Abstract
The activities of the citric acid cycle enzymes were determined in mitochondria isolated from kidneys of relatively young, middle age, and old mice. Aconitase exhibited the most significant decrease in activity with age. The activity of α-ketoglutarate dehydrogenase exhibited a modest decrease in activity, while NADP+-isocitrate dehydrogenase (NADP+-ICD) activity increased moderately with age. Activities of citrate synthase, NAD+-isocitrate dehydrogenase (NAD+-ICD), succinyl-CoA synthetase (SCS), succinate dehydrogenase (SD), fumarase (FUM), and malate dehydrogenase (MD) were not affected. The molar ratio of the intra-mitochondrial redox indicator, NADPH:NADP+, was higher in young compared to old animals, while the NADH:NAD+ molar ratio remained unchanged. It is suggested that an age-related decrease in aconitase activity along with relatively subtle alterations in activities of some other citric acid cycle enzymes are likely to contribute to a decline in the overall efficiency of mitochondrial bioenergetics. The biological consequences of such alterations include age-related fluctuations in the citric acid cycle intermediates, which are precursors of protein synthesis, activators of fatty acid synthesis, and can also act as ligands for orphan G-protein coupled receptors.
Keywords: Citric acid cycle, Aconitase, Aging
1. Introduction
One of the main features of the aging process is that the functional capacity of many physiological systems undergoes progressive decline, most notably in the post-reproductive phase of life. An age associated decrease in stamina, or capacity for sustained work, is observed in virtually all animal species tested, and is suspected to emanate from impairments of the mechanisms of mitochondrial bioenergetics (Sohal and Weindruch, 1996; Hagen et al., 1997). The main reasons for the implication of mitochondria are that the mitochondrion is the predominant site of ATP production and also the primary producer and target of reactive oxygen species (ROS) such as O2− and H2O2. This notion is supported by the findings that the rates of mitochondrial O2−/H2O2 generation, and amounts of oxidative damage to macromolecules are elevated during aging in different species (Sheldahl and Tappel, 1974; Park and Ames, 1988; Stadtman, 1992; Orr and Sohal, 1994; Sohal and Weindruch, 1996). While it was originally thought that oxidative damage to macromolecules, particularly proteins, occurred randomly and ubiquitously (Harman, 1981), more recent studies indicate that such damage in vivo is highly selective (Yan et al., 1997; Yan and Sohal, 1998; Kanski et al., 2005). However, the identity of specific targets of oxidative damage during aging remains controversial because some of the approaches used for determining oxidative modifications are non-specific and, more importantly, the functional consequences of the oxidatively-damaged proteins have often not been demonstrated or remain unclear, as damaged proteins tend to undergo rapid proteolysis (Grune et al., 1997).
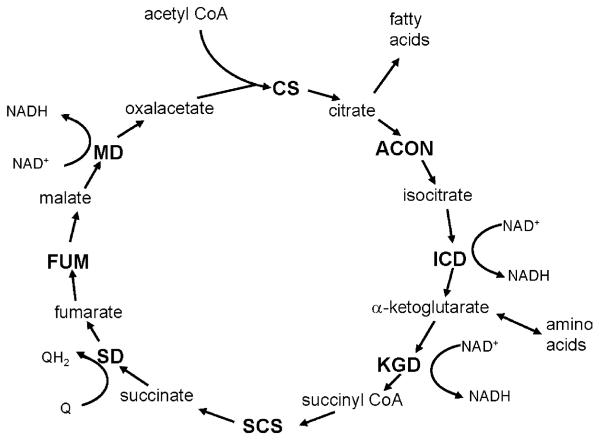
Therefore, in the present study, an alternative, systematic approach was undertaken to identify protein targets of the aging process in mitochondria by determining losses in enzymatic activity of a central metabolic pathway, the citric acid cycle. The rationale is that the citric acid cycle plays a pivotal role in mitochondrial bioenergetics by providing the reducing equivalents, NADH and FADH2 for ATP synthesis, and NADPH for the reduction of H2O2 and glutathione disulfides in mitochondria, as well as supplying intermediates essential for fatty acid and protein synthesis (Fig. 1). Because of its biochemical design, a decrease in the activity of a single enzyme can potentially diminish the turnover rates of the entire citric acid cycle or divert intermediates to other pathways. Metabolism through the citric acid cycle results in the net synthesis of intermediates (anaplerosis), as well as the export of intermediates from the mitochondria (cataplerosis), used in fatty acid and protein synthesis and potentially as extramitochondrial signal molecules.
Fig. 1.
Schematic representation of the citric acid cycle. The citric acid cycle enzymes measured in this study are shown in capital letters. Intermediates, redox products, anaplerotic (replacing intermediates) and cataplerotic (diverting intermediates) reactions are shown in lower-case letters. CS, citrate synthase; ACON, aconitase; ICD, isocitrate dehydrogenase; KGD, α-ketoglutarate dehydrogenase; SCS, succinyl-CoA synthetase; SD, succinate dehydrogenase; FUM, fumarase; MD, malate dehydrogenase.
Experimental studies have shown that specific enzymes of the citric acid cycle are susceptible to oxidation. For instance, aconitase and α-ketoglutarate dehydrogenase can undergo oxidative modification in vitro with a subsequent decrease in enzymatic activity, following exposure of mammalian mitochondria to hydrogen peroxide (Nulton-Persson and Szweda, 2001; Bulteau et al., 2003, 2004). Aconitase has also been shown to be carbonylated in vivo in insects and mammals, resulting in a reduction in activity (Yan et al., 1997; Das et al., 2001; Yarian et al., 2005). NADP+-isocitrate dehydrogenase (NADP+-ICD) is a target of glycation, and subsequent inactivation, in kidney mitochondria from rats during the normal aging process and in a diabetes-induced rat model (Kil et al., 2004). However, a comprehensive analysis of the citric acid cycle enzymes, to determine age-associated changes in activities during the normal aging process has not to our knowledge been previously undertaken using mammalian mitochondria as a model.
In this study, the maximal activities of the citric acid cycle enzymes, citrate synthase (EC 2.3.3.1), aconitase (EC 4.2.1.3), NAD+-isocitrate dehydrogenase (NAD+-ICD, EC 1.1.1.41), NADP+-isocitrate dehydrogenase (EC 1.1.1.42), α-ketoglutarate dehydrogenase complex (EC 1.2.4.2, EC 2.3.1.61, EC 1.8.1.4), succinyl-CoA synthetase (SCS, EC 6.2.1.4), succinate dehydrogenase (SD, EC 1.3.5.1), fumarase (FUM, EC 4.2.1.2) and malate dehydrogenase (MD, EC 1.1.1.37) were assayed in mitochondrial preparations from kidneys of relatively young (6 months), middle age (16 months) and old (24 months) mice. Recent reports suggest that mammalian kidneys exhibit a progressive aging-related decline in filtration efficiency (Hoang et al., 2003), as well as in anti-oxidative capacity (Tian et al., 1998; Rebrin et al., 2003; Kil et al., 2004). Results presented in this study demonstrate that the activities of specific citric acid cycle enzymes are altered during the aging process and thus, define the nature of some of the impairments in bioenergetics during the aging process.
2. Materials and methods
2.1. Chemicals
All chemicals were purchased from Sigma (St. Louis, MO). Acetyl-CoA was synthesized by dissolving the lithium salt of coenzyme A in 0.1 M Tris base to a concentration of 10 mg/mL, followed by the addition of 13 μL of acetic anhydride and an overnight incubation at 4 °C (Robinson et al., 1987).
2.2. Animals
Thirty-six male C57BL/6N mice, aged 6, 16, and 24 months, were obtained from the National Institute on Aging—National Institutes of Health. Mice were fed a Harlan Teklad rodent diet ad libitum and housed, less than four to a cage, at an ambient temperature of 23 ± 1 °C, with a 12-h light:12-h dark cycle for 3 weeks. Nine mice (three from each age) were killed per day on four separate days.
2.3. Preparations of mitochondria from kidneys
Mice were killed by cervical dislocation and their kidneys were quickly removed and placed in ice-cold anti-oxidant buffer containing 50 mM potassium phosphate buffer, pH 7.4, 2 mM EDTA and 0.1 mM butylated hydroxytoluene. For each preparation, kidneys from three mice were pooled and cortical regions of the kidneys were minced and homogenized in 10 vol. (w/v) of isolation buffer (0.22 M mannitol, 70 mM sucrose, 10 mM EGTA, 2 mM Hepes, pH 7.4) in a glass homogenizer with a motor-driven Teflon pestle, as previously described (Lash and Sall, 1993). Low- and high-speed differential centrifugations for mitochondrial isolation were, respectively, 600 × g for 10 min and 15,000 × g for 10 min. The isolation procedure was usually completed within 1–2 h. Mitochondria were resuspended in isolation buffer containing 5 mM citrate and frozen at −80 °C at a concentration of approximately 15 mg/mL. They were disrupted with four freeze–thaw cycles.
2.4. Enzyme assays
All enzyme assays were conducted at 30 °C. Citrate synthase activity was determined by the addition of 0.5 mM oxaloacetate (OAA) to the reaction mixture consisting of 100 mM Tris pH 8.1, 0.1 mM DTNB, 30 mM acetyl-CoA, and 4–13 μg of protein. Activity was calculated by subtracting ΔA412/min before the addition of OAA from ΔA412/min after the addition of OAA (Robinson et al., 1987). Aconitase activity was assayed in a reaction mixture containing 90 mM Tris pH 8.0, 20 mM isocitrate, and 65–75 μg of protein (Henson and Cleland, 1967). NAD+-dependent isocitrate dehydrogenase activity was measured by the addition of 180–230 μg of protein to a mixture of 100 mM Tris, pH 7.2, 1 mM MnCl2, 0.5 mM NAD+, and 0.5 mM ADP (Robinson et al., 1987). NADP+-dependent isocitrate dehydrogenase activity assays (50 mM Tris pH 7.4, 1 mM MnCl2, 0.5 mM EDTA, 0.25 mM NADP+ and 1.6 mM isocitrate) were initiated by the addition of 40–140 μg of protein (Robinson et al., 1987). α-Ketoglutarate dehydrogenase complex was measured by adding 80–110 μg of protein to assay mixtures consisting of 50 mM potassium phosphate (pH 7.4), 0.1 mM coenzyme A, 0.2 mM thiamine pyrophosphate, 1 mM NAD+, 0.5 mM EDTA, 5 mM MgCl2,40 μM rotenone, 0.1% TritonX-100, and 2.5 mM α-ketoglutarate (Robinson et al., 1987). Succinyl-CoA synthetase reactions (50 mM Tris pH 7.2, 110 mM sodium succinate, 10 mM MgCl2, 0.1 mM ATP, 0.1 mM coenzyme A) were initiated by the addition of 45–60 μg of protein (Cha and Parks, 1964). Succinate dehydrogenase was assayed by incubating 50 mM potassium phosphate (pH 7.4), 20 mM sodium succinate and 39–140 μg of protein for 10 min, followed by the addition of 1 mM potassium cyanide, 0.00165% dichlorophenol–indolphenol (DCIP), and 0.033% phenylmethosulfate (PMS) (Ackrell et al., 1978). Fumarase activity was determined by the addition of 80–140 μg of protein to a mixture of 90 mM potassium phosphate (pH 7.4) and 50 mM malate (Robinson et al., 1987). Malate dehydrogenase activity was determined by the addition of 0.48 mM OAA to a reaction mixture containing 100 mM potassium phosphate (pH 7.4), 0.13 mM NADH and 4–14 μg of protein (Robinson et al., 1987). Enzyme activity was determined by the subtraction of ΔA340/min before the addition of OAA from ΔA340/min after the addition of OAA.
The following millimolar extinction coefficients were used to determine activity: 6.22 mM−1 cm−1 for NADH and NADPH at 340 nm, 13.6 mM−1 cm−1 for DTNB at 412 nm, 3.6 mM−1 cm−1 for cis-aconitate at 240 nm, 4 mM−1 cm−1 for succinyl-CoA at 235 nm, 19.1 mM−1 cm−1 for DCIP at 600 nm, and 4.88 mM−1 cm−1 for fumarate at 250 nm.
Enzymes were assayed in four independent preparations of kidney mitochondria, each consisting of tissues pooled from three animals with three or more replicate measurements per preparation. All rates were linear with respect to enzyme concentration for the amount of protein added. Data are presented as mean ± standard error of the mean (S.E.M.).
Protein concentrations were estimated by the BCA Protein Assay (Pierce, Rockford, IL) using bovine serum albumin as standard.
2.5. Measurement of pyridine nucleotides
NADPH, NADP+, NADH, and NAD+ were quantitated using a procedure based on that described by Klaidman et al. (1995). In brief, frozen preparations of kidney mitochondria were homogenized on ice in 200 μL of 0.2 M potassium cyanide, 0.06 M potassium hydroxide, and 1 mM bathophenanthrolinedisulfonic acid, followed by chloroform extraction and centrifugation at 18,000 × g for 5 min in a microcentrifuge at 4 °C. The resulting potassium hydroxide supernatant was extracted twice with chloroform and centrifuged as described above to remove lipids and proteins. The aqueous layer was filtered through 0.45 μm PTFE Acrodisc syringe filters (Gelman Laboratory, Ann Arbor, MI), centrifuged for 10 min to remove RNA and DNA, diluted with mobile phase and injected on an HPLC column. The cyanide reaction, which occurs with NAD+ and NADP+, appears to proceed primarily at the fourth position of the nicotinamide ring (Kaplan, 1960; Oppenheimer et al., 1971). Each reaction produces two isomers (NAD(P)–CN1 and NAD(P)–CN2). As a safety precaution, gloves and a mask were worn while working with potassium cyanide.
The samples were resolved on a 5 μm, 250 mm × 4.6 mm reverse phase C18 column (Phenomemex, Torrance, CA) with the mobile phase consisting of 0.2 M ammonium acetate, pH 6.5, and HPLC-grade methanol. An HPLC gradient time program, with an initial setting of 96% ammonium acetate and 4% methanol with the methanol increasing by 0.2% per minute and a flow rate of 1 mL/min, was used along with a fluorescence detector (excitation, 330 nm; emission, 460 nm).
NADPH, NADP+, NADH and NAD+ standards (0.015–1.5 nmol) were prepared in deionized water and immediately frozen at −80 °C. Immediately before injection, the standards were diluted in 0.2 M KCN, 0.06 M KOH, and 1 mM bathophenanthroline, incubated on ice for 5 min, and diluted with an equal volume of mobile phase. Standard curves, from replicate integrated peaks of 0.015–1.5 nmol of each standard, always had a correlation coefficient of 0.999 or above.
Quantitation of pyridine nucleotides was performed by integrating peaks corresponding to NAD(P)–CN1, NAD(P)–CN2, and NAD(P)H and calculating with the respective standard curve. Molar amounts of NAD(P)–CN1 and NAD(P)–CN2 were combined to represent oxidized pyridine nucleotides. Data for the molar ratios of reduced:oxidized pyridine nucleotides are presented as averages ± standard error of the mean for the ratios at each age.
3. Results
3.1. Age-related changes in activities of the citric acid cycle enzymes
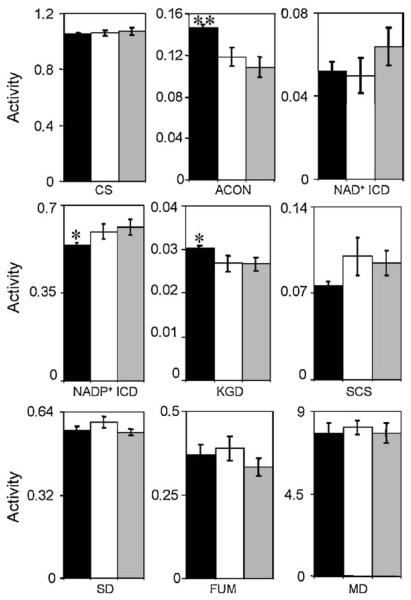
Activities of citrate synthase, aconitase, NAD+-dependent isocitrate degydrogenase, NADP+-isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, succinyl-CoA synthetase, succinate dehydrogenase, fumarase and malate dehydrogenase (Fig. 1) were assayed in four independent mitochondrial preparations, each from kidneys of relatively young (6 months), middle age (16 months), and old (24 months) mice.
Aconitase exhibited the most significant age-related alteration in activity. The maximal activity of aconitase in 16-month-old (118 ± 9 nmol aconitate/min/mg protein) and 24-month-old (108 ± 9 nmol/min/mg protein) mice was consistently less than that from 6-month-old (147 ± 3 nmol aconitate/min/mg protein) mice (Fig. 2).
Fig. 2.
Enzymatic activities in kidney mitochondria from mice of different ages for various stages of the citric acid cycle: CS (μmol CoA/min/mg); ACON (μmol aconitate/min/mg); NAD+-ICD (μmol NADH/min/mg); NADP+-ICD (μmol NADPH/min/mg); KGD (μmol NADH/min/mg); SCS (μmol succinyl-CoA/min/mg); SD (μmol DCIP/min/mg); FUM (μmol fumarate/min/mg); MD (μmol NADH/min/mg). Maximal enzyme activities in 6- (black), 16- (white), and 24- (grey) month-old mice were determined by at least triplicate assays of four independent preparations of kidney mitochondria, as described in Section 2. Statistically significant alterations are indicated by an asterisk, **p < 0.001, *p < 0.05 for 24-month-old vs. 6-month-old, based on a t-test: two-sample, assuming equal variance.
The activity of α-ketoglutarate dehydrogenase was also significantly decreased with age between 6 and 24 months of age (Fig. 2). Conversely, the activity of NADP+-isocitrate dehydrogenase increased significantly between 6 and 24 months of age (Fig. 2). Fumarase, citrate synthase, malate dehydrogenase, NAD+-isocitrate dehydrogenase, succinyl-CoA synthetase, and succinate dehydrogenase did not show a statistically significant age-associated alteration in activity (Fig. 2).
3.2. Pyridine nucleotide levels in kidney mitochondria
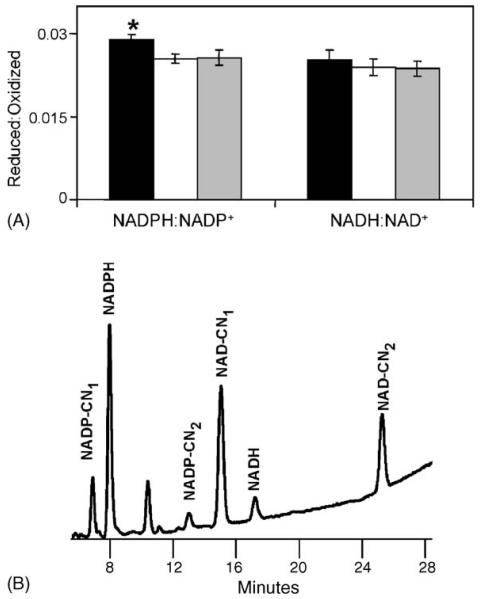
The molar ratios of NADPH:NADP+ and NADH:NAD+ were determined as a general measure of the mitochondrial redox state. The redox state of tissues is determined by the amounts and ratios of the interconvertible reduced and oxidized forms of various redox couples, such as reduced glutathione (GSH):oxidized glutathione disulfides (GSSG), cysteine:cystine, reduced:oxidized thioredoxin, NADH:NAD+ and NADPH:NADP+ (Schafer and Buettner, 2001). The dehydrogenases in the citric acid cycle produce the reducing equivalents NADH, NADPH, and FADH2.
The molar ratio of NADPH:NADP+ was significantly decreased in 24 months compared to 6-month-old animals (Fig. 3A). The ratio of reduced to oxidized pyridine nucleotides was 0.0294 ± 0.0007 for 6-month-old mice while it was 0.0255 ± 0.0009 and 0.0256 ± 0.0010 for 16 and 24 months of age, respectively. While there was a trend of an increase in NADP+ and a decrease in NADPH when comparing 6-month-old mice with those of 24 months, the differences were not statistically significant (Table 1). The ratio of NADH:NAD+ was not significantly altered with age, 0.025 ± 0.002 for 6-month-old, 0.024 ± 0.002 for 16-month-old, and 0.026 ± 0.001 for 24-month-old mice (Fig. 3A). In addition, there was no statistically significant change in the amounts of NAD+ or NAD with age (Table 1).
Fig. 3.
Molar ratios of mitochondrial NADPH:NADP+ and NADH:NAD+. (A) Pyridine nucleotide ratios were determined in kidney mitochondria preparations from 6- (black), 16- (white), and 24- (grey) month-old mice. Statistically significant alterations are indicated by an asterisk, *p < 0.05, for 24-month-old vs. 6-month-old, based on a t-test: two-sample, assuming equal variance. (B) Representative chromatograph for quantitating NADPH, NADP+, NADH, and NAD+. Chromatograph shown here is from 6-month-old kidney mitochondria.
Table 1.
Amounts of reduced, oxidized and ratios of reduced:oxidized pyridine nucleotides with age
| Age (months) |
NAD+ (nmol)/ protein (mg) |
NADH (nmol)/ protein (mg) |
NADH:NAD+ | NADP+ (nmol)/ protein (mg) |
NADPH (nmol)/ protein (mg) |
NADPH:NADP+ |
|---|---|---|---|---|---|---|
| 6 | 0.092 ± 0.002 | 0.0023 ± 0.0002 | 0.0253 ± 0.002 | 0.059 ± 0.005 | 0.0017 ± 0.0001 | 0.0294 ± 0.0007 |
| 16 | 0.096 ± 0.014 | 0.0023 ± 0.0005 | 0.0239 ± 0.002 | 0.069 ± 0.007 | 0.0018 ± 0.0002 | 0.0255 ± 0.0009 |
| 24 | 0.090 ± 0.021 | 0.0024 ± 0.0007 | 0.0257 ± 0.001 | 0.062 ± 0.014 | 0.0016 ± 0.0004 | 0.0256 ± 0.0010 |
Data for the molar ratios of reduced:oxidized pyridine nucleotides are presented as averages ± standard error of the mean for the ratios at each age.
The method used here for quantitating NADPH, NADP+, NADH and NAD+ allows clear separation of the respective peaks in a sample (Fig. 3B) while minimizing the degradation of reduced products. This is accomplished by the addition of cyanide, keeping the samples in a basic solution, addition of a divalent metal chelator, and minimizing the number of steps and time involved from homogenization to detection of the pyridine nucleotides (Klaidman et al., 1995). The cyanide addition reaction occurs during homogenization of the sample, resulting in the fluorescent products NAD–CN and NADP–CN (shown in Fig. 3B as NAD(P)–CN1 and –CN2), which, along with NADPH and NADH, are stable in a basic solution. An additional precaution against oxidation by metal ions, such as Fe3+, is the addition of the divalent metal chelator, bath-ophenanthroline (Klaidman et al., 1995).
4. Discussion
Results of this study indicate that among citric acid cycle enzymes, aconitase exhibits the most significant age-associated decline in activity. A smaller, but significant decline occurred for α-ketoglutarate dehydrogenase while NADP+-isocitrate dehydrogenase showed an elevation in activity. In addition, NADPH:NADP+ molar ratios declined with age.
Aconitase is known to be highly susceptible to oxidative damage. For instance, exposure to superoxide and hydrogen peroxide ultimately causes the release of Fe-α from the [4Fe–4S]2+ cluster, producing [3Fe–4S]1+ and an inactivation of aconitase activity (Verniquet et al., 1991; Brazzolotto et al., 1999; Vasquez-Vivar et al., 2000; Bulteau et al., 2003). However, recent studies suggest that aconitase is likely to be inhibited by a post-translational modification since the amount of Fe-α removal does not reflect the magnitude of enzyme inactivation (Bulteau et al., 2003). In addition, O2− and H2O2 do not appear to directly inhibit aconitase, instead requiring interaction between aconitase and a membrane component responsive to peroxide (Bulteau et al., 2003). The post-translational oxidative modification, carbonylation has previously been shown to result in a decrease in aconitase activity in both the housefly (Yan et al., 1997; Yan and Sohal, 2000; Yarian and Sohal, 2005) and fruitfly, Drosophila melanogaster (Das et al., 2001).
A decrease in aconitase activity is likely to affect the overall turnover efficiency of the citric acid cycle (Fig. 1). Because intermediates produced in the citric acid cycle through anaplerosis do not accumulate in the mitochondria, and are diverted via cataplerosis, the citrate produced by citrate synthase would not be isomerized by aconitase and, consequently, would be exported. The accumulation of citrate in the cytosol would quite likely activate fatty acid synthesis. In addition, a decrease in aconitase activity would ultimately affect the products of the downstream reactions in the cycle. Amino acids can enter the cycle at α-ketoglutarate dehydrogenase, thereby bypassing the requirement for aconitase to isomerize citrate. However, in this study, α-ketoglutarate dehydrogenase also exhibited a decline in activity with age.
Age-related changes in the activities of citric acid cycle enzymes besides aconitase may also potentially affect cellular bioenergetics. There was no measurable alteration in the age-related activity of NAD+-isocitrate dehydrogenase, the isozyme which functions in the mammalian citric acid cycle. However, mitochondrial NADP+-isocitrate dehydrogenase showed a modest age-related increase in activity. This alteration in activity would likely result in the augmentation of mitochondrial NADPH levels. However, the ratios of NADPH:NADP+ decreased with age, which may be associated with an age-related increase in the pro-oxidant state in kidney mitochondria, also demonstrated by a decrease in the GSH:GSSG ratio (Rebrin et al., 2003).
Recent reports, identifying citric acid cycle intermediates as ligands for G-protein coupled receptors (He et al., 2004), suggest a link between the citric acid cycle and certain metabolic diseases common during aging. Succinate has been shown to be a natural ligand for an orphan receptor highly expressed in kidneys with a pro-hypertensive effect involving the rennin-angiotensin system, while α-ketoglutarate is a ligand for a homologous receptor (He et al., 2004). It has been suggested that mitochondrial dysfunction, caused by an imbalance of energy supply and demand or altered metabolism of citric acid cycle intermediates may result in the release of citric acid cycle intermediates (Hebert, 2004), albeit the underlying mechanisms have not as yet been fully elucidated. Nevertheless, it is of interest to point out that aging animals suffer from metabolic diseases such as hypertension, atherosclerosis and diabetes, which may, in part, be affected by an age-associated alteration of the citric acid cycle and, consequently, intermediates acting as signaling molecules.
In conclusion, the results of this study demonstrate that the citric acid cycle is a functional target in the aging process in mammals. Even selective and subtle damage to the citric acid cycle enzymes can potentially result in altered concentrations of intermediates, which may affect secondary metabolism as well as signal transduction mechanisms.
Acknowledgements
This work was funded by the grant R01 AG13563 from National Institute on Aging—National Institutes of Health. The authors thank Catherine Bregere for the preparation of kidney mitochondria.
Abbreviations
- ACON
aconitase
- CS
citrate synthase
- DCIP
dichlorophenol–indolphenol
- DTNB
5,5′-dithio-bis-(2-nitrobenzoic) acid
- FUM
fumarase
- ICD
isocitrate dehydrogenase
- KGD
α-ketoglutarate dehydrogenase
- MD
malate dehydrogenase
- OAA
oxaloacetate
- PMS
phenylmethosulfate
- SCS
succinyl-CoA synthetase
- SD
succinate dehydrogenase
References
- Ackrell BA, Kearney EB, Singer TP. Mammalian succinate dehydrogenase. Methods Enzymol. 1978;53:466–483. doi: 10.1016/s0076-6879(78)53050-4. [DOI] [PubMed] [Google Scholar]
- Brazzolotto X, Gaillard J, Pantopoulos K, Hentze MW, Moulis JM. Human cytoplasmic aconitase (iron regulatory protein 1) is converted into its [3Fe–4S] form by hydrogen peroxide in vitro but is not activated for iron-responsive element binding. J. Biol. Chem. 1999;274:21625–21630. doi: 10.1074/jbc.274.31.21625. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Ikeda-Saito M, Szweda LI. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2003;42:14846–14855. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, O'Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science. 2004;305:242–245. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]
- Cha S, Parks RE., Jr. Succinic thiokinase. I. Purification of the enzyme from pig heart. J. Biol. Chem. 1964;239:1961–1967. [PubMed] [Google Scholar]
- Das N, Levine RL, Orr WC, Sohal RS. Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem. J. 2001;360:209–216. doi: 10.1042/0264-6021:3600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11:526–534. [PubMed] [Google Scholar]
- Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, Ames BN. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc. Natl. Acad. Sci. U.S.A. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- Hebert SC. Physiology: orphan detectors of metabolism. Nature. 2004;429:143–145. doi: 10.1038/429143a. [DOI] [PubMed] [Google Scholar]
- Henson CP, Cleland WW. Purification and kinetic studies of beef liver cytoplasmic aconitase. J. Biol. Chem. 1967;242:3833–3838. [PubMed] [Google Scholar]
- Hoang K, Tan JC, Derby G, Blouch KL, Masek M, Ma I, Lemley KV, Myers BD. Determinants of glomerular hypofiltration in aging humans. Kidney Int. 2003;64:1417–1424. doi: 10.1046/j.1523-1755.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- Kaplan NO. The Enzymes. part 3. Vol. 3. Academic Press; New York: 1960. pp. 105–169. [Google Scholar]
- Kil IS, Lee JH, Shin AH, Park JW. Glycation-induced inactivation of NADP(+)-dependent isocitrate dehydrogenase: implications for diabetes and aging. Free Radic. Biol. Med. 2004;37:1765–1778. doi: 10.1016/j.freeradbiomed.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Klaidman LK, Leung AC, Adams JD., Jr. High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Anal. Biochem. 1995;228:312–317. doi: 10.1006/abio.1995.1356. [DOI] [PubMed] [Google Scholar]
- Lash LH, Sall JM. Mitochondrial isolation from liver and kidney: strategy, techniques, and criteria for purity. In: Lash LH, Jones DP, editors. Methods in Toxicology: Mitochondrial Dysfunction. Vol. 2. Academic Press; San Diego: 1993. pp. 8–12. [Google Scholar]
- Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. J. Biol. Chem. 2001;276:23357–23361. doi: 10.1074/jbc.M100320200. [DOI] [PubMed] [Google Scholar]
- Oppenheimer NJ, Arnold LJ, Kaplan NO. A structure of pyridine nucleotides in solution. Proc. Natl. Acad. Sci. U.S.A. 1971;68:3200–3205. doi: 10.1073/pnas.68.12.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Park JW, Ames BN. 7-Methylguanine adducts in DNA are normally present at high levels and increase on aging: analysis by HPLC with electrochemical detection. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7467–7470. doi: 10.1073/pnas.85.20.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JB, Jr., Brent LG, Sumegi B, Srere PA. An enzymatic approach to the study of the Krebs tricarboxylic acid cycle. In: Darley-Usmar VM, Rickwood D, Wilson MT, editors. Mitochondria: A Practical Approach. IRL Press; Washington, DC: 1987. pp. 153–169. [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Sheldahl JA, Tappel AL. Fluorescent products from aging Drosophila melanogaster: an indicator of free radical lipid peroxidation damage. Exp. Gerontol. 1974;9:33–41. doi: 10.1016/0531-5565(74)90005-9. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Tian L, Cai Q, Wei H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic. Biol. Med. 1998;24:1477–1484. doi: 10.1016/s0891-5849(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Kennedy MC. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J. Biol. Chem. 2000;275:14064–14069. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- Verniquet F, Gaillard J, Neuburger M, Douce R. Rapid inactivation of plant aconitase by hydrogen peroxide. Biochem. J. 1991;276:643–648. doi: 10.1042/bj2760643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Sohal RS. Prevention of flight activity prolongs the life span of the housefly, Musca domestica, and attenuates the age-associated oxidative damage to specific mitochondrial proteins. Free Radic. Biol. Med. 2000;29:1143–1150. doi: 10.1016/s0891-5849(00)00423-8. [DOI] [PubMed] [Google Scholar]
- Yarian CS, Rebrin I, Sohal RS. Aconitase and ATP synthase are targets of malondialdehyde modification and undergo an age-related decrease in activity in mouse heart mitochondria. Biochem. Biophys. Res. Commun. 2005;330:151–156. doi: 10.1016/j.bbrc.2005.02.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarian CS, Sohal RS. In the aging housefly aconitase is the only citric acid cycle enzyme to decline significantly. J. Bioenerg. Biomembr. 2005;37:91–96. doi: 10.1007/s10863-005-4132-z. [DOI] [PubMed] [Google Scholar]