Abstract
Our understanding of the humoral immune response to hepatitis C virus (HCV) is limited because the virus can be studied only in humans and chimpanzees and because previously described neutralization assays have not been robust or simple to perform. Nevertheless, epidemiologic and laboratory studies suggested that neutralizing Ab to HCV might be important in preventing infection. We have recently described a neutralization assay based on the neutralization of pseudotyped murine retrovirus constructs bearing HCV envelope glycoproteins on their surface. We have applied the assay to well characterized clinical samples from HCV-infected patients and chimpanzees, confirmed the existence of neutralizing Ab to HCV, and validated most previously reported neutralizations of the virus. We did not find neutralizing anti-HCV in resolving infections but did find relatively high titers (>1:320) of such Ab in chronic infections. Neutralizing Ab was directed not only to epitope(s) in the hypervariable region of the E2 envelope protein but also to one or more epitopes elsewhere in the envelope of the virus. Neutralizing Ab was broadly reactive and could neutralize pseudotype particles bearing the envelope glycoproteins of two different subgenotypes (1a and 1b). The ability to assay neutralizing anti-HCV should permit an assessment of the prospects for successful Ab-mediated passive and active immunoprophylaxis against hepatitis C.
Hepatitis C virus (HCV) is a small enveloped virus containing single-stranded positive sense RNA. It infects up to 170 million people worldwide and is a major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Although HCV accounts for only ≈12% of acute hepatitis in the United States, its high rate of persistence (70–80%) makes it responsible for almost half of the economic burden of this disease.
A better understanding of the pathogenesis of hepatitis C and its control is hampered by certain characteristics of the virus. (i) HCV is genetically and probably serologically heterogeneous. (ii) The only animal model for HCV infection is the chimpanzee. (iii) Although the virus replicates sparingly in some cell lines, the in vitro method developed for detecting neutralizing antibodies (Nt Ab) to HCV in this system is so difficult to perform that it has not been widely used. Consequently, although the two envelope proteins of HCV, E1 and E2, have been expressed individually and as heterodimers, and Ab to them has been measured, there is no confirmation that such Ab accurately reflects the response to the virus or that it is Nt Ab.
Previously, we identified Nt Ab to HCV by their ability to prevent replication of the virus in a lymphoid cell line (1, 2) or to prevent hepatitis C in chimpanzees (3, 4). These and a small number of similar studies (5) have provided, for almost a decade, the only direct evidence for Ab-mediated neutralization of HCV. Consequently, although considerable new knowledge has been gained about the cellular immune response to HCV, little is known about the role of the humoral immune response. However, there is historical evidence that normal immune globulin manufactured before the screening of donor plasma for HCV infection protected against hepatitis C, whereas globulin manufactured subsequently does not (6).
Recently we described the construction of infectious HCV pseudotype particles (pp) that were assembled in cell culture from three plasmids bearing parts of a truncated murine retrovirus genome encoding genes for the retroviral core proteins and enzymes, a retroviral vector bearing the gene for GFP, and a vector bearing the genes for functional HCV E1 and E2 envelope glycoproteins (7). These pseudotyped retroviruses were infectious for certain cell lines of hepatocyte origin (principally Huh-7 cells), as well as for human primary hepatocytes. Certain murine monoclonal antibodies to the E2 protein of HCV, as well as sera from patients chronically infected with HCV, but not sera from healthy controls, were able specifically to neutralize the infectivity of the pp for Huh-7 cells. The infectivity of pp for similar cells was confirmed by others, as was the ability of rodent monoclonal antibodies to neutralize the virus (8, 9).
Such an in vitro neutralization assay for HCV could be extremely valuable for characterizing the humoral immune response to HCV and for evaluating the potential for developing passive and active immunization for hepatitis C. However, Huh-7 cells, and even primary hepatocytes maintained in culture, are not the same as hepatocytes in vivo. Thus, it is important to validate results obtained in vitro by comparison with in vivo studies. We describe herein attempts to validate an in vitro assay for Nt Ab to HCV, based on pp, by comparison with previously obtained data on neutralization of HCV in in vivo (chimpanzee) and in vitro (lymphoid cell) models and show that the detection of Nt Ab to HCV, based on pp, is sensitive, specific, reproducible, and quantifiable. Furthermore, we show that such Ab is more broadly neutralizing than previously thought.
Materials and Methods
Production and Neutralization of Pseudotype Particles. Pseudotype particles were generated as described (7). Briefly, 293T cells were transfected with expression vectors encoding the viral components, i.e., HCV E1/E2, retroviral core/packaging-component, and GFP/integration signal. Expression plasmids encoding E1E2 glycoproteins of HCV strain H77 (genotype 1a) [ppH77(1a)] (10) or strain J (genotype 1b) [ppJ(1b)] (11) were used. In some experiments, an expression vector encoding the E1E2 glycoproteins of strain H77 from which the hypervariable region (HVR1) of E2 had been deleted [ppH77Δ(1a)] was used (12). The medium was replaced 16 h after transfection. Supernatants containing the pp were harvested 24 h later, filtered through 0.45-μm-pore membranes, and used to infect Huh-7 cells, which had been seeded the day before at a density of 8 × 104 cells per well in 12-well plates. Dilutions of sera to be tested for neutralization were mixed with dilutions of viral supernatants, preincubated at room temperature for 1 h, and added to the target cells. After 3 h, the supernatants were removed and the cells incubated in regular medium for 96 h at 37°C. Input pp transduced ≈2–4% of cells. The infectivity of pp in the presence of sera from healthy seronegative human donors (samples 683 and 691) from France was standardized to 100%. All dilutions of test sera were compared with the same dilutions of these negative control sera. The positive control sample was from a French patient (Vu) who was chronically infected with HCV. Control neutralization experiments were performed by using pp bearing glycoproteins derived from the feline endogenous retrovirus RD114 as described (7).
Anti-HCV Testing. Antibodies to HCV core and nonstructural proteins were determined with a commercially available second-generation ELISA (Abbott). Ab to E1 was tested by ELISA against a truncated form of E1 (amino acids 192–329) of H77 (13), expressed in Huh-7 cells from recombinant vaccinia virus (J.-C.M., unpublished data). Ab to E2 was detected by ELISA against truncated recombinant E2 of strain H77 [amino acids 388–664; differs at six amino acid sites from H77 (13); kindly supplied by Isa Mushahwar (Abbott) (14)]. Ab to HVR1 was measured by ELISA against a synthetic 21-mer peptide representing a truncated form (amino acids 390–410) of the master sequence of the virus quasispecies of strain H77 (4).
Testing for HCV RNA. Total RNA extracted from 100 μl of serum was tested for HCV RNA in RT-PCR by amplification of the 5′ UTR with nested primer pairs as described (13).
Clinical Evaluation. Serial serum samples were tested for alanine aminotransferase by a commercial method (Anilytics, Gaithersburg, MD). Serum samples were stored at –80°C. Informed consent was obtained from the patient, and the housing, maintenance and care of the chimpanzees met or exceeded all relevant guidelines and requirements.
Serum/Plasma Samples. Samples from a chronically infected patient, from chimpanzees that were experimentally infected with HCV, and from a rabbit that was hyperimmunized with a synthetic peptide derived from the HVR1 sequence of HCV were randomly coded and tested for Nt Ab. Based on preliminary data, additional coded samples were tested. The code was not broken until after testing was completed. What follows is a description of the sources of the samples tested.
Patient H (Pt H) was infected with HCV after receipt of transfusions for open-heart surgery in 1977. HCV (strain H77) from this patient is a genotype 1a virus and has been used for numerous studies, including transmission to chimpanzees, infectivity titration of HCV, determination of mutation rate, infectious cDNA clones of HCV, and demonstration of Nt Ab to HCV (refs. 1, 3, and 14–17; Fig. 5A, which is published as supporting information on the PNAS web site).
Chimpanzee (Ch) 1530 was infected with the H77C molecular clone of HCV (ref. 13; J.B., unpublished data). The animal developed acute hepatitis C and became chronically infected (Fig. 5B).
Ch 1494, like Ch 1530, was infected with the H77C molecular clone of HCV (J.B., unpublished data). However, Ch 1494 resolved its infection after experiencing hepatitis and 23 weeks of viremia. The animal was subsequently repeatedly rechallenged with escalating doses of the homologous monoclonal virus, the parent polyclonal wild-type virus, HCV of a different subgenotype (1b), and HCV of a different major genotype (2a). Following each challenge, the chimpanzee had either a transient viremia or, in some cases, sterilizing immunity. Finally, the chimpanzee became chronically infected after rechallenge with the original monoclonal virus with which it was initially infected (Fig. 5C). A pool of three serum samples obtained from Ch 1494 at a time when the animal demonstrated sterilizing immunity was among the sera tested. The pool failed to neutralize HCV in an in vivo neutralization experiment in a separate chimpanzee.
Ch 96A008 was infected with the H77C molecular clone from which the HVR1 had been deleted (18). This chimpanzee developed mild hepatitis and resolved the infection after 17 weeks of viremia. It was rechallenged with the homologous monoclonal virus 1 year later and became chronically infected (Fig. 5D).
Ch 1581 was infected with a different strain of genotype 1a virus. This strain, TN, had been recovered from a patient with fulminant hepatitis C and transmitted previously to a chimpanzee (19). Virus recovered from the chimpanzee was used for initiating infection in Ch 1581 (20). Ch 1581 became chronically infected (Fig. 5E).
Rabbit (Rab) LMF87 was immunized with a synthetic peptide representing the carboxy portion of HVR1 of H77 (4). Immune serum from Rab LMF87, but not preimmunization serum, was shown to neutralize H77 (2, 4).
Results
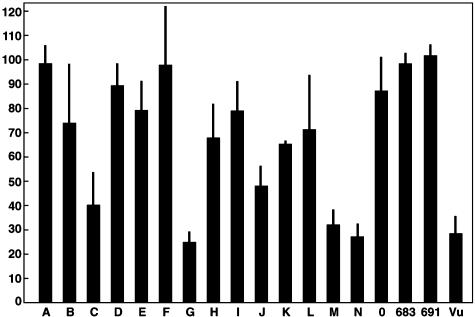
Nt Ab to HCV in Selected Sera/Plasma. Fig. 1 depicts the mean of results obtained with the initial set of sera from the panel of coded samples, at a dilution of 1:50, when tested against two concentrations of a pseudotyped retroviral construct bearing the envelope proteins of HCV strain H77. Samples C, G, J, M, and N (and the positive control, Vu) neutralized the ppH77(1a) (≥50% reduction in percent of GFP-positive cells) in this and other tests when compared with sera from healthy uninfected donors. In contrast, none of the sera neutralized the retroviruses pseudotyped with an irrelevant envelope glycoprotein derived from the feline endogenous virus RD114 (7) (data not shown). Reproducibility of the neutralization assay was acceptable: median standard deviations (as % positive; see Fig. 1) were 9.1 (range 1.6–24.8) for within-test comparisons (Fig. 1) and 11.2 (range 2.2–35.7) between tests (data not shown). These were similar to median standard deviations for duplicate coded samples (B, I; H, K) within (12.8) and between (12.4) tests (data not shown).
Fig. 1.
Results of a single neutralization assay with coded sera (1:50) tested against two concentrations of HCV genotype 1a pp. Shown is the mean percent of cells positive for GFP (determined by FACS analysis), compared with the mean percent infected in the presence of the same dilution of the two normal sera, set to 100%. Samples 683 and 691, normal human sera; Vu, patient with chronic HCV infection; bars, standard deviation of the two determinations.
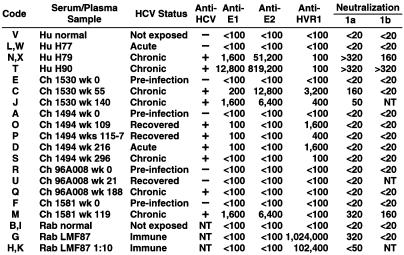
Nt Ab and the Clinical Course of Hepatitis C. Pt H and three chimpanzees (1530, 1494, and 96A008) were infected with HCV strain H77; Ch 1581 was infected with another genotype 1a strain. Nt Ab was not detected before infection, during the acute phase, or during the convalescent phase of resolving HCV infection (Fig. 5 and Table 1). In fact, Nt Ab was detected only in some, but not all, chronic HCV infections. The highest titers of Nt Ab were detected in patient sera, but two of four chronically infected chimpanzees also had relatively high titers. The other two chimpanzees (96A008 and 1494) had previously been challenged 1 and 13 times, respectively, without developing Nt Ab or a chronic infection before being rechallenged with a virus that was identical to the virus with which they were first infected. In both instances, the chimpanzees became chronically infected and failed to develop Nt Ab 2.3 and 1.6 years later, respectively. In Pt H, as well as in the chimpanzees, the chronic phase of infection was characterized by little or no evidence of liver disease, regardless of whether Nt Ab was present. Thus, Nt Ab did not appear to correlate with disease or resolution of the infection and correlated only partially with progression to chronicity.
Table 1. Serum/plasma samples tested under code and results of serologic tests (ELISA and neutralization-reciprocal titers).
Hu, human; NT, not tested.
Nt Ab and Ab to HCV E1, E2, and HVR1. The titers of anti-E1, anti-E2, anti-HVR1, and Nt Ab are shown in Table 1. Antibodies to E1 and E2 were both detected in two chronically infected chimpanzees and in Pt H, but only E1 was detected in one of the two convalescent chimpanzees and neither E1 nor E2 was detected in the other. Anti-HVR1 was detected in one recovered chimpanzee, one chronically infected chimpanzee, and chronically infected Pt H. The highest titers of anti-E1 and anti-E2 were found in Pt H, whereas the highest titers of anti-HVR1 were found in chimpanzees. Pt H had barely detectable Ab (1:100 titer) to the H77 sequence of HVR1 2 years after infection. In contrast, Ch 1530 and Ch 1494 had titers of 1:400 and 1:1,600, respectively, at approximately the same interval postinfection as Pt H. It is not surprising that Ch 96A008 and Ch 1581 did not develop anti-HVR1, because the former was infected with an HVR1 deletion mutant of HCV and the latter was infected with a strain of virus that differed in its HVR1 sequence from that of strain H77.
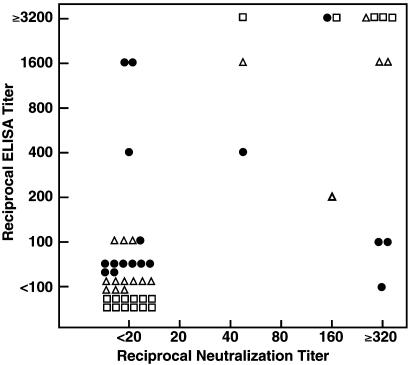
To determine which, if any, of the antibodies tested correlated with Nt Ab, the ELISA titer of each Ab was compared with the titer of Nt Ab for each clinical sample (Fig. 2). There was a strong correlation between the titers of Nt Ab and the ELISA titers of anti-E1 (Spearman rank correlation 0.86; P < 0.001) and anti-E2 (Spearman rank correlation 0.99; P = < 0.001). Similarly, the ELISA titers of anti-E1 and anti-E2 strongly correlated with each other (Spearman rank correlation 0.86; P = < 0.001). In contrast, the titers of anti-HVR1 did not correlate well with Nt Ab titers (Spearman rank correlation 0.32; P = 0.22).
Fig. 2.
Plot of reciprocal titers of Nt Ab on abscissa vs. reciprocal ELISA titers of anti-E1 (▵), anti-E2 (□), and anti-HVR1 (•) on ordinate.
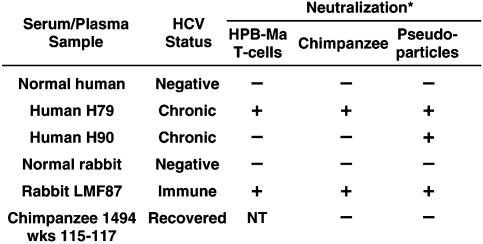
Comparison of Results Obtained with Different Tests for Nt Ab to HCV. We had reported previously that HCV could replicate sparingly in the murine retrovirus-infected human T cell line, HPB-Ma (1). The sample with the greatest infectivity for the cells in vitro was an acute phase plasma sample (H77) obtained from Pt H in 1977, 7 weeks after transfusion and just as he was developing liver enzyme elevations. The patient developed acute hepatitis and his infection became persistent. Plasma H77 has an infectivity titer of 106.5 50% chimpanzee infectious doses (CID50).
Both the in vitro lymphoid cell replication system and the chimpanzee model were used to test the ability of certain serum or plasma samples to neutralize the infectivity of HCV in the H77 plasma. Briefly, the plasma obtained from Pt H 2 years after he became infected (H79) neutralized the homologous H77 virus in both the in vitro and in vivo neutralization systems, but plasma obtained 11 years later from the same patient (H90) did not neutralize the virus in either assay (1, 3). However, it was not possible to identify the neutralization epitopes. Successful neutralization of H77 also was demonstrated in both of these tests with serum from Rab LMF87, which had been hyperimmunized with a peptide representing the carboxy portion of the HVR1 region of strain H77, thus localizing one neutralization epitope to the HVR1. However, this rabbit serum was highly specific for the homologous sequence and it did not neutralize slightly divergent HVR1 variants of the virus (2, 4).
We determined the ability of the same samples to neutralize ppH77(1a) and compared the results with the previous neutralization results. The normal human plasma and normal rabbit serum did not neutralize in any of these three tests, whereas plasma obtained in 1979 from Pt H and Rab LMF87 neutralized in all three tests (Table 1 and Table 2). In addition, a pool of serum samples from Ch 1494, which failed to neutralize strain H77 in a chimpanzee experiment (but was not tested in HPB-Ma cells), also failed to neutralize ppH77(1a) (Tables 1 and 2). The single exception to the uniformity of the neutralization results was sample H90, which strongly neutralized ppH77(1a) but did not neutralize in the T cell and chimpanzee assays, even though all three tests were based on the neutralization of H77-derived viruses (Tables 1 and 2). Thus, the assay based on neutralization of the pp confirmed most, but not all, of the results obtained in previous neutralization assays.
Table 2. Comparison of three assays for detecting Nt Ab to HCV.
*HCV, strain H77, was used for neutralization assays In HPB-Ma cells and chimpanzees; pp bearing H77-derived E1/E2 glycoproteins were used for the third neutralization assay.
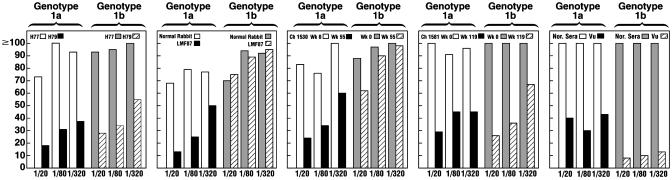
Nt Ab Are Broadly Reactive. We tested dilutions of selected sera against not only ppH77(1a) but also against ppJ(1b). Nt Ab cross-neutralized the ppJ(1b), sometimes almost to the same titer as to the homologous ppH77(1a) (Fig. 3 and Table 1). Interestingly, Nt Ab from Ch 1581, infected with a different 1a virus (TN), cross-neutralized both the ppH77(1a) and ppJ(1b), but sera from Ch 1530, infected with strain H77, neutralized the ppH77(1a) almost as well as did serum from Ch 1581, but it did not cross-neutralize the ppJ(1b). The Rab LMF87 hyperimmune anti-HVR1 strongly neutralized ppH77(1a) but did not neutralize ppJ(1b). This finding is not surprising, because the ppJ(1b) HVR1 sequence differed in approximately two-thirds of its amino acids from that of ppH77(1a). Serum from the positive control patient, Vu, also strongly neutralized both pp but neutralized the ppJ(1b) to a greater extent than it did the ppH77(1a) (Fig. 3). The HCV strain chronically infecting this patient was found to be genotype 1b. Thus, the Nt Ab response to the homologous genotype, whether 1a or 1b, was somewhat stronger than the response to the heterologous genotype (Fig. 3). However, unlike the HCV infections in Pt H and chimpanzees 1530 and 1581, we cannot be sure that patient Vu had been exposed to only one genotype of HCV.
Fig. 3.
Titration of neutralizing anti-HCV titers against pp bearing HCV E1 and E2 glycoproteins of genotype 1a or 1b. Sera/plasma were tested at final dilutions of 1:20, 1:80, and 1:320. Results were adjusted for concentration effects by comparing with comparable dilutions of normal anti-HCV negative sera.
Identification of One or More Additional Neutralization Epitopes on HCV. Hsu et al. (8) have shown that a rat monoclonal Ab (9/27) directed against a synthetic peptide representing the carboxy portion of the HVR1 of the H77 strain of HCV neutralized a pseudotyped virus similar to the one we have described and that a rat monoclonal Ab directed against another region of the E2 glycoprotein of HCV also neutralized the virus. We have also demonstrated that the identical rat monoclonal Ab to HVR1 neutralized our ppH77(1a) and that it did not neutralize ppH77Δ(1a) (an identical pp from which the HVR1 had been deleted). The synthetic peptide used to immunize Rab LMF87 encompassed the 21 C-terminal amino acids of HVR1 (amino acids 390–410) and contained the 12 aa of the peptide from which the 9/27 Ab was generated (amino acids 396–407). Thus, both reagents apparently react with the same or closely overlapping epitopes of HVR1.
To determine whether the pedigreed sera tested herein reacted with epitopes outside of the HVR1 region of the HCV envelope proteins, we tested the panel of sera at a dilution of 1:50 against the ppH77Δ(1a). All of the sera that neutralized ppH77(1a) also neutralized ppH77Δ(1a) with the expected exception of the rabbit hyperimmune anti-HVR1 serum (data not shown). Thus, Nt Ab from patients and chimpanzees persistently infected with HCV reacted with one or more additional epitopes elsewhere in the HCV envelope glycoproteins.
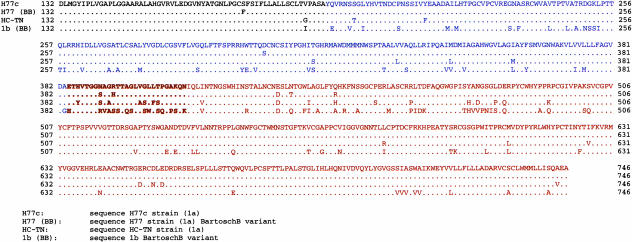
Depicted in Fig. 4 are the predicted amino acid sequences of the E1 and E2 glycoproteins used in this study. The sequence of strain H77 (13) differs from that of ppH77(1a) at one site in E1, two sites in HVR1, and three sites elsewhere in E2. The sites in HVR1 lie within the sequence of the peptide used for hyperimmunization of Rab LMF87 but outside of the sequence used to prepare rat monoclonal Ab 9/27. All three changes elsewhere in E2 lie within neutralization epitopes defined by Hsu et al. (amino acids 412–447) (8) and two of them are in the 16-aa region (amino acids 432–447) associated with strong neutralization of the virus. Genotype 1a strain TN differs from H77 (13) at 7 sites in E1, 7 sites in HVR1, and 18 sites elsewhere in E2 (only 2 within amino acids 432–447).
Fig. 4.
Alignment of predicted amino acid sequence of E1 and E2 of HCV strain H77 (13), E1 and E2 derived from H77 and incorporated into the HCV genotype 1a pseudotype particle (10), E1 and E2 derived from HCV strain TN (genotype 1a) (19, 20), and E1 and E2 derived from HCV strain J (genotype 1b) and incorporated into the HCV genotype 1b pseudotype particle (11). Black, C′ end of core; blue, E1; brown, E2; bold, HVR1 of E2.
The sequence of ppJ(1b) is quite different from the H77 sequence (13) and varies at 32 amino acid positions in E1, 15 amino acid positions in HVR1 (14 and 9 of them within the sequence of the peptide used for immunizing Rab LMF87 and for preparing rat monoclonal Ab 9/27, respectively), and 56 amino acid positions within the rest of E2 (9 of them within the neutralization epitopes defined by other rat monoclonal antibodies and 6 of them within the 16 amino acids comprising the principal identified neutralization epitopes of this region) (8). The lack of cross-neutralization of ppJ(1b) by Rab LMF87 (Fig. 2) is consistent with the significant sequence divergence of HCV 1b in the HVR1 region, but the cross-neutralization observed with sera from the chronic phase of infection of Pt H, patient Vu, and Ch 1581 is not consistent with the degree of genetic heterogeneity between the genotype 1a neutralization epitopes identified by Hsu et al. (8) and the comparable region of ppJ(1b). Thus, one or more previously unrecognized neutralization epitopes may exist in the glycoproteins of HCV.
Discussion
The nature and importance of the humoral immune response to HCV has long been a controversial topic. Because HCV does not grow robustly in cell culture, it has been difficult to demonstrate Nt Ab to the virus and, thus, to determine whether Ab protects against hepatitis C. The demonstration that both experimentally infected chimpanzees and naturally infected humans could be reinfected with HCV suggested that classical humoral immunity did not develop after resolution of hepatitis C (21–23). However, the demonstration by others that the early development of antibodies to the HCV envelope proteins, and especially to the E2 glycoprotein hypervariable region, correlated with recovery from the infection suggested that such antibodies may play a role in clearance of the virus (24–26) and that this region of the HCV envelope was under pressure from the humoral immune system. The demonstration that HCV could replicate, albeit not robustly, in certain lymphoid cells of human origin provided a means for testing for Nt Ab, and Shimizu et al. (1) demonstrated that serum obtained 2 years after onset of HCV infection in a chronically infected patient could neutralize the homologous virus derived from the acute phase of infection and that serum samples obtained >5 years after infection could no longer neutralize the acute-phase virus but could neutralize variants that had emerged subsequently. Farci et al. (3) demonstrated that the same samples that neutralized the virus in vitro also neutralized it when the indicator system was a chimpanzee instead of HPB-Ma cells. However, because HCV replicated so poorly in lymphoid cells and because it never adequately adapted to this substrate, others had trouble reproducing Shimizu's results.
Shimizu et al. (2) and Farci et al. (4) demonstrated that a rabbit hyperimmune serum prepared against a peptide representing the 21 C-terminal amino acids of the HVR1 H77 could neutralize the homologous virus in vitro and in vivo. These studies provided the first identification of a neutralization epitope on the surface of HCV. They also demonstrated that the neutralization was highly strain-specific and that minor variants of HCV bearing divergent sequences in the HVR1 were not neutralized and emerged in the cell culture and the chimpanzee as neutralization escape mutants. The development of a vaccine against HCV, based on stimulating Nt Ab to the HVR1, appeared to be a daunting task (27), but it was not clear whether other, more conserved, neutralization epitopes existed on HCV.
The development of a pseudotyped virus system for studying the interaction between cells and the envelope glycoproteins of HCV has been a long-sought goal. Several groups have reported the construction of pseudotyped vesicular stomatitis virus bearing HCV E1 and E2 glycoproteins that had been modified to contain the transmembrane domain and cytoplasmic tail of the envelope glycoprotein of VSV (28, 29). However, attempts to neutralize such pseudotyped viruses have yielded conflicting results (30). In contrast, retrovirus-based pseudotyped viruses such as those described herein have been useful for defining HCV receptor sites on cells, delineating the mechanism of virus-cell fusion, and detecting Nt Ab that block replication of the virus (7, 8, 12).
In the present study, we found that infection-induced Nt Ab was detected exclusively in chronic HCV infections resulting from the initial exposure: chimpanzees that resolved initial HCV infection and subsequently became chronically infected after a reexposure to the virus failed to develop Nt Ab. Nt Ab from chronic infections were relatively high-titered against the homotypic 1a pp and usually, but not always, cross-neutralized a pseudotyped virus of a different subgenotype (1b). In contrast, Nt Ab directed against a portion of the HVR1 neutralized only the homologous virus. Furthermore, it did not neutralize an HVR1 deletion mutant virus, confirming that the antibodies were directed solely against the HVR1. These and other data indicated that HCV contains at least two neutralization epitopes, as reported by Hsu et al. (8). The fact that sera from Ch 1530 and Ch 1581 both neutralized ppH77(1a), as well as ppH77Δ(1a), indicated that they both contained antibodies to an epitope outside of HVR1, but sera from Ch 1581 also neutralized ppJ(1b), even though its amino acid sequence is quite different in the region encompassing the two recognized neutralization epitopes, suggesting that more than two neutralization epitopes may exist on HCV.
The titer of Nt Ab in the sera tested herein correlated most closely with the titer of anti-E1 and of anti-E2. In contrast, there was a poor correlation with the titer of anti-HVR1. That HVR1 contains a neutralization epitope is unequivocal, but effective neutralization appears to require a very high titer of Ab directed against virtually the homologous HVR1 sequence. Taken together, these data suggest that Nt Ab in clinical samples is directed principally against epitopes that are outside of the HVR1.
It is unclear why plasma H90 neutralized homologous ppH77(1a) but failed to neutralize the homologous virus in two other neutralization systems. In vitro neutralization monitored by inoculation of the virus-Ab mixture into a chimpanzee is a rigorous test for Nt Ab because 100% of the virus must be neutralized. Burton (31) has reported that 100% neutralization of viruses requires 10- to 100-fold more Ab than 50% neutralization. Indeed, in our previous neutralization studies involving chimpanzees, only one of two attempts to neutralize 64 CID50 of H77 by H79 serum was successful, and the failure was deemed a breakthrough, not the emergence of a neutralization escape variant (4). Serum H90 failed to neutralize 64 CID50 of H77 in a single neutralization attempt in a chimpanzee (a second failed neutralization attempt was performed with 640 CID50). The viruses recovered from those failed neutralization tests were shown to be derived from H77, not H90, and similar results were obtained by Shimizu et al. (2). Thus, H90 failed to neutralize in two of three neutralization assays but repeatedly neutralized in the third assay. The reason for this discrepancy is unknown. There are several important differences among the tests. (i) The T cell and chimpanzee-based tests use a quasispecies of H77, although the breadth of the heterogeneity is limited by the small dose of virus used, whereas the pp-based test uses a relatively large dose of monoclonal virus. (ii) The receptor used for binding of the virus to the cell substrate may be different with each test: T cells served as the substrate for one assay, differentiated in vivo hepatocytes presumably were the substrate for the second test, and a continuous cell line of hepatoma cells was the substrate for the third assay [indeed, the pp could not infect cells of lymphoid origin (7)]. (iii) As noted, the chimpanzee-based test requires complete neutralization to be scored positive, and, similarly, the T cell-based assay requires that virtually all of the HCV detected by RT-PCR be neutralized to be scored positive, whereas the pp-based assay requires only that 50% of the ≈103 pp used in the test be neutralized to be scored as positive. Whether one or more of these differences can explain the discrepancy will require additional studies.
The failure of chimpanzees that had resolved HCV infections to develop Nt Ab is surprising but consistent with the observation that chimpanzees (and humans) can be repeatedly infected with HCV. This is also consistent with the hypothesis of Klenerman et al. (32) that the humoral immune response to HCV is a compensatory host response to the failure of cellular immunity to clear the infection.
The observation that chronically infected chimpanzees and humans do make neutralizing anti-HCV and that this Ab is broadly cross-reactive is consistent with the observation that immune globulin prepared from such chronically infected patients can protect against HCV infection (5). This finding has important implications for rethinking the feasibility of developing Ab-based passive and active immunoprophylaxis strategies against HCV, as well as for reassessing the relative importance of humoral and cellular immunity in controlling chronic HCV infections.
Supplementary Material
Acknowledgments
We thank T. Cabezon and H. Alter for sera and S. Chang for excellent clerical assistance. This work was supported by Agence Nationale pour la Recherche contre le SIDA and Institut National de la Santé Et de la Recherche Médicale (INSERM–ATC “Hépatite C”). B.B. was supported by a Marie Curie fellowship from the European Community.
Abbreviations: Ch, chimpanzee; HCV, hepatitis C virus; Nt Ab, neutralizing antibody or antibodies; pp, pseudotype particle(s); Pt H, patient H; Rab, rabbit.
References
- 1.Shimizu, Y. K., Hijikata, M., Iwamoto, A., Alter, H. J., Purcell, R. H. & Yoshikura, H. (1994) J. Virol. 68, 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu, Y. K., Igarashi, H., Kiyohara, T., Cabezon, T., Farci, P., Purcell, R. H. & Yoshikura, H. (1996) Virology 223, 409–412. [DOI] [PubMed] [Google Scholar]
- 3.Farci, P., Alter, H. J., Wong, D. C., Miller, R. H., Govindarajan, S., Engle, R., Shapiro, M. & Purcell, R. H. (1994) Proc. Natl. Acad. Sci. USA 91, 7792–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farci, P., Shimoda, A., Wong, D., Cabezon, T., De Gioannis, D., Strazzera, A., Shimizu, Y., Shapiro, M., Alter, H. J. & Purcell, R. H. (1996) Proc. Natl. Acad. Sci. USA 93, 15394–15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu, M. W., Shen, L., Major, M. E., Feinstone, S. M. & Jong, J. S. (2002) in Viral Hepatitis and Liver Disease, eds. Margolis, H. S., Alter, M. J., Liang, T. J. & Dienstag, J. L. (International Medical Press, Atlanta), pp. 374–377.
- 6.Yu, M. W., Mason, B. L., Guo, Z. P., Tankersley, D. L., Nedjar, S., Mitchell, F. D. & Biswas, R. M. (1995) Lancet 345, 1173–1174. [DOI] [PubMed] [Google Scholar]
- 7.Bartosch, B., Dubuisson, J. & Cosset, F.-L. (2003) J. Exp. Med. 197, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu, M., Zhang, J., Flint, M., Logvinoff, C., Cheng-Mayer, C., Rice, C. M. & McKeating, J. A. (2003) Proc. Natl. Acad. Sci. USA 100, 7271–7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummer, H. E., Maerz, A. & Poumbourios, P. (2003) FEBS Lett. 546, 385–390. [DOI] [PubMed] [Google Scholar]
- 10.Op De Beeck, A., Monserret, R., Duvet, S., Cocquerel, L., Cacan, R., Barberot, B., Le Maire, M., Penin, F. & Dubuisson, J. (2000) J. Biol. Chem. 275, 31428–31437. [DOI] [PubMed] [Google Scholar]
- 11.Thomson, M., Nascimbeni, M., Gonzales, S., Murthy, K., Rehermann, B. & Liang, T. J. (2001) Gastroenterology 121, 1226–1233. [DOI] [PubMed] [Google Scholar]
- 12.Bartosch, B., Vitelli, A., Granier, C., Goujon, C., Dubuisson, J., Pascale, S., Scarselli, E., Cortese, R., Nicosia, A. & Cosset, F.-L. (2003) J. Biol. Chem. 278, 41624–41630. [DOI] [PubMed] [Google Scholar]
- 13.Yanagi, M., Purcell, R. H., Emerson, S. U. & Bukh, J. (1997) Proc. Natl. Acad. Sci. USA 94, 8738–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesniewski, R., Okasinski, G., Carrick, R., Van Sant, C., Desai, S., Johnson, R., Scheffel, J., Moore, B. & Mushahwar, I. (1995) J. Med. Virol. 45, 415–422. [DOI] [PubMed] [Google Scholar]
- 15.Feinstone, S. M., Alter, H. J., Dienes, H. P., Shimizu, Y., Popper, H., Blackmore, D., Sly, D., London, W. T. & Purcell, R. H. (1981) J. Infect. Dis. 144, 588–598. [DOI] [PubMed] [Google Scholar]
- 16.Ogata, N., Alter, H. J., Miller, R. H. & Purcell, R. H. (1991) Proc. Natl. Acad. Sci. USA 88, 3392–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolykhalov, A. A., Agapov, E. V., Blight, K. J., Mihalik, K., Feinstone, S. M. & Rice, C. M. (1997) Science 277, 570–574. [DOI] [PubMed] [Google Scholar]
- 18.Forns, X., Thimme, R., Govindarajan, S., Emerson, S. U., Purcell, R. H., Chisari, F. V. & Bukh, J. (2000) Proc. Natl. Acad. Sci. USA 97, 13318–13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farci, P., Munoz, S. J., Shimoda, A., Govindarajan, S., Wong, D. C., Coiana, A., Peddis, G., Rubin, R. & Purcell, R. H. (1999) J. Infect. Dis. 179, 1007–1011. [DOI] [PubMed] [Google Scholar]
- 20.Thimme, R., Bukh, J., Spangenberg, H. C., Wieland, S., Pemberton, J., Steiger, C., Govindarajan, S., Purcell, R. H. & Chisari, F. V. (2002) Proc. Natl. Acad. Sci. USA 99, 15661–15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince, A. M., Brotman, B., Huima, T., Pascual, D., Jaffery, M. & Inchauspe, G. (1992) J. Infect. Dis. 165, 438–443. [DOI] [PubMed] [Google Scholar]
- 22.Farci, P., Alter, H. J., Govindarajan, S., Wong, D. C., Engle, R., Lesniewski, R. R., Mushahwar, I. K., Desai, S. M., Miller, R. H., Ogata, N., et al. (1992) Science 258, 135–140. [DOI] [PubMed] [Google Scholar]
- 23.Lai, M. E., Mazzoleni, A. P., Argiolu, F., De Virgilis, S., Balestrieri, A., Purcell, R. H., Cao, A. & Farci, P. (1994) Lancet 343, 388–390. [DOI] [PubMed] [Google Scholar]
- 24.Zibert, A., Schreier, E. & Roggendorf, M. (1995) Virology 208, 653–661. [DOI] [PubMed] [Google Scholar]
- 25.Allander, T., Beyene, A., Jacobson, S. H., Grillner, L. & Persson, M. A. (1997) J. Infect. Dis. 175, 26–31. [DOI] [PubMed] [Google Scholar]
- 26.Isaguliants, M. G., Widell, A., Zhang, S. M., Sidorchuk, A., Levi, M., Smirnov, V. D., Santantonio, T., Diepolder, H. M., Pape, G. R. & Nordenfelt, E. (2002) J. Med. Virol. 66, 204–217. [DOI] [PubMed] [Google Scholar]
- 27.Puntoriero, G., Meola, A., Lahm, A., Zucchelli, S., Ercole, B. B., Tafi, R., Pezzanera, M., Mondelli, M. U., Cortese, R., Tramontano, A., et al. (1998) EMBO J. 17, 3521–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagging, L. M., Meyer, K., Owens, R. J. & Ray, R. (1998) J. Virol. 72, 3539–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuura, Y., Tani, H., Suzuki, K., Kimura-Someya, T., Suzuki, R., Aizaki, H., Ishii, K., Moriishi, K., Robison, C. S., Whitt, M. A. & Miyamura, T. (2001) Virology 286, 263–275. [DOI] [PubMed] [Google Scholar]
- 30.Buonocore, L., Blight, K. J., Rice, C. M. & Rose, J. K. (2002) J. Virol. 76, 6865–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton, D. (2002) Nat. Rev. Immunol. 2, 706–713. [DOI] [PubMed] [Google Scholar]
- 32.Klenerman, P., Lechner, F., Kantzanou, M., Ciurea, A., Hengartner, H. & Zinkernagel, R. (2000) Science 289, 2003a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.