the hydrolysis of ATP. The removal of phosphate groups is performed by protein phosphatases, making phosphorylation a reversible process. Attachment of phosphate groups to drug transporters is thought to alter conformational structure, cellular localization, and transport function. Experimental approaches to study transporter phosphorylation include pharmacological stimulation of protein kinases, inhibition of phosphatase activity, and mutation of amino acid residues where phosphorylation occurs. There are differential effects of protein phosphorylation on transport function depending upon the transporter isoform and species. Moreover, the isoform of the protein kinase can contribute to the functional outcome of transporter phosphorylation.
The majority of research regarding the regulation of transporters by phosphorylation has focused on the OCT and OAT uptake transport families. Transport in rat Oct1- and Oct2-HEK293-overexpressing cells is stimulated by pharmacological activation of protein kinases A and C (Mehrens et al., 2000; Wilde et al., 2009). Specifically, protein kinase C phosphorylates rat Oct1 protein at certain serine residues (Mehrens et al., 2000; Ciarimboli et al., 2005a). When individual serine and threonine residues in each of the five putative phosphorylation sites are mutated, protein kinase C activation of rat Oct1 is diminished (Ciarimboli et al., 2005a). In contrast to what has been reported for rat Oct1, protein kinase A activation reduces transport mediated by human OCT1 and OCT2 in HEK293 cells (Cetinkaya et al., 2003; Ciarimboli et al., 2004). Although the exact mechanism underlying this species difference is not entirely clear, it has been proposed that protein kinase A activation differentially affects the association of rat and human Oct2/OCT2 with additional proteins (such as calmodulin) that can influence substrate affinity (Wilde et al., 2009).
Activation of protein kinase C by various pharmacological agents inhibits Oat3 (rat and rabbit) and human OAT4 activity in kidney proximal tubule cells and placental cells, respectively (Takeda et al., 2000; Soodvilai et al., 2004; Zhou et al., 2007a). Phosphorylation of OAT by protein kinase C may have indirect effects on its activity but also has indirect effects on subcellular localization (You et al., 2000; Wolff et al., 2003; Zhou et al., 2007a). Instead, stimulation of protein kinase C causes retrieval of various OAT transporters from the cell membrane, resulting in reduced activity (Wolff et al., 2003; Zhou et al., 2007a). Upstream signals such as angiotensin II can inhibit OAT1 activity in COS-7 kidney-derived cells by activating protein kinase C and retrieving OAT1 from the cell surface to the cytoplasm (Li et al., 2009b). The retrieval of OAT1 from the plasma membrane to recycling endosomes is a constitutive activity that occurs through protein kinase C activation (Zhang et al., 2008a). Alternatively, unconventional protein kinase isoforms (such as protein kinase Cζ) increase Oat3 activity possibly by promoting insertion into the plasma membrane of rodent renal cells (Barros et al., 2009). In contrast, inhibition of protein phosphatase activity reduces mouse Oat1 transport in LLC-PK1 proximal tubule cells, at least partly as a result of direct phosphorylation (You et al., 2000).
There is limited work exploring the role of phosphorylation in regulating hepatic transporters. Incubation of hepatocytes with phosphatase inhibitors reduces Oatp1a1-mediated transport without altering subcellular localization (Glavy et al., 2000). Subsequent analysis revealed that Oatp1a1 can be phosphorylated at two serine residues, and protein kinase C is likely to participate in regulating phosphorylation status (Guo and Klaassen, 2001; Xiao et al., 2006). In a variety of cell types and/or models, the α isoform of protein kinase C increases Bsep (Noe et al., 2001) and Mrp2 (Beuers et al., 2001) transporter activity and/or membrane insertion, whereas the δ/ζ isoforms stimulate human ENT1 (Coe et al., 2002) and rat Ntcp (Sarkar et al., 2006; Schonhoff et al., 2008). It is noteworthy that cAMP stimulates rat Ntcp function by promoting Ntcp dephosphorylation at serine 226 and localization in the plasma membrane rather than the endosome (Mukhopadhayay et al., 1997; Mukhopadhyay, 1998a,b; Anwer et al., 2005). Another study demonstrates the redistribution of MRP2 protein from the canalicular to the basolateral membrane of human HepG2 cells in response to protein kinase C activation by phorbol-12-myristate-13-acetate (Kubitz et al., 2001). Subsequent studies will be needed to determine whether phosphorylation of transporter proteins is necessary for their translocation as well as other conditions responsible for insertion into a particular membrane locale.
Phosphoinositide 3-kinases catalyze the phosphorylation of lipid signaling molecules. Early work demonstrated that lipid products from phosphoinositide 3-kinases participate in the ATP-dependent transport of organic anions (including bile acids) across the canalicular membrane (Misra et al., 1999). Furthermore, phosphoinositide 3-kinases can be activated by cAMP, which stimulates bile acid secretion in vitro (Kagawa et al., 2002). Stimulation of phosphoinositide 3-kinase activity by cAMP may participate in the activation of Bsep function in rat liver but does not influence its subcellular trafficking (Misra et al., 2003). Instead, Bsep protein trafficking to the apical membrane is related to signaling and sorting of myosin-related proteins (Chan et al., 2005) and the Rab11a protein (Wakabayashi et al., 2004, 2005).
B. Glycosylation
Glycosylation is the covalent addition of sugar moieties to newly synthesized proteins. This post-translational protein modification is typically performed by enzymes in the rough endoplasmic reticulum and the Golgi apparatus that sequentially add and modify the sugar groups. Glycosylation can occur at the amide side chain of asparagine (N-linked glycosylation) and at the hydroxyl side chain of serine and threonine residues (O-linked glycosylation). Multiple approaches to investigate glycosylation of transporters are available and include the use of inhibitors of different steps of glycosylation or mutant cells lacking specific glycosylation enzymes. Interruption of glycosylation can have a range of effects on transporter expression and function, including improper protein folding, protein degradation, impaired cellular trafficking to the cell surface, and altered transporter activity.
An example of the multiple and differing effects of glycosylation on transport involves OCT2. OCT2 contains consensus sites for N-glycosylation at amino acid positions 71, 96, and 112 (Pelis et al., 2006). Mutation of these asparagine residues demonstrates that each site is indeed glycosylated (Pelis et al., 2006). Substitution at amino acids 96 and 112, but not 71, reduces the transport rate of OCT2. The reduced transport rate of the 112 mutant OCT2 is due to insufficient plasma membrane expression and intracellular retention (Pelis et al., 2006). The mutant protein containing a substituted amino acid at position 96 traffics properly to the cell membrane, suggesting that glycosylation of this residue reduces the rate of transport by increasing transporter turnover. It is noteworthy that substitution of the three asparagines increases the affinity of OCT2 for its prototypical substrate.
Similar mutagenesis experiments have been performed for OAT1 and OAT4 (Tanaka et al., 2004; Zhou et al., 2005). Mutation of the asparagine residue at position 39 reduces human OAT1 function without altering insertion into the cell membrane of HeLa cells (Tanaka et al., 2004). Although this position is not glycosylated in the mouse Oat1 isoform, mutation of this residue does decrease activity, suggesting that this position is important for substrate recognition by Oat1/OAT1 (Tanaka et al., 2004). Mutation of a number of asparagine residues in OAT4 prevents targeting of de novo protein to the Chinese hamster ovary cell membrane and, in turn, lowers functional transport (Zhou et al., 2005). It is noteworthy that mutant cells with altered processing of glycosylated proteins (sialic acid- and galactose-deficient oligosaccharides) exhibit normal OAT4 expression at the cell surface with reduced transport activity (Zhou et al., 2005). Therefore, glycosylation plays a critical role in not only targeting of OAT isoforms to the cell surface but also enhancing its binding affinity for substrates.
Glycosylation of Oatp1a1 protein occurs at three asparagine sites (Wang et al., 2008c). Hypoglycosylation of Oatp1a1, using the glycosylation inhibitor tunicamycin, causes intracellular retention and diminished transport of taurocholate in X. laevis oocytes (Lee et al., 2003). Both membrane targeting and functional activity of Oatp1a1 are regulated by the extent of N-glycosylation (Lee et al., 2003; Wang et al., 2008c). Similar to the post-translational regulation of uptake transporters, apically expressed efflux transporters Mrp2 (Zhang et al., 2005), BCRP (Mohrmann et al., 2005), Bsep (Mochizuki et al., 2007), and MDR1 (Schinkel et al., 1993) are regulated by glycosylation and cellular trafficking.
C. Ubiquitination and SUMOylation
Ubiquitin is a small regulatory protein of 76 amino acids that is widely expressed. Covalent addition of ubiquitin molecules (also called ubiquitination) to proteins labels them for degradation by the proteasome. Monoubiquitination tends to traffic proteins to the endosome. In addition to targeting proteins for degradation, ubiquitination can influence protein stability, function, and localization. The earliest work examining the ubiquitination of drug and bile acid transporters focused on Pgp and Asbt, respectively (Xia et al., 2004; Zhang et al., 2004e). The half-life of rat Asbt protein is reported to be approximately 6 h in stably transfected cholangiocarcinoma cells (Xia et al., 2004). Treatment of these cells with a proteasome inhibitor increases Asbt protein levels (Xia et al., 2004). In addition, Asbt and ubiquitin associate under basal conditions (Xia et al., 2004). Likewise, exposure to proteasome inhibitors increases the amount of ubiquitinated Pgp (Zhang et al., 2004e). Addition of exogenous ubiquitin to MDR1 (Pgp)-expressing breast cancer cells not only decreases Pgp expression but also enhances cellular accumulation of a Pgp substrate (Zhang et al., 2004e). More recently, it has been suggested that modulation of Pgp ubiquitination may be a novel approach for overcoming drug resistance in cancer cells (Wang et al., 2008d).
NTCP, BSEP, BCRP, and ABCA1 also undergo ubiquitination and proteasomal degradation (Kühlkamp et al., 2005; Nakagawa et al., 2008; Azuma et al., 2009; Hayashi and Sugiyama, 2009; Wakabayashi-Nakao et al., 2009). Inhibition of the proteasome alters the subcellular localization of rat Ntcp from the plasma membrane of HepG2 cells to intracellular compartments (Kühlkamp et al., 2005). NTCP colocalizes with ubiquitin in livers of patients with progressive familial intrahepatic cholestasis 3 (Kühlkamp et al., 2005). Likewise, mutations in ABCB11 (BSEP) are associated with enhanced protein turnover by the ubiquitin-proteasome system (Hayashi and Sugiyama, 2009). It was recently reported that 4-phenylbutyrate, a drug approved in the United States to treat urea cycle disorders, promotes BSEP expression on the canalicular membrane by interfering with ubiquitination (Hayashi and Sugiyama, 2007; Hayashi and Sugiyama, 2009). These findings are of clinical importance, because patients with cholestasis often exhibit reduced or intracellular expression of BSEP protein and may point to a novel therapeutic modality.
Early findings suggest that the Mrp2 transporter can undergo SUMOylation (Minami et al., 2009). Small ubiquitin-like modifier (SUMO) proteins are a family of small proteins (∼100 amino acids) that modify protein function when attached via an isopeptide linkage. Using a protein-protein interaction assay, a number of SUMO-related proteins (including SUMO-1) were pulled down using the linker region of rat Mrp2 protein (Minami et al., 2009). Subsequent work confirmed that this region of Mrp2 can undergo an in vitro SUMOylation reaction. Likewise, inhibition of SUMO-related enzymes in hepatoma cells reduces Mrp2 protein expression but does not alter its mRNA expression or canalicular localization (Minami et al., 2009). Additional research is warranted and should attempt to reveal the functional consequences of transporter SUMOylation.
From the aforementioned studies, it is evident that post-translational modifications can have diverse consequences on transport protein turnover, localization, and function. Events such as glycosylation and phosphorylation can alter localization and function of transporter proteins, whereas ubiquitination and SUMOylation lead to degradation.
D. Membrane Rafts
There is accumulating evidence that the plasma membrane is not a uniform phospholipid bilayer. Instead, it is composed of distinct and specialized regions with varying proportions of lipids and proteins. Such small microdomains (10–200 nm) that are sphingolipid- and cholesterol-enriched are called “lipid rafts” or “membrane rafts” (Zegers and Hoekstra, 1998; Pike, 2006). Because membrane rafts are highly ordered compared with surrounding areas, they are less fluid and more resistant to solubilization by nonionic detergents such as Triton X-100. A number of studies have begun to investigate whether transporters are contained within membrane rafts and, if so, which types of rafts. Early studies focused upon Pgp, which was found to localize to membrane rafts (Luker et al., 2000; Bacso et al., 2004; Radeva et al., 2005). Pgp has been reported in both low-density (Luker et al., 2000) and intermediate-density (Radeva et al., 2005) membrane microdomains. BCRP is found in detergent-resistant membranes (Storch et al., 2007). Likewise, a portion of Ntcp protein localizes to membrane rafts in mouse liver (Molina et al., 2008). Within the mouse intestine, Pept1 protein colocalizes with markers of membrane rafts (Nguyen et al., 2007). Using rat liver, it has been demonstrated that the localization of ABC family transporters (Abcg5, Bsep, Mrp2, Mdr2) is predominantly in “lubrol-microdomains” that contain the majority of canalicular cholesterol and phospholipids, as well as caveolin-1 and dipeptidyl peptidase IV proteins (Ismair et al., 2009). The designation as “lubrol-microdomains” is based upon the detergent used for solubilizing the canalicular plasma membrane.
Cholesterol is important for maintaining the order and packing of lipids and proteins in membrane rafts. Therefore, a common approach to studying membrane compartmentalization is to assess transporter function under cholesterol-depleted and replenished conditions. Depletion of membrane cholesterol enhances rat Ntcp uptake activity in transfected HEK293 cells (Molina et al., 2008). Moreover, reduced membrane cholesterol lowers BCRP (Storch et al., 2007), ASBT (Annaba et al., 2008), and Pgp (Dos Santos et al., 2007; Fenyvesi et al., 2008) transporter activity in overexpressing cell lines. In the cases of BCRP and ASBT, lower cholesterol levels did not alter cell viability or transporter subcellular localization or expression (Storch et al., 2007; Annaba et al., 2008). Supplementation of cholesterol-depleted cells with exogenous cholesterol restores transporter activity (Storch et al., 2007; Annaba et al., 2008). Exactly how cholesterol depletion influences transporter function is not clear, but may involve altered protein-protein interactions within the membrane, modulation of transporter transmembrane domains, disassembly of membrane rafts, or interference of transporter binding to lipophilic substrates (Storch et al., 2007).
The importance of canalicular membrane cholesterol content in biliary excretion was recently revealed in Atp8b1-null mice (Paulusma et al., 2009). Atp8b1-null mice have increased biliary levels of cholesterol because of nonspecific extraction from the canalicular membrane (Groen et al., 2008). Furthermore, biliary bile acid excretion is strongly impaired in Atp8b1-null mice despite normal Bsep protein expression and localization (Paulusma et al., 2006). Recent evidence suggests that the impaired Bsep activity in Atp8b1-null mice is due to the reduced cholesterol content of the canalicular membrane (Paulusma et al., 2009). In support of this hypothesis, repletion of cholesterol to membranes from Atp8b1-null mice restores Bsep transport activity (Paulusma et al., 2009).
Future studies into the functional significance of transporter expression in membrane rafts should provide insight into the complex membrane organization and compartmentalization of the plasma membrane. It should be noted that depletion of cholesterol yields conflicting results on Pept1 activity in polarized intestinal epithelial cells (increased activity) compared with intestinal apical membrane vesicles (decreased activity) (Nguyen et al., 2007). As future studies of transporters and lipid rafts are designed, these findings should be taken into consideration.
E. Scaffold Protein Interactions
A number of transporters contain PDZ domains (postsynaptic density 95/disc-large/zona occludens) for interaction with scaffold proteins (Hung and Sheng, 2002; Kato et al., 2004). Scaffold proteins connect transporters and regulatory components and influence subcellular targeting, transport activity, recruiting additional proteins, and protein stability on the cell surface. PDZ binding motifs are generally three to eight amino acids and occur at internal and/or C-terminal regions (Hung and Sheng, 2002). Cell interactions between PDZ proteins and several transporters, including OCTN, PEPT, OAT, and OATP isoforms, have been shown (Kato et al., 2004). PDZK1 and -2 and Na+/H+ exchanger regulatory factors (NHERF1 and 2) are well characterized PDZ proteins that interact with transporters.
A number of experimental approaches are available to study transporter-PDZ protein interactions including pull-down/direct interaction studies, cotransfection of transporter and PDZ genes (as well as mutant forms), and double immunohistochemical staining. Pull-down experiments demonstrate interaction of PDZK1 with apical URAT1, OCTN1, and OCTN2 proteins but not basolateral OCT1 or OCT2 (Anzai et al., 2004; Kato et al., 2004, 2005). Mutation of the PDZ motifs in URAT1 disrupts the physical interaction of these proteins (Anzai et al., 2004). Protein-protein interaction studies are supported by in vitro overexpression cell systems (Table 21). Cotransfection of cells with PDZK1 and OAT4 (Miyazaki et al., 2005), OCTN1 (Sugiura et al., 2006), OCTN2 (Kato et al., 2005), PEPT1 (Sugiura et al., 2008), PEPT2 (Kato et al., 2004; Noshiro et al., 2006), and URAT (Anzai et al., 2004) increases transport of prototypical substrates. Likewise, cotransfection with NHERF2 and the protein kinase SGK1 enhances activity of PEPT2 in X. laevis oocytes (Boehmer et al., 2008). Other examples of PDZ protein interactions include PDZK2 and OCTN2 (Watanabe et al., 2006) as well as NHERF1 and OAT4 (Miyazaki et al., 2005). In most of these cases, increased transporter activity was due to enhanced cell surface expression. Mutation of either the PDZ protein or the transporter PDZ motif can reduce cell surface expression and/or activity of the transporter (Kato et al., 2005; Miyazaki et al., 2005; Sugiura et al., 2006).
Using immunohistochemical staining, the colocalization of PDZ proteins and transporters has been shown. In various cells, PDZK1 protein colocalizes with OCTN1 (Sugiura et al., 2006), URAT1 (Anzai et al., 2004), and OAT4 (Miyazaki et al., 2005). Likewise, Octn2 protein colocalizes with Pdzk1 (Kato et al., 2005) and Pdzk2 (Watanabe et al., 2006) proteins in mouse kidney. In the intestine, Pdzk1 protein colocalizes with Pept1 (Sugiura et al., 2008) and Octn2 (Kato et al., 2006) on the brush border membrane of mouse enterocytes.
There are differences in the targeting of transporters to the apical membrane by PDZ proteins between cell types. For example, PDZK1 and NHERF1 increase OAT4 cell surface expression and activity in renal cells but do not alter OAT4 activity in human placenta BeWo cells (Miyazaki et al., 2005; Zhou et al., 2008a). In addition, MRP4 protein traffics to the basolateral and apical membranes in MDCK and LLC-PK1 kidney cells, respectively (Hoque et al., 2009). Basolateral trafficking of MRP4 protein in MDCK cells is due to low expression of NHERF1 in the cell line (Hoque et al., 2009). Ectopic expression of NHERF1 in MDCK1 cells redirects MRP4 to the apical membrane (Hoque et al., 2009). These findings suggest that transporters may require an alternate set of adaptor proteins for subcellular trafficking to specific membrane domains (apical versus basolateral) in different cells.
Mice null for Pdzk1 have been developed and exhibit reduced intestinal absorption of cephalexin (Pept1 substrate) and carnitine (Octn2 substrate) (Sugiura et al., 2008). Reduced uptake of both substrates is likely to be related to lower abundance of Pept1 and Octn2 proteins in intestinal brush border membranes (Sugiura et al., 2008). Electron microscopy demonstrates retention of Pept1 protein in intracellular vesicular structures in PDZK1-null mice (Sugiura et al., 2008).
The interaction of Oatp and PDZ proteins has been investigated using in vitro and in vivo approaches. During yeast two-hybrid screening, OATP1A2, -3A1, and -1C1 bind directly to members of the PDZ family (Kato et al., 2004). Oatp1a1 is localized to the basolateral surface of hepatocytes and binds predominantly to the first and third PDZ binding domains of PDZK1. Expression of Oatp1a1 is normal in Pdzk1-null mice, yet is restricted to the intracellular compartment (Wang et al., 2005). Finally, altered localization of Oatp1a1 in Pdzk1-null mice corresponds with impaired hepatic uptake of sulfobromophthalein (Wang et al., 2005).
The current data regarding the interaction of PDZ proteins with MRP2 are unclear. PDZK1 protein interacts with the carboxyl terminal of MRP2 in a yeast two-hybrid system (Kocher et al., 1999). In line with this finding, phosphorylation of the MRP2 C-terminal increases binding to various PDZ proteins (Hegedüs et al., 2003). However, truncation of the carboxyl terminus of MRP2 protein (containing a PDZ motif) does not interfere with trafficking to the apical membrane of polarized MDCK cells (Nies et al., 2002a). Instead, a larger portion (15 amino acids) needs to be removed before cellular trafficking is impaired (Nies et al., 2002a).
Collectively, post-translational modifications, membrane rafts, and protein-protein interactions work in concert to regulate the expression, trafficking, and function of transporters. Additional reviews on intracellular trafficking of transporters are recommended (Kipp and Arias, 2000, 2002; Wakabayashi et al., 2006). A number of other biochemical events, including altered osmolarity and redox signaling, influence transporter trafficking, but they are beyond the scope of this review. Likewise, the formation of cysteine disulfide bridges as well as homoligomerization and hetero-oligomerization are important for transport protein stabilization and function.
V. Sex Differences in Transporter Expression
Transporters exhibit sex differences in their expression, and these discrepancies probably contribute to disparities in drug disposition and toxicity between male and female subjects. Table 22 illustrates examples of sex differences in the expression of transporters in rodents. Data regarding sex differences in human transporters are quite limited; this is likely to be an active area of research.
TABLE 22.
Sex differences in transporter expression in mice and rats
Sex differences in transporter mRNA expression between males and females are provided for various tissues including kidney, liver, lung, brain, and intestine.
| Tissue & Transporter | Mice | Rat | References |
|---|---|---|---|
| Kidneys | |||
| Uptake | |||
| Oatp1a1 | M > F | M > F | Li et al., 2002; Cheng et al., 2005a |
| Oatp3a1 | M > F | N.D. | Cheng et al., 2005a |
| Oatp4c1 | M > F | N.D. | Cheng et al., 2005a |
| Oat1 | M > F | M > F | Buist et al., 2002, 2003; Buist and Klaassen, 2004 |
| Oat2 | M = F | F > M | Buist et al., 2002; Ljubojevic et al., 2007 |
| Oat5 | F > M | N.D. | Cheng and Klaassen, 2009 |
| Urat1 | M > F | N.D. | Hosoyamada et al., 2004 |
| Oct2 | M > F | M > F | Urakami et al., 1999; Slitt et al., 2002; Alnouti et al., 2006 |
| Asbt | F > M | N.D. | Cheng and Klaassen, 2009 |
| Abca1 | F > M | N.D. | Cheng and Klaassen, 2009 |
| Pept2 | M = F | F > M | Lu and Klaassen, 2006 |
| Efflux | |||
| Ent1 | F > M | M = F | Lu et al., 2004 |
| Ent2 | F > M | M = F | Lu et al., 2004 |
| Ent3 | M > F | M = F | Lu et al., 2004 |
| Mrp3 | F > M | N.D. | Maher et al., 2005b |
| Mrp4 | F > M | N.D. | Maher et al., 2005b |
| Mdr1a | F > M | N.D. | Cui et al., 2009c |
| Mdr1b | F > M | N.D. | Cui et al., 2009c |
| Bcrp | M = F | M > F | Tanaka et al., 2005 |
| Ostα | M > F | N.D. | C. Klaassen et al., unpublished |
| Mate1 | M > F | N.D. | Lickteig et al., 2008 |
| Liver | |||
| Uptake | |||
| Oatp1a1 | M > F | M = F | Li et al., 2002; Cheng et al., 2005a |
| Oatp1a4 | F > M | M = F | Guo et al., 2002b; Li et al., 2002; Cheng et al., 2005a |
| Oatp1a6 | F > M | N.D. | Li et al., 2002; Cheng et al., 2005a |
| Oatp2b1 | F > M | N.D. | Cheng et al., 2005a |
| Ntcp | F > M | M > F | Simon et al., 1999; Cheng et al., 2007 |
| Abca1 | M > F | N.D. | Cheng and Klaassen, 2009 |
| Efflux | |||
| Mrp3 | M = F | F > M | Maher et al., 2005b; Rost et al., 2005 |
| Mrp4 | F > M | N.D. | Maher et al., 2005b |
| Bcrp | M > F | M = F | Merino et al., 2005c; Tanaka et al., 2005 |
| Mate1 | F > M | N.D. | Lickteig et al., 2008 |
| Abcg5 | M = F | M > F | Dieter et al., 2004 |
| Lung | |||
| Mdr1b | F > M | N.D. | Cui et al., 2009c |
| Mdr2 | F > M | N.D. | Cui et al., 2009c |
| Brain | |||
| Oct3 | M > F | N.D. | Alnouti et al., 2006 |
| Mdr1b | M > F | N.D. | Cui et al., 2009c |
| Intestine | |||
| Abca1 (duo) | F > M | N.D. | Cheng and Klaassen, 2009 |
| Oatp2b1 (jej) | M > F | N.D. | Cheng et al., 2005a |
| Cnt3 (jej) | M > F | N.D. | Lu et al., 2004 |
| Ostα (jej) | M > F | N.D. | C. Klaassen et al., unpublished |
| Ostβ (jej) | M > F | N.D. | C. Klaassen et al., unpublished |
| Asbt (il) | F > M | N.D. | Cheng and Klaassen, 2009 |
| Mate2 (LI) | M > F | N.D. | Lickteig et al., 2008 |
| Abcg5 (jej) | M = F | M > F | Dieter et al., 2004 |
| Abcg8 (jej, il) | M = F | M > F | Dieter et al., 2004 |
M, male; F, female; duo, duodenum; jej, jejunum; il, ileum; LI, large intestine; N.D., not determined.
A. Sex Differences in Various Tissues
Studies in mice demonstrate tissue-dependent regulation of transporter isoforms between the sexes. The most notable differences exist in the kidneys and liver (Table 22).
Evaluation of transporter patterns in the kidneys of male and female mice reveals an interesting pattern. There is higher mRNA expression of uptake transporters (Oatp1a1, -3a1, -4c1, Oat1, Urat1, Oct2) in the kidneys of male mice, compared with female mice (Urakami et al., 1999; Li et al., 2002; Slitt et al., 2002; Hosoyamada et al., 2004; Cheng et al., 2005a; Alnouti et al., 2006). Conversely, female mice express higher levels of efflux transporters (Mrp3, Mrp4, Mdr1a, Mdr1b, Ent1, Ent2) in their kidneys (Lu et al., 2004; Maher et al., 2005b; Cui et al., 2009c). The net effect of these expression patterns favors lower intracellular accumulation of substrates in the renal tubule cells of female mice and could influence rates of chemical secretion and reabsorption.
A number of liver transporters demonstrate female-predominant (Oatp1a4, -1a6, -2b1, Ntcp, Mrp4, Mate1) or male-predominant (Oatp1a1, Bcrp, Abca1) mRNA expression patterns in mice (Simon et al., 1999; Guo et al., 2002a; Li et al., 2002; Cheng et al., 2005a, 2007; Maher et al., 2005b; Tanaka et al., 2005; Lickteig et al., 2008). There are relatively fewer sex differences in other mouse tissues. Compared with female mice, male mice express higher levels of Oatp2b1, Cnt3, Ostα, and Ostβ mRNA in the jejunum and Oct3 and Mdr1b in the brain (Lu et al., 2004; Cheng et al., 2005a; Alnouti et al., 2006; Lickteig et al., 2008; Cui et al., 2009c). In contrast, female-predominant expression of Mdr1b and Mdr2 mRNA is observed in mouse lungs (Cui et al., 2009c). Collectively, these data highlight the variability in sex differences depending upon the tissue and transporter isoform.
B. Sex Differences among Species
Sex differences are not always consistent among species. Male-predominant expression of Oatp1a1, Oat1, and Oct2 is similarly observed in the kidneys of mice and rats (Urakami et al., 1999; Buist et al., 2002; Slitt et al., 2002; Buist and Klaassen, 2004; Alnouti et al., 2006; Groves et al., 2006). In contrast, hepatic Ntcp demonstrates female-predominant mRNA expression in mice and male-predominant expression in rats (Simon et al., 1999; Cheng et al., 2007). There is more NTCP mRNA in female human livers, although it is not statistically significant because of large interindividual variation (Cheng et al., 2007). Likewise, hepatic Bcrp/BCRP mRNA is higher in male mice and humans, respectively, compared with female counterparts (Merino et al., 2005c). As a result, Bcrp substrates (nitrofurantoin and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) exhibit greater biliary excretion in male wild-type mice relative to female mice (Merino et al., 2005c). It is noteworthy that there are no differences in the pharmacokinetics of these two chemicals in Bcrp-null mice, suggesting that the transporter is indeed responsible for the sex-divergent pharmacokinetics (Merino et al., 2005c). Similarities in the sex-dependent expression of Ntcp/NTCP and Bcrp/BCRP data suggest that mice may be an appropriate model for evaluating functional outcomes of sex differences in human transporters (Merino et al., 2005c; Cheng et al., 2007).
Kidney Oat1 mRNA and protein expression are higher in male mice and rats than in female mice and rats (Buist et al., 2002; Buist and Klaassen, 2004), and corresponds with more p-aminohippurate transport (Cerrutti et al., 2002; Ljubojevic et al., 2004). In contrast, no sex difference in Oat1 is observed in renal proximal tubule suspensions from rabbits (Groves et al., 2006). Evaluation of human OAT1 expression patterns in both sexes is necessary before extrapolating from rodent data.
Whereas the majority of sex difference studies have focused upon mRNA expression, additional work is needed at the protein and functional levels to better understand the in vivo significance. Moreover, there is a clear need for characterization of sex differences in human transporters.
C. Regulatory Mechanisms of Sex Differences
1. Growth Hormone.
Sex differences in the regulation of transporters are dictated by sex hormones as well as sex-dimorphic growth hormone secretion patterns. Experimental data investigating the mechanistic role of hormones in regulating sex-divergent transporter expression are summarized in Table 23. Male rats secrete growth hormone in high-amplitude pulses at regular intervals, whereas female rats exhibit lower and more frequent growth hormone pulses (Terry et al., 1977). In turn, circulating levels of growth hormone exhibit fewer fluctuations in female rats.
TABLE 23.
Hormonal regulation of transporter mRNA expression in rodent livers and kidneys
The tissue, species, and predominant gender are provided for each transporter. Hormonal regulation was determined using gonadectomy (GNX), hypophysectomy (HPX), lit/lit mice, and hormone replacement (estrogen and 5α-dihydroxytesterone, DHT) in gonadectomized mice. Changes in estrogen- and DHT-treated mice should be compared with those in GNX mice.
| Transporter | Tissue | Species | Gender Predominant | Female |
Male |
References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GNX | HPX | Lit/Lit | Estrogen | GNX | HPX | Lit/Lit | DHT | |||||
| Mdr1a | Kidney | Mice | Female | ↓ | ↔ | ↓ | ↔ | ↑ | ↑ | ↑ | ↓ | Cui et al., 2009c |
| Mdr1b | Kidney | Mice | Female | ↔ | ↑ | ↓ | ↔ | ↑ | ↑ | ↑ | ↓ | Cui et al., 2009c |
| Mrp3 | Kidney | Mice | Female | ↓ | ↓ | ↓ | ↑ | ↑ | ↔ | ↑ | ↓ | Maher et al., 2006a |
| Mrp4 | Kidney | Mice | Female | ↔ | ↑ | ↑ | ↔ | ↑ | ↑ | ↑ | ↓ | Maher et al., 2006a |
| Bcrp | Liver | Mice | Male | ↔ | N.D. | N.D. | ↔ | ↓ | N.D. | N.D. | ↑ | Tanaka et al., 2005 |
| Ntcp | Liver | Mice | Female | ↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | Cheng et al., 2007 |
| Oatp1a1 | Liver | Mice | Male | ↓ | ↓ | ↓ | ↔ | ↓ | ↓ | ↓ | ↑ | Cheng et al., 2006 |
| Oatp1a1 | Kidney | Mice | Male | ↔ | ↔ | ↔ | ↔ | ↓ | ↓ | ↓ | ↑ | Cheng et al., 2006 |
| Oatp1a4 | Liver | Mice | Female | ↔ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↓ | Cheng et al., 2006 |
| Oatp3a1 | Kidney | Mice | Male | ↔ | ↔ | ↔ | ↔ | ↓ | ↓ | ↔ | ↑ | Cheng et al., 2006 |
| Oatp4c1 | Kidney | Mice | Male | ↑ | N.D. | ↔ | ↔ | ↓ | N.D. | ↔ | ↑ | Cheng and Klaassen, 2009 |
| Oat1 | Kidney | Rat | Male | ↔ | ↔ | N.D. | N.D. | ↓ | ↓ | N.D. | N.D. | Buist et al., 2003 |
| Oat2 | Kidney | Rat | Female | ↓ | ↓ | N.D. | N.D. | ↔ | ↔ | N.D. | N.D. | Buist et al., 2003 |
| Oat3 | Liver | Rat | Male | ↔ | ↑ | N.D. | N.D. | ↓ | ↓ | N.D. | N.D. | Buist et al., 2003 |
| Oat5 | Kidney | Mice | Female | ↓ | N.D. | ↓ | ↑ | ↑ | N.D. | ↓ | ↓ | Cheng and Klaassen, 2009 |
| Urat1 | Kidney | Mice | Male | ↔ | N.D. | ↔ | ↓ | ↔ | N.D. | ↔ | ↑ | Cheng and Klaassen, 2009 |
| Oct2 | Kidney | Mice | Male | ↔ | N.D. | N.D. | ↔ | ↓ | N.D. | N.D. | ↑ | Alnouti et al., 2006 |
↑, increased mRNA levels; ↓, reduced mRNA levels; ↔, no change in mRNA levels; N.D., not determined.
Surgical removal of the pituitary gland (hypophysectomy) followed by growth hormone supplementation is one approach to assess the contribution of growth hormone secretion patterns to the regulation of renal and hepatic transporters (Table 23). The pituitary gland produces not only growth hormone but also luteinizing hormone, follicle-stimulating hormone, adrenocorticotropic hormone, and prolactin. Therefore, hypophysectomy diminishes multiple hormone signaling pathways. Another approach to assess the contribution of growth hormone to transporter regulation is the use of lit/lit mice (Table 23). Lit/lit mice have a spontaneous mutation in the growth hormone-releasing hormone receptor (Beamer and Eicher, 1976). Using these approaches, it has been shown that the female-predominant expression of hepatic Ntcp and renal Mrp4 is due to the inhibitory effects of male-pattern growth hormone secretion (Maher et al., 2006a; Cheng et al., 2007). In the case of Ntcp, exogenous administration of growth hormone (male-type patten) to hypophysectomized or lit/lit mice reduced hepatic mRNA levels (Cheng et al., 2007). Likewise, male-predominant Oatp1a1 expression in mouse liver is caused by the stimulatory effects of male-pattern growth hormone secretion (Cheng et al., 2006). The influence of growth hormone secretion patterns on the female-predominant expression of hepatic Mrp2 mRNA in rat liver has also been reported (Simon et al., 2006).
Although Oat2 mRNA levels in kidneys are similar in male and female mice (Buist and Klaassen, 2004), protein expression is higher in female mice (Ljubojević et al., 2007). Hypophysectomy of female rats reduces Oat2 mRNA expression in the kidneys that is partially restored by growth hormone injection (Buist et al., 2003).
2. Sex Steroids.
Testosterone and estrogen can have both stimulatory and inhibitory influences on the mRNA expression of various transporters. Gonadectomy is the surgical removal of the testes or ovaries, thereby reducing circulating levels of sex hormones. One approach to confirming the role(s) of individual sex hormones in regulating sex-divergent expression is to include a subset of mice that receive hormone replacement after surgical intervention (Table 23). Using gonadectomy, it has been shown that the female-predominant expression of renal Mdr1a, Mdr1b, and Mrp4, as well as hepatic Oatp1a4 in mice, is due to an inhibitory effect by androgens (Cheng et al., 2006; Maher et al., 2006a; Cui et al., 2009c). In contrast, female-predominant expression of renal Mrp3 and Oat5 in mice and Oat2 in rats is due to estradiol (Buist et al., 2002, 2003; Kobayashi et al., 2002a; Maher et al., 2006a; Ljubojević et al., 2007; Cheng and Klaassen, 2009).
Testosterone stimulates Oat3 mRNA expression in male rat livers (Kobayashi et al., 2002a; Buist et al., 2003) and represses Pgp and Mrp2 protein levels in female rat livers (Suzuki et al., 2006). In kidneys, male-predominant Oatp1a1, Oatp3a1, Oatp4c1, Oat1, Urat1, and Oct2 expression is androgen-dependent (Isern et al., 2001; Buist et al., 2003; Ljubojevic et al., 2004; Alnouti et al., 2006; Cheng et al., 2006; Cheng and Klaassen, 2009). For example, gonadectomy of male rodents reduces expression of kidney Oct2 to levels comparable with that in sham-operated female mice and rats (Slitt et al., 2002; Alnouti et al., 2006). Treatment of gonadectomized male and female mice with 5α-dihydroxytestosterone induces renal Oct2 in both sexes, whereas estradiol has little influence on renal Oct2 (Alnouti et al., 2006). Likewise, treatment of intact male and female rats with testosterone increases Oct2 mRNA, protein, and transport in the kidneys (Urakami et al., 2000).
Researchers have only begun to report sex differences in transporter expression. Mice are the most characterized rodent model. Future emphasis should be placed on quantifying sex differences in humans. Until such efforts are accomplished, only predictions can be made regarding the clinical implications of sex-divergent transporter regulation. For example, Urat1 mRNA and protein levels are expressed at a higher level in male mouse kidneys compared with female mouse kidneys (Hosoyamada et al., 2004). If this sex difference exists in humans, it may explain in part the increased incidence of gout in men compared with women. In addition, a polymorphism in ABCA1 in women is associated with an increased risk of developing late-onset Alzheimer's disease (Sundar et al., 2007). This is a unique risk factor and highlights the future direction of research into sex-specific polymorphisms in transporters.
VI. Ontogeny of Transporters
Developmental changes in metabolism and transport govern drug pharmacokinetics in humans and laboratory animals. These changes critically determine the systemic clearance of drugs and in turn influence the pharmacodynamic responses of newborns. Early studies focused upon the ontogenic development of drug-metabolizing enzymes as the underlying mechanism for altered drug disposition in juveniles. With the discovery of drug and bile acid transporters, attention has been placed on variations in their expression and/or function with age. This section will focus primarily on the perinatal to adulthood mRNA expression of transporters in rodents (mostly, mice) and humans in various tissues (Fig. 12).
Fig. 12.
Ontogeny of mouse basolateral uptake transporter mRNA expression in liver. Tissues from C57BL/6 mice were obtained at −2, 0, 5, 10, 15, 22, 30, and 45 days. Day −2 represents gestational day 17. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. Data are summarized from unpublished observations (C. Klaassen) and previous publications (Cheng et al., 2005a, 2007; Alnouti et al., 2006).
A. Liver
Hepatic drug clearance is low in neonatal rats, leading to increased circulating plasma levels of chemicals and enhanced susceptibility to drug toxicity (such as ouabain) (Klaassen, 1972). Likewise, human newborns and infants are at risk of jaundice because of the physiological immaturity of the liver (Suchy et al., 1981). During ontogeny, hepatocytes become polarized allowing for distinct localization of transporters on the basolateral and apical membranes and the vectorial transport of endo- and xenobiotics. Hepatic uptake transporters vary in the timing at which they reach adult mRNA levels (Fig. 12). Before birth, Oatp2a1 is the only transporter expressed at mature levels in mouse liver (Cheng et al., 2005a). It is noteworthy that Ntcp and Ent1 exhibit a spike in mRNA expression at birth (0 days) and then decrease (Cheng et al., 2007). Likewise, Ntcp mRNA levels increase in the fetal rat liver near birth (St-Pierre et al., 2004). It is hypothesized that the elevation of Ntcp mRNA at day 0 is a response to the development of the enterohepatic recirculation of bile acids in newborns (Cheng et al., 2007).
The remaining Oat/Oatp transporters reach adult mRNA levels at early (5–10 days: Oatp1a4, Oatp1a6, Oat2), middle (15–22 days: Oatp1b2, Oatp2b1, Oct1), and late (30–45 days: Oatp1a1) periods of liver development (Cheng et al., 2005a; Alnouti et al., 2006). The hepatic mRNA patterns of Oatp1a1, -1a4, and -1b2 ontogeny in rats are similar to mice (Li et al., 2002; Gao et al., 2004). For uptake transporters exhibiting sex-divergent expression (Oatp1a1, Oatp1a4, and Ntcp), differences between male and female mice are evident by 30 to 45 days of age (Fig. 12). Hepatic expression of rat Cnt2 mRNA does not increase until 21 days after birth (del Santo et al., 2001).
Liver efflux transporters demonstrate an intriguing initiation of mRNA expression in mice at birth (Figs. 13 and 14). These canalicular and sinusoidal transporters include Mrp2, Mrp4, Mate1, Mdr2, Bcrp, Bsep, Ostα, and Ostβ (Maher et al., 2005b; Cheng et al., 2007; Cui et al., 2009c). It is noteworthy that stimulation of Bsep, Mrp4, Ostα, Ostβ, and Mdr2 expression at birth corresponds with increased Ntcp mRNA and may represent the constitution of the enterohepatic recirculation of bile acids in mice. Likewise, Bsep and Mrp2 mRNA levels increase in the fetal rat liver perinatally and are followed by elevated protein expression between 1 and 4 weeks of age (Zinchuk et al., 2002; Rosati et al., 2003; Tomer et al., 2003; Gao et al., 2004; St-Pierre et al., 2004). Weak canalicular Bsep and Mrp2 protein immunostaining is observed in the fetal rat liver and becomes diffuse in appearance in newborns, suggesting subapical localization (Zinchuk et al., 2002). It is not until after 1 week of age that Bsep and Mrp2 protein are sharply localized to the rat canalicular membrane (Zinchuk et al., 2002).
Fig. 13.
Ontogeny of mouse canalicular efflux transporter mRNA expression in liver. Tissues from C57BL/6 mice were obtained at −2, 0, 5, 10, 15, 22, 30, and 45 days. Day −2 represents gestational day 17. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. Data are summarized from unpublished observations (C. Klaassen) and previous publications (Maher et al., 2005b; Cheng et al., 2007; Cui et al., 2009c).
Fig. 14.
Ontogeny of mouse basolateral efflux transporter mRNA expression in liver. Tissues from C57BL/6 mice were obtained at −2, 0, 5, 10, 15, 22, 30, and 45 days. Day −2 represents gestational day 17. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. Data are summarized from unpublished observations (C. Klaassen) and previous publications (Maher et al., 2005b).
Messenger RNA levels of mouse Mrp4, Ostα, Ostβ, Bsep, Bcrp, and Mate1 quickly decrease by 5 days of age (Figs. 13 and 14). Shortly after birth (10 days), peak levels of Mrp6 mRNA are observed in mouse and rat liver (Gao et al., 2004; Maher et al., 2005b). Maximal mRNA expression of Mate2, Abcg5, and Abcg8 is not observed until later in development (15–22 days). By 30 days, mouse Mrp3 mRNA approximate adult levels (Maher et al., 2005b). Mouse Mdr1b mRNA in liver is unchanged from gestation throughout adulthood (Cui et al., 2009c).
Initial studies have begun to examine the developmental expression of hepatobiliary transporters in humans. Our laboratory recently screened the mRNA expression of uptake and efflux transporters in human livers from three developmental periods: perinatal (gestation through 30 days), 0 to 4 years of age, and 7 to 17 years of age (Figs. 15 and 16). Only a small number of transporters (OCTN1, ENT1, ATP8B1, MRP4, and ABCA1) were detected in the perinatal livers. In general, mRNA expression of uptake and efflux transporters (with the exception of MRP4) increased from 0 to 4 years and was even higher in livers of persons over 7 years old. Similar to our findings, it has previously been reported that canalicular transporters BSEP, MRP2, and MDR3 tend to increase from the fetal period to adulthood (Chen et al., 2005a). As in rat liver development, the immunostaining patterns of MRP2, BSEP, and MDR3 proteins in the human fetal liver are diffuse with intracellular and canalicular staining (Chen et al., 2005a). Insufficient trafficking of canalicular transporters in the immature liver may be one reason for the heightened susceptibility of newborns to cholestasis.
Fig. 15.
Ontogeny of human uptake transporter mRNA expression in liver. Human liver specimens are from various time periods: perinatal (prenatal to postnatal day 30, n = 6), 0 to 4 years (n = 8), and more than 7 years old (n = 6). Data from male and female human livers are combined (C. Klaassen, unpublished observations).
Fig. 16.
Ontogeny of human efflux transporter mRNA expression in liver. Human liver specimens are from various time periods: perinatal (prenatal to postnatal day 30, n = 6), 0 to 4 years (n = 8), and more than 7 years old (n = 6). Data from male and female human livers are combined (C. Klaassen, unpublished observations).
B. Kidneys
Excretion by prenatal and juvenile rodent kidneys is functionally immature. Renal transport of the organic anion p-aminohippurate in rat kidney slices increases after birth and continues through adulthood (Nakajima et al., 2000). Maturation of renal excretion parallels the appearance of organic anion and cation transporters in developing kidneys. In mice, renal uptake transporters on the brush-border membrane seem to develop before those on the basolateral surface (Figs. 17 and 18). Before and at birth, the fetal mouse kidney expresses Oatp1a4, -2a1, and -2b1 (Cheng et al., 2005a). Although the subcellular localization of these transporters has not been determined, Oatp isoforms are presumed to be expressed on the apical surface of the kidneys, suggesting that only reabsorption is functional during gestational development. It is not until 15 to 22 days of postnatal life that the mRNA of mouse Oct1–2, Octn1–2, Oatp1a6, Oatp4c1, Oat5, Cnt1, Pept2, and Urat1 are sufficiently expressed in developing kidneys (Choudhuri et al., 2001; Cheng et al., 2005a; Alnouti et al., 2006; Cheng and Klaassen, 2009). Mice and rats exhibit similar increases in Pept2 mRNA postnatally (Shen et al., 2001). Increased Octn2 mRNA at 16 to 18 days in rat kidney corresponds with increased carnitine transport in brush border membrane vesicles (García-Delgado et al., 2009). By later in development (30+ days), Oat1–3, Oatp1a1, Oatp3a1, and Asbt are detected at adult levels (Choudhuri et al., 2001; Buist and Klaassen, 2004; Cheng et al., 2005a; Cheng and Klaassen, 2009). Similar delays in Oat and Oct expression are observed in developing rat kidneys at which time male-predominant Oat1, Oat2 (mouse only), and Oct2 expression is noticed, typically around 30 days of age (Buist et al., 2002; Slitt et al., 2002; Buist and Klaassen, 2004; Alnouti et al., 2006). Likewise, Oat1 and Oat3 mRNA is higher in renal proximal tubule suspensions from 15- to 20-week-old rabbits compared with 8-week-old rabbits (Groves et al., 2006). Oat1 and Oat3 mRNA is first expressed during late gestation in the renal cortex of sheep and remains elevated postnatally (Wood et al., 2005). In contrast to mice, Asbt mRNA is observed earlier (7 days) in the development of rat kidneys (Christie et al., 1996).
Fig. 17.
Ontogeny of mouse basolateral uptake transporter mRNA expression in kidneys. Tissues from C57BL/6 mice were obtained at −2, 0, 5, 10, 15, 22, 30, and 45 days. Day −2 represents gestational day 17. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. It is noteworthy that Oat1 and Oat3 mRNA expression was not quantified at day −2. Data are summarized from previous publications (Buist and Klaassen, 2004; Cheng et al., 2005a; Alnouti et al., 2006; Cheng and Klaassen, 2009).
Fig. 18.
Ontogeny of mouse apical uptake transporter mRNA expression in kidneys. Tissues from C57BL/6 mice were obtained at −2, 0, 5, 10, 15, 22, 30, and 45 days. Day −2 represents gestational day 17. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. Data are summarized from unpublished observations (C. Klaassen) and previous publications (Cheng et al., 2005a; Alnouti et al., 2006; Cheng and Klaassen, 2009).
The efflux transporters involved in reabsorption appear earlier in mouse renal development compared with those that participate in chemical secretion (Figs. 19 and 20). Ent2 and Mrp6 mRNA are detected in fetal kidneys and are accompanied by increases in Mrp1 and Mrp5 at birth (Fig. 20) (Maher et al., 2005b). Brush border efflux transporters (Mrp2, Mrp4, Mdr1b, Bcrp, and Mate1) and additional retrograde basolateral transporters (Ent3 and Mrp3) mature slowly and are not maximally expressed until the mid-juvenile stage of mouse renal development (15–22 days) (Figs. 19 and 20) (Maher et al., 2005b; Lickteig et al., 2008; Cheng and Klaassen, 2009; Cui et al., 2009c). The ontogeny of Mdr1a and -1b in mouse kidneys corresponds with increased renal excretion of the Pgp substrate, digoxin, from postnatal days 0 to 21 (Pinto et al., 2005). Similar maturation of Mdr1a, Mdr1b, and Mrp2 mRNA in rat kidneys occurs postnatally (Rosati et al., 2003; Garrovo et al., 2006).
Fig. 19.
Ontogeny of mouse apical efflux transporter mRNA expression in kidneys. Tissues from C57BL/6 mice were obtained at −2, 0, 5, 10, 15, 22, 30, and 45 days. Day −2 represents gestational day 17. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. Data are summarized from unpublished observations (C. Klaassen) and previous publications (Maher et al., 2005b; Lickteig et al., 2008; Cheng and Klaassen, 2009; Cui et al., 2009c).
Fig. 20.
Ontogeny of mouse basolateral efflux transporter mRNA expression in kidneys. Tissues from C57BL/6 mice were obtained at −2, 0, 5, 10, 15, 22, 30, and 45 days. Day −2 represents gestational day 17. Male (♂) mRNA is shown on the left, whereas female (♀) mRNA is shown on the right side of each box. Data are summarized from unpublished observations (C. Klaassen) and previous publications (Maher et al., 2005b; Cheng and Klaassen, 2009).
C. Intestine
Development of the small intestine in animals is not complete at birth. Intestinal expression of transporters seems to be elevated after birth and again at weaning (transition from milk to solid food). Dietary exposure to lipids, peptides, and carnitine necessitate the expression of intestinal transporters for efficient absorption. Likewise, enterohepatic circulation of bile acids is important to solubilize dietary lipids.
Expression of Asbt mRNA in rat and mouse ileum is biphasic with increased mRNA prenatally (gestation day 22) and increased levels again at weaning (19–21 days after birth) (Shneider et al., 1995, 1997; Christie et al., 1996; Håkansson et al., 2002). During the intervening period (day 7 after birth), Asbt mRNA expression is suppressed (Shneider et al., 1997). Asbt mRNA expression at all three time points (gestation day 22, postnatal days 7 and 21) corresponds with apical protein expression and taurocholate uptake in isolated ileal vesicles (Christie et al., 1996; Shneider et al., 1997). The second dramatic increase in Asbt mRNA is a result of weaning; early (day 15) or late (day 22) weaning of rat pups accelerates (at day 19) or decelerates (at day 22) Asbt up-regulation, respectively (Hwang and Henning, 2001).
Rat Pept1 mRNA increases in all three segments of the small intestine 1 day after birth and peaks between 3 and 5 days with a dramatic decline afterward (Shen et al., 2001). An additional report suggests that the decline in Pept1 mRNA occurs later in rat development (around postnatal day 50) (Rome et al., 2002). The rise in Pept1 mRNA after birth is mirrored by an increase in protein. It is noteworthy that Pept1 protein expression in the small intestine is biphasic, with a dramatic decrease at day 14 followed by a rise to adult rat levels after weaning (Shen et al., 2001). It should be noted that Pept1 mRNA and protein disappears from the rat colon between 3 and 5 days after birth and remains undetectable through 75 days of age (Shen et al., 2001).
Octn2 mRNA in the rat jejunum and ileum is expressed higher perinatally and decreases 1 day after birth through 6 months of age (García-Miranda et al., 2005). This pattern of mRNA expression parallels the decline in sodium-dependent uptake of l-carnitine with advancing age (García-Miranda et al., 2005).
D. Brain
A number of transporters have been detected in different regions of the developing brain. The protein expression of Pept2 is highest in rat cerebral cortex during embryonic development and steadily decreases through 75 days after birth (Shen et al., 2004). The decline in Pept2 protein is due to loss of the transporter in astrocytes (Shen et al., 2004). Pept2 staining is maintained in the choroid plexus throughout development (Shen et al., 2004).
From gestation through adulthood, there are increases in the mRNA expression of rodent Mdr1a, Mdr1b, and Mrp2 in brain (Rosati et al., 2003; Garrovo et al., 2006; Cui et al., 2009c). Pgp protein reaches adult levels in mouse brain by 21 days (Ose et al., 2008). As Pgp expression increases with age, the brain accumulation of Pgp substrates oseltamivir, digoxin, and cyclosporine declines (Goralski et al., 2006; Ose et al., 2008). It was recently shown that human Pgp protein can be detected on microvessel endothelial cells of the brain as early as 22 weeks of gestation, and staining intensity increases with development (Daood et al., 2008). Conversely, Mrp1 mRNA is similar in the rat fetal, newborn, and adult brain (Garrovo et al., 2006). Human MRP1 protein staining in the choroid plexus and ventricular ependyma is observed early in development (Daood et al., 2008). Human and mouse BCRP protein is also consistently detected in fetal and adult choroid plexus and capillary endothelial cells, respectively (Tachikawa et al., 2005; Daood et al., 2008).
During the prenatal and postnatal periods, organ development is incomplete. Therefore, the pharmacokinetics of drugs changes as various tissues mature. The consequences of variation in drug disposition may be inadequate therapeutic efficacy and/or a heightened incidence of adverse events. Although maturation of drug metabolizing enzyme expression is important, it is likely that establishment of polarized epithelia and vectorial transport across membranes during development also influences drug pharmacokinetics. Furthermore, transporter expression may be a marker of tissue differentiation as newborns develop. Although beyond the scope of this discussion, it is important to consider alterations in transport isoforms with advanced age. There are limited studies addressing transporter expression in older rodents and/or patients.
VII. Regulation of Hepatic Transporters by Xenobiotic-Activated Transcription Factors
A number of transporters are relatively highly expressed in mouse and human livers including Ntcp, Oatp1a1, -1a4, -1b2 (and their human orthologs) as well as Mrp2, Abcg5/8, Mdr2, Bcrp, and Bsep. Other transporters (Mrp3, Mrp4, Ostα, and Ostβ) are expressed at lower levels but have the potential to be induced by chemicals or during pathological conditions. Early work by our laboratory demonstrated that xenobiotics known to induce microsomal enzyme activity also altered the hepatobiliary disposition of chemicals (Klaassen, 1970, 1974, 1976). Although induction of drug metabolizing enzymes is an important pharmacological phenomenon, differential expression of hepatobiliary transporters after chemical treatment also contributes to changes in drug disposition. Coordinated up-regulation of drug-metabolizing enzymes and transporters is mediated by a number of hepatic transcription factors and has been discussed in greater detail in previous review articles (Handschin and Meyer, 2003; Klaassen and Slitt, 2005; Xu et al., 2005). Transcription factor-mediated up-regulation of hepatobiliary transporters has been reported to be mediated by the aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR, NR1I3), pregnane X receptor (PXR, NR1I2), peroxisome proliferator-activated receptor (PPAR, NR1C1), and farnesoid X receptor (FXR, NR1H4). These receptors (with the exception of AhR) function by heterodimerizing with the retinoid X receptor α (RXRα, NR2B1). Other transcription factors involved in transporter regulation include the oxidative stress sensor, NFE2-related factor 2 (Nrf2, NFE2L2) and the liver-specific regulators known as hepatocyte nuclear factors (HNF). Mice lacking these transcription factors have been developed and are useful tools in evaluating the physiological and chemical regulation of transporters (Fig. 21, Table 24).
Fig. 21.
Hepatic transporter mRNA expression in male transcription factor-null mice after chemical inducer treatment. Wild-type (WT) and knockout (KO) mice lacking AhR, CAR, PXR, PPARα, and Nrf2 were treated for 4 days with prototypical inducers (or corn oil vehicle) for each transcription factor. WT and AhR-null mice were administered TCDD (40 μg/kg i.p.). WT and CAR-null mice were administered TCPOBOP (300 μg/kg i.p.). WT and Nrf2-null mice were administered oltipraz (150 mg/kg i.p.). WT and PPARα-null mice were administered clofibrate (500 mg/kg i.p.). WT and PXR-null mice were administered PCN (200 mg/kg i.p.). Livers were removed 24 h after the final chemical inducer treatment. Transporter mRNA expression was quantified using multiplex mRNA expression analysis.
TABLE 24.
Expression of liver, kidney, and duodenal transporters in HNF1α-null mice
Differences in transporter mRNA between wild-type and HNF1α-null mice in liver, kidneys, and duodenum.
| Liver | Kidney | Duodenum | |
|---|---|---|---|
| Oat1 | ↔ | ↓ | N.D. |
| Oat2 | ↓ | ↓ | N.D. |
| Oat3 | ↔ | ↓ | ↑ |
| Urat | N.D. | ↓ | N.D. |
| Oatp1a1 | ↓ | ↓ | N.D. |
| Oatp1a4 | ↑ | ↔ | N.D. |
| Oatp1a5 | ↓ | N.D. | N.D. |
| Oatp1a6 | N.D. | ↑ | N.D. |
| Oatp1b2 | ↓ | ↔ | N.D. |
| Oatp2a1 | ↔ | ↔ | ↓ |
| Oatp2b1 | ↓ | ↑ | ↔ |
| Oatp3a1 | N.D. | ↓ | N.D. |
| Oatp4c1 | N.D. | ↑ | N.D. |
| Oct1 | ↔ | ↔ | ↓ |
| Oct2 | ↑ | ↑ | ↔ |
| Oct3 | ↑ | ↔ | ↔ |
| Octn1 | ↔ | ↔ | ↔ |
| Octn2 | ↑ | ↓ | ↔ |
| Octn3 | ↔ | ↓ | ↔ |
| Mrp1 | ↔ | ↑ | ↔ |
| Mrp2 | ↔ | ↑ | ↔ |
| Mrp3 | ↔ | ↑ | ↔ |
| Mrp4 | ↑ | ↑ | ↔ |
| Mrp5 | ↑ | ↑ | ↔ |
| Mrp6 | ↓ | ↔ | ↓ |
| Mdr1a | ↑ | ↑ | ↑ |
| Mdr1b | ↔ | ↑ | N.D. |
| Mdr2 | ↑ | ↑ | N.D. |
| Ntcp | ↔ | ↔ | N.D. |
| Bsep | ↓ | ↔ | N.D. |
| Asbt | ↓ | ↓ | ↔ |
| Bcrp | ↔ | ↔ | ↔ |
| Abcg5 | ↔ | ↑ | ↑ |
| Abcg8 | ↔ | ↔ | ↓ |
↑, increased mRNA levels in HNF1α-null mice; ↓, mRNA levels in HNF1α-null mice; ↔, no change in mRNA levels between genotypes; N.D., not determined.
A. Aryl Hydrocarbon Receptor
AhR is a transcription factor that typically resides in an inactive form in the cytosol. In response to ligand binding to chemicals, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), AhR dissociates from repressive chaperones and translocates to the nucleus, where it dimerizes with the AhR nuclear translocator, resulting in gene transcription. AhR binds to xenobiotic responsive elements and mediates the induction of cytochrome P450 1A1 by TCDD (Whitlock et al., 1989). In addition to TCDD, other AhR ligands include polychlorinated biphenyls, 3-methylcholanthrene, and β-naphthoflavone (Safe et al., 1985; Köhle and Bock, 2006). AhR ligands elevate hepatic expression of Mrps as well as Oatp2b1 and -3a1 mRNA in mice and decrease Oatp1a4 mRNA in rats (Figs. 21 and 22) (Rausch-Derra et al., 2001; Guo et al., 2002a; Cheng et al., 2005b; Maher et al., 2005a). Down-regulation of hepatic Oatp1a1 and -1a4 mRNA in mouse livers by TCDD is dependent upon AhR expression as demonstrated in Fig. 21 (Cheng et al., 2005b). It is noteworthy that AhR-null mice have elevated constitutive expression of Oatp1a4 and Abcg5 mRNA (Fig. 21). Furthermore, exposure of human hepatocytes to TCDD increases mRNA levels of MDR1 and represses BSEP, OCT1, OATP1B1, OATP1B3, OAT2, and NTCP (Fig. 22) (Jigorel et al., 2006).
Fig. 22.
Transporter regulation by the AhR, CAR, PXR, PPARα, and FXR transcription factors. Hepatic mRNA and/or protein expression of rodent (in vivo studies) and human (in vitro studies) transporters is increased (↑) or decreased (↓) in response to transcription factor activation.
B. Constitutive Androstane Receptor
CAR is a nuclear hormone receptor that is responsible for detoxifying xenobiotics. As its name implies, CAR is constitutively active in the absence of administered ligand but can be regulated by chemical agonists and inverse agonists. Upon ligand activation, CAR translocates to the nucleus where it heterodimerizes with RXRα and transactivates genes containing the phenobarbital response element and various direct repeat sites (Masahiko and Honkakoski, 2000; Handschin and Meyer, 2003). Isoforms from the cytochrome P450 2B subfamily are associated with CAR activation in rodents and humans (Wang and Negishi, 2003). There are numerous CAR activators with differential affinity and activity for rodent and human CAR and include phenobarbital, 1,4-bis-[2-(3,5,dichloropyridyloxy)] benzene (TCPOBOP), diallyl sulfide, and trans-stilbene oxide (Klaassen and Slitt, 2005).
CAR ligands reduce expression of Oatp1a1 mRNA and increase Oatp1a4 and Mrp2–6 mRNA in rat and mouse livers (Fig. 22) (Cherrington et al., 2002; Guo et al., 2002a; Xiong et al., 2002; Cheng et al., 2005b; Maher et al., 2005a; Petrick and Klaassen, 2007). Induction of Oatp1a4 mRNA as well as Mrp2–4 mRNA in livers of mice treated with TCPOBOP is absent in CAR-null mice (Fig. 21) (Maglich et al., 2002). It is noteworthy that phenobarbital induces mouse Mrp3 mRNA independent of CAR (Cherrington et al., 2003), whereas Mrp3 induction in this model depends upon RXRα expression and function (Cherrington et al., 2003).
Incubation of human liver slices with phenobarbital increases the mRNA expression of efflux transporters (BSEP, BCRP, MRP2, MRP3, MDR1) and decreases levels of uptake transporters (OCT1, OATP1B3, OAT2, NTCP) (Kiuchi et al., 1998; Jigorel et al., 2006; Olinga et al., 2008; Richert et al., 2009). In addition, constitutive levels of MRP2 mRNA in normal human livers correlates with CAR mRNA expression (Aleksunes et al., 2009). These findings correspond with the known induction of Mrp2 mRNA in phenobarbital-exposed rat hepatocytes (Kast et al., 2002).
C. Pregnane X Receptor
PXR is a major chemical sensor known to activate expression of cytochrome P450 3A enzymes in humans and rodents. Foreign substances (ligands) trigger PXR heterodimerization with RXRα and binding to response elements in the promoter/enhancer regions of genes involved in detoxification and transport (Staudinger et al., 2001a). The ability of CAR and PXR to coordinately regulate expression of metabolic and transport genes has been linked to pharmacological protection of the liver in a variety of pathological models including bile acid toxicity (Staudinger et al., 2001b; Zhang et al., 2004c; Stedman et al., 2005; Uppal et al., 2005).
Treatment of mice and rats with PXR ligands such as pregnenolone-16α-carbonitrile (PCN) increases mRNA expression of Oatp1a4 in liver (Fig. 22) (Rausch-Derra et al., 2001; Guo et al., 2002a; Cheng et al., 2005b; Cheng and Klaassen, 2006). Up-regulation of Oatp1a4 mRNA is absent in livers from PXR-null mice (Fig. 21) (Staudinger et al., 2001b, 2003; Cheng and Klaassen, 2006). In addition to Oatp1a4, PCN increases Mrp3, Mdr1a, and Mdr1b mRNA in liver and Abca1, Mdr1a, and Mrp2 mRNA in the small intestines of wild-type mice but not in mice lacking PXR expression (Figs. 21 and 22) (Maglich et al., 2002; Staudinger et al., 2003; Teng et al., 2003; Maher et al., 2005a; Cheng and Klaassen, 2006). A wide range of PXR agonists transactivate the Abcc3 (Mrp3) gene (Teng et al., 2003). Furthermore, the fact that PXR ligands alter mRNA expression of Abca1 as well as Abcg5 and -g8 in human and rodent cells suggests that this receptor can regulate cholesterol trafficking (Dieter et al., 2004; Sporstøl et al., 2005).
Not all of the effects of PXR ligands are observed at the transcriptional level. In fact, PCN treatment increases Mrp2 protein expression in rat livers in the absence of mRNA changes (Johnson and Klaassen, 2002). Increases in Mrp2 protein after PCN are due to de novo protein synthesis and not to changes in mRNA stability or protein degradation (Jones et al., 2005). Whether PXR is specifically involved in this response is not known. Additional studies have focused upon the translational regulation of MRP2 and provide insight into the mechanisms the role(s) of various open reading frames (Zhang et al., 2007b, 2009).
PXR activation can up-regulate human MDR1 expression in hepatocytes and intestinal cells in vitro (Synold et al., 2001). Using human liver slices and hepatocytes, it was demonstrated that rifampicin (a PXR ligand) induces MRP2, MRP3, BCRP, and MDR1 mRNA (Fig. 22) (Kast et al., 2002; Jigorel et al., 2006; Olinga et al., 2008; Richert et al., 2009). Exposure of human breast cancer cells to rifampin increases OATP1A2 mRNA levels (Meyer zu Schwabedissen et al., 2008). In addition, rifampicin decreases human BSEP, OCT1, OAT2, and NTCP (Jigorel et al., 2006). More recent work demonstrates the ability of various PXR ligands to alter OATP1B1 and -1B3-mediated transport, which may result in drug-drug interactions at the transporter level (Gui et al., 2008).
D. Peroxisome Proliferator-Activated Receptor
PPARs are a group of nuclear receptors that are important for cell differentiation and metabolism (notably, carbohydrates and lipids). The three main PPAR isoforms include α, γ, and δ (often termed β). Endogenous ligands for PPARs include free fatty acids and eicosanoids (Dreyer et al., 1993; Wahli et al., 1999). The name of this class of receptors reflects the fact that chemicals that activate PPAR isoforms increase the number and size of peroxisomes in cells (Dreyer et al., 1992). Pharmacological activation of the α and γ isoforms can be achieved by fibrate and thiazolidinedione drugs, respectively (Willson and Wahli, 1997). In addition to fibrate drugs (such as clofibrate and ciprofibrate), PPARα is also activated by a number of environmental chemicals, including phthalates [plasticizers such as di(2-ethylhexyl) phthalate] and perfluorinated fatty acids [stain- and heat-resistant chemicals such as perfluorooctanoic acid and perfluorodecanoic acid (PFDA)]. Like CAR and PXR, PPARs heterodimerize with RXRα and bind to peroxisome proliferator response elements in the promoter regions of genes (Dreyer et al., 1993). Of the drug-metabolizing enzymes, the cytochrome P450 4A subfamily is most sensitive to PPAR signaling (Johnson et al., 1996).
The development of PPARα-null mice has greatly enhanced investigations into the regulation of transporters by various exogenous ligands. PPARα ligands decrease expression of multiple Oatp isoforms in mouse livers (Cheng et al., 2005b). Down-regulation of Oatp1a1 mRNA by clofibrate is observed in wild-type but not in PPARα-null mice (Figs. 21 and 22). It is noteworthy that Oatp1a4 mRNA is not altered by clofibrate administration but is more highly expressed in PPARα-null mice (Fig. 21). Treatment of mice with clofibrate increases Mdr1a, Bcrp, Mrp3, and Mrp4 mRNA in a PPARα-dependent manner (Figs. 21 and 22) (Maher et al., 2005a; Moffit et al., 2006). Like clofibrate, ciprofibrate differentially alters transporter expression. Ciprofibrate increases Mdr1a, -1b, and -2 mRNA and protein in wild-type mouse livers but not in PPARα-null mice (Fig. 22) (Kok et al., 2003). In addition, ciprofibrate decreases Bsep, Oatp1a1, and Ntcp protein expression in a PPARα-dependent manner (Kok et al., 2003). Likewise, the perfluorinated chemicals perfluorooctanoic acid and PFDA decrease mRNA levels of Oatp1a1, -1a4, -1b2, and Ntcp (PFDA only) and increase Mrp3 and Mrp4 (Cheng and Klaassen, 2008; Maher et al., 2008). It is noteworthy that these changes are absent in PPARα-null mice treated with PFDA (Cheng and Klaassen, 2008; Maher et al., 2008). PPARα agonists also increase hepatic Bcrp mRNA in a PPARα-dependent manner (Hirai et al., 2007). Taken together, these findings from null mice demonstrate similarity among PPARα agonists in their regulation of hepatobiliary transporters.
In addition to regulating transporters involved in drug disposition, PPARα is important for controlling carnitine homeostasis. Hepatic and intestinal carnitine levels are increased after PPARα agonist (clofibrate and Wy14643) treatment of rodents and pigs (van Vlies et al., 2007; Ringseis et al., 2008). Increased carnitine levels are due to enhanced carnitine biosynthesis and uptake (Ringseis et al., 2008). Treatment of mice with PPARα agonists increase hepatic and intestinal Octn2 mRNA expression and carnitine levels in wild-type mice but not in PPARα-null mice (Fig. 22) (Ringseis et al., 2007; Maeda et al., 2008). Clofibrate up-regulates Octn1 (liver) and Octn2 mRNA (liver, duodenum, jejunum) in mice (Fig. 22) (Ringseis et al., 2007). Likewise, fibrate drugs elevate Octn2 mRNA in rat hepatocytes (Maeda et al., 2008). In line with these findings, freshly isolated hepatocytes from fenofibrate-treated rats demonstrate increased [3H]carnitine uptake (Maeda et al., 2008).
E. Farnesoid X Receptor
FXR is an intracellular bile acid sensor that controls bile acid and lipid homeostasis. By coordinately regulating bile acid synthesis and transport, FXR signaling provides one mechanism for reducing the bile acid burden of liver. FXR heterodimerizes with RXRα and binds to inverted nucleotide repeat motifs in the promoter sequences of SLCO1B3 (OATP1B3) and ABCB11 (BSEP) (Fig. 22) (Ananthanarayanan et al., 2001; Jung et al., 2002a). Inverted repeat response elements are essential for transactivation of the SLCO1B3 and ABCB11 promoters in response to bile acids (Ananthanarayanan et al., 2001; Jung et al., 2002a). Functional transactivation of the SLCO1B3 gene has been demonstrated in hepatocyte-derived cells incubated with bile acids (Ohtsuka et al., 2006). Furthermore, a polymorphism in the FXR promoter corresponds with reduced hepatic mRNA expression of human OATP1B1 and -1B3 (Marzolini et al., 2007). In addition to human OATP1B3 and BSEP, the rat Mrp2 promoter can be transactivated by FXR and its ligands (Fig. 22) (Kast et al., 2002).
Additional clues for identifying transporters regulated by FXR can be ascertained from FXR-null mice. FXR-null mice have reduced levels of hepatic Bsep mRNA and renal Oat3, Oatp1a1, Oatp1a4, Oct2, and Octn1 mRNA (Maeda et al., 2004).
Bile acids stimulate FXR and induce expression of the small heterodimer partner (Shp, NR0B2), which in turn down-regulates Ntcp gene expression in isolated hepatocytes (Denson et al., 2001). Likewise, bile acid treatment and bile-duct ligation repress Ntcp mRNA in wild-type, but not FXR-null mice (Zollner et al., 2005). At the same time, FXR is responsible for the bile acid and oxysterol-induced transactivation of the Abcb11/ABCB11 (Bsep/BSEP) gene in mouse liver (Maeda et al., 2004) and hepatocytes (Plass et al., 2002; Deng et al., 2006). Impaired regulation of Bsep mRNA in FXR-null mice during intrahepatic cholestasis probably contributes to increased hepatic necrosis observed in these animals (Zollner et al., 2003; Cui et al., 2009b). In addition to Ntcp and Bsep, the other bile acid transporters Ostα and Ostβ are regulated by FXR (Fig. 22). In 2006, Ostα and β were identified as novel FXR targets (Boyer et al., 2006; Zollner et al., 2006). Feeding with bile acids increases Ostα and β in livers, kidneys, and ilea of wild-type mice but not FXR-null mice (Zollner et al., 2006). Similar results have been observed in wild-type and FXR-null mice after bile-duct ligation (Boyer et al., 2006). Ostα contains two functional FXR binding motifs, whereas only one motif is found in the Ostβ gene (Landrier et al., 2006). Finally, the fibroblast growth factors 15 and 19, which are FXR target genes, repress the expression of Asbt protein in mouse ileum and gallbladder and in human cholangiocarcinoma cells (Sinha et al., 2008). Secondary signaling of FXR via fibroblast growth factors may be important in the regulation of additional hepatic and ileal transporters.
F. Hepatocyte Nuclear Factors
HNF1α and -4α are liver-enriched transcription factors that regulate the basal expression of many genes and are commonly referred to as “master” transcription factors because of their ability to regulate other nuclear receptors and transcription factors. HNF1α is expressed in livers, kidneys, intestines, and stomach and is implicated in the regulation of bile acid, fatty acid, and drug metabolism.
To better characterize additional roles for HNF1α in regulating transporters, hepatic, renal, and intestinal expression of xenobiotic and bile acid transporters was quantified in HNF1α-null mice (Maher et al., 2006c; Kikuchi et al., 2007) (Table 24). The most dramatic changes in mRNA expression in HNF1α-null mice include down-regulation of hepatic Oatp1a1, 1b2, and Asbt and renal Urat1, Oat1, Oat2, and Oat3 (Shih et al., 2001; Maher et al., 2006c; Kikuchi et al., 2007). A number of genes are up-regulated in HNF1α-null mice including hepatic Mrp4, Oatp1a4, renal Mrp3, Mrp4, Mdr1a, Mdr1b, Abcg5, as well as duodenal Oat3 and Mdr1a (Maher et al., 2006c). Up-regulation of these transporters may reflect repression by HNF1α or compensation to hepatic dysfunction in HNF1α-null mice.
Regulation of Ntcp and Oatp genes by HNF1α may be one mechanism for the liver-predominant expression of these transporters. Moreover, HNF1α seems to regulate these uptake transporters during various pathological conditions of the liver. Rats treated with carbon tetrachloride, endotoxin, or 17α-ethinylestradiol have reduced hepatic HNF1α binding activity and lower Ntcp mRNA levels (Trauner et al., 1998; Geier et al., 2002, 2003a). HNF1α seems to be important in modulating rat Ntcp but not the mouse or human genes (Fig. 23) (Karpen et al., 1996; Jung et al., 2004). HNF1α binding to the mouse Oatp1b2 promoter is decreased within 2 h after endotoxin administration (Li and Klaassen, 2004). HNF1α-mediated regulation of hepatic bile acid and organic anion transport probably has implications in enterohepatic recirculation under basal and pathological conditions.
Fig. 23.
Transporter regulation by the HNF1α, HNF4α, and Nrf2 transcription factors. Hepatic mRNA and/or protein expression of rodent (in vivo studies) and human (in vitro studies) transporters is increased (↑) or decreased (↓) in response to transcription factor activation.
HNF1α and 1β positively regulate OAT1, OAT3, URAT1, OATP1B1, and OATP1B3 by directly binding to and transactivating their promoters (Fig. 23) (Jung et al., 2001; Kikuchi et al., 2006, 2007; Ohtsuka et al., 2006; Furihata et al., 2007; Saji et al., 2008). Furthermore, OATP1B1 mRNA levels correlate with HNF1α gene expression in livers from adult Japanese subjects (Furihata et al., 2007). In addition, a polymorphism in the proximal promoter of human CNT2 increases the binding and transactivation of HNF1α and -1β (Yee et al., 2009).
Like HNF1α, HNF4α is important in regulating drug transporters. HNF4α binds to response elements and transactivates the promoters of the CNT1, OCT1, OAT1, and OAT2 genes (Fig. 23) (Popowski et al., 2005; Saborowski et al., 2006; Ogasawara et al., 2007; Klein et al., 2009). Likewise, HNF4α increases mouse Ntcp promoter transactivation in cultured cells by binding to a conserved distal cis-element (Geier et al., 2008). In addition, down-regulation of HNF4α using small interfering RNA suppresses Ntcp RNA expression up to 95% in mouse Hepa1–6 cells (Geier et al., 2008) and reduces MDR1, BSEP, MRP2, OATP1B1, and OCT1 mRNA in human hepatocytes (Kamiyama et al., 2007). Taken together, these data suggest that HNF1α and -4α are master regulators for the expression of hepatic drug and bile acid transporters and they may be important determinants for interindividual variation in drug pharmacokinetics (Wortham et al., 2007).
G. Nuclear Factor-E2-Related Factor 2
Nrf2 belongs to the basic region-leucine zipper family of transcription factors and is activated in response to electrophiles and oxidative stress. During periods of oxidative stress, Nrf2 is released from sequestration in the cytoplasm and translocates to the nucleus. Nrf2 binds antioxidant response elements in the regulatory regions of target genes and activates transcription (Aleksunes and Manautou, 2007). NADPH:quinone oxidoreductase 1 is a prototypical target gene of Nrf2 signaling (Venugopal and Jaiswal, 1996; Nioi et al., 2003). Antioxidant response element sequences have been identified in mouse Abcc1–4 (Mrp1–4) promoters (Fig. 23) (Hayashi et al., 2003; Vollrath et al., 2006; Maher et al., 2007). Treatment of mice with Nrf2-activating chemicals coordinately induces hepatic Mrp2–6 mRNA (Maher et al., 2005a, 2007) and some Oatp isoforms (Cheng et al., 2005b). The Nrf2 activator oltipraz induces Mrp3, Mrp4, Mdr1a, and Mdr1b mRNA in rat livers (Cherrington et al., 2002; Merrell et al., 2008) and MDR1, MRP2, MRP3, and BCRP in human hepatocytes (Fig. 23) (Jigorel et al., 2006). Small interfering RNA knockdown of Nrf2 in HepG2 cells prevents pharmacological induction of MRP2 mRNA (Adachi et al., 2007). Likewise, induction of BSEP mRNA and protein expression in HepG2 cells after oltipraz treatment is attenuated when small interfering RNAs are used to lower NRF2 expression (Weerachayaphorn et al., 2009). In addition, up-regulation of liver Mrp3 and 4 mRNA and protein after oltipraz treatment is observed in wild-type, but not Nrf2-null mice (Fig. 21) (Maher et al., 2007). Induction of Mrp3 and Mrp4 mRNA and protein during hepatotoxicity has also been shown to be dependent upon Nrf2 expression (Aleksunes et al., 2008c; Maher et al., 2008; Okada et al., 2008). There is additional evidence that Nrf2 can work in concert with CAR to regulate metabolism and transport (Slitt et al., 2006).
Mice with low expression of the Nrf2 repressor protein Kelch-like ECH-associated protein 1 (Keap1), not only have higher Nrf2 activation, but also elevated levels of Mrp2–4 mRNA and protein (Okada et al., 2008; Reisman et al., 2009b). Increased Mrp2–4 expression in Keap1-knockdown mice has functional consequences in the disposition of acetaminophen conjugates (Reisman et al., 2009a). Regulation of Mrp transporters via Nrf2 suggests that enhanced efflux is a component of the coordinated response to cellular oxidative stress.
VIII. Regulation of Hepatic Transporters in Pathophysiological Conditions
Transporters are highly expressed on hepatocytes and cholangiocytes and, in turn, are quite important in the biotransformation and disposition of toxicants. This section discusses the regulation of drug transporters in the liver in response to a variety of pathological conditions. It is generally thought that differential changes in the mRNA, protein, and/or function of transporters in damaged livers are an adaptive response to reduce cellular accumulation of substrates. Evidence supporting this notion includes the similar patterns of transporter expression among diverse conditions including cholestasis, drug-induced hepatotoxicity, liver regeneration, and ischemia-reperfusion injury (Fig. 24). There are likely to be consequences for changes in transporter expression and function. One possibility is drug-disease interactions in which a pathological condition alters the expression of a particular transporter responsible for the excretion of one of the patient's medications. In addition, this section provides examples of how transporters influence susceptibility to liver injury either by interfering with endogenous systems (such as bile acid transport) or directly altering uptake or efflux of toxicants from the liver.
Fig. 24.
Pathophysiological regulation of hepatic transporters. Hepatic mRNA and/or protein expression of rodent uptake and efflux transporters is increased (↑), decreased (↓), and/or unchanged (↔) in response to various toxicants and pathological conditions. The time points at which mRNA and protein transporter changes are observed vary among experimental models.
A. Acetaminophen Hepatotoxicity
Acetaminophen (APAP) hepatotoxicity is the leading cause of drug-induced liver failure in the United States. APAP is a commonly used analgesic and antipyretic that is safe when taken at therapeutic doses. When supratherapeutic doses are ingested, detoxification pathways (sulfation and glucuronidation) can be overwhelmed, and APAP is bioactivated by cytochrome P450 enzymes to a toxic, reactive metabolite. The reactive APAP electrophile is detoxified by conjugation with glutathione; however, when intracellular glutathione stores are depleted, the metabolite reacts with sulfhydryl groups of cellular proteins. The formation of protein covalent adducts, in addition to oxidative stress, results in centrilobular hepatocyte damage that can ultimately lead to fulminant hepatic failure.
Because of the similar responses of rodents and humans to APAP, laboratory animals have been used to study the effects of chemical-induced liver injury on transporter expression and function. In general, hepatobiliary transporters are similarly regulated in rodents and humans in response to APAP. Exposure of rats to APAP increases hepatic Mrp2 and Pgp protein expression (Ghanem et al., 2004). Administration of a single toxic dose of APAP to mice lowers mRNA expression of the basolateral uptake transporters Oatp1a1, Oatp1b2, and Ntcp (Aleksunes et al., 2005, 2007). Concurrently, basolateral (Mrp1, Mrp3, Mrp4) and canalicular (Mrp2, Mdr1a, Mdr1b) efflux transporter mRNA levels are elevated in APAP-treated mice (Aleksunes et al., 2005, 2007). Protein expression patterns mirror mRNA changes. Livers from APAP-treated mice exhibit reduced Oatp1a1, Oatp1b2, and Ntcp and increased Mrp2, Mrp3, and Mrp4 protein expression (Aleksunes et al., 2006; Campion et al., 2008). It is noteworthy that Mrp3 and Mrp4 proteins are selectively up-regulated in hepatocytes surrounding the central vein and adjacent to regions of hepatic damage (Aleksunes et al., 2006). In these studies, transporter expression was largely unchanged by nonhepatotoxic APAP doses, suggesting a dependence on hepatic injury to alter transporter levels (Aleksunes et al., 2005). Like rats and mice, explant liver specimens from patients with fulminant hepatic failure caused by APAP exhibit elevated expression of efflux transporters. Human MRP1 and MRP4 mRNA levels are significantly higher in liver specimens from APAP overdoses compared with normal liver controls (Barnes et al., 2007). In addition, MRP4, MRP5, BCRP, and Pgp proteins are increased in livers from patients after APAP overdose.
A single low dose of APAP protects rodents against a subsequent higher dose of APAP (a process known as autoprotection) (Aleksunes et al., 2008a). It is noteworthy that Mrp4 up-regulation is localized to proliferating hepatocytes and may contribute to APAP resistance in mice (Aleksunes et al., 2008a). Interruption of hepatocyte proliferation using an antimitotic chemical not only inhibits APAP autoprotection, but also prevents induction of Mrp4 (Aleksunes et al., 2008a). Functional studies to dissect the role of Mrp4 in APAP hepatotoxicity are under way, although it is purported that Mrp4 may reduce hepatotoxicity by removing byproducts of cellular injury and/or facilitating hepatocyte recovery by exporting signaling molecules to adjacent hepatocytes and nonparenchymal cells. This hypothesis is supported by the fact that Kupffer cells participate not only in protecting the liver from APAP toxicity but also in up-regulating hepatocellular Mrp4 protein (Campion et al., 2008). Work by Ghanem et al. (2005) has provided an additional explanation for APAP autoprotection in rodents. Pretreatment of rats with increasing doses of APAP alters the excretion of the final high dose of APAP and corresponds to reduced hepatotoxicity (compared with rats receiving only the high dose). Up-regulation of Mrp3 in response to APAP pretreatment shifts excretion of APAP-glucuronide from bile to urine (Ghanem et al., 2005). Using Mrp3-null mice, APAP-glucuronide has been shown to be an in vivo substrate for Mrp3 (Manautou et al., 2005; Zamek-Gliszczynski et al., 2006). More recently, it has been suggested that the diversion of APAP-glucuronide from bile to urine by Mrp3 prevents the enterohepatic recirculation of APAP and reduces exposure of the liver to APAP (Ghanem et al., 2009). Likewise, rat Mrp3 is induced in other pathological conditions such as nonalcoholic fatty liver disease (induced by a methionine and choline-deficient diet) and bile-duct ligation (Lickteig et al., 2007a; Villanueva et al., 2008). Induction of Mrp3 protein in both models corresponds with higher urinary excretion of APAP-glucuronide in rats (Lickteig et al., 2007a; Villanueva et al., 2008). These studies collectively point to potentially novel roles for Mrp3 and Mrp4 transporters in liver adaptation to APAP hepatotoxicity.
B. Carbon Tetrachloride Hepatotoxicity
Carbon tetrachloride (CCl4) is a chemical that was previously used as a dry-cleaning solvent, a refrigerant, and a fire retardant. Its industrial use has been largely abandoned because of well documented adverse health effects, including centrilobular hepatotoxicity. CCl4 is bioactivated to a highly reactive free radical that stimulates lipid peroxidation and subsequent hepatocyte damage (Manibusan et al., 2007).
Like APAP, CCl4 influences the expression of hepatobiliary transporters. Messenger RNA and/or protein expression of uptake transporters (Ntcp, Oatp1a1, Oatp1b2, Oat3, Oct1) is decreased in mouse and rat livers after CCl4 (Geier et al., 2002; Aleksunes et al., 2005; Okumura et al., 2007). Meanwhile, CCl4 increases canalicular efflux transporters (Mdr1a, Mdr1b, Mdr2) mRNA and/or protein expression in rat livers that corresponds with enhanced transport activity (Nakatsukasa et al., 1993; Song et al., 2003; Minami et al., 2005; Okumura et al., 2007). Administration of a single hepatotoxic dose of CCl4 to mice increases Mrp2 mRNA, whereas multiple low doses of CCl4 in rats reduce hepatic Mrp2 mRNA (Aleksunes et al., 2006; Okumura et al., 2007). On the basolateral membrane, Mrp1 and Mrp4 are elevated in livers of CCl4-treated mice and rats (Aleksunes et al., 2006; Okumura et al., 2007). Similar to the immunostaining distribution of Mrp4 protein in APAP livers, up-regulation of Mrp4 protein after CCl4 exposure is observed on hepatocytes adjacent to the central vein (Aleksunes et al., 2006). It is noteworthy that enhanced Mrp1 staining in rat livers exposed to CCl4 for 2 weeks is not only localized to hepatocytes but also to stellate cells (Hannivoort et al., 2008). Therefore, expression of efflux transporters in multiple cell types and in different regions may be important in recovery of the liver from chemical injury. More data in rodent and human livers after chemical-induced injury may help to delineate the extent of transporter changes with varying degrees of hepatotoxicity.
C. α-Naphthylisothiocyanate Cholestasis
Cholestasis is the disruption of bile flow that can occur at the cellular level of the hepatocyte, at the level of the intrahepatic biliary ductules, or as a result of extrahepatic obstruction of the bile ducts. Interruption of bile flow leads to the accumulation of bile acids and other bile components in hepatocytes, and ultimately hepatobiliary toxicity. Cholestasis is often designated as intrahepatic or extrahepatic (i.e., obstructive), depending upon the etiology.
α-Naphthylisothiocyanate (ANIT) damages bileductules and causes intrahepatic cholestasis in rodents. After glutathione conjugation, ANIT is transported into bile by Mrp2 (Dietrich et al., 2001b). Upon release into the bile, ANIT-glutathione rapidly dissociates, and ANIT injures bile duct epithelial cells. Damage to the biliary tract reduces bile flow leading to hepatic accumulation of bile acids and subsequent hepatocyte necrosis and neutrophil infiltration. In addition, ANIT can be reabsorbed into the cell and again conjugated with glutathione leading to depletion of glutathione within the cell.
Because glutathione conjugates have a high affinity for Mrp2 transport, it was not surprising that rats lacking Mrp2 are protected from ANIT-induced injury. However, it was surprising that the liver adapts to ANIT injury by up-regulating Mrp2 mRNA and protein (Cui et al., 2009b). It can be hypothesized that this response would lead to higher exposure of the biliary tract to ANIT upon a subsequent dose. A number of other canalicular (Mdr2, Bsep, Atp8b1) and basolateral transporters (Mrp3 and Ostβ) are also induced by ANIT in rodents (Ogawa et al., 2000; Liu et al., 2005; Cui et al., 2009b; Tanaka et al., 2009). Up-regulation of Mrp3 and Ostβ may redirect bile acids from bile to blood and help to limit the accumulation within hepatocytes. Likewise, Ntcp, Oatp1a1, and Oatp1b2 mRNA are down-regulated in ANIT-treated mice and rats and may aid in preventing uptake of bile acids into the liver (Ogawa et al., 2000; Liu et al., 2005; Cui et al., 2009b; Tanaka et al., 2009).
D. Lipopolysaccharide Cholestasis
One complication of systemic bacterial infections is intrahepatic cholestasis. Lipopolysaccharide (LPS), also termed endotoxin, is a component of the outer cell wall of Gram-negative bacteria that enters the liver through circulating portal blood. LPS exogenously administered to rodents impedes bile flow and biliary excretion of organic anions and leads to cholestatic liver injury. Dramatic reductions in bile flow can be observed within 6 to 12 h after LPS treatment. Because of the rapid decline in bile flow after LPS, it has been hypothesized that LPS exposure alters expression and/or function of transporters that participate in bile salt-dependent (Ntcp, Bsep) and bile salt-independent (Mrp2) bile flow. In line with this hypothesis, Ntcp mRNA is reduced in rodent livers 2 h after LPS administration (Green et al., 1996), and by 16 h, both Ntcp mRNA and protein levels are reduced to 10% of control rats (Trauner et al., 1998; Lee et al., 2000a; Geier et al., 2003b; Cherrington et al., 2004). The response of mice and humans to LPS is similar to that of rats. Down-regulation of Ntcp mRNA and protein occurs in the livers of LPS-treated mice and in human liver slices after incubation with LPS (Elferink et al., 2004; Lickteig et al., 2007b). Bile acid transport is compromised not only on the basolateral membrane, but also on the canalicular surface of rat livers. LPS down-regulates Bsep mRNA and protein in rodent livers (Vos et al., 1998; Lee et al., 2000a; Hojo et al., 2003; Cherrington et al., 2004; Lickteig et al., 2007b). As a consequence, the efflux of bile acids from canalicular plasma membrane vesicles is reduced in livers from LPS-treated rats (Moseley et al., 1996; Bolder et al., 1997).
Administration of LPS to rodents diminishes the biliary excretion of not only bile acids, but also non–bile-acid organic anions. Lower expression of Mrp2 mRNA and protein in livers of LPS-treated rodents corresponds with impaired biliary excretion of Mrp2 substrates (Bolder et al., 1997; Vos et al., 1998; Lee et al., 2000a; Geier et al., 2003b; Hojo et al., 2003; Cherrington et al., 2004; Lickteig et al., 2007b). A decline in biliary organic anion excretion in rats is observed as early as 3 h after LPS and is probably a result of rapid retrieval of Mrp2 protein from the canalicular membrane to subapical vesicles (Kubitz et al., 1999; Zinchuk et al., 2005). Using liver slices from rats, subapical staining of Mrp2 protein after in vitro LPS exposure has also been reported (Elferink et al., 2004). In contrast, MRP2 staining in human liver slices is reduced in response to LPS, but remains localized to the canaliculus with no observable redistribution to other membrane sites (Elferink et al., 2004). From these in vitro findings, it is thought that the mechanisms responsible for protein regulation and trafficking in response to LPS occur in a species-specific manner.
Impaired uptake of chemicals across the basolateral hepatocyte membrane during endotoxemia probably contributes to the compromised excretion of organic anions into bile. Likewise, reduced mRNA expression of mouse and rat Oatp1a1, -1a4, and -1b2 are observed in livers after LPS (Hartmann et al., 2002; Geier et al., 2003b; Cherrington et al., 2004; Li and Klaassen, 2004; Lickteig et al., 2007b). Likewise, mouse Oat2 and rat Oat3 mRNA are decreased after LPS (Cherrington et al., 2004; Lickteig et al., 2007b). Reduced mRNA expression of organic anion transporters is reflected functionally in lower uptake of organic anions into basolateral plasma membrane vesicles from LPS-treated rats (Bolder et al., 1997).
E. Bile-Duct Ligation
Extrahepatic cholestasis is typically observed in patients with gallstones or tumors in the common biliary tract, and it is often recapitulated in rodents by ligating the common bile duct (BDL). BDL prevents bile flow, leading to a backflow of biliary constituents, such as bile acids into hepatocytes, as early as 1 day after surgery (Slitt et al., 2007). The liver adapts to the higher burden of biliary constituents in part by altering the expression of hepatobiliary transporters. In general, BDL decreases mRNA and protein levels of uptake transporters such as Ntcp, Oatp1a1, Oatp1b2, and Oct1 (Gartung et al., 1996; Dumont et al., 1997; Denk et al., 2004a; Donner et al., 2007). Increased serum and urinary bile acid concentrations observed in rodents after BDL are due, to some extent, to decreased Ntcp and Oatp expression (Lee et al., 2001a; Zollner et al., 2002; Kamisako and Ogawa, 2005). Apart from these findings, there are a number of mouse Oatp isoforms (2b1, 4a1, 1c1) for which the mRNA expression does not change 3 days after BDL (Lickteig et al., 2007b). Two Oatp genes (1a4 and 3a1) are induced in mouse livers 3 to 7 days after BDL (Lickteig et al., 2007b; Slitt et al., 2007). Differential mRNA regulation of Oatp isoforms (decreased, increased, and unchanged) during extrahepatic cholestasis may alter the uptake of some organic anions into the liver in a selective manner.
Investigations of the regulation of canalicular transporters after BDL have yielded conflicting findings. Bsep mRNA and protein are unchanged or up-regulated in rodents after BDL (Hyogo et al., 2001; Wagner et al., 2003; Kamisako and Ogawa, 2005; Lickteig et al., 2007a; Slitt et al., 2007). Differing reports suggest that canalicular Mrp2 mRNA and protein can be increased or decreased after BDL, depending on the species (Trauner et al., 1997; Kagawa et al., 1998; Paulusma et al., 2000; Hyogo et al., 2001; Lee et al., 2001a; Wagner et al., 2003; Kamisako and Ogawa, 2005; Donner et al., 2007; Slitt et al., 2007). Mrp2 mRNA and protein are reduced in rats after BDL (Trauner et al., 1997; Kagawa et al., 1998; Paulusma et al., 2000; Donner and Keppler, 2001; Hyogo et al., 2001; Lee et al., 2001a; Denk et al., 2004b; Kamisako and Ogawa, 2005), but remain preserved or slightly increased in mouse livers (Wagner et al., 2003; Slitt et al., 2007). Additional canalicular transporters are differentially expressed after BDL. Mdr mRNA (namely, Mdr1b and Mdr2) and Pgp protein are increased in rodent livers after BDL (Accatino et al., 1996; Kagawa et al., 1998; Hyogo et al., 2001). In contrast, BDL reduces gene expression of the sterol half-transporters (Abcg5 and Abcg8) within 1 day after surgery in rats (Kamisako and Ogawa, 2005). Because BDL causes the complete interruption of bile flow and cannot be overcome, there is probably little functional consequence of compensatory up-regulation of canalicular transporters. Instead, transporter changes probably reflect a general adaptation to liver injury.
Similar to chemical-induced liver injury, basolateral efflux transporters are up-regulated after BDL and probably function as an alternate excretion pathway to prevent intracellular accumulation of bile constituents within hepatocytes and cholangiocytes. Numerous reports have demonstrated elevated mRNA and protein levels of Mrp1, -3, -4, -5, Ostα (protein only), and Ostβ (mRNA only) between 3 to 14 days after BDL (Ogawa et al., 2000; Donner and Keppler, 2001; Soroka et al., 2001; Wagner et al., 2003; Denk et al., 2004b; Kamisako and Ogawa, 2005; Boyer et al., 2006; Slitt et al., 2007). Mrp4-null mice have enhanced liver injury after BDL compared with wild-type mice (Mennone et al., 2006), suggesting that Mrp4 up-regulation is an important compensatory mechanism of the liver (Denk et al., 2004b). The adaptive up-regulation of basolateral efflux transporters entails some isoform selectivity, because Mrp6, -7, and -9 mRNA are unchanged in rodent livers after BDL.
Mrp3 is normally expressed in centrilobular hepatocytes (Aleksunes et al., 2006). In response to BDL, Mrp3 staining is enhanced in centrilobular hepatocytes and extends to periportal cells (Donner and Keppler, 2001; Soroka et al., 2001). Although Asbt mRNA and protein levels are increased approximately 3-fold in livers from rats after BDL, the intensity of Asbt protein staining on proliferating cholangiocytes is reduced (Lee et al., 2001a). One explanation for this discrepancy may be the dramatic increase in cholangiocyte proliferation (greater than 10-fold) after BDL, in effect diluting Asbt protein among a larger number of cells. Coordinated regulation of the efflux transporters Mrp3, Mrp4, Asbt, Ostα, and Ostβ in hepatocytes and cholangiocytes probably reduces the bile acid burden of the liver during extrahepatic cholestasis.
F. Partial Hepatectomy
One of the unique features of the liver is its ability to regenerate. In response to cellular loss, a number of stimuli activate normally quiescent hepatocytes to undergo cell division. Administration of nonlethal doses of toxicants, such as APAP and CCl4, triggers parenchymal cells to undergo mitosis to repopulate the liver lobule. During severe liver injury or when hepatocyte proliferation is inhibited (using 2-acetyl-aminofluorene), a resident population of progenitor cells is activated to replace hepatocytes and cholangiocytes. In addition to toxicant exposure, hepatic regeneration can be modeled in rodents using surgical removal of 66% of the liver (partial hepatectomy; PHx). Appropriate controls for these studies are sham-operated rodents that undergo the same surgical procedure without ligation of lobes and without removal of two thirds of the liver. Within 24 to 36 h of removing three liver lobes, parenchymal cells undergo synchronized DNA synthesis and cell division. By 1 to 2 weeks, the original liver cell mass is restored and hepatocytes return to quiescence.
Within 24 h after PHx, bile flow and biliary secretion of bile acids, cholesterol (mice only), and phospholipids (mice only) in rodents are increased (Vos et al., 1999; Csanaky et al., 2009). In contrast, the secretion of glutathione into bile is reduced. It should be noted that these parameters are expressed as secretion into bile per gram of liver, rather than normalized to total body weight. Likewise, bile acid and total bilirubin levels are elevated in the plasma of rats and mice 24 h after PHx (Vos et al., 1999; Chang et al., 2004; Csanaky et al., 2009). Phenotypic changes in bile flow and the disposition of bile constituents suggest that hepatocytes adapt to cell loss by differentially regulating bile acid transporters (and possibly bile acid synthesis) to limit accumulation of toxic bile acids in remnant hepatocytes.
Compared with sham-operated control rats, remnant livers from PHx rats exhibit early reductions (3–24 h) in the mRNA expression of Oatp1a1, Oatp1a4, and Ntcp that are restored to normal levels by 2 to 4 days (Gerloff et al., 1999; Vos et al., 1999). Similar declines in Ntcp and Oatp1a4 proteins are observed at 24 h in PHx-rats (Vos et al., 1999). In contrast, Oatp1a4 mRNA and protein are elevated in mice 24 to 48 h after PHx (Csanaky et al., 2009). Regulation of Oatp1a1 protein in remnant rodent livers is either unchanged or reduced 1 to 4 days after PHx (Gerloff et al., 1999; Vos et al., 1999; Csanaky et al., 2009). Microarray analysis has confirmed lower mRNA levels of Oatp1a4, Oatp1b2, and Ntcp in rat livers between 12 and 48 h after PHx (Dransfeld et al., 2005). It is noteworthy that Oatp1a6 mRNA is increased by 3 h and remains elevated over a 48-h period (Dransfeld et al., 2005). Lower expression of Ntcp and Oatp isoforms corresponds with reduced taurocholate uptake in basolateral plasma membrane vesicles obtained from rat livers after PHx (Green et al., 1997; Gerloff et al., 1999; Vos et al., 1999).
Canalicular Bsep and Mrp2 transport proteins are either unchanged or elevated after PHx, depending on the species and strain. In Wistar rats, expression of Bsep and Mrp2 mRNA and protein is fairly stable 24 h after PHx (Vos et al., 1999; Ros et al., 2003; Dransfeld et al., 2005). Furthermore, the subcellular localization of Bsep and Mrp2 to the canalicular membrane is not affected by PHx (Dransfeld et al., 2005). In contrast, Sprague-Dawley rats have increased Bsep and Mrp2 protein expression from 12 h to 2 days after PHx (Gerloff et al., 1999). It is noteworthy that increased Bsep and Mrp2 mRNA levels are not observed until 2 to 4 days, suggesting that early protein changes occur through post-transcriptional processes (Gerloff et al., 1999). As in rats, transporter profiling of mouse livers after PHx demonstrates either increased or unchanged Bsep mRNA (Huang et al., 2006b; Csanaky et al., 2009). Normal or enhanced Bsep and Mrp2 expression after PHx is consistent with functional bile flow and bile acid excretion by the remnant liver, as well as the requirement of an animal with a smaller liver mass to transport the same amount of bile acids and endobiotics as animals with a regular liver mass.
Rat Mdr1b mRNA (but not mouse) dramatically increases shortly after PHx and remains elevated through 48 h (Vos et al., 1999; Csanaky et al., 2009). Levels of Mdr1b increase approximately 40-fold in rat liver after PHx, with no change in Mdr1a (Vos et al., 1999; Ros et al., 2003). In mice, PHx stimulates expression of Mdr2 at 24 and 48 h after surgery, which corresponds with increased excretion of phospholipids into bile (Csanaky et al., 2009).
Consistent with toxicant-induced hepatocyte loss, expression of basolateral Mrp1, Mrp3, Mrp4, and Ostβ transporters is increased in rodents after PHx. Within 3 h after PHx, Mrp1 mRNA expression is enhanced and persists at high levels through 48 h (Vos et al., 1999). Mrp3 protein is dramatically up-regulated in remnant livers from rats and mice after 70 and 90% PHx (Chang et al., 2004; Csanaky et al., 2009). PHx enhances the immunostaining of Mrp3 on the basolateral membranes of hepatocytes throughout the liver (Csanaky et al., 2009). Mrp3 protein is increased most notably in hepatocytes surrounding the central and portal veins (Csanaky et al., 2009). The mRNA analysis has also revealed a 2-fold increase in Mrp4 in rat hepatocytes 24 h after PHx (Ros et al., 2003). It has more recently been shown that mRNA expression of Ostβ is elevated in remnant mouse livers at 24 and 48 h (Csanaky et al., 2009). Because Mrp3, Mrp4, and Ostβ transport bile acids, up-regulation of these transporters in response to PHx probably contributes to enhanced basolateral egress and elevated plasma bile acid levels.
As noted above, progenitor cells contribute to the repopulation of the liver after hepatocyte loss. Pretreatment of rats with 2-acetyl-aminofluorene before PHx prevents hepatocytes from dividing, leading to the activation of progenitor oval cells in the liver. By 9 days after PHx, expression of Mdr1b, Mdr2, Mrp1, and Mrp4 mRNA in progenitor cells increases compared with appropriate control rats (Ros et al., 2003). Collectively, these data suggest that efflux transporter expression can be stimulated in hepatocytes and progenitor cells during liver regeneration, and the specific cell type engaged in proliferation and transporter expression is dependent upon the degree of tissue injury.
G. Ischemia-Reperfusion
Hepatic ischemia followed by tissue reperfusion is a complication of liver resection surgery, transplantation, hypovolemic shock, intra-arterial chemotherapy, and embolization. Ischemia with and without reperfusion can be recapitulated surgically in rodents. Prominent features of ischemia-mediated injury include hypoxia, activation of resident macrophages, and cholestasis. Within 6 to 24 h after hepatic ischemia in rats (with or without reperfusion), hypoxia and cytokine signaling are detected (Tanaka et al., 2006; Fouassier et al., 2007). During this period, there are declines in bile flow and biliary bile acid excretion in ischemic rats. Corresponding increases in markers of cholestasis, such as serum transaminases, bilirubin, and bile acids, are also observed.
Similar to other pathogenic conditions within the liver, hepatobiliary transporter expression is altered in rodents after ischemia reperfusion. Ischemia elevates hepatic Mdr1b mRNA and reduces Ntcp, Oatp1a1, Oatp1a4, Oatp1b2, Bsep, and Mrp2 mRNA in rats (Tanaka et al., 2006, 2008; Fouassier et al., 2007). In addition, incubation of cultured hepatocytes under hypoxic conditions decreases mRNA and protein expression of Ntcp, Bsep, and Mrp2 (Fouassier et al., 2007). It is noteworthy that hepatic ischemia-reperfusion injury is accompanied by up-regulation of Mrp2 and Mrp4 mRNA and protein in the kidneys (Tanaka et al., 2008). These data suggest that there is a shift from biliary to renal excretion of chemicals generated by hepatic ischemia and reperfusion.
Collectively, the chemical and surgical models of liver injury presented in this section demonstrate similar changes in the regulation of hepatobiliary transporters. Although beyond the scope of this section, there are a number of other chemicals (bromobenzene and troglitazone) and conditions (fatty liver, nonalcoholic steatohepatitis, hepatocellular carcinoma, and viral hepatitis) that show analogous findings. In general, a diseased liver reduces expression of sinusoidal uptake transporters while increasing efflux transporters on both the basolateral and canalicular membranes (Fig. 24). It is noteworthy that the remarkable induction of basolateral efflux transporters represents an alternate route of excretion for hepatocytes during injury. Future studies are needed to determine whether differential transporter regulation is a beneficial event for the human liver to recover and regenerate. Likewise, the clinical consequences of altered transporter expression in patients with liver disease need to be addressed (i.e., drug disposition).
IX. Future Directions
Since the identification of drug transporters as mediators of multidrug resistance in cancer cells, researchers have demonstrated ubiquitous expression of not only efflux but also uptake carriers in a variety of tissues. In this review, we have attempted to thoroughly describe the expression patterns of rodent and human bile acid and drug transporters throughout the body. It is noteworthy that some transporters are expressed across many tissues, whereas others have very restrictive expression patterns. Although the exact endogenous roles of only some of these transporters are known, initial insights have been gained from the development of knockout mice and the identification of human polymorphic variants.
There are a number of areas for future investigation that will aid in our understanding of the physiological and pharmacological functions of transporters:
A. Transporter Localization
The development of specific antibodies to transporters has aided in the identification of subcellular and tissue distribution. However, there are differences in transporter isoform localization for which a mechanism is not clear. For example, some transporters localize to the apical surface in certain epithelia and to the basolateral membrane in others. Additional work is needed to understand tissue-specific targeting in polarized cells.
B. In Vitro-In Vivo Extrapolation
Extrapolation of in vitro determined transport properties (using recombinantly expressed transporters) needs to be judicious. For example, uptake of fluvastatin by OATP1B1, -1B3, and -2B1 is inhibited by gemfibrozil between 60 and 90% in overexpressed systems, whereas uptake into primary hepatocytes is blocked to a lesser extent (27% inhibition) (Noe et al., 2007). In vivo studies are needed to confirm the functional activity of transporters for the various in vitro substrates.
C. Transport Driving Forces
A better understanding of transport mechanisms and driving forces for transport (cosubstrates, countertransport) may be facilitated by structural modeling and determination of transporter crystal structures.
D. Substrate Binding Domains
Depending on the transporter, there may be more than one substrate binding domain. Chemicals that are transported by a particular pump may not compete for transport if they bind to different domains. Therefore, work is needed to thoroughly characterize the inhibitory potency and cooperativity of cosubstrates in vitro and in vivo to guide clinical decision-making (Noe et al., 2007).
E. Racial Differences
There are limited comparisons of transporter expression and regulation in various ethnic groups. More comprehensive screening of drug transporters in human tissues should aid in predicting whether particular transporters are more influential in drug disposition in different populations. Databases such as HapMap and PharmGKB are particularly useful in estimating the frequency of race-specific polymorphism allelic variants.
F. Pharmacokinetics
The existence of efflux transporters in various cell types necessitates reconsideration of how transporters influence pharmacokinetic drug profiles. Plasma clearance of drugs has traditionally been used to evaluate pharmacokinetics. Although this clinical parameter probably reflects the influence of uptake transporters, plasma drug concentrations do not address tissue accumulation and the function of efflux transporters on opposing domains of the plasma membrane. The expression and/or function of these transporters will influence intracellular concentrations and, consequently, the activation of cytosolic and/or nuclear drug targets. In addition, the intracellular concentrations of drugs will affect susceptibility of cells to toxicity.
G. Redundancy and Cooperativity
Mutations in the expression of some transporters clearly underlie genetic disorders and thereby point to critical roles for these proteins in normal physiology. However, other transporters exhibit overlapping substrate specificity resulting in functional redundancy. As a result, the development of single isoform null mice demonstrated little effect on the pharmacokinetics of some substrates identified from in vitro screens. It was not until double- and triple-knockout mice were generated that the in vivo redundancy of transporters was more clearly elucidated. This has been most evident for shared substrates of Bcrp and Mdr1a/1b. Because of these findings, polymorphisms in redundant transporters may not yield a clinical phenotype. Instead, it may become important to look at combinations of variants in functionally related transport systems to assess variations in drug pharmacokinetics.
H. Novel Polymorphisms
Because transporters are highly expressed in excretory organs, it can be hypothesized that clinically observed variations in drug kinetics and pharmacodynamics involve transporters. Up until now, research has primarily been limited to nonsynonymous polymorphisms in the coding region of transporters. However, future work should aim to characterize the functional consequence of intronic and promoter variants in drug pharmacokinetics and response.
I. Ontogeny
The expression profile of hepatic transporters before and after birth has been well characterized in mice. Additional work is necessary to extend this work to human livers (as well as other tissues) and to identify the “triggers” that turn on or off the expression of transporters during different developmental periods. This work will be particularly important in adjusting dosing regimens for newborns and premature infants to ensure proper efficacy and minimize drug toxicity.
J. Novel Drug Delivery Routes
Drug transporters may be exploited for delivery of therapeutics into the body. For example, Oat6 in the nasal epithelium may be a novel route of administration of drugs to the central nervous system (Nigam et al., 2007). Likewise, the mRNA expression of a number of OATP isoforms (1A2, 2B1, 3A1, and 4A1) is elevated in human breast carcinoma compared with nonmalignant specimens (Miki et al., 2006; Wlcek et al., 2008). The relative expression of OATPs in cancer cells needs to be compared with that of other normal tissues such as liver. Up-regulation of uptake transporters in cancer cells may be used in drug development to improve targeting of chemotherapeutic agents and minimize cytotoxicity to normal cells.
Acknowledgments.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant RR021940]; National Institutes of Health National Institute of Environmental Health Sciences [Grants ES009649, ES009716, ES013714, ES007079]; and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK081461, DK080774].
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.002014.
- ABC
- ATP-binding cassette
- ABCA1
- ATP-binding cassette transporter A1
- ABCG
- ATP-binding cassette subfamily G
- ABCG5/8
- ATP-binding cassette subfamily G members 5 and 8
- ABCP
- ABC transporter highly expressed in placenta
- AhR
- aryl hydrocarbon receptor
- ANIT
- α-naphthylisothiocyanate
- APAP
- acetaminophen
- ASBT
- apical sodium-dependent bile acid transporter
- ATP7B
- ATPase copper-transporting β polypeptide
- ATP8B1
- ATPase class I type 8B member 1
- BCRP
- breast cancer resistance protein
- BDL
- bile-duct ligation
- BQ-123
- cyclo(d-Asp-Pro-d-Val-Leu-d-Trp)
- BSEP
- bile salt export pump
- CAR
- constitutive androstane receptor
- CNT
- concentrative nucleoside transporter
- ENT
- equilibrative nucleoside transporter
- FXR
- farnesoid X receptor
- Gly-Sar
- glycylsarcosine
- GF120918
- elacridar
- HEK
- human embryonic kidney
- HERG
- human ether-à-go-go-related gene
- HNF
- hepatocyte nuclear factor
- JNJ-7706621
- 4-[5-amino-1-(2,6-difluorobenzoyl)-1H-[1,2,4]triazol-3-ylamino]-benzenesulfonamide
- JVS
- juvenile visceral steatosis
- Keap1
- Kelch-like ECH-associated protein 1
- LPS
- lipopolysaccharide
- MATE
- multidrug and toxin extrusion transporter
- MATE2-K
- multidrug and toxin extrusion 2-K
- MDCK
- Madin-Darby canine kidney
- MDR
- multidrug resistance protein
- MRP
- multidrug resistance-associated protein
- MXR
- mitoxantrone resistance
- NHERF
- Na+/H+ exchanger regulatory factor
- Nrf2
- NFE2-related factor 2
- NTCP
- sodium taurocholate cotransporting polypeptide
- OAT
- organic anion transporter
- OATP
- organic anion transporting polypeptide
- OCT
- organic cation transporter
- OCTN
- organic cation/carnitine transporter
- OST
- organic solute transporter
- PCN
- pregnenolone-16α-carbonitrile
- PDZ
- postsynaptic density 95/disc-large/zona occludens
- PDZK
- PDZ kinase
- PEPT
- peptide transporter
- PFDA
- perfluorodecanoic acid
- PFIC
- progressive familial intrahepatic cholestasis
- Pgp
- P-glycoprotein
- PHx
- partial hepatectomy
- PMEA
- 9-(2-(phosphomethoxy)ethyl)-adenine, adefovir
- PPAR
- peroxisome proliferator-activated receptor
- PXR
- pregnane X receptor
- RXRα
- retinoid X receptor α
- SLC
- solute carrier
- SNP
- single-nucleotide polymorphism
- SUMO
- small ubiquitin-like modifier
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- TCPOBOP
- 1,4-bis-[2-(3,5,dichloropyridyloxy)] benzene
- TR−
- transport-deficient
- URAT
- urate transporter
- Wy14643
- [[4-chloro-6-[(2,3-dimethylphenyl)amino]-2-pyrimidinyl]thio]-acetic acid.
References
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, et al. (1999) Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 274:17159–17163 [DOI] [PubMed] [Google Scholar]
- Abla N, Chinn LW, Nakamura T, Liu L, Huang CC, Johns SJ, Kawamoto M, Stryke D, Taylor TR, Ferrin TE, et al. (2008) The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther 325:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accatino L, Pizarro M, Solís N, Koenig CS, Vollrath V, Chianale J. (1996) Modulation of hepatic content and biliary excretion of P-glycoproteins in hepatocellular and obstructive cholestasis in the rat. J Hepatol 25:349–361 [DOI] [PubMed] [Google Scholar]
- Adachi H, Suzuki T, Abe M, Asano N, Mizutamari H, Tanemoto M, Nishio T, Onogawa T, Toyohara T, Kasai S, et al. (2003) Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol 285:F1188–F1197 [DOI] [PubMed] [Google Scholar]
- Adachi T, Nakagawa H, Chung I, Hagiya Y, Hoshijima K, Noguchi N, Kuo MT, Ishikawa T. (2007) Nrf2-dependent and -independent induction of ABC transporters ABCC1, ABCC2, and ABCG2 in HepG2 cells under oxidative stress. J Exp Ther Oncol 6:335–348 [PubMed] [Google Scholar]
- Adachi Y, Suzuki H, Schinkel AH, Sugiyama Y. (2005) Role of breast cancer resistance protein (Bcrp1/Abcg2) in the extrusion of glucuronide and sulfate conjugates from enterocytes to intestinal lumen. Mol Pharmacol 67:923–928 [DOI] [PubMed] [Google Scholar]
- Adkison KK, Vaidya SS, Lee DY, Koo SH, Li L, Mehta AA, Gross AS, Polli JW, Lou Y, Lee EJ. (2008) The ABCG2 C421A polymorphism does not affect oral nitrofurantoin pharmacokinetics in healthy Chinese male subjects. Br J Clin Pharmacol 66:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita H, Suzuki H, Hirohashi T, Takikawa H, Sugiyama Y. (2002) Transport activity of human MRP3 expressed in Sf9 cells: comparative studies with rat MRP3. Pharm Res 19:34–41 [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Augustine LM, Cherrington NJ, Manautou JE. (2007) Influence of acetaminophen vehicle on regulation of transporter gene expression during hepatotoxicity. J Toxicol Environ Health A 70:1870–1872 [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Campion SN, Goedken MJ, Manautou JE. (2008a) Acquired resistance to acetaminophen hepatotoxicity is associated with induction of multidrug resistance-associated protein 4 (Mrp4) in proliferating hepatocytes. Toxicol Sci 104:261–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Cui Y, Klaassen CD. (2008b) Prominent expression of xenobiotic efflux transporters in mouse extraembryonic fetal membranes compared with placenta. Drug Metab Dispos 36:1960–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Manautou JE. (2007) Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol 35:459–473 [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. (2006) Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci 89:370–379 [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AL, Maher JM, Augustine LM, Goedken MJ, Chan JY, Cherrington NJ, Klaassen CD, Manautou JE. (2008c) Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol Appl Pharmacol 226:74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. (2005) Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci 83:44–52 [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Yeager RL, Klaassen CD. (2009) Application of multivariate statistical procedures to identify transcription factors that correlate with MRP2, 3, and 4 mRNA in adult human livers. Xenobiotica 39:514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. (1999) The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 59:4237–4241 [PubMed] [Google Scholar]
- Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, et al. (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323:1718–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. (1998) A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58:5337–5339 [PubMed] [Google Scholar]
- Alnouti Y, Petrick JS, Klaassen CD. (2006) Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos 34:477–482 [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. (1997) Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology 113:1734–1740 [DOI] [PubMed] [Google Scholar]
- Amat di San Filippo C, Longo N. (2004) Tyrosine residues affecting sodium stimulation of carnitine transport in the OCTN2 carnitine/organic cation transporter. J Biol Chem 279:7247–7253 [DOI] [PubMed] [Google Scholar]
- Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S. (2006) Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology 50:941–952 [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. (2001) Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 276:28857–28865 [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M, Ng OC, Boyer JL, Suchy FJ. (1994) Characterization of cloned rat liver Na(+)-bile acid cotransporter using peptide and fusion protein antibodies. Am J Physiol 267:G637–G643 [DOI] [PubMed] [Google Scholar]
- Anderle P, Nielsen CU, Pinsonneault J, Krog PL, Brodin B, Sadée W. (2006) Genetic variants of the human dipeptide transporter PEPT1. J Pharmacol Exp Ther 316:636–646 [DOI] [PubMed] [Google Scholar]
- Anderson CM, Baldwin SA, Young JD, Cass CE, Parkinson FE. (1999a) Distribution of mRNA encoding a nitrobenzylthioinosine-insensitive nucleoside transporter (ENT2) in rat brain. Brain Res Mol Brain Res 70:293–297 [DOI] [PubMed] [Google Scholar]
- Anderson CM, Xiong W, Geiger JD, Young JD, Cass CE, Baldwin SA, Parkinson FE. (1999b) Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. J Neurochem 73:867–873 [DOI] [PubMed] [Google Scholar]
- Ando M, Ando Y, Hasegawa Y, Sekido Y, Shimokata K, Horibe K. (2001) Genetic polymorphisms of thiopurine S-methyltransferase and 6-mercaptopurine toxicity in Japanese children with acute lymphoblastic leukaemia. Pharmacogenetics 11:269–273 [DOI] [PubMed] [Google Scholar]
- Ando T, Kusuhara H, Merino G, Alvarez AI, Schinkel AH, Sugiyama Y. (2007) Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones. Drug Metab Dispos 35:1873–1879 [DOI] [PubMed] [Google Scholar]
- Angeletti RH, Novikoff PM, Juvvadi SR, Fritschy JM, Meier PJ, Wolkoff AW. (1997) The choroid plexus epithelium is the site of the organic anion transport protein in the brain. Proc Natl Acad Sci U S A 94:283–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaba F, Sarwar Z, Kumar P, Saksena S, Turner JR, Dudeja PK, Gill RK, Alrefai WA. (2008) Modulation of ileal bile acid transporter (ASBT) activity by depletion of plasma membrane cholesterol: association with lipid rafts. Am J Physiol Gastrointest Liver Physiol 294:G489–G497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annese V, Valvano MR, Palmieri O, Latiano A, Bossa F, Andriulli A. (2006) Multidrug resistance 1 gene in inflammatory bowel disease: a meta-analysis. World J Gastroenterol 12:3636–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annilo T, Dean M. (2004) Degeneration of an ATP-binding cassette transporter gene, ABCC13, in different mammalian lineages. Genomics 84:34–46 [DOI] [PubMed] [Google Scholar]
- Anwer MS, Gillin H, Mukhopadhyay S, Balasubramaniyan N, Suchy FJ, Ananthanarayanan M. (2005) Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J Biol Chem 280:33687–33692 [DOI] [PubMed] [Google Scholar]
- Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H, et al. (2008) Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 283:26834–26838 [DOI] [PubMed] [Google Scholar]
- Anzai N, Jutabha P, Enomoto A, Yokoyama H, Nonoguchi H, Hirata T, Shiraya K, He X, Cha SH, Takeda M, et al. (2005) Functional characterization of rat organic anion transporter 5 (Slc22a19) at the apical membrane of renal proximal tubules. J Pharmacol Exp Ther 315:534–544 [DOI] [PubMed] [Google Scholar]
- Anzai N, Miyazaki H, Noshiro R, Khamdang S, Chairoungdua A, Shin HJ, Enomoto A, Sakamoto S, Hirata T, Tomita K, et al. (2004) The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem 279:45942–45950 [DOI] [PubMed] [Google Scholar]
- Aoyama N, Takahashi N, Kitaichi K, Ishihara R, Saito S, Maeno N, Ji X, Takagi K, Sekine Y, Iyo M, et al. (2006) Association between gene polymorphisms of SLC22A3 and methamphetamine use disorder. Alcohol Clin Exp Res 30:1644–1649 [DOI] [PubMed] [Google Scholar]
- Apiwattanakul N, Sekine T, Chairoungdua A, Kanai Y, Nakajima N, Sophasan S, Endou H. (1999) Transport properties of nonsteroidal anti-inflammatory drugs by organic anion transporter 1 expressed in Xenopus laevis oocytes. Mol Pharmacol 55:847–854 [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Redeker S, van Vliet EA, Ramkema M, Scheffer GL, Scheper RJ, van der Valk P, Leenstra S, Baayen JC, et al. (2005) Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia 46:849–857 [DOI] [PubMed] [Google Scholar]
- Asashima T, Hori S, Ohtsuki S, Tachikawa M, Watanabe M, Mukai C, Kitagaki S, Miyakoshi N, Terasaki T. (2006) ATP-binding cassette transporter G2 mediates the efflux of phototoxins on the luminal membrane of retinal capillary endothelial cells. Pharm Res 23:1235–1242 [DOI] [PubMed] [Google Scholar]
- Aslamkhan AG, Han YH, Yang XP, Zalups RK, Pritchard JB. (2003) Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol Pharmacol 63:590–596 [DOI] [PubMed] [Google Scholar]
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, et al. (2004) Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279:22250–22257 [DOI] [PubMed] [Google Scholar]
- Augustine LM, Markelewicz RJ, Jr., Boekelheide K, Cherrington NJ. (2005) Xenobiotic and endobiotic transporter mRNA expression in the blood-testis barrier. Drug Metab Dispos 33:182–189 [DOI] [PubMed] [Google Scholar]
- Aust S, Obrist P, Jaeger W, Klimpfinger M, Tucek G, Wrba F, Penner E, Thalhammer T. (2004) Subcellular localization of the ABCG2 transporter in normal and malignant human gallbladder epithelium. Lab Invest 84:1024–1036 [DOI] [PubMed] [Google Scholar]
- Aye IL, Paxton JW, Evseenko DA, Keelan JA. (2007) Expression, localisation and activity of ATP binding cassette (ABC) family of drug transporters in human amnion membranes. Placenta 28:868–877 [DOI] [PubMed] [Google Scholar]
- Azuma Y, Takada M, Maeda M, Kioka N, Ueda K. (2009) The COP9 signalosome controls ubiquitinylation of ABCA1. Biochem Biophys Res Commun 382:145–148 [DOI] [PubMed] [Google Scholar]
- Babu E, Takeda M, Narikawa S, Kobayashi Y, Enomoto A, Tojo A, Cha SH, Sekine T, Sakthisekaran D, Endou H. (2002a) Role of human organic anion transporter 4 in the transport of ochratoxin A. Biochim Biophys Acta 1590:64–75 [DOI] [PubMed] [Google Scholar]
- Babu E, Takeda M, Narikawa S, Kobayashi Y, Yamamoto T, Cha SH, Sekine T, Sakthisekaran D, Endou H. (2002b) Human organic anion transporters mediate the transport of tetracycline. Jpn J Pharmacol 88:69–76 [DOI] [PubMed] [Google Scholar]
- Babusukumar U, Wang T, McGuire E, Broeckel U, Kugathasan S. (2006) Contribution of OCTN variants within the IBD5 locus to pediatric onset Crohn's disease. Am J Gastroenterol 101:1354–1361 [DOI] [PubMed] [Google Scholar]
- Bacq Y, Gendrot C, Perrotin F, Lefrou L, Chrétien S, Vie-Buret V, Brechot MC, Andres CR. (2009) ABCB4 gene mutations and single-nucleotide polymorphisms in women with intrahepatic cholestasis of pregnancy. J Med Genet 46:711–715 [DOI] [PubMed] [Google Scholar]
- Bacso Z, Nagy H, Goda K, Bene L, Fenyvesi F, Matkó J, Szabó G. (2004) Raft and cytoskeleton associations of an ABC transporter: P-glycoprotein. Cytometry A 61:105–116 [DOI] [PubMed] [Google Scholar]
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, Kawamoto M, Johns SJ, Ferrin TE, Carlson EJ, et al. (2006) Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther 318:521–529 [DOI] [PubMed] [Google Scholar]
- Bahadduri PM, D'Souza VM, Pinsonneault JK, Sadée W, Bao S, Knoell DL, Swaan PW. (2005) Functional characterization of the peptide transporter PEPT2 in primary cultures of human upper airway epithelium. Am J Respir Cell Mol Biol 32:319–325 [DOI] [PubMed] [Google Scholar]
- Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, Hagos Y. (2005) Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol 289:C1075–C1084 [DOI] [PubMed] [Google Scholar]
- Bailey DG, Dresser GK, Leake BF, Kim RB. (2007) Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther 81:495–502 [DOI] [PubMed] [Google Scholar]
- Bakhiya A, Bahn A, Burckhardt G, Wolff N. (2003) Human organic anion transporter 3 (hOAT3) can operate as an exchanger and mediate secretory urate flux. Cell Physiol Biochem 13:249–256 [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Mackey JR, Cass CE, Young JD. (1999) Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today 5:216–224 [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, Ritzel MW, Cass CE, Young JD. (2005) Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem 280:15880–15887 [DOI] [PubMed] [Google Scholar]
- Balimane PV, Han YH, Chong S. (2006) Current industrial practices of assessing permeability and P-glycoprotein interaction. AAPS J 8:E1–E13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. (2005) OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42:1270–1279 [DOI] [PubMed] [Google Scholar]
- Ballatori N, Fang F, Christian WV, Li N, Hammond CL. (2008) Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol 295:G179–G186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Swaan PW. (2006) Membrane topology of human ASBT (SLC10A2) determined by dual label epitope insertion scanning mutagenesis. New evidence for seven transmembrane domains. Biochemistry 45:943–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Pucci ML, Chan BS, Lu R, Ito S, Schuster VL. (2002) Prostaglandin transporter PGT is expressed in cell types that synthesize and release prostanoids. Am J Physiol Renal Physiol 282:F1103–F1110 [DOI] [PubMed] [Google Scholar]
- Bardelmeijer HA, Beijnen JH, Brouwer KR, Rosing H, Nooijen WJ, Schellens JH, van Tellingen O. (2000) Increased oral bioavailability of paclitaxel by GF120918 in mice through selective modulation of P-glycoprotein. Clin Cancer Res 6:4416–4421 [PubMed] [Google Scholar]
- Barnes K, Dobrzynski H, Foppolo S, Beal PR, Ismat F, Scullion ER, Sun L, Tellez J, Ritzel MW, Claycomb WC, et al. (2006) Distribution and functional characterization of equilibrative nucleoside transporter-4, a novel cardiac adenosine transporter activated at acidic pH. Circ Res 99:510–519 [DOI] [PubMed] [Google Scholar]
- Barnes SN, Aleksunes LM, Augustine L, Scheffer GL, Goedken MJ, Jakowski AB, Pruimboom-Brees IM, Cherrington NJ, Manautou JE. (2007) Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab Dispos 35:1963–1969 [DOI] [PubMed] [Google Scholar]
- Barrand MA, Heppell-Parton AC, Wright KA, Rabbitts PH, Twentyman PR. (1994) A 190-kilodalton protein overexpressed in non-P-glycoprotein-containing multidrug-resistant cells and its relationship to the MRP gene. J Natl Cancer Inst 86:110–117 [DOI] [PubMed] [Google Scholar]
- Barros SA, Srimaroeng C, Perry JL, Walden R, Dembla-Rajpal N, Sweet DH, Pritchard JB. (2009) Activation of protein kinase Czeta increases OAT1 (SLC22A6)- and OAT3 (SLC22A8)-mediated transport. J Biol Chem 284:2672–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. (2004) The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer 40:2064–2070 [DOI] [PubMed] [Google Scholar]
- Barton A, Eyre S, Bowes J, Ho P, John S, Worthington J. (2005) Investigation of the SLC22A4 gene (associated with rheumatoid arthritis in a Japanese population) in a United Kingdom population of rheumatoid arthritis patients. Arthritis Rheum 52:752–758 [DOI] [PubMed] [Google Scholar]
- Bataille AM, Goldmeyer J, Renfro JL. (2008) Avian renal proximal tubule epithelium urate secretion is mediated by Mrp4. Am J Physiol Regul Integr Comp Physiol 295:R2024–R2033 [DOI] [PubMed] [Google Scholar]
- Beamer WH, Eicher EM. (1976) Stimulation of growth in the little mouse. J Endocrinol 71:37–45 [DOI] [PubMed] [Google Scholar]
- Beck K, Hayashi K, Dang K, Hayashi M, Boyd CD. (2005) Analysis of ABCC6 (MRP6) in normal human tissues. Histochem Cell Biol 123:517–528 [DOI] [PubMed] [Google Scholar]
- Beck K, Hayashi K, Nishiguchi B, Le Saux O, Hayashi M, Boyd CD. (2003) The distribution of Abcc6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem 51:887–902 [DOI] [PubMed] [Google Scholar]
- Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. (2009) Genetic variation in the organic cation transporter 1 is associated with metformin response in patients with diabetes mellitus. Pharmacogenomics J 9:242–247 [DOI] [PubMed] [Google Scholar]
- Béery E, Middel P, Bahn A, Willenberg HS, Hagos Y, Koepsell H, Bornstein SR, Müller GA, Burckhardt G, Steffgen J. (2003) Molecular evidence of organic ion transporters in the rat adrenal cortex with adrenocorticotropin-regulated zonal expression. Endocrinology 144:4519–4526 [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Bain LJ, Balsara BB, Testa JR, Kruh GD. (1998) Characterization of MOAT-C and MOAT-D, new members of the MRP/cMOAT subfamily of transporter proteins. J Natl Cancer Inst 90:1735–1741 [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD. (2002) Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res 62:6172–6177 [PubMed] [Google Scholar]
- Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, et al. (2005) Analysis of the in vivo functions of Mrp3. Mol Pharmacol 68:160–168 [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Guo P, Lee K, Zhou F, Kotova E, Grinberg A, Westphal H, Shchaveleva I, Klein-Szanto A, Gallo JM, et al. (2007) Multidrug resistance protein 4 protects bone marrow, thymus, spleen, and intestine from nucleotide analogue-induced damage. Cancer Res 67:262–268 [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Kruh GD. (1999) MOAT-E (ARA) is a full-length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer 80:1342–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bene J, Magyari L, Talián G, Komlósi K, Gasztonyi B, Tari B, Várkonyi A, Mózsik G, Melegh B. (2006) Prevalence of SLC22A4, SLC22A5 and CARD15 gene mutations in Hungarian pediatric patients with Crohn's disease. World J Gastroenterol 12:5550–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera TK, Lee S, Salvatore G, Lee B, Pastan I. (2001) MRP8, a new member of ABC transporter superfamily, identified by EST database mining and gene prediction program, is highly expressed in breast cancer. Mol Med 7:509–516 [PMC free article] [PubMed] [Google Scholar]
- Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. (2000) Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290:1771–1775 [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. (1999) Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anat Embryol (Berl) 199:439–449 [DOI] [PubMed] [Google Scholar]
- Bergwerk AJ, Shi X, Ford AC, Kanai N, Jacquemin E, Burk RD, Bai S, Novikoff PM, Stieger B, Meier PJ, et al. (1996) Immunologic distribution of an organic anion transport protein in rat liver and kidney. Am J Physiol 271:G231–G238 [DOI] [PubMed] [Google Scholar]
- Bessho Y, Oguri T, Ozasa H, Uemura T, Sakamoto H, Miyazaki M, Maeno K, Sato S, Ueda R. (2009) ABCC10/MRP7 is associated with vinorelbine resistance in non-small cell lung cancer. Oncol Rep 21:263–268 [PubMed] [Google Scholar]
- Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. (2001) Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology 33:1206–1216 [DOI] [PubMed] [Google Scholar]
- Bleasby K, Hall LA, Perry JL, Mohrenweiser HW, Pritchard JB. (2005) Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6). J Pharmacol Exp Ther 314:923–931 [DOI] [PubMed] [Google Scholar]
- Bockman DE, Ganapathy V, Oblak TG, Leibach FH. (1997) Localization of peptide transporter in nuclei and lysosomes of the pancreas. Int J Pancreatol 22:221–225 [DOI] [PubMed] [Google Scholar]
- Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M, et al. (1999) The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 22:347–351 [DOI] [PubMed] [Google Scholar]
- Boehmer C, Palmada M, Klaus F, Jeyaraj S, Lindner R, Laufer J, Daniel H, Lang F. (2008) The peptide transporter PEPT2 is targeted by the protein kinase SGK1 and the scaffold protein NHERF2. Cell Physiol Biochem 22:705–714 [DOI] [PubMed] [Google Scholar]
- Bolder U, Ton-Nu HT, Schteingart CD, Frick E, Hofmann AF. (1997) Hepatocyte transport of bile acids and organic anions in endotoxemic rats: impaired uptake and secretion. Gastroenterology 112:214–225 [DOI] [PubMed] [Google Scholar]
- Bortfeld M, Rius M, König J, Herold-Mende C, Nies AT, Keppler D. (2006) Human multidrug resistance protein 8 (MRP8/ABCC11), an apical efflux pump for steroid sulfates, is an axonal protein of the CNS and peripheral nervous system. Neuroscience 137:1247–1257 [DOI] [PubMed] [Google Scholar]
- Bourdet DL, Pritchard JB, Thakker DR. (2005) Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J Pharmacol Exp Ther 315:1288–1297 [DOI] [PubMed] [Google Scholar]
- Boyer JL, Ng OC, Ananthanarayanan M, Hofmann AF, Schteingart CD, Hagenbuch B, Stieger B, Meier PJ. (1994) Expression and characterization of a functional rat liver Na+ bile acid cotransport system in COS-7 cells. Am J Physiol 266:G382–G387 [DOI] [PubMed] [Google Scholar]
- Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. (2006) Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol 290:G1124–G1130 [DOI] [PubMed] [Google Scholar]
- Brady KP, Dushkin H, Förnzler D, Koike T, Magner F, Her H, Gullans S, Segre GV, Green RM, Beier DR. (1999) A novel putative transporter maps to the osteosclerosis (oc) mutation and is not expressed in the oc mutant mouse. Genomics 56:254–261 [DOI] [PubMed] [Google Scholar]
- Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH. (2005) The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 65:2577–2582 [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, et al. (1999) Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 22:336–345 [DOI] [PubMed] [Google Scholar]
- Brown MH, Paulsen IT, Skurray RA. (1999) The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol 31:394–395 [DOI] [PubMed] [Google Scholar]
- Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC. (1999) Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet 8:1665–1671 [DOI] [PubMed] [Google Scholar]
- Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. (2002) Gender-specific and developmental influences on the expression of rat organic anion transporters. J Pharmacol Exp Ther 301:145–151 [DOI] [PubMed] [Google Scholar]
- Buist SC, Cherrington NJ, Klaassen CD. (2003) Endocrine regulation of rat organic anion transporters. Drug Metab Dispos 31:559–564 [DOI] [PubMed] [Google Scholar]
- Buist SC, Klaassen CD. (2004) Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1–3; Slc22a6–8) mRNA levels. Drug Metab Dispos 32:620–625 [DOI] [PubMed] [Google Scholar]
- Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, Lomri N, Berger R, Scharschmidt BF, et al. (1998) A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet 18:219–224 [DOI] [PubMed] [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 5:327–337 [DOI] [PubMed] [Google Scholar]
- Burckhardt G, Wolff NA. (2000) Structure of renal organic anion and cation transporters. Am J Physiol Renal Physiol 278:F853–F866 [DOI] [PubMed] [Google Scholar]
- Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, Arndt P, Ulzheimer JC, Sonders MS, Baumann C, et al. (1998) Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol 54:342–352 [DOI] [PubMed] [Google Scholar]
- Busch AE, Quester S, Ulzheimer JC, Gorboulev V, Akhoundova A, Waldegger S, Lang F, Koepsell H. (1996a) Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS Lett 395:153–156 [DOI] [PubMed] [Google Scholar]
- Busch AE, Quester S, Ulzheimer JC, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H. (1996b) Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J Biol Chem 271:32599–32604 [DOI] [PubMed] [Google Scholar]
- Buschman E, Arceci RJ, Croop JM, Che M, Arias IM, Housman DE, Gros P. (1992) mdr2 encodes P-glycoprotein expressed in the bile canalicular membrane as determined by isoform-specific antibodies. J Biol Chem 267:18093–18099 [PubMed] [Google Scholar]
- Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. (2002) The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology 123:1649–1658 [DOI] [PubMed] [Google Scholar]
- Cai L, Lumsden A, Guenther UP, Neldner SA, Zäch S, Knoblauch H, Ramesar R, Hohl D, Callen DF, Neldner KH, et al. (2001) A novel Q378X mutation exists in the transmembrane transporter protein ABCC6 and its pseudogene: implications for mutation analysis in pseudoxanthoma elasticum. J Mol Med 79:536–546 [DOI] [PubMed] [Google Scholar]
- Campion SN, Johnson R, Aleksunes LM, Goedken MJ, van Rooijen N, Scheffer GL, Cherrington NJ, Manautou JE. (2008) Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am J Physiol Gastrointest Liver Physiol 295:G294–G304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Soldado P, Lorráyoz IM, Molina-Arcas M, Casado FJ, Martinez-Picado J, Lostao MP, Pastor-Anglada M. (2004) Interaction of nucleoside inhibitors of HIV-1 reverse transcriptase with the concentrative nucleoside transporter-1 (SLC28A1). Antivir Ther 9:993–1002 [PubMed] [Google Scholar]
- Cattori V, van Montfoort JE, Stieger B, Landmann L, Meijer DK, Winterhalter KH, Meier PJ, Hagenbuch B. (2001) Localization of organic anion transporting polypeptide 4 (Oatp4) in rat liver and comparison of its substrate specificity with Oatp1, Oatp2 and Oatp3. Pflugers Arch 443:188–195 [DOI] [PubMed] [Google Scholar]
- Cederbaum SD, Koo-McCoy S, Tein I, Hsu BY, Ganguly A, Vilain E, Dipple K, Cvitanovic-Sojat L, Stanley C. (2002) Carnitine membrane transporter deficiency: a long-term follow up and OCTN2 mutation in the first documented case of primary carnitine deficiency. Mol Genet Metab 77:195–201 [DOI] [PubMed] [Google Scholar]
- Cerrutti JA, Brandoni A, Quaglia NB, Torres AM. (2002) Sex differences in p-aminohippuric acid transport in rat kidney: role of membrane fluidity and expression of OAT1. Mol Cell Biochem 233:175–179 [DOI] [PubMed] [Google Scholar]
- Cetinkaya I, Ciarimboli G, Yalçinkaya G, Mehrens T, Velic A, Hirsch JR, Gorboulev V, Koepsell H, Schlatter E. (2003) Regulation of human organic cation transporter hOCT2 by PKA, PI3K, and calmodulin-dependent kinases. Am J Physiol Renal Physiol 284:F293–F302 [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, Endou H. (2001) Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol 59:1277–1286 [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. (2000) Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem 275:4507–4512 [DOI] [PubMed] [Google Scholar]
- Chan W, Calderon G, Swift AL, Moseley J, Li S, Hosoya H, Arias IM, Ortiz DF. (2005) Myosin II regulatory light chain is required for trafficking of bile salt export protein to the apical membrane in Madin-Darby canine kidney cells. J Biol Chem 280:23741–23747 [DOI] [PubMed] [Google Scholar]
- Chang TH, Hakamada K, Toyoki Y, Tsuchida S, Sasaki M. (2004) Expression of MRP2 and MRP3 during liver regeneration after 90% partial hepatectomy in rats. Transplantation 77:22–27 [DOI] [PubMed] [Google Scholar]
- Che M, Ortiz DF, Arias IM. (1995) Primary structure and functional expression of a cDNA encoding the bile canalicular, purine-specific Na(+)-nucleoside cotransporter. J Biol Chem 270:13596–13599 [DOI] [PubMed] [Google Scholar]
- Chemuturi NV, Donovan MD. (2007) Role of organic cation transporters in dopamine uptake across olfactory and nasal respiratory tissues. Mol Pharm 4:936–942 [DOI] [PubMed] [Google Scholar]
- Chen C, Klaassen CD. (2004) Rat multidrug resistance protein 4 (Mrp4, Abcc4): molecular cloning, organ distribution, postnatal renal expression, and chemical inducibility. Biochem Biophys Res Commun 317:46–53 [DOI] [PubMed] [Google Scholar]
- Chen C, Stock JL, Liu X, Shi J, Van Deusen JW, DiMattia DA, Dullea RG, de Morais SM. (2008) Utility of a novel Oatp1b2 knockout mouse model for evaluating the role of Oatp1b2 in the hepatic uptake of model compounds. Drug Metab Dispos 36:1840–1845 [DOI] [PubMed] [Google Scholar]
- Chen HL, Chen HL, Liu YJ, Feng CH, Wu CY, Shyu MK, Yuan RH, Chang MH. (2005a) Developmental expression of canalicular transporter genes in human liver. J Hepatol 43:472–477 [DOI] [PubMed] [Google Scholar]
- Chen J, Rinaldo L, Lim SJ, Young H, Messing RO, Choi DS. (2007a) The type 1 equilibrative nucleoside transporter regulates anxiety-like behavior in mice. Genes Brain Behav 6:776–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang S, Sorani M, Giacomini KM. (2007b) Transport of paraquat by human organic cation transporters and multidrug and toxic compound extrusion family. J Pharmacol Exp Ther 322:695–700 [DOI] [PubMed] [Google Scholar]
- Chen ZS, Guo Y, Belinsky MG, Kotova E, Kruh GD. (2005b) Transport of bile acids, sulfated steroids, estradiol 17-beta-d-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11). Mol Pharmacol 67:545–557 [DOI] [PubMed] [Google Scholar]
- Chen ZS, Hopper-Borge E, Belinsky MG, Shchaveleva I, Kotova E, Kruh GD. (2003) Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10). Mol Pharmacol 63:351–358 [DOI] [PubMed] [Google Scholar]
- Chen ZS, Lee K, Kruh GD. (2001) Transport of cyclic nucleotides and estradiol 17-beta-d-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem 276:33747–33754 [DOI] [PubMed] [Google Scholar]
- Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, Zeng H, Kruh GD. (2002) Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res 62:3144–3150 [PubMed] [Google Scholar]
- Cheng X, Buckley D, Klaassen CD. (2007) Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol 74:1665–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. (2006) Regulation of mRNA expression of xenobiotic transporters by the pregnane x receptor in mouse liver, kidney, and intestine. Drug Metab Dispos 34:1863–1867 [DOI] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. (2008) Critical role of PPAR-alpha in perfluorooctanoic acid- and perfluorodecanoic acid-induced downregulation of Oatp uptake transporters in mouse livers. Toxicol Sci 106:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. (2009) Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos 37:2178–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. (2005a) Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps). Drug Metab Dispos 33:1062–1073 [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD. (2005b) Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos 33:1276–1282 [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Lu H, Klaassen CD. (2006) Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol 70:1291–1297 [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Hartley DP, Li N, Johnson DR, Klaassen CD. (2002) Organ distribution of multidrug resistance proteins 1, 2, and 3 (Mrp1, 2, and 3) mRNA and hepatic induction of Mrp3 by constitutive androstane receptor activators in rats. J Pharmacol Exp Ther 300:97–104 [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Slitt AL, Li N, Klaassen CD. (2004) Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos 32:734–741 [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Slitt AL, Maher JM, Zhang XX, Zhang J, Huang W, Wan YJ, Moore DD, Klaassen CD. (2003) Induction of multidrug resistance protein 3 (mrp3) in vivo is independent of constitutive androstane receptor. Drug Metab Dispos 31:1315–1319 [DOI] [PubMed] [Google Scholar]
- Childs S, Yeh RL, Georges E, Ling V. (1995) Identification of a sister gene to P-glycoprotein. Cancer Res 55:2029–2034 [PubMed] [Google Scholar]
- Childs S, Yeh RL, Hui D, Ling V. (1998) Taxol resistance mediated by transfection of the liver-specific sister gene of P-glycoprotein. Cancer Res 58:4160–4167 [PubMed] [Google Scholar]
- Chin JE, Soffir R, Noonan KE, Choi K, Roninson IB. (1989) Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol 9:3808–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn LW, Kroetz DL. (2007) ABCB1 pharmacogenetics: progress, pitfalls, and promise. Clin Pharmacol Ther 81:265–269 [DOI] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. (2004) The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci 7:855–861 [DOI] [PubMed] [Google Scholar]
- Choi DS, Handa M, Young H, Gordon AS, Diamond I, Messing RO. (2000) Genomic organization and expression of the mouse equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporter 1 (ENT1) gene. Biochem Biophys Res Commun 277:200–208 [DOI] [PubMed] [Google Scholar]
- Choi JH, Ahn BM, Yi J, Lee JH, Lee JH, Nam SW, Chon CY, Han KH, Ahn SH, Jang IJ, et al. (2007) MRP2 haplotypes confer differential susceptibility to toxic liver injury. Pharmacogenet Genomics 17:403–415 [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Cherrington NJ, Li N, Klaassen CD. (2003) Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab Dispos 31:1337–1345 [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Ogura K, Klaassen CD. (2001) Cloning, expression, and ontogeny of mouse organic anion-transporting polypeptide-5, a kidney-specific organic anion transporter. Biochem Biophys Res Commun 280:92–98 [DOI] [PubMed] [Google Scholar]
- Chowbay B, Li H, David M, Cheung YB, Lee EJ. (2005) Meta-analysis of the influence of MDR1 C3435T polymorphism on digoxin pharmacokinetics and MDR1 gene expression. Br J Clin Pharmacol 60:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie DM, Dawson PA, Thevananther S, Shneider BL. (1996) Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol 271:G377–G385 [DOI] [PubMed] [Google Scholar]
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, et al. (2006) Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2). J Pharmacol Exp Ther 317:579–589 [DOI] [PubMed] [Google Scholar]
- Chung JY, Cho JY, Yu KS, Kim JR, Oh DS, Jung HR, Lim KS, Moon KH, Shin SG, Jang IJ. (2005) Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther 78:342–350 [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Koepsell H, Iordanova M, Gorboulev V, Dürner B, Lang D, Edemir B, Schröter R, Van Le T, Schlatter E. (2005a) Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. J Am Soc Nephrol 16:1562–1570 [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E. (2005b) Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167:1477–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarimboli G, Struwe K, Arndt P, Gorboulev V, Koepsell H, Schlatter E, Hirsch JR. (2004) Regulation of the human organic cation transporter hOCT1. J Cell Physiol 201:420–428 [DOI] [PubMed] [Google Scholar]
- Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. (1999) The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol 56:570–580 [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, et al. (2005) P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest 115:3285–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. (2004) Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res 64:3296–3301 [DOI] [PubMed] [Google Scholar]
- Coe I, Zhang Y, McKenzie T, Naydenova Z. (2002) PKC regulation of the human equilibrative nucleoside transporter, hENT1. FEBS Lett 517:201–205 [DOI] [PubMed] [Google Scholar]
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258:1650–1654 [DOI] [PubMed] [Google Scholar]
- Conrad S, Kauffmann HM, Ito K, Deeley RG, Cole SP, Schrenk D. (2001) Identification of human multidrug resistance protein 1 (MRP1) mutations and characterization of a G671V substitution. J Hum Genet 46:656–663 [DOI] [PubMed] [Google Scholar]
- Conrad S, Kauffmann HM, Ito K, Leslie EM, Deeley RG, Schrenk D, Cole SP. (2002) A naturally occurring mutation in MRP1 results in a selective decrease in organic anion transport and in increased doxorubicin resistance. Pharmacogenetics 12:321–330 [DOI] [PubMed] [Google Scholar]
- Cooray HC, Blackmore CG, Maskell L, Barrand MA. (2002) Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport 13:2059–2063 [DOI] [PubMed] [Google Scholar]
- Couvert P, Giral P, Dejager S, Gu J, Huby T, Chapman MJ, Bruckert E, Carrié A. (2008) Association between a frequent allele of the gene encoding OATP1B1 and enhanced LDL-lowering response to fluvastatin therapy. Pharmacogenomics 9:1217–1227 [DOI] [PubMed] [Google Scholar]
- Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. (1998) Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol 274:G157–G169 [DOI] [PubMed] [Google Scholar]
- Crawford CR, Patel DH, Naeve C, Belt JA. (1998) Cloning of the human equilibrative, nitrobenzylmercaptopurine riboside (NBMPR)-insensitive nucleoside transporter ei by functional expression in a transport-deficient cell line. J Biol Chem 273:5288–5293 [DOI] [PubMed] [Google Scholar]
- Crouthamel MH, Wu D, Yang Z, Ho RJ. (2006) A novel MDR1 G1199T variant alters drug resistance and efflux transport activity of P-glycoprotein in recombinant Hek cells. J Pharm Sci 95:2767–2777 [DOI] [PubMed] [Google Scholar]
- Csanaky IL, Aleksunes LM, Tanaka Y, Klaassen CD. (2009) Role of hepatic transporters in prevention of bile acid toxicity after partial hepatectomy in mice. Am J Physiol Gastrointest Liver Physiol 297:G419–G433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiara S, Latiano A, Palmieri O, Staiano AM, D'Incà R, Guariso G, Vieni G, Rutigliano V, Borrelli O, Valvano MR, et al. (2007) Role of CARD15, DLG5 and OCTN genes polymorphisms in children with inflammatory bowel diseases. World J Gastroenterol 13:1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, et al. (2009a) The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A 106:8043–8048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, König J, Buchholz JK, Spring H, Leier I, Keppler D. (1999) Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol 55:929–937 [PubMed] [Google Scholar]
- Cui Y, König J, Leier I, Buchholz U, Keppler D. (2001) Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem 276:9626–9630 [DOI] [PubMed] [Google Scholar]
- Cui Y, König J, Nies AT, Pfannschmidt M, Hergt M, Franke WW, Alt W, Moll R, Keppler D. (2003) Detection of the human organic anion transporters SLC21A6 (OATP2) and SLC21A8 (OATP8) in liver and hepatocellular carcinoma. Lab Invest 83:527–538 [DOI] [PubMed] [Google Scholar]
- Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD. (2009b) Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci 110:47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YJ, Cheng X, Weaver YM, Klaassen CD. (2009c) Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos 37:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusatis G, Gregorc V, Li J, Spreafico A, Ingersoll RG, Verweij J, Ludovini V, Villa E, Hidalgo M, Sparreboom A, et al. (2006) Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst 98:1739–1742 [DOI] [PubMed] [Google Scholar]
- Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. (1999) OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos 27:866–871 [PubMed] [Google Scholar]
- Cygalova L, Ceckova M, Pavek P, Staud F. (2008) Role of breast cancer resistance protein (Bcrp/Abcg2) in fetal protection during gestation in rat. Toxicol Lett 178:176–180 [DOI] [PubMed] [Google Scholar]
- Dahan A, Sabit H, Amidon GL. (2009) Multiple efflux pumps are involved in the transepithelial transport of colchicine: combined effect of p-glycoprotein and multidrug resistance-associated protein 2 leads to decreased intestinal absorption throughout the entire small intestine. Drug Metab Dispos 37:2028–2036 [DOI] [PubMed] [Google Scholar]
- Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. (2003) Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther 304:1085–1092 [DOI] [PubMed] [Google Scholar]
- Daly AK, Aithal GP, Leathart JB, Swainsbury RA, Dang TS, Day CP. (2007) Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology 132:272–281 [DOI] [PubMed] [Google Scholar]
- Damaraju VL, Elwi AN, Hunter C, Carpenter P, Santos C, Barron GM, Sun X, Baldwin SA, Young JD, Mackey JR, et al. (2007) Localization of broadly selective equilibrative and concentrative nucleoside transporters, hENT1 and hCNT3, in human kidney. Am J Physiol Renal Physiol 293:F200–F211 [DOI] [PubMed] [Google Scholar]
- Damaraju VL, Visser F, Zhang J, Mowles D, Ng AM, Young JD, Jayaram HN, Cass CE. (2005) Role of human nucleoside transporters in the cellular uptake of two inhibitors of IMP dehydrogenase, tiazofurin and benzamide riboside. Mol Pharmacol 67:273–279 [DOI] [PubMed] [Google Scholar]
- Daniel H, Herget M. (1997) Cellular and molecular mechanisms of renal peptide transport. Am J Physiol 273:F1–F8 [DOI] [PubMed] [Google Scholar]
- Daniel H, Kottra G. (2004) The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch 447:610–618 [DOI] [PubMed] [Google Scholar]
- Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. (2008) ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 39:211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. (2003) Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem 278:33920–33927 [DOI] [PubMed] [Google Scholar]
- Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. (2005) The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem 280:6960–6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazert P, Meissner K, Vogelgesang S, Heydrich B, Eckel L, Böhm M, Warzok R, Kerb R, Brinkmann U, Schaeffeler E, et al. (2003) Expression and localization of the multidrug resistance protein 5 (MRP5/ABCC5), a cellular export pump for cyclic nucleotides, in human heart. Am J Pathol 163:1567–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, et al. (1998) Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A 95:282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. (2007) P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res 13:6440–6449 [DOI] [PubMed] [Google Scholar]
- Dean M. (2005) The genetics of ATP-binding cassette transporters. Methods Enzymol 400:409–429 [DOI] [PubMed] [Google Scholar]
- del Santo B, Tarafa G, Felipe A, Casado FJ, Pastor-Anglada M. (2001) Developmental regulation of the concentrative nucleoside transporters CNT1 and CNT2 in rat liver. J Hepatol 34:873–880 [DOI] [PubMed] [Google Scholar]
- Deng R, Yang D, Yang J, Yan B. (2006) Oxysterol 22(R)-hydroxycholesterol induces the expression of the bile salt export pump through nuclear receptor farsenoid X receptor but not liver X receptor. J Pharmacol Exp Ther 317:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk GU, Soroka CJ, Mennone A, Koepsell H, Beuers U, Boyer JL. (2004a) Down-regulation of the organic cation transporter 1 of rat liver in obstructive cholestasis. Hepatology 39:1382–1389 [DOI] [PubMed] [Google Scholar]
- Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL. (2004b) Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol 40:585–591 [DOI] [PubMed] [Google Scholar]
- Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. (2001) The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 121:140–147 [DOI] [PubMed] [Google Scholar]
- Devault A, Gros P. (1990) Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol 10:1652–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir R, Buschman E, Gros P. (1990) Structural and functional characterization of the mouse multidrug resistance gene family. Bull Cancer 77:1125–1129 [PubMed] [Google Scholar]
- Diestra JE, Scheffer GL, Català I, Maliepaard M, Schellens JH, Scheper RJ, Germà-Lluch JR, Izquierdo MA. (2002) Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol 198:213–219 [DOI] [PubMed] [Google Scholar]
- Dieter MZ, Maher JM, Cheng X, Klaassen CD. (2004) Expression and regulation of the sterol half-transporter genes ABCG5 and ABCG8 in rats. Comp Biochem Physiol C Toxicol Pharmacol 139:209–218 [DOI] [PubMed] [Google Scholar]
- Dietrich CG, de Waart DR, Ottenhoff R, Schoots IG, Elferink RP. (2001a) Increased bioavailability of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in MRP2-deficient rats. Mol Pharmacol 59:974–980 [DOI] [PubMed] [Google Scholar]
- Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. (2001b) Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology 167:73–81 [DOI] [PubMed] [Google Scholar]
- Dixon PH, van Mil SW, Chambers J, Strautnieks S, Thompson RJ, Lammert F, Kubitz R, Keitel V, Glantz A, Mattsson LA, et al. (2008) Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut 58:537–544 [DOI] [PubMed] [Google Scholar]
- Donner MG, Keppler D. (2001) Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology 34:351–359 [DOI] [PubMed] [Google Scholar]
- Donner MG, Schumacher S, Warskulat U, Heinemann J, Häussinger D. (2007) Obstructive cholestasis induces TNF-alpha- and IL-1 -mediated periportal downregulation of Bsep and zonal regulation of Ntcp, Oatp1a4, and Oatp1b2. Am J Physiol Gastrointest Liver Physiol 293:G1134–G1146 [DOI] [PubMed] [Google Scholar]
- Döring F, Walter J, Will J, Föcking M, Boll M, Amasheh S, Clauss W, Daniel H. (1998) Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J Clin Invest 101:2761–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos SM, Weber CC, Franke C, Müller WE, Eckert GP. (2007) Cholesterol: coupling between membrane microenvironment and ABC transporter activity. Biochem Biophys Res Commun 354:216–221 [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 95:15665–15670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfeld O, Gehrmann T, Köhrer K, Kircheis G, Holneicher C, Häussinger D, Wettstein M. (2005) Oligonucleotide microarray analysis of differential transporter regulation in the regenerating rat liver. Liver Int 25:1243–1258 [DOI] [PubMed] [Google Scholar]
- Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF. (2002) MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol 53:526–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. (2002) Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther 71:11–20 [DOI] [PubMed] [Google Scholar]
- Dresser MJ, Gray AT, Giacomini KM. (2000) Kinetic and selectivity differences between rodent, rabbit, and human organic cation transporters (OCT1). J Pharmacol Exp Ther 292:1146–1152 [PubMed] [Google Scholar]
- Dreyer C, Keller H, Mahfoudi A, Laudet V, Krey G, Wahli W. (1993) Positive regulation of the peroxisomal beta-oxidation pathway by fatty acids through activation of peroxisome proliferator-activated receptors (PPAR). Biol Cell 77:67–76 [DOI] [PubMed] [Google Scholar]
- Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. (1992) Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68:879–887 [DOI] [PubMed] [Google Scholar]
- Drobnik W, Lindenthal B, Lieser B, Ritter M, Christiansen Weber T, Liebisch G, Giesa U, Igel M, Borsukova H, Büchler C, et al. (2001) ATP-binding cassette transporter A1 (ABCA1) affects total body sterol metabolism. Gastroenterology 120:1203–1211 [DOI] [PubMed] [Google Scholar]
- Droździk M, Białecka M, Myśliwiec K, Honczarenko K, Stankiewicz J, Sych Z. (2003) Polymorphism in the P-glycoprotein drug transporter MDR1 gene: a possible link between environmental and genetic factors in Parkinson's disease. Pharmacogenetics 13:259–263 [DOI] [PubMed] [Google Scholar]
- Duflot S, Calvo M, Casado FJ, Enrich C, Pastor-Anglada M. (2002) Concentrative nucleoside transporter (rCNT1) is targeted to the apical membrane through the hepatic transcytotic pathway. Exp Cell Res 281:77–85 [DOI] [PubMed] [Google Scholar]
- Dumont M, Jacquemin E, D'Hont C, Descout C, Cresteil D, Haouzi D, Desrochers M, Stieger B, Hadchouel M, Erlinger S. (1997) Expression of the liver Na+-independent organic anion transporting polypeptide (oatp-1) in rats with bile duct ligation. J Hepatol 27:1051–1056 [DOI] [PubMed] [Google Scholar]
- Durán JM, Peral MJ, Calonge ML, Ilundáin AA. (2005) OCTN3: a Na+-independent l-carnitine transporter in enterocytes basolateral membrane. J Cell Physiol 202:929–935 [DOI] [PubMed] [Google Scholar]
- Eckhardt U, Schroeder A, Stieger B, Höchli M, Landmann L, Tynes R, Meier PJ, Hagenbuch B. (1999) Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am J Physiol 276:G1037–G1042 [DOI] [PubMed] [Google Scholar]
- Eisenbraun MD, Mosley RL, Teitelbaum DH, Miller RA. (2000) Altered development of intestinal intraepithelial lymphocytes in P-glycoprotein-deficient mice. Dev Comp Immunol 24:783–795 [DOI] [PubMed] [Google Scholar]
- Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, Kanai Y, Sophasan S, Endou H. (2004) Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci 94:297–304 [DOI] [PubMed] [Google Scholar]
- El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. (2007) Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther 320:229–235 [DOI] [PubMed] [Google Scholar]
- Elamiri A, Perwaiz S, Tuchweber B, Yousef IM. (2003) Effect of mdr2 mutation with combined tandem disruption of canalicular glycoprotein transporters by cyclosporine A on bile formation in mice. Pharmacol Res 48:467–472 [DOI] [PubMed] [Google Scholar]
- Elferink MG, Olinga P, Draaisma AL, Merema MT, Faber KN, Slooff MJ, Meijer DK, Groothuis GM. (2004) LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am J Physiol Gastrointest Liver Physiol 287:G1008–G1016 [DOI] [PubMed] [Google Scholar]
- Elferink RP, Ottenhoff R, Liefting W, de Haan J, Jansen PL. (1989) Hepatobiliary transport of glutathione and glutathione conjugate in rats with hereditary hyperbilirubinemia. J Clin Invest 84:476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink RP, Tytgat GN, Groen AK. (1997) Hepatic canalicular membrane 1: the role of mdr2 P-glycoprotein in hepatobiliary lipid transport. FASEB J 11:19–28 [DOI] [PubMed] [Google Scholar]
- Elimrani I, Lahjouji K, Seidman E, Roy MJ, Mitchell GA, Qureshi I. (2003) Expression and localization of organic cation/carnitine transporter OCTN2 in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 284:G863–G871 [DOI] [PubMed] [Google Scholar]
- Elwi AN, Damaraju VL, Kuzma ML, Mowles DA, Baldwin SA, Young JD, Sawyer MB, Cass CE. (2009) Transepithelial fluxes of adenosine and 2′-deoxyadenosine across human renal proximal tubule cells: the roles of nucleoside transporters hENT1, hENT2, and hCNT3. Am J Physiol Renal Physiol 296:F1439–F1451 [DOI] [PubMed] [Google Scholar]
- Endres CJ, Moss AM, Govindarajan R, Choi DS, Unadkat JD. (2009a) The role of nucleoside transporters in the erythrocyte disposition and oral absorption of ribavirin in the wild-type and equilibrative nucleoside transporter 1 (−/−) mice. J Pharmacol Exp Ther 331:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres CJ, Moss AM, Ke B, Govindarajan R, Choi DS, Messing RO, Unadkat JD. (2009b) The role of the equilibrative nucleoside transporter 1 (ENT1) in transport and metabolism of ribavirin by human and wild-type or Ent1(−/−) mouse erythrocytes. J Pharmacol Exp Ther 329:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. (2008) Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab Dispos 36:995–1002 [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. (2007) Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol 72:967–975 [DOI] [PubMed] [Google Scholar]
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, et al. (2002a) Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417:447–452 [DOI] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Shimoda M, Narikawa S, Kobayashi Y, Kobayashi Y, Yamamoto T, Sekine T, Cha SH, Niwa T, et al. (2002b) Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J Pharmacol Exp Ther 301:797–802 [DOI] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Taki K, Takayama F, Noshiro R, Niwa T, Endou H. (2003) Interactions of human organic anion as well as cation transporters with indoxyl sulfate. Eur J Pharmacol 466:13–20 [DOI] [PubMed] [Google Scholar]
- Eppens EF, van Mil SW, de Vree JM, Mok KS, Juijn JA, Oude Elferink RP, Berger R, Houwen RH, Klomp LW. (2001) FIC1, the protein affected in two forms of hereditary cholestasis, is localized in the cholangiocyte and the canalicular membrane of the hepatocyte. J Hepatol 35:436–443 [DOI] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Rieg T, Gangoiti JA, Wikoff WR, Siuzdak G, Barshop BA, Nigam SK. (2008) Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 33:180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, et al. (2006) Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem 281:5072–5083 [DOI] [PubMed] [Google Scholar]
- Erdman AR, Mangravite LM, Urban TJ, Lagpacan LL, Castro RA, de la Cruz M, Chan W, Huang CC, Johns SJ, Kawamoto M, et al. (2006) The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am J Physiol Renal Physiol 290:F905–F912 [DOI] [PubMed] [Google Scholar]
- Ernest S, Rajaraman S, Megyesi J, Bello-Reuss EN. (1997) Expression of MDR1 (multidrug resistance) gene and its protein in normal human kidney. Nephron 77:284–289 [DOI] [PubMed] [Google Scholar]
- Errasti-Murugarren E, Pastor-Anglada M, Casado FJ. (2007) Role of CNT3 in the transepithelial flux of nucleosides and nucleoside-derived drugs. J Physiol 582:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WE, Relling MV. (1999) Pharmacogenomics: translating functional genomics into rational therapeutics. Science 286:487–491 [DOI] [PubMed] [Google Scholar]
- Evers R, Cnubben NH, Wijnholds J, van Deemter L, van Bladeren PJ, Borst P. (1997) Transport of glutathione prostaglandin A conjugates by the multidrug resistance protein 1. FEBS Lett 419:112–116 [DOI] [PubMed] [Google Scholar]
- Eyal S, Chung FS, Muzi M, Link JM, Mankoff DA, Kaddoumi A, O'Sullivan F, Hebert MF, Unadkat JD. (2009) Simultaneous PET imaging of P-glycoprotein inhibition in multiple tissues in the pregnant nonhuman primate. J Nucl Med 50:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faneyte IF, Kristel PM, Maliepaard M, Scheffer GL, Scheper RJ, Schellens JH, van de Vijver MJ. (2002) Expression of the breast cancer resistance protein in breast cancer. Clin Cancer Res 8:1068–1074 [PubMed] [Google Scholar]
- Fang X, Parkinson FE, Mowles DA, Young JD, Cass CE. (1996) Functional characterization of a recombinant sodium-dependent nucleoside transporter with selectivity for pyrimidine nucleosides (cNT1rat) by transient expression in cultured mammalian cells. Biochem J 317:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach T, Cui Y, Faulstich H, Keppler D. (2003) Characterization of the transport of the bicyclic peptide phalloidin by human hepatic transport proteins. Naunyn Schmiedebergs Arch Pharmacol 368:415–420 [DOI] [PubMed] [Google Scholar]
- Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. (1994) Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 368:563–566 [DOI] [PubMed] [Google Scholar]
- Fenyvesi F, Fenyvesi E, Szente L, Goda K, Bacsó Z, Bácskay I, Váradi J, Kiss T, Molnár E, Janáky T, et al. (2008) P-glycoprotein inhibition by membrane cholesterol modulation. Eur J Pharm Sci 34:236–242 [DOI] [PubMed] [Google Scholar]
- Ferraris A, Torres B, Knafelz D, Barabino A, Lionetti P, de Angelis GL, Iacono G, Papadatou B, D'Amato G, Di Ciommo V, et al. (2006) Relationship between CARD15, SLC22A4/5, and DLG5 polymorphisms and early-onset inflammatory bowel diseases: an Italian multicentric study. Inflamm Bowel Dis 12:355–361 [DOI] [PubMed] [Google Scholar]
- Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE. (2006) Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett 235:84–92 [DOI] [PubMed] [Google Scholar]
- Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C, et al. (2006) 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 130:465–481 [DOI] [PubMed] [Google Scholar]
- Fiedler T, Büning C, Reuter W, Pitre G, Gentz E, Schmidt HH, Büttner J, Ockenga J, Gerloff T, Meisel C, et al. (2007) Possible role of MDR1 two-locus genotypes for young-age onset ulcerative colitis but not Crohn's disease. Eur J Clin Pharmacol 63:917–925 [DOI] [PubMed] [Google Scholar]
- Filipski KK, Loos WJ, Verweij J, Sparreboom A. (2008) Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res 14:3875–3880 [DOI] [PubMed] [Google Scholar]
- Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. (2009) Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 86:396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flens MJ, Zaman GJ, van der Valk P, Izquierdo MA, Schroeijers AB, Scheffer GL, van der Groep P, de Haas M, Meijer CJ, Scheper RJ. (1996) Tissue distribution of the multidrug resistance protein. Am J Pathol 148:1237–1247 [PMC free article] [PubMed] [Google Scholar]
- Fouassier L, Beaussier M, Schiffer E, Rey C, Barbu V, Mergey M, Wendum D, Callard P, Scoazec JY, Lasnier E, et al. (2007) Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol 293:G25–G35 [DOI] [PubMed] [Google Scholar]
- Frey IM, Rubio-Aliaga I, Siewert A, Sailer D, Drobyshev A, Beckers J, de Angelis MH, Aubert J, Bar Hen A, Fiehn O, et al. (2007) Profiling at mRNA, protein, and metabolite levels reveals alterations in renal amino acid handling and glutathione metabolism in kidney tissue of Pept2−/− mice. Physiol Genomics 28:301–310 [DOI] [PubMed] [Google Scholar]
- Fritz F, Chen J, Hayes P, Sirotnak FM. (2000) Molecular cloning of the murine cMOAT ATPase. Biochim Biophys Acta 1492:531–536 [DOI] [PubMed] [Google Scholar]
- Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y. (2006) Effects of herbal extracts on the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 34:577–582 [DOI] [PubMed] [Google Scholar]
- Fujita T, Brown C, Carlson EJ, Taylor T, de la Cruz M, Johns SJ, Stryke D, Kawamoto M, Fujita K, Castro R, et al. (2005) Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1). Pharmacogenet Genomics 15:201–209 [DOI] [PubMed] [Google Scholar]
- Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM. (2006) Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci 95:25–36 [DOI] [PubMed] [Google Scholar]
- Fukumura S, Takikawa H, Yamanaka M. (1998) Effects of organic anions and bile acid conjugates on biliary excretion of pravastatin in the rat. Pharm Res 15:72–76 [DOI] [PubMed] [Google Scholar]
- Fukushima-Uesaka H, Maekawa K, Ozawa S, Komamura K, Ueno K, Shibakawa M, Kamakura S, Kitakaze M, Tomoike H, Saito Y, et al. (2004) Fourteen novel single nucleotide polymorphisms in the SLC22A2 gene encoding human organic cation transporter (OCT2). Drug Metab Pharmacokinet 19:239–244 [DOI] [PubMed] [Google Scholar]
- Fukushima-Uesaka H, Saito Y, Maekawa K, Hasegawa R, Suzuki K, Yanagawa T, Kajio H, Kuzuya N, Noda M, Yasuda K, et al. (2007) Genetic variations of the ABC transporter gene ABCC3 in a Japanese population. Drug Metab Pharmacokinet 22:129–135 [DOI] [PubMed] [Google Scholar]
- Furihata T, Satoh T, Yamamoto N, Kobayashi K, Chiba K. (2007) Hepatocyte nuclear factor 1 alpha is a factor responsible for the interindividual variation of OATP1B1 mRNA levels in adult Japanese livers. Pharm Res 24:2327–2332 [DOI] [PubMed] [Google Scholar]
- Furuno T, Landi MT, Ceroni M, Caporaso N, Bernucci I, Nappi G, Martignoni E, Schaeffeler E, Eichelbaum M, Schwab M, et al. (2002) Expression polymorphism of the blood-brain barrier component P-glycoprotein (MDR1) in relation to Parkinson's disease. Pharmacogenetics 12:529–534 [DOI] [PubMed] [Google Scholar]
- Gallo JM, Li S, Guo P, Reed K, Ma J. (2003) The effect of P-glycoprotein on paclitaxel brain and brain tumor distribution in mice. Cancer Res 63:5114–5117 [PubMed] [Google Scholar]
- Ganapathy ME, Brandsch M, Prasad PD, Ganapathy V, Leibach FH. (1995) Differential recognition of beta -lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J Biol Chem 270:25672–25677 [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Huang W, Rajan DP, Carter AL, Sugawara M, Iseki K, Leibach FH, Ganapathy V. (2000) beta-lactam antibiotics as substrates for OCTN2, an organic cation/carnitine transporter. J Biol Chem 275:1699–1707 [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Prasad PD, Mackenzie B, Ganapathy V, Leibach FH. (1997) Interaction of anionic cephalosporins with the intestinal and renal peptide transporters PEPT 1 and PEPT 2. Biochim Biophys Acta 1324:296–308 [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. (2000) Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther 294:73–79 [PubMed] [Google Scholar]
- Gao B, St Pierre MV, Stieger B, Meier PJ. (2004) Differential expression of bile salt and organic anion transporters in developing rat liver. J Hepatol 41:201–208 [DOI] [PubMed] [Google Scholar]
- Gao B, Stieger B, Noé B, Fritschy JM, Meier PJ. (1999) Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem 47:1255–1264 [DOI] [PubMed] [Google Scholar]
- Gao B, Wenzel A, Grimm C, Vavricka SR, Benke D, Meier PJ, Remè CE. (2002) Localization of organic anion transport protein 2 in the apical region of rat retinal pigment epithelium. Invest Ophthalmol Vis Sci 43:510–514 [PubMed] [Google Scholar]
- García-Delgado M, Peral MJ, Durán JM, García-Miranda P, Calonge ML, Ilundáin AA. (2009) Ontogeny of Na(+)/l-carnitine transporter and of gamma-trimethylaminobutyraldehyde dehydrogenase and gamma-butyrobetaine hydroxylase genes expression in rat kidney. Mech Ageing Dev 130:227–233 [DOI] [PubMed] [Google Scholar]
- García-Miranda P, Durán JM, Peral MJ, Ilundáin AA. (2005) Developmental maturation and segmental distribution of rat small intestinal l-carnitine uptake. J Membr Biol 206:9–16 [DOI] [PubMed] [Google Scholar]
- Garrett Q, Xu S, Simmons PA, Vehige J, Flanagan JL, Willcox MD. (2008) Expression and localization of carnitine/organic cation transporter OCTN1 and OCTN2 in ocular epithelium. Invest Ophthalmol Vis Sci 49:4844–4849 [DOI] [PubMed] [Google Scholar]
- Garrovo C, Rosati A, Bartoli F, Decorti G. (2006) St John's wort modulation and developmental expression of multidrug transporters in the rat. Phytother Res 20:468–473 [DOI] [PubMed] [Google Scholar]
- Gartung C, Ananthanarayanan M, Rahman MA, Schuele S, Nundy S, Soroka CJ, Stolz A, Suchy FJ, Boyer JL. (1996) Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology 110:199–209 [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Geyer J, Petzinger E. (2007) In vivo relevance of Mrp2-mediated biliary excretion of the Amanita mushroom toxin demethylphalloin. Biochim Biophys Acta 1768:2070–2077 [DOI] [PubMed] [Google Scholar]
- Gazouli M, Mantzaris G, Archimandritis AJ, Nasioulas G, Anagnou NP. (2005) Single nucleotide polymorphisms of OCTN1, OCTN2, and DLG5 genes in Greek patients with Crohn's disease. World J Gastroenterol 11:7525–7530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedeon C, Anger G, Lubetsky A, Miller MP, Koren G. (2008a) Investigating the potential role of multi-drug resistance protein (MRP) transporters in fetal to maternal glyburide efflux in the human placenta. J Obstet Gynaecol 28:485–489 [DOI] [PubMed] [Google Scholar]
- Gedeon C, Anger G, Piquette-Miller M, Koren G. (2008b) Breast cancer resistance protein: mediating the trans-placental transfer of glyburide across the human placenta. Placenta 29:39–43 [DOI] [PubMed] [Google Scholar]
- Geier A, Dietrich CG, Gerloff T, Haendly J, Kullak-Ublick GA, Stieger B, Meier PJ, Matern S, Gartung C. (2003a) Regulation of basolateral organic anion transporters in ethinylestradiol-induced cholestasis in the rat. Biochim Biophys Acta 1609:87–94 [DOI] [PubMed] [Google Scholar]
- Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C. (2003b) Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology 38:345–354 [DOI] [PubMed] [Google Scholar]
- Geier A, Kim SK, Gerloff T, Dietrich CG, Lammert F, Karpen SJ, Stieger B, Meier PJ, Matern S, Gartung C. (2002) Hepatobiliary organic anion transporters are differentially regulated in acute toxic liver injury induced by carbon tetrachloride. J Hepatol 37:198–205 [DOI] [PubMed] [Google Scholar]
- Geier A, Martin IV, Dietrich CG, Balasubramaniyan N, Strauch S, Suchy FJ, Gartung C, Trautwein C, Ananthanarayanan M. (2008) Hepatocyte nuclear factor-4alpha is a central transactivator of the mouse Ntcp gene. Am J Physiol Gastrointest Liver Physiol 295:G226–G233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerk PM, Kuhn RJ, Desai NS, McNamara PJ. (2001) Active transport of nitrofurantoin into human milk. Pharmacotherapy 21:669–675 [DOI] [PubMed] [Google Scholar]
- Gerloff T, Geier A, Stieger B, Hagenbuch B, Meier PJ, Matern S, Gartung C. (1999) Differential expression of basolateral and canalicular organic anion transporters during regeneration of rat liver. Gastroenterology 117:1408–1415 [DOI] [PubMed] [Google Scholar]
- Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. (1998) The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem 273:10046–10050 [DOI] [PubMed] [Google Scholar]
- Ghanem CI, Gómez PC, Arana MC, Perassolo M, Ruiz ML, Villanueva SS, Ochoa EJ, Catania VA, Bengochea LA, Mottino AD. (2004) Effect of acetaminophen on expression and activity of rat liver multidrug resistance-associated protein 2 and P-glycoprotein. Biochem Pharmacol 68:791–798 [DOI] [PubMed] [Google Scholar]
- Ghanem CI, Ruiz ML, Villanueva SS, Luquita M, Llesuy S, Catania VA, Bengochea LA, Mottino AD. (2009) Effect of repeated administration with subtoxic doses of acetaminophen to rats on enterohepatic recirculation of a subsequent toxic dose. Biochem Pharmacol 77:1621–1628 [DOI] [PubMed] [Google Scholar]
- Ghanem CI, Ruiz ML, Villanueva SS, Luquita MG, Catania VA, Jones B, Bengochea LA, Vore M, Mottino AD. (2005) Shift from biliary to urinary elimination of acetaminophen-glucuronide in acetaminophen-pretreated rats. J Pharmacol Exp Ther 315:987–995 [DOI] [PubMed] [Google Scholar]
- Gigliozzi A, Fraioli F, Sundaram P, Lee J, Mennone A, Alvaro D, Boyer JL. (2000) Molecular identification and functional characterization of Mdr1a in rat cholangiocytes. Gastroenterology 119:1113–1122 [DOI] [PubMed] [Google Scholar]
- Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, et al. (2007) Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther 81:362–370 [DOI] [PubMed] [Google Scholar]
- Glavy JS, Wu SM, Wang PJ, Orr GA, Wolkoff AW. (2000) Down-regulation by extracellular ATP of rat hepatocyte organic anion transport is mediated by serine phosphorylation of oatp1. J Biol Chem 275:1479–1484 [DOI] [PubMed] [Google Scholar]
- Glube N, Langguth P. (2008) Caki-1 cells as a model system for the interaction of renally secreted drugs with OCT3. Nephron Physiol 108:p18–28 [DOI] [PubMed] [Google Scholar]
- Goralski KB, Acott PD, Fraser AD, Worth D, Sinal CJ. (2006) Brain cyclosporin A levels are determined by ontogenic regulation of mdr1a expression. Drug Metab Dispos 34:288–295 [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. (1997) Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol 16:871–881 [DOI] [PubMed] [Google Scholar]
- Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, van Kuppevelt TH, Levelt CN, de Wolf A, Loves WJ, et al. (2005) Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet 14:1763–1773 [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Suzuki H, Kinoshita S, Hirohashi T, Kato Y, Sugiyama Y. (2000) Involvement of an organic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. J Pharmacol Exp Ther 292:433–439 [PubMed] [Google Scholar]
- Govindarajan R, Bakken AH, Hudkins KL, Lai Y, Casado FJ, Pastor-Anglada M, Tse CM, Hayashi J, Unadkat JD. (2007) In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta. Am J Physiol Regul Integr Comp Physiol 293:R1809–R1822 [DOI] [PubMed] [Google Scholar]
- Govindarajan R, Endres CJ, Whittington D, LeCluyse E, Pastor-Anglada M, Tse CM, Unadkat JD. (2008) Expression and hepatobiliary transport characteristics of the concentrative and equilibrative nucleoside transporters in sandwich-cultured human hepatocytes. Am J Physiol Gastrointest Liver Physiol 295:G570–G580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan R, Leung GP, Zhou M, Tse CM, Wang J, Unadkat JD. (2009) Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol 296:G910–G922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow JM, Hodges LM, Chinn LW, Kroetz DL. (2008) Substrate-dependent effects of human ABCB1 coding polymorphisms. J Pharmacol Exp Ther 325:435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradhand U, Kim RB. (2008) Pharmacogenomics of MRP transporters (ABCC1–5) and BCRP (ABCG2). Drug Metab Rev 40:317–354 [DOI] [PubMed] [Google Scholar]
- Gradhand U, Lang T, Schaeffeler E, Glaeser H, Tegude H, Klein K, Fritz P, Jedlitschky G, Kroemer HK, Bachmakov I, et al. (2008) Variability in human hepatic MRP4 expression: influence of cholestasis and genotype. Pharmacogenomics J 8:42–52 [DOI] [PubMed] [Google Scholar]
- Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. (2003) ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem 278:48275–48282 [DOI] [PubMed] [Google Scholar]
- Graham KA, Leithoff J, Coe IR, Mowles D, Mackey JR, Young JD, Cass CE. (2000) Differential transport of cytosine-containing nucleosides by recombinant human concentrative nucleoside transporter protein hCNT1. Nucleosides Nucleotides Nucleic Acids 19:415–434 [DOI] [PubMed] [Google Scholar]
- Grant CE, Valdimarsson G, Hipfner DR, Almquist KC, Cole SP, Deeley RG. (1994) Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res 54:357–361 [PubMed] [Google Scholar]
- Green RM, Beier D, Gollan JL. (1996) Regulation of hepatocyte bile salt transporters by endotoxin and inflammatory cytokines in rodents. Gastroenterology 111:193–198 [DOI] [PubMed] [Google Scholar]
- Green RM, Gollan JL, Hagenbuch B, Meier PJ, Beier DR. (1997) Regulation of hepatocyte bile salt transporters during hepatic regeneration. Am J Physiol 273:G621–G627 [DOI] [PubMed] [Google Scholar]
- Green RM, Hoda F, Ward KL. (2000) Molecular cloning and characterization of the murine bile salt export pump. Gene 241:117–123 [DOI] [PubMed] [Google Scholar]
- Griffiths M, Beaumont N, Yao SY, Sundaram M, Boumah CE, Davies A, Kwong FY, Coe I, Cass CE, Young JD, et al. (1997) Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nat Med 3:89–93 [DOI] [PubMed] [Google Scholar]
- Grisk O, Steinbach AC, Ciecholewski S, Schlüter T, Klöting I, Schmidt H, Dazert E, Schaeffeler E, Steil L, Gauer S, et al. (2009) Multidrug resistance-related protein 2 genotype of the donor affects kidney graft function. Pharmacogenet Genomics 19:276–288 [DOI] [PubMed] [Google Scholar]
- Groen A, Kunne C, Jongsma G, van den Oever K, Mok KS, Petruzzelli M, Vrins CL, Bull L, Paulusma CC, Oude Elferink RP. (2008) Abcg5/8 independent biliary cholesterol excretion in Atp8b1-deficient mice. Gastroenterology 134:2091–2100 [DOI] [PubMed] [Google Scholar]
- Groen A, Kunne C, Oude Elferink RP. (2006) Increased serum concentrations of secondary bile salts during cholate feeding are due to coprophagy. A study with wild-type and Atp8b1-deficient mice. Mol Pharm 3:756–761 [DOI] [PubMed] [Google Scholar]
- Groen A, Kunne C, Paulusma CC, Kramer W, Agellon LB, Bull LN, Oude Elferink RP. (2007) Intestinal bile salt absorption in Atp8b1 deficient mice. J Hepatol 47:114–122 [DOI] [PubMed] [Google Scholar]
- Groen AK, Bloks VW, Bandsma RH, Ottenhoff R, Chimini G, Kuipers F. (2001) Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J Clin Invest 108:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg DA, Döring F, Theis S, Nickolaus M, Fischer A, Daniel H. (2002) Peptide transport in the mammary gland: expression and distribution of PEPT2 mRNA and protein. Am J Physiol Endocrinol Metab 282:E1172–E1179 [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Nickolaus M, Springer J, Döring F, Daniel H, Fischer A. (2001) Localization of the peptide transporter PEPT2 in the lung: implications for pulmonary oligopeptide uptake. Am J Pathol 158:707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves CE, Suhre WB, Cherrington NJ, Wright SH. (2006) Sex differences in the mRNA, protein, and functional expression of organic anion transporter (Oat) 1, Oat3, and organic cation transporter (Oct) 2 in rabbit renal proximal tubules. J Pharmacol Exp Ther 316:743–752 [DOI] [PubMed] [Google Scholar]
- Grube M, Köck K, Oswald S, Draber K, Meissner K, Eckel L, Böhm M, Felix SB, Vogelgesang S, Jedlitschky G, et al. (2006a) Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther 80:607–620 [DOI] [PubMed] [Google Scholar]
- Grube M, Meyer zu Schwabedissen HE, Präger D, Haney J, Möritz KU, Meissner K, Rosskopf D, Eckel L, Böhm M, Jedlitschky G, et al. (2006b) Uptake of cardiovascular drugs into the human heart: expression, regulation, and function of the carnitine transporter OCTN2 (SLC22A5). Circulation 113:1114–1122 [DOI] [PubMed] [Google Scholar]
- Grube M, Reuther S, Meyer Zu Schwabedissen H, Köck K, Draber K, Ritter CA, Fusch C, Jedlitschky G, Kroemer HK. (2007) Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos 35:30–35 [DOI] [PubMed] [Google Scholar]
- Grube M, Schwabedissen HM, Draber K, Präger D, Möritz KU, Linnemann K, Fusch C, Jedlitschky G, Kroemer HK. (2005) Expression, localization, and function of the carnitine transporter octn2 (slc22a5) in human placenta. Drug Metab Dispos 33:31–37 [DOI] [PubMed] [Google Scholar]
- Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. (1994) Drug excretion mediated by a new prototype of polyspecific transporter. Nature 372:549–552 [DOI] [PubMed] [Google Scholar]
- Gründemann D, Schechinger B, Rappold GA, Schömig E. (1998) Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci 1:349–351 [DOI] [PubMed] [Google Scholar]
- Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, Hagenbuch B. (2008) Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol 584:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo GL, Choudhuri S, Klaassen CD. (2002a) Induction profile of rat organic anion transporting polypeptide 2 (oatp2) by prototypical drug-metabolizing enzyme inducers that activate gene expression through ligand-activated transcription factor pathways. J Pharmacol Exp Ther 300:206–212 [DOI] [PubMed] [Google Scholar]
- Guo GL, Johnson DR, Klaassen CD. (2002b) Postnatal expression and induction by pregnenolone-16alpha-carbonitrile of the organic anion-transporting polypeptide 2 in rat liver. Drug Metab Dispos 30:283–288 [DOI] [PubMed] [Google Scholar]
- Guo GL, Klaassen CD. (2001) Protein kinase C suppresses rat organic anion transporting polypeptide 1- and 2-mediated uptake. J Pharmacol Exp Ther 299:551–557 [PubMed] [Google Scholar]
- Guo Y, Kotova E, Chen ZS, Lee K, Hopper-Borge E, Belinsky MG, Kruh GD. (2003) MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem 278:29509–29514 [DOI] [PubMed] [Google Scholar]
- Guo Y, Nyasae L, Braiterman LT, Hubbard AL. (2005) NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol 289:G904–G916 [DOI] [PubMed] [Google Scholar]
- Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J. (2005) Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem Pharmacol 70:695–699 [DOI] [PubMed] [Google Scholar]
- Haenisch S, Zimmermann U, Dazert E, Wruck CJ, Dazert P, Siegmund W, Siegmund S, Kroemer HK, Warzok RW, Cascorbi I. (2007) Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenomics J 7:56–65 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Adler ID, Schmid TE. (2000) Molecular cloning and functional characterization of the mouse organic-anion-transporting polypeptide 1 (Oatp1) and mapping of the gene to chromosome X. Biochem J 345:115–120 [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38:778–801 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Lübbert H, Stieger B, Meier PJ. (1990) Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J Biol Chem 265:5357–5360 [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (1994) Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest 93:1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2003) The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 1609:1–18 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Scharschmidt BF, Meier PJ. (1996) Effect of antisense oligonucleotides on the expression of hepatocellular bile acid and organic anion uptake systems in Xenopus laevis oocytes. Biochem J 316:901–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Stieger B, Foguet M, Lübbert H, Meier PJ. (1991) Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A 88:10629–10633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson P, Andersson I, Nyström S, Löfgren L, Amrot LF, Li H. (2002) Ontogenetic development and spatial distribution of the ileal apical sodium-dependent bile acid transporter and the ileal lipid-binding protein in apoE knockout and C57BL/6 mice. Scand J Gastroenterol 37:1089–1096 [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Yao SY, Ingram JC, Hadden DA, Ritzel MW, Gallagher MP, Henderson PJ, Cass CE, Young JD, Baldwin SA. (2001) Subcellular distribution and membrane topology of the mammalian concentrative Na+-nucleoside cotransporter rCNT1. J Biol Chem 276:27981–27988 [DOI] [PubMed] [Google Scholar]
- Handschin C, Meyer UA. (2003) Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev 55:649–673 [DOI] [PubMed] [Google Scholar]
- Hannivoort RA, Dunning S, Vander Borght S, Schroyen B, Woudenberg J, Oakley F, Buist-Homan M, van den Heuvel FA, Geuken M, Geerts A, et al. (2008) Multidrug resistance-associated proteins are crucial for the viability of activated rat hepatic stellate cells. Hepatology 48:624–634 [DOI] [PubMed] [Google Scholar]
- Hardman B, Manuelpillai U, Wallace EM, van de Waasenburg S, Cater M, Mercer JF, Ackland ML. (2004) Expression and localization of Menkes and Wilson copper transporting ATPases in human placenta. Placenta 25:512–517 [DOI] [PubMed] [Google Scholar]
- Hardman B, Michalczyk A, Greenough M, Camakaris J, Mercer J, Ackland L. (2007) Distinct functional roles for the Menkes and Wilson copper translocating P-type ATPases in human placental cells. Cell Physiol Biochem 20:1073–1084 [DOI] [PubMed] [Google Scholar]
- Hartmann G, Cheung AK, Piquette-Miller M. (2002) Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Ther 303:273–281 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. (2007) Multidrug resistance-associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol 18:37–45 [DOI] [PubMed] [Google Scholar]
- Hassan HE, Myers AL, Lee IJ, Coop A, Eddington ND. (2007) Oxycodone induces overexpression of P-glycoprotein (ABCB1) and affects paclitaxel's tissue distribution in Sprague Dawley rats. J Pharm Sci 96:2494–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. (2003) Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun 310:824–829 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sugiyama Y. (2007) 4-Phenylbutyrate enhances the cell surface expression and the transport capacity of wild-type and mutated bile salt export pumps. Hepatology 45:1506–1516 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sugiyama Y. (2009) Short-chain ubiquitination is associated with the degradation rate of a cell-surface-resident bile salt export pump (BSEP/ABCB11). Mol Pharmacol 75:143–150 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Takada T, Suzuki H, Akita H, Sugiyama Y. (2005) Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology 41:916–924 [DOI] [PubMed] [Google Scholar]
- Hegedüs T, Sessler T, Scott R, Thelin W, Bakos E, Váradi A, Szabó K, Homolya L, Milgram SL, Sarkadi B. (2003) C-terminal phosphorylation of MRP2 modulates its interaction with PDZ proteins. Biochem Biophys Res Commun 302:454–461 [DOI] [PubMed] [Google Scholar]
- Henriksen U, Fog JU, Litman T, Gether U. (2005a) Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem 280:36926–36934 [DOI] [PubMed] [Google Scholar]
- Henriksen U, Gether U, Litman T. (2005b) Effect of Walker A mutation (K86M) on oligomerization and surface targeting of the multidrug resistance transporter ABCG2. J Cell Sci 118:1417–1426 [DOI] [PubMed] [Google Scholar]
- Hernandez S, Tsuchiya Y, García-Ruiz JP, Lalioti V, Nielsen S, Cassio D, Sandoval IV. (2008) ATP7B copper-regulated traffic and association with the tight junctions: copper excretion into the bile. Gastroenterology 134:1215–1223 [DOI] [PubMed] [Google Scholar]
- Herrera-Ruiz D, Wang Q, Gudmundsson OS, Cook TJ, Smith RL, Faria TN, Knipp GT. (2001) Spatial expression patterns of peptide transporters in the human and rat gastrointestinal tracts, Caco-2 in vitro cell culture model, and multiple human tissues. AAPS PharmSci 3:E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett M, Oliver DE, Rubin DL, Easton KL, Stuart JM, Altman RB, Klein TE. (2002) PharmGKB: the Pharmacogenetics Knowledge Base. Nucleic Acids Res 30:163–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa M, Matsumoto T, Komatsu T, Omote H, Moriyama Y. (2007) Functional characterization of testis-specific rodent multidrug and toxic compound extrusion 2, a class III MATE-type polyspecific H+/organic cation exporter. Am J Physiol Cell Physiol 293:C1437–C1444 [DOI] [PubMed] [Google Scholar]
- Hirai T, Fukui Y, Motojima K. (2007) PPARalpha agonists positively and negatively regulate the expression of several nutrient/drug transporters in mouse small intestine. Biol Pharm Bull 30:2185–2190 [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Hayashi H, Kusuhara H, Sugiyama Y. (2005) Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J Pharmacol Exp Ther 314:876–882 [DOI] [PubMed] [Google Scholar]
- Hirano T, Yasuda S, Osaka Y, Kobayashi M, Itagaki S, Iseki K. (2006) Mechanism of the inhibitory effect of zwitterionic drugs (levofloxacin and grepafloxacin) on carnitine transporter (OCTN2) in Caco-2 cells. Biochim Biophys Acta 1758:1743–1750 [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Sugiyama Y. (1999) Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3). J Biol Chem 274:15181–15185 [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. (2000) ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J Biol Chem 275:2905–2910 [DOI] [PubMed] [Google Scholar]
- Hirouchi M, Suzuki H, Itoda M, Ozawa S, Sawada J, Ieiri I, Ohtsubo K, Sugiyama Y. (2004) Characterization of the cellular localization, expression level, and function of SNP variants of MRP2/ABCC2. Pharm Res 21:742–748 [DOI] [PubMed] [Google Scholar]
- Hitzl M, Schaeffeler E, Hocher B, Slowinski T, Halle H, Eichelbaum M, Kaufmann P, Fritz P, Fromm MF, Schwab M. (2004) Variable expression of P-glycoprotein in the human placenta and its association with mutations of the multidrug resistance 1 gene (MDR1, ABCB1). Pharmacogenetics 14:309–318 [DOI] [PubMed] [Google Scholar]
- Ho GT, Soranzo N, Nimmo ER, Tenesa A, Goldstein DB, Satsangi J. (2006a) ABCB1/MDR1 gene determines susceptibility and phenotype in ulcerative colitis: discrimination of critical variants using a gene-wide haplotype tagging approach. Hum Mol Genet 15:797–805 [DOI] [PubMed] [Google Scholar]
- Ho RH, Choi L, Lee W, Mayo G, Schwarz UI, Tirona RG, Bailey DG, Michael Stein C, Kim RB. (2007) Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics 17:647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. (2004) Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem 279:7213–7222 [DOI] [PubMed] [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. (2006b) Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology 130:1793–1806 [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, et al. (2000) Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A 97:3473–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M, Sano N, Takikawa H. (2003) Effects of lipopolysaccharide on the biliary excretion of bile acids and organic anions in rats. J Gastroenterol Hepatol 18:815–821 [DOI] [PubMed] [Google Scholar]
- Honjo Y, Morisaki K, Huff LM, Robey RW, Hung J, Dean M, Bates SE. (2002) Single-nucleotide polymorphism (SNP) analysis in the ABC half-transporter ABCG2 (MXR/BCRP/ABCP1). Cancer Biol Ther 1:696–702 [DOI] [PubMed] [Google Scholar]
- Hopper-Borge E, Chen ZS, Shchaveleva I, Belinsky MG, Kruh GD. (2004) Analysis of the drug resistance profile of multidrug resistance protein 7 (ABCC10): resistance to docetaxel. Cancer Res 64:4927–4930 [DOI] [PubMed] [Google Scholar]
- Hopper-Borge E, Xu X, Shen T, Shi Z, Chen ZS, Kruh GD. (2009) Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res 69:178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper E, Belinsky MG, Zeng H, Tosolini A, Testa JR, Kruh GD. (2001) Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily. Cancer Lett 162:181–191 [DOI] [PubMed] [Google Scholar]
- Hoque MT, Conseil G, Cole SP. (2009) Involvement of NHERF1 in apical membrane localization of MRP4 in polarized kidney cells. Biochem Biophys Res Commun 379:60–64 [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. (2004) Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s). J Neurochem 90:526–536 [DOI] [PubMed] [Google Scholar]
- Horvath G, Mendes ES, Schmid N, Schmid A, Conner GE, Salathe M, Wanner A. (2007) The effect of corticosteroids on the disposal of long-acting beta2-agonists by airway smooth muscle cells. J Allergy Clin Immunol 120:1103–1109 [DOI] [PubMed] [Google Scholar]
- Hosoya K, Makihara A, Tsujikawa Y, Yoneyama D, Mori S, Terasaki T, Akanuma S, Tomi M, Tachikawa M. (2009) Roles of inner blood-retinal barrier organic anion transporter 3 in the vitreous/retina-to-blood efflux transport of p-aminohippuric acid, benzylpenicillin, and 6-mercaptopurine. J Pharmacol Exp Ther 329:87–93 [DOI] [PubMed] [Google Scholar]
- Hosoyamada M, Ichida K, Enomoto A, Hosoya T, Endou H. (2004) Function and localization of urate transporter 1 in mouse kidney. J Am Soc Nephrol 15:261–268 [DOI] [PubMed] [Google Scholar]
- Hosoyamada M, Sekine T, Kanai Y, Endou H. (1999) Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol 276:F122–F128 [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. (1999) A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem 274:37161–37168 [DOI] [PubMed] [Google Scholar]
- Hu Y, Shen H, Keep RF, Smith DE. (2007) Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J Neurochem 103:2058–2065 [DOI] [PubMed] [Google Scholar]
- Hu Y, Smith DE, Ma K, Jappar D, Thomas W, Hillgren KM. (2008) Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol Pharm 5:1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Wang Y, Grimm S. (2006a) ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos 34:738–742 [DOI] [PubMed] [Google Scholar]
- Huang QQ, Yao SY, Ritzel MW, Paterson AR, Cass CE, Young JD. (1994) Cloning and functional expression of a complementary DNA encoding a mammalian nucleoside transport protein. J Biol Chem 269:17757–17760 [PubMed] [Google Scholar]
- Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. (2006b) Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312:233–236 [DOI] [PubMed] [Google Scholar]
- Hung AY, Sheng M. (2002) PDZ domains: structural modules for protein complex assembly. J Biol Chem 277:5699–5702 [DOI] [PubMed] [Google Scholar]
- Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S. (2006) Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am J Pathol 168:423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster D, Purnat TD, Burkhead JL, Ralle M, Fiehn O, Stuckert F, Olson NE, Teupser D, Lutsenko S. (2007) High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem 282:8343–8355 [DOI] [PubMed] [Google Scholar]
- Hwang ST, Henning SJ. (2001) Ontogenic regulation of components of ileal bile acid absorption. Exp Biol Med (Maywood) 226:674–680 [DOI] [PubMed] [Google Scholar]
- Hyogo H, Tazuma S, Nishioka T, Ochi H, Yamaguchi A, Numata Y, Kanno K, Sakomoto M, Asamoto Y, Tsuboi K, et al. (2001) Phospholipid alterations in hepatocyte membranes and transporter protein changes in cholestatic rat model. Dig Dis Sci 46:2089–2097 [DOI] [PubMed] [Google Scholar]
- Iliás A, Urbán Z, Seidl TL, Le Saux O, Sinkó E, Boyd CD, Sarkadi B, Váradi A. (2002) Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J Biol Chem 277:16860–16867 [DOI] [PubMed] [Google Scholar]
- Imaoka T, Kusuhara H, Adachi-Akahane S, Hasegawa M, Morita N, Endou H, Sugiyama Y. (2004) The renal-specific transporter mediates facilitative transport of organic anions at the brush border membrane of mouse renal tubules. J Am Soc Nephrol 15:2012–2022 [DOI] [PubMed] [Google Scholar]
- Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. (2007) Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol 71:619–627 [DOI] [PubMed] [Google Scholar]
- Inazu M, Takeda H, Maehara K, Miyashita K, Tomoda A, Matsumiya T. (2006) Functional expression of the organic cation/carnitine transporter 2 in rat astrocytes. J Neurochem 97:424–434 [DOI] [PubMed] [Google Scholar]
- Isern J, Hagenbuch B, Stieger B, Meier PJ, Meseguer A. (2001) Functional analysis and androgen-regulated expression of mouse organic anion transporting polypeptide 1 (Oatp1) in the kidney. Biochim Biophys Acta 1518:73–78 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Esterbauer H, Sies H. (1986) Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J Biol Chem 261:1576–1581 [PubMed] [Google Scholar]
- Ishikawa T, Kobayashi K, Sogame Y, Hayashi K. (1989) Evidence for leukotriene C4 transport mediated by an ATP-dependent glutathione S-conjugate carrier in rat heart and liver plasma membranes. FEBS Lett 259:95–98 [DOI] [PubMed] [Google Scholar]
- Islinger F, Gekle M, Wright SH. (2001) Interaction of 2,3-dimercapto-1-propane sulfonate with the human organic anion transporter hOAT1. J Pharmacol Exp Ther 299:741–747 [PubMed] [Google Scholar]
- Ismair MG, Häusler S, Stuermer CA, Guyot C, Meier PJ, Roth J, Stieger B. (2009) ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology 49:1673–1682 [DOI] [PubMed] [Google Scholar]
- Ismair MG, Stieger B, Cattori V, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. (2001) Hepatic uptake of cholecystokinin octapeptide by organic anion-transporting polypeptides OATP4 and OATP8 of rat and human liver. Gastroenterology 121:1185–1190 [DOI] [PubMed] [Google Scholar]
- Ito A, Yamaguchi K, Onogawa T, Unno M, Suzuki T, Nishio T, Suzuki T, Sasano H, Abe T, Tamai M. (2002) Distribution of organic anion-transporting polypeptide 2 (oatp2) and oatp3 in the rat retina. Invest Ophthalmol Vis Sci 43:858–863 [PubMed] [Google Scholar]
- Ito A, Yamaguchi K, Tomita H, Suzuki T, Onogawa T, Sato T, Mizutamari H, Mikkaichi T, Nishio T, Suzuki T, et al. (2003) Distribution of rat organic anion transporting polypeptide-E (oatp-E) in the rat eye. Invest Ophthalmol Vis Sci 44:4877–4884 [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki H, Hirohashi T, Kume K, Shimizu T, Sugiyama Y. (1997) Molecular cloning of canalicular multispecific organic anion transporter defective in EHBR. Am J Physiol 272:G16–G22 [DOI] [PubMed] [Google Scholar]
- Itoda M, Saito Y, Soyama A, Saeki M, Murayama N, Ishida S, Sai K, Nagano M, Suzuki H, Sugiyama Y, et al. (2002) Polymorphisms in the ABCC2 (cMOAT/MRP2) gene found in 72 established cell lines derived from Japanese individuals: an association between single nucleotide polymorphisms in the 5′-untranslated region and exon 28. Drug Metab Dispos 30:363–364 [DOI] [PubMed] [Google Scholar]
- Iwai M, Suzuki H, Ieiri I, Otsubo K, Sugiyama Y. (2004a) Functional analysis of single nucleotide polymorphisms of hepatic organic anion transporter OATP1B1 (OATP-C). Pharmacogenetics 14:749–757 [DOI] [PubMed] [Google Scholar]
- Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H. (2004b) A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int 66:935–944 [DOI] [PubMed] [Google Scholar]
- Iwata D, Kato Y, Wakayama T, Sai Y, Kubo Y, Iseki S, Tsuji A. (2008) Involvement of carnitine/organic cation transporter OCTN2 (SLC22A5) in distribution of its substrate carnitine to the heart. Drug Metab Pharmacokinet 23:207–215 [DOI] [PubMed] [Google Scholar]
- Izzedine H, Hulot JS, Villard E, Goyenvalle C, Dominguez S, Ghosn J, Valantin MA, Lechat P, Deray AG. (2006) Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis 194:1481–1491 [DOI] [PubMed] [Google Scholar]
- Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. (1994) Expression cloning of a rat liver Na(+)-independent organic anion transporter. Proc Natl Acad Sci U S A 91:133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, De Jager-Krikken A, Kuipers F, Stellaard F, et al. (1999) Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 117:1370–1379 [DOI] [PubMed] [Google Scholar]
- Jariyawat S, Sekine T, Takeda M, Apiwattanakul N, Kanai Y, Sophasan S, Endou H. (1999) The interaction and transport of beta-lactam antibiotics with the cloned rat renal organic anion transporter 1. J Pharmacol Exp Ther 290:672–677 [PubMed] [Google Scholar]
- Jedlitschky G, Burchell B, Keppler D. (2000) The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275:30069–30074 [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Hoffmann U, Kroemer HK. (2006) Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin Drug Metab Toxicol 2:351–366 [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. (1996) Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res 56:988–994 [PubMed] [Google Scholar]
- Jedlitschky G, Tirschmann K, Lubenow LE, Nieuwenhuis HK, Akkerman JW, Greinacher A, Kroemer HK. (2004) The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood 104:3603–3610 [DOI] [PubMed] [Google Scholar]
- Jennings LL, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, Cass CE. (2001) Distinct regional distribution of human equilibrative nucleoside transporter proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacology 40:722–731 [DOI] [PubMed] [Google Scholar]
- Jetté L, Beaulieu E, Leclerc JM, Béliveau R. (1996) Cyclosporin A treatment induces overexpression of P-glycoprotein in the kidney and other tissues. Am J Physiol 270:F756–F765 [DOI] [PubMed] [Google Scholar]
- Jiang H, Hu Y, Keep RF, Smith DE. (2009) Enhanced antinociceptive response to intracerebroventricular kyotorphin in Pept2 null mice. J Neurochem 109:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. (2006) Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos 34:1756–1763 [DOI] [PubMed] [Google Scholar]
- Johnson DR, Klaassen CD. (2002) Regulation of rat multidrug resistance protein 2 by classes of prototypical microsomal enzyme inducers that activate distinct transcription pathways. Toxicol Sci 67:182–189 [DOI] [PubMed] [Google Scholar]
- Johnson EF, Palmer CN, Griffin KJ, Hsu MH. (1996) Role of the peroxisome proliferator-activated receptor in cytochrome P450 4A gene regulation. FASEB J 10:1241–1248 [DOI] [PubMed] [Google Scholar]
- Jones BR, Li W, Cao J, Hoffman TA, Gerk PM, Vore M. (2005) The role of protein synthesis and degradation in the post-transcriptional regulation of rat multidrug resistance-associated protein 2 (Mrp2, Abcc2). Mol Pharmacol 68:701–710 [DOI] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, et al. (2002) The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A 99:15649–15654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Freeman J, Bolscher E, Musters S, Alvi AJ, Titley I, Schinkel AH, Dale TC. (2005a) Contribution of the ABC transporters Bcrp1 and Mdr1a/1b to the side population phenotype in mammary gland and bone marrow of mice. Stem Cells 23:1059–1065 [DOI] [PubMed] [Google Scholar]
- Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, Schinkel AH. (2005b) The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med 11:127–129 [DOI] [PubMed] [Google Scholar]
- Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH. (2000) Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst 92:1651–1656 [DOI] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Mol CA, Buitelaar M, Koepsell H, Smit JW, Schinkel AH. (2001) Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol 21:5471–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. (2003) Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol 23:7902–7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL, Ling V. (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455:152–162 [DOI] [PubMed] [Google Scholar]
- Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. (2004) Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol 286:G752–G761 [DOI] [PubMed] [Google Scholar]
- Jung D, Hagenbuch B, Gresh L, Pontoglio M, Meier PJ, Kullak-Ublick GA. (2001) Characterization of the human OATP-C (SLC21A6) gene promoter and regulation of liver-specific OATP genes by hepatocyte nuclear factor 1 alpha. J Biol Chem 276:37206–37214 [DOI] [PubMed] [Google Scholar]
- Jung D, Inagaki T, Gerard RD, Dawson PA, Kliewer SA, Mangelsdorf DJ, Moschetta A. (2007) FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res 48:2693–2700 [DOI] [PubMed] [Google Scholar]
- Jung D, Podvinec M, Meyer UA, Mangelsdorf DJ, Fried M, Meier PJ, Kullak-Ublick GA. (2002a) Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology 122:1954–1966 [DOI] [PubMed] [Google Scholar]
- Jung KY, Takeda M, Shimoda M, Narikawa S, Tojo A, Kim DK, Chairoungdua A, Choi BK, Kusuhara H, Sugiyama Y, et al. (2002b) Involvement of rat organic anion transporter 3 (rOAT3) in cephaloridine-induced nephrotoxicity: in comparison with rOAT1. Life Sci 70:1861–1874 [DOI] [PubMed] [Google Scholar]
- Jung N, Lehmann C, Rubbert A, Knispel M, Hartmann P, van Lunzen J, Stellbrink HJ, Faetkenheuer G, Taubert D. (2008) Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos 36:1616–1623 [DOI] [PubMed] [Google Scholar]
- Jungsuwadee P, Cole MP, Sultana R, Joshi G, Tangpong J, Butterfield DA, St Clair DK, Vore M. (2006) Increase in Mrp1 expression and 4-hydroxy-2-nonenal adduction in heart tissue of Adriamycin-treated C57BL/6 mice. Mol Cancer Ther 5:2851–2860 [DOI] [PubMed] [Google Scholar]
- Jungsuwadee P, Nithipongvanitch R, Chen Y, Oberley TD, Butterfield DA, St Clair DK, Vore M. (2009) Mrp1 localization and function in cardiac mitochondria after doxorubicin. Mol Pharmacol 75:1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T, Varticovski L, Sai Y, Arias IM. (2002) Mechanism by which cAMP activates PI3-kinase and increases bile acid secretion in WIF-B9 cells. Am J Physiol Cell Physiol 283:C1655–C1666 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Watanabe N, Sato M, Nakano A, Nishizaki Y, Hosoi K, Takashimizu S, Uchiyama J, Kimura M, Matsuzaki S. (1998) Differential expression of multidrug resistance (mdr) and canalicular multispecific organic anion transporter (cMOAT) genes following extrahepatic biliary obstruction in rats. Biochem Mol Biol Int 44:443–452 [DOI] [PubMed] [Google Scholar]
- Kai S, Yakushiji K, Yamauchi M, Ito C, Kuwajima M, Osada Y, Toshimori K. (2005) Expression of novel organic cation/carnitine transporter (OCTN2) in the mouse pancreas. Tissue Cell 37:309–315 [DOI] [PubMed] [Google Scholar]
- Kakyo M, Sakagami H, Nishio T, Nakai D, Nakagomi R, Tokui T, Naitoh T, Matsuno S, Abe T, Yawo H. (1999) Immunohistochemical distribution and functional characterization of an organic anion transporting polypeptide 2 (oatp2). FEBS Lett 445:343–346 [DOI] [PubMed] [Google Scholar]
- Kamal MA, Jiang H, Hu Y, Keep RF, Smith DE. (2009) Influence of genetic knockout of Pept2 on the in vivo disposition of endogenous and exogenous carnosine in wild-type and Pept2 null mice. Am J Physiol Regul Integr Comp Physiol 296:R986–R991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. (2005) Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics 15:513–522 [DOI] [PubMed] [Google Scholar]
- Kamisako T, Ogawa H. (2005) Alteration of the expression of adenosine triphosphate-binding cassette transporters associated with bile acid and cholesterol transport in the rat liver and intestine during cholestasis. J Gastroenterol Hepatol 20:1429–1434 [DOI] [PubMed] [Google Scholar]
- Kamiyama Y, Matsubara T, Yoshinari K, Nagata K, Kamimura H, Yamazoe Y. (2007) Role of human hepatocyte nuclear factor 4alpha in the expression of drug-metabolizing enzymes and transporters in human hepatocytes assessed by use of small interfering RNA. Drug Metab Pharmacokinet 22:287–298 [DOI] [PubMed] [Google Scholar]
- Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. (1995) Identification and characterization of a prostaglandin transporter. Science 268:866–869 [DOI] [PubMed] [Google Scholar]
- Kang HJ, Song IS, Shin HJ, Kim WY, Lee CH, Shim JC, Zhou HH, Lee SS, Shin JG. (2007) Identification and functional characterization of genetic variants of human organic cation transporters in a Korean population. Drug Metab Dispos 35:667–675 [DOI] [PubMed] [Google Scholar]
- Kao HH, Huang JD, Chang MS. (2002) cDNA cloning and genomic organization of the murine MRP7, a new ATP-binding cassette transporter. Gene 286:299–306 [DOI] [PubMed] [Google Scholar]
- Karbach U, Kricke J, Meyer-Wentrup F, Gorboulev V, Volk C, Loffing-Cueni D, Kaissling B, Bachmann S, Koepsell H. (2000) Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am J Physiol Renal Physiol 279:F679–F687 [DOI] [PubMed] [Google Scholar]
- Karpen SJ, Sun AQ, Kudish B, Hagenbuch B, Meier PJ, Ananthanarayanan M, Suchy FJ. (1996) Multiple factors regulate the rat liver basolateral sodium-dependent bile acid cotransporter gene promoter. J Biol Chem 271:15211–15221 [DOI] [PubMed] [Google Scholar]
- Kartenbeck J, Leuschner U, Mayer R, Keppler D. (1996) Absence of the canalicular isoform of the MRP gene-encoded conjugate export pump from the hepatocytes in Dubin-Johnson syndrome. Hepatology 23:1061–1066 [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. (2002) Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277:2908–2915 [DOI] [PubMed] [Google Scholar]
- Kato S, Ito K, Kato Y, Wakayama T, Kubo Y, Iseki S, Tsuji A. (2009) Involvement of multidrug resistance-associated protein 1 in intestinal toxicity of methotrexate. Pharm Res 26:1467–1476 [DOI] [PubMed] [Google Scholar]
- Kato Y, Sai Y, Yoshida K, Watanabe C, Hirata T, Tsuji A. (2005) PDZK1 directly regulates the function of organic cation/carnitine transporter OCTN2. Mol Pharmacol 67:734–743 [DOI] [PubMed] [Google Scholar]
- Kato Y, Sugiura M, Sugiura T, Wakayama T, Kubo Y, Kobayashi D, Sai Y, Tamai I, Iseki S, Tsuji A. (2006) Organic cation/carnitine transporter OCTN2 (Slc22a5) is responsible for carnitine transport across apical membranes of small intestinal epithelial cells in mouse. Mol Pharmacol 70:829–837 [DOI] [PubMed] [Google Scholar]
- Kato Y, Takahara S, Kato S, Kubo Y, Sai Y, Tamai I, Yabuuchi H, Tsuji A. (2008) Involvement of multidrug resistance-associated protein 2 (Abcc2) in molecular weight-dependent biliary excretion of beta-lactam antibiotics. Drug Metab Dispos 36:1088–1096 [DOI] [PubMed] [Google Scholar]
- Kato Y, Yoshida K, Watanabe C, Sai Y, Tsuji A. (2004) Screening of the interaction between xenobiotic transporters and PDZ proteins. Pharm Res 21:1886–1894 [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chen ZS, Wada M, Uchiumi T, Ono M, Akiyama S, Kuwano M. (1999) Enhanced transport of anticancer agents and leukotriene C4 by the human canalicular multispecific organic anion transporter (cMOAT/MRP2). FEBS Lett 456:327–331 [DOI] [PubMed] [Google Scholar]
- Kawahara M, Sakata A, Miyashita T, Tamai I, Tsuji A. (1999) Physiologically based pharmacokinetics of digoxin in mdr1a knockout mice. J Pharm Sci 88:1281–1287 [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kato Y, Sai Y, Tsuji A. (2004) Functional characterization of human organic cation transporter OCTN1 single nucleotide polymorphisms in the Japanese population. J Pharm Sci 93:2920–2926 [DOI] [PubMed] [Google Scholar]
- Keitel V, Burdelski M, Vojnisek Z, Schmitt L, Häussinger D, Kubitz R. (2009) De novo bile salt transporter antibodies as a possible cause of recurrent graft failure after liver transplantation: a novel mechanism of cholestasis. Hepatology 50:510–517 [DOI] [PubMed] [Google Scholar]
- Keitel V, Burdelski M, Warskulat U, Kühlkamp T, Keppler D, Häussinger D, Kubitz R. (2005) Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology 41:1160–1172 [DOI] [PubMed] [Google Scholar]
- Keitel V, Kartenbeck J, Nies AT, Spring H, Brom M, Keppler D. (2000) Impaired protein maturation of the conjugate export pump multidrug resistance protein 2 as a consequence of a deletion mutation in Dubin-Johnson syndrome. Hepatology 32:1317–1328 [DOI] [PubMed] [Google Scholar]
- Keitel V, Vogt C, Häussinger D, Kubitz R. (2006) Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology 131:624–629 [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, Ganapathy V. (1998) Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem 273:15971–15979 [DOI] [PubMed] [Google Scholar]
- Keppler D, König J, Büchler M. (1997) The canalicular multidrug resistance protein, cMRP/MRP2, a novel conjugate export pump expressed in the apical membrane of hepatocytes. Adv Enzyme Regul 37:321–333 [DOI] [PubMed] [Google Scholar]
- Kerb R, Brinkmann U, Chatskaia N, Gorbunov D, Gorboulev V, Mornhinweg E, Keil A, Eichelbaum M, Koepsell H. (2002) Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics 12:591–595 [DOI] [PubMed] [Google Scholar]
- Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. (2009) ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther 86:197–203 [DOI] [PubMed] [Google Scholar]
- Khamdang S, Takeda M, Babu E, Noshiro R, Onozato ML, Tojo A, Enomoto A, Huang XL, Narikawa S, Anzai N, et al. (2003) Interaction of human and rat organic anion transporter 2 with various cephalosporin antibiotics. Eur J Pharmacol 465:1–7 [DOI] [PubMed] [Google Scholar]
- Kido Y, Tamai I, Ohnari A, Sai Y, Kagami T, Nezu J, Nikaido H, Hashimoto N, Asano M, Tsuji A. (2001) Functional relevance of carnitine transporter OCTN2 to brain distribution of l-carnitine and acetyl-l-carnitine across the blood-brain barrier. J Neurochem 79:959–969 [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, Sugiyama Y. (2007) Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol 72:1619–1625 [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Hattori N, Shiota K, Kim I, Gonzalez FJ, Sugiyama Y. (2006) Regulation of the expression of human organic anion transporter 3 by hepatocyte nuclear factor 1alpha/beta and DNA methylation. Mol Pharmacol 70:887–896 [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Sugiyama D, Sugiyama Y. (2003) Contribution of organic anion transporter 3 (Slc22a8) to the elimination of p-aminohippuric acid and benzylpenicillin across the blood-brain barrier. J Pharmacol Exp Ther 306:51–58 [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim KH, Lee JA, Namkung W, Sun AQ, Ananthanarayanan M, Suchy FJ, Shin DM, Muallem S, Lee MG. (2002a) Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology 122:1941–1953 [DOI] [PubMed] [Google Scholar]
- Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. (2002b) The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res 8:22–28 [PubMed] [Google Scholar]
- Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, et al. (2001) Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther 70:189–199 [DOI] [PubMed] [Google Scholar]
- Kim SR, Saito Y, Itoda M, Maekawa K, Kawamoto M, Kamatani N, Ozawa S, Sawada J. (2009) Genetic variations of the ABC transporter gene ABCB11 encoding the human bile salt export pump (BSEP) in a Japanese population. Drug Metab Pharmacokinet 24:277–281 [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Gribar JJ, Gottesman MM. (2002) Functional characterization of coding polymorphisms in the human MDR1 gene using a vaccinia virus expression system. Mol Pharmacol 62:1–6 [DOI] [PubMed] [Google Scholar]
- Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, Inui K. (2005) Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet 20:379–386 [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Suzuki H, Ito K, Kume K, Shimizu T, Sugiyama Y. (1998) Transfected rat cMOAT is functionally expressed on the apical membrane in Madin-Darby canine kidney (MDCK) cells. Pharm Res 15:1851–1856 [DOI] [PubMed] [Google Scholar]
- Kipp H, Arias IM. (2000) Intracellular trafficking and regulation of canalicular ATP-binding cassette transporters. Semin Liver Dis 20:339–351 [DOI] [PubMed] [Google Scholar]
- Kipp H, Arias IM. (2002) Trafficking of canalicular ABC transporters in hepatocytes. Annu Rev Physiol 64:595–608 [DOI] [PubMed] [Google Scholar]
- Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. (2008) Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr 47:298–303 [DOI] [PubMed] [Google Scholar]
- Kiss A, Farah K, Kim J, Garriock RJ, Drysdale TA, Hammond JR. (2000) Molecular cloning and functional characterization of inhibitor-sensitive (mENT1) and inhibitor-resistant (mENT2) equilibrative nucleoside transporters from mouse brain. Biochem J 352:363–372 [PMC free article] [PubMed] [Google Scholar]
- Kitaichi K, Fukuda M, Nakayama H, Aoyama N, Ito Y, Fujimoto Y, Takagi K, Takagi K, Hasegawa T. (2005) Behavioral changes following antisense oligonucleotide-induced reduction of organic cation transporter-3 in mice. Neurosci Lett 382:195–200 [DOI] [PubMed] [Google Scholar]
- Kitamura S, Maeda K, Wang Y, Sugiyama Y. (2008) Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos 36:2014–2023 [DOI] [PubMed] [Google Scholar]
- Kitano T, Yuasa I, Yamazaki K, Nakayashiki N, Miyoshi A, Park KS, Umetsu K. (2008) Allele frequencies of a SNP and a 27-bp deletion that are the determinant of earwax type in the ABCC11 gene. Leg Med (Tokyo) 10:113–114 [DOI] [PubMed] [Google Scholar]
- Kiuchi Y, Suzuki H, Hirohashi T, Tyson CA, Sugiyama Y. (1998) cDNA cloning and inducible expression of human multidrug resistance associated protein 3 (MRP3). FEBS Lett 433:149–152 [DOI] [PubMed] [Google Scholar]
- Kivistö KT, Grisk O, Hofmann U, Meissner K, Möritz KU, Ritter C, Arnold KA, Lutjöohann D, von Bergmann K, Klöting I, et al. (2005) Disposition of oral and intravenous pravastatin in MRP2-deficient TR- rats. Drug Metab Dispos 33:1593–1596 [DOI] [PubMed] [Google Scholar]
- Kivistö KT, Zukunft J, Hofmann U, Niemi M, Rekersbrink S, Schneider S, Luippold G, Schwab M, Eichelbaum M, Fromm MF. (2004) Characterisation of cerivastatin as a P-glycoprotein substrate: studies in P-glycoprotein-expressing cell monolayers and mdr1a/b knock-out mice. Naunyn Schmiedebergs Arch Pharmacol 370:124–130 [DOI] [PubMed] [Google Scholar]
- Kiyotani K, Mushiroda T, Kubo M, Zembutsu H, Sugiyama Y, Nakamura Y. (2008) Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci 99:967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD. (1970) Plasma disappearance and biliary excretion of sulfobromophthalein and phenol-3,6-dibromphthalein disulfonate after microsomal enzyme induction. Biochem Pharmacol 19:1241–1249 [DOI] [PubMed] [Google Scholar]
- Klaassen CD. (1972) Immaturity of the newborn rat's hepatic excretory function for ouabain. J Pharmacol Exp Ther 183:520–526 [PubMed] [Google Scholar]
- Klaassen CD. (1974) Effect of microsomal enzyme inducers on the biliary excretion of cardiac glycosides. J Pharmacol Exp Ther 191:201–211 [PubMed] [Google Scholar]
- Klaassen CD. (1976) Effect of microsomal enzyme inducers on the biliary excretion of an exogenous load of bilirubin in newborn rats. Proc Soc Exp Biol Med 153:370–373 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Lu H. (2008) Xenobiotic transporters: ascribing function from gene knockout and mutation studies. Toxicol Sci 101:186–196 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL. (2005) Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab 6:309–328 [DOI] [PubMed] [Google Scholar]
- Kleemann P, Casper B, Huber M, Schuetz JD, Lohoff M. (2006) Multidrug-resistance-associated protein 1 (Mrp1) is probably not required for murine Th cell activation. Int Immunol 18:1603–1606 [DOI] [PubMed] [Google Scholar]
- Klein K, Kullak-Ublick GA, Wagner M, Trauner M, Eloranta JJ. (2009) Hepatocyte nuclear factor-4alpha and bile acids regulate human concentrative nucleoside transporter-1 gene expression. Am J Physiol Gastrointest Liver Physiol 296:G936–G947 [DOI] [PubMed] [Google Scholar]
- Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. (2004a) Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol 4:21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett EL, Lu K, Kosters A, Vink E, Lee MH, Altenburg M, Shefer S, Batta AK, Yu H, Chen J, et al. (2004b) A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med 2:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AC, Todesco L, Torok M, Beier K, Krähenbühl S. (2008) Effect of carnitine deprivation on carnitine homeostasis and energy metabolism in mice with systemic carnitine deficiency. Ann Nutr Metab 52:136–144 [DOI] [PubMed] [Google Scholar]
- Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikçi B, Ozçay F, et al. (2006) Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44:478–486 [DOI] [PubMed] [Google Scholar]
- Knütter I, Rubio-Aliaga I, Boll M, Hause G, Daniel H, Neubert K, Brandsch M. (2002) H+-peptide cotransport in the human bile duct epithelium cell line SK-ChA-1. Am J Physiol Gastrointest Liver Physiol 283:G222–G229 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Goto A, Maeda T, Nezu J, Tsuji A, Tamai I. (2005a) OCTN2-mediated transport of carnitine in isolated Sertoli cells. Reproduction 129:729–736 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Ieiri I, Hirota T, Takane H, Maegawa S, Kigawa J, Suzuki H, Nanba E, Oshimura M, Terakawa N, et al. (2005b) Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos 33:94–101 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Irokawa M, Maeda T, Tsuji A, Tamai I. (2005c) Carnitine/organic cation transporter OCTN2-mediated transport of carnitine in primary-cultured epididymal epithelial cells. Reproduction 130:931–937 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2003) Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther 306:703–708 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Tamai I, Sai Y, Yoshida K, Wakayama T, Kido Y, Nezu J, Iseki S, Tsuji A. (2007) Transport of carnitine and acetylcarnitine by carnitine/organic cation transporter (OCTN) 2 and OCTN3 into epididymal spermatozoa. Reproduction 134:651–658 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ito K, Takada T, Sugiyama Y, Suzuki H. (2008) Functional analysis of nonsynonymous single nucleotide polymorphism type ATP-binding cassette transmembrane transporter subfamily C member 3. Pharmacogenet Genomics 18:823–833 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Saitoh H, Kobayashi M, Tadano K, Takahashi Y, Hirano T. (2004) Cyclosporin A, but not tacrolimus, inhibits the biliary excretion of mycophenolic acid glucuronide possibly mediated by multidrug resistance-associated protein 2 in rats. J Pharmacol Exp Ther 309:1029–1035 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Hirokawa N, Ohshiro N, Sekine T, Sasaki T, Tokuyama S, Endou H, Yamamoto T. (2002a) Differential gene expression of organic anion transporters in male and female rats. Biochem Biophys Res Commun 290:482–487 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Ohshiro N, Shibusawa A, Sasaki T, Tokuyama S, Sekine T, Endou H, Yamamoto T. (2002b) Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. Mol Pharmacol 62:7–14 [DOI] [PubMed] [Google Scholar]
- Kocher O, Comella N, Gilchrist A, Pal R, Tognazzi K, Brown LF, Knoll JH. (1999) PDZK1, a novel PDZ domain-containing protein up-regulated in carcinomas and mapped to chromosome 1q21, interacts with cMOAT (MRP2), the multidrug resistance-associated protein. Lab Invest 79:1161–1170 [PubMed] [Google Scholar]
- Koepsell H, Endou H. (2004) The SLC22 drug transporter family. Pflugers Arch 447:666–676 [DOI] [PubMed] [Google Scholar]
- Koh AS, Simmons-Willis TA, Pritchard JB, Grassl SM, Ballatori N. (2002) Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: transport by the renal organic anion transporter-1. Mol Pharmacol 62:921–926 [DOI] [PubMed] [Google Scholar]
- Köhle C, Bock KW. (2006) Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem Pharmacol 72:795–805 [DOI] [PubMed] [Google Scholar]
- Koizumi A, Nozaki J, Ohura T, Kayo T, Wada Y, Nezu J, Ohashi R, Tamai I, Shoji Y, Takada G, et al. (1999) Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency. Hum Mol Genet 8:2247–2254 [DOI] [PubMed] [Google Scholar]
- Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. (2002) Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol 13:848–857 [DOI] [PubMed] [Google Scholar]
- Kok T, Bloks VW, Wolters H, Havinga R, Jansen PL, Staels B, Kuipers F. (2003) Peroxisome proliferator-activated receptor alpha (PPARalpha)-mediated regulation of multidrug resistance 2 (Mdr2) expression and function in mice. Biochem J 369:539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoda F, Sekine T, Inatomi J, Enomoto A, Endou H, Ota T, Matsuyama T, Ogata T, Ikeda M, Awazu M, et al. (2004) The W258X mutation in SLC22A12 is the predominant cause of Japanese renal hypouricemia. Pediatr Nephrol 19:728–733 [DOI] [PubMed] [Google Scholar]
- Kondo C, Suzuki H, Itoda M, Ozawa S, Sawada J, Kobayashi D, Ieiri I, Mine K, Ohtsubo K, Sugiyama Y. (2004) Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res 21:1895–1903 [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000a) Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275:23161–23168 [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000b) A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol 278:G156–G164 [DOI] [PubMed] [Google Scholar]
- König J, Rost D, Cui Y, Keppler D. (1999) Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology 29:1156–1163 [DOI] [PubMed] [Google Scholar]
- Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P. (1997) Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 57:3537–3547 [PubMed] [Google Scholar]
- Kool M, van der Linden M, de Haas M, Baas F, Borst P. (1999a) Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res 59:175–182 [PubMed] [Google Scholar]
- Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ, et al. (1999b) MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A 96:6914–6919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. (2004) The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem 279:24218–24225 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Schwab M, Takenaka K, Nachagari D, Morgan J, Leslie M, Du W, Boyd K, Cheok M, Nakauchi H, et al. (2008) Transporter-mediated protection against thiopurine-induced hematopoietic toxicity. Cancer Res 68:4983–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh GD, Belinsky MG, Gallo JM, Lee K. (2007) Physiological and pharmacological functions of Mrp2, Mrp3 and Mrp4 as determined from recent studies on gene-disrupted mice. Cancer Metastasis Rev 26:5–14 [DOI] [PubMed] [Google Scholar]
- Kubitz R, Huth C, Schmitt M, Horbach A, Kullak-Ublick G, Häussinger D. (2001) Protein kinase C-dependent distribution of the multidrug resistance protein 2 from the canalicular to the basolateral membrane in human HepG2 cells. Hepatology 34:340–350 [DOI] [PubMed] [Google Scholar]
- Kubitz R, Keitel V, Häussinger D. (2005) Inborn errors of biliary canalicular transport systems. Methods Enzymol 400:558–569 [DOI] [PubMed] [Google Scholar]
- Kubitz R, Wettstein M, Warskulat U, Häussinger D. (1999) Regulation of the multidrug resistance protein 2 in the rat liver by lipopolysaccharide and dexamethasone. Gastroenterology 116:401–410 [DOI] [PubMed] [Google Scholar]
- Kühlkamp T, Keitel V, Helmer A, Häussinger D, Kubitz R. (2005) Degradation of the sodium taurocholate cotransporting polypeptide (NTCP) by the ubiquitin-proteasome system. Biol Chem 386:1065–1074 [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. (1995) Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology 109:1274–1282 [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. (2001) Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120:525–533 [DOI] [PubMed] [Google Scholar]
- Kummer W, Wiegand S, Akinci S, Schinkel AH, Wess J, Koepsell H, Haberberger RV, Lips KS. (2006) Role of acetylcholine and muscarinic receptors in serotonin-induced bronchoconstriction in the mouse. J Mol Neurosci 30:67–68 [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H. (1999) Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem 274:13675–13680 [DOI] [PubMed] [Google Scholar]
- Kwak JO, Kim HW, Oh KJ, Ko CB, Park H, Cha SH. (2005) Characterization of mouse organic anion transporter 5 as a renal steroid sulfate transporter. J Steroid Biochem Mol Biol 97:369–375 [DOI] [PubMed] [Google Scholar]
- Lagas JS, van Waterschoot RA, van Tilburg VA, Hillebrand MJ, Lankheet N, Rosing H, Beijnen JH, Schinkel AH. (2009) Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res 15:2344–2351 [DOI] [PubMed] [Google Scholar]
- Lagas JS, Vlaming ML, van Tellingen O, Wagenaar E, Jansen RS, Rosing H, Beijnen JH, Schinkel AH. (2006) Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics. Clin Cancer Res 12:6125–6132 [DOI] [PubMed] [Google Scholar]
- Lahjouji K, Elimrani I, Lafond J, Leduc L, Qureshi IA, Mitchell GA. (2004) L-Carnitine transport in human placental brush-border membranes is mediated by the sodium-dependent organic cation transporter OCTN2. Am J Physiol Cell Physiol 287:C263–C269 [DOI] [PubMed] [Google Scholar]
- Lai L, Tan TM. (2002) Role of glutathione in the multidrug resistance protein 4 (MRP4/ABCC4)-mediated efflux of cAMP and resistance to purine analogues. Biochem J 361:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Tse CM, Unadkat JD. (2004) Mitochondrial expression of the human equilibrative nucleoside transporter 1 (hENT1) results in enhanced mitochondrial toxicity of antiviral drugs. J Biol Chem 279:4490–4497 [DOI] [PubMed] [Google Scholar]
- Lam P, Wang R, Ling V. (2005) Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry 44:12598–12605 [DOI] [PubMed] [Google Scholar]
- Lamhonwah AM, Ackerley CA, Tilups A, Edwards VD, Wanders RJ, Tein I. (2005) OCTN3 is a mammalian peroxisomal membrane carnitine transporter. Biochem Biophys Res Commun 338:1966–1972 [DOI] [PubMed] [Google Scholar]
- Lamhonwah AM, Hawkins CE, Tam C, Wong J, Mai L, Tein I. (2008) Expression patterns of the organic cation/carnitine transporter family in adult murine brain. Brain Dev 30:31–42 [DOI] [PubMed] [Google Scholar]
- Lamhonwah AM, Skaug J, Scherer SW, Tein I. (2003) A third human carnitine/organic cation transporter (OCTN3) as a candidate for the 5q31 Crohn's disease locus (IBD5). Biochem Biophys Res Commun 301:98–101 [DOI] [PubMed] [Google Scholar]
- Lamhonwah AM, Tein I. (2006) Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria. Biochem Biophys Res Commun 345:1315–1325 [DOI] [PubMed] [Google Scholar]
- Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. (2006) The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol 290:G476–G485 [DOI] [PubMed] [Google Scholar]
- Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, Kullak-Ublick GA, Meier PJ, Pauli-Magnus C. (2007) Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics 17:47–60 [DOI] [PubMed] [Google Scholar]
- Lang T, Hitzl M, Burk O, Mornhinweg E, Keil A, Kerb R, Klein K, Zanger UM, Eichelbaum M, Fromm MF. (2004) Genetic polymorphisms in the multidrug resistance-associated protein 3 (ABCC3, MRP3) gene and relationship to its mRNA and protein expression in human liver. Pharmacogenetics 14:155–164 [DOI] [PubMed] [Google Scholar]
- Langheim S, Yu L, von Bergmann K, Lütjohann D, Xu F, Hobbs HH, Cohen JC. (2005) ABCG5 and ABCG8 require MDR2 for secretion of cholesterol into bile. J Lipid Res 46:1732–1738 [DOI] [PubMed] [Google Scholar]
- Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G. (1999) Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun 257:29–33 [DOI] [PubMed] [Google Scholar]
- Lankas GR, Wise LD, Cartwright ME, Pippert T, Umbenhauer DR. (1998) Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod Toxicol 12:457–463 [DOI] [PubMed] [Google Scholar]
- LaRusso J, Li Q, Jiang Q, Uitto J. (2009) Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(−/−)). J Invest Dermatol 129:1388–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau YY, Huang Y, Frassetto L, Benet LZ. (2007) Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther 81:194–204 [DOI] [PubMed] [Google Scholar]
- Lazar A, Zimmermann T, Koch W, Gründemann D, Schömig A, Kastrati A, Schömig E. (2006) Lower prevalence of the OCT2 Ser270 allele in patients with essential hypertension. Clin Exp Hypertens 28:645–653 [DOI] [PubMed] [Google Scholar]
- Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. (1997) Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest 100:2714–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, Pasquali-Ronchetti I, Pope FM, Richards A, Terry S, et al. (2000) Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 25:223–227 [DOI] [PubMed] [Google Scholar]
- Leabman MK, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, DeYoung J, Taylor T, Clark AG, Herskowitz I, et al. (2002) Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function. Pharmacogenetics 12:395–405 [DOI] [PubMed] [Google Scholar]
- Lecureur V, Sun D, Hargrove P, Schuetz EG, Kim RB, Lan LB, Schuetz JD. (2000) Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol Pharmacol 57:24–35 [PubMed] [Google Scholar]
- Lee CG, Tang K, Cheung YB, Wong LP, Tan C, Shen H, Zhao Y, Pavanni R, Lee EJ, Wong MC, et al. (2004a) MDR1, the blood-brain barrier transporter, is associated with Parkinson's disease in ethnic Chinese. J Med Genet 41:e60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. (2001a) Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology 121:1473–1484 [DOI] [PubMed] [Google Scholar]
- Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. (2000a) Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology 118:163–172 [DOI] [PubMed] [Google Scholar]
- Lee K, Belinsky MG, Bell DW, Testa JR, Kruh GD. (1998) Isolation of MOAT-B, a widely expressed multidrug resistance-associated protein/canalicular multispecific organic anion transporter-related transporter. Cancer Res 58:2741–2747 [PubMed] [Google Scholar]
- Lee K, Klein-Szanto AJ, Kruh GD. (2000b) Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J Natl Cancer Inst 92:1934–1940 [DOI] [PubMed] [Google Scholar]
- Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, et al. (2001b) Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet 27:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Koh AS, Cui Z, Pierce RH, Ballatori N. (2003) N-glycosylation controls functional activity of Oatp1, an organic anion transporter. Am J Physiol Gastrointest Liver Physiol 285:G371–G381 [DOI] [PubMed] [Google Scholar]
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. (2005a) Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem 280:9610–9617 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. (2005b) Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther 312:44–52 [DOI] [PubMed] [Google Scholar]
- Lee YM, Cui Y, König J, Risch A, Jäger B, Drings P, Bartsch H, Keppler D, Nies AT. (2004b) Identification and functional characterization of the natural variant MRP3-Arg1297His of human multidrug resistance protein 3 (MRP3/ABCC3). Pharmacogenetics 14:213–223 [DOI] [PubMed] [Google Scholar]
- Leier I, Jedlitschky G, Buchholz U, Center M, Cole SP, Deeley RG, Keppler D. (1996) ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J 314:433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, Keppler D. (1994) The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem 269:27807–27810 [PubMed] [Google Scholar]
- Leslie EM, Létourneau IJ, Deeley RG, Cole SP. (2003) Functional and structural consequences of cysteine substitutions in the NH2 proximal region of the human multidrug resistance protein 1 (MRP1/ABCC1). Biochemistry 42:5214–5224 [DOI] [PubMed] [Google Scholar]
- Leslie EM, Watkins PB, Kim RB, Brouwer KL. (2007) Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1)by bosentan: a mechanism for species differences in hepatotoxicity. J Pharmacol Exp Ther 321:1170–1178 [DOI] [PubMed] [Google Scholar]
- Létourneau IJ, Deeley RG, Cole SP. (2005) Functional characterization of non-synonymous single nucleotide polymorphisms in the gene encoding human multidrug resistance protein 1 (MRP1/ABCC1). Pharmacogenet Genomics 15:647–657 [DOI] [PubMed] [Google Scholar]
- Letschert K, Keppler D, König J. (2004) Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8). Pharmacogenetics 14:441–452 [DOI] [PubMed] [Google Scholar]
- Leung E, Hong J, Fraser AG, Merriman TR, Vishnu P, Krissansen GW. (2006) Polymorphisms in the organic cation transporter genes SLC22A4 and SLC22A5 and Crohn's disease in a New Zealand Caucasian cohort. Immunol Cell Biol 84:233–236 [DOI] [PubMed] [Google Scholar]
- Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. (2009) Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am J Physiol Cell Physiol 296:C570–C582 [DOI] [PubMed] [Google Scholar]
- Leveille-Webster CR, Arias IM. (1994) Mdr 2 knockout mice link biliary phospholipid deficiency with small bile duct destruction. Hepatology 19:1528–1531 [DOI] [PubMed] [Google Scholar]
- Lévesque E, Benoit-Biancamano MO, Delage R, Couture F, Guillemette C. (2008) Pharmacokinetics of mycophenolate mofetil and its glucuronide metabolites in healthy volunteers. Pharmacogenomics 9:869–879 [DOI] [PubMed] [Google Scholar]
- Li J, Cusatis G, Brahmer J, Sparreboom A, Robey RW, Bates SE, Hidalgo M, Baker SD. (2007a) Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol Ther 6:432–438 [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Meier PJ, Ballatori N. (1998) Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem 273:16184–16191 [DOI] [PubMed] [Google Scholar]
- Li L, Meier PJ, Ballatori N. (2000) Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol 58:335–340 [DOI] [PubMed] [Google Scholar]
- Li N, Cui Z, Fang F, Lee JY, Ballatori N. (2007b) Heterodimerization, trafficking and membrane topology of the two proteins, Ost alpha and Ost beta, that constitute the organic solute and steroid transporter. Biochem J 407:363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Hartley DP, Cherrington NJ, Klaassen CD. (2002) Tissue expression, ontogeny, and inducibility of rat organic anion transporting polypeptide 4. J Pharmacol Exp Ther 301:551–560 [DOI] [PubMed] [Google Scholar]
- Li N, Klaassen CD. (2004) Role of liver-enriched transcription factors in the down-regulation of organic anion transporting polypeptide 4 (oatp4; oatplb2; slc21a10) by lipopolysaccharide. Mol Pharmacol 66:694–701 [DOI] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Larusso J, Klement JF, Sartorelli AC, Belinsky MG, Kruh GD, Uitto J. (2007c) Targeted ablation of Abcc1 or Abcc3 in Abcc6(−/−) mice does not modify the ectopic mineralization process. Exp Dermatol 16:853–859 [DOI] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Pfendner E, Váradi A, Uitto J. (2009a) Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol 18:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Uitto J. (2008) Pseudoxanthoma elasticum: oxidative stress and antioxidant diet in a mouse model (Abcc6−/−). J Invest Dermatol 128:1160–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Duan P, You G. (2009b) Regulation of human organic anion transporter 1 by ANG II: involvement of protein kinase Calpha. Am J Physiol Endocrinol Metab 296:E378–E383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orrù M, Albai G, et al. (2007d) The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet 3:e194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ito K, Horie T. (2003) Transport of fluorescein methotrexate by multidrug resistance-associated protein 3 in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol 285:G602–G610 [DOI] [PubMed] [Google Scholar]
- Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. (1995) Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem 270:6456–6463 [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Cheng X, Augustine LM, Klaassen CD, Cherrington NJ. (2008) Tissue distribution, ontogeny and induction of the transporters Multidrug and toxin extrusion (MATE) 1 and MATE2 mRNA expression levels in mice. Life Sci 83:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, Cherrington NJ. (2007a) Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 35:1970–1978 [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Slitt AL, Arkan MC, Karin M, Cherrington NJ. (2007b) Differential regulation of hepatic transporters in the absence of tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and nuclear factor-kappaB in two models of cholestasis. Drug Metab Dispos 35:402–409 [DOI] [PubMed] [Google Scholar]
- Lin ZP, Zhu YL, Johnson DR, Rice KP, Nottoli T, Hains BC, McGrath J, Waxman SG, Sartorelli AC. (2008) Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Mol Pharmacol 73:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincke CR, Smit JJ, van der Velde-Koerts T, Borst P. (1991) Structure of the human MDR3 gene and physical mapping of the human MDR locus. J Biol Chem 266:5303–5310 [PubMed] [Google Scholar]
- Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, Ermert L, Kummer W, Koepsell H. (2005) Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol 33:79–88 [DOI] [PubMed] [Google Scholar]
- Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. (2000) The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2). J Cell Sci 113:2011–2021 [DOI] [PubMed] [Google Scholar]
- Liu J, He YY, Chignell CF, Clark J, Myers P, Saavedra JE, Waalkes MP. (2005) Limited protective role of V-PYRRO/NO against cholestasis produced by alpha-naphthylisothiocyanate in mice. Biochem Pharmacol 70:144–151 [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Powell DA, Waalkes MP, Klaassen CD. (2002) Multidrug-resistance mdr1a/1b double knockout mice are more sensitive than wild type mice to acute arsenic toxicity, with higher arsenic accumulation in tissues. Toxicology 170:55–62 [DOI] [PubMed] [Google Scholar]
- Liu W, Liang R, Ramamoorthy S, Fei YJ, Ganapathy ME, Hediger MA, Ganapathy V, Leibach FH. (1995) Molecular cloning of PEPT 2, a new member of the H+/peptide cotransporter family, from human kidney. Biochim Biophys Acta 1235:461–466 [DOI] [PubMed] [Google Scholar]
- Ljubojević M, Balen D, Breljak D, Kusan M, Anzai N, Bahn A, Burckhardt G, Sabolić I. (2007) Renal expression of organic anion transporter OAT2 in rats and mice is regulated by sex hormones. Am J Physiol Renal Physiol 292:F361–F372 [DOI] [PubMed] [Google Scholar]
- Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I. (2004) Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol 287:F124–F138 [DOI] [PubMed] [Google Scholar]
- Longhurst TJ, O'Neill GM, Harvie RM, Davey RA. (1996) The anthracycline resistance-associated (ara) gene, a novel gene associated with multidrug resistance in a human leukaemia cell line. Br J Cancer 74:1331–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. (1997) Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem 272:6471–6478 [DOI] [PubMed] [Google Scholar]
- Lu H, Chen C, Klaassen C. (2004) Tissue distribution of concentrative and equilibrative nucleoside transporters in male and female rats and mice. Drug Metab Dispos 32:1455–1461 [DOI] [PubMed] [Google Scholar]
- Lu H, Choudhuri S, Ogura K, Csanaky IL, Lei X, Cheng X, Song PZ, Klaassen CD. (2008) Characterization of organic anion transporting polypeptide 1b2-null mice: essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol Sci 103:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Klaassen C. (2006) Tissue distribution and thyroid hormone regulation of Pept1 and Pept2 mRNA in rodents. Peptides 27:850–857 [DOI] [PubMed] [Google Scholar]
- Lu K, Lee MH, Hazard S, Brooks-Wilson A, Hidaka H, Kojima H, Ose L, Stalenhoef AF, Mietinnen T, Bjorkhem I, Bruckert E, Pandya A, Brewer HB, Jr., Salen G, Dean M, Srivastava A, Patel SB. (2001) Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet 69:278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Lee MH, Yu H, Zhou Y, Sandell SA, Salen G, Patel SB. (2002) Molecular cloning, genomic organization, genetic variations, and characterization of murine sterolin genes Abcg5 and Abcg8. J Lipid Res 43:565–578 [PMC free article] [PubMed] [Google Scholar]
- Lu K, Nishimori H, Nakamura Y, Shima K, Kuwajima M. (1998) A missense mutation of mouse OCTN2, a sodium-dependent carnitine cotransporter, in the juvenile visceral steatosis mouse. Biochem Biophys Res Commun 252:590–594 [DOI] [PubMed] [Google Scholar]
- Ludwig T, Riethmüller C, Gekle M, Schwerdt G, Oberleithner H. (2004) Nephrotoxicity of platinum complexes is related to basolateral organic cation transport. Kidney Int 66:196–202 [DOI] [PubMed] [Google Scholar]
- Luker GD, Pica CM, Kumar AS, Covey DF, Piwnica-Worms D. (2000) Effects of cholesterol and enantiomeric cholesterol on P-glycoprotein localization and function in low-density membrane domains. Biochemistry 39:7651–7661 [DOI] [PubMed] [Google Scholar]
- Lutsenko S. (2008) Atp7b−/− mice as a model for studies of Wilson's disease. Biochem Soc Trans 36:1233–1238 [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. (2007) Function and regulation of human copper-transporting ATPases. Physiol Rev 87:1011–1046 [DOI] [PubMed] [Google Scholar]
- Mackey JR, Yao SY, Smith KM, Karpinski E, Baldwin SA, Cass CE, Young JD. (1999) Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Natl Cancer Inst 91:1876–1881 [DOI] [PubMed] [Google Scholar]
- Madon J, Hagenbuch B, Landmann L, Meier PJ, Stieger B. (2000) Transport function and hepatocellular localization of mrp6 in rat liver. Mol Pharmacol 57:634–641 [DOI] [PubMed] [Google Scholar]
- Maeda T, Goto A, Kobayashi D, Tamai I. (2007a) Transport of organic cations across the blood-testis barrier. Mol Pharm 4:600–607 [DOI] [PubMed] [Google Scholar]
- Maeda T, Miyata M, Yotsumoto T, Kobayashi D, Nozawa T, Toyama K, Gonzalez FJ, Yamazoe Y, Tamai I. (2004) Regulation of drug transporters by the farnesoid X receptor in mice. Mol Pharm 1:281–289 [DOI] [PubMed] [Google Scholar]
- Maeda T, Takahashi K, Ohtsu N, Oguma T, Ohnishi T, Atsumi R, Tamai I. (2007b) Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm 4:85–94 [DOI] [PubMed] [Google Scholar]
- Maeda T, Wakasawa T, Funabashi M, Fukushi A, Fujita M, Motojima K, Tamai I. (2008) Regulation of Octn2 transporter (SLC22A5) by peroxisome proliferator activated receptor alpha. Biol Pharm Bull 31:1230–1236 [DOI] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. (2002) Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol 62:638–646 [DOI] [PubMed] [Google Scholar]
- Magyari L, Bene J, Komlósi K, Talián G, Faragó B, Csöngei V, Járomi L, Sáfrány E, Sipeky C, Lakner L, et al. (2007) Prevalence of SLC22A4 1672T and SLC22A5 −207C combination defined TC haplotype in Hungarian ulcerative colitis patients. Pathol Oncol Res 13:53–56 [DOI] [PubMed] [Google Scholar]
- Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. (2007) Human organic anion transporter 1B1 and 1B3 function as bidirectional carriers and do not mediate GSH-bile acid cotransport. Am J Physiol Gastrointest Liver Physiol 293:G271–G278 [DOI] [PubMed] [Google Scholar]
- Maher JM, Aleksunes LM, Dieter MZ, Tanaka Y, Peters JM, Manautou JE, Klaassen CD. (2008) Nrf2- and PPAR alpha-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol Sci 106:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. (2005a) Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos 33:956–962 [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Tanaka Y, Scheffer GL, Klaassen CD. (2006a) Hormonal regulation of renal multidrug resistance-associated proteins 3 and 4 (Mrp3 and Mrp4) in mice. Biochem Pharmacol 71:1470–1478 [DOI] [PubMed] [Google Scholar]
- Maher JM, Cherrington NJ, Slitt AL, Klaassen CD. (2006b) Tissue distribution and induction of the rat multidrug resistance-associated proteins 5 and 6. Life Sci 78:2219–2225 [DOI] [PubMed] [Google Scholar]
- Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, et al. (2007) Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology 46:1597–1610 [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Callaghan TN, Cheng X, Cheung C, Gonzalez FJ, Klaassen CD. (2006c) Alterations in transporter expression in liver, kidney, and duodenum after targeted disruption of the transcription factor HNF1alpha. Biochem Pharmacol 72:512–522 [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. (2005b) Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos 33:947–955 [DOI] [PubMed] [Google Scholar]
- Makhseed N, Vallance HD, Potter M, Waters PJ, Wong LT, Lillquist Y, Pasquali M, Amat di San Filippo C, Longo N. (2004) Carnitine transporter defect due to a novel mutation in the SLC22A5 gene presenting with peripheral neuropathy. J Inherit Metab Dis 27:778–780 [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. (2001) Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 61:3458–3464 [PubMed] [Google Scholar]
- Manautou JE, de Waart DR, Kunne C, Zelcer N, Goedken M, Borst P, Elferink RO. (2005) Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology 42:1091–1098 [DOI] [PubMed] [Google Scholar]
- Mangravite LM, Xiao G, Giacomini KM. (2003) Localization of human equilibrative nucleoside transporters, hENT1 and hENT2, in renal epithelial cells. Am J Physiol Renal Physiol 284:F902–F910 [DOI] [PubMed] [Google Scholar]
- Manibusan MK, Odin M, Eastmond DA. (2007) Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 25:185–209 [DOI] [PubMed] [Google Scholar]
- Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, Mazzanti R, van Tellingen O, Schellens JH. (2008) Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1−/−/Mdr1a/1b−/− (triple-knockout) and wild-type mice. Mol Cancer Ther 7:2280–2287 [DOI] [PubMed] [Google Scholar]
- Marcil M, Brooks-Wilson A, Clee SM, Roomp K, Zhang LH, Yu L, Collins JA, van Dam M, Molhuizen HO, Loubster O, Ouellette BF, Sensen CW, Fichter K, Mott S, Denis M, Boucher B, Pimstone S, Genest J, Jr., Kastelein JJ, Hayden MR. (1999) Mutations in the ABC1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet 354:1341–1346 [DOI] [PubMed] [Google Scholar]
- Mareninova O, Shin JM, Vagin O, Turdikulova S, Hallen S, Sachs G. (2005) Topography of the membrane domain of the liver Na+-dependent bile acid transporter. Biochemistry 44:13702–13712 [DOI] [PubMed] [Google Scholar]
- Martínez A, Valdivia A, Pascual-Salcedo D, Balsa A, Fernández-Gutiérrez B, De la Concha E, Urcelay E. (2006) Role of SLC22A4, SLC22A5, and RUNX1 genes in rheumatoid arthritis. J Rheumatol 33:842–846 [PubMed] [Google Scholar]
- Marzolini C, Tirona RG, Gervasini G, Poonkuzhali B, Assem M, Lee W, Leake BF, Schuetz JD, Schuetz EG, Kim RB. (2007) A common polymorphism in the bile acid receptor farnesoid X receptor is associated with decreased hepatic target gene expression. Mol Endocrinol 21:1769–1780 [DOI] [PubMed] [Google Scholar]
- Masahiko N, Honkakoski P. (2000) Induction of drug metabolism by nuclear receptor CAR: molecular mechanisms and implications for drug research. Eur J Pharm Sci 11:259–264 [DOI] [PubMed] [Google Scholar]
- Masuda M, I'izuka Y, Yamazaki M, Nishigaki R, Kato Y, Ni'inuma K, Suzuki H, Sugiyama Y. (1997) Methotrexate is excreted into the bile by canalicular multispecific organic anion transporter in rats. Cancer Res 57:3506–3510 [PubMed] [Google Scholar]
- Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, Ogawa O, Inui K. (2006) Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol 17:2127–2135 [DOI] [PubMed] [Google Scholar]
- Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, Wiriyasermkul P, Kikuchi Y, Oda T, Nishiyama J, et al. (2008) Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet 83:744–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP. (1994) Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol 145:1237–1245 [PMC free article] [PubMed] [Google Scholar]
- Mayatepek E, Nezu J, Tamai I, Oku A, Katsura M, Shimane M, Tsuji A.(2000) Two novel missense mutations of the OCTN2 gene (W283R and V446F) in a patient with primary systemic carnitine deficiency. Hum Mutat 15:118 [DOI] [PubMed] [Google Scholar]
- McAleer MA, Breen MA, White NL, Matthews N. (1999) pABC11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J Biol Chem 274:23541–23548 [DOI] [PubMed] [Google Scholar]
- McArdle PF, Parsa A, Chang YP, Weir MR, O'Connell JR, Mitchell BD, Shuldiner AR. (2008) Association of a common nonsynonymous variant in GLUT9 with serum uric acid levels in old order amish. Arthritis Rheum 58:2874–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride BF, Yang T, Liu K, Urban TJ, Giacomini KM, Kim RB, Roden DM. (2009) The organic cation transporter, OCTN1, expressed in the human heart, potentiates antagonism of the HERG potassium channel. J Cardiovasc Pharmacol 54:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, et al. (2000) High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A 97:4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrens T, Lelleck S, Cetinkaya I, Knollmann M, Hohage H, Gorboulev V, Bokník P, Koepsell H, Schlatter E. (2000) The affinity of the organic cation transporter rOCT1 is increased by protein kinase C-dependent phosphorylation. J Am Soc Nephrol 11:1216–1224 [DOI] [PubMed] [Google Scholar]
- Meier-Abt F, Faulstich H, Hagenbuch B. (2004) Identification of phalloidin uptake systems of rat and human liver. Biochim Biophys Acta 1664:64–69 [DOI] [PubMed] [Google Scholar]
- Meier-Abt F, Hammann-Hänni A, Stieger B, Ballatori N, Boyer JL. (2007) The organic anion transport polypeptide 1d1 (Oatp1d1) mediates hepatocellular uptake of phalloidin and microcystin into skate liver. Toxicol Appl Pharmacol 218:274–279 [DOI] [PubMed] [Google Scholar]
- Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. (1997) Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology 26:1667–1677 [DOI] [PubMed] [Google Scholar]
- Meier Y, Zodan T, Lang C, Zimmermann R, Kullak-Ublick GA, Meier PJ, Stieger B, Pauli-Magnus C. (2008) Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenterol 14:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K, Heydrich B, Jedlitschky G, Meyer Zu Schwabedissen H, Mosyagin I, Dazert P, Eckel L, Vogelgesang S, Warzok RW, Böhm M, et al. (2006) The ATP-binding cassette transporter ABCG2 (BCRP), a marker for side population stem cells, is expressed in human heart. J Histochem Cytochem 54:215–221 [DOI] [PubMed] [Google Scholar]
- Melaine N, Liénard MO, Dorval I, Le Goascogne C, Lejeune H, Jégou B. (2002) Multidrug resistance genes and p-glycoprotein in the testis of the rat, mouse, guinea pig, and human. Biol Reprod 67:1699–1707 [DOI] [PubMed] [Google Scholar]
- Melegh B, Bene J, Mogyorósy G, Havasi V, Komlósi K, Pajor L, Oláh E, Kispál G, Sumegi B, Méhes K. (2004) Phenotypic manifestations of the OCTN2 V295X mutation: sudden infant death and carnitine-responsive cardiomyopathy in Roma families. Am J Med Genet A 131:121–126 [DOI] [PubMed] [Google Scholar]
- Meng LJ, Wang P, Wolkoff AW, Kim RB, Tirona RG, Hofmann AF, Pang KS. (2002) Transport of the sulfated, amidated bile acid, sulfolithocholyltaurine, into rat hepatocytes is mediated by Oatp1 and Oatp2. Hepatology 35:1031–1040 [DOI] [PubMed] [Google Scholar]
- Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. (2006) Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology 43:1013–1021 [DOI] [PubMed] [Google Scholar]
- Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. (2006) Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos 34:690–695 [DOI] [PubMed] [Google Scholar]
- Merino G, Jonker JW, Wagenaar E, Pulido MM, Molina AJ, Alvarez AI, Schinkel AH. (2005a) Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2). Drug Metab Dispos 33:614–618 [DOI] [PubMed] [Google Scholar]
- Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. (2005b) The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol 67:1758–1764 [DOI] [PubMed] [Google Scholar]
- Merino G, van Herwaarden AE, Wagenaar E, Jonker JW, Schinkel AH. (2005c) Sex-dependent expression and activity of the ATP-binding cassette transporter breast cancer resistance protein (BCRP/ABCG2) in liver. Mol Pharmacol 67:1765–1771 [DOI] [PubMed] [Google Scholar]
- Merrell MD, Augustine LM, Slitt AL, Cherrington NJ. (2008) Induction of drug metabolism enzymes and transporters by oltipraz in rats. J Biochem Mol Toxicol 22:128–135 [DOI] [PubMed] [Google Scholar]
- Meyer-Wentrup F, Karbach U, Gorboulev V, Arndt P, Koepsell H. (1998) Membrane localization of the electrogenic cation transporter rOCT1 in rat liver. Biochem Biophys Res Commun 248:673–678 [DOI] [PubMed] [Google Scholar]
- Meyer Zu Schwabedissen HE, Grube M, Heydrich B, Linnemann K, Fusch C, Kroemer HK, Jedlitschky G. (2005) Expression, localization, and function of MRP5 (ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: effects of gestational age and cellular differentiation. Am J Pathol 166:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. (2008) Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res 68:9338–9347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczyk AA, Rieger J, Allen KJ, Mercer JF, Ackland ML. (2000) Defective localization of the Wilson disease protein (ATP7B) in the mammary gland of the toxic milk mouse and the effects of copper supplementation. Biochem J 352 Pt2:565–571 [PMC free article] [PubMed] [Google Scholar]
- Michalski C, Cui Y, Nies AT, Nuessler AK, Neuhaus P, Zanger UM, Klein K, Eichelbaum M, Keppler D, Konig J. (2002) A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J Biol Chem 277:43058–43063 [DOI] [PubMed] [Google Scholar]
- Miecz D, Januszewicz E, Czeredys M, Hinton BT, Berezowski V, Cecchelli R, Nałecz KA. (2008) Localization of organic cation/carnitine transporter (OCTN2) in cells forming the blood-brain barrier. J Neurochem 104:113–123 [DOI] [PubMed] [Google Scholar]
- Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. (2006) Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res 66:535–542 [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, et al. (2004) Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci U S A 101:3569–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. (2000) Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 58:1357–1367 [DOI] [PubMed] [Google Scholar]
- Minami K, Saito T, Narahara M, Tomita H, Kato H, Sugiyama H, Katoh M, Nakajima M, Yokoi T. (2005) Relationship between hepatic gene expression profiles and hepatotoxicity in five typical hepatotoxicant-administered rats. Toxicol Sci 87:296–305 [DOI] [PubMed] [Google Scholar]
- Minami S, Ito K, Honma M, Ikebuchi Y, Anzai N, Kanai Y, Nishida T, Tsukita S, Sekine S, Horie T, et al. (2009) Posttranslational regulation of Abcc2 expression by SUMOylation system. Am J Physiol Gastrointest Liver Physiol 296:G406–G413 [DOI] [PubMed] [Google Scholar]
- Misra S, Ujházy P, Varticovski L, Arias IM. (1999) Phosphoinositide 3-kinase lipid products regulate ATP-dependent transport by sister of P-glycoprotein and multidrug resistance associated protein 2 in bile canalicular membrane vesicles. Proc Natl Acad Sci U S A 96:5814–5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Varticovski L, Arias IM. (2003) Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol 285:G316–G324 [DOI] [PubMed] [Google Scholar]
- Mita S, Suzuki H, Akita H, Hayashi H, Onuki R, Hofmann AF, Sugiyama Y. (2006) Inhibition of bile acid transport across Na+/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metab Dispos 34:1575–1581 [DOI] [PubMed] [Google Scholar]
- Miura K, Yoshiura K, Miura S, Shimada T, Yamasaki K, Yoshida A, Nakayama D, Shibata Y, Niikawa N, Masuzaki H. (2007) A strong association between human earwax-type and apocrine colostrum secretion from the mammary gland. Hum Genet 121:631–633 [DOI] [PubMed] [Google Scholar]
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, et al. (1999) Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res 59:8–13 [PubMed] [Google Scholar]
- Miyazaki H, Anzai N, Ekaratanawong S, Sakata T, Shin HJ, Jutabha P, Hirata T, He X, Nonoguchi H, Tomita K, et al. (2005) Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J Am Soc Nephrol 16:3498–3506 [DOI] [PubMed] [Google Scholar]
- Mizuarai S, Aozasa N, Kotani H. (2004) Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int J Cancer 109:238–246 [DOI] [PubMed] [Google Scholar]
- Mizuno N, Suzuki M, Kusuhara H, Suzuki H, Takeuchi K, Niwa T, Jonker JW, Sugiyama Y. (2004) Impaired renal excretion of 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl) benzothiazole (E3040) sulfate in breast cancer resistance protein (BCRP1/ABCG2) knockout mice. Drug Metab Dispos 32:898–901 [PubMed] [Google Scholar]
- Mizuno N, Takahashi T, Iwase Y, Kusuhara H, Niwa T, Sugiyama Y. (2007a) Human organic anion transporters 1 (hOAT1/SLC22A6) and 3 (hOAT3/SLC22A8) transport edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one) and its sulfate conjugate. Drug Metab Dispos 35:1429–1434 [DOI] [PubMed] [Google Scholar]
- Mizuno N, Takahashi T, Kusuhara H, Schuetz JD, Niwa T, Sugiyama Y. (2007b) Evaluation of the role of breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance-associated protein 4 (MRP4/ABCC4) in the urinary excretion of sulfate and glucuronide metabolites of edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one). Drug Metab Dispos 35:2045–2052 [DOI] [PubMed] [Google Scholar]
- Mochizuki K, Kagawa T, Numari A, Harris MJ, Itoh J, Watanabe N, Mine T, Arias IM. (2007) Two N-linked glycans are required to maintain the transport activity of the bile salt export pump (ABCB11) in MDCK II cells. Am J Physiol Gastrointest Liver Physiol 292:G818–G828 [DOI] [PubMed] [Google Scholar]
- Moffit JS, Aleksunes LM, Maher JM, Scheffer GL, Klaassen CD, Manautou JE. (2006) Induction of hepatic transporters multidrug resistance-associated proteins (Mrp) 3 and 4 by clofibrate is regulated by peroxisome proliferator-activated receptor alpha. J Pharmacol Exp Ther 317:537–545 [DOI] [PubMed] [Google Scholar]
- Mohrmann K, van Eijndhoven MA, Schinkel AH, Schellens JH. (2005) Absence of N-linked glycosylation does not affect plasma membrane localization of breast cancer resistance protein (BCRP/ABCG2). Cancer Chemother Pharmacol 56:344–350 [DOI] [PubMed] [Google Scholar]
- Molho-Pessach V, Lerer I, Abeliovich D, Agha Z, Abu Libdeh A, Broshtilova V, Elpeleg O, Zlotogorski A. (2008) The H syndrome is caused by mutations in the nucleoside transporter hENT3. Am J Hum Genet 83:529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H, Azocar L, Ananthanarayanan M, Arrese M, Miquel JF. (2008) Localization of the Sodium-Taurocholate cotransporting polypeptide in membrane rafts and modulation of its activity by cholesterol in vitro. Biochim Biophys Acta 1778:1283–1291 [DOI] [PubMed] [Google Scholar]
- Monte JC, Nagle MA, Eraly SA, Nigam SK. (2004) Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem Biophys Res Commun 323:429–436 [DOI] [PubMed] [Google Scholar]
- Mori K, Ogawa Y, Ebihara K, Aoki T, Tamura N, Sugawara A, Kuwahara T, Ozaki S, Mukoyama M, Tashiro K, et al. (1997) Kidney-specific expression of a novel mouse organic cation transporter-like protein. FEBS Lett 417:371–374 [DOI] [PubMed] [Google Scholar]
- Morikawa A, Goto Y, Suzuki H, Hirohashi T, Sugiyama Y. (2000) Biliary excretion of 17beta-estradiol 17beta-D-glucuronide is predominantly mediated by cMOAT/MRP2. Pharmacol Res 17:546–552 [DOI] [PubMed] [Google Scholar]
- Morimoto K, Oishi T, Ueda S, Ueda M, Hosokawa M, Chiba K. (2004) A novel variant allele of OATP-C (SLCO1B1) found in a Japanese patient with pravastatin-induced myopathy. Drug Metab Pharmacokinet 19:453–455 [DOI] [PubMed] [Google Scholar]
- Morisaki K, Robey RW, Ozvegy-Laczka C, Honjo Y, Polgar O, Steadman K, Sarkadi B, Bates SE. (2005) Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol 56:161–172 [DOI] [PubMed] [Google Scholar]
- Morita N, Yasumori T, Nakayama K. (2003a) Human MDR1 polymorphism: G2677T/A and C3435T have no effect on MDR1 transport activities. Biochem Pharmacol 65:1843–1852 [DOI] [PubMed] [Google Scholar]
- Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. (1998) NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother 42:1778–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Sakaeda T, Horinouchi M, Nakamura T, Kuroda K, Miki I, Yoshimura K, Sakai T, Shirasaka D, Tamura T, et al. (2003b) MDR1 genotype-related duodenal absorption rate of digoxin in healthy Japanese subjects. Pharm Res 20:552–556 [DOI] [PubMed] [Google Scholar]
- Moriya Y, Nakamura T, Horinouchi M, Sakaeda T, Tamura T, Aoyama N, Shirakawa T, Gotoh A, Fujimoto S, Matsuo M, et al. (2002) Effects of polymorphisms of MDR1, MRP1, and MRP2 genes on their mRNA expression levels in duodenal enterocytes of healthy Japanese subjects. Biol Pharm Bull 25:1356–1359 [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Hiasa M, Matsumoto T, Omote H. (2008) Multidrug and toxic compound extrusion (MATE)-type proteins as anchor transporters for the excretion of metabolic waste products and xenobiotics. Xenobiotica 38:1107–1118 [DOI] [PubMed] [Google Scholar]
- Moseley RH, Wang W, Takeda H, Lown K, Shick L, Ananthanarayanan M, Suchy FJ. (1996) Effect of endotoxin on bile acid transport in rat liver: a potential model for sepsis-associated cholestasis. Am J Physiol 271:G137–G146 [DOI] [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, Inui K. (2002) Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol 13:866–874 [DOI] [PubMed] [Google Scholar]
- Mottino AD, Hoffman T, Jennes L, Vore M. (2000) Expression and localization of multidrug resistant protein mrp2 in rat small intestine. J Pharmacol Exp Ther 293:717–723 [PubMed] [Google Scholar]
- Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. (1997) cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am J Physiol 273:G842–G848 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. (1998a) Sodium taurocholate cotransporting polypeptide is a serine, threonine phosphoprotein and is dephosphorylated by cyclic adenosine monophosphate. Hepatology 28:1629–1636 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Webster CR, Anwer MS. (1998b) Role of protein phosphatases in cyclic AMP-mediated stimulation of hepatic Na+/taurocholate cotransport. J Biol Chem 273:30039–30045 [DOI] [PubMed] [Google Scholar]
- Müller J, Lips KS, Metzner L, Neubert RH, Koepsell H, Brandsch M. (2005) Drug specificity and intestinal membrane localization of human organic cation transporters (OCT). Biochem Pharmacol 70:1851–1860 [DOI] [PubMed] [Google Scholar]
- Musa H, Dobrzynski H, Berry Z, Abidi F, Cass CE, Young JD, Baldwin SA, Boyett MR. (2002) Immunocytochemical demonstration of the equilibrative nucleoside transporter rENT1 in rat sinoatrial node. J Histochem Cytochem 50:305–309 [DOI] [PubMed] [Google Scholar]
- Mutch DM, Anderle P, Fiaux M, Mansourian R, Vidal K, Wahli W, Williamson G, Roberts MA. (2004) Regional variations in ABC transporter expression along the mouse intestinal tract. Physiol Genomics 17:11–20 [DOI] [PubMed] [Google Scholar]
- Mwinyi J, Johne A, Bauer S, Roots I, Gerloff T. (2004) Evidence for inverse effects of OATP-C (SLC21A6) 5 and 1b haplotypes on pravastatin kinetics. Clin Pharmacol Ther 75:415–421 [DOI] [PubMed] [Google Scholar]
- Myllynen P, Kummu M, Kangas T, Ilves M, Immonen E, Rysä J, Pirilä R, Lastumäki A, Vähäkangas KH. (2008) ABCG2/BCRP decreases the transfer of a food-born chemical carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in perfused term human placenta. Toxicol Appl Pharmacol 232:210–217 [DOI] [PubMed] [Google Scholar]
- Nabulsi NB, Smith DE, Kilbourn MR. (2005) [11C]Glycylsarcosine: synthesis and in vivo evaluation as a PET tracer of PepT2 transporter function in kidney of PepT2 null and wild-type mice. Bioorg Med Chem 13:2993–3001 [DOI] [PubMed] [Google Scholar]
- Naesens M, Kuypers DR, Verbeke K, Vanrenterghem Y. (2006) Multidrug resistance protein 2 genetic polymorphisms influence mycophenolic acid exposure in renal allograft recipients. Transplantation 82:1074–1084 [DOI] [PubMed] [Google Scholar]
- Nagai K, Nagasawa K, Kyotani Y, Hifumi N, Fujimoto S. (2007) Mouse equilibrative nucleoside transporter 2 (mENT2) transports nucleosides and purine nucleobases differing from human and rat ENT2. Biol Pharm Bull 30:979–981 [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Tamura A, Wakabayashi K, Hoshijima K, Komada M, Yoshida T, Kometani S, Matsubara T, Mikuriya K, Ishikawa T. (2008) Ubiquitin-mediated proteasomal degradation of non-synonymous SNP variants of human ABC transporter ABCG2. Biochem J 411:623–631 [DOI] [PubMed] [Google Scholar]
- Nakagomi-Hagihara R, Nakai D, Kawai K, Yoshigae Y, Tokui T, Abe T, Ikeda T. (2006) OATP1B1, OATP1B3, and mrp2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker. Drug Metab Dispos 34:862–869 [DOI] [PubMed] [Google Scholar]
- Nakahara S, Arimura Y, Saito K, Goto A, Motoya S, Shinomura Y, Miyamoto A, Imai K. (2008) Association of SLC22A4/5 polymorphisms with steroid responsiveness of inflammatory bowel disease in Japan. Dis Colon Rectum 51:598–603 [DOI] [PubMed] [Google Scholar]
- Nakai D, Nakagomi R, Furuta Y, Tokui T, Abe T, Ikeda T, Nishimura K. (2001) Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J Pharmacol Exp Ther 297:861–867 [PubMed] [Google Scholar]
- Nakajima N, Sekine T, Cha SH, Tojo A, Hosoyamada M, Kanai Y, Yan K, Awa S, Endou H. (2000) Developmental changes in multispecific organic anion transporter 1 expression in the rat kidney. Kidney Int 57:1608–1616 [DOI] [PubMed] [Google Scholar]
- Nakakariya M, Shimada T, Irokawa M, Koibuchi H, Iwanaga T, Yabuuchi H, Maeda T, Tamai I. (2008) Predominant contribution of rat organic anion transporting polypeptide-2 (Oatp2) to hepatic uptake of beta-lactam antibiotics. Pharm Res 25:578–585 [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H, Silverman JA, Gant TW, Evarts RP, Thorgeirsson SS. (1993) Expression of multidrug resistance genes in rat liver during regeneration and after carbon tetrachloride intoxication. Hepatology 18:1202–1207 [PubMed] [Google Scholar]
- Nakken KE, Labori KJ, Rødningen OK, Nakken S, Berge KE, Eiklid K, Raeder MG. (2009) ABCB4 sequence variations in young adults with cholesterol gallstone disease. Liver Int 29:743–747 [DOI] [PubMed] [Google Scholar]
- Naramoto H, Uematsu T, Uchihashi T, Doto R, Matsuura T, Usui Y, Uematsu S, Li X, Takahashi M, Yamaoka M, et al. (2007) Multidrug resistance-associated protein 7 expression is involved in cross-resistance to docetaxel in salivary gland adenocarcinoma cell lines. Int J Oncol 30:393–401 [PubMed] [Google Scholar]
- Neufeld EB, Demosky SJ, Jr, Stonik JA, Combs C, Remaley AT, Duverger N, Santamarina-Fojo S, Brewer HB., Jr (2002) The ABCA1 transporter functions on the basolateral surface of hepatocytes. Biochem Biophys Res Commun 297:974–979 [DOI] [PubMed] [Google Scholar]
- Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, Dwyer NK, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S, et al. (2001) Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem 276:27584–27590 [DOI] [PubMed] [Google Scholar]
- Newman B, Wintle RF, van Oene M, Yazdanpanah M, Owen J, Johnson B, Gu X, Amos CI, Keystone E, Rubin LA, et al. (2005) SLC22A4 polymorphisms implicated in rheumatoid arthritis and Crohn's disease are not associated with rheumatoid arthritis in a Canadian Caucasian population. Arthritis Rheum 52:425–429 [DOI] [PubMed] [Google Scholar]
- Nezu J, Tamai I, Oku A, Ohashi R, Yabuuchi H, Hashimoto N, Nikaido H, Sai Y, Koizumi A, Shoji Y, et al. (1999) Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat Genet 21:91–94 [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Charrier-Hisamuddin L, Dalmasso G, Hiol A, Sitaraman S, Merlin D. (2007) Association of PepT1 with lipid rafts differently modulates its transport activity in polarized and nonpolarized cells. Am J Physiol Gastrointest Liver Physiol 293:G1155–G1165 [DOI] [PubMed] [Google Scholar]
- Niemi M, Arnold KA, Backman JT, Pasanen MK, Gödtel-Armbrust U, Wojnowski L, Zanger UM, Neuvonen PJ, Eichelbaum M, Kivistö KT, et al. (2006a) Association of genetic polymorphism in ABCC2 with hepatic multidrug resistance-associated protein 2 expression and pravastatin pharmacokinetics. Pharmacogenet Genomics 16:801–808 [DOI] [PubMed] [Google Scholar]
- Niemi M, Kivistö KT, Diczfalusy U, Bodin K, Bertilsson L, Fromm MF, Eichelbaum M. (2006b) Effect of SLCO1B1 polymorphism on induction of CYP3A4 by rifampicin. Pharmacogenet Genomics 16:565–568 [DOI] [PubMed] [Google Scholar]
- Nies AT, Jedlitschky G, König J, Herold-Mende C, Steiner HH, Schmitt HP, Keppler D. (2004) Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience 129:349–360 [DOI] [PubMed] [Google Scholar]
- Nies AT, König J, Cui Y, Brom M, Spring H, Keppler D. (2002a) Structural requirements for the apical sorting of human multidrug resistance protein 2 (ABCC2). Eur J Biochem 269:1866–1876 [DOI] [PubMed] [Google Scholar]
- Nies AT, Schwab M, Keppler D. (2008) Interplay of conjugating enzymes with OATP uptake transporters and ABCC/MRP efflux pumps in the elimination of drugs. Expert Opin Drug Metab Toxicol 4:545–568 [DOI] [PubMed] [Google Scholar]
- Nies AT, Spring H, Thon WF, Keppler D, Jedlitschky G. (2002b) Immunolocalization of multidrug resistance protein 5 in the human genitourinary system. J Urol 167:2271–2275 [PubMed] [Google Scholar]
- Nigam SK, Bush KT, Bhatnagar V. (2007) Drug and toxicant handling by the OAT organic anion transporters in the kidney and other tissues. Nat Clin Pract Nephrol 3:443–448 [DOI] [PubMed] [Google Scholar]
- Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. (2003) Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J 374:337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara K, Masuda S, Ji L, Katsura T, Inui K. (2007) Pharmacokinetic significance of luminal multidrug and toxin extrusion 1 in chronic renal failure rats. Biochem Pharmacol 73:1482–1490 [DOI] [PubMed] [Google Scholar]
- Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, Takane H, Irie S, Kusuhara H, Urasaki Y, et al. (2003) Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther 73:554–565 [DOI] [PubMed] [Google Scholar]
- Noé B, Hagenbuch B, Stieger B, Meier PJ. (1997) Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. Proc Natl Acad Sci U S A 94:10346–10350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe J, Hagenbuch B, Meier PJ, St-Pierre MV. (2001) Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology 33:1223–1231 [DOI] [PubMed] [Google Scholar]
- Noe J, Kullak-Ublick GA, Jochum W, Stieger B, Kerb R, Haberl M, Müllhaupt B, Meier PJ, Pauli-Magnus C. (2005) Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J Hepatol 43:536–543 [DOI] [PubMed] [Google Scholar]
- Noé J, Portmann R, Brun ME, Funk C. (2007) Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos 35:1308–1314 [DOI] [PubMed] [Google Scholar]
- Noé J, Stieger B, Meier PJ. (2002) Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology 123:1659–1666 [DOI] [PubMed] [Google Scholar]
- Noshiro R, Anzai N, Sakata T, Miyazaki H, Terada T, Shin HJ, He X, Miura D, Inui K, Kanai Y, et al. (2006) The PDZ domain protein PDZK1 interacts with human peptide transporter PEPT2 and enhances its transport activity. Kidney Int 70:275–282 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2004a) Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther 308:438–445 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. (2005) Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos 33:434–439 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, Tsuji A, Yokoi T. (2002) Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther 302:804–813 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Sugiura S, Nakajima M, Goto A, Yokoi T, Nezu J, Tsuji A, Tamai I. (2004b) Involvement of organic anion transporting polypeptides in the transport of troglitazone sulfate: implications for understanding troglitazone hepatotoxicity. Drug Metab Dispos 32:291–294 [DOI] [PubMed] [Google Scholar]
- O'Neill GM, Peters GB, Harvie RM, MacKenzie HB, Henness S, Davey RA. (1998) Amplification and expression of the ABC transporters ARA and MRP in a series of multidrug-resistant leukaemia cell sublines. Br J Cancer 77:2076–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocheltree SM, Shen H, Hu Y, Keep RF, Smith DE. (2005) Role and relevance of peptide transporter 2 (PEPT2) in the kidney and choroid plexus: in vivo studies with glycylsarcosine in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther 315:240–247 [DOI] [PubMed] [Google Scholar]
- Ocheltree SM, Shen H, Hu Y, Xiang J, Keep RF, Smith DE. (2004a) Mechanisms of cefadroxil uptake in the choroid plexus: studies in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther 308:462–467 [DOI] [PubMed] [Google Scholar]
- Ocheltree SM, Shen H, Hu Y, Xiang J, Keep RF, Smith DE. (2004b) Role of PEPT2 in the choroid plexus uptake of glycylsarcosine and 5-aminolevulinic acid: studies in wild-type and null mice. Pharm Res 21:1680–1685 [DOI] [PubMed] [Google Scholar]
- Oelkers P, Kirby LC, Heubi JE, Dawson PA. (1997) Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J Clin Invest 99:1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K, Terada T, Asaka J, Katsura T, Inui K. (2007) Hepatocyte nuclear factor-4{alpha} regulates the human organic anion transporter 1 gene in the kidney. Am J Physiol Renal Physiol 292:F1819–F1826 [DOI] [PubMed] [Google Scholar]
- Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, Akizawa T, Yoshioka M, Sugiyama Y. (2000) Characterization of inducible nature of MRP3 in rat liver. Am J Physiol Gastrointest Liver Physiol 278:G438–G446 [DOI] [PubMed] [Google Scholar]
- Ogihara H, Suzuki T, Nagamachi Y, Inui K, Takata K. (1999) Peptide transporter in the rat small intestine: ultrastructural localization and the effect of starvation and administration of amino acids. Histochem J 31:169–174 [DOI] [PubMed] [Google Scholar]
- Ogihara T, Kamiya M, Ozawa M, Fujita T, Yamamoto A, Yamashita S, Ohnishi S, Isomura Y. (2006) What kinds of substrates show P-glycoprotein-dependent intestinal absorption? Comparison of verapamil with vinblastine. Drug Metab Pharmacokinet 21:238–244 [DOI] [PubMed] [Google Scholar]
- Oguri T, Ozasa H, Uemura T, Bessho Y, Miyazaki M, Maeno K, Maeda H, Sato S, Ueda R. (2008) MRP7/ABCC10 expression is a predictive biomarker for the resistance to paclitaxel in non-small cell lung cancer. Mol Cancer Ther 7:1150–1155 [DOI] [PubMed] [Google Scholar]
- Ohashi R, Tamai I, Yabuuchi H, Nezu JI, Oku A, Sai Y, Shimane M, Tsuji A. (1999) Na(+)-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J Pharmacol Exp Ther 291:778–784 [PubMed] [Google Scholar]
- Ohta KY, Inoue K, Hayashi Y, Yuasa H. (2006) Molecular identification and functional characterization of rat multidrug and toxin extrusion type transporter 1 as an organic cation/H+ antiporter in the kidney. Drug Metab Dispos 34:1868–1874 [DOI] [PubMed] [Google Scholar]
- Ohtsuka H, Abe T, Onogawa T, Kondo N, Sato T, Oshio H, Mizutamari H, Mikkaichi T, Oikawa M, Rikiyama T, et al. (2006) Farnesoid X receptor, hepatocyte nuclear factors 1alpha and 3beta are essential for transcriptional activation of the liver-specific organic anion transporter-2 gene. J Gastroenterol 41:369–377 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Kikkawa T, Mori S, Hori S, Takanaga H, Otagiri M, Terasaki T. (2004a) Mouse reduced in osteosclerosis transporter functions as an organic anion transporter 3 and is localized at abluminal membrane of blood-brain barrier. J Pharmacol Exp Ther 309:1273–1281 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Takizawa T, Takanaga H, Hori S, Hosoya K, Terasaki T. (2004b) Localization of organic anion transporting polypeptide 3 (oatp3) in mouse brain parenchymal and capillary endothelial cells. J Neurochem 90:743–749 [DOI] [PubMed] [Google Scholar]
- Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, et al. (2008) Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol 295:G735–G747 [DOI] [PubMed] [Google Scholar]
- Okamura T, Kikuchi T, Okada M, Toramatsu C, Fukushi K, Takei M, Irie T. (2009) Noninvasive and quantitative assessment of the function of multidrug resistance-associated protein 1 in the living brain. J Cereb Blood Flow Metab 29:504–511 [DOI] [PubMed] [Google Scholar]
- Okuda M, Saito H, Urakami Y, Takano M, Inui K. (1996) cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem Biophys Res Commun 224:500–507 [DOI] [PubMed] [Google Scholar]
- Okumura H, Katoh M, Minami K, Nakajima M, Yokoi T. (2007) Change of drug excretory pathway by CCl4-induced liver dysfunction in rat. Biochem Pharmacol 74:488–495 [DOI] [PubMed] [Google Scholar]
- Olinga P, Elferink MG, Draaisma AL, Merema MT, Castell JV, Pérez G, Groothuis GM. (2008) Coordinated induction of drug transporters and phase I and II metabolism in human liver slices. Eur J Pharm Sci 33:380–389 [DOI] [PubMed] [Google Scholar]
- Ono N, Van der Heijden I, Scheffer GL, Van de Wetering K, Van Deemter E, De Haas M, Boerke A, Gadella BM, De Rooij DG, Neefjes JJ, et al. (2007) Multidrug resistance-associated protein 9 (ABCC12) is present in mouse and boar sperm. Biochem J 406:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo CY, Kuhn RJ, Desai N, McNamara PJ. (1995) Active transport of cimetidine into human milk. Clin Pharmacol Ther 58:548–555 [DOI] [PubMed] [Google Scholar]
- Oostenbrug LE, Dijkstra G, Nolte IM, van Dullemen HM, Oosterom E, Faber KN, de Jong DJ, van der Linde K, te Meerman GJ, van der Steege G, et al. (2006) Absence of association between the multidrug resistance (MDR1) gene and inflammatory bowel disease. Scand J Gastroenterol 41:1174–1182 [DOI] [PubMed] [Google Scholar]
- Oostendorp RL, Buckle T, Beijnen JH, van Tellingen O, Schellens JH. (2009) The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest New Drugs 27:31–40 [DOI] [PubMed] [Google Scholar]
- Orsó E, Broccardo C, Kaminski WE, Böttcher A, Liebisch G, Drobnik W, Götz A, Chambenoit O, Diederich W, Langmann T, et al. (2000) Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat Genet 24:192–196 [DOI] [PubMed] [Google Scholar]
- Ose A, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, Fujita T, Yamamoto A, Sugiyama Y. (2008) P-glycoprotein restricts the penetration of oseltamivir across the blood-brain barrier. Drug Metab Dispos 36:427–434 [DOI] [PubMed] [Google Scholar]
- Oswald S, König J, Lütjohann D, Giessmann T, Kroemer HK, Rimmbach C, Rosskopf D, Fromm MF, Siegmund W. (2008) Disposition of ezetimibe is influenced by polymorphisms of the hepatic uptake carrier OATP1B1. Pharmacogenet Genomics 18:559–568 [DOI] [PubMed] [Google Scholar]
- Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. (2005) A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A 102:17923–17928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Elferink RP, Ottenhoff R, van Wijland M, Smit JJ, Schinkel AH, Groen AK. (1995) Regulation of biliary lipid secretion by mdr2 P-glycoprotein in the mouse. J Clin Invest 95:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri O, Latiano A, Valvano R, D'Incà R, Vecchi M, Sturniolo GC, Saibeni S, Peyvandi F, Bossa F, Zagaria C, et al. (2006) Variants of OCTN1–2 cation transporter genes are associated with both Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther 23:497–506 [DOI] [PubMed] [Google Scholar]
- Pan G, Giri N, Elmquist WF. (2007) Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine. Drug Metab Dispos 35:1165–1173 [DOI] [PubMed] [Google Scholar]
- Panwala CM, Jones JC, Viney JL. (1998) A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol 161:5733–5744 [PubMed] [Google Scholar]
- Pariante CM, Kim RB, Makoff A, Kerwin RW. (2003) Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters. Br J Pharmacol 139:1111–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Makoff A, Lovestone S, Feroli S, Heyden A, Miller AH, Kerwin RW. (2001) Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. Br J Pharmacol 134:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolo L, Fernetti C, Pirulli D, Crovella S, Amoroso A, Tiribelli C. (2003) Effects of maturation on RNA transcription and protein expression of four MRP genes in human placenta and in BeWo cells. Biochem Biophys Res Commun 303:259–265 [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M, Cano-Soldado P, Errasti-Murugarren E, Casado FJ. (2008) SLC28 genes and concentrative nucleoside transporter (CNT) proteins. Xenobiotica 38:972–994 [DOI] [PubMed] [Google Scholar]
- Patel DH, Crawford CR, Naeve CW, Belt JA. (2000) Cloning, genomic organization and chromosomal localization of the gene encoding the murine sodium-dependent, purine-selective, concentrative nucleoside transporter (CNT2). Gene 242:51–58 [DOI] [PubMed] [Google Scholar]
- Patil SD, Ngo LY, Glue P, Unadkat JD. (1998) Intestinal absorption of ribavirin is preferentially mediated by the Na+-nucleoside purine (N1) transporter. Pharm Res 15:950–952 [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. (1996) Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271:1126–1128 [DOI] [PubMed] [Google Scholar]
- Paulusma CC, de Waart DR, Kunne C, Mok KS, Elferink RP. (2009) Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content. J Biol Chem 284:9947–9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulusma CC, Groen A, Kunne C, Ho-Mok KS, Spijkerboer AL, Rudi de Waart D, Hoek FJ, Vreeling H, Hoeben KA, van Marle J, et al. (2006) Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology 44:195–204 [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. (1997) A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology 25:1539–1542 [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Kothe MJ, Bakker CT, Bosma PJ, van Bokhoven I, van Marle J, Bolder U, Tytgat GN, Oude Elferink RP. (2000) Zonal down-regulation and redistribution of the multidrug resistance protein 2 during bile duct ligation in rat liver. Hepatology 31:684–693 [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Oude Elferink RP. (2005) The type 4 subfamily of P-type ATPases, putative aminophospholipid translocases with a role in human disease. Biochim Biophys Acta 1741:11–24 [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Groen A, Eppens EF, Kunne C, Ottenhoff R, Looije N, Knisely AS, Killeen NP, Bull LN, Elferink RP, et al. (2004) A mouse genetic model for familial cholestasis caused by ATP8B1 mutations reveals perturbed bile salt homeostasis but no impairment in bile secretion. Hum Mol Genet 13:881–892 [DOI] [PubMed] [Google Scholar]
- Pelis RM, Suhre WM, Wright SH. (2006) Functional influence of N-glycosylation in OCT2-mediated tetraethylammonium transport. Am J Physiol Renal Physiol 290:F1118–F1126 [DOI] [PubMed] [Google Scholar]
- Peng KC, Cluzeaud F, Bens M, Van Huyen JP, Wioland MA, Lacave R, Vandewalle A. (1999) Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem 47:757–768 [DOI] [PubMed] [Google Scholar]
- Pennycooke M, Chaudary N, Shuralyova I, Zhang Y, Coe IR. (2001) Differential expression of human nucleoside transporters in normal and tumor tissue. Biochem Biophys Res Commun 280:951–959 [DOI] [PubMed] [Google Scholar]
- Pérez M, Real R, Mendoza G, Merino G, Prieto JG, Alvarez AI. (2009) Milk secretion of nitrofurantoin, as a specific BCRP/ABCG2 substrate, in assaf sheep: modulation by isoflavones. J Vet Pharmacol Ther 32:498–502 [DOI] [PubMed] [Google Scholar]
- Petrick JS, Klaassen CD. (2007) Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metab Dispos 35:1806–1815 [DOI] [PubMed] [Google Scholar]
- Petrukhin K, Lutsenko S, Chernov I, Ross BM, Kaplan JH, Gilliam TC. (1994) Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Hum Mol Genet 3:1647–1656 [DOI] [PubMed] [Google Scholar]
- Pike LJ. (2006) Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res 47:1597–1598 [DOI] [PubMed] [Google Scholar]
- Pinsonneault J, Nielsen CU, Sadée W. (2004) Genetic variants of the human H+/dipeptide transporter PEPT2: analysis of haplotype functions. J Pharmacol Exp Ther 311:1088–1096 [DOI] [PubMed] [Google Scholar]
- Pinto N, Halachmi N, Verjee Z, Woodland C, Klein J, Koren G. (2005) Ontogeny of renal P-glycoprotein expression in mice: correlation with digoxin renal clearance. Pediatr Res 58:1284–1289 [DOI] [PubMed] [Google Scholar]
- Pippert TR, Umbenhauer DR. (2001) The subpopulation of CF-1 mice deficient in P-glycoprotein contains a murine retroviral insertion in the mdr1a gene. J Biochem Mol Toxicol 15:83–89 [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. (2002) Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol 16:2283–2296 [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV. (2003) Identification of steroid sulfate transport processes in the human mammary gland. J Clin Endocrinol Metab 88:3902–3912 [DOI] [PubMed] [Google Scholar]
- Plass JR, Mol O, Heegsma J, Geuken M, Faber KN, Jansen PL, Müller M. (2002) Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology 35:589–596 [DOI] [PubMed] [Google Scholar]
- Plösch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, Van Der Sluijs F, Kema IP, Groen AK, Shan B, Kuipers F, et al. (2004) Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology 126:290–300 [DOI] [PubMed] [Google Scholar]
- Polli JW, Humphreys JE, Harmon KA, Castellino S, O'Mara MJ, Olson KL, John-Williams LS, Koch KM, Serabjit-Singh CJ. (2008) The role of efflux and uptake transporters in N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethy l]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos 36:695–701 [DOI] [PubMed] [Google Scholar]
- Polli JW, Olson KL, Chism JP, St John-Williams L, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE. (2009) An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos 37:439–442 [DOI] [PubMed] [Google Scholar]
- Pombrio JM, Giangreco A, Li L, Wempe MF, Anders MW, Sweet DH, Pritchard JB, Ballatori N. (2001) Mercapturic acids (N-acetylcysteine S-conjugates) as endogenous substrates for the renal organic anion transporter-1. Mol Pharmacol 60:1091–1099 [DOI] [PubMed] [Google Scholar]
- Popowski K, Eloranta JJ, Saborowski M, Fried M, Meier PJ, Kullak-Ublick GA. (2005) The human organic anion transporter 2 gene is transactivated by hepatocyte nuclear factor-4 alpha and suppressed by bile acids. Mol Pharmacol 67:1629–1638 [DOI] [PubMed] [Google Scholar]
- Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, 3rd, Dantzig AH. (2005) The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther 4:855–863 [DOI] [PubMed] [Google Scholar]
- Prechtl S, Roellinghoff M, Scheper R, Cole SP, Deeley RG, Lohoff M. (2000) The multidrug resistance protein 1: a functionally important activation marker for murine Th1 cells. J Immunol 164:754–761 [DOI] [PubMed] [Google Scholar]
- Preitner F, Bonny O, Laverrière A, Rotman S, Firsov D, Da Costa A, Metref S, Thorens B. (2009) Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A 106:15501–15506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulaski L, Jedlitschky G, Leier I, Buchholz U, Keppler D. (1996) Identification of the multidrug-resistance protein (MRP) as the glutathione-S-conjugate export pump of erythrocytes. Eur J Biochem 241:644–648 [DOI] [PubMed] [Google Scholar]
- Pulido MM, Molina AJ, Merino G, Mendoza G, Prieto JG, Alvarez AI. (2006) Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): influence of flavonoids and role in milk secretion in sheep. J Vet Pharmacol Ther 29:279–287 [DOI] [PubMed] [Google Scholar]
- Pulkkinen L, Nakano A, Ringpfeil F, Uitto J. (2001) Identification of ABCC6 pseudogenes on human chromosome 16p: implications for mutation detection in pseudoxanthoma elasticum. Hum Genet 109:356–365 [DOI] [PubMed] [Google Scholar]
- Quarcoo D, Fischer TC, Heppt W, Lauenstein HD, Pilzner C, Welte T, Groneberg DA. (2009) Expression, localisation and functional implications of the transporter protein PEPT2 in the upper respiratory tract. Respiration 77:440–446 [DOI] [PubMed] [Google Scholar]
- Race JE, Grassl SM, Williams WJ, Holtzman EJ. (1999) Molecular cloning and characterization of two novel human renal organic anion transporters (hOAT1 and hOAT3). Biochem Biophys Res Commun 255:508–514 [DOI] [PubMed] [Google Scholar]
- Radeva G, Perabo J, Sharom FJ. (2005) P-Glycoprotein is localized in intermediate-density membrane microdomains distinct from classical lipid rafts and caveolar domains. FEBS J 272:4924–4937 [DOI] [PubMed] [Google Scholar]
- Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. (2008) The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A 105:3891–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. (1999) Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A 96:3900–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau T, Erney B, Göres R, Eschenhagen T, Beck J, Langer T. (2006) High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin Pharmacol Ther 80:468–476 [DOI] [PubMed] [Google Scholar]
- Rausch-Derra LC, Hartley DP, Meier PJ, Klaassen CD. (2001) Differential effects of microsomal enzyme-inducing chemicals on the hepatic expression of rat organic anion transporters, OATP1 and OATP2. Hepatology 33:1469–1478 [DOI] [PubMed] [Google Scholar]
- Raymond M, Rose E, Housman DE, Gros P. (1990) Physical mapping, amplification, and overexpression of the mouse mdr gene family in multidrug-resistant cells. Mol Cell Biol 10:1642–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic ZB, Biringer J, Barnes K, Baldwin SA, Al-Sarraf H, Nicola PA, Young JD, Cass CE, Barrand MA, Hladky SB. (2005) Polarized distribution of nucleoside transporters in rat brain endothelial and choroid plexus epithelial cells. J Neurochem 94:1420–1426 [DOI] [PubMed] [Google Scholar]
- Reichel C, Gao B, Van Montfoort J, Cattori V, Rahner C, Hagenbuch B, Stieger B, Kamisako T, Meier PJ. (1999) Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology 117:688–695 [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. (2003a) Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol 63:1094–1103 [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, Wijnholds J, Borst P. (2003b) The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A 100:9244–9249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Wolff NA, Dautzenberg FM, Burckhardt G. (1998) Cloning of a human renal p-aminohippurate transporter, hROAT1. Kidney Blood Press Res 21:233–237 [DOI] [PubMed] [Google Scholar]
- Reisman SA, Csanaky IL, Aleksunes LM, Klaassen CD. (2009a) Altered disposition of acetaminophen in Nrf2-null and Keap1-knockdown mice. Toxicol Sci 109:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman SA, Yeager RL, Yamamoto M, Klaassen CD. (2009b) Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol Sci 108:35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaley AT, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, Peterson KM, Koch C, Arnould I, Prades C, et al. (1999) Human ATP-binding cassette transporter 1 (ABC1): genomic organization and identification of the genetic defect in the original Tangier disease kindred. Proc Natl Acad Sci U S A 96:12685–12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert L, Tuschl G, Abadie C, Blanchard N, Pekthong D, Mantion G, Weber JC, Mueller SO. (2009) Use of mRNA expression to detect the induction of drug metabolising enzymes in rat and human hepatocytes. Toxicol Appl Pharmacol 235:86–96 [DOI] [PubMed] [Google Scholar]
- Ringseis R, Luci S, Spielmann J, Kluge H, Fischer M, Geissler S, Wen G, Hirche F, Eder K. (2008) Clofibrate treatment up-regulates novel organic cation transporter (OCTN)-2 in tissues of pigs as a model of non-proliferating species. Eur J Pharmacol 583:11–17 [DOI] [PubMed] [Google Scholar]
- Ringseis R, Pösel S, Hirche F, Eder K. (2007) Treatment with pharmacological peroxisome proliferator-activated receptor alpha agonist clofibrate causes upregulation of organic cation transporter 2 in liver and small intestine of rats. Pharmacol Res 56:175–183 [DOI] [PubMed] [Google Scholar]
- Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, Ritzel RG, Mowles DA, Carpenter P, Chen XZ, et al. (2001) Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). J Biol Chem 276:2914–2927 [DOI] [PubMed] [Google Scholar]
- Ritzel MW, Yao SY, Huang MY, Elliott JF, Cass CE, Young JD. (1997) Molecular cloning and functional expression of cDNAs encoding a human Na+-nucleoside cotransporter (hCNT1). Am J Physiol 272:C707–C714 [DOI] [PubMed] [Google Scholar]
- Ritzel MW, Yao SY, Ng AM, Mackey JR, Cass CE, Young JD. (1998) Molecular cloning, functional expression and chromosomal localization of a cDNA encoding a human Na+/nucleoside cotransporter (hCNT2) selective for purine nucleosides and uridine. Mol Membr Biol 15:203–211 [DOI] [PubMed] [Google Scholar]
- Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. (2006) Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am J Physiol Gastrointest Liver Physiol 290:G640–G649 [DOI] [PubMed] [Google Scholar]
- Rius M, Hummel-Eisenbeiss J, Keppler D. (2008) ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4). J Pharmacol Exp Ther 324:86–94 [DOI] [PubMed] [Google Scholar]
- Rius M, Thon WF, Keppler D, Nies AT. (2005) Prostanoid transport by multidrug resistance protein 4 (MRP4/ABCC4) localized in tissues of the human urogenital tract. J Urol 174:2409–2414 [DOI] [PubMed] [Google Scholar]
- Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. (2008) Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 155:423–438 [DOI] [PubMed] [Google Scholar]
- Robey RW, Steadman K, Polgar O, Bates SE. (2005) ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol Ther 4:187–194 [PubMed] [Google Scholar]
- Rodríguez CM, Labus JC, Hinton BT. (2002) Organic cation/carnitine transporter, OCTN2, is differentially expressed in the adult rat epididymis. Biol Reprod 67:314–319 [DOI] [PubMed] [Google Scholar]
- Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ. (2000) Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119:782–793 [DOI] [PubMed] [Google Scholar]
- Rome S, Barbot L, Windsor E, Kapel N, Tricottet V, Huneau JF, Reynes M, Gobert JG, Tomé D. (2002) The regionalization of PepT1, NBAT and EAAC1 transporters in the small intestine of rats are unchanged from birth to adulthood. J Nutr 132:1009–1011 [DOI] [PubMed] [Google Scholar]
- Ros JE, Roskams TA, Geuken M, Havinga R, Splinter PL, Petersen BE, LaRusso NF, van der Kolk DM, Kuipers F, Faber KN, et al. (2003) ATP binding cassette transporter gene expression in rat liver progenitor cells. Gut 52:1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati A, Maniori S, Decorti G, Candussio L, Giraldi T, Bartoli F. (2003) Physiological regulation of P-glycoprotein, MRP1, MRP2 and cytochrome P450 3A2 during rat ontogeny. Dev Growth Differ 45:377–387 [DOI] [PubMed] [Google Scholar]
- Rosenberg MF, Callaghan R, Ford RC, Higgins CF. (1997) Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem 272:10685–10694 [DOI] [PubMed] [Google Scholar]
- Rosenberg MF, Kamis AB, Callaghan R, Higgins CF, Ford RC. (2003) Three-dimensional structures of the mammalian multidrug resistance P-glycoprotein demonstrate major conformational changes in the transmembrane domains upon nucleotide binding. J Biol Chem 278:8294–8299 [DOI] [PubMed] [Google Scholar]
- Rosenberg MF, Velarde G, Ford RC, Martin C, Berridge G, Kerr ID, Callaghan R, Schmidlin A, Wooding C, Linton KJ, et al. (2001) Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J 20:5615–5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross DD, Yang W, Abruzzo LV, Dalton WS, Schneider E, Lage H, Dietel M, Greenberger L, Cole SP, Doyle LA. (1999) Atypical multidrug resistance: breast cancer resistance protein messenger RNA expression in mitoxantrone-selected cell lines. J Natl Cancer Inst 91:429–433 [DOI] [PubMed] [Google Scholar]
- Rost D, König J, Weiss G, Klar E, Stremmel W, Keppler D. (2001) Expression and localization of the multidrug resistance proteins MRP2 and MRP3 in human gallbladder epithelia. Gastroenterology 121:1203–1208 [DOI] [PubMed] [Google Scholar]
- Rost D, Kopplow K, Gehrke S, Mueller S, Friess H, Ittrich C, Mayer D, Stiehl A. (2005) Gender-specific expression of liver organic anion transporters in rat. Eur J Clin Invest 35:635–643 [DOI] [PubMed] [Google Scholar]
- Rost D, Mahner S, Sugiyama Y, Stremmel W. (2002) Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am J Physiol Gastrointest Liver Physiol 282:G720–G726 [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Boll M, Daniel H. (2000) Cloning and characterization of the gene encoding the mouse peptide transporter PEPT2. Biochem Biophys Res Commun 276:734–741 [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Daniel H. (2008) Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica 38:1022–1042 [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Frey I, Boll M, Groneberg DA, Eichinger HM, Balling R, Daniel H. (2003) Targeted disruption of the peptide transporter Pept2 gene in mice defines its physiological role in the kidney. Mol Cell Biol 23:3247–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetz S, Gros P. (1994) Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell 77:1071–1081 [DOI] [PubMed] [Google Scholar]
- Rühl A, Hoppe S, Frey I, Daniel H, Schemann M. (2005) Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system. J Comp Neurol 490:1–11 [DOI] [PubMed] [Google Scholar]
- Russell RK, Drummond HE, Nimmo ER, Anderson NH, Noble CL, Wilson DC, Gillett PM, McGrogan P, Hassan K, Weaver LT, et al. (2006) Analysis of the influence of OCTN1/2 variants within the IBD5 locus on disease susceptibility and growth indices in early onset inflammatory bowel disease. Gut 55:1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P, et al. (1999) Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 22:352–355 [DOI] [PubMed] [Google Scholar]
- Saborowski M, Kullak-Ublick GA, Eloranta JJ. (2006) The human organic cation transporter-1 gene is transactivated by hepatocyte nuclear factor-4alpha. J Pharmacol Exp Ther 317:778–785 [DOI] [PubMed] [Google Scholar]
- Saeki T, Takahashi N, Kanamoto R, Iwami K. (2002) Characterization of cloned mouse Na+/taurocholate cotransporting polypeptide by transient expression in COS-7 cells. Biosci Biotechnol Biochem 66:1116–1118 [DOI] [PubMed] [Google Scholar]
- Safe S, Bandiera S, Sawyer T, Zmudzka B, Mason G, Romkes M, Denomme MA, Sparling J, Okey AB, Fujita T. (1985) Effects of structure on binding to the 2,3,7,8-TCDD receptor protein and AHH induction—halogenated biphenyls. Environ Health Perspect 61:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai K, Saito Y, Itoda M, Fukushima-Uesaka H, Nishimaki-Mogami T, Ozawa S, Maekawa K, Kurose K, Kaniwa N, Kawamoto M, et al. (2008) Genetic variations and haplotypes of ABCC2 encoding MRP2 in a Japanese population. Drug Metab Pharmacokinet 23:139–147 [DOI] [PubMed] [Google Scholar]
- Saito H, Okuda M, Terada T, Sasaki S, Inui K. (1995) Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of beta-lactam antibiotics in the intestine and kidney. J Pharmacol Exp Ther 275:1631–1637 [PubMed] [Google Scholar]
- Saito H, Terada T, Okuda M, Sasaki S, Inui K. (1996) Molecular cloning and tissue distribution of rat peptide transporter PEPT2. Biochim Biophys Acta 1280:173–177 [DOI] [PubMed] [Google Scholar]
- Saji T, Kikuchi R, Kusuhara H, Kim I, Gonzalez FJ, Sugiyama Y. (2008) Transcriptional regulation of human and mouse organic anion transporter 1 by hepatocyte nuclear factor 1 alpha/beta. J Pharmacol Exp Ther 324:784–790 [DOI] [PubMed] [Google Scholar]
- Sakaeda T, Nakamura T, Horinouchi M, Kakumoto M, Ohmoto N, Sakai T, Morita Y, Tamura T, Aoyama N, Hirai M, et al. (2001) MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm Res 18:1400–1404 [DOI] [PubMed] [Google Scholar]
- Sakata T, Anzai N, Shin HJ, Noshiro R, Hirata T, Yokoyama H, Kanai Y, Endou H. (2004) Novel single nucleotide polymorphisms of organic cation transporter 1 (SLC22A1) affecting transport functions. Biochem Biophys Res Commun 313:789–793 [DOI] [PubMed] [Google Scholar]
- Salama NN, Yang Z, Bui T, Ho RJ. (2006) MDR1 haplotypes significantly minimize intracellular uptake and transcellular P-gp substrate transport in recombinant LLC-PK1 cells. J Pharm Sci 95:2293–2308 [DOI] [PubMed] [Google Scholar]
- Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. (1992) Sitosterolemia. J Lipid Res 33:945–955 [PubMed] [Google Scholar]
- Sarkar S, Bananis E, Nath S, Anwer MS, Wolkoff AW, Murray JW. (2006) PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic 7:1078–1091 [DOI] [PubMed] [Google Scholar]
- Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, Uchiumi T, Kuwano M, Nagata H, Tsukimori K, et al. (2005) Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharmacol Exp Ther 315:888–895 [DOI] [PubMed] [Google Scholar]
- Sato K, Sugawara J, Sato T, Mizutamari H, Suzuki T, Ito A, Mikkaichi T, Onogawa T, Tanemoto M, Unno M, et al. (2003) Expression of organic anion transporting polypeptide E (OATP-E) in human placenta. Placenta 24:144–148 [DOI] [PubMed] [Google Scholar]
- Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. (2005) Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 33:518–523 [DOI] [PubMed] [Google Scholar]
- Schaefer M, Roots I, Gerloff T. (2006) In-vitro transport characteristics discriminate wild-type ABCB1 (MDR1) from ALA893SER and ALA893THR polymorphisms. Pharmacogenet Genomics 16:855–861 [DOI] [PubMed] [Google Scholar]
- Schaner ME, Wang J, Zhang L, Su SF, Gerstin KM, Giacomini KM. (1999) Functional characterization of a human purine-selective, Na+-dependent nucleoside transporter (hSPNT1) in a mammalian expression system. J Pharmacol Exp Ther 289:1487–1491 [PubMed] [Google Scholar]
- Schaub TP, Kartenbeck J, König J, Vogel O, Witzgall R, Kriz W, Keppler D. (1997) Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol 8:1213–1221 [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. (2002a) MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest 82:515–518 [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. (2002b) Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest 82:193–201 [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Kool M, Heijn M, de Haas M, Pijnenborg AC, Wijnholds J, van Helvoort A, de Jong MC, Hooijberg JH, Mol CA, et al. (2000) Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5, and MDR3 P-glycoprotein with a panel of monoclonal antibodies. Cancer Res 60:5269–5277 [PubMed] [Google Scholar]
- Scheffer GL, Pijnenborg AC, Smit EF, Müller M, Postma DS, Timens W, van der Valk P, de Vries EG, Scheper RJ. (2002c) Multidrug resistance related molecules in human and murine lung. J Clin Pathol 55:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Kemp S, Dollé M, Rudenko G, Wagenaar E. (1993) N-glycosylation and deletion mutants of the human MDR1 P-glycoprotein. J Biol Chem 268:7474–7481 [PubMed] [Google Scholar]
- Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, van der Valk MA, Voordouw AC, Spits H, van Tellingen O, et al. (1997) Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A 94:4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Roelofs EM, Borst P. (1991) Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res 51:2628–2635 [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP. (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502 [DOI] [PubMed] [Google Scholar]
- Schippling S, Orth M, Beisiegel U, Rosenkranz T, Vogel P, Münchau A, Hagel C, Seedorf U. (2008) Severe Tangier disease with a novel ABCA1 gene mutation. Neurology 71:1454–1455 [DOI] [PubMed] [Google Scholar]
- Schmitz G, Kaminski WE, Porsch-Ozcürümez M, Klucken J, Orsó E, Bodzioch M, Büchler C, Drobnik W. (1999) ATP-binding cassette transporter A1 (ABCA1) in macrophages: a dual function in inflammation and lipid metabolism? Pathobiology 67:236–240 [DOI] [PubMed] [Google Scholar]
- Schnabolk GW, Youngblood GL, Sweet DH. (2006) Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20). Am J Physiol Renal Physiol 291:F314–F321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff CM, Gillin H, Webster CR, Anwer MS. (2008) Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology 47:1309–1316 [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A. (1999) MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med 5:1048–1051 [DOI] [PubMed] [Google Scholar]
- Schultz MJ, Wijnholds J, Peppelenbosch MP, Vervoordeldonk MJ, Speelman P, van Deventer SJ, Borst P, van der Poll T. (2001) Mice lacking the multidrug resistance protein 1 are resistant to Streptococcus pneumoniae-induced pneumonia. J Immunol 166:4059–4064 [DOI] [PubMed] [Google Scholar]
- Schulz T, Schumacher U, Prehm P. (2007) Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. J Biol Chem 282:20999–21004 [DOI] [PubMed] [Google Scholar]
- Schweifer N, Barlow DP. (1996) The Lx1 gene maps to mouse chromosome 17 and codes for a protein that is homologous to glucose and polyspecific transmembrane transporters. Mamm Genome 7:735–740 [DOI] [PubMed] [Google Scholar]
- Seamon JA, Rugg CA, Emanuel S, Calcagno AM, Ambudkar SV, Middleton SA, Butler J, Borowski V, Greenberger LM. (2006) Role of the ABCG2 drug transporter in the resistance and oral bioavailability of a potent cyclin-dependent kinase/Aurora kinase inhibitor. Mol Cancer Ther 5:2459–2467 [DOI] [PubMed] [Google Scholar]
- SEARCH Collaborative Group. Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. (2008) SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 359:789–799 [DOI] [PubMed] [Google Scholar]
- Seithel A, Eberl S, Singer K, Auge D, Heinkele G, Wolf NB, Dörje F, Fromm MF, König J. (2007) The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos 35:779–786 [DOI] [PubMed] [Google Scholar]
- Seki S, Kobayashi M, Itagaki S, Hirano T, Iseki K. (2009) Contribution of organic anion transporting polypeptide OATP2B1 to amiodarone accumulation in lung epithelial cells. Biochim Biophys Acta 1788:911–917 [DOI] [PubMed] [Google Scholar]
- Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. (1998) Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett 429:179–182 [DOI] [PubMed] [Google Scholar]
- Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. (1997) Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem 272:18526–18529 [DOI] [PubMed] [Google Scholar]
- Seth P, Wu X, Huang W, Leibach FH, Ganapathy V. (1999) Mutations in novel organic cation transporter (OCTN2), an organic cation/carnitine transporter, with differential effects on the organic cation transport function and the carnitine transport function. J Biol Chem 274:33388–33392 [DOI] [PubMed] [Google Scholar]
- Seward DJ, Koh AS, Boyer JL, Ballatori N. (2003) Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem 278:27473–27482 [DOI] [PubMed] [Google Scholar]
- Sharma RP, Bhandari N, Tsunoda M, Riley RT, Voss KA, Meredith FI. (2000) Fumonisin toxicity in a transgenic mouse model lacking the mdr1a/1b P-glycoprotein genes. Environ Toxicol Pharmacol 8:173–182 [DOI] [PubMed] [Google Scholar]
- Shekhawat PS, Srinivas SR, Matern D, Bennett MJ, Boriack R, George V, Xu H, Prasad PD, Roon P, Ganapathy V. (2007) Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(−/−)) mice. Mol Genet Metab 92:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat PS, Yang HS, Bennett MJ, Carter AL, Matern D, Tamai I, Ganapathy V. (2004) Carnitine content and expression of mitochondrial beta-oxidation enzymes in placentas of wild-type (OCTN2(+/+)) and OCTN2 Null (OCTN2(−/−)) Mice. Pediatr Res 56:323–328 [DOI] [PubMed] [Google Scholar]
- Shen H, Ocheltree SM, Hu Y, Keep RF, Smith DE. (2007) Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metab Dispos 35:1209–1216 [DOI] [PubMed] [Google Scholar]
- Shen H, Smith DE, Brosius FC., 3rd (2001) Developmental expression of PEPT1 and PEPT2 in rat small intestine, colon, and kidney. Pediatr Res 49:789–795 [DOI] [PubMed] [Google Scholar]
- Shen H, Smith DE, Keep RF, Brosius FC., 3rd (2004) Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol Pharm 1:248–256 [DOI] [PubMed] [Google Scholar]
- Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC., 3rd (1999) Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol 276:F658–F665 [DOI] [PubMed] [Google Scholar]
- Shih DQ, Bussen M, Sehayek E, Ananthanarayanan M, Shneider BL, Suchy FJ, Shefer S, Bollileni JS, Gonzalez FJ, Breslow JL, et al. (2001) Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet 27:375–382 [DOI] [PubMed] [Google Scholar]
- Shikata E, Yamamoto R, Takane H, Shigemasa C, Ikeda T, Otsubo K, Ieiri I. (2007) Human organic cation transporter (OCT1 and OCT2) gene polymorphisms and therapeutic effects of metformin. J Hum Genet 52:117–122 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Taniguchi H, Hippo Y, Hayashizaki Y, Aburatani H, Ishikawa T. (2003) Characterization of the mouse Abcc12 gene and its transcript encoding an ATP-binding cassette transporter, an orthologue of human ABCC12. Gene 310:17–28 [DOI] [PubMed] [Google Scholar]
- Shin HC, Landowski CP, Sun D, Vig BS, Kim I, Mittal S, Lane M, Rosania G, Drach JC, Amidon GL. (2003) Functional expression and characterization of a sodium-dependent nucleoside transporter hCNT2 cloned from human duodenum. Biochem Biophys Res Commun 307:696–703 [DOI] [PubMed] [Google Scholar]
- Shin HJ, Anzai N, Enomoto A, He X, Kim do K, Endou H, Kanai Y. (2007) Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology 45:1046–1055 [DOI] [PubMed] [Google Scholar]
- Shitara Y, Hirano M, Sato H, Sugiyama Y. (2004) Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/OATP1B1:SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin: analysis of the mechanism of the clinically relevant drug-drug interaction between cerivastatin and gemfibrozil. J Pharmacol Exp Ther 311:228–236 [DOI] [PubMed] [Google Scholar]
- Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. (1995) Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest 95:745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider BL, Setchell KD, Crossman MW. (1997) Fetal and neonatal expression of the apical sodium-dependent bile acid transporter in the rat ileum and kidney. Pediatr Res 42:189–194 [DOI] [PubMed] [Google Scholar]
- Shu C, Shen H, Teuscher NS, Lorenzi PJ, Keep RF, Smith DE. (2002) Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: studies in rat choroid plexus epithelial cells in primary culture. J Pharmacol Exp Ther 301:820–829 [DOI] [PubMed] [Google Scholar]
- Shu Y, Leabman MK, Feng B, Mangravite LM, Huang CC, Stryke D, Kawamoto M, Johns SJ, DeYoung J, Carlson E, et al. (2003) Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc Natl Acad Sci U S A 100:5902–5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, et al. (2007) Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 117:1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon FR, Fortune J, Iwahashi M, Bowman S, Wolkoff A, Sutherland E. (1999) Characterization of the mechanisms involved in the gender differences in hepatic taurocholate uptake. Am J Physiol 276:G556–G565 [DOI] [PubMed] [Google Scholar]
- Simon FR, Iwahashi M, Hu LJ, Qadri I, Arias IM, Ortiz D, Dahl R, Sutherland E. (2006) Hormonal regulation of hepatic multidrug resistance-associated protein 2 (Abcc2) primarily involves the pattern of growth hormone secretion. Am J Physiol Gastrointest Liver Physiol 290:G595–G608 [DOI] [PubMed] [Google Scholar]
- Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. (1994) Molecular cloning and characterization of a novel liver-specific transport protein. J Cell Sci 107:1065–1072 [DOI] [PubMed] [Google Scholar]
- Sinha J, Chen F, Miloh T, Burns RC, Yu Z, Shneider BL. (2008) beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am J Physiol Gastrointest Liver Physiol 295:G996–G1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkó E, Iliás A, Ujhelly O, Homolya L, Scheffer GL, Bergen AA, Sarkadi B, Váradi A. (2003) Subcellular localization and N-glycosylation of human ABCC6, expressed in MDCKII cells. Biochem Biophys Res Commun 308:263–269 [DOI] [PubMed] [Google Scholar]
- Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. (2007) Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta 1768:637–647 [DOI] [PubMed] [Google Scholar]
- Slitt AL, Cherrington NJ, Dieter MZ, Aleksunes LM, Scheffer GL, Huang W, Moore DD, Klaassen CD. (2006) trans-Stilbene oxide induces expression of genes involved in metabolism and transport in mouse liver via CAR and Nrf2 transcription factors. Mol Pharmacol 69:1554–1563 [DOI] [PubMed] [Google Scholar]
- Slitt AL, Cherrington NJ, Hartley DP, Leazer TM, Klaassen CD. (2002) Tissue distribution and renal developmental changes in rat organic cation transporter mRNA levels. Drug Metab Dispos 30:212–219 [DOI] [PubMed] [Google Scholar]
- Smit JJ, Schinkel AH, Mol CA, Majoor D, Mooi WJ, Jongsma AP, Lincke CR, Borst P. (1994) Tissue distribution of the human MDR3 P-glycoprotein. Lab Invest 71:638–649 [PubMed] [Google Scholar]
- Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA. (1993) Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75:451–462 [DOI] [PubMed] [Google Scholar]
- Smit JW, Huisman MT, van Tellingen O, Wiltshire HR, Schinkel AH. (1999) Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure. J Clin Invest 104:1441–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Timmermans-Hereijgers JL, Roelofsen B, Wirtz KW, van Blitterswijk WJ, Smit JJ, Schinkel AH, Borst P. (1994) The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett 354:263–266 [DOI] [PubMed] [Google Scholar]
- Song IS, Lee YM, Chung SJ, Shim CK. (2003) Multiple alterations of canalicular membrane transport activities in rats with CCl(4)-induced hepatic injury. Drug Metab Dispos 31:482–490 [DOI] [PubMed] [Google Scholar]
- Song IS, Shin HJ, Shin JG. (2008) Genetic variants of organic cation transporter 2 (OCT2) significantly reduce metformin uptake in oocytes. Xenobiotica 38:1252–1262 [DOI] [PubMed] [Google Scholar]
- Soodvilai S, Chatsudthipong V, Evans KK, Wright SH, Dantzler WH. (2004) Acute regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol 287:F1021–F1029 [DOI] [PubMed] [Google Scholar]
- Sookoian S, Castaño G, Burgueño A, Gianotti TF, Pirola CJ. (2008) Association of the multidrug-resistance-associated protein gene (ABCC2) variants with intrahepatic cholestasis of pregnancy. J Hepatol 48:125–132 [DOI] [PubMed] [Google Scholar]
- Soontornmalai A, Vlaming ML, Fritschy JM. (2006) Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier. Neuroscience 138:159–169 [DOI] [PubMed] [Google Scholar]
- Soroka CJ, Lee JM, Azzaroli F, Boyer JL. (2001) Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 33:783–791 [DOI] [PubMed] [Google Scholar]
- Spears KJ, Ross J, Stenhouse A, Ward CJ, Goh LB, Wolf CR, Morgan P, Ayrton A, Friedberg TH. (2005) Directional trans-epithelial transport of organic anions in porcine LLC-PK1 cells that co-express human OATP1B1 (OATP-C) and MRP2. Biochem Pharmacol 69:415–423 [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Huener G, Baykal T, Demirkol M, Duran M, Wanders R, Nezu J, Mayatepek E. (2003) Silent and symptomatic primary carnitine deficiency within the same family due to identical mutations in the organic cation/carnitine transporter OCTN2. J Inherit Metab Dis 26:613–615 [DOI] [PubMed] [Google Scholar]
- Sporstøl M, Tapia G, Malerød L, Mousavi SA, Berg T. (2005) Pregnane X receptor-agonists down-regulate hepatic ATP-binding cassette transporter A1 and scavenger receptor class B type I. Biochem Biophys Res Commun 331:1533–1541 [DOI] [PubMed] [Google Scholar]
- Srimaroeng C, Perry JL, Pritchard JB. (2008) Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica 38:889–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. (2002) Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab 87:1856–1863 [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, Lauper U, Meier PJ, Marin JJ. (2000) Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol 279:R1495–R1503 [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Stallmach T, Freimoser Grundschober A, Dufour JF, Serrano MA, Marin JJ, Sugiyama Y, Meier PJ. (2004) Temporal expression profiles of organic anion transport proteins in placenta and fetal liver of the rat. Am J Physiol Regul Integr Comp Physiol 287:R1505–R1516 [DOI] [PubMed] [Google Scholar]
- Stapelbroek JM, Peters TA, van Beurden DH, Curfs JH, Joosten A, Beynon AJ, van Leeuwen BM, van der Velden LM, Bull L, Oude Elferink RP, et al. (2009) ATP8B1 is essential for maintaining normal hearing. Proc Natl Acad Sci U S A 106:9709–9714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. (2001a) Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos 29:1467–1472 [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. (2001b) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A 98:3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Madan A, Carol KM, Parkinson A. (2003) Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos 31:523–527 [DOI] [PubMed] [Google Scholar]
- Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. (2005) Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A 102:2063–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger B, Hagenbuch B, Landmann L, Höchli M, Schroeder A, Meier PJ. (1994) In situ localization of the hepatocytic Na+/Taurocholate cotransporting polypeptide in rat liver. Gastroenterology 107:1781–1787 [DOI] [PubMed] [Google Scholar]
- Storch CH, Ehehalt R, Haefeli WE, Weiss J. (2007) Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J Pharmacol Exp Ther 323:257–264 [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, et al. (1998) A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet 20:233–238 [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerová D, Rayner A, Dutton L, Meier Y, Antoniou A, Stieger B, Arnell H, et al. (2008) Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology 134:1203–1214 [DOI] [PubMed] [Google Scholar]
- Stride BD, Grant CE, Loe DW, Hipfner DR, Cole SP, Deeley RG. (1997) Pharmacological characterization of the murine and human orthologs of multidrug-resistance protein in transfected human embryonic kidney cells. Mol Pharmacol 52:344–353 [DOI] [PubMed] [Google Scholar]
- Suchy FJ, Balistreri WF, Heubi JE, Searcy JE, Levin RS. (1981) Physiologic cholestasis: elevation of the primary serum bile acid concentrations in normal infants. Gastroenterology 80:1037–1041 [PubMed] [Google Scholar]
- Sugiura T, Kato Y, Kubo Y, Tsuji A. (2006) Mutation in an adaptor protein PDZK1 affects transport activity of organic cation transporter OCTNs and oligopeptide transporter PEPT2. Drug Metab Pharmacokinet 21:375–383 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kato Y, Wakayama T, Silver DL, Kubo Y, Iseki S, Tsuji A. (2008) PDZK1 regulates two intestinal solute carriers (Slc15a1 and Slc22a5) in mice. Drug Metab Dispos 36:1181–1188 [DOI] [PubMed] [Google Scholar]
- Sun AQ, Ananthanarayanan M, Soroka CJ, Thevananther S, Shneider BL, Suchy FJ. (1998) Sorting of rat liver and ileal sodium-dependent bile acid transporters in polarized epithelial cells. Am J Physiol 275:G1045–G1055 [DOI] [PubMed] [Google Scholar]
- Sun D, Landowski CP, Chu X, Wallsten R, Komorowski TE, Fleisher D, Amidon GL. (2001a) Drug inhibition of Gly-Sar uptake and hPepT1 localization using hPepT1-GFP fusion protein. AAPS PharmSci 3:E2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. (2006) Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta 27:602–609 [DOI] [PubMed] [Google Scholar]
- Sun W, Wu RR, van Poelje PD, Erion MD. (2001b) Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun 283:417–422 [DOI] [PubMed] [Google Scholar]
- Sundar PD, Feingold E, Minster RL, DeKosky ST, Kamboh MI. (2007) Gender-specific association of ATP-binding cassette transporter 1 (ABCA1) polymorphisms with the risk of late-onset Alzheimer's disease. Neurobiol Aging 28:856–862 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Onogawa T, Asano N, Mizutamari H, Mikkaichi T, Tanemoto M, Abe M, Satoh F, Unno M, Nunoki K, et al. (2003) Identification and characterization of novel rat and human gonad-specific organic anion transporters. Mol Endocrinol 17:1203–1215 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Zhao YL, Nadai M, Naruhashi K, Shimizu A, Takagi K, Takagi K, Hasegawa T. (2006) Gender-related differences in expression and function of hepatic P-glycoprotein and multidrug resistance-associated protein (Mrp2) in rats. Life Sci 79:455–461 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB. (2001) Ventricular choline transport: a role for organic cation transporter 2 expressed in choroid plexus. J Biol Chem 276:41611–41619 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. (2002) Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem 277:26934–26943 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Wolff NA, Pritchard JB. (1997) Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J Biol Chem 272:30088–30095 [DOI] [PubMed] [Google Scholar]
- Sykes D, Sweet DH, Lowes S, Nigam SK, Pritchard JB, Miller DS. (2004) Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am J Physiol Renal Physiol 286:F972–F978 [DOI] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM. (2001) The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med 7:584–590 [DOI] [PubMed] [Google Scholar]
- Tachibana-Iimori R, Tabara Y, Kusuhara H, Kohara K, Kawamoto R, Nakura J, Tokunaga K, Kondo I, Sugiyama Y, Miki T. (2004) Effect of genetic polymorphism of OATP-C (SLCO1B1) on lipid-lowering response to HMG-CoA reductase inhibitors. Drug Metab Pharmacokinet 19:375–380 [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Toki H, Tomi M, Hosoya K. (2008) Gene expression profiles of ATP-binding cassette transporter A and C subfamilies in mouse retinal vascular endothelial cells. Microvasc Res 75:68–72 [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Watanabe M, Hori S, Fukaya M, Ohtsuki S, Asashima T, Terasaki T. (2005) Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem 95:294–304 [DOI] [PubMed] [Google Scholar]
- Tahara H, Kusuhara H, Fuse E, Sugiyama Y. (2005) P-glycoprotein plays a major role in the efflux of fexofenadine in the small intestine and blood-brain barrier, but only a limited role in its biliary excretion. Drug Metab Dispos 33:963–968 [DOI] [PubMed] [Google Scholar]
- Takahashi R, Asai T, Murakami H, Murakami R, Tsuzuki M, Numaguchi Y, Matsui H, Murohara T, Okumura K. (2007) Pressure overload-induced cardiomyopathy in heterozygous carrier mice of carnitine transporter gene mutation. Hypertension 50:497–502 [DOI] [PubMed] [Google Scholar]
- Takata Y, Inoue H, Sato A, Tsugawa K, Miyatake K, Hamada D, Shinomiya F, Nakano S, Yasui N, Tanahashi T, et al. (2008) Replication of reported genetic associations of PADI4, FCRL3, SLC22A4 and RUNX1 genes with rheumatoid arthritis: results of an independent Japanese population and evidence from meta-analysis of East Asian studies. J Hum Genet 53:163–173 [DOI] [PubMed] [Google Scholar]
- Takeda M, Khamdang S, Narikawa S, Kimura H, Kobayashi Y, Yamamoto T, Cha SH, Sekine T, Endou H. (2002) Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther 300:918–924 [DOI] [PubMed] [Google Scholar]
- Takeda M, Noshiro R, Onozato ML, Tojo A, Hasannejad H, Huang XL, Narikawa S, Endou H. (2004) Evidence for a role of human organic anion transporters in the muscular side effects of HMG-CoA reductase inhibitors. Eur J Pharmacol 483:133–138 [DOI] [PubMed] [Google Scholar]
- Takeda M, Sekine T, Endou H. (2000) Regulation by protein kinase C of organic anion transport driven by rat organic anion transporter 3 (rOAT3). Life Sci 67:1087–1093 [DOI] [PubMed] [Google Scholar]
- Takenaka K, Morgan JA, Scheffer GL, Adachi M, Stewart CF, Sun D, Leggas M, Ejendal KF, Hrycyna CA, Schuetz JD. (2007) Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res 67:6965–6972 [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Motohashi H, Okuda M, Inui K. (2003) Decreased function of genetic variants, Pro283Leu and Arg287Gly, in human organic cation transporter hOCT1. Drug Metab Pharmacokinet 18:409–412 [DOI] [PubMed] [Google Scholar]
- Tamai I, China K, Sai Y, Kobayashi D, Nezu J, Kawahara E, Tsuji A. (2001a) Na(+)-coupled transport of L-carnitine via high-affinity carnitine transporter OCTN2 and its subcellular localization in kidney. Biochim Biophys Acta 1512:273–284 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nakanishi T, Kobayashi D, China K, Kosugi Y, Nezu J, Sai Y, Tsuji A. (2004) Involvement of OCTN1 (SLC22A4) in pH-dependent transport of organic cations. Mol Pharm 1:57–66 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. (2000a) Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun 273:251–260 [DOI] [PubMed] [Google Scholar]
- Tamai I, Nozawa T, Koshida M, Nezu J, Sai Y, Tsuji A. (2001b) Functional characterization of human organic anion transporting polypeptide B (OATP-B) in comparison with liver-specific OATP-C. Pharm Res 18:1262–1269 [DOI] [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. (1998) Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem 273:20378–20382 [DOI] [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu JI, Sai Y, Kobayashi D, Oku A, Shimane M, Tsuji A. (2000b) Molecular and functional characterization of organic cation/carnitine transporter family in mice. J Biol Chem 275:40064–40072 [DOI] [PubMed] [Google Scholar]
- Tamai I, Yabuuchi H, Nezu J, Sai Y, Oku A, Shimane M, Tsuji A. (1997) Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett 419:107–111 [DOI] [PubMed] [Google Scholar]
- Tammur J, Prades C, Arnould I, Rzhetsky A, Hutchinson A, Adachi M, Schuetz JD, Swoboda KJ, Ptácek LJ, Rosier M, et al. (2001) Two new genes from the human ATP-binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene 273:89–96 [DOI] [PubMed] [Google Scholar]
- Tamura A, Onishi Y, An R, Koshiba S, Wakabayashi K, Hoshijima K, Priebe W, Yoshida T, Kometani S, Matsubara T, et al. (2007a) In vitro evaluation of photosensitivity risk related to genetic polymorphisms of human ABC transporter ABCG2 and inhibition by drugs. Drug Metab Pharmacokinet 22:428–440 [DOI] [PubMed] [Google Scholar]
- Tamura A, Wakabayashi K, Onishi Y, Nakagawa H, Tsuji M, Matsuda Y, Ishikawa T. (2006a) Genetic polymorphisms of human ABC transporter ABCG2: development of the standard method for functional validation of SNPs by using the Flp recombinase system. J Exp Ther Oncol 6:1–11 [PubMed] [Google Scholar]
- Tamura A, Wakabayashi K, Onishi Y, Takeda M, Ikegami Y, Sawada S, Tsuji M, Matsuda Y, Ishikawa T. (2007b) Re-evaluation and functional classification of non-synonymous single nucleotide polymorphisms of the human ATP-binding cassette transporter ABCG2. Cancer Sci 98:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, Watanabe M, Saito H, Nakagawa H, Kamachi T, Okura I, Ishikawa T. (2006b) Functional validation of the genetic polymorphisms of human ATP-binding cassette (ABC) transporter ABCG2: identification of alleles that are defective in porphyrin transport. Mol Pharmacol 70:287–296 [DOI] [PubMed] [Google Scholar]
- Tan EK, Chan DK, Ng PW, Woo J, Teo YY, Tang K, Wong LP, Chong SS, Tan C, Shen H, et al. (2005) Effect of MDR1 haplotype on risk of Parkinson disease. Arch Neurol 62:460–464 [DOI] [PubMed] [Google Scholar]
- Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, Takahashi M, Kurata Y, Kigawa J, Higuchi S, et al. (2001) Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther 297:1137–1143 [PubMed] [Google Scholar]
- Tanaka K, Xu W, Zhou F, You G. (2004) Role of glycosylation in the organic anion transporter OAT1. J Biol Chem 279:14961–14966 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD. (2009) ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2 dependent and independent signaling. Toxicol Sci 108:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Chen C, Maher JM, Klaassen CD. (2006) Kupffer cell-mediated downregulation of hepatic transporter expression in rat hepatic ischemia-reperfusion. Transplantation 82:258–266 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Chen C, Maher JM, Klaassen CD. (2008) Ischemia-reperfusion of rat livers decreases liver and increases kidney multidrug resistance associated protein 2 (Mrp2). Toxicol Sci 101:171–178 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. (2005) Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun 326:181–187 [DOI] [PubMed] [Google Scholar]
- Tang-Wai DF, Kajiji S, DiCapua F, de Graaf D, Roninson IB, Gros P. (1995) Human (MDR1) and mouse (mdr1, mdr3) P-glycoproteins can be distinguished by their respective drug resistance profiles and sensitivity to modulators. Biochemistry 34:32–39 [DOI] [PubMed] [Google Scholar]
- Taniguchi A, Urano W, Yamanaka M, Yamanaka H, Hosoyamada M, Endou H, Kamatani N. (2005) A common mutation in an organic anion transporter gene, SLC22A12, is a suppressing factor for the development of gout. Arthritis Rheum 52:2576–2577 [DOI] [PubMed] [Google Scholar]
- Tanihara Y, Masuda S, Katsura T, Inui K. (2009) Protective effect of concomitant administration of imatinib on cisplatin-induced nephrotoxicity focusing on renal organic cation transporter OCT2. Biochem Pharmacol 78:1263–1271 [DOI] [PubMed] [Google Scholar]
- Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. (2007) Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol 74:359–371 [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM. (1993) The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 5:344–350 [DOI] [PubMed] [Google Scholar]
- Taubert D, Jung N, Goeser T, Schömig E. (2009) Increased ergothioneine tissue concentrations in carriers of the Crohn's disease risk-associated 503F variant of the organic cation transporter OCTN1. Gut 58:312–314 [DOI] [PubMed] [Google Scholar]
- Teng S, Jekerle V, Piquette-Miller M. (2003) Induction of ABCC3 (MRP3) by pregnane X receptor activators. Drug Metab Dispos 31:1296–1299 [DOI] [PubMed] [Google Scholar]
- Terada T, Inui K. (2008) Physiological and pharmacokinetic roles of H+/organic cation antiporters (MATE/SLC47A). Biochem Pharmacol 75:1689–1696 [DOI] [PubMed] [Google Scholar]
- Terada T, Irie M, Okuda M, Inui K. (2004) Genetic variant Arg57His in human H+/peptide cotransporter 2 causes a complete loss of transport function. Biochem Biophys Res Commun 316:416–420 [DOI] [PubMed] [Google Scholar]
- Terada T, Masuda S, Asaka J, Tsuda M, Katsura T, Inui K. (2006) Molecular cloning, functional characterization and tissue distribution of rat H+/organic cation antiporter MATE1. Pharm Res 23:1696–1701 [DOI] [PubMed] [Google Scholar]
- Terada T, Sawada K, Irie M, Saito H, Hashimoto Y, Inui K. (2000) Structural requirements for determining the substrate affinity of peptide transporters PEPT1 and PEPT2. Pflugers Arch 440:679–684 [DOI] [PubMed] [Google Scholar]
- Terada T, Shimada Y, Pan X, Kishimoto K, Sakurai T, Doi R, Onodera H, Katsura T, Imamura M, Inui K. (2005) Expression profiles of various transporters for oligopeptides, amino acids and organic ions along the human digestive tract. Biochem Pharmacol 70:1756–1763 [DOI] [PubMed] [Google Scholar]
- Terry LC, Saunders A, Audet J, Willoughby JO, Brazeau P, Martin JB. (1977) Physiologic secretion of growth hormone and prolactin in male and female rats. Clin Endocrinol (Oxf) 6Suppl:19S–28S [DOI] [PubMed] [Google Scholar]
- Tian Q, Zhang J, Tan TM, Chan E, Duan W, Chan SY, Boelsterli UA, Ho PC, Yang H, Bian JS, et al. (2005) Human multidrug resistance associated protein 4 confers resistance to camptothecins. Pharm Res 22:1837–1853 [DOI] [PubMed] [Google Scholar]
- Tian X, Li J, Zamek-Gliszczynski MJ, Bridges AS, Zhang P, Patel NJ, Raub TJ, Pollack GM, Brouwer KL. (2007) Roles of P-glycoprotein, Bcrp, and Mrp2 in biliary excretion of spiramycin in mice. Antimicrob Agents Chemother 51:3230–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Zamek-Gliszczynski MJ, Li J, Bridges AS, Nezasa K, Patel NJ, Raub TJ, Brouwer KL. (2008) Multidrug resistance-associated protein 2 is primarily responsible for the biliary excretion of fexofenadine in mice. Drug Metab Dispos 36:61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, et al. (2005) Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest 115:1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Merino G, Kim RB. (2001) Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem 276:35669–35675 [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. (2003) Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther 304:223–228 [DOI] [PubMed] [Google Scholar]
- Toan SV, To KK, Leung GP, de Souza MO, Ward JL, Tse CM. (2003) Genomic organization and functional characterization of the human concentrative nucleoside transporter-3 isoform (hCNT3) expressed in mammalian cells. Pflugers Arch 447:195–204 [DOI] [PubMed] [Google Scholar]
- Tojo A, Sekine T, Nakajima N, Hosoyamada M, Kanai Y, Kimura K, Endou H. (1999) Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J Am Soc Nephrol 10:464–471 [DOI] [PubMed] [Google Scholar]
- Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, Suzuki M, Nagasaki M, Ohtsuki M, Ono M, et al. (2003) An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet 35:341–348 [DOI] [PubMed] [Google Scholar]
- Tomer G, Ananthanarayanan M, Weymann A, Balasubramanian N, Suchy FJ. (2003) Differential developmental regulation of rat liver canalicular membrane transporters Bsep and Mrp2. Pediatr Res 53:288–294 [DOI] [PubMed] [Google Scholar]
- Török HP, Glas J, Tonenchi L, Lohse P, Müller-Myhsok B, Limbersky O, Neugebauer C, Schnitzler F, Seiderer J, Tillack C, et al. (2005) Polymorphisms in the DLG5 and OCTN cation transporter genes in Crohn's disease. Gut 54:1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Hagiya Y, Adachi T, Hoshijima K, Kuo MT, Ishikawa T. (2008) MRP class of human ATP binding cassette (ABC) transporters: historical background and new research directions. Xenobiotica 38:833–862 [DOI] [PubMed] [Google Scholar]
- Trauner M, Arrese M, Lee H, Boyer JL, Karpen SJ. (1998) Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J Clin Invest 101:2092–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, Keppler D, Boyer JL. (1997) The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology 113:255–264 [DOI] [PubMed] [Google Scholar]
- Treiber A, Schneiter R, Häusler S, Stieger B. (2007) Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos 35:1400–1407 [DOI] [PubMed] [Google Scholar]
- Tribull TE, Bruner RH, Bain LJ. (2003) The multidrug resistance-associated protein 1 transports methoxychlor and protects the seminiferous epithelium from injury. Toxicol Lett 142:61–70 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Sekine T, Takeda M, Cha SH, Kanai Y, Kimura M, Endou H. (1999) Transport of ochratoxin A by renal multispecific organic anion transporter 1. J Pharmacol Exp Ther 289:1301–1305 [PubMed] [Google Scholar]
- Tsuda M, Terada T, Mizuno T, Katsura T, Shimakura J, Inui K. (2009) Targeted disruption of the multidrug and toxin extrusion 1 (mate1) gene in mice reduces renal secretion of metformin. Mol Pharmacol 75:1280–1286 [DOI] [PubMed] [Google Scholar]
- Tsujii H, König J, Rost D, Stöckel B, Leuschner U, Keppler D. (1999) Exon-intron organization of the human multidrug-resistance protein 2 (MRP2) gene mutated in Dubin-Johnson syndrome. Gastroenterology 117:653–660 [DOI] [PubMed] [Google Scholar]
- Tzvetkov MV, Vormfelde SV, Balen D, Meineke I, Schmidt T, Sehrt D, Sabolić I, Koepsell H, Brockmöller J. (2009) The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther 86:299–306 [DOI] [PubMed] [Google Scholar]
- Uchida N, Leung FY, Eaves CJ. (2002) Liver and marrow of adult mdr-1a/1b(−/−) mice show normal generation, function, and multi-tissue trafficking of primitive hematopoietic cells. Exp Hematol 30:862–869 [DOI] [PubMed] [Google Scholar]
- Uchiumi T, Hinoshita E, Haga S, Nakamura T, Tanaka T, Toh S, Furukawa M, Kawabe T, Wada M, Kagotani K, et al. (1998) Isolation of a novel human canalicular multispecific organic anion transporter, cMOAT2/MRP3, and its expression in cisplatin-resistant cancer cells with decreased ATP-dependent drug transport. Biochem Biophys Res Commun 252:103–110 [DOI] [PubMed] [Google Scholar]
- Ugele B, Bahn A, Rex-Haffner M. (2008) Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta. J Steroid Biochem Mol Biol 111:1–6 [DOI] [PubMed] [Google Scholar]
- Uhr M, Holsboer F, Müller MB. (2002) Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol 14:753–759 [DOI] [PubMed] [Google Scholar]
- Ujhazy P, Ortiz D, Misra S, Li S, Moseley J, Jones H, Arias IM. (2001) Familial intrahepatic cholestasis 1: studies of localization and function. Hepatology 34:768–775 [DOI] [PubMed] [Google Scholar]
- Umbenhauer DR, Lankas GR, Pippert TR, Wise LD, Cartwright ME, Hall SJ, Beare CM. (1997) Identification of a P-glycoprotein-deficient subpopulation in the CF-1 mouse strain using a restriction fragment length polymorphism. Toxicol Appl Pharmacol 146:88–94 [DOI] [PubMed] [Google Scholar]
- Uppal H, Toma D, Saini SP, Ren S, Jones TJ, Xie W. (2005) Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology 41:168–176 [DOI] [PubMed] [Google Scholar]
- Urakami Y, Nakamura N, Takahashi K, Okuda M, Saito H, Hashimoto Y, Inui K. (1999) Gender differences in expression of organic cation transporter OCT2 in rat kidney. FEBS Lett 461:339–342 [DOI] [PubMed] [Google Scholar]
- Urakami Y, Okuda M, Masuda S, Saito H, Inui KI. (1998) Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Exp Ther 287:800–805 [PubMed] [Google Scholar]
- Urakami Y, Okuda M, Saito H, Inui K. (2000) Hormonal regulation of organic cation transporter OCT2 expression in rat kidney. FEBS Lett 473:173–176 [DOI] [PubMed] [Google Scholar]
- Urban TJ, Brown C, Castro RA, Shah N, Mercer R, Huang Y, Brett CM, Burchard EG, Giacomini KM. (2008) Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin Pharmacol Ther 83:416–421 [DOI] [PubMed] [Google Scholar]
- Urban TJ, Gallagher RC, Brown C, Castro RA, Lagpacan LL, Brett CM, Taylor TR, Carlson EJ, Ferrin TE, Burchard EG, et al. (2006) Functional genetic diversity in the high-affinity carnitine transporter OCTN2 (SLC22A5). Mol Pharmacol 70:1602–1611 [DOI] [PubMed] [Google Scholar]
- Urban TJ, Yang C, Lagpacan LL, Brown C, Castro RA, Taylor TR, Huang CC, Stryke D, Johns SJ, Kawamoto M, et al. (2007) Functional effects of protein sequence polymorphisms in the organic cation/ergothioneine transporter OCTN1 (SLC22A4). Pharmacogenet Genomics 17:773–782 [DOI] [PubMed] [Google Scholar]
- Urquhart BL, Ware JA, Tirona RG, Ho RH, Leake BF, Schwarz UI, Zaher H, Palandra J, Gregor JC, Dresser GK, et al. (2008) Breast cancer resistance protein (ABCG2) and drug disposition: intestinal expression, polymorphisms and sulfasalazine as an in vivo probe. Pharmacogenet Genomics 18:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushigome F, Koyabu N, Satoh S, Tsukimori K, Nakano H, Nakamura T, Uchiumi T, Kuwano M, Ohtani H, Sawada Y. (2003) Kinetic analysis of P-glycoprotein-mediated transport by using normal human placental brush-border membrane vesicles. Pharm Res 20:38–44 [DOI] [PubMed] [Google Scholar]
- Vallon V, Eraly SA, Wikoff WR, Rieg T, Kaler G, Truong DM, Ahn SY, Mahapatra NR, Mahata SK, Gangoiti JA, et al. (2008a) Organic anion transporter 3 contributes to the regulation of blood pressure. J Am Soc Nephrol 19:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK. (2008b) Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol 294:F867–F873 [DOI] [PubMed] [Google Scholar]
- van Asperen J, Schinkel AH, Beijnen JH, Nooijen WJ, Borst P, van Tellingen O. (1996) Altered pharmacokinetics of vinblastine in Mdr1a P-glycoprotein-deficient Mice. J Natl Cancer Inst 88:994–999 [DOI] [PubMed] [Google Scholar]
- van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. (2002) The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol 13:595–603 [DOI] [PubMed] [Google Scholar]
- Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. (2005) Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol 288:F327–F333 [DOI] [PubMed] [Google Scholar]
- van de Wetering K, Burkon A, Feddema W, Bot A, de Jonge H, Somoza V, Borst P. (2009a) Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol Pharmacol 75:876–885 [DOI] [PubMed] [Google Scholar]
- van de Wetering K, Feddema W, Helms JB, Brouwers JF, Borst P. (2009b) Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology 137:1725–1735 [DOI] [PubMed] [Google Scholar]
- van de Wetering K, Zelcer N, Kuil A, Feddema W, Hillebrand M, Vlaming ML, Schinkel AH, Beijnen JH, Borst P. (2007) Multidrug resistance proteins 2 and 3 provide alternative routes for hepatic excretion of morphine-glucuronides. Mol Pharmacol 72:387–394 [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Kooiman PM, Schneider C, Borst P. (1988) Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene 71:401–411 [DOI] [PubMed] [Google Scholar]
- van der Deen M, Timens W, Timmer-Bosscha H, van der Strate BW, Scheper RJ, Postma DS, de Vries EG, Kerstjens HA. (2007) Reduced inflammatory response in cigarette smoke exposed Mrp1/Mdr1a/1b deficient mice. Respir Res 8:49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. (1996) MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 87:507–517 [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Wagenaar E, Karnekamp B, Merino G, Jonker JW, Schinkel AH. (2006) Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis 27:123–130 [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, Schinkel AH. (2007) Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cell Biol 27:1247–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfoort JE, Schmid TE, Adler ID, Meier PJ, Hagenbuch B. (2002) Functional characterization of the mouse organic-anion-transporting polypeptide 2. Biochim Biophys Acta 1564:183–188 [DOI] [PubMed] [Google Scholar]
- van Nieuwerk CM, Groen AK, Ottenhoff R, van Wijland M, van den Bergh Weerman MA, Tytgat GN, Offerhaus JJ, Oude Elferink RP. (1997) The role of bile salt composition in liver pathology of mdr2 (−/−) mice: differences between males and females. J Hepatol 26:138–145 [DOI] [PubMed] [Google Scholar]
- van Vlies N, Ferdinandusse S, Turkenburg M, Wanders RJ, Vaz FM. (2007) PPAR alpha-activation results in enhanced carnitine biosynthesis and OCTN2-mediated hepatic carnitine accumulation. Biochim Biophys Acta 1767:1134–1142 [DOI] [PubMed] [Google Scholar]
- Vanwert AL, Bailey RM, Sweet DH. (2007) Organic anion transporter 3 (Oat3/Slc22a8) knockout mice exhibit altered clearance and distribution of penicillin G. Am J Physiol Renal Physiol 293:F1332–F1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanwert AL, Srimaroeng C, Sweet DH. (2008) Organic anion transporter 3 (oat3/slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin. Mol Pharmacol 74:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWert AL, Sweet DH. (2008) Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharm Res 25:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. (2002) Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology 36:164–172 [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. (1996) Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A 93:14960–14965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbon A, Leemans JC, Weijer S, Florquin S, Van Der Poll T. (2002) Mice lacking the multidrug resistance protein 1 have a transiently impaired immune response during tuberculosis. Clin Exp Immunol 130:32–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaagh S, Schweifer N, Barlow DP, Zwart R. (1999) Cloning of the mouse and human solute carrier 22a3 (Slc22a3/SLC22A3) identifies a conserved cluster of three organic cation transporters on mouse chromosome 17 and human 6q26–q27. Genomics 55:209–218 [DOI] [PubMed] [Google Scholar]
- Verstuyft C, Schwab M, Schaeffeler E, Kerb R, Brinkmann U, Jaillon P, Funck-Brentano C, Becquemont L. (2003) Digoxin pharmacokinetics and MDR1 genetic polymorphisms. Eur J Clin Pharmacol 58:809–812 [DOI] [PubMed] [Google Scholar]
- Vethanayagam RR, Wang H, Gupta A, Zhang Y, Lewis F, Unadkat JD, Mao Q. (2005) Functional analysis of the human variants of breast cancer resistance protein: I206L, N590Y, and D620N. Drug Metab Dispos 33:697–705 [DOI] [PubMed] [Google Scholar]
- Vialou V, Amphoux A, Zwart R, Giros B, Gautron S. (2004) Organic cation transporter 3 (Slc22a3) is implicated in salt-intake regulation. J Neurosci 24:2846–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva SS, Ruiz ML, Ghanem CI, Luquita MG, Catania VA, Mottino AD. (2008) Hepatic synthesis and urinary elimination of acetaminophen glucuronide are exacerbated in bile duct-ligated rats. Drug Metab Dispos 36:475–480 [DOI] [PubMed] [Google Scholar]
- Vlaming ML, Lagas JS, Schinkel AH. (2009a) Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev 61:14–25 [DOI] [PubMed] [Google Scholar]
- Vlaming ML, Mohrmann K, Wagenaar E, de Waart DR, Elferink RP, Lagas JS, van Tellingen O, Vainchtein LD, Rosing H, Beijnen JH, et al. (2006) Carcinogen and anticancer drug transport by Mrp2 in vivo: studies using Mrp2 (Abcc2) knockout mice. J Pharmacol Exp Ther 318:319–327 [DOI] [PubMed] [Google Scholar]
- Vlaming ML, Pala Z, van Esch A, Wagenaar E, de Waart DR, van de Wetering K, van der Kruijssen CM, Oude Elferink RP, van Tellingen O, Schinkel AH. (2009b) Functionally overlapping roles of Abcg2 (Bcrp1) and Abcc2 (Mrp2) in the elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate in vivo. Clin Cancer Res 15:3084–3093 [DOI] [PubMed] [Google Scholar]
- Vollrath V, Wielandt AM, Iruretagoyena M, Chianale J. (2006) Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem J 395:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos TA, Hooiveld GJ, Koning H, Childs S, Meijer DK, Moshage H, Jansen PL, Müller M. (1998) Up-regulation of the multidrug resistance genes, Mrp1 and Mdr1b, and down-regulation of the organic anion transporter, Mrp2, and the bile salt transporter, Spgp, in endotoxemic rat liver. Hepatology 28:1637–1644 [DOI] [PubMed] [Google Scholar]
- Vos TA, Ros JE, Havinga R, Moshage H, Kuipers F, Jansen PL, Müller M. (1999) Regulation of hepatic transport systems involved in bile secretion during liver regeneration in rats. Hepatology 29:1833–1839 [DOI] [PubMed] [Google Scholar]
- Voshol PJ, Havinga R, Wolters H, Ottenhoff R, Princen HM, Oude Elferink RP, Groen AK, Kuipers F. (1998) Reduced plasma cholesterol and increased fecal sterol loss in multidrug resistance gene 2 P-glycoprotein-deficient mice. Gastroenterology 114:1024–1034 [DOI] [PubMed] [Google Scholar]
- Wada S, Tsuda M, Sekine T, Cha SH, Kimura M, Kanai Y, Endou H. (2000) Rat multispecific organic anion transporter 1 (rOAT1) transports zidovudine, acyclovir, and other antiviral nucleoside analogs. J Pharmacol Exp Ther 294:844–849 [PubMed] [Google Scholar]
- Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, et al. (2003) Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology 125:825–838 [DOI] [PubMed] [Google Scholar]
- Wahli W, Devchand PR, IJpenberg A, Desvergne B. (1999) Fatty acids, eicosanoids, and hypolipidemic agents regulate gene expression through direct binding to peroxisome proliferator-activated receptors. Adv Exp Med Biol 447:199–209 [DOI] [PubMed] [Google Scholar]
- Wakabayashi-Nakao K, Tamura A, Furukawa T, Nakagawa H, Ishikawa T. (2009) Quality control of human ABCG2 protein in the endoplasmic reticulum: ubiquitination and proteasomal degradation. Adv Drug Deliv Rev 61:66–72 [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Dutt P, Lippincott-Schwartz J, Arias IM. (2005) Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc Natl Acad Sci U S A 102:15087–15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Kipp H, Arias IM. (2006) Transporters on demand: intracellular reservoirs and cycling of bile canalicular ABC transporters. J Biol Chem 281:27669–27673 [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Lippincott-Schwartz J, Arias IM. (2004) Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell 15:3485–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller S, Tremelling M, Bredin F, Godfrey L, Howson J, Parkes M. (2006) Evidence for association of OCTN genes and IBD5 with ulcerative colitis. Gut 55:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters HC, Craddock AL, Fusegawa H, Willingham MC, Dawson PA. (2000) Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. Am J Physiol Gastrointest Liver Physiol 279:G1188–G1200 [DOI] [PubMed] [Google Scholar]
- Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. (2002a) Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther 302:510–515 [DOI] [PubMed] [Google Scholar]
- Wang DS, Kusuhara H, Kato Y, Jonker JW, Schinkel AH, Sugiyama Y. (2003a) Involvement of organic cation transporter 1 in the lactic acidosis caused by metformin. Mol Pharmacol 63:844–848 [DOI] [PubMed] [Google Scholar]
- Wang H, Lee EW, Cai X, Ni Z, Zhou L, Mao Q. (2008a) Membrane topology of the human breast cancer resistance protein (BCRP/ABCG2) determined by epitope insertion and immunofluorescence. Biochemistry 47:13778–13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Negishi M. (2003) Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr Drug Metab 4:515–525 [DOI] [PubMed] [Google Scholar]
- Wang J, Near S, Young K, Connelly PW, Hegele RA. (2001a) ABCC6 gene polymorphism associated with variation in plasma lipoproteins. J Hum Genet 46:699–705 [DOI] [PubMed] [Google Scholar]
- Wang J, Su SF, Dresser MJ, Schaner ME, Washington CB, Giacomini KM. (1997) Na(+)-dependent purine nucleoside transporter from human kidney: cloning and functional characterization. Am J Physiol 273:F1058–F1065 [DOI] [PubMed] [Google Scholar]
- Wang L, Leggas M, Goswami M, Empey PE, McNamara PJ. (2008b) N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9, 10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) as a chemical ATP-binding cassette transporter family G member 2 (Abcg2) knockout model to study nitrofurantoin transfer into milk. Drug Metab Dispos 36:2591–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Soroka CJ, Boyer JL. (2002b) The role of bile salt export pump mutations in progressive familial intrahepatic cholestasis type II. J Clin Invest 110:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Silver DL, Thiele C, Tall AR. (2001b) ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem 276:23742–23747 [DOI] [PubMed] [Google Scholar]
- Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. (2008c) Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. Am J Physiol Gastrointest Liver Physiol 294:G1052–G1059 [DOI] [PubMed] [Google Scholar]
- Wang P, Wang JJ, Xiao Y, Murray JW, Novikoff PM, Angeletti RH, Orr GA, Lan D, Silver DL, Wolkoff AW. (2005) Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface. J Biol Chem 280:30143–30149 [DOI] [PubMed] [Google Scholar]
- Wang R, Chen HL, Liu L, Sheps JA, Phillips MJ, Ling V. (2009) Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump. Hepatology 50:948–956 [DOI] [PubMed] [Google Scholar]
- Wang R, Lam P, Liu L, Forrest D, Yousef IM, Mignault D, Phillips MJ, Ling V. (2003b) Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology 38:1489–1499 [DOI] [PubMed] [Google Scholar]
- Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V. (2001c) Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A 98:2011–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Seward DJ, Li L, Boyer JL, Ballatori N. (2001d) Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci U S A 98:9431–9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Li QQ, Xu JD, Cao XX, Li HX, Tang F, Chen Q, Yang JM, Xu ZD, Liu XP. (2008d) Interaction between CD147 and P-glycoprotein and their regulation by ubiquitination in breast cancer cells. Chemotherapy 54:291–301 [DOI] [PubMed] [Google Scholar]
- Wang X, Furukawa T, Nitanda T, Okamoto M, Sugimoto Y, Akiyama S, Baba M. (2003c) Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Mol Pharmacol 63:65–72 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kelly MA, Cowan TM, Longo N. (2000a) A missense mutation in the OCTN2 gene associated with residual carnitine transport activity. Hum Mutat 15:238–245 [DOI] [PubMed] [Google Scholar]
- Wang Y, Taroni F, Garavaglia B, Longo N. (2000b) Functional analysis of mutations in the OCTN2 transporter causing primary carnitine deficiency: lack of genotype-phenotype correlation. Hum Mutat 16:401–407 [DOI] [PubMed] [Google Scholar]
- Wang Y, Ye J, Ganapathy V, Longo N. (1999) Mutations in the organic cation/carnitine transporter OCTN2 in primary carnitine deficiency. Proc Natl Acad Sci U S A 96:2356–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Yin OQ, Tomlinson B, Chow MS. (2008e) OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics 18:637–645 [DOI] [PubMed] [Google Scholar]
- Wasmuth HE, Glantz A, Keppeler H, Simon E, Bartz C, Rath W, Mattsson LA, Marschall HU, Lammert F. (2007) Intrahepatic cholestasis of pregnancy: the severe form is associated with common variants of the hepatobiliary phospholipid transporter ABCB4 gene. Gut 56:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C, Kato Y, Sugiura T, Kubo Y, Wakayama T, Iseki S, Tsuji A. (2006) PDZ adaptor protein PDZK2 stimulates transport activity of organic cation/carnitine transporter OCTN2 by modulating cell surface expression. Drug Metab Dispos 34:1927–1934 [DOI] [PubMed] [Google Scholar]
- Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. (2009) Nuclear factor erythroid 2-related factor 2 is a positive regulator of human bile salt export pump expression. Hepatology 50:1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KH, Wurz J, Gotthardt D, Merle U, Stremmel W, Füllekrug J. (2008) Localization of the Wilson disease protein in murine intestine. J Anat 213:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel U, Gebert I, Weintraut H, Weber WM, Clauss W, Daniel H. (1996) Transport characteristics of differently charged cephalosporin antibiotics in oocytes expressing the cloned intestinal peptide transporter PepT1 and in human intestinal Caco-2 cells. J Pharmacol Exp Ther 277:831–839 [PubMed] [Google Scholar]
- Westley IS, Brogan LR, Morris RG, Evans AM, Sallustio BC. (2006) Role of Mrp2 in the hepatic disposition of mycophenolic acid and its glucuronide metabolites: effect of cyclosporine. Drug Metab Dispos 34:261–266 [DOI] [PubMed] [Google Scholar]
- Whitlock JP, Jr., Denison MS, Fisher JM, Shen ES. (1989) Induction of hepatic cytochrome P450 gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Biol Med 6:169–178 [PubMed] [Google Scholar]
- Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. (2007) Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol 27:762–768 [DOI] [PubMed] [Google Scholar]
- Wielinga P, Hooijberg JH, Gunnarsdottir S, Kathmann I, Reid G, Zelcer N, van der Born K, de Haas M, van der Heijden I, Kaspers G, et al. (2005) The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res 65:4425–4430 [DOI] [PubMed] [Google Scholar]
- Wielinga PR, Reid G, Challa EE, van der Heijden I, van Deemter L, de Haas M, Mol C, Kuil AJ, Groeneveld E, Schuetz JD, et al. (2002) Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol 62:1321–1331 [DOI] [PubMed] [Google Scholar]
- Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. (2003) Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem 278:17664–17671 [DOI] [PubMed] [Google Scholar]
- Wijnholds J, deLange EC, Scheffer GL, van den Berg DJ, Mol CA, van der Valk M, Schinkel AH, Scheper RJ, Breimer DD, Borst P. (2000a) Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J Clin Invest 105:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P. (1997) Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med 3:1275–1279 [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, et al. (2000b) Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A 97:7476–7481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, Borst P. (1998) Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med 188:797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde S, Schlatter E, Koepsell H, Edemir B, Reuter S, Pavenstädt H, Neugebauer U, Schröter R, Brast S, Ciarimboli G. (2009) Calmodulin-associated post-translational regulation of rat organic cation transporter 2 in the kidney is gender dependent. Cell Mol Life Sci 66:1729–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson TM, Wahli W. (1997) Peroxisome proliferator-activated receptor agonists. Curr Opin Chem Biol 1:235–241 [DOI] [PubMed] [Google Scholar]
- Wlcek K, Svoboda M, Thalhammer T, Sellner F, Krupitza G, Jaeger W. (2008) Altered expression of organic anion transporter polypeptide (OATP) genes in human breast carcinoma. Cancer Biol Ther 7:1450–1455 [DOI] [PubMed] [Google Scholar]
- Wolff NA, Philpot RM, Miller DS, Pritchard JB. (1992) Functional expression of renal organic anion transport in Xenopus laevis oocytes. Mol Cell Biochem 114:35–41 [DOI] [PubMed] [Google Scholar]
- Wolff NA, Thies K, Kuhnke N, Reid G, Friedrich B, Lang F, Burckhardt G. (2003) Protein kinase C activation downregulates human organic anion transporter 1-mediated transport through carrier internalization. J Am Soc Nephrol 14:1959–1968 [DOI] [PubMed] [Google Scholar]
- Wong MH, Oelkers P, Craddock AL, Dawson PA. (1994) Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem 269:1340–1347 [PubMed] [Google Scholar]
- Wong MH, Oelkers P, Dawson PA. (1995) Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem 270:27228–27234 [DOI] [PubMed] [Google Scholar]
- Wood CE, Cousins R, Zhang D, Keller-Wood M. (2005) Ontogeny of expression of organic anion transporters 1 and 3 in ovine fetal and neonatal kidney. Exp Biol Med (Maywood) 230:668–673 [DOI] [PubMed] [Google Scholar]
- Woodahl EL, Crouthamel MH, Bui T, Shen DD, Ho RJ. (2009) MDR1 (ABCB1) G1199A (Ser400Asn) polymorphism alters transepithelial permeability and sensitivity to anticancer agents. Cancer Chemother Pharmacol 64:183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward OM, Köttgen A, Coresh J, Boerwinkle E, Guggino WB, Köttgen M. (2009) Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A 106:10338–10342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortham M, Czerwinski M, He L, Parkinson A, Wan YJ. (2007) Expression of constitutive androstane receptor, hepatic nuclear factor 4 alpha, and P450 oxidoreductase genes determines interindividual variability in basal expression and activity of a broad scope of xenobiotic metabolism genes in the human liver. Drug Metab Dispos 35:1700–1710 [DOI] [PubMed] [Google Scholar]
- Wright SR, Boag AH, Valdimarsson G, Hipfner DR, Campling BG, Cole SP, Deeley RG. (1998) Immunohistochemical detection of multidrug resistance protein in human lung cancer and normal lung. Clin Cancer Res 4:2279–2289 [PubMed] [Google Scholar]
- Wu SP, Shyu MK, Liou HH, Gau CS, Lin CJ. (2004) Interaction between anticonvulsants and human placental carnitine transporter. Epilepsia 45:204–210 [DOI] [PubMed] [Google Scholar]
- Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, Ganapathy V. (2000a) Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta 1466:315–327 [DOI] [PubMed] [Google Scholar]
- Wu X, Huang W, Ganapathy ME, Wang H, Kekuda R, Conway SJ, Leibach FH, Ganapathy V. (2000b) Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. Am J Physiol Renal Physiol 279:F449–F458 [DOI] [PubMed] [Google Scholar]
- Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. (1999) Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J Pharmacol Exp Ther 290:1482–1492 [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. (1998) Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem 273:32776–32786 [DOI] [PubMed] [Google Scholar]
- Wultsch T, Grimberg G, Schmitt A, Painsipp E, Wetzstein H, Breitenkamp AF, Gründemann D, Schömig E, Lesch KP, Gerlach M, et al. (2009) Decreased anxiety in mice lacking the organic cation transporter 3. J Neural Transm 116:689–697 [DOI] [PubMed] [Google Scholar]
- Xia L, Engel K, Zhou M, Wang J. (2007) Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Renal Physiol 292:F682–F690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Roundtree M, Merikhi A, Lu X, Shentu S, Lesage G. (2004) Degradation of the apical sodium-dependent bile acid transporter by the ubiquitin-proteasome pathway in cholangiocytes. J Biol Chem 279:44931–44937 [DOI] [PubMed] [Google Scholar]
- Xiang J, Hu Y, Smith DE, Keep RF. (2006) PEPT2-mediated transport of 5-aminolevulinic acid and carnosine in astrocytes. Brain Res 1122:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Han Y, Neuvonen M, Pasanen MK, Kalliokoski A, Backman JT, Laitila J, Neuvonen PJ, Niemi M. (2009) Effect of SLCO1B1 polymorphism on the plasma concentrations of bile acids and bile acid synthesis marker in humans. Pharmacogenet Genomics 19:447–457 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Nieves E, Angeletti RH, Orr GA, Wolkoff AW. (2006) Rat organic anion transporting protein 1A1 (Oatp1a1): purification and phosphopeptide assignment. Biochemistry 45:3357–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaofei E, Wada Y, Dakeishi M, Hirasawa F, Murata K, Masuda H, Sugiyama T, Nikaido H, Koizumi A. (2002) Age-associated cardiomyopathy in heterozygous carrier mice of a pathological mutation of carnitine transporter gene, OCTN2. J Gerontol A Biol Sci Med Sci 57:B270–B278 [DOI] [PubMed] [Google Scholar]
- Xiong H, Callaghan D, Jones A, Bai J, Rasquinha I, Smith C, Pei K, Walker D, Lue LF, Stanimirovic D, et al. (2009) ABCG2 is upregulated in Alzheimer's brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1–40) peptides. J Neurosci 29:5463–5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Yoshinari K, Brouwer KL, Negishi M. (2002) Role of constitutive androstane receptor in the in vivo induction of Mrp3 and CYP2B1/2 by phenobarbital. Drug Metab Dispos 30:918–923 [DOI] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. (2005) Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res 28:249–268 [DOI] [PubMed] [Google Scholar]
- Xu J, Liu Y, Yang Y, Bates S, Zhang JT. (2004) Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem 279:19781–19789 [DOI] [PubMed] [Google Scholar]
- Yabuuchi H, Takayanagi S, Yoshinaga K, Taniguchi N, Aburatani H, Ishikawa T. (2002) ABCC13, an unusual truncated ABC transporter, is highly expressed in fetal human liver. Biochem Biophys Res Commun 299:410–417 [DOI] [PubMed] [Google Scholar]
- Yabuuchi H, Tamai I, Nezu J, Sakamoto K, Oku A, Shimane M, Sai Y, Tsuji A. (1999) Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther 289:768–773 [PubMed] [Google Scholar]
- Yakushiji K, Kai S, Yamauchi M, Kuwajima M, Osada Y, Toshimori K. (2006) Expression and distribution of OCTN2 in mouse epididymis and its association with obstructive azoospermia in juvenile visceral steatosis mice. Int J Urol 13:420–426 [DOI] [PubMed] [Google Scholar]
- Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. (2007) Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metab Dispos 35:1142–1148 [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Ieiri I, Kusuhara H, Sasaki T, Kimura M, Tabuchi H, Ando Y, Irie S, Ware J, Nakai Y, et al. (2008) Pharmacogenetic characterization of sulfasalazine disposition based on NAT2 and ABCG2 (BCRP) gene polymorphisms in humans. Clin Pharmacol Ther 84:95–103 [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Akiyama S, Ni'inuma K, Nishigaki R, Sugiyama Y. (1997) Biliary excretion of pravastatin in rats: contribution of the excretion pathway mediated by canalicular multispecific organic anion transporter. Drug Metab Dispos 25:1123–1129 [PubMed] [Google Scholar]
- Yamazaki M, Li B, Louie SW, Pudvah NT, Stocco R, Wong W, Abramovitz M, Demartis A, Laufer R, Hochman JH, et al. (2005) Effects of fibrates on human organic anion-transporting polypeptide 1B1-, multidrug resistance protein 2- and P-glycoprotein-mediated transport. Xenobiotica 35:737–753 [DOI] [PubMed] [Google Scholar]
- Yao SY, Ng AM, Muzyka WR, Griffiths M, Cass CE, Baldwin SA, Young JD. (1997) Molecular cloning and functional characterization of nitrobenzylthioinosine (NBMPR)-sensitive (es) and NBMPR-insensitive (ei) equilibrative nucleoside transporter proteins (rENT1 and rENT2) from rat tissues. J Biol Chem 272:28423–28430 [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Thomas S, Kerwin R, Morgan PE, Lightman S, Seckl JR, Pariante CM. (2007) The antidepressant desipramine requires the ABCB1 (Mdr1)-type p-glycoprotein to upregulate the glucocorticoid receptor in mice. Neuropsychopharmacology 32:2520–2529 [DOI] [PubMed] [Google Scholar]
- Yeboah D, Kalabis GM, Sun M, Ou RC, Matthews SG, Gibb W. (2008) Expression and localisation of breast cancer resistance protein (BCRP) in human fetal membranes and decidua and the influence of labour at term. Reprod Fertil Dev 20:328–334 [DOI] [PubMed] [Google Scholar]
- Yee SW, Shima JE, Hesselson S, Nguyen L, De Val S, Lafond RJ, Kawamoto M, Johns SJ, Stryke D, Kwok PY, et al. (2009) Identification and characterization of proximal promoter polymorphisms in the human concentrative nucleoside transporter 2 (SLC28A2). J Pharmacol Exp Ther 328:699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SY, Hong KS, Lim HS, Chung JY, Oh DS, Kim JR, Jung HR, Cho JY, Yu KS, Jang IJ, et al. (2004) A variant 2677A allele of the MDR1 gene affects fexofenadine disposition. Clin Pharmacol Ther 76:418–427 [DOI] [PubMed] [Google Scholar]
- Yokogawa K, Yonekawa M, Tamai I, Ohashi R, Tatsumi Y, Higashi Y, Nomura M, Hashimoto N, Nikaido H, Hayakawa J, et al. (1999) Loss of wild-type carrier-mediated l-carnitine transport activity in hepatocytes of juvenile visceral steatosis mice. Hepatology 30:997–1001 [DOI] [PubMed] [Google Scholar]
- Yokoo S, Masuda S, Yonezawa A, Terada T, Katsura T, Inui K. (2008) Significance of organic cation transporter 3 (SLC22A3) expression for the cytotoxic effect of oxaliplatin in colorectal cancer. Drug Metab Dispos 36:2299–2306 [DOI] [PubMed] [Google Scholar]
- Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. (2007) Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol 74:477–487 [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. (2006) Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1–3 and multidrug and toxin extrusion family). J Pharmacol Exp Ther 319:879–886 [DOI] [PubMed] [Google Scholar]
- Yoshiura K, Kinoshita A, Ishida T, Ninokata A, Ishikawa T, Kaname T, Bannai M, Tokunaga K, Sonoda S, Komaki R, et al. (2006) A SNP in the ABCC11 gene is the determinant of human earwax type. Nat Genet 38:324–330 [DOI] [PubMed] [Google Scholar]
- You G, Kuze K, Kohanski RA, Amsler K, Henderson S. (2000) Regulation of mOAT-mediated organic anion transport by okadaic acid and protein kinase C in LLC-PK(1) cells. J Biol Chem 275:10278–10284 [DOI] [PubMed] [Google Scholar]
- Young JD, Yao SY, Sun L, Cass CE, Baldwin SA. (2008) Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins. Xenobiotica 38:995–1021 [DOI] [PubMed] [Google Scholar]
- Youngblood GL, Sweet DH. (2004) Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol 287:F236–F244 [DOI] [PubMed] [Google Scholar]
- Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. (2002) Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A 99:16237–16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. (2004) Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J Lipid Res 45:301–307 [DOI] [PubMed] [Google Scholar]
- Yu L, von Bergmann K, Lütjohann D, Hobbs HH, Cohen JC. (2005) Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J Lipid Res 46:1739–1744 [DOI] [PubMed] [Google Scholar]
- Zaher H, Khan AA, Palandra J, Brayman TG, Yu L, Ware JA. (2006) Breast cancer resistance protein (Bcrp/abcg2) is a major determinant of sulfasalazine absorption and elimination in the mouse. Mol Pharm 3:55–61 [DOI] [PubMed] [Google Scholar]
- Zaher H, zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, Palandra J, Stock JL, Kim RB, Ware JA. (2008) Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol 74:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. (2005a) Handling of cysteine S-conjugates of methylmercury in MDCK cells expressing human OAT1. Kidney Int 68:1684–1699 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. (2005b) Handling of the homocysteine S-conjugate of methylmercury by renal epithelial cells: role of organic anion transporter 1 and amino acid transporters. J Pharmacol Exp Ther 315:896–904 [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. (2005c) Transport of N-acetylcysteine S-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J Pharmacol Exp Ther 314:1158–1168 [DOI] [PubMed] [Google Scholar]
- Zaman GJ, Cnubben NH, van Bladeren PJ, Evers R, Borst P. (1996) Transport of the glutathione conjugate of ethacrynic acid by the human multidrug resistance protein MRP. FEBS Lett 391:126–130 [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Nezasa K, Tian X, Bridges AS, Lee K, Belinsky MG, Kruh GD, Brouwer KL. (2006) Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3−/− and Abcc4−/− mice. J Pharmacol Exp Ther 319:1485–1491 [DOI] [PubMed] [Google Scholar]
- Zegers MM, Hoekstra D. (1998) Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells. Biochem J 336:257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. (2003a) Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4). Biochem J 371:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Saeki T, Bot I, Kuil A, Borst P. (2003b) Transport of bile acids in multidrug-resistance-protein 3-overexpressing cells co-transfected with the ileal Na+-dependent bile-acid transporter. Biochem J 369:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, van de Wetering K, de Waart R, Scheffer GL, Marschall HU, Wielinga PR, Kuil A, Kunne C, Smith A, van der Valk M, et al. (2006) Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol 44:768–775 [DOI] [PubMed] [Google Scholar]
- Zelcer N, van de Wetering K, Hillebrand M, Sarton E, Kuil A, Wielinga PR, Tephly T, Dahan A, Beijnen JH, Borst P. (2005) Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc Natl Acad Sci U S A 102:7274–7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Bain LJ, Belinsky MG, Kruh GD. (1999) Expression of multidrug resistance protein-3 (multispecific organic anion transporter-D) in human embryonic kidney 293 cells confers resistance to anticancer agents. Cancer Res 59:5964–5967 [PubMed] [Google Scholar]
- Zeng H, Chen ZS, Belinsky MG, Rea PA, Kruh GD. (2001) Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: effect of polyglutamylation on MTX transport. Cancer Res 61:7225–7232 [PubMed] [Google Scholar]
- Zeng H, Liu G, Rea PA, Kruh GD. (2000) Transport of amphipathic anions by human multidrug resistance protein 3. Cancer Res 60:4779–4784 [PubMed] [Google Scholar]
- Zhang EY, Emerick RM, Pak YA, Wrighton SA, Hillgren KM. (2004a) Comparison of human and monkey peptide transporters: PEPT1 and PEPT2. Mol Pharm 1:201–210 [DOI] [PubMed] [Google Scholar]
- Zhang EY, Fu DJ, Pak YA, Stewart T, Mukhopadhyay N, Wrighton SA, Hillgren KM. (2004b) Genetic polymorphisms in human proton-dependent dipeptide transporter PEPT1: implications for the functional role of Pro586. J Pharmacol Exp Ther 310:437–445 [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Qatanani M, Evans RM, Moore DD. (2004c) The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem 279:49517–49522 [DOI] [PubMed] [Google Scholar]
- Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. (1997) Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol 51:913–921 [DOI] [PubMed] [Google Scholar]
- Zhang P, Tian X, Chandra P, Brouwer KL. (2005) Role of glycosylation in trafficking of Mrp2 in sandwich-cultured rat hepatocytes. Mol Pharmacol 67:1334–1341 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. (2008a) Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem 283:32570–32579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, et al. (2006a) Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res 66:8847–8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yu BN, He YJ, Fan L, Li Q, Liu ZQ, Wang A, Liu YL, Tan ZR, Fen-Jiang, et al. (2006b) Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta 373:99–103 [DOI] [PubMed] [Google Scholar]
- Zhang WX, Chen B, Jin Z, Yu Z, Wang X, Chen H, Mao A, Cai W. (2008b) Influence of uridine diphosphate (UDP)-glucuronosyltransferases and ABCC2 genetic polymorphisms on the pharmacokinetics of mycophenolic acid and its metabolites in Chinese renal transplant recipients. Xenobiotica 38:1422–1436 [DOI] [PubMed] [Google Scholar]
- Zhang X, Cherrington NJ, Wright SH. (2007a) Molecular identification and functional characterization of rabbit MATE1 and MATE2-K. Am J Physiol Renal Physiol 293:F360–F370 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li W, Vore M. (2007b) Translational regulation of rat multidrug resistance-associated protein 2 expression is mediated by upstream open reading frames in the 5′ untranslated region. Mol Pharmacol 71:377–383 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuetz JD, Elmquist WF, Miller DW. (2004d) Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther 311:449–455 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Unadkat JD, Mao Q. (2007c) Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab Dispos 35:2154–2158 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao T, Li W, Vore M. (2009) The 5′-untranslated region of multidrug resistance associated protein 2 (MRP2; ABCC2) regulates downstream open reading frame expression through translational regulation. Mol Pharmacol doi: 10.1124/mol.109.058982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu JY, Hait WN, Yang JM. (2004e) Regulation of the stability of P-glycoprotein by ubiquitination. Mol Pharmacol 66:395–403 [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Saito T, Kimura Y, Sugimoto C, Ohtsubo T, Saito H. (2000) Disruption of mdr1a p-glycoprotein gene results in dysfunction of blood-inner ear barrier in mice. Brain Res 852:116–126 [DOI] [PubMed] [Google Scholar]
- Zhou F, Hong M, You G. (2007a) Regulation of human organic anion transporter 4 by progesterone and protein kinase C in human placental BeWo cells. Am J Physiol Endocrinol Metab 293:E57–E61 [DOI] [PubMed] [Google Scholar]
- Zhou F, Illsley NP, You G. (2006) Functional characterization of a human organic anion transporter hOAT4 in placental BeWo cells. Eur J Pharm Sci 27:518–523 [DOI] [PubMed] [Google Scholar]
- Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, You G. (2005) The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol 67:868–876 [DOI] [PubMed] [Google Scholar]
- Zhou F, Xu W, Tanaka K, You G. (2008a) Comparison of the interaction of human organic anion transporter hOAT4 with PDZ proteins between kidney cells and placental cells. Pharm Res 25:475–480 [DOI] [PubMed] [Google Scholar]
- Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. (2008b) The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol 73:949–959 [DOI] [PubMed] [Google Scholar]
- Zhou M, Xia L, Engel K, Wang J. (2007b) Molecular determinants of substrate selectivity of a novel organic cation transporter (PMAT) in the SLC29 family. J Biol Chem 282:3188–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. (2002) Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A 99:12339–12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7:1028–1034 [DOI] [PubMed] [Google Scholar]
- Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. (2008c) Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem 15:1981–2039 [DOI] [PubMed] [Google Scholar]
- Zhou X, Thamotharan M, Gangopadhyay A, Serdikoff C, Adibi SA. (2000) Characterization of an oligopeptide transporter in renal lysosomes. Biochim Biophys Acta 1466:372–378 [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Gutmann H, Hruz P, Gutzwiller JP, Beglinger C, Drewe J. (2005) Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab Dispos 33:219–224 [DOI] [PubMed] [Google Scholar]
- Zinchuk V, Zinchuk O, Okada T. (2005) Experimental LPS-induced cholestasis alters subcellular distribution and affects colocalization of Mrp2 and Bsep proteins: a quantitative colocalization study. Microsc Res Tech 67:65–70 [DOI] [PubMed] [Google Scholar]
- Zinchuk VS, Okada T, Akimaru K, Seguchi H. (2002) Asynchronous expression and colocalization of Bsep and Mrp2 during development of rat liver. Am J Physiol Gastrointest Liver Physiol 282:G540–G548 [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H, et al. (2003) Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol 39:480–488 [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Silbert D, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H, Trauner M. (2002) Induction of short heterodimer partner 1 precedes downregulation of Ntcp in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol 282:G184–G191 [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Fickert P, Geier A, Fuchsbichler A, Silbert D, Gumhold J, Zatloukal K, Kaser A, Tilg H, et al. (2005) Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am J Physiol Gastrointest Liver Physiol 289:G798–G805 [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, et al. (2006) Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol 290:G923–G932 [DOI] [PubMed] [Google Scholar]