Abstract
Phospholipid transfer protein (PLTP) plays an important role in atherogenesis, and its function goes well beyond that of transferring phospholipids between lipoprotein particles. Previous studies showed that genetic deficiency of PLTP in mice causes a substantially impaired hepatic secretion of apolipoprotein-B (apoB), the major protein of atherogenic lipoproteins. To understand whether the impaired apoB secretion is a direct result from lack of PLTP activity, in this study, we further investigated the function of PLTP in apoB secretion by using PLTP inhibitors. We identified a series of compounds containing a 3-benzazepine core structure that inhibit PLTP activity. Compound A, the most potent inhibitor, was characterized further and had little cross-reactivity with microsomal triglyceride transfer protein. Compound A reduced apoB secretion in human hepatoma cell lines and mouse primary hepatocytes. Furthermore, we confirmed that the reduction of apoB secretion mediated by compound A is PLTP-dependent, because the PLTP inhibitor had no effect on apoB secretion from PLTP-deficient hepatocytes. These studies provided evidence that PLTP activity regulates apoB secretion and pharmacologic inhibition of PLTP may be a new therapy for dyslipidemia by reducing apoB secretion.
Accumulating evidence has shown that phospholipid transfer protein (PLTP) plays an important role in the metabolism of lipoproteins (Tall and Lalanne, 2003). PLTP belongs to the family of lipid transfer/lipopolysaccharide-binding proteins, including cholesteryl ester transfer protein (CETP), lipopolysaccharide-binding protein, and bactericidal permeability increasing protein (Tollefson et al., 1988; Day et al., 1994). It has been shown that PLTP facilitates the transfer and exchange of phospholipids between VLDL and HDL (Tall et al., 1985). It also transfers phospholipids between HDL particles, resulting in the conversion of HDL3 into larger and smaller HDL particles (Jauhiainen et al., 1993; Huuskonen et al., 2000). PLTP can also bind several other amphipathic molecules, including α-tocopherol, diacylglycerides, cerebrosides, and lipopolysaccharides (Lagrost et al., 1998). Several clinical studies suggest that high plasma PLTP activity is a risk factor for coronary artery disease and a determinant of carotid intima-media thickness in type 2 diabetes mellitus (Schlitt et al., 2003; de Vries et al., 2006).
Studies using genetically modified mice strongly suggest that PLTP functions as a proatherogenic factor (Jiang et al., 2001; van Haperen et al., 2002; Yang et al., 2003). PLTP has complex effects on lipoprotein metabolism and atherogenesis. PLTP deficiency causes a marked decrease in HDL lipids and apoA-I, due to higher catabolism, in both chow and 2-week Western diet-fed mice (Jiang et al., 1999; Kawano et al., 2002). Deletion of PLTP in hyperlipidemic apoE-deficient and human apoB transgenic mouse strains results in reduced LDL, attributable to decreased production of apoB, which was measured by Triton WR1339 injection (Jiang et al., 2001). Atherosclerotic lesion areas are also decreased in PLTP knockout mice in LDL receptor or apoE-deficient or apoB transgenic background, despite decreased HDL (Jiang et al., 2001). The proatherogenic role of PLTP is further supported by results from PLTP transgenic approaches. It has been reported that overexpression of PLTP in hyperlipidemic mouse models increased susceptibility to atherosclerosis, mainly due to reduced HDL (van Haperen et al., 2002, 2008; Yang et al., 2003; Samyn et al., 2008). Elevation of PLTP in adult mice increased VLDL and reduced HDL, leading to aggravation of preexisting atherosclerotic lesions (Moerland et al., 2008).
In addition to its function in the circulation, intracellular PLTP has been shown to regulate apoB-containing lipoprotein secretion in murine hepatocytes (Jiang et al., 2001). PLTP deficiency reduces apoB secretion from mouse primary hepatocytes; however, the mechanism remains unknown. Because the previous studies were carried out using hepatocytes isolated from PLTP-deficient mice, it is not known whether it is PLTP activity or PLTP protein per se that plays a role in apoB secretion. To address these questions, in this study we developed compounds that inhibit phospholipid transfer activity of PLTP. We found that inhibition of PLTP activity reduces the secretion of apoB from human hepatoma cells and mouse primary hepatocytes. Moreover, the PLTP inhibitor had no effect on apoB secretion from PLTP-deficient hepatocytes. These studies support the hypothesis that PLTP-mediated phospholipid transfer activity regulates apoB-containing lipoprotein secretion.
Materials and Methods
Materials
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC; 25 mg/ml) was from Avanti Polar Lipids (Alabaster, AL). BODIPY fluorescently labeled PC (BODIPY-PC; β-BODIPY 500/510 C12-HPC) was from Invitrogen (Carlsbad, CA). Human apoA-I (10 mg/ml) was from Biodesign International (Kennebunk, ME). Fluorescent quencher QSY9 carboxylic acid, succinimidyl ester was from Invitrogen. [3H]Phosphatidylcholine (l-α-dipalmitoyl [choline-methyl-3H]) was from PerkinElmer Life and Analytical Sciences, Boston, MA).
Generation of Recombinant Human PLTP Protein
DNA fragments containing full-length PLTP coding sequence were obtained by reverse transcription-polymerase chain reaction from human liver cDNA (Day et al., 1994; Jauhiainen et al., 1999) and cloned into pCDNA3.1 (Invitrogen) to express PLTP protein in C cells. Secreted recombinant PLTP proteins were purified as described previously (Jauhiainen et al., 1999).
High-Throughput Fluorescent Label-Based PLTP Activity Assay
Preparation of 12X Donor and 20X Acceptor Vesicles.
Quenched donor vesicles (molar ratio BODIPY-PC/PC, 1:25 and molar ratio apoA-I/PC, 1:264) contain POPC and BODIPY-PC and human apoA-I derivatized with amino-reactive fluorescent quencher QSY9 carboxylic acid. Unlabeled donor vesicles are made and used for quantitation. Acceptor vesicles (molar ratio apoA-I/PC, 1:100) contain unlabeled apoA-I and POPC. Human apoA-I was dialyzed (75 mg) in dialysis buffer (PBS containing 1 mM EDTA and 0.1% sodium azide, pH 7.4) to remove guanidine HCl and dithiothreitol. Protein concentration was determined after dialysis using bicinchoninic acid protein assay (Pierce Chemical, Rockford, IL). POPC (for acceptor) or mixed POPC and BODIPY-PC (for donor, 1:25 M ratio) dissolved in chloroform were dried down under a stream of nitrogen. Dried lipids were dissolved in PBS containing 16.5 mg/ml cholate. Lipids were vigorously vortexed and then sonicated in a bath sonicator until the solution was uniform. Dissolved lipids were incubated for 2 h at 4°C with gentle shaking until solutions become clear. The appropriate amount of apoA-I was added to each lipid solution with gentle stirring. The solutions were incubated at 4°C with gentle shaking for at least 16 h and were dialyzed in PBS buffer. After dialysis, the BODIPY-PC-labeled donors were derivatized with QSY9 quencher by dissolving 10 mg of QSY9 in 500 μl of water. For 3-ml donor solution, a 560-μl volume of 1 M carbonate, pH 8.75 (filtered), was added to the labeled donor solution. Then, 340 μl of QSY9 solution was added slowly with stirring to donor vesicles. The solution was incubated for 1 h at room temperature with continuous stirring. The reaction was stopped with 320 μl of 2 M Tris, pH 8.5. Unreacted dye was separated from donor vesicles by running 650-μl aliquots on an FPLC by using a Superdex 200 10/300 GL column (GE Healthcare, Little Chalfont, Buckinghamshire, UK) pre-equilibrated with column buffer (10 mM Tris, pH 8.0, 154 mM NaCl, 1 mM EDTA, and 0.1% sodium azide) at 4°C. Fractions (0.4 ml) then were collected, and the peak fractions, determined by measuring protein and phospholipid levels, were pooled.
Activity Assay.
The assay was modified based on the method described by Rao et al. (1997). We added 10 μl of Acceptor (75 μM final), 10 μl of prediluted recombinant PLTP (10 ng), and 1 μl of compounds to each well of a 384-well assay plate followed by addition of 10 μl of BODIPY-PC containing donor vesicles (10 μM final; (total volume, 30 μl). The plates were incubated at room temperature for 45 min. Labeled phospholipids were transferred by PLTP to unlabeled acceptors. Unquenching of BODIPY fluorescent signal upon transfer to acceptors generated a fluorescent signal. The plates were read on Analyst GT plate reader (Molecular Devices, Sunnyvale, CA) at an excitation of 580 nm and an emission of 620 nm. Reaction without PLTP protein was used as the baseline control for calculation of PLTP activity. A quenching assay using vesicles containing PC and BODIPY-PC was used to assess the fluorescent quenching activity of compounds. One microliter each of compounds and vesicles were incubated for 45 min and read at 530 nm.
Radioactive Label-Based PLTP Activity Assay
PLTP activity was measured as described previously with the following modifications (Jiang et al., 1999): Egg PC (5000 nmol; Sigma-Aldrich, St. Louis, MO) containing 10 μCi of [3H]PC and 20 nmol of butyrated hydroxytoluene (Sigma-Aldrich) in chloroform was dried under a stream of nitrogen and then resuspended in 1 ml of a solution of 10 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.02% sodium azide, pH 7.4. The solution was probe-sonicated until clear and centrifuged at 10,000 rpm to remove debris. Transfer of radiolabeled phospholipid was measured by incubating 0.25 μl (20 ng) of purified recombinant PLTP protein with radiolabeled phospholipid vesicles (20 μg of phospholipid) and HDL3 (20 μg of phospholipid), in the presence of 1% DMSO or compounds in assay buffer [10 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.02% NaN3, and 0.1% bovine serum albumin (BSA), pH 7.4] to a final volume of 100 μl in a conical-bottomed 96-well polypropylene plate at room temperature for 15 min. Vesicles were subsequently precipitated by the addition of 75 μl of a solution of 500 mM NaCl, 215 mM MnCl2, and 450 U/ml heparin; vortexed on a plate shaker; and allowed to sit at room temperature for 10 min before being centrifuged at 3000 rpm for 20 min to pellet the precipitate. A 50-μl aliquot of the supernatant was transferred to an isoplate (PerkinElmer Wallac, Gaithersburg, MD) and 100 μl of Optiphase Supermix scintillation fluid (PerkinElmer Wallac) was added. The plate was shaken on a plate shaker until uniformly opaque then radioactivity was counted on a Microbeta scintillation counter (PerkinElmer Wallac). Nonspecific transfer wells (−PLTP) were included for background subtraction. Transfer rate was calculated as [[(total dpm − background dpm) × 3.5]/specific activity (dpm/nmol)/assay time (hours)].
MTP Activity Assay
MTP activity was measured as described previously (Chandler et al., 2003), with minor modification. Human microsomes purchased from Sigma-Aldrich were extracted as described to obtain soluble MTP protein (Haghpassand et al., 1996). Solubilized MTP protein was dialyzed and used as the source for MTP activity. Donor and acceptor liposomes were prepared as described previously (Haghpassand et al., 1996). Donor liposomes were prepared by bath sonication of a mixture containing 447 μM egg phosphatidylcholine, 83 μM bovine heart cardiolipin, and 0.91 μM [14C]triolein (110 Ci/mol). Acceptor liposomes were prepared by bath sonication of a dispersion containing 1.3 mM egg phosphatidylcholine, 2.6 μM triolein, and 0.5 nM [3H]egg phosphatidylcholine in assay buffer. The donor and acceptor liposomes were centrifuged at 160,000g for 2 h at 7°C. MTP activity was determined by adding 200 μl of buffer containing 5% BSA with either DMSO alone or compounds to a mixture containing 50 μl of donor liposomes, 100 μl of acceptor liposomes and 150 μl of MTP protein. After incubation at 37°C for 45 min, triglyceride transfer was terminated by addition of 300 μl of a 50% (wt/v) DEAE-cellulose suspension in assay buffer. After thorough mixing, the donor liposomes, bound to DEAE-cellulose, were selectively precipitated by centrifugation at 3000g for 5 min. An aliquot of the supernatant containing the acceptor liposomes was collected to determine 14C and 3H counts. The obtained radioactivities were used to calculate the percentage of triglyceride transfer using first-order kinetics.
PLTP Gene Expression
Human liver and primary hepatocyte RNA samples were obtained from Invitrogen. RNA was isolated from HepG2 and Huh7 cells. PLTP gene expression was analyzed by TaqMan as described previously (Shelly et al., 2008).
ApoB and Triglyceride Secretion
HepG2 or Huh 7 cells were maintained in DMEM with 10% fetal bovine serum. Cells (200,000) were seeded and cultured for 24 h in a 24-well plate. Cells were then treated with DMEM containing 1% BSA and 0.4 mM oleic acid in the presence or absence of compounds for 24 h. The compounds were dissolved in DMSO and added in culture medium at a final concentration of 0.5% DMSO. Vehicle control contains 0.5% DMSO. Secreted apoB and transferrin were measured by ELISA. ApoB ELISA was performed as described previously (Chandler et al., 2003). Transferrin levels were measured as suggested by the manufacturer (Bethyl Laboratories, Montgomery, TX). Triglyceride levels in medium were measured using L-Type Triglyceride M assay with 180 μl of Color A (Wako Diagnostics, Richmond, VA), 60 μl of Color B (Wako Diagnostics), and the Multi-Calibrator Lipid standard (Wako Diagnostics). Then, 100 μl of medium was used for the assay. Absorbance was measured at 600 nm, with a reference wavelength of 700 nm.
ApoB Secretion in Mouse Primary Hepatocytes
Hepatocytes were incubated for 1 h in methionine/cysteine-free DMEM supplemented with 0.2 mM oleic acid/BSA. Then, 100 μCi of [35S]methionine/cysteine was added, and the cells were incubated in the presence of DMSO or compound for 4 h and medium was collected. Cells were then washed once with DMEM and twice with PBS. ApoB in the medium and cell lysate was then immunoprecipitated by an antibody against apoB (Abcam Inc., Cambridge, MA) plus protein A/G-agarose, and precipitates were analyzed by 4% SDS-polyacrylamide gel electrophoresis. Incorporation of [35S]methionine/cysteine into apoB48 and apoB100 was assessed with a bio-imaging analyzer (Fujifilm, Tokyo, Japan).
Results
Identification and Characterization of PLTP Inhibitors.
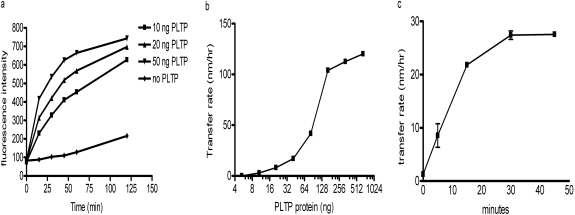
A high-throughput fluorescence-labeled phospholipid transfer activity assay was developed based on the methods described by Rao et al. (1997). Under the conditions of donor/acceptor ratio of 1:7.5, recombinant PLTP dose-dependently increased the transfer of labeled BODIPY-PC from quenched donor to acceptor and led to increased fluorescence (Fig. 1a). Non-PLTP-dependent phospholipid transfer is negligible during the 1st h of incubation at room temperature. By using this assay under conditions described under Materials and Methods, we screened the Pfizer compound collection of approximately 2 million compounds, and 4500 compounds were identified that reduced the fluorescence signal. A fluorescence quenching assay was performed to filter out the compounds that reduce fluorescence intensity by quenching the fluorescence signal rather than inhibiting PLTP activity, resulting in confirmation of 50 compounds as PLTP inhibitors.
Fig. 1.
Optimization of PLTP activity assay conditions. a, characterization of fluorescence-based phospholipid transfer activity assay. Various amounts of purified recombinant PLTP were incubated with donor and acceptor vesicles at room temperature as described under Materials and Methods. Reactions without PLTP enzyme were used as controls for baseline nonspecific PC transfer. Fluorescence intensity was measured at indicated time points in triplicate. b, dose-response characterization of radioactive-labeled phospholipid transfer assay. Various amounts of purified recombinant PLTP were incubated with donor and acceptor vesicles at room temperature for 15 min. The assay was performed, and the transfer rate was calculated as described under Materials and Methods. c, time course characterization of radioactive-labeled phospholipid transfer assay. We incubated 100 ng of PLTP protein with donor and acceptor vesicles at room temperature for the indicated times. The assay was performed and the transfer rate was calculated as described under Materials and Methods.
To avoid the complication of compound influence on fluorescence signal, a secondary lower throughput radioactive label based PLTP activity assay was also developed to further characterize the hits obtained from high-throughput screening. In this assay, PLTP transfers [3H]phosphatidylcholine from donor liposomes to HDL3 acceptors. Donor vesicles were removed by precipitation, and the radioactive signal in the supernatant containing the acceptors was counted. Under the assay conditions described under Materials and Methods, PLTP dose-dependently increased phospholipid transfer and reached saturation with approximately 128 ng of PLTP/reaction (Fig. 1b). In a time course experiment, a linear transfer rate was obtained during the 15-min incubation at room temperature (Fig. 1c). An activity assay condition using 100 ng of PLTP protein/reaction and 15-min incubation time was developed to test the activity of the compounds.
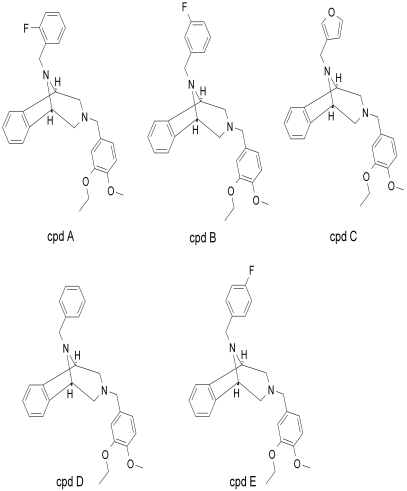
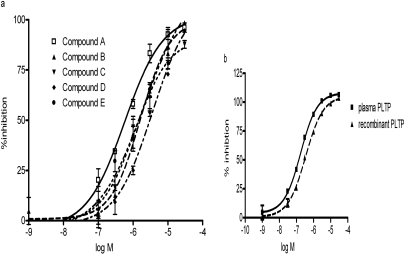
Five compounds that contain 3-benzazepine core structure were identified as potent inhibitors of PLTP activity (Fig. 2). All of these compounds inhibited the phospholipid transfer activity of recombinant PLTP in a dose-dependent manner (Fig. 3a). The IC50 values of the compounds are summarized in Table 1. Compound A, which is the most potent inhibitor, was chosen for further characterization and biological functional studies. To confirm that the compound can inhibit the activity of native PLTP, we compared the inhibitory activity of compound A on recombinant human PLTP to its activity on human plasma PLTP. Compound A showed similar potency in inhibiting plasma PLTP and recombinant PLTP (Fig. 3b).
Fig. 2.
Structures of 3-benzazepine compounds that inhibit PLTP activity.
Fig. 3.
Inhibition of human PLTP mediated phospholipid transfer activity by test compounds. a, phospholipid transfer activity of human recombinant PLTP was measured in the presence of 0 to 30 μM compounds as described under Materials and Methods. Reactions excluding PLTP protein were used as baseline control. The data are expressed as percentage of inhibition of phospholipid transfer rate. b, inhibition of recombinant PLTP and human plasma PLTP by compound A. The phospholipid transfer activity was measured in the presence of 0 to 30 μM compound A as described under Materials and Methods. We used 10 ng of recombinant PLTP protein or 3 μl of fresh human plasma as the enzyme source. The data are expressed as percentage of inhibition of phospholipid transfer rate. Reactions without enzyme were used as a complete inhibition control in the calculation.
TABLE 1.
Inhibition of PLTP activity by PLTP inhibitors
| Compound | PLTP Activity, IC50 |
|---|---|
| μM | |
| A | 0.6 |
| B | 1.6 |
| C | 1.2 |
| D | 3.6 |
| E | 2.0 |
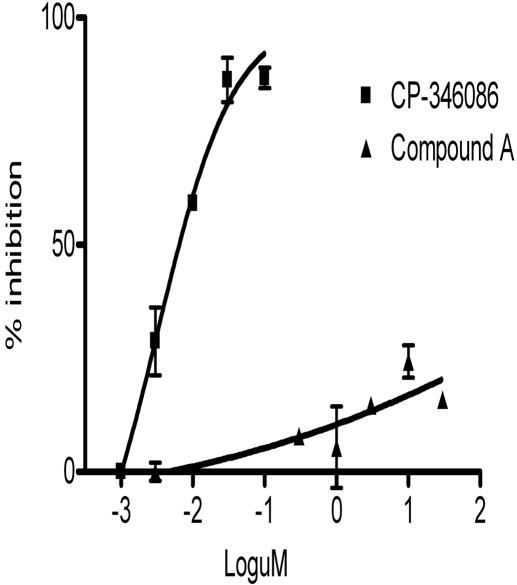
Furthermore, we analyzed the selectivity of the compounds against other lipid transfer protein activities that are involved in lipoprotein metabolism. A close homolog of PLTP is CETP, which transfers cholesterol ester from HDL to VLDL (Tall, 1995). Compound A does not inhibit cholesterol ester transfer activity at a concentration of 30 μM (Supplemental Fig. 1). Microsomal triglyceride transfer protein (MTP) is an important intracellular lipid transfer protein that regulates apoB secretion (Jamil et al., 1996; Athar et al., 2004, Rava et al., 2005). Compound A had little effect on MTP triglyceride transfer activity, with an IC50 value >1000 μM (Fig. 4). CP-346086, an MTP inhibitor with an IC50 value of 3 nM, was used as a positive control in the MTP activity assay (Chandler et al., 2003).
Fig. 4.
Inhibition of human MTP-mediated triglyceride transfer activity by compound A. Triglyceride transfer activity of human MTP was measured in the presence of 0 to 30 μM compound A and MTP inhibitor CP-346086 as described under Materials and Methods. Reactions without enzyme were used as a complete inhibition control in the calculation. The data are expressed as percentage of inhibition of transfer rate.
Effects of PLTP Inhibitors on ApoB Secretion in Human Hepatoma Cells.
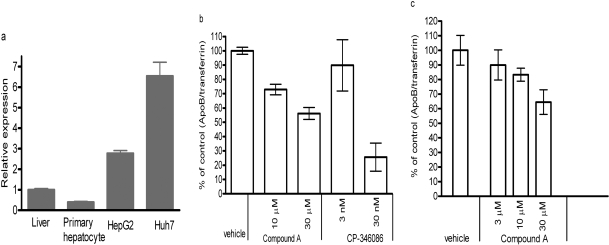
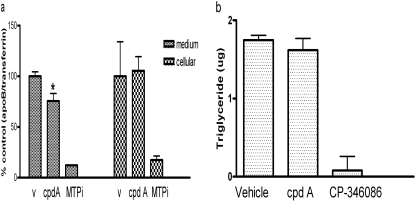
PLTP deficiency in mice reduces apoB secretion from hepatocytes by up to 50% and leads to lowering of plasma LDL and apoB levels without causing fatty liver (Jiang et al., 2001). The PLTP gene is highly expressed in human hepatoma cell lines HepG2 and Huh7 (Fig. 5a). The relative expression levels in cell lines are higher than that in liver and primary hepatocytes. We analyzed the effects of compound A on apoB secretion in Huh7 and HepG2 cells (Fig. 5, b and c). The compound had no overt toxicity when tested in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium cytotoxicity assay (data not shown). HepG2 and Huh7 showed a similar response to compound treatment. The secretion of apoB was normalized to the secreted transferrin levels to eliminate any compound-mediated effects on protein secretion. There was no significant change in transferrin secretion upon compound treatment (Supplemental Fig. 2, a and b). Compound A dose-dependently reduced apoB secretion by up to 40%. Inhibition of MTP by CP-346086 resulted in a more dramatic reduction of apoB secretion (−75% at 30 nM; Fig. 5b), consistent with previous reports (Jamil et al., 1996; Chandler et al., 2003). CP-346086, an MTP inhibitor, reduced both cellular intracellular and medium-secreted apoB levels, whereas compound A only reduced apoB secretion (Fig. 6a). It is interesting to note that compound A did not reduce secreted triglyceride levels in medium, unlike MTP inhibitor CP-346086, which abolished triglyceride secretion (Fig. 6b). Compound A had no effect on PLTP protein levels in cells or medium (data not shown).
Fig. 5.
a, relative expression of PLTP mRNA in human liver, primary hepatocytes, HepG2, and Huh7 cells. Gene expression was analyzed by TaqMan real-time polymerase chain reaction as described under Materials and Methods. The relative expression is presented relative to the expression level in liver. b, inhibition of apoB secretion by compound A and MTP inhibitor CP-346086 in Huh7 cells. c, dose-dependent inhibition of apoB secretion by compound A in HepG2 cells. HepG2 or Huh7 cells were treated with the compounds for 24 h, and medium was collected for apoB and transferrin measurement by ELISA. Data are the average of triplicates ± S.D.
Fig. 6.
a, effects of compound A and MTP inhibitor on secreted and cellular levels of apoB in HepG2 cells. The cells were treated with 30 μM compound A and 10 nM MTP inhibitor CP-346086 for 24 h. ApoB levels in medium and cell lysate were determined by ELISA. v, vehicle; cpd A, compound A; MTPi, CP-346036. *, p < 0.01. b, triglyceride levels in medium from HepG2 cells treated with compounds. Cells were treated with 30 μM compound A and 10 nM MTP inhibitor CP-346086, and medium triglyceride levels were measured as described under Materials and Methods.
Effects of PLTP Inhibitors on ApoB Secretion in Mouse Primary Hepatocytes.
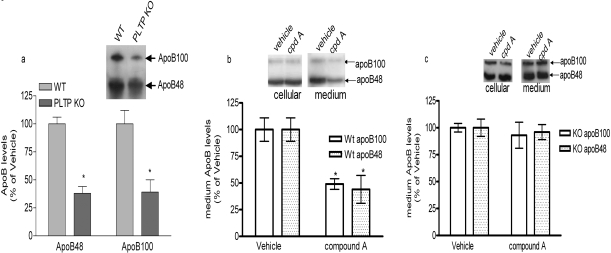
To further confirm that the reduced apoB secretion in the presence of compound A is mediated through inhibition of PLTP activity, we analyzed the effect of compound A on apoB secretion in primary hepatocytes isolated from wild-type or PLTP-deficient mice. Newly synthesized protein was labeled by incubation with [35S]Met for 4 h, and secreted apoB was determined as described under Materials and Methods. PLTP deficiency diminished both apoB100 and apoB48 secretion from primary hepatocytes by approximately 50% (Fig. 7a), consistent with a previous report (Jiang et al., 2001). At 30 μM concentration, compound A reduced apoB100 and apoB48 secretion by approximately 50% in hepatocytes from wild-type mice (Fig. 7b). No significant change in cellular apoB levels was observed (Fig. 7b, inset). However, in hepatocytes isolated from PLTP-deficient mice, compound A had no effect on apoB secretion (Fig. 7c). These data strongly suggest that the reduction of apoB secretion by compound A is specifically mediated by inhibiting PLTP activity.
Fig. 7.
Effects of PLTP inhibitors on apoB secretion in mouse primary hepatocytes. a, apoB secretion in mouse primary hepatocytes isolated from wild-type and PLTP-deficient mice. ApoB secretion was measured as described under Materials and Methods. Data are the average of triplicates ± S.D. (*, p < 0.01 compared with wild type). Representative image is shown in inset. b and c, effects of compound A on apoB secretion in hepatocytes isolated from wild-type mice (b) or PLTP-deficient mice (c). Cells were labeled with [35S]methionine/cysteine in the presence of vehicle (DMSO) or 30 μM compounds for 4 h. Medium and cells were collected to detect labeled apoB as described under Materials and Methods. Data are average of triplicate experiments ± S.D. (p < 0.01 compared with vehicle group). Representative images are shown in insets.
Discussion
This is the first report on the identification of compounds that potently inhibit PLTP-mediated phospholipid transfer from liposomes to HDL. We demonstrated that inhibition of PLTP activity can significantly diminish apoB secretion from human hepatoma cell lines, as well as mouse primary hepatocytes. These results again indicate that PLTP activity plays an important role in apoB secretion from the liver and that the mechanism seems to be conserved from mice to humans.
We cross-examined the selectivity of these compounds on the activities of other lipid transfer proteins, MTP (triglyceride transfer activity) and CETP (cholesteryl ester transfer activity). CETP and PLTP belong to the family of lipid transfer/lipopolysaccharide-binding proteins, including lipopolysaccharide-binding protein and bactericidal permeability increasing protein (Tollefson et al., 1988; Day et al., 1994). Although CETP is a close homolog of PLTP, none of these compounds inhibits CETP activity. MTP is essential for the assembly and secretion of apoB-containing lipoproteins in hepatocytes (Jamil et al., 1996). MTP has been reported to transfer triglyceride as well as phospholipids as well between liposomes (Athar et al., 2004; Rava et al., 2005). Compound A had little effect on MTP triglyceride transfer activity (Fig. 4). Similar liposome donor vesicles were used in MTP and PLTP assays, although the MTP assay used [14C]triolein as the substrate, whereas the PLTP assay used [3H]PC. Compound A specifically inhibited PLTP but not MTP, indicating that the compound does not function by nonspecifically altering the property of vesicles. Compound A did not affect the low levels of spontaneous transfer of labeled phospholipids from donor to acceptor in the absence of PLTP (data not shown). To test whether the compound affects vesicle properties and interferes with the precipitation step in the radioactive labeled assay, the compound was added 30 min after the lipid transfer reaction and did not reduce the transfer rate (data not shown). At this time, it is not clear how the compound exerts its inhibitory function. It could bind directly to the PLTP phospholipid binding pocket and inhibit the activity, or it is possible that the compound may specifically block the interaction between PLTP and the vesicles. The mechanism of action is under further investigation.
We report for the first time that inhibition of PLTP activity reduces the secretion of apoB from human hepatoma cells and mouse primary hepatocytes by approximately 50%, similar to the extent of reduction obtained by deleting PLTP in hepatocytes (Jiang et al., 2001). Furthermore, we confirmed that the inhibitor-mediated reduction of apoB secretion requires the presence of PLTP (Fig. 7, b and c). These results support the hypothesis that PLTP-mediated phospholipid transfer activity regulates the secretion of apoB-containing lipoproteins. This regulatory mechanism, at least at the cellular level, appears to be conserved in mice and humans. These findings indicate that PLTP inhibitors could potentially be developed as a new class of therapeutic agents to reduce apoB secretion and lower LDL. Unlike MTP inhibitors, PLTP-specific inhibitors may not exhibit the fatty liver side effect as suggested by studies in PLTP knockout mice (Jiang et al., 2001; Shelly et al., 2008). Furthermore, compound A reduced the secretion of apoB but not triglyceride in cultured cells (Fig. 6). We have tested the metabolic properties of these compounds and found that all of the compounds have high hepatocyte and microsomal clearance rates (data not shown). Because of the high catabolism, we were unable to obtain optimal exposure in mice to test the efficacy in vivo. Compounds with improved pharmacokinetic properties are waiting to be developed to test this mechanism in vivo.
PLTP plays complex regulatory roles in plasma lipoprotein metabolism. Intracellular PLTP modulates plasma apoB-containing lipoproteins by increasing apoB secretion (Jiang et al., 2001, 2005) and plasma PLTP regulates VLDL catabolism (Moerland et al., 2008; van Haperen et al., 2008). Due to the complex regulation of PLTP on VLDL/LDL metabolism, PLTP deficiency results in various effects on plasma VLDL levels in mice with different genetic modifications. ApoB-containing lipoprotein VLDL/LDL levels are reduced by PLTP deficiency in human apoB transgenic mice and apoE knockout mice, but not in LDLR knockout mice (Jiang et al., 2001). It appears that LDL receptor is required for the function of PLTP in regulating apoB secretion in hepatocytes, because LDL catabolism is not affected by PLTP deficiency (Jiang et al., 2001). Elevation of PLTP in adult mice by inducible transgene expression or bone marrow transplantation leads to plasma VLDL accumulation resulting from reduced catabolism (Moerland et al., 2008; van Haperen et al., 2008). Due to the complex functions of PLTP in regulating lipoprotein metabolism and atherogenesis, it remains to be determined whether PLTP inhibition is a viable approach to treat dyslipidemia and atherosclerosis.
Supplementary Material
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL64735, HL69817] (to X.-C.J.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161232.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- PLTP
- phospholipid transfer protein
- CETP
- cholesteryl ester transfer protein
- VLDL
- very low-density lipoprotein
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- apo
- apolipoprotein
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- BODIPY-PC
- 2-(4,4-difluoro-5-methyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine
- PC
- phosphatidylcholine
- PBS
- phosphate-buffered saline
- DMSO
- dimethyl sulfoxide
- BSA
- bovine serum albumin
- MTP
- microsomal triglyceride transfer protein
- DMEM
- Dulbecco's modified Eagle's medium
- ELISA
- enzyme-linked immunosorbent assay
- CP-346086
- 4′-trifluorometthyl-biphenyl-2-carboylic acid(2-(2H-(1,2,4)trizol-3-ylmethyl)-1,2,3,4-tetrahydro-isoquinolin-6-yl).
References
- Athar H, Iqbal J, Jiang XC, Hussain MM. (2004) A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J Lipid Res 45:764–772 [DOI] [PubMed] [Google Scholar]
- Chandler CE, Wilder DE, Pettini JL, Savoy YE, Petras SF, Chang G, Vincent J, Harwood HJ., Jr (2003) CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J Lipid Res 44:1887–1901 [DOI] [PubMed] [Google Scholar]
- Day JR, Albers JJ, Lofton-Day CE, Gilbert TL, Ching AF, Grant FJ, O'Hara PJ, Marcovina SM, Adolphson JL. (1994) Complete cDNA encoding human phospholipid transfer protein from human endothelial cells. J Biol Chem 269:9388–9391 [PubMed] [Google Scholar]
- de Vries R, Dallinga-Thie GM, Smit AJ, Wolffenbuttel B, van Tol A, Dullaart RP. (2006) Elevated plasma phospholipid transfer protein activity is a determinant of carotid intima-media thickness in type 2 diabetes mellitus. Diabetologia 49:398–404 [DOI] [PubMed] [Google Scholar]
- Haghpassand M, Wilder D, Moberly JB. (1996) Inhibition of apolipoprotein B and triglyceride secretion in human hepatoma cells (HepG2). J Lipid Res 37:1468–1480 [PubMed] [Google Scholar]
- Huuskonen J, Olkkonen VM, Ehnholm C, Metso J, Julkunen I, Jauhiainen M. (2000) Phospholipid transfer is a prerequisite for PLTP-mediated HDL conversion. Biochemistry 39:16092–16098 [DOI] [PubMed] [Google Scholar]
- Jamil H, Gordon DA, Eustice DC, Brooks CM, Dickson JK, Jr, Chen Y, Ricci B, Chu CH, Harrity TW, Ciosek CP, Jr, et al. (1996) An inhibitor of the microsomal triglyceride transfer protein inhibits apoB secretion from HepG2 cells. Proc Natl Acad Sci U S A 93:11991–11995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhiainen M, Huuskonen J, Baumann M, Metso J, Oka T, Egashira T, Hattori H, Olkkonen VM, Ehnholm C. (1999) Phospholipid transfer protein (PLTP) causes proteolytic cleavage of apolipoprotein A-I. J Lipid Res 40:654–664 [PubMed] [Google Scholar]
- Jauhiainen M, Metso J, Pahlman R, Blomqvist S, van Tol A, Ehnholm C. (1993) Human plasma phospholipid transfer protein causes high density lipoprotein conversion. J Biol Chem 268:4032–4036 [PubMed] [Google Scholar]
- Jiang XC, Li Z, Liu R, Yang XP, Pan M, Lagrost L, Fisher EA, Williams KJ. (2005) Phospholipid transfer protein deficiency impairs apolipoprotein-B secretion from hepatocytes by stimulating a proteolytic pathway through a relative deficiency of vitamin E and an increase in intracellular oxidants. J Biol Chem 280:18336–18340 [DOI] [PubMed] [Google Scholar]
- Jiang XC, Bruce C, Mar J, Lin M, Ji Y, Francone OL, Tall AR. (1999) Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels. J Clin Invest 103:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XC, Qin S, Qiao C, Kawano K, Lin M, Skold A, Xiao X, Tall AR. (2001) Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency. Nat Med 7:847–852 [DOI] [PubMed] [Google Scholar]
- Kawano K, Qin S, Vieu C, Collet X, Jiang XC. (2002) Role of hepatic lipase and scavenger receptor BI in clearing phospholipid/free cholesterol-rich lipoproteins in PLTP-deficient mice. Biochim Biophys Acta 1583:133–140 [DOI] [PubMed] [Google Scholar]
- Lagrost L, Desrumaux C, Masson D, Deckert V, Gambert P. (1998) Structure and function of the plasma phospholipid transfer protein. Curr Opin Lipidol 9:203–209 [DOI] [PubMed] [Google Scholar]
- Moerland M, Samyn H, van Gent T, van Haperen R, Dallinga-Thie G, Grosveld F, van Tol A, de Crom R. (2008) Acute elevation of plasma PLTP activity strongly increases pre-existing atherosclerosis. Arterioscler Thromb Vasc Biol 28:1277–1282 [DOI] [PubMed] [Google Scholar]
- Rao R, Albers JJ, Wolfbauer G, Pownall HJ. (1997) Molecular and macromolecular specificity of human plasma phospholipid transfer protein. Biochemistry 36:3645–3653 [DOI] [PubMed] [Google Scholar]
- Rava P, Athar H, Johnson C, Hussain MM. (2005) Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J Lipid Res 46:1779–1785 [DOI] [PubMed] [Google Scholar]
- Samyn H, Moerland M, van Gent T, van Haperen R, Metso J, Grosveld F, Jauhiainen M, van Tol A, de Crom R. (2008) Plasma phospholipid transfer activity is essential for increased atherogenesis in PLTP transgenic mice: a mutation-inactivation study. J Lipid Res 49:2504–2512 [DOI] [PubMed] [Google Scholar]
- Schlitt A, Bickel C, Thumma P, Blankenberg S, Rupprecht HJ, Meyer J, Jiang XC. (2003) High plasma phospholipid transfer protein levels as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol 23:1857–1862 [DOI] [PubMed] [Google Scholar]
- Shelly L, Royer L, Sand T, Jensen H, Luo Y. (2008) Phospholipid transfer protein deficiency ameliorates diet-induced hypercholesterolemia and inflammation in mice. J Lipid Res 49:773–781 [DOI] [PubMed] [Google Scholar]
- Tall AR. (1995) Plasma cholesteryl ester transfer protein and high-density lipoproteins: new insights from molecular genetic studies. J Intern Med 237:5–12 [DOI] [PubMed] [Google Scholar]
- Tall AR, Krumholz S, Olivecrona T, Deckelbaum RJ. (1985) Plasma phospholipid transfer protein enhances transfer and exchange of phospholipids between very low density lipoproteins and high density lipoproteins during lipolysis. J Lipid Res 26:842–851 [PubMed] [Google Scholar]
- Tall AR, Lalanne F. (2003) Phospholipid transfer protein and atherosclerosis. Arterioscler Thromb Vasc Biol 23:1484–1485 [DOI] [PubMed] [Google Scholar]
- Tollefson JH, Ravnik S, Albers JJ. (1988) Isolation and characterization of a phospholipid transfer protein (LTP-II) from human plasma. J Lipid Res 29:1593–1602 [PubMed] [Google Scholar]
- van Haperen R, Samyn H, Moerland M, van Gent T, Peeters M, Grosveld F, van Tol A, de Crom R. (2008) Elevated expression of phospholipid transfer protein in bone marrow derived cells causes atherosclerosis. PLoS ONE 3:e2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haperen R, van Tol A, van Gent T, Scheek L, Visser P, van der Kamp A, Grosveld F, de Crom R. (2002) Increased risk of atherosclerosis by elevated plasma levels of phospholipid transfer protein. J Biol Chem 277:48938–48943 [DOI] [PubMed] [Google Scholar]
- Yang XP, Yan D, Qiao C, Liu RJ, Chen JG, Li J, Schneider M, Lagrost L, Xiao X, Jiang XC. (2003) Increased atherosclerotic lesions in apoE mice with plasma phospholipid transfer protein overexpression. Arterioscler Thromb Vasc Biol 23:1601–1607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.